Abstract
Diabetes and undernutrition are common risk factors for TB, associated with poor treatment outcomes and exacerbated by TB. We aimed to assess non-communicable multimorbidity (co-occurrence of two or more medical conditions) in Filipino TB outpatients, focusing on malnutrition and diabetes. In a cross-sectional study, 637 adults (70% male) from clinics in urban Metro Manila (N = 338) and rural Negros Occidental (N = 299) were enrolled. Diabetes was defined as HbA1c of ≥6.5% and/or current diabetes medication. Study-specific HIV screening was conducted. The prevalence of diabetes was 9.2% (54/589, 95%CI: 7.0–11.8%) with 52% newly diagnosed. Moderate/severe undernutrition (body mass index (BMI) <17 kg/2) was 20.5% (130/634, 95%CI: 17.4–23.9%). Forty percent of participants had at least one co-morbidity (diabetes, moderate/severe undernutrition or moderate/severe anaemia (haemoglobin <11 g/dL)). HIV infection (24.4%, 74/303) was not associated with other co-morbidities (but high refusal in rural clinics). Central obesity assessed by waist-to-hip ratio was more strongly associated with diabetes (Adjusted Odds Ratio (AOR) = 6.16, 95%CI: 3.15–12.0) than BMI. Undernutrition was less common in men (AOR = 0.44, 95%CI: 0.28–0.70), and associated with previous history of TB (AOR = 1.97, 95%CI: 1.28–3.04) and recent reduced food intake. The prevalence of multimorbidity was high demonstrating a significant unmet need. HIV was not a risk factor for increased non-communicable multimorbidity.
Subject terms: Tuberculosis, Malnutrition, Type 2 diabetes, Epidemiology, Epidemiology
Introduction
Tuberculosis (TB) remains a global health burden despite advances in diagnosis and treatment and is the leading cause of death from a single infectious agent1. Undernutrition is both a risk factor and a consequence of active TB disease and is associated with mortality and adverse outcomes2,3. Diabetes is another known risk factor for active TB4 and diabetes increases the risk of death and relapse during TB disease5 whilst TB can also negatively affect glycaemic control6 and may cause the development of diabetes, although this may sometimes be a transient effect6. Thus two conditions, both largely nutritional in origin, present a significant challenge to the End TB Strategy targets due to the increasing double burden of under and over nutrition occurring in many high burden, low and middle-income countries7,8. For implementation of the END TB strategy, it is recommended that “All persons with TB need to be assessed for nutritional status and receive nutritional counselling and care according to need” and additionally, “all persons with TB should be screened for diabetes” and, “in addition to HIV/AIDS, other co-morbidities and health risks associated with TB require integrated management”9. Both diabetes and TB can increase the short and long-term risk of cardiovascular10 and respiratory conditions11 requiring lifelong management to reduce population health and economic impacts and are recognised as priorities for cost-effective disease control12,13. As well as increasing the risk of developing active TB disease, untreated co-morbid undernutrition and diabetes may also increase the duration of infectivity and risk of relapse, thus contributing to ongoing transmission14. Undernutrition and anaemia during and after treatment are also likely to affect quality of life and productivity of TB affected households, contributing to a cycle of poverty and risk of catastrophic costs to households; the reduction of which is another goal of the End TB Strategy8. However, there are relatively few studies or data specifically documenting the prevalence of these and other co-morbid conditions in persons with TB or of the extent of overlap between them; information needed for the planning of services.
The Philippines, located in the Western-Pacific region is one of the WHO high burden countries for TB and multi-drug resistant TB (MDR-TB) and has the third highest TB incidence of 554/100,000 occurring in the context of a low HIV prevalence, estimated at 2% of incident TB cases, although HIV testing is low in many areas1. The prevalence of chronic energy deficiency (BMI < 18.5 kg/m2) in Filipino adults was 10% in the most recent National Nutrition Survey completed in 201415. Nationally, the population attributable risk fraction of undernutrition (BMI < 18.5 kg/m2) for TB has been estimated at 30%16. The prevalence of diabetes in the Philippines is increasing, with the best estimate from 2008 of 7.2%17.
There are currently very limited data of the prevalence of co-morbidities in Filipino persons with TB disease. Limited data of diabetes in TB has been identified as a barrier to planning a coordinated response18. The Philippine’s Plan of Action to Control Tuberculosis identifies the need to develop guidelines for persons living with diabetes as a vulnerable group, including screening for diabetes in TB, but undernutrition is not mentioned and no TB programme guidelines for its diagnosis and treatment exist19. In the general population obesity and in particular central obesity, are common risk factors for diabetes and are important components of various risk scores to detect undiagnosed diabetes. However, the relationship of these in persons with active TB disease is likely to differ, due to TB-associated weight loss.
The aim of this cross-sectional study was to provide a quantitative assessment of non-communicable multimorbidity (co-occurrence of two or more medical conditions)20 in TB DOTS outpatients in a rural and an urban setting in the Philippines, focusing on malnutrition and diabetes and on the degree of overlap between co-morbid conditions including HIV infection. The primary objective was to determine the prevalence of moderate or severe undernutrition (body mass index, BMI, <17.0 kg/m2) and diabetes (HbA1c ≥ 6.5% or receiving treatment for diabetes) among adults, aged 18 years and above enrolled in our participating TB-DOTS sites.
Methods
Study design
A cross-sectional survey of persons enrolled in TB-DOTS clinics (ISRCTN12506117).
Setting
Three TB-DOTS clinics in Metro Manila provided data from an urban setting, including the TB-DOTS and programmatic management of drug resistant TB (PMDT) centres at San Lazaro Hospital, an infectious disease referral hospital and HIV treatment centre, serving a predominantly poor population. Metro Manila is identified as a high HIV category area compared to other areas of the country. Two sites on the island of Negros Occidental provided data from a rural setting.
Participants
All non-pregnant adults (≥18 years) whose TB-DOTS registration date indicated that TB treatment should be ongoing at each study site, were eligible to participate. This included any patients who had stopped attending the TB DOTS clinic or visited infrequently. Individuals were approached to participate and enrolled at the clinics, or were traced and approached by research nurses in their Barangay (neighbourhood). This was done to attempt to locate all those whose treatment was supposed to be ongoing, to minimize selection bias.
Outcomes
The primary outcome of interest was co-morbid acute undernutrition (BMI <18.5 kg/m2)21 and the secondary outcome was diabetes, defined as HBA1c >6.5% or receiving treatment for diabetes at the time of enrolment22. However, analyses of undernutrition focussed on moderate and severe undernutrition (BMI <17 kg/m2), due to this being a level at which intervention is generally recommended. Other secondary outcomes of interest were moderate or severe anaemia (haemoglobin <11 g/dL)23, HIV and reported hypertension.
Data collection
Trained research nurses interviewed participants and completed all study assessments using structured questionnaires and extracted information recorded on individuals’ National TB Program treatment cards using direct electronic data capture with tablets using Open Data Kit software24. Data were uploaded to a secure server daily. Household food security was assessed using the Adapted U.S. Household Food Security Survey Module (US HFSSM)25. Research nurses conducted anthropometry, including weight (to the nearest 0.1 kg; Seca 803 Clara Digital Personal Non-Medical Scale) on a flat surface with the patient standing upright and unassisted without shoes. Heights were taken (to nearest 0.5 cm; Seca 216 Mechanical Stadiometer) without shoes or socks, with the patient standing unsupported and positioned fully upright with the lower border of the left orbit and the upper margin of the external auditory meatus horizontal. Waist and hip circumferences were measured (to the nearest 0.5 cm; Seca 201 measuring tape), midway between the uppermost border of the iliac crest and lower border of costal margin with tape parallel to the floor26. Fingerprick blood samples were used to obtain haemoglobin (HemoCue 301, Ängelholm, Sweden), HbA1C, C-reactive protein (CRP) (Alere Afinion AS100 Analyzer) and conduct HIV screening (Standard Diagnostics Bioline HIV-1/2 Ag/Ab Combo Rapid Test kits) for those with unknown status and who provided additional consent.
