Abstract
Eosinophilia is a hallmark of airway allergic inflammation (AAI). Identifying key molecules and specific signaling pathways that regulate eosinophilic inflammation is critical for development of novel therapeutics. Tropomyosin Receptor Kinase (Trk) A is the high affinity receptor for nerve growth factor. AAI is associated with increased expression of TrkA by eosinophils; however, the functional role of TrkA in regulating eosinophil recruitment and contributing to AAI is poorly understood. This study identifies a novel mechanism of eotaxin-mediated activation of TrkA and its role in regulating eosinophil recruitment by using a chemical-genetic approach to specifically inhibit TrkA kinase activity with 1-NM-PP1 in TrkAF592A-knock-in (TrkA-KI) eosinophils. Blockade of TrkA by 1-NM-PP1 enhanced eosinophil spreading on vascular cell adhesion molecule-1 but inhibited eotaxin-1 (CCL11)-mediated eosinophil migration, calcium flux, cell polarization and ERK1/2 activation, suggesting that TrkA is an important player in the signaling pathway activated by eotaxin-1 during eosinophil migration. Further, blockade of matrix metalloprotease (MMP) with BB-94 inhibited eotaxin-1-induced TrkA activation and eosinophil migration, additively with 1-NM-PP1, indicating a role for MMPs in TrkA activation. TrkA inhibition in A. alternata-challenged TrkA-KI mice markedly inhibited eosinophilia and attenuated various features of AAI. These findings are indicative of a distinctive eotaxin-mediated TrkA-dependent signaling pathway, which in addition to other TrkA-activating mediators, contributes to eosinophil recruitment during AAI and suggests that targeting the TrkA signaling pathway to inhibit eosinophil recruitment may serve as a therapeutic strategy for management of eosinophilic inflammation in allergic airway disease including asthma.
Keywords: TrkA, eosinophils, cell adhesion, migration, activation, allergic airway inflammation, asthma
Introduction
Tropomycin Receptor Kinase A (TrkA), also known as neurotrophic tyrosine kinase receptor type 1 (NTRK1), is a member of the receptor tyrosine kinase (RTK) family and the high-affinity receptor for nerve growth factor (NGF) (1). NGF/TrkA signaling plays a significant role in regulating neuronal survival, development, and function (2) and has been extensively studied in the context of pain management (3, 4) as well as tumor development (5, 6). Previous studies have also indicated a role for NGF and TrkA signaling in allergic airway inflammation (AAI). AAI is an inflammatory disease of the airways characterized by increased recruitment of inflammatory cells, especially eosinophils, elevated levels of Th2 cytokines, pro-inflammatory chemokines and growth factors that together contribute to the overall disease pathogenesis (7). TrkA is expressed in many cell types in the lungs such as inflammatory cells, including eosinophils, bronchial and alveolar epithelial cells, smooth muscle cells, and vascular endothelial cells (8). Elevated NGF levels have been detected in the bronchoalveolar lavage fluid (BALF) and serum of patients with AAI and asthma (9). Evidence from animal models of AAI supports an important role for NGF and TrkA signaling in allergic asthma [reviewed in (10)]. Recent studies have also shown that TrkA/NTRK1 is a transcriptional target of the Th2 cytokine IL-13 in human epithelial cells and is strongly upregulated in patients with eosinophilic esophagitis, conferring increased responsiveness to NGF and is thus likely to contribute to allergic inflammation (11).
While it is clear that NGF/TrkA signaling plays a role in AAI, the causal relationship between NGF/TrkA signaling and allergic asthma or the mechanism involved remains unclear due to the lack of a specific method thus far to interrogate the NGF/TrkA signaling pathway. Both NGF and TrkA gene-knock out in mice leads to a lethal phenotype due to defective neuron development (12, 13). TrkA inhibitors, like other receptor tyrosine kinase inhibitors, have limited drug specificity. TrkA kinase inhibitors used in previous studies also inhibit other kinases of the RTK family (14). The most commonly used TrkA inhibitor K252a blocks the activities of other kinases such as myosin light chain kinase and protein kinase C (PKC)(15). Anti-NGF antibody or NGF-siRNA cannot elucidate the functional importance of the TrkA kinase activity. Further, anti-NGF antibody cannot distinguish the functional roles of its two receptors TrkA and p75NTR, nor completely block the activation of the receptors if they are activated through cross-talk with other receptors, e.g., G-protein-coupled receptors (GPCRs) (16-18).
Eotaxin-1 (CCL11) is the predominant chemokine contributing to airway eosinophil recruitment and exerts its activity on eosinophils via the GPCR C-C chemokine receptor type 3 (CCR3) (19). In this study we have delineated a functional role for TrkA signaling in regulating eotaxin-1-induced eosinophil recruitment and the development of AAI in mice by using a combined genetic-chemical approach to specifically control the TrkA kinase activity in vitro and in vivo. An F592A mutation within the ATP-binding pocket in the kinase subdomain V allows the selective and reversible inhibition of TrkA by an ATP analog such as 1-tert-Butyl-3-(naphthalen-1-ylmethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine (1-NM-PP1) (20). Using the TrkAF592A-knock-in (TrkA-KI) mouse strain (21) in an Alternaria alternata-induced AAI model, we have established that TrkA signaling plays an important role in AAI development and uncovered a distinct signaling mechanism wherein TrkA mediates the effects of eotaxin on cellular recruitment.
Materials and Methods
Mouse model of allergic airway inflammation
A TrkA-KI mouse strain (TrkAF592A) wherein the ATP-binding pocket in the kinase subdomain V is mutated by replacing the phenylalanine residue with alanine resulting in the generation of an ATP-analog-sensitive kinase model which undergoes specific inhibition by an ATP analog such as 1-NM-PP1 ((21)) was used for in vivo studies. TrkA-KI mice were obtained from Dr. Ye, Pathology and laboratory Medicine, Emory University School of Medicine, Atlanta, GA, and subsequently bred and maintained at University of Minnesota. TrkA-KI mice (8-12 weeks, male and female) were challenged with 25 μg of A. alternata extract (Greer Laboratories) in 50 μl of PBS on days 0, 3 and 6 or with PBS (control) as described previously (22). Allergen-exposed mice were divided into three groups; one group received no treatment, the second group received TrkA kinase inhibitor 1-NM-PP1 (Axon Medchem) in DMSO/PBS intra-muscularly at a dosage of 10 mg/kg/day on days 0, 3 and 6, and the third group received the same dosage on days 0, 3, 4, 5 and 6. In pilot studies, DMSO/PBS was not found to exert non-specific pathological effects in the lung (Supplemental Fig. 1 and Supplemental Fig. 2); thus allergen-exposed mice were administered DMSO/PBS as vehicle only in select experiments. Wild-type (WT) mice (Charles River Laboratories) were bred in house for culture of bone marrow (BM)-derived eosinophils. All studies involving mice were performed following standards and procedures approved by the Institutional Animal Care and Use Committee at the University of Minnesota.
Sample collection and analysis
Mice were sacrificed 24 h after the last allergen challenge. Total number of cells in the BALF was counted in a hemocytometer and differential cell counts in the BALF and blood were determined from cytocentrifuged samples based on morphologic and histologic criteria after staining with Hema 3 System (Thermo Fisher Scientific). BALF supernatants were stored at −80°C for later analysis. Right lungs were snap-frozen and left lungs were first perfused with 4% paraformaldehyde (PFA), fixed in 4% PFA and paraffin embedded. Lung tissue harvested from WT C57BL/6 mice challenged with A. alternata (22), ovalbumin (23) or cockroach allergen (24) and paraffin embedded in a manner similar was used in some studies.
Lung Histology
Paraffin-embedded lung tissue sections (4 μm) were stained with H&E (Leica Biosystems Inc.) to determine cellular infiltration. After antigen retrieval, expression of total and phosphorylated TrkA (t-TrkA and p-TrkA, respectively) in lung tissue sections was detected by immunofluorescence staining using rabbit polyclonal antibodies against t-TrkA (#2505S, Cell Signaling Technology, at 2 μg/mL) and rabbit monoclonal antibodies (mAb) against p-TrkA (#4168S, detects TrkA phosphorylated at Tyr785, Cell Signaling Technology, at 2 μg/mL), respectively. Rabbit IgG was used as a control for both antibodies. Bound antibodies were detected using Rhodamine-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories). To examine p-TrkA expression by lung tissue eosinophils, sections were dual-stained with mAb against p-TrkA and rat mAb against eosinophil-specific major basic protein (MBP, 5 μg/ml) (25), followed by FITC-conjugated anti-rat and Rhodamine-conjugated anti-rabbit secondary antibodies (Jackson ImmunoResearch Laboratories). 4,6-diamidino-2-phenylindole (DAPI) was used to visualize nuclei and slides were examined by confocal microscopy.
For all immunohistochemical (IHC) analysis, tissue sections were subjected to antigen retrieval followed by quenching of endogenous peroxidase activity prior to staining with specific antibodies. VECTASTAIN ABC Kits containing biotinylated secondary antibodies and avidin-biotin horseradish peroxidase complex (Vector Laboratories) were used along with the Peroxidase AEC (3-amino-9-ethylcarbazole) substrate kit (Vector Laboratories) for detection. Evaluation and quantitation of lung tissue eosinophil infiltration were performed by IHC staining for MBP with rat mAb against murine MBP (2 μg/mL) as described previously (25). Expression of α-smooth muscle actin (α-SMA) was assessed using a mAb against α-SMA (2 μg/mL; Sigma-Aldrich), and the area of the α-SMA in peribronchial smooth muscle layer was quantitated from captured images (four to five airways per mouse) using ImageJ image analysis software as described previously (24). Results were expressed as α-SMA–positive area (μm2) per mm basement membrane length (BML). Rat IgG (for MBP) and mouse IgG (for α-SMA) were used as control antibodies. Airway mucus accumulation was evaluated by staining lung sections with periodic acid–Schiff (PAS) reagent (Sigma-Aldrich). The PAS-positive area (mucus secretion) in airways of relatively similar size (three to seven airways per mouse) was quantitated by ImageJ analysis and expressed as PAS-positive area (μm2) per 100 μm BML(24). Stained slides were examined using a Nikon Microphot EPI-FL microscope (Nikon Instruments Inc.) and images were captured with an Olympus DP71 camera.
Measurement of airway hyperresponsiveness (AHR)
AHR was measured in anesthetized allergen-challenged TrkA-KI mice treated with 1-NM-PP1 (10 mg/kg/day on days 0, 3, 4, 5 and 6) or DMSO/PBS (as vehicle control) using the FinePointe RC System (Buxco) as described previously (26). Allergen-challenged mice without any treatment (1-NM-PP1 or DMSO), and naïve mice treated with DMSO/PBS or with 1-NM-PP1 served as additional control groups. Changes in pulmonary resistance (RL) in response to saline followed by increasing concentrations of inhaled methacholine (3-50 mg/ml) were continuously monitored and expressed as mean RL values for each dose.
