Abstract
Treatment guidelines for type 2 diabetes (T2D) recommend avoidance of hypoglycemia and less stringent glycemic control in older patients. We examined the relation of glycemic control to glucose-lowering medications use in a cohort of patients aged>80 years with a diagnosis of T2D and a hospital admission in the Capital Region of Denmark in 2012–2016. We extracted data on medication use, diagnoses, and biochemistry from the hospitals’ records. We identified 5,172 T2D patients with high degree of co-morbidity and where 17% had an HbA1c in the range recommended for frail, comorbid, older patients with type 2 diabetes (58–75 mmol/mol (7.5–9%)). Half of the patients (n = 2,575) had an HbA1c <48 mmol/mol (<6.5%), and a majority of these (36% of all patients) did not meet the diagnostic criteria for T2D. Of patients treated with one or more glucose-lowering medications (n = 1,758), 20% had HbA1c-values <42 mmol/mol (<6%), and 1% had critically low Hba1c values <30 mmol/mol (<4.9%), In conclusion, among these hospitalized T2D patients, few had an HbA1c within the generally recommended glycemic targets. One third of patients did not meet the diagnostic criteria for T2D, and of the patients who were treated with glucose-lowering medications, one-fifth had HbA1c-values suggesting overtreatment.
Subject terms: Type 2 diabetes, Epidemiology
Introduction
For patients with type 2 diabetes, it is important to maintain blood glucose levels as close to normal as possible in order to reduce the risk of micro- and macrovascular complications1–4. Treatment should, however, be individualized according to comorbidities, disease duration, risk of adverse events and in particular hypoglycemia, life expectancy as well as the patient’s own preferences, resources and support system1. Elderly people with type 2 diabetes will generally have co-existing illness and relatively few resources5. Life expectancy will often be shorter than the time it takes for micro- and macrovascular disease complications to develop and manifest6,7. This is in contrast to the potential adverse effects of glucose-lowering medications that often appear in the short term. Hypoglycemia is the most important example of an acute and potentially fatal adverse effect to which elderly are particularly vulnerable8–15. Less effective counterregulatory mechanisms, decreased drug elimination, motor and cognitive impairment as well as unspecific/uncharacteristic symptoms all contribute to the heightened risk in elderly patients16. Thus, the overall goal with treatment individualization should be to weigh the typically long-term benefits vs. therapy burden and risk of adverse events on the shorter term7,15,17,18. Available evidence from the few clinical trials enrolling elderly patients with type 2 diabetes support that the benefits of intensive glycemic control targeting near-normal glycemia may not outweigh potential risks in this population8,19–22. This is also reflected in several international guidelines which generally advocate a less stringent treatment approach for older people with coexisting illnesses. An HbA1c target of 58–75 mmol/mol (7.5–9%) after pharmacological intervention, is generally recommended1,6,7,17. Recent studies have, however, questioned the extent to which these recommendations have been adopted and implemented in clinical practice12,23,24.
Previous studies examining trends in use, effects (glycemic control as measured by HbA1c) and harms (e.g. hypoglycemia) of glucose-lowering medications have predominantly focused on the general type 2 diabetes population25–31. This study focuses on a cohort of patients aged 80 years or older with a diagnosis of type 2 diabetes and a hospital-based health record in the period 2012–2016. The main objective was to examine glycemic control in relation to use of glucose-lowering medications; secondary objectives included characterizing the patient cohort with regards to comorbidity, drug administration and biochemical status at the time of hospital admission.
Results
Patient characteristics and admission diagnoses
A total of 5,172 patients with type 2 diabetes were included in the study (Table 1). The median age was 84 years (IQR 82–88 years) and 54% of the patients were female. Based on Body Mass Index (BMI), 41% were normal weight (BMI 18.5–25 kg/m2) and 55% were overweight or obese (BMI >25 kg/m2) (Table 1). Regarding biochemical status, LDL-cholesterol was >2,5 mmol/L for 25% of the patients. The estimated glomerular filtration rate (eGFR) was ≤60 mmol/L for 57% of the patients and 56% had a hemoglobin below the reference level calculated for men and women respectively (Table 1). The median duration of hospital admission was four days with pneumonia being the most common cause of admission (4%, n = 211). Diabetes related diagnoses were registered as the primary cause of admission for 2% (n = 78) of all patients and 1% (n = 70) had hypoglycemia as the primary cause of admission.
Table 1.
