Abstract
The cannabinoid cannabidiol (CBD) is characterised in this study as a helper compound against resistant bacteria. CBD potentiates the effect of bacitracin (BAC) against Gram-positive bacteria (Staphylococcus species, Listeria monocytogenes, and Enterococcus faecalis) but appears ineffective against Gram-negative bacteria. CBD reduced the MIC value of BAC by at least 64-fold and the combination yielded an FIC index of 0.5 or below in most Gram-positive bacteria tested. Morphological changes in S. aureus as a result of the combination of CBD and BAC included several septa formations during cell division along with membrane irregularities. Analysis of the muropeptide composition of treated S. aureus indicated no changes in the cell wall composition. However, CBD and BAC treated bacteria did show a decreased rate of autolysis. The bacteria further showed a decreased membrane potential upon treatment with CBD; yet, they did not show any further decrease upon combination treatment. Noticeably, expression of a major cell division regulator gene, ezrA, was reduced two-fold upon combination treatment emphasising the impact of the combination on cell division. Based on these observations, the combination of CBD and BAC is suggested to be a putative novel treatment in clinical settings for treatment of infections with antibiotic resistant Gram-positive bacteria.
Subject terms: Drug discovery, Microbiology, Antimicrobials
Introduction
Since the discovery of penicillin in 1928 by Sir Alexander Fleming, antibiotics have saved millions of lives from fatal infections world-wide. However, with time bacteria have developed mechanisms to escape the effects of antibiotics. The amount of antibiotics used seems to be directly related to development of antibiotic resistance. Similarly, a growing number of multi drug resistant (MDR) bacteria is a result of inadequate intentions to solve the resistance problem and increasing unmet demands for new antibacterial drugs1.
With fewer antibiotics available to treat MDR bacterial infections, the possibility of entering a pre-antibiotic era is looming ahead. Alternative strategies are being explored and helper compounds, also known as antibiotic potentiators or resistant breakers, are attracting attention2. Helper compounds are non-antibiotic compounds functioning as adjuvants for antibiotics to operate in synergy through various mechanisms including efflux pump inhibition, inhibition of enzymes, and changes in membrane permeability, all of which may contribute to increasing the efficacy of a specific antibiotic 3,4. Drugs found to contain helper compound properties are normally used for treatment of non-infectious diseases but may contain some degree of antibacterial activity itself5. Helper compounds are usually associated with side-of-action in the central and peripheral nervous system as local anaesthetics and in psychopharmaceutic practice, where they usually block membrane associated transporter activity5.
Overuse of antibiotics is the main cause of antibiotic resistance. Therefore, by combining an antibiotic with a helper compound less antibiotic is needed in order to achieve bacterial growth inhibition or killing compared to using the antibiotic alone. This strategy may therefore decrease the likelihood of resistance development, and investigations to identify efficient helper compounds are thus important.
Cannabinoids are categorised as either endogenous cannabinoids, which are cannabinoids produced by the human body, or exogenous cannabinoids, which are produced either by plants such as Cannabis sativa or synthetically. Cannabinoids act on the endocannabinoid system of the human body6 consisting of two G-protein coupled receptors (GPCR). These are named cannabinoid type 1 and 2 (CB1 and CB2) receptors, and, depending on the specific cannabinoid, the binding results in either an agonistic or antagonistic downstream effect7. Besides endocannabinoids being ligands for the endocannabinoid receptors, exogenous cannabinoids are also ligands for the receptors. One of the best characterised exogenous ligands is tetrahydrocannabinol (THC). It is a partial agonist for both CB1 and CB2 receptor mediating effects such as analgesia, muscle relaxation, and antiemetic effects, but also results in negative effects such as anxiety, psychosis, and sedation. Another exogenous cannabinoid is cannabidiol (CBD), which has been observed to decrease the adverse negative effects of THC. CBD is an antagonist of both CB1 and CB2 receptor leading to anti-sedative, anti-psychotic, and anxiolytic effects7. However, these are not the only known effects of CBD, as it is able to cause a variety of different effects such as inhibition of cancer cell growth8, neuroprotection in both neuro-degenerative diseases such as Parkinson’s Disease9 and post-ischemia10, and anti-inflammatory effects as in type-1 diabetes11. Not much is known regarding antimicrobial effects of cannabinoids and even less on the mechanism of action. Endocannabinoids and exogenous cannabinoids such as CBD have been observed to inhibit growth of bacteria12–14, yet the use of cannabidiol as an antibiotic adjuvant has not been studied so far.
In the present study, we aim to characterise cannabidiol as a potential helper compound against resistant bacteria in combination with the cyclic peptide antibiotic bacitracin (BAC). BAC is a mixture of related cyclic peptides operating as a bactericidal antibiotic by interfering with the cell wall and interrupting the biosynthesis of the peptidoglycan leading to cell lysis15.
Results
The combination of CBD and BAC is effective against Gram-positive bacteria
Initially, we validated the antimicrobial effect of cannabidiol (CBD) against the Gram-positive bacterium Methicillin-Resistant Staphylococcus aureus (MRSA) as previously published by Appendino and colleagues14 but also for Enterococcus faecalis (E. faecalis), Listeria monocytogenes (L. monocytogenes), and Methicillin-Resistant Staphylococcus epidermidis (MRSE). We found the Minimum Inhibitory Concentration (MIC) to be 4 µg/mL for S. aureus, L. monocytogenes, and the MRSE strain and 8 µg/mL for E. faecalis, indicating that Gram-positive bacteria are sensitive towards CBD (Table 1).
Table 1.
*FIC index indicates synergy when FIC index ≤ 0.5, indifference when 0.5 < FIC index < 4, and antagonism when FIC index > 437.
| Strain | MIC CBD (µg/mL) | MIC BAC (µg/mL) | Fold reduction in MIC of BAC when combined with ½xMIC CBD | FIC Index* |
|---|---|---|---|---|
| MRSA USA300 FPR3757 | 4 | 64 | 32–64 | 0.5 |
| E. faecalis (13–327129) | 8 | 64 | ≥64 | 0.375 |
| L. monocytogenes (EGD) | 4 | 512 | 8 | 0.625 |
| MRSE (933010 3F-16 b4) | 4 | 32 | 64 | 0.5 |
To determine whether CBD would induce a higher susceptibility of BAC in Gram-positive bacteria, MICs of BAC were determined for the four Gram-positive bacteria in the presence of CBD. Remarkably, the MIC of BAC was decreased by 8 to at least 64-fold when combined with 1/2 x MIC of CBD compared to MIC of BAC alone in the different Gram-positive strains (Table 1). Furthermore, the Fractional Inhibitory Concentration (FIC) index was determined for each Gram-positive bacteria. The results showed a FIC index at 0.5 for both MRSA USA300 and MRSE and 0.375 for E. faecalis indicating weak synergistic effect between the compounds CBD and BAC (Table 1). After combining CBD with other antibiotics, both similar and different types, we concluded that CBD had the best effect together with BAC (see Supplementary Figure S1).