Measurement of lung cytokines, chemokines and IgE
Th1 (IL-2, IFN-γ)/Th2 (IL-4, IL-5, IL-13) cytokine and TNF-α levels in supernatants of lung tissue lysates were determined using CBA Flex Set Kits (BD Biosciences) with a FACScan flow cytometer equipped with CellQuest Pro™ (BD Biosciences) for data acquisition and FlowJo Software (Tree Star, Inc.) for analysis. Eotaxin-1 and eotaxin-2 in the BALF and IL-33 in lung lysates was measured using ELISA kits (R&D Systems). IgE level in the BALF was measured using a mouse IgE ELISA kit (BioLegend). All kits were used as per the manufacturer’s recommendation.
Culture of murine eosinophils
Eosinophils were routinely cultured from BM of naïve male or female TrkA-KI or WT mice as described previously (26, 27). Cells between day 12 and 18 of culture stained were used in studies after assessing purity with Hema 3 (i.e., differentiation of granulocyte populations based on pink, granular cytoplasm and bilobed purple nuclei) and expression of eosinophil-specific MBP and Siglec-F by immunofluorescent staining and flow cytometry, respectively.
Immunofluorescent staining and actin polymerization
BM-derived eosinophils (2 × 105) were treated with eotaxin-1 (PeproTech) at 100 nM (or PBS) for 5 min at 37°C, cytocentrifuged on to glass slides, fixed (4% PFA in PBS, 20 min at room temperature) and blocked with goat serum in TBS for 30 min. Cells were then incubated overnight at 4°C with polyclonal antibodies against t-TrkA (5 μg/ml), rabbit mAb against p-TrkA (2 μg/ml, both from Cell Signaling Technology) or rabbit mAb against Cdc42 (Abcam, 2 μg/ml) followed by Rhodamine Red-X-affinity purified goat anti rabbit IgG (Jackson ImmunoResearch Laboratories, 15 μg/ml, 1 h at room temperature). Cells treated with rabbit IgG served as the negative control. In some experiments, cells were first incubated with 1-NM-PP1 (10 μM, 15 min), BB-94, a pan matrix metalloproteases (MMP) inhibitor (Sigma Aldrich, 10 μM, 10 min), or DMSO as the vehicle control at 37°C and then exposed to eotaxin-1 for an additional 5 min before staining as described above. Eosinophils treated with Z-VAD-FMK (R&D Systems), a pan-caspase inhibitor, PMSF (Sigma Aldrich), an inhibitor of serine proteases (both at 10 μM), or appropriate vehicle controls (DMSO or isopropyl alcohol, respectively) before exposure to eotaxin-1 served as negative controls. Cells were examined by confocal microscopy after counterstaining with 4,6-diamidino-2-phenylindole (DAPI) using a FLUOVIEW FV1000/BX61 – Confocal Laser Scanning Biological Microscope (Olympus) equipped with an UPlanSApo lens (20×/0.85 [oil]) and a PlanApo N lens (60×/1.42 [oil]), at ambient temperature. FV10-ASW 2.0 software (Olympus) was used for image acquisition.
Actin polymerization in eotaxin-treated eosinophils was evaluated as described (23). Briefly, 2 × 105 eosinophils seeded onto poly-L-lysine-coated cover-slips were incubated with 10 μM 1-NM-PP1 or DMSO for 20 min and then exposed to eotaxin-1 (100 nM, 2 or 10 min) or left untreated. At the end of incubation, cells were fixed and permeabilized with 4% PFA containing 0.1% saponin, incubated with Alexa Fluor 488 Phalloidin (Thermo Fisher Scientific, 20-30 min in the dark), counterstained with DAPI and evaluated by confocal microscopy.
Adhesion and migration assays
Role of TrkA in regulating eosinophil adhesion to vascular cell adhesion molecule (VCAM)-1 and eotaxin-1-induced migration was evaluated as described previously (23). In the adhesion assays, the number of adherent cells per field in randomly selected fields (~20 fields/coverslip at ×400 magnification) was manually determined. Next, the number of adherent cells exhibiting spreading was counted and expressed as a percentage of the total number of adherent cells in the field. In the case of migration, migrated cells in a fixed number of randomly selected non-overlapping fields were counted using an Olympus CK2 inverted microscope (200× magnification) and expressed as percent migration relative to migration of vehicle (DMSO)-treated cells.
Cell surface receptor expression
Cell surface adhesion molecule expression (CD49d, CD11a, CD11b, L-selectin and CD18) in cells treated with 1-NM-PP1 (10 μM) or DMSO was examined by flow cytometry as described previously (23). All antibodies were used at 10 μg/mL. To examine whether TrkA regulates basal and/or agonist-induced expression of CCR3, the receptor for eotaxin-1, eosinophils were first treated with 1-NM-PP1 or DMSO and then exposed to eotaxin-1 (100 nM) or PBS (control) for 5 min at room temperature before flow cytometry using FITC-conjugated rat anti-mouse CCR3 (2.5 μg/mL, R&D Systems) with FITC-conjugated rat IgG2a as the isotype control.
Calcium imaging assay
Role of TrkA in regulating eotaxin-1-induced changes in intracellular calcium ([Ca2+]i) concentration was evaluated by real-time digital video fluorescence imaging using Fura-2 AM (Sigma-Aldrich) as described previously (24). Basal [Ca2+]i was first measured for 2 min after which eosinophils were treated with 1-NM-PP1 or vehicle and evaluated for an additional 3 min. Cells were then stimulated with eotaxin-1 and monitored for 2 min to assess agonist-induced [Ca2+]i responses. Cells were finally exposed to 2 μM Ionomycin, free acid (BioVision) as a positive control and monitored for an additional 3 min. The ratio of fluorescence emissions at 340 nm and 380 nm was determined at each time point which is directly reflective of the amount of [Ca2+]i.
Western blot analysis
WT BM-derived murine eosinophils (18 day culture, 7-10 × 106/treatment) were treated with eotaxin-1 (100 nM) or NGF (100 ng/ml, based on previous studies using human eosinophils(28)) for 5 min. Cells were collected by centrifugation and cell pellets were resuspended in RIPA buffer containing 1 mM orthovanadate as well as protease and phosphatase inhibitors. Cell suspensions were subjected to three freeze-thaw cycles followed by three cycles of sonication (5-7 sec each) on ice with 2 min intervals. Cell lysates were centrifuged and total protein in the supernatants was quantified using the BCA Protein Assay Kit (Thermo Fisher Scientific Co.). Expression of p-TrkA and t-TrkA in cell lysates (30-60 μg/lane) was evaluated by SDS/PAGE (8%) under reduced conditions followed by immunoblotting with mAb against p-TrkA (sc-8058, Santa Cruz Biotechnology, 1 μg/ml) in the presence or absence of a p-TrkA mAb blocking peptide (5× concentration of primary antibody, Santa Cruz Biotechnology) or polyclonal antibodies against t-TrkA (Cell Signaling Technology, 0.15 μg/ml). Membranes were incubated with the primary antibody overnight at 4°C and then exposed to HRP-conjugated goat anti-mouse or anti-rabbit IgG (0.16 μg/ml, Jackson ImmunoResearch Laboratories). Expression levels of actin were monitored as an internal control using HRP-conjugated mAb against actin (Santa Cruz Biotechnology, 66 ng/ml,).
For detection of t- and p-ERK(1/2), TrkA-KI BM-derived eosinophils were first treated with 10 μM 1-NM-PP1 or DMSO followed by eotaxin-1 (100 nM) for an additional 5 min. Cell lysates (20 μg per lane) were electrophoresed (10% SDS/PAGE) and subjected to immunoblotting using rabbit mAb against t-ERK(1/2) (0.04 μg/ml) and rabbit polyclonal antibodies against p-ERK(1/2) (0.088 μg/ml) (both from Cell Signaling Technology), respectively. Bound antibodies were detected using HRP-conjugated goat anti-rabbit IgG (0.16 μg/ml, Jackson ImmunoResearch Laboratories). Protein bands were detected using Western Bright™ ECL (Advansta) and visualized on X-ray films. Intensity of detected bands was quantified using ImageJ image analysis software. Expression level of p-TrkA and t-TrkA was normalized against actin, while the expression level of p-ERK(1/2) was normalized against that of t-ERK(1/2).
Statistical Analysis
All data are presented as mean ± SEM unless otherwise stated. Representative or combined data of experiments performed at least three times are shown for in vitro studies. Statistical significance between two groups was determined using a two-tail unpaired Student’s t-test. Statistical significance between multiple groups was determined by one-way ANOVA followed by Tukey post-hoc analysis. Statistical analysis of AHR studies was performed by two-way ANOVA followed by Tukey post-hoc analysis. Differences were considered significant at p < 0.05.
Results
Exposure to allergens leads to activation of TrkA in the lungs
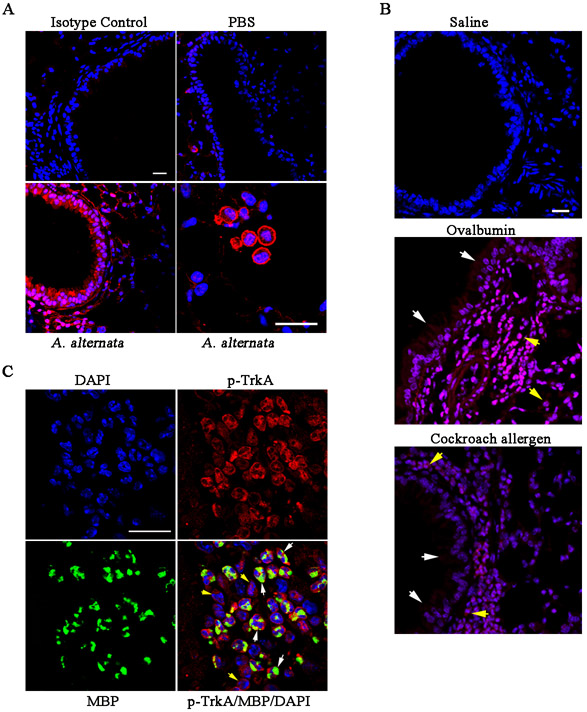
Previous studies have indicated a role for NGF/TrkA signaling in allergic asthma (reviewed in (10)); however, the causal relationship and the underlying mechanism(s) involved are not well understood. To understand the significance and role of TrkA signaling in promoting allergic asthma, we first investigated whether allergen exposure leads to activation of TrkA in the lung. Lung tissue of mice exposed to A. alternata, an airborne fungal allergen known to induce and/or exacerbate allergic asthma in humans (29), was analyzed for expression of p-TrkA by immunofluorescent staining (Fig. 1, A). Epithelial cells lining the airways as well as inflammatory cells recruited to the lungs were positive for expression of p-TrkA. Epithelial cells lining the airways of control mice showed minimal p-TrkA staining. Activation of TrkA in airway epithelial cells and recruited inflammatory cells was also noted in the lungs of mice exposed to other allergens such as ovalbumin or cockroach allergen (Fig. 1, B). We next examined whether TrkA is activated in eosinophils, the predominant inflammatory cells recruited to allergic airways. Dual immunofluorescence staining of lung sections from A. alternata-challenged WT mice for expression of MBP, an eosinophil-specific granule protein, and p-TrkA showed that several eosinophils (based on MBP expression) in the peribronchial region were positive for expression of p-TrkA (Fig. 1, C, white arrows), thus demonstrating that TrkA in eosinophils is activated under inflammatory conditions. In addition, cells positive for p-TrkA but not MBP were also detectable (Fig. 1, C, yellow arrows), indicating that TrkA is also activated in other inflammatory cells recruited to the inflamed lungs.