Sample size
Sample sizes were calculated based on formulae to estimate prevalence with precision27. A sample size of 600 (300 urban, 300 rural participants) was determined to provide 90% power to estimate prevalence of undernutrition (BMI < 18.5 kg/m2) within ±6% if the true prevalence was between 40–60%, and to estimate the prevalence of diabetes within ±5% if the true prevalence was between 5–15%, or within ±4% if the true prevalence was between 5–10%. This sample size was also predicted to provide 90% power to detect an absolute difference of at least 15% in the prevalence of acute undernutrition between urban and rural areas if the prevalence in the urban area of Metro Manila was between 30% and 60%.
Statistical methods
Data were analyzed using Stata, version 14.1 (College Station TX: StataCorp LP) and R28. Characteristics of those enrolled were summarized overall and by area (Metro Manila (urban) and Negros Occidental (rural)). Differences in characteristics between areas were tested with either a Fisher’s exact test, t-test or Wilcoxon rank sum test as appropriate for the data type. Prevalence of moderate or severe undernutrition, diabetes and moderate or severe anaemia are presented with corresponding 95% CIs overall and by area. Differences in prevalence of comorbidities by area were tested with Fisher’s exact tests. The distribution of HbA1c versus BMI by previous diagnosis status and by area are shown graphically, with associations between BMI and HbA1c tested using Spearman’s correlation coefficient. The extent of overlap between diabetes, moderate or severe undernutrition, moderate or severe anaemia and HIV are illustrated with Venn diagrams.
Associations with outcomes of undernutrition and diabetes were investigated using logistic regression. A multivariable model was built for each outcome using a forward stepwise approach, including factors associated with each outcome in univariable analyses based on a likelihood ratio test (LRT) p < 0.1, with retention and exclusion criteria of p < 0.1 from a likelihood ratio test comparing models at each model building step.
Ethics
Ethical approval was obtained from the ethical review boards of The London School of Hygiene & Tropical Medicine (REF: 11995), The School of Tropical Medicine and Global Health, Nagasaki University (14th October 2016) and the Philippines National Ethics Committee (REF: 2016–021-Cox-Malnutrition and Tuberculosis) and San Lazaro Hospital, Santa Cruz, Manila, the Philippines (23rd February 2017). Written informed consent was obtained from all participants prior to study enrolment and additional written consent was obtained for optional HIV screening following Dept of Health procedures. All study procedures were conducted in accordance with UK, Japan and Filipino laws and regulations including the Philippines Data Privacy Act.
Results
Participants
A total of 637 persons were recruited into the study across the five sites; 338 (53.1%) from three urban sites (Metro Manila) and 299 (46.9%) from two rural sites (Table 1) during the period 8th May to 18th September 2017. This represented 63.4% of those identified from the registers in the rural sites and 73.2% in the urban sites. Of those enrolled, 37.9% (240/633) had a history of previous tuberculosis treatment, classified as either relapse, treatment after loss to follow-up, treatment failure or previous treatment outcome unknown. Thirty-five percent of participants had bacteriological confirmation (220/628) of tuberculosis disease. Compared to rural areas, participants in urban areas were notably younger (Fisher’s exact test test p < 0.001, mean age 41 years vs 50 years), more likely to be unmarried (Fisher’s exact test p < 0.001), more likely to have some level of education or training beyond primary education (Fisher’s exact test p < 0.001), not have health insurance (Fisher’s exact test p = 0.002), have a longer transportation time to the clinic (Fisher’s exact test p < 0.001), and less likely to be current smokers (Fisher’s exact test p < 0.001) or frequent drinkers (Fisher’s exact test p < 0.001). There was substantially more food insecurity reported in those enrolled at rural sites (Fisher’s exact test p < 0.001), compared to urban along with substantially more reported weight loss in the last 3–6 months (Fisher’s exact test p < 0.001) and severe decreases in food intake in the last month (Fisher’s exact test p < 0.001). In urban sites, slightly more participants were in the intensive rather than continuation phase of treatment (Fisher’s exact test p = 0.068). Only one study site (San Lazaro Hospital) in Metro Manila managed persons with MDR-TB and all those registered and alive were enrolled making up 10% of participants in the urban sites. There was no difference in the proportion of new cases between the urban and rural sites, but a higher rate of bacteriologically confirmed cases in the urban sites (Table 1).
Table 1.
Number of patients per site and characteristics of enrolled TB DOTS patients.
| Metro Manila region (N = 338) | Negros Occidental region (N = 299) | Total (N = 637) | p-value1 | ||
|---|---|---|---|---|---|
| Centre | San Nicolas | 108 | |||
| Pedro Gil | 102 | ||||
| San Lazaro Hospital | 128 | ||||
| Bago City | 222 | ||||
| Valladolid | 77 | ||||
| Sex | Female | 97 (28.7) | 94 (31.4) | 191 (30.0) | 0.488 |
| Male | 241 (71.3) | 205 (68.6) | 446 (70.0) | ||
| Age (years) | 18–40 | 183 (54.1) | 89 (29.8) | 272 (42.7) | <0.001 |
| 41–65 | 127 (37.6) | 154 (51.5) | 281 (44.1) | ||
| >65 | 28 (8.3) | 56 (18.7) | 84 (13.2) | ||
| Household size | mean (SD) | 4.7 (3.1) | 4.7 (2.2) | 4.7 (2.7) | 0.972 |
| Marital status | Single | 171 (50.6) | 87 (29.1) | 258 (40.5) | <0.001 |
| Married | 132 (39.1) | 165 (55.2) | 297 (46.6) | ||
| Divorced/separated | 8 (2.4) | 6 (2.0) | 14 (2.2) | ||
| Widowed | 27 (8.0) | 41 (13.7) | 68 (10.7) | ||
| Highest level of education obtained | Primary | 63 (18.6) | 155 (51.8) | 218 (34.2) | <0.001 |
| Secondary | 147 (43.5) | 104 (34.8) | 251 (39.4) | ||
| Tertiary | 120 (35.5) | 27 (9.0) | 147 (23.1) | ||
| Vocational | 8 (2.4) | 13 (4.3) | 21 (3.3) | ||
| Health insurance2 | No | 145 (45.7) | 98 (33.2) | 243 (39.7) | 0.002 |
| Yes | 172 (54.3) | 197 (66.8) | 369 (60.3) | ||
| Time to health clinic (minutes), median (range) | 15 (0–300) | 6 (1–120) | 10 (0–300) | <0.001 | |
| Smoking status | No or Ex | 304 (89.9) | 209 (69.9) | 513 (80.5) | <0.001 |
| Yes | 34 (10.1) | 90 (30.1) | 124 (19.5) | ||
| Alcohol consumption | Daily | 25 (7.4) | 92 (30.8) | 117 (18.4) | <0.001 |
| Weekly or monthly | 78 (23.1) | 61 (20.4) | 139 (21.8) | ||
| Rarely/Never | 235 (69.5) | 146 (48.8) | 381 (59.8) | ||
| Weight change last 3–6 months3 | No change | 70 (20.8) | 32 (10.7) | 102 (16.0) | <0.001 |
| Increase | 73 (21.7) | 18 (6.0) | 91 (14.3) | ||
| Decrease | 194 (57.6) | 249 (83.3) | 443 (69.7) | ||
| Food intake last 1 month3 | No change | 196 (58.2) | 43 (14.4) | 239 (37.6) | <0.001 |
| Increase | 66 (19.6) | 79 (26.4) | 145 (22.8) | ||
| Moderate decrease | 65 (19.3) | 98 (32.8) | 163 (25.6) | ||
| Severe decrease | 10 (3.0) | 79 (26.4) | 89 (14.0) | ||
| Household Food security score | Secure | 218 (64.5) | 108 (36.1) | 326 (51.2) | <0.001 |
| Moderate insecurity | 69 (20.4) | 98 (32.8) | 167 (26.2) | ||
| Severe insecurity | 51 (15.1) | 93 (31.1) | 144 (22.6) | ||
| Multi-drug resistant TB | No | 305 (90.2) | 299 (100.0) | 604 (94.8) | <0.001 |
| Yes | 33 (9.8) | 0 (0) | 33 (5.2) | ||
| Previous TB4 | New case | 206 (61.5) | 187 (62.8) | 393 (62.1) | 0.806 |
| Relapse case | 129 (38.5) | 111 (37.3) | 240 (37.9) | ||
| Bacteriologically confirmed TB5 | No | 198 (60.2) | 210 (70.2) | 408 (65.0) | 0.009 |
| Yes | 131 (39.8) | 89 (29.8) | 220 (35.0) | ||
| Treatment phase6 | Intensive | 199 (58.9) | 133 (44.5) | 332 (52.1) | <0.001 |
| Continuation | 139 (41.1) | 166 (55.5) | 305 (47.9) | ||
Data are n (%) of non-missing values for each characteristic, unless otherwise indicated as mean (SD) or median (range).