Patient characteristics for all patients with type 2 diabetes ≥80 years included in the study.
| n (%) | |
|---|---|
| Unique patients, number | 5172 (100%) |
| Gender | |
| Male | 2392 (46%) |
| Female | 2780 (54%) |
| Age in years (median, IQR) | 84 (81.5–87.6) |
| Days of admission (median, IQR) | 4 (1–9) |
| BMI (n = 4139) | |
| <18.5 | 163 (4%) |
| 18.5 – <25 | 1685 (41%) |
| 25 – <30 | 1454 (35%) |
| 30 – <40 | 766 (19%) |
| ≥40 | 71 (2%) |
| Charlson Comorbidity Index | |
| 0 | 0 (0%) |
| 1 | 342 (7%) |
| 2 | 602 (12%) |
| >2 | 4228 (82%) |
| HbA1c (mmol/mol) | |
| <30 | 57 (1%) |
| 30–41 | 1304 (25%) |
| 42–47 | 1214 (23%) |
| 48–52 | 757 (15%) |
| 53–57 | 544 (11%) |
| 58–74 | 891 (17%) |
| ≥75 | 405 (8%) |
| LDL (mmol/L) | (n = 2983) |
| <1.8 | 1379 (46%) |
| 1.8–2.5 | 856 (29%) |
| >2.5 | 748 (25%) |
| Total cholesterol (mmol/L) | (n = 2222) |
| <5 | 1820 (82%) |
| ≥ 5 | 402 (18%) |
| HDL (mmol/L) | (n = 3105) |
| ≤1 | 842 (27%) |
| >1 | 2263 (73%) |
| Creatinine (normal range men: 50–90, women: 60–105) (µmol/L) | (n = 5154) |
| Within range | 2386 (46%) |
| Above range | 2541 (49%) |
| Under range | 227 (4%) |
| eGFR (mL/min/1,73m2) | (n = 4221) |
| ≤60 | 2426 (57%) |
| >60 | 1795 (43%) |
| Haemoglobin (normal range women: 7.3–9.5, men: 8.3–10.5) (mmol/L) | (n = 5118) |
| Within range | 2191 (43%) |
| Above range | 64 (1%) |
| Under range | 2863 (56%) |
| TSH (normal range 0.35–4.0 or 0.65–4.80) (IU/L) | (n = 3862) |
| Within range | 3278 (85%) |
| Above range | 295 (8%) |
| Under range | 289 (7%) |
Values are displayed in absolute numbers, percentages and median (inter-quartile range). For haemoglobin and creatinine, the reference values are displayed for men and women separately.
Comorbidities
The majority (82%, n = 4,228) of patients had a high level of comorbidity with a value >2 on the Charlson Comorbidity Index (Table 1). Detailed data on the cognitive status of the patients was not available, but 16% had a diagnosis of dementia (Table 2). Hypertension was the most commonly registered comorbidity (71%), followed by congestive heart failure (32%), peripheral vascular disease (18%) and previous myocardial infarction (13%) (Table 2).
Table 2.
Number of patients with co-morbidities, using all available data for each individual.
| Co-morbidities | n (%) |
|---|---|
| Hypertension | 3648 (71%) |
| Atrial fibrillation | 1990 (38%) |
| Congestive heart failure | 1650 (32%) |
| Cerebrovascular disease | 1547 (30%) |
| Chronic pulmonary disease | 1207 (23%) |
| Moderate to severe renal disease | 1079 (21%) |
| Non-skin malignancy | 984 (19%) |
| Peripheral vascular disease | 930 (18%) |
| Dementia | 831 (16%) |
| Myocardial infarction | 689 (13%) |
| Thyroid disorders | 524 (10%) |
| Depression | 455 (9%) |
| Peptic ulcer disease | 400 (8%) |
| Rheumatologic disease | 137 (3%) |
| Metastatic solid tumor | 124 (2%) |
| Moderate or severe liver disease | 41 (1%) |
| Schizophrenia | 10 (0%) |
Glycemic control
The distribution of HbA1c values is shown in Fig. 1. Most patients (91%, n = 4,710) had an HbA1c between 30–75 mmol/mol (4.9–9%). Half of the patients (n = 2,575) had an HbA1c <48 mmol/mol (<6.5%), and 26% (n = 1,361) had an HbA1c <42 mmol/mol (<6%). In the other end of the spectrum, 8% (n = 405) had HbA1c-values >75 mmol/mol (>9%) (Table 1). A total of 17% (n = 891) had an HbA1c between 58–75 mmol/mol (7.5–9%), i.e. within the interval recommended for elderly, comorbid patients with overt type 2 diabetes (Table 1).
