Abstract
Ozone (O3) plays an extremely important role in airway inflammation by generating reactive oxygen species (ROS) including hydrogen peroxide, then promoting redox actions and causing oxidative stress. Evidences indicate that TRPC6 (canonical transient receptor potential channel 6) is a redox-regulated Ca2+ permeable nonselective cation channel, but its role in the setting of oxidative stress-related airway inflammation remains unknown. Here, we found that both TRPC6−/− mice and mice pretreated with SAR7334, a potent TRPC6 inhibitor, were protected from O3-induced airway inflammatory responses. In vitro, both knockdown of TRPC6 expression with shRNA and TRPC6 blockage markedly attenuated the release of cytokines IL-6 and IL-8 induced by O3 or H2O2 in 16HBE cells (human bronchial epithelial cell line). Treatment with O3 or H2O2 enhanced TRPC6 protein expression in vivo and vitro. We also observed that TRPC6-dependent increase of intracellular Ca2+ concentration ([Ca2+]i) was triggered by H2O2, which consisted of the release from intracellular calcium store and the influx of extracellular Ca2+ and could be further strengthened by 6-h O3 exposure in both 16HBE cells and HBEpiCs (primary human bronchial epithelial cells). Moreover, we confirmed that the activation of MAPK signals (ERK1/2, p38, JNK) was required for the inflammatory response induced by O3 or H2O2 while only the phosphorylation of ERK pathway was diminished in the TRPC6-knockdown situation. These results demonstrate that oxidative stress regulates TRPC6-mediated Ca2+ cascade, which leads to the activation of ERK pathway and inflammation and could become a potential target to treat oxidative stress-associated airway inflammatory diseases.
Subject terms: Ion channels, Molecular biology
Introduction
Abnormal airway inflammation resulting from exposure to various oxidizing ambient pollutants is one of the most common and significant pathogenesis for numerous respiratory diseases, including asthma, chronic obstructive pulmonary disease (COPD), and lung cancer. Ambient pollutants, such as inhalable dusts, particulate matter (PM), tobacco smoke and ozone (O3), have strong ability to generate reactive oxygen species (ROS), accelerate redox actions and trigger oxidative stress. As the first line of defense and major target of inhaled harmful environmental pollutants, bronchial epithelium produces a series of pro-inflammatory molecules and recruits inflammatory cells into interstitium and airways after suffering oxidative stress, which could further aggravate airway inflammation. However, the molecular mechanisms by which oxidative air pollutants trigger pulmonary inflammation are still elusive.
Ca2+, an essential secondary messenger relevant to a variety of cellular processes, plays a key role in mediating airway inflammatory responses. Rises in [Ca2+]i in pulmonary cells are essential for the activation of inflammatory signal transduction proteins and transcriptions factors1–3. Dysregulation of [Ca2+]i homeostasis in bronchial epithelia contributes to pulmonary disease4–6. In addition, ROS has been found to be responsible for the activity of various calcium channels7. TRPM2, a plasma membrane Ca2+-permeable channel, mediates ROS-induced chemokine production in monocytes8. Compared with WT mice, TRPM2−/− mice exhibits enhanced gastric inflammation after infecting with Helicobacter pylori, which is owing to intracellular calcium overloading and augmented oxidative stress9. These findings suggest that the abnormality of [Ca2+]i suffered from ROS in pulmonary cells may be involved in airway inflammation.
TRPC6, a Ca2+-permeable non-selective cation channel of the canonical transient receptor potential (TRPC) family, is widely expressed in a number of tissues including brain, heart, lung, ovary, kidney, and vascular tissues10. Consistent with its broad expression in lungs, including bronchial epithelial cells, alveolar macrophages and pulmonary vasculature11–13, TRPC6 contributes to pulmonary disorders, such as cystic fibrosis, asthma, pulmonary hypertension, COPD, lung edema, and lung fibrosis11,14,15. Via analyzing the TRPC6 gene promoter of pulmonary artery smooth muscle cells from patients with idiopathic pulmonary arterial hypertension (IPAH), three single-nucleotide polymorphisms are identified and one of them are found to increase basal gene promoter activity, which may link abnormal transcription of TRPC6 to the activation of NF-κB and lead to upregulated risk of IPAH16. The expression of TRPC6 mRNA in alveolar macrophages isolated from COPD patients is significantly more than healthy controls12. Particularly, as a modulator of membrane calcium currents, TRPC6 is newly considered as an essential element in the regulation of inflammatory response17. TRPC6 channels have been reported to regulate CXCR2-related chemotaxis via mediating calcium supply18. After the activation of TLR4 and generation of DAG, TRPC6-dependent Ca2+ influx into endothelial cells is triggered and cooperated in endotoxin-induced lung inflammation19. Moreover, growing evidence points out that TRPC6 acts as a redox-related channel, while the definite relation between TRPC6 and ROS seems to be affected by cell specific difference20–23. Recently, we reported that TRPC6 is a key element in the regulation of adhesion of neutrophils to bronchial epithelial cell with O3 exposure24, while the role and regulatory mechanisms of TRPC6 channel in oxidative stress-induced airway inflammation are still unclear.
Here, we investigated the relevance of TRPC6 in O3-induced airway inflammation in mice and inflammatory response in bronchial epithelial cells. We further explored the involved underlying mechanisms to extrapolate the potential of TRPC6 as target to treat oxidative stress-associated airway inflammation.
Results
TRPC6 is required for O3-induced airway inflammatory responses in mice
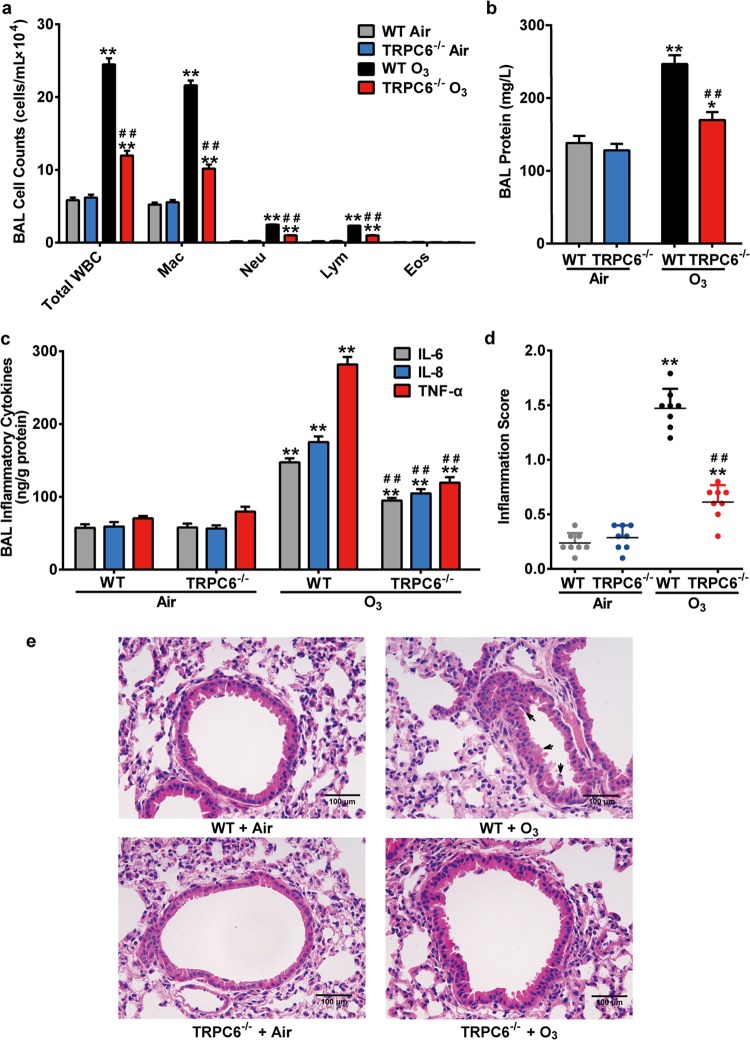
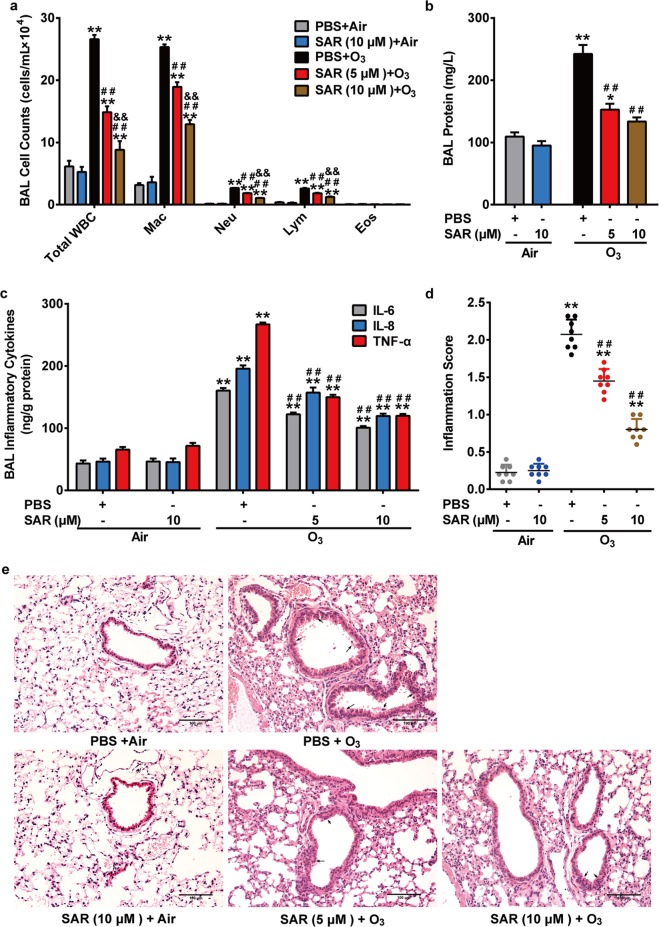
We sought to investigate the function of TRPC6 in O3-induced airway inflammatory response by employing TRPC6−/− mice and SAR7334, a TRPC6-selective inhibitor. As shown in Figs. 1, 2, both TRPC6−/− mice and mice pretreated with SAR7334 failed to respond to O3 exposure entirely as exhibiting mild airway inflammatory response. TRPC6-deficiency as well as SAR7334 significantly inhibited O3-induced inflammatory cell recruitment in BAL fluid, as reflected by the reduced numbers of neutrophils, macrophages and lymphocytes but not eosinophils, compared with that in WT + O3 group or PBS + O3 group (Figs. 1a, 2a). Not only TRPC6−/− mice but also mice pretreated with SAR7334 had lower total protein, IL-6, IL-8, and TNF-α content in BAL fluid than WT mice had after O3 exposure (Figs. 1b, c, 2b, c). The O3-induced increased lung inflammation scores and inflammatory changes of lung sections were also significantly inhibited by TRPC6-deficiency or SAR7334 (Figs. 1d, e, 2d, e). Given the above, TRPC6 contributes to the development of O3-induced airway inflammation in mice.