1p-value from Fishers exact test for categorical variables, t-test to compare means or Wilcoxon rank sum test to compare medians.
221 missing values in Metro Manila region and 4 missing values in Negros Occidental region.
31 missing value in Metro Manila region.
43 missing value in Metro Manila and 1 missing value in Negros Occidental region.
59 missing values in Metro Manila region.
6Intensive treatment phase, unless extended, is the first 2 months of the 6 month standard TB-DOTS shorter regimen comprising isoniazid, rifampicin, pyrazinamide and ethambutol, followed by continuation phase comprising isoniazid and rifampicin only. The intensive phase of the 9/12 months WHO shorter regimen for multi-drug resistant TB is the first 4 months (unless extended) comprising kanamycin, moxifloxacin, prothionamide, clofazimine, pyrazinamide and high dose isoniazid and ethambutol followed by continuation phase comprising moxifloxacin, clofazimine, pyrazinamide and ethambutol only.
Prevalence of co-morbidities
Amongst 634 individuals with BMI measurements, the prevalence of all undernutrition (BMI < 18.5 kg/m2) was 36.6% (95% CI: 32.8–40.5%), with 32.1% (95% CI: 27.2–37.4%) in the urban sites compared to 41.6% (95% CI: 36.0–47.4%) in the rural sites (Fisher’s exact test p = 0.017). The prevalence of moderate or severe undernutrition (BMI < 17 kg/m2) was 20.5% (95% CI: 17.4–23.9%) and more common in rural sites (Table 2, 25% vs 17%, Fisher’s exact test p = 0.014). The prevalence of moderate undernutrition (16 kg/m2 < BMI < 17 kg/m2) was 8.7% and the prevalence of severe undernutrition (BMI < 16 kg/m2) was 11.8%. (Table 2). Overall, 29 (4.6%) participants were overweight (BMI 25–30 kg/m2) and 6 (1.0%) were obese (BMI ≥ 30 kg/m2).
Table 2.
Prevalence of co-morbidities amongst enrolled TB DOTS patients.
| Characteristic | Metro Manila Region (N = 338) | Negros Occidental Region (N = 299) | Total | p-value1 | |
|---|---|---|---|---|---|
| Undernutrition2 | None or mild | 280/336, 83.3% (78.9–87.2%) | 224/298, 75.2% (69.9–80.0%) | 504/634, 79.5% (76.1–82.6%) | 0.014 |
| Moderate or severe | 56/336, 16.7% (12.8–21.1%) | 74/298, 24.8% (20.0–30.1%) | 130/634, 20.5% (17.4–23.9%) | ||
| Undernutrition2 | None | 228/336, 67.9% (62.6–72.8%) | 174/298, 58.4% (52.6–64.0%) | 402/634, 63.4% (59.5–67.2%) | 0.048 |
| Mild | 52/336, 15.5% (11.8–19.8%) | 50/298, 16.8% (12.7–21.5%) | 102/634, 16.1% (13.3–19.2%) | ||
| Moderate | 22/336, 6.5% (4.1–9.7%) | 33/298, 11.1% (7.7–15.2%) | 55/634, 8.7% (6.6–11.1%) | ||
| Severe | 34/336, 10.1% (7.1–13.9%) | 41/298, 13.8% (10.1–18.2%) | 75/634, 11.8% (9.4–14.6%) | ||
| Diabetes3 | No | 299/325, 92.0% (88.5–94.7%) | 236/264, 89.4% (85.0–92.8%) | 535/589, 90.8% (88.2–93.0%) | 0.316 |
| Yes | 26/325, 8.0% (5.3–11.5%) | 28/264, 10.6% (7.2–15.0%) | 54/589, 9.2% (7.0–11.8%) | ||
| Anaemia4 | None or mild | 297/336, 88.4% (84.5–91.6%) | 249/296, 84.1% (79.5–88.1%) | 546/632, 86.4% (83.5–89.0%) | 0.131 |
| Moderate or severe | 39/336, 11.6% (8.4–15.5%) | 47/296, 15.9% (11.9–20.5%) | 86/632, 13.6% (11.0–16.5%) | ||
| HIV5 | No | 229/303, 75.6% (70.3–80.3%) | 39/41 | – | |
| Yes | 74/303, 24.4% (19.7–29.7%) | 2/41 | |||
| Reported hypertension | No | 312/338, 92.3% (88.9–94.9%) | 270/299, 90.3% (86.4–93.4%) | 582/637, 91.4% (88.9–93.4%) | 0.398 |
| Yes | 26/338, 7.7% (5.1–11.1%) | 29/299, 9.7% (6.6–13.6%) | 55/637, 8.6% (6.6–11.1%) | ||
| Reported chronic obstructive pulmonary disease | No | 338/338, 100.0% (98.9–100.0%) | 296/299, 99.0% (97.1–99.8%) | 634/637, 99.5% (98.6–99.9%) | 0.103 |
| Yes | 0/338, 0.0% (0.0–1.1%) | 3/299, 1.0% (0.2–2.9%) | 3/637, 0.5% (0.1–1.4%) | ||
| Reported Chronic kidney disease | No | 336/338, 99.4% (97.9–99.9%) | 298/299, 99.7% (98.2–100.0%) | 634/637, 99.5% (98.6–99.9%) | 1.000 |
| Yes | 2/338, 0.6% (0.1–2.1%) | 1/299, 0.3% (0.0–1.8%) | 3/637, 0.5% (0.1–1.4%) | ||
| Inflammatory status (CRP)6 | <5 mg/l | 290/318, 91.2% (87.5–94.1%) | 213/227, 93.8% (89.9–96.6%) | 503/545, 92.3% (89.7–94.4%) | 0.328 |
| ≥5 mg/l | 28/318, 8.8% (5.9–12.5%) | 14/227, 6.2% (3.4–10.1%) | 42/545, 7.7% (5.6–10.3%) | ||
1p-value from Fishers exact test for categorical variables.
22 missing values in Metro Manila region and 1 missing value in Negros Occidental region. Undernutrition is defined using BMI kg/m2: mild: <18.5–17; moderate: <17–16; severe: <16; none: ≥18.5.
313 missing values in Metro Manila region and 35 missing values in Negros Occidental region. Diabetes defined as HbA1c ≥ 6–5% or on current diabetes medication.
42 missing values in Metro Manila region and 3 missing values in Negros Occidental region. Moderate or Severe anaemia defined as haemoglobin<10 g/dL.
5only 41/299 patients were tested for HIV in Negros Occidental region, 35 missing values in Metro Manila.
6C-REactive Protein; Ref. 45.
The prevalence of diabetes was 9.2% (95% CI: 7.0–11.8%) with 52% previously undiagnosed. The prevalence of severe or moderate anaemia was 13.6% (95% CI: 11.0–16.5%). The prevalence of self-reported hypertension was 8.6% (95% CI: 6.6–11.1). There was no evidence of a difference in prevalence between rural and urban areas for these conditions (Table 2).
In the urban sites (Metro Manila), HIV testing is a routine component of TB DOTS clinic enrolment. Of the 90% of urban participants whose HIV status was known, 74/303 (24%) were HIV positive. HIV status was unknown for 85% of participants in rural sites due to a low number providing consent for study-specific HIV testing (not a routine part of TB DOTS clinic enrolment), therefore subsequent analysis of data including HIV status is reported for urban areas only. Less than 1% of all enrolled participants reported previous doctor-diagnosed chronic obstructive lung disease or chronic kidney disease.