Figure 1.
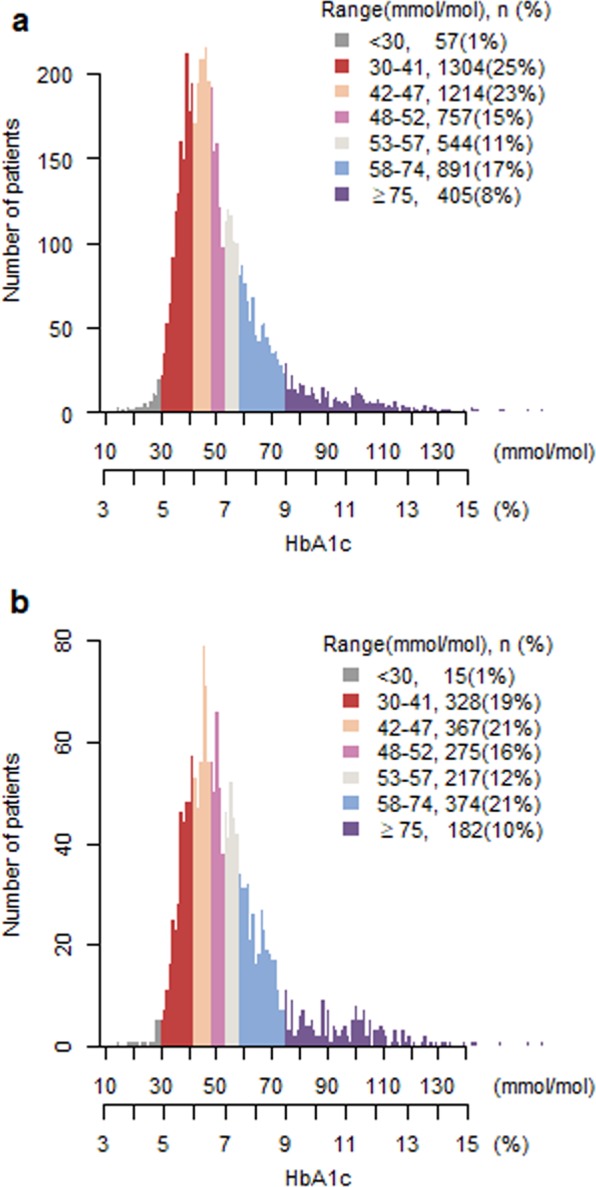
Distribution of individual HbA1c-values for patients with type 2 diabetes >80 years with and without glucose-lowering medications divided into HbA1c-categories. HbA1c-values on the x-axis are displayed in both percentage and in mmol/mol. (a) Displays HbA1c-values for all patients (n = 5172). (b) Displays HbA1c-values for patients treated with glucose-lowering medications (n = 1758).
Glucose-lowering medications
Close to one third of patients (34%, n = 1,758) were treated with at least one glucose-lowering medication at discharge (Table 3); 41% (n = 2,100) were administered at least one glucose-lowering medication, including sliding scale bolus insulin, during the index hospital admission (data not shown). Among the patients treated with glucose-lowering medication at discharge, one fourth (25%, n = 448) were treated with two or more glucose-lowering medications (Table 3). The most commonly used glucose-lowering medications were metformin (50%), basal insulin (32%), bolus insulin (10%), sulphonylureas (14%) and dipeptidyl peptidase-4 inhibitors (14%) (Table 4).
Table 3.
Number of patients grouped by number of glucose-lowering medications administered at the time of hospital discharge and HbA1c-value (obtained ±90 days before hospital admission).
| Number of glucose-lowering medications | HbA1c | |||||||
|---|---|---|---|---|---|---|---|---|
| <30 | 30–41 | 42–47 | 48–52 | 53–57 | 58–74 | 75+ | Total | |
| 0 | 42 (74%) | 976 (75%) | 847 (70%) | 482 (64%) | 326 (60%) | 518 (58%) | 223 (55%) | 3414 (66%) |
| 1 | 12 (21%) | 279 (21%) | 284 (23%) | 217 (29%) | 155 (29%) | 250 (28%) | 113 (28%) | 1310 (25%) |
| 2 | 2 (4%) | 43 (3%) | 75 (6%) | 50 (7%) | 55 (10%) | 103 (12%) | 60 (15%) | 388 (8%) |
| 3 | 0 (0%) | 5 (0%) | 8 (1%) | 7 (1%) | 7 (1%) | 19 (2%) | 8 (2%) | 54 (1%) |
| 4 | 1 (2%) | 1 (0%) | 0 (0%) | 1 (0%) | 0 (0%) | 2 (0%) | 1 (0%) | 6 (0%) |
| Total | 57 (100%) | 1304 (100%) | 1214 (100%) | 757 (100%) | 543 (100%) | 892 (100%) | 405 (100%) | 5172 (100%) |
HbA1c-values are divided into categories and displayed in mmol/mol.