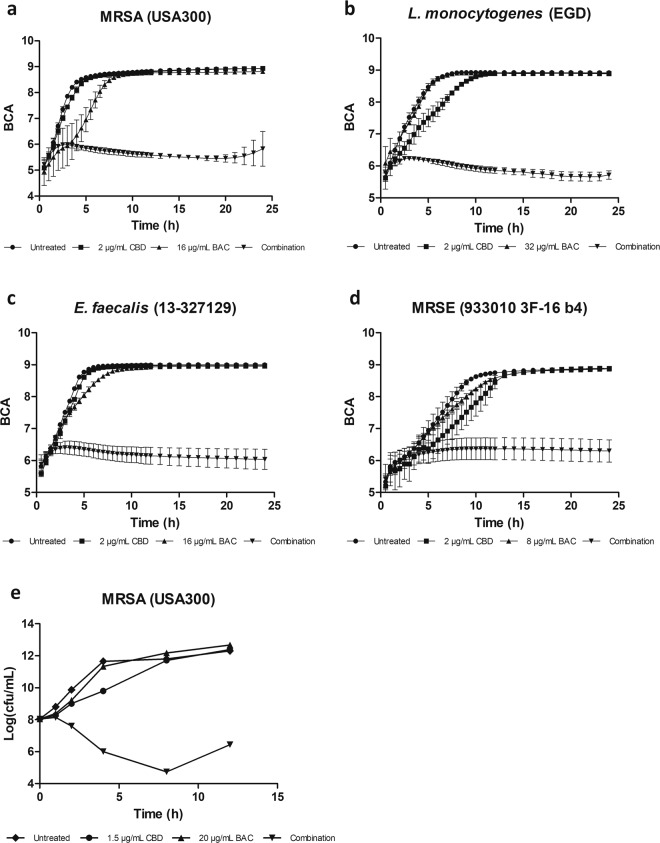
To assess the potentiating effect of CBD on BAC over time, measurements of bacterial growth over 24 hours in the presence of either CBD alone or in combination with BAC were performed. The assessment concentrations of CBD were at 2 µg/mL and 8, 16, and 32 µg/mL for BAC. As seen in Fig. 1a, growth of S. aureus is inhibited by 2 µg/mL CBD and 16 µg/mL BAC combined compared to monotherapies of the individual compounds. The results suggest that CBD can potentiate the antimicrobial effects of BAC. Similarly, growth measurements of E. faecalis, MRSE, and L. monocytogenes on monotherapies and combination (Fig. 1b–d), suggests that the combination of CBD and BAC is useful against other Gram-positive bacteria.
Figure 1.
Growth curves of cannabidiol (CBD) in combination with bacitracin (BAC). Bacterial density (BCA: Background corrected absorption) was measured using an oCelloScope for 24 hours; (a) Methicillin-resistant Staphylococcus aureus USA300 FPR 3757, (b) Enterococcus faecalis (13-327129), (c) Listeria monocytogenes EGD, (d) Methicillin-resistant Staphylococcus epidermidis. (e) Time-kill assay showing the effect of CBD and BAC in monotherapy and combination in MRSA USA300 FPR3757. Viability was monitored by OD600 readings and CFU/mL determinations. Growth experiments were repeated at least three times and the time-kill assay was repeated twice with similar results.
To clarify whether CBD and BAC act in synergy, time-kill assays were performed (Fig. 1e). CBD and BAC reduced the viability by 6 log10 CFU/mL compared to CBD alone. The result shows that a clear synergistic effect in fact exists between CBD and BAC, and that the effect is bactericidal. The slight re-initiation of growth after 8 hours is almost certainly caused by degradation or oxidation of the cannabinoid16. To verify that the decreased CFU upon combination treatment is caused by killing of the bacteria and not due to clustering of the cells, microscopy was performed at the time 1, 2, 4, and 8 hours post treatment (Supplementary Figure S2). Images show no additional clustering of the cells treated with the combination compared to the other treatments.
To further assess the spectrum of use for the combination of CBD and BAC, growth of Gram-negative bacteria upon treatment was measured as well. The Gram-negative bacteria tested were strains of Pseudomonas aeruginosa, Salmonella typhimurium, Klebsiella pneumoniae, and Escherichia coli (Supplementary Figure S3). Experiments for CBD and BAC against the Gram-negative bacteria revealed MIC values above 128 µg/mL for all tested bacteria, presumably due to the outer membrane. In addition, the experiments did not reveal any synergy between CBD and BAC in the concentrations tested, limiting the use of the combination to Gram-positive bacteria.
CBD and BAC causes morphological changes
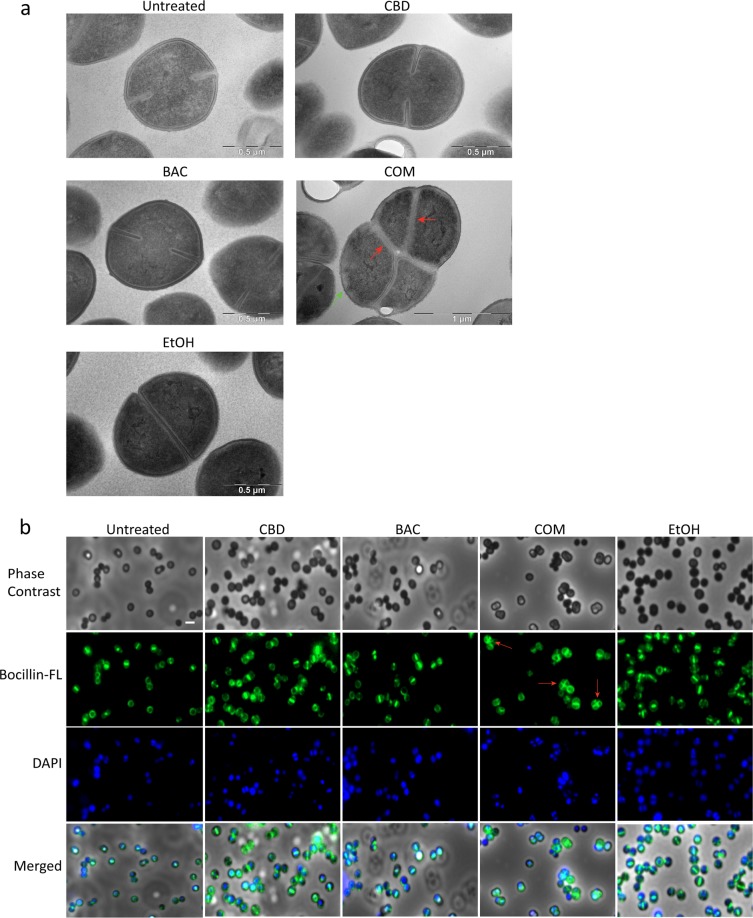
We have established that CBD can potentiate the effect of BAC in Gram-positive bacteria. The next step is to study the mechanism underlying this synergy. First, we looked at the morphological changes of S. aureus USA300 upon exposure to CBD and/or BAC by treating a culture at start exponential phase for 2.5 hours and then performing transmission electron microscopy (TEM) of the cells. Results showed that CBD and BAC alone caused no morphological changes, as they resembled untreated control and EtOH control. However, as seen in Fig. 2a, treatment with the combination of CBD and BAC resulted in large undivided cells with several septa formations or several initiations of septum formation indicating severe defects in cell division (red arrows) and irregularities around cell envelope (green arrow) (Supplementary Figure S4). The result was confirmed by staining the Penicillin Binding Proteins (PBPs) in the membrane using Bocillin-FL, a fluorescence-conjugated penicillin V derivative (Fig. 2b) which showed similar morphology (red arrows). As peptidoglycan synthesis occurs both at the septal and peripheral cell wall, we can observe irregularities concerning the peptidoglycan all over the cell surface17. Upon exposure to either CBD or BAC alone regular septum formations were visualised, however, when treated with the combination, several septa formations appeared for some of the cells as visualised by Bocillin-FL as in the TEM images. This suggests that the combination of CBD and BAC affects the cell envelope causing irregular cell division visualised by multiple septa formations and irregular cell membrane. To study whether the cell division defect is specific for the combination of CBD and BAC, microscopy analysed using higher concentrations of CBD and BAC at 4 and 64 µg/mL, respectively, was performed (Supplementary Information Figure S5). Images show cells with multiple septa upon treatment with 64 µg/mL BAC, indicating that the effect visualised is not specific to the combination of CBD and BAC. However, it further emphasises the CBD mediated potentiation of BAC, since this phenotype did not appear at lower BAC concentration. Adding a higher concentration of CBD did not seem to cause any division defects.
