Abstract
Background
Volume-controlled ventilation (VCV) in one-lung ventilation (OLV) is most commonly used in thoracotomy, but pressure-controlled ventilation-volume guaranteed (PCV-VG) is used in elderly patients to improve arterial oxygenation, reduce inflammatory factors, and decrease acute lung injury (ALI). The purpose of this study was to investigate the effects of these 2 different ventilation modes – VCV versus PCV-VG – during OLV in elderly patients undergoing thoracoscopic lobectomy.
Material/Methods
Sixty patients undergoing thoracoscopic lobectomy from September 2018 to February 2019 at Cangzhou Central Hospital, Hebei, China were randomly assigned to a VCV group or a PCV-VG group. Pulmonary dynamic compliance (Cdyn), peak inspiratory pressure (PIP), arterial blood gas, and inflammatory factors were monitored to assess lung function. The Clinical Trial Registration Identifier number is ChiCTR1800017835.
Results
Compared with the VCV group, PIP in the PCV-VG group was significantly lower (P=0.01) and Cdyn was significantly higher at 30 min after one-lung ventilation (P=0.01). MAP of the PCV-VG group was higher than in the VCV group (P=0.01). MAP of the PCV-VG group was also higher than in the VCV group at 30 min after one-lung ventilation (P=0.01). The concentration of neutrophil elastase (NE) in the PCV-VG group was significantly lower than in the VCV group (P=0.01).
Conclusions
Compared with VCV, PCV-VG mode reduced airway pressure in patients undergoing thoracotomy and also decreased the release of NE and reduced inflammatory response and lung injury. We conclude that PCV-VG mode can protect the lung function of elderly patients undergoing thoracotomy.
MeSH Keywords: One-Lung Ventilation, Pneumonia, Ventilator-Induced Lung Injury
Background
The incidence of lung cancer ranks first among malignant tumors and is more common in elderly patients [1]. Thoracic surgery is still the main treatment for early lung cancer. The incidence of pulmonary complications after thoracotomy is closely related to the decrease of lung function before surgery and intraoperative one-lung ventilation (OLV), which can affect the prognosis [2]. Elderly patients can have physiological hypoxia due to decreased preoperative pulmonary function and pulmonary fibrosis; therefore, the risk of lung injury after OLV in elderly patients is higher [3,4]. OLV in thoracotomy has adverse effects on the respiratory system, and improper ventilation can cause or aggravate lung injury. Therefore, it is very important to choose appropriate ventilation strategies for elderly patients to reduce lung injury [5].
Volume-controlled ventilation (VCV) in OLV is most commonly used mode in thoracotomy, but it causes inflammatory cells to activate and release large amounts of inflammatory factors, which can lead to pulmonary inflammatory response and pulmonary complications. In recent years, pressure-controlled ventilation-volume guaranteed (PCV-VG) ventilation had been used in elderly patients to improve arterial oxygenation, reduce inflammatory factors, and decrease acute lung injury (ALI) [6–11]. The present study investigated the effects of 2 ventilation modes – VCV and PCV-VG – on arterial oxygenation and release of inflammatory factors during thoracic surgery anesthesia in elderly patients.
Material and Methods
Study design and objectives
This was a single-center, randomized, controlled study, approved by Cangzhou Central Hospital, China (ethics approval no. 2018-024-01, Clinical Trial Registration Identifier: ChiCTR1800017835). Informed consent was signed by patients and their relatives. We enrolled 60 patients with lung cancer who underwent thoracoscopic lobectomy from September 2018 to February 2019. They were randomly divided into 2 groups: a volume-controlled ventilation mode group (VCV group, N=30) and a pressure-controlled ventilation-volume guaranteed mode group (PCV-VG group, N=30). The inclusion criteria were: lung cancer patients who underwent thoracoscopic lobectomy; age ≥65 years; either sex; ASA grade: I–II; forced expiratory volume 1% (FEV1%) ≥60%; PaO2 >70 mmHg. The exclusion criteria were: circulatory disease; acute or chronic pulmonary inflammation; history of chest surgery or trauma; history of psychiatric or neurological disorders.
