Abstract
Background
Surgery has been the treatment of choice for patients with localized esophageal cancer. Several studies have investigated whether preoperative chemotherapy followed by surgery leads to improvement in cure rates, but individual reports have provided conflicting results. An explicit systematic update of the role of preoperative chemotherapy in the treatment of patients with resectable thoracic esophageal cancer is, therefore, warranted.
Objectives
The objective of this review is to determine the role of preoperative chemotherapy in the treatment of patients with resectable thoracic esophageal carcinoma.
Search methods
We identified trials by searching the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (1966 to 2013), EMBASE (1988 to 2013), and CANCERLIT (1993 to 2013). We did not confine our search to English language publications. We updated searches in CENTRAL, MEDLINE, and EMBASE in October 2013.
Selection criteria
All trials of patients with potentially resectable carcinoma of the esophagus (of any histologic type) who were randomly assigned to chemotherapy or no chemotherapy before surgery.
Data collection and analysis
The primary outcome was survival, which was assessed with the use of hazard ratios. This is an amendment to the original review, which used risk ratios to assess survival at yearly intervals. Hazard ratios (HRs) have now been introduced to summarize the complete survival experience in a single analysis. Risk ratios (RRs) were used to compare rates of resection, tumor recurrences, and treatment morbidity and mortality.
Main results
We identified a total of 13 randomized trials involving 2362 participants. Ten trials (2122 participants) reported sufficient detail on survival to be included in a meta‐analysis for the primary outcome. Preoperative chemotherapy improves overall survival (HR 0.88, 95% confidence interval (CI) 0.80 to 0.96) and is associated with a significantly higher rate of complete (R0) resection (RR 1.11, 95% CI 1.03 to 1.19).
No evidence suggests that the overall rate of resection (RR 0.96, 95% CI 0.92 to 1.01), tumor recurrence (RR 0.81, 95% CI 0.54 to 1.22) or nonfatal complications (RR 0.90; 95% CI 0.76 to 1.06) was different for preoperative chemotherapy compared with surgery alone. Trials reported risks of toxicity with chemotherapy that ranged from 11% to 90%.
Authors' conclusions
In summary, preoperative chemotherapy plus surgery offers a survival advantage compared with surgery alone for patients with resectable thoracic esophageal cancer, but the evidence is of moderate quality. Some evidence of toxicity and preoperative mortality have been associated with chemotherapy.
Keywords: Humans; Preoperative Care; Antineoplastic Agents; Antineoplastic Agents/therapeutic use; Cisplatin; Cisplatin/therapeutic use; Combined Modality Therapy; Combined Modality Therapy/methods; Combined Modality Therapy/mortality; Esophageal Neoplasms; Esophageal Neoplasms/drug therapy; Esophageal Neoplasms/mortality; Esophageal Neoplasms/surgery; Fluorouracil; Fluorouracil/therapeutic use; Life Expectancy; Neoplasm Recurrence, Local; Quality of Life; Randomized Controlled Trials as Topic
Plain language summary
Chemotherapy before surgery for patients with surgically removable cancer of the esophagus
Question
In patients who have cancer of the esophagus that is potentiallly removable by surgery, does the use of chemotherapy before surgery result in improved survival?
Background
Cancer of the esophagus often is not discovered until it is at quite an advanced stage. This means that even removing the tumor through surgery is not very successful, and many people die within five years. Chemotherapy (cancer‐fighting drugs such as cisplatin) has been used before surgery to try to shrink the tumor, making it easier to operate on and stopping it from spreading. Therefore, chemotherapy may help people to live longer.
Study characteristics
This review included information from 13 randomized studies and combined results from 2122 patients to answer our question regarding survival.
Key results
This review of 13 trials, including patients with esophageal cancer of any cell type, found some evidence that cisplatin‐based chemotherapy may help them to live longer. However, chemotherapy may introduce side effects.
Quality of the evidence
This review used information from randomized studies that is considered to represent the highest quality of evidence.