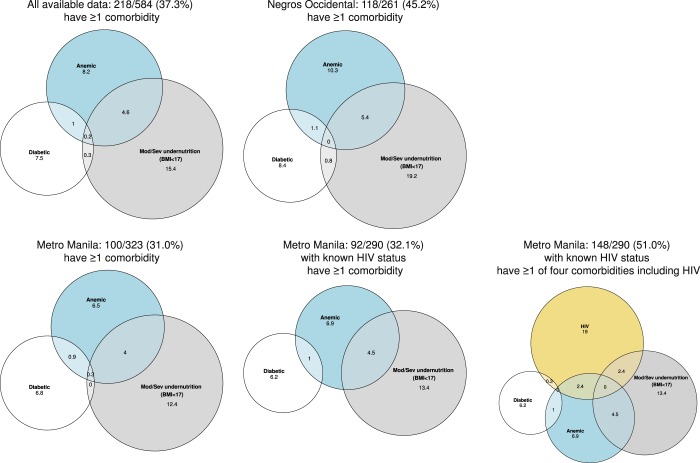
Multimorbidity
Approximately 40% of enrolled participants had at least one non-communicable clinical condition (moderate or severe undernutrition, diabetes and, or moderate or severe anaemia), as measured in 584 participants with non-missing data for each condition (Fig. 1, Supplementary Fig. 1). In urban sites 100/323 participants (31.0%) had at least one of these non-communicable conditions compared to 118/261 (45.2%) in rural sites (Fisher’s exact test p < 0.001). In urban sites, limited to those with known HIV status, the prevalence of at least one non-communicable condition was 32.1%. Fewer persons enrolled in urban sites were affected by one of the non-communicable conditions but 24.4% (74/303) of participants with known status had HIV-TB co-infection, only 8 of whom were newly diagnosed as the result of study screening. The most common combination of two conditions was undernutrition and anaemia in both the urban sites when limited to those with HIV data and in all participants in the rural sites (Supplementary Table 1).
Figure 1.
Percentage of persons with TB with co-morbidities1 overall and by area1 amongst participants with non-missing values for each comorbidity shown.
Diabetes in persons with TB disease
Of 54 participants with diabetes, 26 (48%) reported a previous diagnosis of diabetes, 18 of whom (69%) reported regular diabetes follow-up visits at a health centre or with a doctor, whilst 24/26 (92%) reported currently taking diabetes medication (21 metformin or metformin + insulin). However, amongst those reporting diabetic medication, only 9 (38%) were controlled (HbA1c ≤7.0%) with HbA1c ranging to over 14% in those remaining (Fig. 2A). There was some evidence of higher HbA1c values in those with newly diagnosed versus previously diagnosed diabetes (median HbA1c in newly diagnosed = 10.5% and existing = 8.9%, Wilcoxon rank sum test p = 0.040, Fig. 2A). Although the majority of diabetes cases had normal BMI (38/54, 70.4%), or were overweight/obese (8/54, 14.8%), there were three diabetes cases in those with a BMI < 17 kg/m2, one of whom had severe undernutrition with a BMI < 16 kg/m2 (Fig. 2A). There was no apparent correlation between BMI and HbA1c levels (Fig. 2A–C) in those with previously diagnosed diabetes (Spearman p = 0.390), or newly diagnosed diabetes (Spearman p = 0.816). In participants with diabetes, hypertension was the most common second co-morbidity (11/54, 20.4%) with the majority (10/11) reporting current anti-hypertensive medication. This pattern of co-morbidity in diabetic participants was also observed when limited to those in the urban sites with complete data for all conditions including HIV (Supplementary Table 1).
Figure 2.
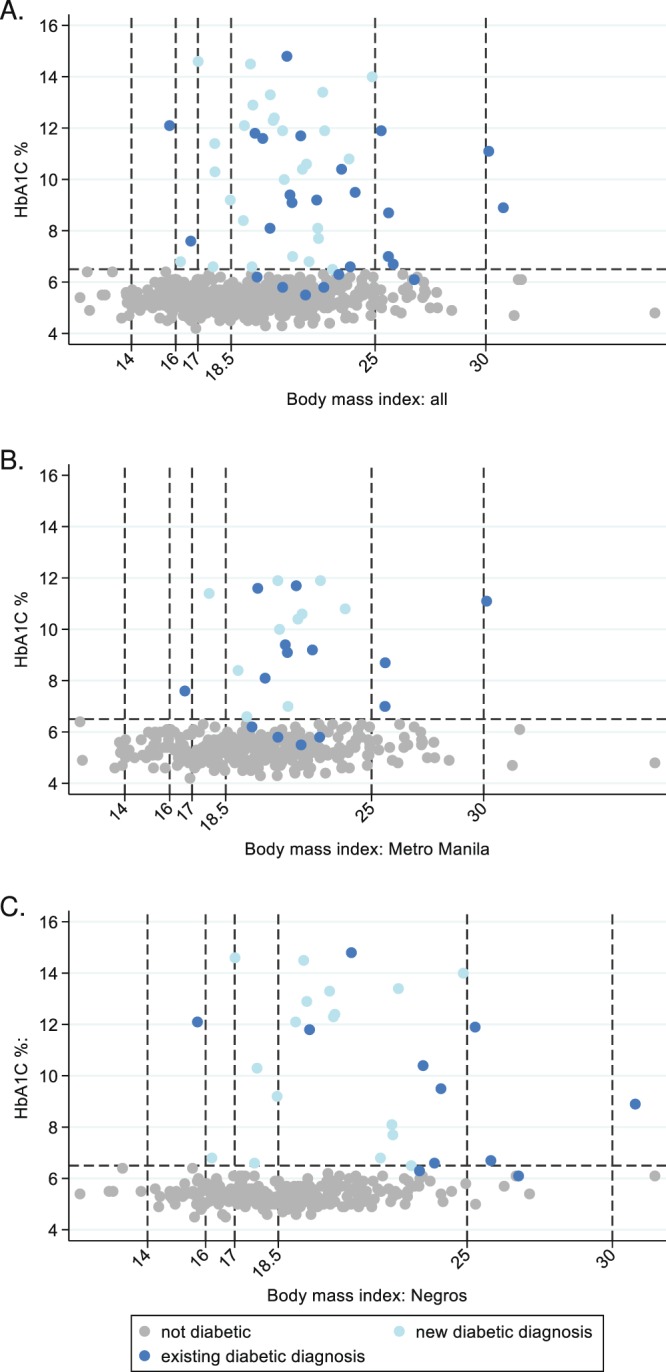
HbA1c by body mass index in new and previously diagnosed diabetes cases, overall and by urban/rural area.
Factors associated with undernutrition
In univariable analyses (Table 3), participants were more likely to be undernourished if they were female (LRT p = 0.002), single or widowed compared to married (LRT p = 0.003), did not have health insurance (LRT p = 0.051), experienced weight loss in the previous 3–6 months (LRT p = 0.007), reported a moderate or severe reduction in food intake in the previous month (LRT p < 0.001), were classified as a relapse/treatment failure, loss to follow-up or previous treatment outcome unknown (LRT p = 0.004), were in the intensive versus continuation phase of TB treatment (LRT p = 0.037), had inflammation (CRP ≥ 5 mg/L; LRT p = 0.030), or were enrolled in the rural sites (LRT p = 0.011).
Table 3.
Univariable and multivariable analysis of factors associated with undernutrition.