Figure 2.
Morphology of USA300 FPR3757 following treatment with CBD and/or BAC. Cultures were subjected to the drugs for 2.5 hours as described in Methods. (a) Morphology imaged by transmission electron microscopy. TEM overview images are shown in Supplementary Figure S4. (b) Bocillin-FL labelled PBPs visualised using Olympus IX83 fluorescence microscope. Images merged with DAPI stain solution to localise PBPs in the bacteria. Defects in cell division are marked by red arrows and irregularities around cell envelope are marked by a green arrow.
The combination of CBD and BAC decreases autolysis in S. aureus
Since treatment with the combination of CBD and BAC shows impaired cell division, probably causing an arrest in cell division and potentially decreased cell wall turnover, one could speculate if this would result in decreased autolysis as well. Therefore, a Triton X-100 induced autolysis assay was performed. S. aureus USA300 were grown until start exponential phase and stressed for one hour with CBD, BAC, CBD+BAC, EtOH or left untreated. Cells were then washed and incubated with or without triton X-100. As suspected, upon treatment with the combination of 1 µg/mL CBD and 16 µg/mL BAC a significant decreased autolysis was observed (Fig. 3) compared to the untreated control from 90 to 300 minutes except at the 150 minute timepoint, indicating cell division arrest.
Figure 3.
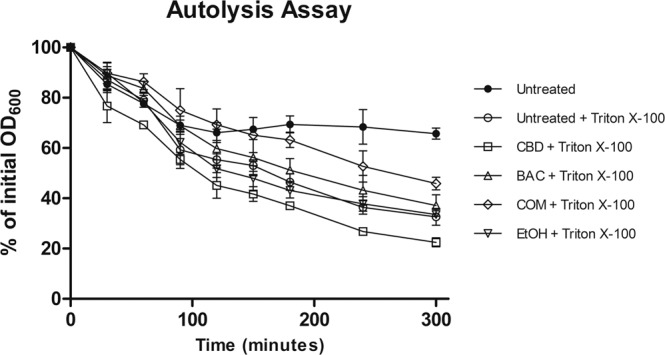
Effects of CBD and BAC on autolysis. Unstimulated and Triton X-100 stimulated autolysis of USA300 grown in BHI to early exponential phase. Statistical analysis by 2-way ANOVA with Bonferroni’s Multiple Comparison Test shows P < 0.05 when comparing Triton X-100 stimulated untreated samples with combination of CBD and BAC samples after 90 minutes, except at the 150 minutes timepoint. Detailed statistical analysis and figure including all controls are shown in Supplementary Figure S6.
The combination of CBD and BAC does not change the cell wall composition or the degree of cross-linking
To further asses the irregularities around the cell envelope and the possible effect on the cell wall biosynthesis, the muropeptide composition of the peptidoglycan was analysed. Peptidoglycan was purified from S. aureus USA300 grown in either CBD, BAC, the combination of CBD and BAC, EtOH or left untreated and further digested using mutanolysin and analysed using HPLC. The chromatogram of purified digested muropeptides revealed the typical pattern of S. aureus18 with the highest peak found in the dimeric fraction (peak 4). Treating the bacteria with both CBD and BAC alone or in combination did not change the pattern of the HPLC chromatogram of the muropeptides (Fig. 4) indicating no change in the muropeptide composition. Even though the relative amount of some of the muropeptide fractions were significantly different, the degree of cross-linking was unaltered when compared to the untreated control (Supplementary Tables S3, S4 and S5). Based on these observations, CBD or the combination of CBD and BAC does not seem to cause changes in the cell wall composition.
Figure 4.
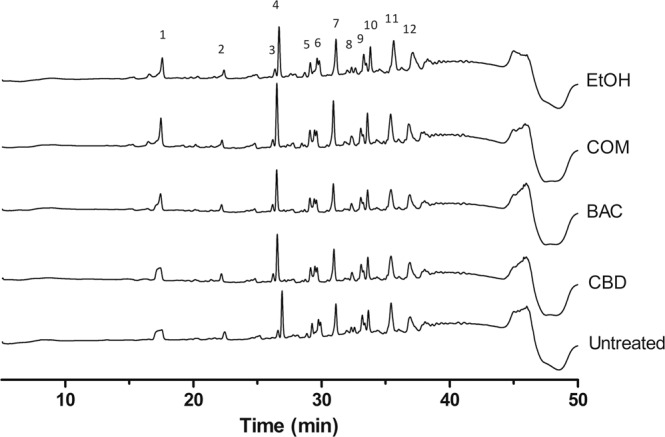
Effect of CBD and BAC on the muropeptide composition of USA300 peptidoglycan. Peptidoglycan was isolated from cultures grown to exponential phase in the absence or presence of CBD or BAC and muropeptide compositions were analysed by HPLC as described in Methods. Muropeptide analysis was performed twice with similar profiles. X-axis show retention time in minutes, Y-axis show milli absorbance units (mAU). One biological replicate is depicted in the figure. Two other replicates are shown in Supplementary Figure S7.
CBD causes depolarisation of the cytoplasmatic membrane
Since the analysis of the muropeptide composition did not reveal any changes, we investigated the membrane. To evaluate effects on the bacterial membrane, the membrane potential was measured when exposed to either CBD, BAC, or the combination of the two (Fig. 5). Accumulation of the fluorescent dye DiOC2(3) in healthy bacteria cells with intact membrane potential results in red fluorescence (high red/green ratio), whereas lower concentrations of the dye, due to membrane potential disruption, exhibit green fluorescence (low red/green ratio), as visualised for the depolarised control using CCCP. Thus, the ratio between red and green fluorescence can reveal the state of the membrane potential. As shown in Fig. 5, even very low concentrations of CBD at 0.1 and 0.2 µg/mL as well as concentration of BAC at 16 µg/mL resulted in a significant lower red/green fluorescence ratio compared to either the untreated or the EtOH control indicating disruption of the membrane potential. However, combining BAC with CBD at either 0.1 or 0.2 µg/mL did not show any significant further membrane depolarisation compared to either CBD or BAC alone.