Fig. 1. Allergen-exposure leads to activation of TrkA in the lungs.
(A) Expression of p-TrkA in the airway epithelium (lower left panel) and inflammatory cells (lower right panel) of WT mice exposed to A. alternata challenge or PBS (control, upper right panel) after IHC with anti-p-TrkA. IHC of lung sections from allergen-challenged mice with a control antibody (upper left panel) is shown as a negative control. (B) Expression of p-TrkA in the airway epithelium (white arrows) and inflammatory cells (yellow arrows) of WT mice exposed to saline alone or challenged with OVA or CRA after IHC staining with anti-p-TrkA. (C) Dual-immunostaining of lung sections from A. alternata-exposed WT mice with antibodies against eosinophil-specific MBP (green) and p-TrkA (red). Yellow arrows, cells positive for p-TrkA only; white arrows, cells positive for p-TrkA and MBP. Scale bar, 20 μm. Data representative of three mice/group is shown in A-C.
Eotaxin-1 activates TrkA in eosinophils
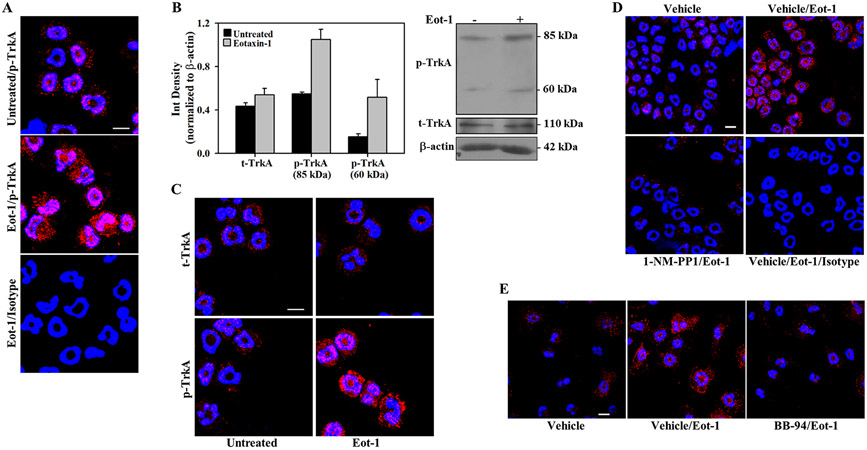
Eotaxin-1 is a major chemoattractant for eosinophils during allergic inflammation (19). Since eotaxin-1 levels are elevated in the lungs of A. alternata-challenged mice (30) and TrkA in eosinophils in the inflamed lungs of A. alternata-challenged mice is activated (Fig. 1, C), we examined whether eotaxin-1 can activate TrkA in eosinophils and thereby regulate eosinophil recruitment. Eosinophils cultured from the BM of WT mice were treated with eotaxin-1 and examined for activation of TrkA by immunofluorescence staining (i.e., p-TrkA expression). Relative to untreated cells, eosinophils exposed to eotaxin-1 exhibited increased expression of p-TrkA (Fig. 2, A), similar to that noted with NGF, a well-known ligand of TrkA (Supplemental Fig. 3, A). This finding was confirmed by Western blot analysis. WT eosinophils showed two immunoreactive bands with molecular weights of ~85 kDa and ~60 kDa with p-TrkA antibody, which increased in intensity after eotaxin-1 treatment. The expression level of t-TrkA (~110 kDa) on the other hand remained similar in the two groups (Fig. 2, B, upper and lower panels).
Fig. 2. Activation of TrkA by eotaxin-1 in murine eosinophils is MMP-mediated.
(A) Expression of p-TrkA in eotaxin-1-treated and control (untreated) BM eosinophils from WT mice after immunofluorescence staining with anti-p-TrkA. (B) Expression of p- and t-TrkA in eotaxin-1-treated (5 min) and control WT BM eosinophils by Western blot followed by densitometric analysis of protein bands. Combined data (mean ± STD) of two out of four experiments with similar results is shown. A representative blot is shown below graph. (C) p- and t-TrkA expression in eosinophils from TrkA-KI mice after exposure to eotaxin-1 by immunofluorescence staining. (D) Expression of p-TrkA in TrkA-KI eosinophils pre-treated with 1-NM-PP1 (10 μM) or vehicle (DMSO) and then exposed to eotaxin-1 (100 nM). (E) Effect of the broad spectrum MMP inhibitor BB-94 versus vehicle on eotaxin-1-induced activation of TrkA by immunofluorescence staining. Scale bar, 10 μm. Representative data of experiments repeated at least three times with similar results using eosinophils from different mice is shown in A-E.
Because of the limited drug specificity of known TrkA inhibitors, we used a genetic-chemical approach to further examine the role of eotaxin-1-induced TrkA activation. In these studies, eosinophils cultured from BM of TrkA-KI mice, that have a point mutation in the ATP-binding pocket allowing specific inhibition of TrkA kinase activity by an ATP analog such as 1-NM-PP1 (21) were used. Treatment with eotaxin-1 or NGF activated TrkA in TrkA-KI eosinophils exhibiting two p-TrkA mAb immunoreactive bands of ~85 kDa and ~60 kDa by Western blot analysis similar to that observed in WT eosinophils (Supplemental Fig. 3, B). Incubation of eotaxin-1-treated eosinophil lysates with the primary antibody in the presence of a p-TrkA antibody blocking peptide markedly decreased intensity of the 85 and 60 kDa bands, demonstrating the specificity of the bands detected by the antibody (Supplemental Fig. 3, C). Additionally, immunofluorescence staining revealed that relative to untreated cells, TrkA-KI eosinophils exhibit increased expression of p-TrkA when exposed to eotaxin-1, thus confirming that eotaxin-1 activates TrkA in eosinophils and demonstrating that the point mutation in TrkA in TrkA-KI eosinophils does not alter its ability to undergo phosphorylation (Fig. 2, C, lower panels). Expression of t-TrkA was similar in control and eotaxin-1-treated cells (Fig. 2, C, upper panels). Further, treatment of TrkA-KI eosinophils with 1-NM-PP1 prior to eotaxin-1 inhibited TrkA activation when compared to cells pre-treated with DMSO, the vehicle control, based on reduced expression of p-TrkA by immunofluorescence staining (Fig. 2, D). Next, since metalloproteases have been shown to play a role in activation of TrkA through cleavage in neuronal cells (31), we examined whether TrkA activation in eosinophils is mediated by metalloproteases. Eosinophils were treated with the potent broad-spectrum MMP inhibitor BB-94 before exposure to eotaxin-1 and analyzed for p-TrkA expression by immunofluorescence staining. Cells pre-treated with BB-94 showed markedly decreased p-TrkA expression after eotaxin-1 treatment compared to cells treated with vehicle (Fig. 2, E). Further, on Western blots, decreased generation of the ~85 and ~60 kDa p-TrkA immunoreactive bands was noted in eosinophils pre-treated with BB-94 relative to vehicle before eotaxin-1 exposure (Supplemental Fig. 4, A). In contrast, cells pre-treatment with a pan-caspase inhibitor Z-VAD-FMK or PMSF did not inhibit eotaxin-1-induced TrkA activation (Supplemental Fig. 4, B). These findings indicate that activation of TrkA by eotaxin-1 involves MMPs.
TrkA regulates eosinophil adhesion and migration
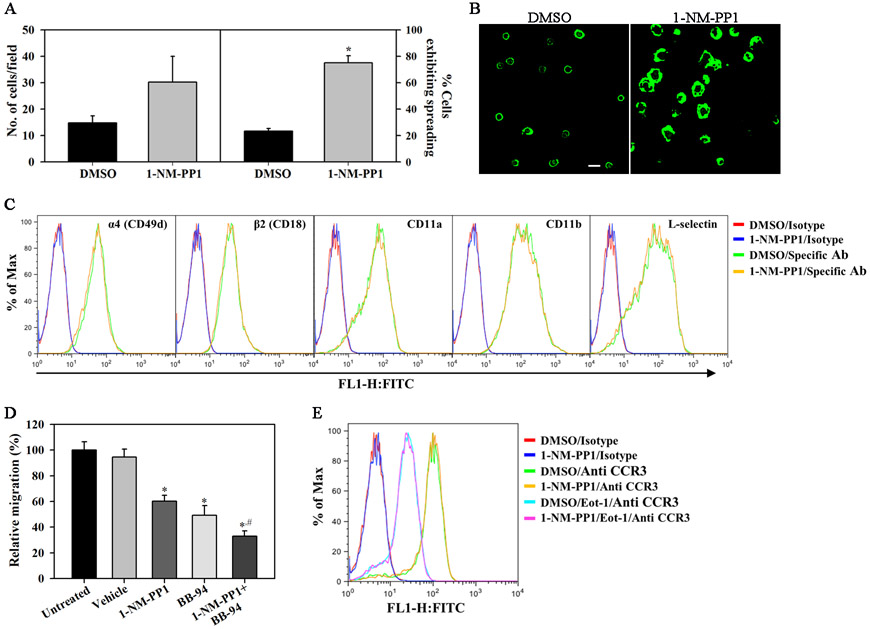
Given the role of TrkA in migration of neuronal (32) and non-neuronal cells (18, 33), we examined whether TrkA signaling is involved in eotaxin-1-induced eosinophil migration. Since the endothelial adhesion molecule VCAM-1 supports eosinophil trafficking (rolling and stable adhesion) in vivo (34) prior to transmigration, we first evaluated the ability of TrkA-KI eosinophils pre-treated with 1-NM-PP1 or DMSO to adhere to VCAM-1. No statistically significant difference was noted between 1-NM-PP1- and vehicle-treated cells in the number of adherent cells on VCAM-1 (Fig. 3, A, left panel). Whereas majority of the adherent control cells retained a compact round cell body with F-actin localized at the cell periphery, most of the adherent 1-NM-PP1-treated cells exhibited increased actin polymerization and augmented spreading (Fig. 3, A, right panel and B). To determine whether the increased spreading noted in 1-NM-PP1-treated cells was due to changes in expression of cell surface adhesion molecules, 1-NM-PP1- or vehicle-treated cells were analyzed for expression of CD49d, CD11a, CD11b, L-selectin or CD18 (Fig. 3, C). No difference in expression of these molecules was noted between the two groups. In addition to altered cell spreading on VCAM-1, treatment with 1-NM-PP1 strongly inhibited migration of TrkA-KI eosinophils towards eotaxin-1 compared to DMSO-treated cells (Fig. 3, D). Since MMPs play a role in eotaxin-1-induced activation of TrkA (Fig. 2, E), we examined the effect of BB-94 independently and in combination with 1-NM-PP1 on eotaxin-1-induced migration of TrkA-KI eosinophils. While both 1-NM-PP1 and BB-94 significantly inhibited migration independently, an additive inhibitory effect was noted when 1-NM-PP1 and BB-94 were used in combination which was significantly higher than 1-NM-PP1 alone. Expression level of the eotaxin-1 receptor CCR3 was unaffected by 1-NM-PP1 treatment at baseline and in the presence of eotaxin-1 (Fig. 3, E), suggesting that the level of expression of CCR3 is not regulated by TrkA.