| Characteristic | N | Undernourished, n (%) | Unadjusted OR (95% CI) | p-value1 | Adjusted OR2 (95% CI) | p-value1 | |
|---|---|---|---|---|---|---|---|
| Sex | Female | 190 | 54 (28.4) | 1 | 0.002 | 1 | 0.001 |
| Male | 444 | 76 (17.1) | 0.52 (0.35df0.78) | 0.44 (0.28–0.70) | |||
| Age category (years) | 18–40 | 271 | 47 (17.3) | 1 | 0.216 | — | |
| 41–65 | 279 | 65 (23.3) | 1.45 (0.95–2.20) | — | |||
| >65 | 84 | 18 (21.4) | 1.30 (0.71–2.39) | — | |||
| Family size | 634 | 0.96 (0.89–1.03) | 0.282 | — | |||
| Marital status | Married | 295 | 45 (15.3) | 1 | 0.003 | 1 | 0.001 |
| Single | 257 | 63 (24.5) | 1.80 (1.18–2.76) | 2.30 (1.44–3.67) | |||
| Divorced/separated | 14 | 1 (7.1) | 0.43 (0.05–3.35) | 0.61 (0.07–4.95) | |||
| Widowed | 68 | 21 (30.9) | 2.48 (1.36–4.54) | 2.29 (1.18–4.46) | |||
| Highest level of education obtained | Primary | 218 | 48 (22.0) | 1 | 0.684 | — | |
| Secondary | 249 | 53 (21.3) | 0.96 (0.62–1.49) | — | |||
| Tertiary | 146 | 25 (17.1) | 0.73 (0.43–1.25) | — | |||
| Vocational | 21 | 4 (19.0) | 0.83 (0.27–2.59) | — | |||
| Health insurance | No | 242 | 60 (24.8) | 1 | 0.051 | — | |
| Yes | 368 | 67 (18.2) | 0.68 (0.46–1.00) | — | |||
| Time to health clinic (minutes) | 634 | 1.00 (0.99–1.00) | 0.281 | — | |||
| Smoking status | No or Ex | 510 | 110 (21.6) | 1 | 0.169 | — | |
| Yes | 124 | 20 (16.1) | 0.70 (0.41–1.18) | — | |||
| Alcohol consumption | Daily | 117 | 28 (23.9) | 1 | 0.503 | — | |
| Weekly or monthly | 139 | 25 (18.0) | 0.70 (0.38–1.28) | — | |||
| Rarely/Never | 378 | 77 (20.4) | 0.81 (0.50–1.33) | — | |||
| Weight change last 3–6 months | No change | 102 | 16 (15.7) | 1 | 0.007 | — | |
| Increase | 91 | 10 (11.0) | 0.66 (0.28–1.55) | — | |||
| Decrease | 441 | 104 (23.6) | 1.66 (0.93–2.95) | — | |||
| Food intake last 1 month | No change | 238 | 33 (13.9) | 1 | <0.001 | 1 | <0.001 |
| Increase | 145 | 24 (16.6) | 1.23 (0.70–2.18) | 1.47 (0.79–2.72) | |||
| Moderate decrease | 163 | 42 (25.8) | 2.16 (1.30–3.58) | 2.41 (1.39–4.17) | |||
| Severe decrease | 88 | 31 (35.2) | 3.38 (1.91–5.98) | 4.48 (2.40–8.37) | |||
| Household Food security | Secure | 324 | 57 (17.6) | 1 | 0.147 | — | |
| Moderate insecurity | 167 | 37 (22.2) | 1.33 (0.84–2.12) | — | |||
| Severe insecurity | 143 | 36 (25.2) | 1.58 (0.98–2.53) | — | |||
| TB treatment phase1 | Intensive | 329 | 78 (23.7) | 1 | 0.037 | — | |
| Continuation | 305 | 52 (17.0) | 0.66 (0.45–0.98) | — | |||
| Previous TB | New case | 393 | 67 (17.1) | 1 | 0.005 | 1 | 0.002 |
| Relapse case | 237 | 63 (26.6) | 1.76 (1.19–2.60) | 1.97 (1.28–3.04) | |||
| Bacteriologically confirmed TB | No | 407 | 74 (18.2) | 1 | 0.072 | 1 | 0.050 |
| Yes | 218 | 53 (24.3) | 1.45 (0.97–2.15) | 1.56 (1.00–2.42) | |||
| Inflammatory status (CRP)3 | <5 mg/l | 502 | 96 (19.1) | 1 | 0.030 | — | |
| ≥5 mg/l | 41 | 14 (34.2) | 2.19 (1.11–4.34) | — | |||
| Area | Urban | 336 | 56 (16.7) | 1 | 0.011 | — | |
| Rural | 298 | 74 (24.8) | 1.65 (1.12–2.44) | — | |||
OR = odds ratio, CI = confidence interval. 1p-value from likelihood ratio test.
2Adjusted ORs shown for covariates retained in the final multivariable model (if effects are not reported, covariate was not included).
3C-reactive protein Ref. 45.
In a final multivariable model (Table 3), reduced food intake reported in the previous month, phase of TB treatment, marital status and sex, remained associated with undernutrition after adjustment. After adjustment for these four variables, none of; weight change in the previous 3–6 months (LRT p = 0.190), health insurance (LRT p = 0.165) or area (LRT p = 0.241) were associated with undernutrition and so were not retained in the final model. Differences by area observed in Table 2 were mainly explained by reduced food intake in the previous month being more common in the rural sites. In a reduced dataset due to missing data, inflammation was not associated (LRT p = 0.129) with undernutrition after adjustment for food intake in the previous month, phase of TB treatment, marital status and sex. There was no evidence of interaction between sex and any of the other covariates in the final model (LRT p > 0.1). Fitting the final model in just the urban sites where HIV was tested and those with MDR-TB were enrolled, there was no evidence of increased odds of undernutrition in HIV positive versus negative participants (adjusted odds ratio, AOR = 0.54; 95% CI: 0.22–1.34, LRT p = 0.177), or in those with MDR-TB versus drug sensitive TB (AOR = 1.66, 95% CI: 0.69–4.01, LRT p = 0.274).
Undernutrition and anaemia in persons with drug sensitive TB
Although the prevalence of moderate/severe undernutrition was lower in persons in the continuation phase compared to intensive phase of TB treatment (Table 3), a relatively high prevalence was still observed in persons nearing completion of their DS-TB treatment in the 5th or 6th month at 13.9% (26/187). Similarly, the prevalence of moderate and severe anaemia was also lower in persons in continuation compared to intensive phase of treatment (9.6% vs. 17.3% Fisher’s exact test p = 0.005). However, in months 5 or 6 of drug sensitive treatment when TB/inflammatory associated anaemia is expected to have resolved it was observed that of 186 participants: 29 (15.6%) had mild, 14 (7.5%) moderate and 2 (1.1%) had severe anaemia. Of 15 cases of moderate/severe anaemia in month 5 or more of treatment and with CRP measurements, only 3 (20%) had inflammation (CRP > 5 mg/L). Thus, anaemia in the final months of treatment did not appear to be fully explained by remaining inflammation, suggesting a role for iron deficiency in these cases of anaemia.
Factors associated with diabetes
In univariable analyses, increasing age (LRT p < 0.001), having health insurance (LRT p = 0.051), being married or widowed rather than being single (LRT p = 0.003), and central obesity (waist-to-hip ratio >0.9 for men and >0.85 for women26) (LRT p < 0.001) were associated with having existing or newly diagnosed diabetes (Table 4). BMI categorization into normal, underweight, overweight or obese was associated with odds of diabetes (LRT p < 0.001), mostly due to the odds of diabetes being much lower in those who were undernourished compared to those with normal weight. Few participants were overweight or obese (n = 34) and whilst odds of diabetes were higher than for those with normal weight, these did not reach statistical significance. Marital status was no longer associated with diabetes after adjustment for age (LRT p = 0.680) and the final multivariable model included age group, health insurance and central obesity based on waist-to-hip ratio (Table 4). Fitting the final model in just the urban sites including HIV status and participants with MDR-TB were enrolled, there was no evidence of increased odds of diabetes in HIV positive versus negative persons (AOR = 0.21, 95% CI: 0.02–1.98, LRT p = 0.126), or in those with MDR-TB versus drug sensitive TB (AOR = 2.91, 95% CI: 0.85–10.0, LRT p = 0.103).
Table 4.