Fig. 1. Effect of TRPC6-deficiency on O3-induced airway inflammation.
WT and TRPC6−/− mice were exposed to O3 (1 ppm) for 3 h every other day (day 1, 3, 5).The mice were anesthetized 24 h after the last exposure. a–c Total white blood cell counts (Total WBC), macrophage counts (Mac), neutrophil counts (Neu), lymphocyte counts (Lym), eosinophil counts (Eos) (a), total protein content (b) and the release of inflammatory mediators IL-6, IL-8, TNF-α (c) in BAL fluid of different groups were compared. d Inflammation scores in air control and O3-exposed mice. e Representative histological sections of mouse lungs (H&E staining) after exposure to O3 or air. Black arrows: inflammatory changes. Scale bar: 100 μm. Results are presented as mean ± SEM, n = 8. *P < 0.05 or **P < 0.01 compared with WT + Air group, ##P < 0.01 compared with WT + O3 group.
Fig. 2. Effect of TRPC6-blockage on O3-induced airway inflammation in mice.
WT mice were exposed to O3 (1 ppm) for 3 h every other day (day 1, 3, 5). Mice received PBS or SAR7334 by oral gavage 4 h before exposure. The mice were anesthetized 24 h after the last exposure. a–c Total white blood cell counts (Total WBC), macrophage counts (Mac), neutrophil counts (Neu), lymphocyte counts (Lym), eosinophil counts (Eos) (a), total protein content (b) and the release of inflammatory mediators IL-6, IL-8, TNF-α (c) in BAL fluid of different groups were compared. d Inflammation scores in air control and O3-exposed mice. e Representative histological sections of mouse lungs (H&E staining) after exposure to O3 or air. Black arrows: inflammatory changes. Scale bar: 100 μm. Results are presented as mean ± SEM, n = 8. *P < 0.05 or **P < 0.01 compared with PBS + Air group, ##P < 0.01 compared with PBS + O3 group, &&P < 0.01 compared with SAR (5 μM) + O3 group.
Ca2+ signal and TRPC6 is required for oxidative stress-induced inflammatory responses in human bronchial epithelial cells
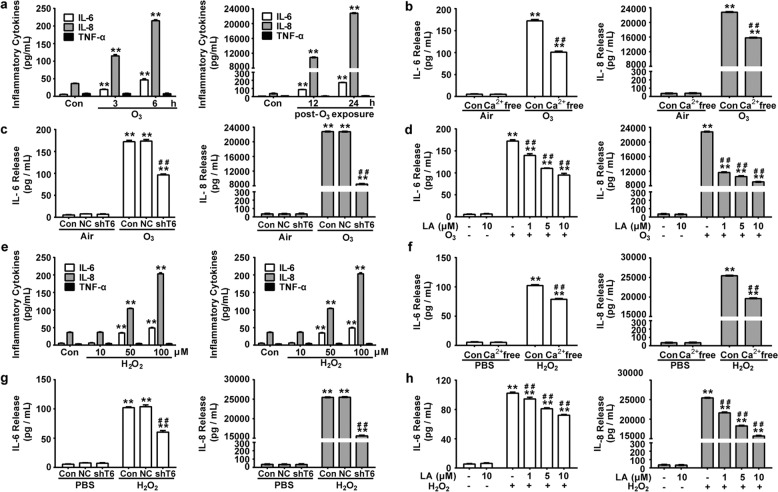
Ahead of studying the effect of Ca2+ and TRPC6 in oxidative stress-induced inflammatory response, we applied O3 (100 ppb) exposure on 16HBE cells and found that it made no difference to cell viability when it lasted for ≤12 h (Fig. S1a). The releases of IL-6 and IL-8 increased after exposure for 6 h and further augmented till 24 h post-exposure, but the release level of TNF-α remained unchanged (Fig. 3a). The levels of O3-induced production of IL-6 and IL-8 were significantly attenuated by removal of extracellular Ca2+ (Fig. 3b), which indicated that the influx of extracellular Ca2+ was a key step in O3-induced releases of inflammatory cytokines. We surmised that TRPC6-mediated O3-induced influx of extracellular Ca2+ as it is a potent monitor of membrane calcium currents. TRPC6 mRNA and protein expression in 16HBE cells was strikingly reduced after transducing with TRPC6 shRNA (shTRPC6) while that in the non-silenced negative control (NC) shRNA group had no difference with that in the control group (Fig. S2.). In parallel, Larixyl Acetate (LA), a potent and specific blocker of TRPC6 channels25, was administrated at 1, 5, 10 μM concentrations. Importantly, O3-induced releases of IL-6 and IL-8 were significantly reduced in shTRPC6-treated or LA-treated cells (Fig. 3c, d), suggesting an involvement of TRPC6 in O3-induced production of IL-6 and IL-8 in 16HBE cells.
Fig. 3. Role of Ca2+ and TRPC6 in oxidative stress-induced inflammatory responses in 16HBE cells.
a Release levels of IL-6, IL-8, and TNF-α were detected after 16HBE cells were exposed to O3 (100 ppb) for 0, 3, 6 h (a, Left) or cultured in fresh atmosphere for another 12, 24 h following 6-hour O3 (100 ppb) exposure (a, Right). Data represent the mean ± SEM, n = 5. **P < 0.01 compared with the Control group. b 16HBE cells incubated with or without Ca2+-free bath solution containing 100 μM EGTA were exposed to O3 (100 ppb) for 6 h and then cultured in fresh atmosphere for another 24 h. Release levels of IL-6 and IL-8 were detected. Data represent the mean ± SEM, n = 5. **P < 0.01 compared with Control group, ##P < 0.01 compared with O3 group. c After NC or shTRPC6 infection, 16 HBE cells were exposed to O3 (100 ppb) for 6 h and then cultured in fresh atmosphere for another 24 h. Release levels of IL-6 and IL-8 were detected. Data represent the mean ± SEM, n = 5. **P < 0.01 compared with Control group, ##P < 0.01 compared with O3 group, &&P < 0.01 compared with NC + O3 group. Con: Control, NC: Negative Control, shT6: shRNA TRPC6. d After pretreatment with LA (0, 1, 5, 10 μM) for 1 h, 16HBE cells were exposed to O3 (100 ppb) for 6 h and then cultured in fresh atmosphere for another 24 h. Release levels of IL-6 and IL-8 were detected. Data represent the mean ± SEM, n = 5. **P < 0.01 compared with Control group, ##P < 0.01 compared with O3 group. e Release levels of IL-6, IL-8, and TNF-α were detected after 16HBE cells were stimulated by H2O2 (0, 10, 50, 100 μM) for 12 h (a, Left) or H2O2 (100 μM) for 0, 3, 6, 12, 24 h (a, Right). Data represent the mean ± SEM, n = 5. **P < 0.01 compared with Control group. f 16HBE cells incubated with or without Ca2+-free bath solution containing 100 μM EGTA were exposed to H2O2 (100 μM) for 24 h. Release levels of IL-6 and IL-8 were detected. Data represent the mean ± SEM, n = 5. **P < 0.01 compared with Control group, ##P < 0.01 compared with H2O2 group. g After NC or shTRPC6 infection, 16HBE cells were exposed to H2O2 (100 μM) for 24 h. Release levels of IL-6 and IL-8 were detected. Data represent the mean ± SEM, n = 5. **P < 0.01 compared with Control group, ##P < 0.01 compared with H2O2 group, &&P < 0.01 compared with NC + H2O2 group. Con: Control, NC: Negative Control, shTRPC6: shRNA TRPC6. h After pretreatment with or without LA (1, 5, 10 μM) for 1 h, 16HBE cells were exposed to H2O2 (100 μM) for 24 h. Release levels of IL-6 and IL-8 were detected. Data represent the mean ± SEM, n = 5. **P < 0.01 compared with Control group, ##P < 0.01 compared with H2O2 group.