Figure 5.
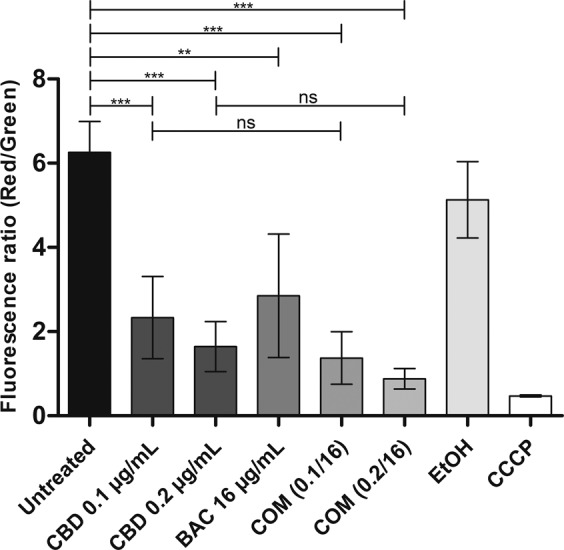
Measurements of membrane potential in USA300 treated with CBD, BAC and the combination using BacLight Bacterial Membrane Potential Kit as described in Methods. The ratio between the mean red fluorescence and mean green fluorescence was calculated as a measure of membrane potential for each sample since the dye will accumulate in unaffected cells thus emitting a red fluorescence, whereas in cells with affected membranes less accumulations will occur resulting in emission of green fluorescence. CCCP is a depolarised control. Statistical analysis was done by one-way ANOVA with Bonferroni’s Multiple Comparison Test and is shown in the upperpart of the figure. ns (not significant) is P-values above 0.05. ** is P-values below or equal to 0.01. *** is P-values below or equal to 0.001.
Transcriptional expression analysis by qPCR
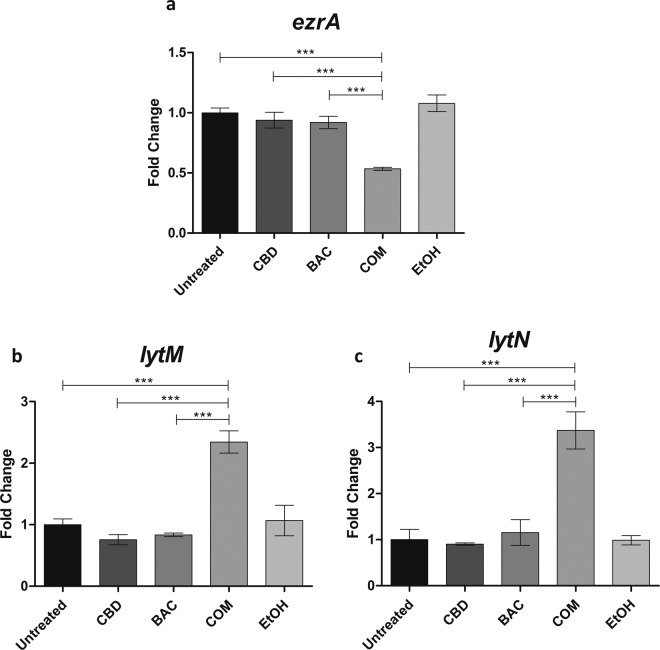
With the shown defects in cell division and septum formation observed by TEM as well as decreased autolysis, we wished to identify whether expression of specific genes encoding proteins important for cell division and formation of the divisome as well as autolysis were affected by CBD and BAC. Similar to the TEM experiment, S. aureus USA300 was grown for 2.5 hours after exposure to CBD and/or BAC in the exponential growth phase. Analysis of transcriptional changes of selected genes (see Supplementary Table S1) involved in the divisome, cell division and autolysis of S. aureus upon treatment was performed by Reverse Transcriptase qPCR. Regarding the divisome and cell division genes, ezrA was shown to be the most regulated gene upon combination treatment at approximately 2-fold down-regulation (Fig. 6a). EzrA is an important multifunctional component of the bacterial cell divisome implicated in peptidoglycan synthesis and assembly of the cell division apparatus19. The results for the remaining genes analysed can be seen in Supplementary Figure S8. These data support the TEM images by showing that CBD in combination with BAC disrupts the cell division. As autolysis was shown to be decreased upon treatment with the combination of CBD and BAC, we studied the expression of selected autolysis genes. Of the genes studied, the expression of lytM and lytN showed to be highly upregulated upon combination treatment at approximately 2.5 (Fig. 6b) and 3.5 (Fig. 6c), respectively, whereas the combination treatment did not seem to affect the expression of the other genes autolysis genes (atl, sle1, lytA)) compared to treatment with either CBD or BAC alone (see Supplementary Information Figure S8).
Figure 6.
qPCR data of the divisome gene ezrA and autolysis genes lytM and lytN studied upon 2.5 hours treatment with either CBD, BAC, combination, EtOH or left untreated in USA300. Data was obtained using the Roche LightCycler 480 Instrument as described in Methods. Experiments were performed in four biological replicates and Cp values were generated in technical replicates. Statistical analysis was done by one-way ANOVA with Bonferroni’s Multiple Comparison Test. *** is P-values below or equal to 0.001.
Discussion
The limited availability of effective therapies against S. aureus has increased the pursuit to discover new treatment strategies. Development of new antibiotics is currently undergoing an innovation gap, while research in the use of helper compound in combination with antibiotics is becoming more intense. It has previously been reviewed that many natural compounds such as flavonoids and compounds from manuka honey and teas have been reported to potentiate antibiotics20–22. In this study, we found that the antibacterial effects of BAC against S. aureus as well as other Gram-positive bacteria can be enhanced by cannabidiol originating from the Cannabis plant. The potentiation was confirmed through MIC determinations, standard growth experiments, fractional inhibitory concentration determination and time-kill assays. As expected, the combination turned out to be ineffective in Gram-negative bacteria, as BAC is a mixture of related cyclic peptides which interrupt cell wall synthesis in Gram-positive bacteria and is probably unable to cross the outer membrane in Gram-negative bacteria. BAC interferes with the dephosphorylation of bactoprenol (C55-isoprenyl pyrophosphate); a membrane lipid-carrier that transports peptidoglycan-precursors across the membrane for peptidoglycan biosynthesis23,24. The use of BAC in combination with other compounds against S. aureus has been studied before such as in combination with colistin25 and alkyl gallates26,27. Colistin is believed to damage the cell membrane thus increasing entry of BAC into the cell or by increasing availability of divalent ions such as Zn2+. This is important for the functionality of BAC25, whereas the mechanism underlying the alkyl gallate mediated potentiation of bacitracin is unknown. However, it has been shown to bind the bacterial membrane affecting the membrane integrity suggesting similar mechanism for synergy as suggested for colistin28.