IL-6 was used as a plasma biomarker in this study. The mean±standard deviation of IL-6 at T4 was 55.00±10.00 and 61.00±4.58 according to a pilot study of 6 patients. With a significant reduction of 20% in level of IL-6 at a power of 95% and two-sided significance level of 0.05, 23 patients were required to be enrolled in this study. A total of 60 patients were recruited to compensate for the possibility of dropout.
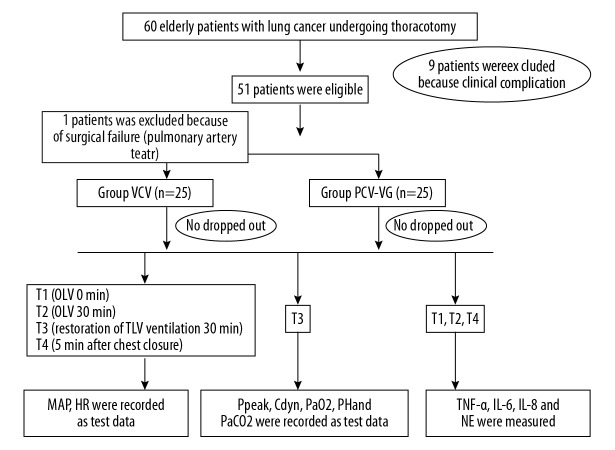
This study was performed by an investigator who was aware of the randomization results. Before entering the operating room, patients were allocated to study groups. An independent statistician prepared the computer-generated randomization list in a 1: 1 ratio. Figure 1 shows the references and consulted experts.
Figure 1.
CONSORT diagram.
Anesthesia method
Electrocardiography (ECG), pulse oximetry, and bispectral index (BIS) assessment were performed after the patients entered the operating room. Then, peripheral veins were opened and radial artery catheterization was performed. Blood pressures were monitored. General anesthesia was induced by midazolam 0.05 mg/kg, propofol 1–2 mg/kg, sufentanil 0.4~0.6 μg/kg, and cisatracurium 0.3 mg/kg. After induction, a double-lumen bronchial catheter (Broncho-part, Rush, Kermen, Germany) was inserted in place (size 37 for men and size 35 for women). Auscultation revealed breath sounds were clear and good bilateral lung isolation. Fiberoptic bronchoscopy was performed to judge the position of the double-lumen tube, then we fixed the catheter and connected it to the GE Dragger anesthesia machine (Perseus A500) for mechanical ventilation. Intraoperative maintenance was performed with inhalation of 2% sevoflurane, dexmedetomidine 0.01 μg·kg−1·min−1, remifentanil 0.1~0.3 μg·kg−1·min−1, and cisatracurium 5 mg/h to maintain anesthesia depth and muscle relaxation. The intraoperative BIS value was maintained at 40~50 and mean arteria pressure (MAP) was maintained at around 20% of the base value. The parameters of the two-lung ventilation (TLV) were set at 12~16 breaths/min, with a volume of 8~10 ml/kg, I: E=1: 2, and oxygen concentration of 100%. The parameters of OLV were set at 14~18 breaths/min, with a volume of 6 ml/kg, positive end-expiratory pressure (PEEP) of 5 mmHg, and I: E=1: 2 at inspired oxygen concentration of 100%. PaCO2 was maintained at 35~45 mmHg and SPO2 was maintained at above 90%.
Data collection and measurement
Peak inspiratory pressure (PIP) and pulmonary dynamic compliance (Cdyn) were continuously monitored with an Ohmeda S/5 monitor and bypass airflow monitoring system. Arterial blood was collected at T1 (OLV 0 min), T2 (OLV 30 min), and T3 (restoration of TLV ventilation 30 min) for artery blood gas analysis (Cobqsb221 Roche, Switzerland). PaO2, PaCO2, and pH were assessed and recorded. Venous blood was extracted and concentrations of neutrophil elastase (NE), interleukin-6 (IL-6), interleukin-8 (IL-8), and tumor necrosis factor-α (TNF-α) in plasma were detected by enzyme-linked immunosorbent assay (ELISA) at T1, T2, and T4 (5 min after chest closure). Changes in chest signs and chest radiographs on Day 1, Day 3, and Day 5 after the operation were monitored to determine the prognosis.