Summary of findings
Summary of findings for the main comparison. Preoperative chemotherapy for resectable thoracic esophageal cancer.
| Preoperative chemotherapy for resectable thoracic esophageal cancer | ||||||
| Patient or population: patients with resectable thoracic esophageal cancer Settings: in‐hospital/outpatient Intervention: preoperative chemotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Preoperative chemotherapy | |||||
| Overall survival Hazard ratio Follow‐up: 5 years | 775 per 1000 | 731 per 1000 (697 to 761) | HR 0.88 (0.8 to 0.96) | 2122 (10 studies) | ⊕⊕⊕⊝ Moderate | |
| Complete resection (R0) Histology | 523 per 1000 | 580 per 1000 (538 to 622) | RR 1.11 (1.03 to 1.19) | 2135 (9 studies) | ⊕⊕⊕⊝ Moderatea | |
| Local‐regional recurrence Radiology Follow‐up: 5 years | 152 per 1000 | 131 per 1000 (96 to 178) | RR 0.86 (0.63 to 1.17) | 2047 (8 studies) | ⊕⊕⊕⊝ Moderatea | |
| Distant recurrence Radiology Follow‐up: 5 years | 188 per 1000 | 177 per 1000 (147 to 213) | RR 0.94 (0.78 to 1.13) | 1947 (7 studies) | ⊕⊕⊕⊝ Moderatea | |
| Any complication Clinical | 358 per 1000 | 333 per 1000 (290 to 387) | RR 0.93 (0.81 to 1.08) | 1340 (5 studies) | ⊕⊕⊕⊝ Moderatea | |
| Postoperative death Clinical Follow‐up: mean 30 days | 68 per 1000 | 63 per 1000 (46 to 87) | RR 0.93 (0.68 to 1.28) | 2196 (10 studies) | ⊕⊕⊝⊝ Lowa,b | |
| Anastomotic leaks Clinical/radiology Follow‐up: mean 30 days | 63 per 1000 | 58 per 1000 (39 to 86) | RR 0.92 (0.62 to 1.37) | 1501 (8 studies) | ⊕⊕⊕⊝ Moderatea | |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; HR: Hazard ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aAbout half of the trials did not report explicitly their methods of randomization and allocation. Less than half of the trials appear to have performed a true intention‐to‐treat analysis. bLow event rate well below the optimal information size.
Background
Description of the condition
Esophageal cancer is the sixth most frequent cause of cancer death and is responsible for more than 286,000 deaths worldwide (Pisani 1999). Age‐standardized mortality rates (world population) in males range from 1.8 per 100,000 in West Africa to 19.8 per 100,000 in China and 28.9 per 100,000 in South Africa (Pisani 1999). Although it accounts for only 2.2% of all cancers, the incidence of esophageal cancer is increasing (Cancer Care 2009). Blot et al reported a greater than 100% increase in the occurrence of adenocarcinoma of the esophagus, and the rise in rate among white North American males has exceeded that of any other malignancy (Blot 1991).
Description of the intervention
Unfortunately, many patients present with widespread disease such that only palliative therapy is possible. Little controversy exists regarding treatment of this patient group; however, those who are potentially curable present as a challenge for both patient and physician. Surgery is the treatment of choice for most patients with localized esophageal cancer (DeMeester 1988; Lerut 1998), but curative resection is possible in only 15% to 39% (Law 1992; Lerut 1992; Lieberman 1995; Orringer 1993a). Failure of surgery to cure clinically localized esophageal cancer is due to the high frequency of lymph node involvement and distant metastases before symptoms occur. Preoperative (neoadjuvant or induction) chemotherapy has been used in an attempt to decrease tumor activity, increase resectability, and improve disease‐free and overall survival. Treatment is aimed at eradicating micrometastatic disease (Goldie 1984) and potentially downstaging cancers to enhance resectability.