Fig. 3. Activation of TrkA promotes eosinophil migration.
(A and B) Adhesion and morphological change (cell spreading) in TrkA-KI eosinophils adherent on rmVCAM-1-coated coverslips after treatment with 1-NM-PP1 (10 μM, 20 min). Scale bar, 20 μm. (C) Effect of 1-NM-PP1 on expression of cell adhesion molecules in TrkA-KI eosinophils by flow cytometry. (D) In vitro migration of TrkA-KI eosinophils towards eotaxin-1 (100 nM) across membranes in Transwell® Chambers after pre-treatment with 1-NM-PP1 and BB-94 (both at 10 μM) independently as well as in combination or with vehicle. (E) Effect of 1-NM-PP1 on CCR3 expression in TrkA-KI eosinophils with or without exposure to eotaxin-1 (5 min) by flow cytometry. Combined data (mean ± SEM) of three-four experiments in duplicate is shown in A and D. Representative data of three independent experiments with eosinophils from three different mice is shown in B, C and E. *p < 0.01 in A and D for comparison with vehicle-treated cells; #p < 0.05 in D for comparison with 1-NM-PP1-treated cells.
Blockade of TrkA activation inhibits eotaxin-1-induced calcium flux, cytoskeletal changes and ERK1/2 activation
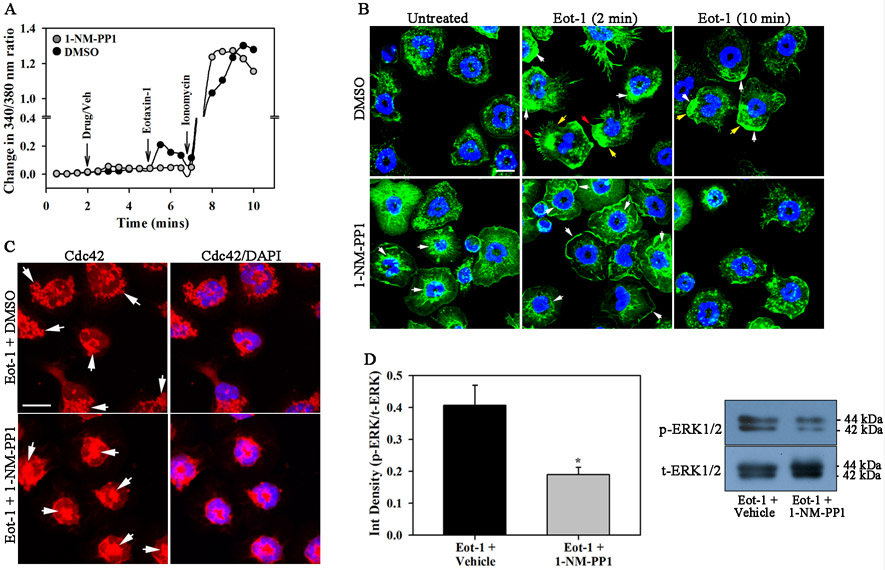
We examined the effect of 1-NM-PP1 on signaling events downstream of CCR3. Eotaxin-1 is known to induce calcium flux in eosinophils (35). Baseline (unstimulated) [Ca2+]i levels were similar in 1-NM-PP1- and DMSO-treated eosinophils by real-time digital video fluorescence imaging (Fig. 4, A). In response to eotaxin-1, DMSO-treated eosinophils demonstrated a surge in free [Ca2+]i within few seconds followed by a decline. In contrast, [Ca2+]i levels in l-NM-PP1-treated cells remained similar to baseline levels even after eotaxin-1 treatment. Both groups of cells demonstrated a similar response to ionomycin, indicating that Ca2+ release as such is not affected and that it is the eotaxin-1-induced Ca2+ response that is abrogated by TrkA inactivation. Ca2+ signaling in response to cell activation is known to play a role in cell migration through cytoskeletal reorganization (36). Kinetics of cytoskeletal changes in TrkA-KI eosinophils in response to cell activation (i.e., eotaxin-1) was examined by confocal microscopy based on phalloidin binding (indicative of F-actin polymerization). In the absence of eotaxin-1, vehicle-treated cells exhibited a diffused pattern of F-actin in the cytoplasm. Upon exposure to eotaxin-1 for 2 min, most cells showed increased phalloidin staining at one end of the cell along with formation of distinct lamellipodia and filopodia. After 10 min, most cells continued to show increased phalloidin binding at one end of the cell but were of irregular shape. In contrast, 1-NM-PP1-treated cells showed increased baseline phalloidin binding mostly around the nucleus and were more spread out even in the absence of eotaxin-1. After 2 min of exposure to eotaxin-1, cells continued to exhibit increased phalloidin binding around the nucleus and at the periphery but with irregular spreading lacking clear lamellipodia and filopodia formation and acquiring a multipolar shape. After 10 min of exposure to eotaxin-1, most of the 1-NM-PP1-treated cells showed diffused phalloidin binding and less spreading relative to non-eotaxin-treated cells (Fig. 4, B). Thus, blockade of TrkA activation with 1-NM-PP1 appears to result in elevated F-actin levels and irregular kinetics of F-actin polymerization/depolymerization resulting in altered cytoskeletal changes relative to control cells upon stimulation with eotaxin-1.
Fig. 4. Blockade of TrkA activation inhibits eotaxin-1-induced calcium flux, cytoskeletal changes and ERK(1/2) activation in eosinophils.
(A) [Ca2+]i levels in eosinophils treated with 10 μM 1-NM-PP1 or vehicle followed by eotaxin-1 (100 nM) and then 2 μM ionomycin (positive control) at the indicated time points by digital video fluorescence imaging using Fura-2 AM. >200 cells were analyzed for each treatment. (B) FITC-Phalloidin staining of TrkA-KI eosinophils adherent on poly-L-Lysine, treated with 1-NM-PP1 (20 min) or vehicle and exposed to eotaxin-1 for the indicated time points. White arrows, regions of increased phalloidin binding; red arrows, filopodia; yellow arrows, lamellipodia. (C) Immunofluorescence staining for Cdc42 expression in adherent TrkA-KI eosinophils treated with 1-NM-PP1 (or vehicle) and then eotaxin-1 as in B. Arrows indicate Cdc42 expression. (D) Total and phosho-ERK(1/2) expression in eotaxin-1-treated TrkA-KI eosinophils after pre-treatment with 1-NM-PP1 or vehicle by densitometric analysis of protein bands after Western blot analysis. A representative blot is shown. Scale bar, 10 μm. Combined data (mean ± SEM) of four experiments is shown in A and D and data representative of three independent experiments with eosinophils from three different mice is shown in B and C. *p < 0.05 in D for comparison with vehicle-treated cells.
The Rho family of small GTPases (Rac1, RhoA, and Cdc42) plays an important role in cellular actin organization and cytoskeletal changes (37), with Cdc42 being involved in regulating cell polarity (38). Changes in expression of Cdc42 in 1-NM-PP1-treated TrkA-KI eosinophils after exposure to eotaxin-1 (2 min) were examined by immunofluorescence staining. A larger number of vehicle-treated cells showed Cdc42 expression localized at one end of the cell at the cell periphery relative to cells pre-treated with 1-NM-PP1 where Cdc42 expression was mostly noted in the center of the cell with minimal staining at the cell periphery (Fig. 4, C, left and right panels). These findings suggest that blockade of TrkA activation interferes with rearrangement/redistribution of Cdc42. Finally, activation of ERK1/2, an important signaling event in eotaxin-1-exposed eosinophils that is required for cell migration in vitro (39) and recruitment in vivo (40), was strongly inhibited in TrkA-KI eosinophils treated with 1-NM-PP1 relative to vehicle treated cells (Fig. 4, D). Overall, these data indicate that TrkA signaling is essential for eotaxin-induced migration of eosinophils.