Statistical analysis
SPSS17.0 statistical software was used for analysis. Measurement data are expressed as mean±standard deviation. The independent-samples t test was used in intergroup comparison of results. Repeated-measures analysis of variance was used to compare serially measured variables with the group and time factors. The Fisher chi-square test was used for comparisons of enumeration data. P≤0.05 was considered statistically significant. Quantitative data were tested for normal distribution.
Results
Figure 1 shows a diagram of this trial. There were 60 patients who met the inclusion criteria and agreed to participate. Nine patients were excluded because of clinical complications and 1 patient in the VCV group was excluded due to a pulmonary artery tear. The characteristics of the enrolled subjects are summarized in Table 1. There were no significant differences in sex, age, body mass index, preoperative lung function, and artery blood gas values between the 2 groups (P>0.05).
Table 1.
Demographic and blood gas analysis before surgery in both groups.
| Variables | Group VCV (n=25) | Group PCV-VG (n=25) | P value |
|---|---|---|---|
| Age(years) | 68.96±3.40 | 69.44±3.86 | 0.64 |
| Female(%) | 10 (40) | 12 (48) | 0.38 |
| Weight | 68.35±18.86 | 73.79±23.45 | 0.25 |
| Smoke history | 18.35±7.18 | 16.97±8.98 | 0.14 |
| Body mass index | 25.49±3.12 | 24.42±2.04 | 0.16 |
| Hypertension (%) | 4 (16) | 6 (24) | 0.36 |
| FEV1 (%) | 71.57±5.59 | 71.22±2.52 | 0.78 |
| ASAi (%) | 12 (48) | 16 (64) | 0.19 |
| ASAii (%) | 13 (52) | 9 (36) | 0.20 |
| PH | 7.39±0.03 | 7.40±0.04 | 0.52 |
| PO2 (mmHg) | 80.95±7.82 | 78.06±5.34 | 0.13 |
| PCO2 (mmHg) | 40.49±3.32 | 40.48±3.47 | 0.99 |
Numerical data are expressed as means±SD. There was no significant difference in gender, age, body mass index, preoperative lung function and artery blood gas values before surgery between two groups (P>0.05). FEV1 – forced expiratory volume 1%; ASA – American Society of Anesthesiologists; PH – pondus hydrogenii; PO2 – partial pressure of oxygen; PCO2 – partial pressure of carbon dioxide.
Compared with the VCV group, PIP in the PCV-VG group was significantly lower (P=0.01) and Cdyn was significantly higher at T2 (P=0.01). There were no significant differences in artery blood gas between the 2 groups at T2 (P>0.05) (Table 2).
Table 2.
Mechanical ventilation and blood gas index at 30 minutes after one-lung ventilation.
| Variables | Group VCV | Group PCV-VG | P value | 95% CI |
|---|---|---|---|---|
| PIP (cmH2O) | 22.36±2.56 | 19.72±2.64* | 0.01 | 1.16, 4.11 |
| Cdyn | 27.00±2.43 | 31.74±2.71* | 0.01 | −6.2, −3.27 |
| PO2 (mmHg) | 162.64±21.09 | 169.38±25.93 | 0.13 | −20.18, 6.69 |
| PCO2 (mmHg) | 39.13±2.97 | 40.93±4.31 | 0.20 | −3.90, 0.30 |
| PH | 7.408±0.03 | 7.398±0.03 | 0.05 | −0.005, 0.025 |
Numerical data are expressed as means±SD.