Hydrogen peroxide (H2O2), an oxidant generated during exposure to ambient oxidizing pollutants26, is deemed as an intermediate capable of exerting some of its biological effects owing to its diffusibility over membranes and longer half-life than most other ROS27. Thus, H2O2 was used in current study to further explore the mechanism regarding O3-induced inflammatory response. The viability of 16HBE cells was not affected by H2O2 treatment with ≤100 μM of dose and ≤24 h of time (Fig. S1b, c). Similar with O3 treatment, H2O2 increased the releases of inflammatory mediators IL-6 and IL-8 in a concentration-dependent and time-dependent manner but not that of TNF-α (Fig. 3e). Consistently, absence of extracellular Ca2+, transfection with shTRPC6 or pretreatment with LA significantly reduced the release levels of IL-6 and IL-8 evoked by H2O2 (Fig. 3f–h), which indicated that Ca2+ signal and TRPC6 contributed to H2O2-induced production of IL-6 and IL-8 in 16HBE cells. Together, these results suggest that TRPC6 mediates oxidative stress-induced inflammatory responses in human bronchial epithelial cells.
TRPC6 expression is increased by oxidative stress in vivo and vitro
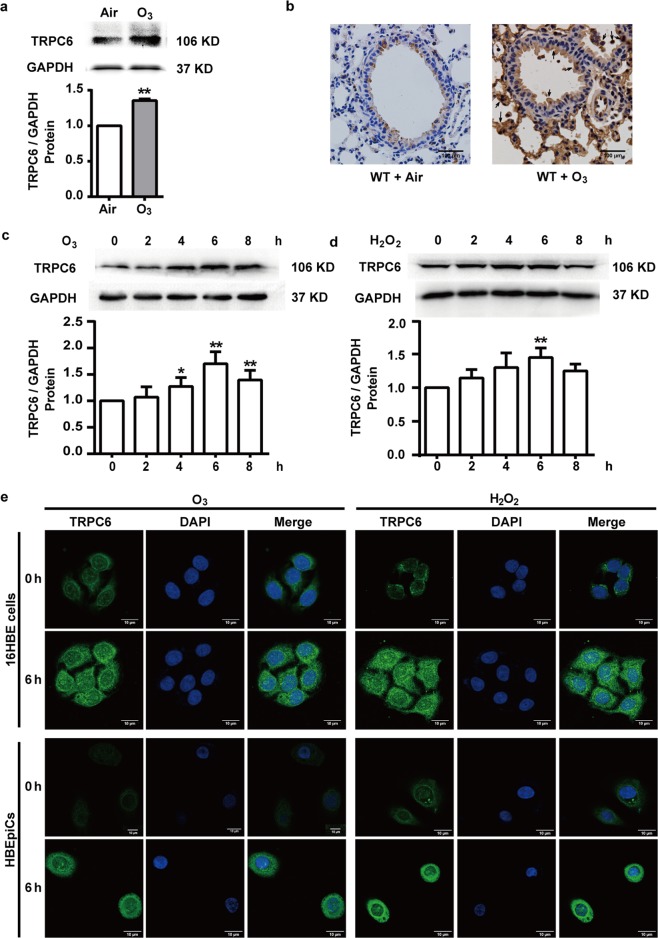
Since TRPC6-mediated oxidative stress-induced inflammatory responses, we next assessed TRPC6 protein expression in lungs of O3-exposed mice and human bronchial epithelial cells administrated with O3 or H2O2. Indeed, repeated O3 exposure increased TRPC6 protein expression in lungs (Fig. 4a). Lung section IHC also showed an enhanced TRPC6 protein expression after O3 exposure, mainly localized in bronchial epithelial cells and macrophages (Fig. 4b). As shown in Fig. 4c, d, TRPC6 protein expression was increased and reached a maximum at 6 h by O3 (100 ppb) exposure or H2O2 (100 μM) stimulation in 16HBE cells. In view of reports highlighting the differences between primary cells with hTERT or viral genes transduced human cells28,29, primary bronchial epithelial cells (HBEpiCs) were employed in parts of our study to further confirm the results obtained from 16HBE cells. Consistently, in both 16HBE cells and HBEpiCs, the expression of TRPC6 protein located in both cytomembrane and cytoplasm was upregulated after O3 (100 ppb) or H2O2 (100 μM) exposure for 6 h (Fig. 4e, f).
Fig. 4. Effect of oxidative stress on TRPC6 expression in lung tissue and human bronchial epithelial cells.
a, b WT mice were exposed to O3 (1 ppm) for 3 h every other day (day 1, 3, 5) and were anesthetized 24 h after the last exposure. TRPC6 expression in lung tissues from mice were analyzed with western blot (a) and immunohistochemistry-stained lung sections (b). Black arrows: epithelial cells and macrophages. Scale bar: 100 μm. Data represent the mean ± SEM, n = 8. **P < 0.01 compared with Air group. c, d TRPC6 expression in 16HBE cells was analyzed by western blot after exposure to O3 (100 ppb) (c) or H2O2 (100 μM) (d) for 0, 2, 4, 6, 8 h. Data represent the mean ± SEM, n = 5. *P < 0.05 or **P < 0.01 compared with 0 h group. e TRPC6 expression in 16HBE cells and primary HBEpiCs was analyzed by immunofluorescence after exposure to O3 (100 ppb) or H2O2 (100 μM) for 6 h. Scale bar: 10 μm. n = 5.
TRPC6 mediates H2O2-induced increase of intracellular calcium in human bronchial epithelial cells
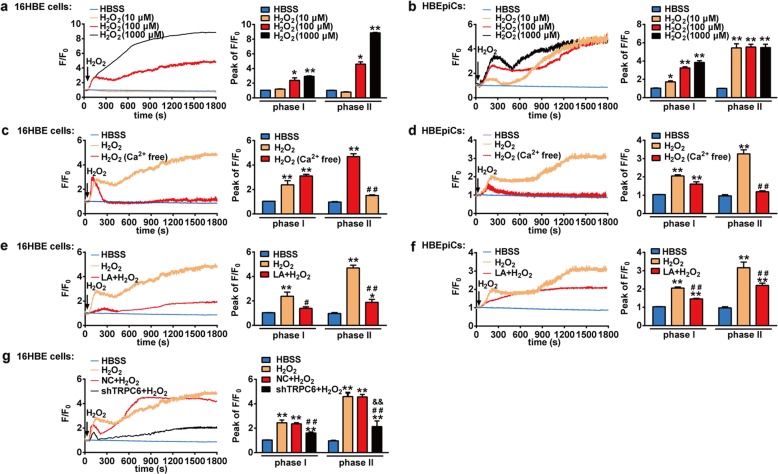
Previous studies have shown that oxidative stress leads to the disruption of intracellular calcium homeostasis which is capable of activating signal pathway associated with inflammatory response7,30. Therefore, we next aimed to study the effect of H2O2 on the intracellular calcium homeostasis in bronchial epithelial cells and the role of TRPC6 in this progress. Both 16HBE cells and HBEpiCs were stimulated with different concentrations of H2O2 and calcium-dependent fluorescence was monitored. Calcium imaging results showed that H2O2-induced intracellular calcium ([Ca2+]i) increase in a concentration-dependent manner (Fig. 5a, b). Interestingly, treatment with 100 μM H2O2 caused a fast [Ca2+]i increase (phase Ι) attaining 2.4 ± 0.3 folds within 2 min in 16HBE cells. A slight decline to 2.1 ± 0.1 folds followed and lasted for about 2 min. Beyond this phase, [Ca2+]i increased slowly and reached a plateau of 4.6 folds 25 min after stimulation (phase II). 1000 μM H2O2 caused a faster and more significant [Ca2+]i increase in cells which reached the plateau of 8.8 folds 25 min after stimulation (Fig. 5a). Intriguingly, compared with 16HBE cells, HBEpiCs were more sensitive to oxidative stress that 10 μM H2O2 was capable to trigger this increase (Fig. 5b). To analyze the source of increased [Ca2+]i, Ca2+-free buffer (containing 5 mM EGTA) was applied. Notably, the initial increase of [Ca2+]i (phase Ι) induced by H2O2 (100 μM) was not influenced while the late [Ca2+]i increase (phase II) was significantly inhibited (Fig. 5c, d), suggesting that 100 μM H2O2-induced increase of [Ca2+]i was composed by intracellular calcium store release (phase Ι) and extracellular Ca2+ influx (phase II), mainly the latter. Moreover, both the phase Ι and phase II of the H2O2-induced [Ca2+]i increase were attenuated by pretreatment with the TRPC6 inhibitor LA (10 μM) and the inhibition of phase II was more salient (Fig. 5e, f). Similarly, knockdown of TRPC6 markedly inhibited 100 μM H2O2-induced [Ca2+]i increase in 16HBE cells (Fig. 5g). Owing to the relatively short lifespan of primary HBEpiCs, which limits total number of times of subculturing, the experiment in the effect of shTRPC6 on H2O2-induced [Ca2+]i increase was unable to carry on. Taken together, these results show that TRPC6 mediates H2O2-evoked increase in [Ca2+]i via the release of intracellular calcium store and the influx of extracellular Ca2+.