1-NM-PP1 inhibits allergen-induced cellular airway inflammation
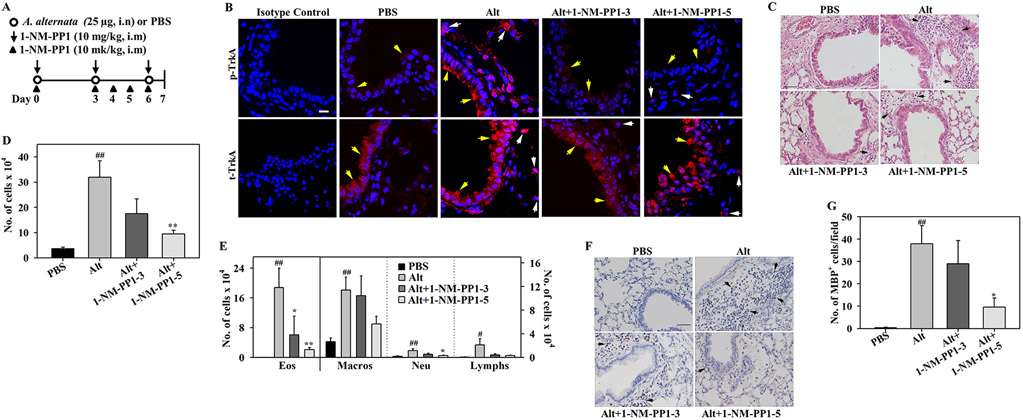
To evaluate the role of TrkA in regulating airway eosinophil recruitment in vivo and determine whether specific blockade of TrkA signaling can inhibit the development of airway inflammation, we used an experimental model of allergic asthma wherein TrkA-KI mice were challenged with A. alternata (22) and administered with 1-NM-PP1 or left untreated as outlined in (Fig. 5, A). Analysis by immunofluorescence staining demonstrated marked TrkA activation in the airway epithelium and inflammatory cells of lung sections from A. alternata-challenged TrkA-KI mice, while only minimal activation of TrkA was noted in the lungs of control mice. Activation of TrkA was markedly inhibited in allergen-exposed TrkA-KI mice treated with 3 or 5 doses of 1-NM-PP1 (Alt+1-NM-PP1-3 and Alt+1-NM-PP1-5, respectively) compared to untreated counterparts. Total TrkA expression level in the airway epithelium and inflammatory cells was similar in the lungs of all groups of mice (Fig. 5, B). Thus, TrkA-KI mice exhibit activation of TrkA in the lungs in a manner similar to WT mice in response to A. alternata challenge (Fig. 1, A) and 1-NM-PP1 specifically inhibits this activation. Additionally, while untreated allergen-challenged TrkA-KI mice showed significantly increased recruitment of inflammatory cells in the lung tissue based on H & E staining and in the BALF compared to control mice, allergen-challenged TrkA-KI mice administered with 1-NM-PP1 at three or five doses exhibited markedly reduced recruitment of inflammatory cells in the peribronchial area of the lungs (Fig 5, C) and in the BALF (Fig 5, D). Further, in the Alt+1-NM-PP1-5 group, a significantly lower number of eosinophils and neutrophils were present in the BALF relative to untreated mice (Fig. 5, E). The number of macrophages and lymphocytes in the BALF were also lower in this group, although the reduction was not statistically significant. The effect of blockade of TrkA on lung tissue eosinophilia was examined by IHC for the expression of eosinophil-specific MBP. As expected, allergen-challenged TrkA-KI mice showed significantly increased infiltration of lung tissue with eosinophils compared to control mice. While administration of three doses of 1-NM-PP1 did not have an effect on lung tissue eosinophils, the number of eosinophils in the lung tissue of Alt+1-NM-PP1-5 group was significantly lower than in the untreated A. alternata-challenged group (Fig. 5, F and G). These findings indicate that TrkA signaling plays a significant role in the recruitment of inflammatory cells, specifically eosinophils, to allergic airways and inhibiting TrkA activation in vivo prevents eosinophil recruitment to allergen-exposed lungs. Eosinophil counts in the peripheral blood of allergen-challenged TrkA-KI mice administered with 1-NM-PP1 were also lower compared to untreated mice (data not shown). Since eotaxin-1 signaling is affected in the presence of 1-NM-PP1, recruitment of generated eosinophils from the bone marrow milieu is also likely to be affected resulting in low numbers in the circulation.
Fig. 5. TrkA kinase inhibition attenuates allergen-induced airway cellular inflammation.
(A) Outline of protocol for treatment of A. alternata-challenged TrkA-KI mice with 1-NM-PP1. i.n; intranasal, i.m; intramuscular. (B) t- and p-TrkA expression in lung sections from PBS (control) and A. alternata-challenged TrkA-KI mice with or without 1-NM-PP1 treatment. Airway epithelium: yellow arrows, inflammatory cells: white arrows. (C) H & E staining of lung sections from mice described in B. (D and E) Total cells and differential cell counts in BALF of mice described in B. (F and G) Infiltrated eosinophils in lung tissue of mice described in B detected by IHC staining for MBP (stained dark brown, black arrows) and quantitation of MBP-positive cells, respectively. A representative image for each group is shown in B, C and F. Scale bar, 10 μm in B; 50 μm in C and F. Combined data (mean ± SEM) of mice from at least three independent experiments is shown in D, E and G (n = 7-12 mice/group in D, 6-10 mice/group in E and 5-7 mice/group in G). #p <0.05 and ##p <0.01 for comparison with control group; *p < 0.05 and **p<0.01 for comparison with untreated A. alternata-challenged group.
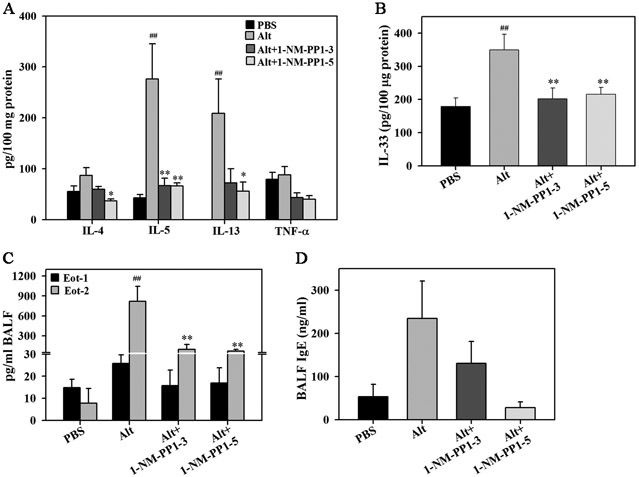
Effect of 1-NM-PP1 on allergen-induced Th2 cytokines, eotaxins and IgE
Lung tissue from allergen-challenged TrkA–KI mice with or without 1-NM-PP1 treatment was evaluated for expression of IL-2, IFN-γ, IL-4, IL-5, IL-13 and TNF-α. Allergen challenge significantly induced level of IL-5 and IL-13 in the lungs. Level of IL-4, on the other hand, was only marginally higher than in the control group. Compared to untreated allergen-challenged mice, IL-5 levels were significantly lower in mice treated with 1-NM-PP1 at three or five doses. While IL-13 levels were also substantially lower in both 1-NM-PP1-treated groups, a statistically significant reduction was noted only in allergen-challenged mice that received 5 doses of 1-NM-PP1. Although TNF-α expression was not induced by A. alternata challenge, level of this cytokine in 1-NM-PP1-treated mice (three or five doses) tended to be lower than in untreated mice (Fig. 6, A). IL-2 and IFN-γ levels were below detection limit. In addition to reducing levels of Th2 cytokines, administration of 1-NM-PP1 at three or five doses significantly inhibited IL-33 levels in the lungs of A. alternata-challenged mice (Fig. 6, B).
Fig. 6. Effect of TrkA kinase inhibition on allergen-induced lung cytokines, chemokines and IgE.
(A) Th2 cytokine and TNF-α level in lung lysates from control and A. alternata-challenged TrkA-KI mice with or without 1-NM-PP1 treatment. (B) IL-33 levels in lung lysates from mice described in A. (C) Eotaxin-1 and −2 levels in BALF of mice described in A. (D) Total IgE levels in BALF of mice described in A. Combined data (mean ± SEM) of n = 6-10 mice for control and allergen-challenged groups and 4-10 mice for 1-NM-PP1-treated groups in A-D is shown. ##p <0.01 for comparison with control group; *p < 0.05 and **p<0.01 for comparison with untreated A. alternata-challenged group.
Eotaxin-1 and −2 are predominant chemokines responsible for recruitment of eosinophils to allergic airways (19). Since eosinophil infiltration in the lungs of 1-NM-PP1-treated allergen-challenged mice was significantly reduced, we examined whether this effect was due to reduced levels of eotaxins in the lungs of these mice. Expression of eotaxin-1 and −2 in the BALF from A. alternata-challenged mice with or without 1-NM-PP1 treatment was measured by ELISA. Eotaxin-1 levels in the allergen-challenged mice tended to be higher than in control mice and marginally reduced by 1-NM-PP1 treatment (not statistically significant) (Fig. 6, C). Eotaxin-2 levels, on the other hand, were significantly higher after allergen challenge. Importantly, blockade of TrkA activation with 1-NM-PP1 at three or five doses significantly reduced the expression of allergen-induced eotaxin-2 levels (Fig. 6, C). Since elevated IgE levels are a distinguishing feature of allergic inflammation, we examined the effect of 1-NM-PP1 treatment on allergen-induced IgE levels in the BALF by ELISA. Consistent with the allergic disease phenotype, IgE levels in A. alternata-challenged mice were higher than in control mice. A trend in reduction of allergen-induced IgE levels was observed in A. alternata-challenged mice treated with five doses of 1-NM-PP1 (Fig. 6, D).
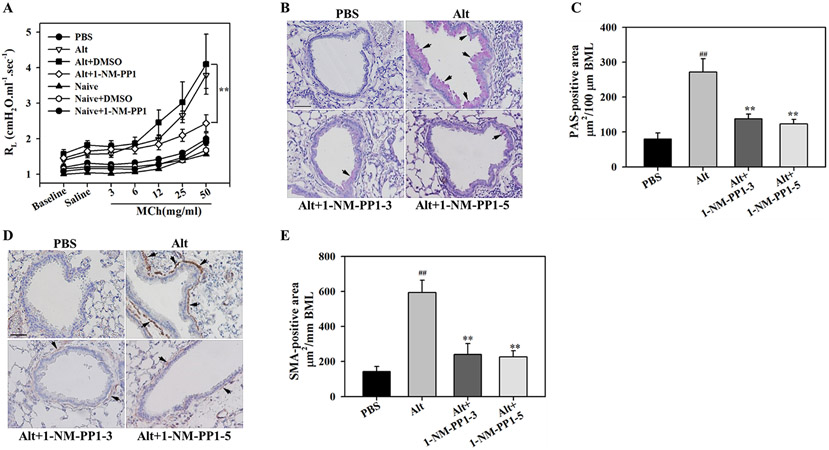
1-NM-PP1 attenuates allergen-induced airway structural changes
Allergen exposure leads to structural changes in the airways such as increased mucus production and airway smooth muscle hyperplasia/hypertrophy, which in turn can contribute to airflow obstruction and AHR (41). We examined whether blockade of TrkA activation alters A. alternata-induced AHR. A. alternata-challenged mice treated with 1-NM-PP1 at 5 doses showed improved airway function with a significant reduction in pulmonary resistance to inhaled methacholine at 50 mg/ml compared to vehicle-treated mice (Fig. 7, A). Administration of 1-NM-PP1 to naïve mice did not exert any non-specific effects on pulmonary resistance to inhaled methacholine, which was similar to that observed in PBS-exposed mice. Based on PAS staining and immunohistology for expression of α-SMA (a regulator of smooth muscle contraction), A. alternata-challenged mice exhibited significantly increased airway mucus secretion and smooth muscle thickening, respectively, compared to control mice. Blockade of TrkA activation in the allergen-challenged mice with three or five doses of 1-NM-PPP1 significantly reduced allergen-induced airway mucus secretion (Fig. 7, B and C) and smooth muscle mass (Fig. 7, D and E) relative to untreated mice. These studies suggest that activation of TrkA promotes structural changes and the development of AHR in allergic airways and inhibition of this signaling pathway attenuates the development of these changes along with improved airway function.