Table 4.
Antidiabetic medication at the time of hospital discharge in relation to HbA1c-values (obtained ±90 days before hospital admission) for very old patients with type 2 diabetes.
| Type of glucose-lowering medication | HbA1c | |||||||
|---|---|---|---|---|---|---|---|---|
| <30 | 30–41 | 42–47 | 48–52 | 53–57 | 58–74 | 75+ | Total | |
| Acarbose | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (100%) | 0 (0%) | 0 (0%) | 2 (100%) |
| Basal Insulin | 1 (0%) | 51 (9%) | 64 (11%) | 75 (13%) | 79 (14%) | 188 (33%) | 105 (19%) | 563 (100%) |
| Bolus Insulin | 3 (2%) | 26 (15%) | 23 (14%) | 21 (12%) | 28 (17%) | 44 (26%) | 24 (14%) | 169 (100%) |
| DPP-4i | 2 (1%) | 30 (12%) | 56 (22%) | 34 (13%) | 36 (14%) | 56 (22%) | 38 (15%) | 252 (100%) |
| GLP-1 RA | 0 (0%) | 5 (16%) | 7 (23%) | 7 (23%) | 1 (3%) | 10 (32%) | 1 (3%) | 31 (100%) |
| Metformin | 10 (1%) | 204 (21%) | 254 (26%) | 163 (17%) | 110 (11%) | 169 (17%) | 75 (8%) | 985 (100%) |
| SGLT-2i | 0 (0%) | 2 (12%) | 1 (6%) | 2 (12%) | 4 (24%) | 6 (35%) | 2 (12%) | 17 (100%) |
| SU | 4 (2%) | 66 (26%) | 53 (21%) | 40 (16%) | 26 (10%) | 48 (19%) | 16 (6%) | 253 (100%) |
| Total | 20 (1%) | 384 (17%) | 458 (20%) | 342 (15%) | 286 (13%) | 521 (23%) | 261 (11%) | 2272 (100%) |
Values are displayed in absolute numbers. HbA1c-values are divided into categories and displayed in mmol/mol. Patients count more than once if administered more than one kind of antidiabetic.
DPP-4i: dipeptidylpeptidase-4 inhibitor, SGLT-2i: sodium-glucose cotransporter-2 inhibitor, SU: sulfonylurea, GLP-1RA: Glucagon-like peptide-1 receptor agonist.
Glucose-lowering medications in relation to glycemic control
Of those treated with a glucose-lowering medication at discharge (n = 1,758), close to half 48%, n = 844) had an HbA1c within the interval recommended for elderly without significant comorbidity (43–57 mmol/mol (6.0–7.5%)). One third had higher HbA1c-values, 21% (n = 374) had a Hba1c between 58–74 mmol/mol (7.5–9), and 10% (n = 182) had Hba1c >75 mmol/mol (9%); while the remaining 20% (n = 343) had near-normalized Hba1c (<42 mmol/mol (6%)) while continuing glucose-lowering medication at discharge. Of the patients with near-normalization of Hba1c values, 15% (n = 52) took two or more glucose-lowering medications (Table 3, Fig. 1) most frequently metformin, insulin and sulphonylureas (Table 4). One percent (n = 15) of the patients treated with an glucose-lowering medication at discharge had very low Hba1c-values <30 mmol/mol (<4.9%) (Table 3, Fig. 1).
For those patients who did not receive a glucose-lowering medication at discharge (n = 3,414), 55% (n = 1,865) had HbA1c-values that did not justify a diagnosis of type 2 diabetes (i.e. HbA1c <48 mmol/mol (<6,5%)) (Table 3, Fig. 1). At the other end of the spectrum, 7% (n = 223) had Hba1c levels for which glucose-lowering medications are generally recommended (i.e.>75 mmol/mol (9%)).