Why it is important to do this review
Several studies have investigated whether preoperative chemotherapy followed by surgery leads to improved cure rates, but individual reports have been conflicting. Six meta‐analyses of randomized trials have been performed in an effort to resolve the dispute. The review by Bhansali et al of 12 randomized trials and eight historical control studies of chemotherapy, with and without radiation, found a gross overestimation of treatment effect in studies using historical controls as compared with randomized trials (Bhansali 1996). These review authors found no survival benefit from cisplatin‐based adjuvant or neoadjuvant chemotherapy in esophageal cancer. Unfortunately, this review included a heterogeneous mix of trials that were exploring very different questions about the treatment of patients with esophageal cancer and should not have been combined. Our initial Cochrane review of preoperative chemotherapy for resectable esophageal cancer (Malthaner 2001) concluded that no survival advantage was associated with chemotherapy. Subsequently, Urschel et al summarized 11 randomized trials of preoperative neoadjuvant chemotherapy for esophageal cancer and similarly concluded that no survival benefit was apparent for chemotherapy (Urschel 2002). The Medical Research Council (MRC) has published the largest randomized trial to date, which found a dramatically significant survival advantage for individuals treated with preoperative chemotherapy (MRC Allum 2009). Our subsequent Cochrane review concluded that preoperative chemotherapy plus surgery appeared to offer a late (five‐year) survival advantage compared with surgery alone for resectable thoracic esophageal cancer (Malthaner 2003). However, induction chemotherapy has associated toxicity and treatment morbidity that may contribute to perioperative mortality. Kaklamanos and colleagues summarized seven trials and concluded that a modest survival advantage is suggested for patients who receive neoadjuvant chemotherapy followed by surgery, as compared with surgery alone (Kaklamanos 2003). An apparent increase in treatment‐related mortality was noted, mainly for patients who received neoadjuvant chemoradiotherapy. Gebski and colleagues summarized eight randomized controlled trials that compared survival benefits of neoadjuvant chemotherapy followed by surgery versus surgery alone (Gebski 2007). Results of this analysis suggest an overall survival benefit at two years with neoadjuvant chemotherapy plus surgery compared with surgery alone.
In all reviews conducted before 2005, estimates at yearly time points were used when trials were combined. When a randomized controlled trial with survival‐type data is described, it is recommended that appropriate summary statistics include the log hazard ratio and its variance (Parmar 1998). The log hazard ratio has been specifically designed for comparison of two survival curves, because it is the only summary statistic that allows for both censoring and time to an event. A method that compares the numbers of events in each group or the odds of survival at one or two fixed points in time is inefficient and could lead to inappropriate conclusions. An explicit systematic update of the role of preoperative chemotherapy in the treatment of patients with resectable thoracic esophageal cancer using hazard ratios (HRs) was therefore warranted. Our subsequent Cochrane review presented data as a summary HR, and review authors concluded that preoperative chemotherapy plus surgery may offer a survival advantage over surgery alone, but that the evidence was still inconclusive (Malthaner 2006).
Since the publication of our most recent Cochrane review, a large trial (Ychou 2011) and updates of two large previously published trials (Boonstra 2011; MRC Allum 2009) have been published, demonstrating a survival advantage of chemotherapy plus surgery over surgery alone. In addition, a large individual patient meta‐analysis, considered to represent the highest level of evidence, was reported in abstract form, demonstrating a small, but significant, benefit of chemotherapy plus surgery versus surgery alone for both overall and disease‐free survival (Thirion 2007). In light of these new data, an update of our previous review is warranted.
Objectives
The objective of this review is to determine the role of preoperative chemotherapy in the treatment of patients with resectable thoracic esophageal carcinoma.
Methods
Criteria for considering studies for this review
Types of studies
We included in this review studies (published or unpublished) that randomly assigned participants with potentially resectable carcinoma of the esophagus (of any histologic type) to chemotherapy or no chemotherapy before surgery. We excluded studies if they were not truly randomized, earlier versions of updated trials reporting on the same participants, if other treatment modalities (eg, radiotherapy, hyperthermia) were used, or if no surgery alone control arms were included.