The use of CBD and other cannabinoids as antibacterial agents was first described in 1976 by Van Klingeren and Ten Ham13 and again in 2008 by Appendino and colleagues14, however, since then very little has been published on this topic. CBD is a quite effective antimicrobial compound with a MIC value of 4 µg/mL against S. aureus USA300 and other Gram-positive bacteria. Appendino and colleagues14 found MIC values of CBD extracted from powdered plant material in the 0.5-1 µg/mL range towards various drug-resistant strains of S. aureus. However, the mechanism or how CBD and other cannabinoids affects the bacteria has not been studied so far. Endogenous endocannabinoids as anandamide (AEA) and endocannabinoid-like arachidonoyl serine (AraS) has been shown to contain poor antimicrobial properties but have a pronounced dose-dependent inhibitory effects on biofilm formation of all tested MRSA strains12. In this study, we have shown that the cannabinoid CBD is able to potentiate the antibacterial properties of the cell wall targeting BAC. Nevertheless, unlike in the case of the endocannabinoids, we did not find any effect on biofilm formation and breakdown in our experimental setup (Supplementary Figure S9). This may indicate a different mechanism of action for CBD.
Cell imaging is an approach to obtain indications of the mechanism or site of action of an antimicrobial compound. Cells grown in the presence of either CBD or BAC did not reveal any phenotypical changes compared to the untreated or the EtOH control. However, treatment with the combination of CBD and BAC revealed a remarkable phenotype visualised by transmission electron microscopy. The TEM images showed bacteria with several septa causing lack of cell separation during cell cycle and a distorted cell membrane. The lack of cell separation and termination of the cell cycle is consistent with the Triton X-100 induced autolysis assay showing a decrease in autolysis upon combination treatment. We therefore thought to study the expression of genes encoding proteins involved in the autolysis and found the expression of lytM and lytN to be upregulated upon combination treatment. In a study of gene silencing of the major regulator of cell wall metabolism walRK, S. aureus was shown to have similar phenotype as observed in this study; several septa and initiation of septa29. In addition, they found that by increasing the expression of lytM, they could restore the viability of the cells even though the cells had still formed several septa. Based on this, the increased expression of lytM, and perhaps also lytN, might be due to a kind of self-defense mechanism trying to restore the cell viability. Regarding the several septa formation, similar characteristics have been recorded by others as well, e.g. by treating S. aureus with the wall teichoic acid biosynthesis inhibitor targocil 30,31, causing both decreased autolysis and impaired cell division as visualised by several septa formations. In addition, formation of several septa has been visualised by others by creation of gene knockouts or by performing gene silencing of genes important for cell cycle regulation. Pang and colleagues showed this phenotype in a Δnoc strain, lacking a very important cell division regulator32. In addition, Stamsas and colleagues found effects on septum formation in a ΔcozEa strain upon gene silencing of cozEb, encoding proteins which together are important for proper cell division in S. aureus and which interacts with the major cell division protein EzrA33. Furthermore, construction of a conditional ezrA mutant has also shown to cause impaired cell division and several septa formations in S. aureus34. The fact that ezrA is downregulated upon exposure to the combination of CBD and BAC by approximately two-fold, indicates that the combination of CBD and BAC affects the cell division in S. aureus. Steele and colleagues19 showed that S. aureus cells partially depleted of EzrA cannot divide without sufficient levels of EzrA. The authors also showed that EzrA is required for peptidoglycan synthesis. Nevertheless, the combination of CBD and BAC in our experimental setup does not seem to have a noteworthy effect on peptidoglycan synthesis, or at least not on the composition of the peptidoglycan nor the degree of cross-linking, as the muropeptide analysis showed a similar pattern in the chromatograms of the untreated cells as for the CBD and BAC treated cells. However, whether the decreased expression of ezrA is a direct or a secondary effect of the combination treatment is unknown and will be studied further in the future.
The exact mechanism of CBD potentiation of BAC is not yet fully understood; however, it was visualised that the combination did cause cell division complications and envelope irregularities. As mentioned above regarding the combination of colistin and BAC and presumably alkyl gallates, one would argue that CBD could have similar mechanism, that is affecting the membrane as visualised by the membrane potential disruption. This causes either increase of BAC entry into the cell or increased divalent ion availability for BAC. On the other hand, the mechanism of potentiation seems to be specific for bacitracin, since no particular synergy was observed when combining CBD with either dicloxacillin, daptomycin, nisin or tetracycline indicating other mechanisms for CBD mediated potentiation of BAC than an increased uptake due to disrupted membrane.
Conclusion
In this study, we present a putative novel antimicrobial combination for treatment of Gram-positive bacterial infections using the cannabinoid cannabidiol and the cell wall targeting antibiotic bacitracin.
Through growth experiments, it was interestingly found that CBD was able to potentiate the effects of BAC against S. aureus USA300 and other Gram-positive bacteria. However, it was found to be ineffective against Gram-negative bacteria. Upon treatment with the combination of CBD and BAC, it was revealed by TEM that the morphology of the cells had changed compared to cells treated with either CBD or BAC alone or left untreated. The cells showed several septa formations indicating lack of cell separation during cell division causing reduced autolysis, as well as an irregular membrane. In addition to this, a very important cell division gene, ezrA, turned out to be transcriptionally down regulated upon combination treatment. Changes observed in morphology was not caused by compositional changes in the cell wall muropeptide composition. Membrane potential changes for the combination of CBD and BAC compared to either CBD or BAC treatment alone did not reveal the mechanism of action for the combination of CBD and BAC. Future studies are therefore focused on the cell division and cell envelope to identify the mechanism of action.
Methods
Bacteria and growth conditions
The resistant Staphylococcus aureus strain, MRSA USA300 FPR375735, was the main bacterium used throughout this study. MRSA was grown in Brain Heart Infusion (BHI) or Müeller Hinton (MH) media on plate or in liquid cultures with agitation at 37 °C. Additional bacteria Enterococcus faecalis (13-327129), Methicillin-resistant Staphylococcus epidermidis (933010 3F-16 b4), Listeria monocytogenes EGD, Pseudomonas aeruginosa (PA01), Salmonella typhimurium (14028), Klebsiella pneumoniae (CAS55), and Escherichia coli (UTI89) were either grown in BHI, MH or Lysogeny Broth (LB) media on plate or in liquid cultures with agitation at 37 °C. As CBD (Sigma Aldrich) was dissolved in EtOH, a control using same volume of 96% EtOH was constructed.