P<0.05. Compared with Group VCV, PIP in Group PCV-VG was significantly reduced (P=0.01) and Cdyn was significantly increased at T2 (P=0.01). There were no significant differences in artery blood gas between both groups at T2 (P>0.05). PIP – peak inspiratory pressure; Cdyn – pulmonary dynamic compliance. T2 – one-lung ventilation 30 min.
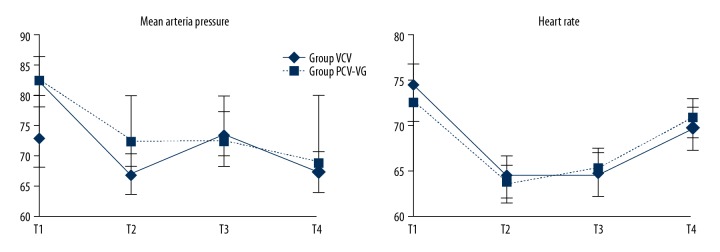
According to repeated-measures analysis of variance, there was no significant difference in HR between the 2 groups (P=0.65). The MAP of the PCV-VG group was higher than in the VCV group (P=0.01). The MAP of the PCV-VG group was also higher than in the VCV group at T2 (P=0.01). HR and MAP values of other time points in each group were significantly different compared with T1 (P<0.01) according to the independent-samples t test (Figure 2).
Figure 2.
Mean arterial pressure and heart rate in both groups suring study. According to repeated measures analysis of variance, there was significant difference in MAP between two groups (P=0.01), MAP to group PCV-VG was higher than group VCV at T2 (P=0.01), compared with T1, MAP values of other time points in each group were statistically different (P=0.01). There was no significant difference in HR between two groups (P=0.65), but compared with T1, HR values of other time points in each groups were statistically different (P=0.01). T1 – one-lung ventilation 0 min; T2 – one-lung ventilation 30 min; T3 – restoration of two-lung ventilation 30 min; T4 – 5 min after chest closure.
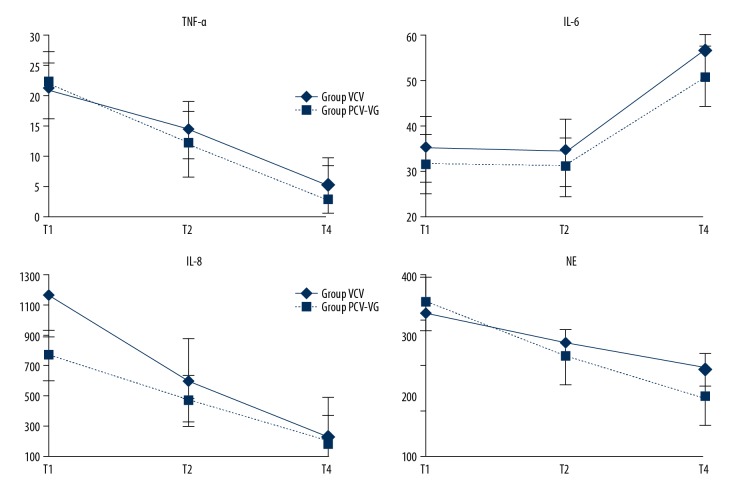
Compared with the VCV group, the concentration of NE in the PCV-VG group was significantly lower (P=0.01). The concentrations of NE at other time points in each group were significantly different compared with T1 (P<0.01). There were no significant differences in TNF-α, IL-6, or IL-8 between the 2 groups (P=0.76, P=0.55, P=0.35). The concentrations of TNF-α, IL-6, and IL-8 at other time points in each group were significantly different compared with T1 (P<0.01) (Figure 3).
Figure 3.
Inflammatory factors in both groups. Compared with group VCV, concentration of NE in group PCV-VG was significantly different, at T4 has a significantly decreased (P=0.01). Compared with T1, concentration of NE other time points in each group were statistically different (P=0.01). There was no significant difference in TNF-α, and IL-8 between two groups (P=0.76, P=0.35). But compared with T1, concentration of TNF-α, and IL-8 other time points in each group were statistically different (P=0.01). There was no significant difference IL-6 between two groups (P=0.55). But compared with T1, concentration of IL-6 T4 points in each group were statistically different (P=0.01). T1 – one-lung ventilation 0 min; T2 – one-lung ventilation 30 min; T4 – 5 min after chest closure.