Fig. 7. TrkA kinase inhibition attenuates allergen-induced AHR, mucus secretion and smooth muscle thickening.
(A) Measurement of AHR following exposure to increasing concentrations of aerosolized methacholine (Mch) in mechanically ventilated control and A. alternata-challenged TrkA-KI mice that were untreated or treated with 1-NM-PP1 (5 doses) or vehicle. (B and C) Airway mucus secretion in control and A. alternata-challenged TrkA-KI mice with or without 1-NM-PP1 treatment assessed by PAS staining (stained dark-pink, black arrows) and quantitation of PAS-positive area, respectively. (D and E) Airway smooth muscle thickening in mice described in B assessed by IHC staining for α-SMA expression (stained brown, black arrows) and quantitation of α-SMA-positive area, respectively. Representative image for each group is shown in B and D. Scale bar, 50 μm. Combined data (mean ± SEM) of n = 5-7 mice/group in A, and 6-7 mice/group C and E is shown. ##p <0.01 for comparison with control group; **p<0.01 for comparison with untreated A. alternata-challenged group.
Discussion
Eosinophilia is the hallmark of airway inflammation in asthma (42). Eosinophils are a source of various cytokines, chemokines, growth factors and highly cytotoxic cationic granule proteins that are secreted upon activation (43) and known to exert various pro-inflammatory effects (reviewed in (44)). Thus, identifying key players involved in selective eosinophil recruitment and the specific pathways promoting airway eosinophilic inflammation is critical for the identification of novel therapeutic targets. Previous studies indicate a role for TrkA in experimental models of AAI, albeit in the context of NGF-mediated effects. Studies using blocking antibodies or siRNA specific for NGF have demonstrated the contributory role of NGF (reviewed in (10)) and TrkA (45) in the development of experimental allergic asthma but the importance of TrkA receptor signaling or its functional mechanism by targeted inactivation of TrkA kinase activity was not established. In the current study, we have used a combined genetic-chemical approach (TrkA-KI mouse strain (21)) to specifically control the TrkA kinase activity and established a distinct role for TrkA signaling in eosinophil recruitment in vitro and in vivo.
Various cells in the lung, including eosinophils, bronchial and alveolar epithelial cells, smooth muscle cells, and vascular endothelial cells, express TrkA (8). In the current study, we found that exposure to not only the fungal allergen A. alternata but also allergens such as OVA and CRA leads to activation of TrkA in the airway epithelium and in inflammatory cells recruited to the lungs of WT mice. While TrkA activation and signaling mediated by NGF is extensively characterized in neuronal cells (46), its activation in other cell types, specifically eosinophils, is less investigated. TrkA is expressed by human peripheral blood eosinophils (28) and its expression on BALF eosinophils is upregulated after allergen challenge (47). Along these lines, in the current study, eosinophils recruited to the inflamed lungs of A. alternata-exposed mice exhibited activation of TrkA. Eotaxin-1, the predominant chemokine contributing to airway eosinophil recruitment, is elevated in the lungs of humans with allergic asthma (48) and in animal models of AAI (49), including A. alternata-induced models (30). In the current study, WT BM-derived eosinophils exposed to eotaxin-1 showed rapid (5 min) activation of TrkA by immunofluorescence staining, a finding that has hitherto not been reported. Additionally, on Western blots, while expression level of t-TrkA remained unaltered after eotaxin-1 treatment showing a single protein band of ~110 kDa, increased intensity of two bands of molecular weight ~85 and ~60 kDa immunoreactive for p-TrkA (confirmed by antibody blocking peptide studies) was noted. This observation was confirmed in eosinophils from TrkA-KI mice wherein 1-NM-PP1, an ATP analog that selectively inhibits TrkA activation in these mice, prevented eotaxin-1-induced activation of TrkA in immunofluorescence staining studies. Overall, these findings identify eotaxin-1 as a new player involved in TrkA activation.
TrkA signaling initiated by direct binding of ligands such as NGF involves auto-phosphorylation of specific tyrosine residues located intracellularly in the kinase domain of the receptor which then creates docking sites for effector proteins that initiate activation of various intracellular signaling pathways (1). However, studies have shown that TrkA can be transactivated by ligands of the GPCR family of transmembrane receptors even in the absence of NGF via second messengers or receptor co-localization (16-18). In this context, studies have suggested a role for MMPs, specifically MMP-9, in inducing activation of TrkA upon agonist/ligand binding to associated GPCRs (reviewed in (50)). Since eotaxin-1 exerts its activity on eosinophils via the GPCR CCR3 (19), we investigated whether MMPs play a role in eotaxin-1-induced activation of TrkA. In eosinophils pre-treated with a pan-MMP inhibitor (BB-94), but not with a pan-caspase inhibitor or PMSF, eotaxin-1 failed to activate TrkA suggesting that eotaxin-1 induced activation of TrkA is MMP-dependent. Eotaxin-1 has been shown to induce expression of MMPs in eosinophils (51) and other cell types (52, 53). In neuronal cells, MMPs can cleave the ectodomain of TrkA generating cell-bound, tyrosine phosphorylated, signaling-competent fragments (31). Furthermore, TrkA has many predicted MMP cleavage sites in the extracellular and intracellular regions based on CleavPredict (http://cleavpredict.sanfordburnham.org), a platform for substrate cleavage prediction for MMPs (54). Our observation of two p-TrkA immunoreactive protein bands in eotaxin-1-treated cells suggestive of cleaved fragments together with blockade of TrkA activation by an inhibitor of MMPs, indicates that eotaxin-1-induced activation of TrkA in eosinophils is likely to be mediated by MMPs.
Studies have shown that TrkA signaling is involved in regulating NGF-driven migration of neuronal and non-neuronal cells (32, 33) as well as non-NGF-mediated migration via GPCR transactivation (18). Adhesion to the vascular endothelium and chemoattractant-induced migration are key steps involved in eosinophil trafficking and recruitment to sites of inflammation (55). In the current study, blockade of TrkA signaling with 1-NM-PP1 did not alter the number of eosinophils that adhered to VCAM-1 in a statistically significant manner, but induced striking changes in cell shape. A large number of 1-NM-PP1-treated cells demonstrating exaggerated spreading and increased actin polymerization, although expression level of several cell surface adhesion molecules, including CD49d (the counter receptor for VCAM-1), remained unaffected. Despite the enhanced spreading on VCAM-1, inhibition of TrkA activation significantly reduced eotaxin-1-induced migration. Additionally, MMP inhibitor BB94 independently, and additively with 1-NM-PP1, reduced eosinophil migration, further supporting a role for MMPs in eotaxin-1-induced activation of TrkA. Expression level of CCR3 in the absence or presence of eotaxin-1 remained unaltered by blockade of TrkA activation. Thus, inactivation of TrkA signaling appears to inhibit migration by impeding cell motility via regulation of signaling events downstream of CCR3 rather than by enhanced adhesion or receptor expression level.
Eotaxin-1 induces a calcium flux in eosinophils and impaired Ca2+ signaling in eosinophils in response to eotaxin-1 is associated with altered actin polymerization and reduced migration (24, 26). In the current study, inhibition of TrkA signaling specifically blocked eotaxin-1-induced Ca2+ flux. Studies have previously shown that TrkA participates in NGF-induced Ca2+ signaling in other cell types (56). Cell polarization and migration are coordinated by Ca2+ signaling via regulation of integrin activation and modulation of actin dynamics, i.e., coordinated polymerization and depolymerization of F-actin, through activation of signaling molecules that interact with actin (PKC and calmodulin-dependent kinases) (36). In support of this, along with inhibition of agonist-induced [Ca2+]i, blockade of TrkA signaling with 1-NM-PP1 resulted in altered F-actin polymerization and failure of cells to form distinct lamellipodia and filopodia. Given the role played by the Rho family of small GTPases in regulating the actin cytoskeleton (37) and specifically that of Cdc42 in triggering filopodia formation and steering cell polarity during migration (38, 57), we examined whether TrkA regulates Cdc42 expression. Consistent with the inability of 1-NM-PP1-treated eosinophils to form clear lamellipodia and filopodia after activation with eotaxin-1, translocation of Cdc42 to the leading edge was abrogated in these cells in contrast to control cells with Cdc42 expression largely localized to the peri-nuclear region or dispersed all over the cell. Another signaling pathway activated by CCR3 stimulation in human and mouse eosinophils is the ERK(1/2) MAPK pathway (24, 58). ERK(1/2) activation is an important signaling event necessary for eotaxin-1-induced shape change and migration of eosinophils (39, 59). Further, in the context of NGF stimulation, TrkA is known to engage ERK(1/2) downstream to promote various events during neuronal development (1). In our study, inhibition of TrkA signaling significantly reduced activation of ERK(1/2) in response to eotaxin-1. Taken together, these studies suggest that TrkA signaling participates in eosinophil migration by regulating the actin cytoskeleton and cell polarization via engagement of other intracellular signaling molecules such as [Ca2+]i, Cdc42 and ERK(1/2).
Once recruited to allergic airways, eosinophils play a critical role in the development and maintenance of allergic asthma by participating in events such as antigen presentation and release of stored pro-inflammatory mediators (cytokines, chemokines, reactive oxygen species, lipid mediators, granule proteins) (60). Therefore, strategies to inhibit airway eosinophil recruitment and suppress Th2 cytokines to mitigate asthma symptoms remain the focus of active research (61). Consistent with our findings that blockade of TrkA signaling inhibits eosinophil migration in vitro, administration of 1-NM-PP1 to TrkA-KI mice significantly inhibited recruitment of inflammatory cells, specifically eosinophils, to the airways and in the lung tissue in a model of A. alternata-induced AAI. Along with the reduced eosinophilia, 1-NM-PP1-treated mice displayed significantly lower levels of allergen-induced Th2 cytokines (IL-5, IL-4 and IL-13), IL-33, IgE and eotaxin-2 in the lungs. In addition to eosinophils, lymphocyte recruitment to the lungs was lower in 1-NM-PP1-treated mice, which is likely to be in part responsible for the decreased levels of these Th2 cytokines. Since TrkA is expressed by many cell types in the lung in addition to eosinophils, e.g., bronchial and alveolar epithelial cells, smooth muscle cells, and vascular endothelial cells, in vivo administration of 1-NM-PP1 can target TrkA signaling that is ligand-induced (via mediators such as NGF) or by transactivation (via mediators such as eotaxin-1) in all these cell types. In the current study, exposure of mice to A. alternata resulted in activation of TrkA in airway epithelial cells, which was markedly inhibited by 1-NM-PP1. Airway epithelial cells contribute to the development of AAI through release of various pro-inflammatory mediators including IL-33 (62), a Th2-phenotype promoting and maintaining cytokine (through IL-5 and IL-13 production by Th2 cells and innate lymphoid cells) (63), and eotaxin-2 (in response to IL-4 and IL-13) (64), both of which were significantly inhibited in A. alternata-challenged mice treated with 1-NM-PP1. Reduced levels of IL-33 in 1-NM-PP1-treated A. alternata-challenged mice may additionally be responsible for lower Th2 cytokine levels in these mice. Studies have also shown that eotaxin-2 cooperates with IL-5 in regulating recruitment of eosinophils, which in turn can serve as an important source of IL-13 to induce AHR (65). Thus, blockade of TrkA signaling in vivo not only inhibits eosinophilic inflammation by impeding eosinophil motility and decreased eotaxin-2 levels but also results in a muted Th2-response.