Fig. 5. Role of TRPC6 in H2O2-induced [Ca2+]i increase in human bronchial epithelial cells.
16HBE cells or primary HBEpiCs were loaded with the Ca2+ indicator Fluo-4/AM (5 μM) in Hanks’ solution for 30 min to measure [Ca2+]i. The [Ca2+]i in the cells was expressed as a pseudo-ratio value of the relative fluorescence intensity (F/F0) (Left). Bar graph shows the mean peak value of F/F0 traces (Peak of F/F0) of the experiments (Right). a, b 16HBE cells (a) and primary HBEpiCs (b) were exposed to variable concentrations of H2O2 (0, 10, 100, 1000 μM). *P < 0.05 or **P < 0.01 compared with HBSS control group. c, d 16HBE cells (c) and primary HBEpiCs (d) incubated with or without Ca2+-free bath solution (100 μM EGTA) were exposed to H2O2 (100 μM). **P < 0.01 compared with HBSS control group, ##P < 0.01 compared with H2O2 group. e, f After pretreatment with or without Larixyl Acetate (LA, 10 μM) for 30 min, 16HBE cells (e) and primary HBEpiCs (f) were exposed to H2O2 (100 μM). *P < 0.05 or **P < 0.01 compared with HBSS control group, #P < 0.05, ##P < 0.01 compared with H2O2 group. g After NC or shTRPC6 infection, 16HBE cells were exposed to H2O2 (100 μM). **P < 0.01 compared with HBSS control group, ##P < 0.01 compared with H2O2 group, &&P < 0.01 compared with NC + H2O2 group. NC: Negative Control, shTRPC6: shRNA TRPC6. Each trace represents the mean from three independent experiments that were derived from 20 to 40 cells in each single experiment.
O3 amplifies H2O2-triggered increase of [Ca2+]i via TRPC6 in human bronchial epithelial cells
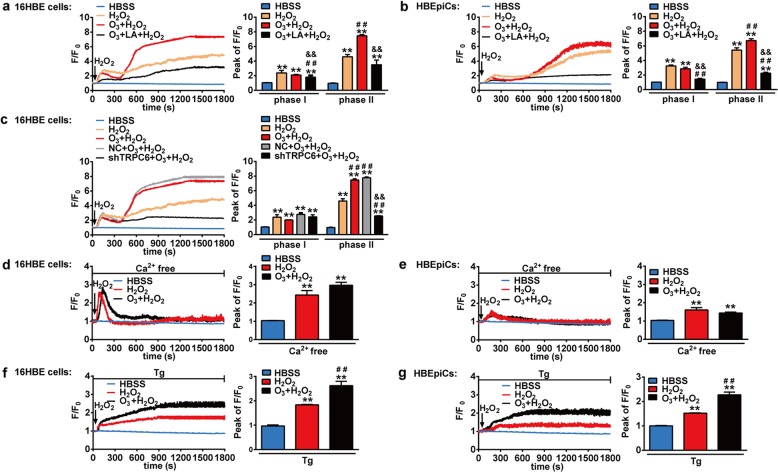
We then speculated that O3 exposure made the cells more sensitive to oxidative stress which resulted in more serious inflammatory responses and oxidative injury. As shown in Fig. 6a, b, O3 (100 ppb) exposure for 6 h further amplified H2O2-triggered [Ca2+]i increase in 16HBE cells and primary HBEpiCs, displayed as augment of the phase II but no change in the phase Ι, and both phase Ι and phase ΙΙ were significantly abolished by pretreatment with LA (10 μM) for 1 h. The phase II of H2O2-induced [Ca2+]i increase after O3 (100 ppb) exposure was inhibited by shTRPC6 in 16HBE cells (Fig. 6c). These results suggest that TRPC6 has a major role in O3-induced augmenting sensitivity to oxidative stress in bronchial epithelial cells.
Fig. 6. Role of TRPC6 in O3 augmenting H2O2-induced [Ca2+]i increase in human bronchial epithelial cells.
16HBE cells or primary HBEpiCs were loaded with the Ca2+ indicator Fluo-4/AM (5 μM) in Hanks’ solution for 30 min to measure [Ca2+]i. The [Ca2+]i in the cells was expressed as a pseudo-ratio value of the relative fluorescence intensity (F/F0) (Left). Bar graphs show the mean peak value of F/F0 traces (Peak of F/F0) of the experiments (Right). a, b After exposure to air or O3 (100 ppb) for 6 h, 16HBE cells (a) and primary HBEpiCs (b) were pretreated with LA (10 μM) for 30 min and then stimulated with H2O2 (100 μM). **P < 0.01 compared with HBSS control group, ##P < 0.01 compared with H2O2 group, &&P < 0.01 compared with O3 + H2O2 group. c After NC or shTRPC6 infection, 16HBE cells were exposed to air or O3 (100 ppb) for 6 h and then stimulated with H2O2 (100 μM). **P < 0.01 compared with HBSS control group, ##P < 0.01 compared with O3 + H2O2 group, &&P < 0.01 compared with NC + O3 + H2O2 group. d, e After exposure to air or O3 (100 ppb) for 6 h, 16HBE cells (d) and primary HBEpiCs (e) were incubated with or without Ca2+-free bath solution containing 100 μM EGTA and then stimulated with H2O2 (100 μM). **P < 0.01 compared with HBSS control group, ##P < 0.01 compared with H2O2 group. f, g After exposure to air or O3 (100 ppb) for 6 h, 16HBE cells (f) and primary HBEpiCs (g) were incubated with or without thapsigargin (Tg, 2 μM) and then stimulated with H2O2 (100 μM). **P < 0.01 compared with HBSS control group, ##P < 0.01 compared with H2O2 group. NC: Negative Control, shTRPC6: shRNA TRPC6. Each trace represents the mean from three independent experiments that were derived from 20 to 40 cells in each single experiment.
Then we sought to further address the source of 6-h O3 exposure-induced augment of [Ca2+]i increase. By incubating cells with Ca2+-free bath solution containing 100 μM EGTA, we found that O3 exposure made no difference to H2O2-induced release of intracellular calcium storage (Fig. 6d, e) but enhanced the influx of extracellular Ca2+ when the intracellular calcium storage was depleted by pretreatment with thapsigargin (Tg, 2 μM) (Fig. 6f, g). Therefore, O3-amplified sensibility to oxidative stress via TRPC6 is mainly dependent on enhancing the influx of extracellular Ca2+ in the bronchial epithelial cells.
MAPK signal pathway contributes to oxidative stress-induced inflammatory response in bronchial epithelial cells
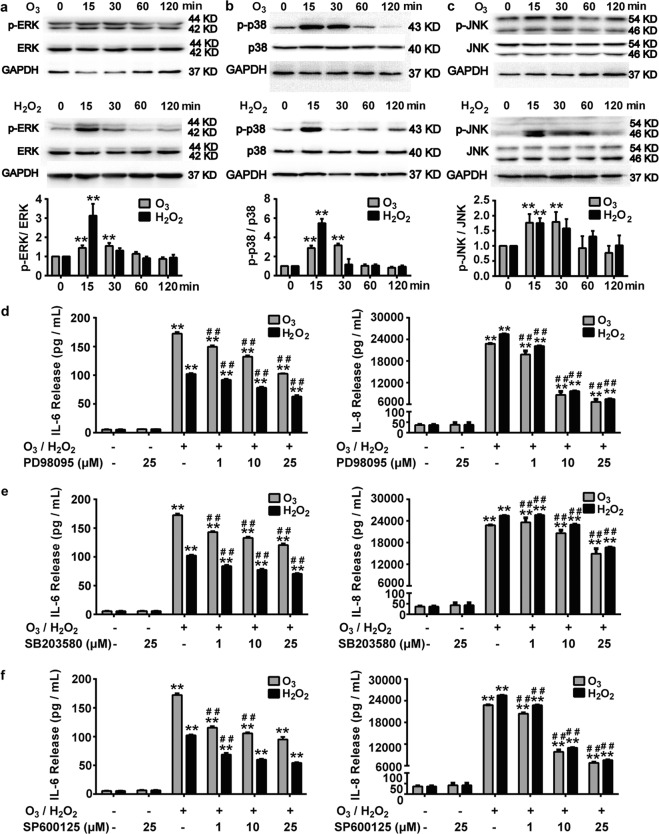
It has been established that MAPK (ERK, p38, JNK) was involved in oxidative stress-induced inflammatory response in lungs31. Thus we investigated whether the inflammatory response in this research was mediated by MAPK signal pathway. Results unveiled that after exposure to O3 (100 ppb) or H2O2 (100 μM) for 15 or 30 min, the phosphorylation levels of ERK1/2, p38 and JNK signaling pathways were significantly increased in 16HBE cells (Fig. 7a–c). Pretreatment with PD98059 (ERK inhibitor), SB203580 (p38 inhibitor) or SP600125 (JNK inhibitor) inhibited O3 or H2O2-augmented release of inflammatory factors IL-6 and IL-8 in 16HBE cells in a dose-dependent manner (Fig. 7d–f), suggesting these MAPK signals participated in O3 or H2O2-induced inflammatory response. These data demonstrate that MAPK signal pathway is responsible for oxidative stress-induced inflammatory response in bronchial epithelial cells.