Univariable and multivariable analysis of factors associated with Type 2 diabetes.
| Characteristic | N | Type 2 diabetes, n (%) | Unadjusted OR (95% CI) | p-value1 | Adjusted OR2 (95% CI) | p-value1 | |
|---|---|---|---|---|---|---|---|
| Sex | Female | 173 | 17 (9.8) | 1 | 0.723 | — | |
| Male | 416 | 37 (8.9) | 0.90 (0.49–1.64) | — | |||
| Age category (years) | 18–40 | 256 | 6 (2.3) | 1 | <0.001 | 1 | <0.001 |
| 41–65 | 258 | 41 (15.9) | 7.87 (3.28–18.9) | 5.93 (2.41–14.6) | |||
| >65 | 75 | 7 (9.3) | 4.29 (1.40–13.2) | 3.07 (0.97–9.70) | |||
| Family size | 589 | 0.93 (0.83–1.05) | 0.219 | — | |||
| Marital status | Single | 243 | 10 (4.1) | 1 | 0.003 | — | |
| Married | 273 | 36 (13.2) | 3.54 (1.72–7.30) | — | |||
| Divorced/separated | 11 | 1 (9.1) | 2.33 (0.27–20.0) | — | |||
| Widowed | 62 | 7 (11.3) | 2.97 (1.08–8.14) | — | |||
| Highest level of education obtained | Primary | 197 | 16 (8.1) | 1 | 0.221 | — | |
| Secondary | 231 | 28 (12.1) | 1.56 (0.82–2.98) | — | |||
| Tertiary | 142 | 9 (6.3) | 0.77 (0.33–1.79) | — | |||
| Vocational | 19 | 1 (5.3) | 0.63 (0.08–5.02) | — | |||
| Health insurance | No | 226 | 14 (6.2) | 1 | 0.051 | 1 | 0.032 |
| Yes | 350 | 38 (10.9) | 1.84 (0.98–3.49) | 2.05 (1.05–4.05) | |||
| Time to health clinic (minutes) | 589 | 0.99 (0.98–1.00) | 0.128 | — | |||
| Smoking status | No or Ex | 476 | 44 (9.2) | 1 | 0.896 | — | |
| Yes | 113 | 10 (8.8) | 0.95 (0.46–1.96) | — | |||
| Alcohol consumption | Daily | 111 | 12 (10.8) | 1 | 0.783 | — | |
| Weekly or monthly | 133 | 11 (8.3) | 0.74 (0.31–1.76) | — | |||
| Rarely/Never | 345 | 31 (9.0) | 0.81 (0.40–1.65) | — | |||
| Weight change last 3–6 months | No change | 97 | 8 (8.2) | 1 | 0.730 | — | |
| Increase | 82 | 6 (7.3) | 0.88 (0.29–2.64) | — | |||
| Decrease | 410 | 40 (9.8) | 1.20 (0.54–2.66) | — | |||
| Food intake last 1 month | No change | 223 | 18 (8.1) | 1 | 0.553 | — | |
| Increase | 136 | 10 (7.4) | 0.90 (0.40–2.02) | — | |||
| Moderate decrease | 152 | 17 (11.2) | 1.43 (0.71–2.88) | — | |||
| Severe decrease | 78 | 9 (11.5) | 1.49 (0.64–3.46) | — | |||
| Food security | Secure | 308 | 30 (9.7) | 1 | 0.689 | — | |
| Moderate insecurity | 148 | 11 (7.4) | 0.74 (0.36–1.53) | — | |||
| Severe insecurity | 133 | 13 (9.8) | 1.00 (0.51–1.99) | — | |||
| TB treatment phase | Intensive | 307 | 32 (10.4) | 1 | 0.269 | — | |
| Continuation | 282 | 22 (7.8) | 0.73 (0.41–1.28) | — | |||
| Previous TB | New case | 366 | 31 (8.5) | 1 | 0.523 | — | |
| Relapse case | 219 | 22 (10.1) | 1.21 (0.68–2.14) | — | |||
| Bacteriologically confirmed TB | No | 373 | 29 (7.8) | 1 | 0.136 | — | |
| Yes | 208 | 24 (11.5) | 1.55 (0.88–2.74) | — | |||
| Inflammatory status (CRP) | <5 g/l | 502 | 44 (8.8) | 1 | 0.121 | — | |
| ≥5 g/l | 42 | 7 (16.7) | 2.08 (0.87–4.96) | — | |||
| BMI group3 | Normal | 216 | 8 (3.7) | 1 | <0.001 | — | |
| Underweight | 337 | 38 (11.3) | 0.30 (0.14–0.66) | — | |||
| Overweight | 28 | 6 (21.4) | 2.15 (0.82–5.63) | — | |||
| Obese | 6 | 2 (33.3) | 3.93 (0.70–22.2) | — | |||
| Central obesity: waist-to-hip ratio4 | No | 393 | 13 (3.3) | 1 | <0.001 | 1 | <0.001 |
| Yes | 195 | 41(21.0) | 7.78 (4.06–14.9) | 6.16 (3.15–12.0) | |||
| Area | Urban | 325 | 26 (8.0) | 1 | 0.277 | — | |
| Rural | 264 | 28 (10.6) | 1.36 (0.78–2.39) | — | |||
OR = odds ratio, CI = confidence interval. 1p-value from likelihood ratio test.
2Adjusted ORs shown for covariates retained in the final multivariable model (if effects are not reported, covariate was not included).
3underweight: Body Mass Index underweight: <18.5, normal: 18.5–24.9, overweight: 25.0–29.9, obese ≥30 kg/m2.
4based on waist-to-hip ratio >0.85 for women and >0.9 for men.
Given the strong association between central obesity based on waist-to-hip ratio cut-off value of >0.85 for women and >0.9 for men, an analysis was conducted to assess the sensitivity and specificity of different waist-to-hip ratio thresholds to correspond to diabetes. Thresholds were considered within a range of 0.7–1.1 in increments of 0.05. Results of these simple, unadjusted analyses suggest a threshold of >0.85 for persons of both sexes in this study would have sensitivity of 87% (95% CI: 0.75–95%) and specificity of 54% (95% CI: 50–58%, Supplementary Table 2).
Discussion
The aim of this cross-sectional study was to evaluate the burden and predictors of diabetes, undernutrition and anaemia co-morbidities in TB DOTS outpatients in a rural and an urban setting in the Philippines and to describe co-morbidity inter-relationships including with HIV-infection. To our knowledge, this study provides the first published estimates of the prevalence of malnutrition and diabetes among persons receiving anti-TB treatment (ATT) in routine outpatient TB-DOTs clinics in the Philippines. The prevalence of diabetes, at 9.1%, with more than 50% of diabetes cases newly diagnosed by the study screening was higher than current national estimates for the Philippines general population (6.1%,WHO 2016)29. but lower than the 14.1% reported in 634 urban HIV-negative Indonesians with newly diagnosed pulmonary TB, systematically assessed for diabetes by fasting blood glucose30 and more recently of 19.7% in the Indonesian participants of the multi-country “TANDEM study”31. Previous limited reports in the Philippines include a diabetes prevalence of 18% in pulmonary TB patients in Quezon City (N = 38)32, and 17.7% in a cohort of acutely unwell admissions to a TB ward in Manila (N = 348)33.
Undernutrition (BMI < 18.5 kg/m2) was the most common comorbidity affecting just over a third of all participants and was more common in the rural sites where greater household food insecurity, decreased food intake and recent weight loss were also reported. There was relatively little clustering of multimorbidity within individuals, with surprisingly little overlap between moderate/severe undernutrition and moderate/severe anaemia or between HIV and non-communicable conditions, for which HIV is a known risk factor. On further exploration, the participants in urban areas living with HIV were younger than those without HIV (31.7 vs 44.4 years) and only 2 were new diagnoses not already enrolled in HIV care programmes, which may explain these observations.
The high prevalence of moderate and severe undernutrition, including in the final months of treatment, when possible TB-mediated catabolic effects should have resolved, combined with frequent household food insecurity, indicate an important unmet need for nutritional support in persons with TB. Similar findings have been reported in a rural TB cohort in India, but at a higher prevalence and degree of severity, with 47% of males and 62% of females being severely undernourished (BMI < 16 kg/m2) at the start of treatment and 17% of males and 35% of females at treatment completion. The prevalence of severe anaemia (haemoglobin <8 g/dL) at start of treatment was 20%, whilst diabetes was reported in 11%. HIV prevalence was 4.4%. Unfortunately, this study did not report the proportions of participants with multiple co-morbidities34. The WHO guideline on Nutritional care and support for patients with tuberculosis states that “because of the clear bidirectional causal link between undernutrition and active TB, nutrition screening, assessment and management are integral components of TB treatment and care”35. These study results can be utilized in the Philippines to inform planning of TB treatment and care related to undernutrition, in which currently height is not assessed to allow calculation of BMI and no anaemia screening occurs. Many countries offer cash transfers or some kind of nutritional support to those undergoing treatment for MDR-TB, but do not extend this to drug sensitive TB (including the Philippines). Participants with a previous history of TB were almost twice as likely to be undernourished, indicating a probable downward spiral effect of undernutrition and TB. Thus, not addressing undernutrition during TB treatment may be contributing to the high rates of TB recurrence (38% of participants reported previous TB disease and were classified as relapse, treatment failure, loss to follow-up or previous treatment unknown). It remains to be determined if effectively treating undernutrition during an initial TB treatment can reduce risk of TB recurrence and thus be a cost-effective intervention. In this outpatient population, men had a lower risk of undernutrition than women. This is in contrast to our observation in a Filipino inpatient cohort (also 70% male) in whom there was no difference in the prevalence of moderate/severe undernutrition by sex, but in whom there was a statistically significant difference in the risk of inpatient death between male and female undernourished patients, with a greater risk in men33.