There were no significant differences in procedure duration, time of recovery, re-intubation, lung infections after surgery, and hospital stays between the 2 groups (P>0.05) (Table 3).
Table 3.
Perioperative characteristics in both groups.
| Variables | Group VCV (n=25) | Group PCV-VG (n=25) | P value |
|---|---|---|---|
| Procedures duration (h) | 1.97±0.25 | 2.06±0.31 | 0.21 |
| Time of recovery room (h) | 1.56±0.61 | 1.79±0.54 | 0.15 |
| Re-intubation n (%) | 1 (4) | 1 (4) | 0.75 |
| Lung infection n (%) | 2 (8) | 1 (4) | 0.50 |
| Hospital stay d (x±s) | 7.52±1.45 | 8.20±1.44 | 0.10 |
Numerical data are expressed as means±SD. There was no statistical difference in procedure duration, time of recovery room, re-intubation, lung infection after operation and hospital stays between two groups (P>0.05).
Discussion
In recent years, the number of elderly patients undergoing thoracotomy has gradually increased. Elderly patients are more likely to suffer lung injury during OLV due to decreased lung compliance and pulmonary function. Therefore, it is very important to implement appropriate protective pulmonary ventilation strategies for elderly patients to reduce lung injury [4].
VCV is the most commonly used mechanical ventilation mode in clinical practice, ventilating the airway by gradually increasing the air flow rate and airway pressure. However, small airways with low compliance cannot be adequately ventilated by VCV and may also cause increased shunt in the lung and ventilation/perfusion ratio imbalance. In addition, it is easy to cause a patient’s airway pressure to become too high, thus causing lung injury [6–9].
PRV-VG is a new ventilation mode set up by the anesthesia machine. This ventilation mode can deliver tidal volume at the lowest preset pressure, which has the efficiency and clinical benefits of pressure-controlled ventilation, and also compensates for the change in tidal volume compliance of VCV. It allows for a more uniform distribution of air in the lung, increasing effective ventilation in the alveoli and reducing airway pressure. Many studies had shown that the PCV-VG mode can reduce lung injury caused by mechanical ventilation in intensive care unit patients, especially in patients with chronic obstructive pulmonary diseases with poor lung compliance. Jun et al. found that, compared with VCV mode, the PCV-VG mode can reduce expiratory pressure and improve arterial oxygenation in patients undergoing thoracotomy [12]. Seok et al. found that, compared with VCV, PCV-VG mode can reduce expiratory pressure, but arterial oxygenation was not significantly improved [13]. A study found that PCV-VG mode provides minimal PIP compared with VCV mode, but there was no difference in PaO2, PaCO2, and hemodynamic parameters between these 2 modes of ventilation [14]. In the present study, PIP in the PCV-VG group was significantly reduced (P=0.01) and Cdyn was significantly increased at T2 compared with the VCV group (P=0.01), which suggests that PCV-VG mode can reduce airway pressure in patients undergoing thoracotomy compared with VCV mode, while the arterial oxygenation was not improved obviously.
OLV is an essential ventilation method for thoracotomy. However, recent studies have shown that improper use of OLV can cause lung injury and promote the release of inflammatory mediators in the lungs, which is also an important factor affecting patient prognosis after surgery [15].