Minimum inhibitory concentration (MIC) and Fractional Inhibitory concentration (FIC)
The MIC was determined using the broth microdilution method36. The MIC was interpreted as the lowest concentration at which no growth was observed. Briefly, MIC measurements were performed using the MH or BHI medium in 96-well plates (Nunc A/S or Sarstedt). A total volume of 100 μl with a bacterial inoculum of approximately 5 × 105 CFU/mL was incubated with two-fold dilution series of the compound or antibiotic of interest for the MIC determination and incubated at 37 °C for 16–22 hours with agitation. For the FIC index determination, ¼ volume of each compound or liquid media was added to the wells in the 96-well plate and final ½ volume with same bacterial inoculum was added to the wells afterwards. The plate was incubated as mentioned above. The MIC and FIC determination were performed using at least three biological replicates. Growth was determined using a Synergy H1 Plate Reader (BioTek). The FIC index was calculated using this formula:
and can be used to determine presence of synergy between two compounds. MICA and MICB in combination indicate the MIC value of compound A or B, when combined with the other compound (A or B). The FIC index were defined as follow: FICI ≤ 0.5 shows synergy, FICI 0.5-4 shows indifference, and FICI > 4 shows antagonism37.
Growth experiments and time-kill assay
For micro dilution growth experiments, a 96-well plate (Nunc Edge) was prepared with different concentrations of the compounds of interest in MH media. Diluted overnight cultures (OD600 nm of 0.005) were added to each well. The plate was incubated at 37 °C (without agitation) for 24 hours. Using an oCelloScope (BioSense Solutions ApS), the bacterial density was measured over a period of 24 hours using the UniExplorer software. Data retrieved was obtained as Background Corrected Absorption (BCA), calculated by an algorithm enabling determination of bacterial growth kinetics resulting from images taken with the oCelloScope camera38,39. Experiments were performed with at least three biological replicates.
For macro dilution growth experiment, an ON culture was diluted to OD600 0.02 in BHI media in an Erlenmeyer Flask and placed in a water bath at 37 °C with agitation. Bacterial growth was determined by turbidity measurements at OD600 nm.
The time-kill assay was performed as previously described40. Briefly, a culture grown to OD600 0.2 in BHI, was split, and treated with CBD or BAC alone and in combination. An untreated control was included. Viability was monitored by OD600 readings and CFU/mL determinations by spotting 10-fold serial dilutions on MH agar plates. The viability assay was performed twice with similar results.
Transmission electron microscopy (TEM)
Cultures were prepared for TEM according to Thorsing et al.40. Briefly, USA300 was grown in BHI media at 37 °C with agitation from OD600 0.02 to start exponential growth at 0.2. The culture was diluted 5 times in BHI media and split into different flasks. The cultures were either left untreated or treated with 1 µg/mL CBD and/or 16 µg/mL BAC or ethanol. The cells were incubated at 37 °C with agitation for 2 hours and 30 minutes. Treated cells were harvested and the pellet was washed twice in PBS followed by fixation ON in 2% glutaraldehyde diluted in 0.04 M phosphate buffer pH = 7.4. The fixated cells were washed in 0.1 M phosphate buffer pH = 7.4 and the pellet was resuspended in 15% bovine serum albumin and incubated at 20 °C for 1 hour and 15 minutes. The cells were centrifuged and fixed again ON in 2% glutaraldehyde at 4 °C. Fixated cell pellets were cut into pieces and washed three times using 0.1 M phosphate buffer pH 7.4, followed by staining with 1% OsO4 for 60 minutes at 4 °C. The samples were dehydrated using increasing concentrations of ethanol (50–99%) at 4 °C and then embedded in epon TAAB-812. The samples were cut into ultra-thin sections using an ultra-microtome and collected on a nickel grid. The sections were stained using 3% uranyl acetate for 14 minutes at 60 °C followed by a wash using water and then stained using lead citrate for 6 minutes at room temperature. Finally, the samples were washed in 20 mM NaOH and water and then dried. The sections were analysed by transmission electron microscopy using a Philips EM 208 Microscope equipped with a Quemsa TEM CCD camera and an iTEM Digital Imaging Platform software. The experiment was carried out using two biological replicates.
Fluorescence microscopy
Cells were grown and treated as mentioned for transmission electron microscopy. After treatment, cells were washed in PBS and incubated at room temperature with 5 µg/mL Bocillin-FL for 4 minutes followed by wash in PBS. The cells were then incubated with 100 µg/mL DAPI for 4 minutes followed by PBS wash. Stained cells were added to a Poly-L-Lysine treated glass slide and visualised using Olympus IX83 fluorescence microscope with 405 nm for DAPI and 488 nm for Bocillin-FL. Images were processed using ImageJ (NIH).
Autolysis assay
The autolysis assay was performed according to Campbell et al.31. Briefly, ON culture of S. aureus USA300 was diluted in BHI to OD600 0.02 and grown to early exponential phase OD600 0.2 at 37 °C with agitation. The culture was treated with either 1 µg/mL CBD, 16 µg/mL BAC, the combination of CBD and BAC, the solvent EtOH, or left untreated and incubated for one hour at 37 °C degrees with agitation. After incubation, cells were washed in PBS pH 7.2 and pellet was resuspended in 50 mM Tris-HCl with or without 0.05% Triton X-100 and adjusted to OD600 1.0 and incubated at 30 °C with gentle agitation. Turbidity measurements were performed every 30 or 60 minutes for five hours at OD600. The autolysis assay was carried out in three biological replicates.
Muropeptide isolation and analysis by reverse phase HPLC
Muropeptides were isolated according to Kühner et al.18 with minor changes. Briefly, an ON culture of S. aureus USA300 was treated and grown as mentioned above for TEM. After 2.5 hours treatment, the cells were harvested at 10.000x G and the pellet was resuspended in 0.1 M tris/HCl containing 0.25% SDS and heated to 100 °C for 30 minutes. To remove SDS the samples were washed at least 15 times in sterile ddH2O and absence of residual SDS was confirmed according to Heyashi, 197541. The samples were sonicated for 30 minutes in a sonicator bath followed by DNase and RNase treatment using DNase I (15 µg/mL) and RNase A (60 µg/mL) and incubated at 37 °C for 1 hour followed by trypsin digestion (50 µg/mL) for 1 hour at 37 °C. Enzymes were inactivated at 100 °C for 3 minutes. To remove wall teichoic acids, the samples were incubated with 1 M HCl at 37 °C for 4 hours with agitation. The samples were washed to pH = 5–6 and resuspended in digestion buffer. Cell walls were digested with mutanolysin (5000 U/mL) (Sigma) at 37 °C with agitation for 17 hours. The samples were then centrifuged, and supernatant was moved to a fresh tube followed by addition of 50 µL reduction solution containing 10 mg/mL NaBH4 and left at room temperature for 20 minutes with lids open to reduce MurNac. The reaction was stopped using 15 µL of 85% phosphoric acid.
Separation of the samples were carried out with a flow rate of 250 µL/min using an Agilent 1260 Infinity RP-HPLC (Agilent Technologies) and an Xselect Peptide CSH C18, 130 A, 3.5 µm, 2.1 mm ×150 mm column (Waters) heated to 52 °C. The peptides were eluted by a gradient of solvent B (0.06% trifluoroacetic acid (TFA)/35% methanol) and solvent A (0.06% TFA) as described18. The muropeptide isolation and subsequent separation was carried out in three biological replicates.