Poor glucose control is associated with poorer treatment outcomes36–38 (reviewed in39), and increased risk of developing diabetes associated complications and recurrent TB40. Although there is currently limited direct evidence that improved glucose control improves TB treatment outcomes, the high prevalence of poorly controlled blood glucose; even when using the more realistic target of HbA1c% <8%40, and including in those reporting to be on regular diabetes medication is worrying. The data suggests that both tuberculosis and non-communicable disease programmes have a large task of ensuring glycemic control among their program clients. The risk of diabetes was the greatest in the middle-aged group (40–65 years). However, unlike recent data from a multi-country study investigating diabetes in TB (the TANDEM study), we did not observe an association between diabetes and previous TB or with smear positive cases31. The distribution of HbA1c values for our diabetic population (new and previously diagnosed cases) was also similar to that from the Indonesian TANDEM study population, in whom a single point of care (POC) HbA1c test was shown to have high sensitivity and specificity to detect new diabetes cases, adding confidence to our diagnoses of diabetes using this single POC measurement41.
Our data demonstrate that diabetes whether previously or newly diagnosed was not limited to those with higher BMI, and could also occur in those with severe or moderate undernutrition. In multivariable analysis, waist to hip ratio was strongly associated with diabetes, whilst BMI was not significant. We thus investigated how, in resource-limited settings, waist-to-hip ratio might be useful as a screening tool for prioritising individuals for diabetes testing and observed a high sensitivity of 87% for a single cut-off for both sexes, supporting its potential use, at least in the Filipino context. Mean waist to hip ratio was also significantly increased in the TANDEM study, and most significantly in Indonesia31, although neither waist to hip ratio or waist circumference were included in the final multicountry predictive risk score for diabetes in persons with TB41.
TB programmes, in which individuals are in regular contact with local health services, provide an opportunity to increase rates of diabetes diagnosis (and other important NCD conditions like hypertension), and to re-engage with persons with diabetes who may have dropped out of diabetes care. Although there is still limited evidence to support how persons with TB-diabetes should be managed and how integration of services can be achieved, specific guidelines on management have recently been released based on best available evidence and expert opinion42. Diabetes management should include drug management, nutritional status assessment, dietary and lifestyle counselling (healthy diet, weight management, physical activity, smoking cessation and avoiding excess alcohol), which are also recommended as part of routine TB care. Training of TB health workers in diagnosing and managing diabetes, undernutrition and anaemia would provide a more holistic and patient-centered approach to TB management and hopefully avoid conflicting advice being given. Integration of services, which are currently split between infectious and non-infectious, will present significant challenges but also opportunities for leveraging of resources and investments between domestic and international funding sources.
This study did not rely on routine testing or self-reporting of diabetes or undernutrition and used trained research nurses to conduct all assessments and anthropometry. Although the use of a single POC test for HbA1c is not currently recommended for diabetes diagnosis by regulatory bodies, its agreement with accredited laboratory measurement using high performance liquid chromatography in persons with TB has recently been shown to be high, with minimal effects on clinical misclassification43. However, the higher proportion of participants with severe anaemia in our setting may have resulted in some misclassification in this group. It has been reported that severe anaemia can affect HbA1c, with possible over-estimation in iron deficiency anaemia and under-estimation with haemolytic anaemia44. In the small number of those with severe anaemia in the study comparing POC HbA1c with laboratory HbA1c, the POC test showed a mean difference of +1.1%43. The amount of missing information for HIV status is a limitation, especially for the rural sites. HIV in TB in the Philippines is still low at 2%1. The prevalence reported in the urban sites of this study is not representative of the majority of Filipino persons with TB due to the inclusion of a hospital site which is one of the largest HIV referral sites in the city. This can be seen as another limitation, but also allowed us, with a reasonable study sample size, to explore the effect of HIV on the frequency and overlap of our other clinical conditions of interest. Finally, hypertension was not directly assessed but was based on patient report. Our diabetes prevalence estimate could be overestimated due to TB induced transient hyperglycemia, greater in the early phase of treatment; or underestimated if those with diabetes were less likely to be included due to more frequent poor TB treatment outcomes, or were of higher social status and less likely to be treated for TB in public facilities. The cross-sectional study design thus limits the conclusions that can be drawn regarding possible causality between changes in the prevalence of co-morbid conditions over TB treatment duration.
Future studies should directly assess hypertension, other respiratory conditions and diabetes associated co-morbidities such as peripheral neuropathy, retinopathy, kidney function and blood lipids to further inform specific management strategies and resources for diabetes in management in TB programmes. The evidence base also requires further strengthening, in relation to context-specific dietary management strategies, especially of diabetes in those who are also undernourished, and of the effects of nutritional supplementation on TB, multimorbidity and long-term health sequalae of TB.
Conclusions
This study provides evidence of a high burden of co-morbidities in Filipino persons with drug-sensitive TB, including in rural areas. In this study, multimorbidity was not a problem limited to those with HIV co-infection. Moderate and severe undernutrition and anaemia were also observed in the final months of TB treatment indicating the need for additional interventions to detect and treat these conditions. Our findings are likely applicable to other country settings with a double burden of under-and overnutrition and low HIV. For effective management of multimorbidity, health care programmes need to move towards integration of services and include support for nutrition and lifestyle management.
Supplementary information
Acknowledgements
This work was funded by Nagasaki University funding awarded to S.E. Cox. The funder had no role in the design, conduct, analysis, interpretation or writing of the final manuscript. We are grateful to the participants, their families, TB-DOTS clinic staff and research nurses: Alan Kevin Q. Llantada, Reby Marie T. Garcia, Michelle A. Savaadra, Cristielyn D. Alvarez, Romil J. Juson and Malou Lourdes with support from Clarinda Berido for their contributions in making this work possible. Electronic data solutions were provided by LSHTM Open Research Kits (odk.lshtm.ac.uk).
Author contributions
S.E.C., L.V.W., T.E., N.L. and J.A.S. conceived the idea and designed the study with input from K.A. and A.M.C.G.G. L.V.W. M.C.C., N.R.S. R.W.C., N.D.F., F.M. and N.S. conducted the study and collected the data. L.V.W. managed the data. T.E., L.V.W. B.F. and S.E.C. analysed the data. S.E.C., T.E. and L.V.W. wrote the report. All authors approved the manuscript.
Data availability
The complete dataset and associated meta-data and data dictionary representing the data generated and analysed during the current study are available in the FigShare repository: 10.6084/m9.figshare.11695467. The study and protocol are registered at ISRCTN12506117.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-60942-2.
References
- 1.WHO. Global TB Report. (2019).
- 2.Lonnroth K, Williams BG, Cegielski P, Dye C. A consistent log-linear relationship between tuberculosis incidence and body mass index. Int. J. Epidemiol. 2010;39:149–155. doi: 10.1093/ije/dyp308. [DOI] [PubMed] [Google Scholar]
- 3.Waitt CJ, Squire SB. A systematic review of risk factors for death in adults during and after tuberculosis treatment. Int. J. Tuberc. Lung Dis. 2011;15:871–885. doi: 10.5588/ijtld.10.0352. [DOI] [PubMed] [Google Scholar]
- 4.Al-Rifai RH, Pearson F, Critchley JA, Abu-Raddad LJ. Association between diabetes mellitus and active tuberculosis: A systematic review and meta-analysis. PLoS One. 2017;12:e0187967. doi: 10.1371/journal.pone.0187967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker MA, et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med. 2011;9:81. doi: 10.1186/1741-7015-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect. Dis. 2009;9:737–746. doi: 10.1016/S1473-3099(09)70282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Odone A, Houben RM, White RG, Lonnroth K. The effect of diabetes and undernutrition trends on reaching 2035 global tuberculosis targets. Lancet Diabetes Endocrinol. 2014;2:754–764. doi: 10.1016/S2213-8587(14)70164-0. [DOI] [PubMed] [Google Scholar]
- 8.WHO. The end TB Strategy: Global strategy and targets for tuberculosis prevention, care and control after 2015. (Geneva, 2014).