Fig. 7. Role of MAPK signal pathway in oxidative stress-induced inflammatory response in bronchial epithelial cells.
a–c Western blot analysis of phosphorylation protein expression of ERK (a), p38 (b) and JNK (c) after 16HBE cells were stimulated with O3 (100 ppb) or H2O2 (100 μM) for 0, 15, 30, 60, 120 min. **P < 0.01 compared with 0-min group. d–f After pretreatment with or without PD98059 (1, 10, 25 μM) (d), SB203580 (1, 10, 25 μM) (e) or SP600125 (1, 10, 25 μM) (f) for 1 h, 16HBE cells were stimulated with O3 (100 ppb) for 6 h followed by continuing culture for another 24 h or stimulated with H2O2 (100 μM) for 24 h. Release levels of IL-6 and IL-8 were detected. Data represent the mean ± SEM, n = 5. **P < 0.01 compared with Control group, ##P < 0.01 compared with H2O2 or O3 group.
TRPC6 is required for the oxidative stress-induced activation of ERK pathway
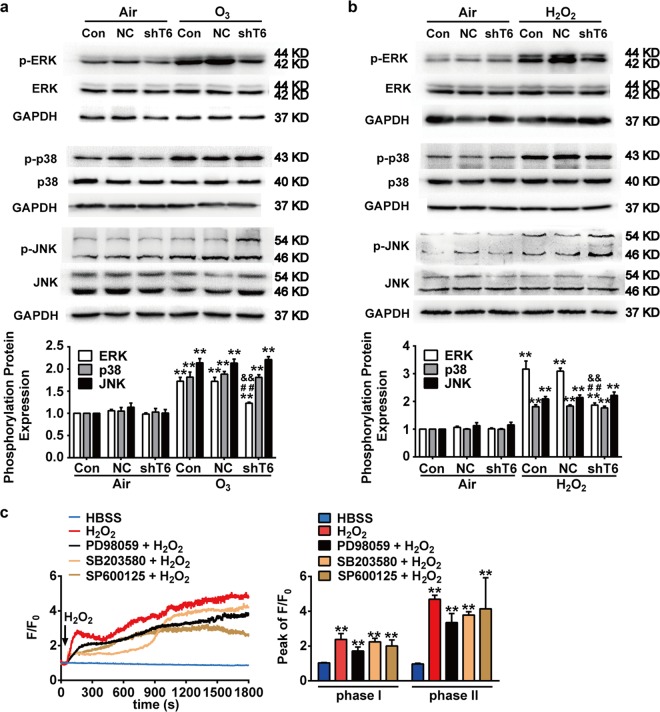
We next investigated the role of TRPC6 in oxidative stress-induced activation of MAPK pathways. Deficiency of TRPC6 by shTRPC6 significantly reversed O3 or H2O2-induced increase of phosphorylation levels of ERK but made no difference to those of p38 and JNK (Fig. 8a, b), indicating that TRPC6-mediated oxidative stress-induced activation of ERK pathway rather than p38 or JNK in bronchial epithelial cells. Additionally, pretreatment with MAPK inhibitors (PD98059, SB203580, or SP600125) did not affect the H2O2-induced [Ca2+]i increase (Fig. 8c), which suggested that TRPC6-mediated [Ca2+]i increase was not dependent on the MAPK pathways. Taken together, these experiments suggest that ERK acts as the downstream of TRPC6 in oxidative stress-induced inflammatory response.
Fig. 8. Role of TRPC6 in oxidative stress-induced activation of MAPK signal pathway.
a, b After NC or shTRPC6 infection, 16HBE cells were exposed to O3 (100 ppb) for 30 min (a) or H2O2 (100 μM) for 15 min (b) respectively. Phosphorylation levels of ERK, p38, JNK were analyzed by western blot. Data represent the mean ± SEM, n = 5. **P < 0.01 compared with Control group, ##P < 0.01 compared with O3 or H2O2 group, &&P < 0.01 compared with NC + O3 or NC + H2O2 group. c 16HBE cells were loaded with the Ca2+ indicator Fluo-4/AM (5 μM) in Hanks’ solution for 30 min to measure [Ca2+]i. The [Ca2+]i in the cells was expressed as a pseudo-ratio value of the relative fluorescence intensity (F/F0) (Left). Bar graph shows the mean peak value of F/F0 traces (Peak of F/F0) of the experiments (Right). After pretreatment with or without PD98059 (25 μM), SB203580 (25 μM) or SP600125 (25 μM) for 1 h, 16HBE cells were stimulated with H2O2 (100 μM). **P < 0.01 compared with HBSS control group. Each trace represents the mean from three independent experiments that were derived from 20 to 40 cells in each single experiment. NC: Negative Control, shT6: shRNA TRPC6.
Discussion
The major findings of our study demonstrated for the first time that TRPC6 acted as an oxidative stress sensor in bronchial epithelium and mediated oxidants-induced inflammatory responses via activating ERK. Exposure to oxidizing air pollutants (e.g. O3) and the following increase of ROS in lungs (e.g. H2O2) are key causative factors in the development of chronic inflammatory respiratory diseases. Here we found that O3 exposure to mice motivated severe airway inflammation and potentiated the expression of TRPC6 protein in lungs, especially in bronchial epithelium and alveolar macrophages. Utilizing TRPC6−/− mice and TRPC6-selective inhibitor, we found that TRPC6 contributed to O3 inhalation-induced airway inflammation. In vitro experiments we confirmed the requirement of TRPC6 for oxidative stress-induced inflammatory responses and the upregulation of the TRPC6 protein expression by O3 or H2O2 stimulation in human bronchial epithelial cells. Furthermore, H2O2-triggered [Ca2+]i increase composing of the release of intracellular Ca2+ store and influx of extracellular Ca2+ was mediated by TRPC6 channels. Importantly, O3 exposure enhanced the influx of extracellular Ca2+ triggered by H2O2, which was abolished by TRPC6 knockdown or blockage. Moreover, MAPK signal pathway (ERK, p38, JNK) was responsible for the inflammatory response and ERK pathway acted as the downstream of TRPC6 in these experiments. Therefore, we concluded that TRPC6 regulated oxidative inflammatory responses induced by O3 or H2O2 through activating ERK pathway.
It is well known that exposure to O3 induces oxidative injury to the respiratory tract, causes inflammatory responses and triggers clinical symptoms of a series of chronic respiratory disease such as asthma, bronchitis and COPD32,33. However, the underlying mechanisms of O3-induced oxidative injury have not yet been fully defined. TRPC6, a lipid-dependent membrane protein acting as non-selective cation channels conducting Na+ and Ca2+, is highly expressed in the lung and most studied in pulmonary diseases among TRPC channels34,35. We previously reported that TRPC6 channels contribute to LPS-induced inflammatory response in human bronchial epithelial cells, which implies that TRPC6 may be a therapeutic target in bronchial epithelial inflammation36. It has been well described in many cell types and tissues that the activity of TRPC6 channel is redox-sensitive, while it seems to have different phenotype according to different cell types23,37. In podocytes, HEK 293T cells and vascular myocytes, ROS not only activates TRPC6 already in the plasma membrane but also upregulates the expression of TRPC6 in the surface38–42. Some other studies show that ROS decreases the activity and abundance of TRPC6 protein in mesangial cells20,43. However, it has been rarely described whether ROS activate TRPC6 channel, the underlying mechanisms of the following Ca2+ transportation and the further effects in respiratory system. Here, we found that O3 exposure led to the increase of TRPC6 protein expression in mice lungs (Fig. 4a, b). H2O2, a diffusible and ubiquitous second messenger, contributes to the pathogenesis of several respiratory disease and their exacerbations44. Generated in lungs during oxidative pollutants exposure and more stable to readily penetrate the cells, H2O2 is adopted in many studies to further investigate the mechanism related to ambient oxidants-induced injury. We found that both O3 and H2O2 enhanced TRPC6 expression in human bronchial epithelial cells (Fig. 4c–e). TRPC6−/− mice or mice pretreated with TRPC6 inhibitor SAR7334 exposed to O3 revealed attenuated recruitment of neutrophils, macrophages and lymphocytes into airway, release of inflammatory factor IL-6, IL-8 and TNF-α in BAL fluid and damage of the lungs (Figs. 1, 2). We also confirmed that activation of TRPC6 was required for the release of cytokines after stimulating with O3 or H2O2 in HBECs (Fig. 3). Although we did not detect the levels of ROS in vivo after O3 exposure, it is conceivable that oxidative stress generated by O3 activates TRPC6 channels and upregulates the expression of TRPC6, which leads to the disruptions of intracellular Ca2+ homeostasis and triggers inflammatory response based on the results in vitro (Figs. 3, 5, 6). Surprisingly, the levels of released TNF-α increased after O3 exposure in BAL fluid (Figs. 1c, 2c) but it remained unchanged after stimulating with O3 or H2O2 in epithelial cells (Fig. 3a, e). We speculated that TNF-α was secreted by other cells but not bronchial epithelial cells.