Types of participants
Participants consisted of patients with localized potentially resectable thoracic esophageal carcinoma. Trials involving patients with carcinoma of the cervical esophagus were excluded.
Types of interventions
We included trials that compared chemotherapy before surgery (esophagectomy) versus surgical resection alone. For the chemotherapy arm, we recorded drugs used, dosages, and routes of administration. For each study, we documented the type of esophagectomy performed (transhiatal or transthoracic), the number of lymph nodes removed, and the replacement organs (stomach or colon).
Types of outcome measures
Primary outcomes
The primary outcome was overall survival after randomization.
Secondary outcomes
Secondary outcomes of interest included rates of resection, response to chemotherapy, rates of local and distant recurrence, quality of life, preoperative mortality, and treatment morbidity and mortality.
Search methods for identification of studies
Electronic searches
We searched:
the Cochrane Central Register of Controlled Trials (CENTRAL) (Appendix 1);
MEDLINE (1966 to 2013) (Appendix 2);
EMBASE (1988 to 2013) (Appendix 3); and
CANCERLIT (1993 to 2013).
We did not confine our search to English language publications. In March 2009 we updated searches in CENTRAL, MEDLINE, and EMBASE.
We combined the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE, Sensitivity Maximizing Version, Ovid format (Higgins 2008), with the search terms in Appendix 2 to identify randomized controlled trials in MEDLINE. We adapted the MEDLINE search strategy for use in the other databases searched.
Searching other resources
We examined the references of all identified studies, review articles, and standard textbooks. We contacted members of the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group and experts in the oncology field and asked them to supply details of outstanding clinical trials and relevant unpublished materials. We asked the investigators in all identified trials to provide additional information that they might have about their trials and other published or unpublished trials of preoperative chemotherapy. We applied no language restrictions. We updated the searches in February 2006, March 2009, and October 2013. We consulted the clinical trial registers of the National Cancer Institute and the Radiation Therapy Oncology Group for ongoing trials.
Data collection and analysis
Selection of studies
Two review authors (KV, RM) assessed titles and abstracts and retrieved studies for full review for potential inclusion. They retrieved any trial identified by either reviewer.
Data extraction and management
We developed data extraction forms and pilot tested them to verify definitions of terms. One review author (KV) screened the retrieved trials. Two review authors (KV, RM) used standardized data extraction forms to independently summarize trials that met the inclusion criteria. Review authors were not blinded to the source of the document for article selection or data extraction. We had articles translated into English when needed. We compared the data and resolved discrepancies by consensus. Two review authors (RM, KV) conducted the most recent update..
Assessment of risk of bias in included studies
As planned in the protocol, we used a scale validated for assessing the quality of randomized controlled trials (Jadad 1996). This scale assesses the methods used for randomization, blinding and handling of dropouts in a trial. Two review authors independently evaluated trials meeting the inclusion criteria by using the Jadad method. Each appraiser was given a scoring sheet that included a detailed description of the scoring system. We resolved discrepancies by consensus. We did not use the scores to weigh the results in any way. We used quality scores to categorize trials in the sensitivity analysis. This type of scoring system is associated with danger of confusing the quality of reporting of trials with the quality of the trial design. We used this scale cautiously and with reservations.
Measures of treatment effect
For the primary outcome of survival, we extracted HRs and their standard errors, when possible, for each trial. When these were not reported directly, we estimated the HR and associated statistics by using an Excel spreadsheet developed by Matthew Sydes (Cancer Division) in collaboration with the Meta‐analysis Group of the MRC Clinical Trials Unit, London.
We used the HR to compare survival, so the complete survival experience can be summarized in a single statistic. This was an amendment to the original protocol and review, wherein survival was compared by using risk ratios (RRs) at five time points at yearly intervals. The amendment means that the primary outcome can now be analyzed using a single test, reducing the risk of spurious false‐positive results obtained from multiple testing. An HR summarizes the survival rate over the entire period of follow‐up and so can be used to estimate the treatment effect at any time point. The HR is a measure of risk of death in the treatment group (preoperative chemotherapy and surgery) versus the control group (surgery alone), such that a number less than one favors treatment. Secondary outcomes of local and distant recurrences may also have been analyzed using HRs, but unfortunately very few trials reported a time‐to‐event analysis of these outcomes, so they were summarized using RRs.