Cross-linking was calculated as -described in40:
Membrane potential
The bacterial membrane potential was analysed using BacLight Bacterial Membrane Potential Kit (ThermoFisher) and the experiment was performed according to the manufacturer’s recommendations. Briefly, an ON cultures of USA300 was diluted to OD600 0.02 and grown to 0.3 in BHI media at 37 °C with agitation. The culture was diluted 1:100 in PBS and split into separate tubes. The samples including an ethanol control were either left untreated or treated with either 5 µM CCCP (depolarised control), 0.1 or 0.2 µg/mL CBD and/or 16 µg/mL BAC and incubated at room temperature for 5 minutes. Following the incubation, the dye diOC2(3) was added to a final concentration of 0.03 mM in each sample except for an unstained control and left to stain for at least 15 minutes at room temperature protected from light. The samples were analysed using BD FACSAria II flow cytometer and FACSDiva Version 6.1.2 software. For each sample, 104 events were analysed using a laser emitting at 488 nm and fluorescence was collected in the red and green channels. The experiment was carried out in three biological replicates.
Isolation of total cellular RNA, DNase treatment and cDNA synthesis
Cultures of S. aureus USA300 were prepared, diluted and treated as described for TEM. After 2.5 hours of treatment, samples were harvested for RNA purification. RNA was purified by a hot acid-phenol procedure42 using FastPrep and FastPrep beads and treated with 0.2 units of DNase I (NEB) for 15 minutes at 37 °C followed by heat inactivation. cDNA was synthesised using the High-Capacity cDNA Reverse Transcriptase Kit (ThermoFisher Scientific) and was performed according to the manufacturer’s recommendations. Briefly, the cDNA synthesis was carried out at 25 °C for 10 minutes followed by 37 °C for 45 minutes and finally the enzyme was inactivated at 85 °C for 5 minutes. A No Template Control (NTC) and a No Reverse Transcriptase Control (NRT) was created as well. The experiment was performed using four biological replicates.
Quantitative polymerase chain reaction
Reverse Transcriptase qPCR was performed using the Roche LightCycler 480 Instrument. For each reaction, 5 µL RealQ Plus Master Mix Green 2×(Ampliqon), 0.75 µL 10 µM primers (Supplementary Table S1), 1 µL sterile ddH2O and 2.5 µL sample were used. Each reaction was made in technical duplicates. Pre-incubation was set at 95 °C for 15 minutes, the amplification cycle was set at 95 °C for 15 seconds followed by 60 °C for 45 seconds and then 72 °C for 45 seconds for 45 cycles. A melting curve was created in the end of the procedure by heating to 95 °C for 5 seconds 60 °C for 20 seconds and then 97 °C continuously. Data was retrieved using LightCycler 480 Software version 1.5.1.62. Data were normalised using gyrB as a reference gene.
Statistical analysis
P-values were calculated by a one-way ANOVA with Bonferroni’s Multiple Comparison Test for qPCR and membrane potential data. A 2-way ANOVA with Bonferroni’s Multiple Comparison Test was used for the autolysis assay. Significance was determined based on the P-values visualised in the figures. ns (not significant) is P-values above 0.05. * is P-values below or equal to 0.05. ** is P-values below or equal to 0.01. *** is P-values below or equal to 0.001.
Supplementary information
Acknowledgements
We thank Tina Kronborg for excellent technical assistance in the laboratory and Professor Birgitte Kallipolitis for scientific discussions.
Author contributions
C.S.W. and J.K.K. designed the experiments. C.S.W. performed the experiments, analysed data, and prepared figures. C.S.W. and J.K.K. performed interpretation of data. P.H. and J.K.K. supervised the research. C.S.W. and J.K.K. wrote the manuscript. PH edited the manuscript. All authors approved the final manuscript.
Competing interests
C.S.W. and J.K.K. are inventors on the patent application WO 2018/234301 A1 entitled “Bacitracin and/or daptomycin combined with cannabidiol for treatment of bacterial infections”.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-60952-0.
References
- 1.Zaman SB, et al. A Review on Antibiotic Resistance: Alarm Bells are Ringing. Cureus. 2017;9:e1403. doi: 10.7759/cureus.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tyers M, Wright GD. Drug combinations: a strategy to extend the life of antibiotics in the 21st century. Nat. reviews. Microbiology. 2019;17:141–155. doi: 10.1038/s41579-018-0141-x. [DOI] [PubMed] [Google Scholar]
- 3.Douafer H, Andrieu V, Phanstiel O, Brunel JM. Antibiotic Adjuvants: Make Antibiotics Great Again! J. Medicinal Chem. 2019;62:8665–8681. doi: 10.1021/acs.jmedchem.8b01781. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez-Bello C. Antibiotic adjuvants - A strategy to unlock bacterial resistance to antibiotics. Bioorg Med. Chem. Lett. 2017;27:4221–4228. doi: 10.1016/j.bmcl.2017.08.027. [DOI] [PubMed] [Google Scholar]
- 5.Veje CT, Willatzen M, Hendricks O, Pagès J-M, Kristiansen J. Population Dynamics Approach for the Study of Synergetic Coupling between Antibiotic and Helper Compounds. Computational Mol. Biosci. 2012;2:1–6. doi: 10.4236/cmb.2012.21001. [DOI] [Google Scholar]
- 6.Pacher P, BÁTkai S, Kunos G. The Endocannabinoid System as an Emerging Target of Pharmacotherapy. Pharmacol. Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howard P, Twycross R, Shuster J, Mihalyo M, Wilcock A. Cannabinoids. J. Pain. Symptom Manag. 2013;46:142–149. doi: 10.1016/j.jpainsymman.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Massi P, Solinas M, Cinquina V, Parolaro D. Cannabidiol as potential anticancer drug. Br. J. Clin. Pharmacology. 2013;75:303–312. doi: 10.1111/j.1365-2125.2012.04298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lastres-Becker I, Molina-Holgado F, Ramos JA, Mechoulam R, Fernández-Ruiz J. Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: Relevance to Parkinson’s disease. Neurobiol. Dis. 2005;19:96–107. doi: 10.1016/j.nbd.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Braida D, et al. Post-ischemic treatment with cannabidiol prevents electroencephalographic flattening, hyperlocomotion and neuronal injury in gerbils. Neurosci. Lett. 2003;346:61–64. doi: 10.1016/S0304-3940(03)00569-X. [DOI] [PubMed] [Google Scholar]
- 11.Weiss L, et al. Cannabidiol lowers incidence of diabetes in non-obese diabetic mice. Autoimmunity. 2006;39:143–151. doi: 10.1080/08916930500356674. [DOI] [PubMed] [Google Scholar]
- 12.Feldman M, Smoum R, Mechoulam R, Steinberg D. Antimicrobial potential of endocannabinoid and endocannabinoid-like compounds against methicillin-resistant Staphylococcus aureus. Sci. Rep. 2018;8:17696. doi: 10.1038/s41598-018-35793-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Klingeren B, Ten Ham M. Antibacterial activity of delta9-tetrahydrocannabinol and cannabidiol. Antonie Van. Leeuwenhoek. 1976;42:9–12. doi: 10.1007/bf00399444. [DOI] [PubMed] [Google Scholar]