Although the functional significance of TRPC6-mediated Ca2+ transport system in health and disease has gained widespread attention, its activation mechanism is not completely elucidated yet. H2O2 has been reported to behave pathophysiologically relevantly with a concentration above 100 μM while a much lower concentration (10 μM) displays a nearly maximal activating effect on TRPC6 channel in HEK293T cells42. In the present study, we found that H2O2 was able to cause concentration-dependently increase of [Ca2+]i in 16HBE cells and primary HBEpiCs (Fig. 5a, b), which was mainly resulted from the activation of TRPC6 (Fig. 5e–g). Interestingly, H2O2-induced increase of [Ca2+]i occurred under the concentration of 10 μM in primary HBEpiCs while that occurred under 100 μM in 16HBE cells (Fig. 5a, b), suggesting primary HBEpiCs were more sensitive to oxidative stress than16HBE cells were.
Intracellular Ca2+ signal is the most universal and versatile mechanism regulating a wide range of physiological and pathophysiological processes. Store-operated Ca2+ entry and receptor-operated Ca2+ entry are pharmacologically and molecularly distinctive Ca2+ pathways in non-excitable cells. Hence we aimed to illuminate the source of H2O2-induced increase of [Ca2+]i in human bronchial epithelial cells. In this study we presented that H2O2 triggered [Ca2+]i increase in two ways: release from intracellular Ca2+ stores and Ca2+ influx into the cells (Fig. 5c, d). To date, although ample evidence suggests that TRPC6 is a DAG-sensitive receptor-operated Ca2+ entry channel45, studies about the source of TRPC6-mediated Ca2+ entry generate discordant findings46. Indeed, we found that TRPC6-mediated [Ca2+]i increase after H2O2 exposure consisted of the release of intracellular Ca2+ stores and the influx of extracellular Ca2+ since both phase I and phase II of H2O2-induced [Ca2+]i increase were inhibited under the situation of TRPC6 blockage or deficiency (Fig. 5e–g). Moreover, the profound augment of H2O2-induced influx of extracellular Ca2+ by O3 exposure was mainly dependent on TRPC6 channels as the potentiated phase II was abolished by TRPC6 blockage or deficiency (Fig. 6a–c). According to our finding that TRPC6 channels mediated the release of intracellular Ca2+ stores, we speculated that TRPC6 channels participated in store-operated Ca2+ entry although the detailed process still needs further exploration.
MAPK signal pathway is implicated in inflammatory response induced by oxidative stress31. Furthermore, previous studies show relationships between the activation of TRPC6 and MAPK signals, which seemed to be context-dependent47,48. Here, we found that treatments with H2O2 or O3 evoked the release of inflammatory factors IL-6 and IL-8 via activating MAPKs (ERK, p38, JNK) (Fig. 7). In addition, deficiency of TRPC6 by shTRPC6 significantly reversed the oxidative stress-induced phosphorylation of ERK but made no effect to that of p38 or JNK (Fig. 8a, b). However, pretreatments with MAPKs inhibitors (PD98059, SB203580, SP600125) did not influence the H2O2-induced increase of [Ca2+]i in 16HBE cells (Fig. 8c), excluding the possibility that MAPK signal pathway acted as the upstream of TRPC6. Therefore, it is suggested that in human bronchial epithelial cells oxidative stress-aroused inflammatory response was mediated by TRPC6 through regulating [Ca2+]i increase and subsequently activating ERK signal pathway.
In summary, this is the first report demonstrating that TRPC6 is an oxidative stress-sensitive channel which can further mediate inflammatory response via ERK pathway in bronchial epithelial cells. Our result provides a mechanistic understanding of how oxidizing air pollutants lead to airway inflammation. Moreover, we revealed a critical role of intracellular calcium homeostasis in the pathogenesis of such diseases and proposed that targeting TRPC6 might provide a novel therapeutic approach to prevent and treat oxidative stress-induced airway inflammation.
Materials and methods
Cell culture
16HBE cells (Jennio Biotechnology, CHN), a transformed human bronchial epithelial cell line were cultured with 10% fetal bovine serum (FBS) (10099141; Gibco, USA) as previously outlined36. Normal primary human bronchial epithelial cells (HBEpiCs) (3210; ScienCell, USA) were maintained in Bronchial Epithelial Cell Medium (BEpiCM) (3211; ScienCell, USA) with 1% bronchial epithelial cell growth supplement. Cells were cultured in humidified air with 5% CO2 at 37 °C. Prior to O3 or H2O2 (H6520; Sigma-Aldrich, CHN) exposure, cells were equilibrated by medium containing 1% FBS or 0.1% supplement medium.
Animals
Eight-week-old female wild-type (WT) and TRPC6-deficient (TRPC6−/−) mice, on 129SvEv:C57BL/6 J (50:50) crossbred background, were generously provided by Dr. Lutz Birnbaumer (National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina) and Dr. Yizheng Wang (Shanghai Institutes of Biological Sciences, State Key Laboratory of Neuroscience, CHN). The TRPC6 knockout genotype was confirmed by RT-PCR as previously described24,49. Mice were maintained under specific-pathogen-free conditions in the Laboratory Animal Center of Guangzhou Medical University. All animal experiments were carried out under the protocol approved by the Institutional Animal Care and Use Committee of Guangzhou Medical University.
ShRNA experiments to stably silence TRPC6 and quantitative real-time PCR analysis in 16HBE cells
16HBE cells were transfected with lentivectors expressing TRPC6 shRNA (5′-CCGCUAUGAACUCCU-UGAA-3′) or negative control (5′-TTCTCCGAACGTGTCACGT-3′) (Oboi Techonology, CHN). The rate of effective transfection in the cells was monitored by fluorescence microscopy. Over 95% of cells transfected with lentiviral vectors showed red fluorescence in the experimental and NC groups. The stably transfected cells were picked out with puromycin (4 μg/mL) after being cultured with the lentivirus (1.66 × 10E9 TU/mL) at an infection multiplicity of 80 for 24 h. Deficiency of TRPC6 expression was analyzed with real-time RT PCR and western blot.
Total RNAs were obtained from lungs or cultured cells with the TRIzol reagent (15596018; Invitrogen, USA). Real-time PCR was performed with SYBR Premix Taq Kit (DRR081, TaKaRa, CHN) and analyzed with ABI PRISM 7000 Sequence Detection System (Applied Biosystems, USA). All primers were purchased from Invitrogen Corporation (as illustrated in Table 1). The qPCR results were presented as threshold cycle (Ct) value and the relative mRNA quantification was determined using the 2-∆∆ct method GAPDH as the endogenous control and normalized to a control group.
Table 1.
Sequences of the primers used for quantitative real-time PCR.
| Name | Forward primer (5'−3') | Reverse primer (5'−3') | Product size |
|---|---|---|---|
| TRPC6 | GGTGAGCCAGTCTGTTGTCA | TATCTGCTCATGGACTCGGA | 109 bp |
| GAPDH | GAAGGTCGGAGTCAACGG | GGAAGATGGTGATGGGATT | 221 bp |
O3 exposure
Experiments of O3 exposure in vivo were performed in reference to similar exposure study50. Mice were randomly assigned into different groups (eight mice per group) and placed awake in whole-body Plexiglas exposure chamber (0.55 m wide, 0.75 m long, 0.65 m high) to be exposed to O3 (1 ppm) for 3 h. Another identical exposure chamber was applied for air exposure. Exposure was repeated every other day (day 1, 3, and 5) to optimize airway inflammation and did not induce any weight change in the mice (unpublished data). Mice received SAR7334 (HY-15699; MedChem Express, USA), a TRPC6-selective inhibitor, by oral gavage 4 h before O3 exposure, as reported by Maier and colleagues that pharmacologically effective concentrations of SAR7334 reached optimum 4 hours after oral administration and maintained for several hours51. No animals were excluded from the analysis and no blinding was carried out for animal experiments.
Cells were exposed to O3 (100 ppb) with 5% CO2 humidified air at 37 °C in the incubator. During exposure, cells were placed on the 3D rocking platform and tilted gently to an angle of 10° from the horizontal to each quarter to ensure direct contact with O3. At the end of the exposure, cells were relocated to another incubator with fresh atmosphere. Assays were performed 0-, 12- or 24-hour after the exposure. Experiments of O3 exposure in vitro were done referred to similar exposure study52. Before O3 exposure, cells were pre-treated with TRPC6-selective inhibitor Larixyl Acetate (LA) (02730595; Sigma-Aldrich, CHN), ERK inhibitor PD98059 (9900; CST, CHN), p38 inhibitor SB203580 (5633; CST, CHN) or JNK inhibitor SP600125 (8177; CST, CHN) for 30 or 60 min.
O3 were generated by HAILEA model HLO-800 ozonizers (HAILEA, CHN) and its concentration within the chamber or incubator was monitored over the exposing period by ambient-air O3 motors (model 106 L; T2B, USA). The mean concentration of O3 within the chambers remained 1 ± 0.05 ppm or 100 ± 10 ppb within the incubator during the exposure.
Measurement of inflammation by collection and analysis of BAL fluid or supernatants of cells
Mice were anesthetized by intraperitoneal injection of pentobarbital sodium (50 mg/kg) 24 h after the last exposure. To collect bronchoalveolar lavage (BAL) fluid, the left bronchus was clamped, the trachea cannulated and then the right lung lavaged three times slowly with 0.6 mL ice-cold phosphate-buffered saline (PBS). BAL fluids were centrifuged to isolate cells from samples. The supernatants were collected and stored at −80 °C till analysis. To quantify total cell numbers, cell pellets were resuspended with 200 μl PBS and multiplied hemacytometer cell counts excluding red blood cells. Differential cell counts stained by Wright-Giema stain set (D10; Jiancheng Bioengineering Institute, CHN) were determined under an optical microscope (EVOS FI; Advanced Microscope Group, USA). At least 200 cells per mouse were counted under ×200 magnification.