AHR is a characteristic feature of allergic asthma. Increased airway smooth muscle mass and mucus hypersecretion, which are recognized features in allergic asthma, are known to contribute to the development of AHR (66, 67). In the current study, A. alternata-challenged mice treated with 1-NM-PP1 had significantly decreased AHR associated with reduced airway smooth muscle mass and mucus secretion relative to untreated allergen-challenged mice. Studies have shown that activation of TrkA signaling promotes human airway smooth muscle cell proliferation (68) and smooth muscle cells can be a source of eotaxins during asthma (69). Mediators such as granule proteins (e.g., MBP, eosinophil peroxidase), cytokines (e.g., IL-13) and leukotrienes released by recruited eosinophils upon activation are known to directly or indirectly (via activation of mast cells and basophils) cause AHR and induce mucus hypersecretion (reviewed in (70)). Thus, in addition to eosinophils, specific blockade of TrkA signaling during allergic asthma can target other cell types such as smooth muscle cells and epithelial cells resulting in attenuation of various features of airway inflammation.
In summary, we have established the functional role of TrkA signaling in AAI in a disease model by demonstrating that specific inactivation of TrkA signaling significantly diminishes the cardinal features of asthma such as airway eosinophilia, Th2 cytokine and eosinophil-specific chemokine levels, structural changes in the airways and AHR. Importantly, our studies have uncovered a novel mechanism of TrkA activation and function in eotaxin-mediated signaling and migration of eosinophils. Together these studies further advance current knowledge of the role of TrkA signaling in asthma pathogenesis and suggest that manipulation of the TrkA signaling pathway may serve as a therapeutic target in the management of allergic airway disease.
Supplementary Material
Key points.
Eosinophilia is a characteristic feature of allergic airway inflammation and asthma.
TrkA activation by eotaxin-1 promotes eosinophil migration and airway inflammation.
Inhibiting TrkA kinase mitigates airway eosinophilia and suppresses a Th2 phenotype.
Acknowledgment
The authors wish to thank Stephanie Rastle-Simpson for excellent technical assistance and culture of murine eosinophils.
Supported by National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases, Grant no. AI137487 to S.P.R
Abbreviations used
- TrkA
Tropomycin receptor kinase A
- NRTK1
Neurotrophic tyrosine kinase receptor type 1
- NGF
Nerve growth factor
- RTK
Receptor tyrosine kinase
- AAI
Allergic airway inflammation
- BALF
Bronchoalveolar lavage fluid
- 1-NM-PP1
1-tert-Butyl-3-(naphthalen-1-ylmethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine
- GPCRs
G-protein-coupled receptors
- CCR3
C-C chemokine receptor type 3
- WT
wild-type
- PFA
Paraformaldehyde
- IHC
Immunohistochemical staining
- MBP
Major basic protein
- α-SMA
α-smooth muscle actin
- PAS
Periodic acid–Schiff
- AHR
Airway hyperresponsiveness
- MMP
Matrix metalloproteases
- DAPI
4,6-diamidino-2-phenylindole
- VCAM-1
Vascular cell adhesion molecule-1
References
- 1.Deinhardt K, Chao MV. 2014. Trk receptors In: Lewin GR, Carter BD, editors, Neurotrophic Factors: Springer-Verlag Berlin Heidelberg, p. 103–119. [Google Scholar]
- 2.Marlin MC, and Li G. 2015. Biogenesis and function of the NGF/TrkA signaling endosome. Int. Rev. Cell Mol. Biol 314:239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar V, and Mahal BA. 2012. NGF - the TrkA to successful pain treatment. J. Pain Res 5:279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nwosu LN, Mapp PI, Chapman V, Walsh DA. 2016. Blocking the tropomyosin receptor kinase A (TrkA) receptor inhibits pain behaviour in two rat models of osteoarthritis. Ann. Rheum. Dis 75:1246–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aubert L, Guilbert M, Corbet C, Genot E, Adriaenssens E, Chassat T, Bertucci F, Daubon T, Magne N, Le Bourhis X, and Toillon R. 2015. NGF-induced TrkA/CD44 association is involved in tumor aggressiveness and resistance to lestaurtinib. Oncotarget. 6:9807–9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demont Y, Corbet C, Page A, Ataman-Önal Y, Choquet-Kastylevsky G, Fliniaux I, Le Bourhis X, Toillon R, Bradshaw R, and Hondermarck H. 2012. Pro-nerve growth factor induces autocrine stimulation of breast cancer cell invasion through tropomyosin-related kinase A (TrkA) and sortilin protein. J. Biol. Chem 287:1923–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambrecht BN, and Hammad H. The immunology of asthma. 2015. Nat. Immunol 16:45–56. [DOI] [PubMed] [Google Scholar]
- 8.Freund-Michel V, and Frossard N. 2008. The nerve growth factor and its receptors in airway inflammatory diseases. Pharmacol. Ther 117:52–76. [DOI] [PubMed] [Google Scholar]
- 9.Prakash YS, Thompson MA, Meuchel L, Pabelick CM, Mantilla CB, Zaidi S, and Martin R. 2010. Neurotrophins in lung health and disease. Expert Rev. Respir. Med 4:395–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manti S, Brown P, Perez MK, Piedimonte G, and Litwack G. 2017. The role of neurotrophins in inflammation and allergy. Vitam. Horm 104:313–341. [DOI] [PubMed] [Google Scholar]
- 11.Rochman M, Kartashov AV, Caldwell JM, Collins MH, Stucke EM, Kc K, Sherrill JD, Herren J, Barski A, and Rothenberg ME. 2015. Neurotrophic tyrosine kinase receptor 1 is a direct transcriptional and epigenetic target of IL-13 involved in allergic inflammation. Mucosal Immunol. 8:785–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crowley C, Spencer SD, Nishimura MC, Chen KS, Pitts-Meek S, Armanini MP, Ling LH, McMahon SB, Shelton DL, Levinson AD, and Phillips HS. 1994. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell; 76:1001–1011. [DOI] [PubMed] [Google Scholar]
- 13.Smeyne RJ, Klein R, Schnapp A, Long LK, Bryant S, Lewin A, Lira SA, and Barbacid M. 1994. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature. 368:246–249. [DOI] [PubMed] [Google Scholar]
- 14.Nye SH, Squinto SP, Glass DJ, Stitt TN, Hantzopoulos P, Macchi MJ, Lindsay NS, Ip NY, and Yancopoulos GD. 1992. K-252a and staurosporine selectively block autophosphorylation of neurotrophin receptors and neurotrophin-mediated responses. Mol. Biol. Cell 3:677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchholz RA, Dundore RL, Cumiskey WR, Harris AL, and Silver PJ. 1991. Protein kinase inhibitors and blood pressure control in spontaneously hypertensive rats. Hypertension. 17:91–100. [DOI] [PubMed] [Google Scholar]
- 16.El Zein N, D’Hondt S, and Sariban E. 2010. Crosstalks between the receptors tyrosine kinase EGFR and TrkA and the GPCR, FPR, in human monocytes are essential for receptors-mediated cell activation. Cell Signal. 22:1437–1447. [DOI] [PubMed] [Google Scholar]
- 17.Rajagopal R, and Chao MV. 2006. A role for Fyn in Trk receptor transactivation by G-protein-coupled receptor signaling. Mol. Cell Neurosci 33:36–46. [DOI] [PubMed] [Google Scholar]
- 18.Nan L, Wei J, Jacko AM, Culley MK, Zhao J, Natarajan V, Ma H, and Zhao Y. 2016. Cross-talk between lysophosphatidic acid receptor 1 and tropomyosin receptor kinase A promotes lung epithelial cell migration. Biochim. Biophys. Acta 1863:229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pope SM, Zimmermann N, Stringer KF, Karow ML, and Rothenberg ME. 2005. The eotaxin chemokines and CCR3 are fundamental regulators of allergen-induced pulmonary eosinophilia. J. Immunol 175:5341–5350. [DOI] [PubMed] [Google Scholar]
- 20.Bishop AC, Ubersax JA, Petsch DT, Matheos DP, Gray NS, Blethrow J, Shimizu E, Tsien JZ, Schultz PG, Rose MD, Wood JL, Morgan DO, Shokat KM. 2000. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature. 407:395–401. [DOI] [PubMed] [Google Scholar]
- 21.Chen X, Ye H, Kuruvilla R, Ramanan N, Scangos KW, Zhang C, Johnson NM, England PM, Shokat KM, and Ginty DD. 2005. A chemical-genetic approach to studying neurotrophin signaling. Neuron. 46:13–21. [DOI] [PubMed] [Google Scholar]
- 22.Ha S, Ge XN, Bahaie NS, Kang BN, Rao A, Rao SP, and Sriramarao P. 2013. ORMDL3 promotes eosinophil trafficking and activation via regulation of integrins and CD48. Nat Commun. 4:2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ge XN, Ha SG, Greenberg YG, Rao A, Bastan I, Blidner AG, Rao SP, Rabinovich GA, and Sriramarao P. 2016. Regulation of eosinophilia and allergic airway inflammation by the glycan-binding protein galectin-1. Proc Natl Acad Sci. USA. 113:E4837–E4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ge XN, Bastan I, Dileepan M, Greenberg YG, Ha SG, Steen KA, Bernlohr DA, Rao SP, and Sriramarao P. 2018. FABP4 regulates eosinophil recruitment and activation in allergic airway inflammation. Am. J. Physiol. Lung Cell Mol. Physiol 315:L227–L240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zuberi RI, Ge X, Jiang S, Bahaie NS, Kang BN, Hosseinkhani RM, Frenzel EM, Fuster MM, Esko JD, Rao SP, and Sriramarao P. 2009. Deficiency of endothelial heparan sulfates attenuates allergic airway inflammation. J. Immunol 183:3971–3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bahaie NS, Hosseinkhani RM, Ge XN, Kang BN, Ha SG, Blumenthal MN, Jessberger R, Rao SP, and Sriramarao P. 2012. Regulation of eosinophil trafficking by SWAP-70 and its role in allergic airway inflammation. J. Immunol 188:1479–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dyer KD, Moser JM, Czapiga M, Siegel SJ, Percopo CM, and Rosenberg HF. 2008. Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J. Immunol 181:4004–4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noga O, Englmann C, Hanf G, Grützkau A, Guhl S, and Kunkel G. 2002. Activation of the specific neurotrophin receptors TrkA, TrkB and TrkC influences the function of eosinophils. Clin. Exp. Allergy 32:1348–1354. [DOI] [PubMed] [Google Scholar]