Resection was defined as any resection ‐ curative or palliative. Complete resection was defined as absence of microscopic disease at the resection margins (R0). Response to chemotherapy was defined as a clinical decrease in the size of the tumor while a complete (pathologic) response occurred, when no microscopic disease was found in the resected specimen. Local‐regional recurrence was defined as isolated local recurrence, and distant recurrence was defined as recurrence only at a distant site. Local and distant recurrence occurred when tumors were found at both locations. Preoperative death was defined as death occurring before surgery, and postoperative death included 30‐day mortality and death occurring during the same hospitalization period. Anastomotic leaks included both clinical and radiographic leaks. Pulmonary complications included pneumonia, acute respiratory distress syndrome (ARDS), and respiratory failure. Cardiac complications included dysrhythmia, infarction, and congestive failure. Infection involved the wound, the urinary tract, or sepsis. Quality of life results included any assessment of dysphagia.
The risk ratio (RR) was the measure of effect for rates of resection, tumor recurrences, and treatment morbidity and mortality. It was selected as the most 'user friendly' outcome for participants and clinicians. The RR was calculated as treatment (preoperative chemotherapy and surgery) versus control (surgery alone), such that a number greater than one favored treatment for good outcomes (resection rate), and a number less than one favored treatment for bad outcomes (recurrence, morbidity). Each outcome (HR or RR) is presented as a point estimate along with the 95% confidence interval (CI) and P value.
Dealing with missing data
We sought from study authors data missing for trials meeting the inclusion criteria, when applicable. We obtained no additional data despite multiple attempts to contact the primary authors.
Assessment of heterogeneity
We assessed the heterogeneity of trial results by using a visual plot as described by L'Abbe (L'Abbe 1987) and by conducting formal statistical testing. We assessed clinical homogeneity by examining baseline and demographic variables to ensure that studies included similar populations. Qualitatively, we examined tables of comparisons and graphic displays of trial outcomes to look for similarities. We summarized outcomes by using HRs and RRs. We examined the plots to identify possible outliers and to explore trends in outcomes due to differences in methods, participant populations, or interventions.
We carried out a formal test of heterogeneity by using a Chi2 statistic (Q) for homogeneity, as described by Peto (Petitti 1994), as well as the I2 statistic. The number of degrees of freedom of Q was equal to the number of studies minus one. An arbitrary P value < 0.1 for the Chi2 test or I2 > 50% or both were selected to denote potential lack of homogeneity such that care was needed in interpreting the results.
Data synthesis
We combined data from various trials by calculating a pooled estimate of HRs and RRs using The Review Manager software of The Cochrane Collaboration. We used the more conservative random‐effects model, as specified in the protocol, because clinical heterogeneity among trials was observed (DerSimonian 1986). The main analysis included all trials fulfilling the inclusion criteria, wherever the relevant outcome was reported. This was possible for the primary outcome of survival and for secondary outcomes of resection, rates of resection, tumor recurrences, and greatest number of treatment morbidities. We carried out a sensitivity analysis to determine whether conclusions were changed when different trials were included in the analysis: Inclusion criteria were varied and the analysis repeated.
Sensitivity analysis
We performed a sensitivity analysis to test the robustness of the results. The a priori criteria used in the analysis included:
study quality (Jadad score < 3 vs > 3);
full publication versus abstract only publication;
histologic subtypes (squamous cell carcinoma vs adenocarcinoma);
chemotherapeutic agents (cisplatin and 5‐fluorouracil (FU)‐based vs others);
year of trial (arbitrarily before 1990 vs after 1990); and
tumor location (esophageal only vs esophageal plus gastric).