The secretion levels of IL-6, IL-8 and TNF-α in the mentioned supernatants as well as cell medium were detected with commercially available enzyme-linked immunosorbent assay (ELISA) kits (NeoBioscience Technology, CHN) according to the instruction manual. Total protein content was determined using a Pierce BCA Protein Assay kit (23225; Thermo Fisher Scientific, USA). The results were expressed as pg/mg protein.
Histological analysis
Following BAL fluid collection, the non-lavaged lungs were cut out and immediately inflated in fresh 4% paraformaldehyde buffer. Paraffin blocks were prepared from dehydrated tissues and histological sections (5 μm) were stained with hematoxylin and eosin (H&E) for evaluation with a light microscopic (Olympus BX51, JPN).
The severity of lung inflammation in peribronchial and perivascular in H&E sections was scored on a scale ranging from 0 to 3 where 0 means no inflammation; 1 means mild inflammation with only a few inflammatory cells around bronchial or vascular wall and in alveolar space; 2 means moderate inflammation with patchy inflammatory cells infiltration or localized inflammation around bronchial or vascular wall and in alveolar space and less than one-third of lung cross-sectional area involved; 3 means severe inflammation with diffuse inflammatory cells infiltration and more than one-third of the lung area involved. The scale was made referred to similar study53.
Western blot
After different treatments, 16HBE cells as well as the lung tissue were rinsed with ice-cold PBS. Following steps were performed as previously described36. Immunoblotting was detected using Immobilon Western Chemiluminescent HRP Substrate (Millipore, USA) and imaged on ChemiDoc XRS + (Bio-Rad, USA). Western blot bands were quantified by ImageJ 1.41 software and expressed as fold compared to control. Primary specific antibodies used were TRPC6 (CST, #16716), p44/42 MAPK (ERK1/2) (CST, #4695) and Phospho-p44/42 MAPK (ERK1/2) (CST, #4370), p38 MAPK 2103(CST, #4671), GAPDH (Proteintech, 60004).
Immunohistochemistry (IHC)
IHC was performed on paraffin-embedded lung tissue sections following standard methods. Lung sections (5 μm) were deparaffinized, rehydrated, treated for endogenous peroxidase inhibition and antigen retrieval and then incubated overnight at 4 °C with primary anti-TRPC6 antibody (dilution 1:100) (ACC-017; Alomone Labs, Israel), followed by 30-min incubation with Horseradish peroxidase-conjugated secondary antibody (dilution 1:500). Binding was visualized with DAB and counterstained with hematoxylin. Staining images were taken by a confocal laser scanning microscopy (BX51; Olympus, JPN).
Fluorescence measurement of intracellular free calcium ([Ca2+]i)
16HBE cells and HBEpiCs grown on confocal dishes were washed three times with fresh HBSS and 200 μl HBSS was added per dish. The fluorescence intensity of Fluo-4 (5 μM, Molecular Probes Eugene, USA) in the cells was recorded by laser scanning confocal microscopy (Leica TCS SP8, GER). Detailed steps were performed as previously described36. H2O2 was used to stimulate the cells when the baseline was stable. The [Ca2+]i was expressed as a pseudo-ratio value (F/F0) of the actual fluorescence intensity (F) divided by the average baseline fluorescence intensity (F0). The Ca2+-free bath solution contained 100 μM EGTA and no CaCl2. Data from 20 to 40 cells were compiled from a single run, and at least three independent experiments were conducted.
Analysis of the expression of TRPC6 protein in the cells by immunofluorescence
The cells cultured on confocal dishes were fixed with 4% formaldehyde in 0.1 M PBS for 15 min and washed three times with 0.1 M PBS. Further details were performed as previously described 366. The cells were incubated with primary specific antibodies against TRPC6 (ACC-017; Alomone Labs, ISR) at 4 °C overnight and rewarmed for 30 min subsequently. Following 3 washes with PBS, the cells were incubated with fluorescein isothiocyanate (FITC)-conjugated secondary antibody (Life Technology, USA) for another 1 h. The nuclei were stained by the fluorescent DNA-binding dye 136 4′, 6-diamidino-2-phenylindole dihydrochloride (DAPI) (Roche, CHN) for 15 min. Fluorescence pictures were taken under a confocal laser scanning microscopy (SP8; Leica TCS, GER).
Statistical analysis
Statistical analysis was performed with SPSS 13.0 software. Data expressed as mean ± SEM represented at least five independent experiments. Statistical significance was determined using Student’s test or one-way analysis of variance followed by ANOVA and post hoc Bonferroni or Dunnett T3 test. Differences were considered statistically significant when the probability value <0.05 or <0.01.
Supplementary information
Acknowledgements
Present address of J.-R. Huang: The Fifth Affiliated Hospital of Guangzhou Medical University. No.621, Gangwan Road, Huangpu District, Guangzhou City, Guangdong Province 510700, China (e-mail: guke16@163.com). In addition, we thank the anonymous reviewers and the editors for their constructive suggestions in order to improve our manuscript. We thank Dr. Lutz Birnbaumer (National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina) and Dr. Yizheng Wang (Shanghai Institutes of Biological Sciences, State Key Laboratory of Neuroscience, China) for TRPC6−/− mice. This work was supported by National Natural Science Foundation of China (81470205), Key Scientific Research Project of Guangzhou Municipal Colleges and Universities (2012C043) and Guangzhou Key Medical Discipline Construction Project Fund.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Edited by J.-E. Ricci
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Qingzi Chen, Yubo Zhou, Lifen Zhou
These authors jointly supervised this work: Jianrong Huang, Jianhua Li
Contributor Information
Jianrong Huang, Email: guke16@163.com.
Jianhua Li, Email: lijianh@gzhmu.edu.cn.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41419-020-2360-0).
References
- 1.Hsuan SL, et al. Pasteurella haemolytica leukotoxin and endotoxin induced cytokine gene expression in bovine alveolar macrophages requires NF-kappaB activation and calcium elevation. Micro. Pathog. 1999;26:263–273. doi: 10.1006/mpat.1998.0271. [DOI] [PubMed] [Google Scholar]
- 2.Ribeiro CM. The role of intracellular calcium signals in inflammatory responses of polarised cystic fibrosis human airway epithelia. Drugs R. D. 2006;7:17–31. doi: 10.2165/00126839-200607010-00002. [DOI] [PubMed] [Google Scholar]
- 3.Ratner AJ, et al. Cystic fibrosis pathogens activate Ca2+-dependent mitogen-activated protein kinase signaling pathways in airway epithelial cells. J. Biol. Chem. 2001;276:19267–19275. doi: 10.1074/jbc.M007703200. [DOI] [PubMed] [Google Scholar]
- 4.Chun J, Prince A. Ca2+ signaling in airway epithelial cells facilitates leukocyte recruitment and transepithelial migration. J. Leukoc. Biol. 2009;86:1135–1144. doi: 10.1189/jlb.0209072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis CW, Dickey BF. Regulated airway goblet cell mucin secretion. Annu. Rev. Physiol. 2008;70:487–512. doi: 10.1146/annurev.physiol.70.113006.100638. [DOI] [PubMed] [Google Scholar]
- 6.Samanta K, Bakowski D, Parekh AB. Key role for store-operated Ca2+ channels in activating gene expression in human airway bronchial epithelial cells. PLoS ONE. 2014;9:e105586. doi: 10.1371/journal.pone.0105586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorlach A, Bertram K, Hudecova S, Krizanova O. Calcium and ROS: A mutual interplay. Redox Biol. 2015;6:260–271. doi: 10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto S, et al. TRPM2-mediated Ca2+influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat. Med. 2008;14:738–747. doi: 10.1038/nm1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beceiro S, et al. TRPM2 ion channels regulate macrophage polarization and gastric inflammation during Helicobacter pylori infection. Mucosal Immunol. 2017;10:493–507. doi: 10.1038/mi.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abramowitz J, Birnbaumer L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 2009;23:297–328. doi: 10.1096/fj.08-119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corteling RL, et al. Expression of transient receptor potential C6 and related transient receptor potential family members in human airway smooth muscle and lung tissue. Am. J. Respir. Cell Mol. Biol. 2004;30:145–154. doi: 10.1165/rcmb.2003-0134OC. [DOI] [PubMed] [Google Scholar]
- 12.Finney-Hayward TK, et al. Expression of transient receptor potential C6 channels in human lung macrophages. Am. J. Respir. Cell Mol. Biol. 2010;43:296–304. doi: 10.1165/rcmb.2008-0373OC. [DOI] [PubMed] [Google Scholar]
- 13.Malczyk M, et al. The role of transient receptor potential channel 6 channels in the pulmonary vasculature. Front Immunol. 2017;8:707. doi: 10.3389/fimmu.2017.00707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Preti D, Szallasi A, Patacchini R. TRP channels as therapeutic targets in airway disorders: a patent review. Expert Opin. Ther. Pat. 2012;22:663–695. doi: 10.1517/13543776.2012.696099. [DOI] [PubMed] [Google Scholar]