IL-6, among many inflammatory factors, can be a manifestation of the acute phase, which is closely related to lung injury and pulmonary complications [16,17]. NE is stored in neutrophils under physiological conditions and released during inflammation. NE can break down almost all extracellular matrices and many important plasma proteins when it is released out of the cell, and is considered to be one of the most destructive enzymes [18]. NE is the final effector of inflammatory cascades that cause ALI [19]. It is mainly involved in and initiates the occurrence of acute lung injury through injury to capillary endothelial cells and alveolar epithelial cells, as well as digestion and degradation of extracellular matrix and epithelial junction structures. Studies have shown that, in patients and animal models with ALI, NE is increased in serum and bronchoalveolar lavage fluid, which was found to cause ALI in in vitro experiments. Early use of NE inhibitor can decrease the success rate of ALI modeling and alleviate symptoms for ALI patients or animals [20,21]. TNF-α is produced by alveolar macrophages and can induce and amplify the acute inflammatory response, which causes lung injury [22]. A previous study found that the blood concentration of TNF-α was increased in OLV patients, indicating an inflammatory response in the body [23]. In the present study, the concentration of NE in the PCV-VG group was significantly lower at T4 compared with the VCV group (P=0.01), which suggests that PCV-VG can decrease the release of inflammatory factors and reduce inflammatory response and lung injury.
Our study has certain limitations that need to be considered. First, only 60 patients were studied in a single center. Second, surgical manipulation may have affected the arterial oxygenation and cardiac function, which could have influenced our results. Third, more sample time is needed to compare the effect on arterial oxygenation and release of inflammatory factors during the peri-operative period in elderly patients.
Conclusions
Compared with VCV, PCV-VG mode not only reduces airway pressure in patients undergoing thoracotomy, but also decreases the release of NE and reduces inflammatory response and lung injury. PCV-VG mode can protect the lung function of elderly patients undergoing thoracotomy.
Abbreviations
- VCV
volume-controlled ventilation
- OLV
one-lung ventilation
- PCV-VG
pressure-controlled ventilation-volume guaranteed
- TLV
two-lung ventilation
- ALI
acute lung injury
- Cdyn
pulmonary dynamic compliance
- PIP
peak inspiratory pressure
- NE
neutrophil elastase
- IL-6
interleukin-6
- IL-8
interleukin-8
- TNF-α
tumor necrosis factor-α
- ECG
electrocardiogram
- BIS
bispectral index
- MAP
mean arteria pressure
- HR
heart rate
- PEEP
positive end-expiratory pressure
Footnotes
Source of support: This study was supported by the National Natural Science Foundation of China (No. 81471902 and No. 81871592) and the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding (code: ZYLX201810)
Trial registration link: http://www.chictr.org.cn/showprojen.aspx?proj=29116
Registration number: ChiCTR1800017835
Conflicts of interest
None.
References
- 1.Radovic M, Kanesvaran R, Rittmeyer A, et al. Multidisciplinary treatment of lung cancer in older patients: A review. J Geriatr Oncol. 2019;10:405–10. doi: 10.1016/j.jgo.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Cheng YD, Duan CJ, Dong S, et al. Clinical controlled comparison between lobectomy and segmental resection for patients over 70 years of age with clinical stage I non-small cell lung cancer. Eur J Surg Oncol. 2012;38:1149–55. doi: 10.1016/j.ejso.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Sprung J, Gajic O, Warner DO. Review article: Age related alterations in respiratory function – anesthetic considerations. Can J Anaesth. 2006;53:1244–57. doi: 10.1007/BF03021586. [DOI] [PubMed] [Google Scholar]
- 4.Ruivo S, Viana P, Martins C, et al. Effects of aging on lung function. A comparison of lung function in healthy adults and the elderly. Rev Port Pneumol. 2009;15:629–53. [PubMed] [Google Scholar]