Results
Description of studies
Results of the search
Results of the search are shown in Figure 1.
1.
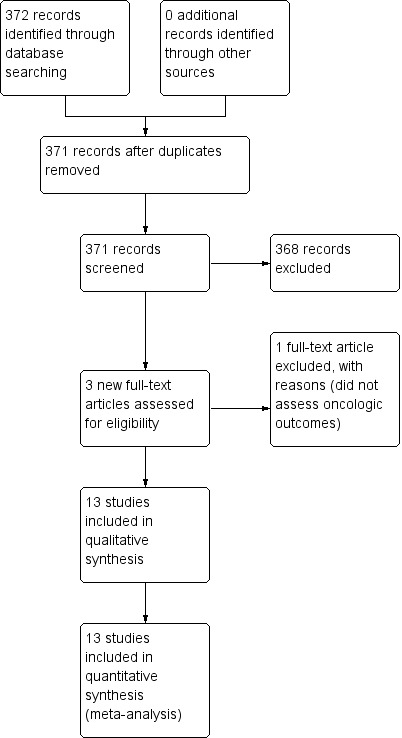
Study flow diagram for the 2014 update only.
We identified as potentially eligible for inclusion in the review a total of 22 randomized controlled trials and four meta‐analyses of preoperative chemotherapy versus surgery alone for esophageal carcinoma (Ancona 1995; Ancona 2001; Baba 1999; Baba 2000; Bhansali 1996; Boige 2007; Clark 2000; Fietkau 1999; Gebski 2007; Hohenberger 2003; Kelsen 1998; Kelsen 2007; Kok 1997; Law 1997; Maipang 1994; MRC Allum 2009; Nygaard 1992; Roth 1988; Roth 1992; Schlag 1992a; Schlag 1992b; Stilidi 2006; Thirion 2007; Urschel 2002; Wang 1986; Wang 2001). Please see Characteristics of included studies. Five trials that were previously published (Ancona 1995; Ancona 1998; Baba 1999; Clark 2000; Kelsen 1998; MRC 2002; Wang 1986) were re‐published with updated data (Ancona 2001; Baba 2000; Kelsen 2007; MRC Allum 2009; Wang 2001). We identified two additional trials (Boonstra 2011; Ychou 2011), of which the former was the full publication of the abstract from Kok 1997. We sought additional information from the investigator about the one trial not yet published in full (Stilidi 2006) and the individual participant meta‐analysis not yet published in full (Thirion 2007), but investigators failed to respond before the review was completed.
Included studies
This review is based on 13 randomized trials and 2307 participants (Ancona 2001; Baba 2000; Boonstra 2011; Kelsen 1998; Law 1997; Maipang 1994; MRC Allum 2009; Nygaard 1992; Roth 1988; Schlag 1992a; Stilidi 2006; Wang 2001; Ychou 2011) (see Characteristics of included studies). Sample sizes in the included trials ranged from 36 to 802 participants. One trial, which was reported only in abstract form, found a disease‐free survival advantage for chemotherapy but reported percent survival only at three years (Stilidi 2006). Roth et al randomly assigned 39 participants with squamous cell carcinoma of the esophagus from a single center (Roth 1988). The experimental group received cisplatin, vindesine, and bleomycin chemotherapy before surgery, and cisplatin and vindesine after surgery, and control groups underwent surgery alone. This is the only included trial in which all participants received postoperative chemotherapy in addition to preoperative chemotherapy. Study authors reported no overall difference in survival but a survival benefit in the subgroup of participants who responded to chemotherapy and did not have weight loss greater than 10%. This post hoc subgroup analysis must be interpreted with caution because it involved small numbers of participants and limited power. Postoperative complications and resectability rates were similar in these groups. This small study used an old chemotherapy protocol. Bleomycin is toxic to the lungs and is no longer used for treatment of patients with esophageal cancer.