Discussion
Based on hospital electronic health records covering the entire population of the Capital Region of Denmark (1.8 million inhabitants) from 2012 to 2016, we investigated the demographics and the degree of glycemic control in relation to glucose-lowering medications in patients with type 2 diabetes aged 80 years or more. Our main findings were (1) almost half of the patients had an HbA1c<48 mmol/mol (<6,5%), and of these 72% (n = 1865, 36% of all patients) were not treated with a glucose-lowering medication and thus did not fulfil the diagnostic criteria of type 2 diabetes; (2) of the patients treated with one or more glucose-lowering medications (often including insulin and/or sulphonylureas), 20% had HbA1c-values below 42 mmol/mol (6%) and 1% had critically low HbA1c values <30 mmol/mol (<4.9%), indicating overtreatment. Conversely, 8% of all patients had Hba1c values >75 mmol/mol (>9%), indicating possible undertreatment.
A surprising finding was that based on HbA1c-value, 36% (n = 1,865) of all the admitted patients did meet the criteria for their diagnosis of type 2 diabetes. The diagnoses were all registered by a physician authorized in Denmark and could have been registered many years prior to the index admission. Thus, one potential explanation for our finding could be that type 2 diabetes is not a chronic disease but rather a condition that may in some cases remit with old age – a notion that has been proposed before32,33. Hence, Abdelhafiz et al. proposed that frailty among older people with type 2 diabetes might lead to the remission of type 2 diabetes with the suggested mechanisms being weight loss accompanied by reduced amounts of visceral fat and thereby improved insulin sensitivity32. Such a mechanism bears resemblance to that described for patients having bariatric surgery and/or substantial weight loss and afterwards experience remission of their type 2 diabetes34,35.
We report that only 17% of included patients had an HbA1c between 58–75 mmol/mol (7.5–9%), the interval generally recommended for elderly with significant comorbidities and limited life expectancy. That our patients were indeed highly comorbid is evidenced by the Charlson comorbidity score, where 94% scored 2 or more36. Of those with an HbA1c <42 mmol/mol (<6.0%), 25% were treated with one or more glucose-lowering medications. These findings are in line with findings from other studies that have raised concerns about the potential overtreatment of older people with type 2 diabetes12,23,24,37–39. Among these is a large register-based study by Tseng et al. including 652,738 patients from the Veteran Health Administration. They reported that approximately 50% of patients aged 75 years or older, who were treated with insulin and/or sulphonylureas, had an HbA1c <53 mmol/mol (<7%)12. Similarly, results from The Fremantle Diabetes Cohort Study, which included 367 patients over the age of 75 with type 2 diabetes showed that approximately three of five (61%) of the patients had an HbA1c <53 mmol/mol (<7%)37. As treatment needs to be individualized according to a patient’s preferences and resources as well as life expectancy it is of interest that in our cohort dementia was registered as a diagnosis for 16% and non-skin malignancy for 19% of the included patients. Studies of frail patients with type 2 diabetes and limited life expectancy, such as nursing home residents, have suggested that particularly elderly with dementia are overtreated with glucose-lowering medications. Thus, in a nursing home population, 46–74% of the patients had an HbA1c <53 mmol/mol (<7%)24,39,40. Although the distributions of Hba1c-values in the mentioned nursing home studies were similar to ours, cognitive and functional impairment may be more frequent in the nursing home setting. One percent (n = 70) of our population had hypoglycemia as the primary cause of admission. However, this is likely an underestimate of the number of patients at high risk of hypoglycemia. In older people, hypoglycemia can go undiscovered and be difficult to recognize due to unspecific symptoms11. Thus, the substantial proportion of patients, who in the context of near-normal Hba1c (i.e. below 42 mmol/mol (6%)) continued treatment with a sulphonylurea (n = 70) or insulin (n = 82) could be considered at high risk of hypoglycemic events11,16. Thus, our study adds to the evidence suggesting that the recommendations favoring looser glycemic control in elderly, comorbid people similar to our population has not been fully adopted into clinical practice.