- 14.Appendino G, et al. Antibacterial cannabinoids from Cannabis sativa: A structure-activity study. J. Nat. Prod. 2008;71:1427–1430. doi: 10.1021/Np8002673. [DOI] [PubMed] [Google Scholar]
- 15.Siewert G, Strominger JL. Bacitracin: an inhibitor of the dephosphorylation of lipid pyrophosphate, an intermediate in the biosynthesis of the peptidoglycan of bacterial cell walls. Proc. Natl Acad. Sci. U S Am. 1967;57:767–773. doi: 10.1073/pnas.57.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mechoulam, R. & Hanus, L. Cannabidiol: an overview of some chemical and pharmacological aspects. Part I: chemical aspects. Chem Phys Lipids121, 35-43, doi:Pii S0009-3084(02)00144-5Doi 10.1016/S0009-3084(02)00144-5 (2002). [DOI] [PubMed]
- 17.Lund VA, et al. Molecular coordination of Staphylococcus aureus cell division. elife. 2018;7:e32057. doi: 10.7554/eLife.32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kühner, D., Stahl, M., Demircioglu, D. D. & Bertsche, U. From cells to muropeptide structures in 24 h: Peptidoglycan mapping by UPLC-MS. (2014). [DOI] [PMC free article] [PubMed]
- 19.Steele VR, Bottomley AL, Garcia-Lara J, Kasturiarachchi J, Foster SJ. Multiple essential roles for EzrA in cell division of Staphylococcus aureus. Mol. Microbiology. 2011;80:542–555. doi: 10.1111/j.1365-2958.2011.07591.x. [DOI] [PubMed] [Google Scholar]
- 20.Maddocks SE, Jenkins RE. Honey: a sweet solution to the growing problem of antimicrobial resistance? Future Microbiol. 2013;8:1419–1429. doi: 10.2217/fmb.13.105. [DOI] [PubMed] [Google Scholar]
- 21.Malongane F, McGaw LJ, Mudau FN. The synergistic potential of various teas, herbs and therapeutic drugs in health improvement: a review. J. Sci. Food Agric. 2017;97:4679–4689. doi: 10.1002/jsfa.8472. [DOI] [PubMed] [Google Scholar]
- 22.Miklasinska-Majdanik M, Kepa M, Wojtyczka RD, Idzik D, Wasik TJ. Phenolic Compounds Diminish Antibiotic Resistance of Staphylococcus Aureus Clinical Strains. Int. J. Environ. Res. Public. Health. 2018;15:18. doi: 10.3390/ijerph15102321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stone KJ, Strominger JL. Mechanism of action of bacitracin: complexation with metal ion and C 55 -isoprenyl pyrophosphate. Proc. Natl Acad. Sci. U S Am. 1971;68:3223–3227. doi: 10.1073/pnas.68.12.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Storm DR. Mechanism of bacitracin action: a specific lipid-peptide interaction. Ann. N. Y. Acad. Sci. 1974;235:387–398. doi: 10.1111/j.1749-6632.1974.tb43278.x. [DOI] [PubMed] [Google Scholar]
- 25.Si W, Wang L, Usongo V, Zhao X. Colistin Induces S. aureus Susceptibility to Bacitracin. Front. Microbiol. 2018;9:2805. doi: 10.3389/fmicb.2018.02805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J-C, Jeon B. Novel adjuvant strategy to potentiate bacitracin against MDR MRSA. J. Antimicrobial Chemotherapy. 2016;71:1260–1263. doi: 10.1093/jac/dkv463. [DOI] [PubMed] [Google Scholar]
- 27.Oh E, Bae J, Kumar A, Choi H-J, Jeon B. Antioxidant-based synergistic eradication of methicillin-resistant Staphylococcus aureus (MRSA) biofilms with bacitracin. Int. J. Antimicrobial Agents. 2018;52:96–99. doi: 10.1016/j.ijantimicag.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Krol E, et al. Antibacterial activity of alkyl gallates is a combination of direct targeting of FtsZ and permeabilization of bacterial membranes. Front. Microbiology. 2015;6:ARTN 390. doi: 10.3389/fmicb.2015.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delaune A, et al. Peptidoglycan Crosslinking Relaxation Plays an Important Role in Staphylococcus aureus WalKR-Dependent Cell Viability. PLoS One. 2011;6:ARTN e17054. doi: 10.1371/journal.pone.0017054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell J, et al. Synthetic lethal compound combinations reveal a fundamental connection between wall teichoic acid and peptidoglycan biosyntheses in Staphylococcus aureus. ACS Chem. Biol. 2011;6:106–116. doi: 10.1021/cb100269f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell J, et al. An antibiotic that inhibits a late step in wall teichoic acid biosynthesis induces the cell wall stress stimulon in Staphylococcus aureus. Antimicrob. Agents Chemother. 2012;56:1810–1820. doi: 10.1128/AAC.05938-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pang T, Wang X, Lim HC, Bernhardt TG, Rudner DZ. The nucleoid occlusion factor Noc controls DNA replication initiation in Staphylococcus aureus. PLOS Genet. 2017;13:e1006908. doi: 10.1371/journal.pgen.1006908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stamsas GA, et al. CozEa and CozEb play overlapping and essential roles in controlling cell division in Staphylococcus aureus. Mol. microbiology. 2018;109:615–632. doi: 10.1111/mmi.13999. [DOI] [PubMed] [Google Scholar]
- 34.Jorge AM, Hoiczyk E, Gomes JP, Pinho MG. EzrA Contributes to the Regulation of Cell Size in Staphylococcus aureus. PLoS One. 2011;6:e27542. doi: 10.1371/journal.pone.0027542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diep BA, et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 2006;367:731–739. doi: 10.1016/s0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- 36.Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008;3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 37.Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrobial Chemotherapy. 2003;52:1–1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 38.Kjeldsen, T. S. B., Sommer, M. O. A. & Olsen, J. E. Extended spectrum β-lactamase-producing Escherichia coli forms filaments as an initial response to cefotaxime treatment. BMC Microbiol15, 10.1186/s12866-015-0399-3 (2015). [DOI] [PMC free article] [PubMed]
- 39.Fredborg M, et al. Real-Time Optical Antimicrobial Susceptibility Testing. J. Clin. Microbiology. 2013;51:2047–2053. doi: 10.1128/jcm.00440-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thorsing, M. et al. Thioridazine Induces Major Changes in Global Gene Expression and Cell Wall Composition in Methicillin-Resistant Staphylococcus aureus USA300. Plos One8, 10.1371/journal.pone.0064518 (2013). [DOI] [PMC free article] [PubMed]
- 41.Hayashi K. A rapid determination of sodium dodecyl sulfate with methylene blue. Anal. Biochem. 1975;67:503–506. doi: 10.1016/0003-2697(75)90324-3. [DOI] [PubMed] [Google Scholar]
- 42.Moazed D, Stern S, Noller HF. Rapid chemical probing of conformation in 16 S ribosomal RNA and 30 S ribosomal subunits using primer extension. J. Mol. Biol. 1986;187:399–416. doi: 10.1016/0022-2836(86)90441-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.