- 9.WHO. Implementing the END TB Strategy: The Essentials. (World Health Organisation, Geneva, 2015).
- 10.Chung WS, et al. Tuberculosis increases the subsequent risk of acute coronary syndrome: a nationwide population-based cohort study. Int. J. Tuberc. Lung Dis. 2014;18:79–83. doi: 10.5588/ijtld.13.0288. [DOI] [PubMed] [Google Scholar]
- 11.Hnizdo E, Singh T, Churchyard G. Chronic pulmonary function impairment caused by initial and recurrent pulmonary tuberculosis following treatment. Thorax. 2000;55:32–38. doi: 10.1136/thorax.55.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prabhakaran D, et al. Cardiovascular, respiratory, and related disorders: key messages from <em>Disease Control Priorities</em>, 3rd edition. Lancet. 2018;391:1224–1236. doi: 10.1016/S0140-6736(17)32471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmes, K. K., Bertozzi, S., Bloom, B. R. & Jha, P. Vol. 6 (World Bank, Washington D.C., 2017).
- 14.Khan A, Sterling TR, Reves R, Vernon A, Horsburgh CR. Lack of weight gain and relapse risk in a large tuberculosis treatment trial. Am. J. Respir. Crit. Care Med. 2006;174:344–348. doi: 10.1164/rccm.200511-1834OC. [DOI] [PubMed] [Google Scholar]
- 15.Food and Nutrition Research Institute, D. o. S. a. T., the Philippines,. 8th National Nutrition Survey, http://www.fnri.dost.gov.ph/index.php/nutrition-statistic/19-nutrition-statistic/118-8th-national-nutrition-survey (2015).
- 16.Bhargava A, Benedetti A, Oxlade O, Pai M, Menzies D. Undernutrition and the incidence of tuberculosis in India: national and subnational estimates of the population-attributable fraction related to undernutrition. Natl Med. J. India. 2014;27:128–133. [PubMed] [Google Scholar]
- 17.Jimeno CA, Kho SA, Matawaran BJ, Duante CA, Jasul GV. Prevalence of Diabetes Mellitus and Pre-Diabetes in the Philippines: A Sub-study of the 7th National Nutrition and Health Survey (2008) Philippine J. Intern. Med. 2015;53:1–8. [Google Scholar]
- 18.Baja, E. Tuberculosis and Diabetes Mellitus Control and Care: A Rapid Situational Analysis for Planning a Coordinated Program Response (Philippine Institute for Development Studies Manila, 2014).
- 19.Dept of Health, T. P. National TB Control Programme: Updated 2010–2016 Philippine plan of action ot control Tuberculosis, https://www.doh.gov.ph/sites/default/files/publications/Updated_PhilPACT_2013-2016_v080715.pdf (2014).
- 20.The Academy of Medical Sciences. Multimorbidity: a priority for global health research (2018).
- 21.WHO. Physical status: The use and interpretation of anthropometry: Report of a WHO expert committee. (World Health Organisation, 1995). [PubMed]
- 22.WHO. Use of Gycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation. (World Health Organisation, 2011). [PubMed]
- 23.WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. (World Health Organisation, Geneva, 2011).
- 24.Hartung, C. et al. Open Data Kit: Tools to build information services for developing regions. ICTD (2010).
- 25.Melgar-Quinonez HR, et al. Household food insecurity and food expenditure in Bolivia, Burkina Faso, And the Philippines. J. Nutr. 2006;136:1431S–1437S. doi: 10.1093/jn/136.5.1431S. [DOI] [PubMed] [Google Scholar]
- 26.WHO. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation. (World Health Organisation, Geneva, 2008).
- 27.Kirkwood, B. R. & Kirkwood, J. S. Essentials of Medical Statistics. 2nd Edition edn, (Wiley-Blackwell, 2013).
- 28.R: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria, 2013).
- 29.WHO. (2016).
- 30.Alisjahbana B, et al. The effect of type 2 diabetes mellitus on the presentation and treatment response of pulmonary tuberculosis. Clin. Infect. Dis. 2007;45:428–435. doi: 10.1086/519841. [DOI] [PubMed] [Google Scholar]
- 31.Ugarte-Gil, C. et al. Diabetes mellitus among pulmonary tuberculosis patients from four TB-endemic countries: the TANDEM study. Clin Infect Dis, 10.1093/cid/ciz284 (2019). [DOI] [PubMed]
- 32.Pablo-Villamor, M. P., Benedicto, J. P., Benedicto, T. J. U. & Perez, V. M. Screening for diabetes mellitus in patients diagnosed with pulmonary tuberculosis. Philippines Journal of Medicine52 (2014).
- 33.Lee N, et al. Mid-upper arm circumference predicts death in adult patients admitted to a TB ward in the Philippines: A prospective cohort study. PLoS One. 2019;14:e0218193. doi: 10.1371/journal.pone.0218193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhargava A, et al. Nutritional status of adult patients with pulmonary tuberculosis in rural central India and its association with mortality. PLoS One. 2013;8:e77979. doi: 10.1371/journal.pone.0077979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.WHO. Guideline: Nutritional Care and Support for Patients with Tuberculosis. Report No. 9789241506410, (Geneva, 2013). [PubMed]
- 36.Mahishale V, et al. Effect of Poor Glycemic Control in Newly Diagnosed Patients with Smear-Positive Pulmonary Tuberculosis and Type-2 Diabetes Mellitus. Iran. J. Med. Sci. 2017;42:144–151. [PMC free article] [PubMed] [Google Scholar]
- 37.Yoon YS, et al. The effect of diabetes control status on treatment response in pulmonary tuberculosis: a prospective study. Thorax. 2017;72:263–270. doi: 10.1136/thoraxjnl-2015-207686. [DOI] [PubMed] [Google Scholar]
- 38.Chiang CY, et al. The influence of diabetes, glycemic control, and diabetes-related comorbidities on pulmonary tuberculosis. PLoS One. 2015;10:e0121698. doi: 10.1371/journal.pone.0121698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shewade HD, et al. Effect of glycemic control and type of diabetes treatment on unsuccessful TB treatment outcomes among people with TB-Diabetes: A systematic review. PLoS One. 2017;12:e0186697. doi: 10.1371/journal.pone.0186697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Crevel R, Koesoemadinata R, Hill PC, Harries AD. Clinical management of combined tuberculosis and diabetes. Int. J. Tuberc. Lung Dis. 2018;22:1404–1410. doi: 10.5588/ijtld.18.0340. [DOI] [PubMed] [Google Scholar]
- 41.Grint D, et al. Accuracy of diabetes screening methods used for people with tuberculosis, Indonesia, Peru, Romania, South Africa. Bull. World Health Organ. 2018;96:738–749. doi: 10.2471/BLT.17.206227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin, Y. et al. Management of diabetes mellitus-tuberculosis: A guide to the essential practice. (International Union Against Tuberculosis and Lung Disease (The Union), Paris, 2019). [DOI] [PubMed]
- 43.Huangfu P, et al. Point of care HbA1c level for diabetes mellitus management and its accuracy among tuberculosis patients: a study in four countries. Int. J. Tuberc. Lung Dis. 2019;23:283–292. doi: 10.5588/ijtld.18.0359. [DOI] [PubMed] [Google Scholar]
- 44.English E, et al. The effect of anaemia and abnormalities of erythrocyte indices on HbA1c analysis: a systematic review. Diabetologia. 2015;58:1409–1421. doi: 10.1007/s00125-015-3599-3. [DOI] [PubMed] [Google Scholar]
- 45.WHO. C-reactive protein concentrations as a marker of inflammation or infection for interpreting biomarkers of micronutrient status. (World Health Organisation, Geneva, 2014).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The complete dataset and associated meta-data and data dictionary representing the data generated and analysed during the current study are available in the FigShare repository: 10.6084/m9.figshare.11695467. The study and protocol are registered at ISRCTN12506117.