Our study has important strengths such as the large sample size, the high data quality from rather accurate national registers with the possibility of linking biochemical data with health record data and drug use. Nonetheless, this register-based study also has some limitations. In our study, only 34% of elderly patients with a diagnosis of type 2 diabetes were treated with glucose-lowering medications. Other studies on glycemic control in older people, including the mentioned studies of nursing home residents and larger cohort studies report a much higher proportion of patients treated with glucose-lowering medication. Thus, between 85–100% of the patients received glucose-lowering medication in other cohort studies of a general population with type 2 diabetes12,23,37, and up to 86% were pharmacologically treated in studies investigating glycemic control in nursing home residents24,39,40. Our lower treatment prevalence is most likely due to the fact that many patients in our cohort did not meet the criteria for type 2 diabetes at the time of study. Since our study was based on a cohort identified by a hospital admission, and data analyses were limited to the time around hospital admission, we did not have information on the duration of diabetes or the glycemic control and use of antidiabetic medication over time. Access to this information could have strengthened our interpretation particularly the reason for the high proportion of patients not fulfilling the diagnostic criteria for type 2 diabetes. There is some indication that our cohort does not fully reflect the population in the capital region of Denmark. Thus, in our cohort, 54% were female, while the concurrent female proportion in the general population was 65%. The reason for such relative underrepresentation of females in our cohort is unclear. Another issue is that 56% had a hemoglobin below reference level, which theoretically could lead to an underestimation of the HbA1c-values. However, as proposed by samples from another Danish population, mild to moderate anemia does not seem have significant impact on the interpretation of HbA1c-values41.
In this hospital-based cohort consisting of more than 5000 patients, few patients ≥80 years with type 2 diabetes had an HbA1c within the limits generally recommended for this population. Many patients were not treated with glucose-lowering medications and had HbA1c-values that could not justify a diagnosis of type 2 diabetes. Of those treated with one or more glucose-lowering medications, quite many had either high or low HbA1c-values, suggesting under- and overtreatment, respectively. Our study supports the assumption that a diagnosis of type 2 diabetes may remit with age. Moreover, it suggests that recommendations for glycemic control in elderly patients with type 2 diabetes are not fully implemented in clinical practice.
Methods
Study cohort and data sources
The study was a retrospective cohort study using data from the Capital Region of Denmark from January 1, 2012 to May 15, 2016. We analyzed the first hospital admission for each patient, where an HbA1c measurement in proximity to the hospital admission (±90 days) was available. On admission, patients were required to be at least 80 years of age and have a prior diagnosis of type 2 diabetes (ICD-10 code DE11). Diagnoses were obtained from the regional system feeding data to The Danish National Patient Register42. Drug utilization was obtained from The Electronic Patient Medication module, which is a database for in-hospital drug-use in the Capital Region of Denmark43. HbA1c-values, as well as biochemical status (blood lipids (cholesterol, LDL and HDL), kidney function (creatinine, eGFR), hemoglobin levels and TSH), were gathered from The Clinical Laboratory Information System44. Body Mass Index (BMI) was obtained from the medical health records. Data sources were linked using the unique and permanent Danish identification number45.
Exposure and comorbidity
Exposure to a glucose-lowering medication was defined as an active prescription of a glucose-lowering medication (Anatomical Therapeutic Chemical classification (ATC)-code A10) at the time of discharge from the hospital and with at least one administration during the hospital admission. To evaluate patient comorbidity, we used diagnoses to calculate The Charlson Comorbidity Index, which is a measure of comorbidity burden and has been shown to be correlated with life expectancy36.
Statistical methods
Data are presented using standard descriptive statistics including median and interquartile ranges. Data management was conducted using R46.
Ethics
According to the Danish “Act on Research Ethics Review of Health Research Projects” section 14 (2), retrospective register-based studies do not require ethical approval in Denmark. The study was approved by The Danish Data Protection Agency (BFH-2016–058, I-Suite nr.: 04906) and The Danish Patient Safety Authority (3-3013-1884/1/).
Compliance with ethics guidelines
This article is based on previously conducted health data and does not contain any studies with human participants or animals performed by any of the authors.
Acknowledgements
The study was funded by the Department of Clinical Pharmacology, Bispebjerg and Frederiksberg Hospital, University of Copenhagen.
Author contributions
D.R.G. and S.V. contributed to drafting of the manuscript and data analyses. T.S.P., T.B.J. and R.C. contributed to study design and data analyses. E.J.S. and M.C. designed the study and contributed to data analyses and manuscript drafting. All authors edited and approved the manuscript.