In a multicenter Scandinavian trial, Nygaard et al randomly assigned 186 participants with squamous cell carcinoma of the esophagus to four treatment groups: Group 1, surgery alone; Group 2, preoperative chemotherapy with cisplatin and bleomycin and surgery; Group 3, preoperative radiation (35 Gy) and surgery; or Group 4, preoperative chemotherapy, radiation, and surgery (Nygaard 1992). Reported three‐year survival was significantly higher in the pooled groups receiving radiotherapy as compared with the pooled groups not receiving radiotherapy. No significant difference in survival was noted in the group given preoperative chemotherapy versus group not given chemotherapy. Only Groups 1 and 2 were analyzed in this review. This was another small study that used the outdated bleomycin chemotherapy.
In another single‐center trial from Germany, Schlag randomly assigned 46 participants with squamous cell carcinoma to receive surgery alone or preoperative cisplatin and fluorouracil followed by surgery (Schlag 1992a). The chemotherapy did not influence resectability nor overall survival of participants with localized esophageal cancer, but it was associated with considerable side effects and a high postoperative mortality rate. This was also a small study, but investigators used a more current standard chemotherapy combination of cisplatin and 5‐fluorouracil.
In the fourth trial, Maipang et al reported on a single‐center experience from Thailand that included 24 randomly assigned participants with squamous cell carcinoma (Maipang 1994). The experimental group received preoperative cisplatin, bleomycin, and vinblastine followed by surgery, and the control group underwent surgery alone. Early survival appeared better in the control group, but overall survival time differences were not statistically significant. This was another small study that used bleomycin and contributed to the lack of encouraging results.
Law et al randomly assigned 147 participants with squamous cell carcinoma to preoperative cisplatin and 5‐fluorouracil followed by surgery or surgery alone in Hong Kong (Law 1997). They reported no differences in reported perioperative mortality and no overall reported survival advantage. Analysis of extracted data showed no differences at one year and at two years but significant improvement in survival with chemotherapy at three, four, and five years. Participants who responded to chemotherapy had improved survival, and nonresponding participants had increased mortality. Local‐regional disease was reduced with chemotherapy. The complete pathologic response was only 7%.
The second‐largest trial was a multicenter North American Intergroup trial (0013) that randomly assigned 467 participants (Kelsen 1998). An update on the results of this trial was recently published (Kelsen 2007); however fewer participants were reported on in this update than in the original analysis, and the overall conclusions were unchanged. Therefore we have included the results of the original analysis in this review (Kelsen 1998). Three cycles of preoperative chemotherapy, consisting of cisplatin and 5‐fluorouracil, were repeated postoperatively if participants responded. Participants with positive margins, residual disease, or recurrence may also have received radiation at the discretion of the investigator. Both adenocarcinoma (51%) and squamous cell carcinoma (44%) were included and randomization allocation was concealed at the coordinating center. No difference in survival was reported with or without preoperative chemotherapy. The only factor found to improve long‐term survival was achievement of a complete (R0) resection.
Baba et al published on a randomized trial of 42 participants from Japan (Baba 2000). They compared preoperative cisplatin, 5‐fluorouracil, and leucovorin for two cycles versus esophagectomy alone. Investigators found that T3 tumors fared poorly with chemotherapy, and a multivariate analysis identified that a partial response to the first course of chemotherapy was a favorable prognostic indicator. Study authors provided only data on distant and local recurrences and treatment complications. Unfortunately they presented no overall survival data. . We requested additional details from the study authors, but they have yet to respond.
Wang et al reported in Chinese on 100 participants randomly assigned to preoperative cisplatin chemotherapy versus surgery alone (Wang 2001). They found a significant survival advantage for the neoadjuvant chemotherapy at five years. Unfortunately, investigators did not provide details of chemotherapy, type of surgery, and other survival information. We contacted the trial authors and are awaiting their reply.
Ancona et al described a randomized single‐center trial from Italy using preoperative cisplatin and 5‐fluorouracil chemotherapy versus esophagectomy alone (Ancona 2001). The trial was stopped after 96 of an expected 240 participants were enrolled because of slow accrual. Researchers reported no overall survival benefit for chemotherapy except in participants who had a complete response.