- 5.Lohser J. Evidence-based management of one-lung ventilation. Anesthesiol Clin. 2008;26:241–72. v. doi: 10.1016/j.anclin.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Lin F, Pan L, Huang B, et al. Pressure-controlled versus volume-controlled ventilation during one-lung ventilation in elderly patients with poor pulmonary function. Ann Thorac Med. 2014;9:203–8. doi: 10.4103/1817-1737.140125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tugrul M, Camci E, Karadeniz H, et al. Comparison of volume controlled with pressure controlled ventilation during one-lung anaesthesia. Br J Anaesth. 1997;79:306–10. doi: 10.1093/bja/79.3.306. [DOI] [PubMed] [Google Scholar]
- 8.Senturk NM, Dilek A, Camci E, et al. Effects of positive end-expiratory pressure on ventilatory and oxygenation parameters during pressure-controlled one-lung ventilation. J Cardiothorac Vasc Anesth. 2005;19:71–75. doi: 10.1053/j.jvca.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Heimberg C, Winterhalter M, Struber M, et al. Pressure-controlled versus volume-controlled one-lung ventilation for MIDCAB. Thorac Cardiovasc Surg. 2006;54:516–20. doi: 10.1055/s-2006-924413. [DOI] [PubMed] [Google Scholar]
- 10.Senturk M. New concepts of the management of one-lung ventilation. Curr Opin Anaesthesiol. 2006;19:1–4. doi: 10.1097/01.aco.0000192778.17151.2c. [DOI] [PubMed] [Google Scholar]
- 11.Pardos PC, Garutti I, Pineiro P, et al. Effects of ventilatory mode during one-lung ventilation on intraoperative and postoperative arterial oxygenation in thoracic surgery. J Cardiothorac Vasc Anesth. 2009;23:770–74. doi: 10.1053/j.jvca.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Pu J, Liu Z, Yang L, et al. Applications of pressure control ventilation volume guaranteed during one-lung ventilation in thoracic surgery. Int J Clin Exp Med. 2014;7:1094–98. [PMC free article] [PubMed] [Google Scholar]
- 13.Song SY, Jung JY, Cho MS, et al. Volume-controlled versus pressure-controlled ventilation-volume guaranteed mode during one-lung ventilation. Korean J Anesthesiol. 2014;67:258–63. doi: 10.4097/kjae.2014.67.4.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dion JM, McKee C, Tobias JD, et al. Ventilation during laparoscopic-assisted bariatric surgery: Volume-controlled, pressure-controlled or volume-guaranteed pressure-regulated modes. Int J Clin Exp Med. 2014;7:2242–47. [PMC free article] [PubMed] [Google Scholar]
- 15.Schilling T, Kozian A, Kretzschmar M, et al. Effects of propofol and desflurane anaesthesia on the alveolar inflammatory response to one-lung ventilation. Br J Anaesth. 2007;99:368–75. doi: 10.1093/bja/aem184. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6:a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao X, Huang J, Zhong H, et al. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther. 2014;141:125–39. doi: 10.1016/j.pharmthera.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Lerman I, Hammes SR. Neutrophil elastase in the tumor microenvironment. Steroids. 2018;133:96–101. doi: 10.1016/j.steroids.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polverino E, Rosales-Mayor E, Dale GE, et al. The role of neutrophil elastase inhibitors in lung diseases. Chest. 2017;152:249–62. doi: 10.1016/j.chest.2017.03.056. [DOI] [PubMed] [Google Scholar]
- 20.Muley MM, Krustev E, Reid AR, et al. Prophylactic inhibition of neutrophil elastase prevents the development of chronic neuropathic pain in osteoarthritic mice. J Neuroinflammation. 2017;14:168. doi: 10.1186/s12974-017-0944-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinod K, Witsch T, Farley K, et al. Neutrophil elastase-deficient mice form neutrophil extracellular traps in an experimental model of deep vein thrombosis. J Thromb Haemost. 2016;14:551–58. doi: 10.1111/jth.13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim GY, Roh SI, Park SK, et al. Alleviation of experimental septic shock in mice by acidic polysaccharide isolated from the medicinal mushroom Phellinus linteus. Biol Pharm Bull. 2003;26:1418–23. doi: 10.1248/bpb.26.1418. [DOI] [PubMed] [Google Scholar]
- 23.Bradley JR. TNF-mediated inflammatory disease. J Pathol. 2008;214:149–60. doi: 10.1002/path.2287. [DOI] [PubMed] [Google Scholar]