Data availability
The dataset used in this study is not available due to local law.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American Diabetes Association. 12. Older Adults: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42:S139–S147. doi: 10.2337/dc19-S012. [DOI] [PubMed] [Google Scholar]
- 2.International Diabetes Federation Guideline Development Group. Global guideline for type 2 diabetes. Diabetes Res. Clin. Pract. 2014;104:1–52. doi: 10.1016/j.diabres.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 3.McGuire H, et al. Management of type 2 diabetes in adults: summary of updated NICE guidance. BMJ. 2016;353:i1575. doi: 10.1136/bmj.i1575. [DOI] [PubMed] [Google Scholar]
- 4.Handelsman Y, et al. American Association Of Clinical Endocrinologists And American College Of Endocrinology – Clinical practice guidelines for developing a diabetes mellitus comprehensive care plan. Endocr. Pract. 2015;21:1–87. doi: 10.4158/EP15672.GLSUPPL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnett K, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet Lond. Engl. 2012;380:37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 6.Sinclair A, et al. Diabetes mellitus in older people: position statement on behalf of the International Association of Gerontology and Geriatrics (IAGG), the European Diabetes Working Party for Older People (EDWPOP), and the International Task Force of Experts in Diabetes. J. Am. Med. Dir. Assoc. 2012;13:497–502. doi: 10.1016/j.jamda.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 7.American Geriatrics Society Expert Panel on Care of Older Adults with Diabetes Mellitus, Moreno, G., Mangione, C. M., Kimbro, L. & Vaisberg, E. Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 update. J. Am. Geriatr. Soc. 61, 2020–2026 (2013). [DOI] [PMC free article] [PubMed]
- 8.Action to Control Cardiovascular Risk in Diabetes Study Group et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 358, 2545–2559 (2008). [DOI] [PMC free article] [PubMed]
- 9.Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N. Engl. J. Med. 2011;365:2002–2012. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- 10.Duckworth W, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 11.Bremer JP, Jauch-Chara K, Hallschmid M, Schmid S, Schultes B. Hypoglycemia unawareness in older compared with middle-aged patients with type 2 diabetes. Diabetes Care. 2009;32:1513–1517. doi: 10.2337/dc09-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tseng C-L, Soroka O, Maney M, Aron DC, Pogach LM. Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Intern. Med. 2014;174:259–268. doi: 10.1001/jamainternmed.2013.12963. [DOI] [PubMed] [Google Scholar]
- 13.Abbatecola AM, et al. Severe hypoglycemia is associated with antidiabetic oral treatment compared with insulin analogs in nursing home patients with type 2 diabetes and dementia: results from the DIMORA study. J. Am. Med. Dir. Assoc. 2015;16:349.e7–12. doi: 10.1016/j.jamda.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Zoungas S, et al. Severe hypoglycemia and risks of vascular events and death. N. Engl. J. Med. 2010;363:1410–1418. doi: 10.1056/NEJMoa1003795. [DOI] [PubMed] [Google Scholar]
- 15.Lipska KJ, Krumholz H, Soones T, Lee SJ. Polypharmacy in the Aging Patient: A Review of Glycemic Control in Older Adults With Type 2 Diabetes. JAMA. 2016;315:1034–1045. doi: 10.1001/jama.2016.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdelhafiz AH, Rodríguez-Mañas L, Morley JE, Sinclair AJ. Hypoglycemia in older people - a less well recognized risk factor for frailty. Aging Dis. 2015;6:156–167. doi: 10.14336/AD.2014.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirkman MS, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650–2664. doi: 10.2337/dc12-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boulin M, Diaby V, Tannenbaum C. Preventing Unnecessary Costs of Drug-Induced Hypoglycemia in Older Adults with Type 2 Diabetes in the United States and Canada. PloS One. 2016;11:e0162951. doi: 10.1371/journal.pone.0162951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Currie CJ, et al. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet Lond. Engl. 2010;375:481–489. doi: 10.1016/S0140-6736(09)61969-3. [DOI] [PubMed] [Google Scholar]
- 20.McCoy RG, et al. Intensive Treatment and Severe Hypoglycemia Among Adults With Type 2 Diabetes. JAMA Intern. Med. 2016;176:969–978. doi: 10.1001/jamainternmed.2016.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SJ, et al. The risks and benefits of implementing glycemic control guidelines in frail older adults with diabetes mellitus. J. Am. Geriatr. Soc. 2011;59:666–672. doi: 10.1111/j.1532-5415.2011.03362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang ES. Potential Overtreatment of Older, Complex Adults With Diabetes. JAMA. 2015;314:1280–1281. doi: 10.1001/jama.2015.9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lipska KJ, et al. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern. Med. 2015;175:356–362. doi: 10.1001/jamainternmed.2014.7345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andreassen LM, Sandberg S, Kristensen GBB, Sølvik UØ, Kjome RLS. Nursing home patients with diabetes: prevalence, drug treatment and glycemic control. Diabetes Res. Clin. Pract. 2014;105:102–109. doi: 10.1016/j.diabres.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 25.Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994–2007. Arch. Intern. Med. 2008;168:2088–2094. doi: 10.1001/archinte.168.19.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christensen DH, Rungby J, Thomsen RW. Nationwide trends in glucose-lowering drug use, Denmark, 1999–2014. Clin. Epidemiol. 2016;8:381–387. doi: 10.2147/CLEP.S113211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beard HA, Markides KS, Al Ghatrif M, Kuo Y-F, Raji MA. Trends in diabetes medication use and prevalence of geriatric syndromes in older Mexican Americans from 1993/1994 to 2004/2005. Ann. Pharmacother. 2010;44:1376–1383. doi: 10.1345/aph.1M724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lipska KJ, et al. Trends in Drug Utilization, Glycemic Control, and Rates of Severe Hypoglycemia, 2006-2013. Diabetes Care. 2017;40:468–475. doi: 10.2337/dc16-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patrick AR, et al. Trends in insulin initiation and treatment intensification among patients with type 2 diabetes. J. Gen. Intern. Med. 2014;29:320–327. doi: 10.1007/s11606-013-2643-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oishi M, et al. Changes in oral antidiabetic prescriptions and improved glycemic control during the years 2002–2011 in Japan (JDDM32) J. Diabetes Investig. 2014;5:581–587. doi: 10.1111/jdi.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geller AI, et al. National estimates of insulin-related hypoglycemia and errors leading to emergency department visits and hospitalizations. JAMA Intern. Med. 2014;174:678–686. doi: 10.1001/jamainternmed.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdelhafiz AH, Koay L, Sinclair AJ. The Emergence of Frailty May Lead to a State of Burnt Out Type 2 Diabetes. J. Frailty. Aging. 2016;5:162–167. [PubMed] [Google Scholar]
- 33.Abdelhafiz, A., Koay, L. & Sinclair, A. Frailty and hypoglycaemia in older people with type 2 diabetes: Therapeutic implications. 2.
- 34.Taylor R. Type 2 diabetes: etiology and reversibility. Diabetes Care. 2013;36:1047–1055. doi: 10.2337/dc12-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ang GY. Reversibility of diabetes mellitus: Narrative review of the evidence. World J. Diabetes. 2018;9:127–131. doi: 10.4239/wjd.v9.i7.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 37.Bruce DG, Davis WA, Davis TME. Glycaemic control and mortality in older people with type 2 diabetes: The Fremantle Diabetes Study Phase II. Diabetes Obes. Metab. 2018;20:2852–2859. doi: 10.1111/dom.13469. [DOI] [PubMed] [Google Scholar]
- 38.McCoy RG, Van Houten HK, Ross JS, Montori VM, Shah ND. HbA1c overtesting and overtreatment among US adults with controlled type 2 diabetes, 2001-13: observational population based study. BMJ. 2015;351:h6138. doi: 10.1136/bmj.h6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bo M, et al. Prevalence, Clinical Correlates, and Use of Glucose-Lowering Drugs among Older Patients with Type 2 Diabetes Living in Long-Term Care Facilities. J. Diabetes Res. 2015;2015:174316. doi: 10.1155/2015/174316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Basso A, Peruzzi P, Carollo MC, Improta G, Fedeli U. Assessment of glycemic control among diabetic residents in nursing homes. Diabetes Res. Clin. Pract. 2012;96:e80–83. doi: 10.1016/j.diabres.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 41.Borg R, et al. Interpretation of HbA1c in primary care and potential influence of anaemia and chronic kidney disease: an analysis from the Copenhagen Primary Care Laboratory (CopLab) Database. Diabet. Med. J. Br. Diabet. Assoc. 2018;35:1700–1706. doi: 10.1111/dme.13776. [DOI] [PubMed] [Google Scholar]
- 42.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand. J. Public Health. 2011;39:30–33. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 43.Jensen, T. B. et al. Content and validation of the Electronic Patient Medication module (EPM)—the administrative in-hospital drug use database in the Capital Region of Denmark. Scand. J. Public Health 140349481876005 10.1177/1403494818760050 (2018). [DOI] [PubMed]
- 44.Grann, Erichsen, R., Nielsen, Frøslev & Thomsen, R. Existing data sources for clinical epidemiology: The clinical laboratory information system (LABKA) research database at Aarhus University, Denmark. Clin. Epidemiol. 133 10.2147/CLEP.S17901 (2011). [DOI] [PMC free article] [PubMed]
- 45.Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur. J. Epidemiol. 2014;29:541–549. doi: 10.1007/s10654-014-9930-3. [DOI] [PubMed] [Google Scholar]
- 46.Team, R. C. R: A Language and Environment for Statistical Computing. (2018).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset used in this study is not available due to local law.