- 29.Rick EM, Woolnough K, Pashley CH, and Wardlaw AJ. 2016. Allergic fungal airway disease. J. Investig. Allergol. Clin. Immunol 26:344–354. [DOI] [PubMed] [Google Scholar]
- 30.Ge XN, Bastan I, Ha SG, Greenberg YG, Esko JD, Rao SP, and Sriramarao P. 2018. Regulation of eosinophil recruitment and allergic airway inflammation by heparan sulfate proteoglycan (HSPG) modifying enzymes. Exp. Lung Res 44:98–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Díaz-Rodríguez E, Cabrera N, Esparís-Ogando A, Montero JC, Pandiella A. 1999. Cleavage of the TrkA neurotrophin receptor by multiple metalloproteases generates signalling-competent truncated forms. Eur. J. Neurosci 11:1421–1430. [DOI] [PubMed] [Google Scholar]
- 32.Altun-Gultekin ZF, and Wagner JA. 1996. Src, ras, and rac mediate the migratory response elicited by NGF and PMA in PC12 cells. J. Neurosci. Res 44:308–327 [DOI] [PubMed] [Google Scholar]
- 33.Dolle J-P, Rezvan A, Allen FD, Lazarovici P, and Lelkes PI. 2005. Nerve growth factor-induced migration of endothelial cells. J. Pharmacol. Exp. Ther 315:1220–1227. [DOI] [PubMed] [Google Scholar]
- 34.Sriramarao P, DiScipio RG, Cobb RR, Cybulsky M, Stachnick G, Castenada D, Elices M, and Broide DH. 2000. VCAM-1 is more effective than MAdCAM-1 in supporting eosinophil rolling under conditions of flow. Blood. 95:592–601. [PubMed] [Google Scholar]
- 35.Rothenberg ME, Ownbey R, Mehlhop PD, Loiselle PM, van de Rijn M, Bonventre JV, Oettgen HC, Leder P, and Luster AD. 1996. Eotaxin triggers eosinophil-selective chemotaxis and calcium flux via a distinct receptor and induces pulmonary eosinophilia in the presence of interleukin 5 in mice. Mol. Med 2:334–348. [PMC free article] [PubMed] [Google Scholar]
- 36.Tsai F-C, Kuo G-H, Chang S-W, and Tsai P-J. 2015. Ca2+ signaling in cytoskeletal reorganization, cell migration, and cancer metastasis. Biomed. Res. Int 2015: 409245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murali A, and Rajalingam K. 2014. Small Rho GTPases in the control of cell shape and mobility. Cell Mol. Life Sci 71:1703–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hall A 2005. Rho GTPases and the control of cell behaviour. Biochem. Soc. Trans 33:891–895. [DOI] [PubMed] [Google Scholar]
- 39.Boehme SA, Sullivan SK, Crowe PD, Santos M, Conlon PJ, Sriramarao P, and Bacon KB. 1999. Activation of mitogen-activated protein kinase regulates eotaxin-induced eosinophil migration. J Immunol. 163:1611–1618. [PubMed] [Google Scholar]
- 40.Duan W, Chan JHP, Wong CH, Leung BP, and Wong WSF. 2004. Anti-inflammatory effects of mitogen-activated protein kinase kinase inhibitor U0126 in an asthma mouse model. J. Immunol 172:7053–7059. [DOI] [PubMed] [Google Scholar]
- 41.Bai TR, and Knight DA. 2005. Structural changes in the airways in asthma: observations and consequences. Clin. Sci 108:463–477. [DOI] [PubMed] [Google Scholar]
- 42.George L, and Brightling CE. 2016. Eosinophilic airway inflammation: role in asthma and chronic obstructive pulmonary disease. Ther. Adv. Chronic Dis 7:34–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davoine F, and Lacy P. 2014. Eosinophil cytokines, chemokines, and growth factors: emerging roles in immunity. Front. Immunol 5:570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Acharya KR, and Ackerman SJ. 2014. Eosinophil granule proteins: form and function. J. Biol. Chem 289:17406–17415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang LW, Sun G, Wang DL, and Kong LF. 2015. Inhibition of nerve growth factor/tyrosine kinase receptor A signaling ameliorates airway remodeling in chronic allergic airway inflammation. Eur. Rev. Med. Pharmacol. Sci; 19:2261–2268. [PubMed] [Google Scholar]
- 46.Minnone G, De Benedetti F, and Bracci-Laudiero L. 2017. NGF and its receptors in the regulation of inflammatory response. Int. J. Mol. Sci 18:1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nassenstein C, Braun A, Erpenbeck VJ, Lommatzsch M, Schmidt S, Krug N, Luttman W, Renz H, and Virchow JC. 2003. The neurotrophins nerve growth factor, brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4 are survival and activation factors for eosinophils in patients with allergic bronchial asthma. J. Exp. Med 198:455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahmadi Z, Hassanshahi G, Khorramdelazad H, Zainodini N, and Koochakzadeh L. 2016. An overlook to the characteristics and roles played by eotaxin network in the pathophysiology of food allergies: allergic asthma and atopic dermatitis. Inflammation; 39:1253–1267. [DOI] [PubMed] [Google Scholar]
- 49.Rothenberg ME, and Hogan SP. 2006. The eosinophil. Ann. Rev. Immunol 24:147–174. [DOI] [PubMed] [Google Scholar]
- 50.Qorri B, Kalaydina R-V, Velickovic A, Kaplya Y, Decarlo A, and Szewczuk MR. 2018. Agonist-biased signaling via matrix metalloproteinase-9 promotes extracellular matrix remodeling. Cells. 7:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kurosaki I, Satoh T, Murakami K, Tastumi T, Mitani N, and Saiki I. 2000. Eotaxin-induced expression of membrane type 1 matrix metalloproteinase mRNA in human eosinophils. Allergol. Int 49:111–116. [Google Scholar]
- 52.Chao P-Z, Hsieh M-S, Cheng C-W, Lin Y-F, and Chen C-H 2011. Regulation of MMP-3 expression and secretion by the chemokine eotaxin-1 in human chondrocytes. J. Biomed. Sci 18:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu F, Liu P, Li J, and Zhang Y. 2014. Eotaxin-1 promotes prostate cancer cell invasion via activation of the CCR3-ERK pathway and upregulation of MMP-3 expression. Oncol. Rep 31:2049–2054 [DOI] [PubMed] [Google Scholar]
- 54.Kumar S, Ratnikov BI, Kazanov MD, Smith JW, and Cieplak P. 2015. CleavPredict: A Platform for reasoning about matrix metalloproteinases proteolytic events. PLoS One. 10:e0131952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosenberg HF, Phipps S, and Foster PS. 2007. Eosinophil trafficking in allergy and asthma. J. Allergy Clin. Immunol 119:1303–1310. [DOI] [PubMed] [Google Scholar]
- 56.De Bernardi MA, Rabin SJ, Colangelo AM, Brooker G, and Mocchetti I. 1996. TrkA mediates the nerve growth factor-induced intracellular calcium accumulation. J. Biol Chem 271:6092–6098. [DOI] [PubMed] [Google Scholar]
- 57.Yang HW, Collins SR, and Meyer T. 2016. Locally excitable Cdc42 signals steer cells during chemotaxis. Nat. Cell Biol 18:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kampen GT, Stafford S, Adachi T, Jinquan T, Quan S, Grant JA, Skov PS, Poulsen LK, and Alam R. 2000. Eotaxin induces degranulation and chemotaxis of eosinophils through the activation of ERK2 and p38 mitogen-activated protein kinases. Blood. 95:1911–1917. [PubMed] [Google Scholar]
- 59.Choi EN, Choi MK, Park C-S, and Chung IY. 2003. A parallel signal-transduction pathway for eotaxin- and interleukin-5-induced eosinophil shape change. Immunology. 108:245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walsh ER and August A. Eosinophils and allergic airway disease: there is more to the story. 2010. Trends Immunol. 31:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buzney CD, Gottlieb AB, and Rosmarin D. 2016. Asthma and atopic dermatitis: a review of targeted inhibition of interleukin-4 and interleukin-13 as therapy for atopic disease. J. Drugs Dermatol 15:165–171. [PubMed] [Google Scholar]
- 62.Bartemes KR, and Kita H. 2012. Dynamic role of epithelium-derived cytokines in asthma. Clin. Immunol 143:222–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murakami-Satsutani N, Ito T, Nakanishi T, Inagaki N, Tanaka A, Vien PT, Kibata K, Inaba M, and Nomura S. 2014. IL-33 promotes the induction and maintenance of Th2 immune responses by enhancing the function of OX40 ligand. Allergol. Int 63:443–455. [DOI] [PubMed] [Google Scholar]
- 64.van Wetering S, Zuyderduyn S, Ninaber DK, van Sterkenburg MA, Rabe KF, and Hiemstra PS. 2007. Epithelial differentiation is a determinant in the production of eotaxin-2 and −3 by bronchial epithelial cells in response to IL-4 and IL-13. Mol. Immunol 44:803–811. [DOI] [PubMed] [Google Scholar]
- 65.Yang M, Hogan SP, Mahalingam S, Pope SM, Zimmermann N, Fulkerson P, Dent LA, Young IA, Matthaei KI, Rothenberg ME, and Foster PS. 2003. Eotaxin-2 and IL-5 cooperate in the lung to regulate IL-13 production and airway eosinophilia and hyperreactivity. J. Allergy Clin. Immunol 112:935–943. [DOI] [PubMed] [Google Scholar]
- 66.Doeing DC, and Solway J. 2013. Airway smooth muscle in the pathophysiology and treatment of asthma. J. Appl. Physiol 114:834–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Evans CM, Kim K, Tuvim MJ, and Dickey BF. 2009. Mucus hypersecretion in asthma: causes and effects. Curr. Opin. Pulm. Med 15:4–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Freund-Michel V, Bertrand C, and Frossard N. 2006. TrkA signalling pathways in human airway smooth muscle cell proliferation. Cell Signal. 18:621–627 [DOI] [PubMed] [Google Scholar]
- 69.Moore PE, Church TL, Chism DD, Panettieri RA, and Shore SA. 2002. IL-13 and IL-4 cause eotaxin release in human airway smooth muscle cells: a role for ERK. Am. J. Physiol. Lung Cell Mol. Physiol 282:L847–L853. [DOI] [PubMed] [Google Scholar]
- 70.McBrien CN, and Menzies-Gow A. 2017. The biology of eosinophils and their role in asthma. Front. Med 4:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.