- 15.Dietrich Alexander. Modulators of Transient Receptor Potential (TRP) Channels as Therapeutic Options in Lung Disease. Pharmaceuticals. 2019;12(1):23. doi: 10.3390/ph12010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu Y, et al. A functional single-nucleotide polymorphism in the TRPC6 gene promoter associated with idiopathic pulmonary arterial hypertension. Circulation. 2009;119:2313–2322. doi: 10.1161/CIRCULATIONAHA.108.782458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramirez Giuseppe, Coletto Lavinia, Sciorati Clara, Bozzolo Enrica, Manunta Paolo, Rovere-Querini Patrizia, Manfredi Angelo. Ion Channels and Transporters in Inflammation: Special Focus on TRP Channels and TRPC6. Cells. 2018;7(7):70. doi: 10.3390/cells7070070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindemann O, et al. TRPC6 regulates CXCR2-mediated chemotaxis of murine neutrophils. J. Immunol. 2013;190:5496–5505. doi: 10.4049/jimmunol.1201502. [DOI] [PubMed] [Google Scholar]
- 19.Tauseef M, et al. TLR4 activation of TRPC6-dependent calcium signaling mediates endotoxin-induced lung vascular permeability and inflammation. J. Exp. Med. 2012;209:1953–1968. doi: 10.1084/jem.20111355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graham S, et al. Abundance of TRPC6 protein in glomerular mesangial cells is decreased by ROS and PKC in diabetes. Am. J. Physiol. Cell Physiol. 2011;301:C304–C315. doi: 10.1152/ajpcell.00014.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding Y, et al. Reactive oxygen species-mediated TRPC6 protein activation in vascular myocytes, a mechanism for vasoconstrictor-regulated vascular tone. J. Biol. Chem. 2011;286:31799–31809. doi: 10.1074/jbc.M111.248344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, et al. Nuclear factor kappaB mediates suppression of canonical transient receptor potential 6 expression by reactive oxygen species and protein kinase C in kidney cells. J. Biol. Chem. 2013;288:12852–12865. doi: 10.1074/jbc.M112.410357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma R, Chaudhari S, Li W. Canonical transient receptor potential 6 channel: a new target of reactive oxygen species in renal physiology and pathology. Antioxid. Redox Signal. 2016;25:732–748. doi: 10.1089/ars.2016.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen QZ, et al. TRPC6 modulates adhesion of neutrophils to airway epithelial cells via NF-kappaB activation and ICAM-1 expression with ozone exposure. Exp. Cell Res. 2019;377:56–66. doi: 10.1016/j.yexcr.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Urban N, et al. Identification and validation of larixyl acetate as a potent TRPC6 inhibitor. Mol. Pharm. 2016;89:197–213. doi: 10.1124/mol.115.100792. [DOI] [PubMed] [Google Scholar]
- 26.Charrier JG, McFall AS, Richards-Henderson NK, Anastasio C. Hydrogen peroxide formation in a surrogate lung fluid by transition metals and quinones present in particulate matter. Environ. Sci. Technol. 2014;48:7010–7017. doi: 10.1021/es501011w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Groeger G, Quiney C, Cotter TG. Hydrogen peroxide as a cell-survival signaling molecule. Antioxid. Redox Signal. 2009;11:2655–2671. doi: 10.1089/ars.2009.2728. [DOI] [PubMed] [Google Scholar]
- 28.McCullough SD, et al. Ozone induces a proinflammatory response in primary human bronchial epithelial cells through mitogen-activated protein kinase activation without nuclear factor-kappaB activation. Am. J. Respir. Cell Mol. Biol. 2014;51:426–435. doi: 10.1165/rcmb.2013-0515OC. [DOI] [PubMed] [Google Scholar]
- 29.Feng W, et al. Human normal bronchial epithelial cells: a novel in vitro cell model for toxicity evaluation. PLoS ONE. 2015;10:e0123520. doi: 10.1371/journal.pone.0123520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 31.Park HS, Kim SR, Lee YC. Impact of oxidative stress on lung diseases. Respirology. 2009;14:27–38. doi: 10.1111/j.1440-1843.2008.01447.x. [DOI] [PubMed] [Google Scholar]
- 32.Nuvolone D, Petri D, Voller F. The effects of ozone on human health. Environ. Sci. Pollut. Res. Int. 2018;25:8074–8088. doi: 10.1007/s11356-017-9239-3. [DOI] [PubMed] [Google Scholar]
- 33.Jerrett M, et al. Long-term ozone exposure and mortality. N. Engl. J. Med. 2009;360:1085–1095. doi: 10.1056/NEJMoa0803894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weissmann N, et al. Activation of TRPC6 channels is essential for lung ischaemia-reperfusion induced oedema in mice. Nat. Commun. 2012;3:649. doi: 10.1038/ncomms1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Preti D, Szallasi A, Patacchini R. TRP channels as therapeutic targets in airway disorders: a patent review. Expert Opin. Ther. Pat. 2012;22:663–695. doi: 10.1517/13543776.2012.696099. [DOI] [PubMed] [Google Scholar]
- 36.Zhou LF, et al. TRPC6 contributes to LPS-induced inflammation through ERK1/2 and p38 pathways in bronchial epithelial cells. Am. J. Physiol. Cell Physiol. 2018;314:C278–C288. doi: 10.1152/ajpcell.00117.2017. [DOI] [PubMed] [Google Scholar]
- 37.Dryer SE, Kim EY. Permeation and rectification in canonical transient receptor potential-6 (TRPC6) channels. Front. Physiol. 2018;9:1055. doi: 10.3389/fphys.2018.01055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding Y, et al. Reactive oxygen species-mediated TRPC6 protein activation in vascular myocytes, a mechanism for vasoconstrictor-regulated vascular tone. J. Biol. Chem. 2011;286:31799–31809. doi: 10.1074/jbc.M111.248344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson M, Roshanravan H, Khine J, Dryer SE. Angiotensin II activation of TRPC6 channels in rat podocytes requires generation of reactive oxygen species. J. Cell Physiol. 2014;229:434–442. doi: 10.1002/jcp.24461. [DOI] [PubMed] [Google Scholar]
- 40.Kim EY, et al. NOX2 interacts with podocyte TRPC6 channels and contributes to their activation by diacylglycerol: essential role of podocin in formation of this complex. Am. J. Physiol. Cell Physiol. 2013;305:C960–C971. doi: 10.1152/ajpcell.00191.2013. [DOI] [PubMed] [Google Scholar]
- 41.Kim EY, Anderson M, Dryer SE. Insulin increases surface expression of TRPC6 channels in podocytes: role of NADPH oxidases and reactive oxygen species. Am. J. Physiol. Ren. Physiol. 2012;302:F298–F307. doi: 10.1152/ajprenal.00423.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graham S, et al. Canonical transient receptor potential 6 (TRPC6), a redox-regulated cation channel. J. Biol. Chem. 2010;285:23466–23476. doi: 10.1074/jbc.M109.093500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, et al. Nuclear factor kappaB mediates suppression of canonical transient receptor potential 6 expression by reactive oxygen species and protein kinase C in kidney cells. J. Biol. Chem. 2013;288:12852–12865. doi: 10.1074/jbc.M112.410357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drost EM, et al. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax. 2005;60:293–300. doi: 10.1136/thx.2004.027946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hofmann T, et al. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- 46.Hou X, et al. Transient receptor potential channel 6 knockdown prevents apoptosis of renal tubular epithelial cells upon oxidative stress via autophagy activation. Cell Death Dis. 2018;9:1015. doi: 10.1038/s41419-018-1052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shirakawa H, et al. Sphingosine-1-phosphate induces Ca(2+) signaling and CXCL1 release via TRPC6 channel in astrocytes. Glia. 2017;65:1005–1016. doi: 10.1002/glia.23141. [DOI] [PubMed] [Google Scholar]
- 48.Shen B, et al. cAMP activates TRPC6 channels via the phosphatidylinositol 3-kinase (PI3K)-protein kinase B (PKB)-mitogen-activated protein kinase kinase (MEK)-ERK1/2 signaling pathway. J. Biol. Chem. 2011;286:19439–19445. doi: 10.1074/jbc.M110.210294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dietrich A, et al. Increased vascular smooth muscle contractility in TRPC6-/- mice. Mol. Cell Biol. 2005;25:6980–6989. doi: 10.1128/MCB.25.16.6980-6989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pichavant M, et al. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J. Exp. Med. 2008;205:385–393. doi: 10.1084/jem.20071507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maier T, et al. Discovery and pharmacological characterization of a novel potent inhibitor of diacylglycerol-sensitive TRPC cation channels. Br. J. Pharm. 2015;172:3650–3660. doi: 10.1111/bph.13151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu W, et al. SRC-mediated EGF receptor activation regulates ozone-induced interleukin 8 expression in human bronchial epithelial cells. Environ. Health Perspect. 2015;123:231–236. doi: 10.1289/ehp.1307379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michaudel C, et al. Interleukin-1alpha mediates ozone-induced myeloid differentiation factor-88-dependent epithelial tissue injury and inflammation. Front Immunol. 2018;9:916. doi: 10.3389/fimmu.2018.00916. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.