The largest and most recent trial with a complete manuscript has now been published (MRC Allum 2009). We have included updated long‐term results from the original MRC trial. The Medical Research Council group randomly assigned 802 participants with squamous cell carcinoma (31%) and adenocarcinoma (66%) of the esophagus from 42 European centers to two cycles of preoperative chemotherapy with cisplatin and 5‐fluorouracil. Nine percent received preoperative radiation as per local practice. Overall survival was better in the chemotherapy group (HR 0.84, 95% CI 0.72 to 0.99; P value = 0.03).
Published in abstract form only, Stilidi et al reported on 78 participants randomly assigned at a single center to receive preoperative chemotherapy with cisplatin, etoposide, leucovorin, and 5‐fluorouracil plus esophagectomy or esophagectomy alone (Stilidi 2006). Although investigators noted improvement in disease‐free survival, they reported no statistically significant difference in three‐year overall survival between the chemotherapy plus surgery group and the surgery group (62.9% vs 39.8%; P value = 0.08). Published results did not provide enough information for calculation of a hazard ratio, so it could not be included in our analysis of the primary outcome. Investigators reported the rate of R0 resection as higher in the chemotherapy plus surgery group (86.8%) than in the surgery alone group (65%; P value = 0.03). We contacted the study authors to ask for further details, and we are awaiting their reply. It appears that this trial is ongoing, as the abstract report states that accrual is continuing.
Excluded studies
Four trial reports were found to be duplications of published results and were excluded (Fietkau 1999; Hohenberger 2003; Roth 1992; Schlag 1992b) (Characteristics of excluded studies). Kelsen et al published the long‐term results of their trial (Kelsen 2007); however, these results included fewer participants than were included in the initial publication; we therefore chose to include the more complete analysis as it was initially published (Kelsen 1998).
A large trial completed by Cunningham et al compared preoperative and postoperative chemotherapy plus surgery versus surgery alone in 503 participants with operable gastric, gastroesophageal, or lower esophageal cancer (Cunningham 2006). Results of this trial showed a significant survival benefit among participants who received chemotherapy (HR 0.75, 95% CI 0.60 to 0.93), with no significant subgroup effect based on the site of the primary tumor (lower esophagus, esophagogastric junction, and stomach; P value for interaction = 0.25). Only 37 participants in the chemotherapy plus surgery arm and 36 in the surgery alone arm had lower rates of esophageal cancer (15%). A hazard ratio for survival among only participants with esophageal cancer could not be calculated; therefore the trial was not included for analysis.
Risk of bias in included studies
A summary of the risk of bias is shown in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Of the 13 trials included in this review, six did not report the method of randomization (Law 1997; Maipang 1994; Nygaard 1992; Schlag 1992a; Stilidi 2006; Wang 2001); five described a stratified method of randomization (Baba 2000; Boonstra 2011; Kelsen 1998; Roth 1988; Ychou 2011); one described a method of blocked randomization using a computer algorithm (Ancona 2001); and one used a minimization method (MRC Allum 2009).
Only one trial explicitly reported concealment of allocation (MRC Allum 2009); however, all of the multicenter trials used central randomization and thus were likely to have accomplished concealed allocation.
Five trials reported no information on the timing of randomization (Baba 2000; Law 1997; Nygaard 1992; Schlag 1992a; Wang 2001); three trials reported that randomization was performed after eligibility criteria were determined (Boonstra 2011; Maipang 1994; Ychou 2011); one trial reported that randomization was performed after clinical evaluation (Stilidi 2006); and the remaining four reported that it was performed immediately before treatment was commenced (Ancona 2001; Kelsen 1998; MRC Allum 2009; Roth 1988).
Power calculations
Seven trials included in their reports details of prospective power calculations (Ancona 2001; Boonstra 2011; Kelsen 1998; Law 1997; MRC Allum 2009; Schlag 1992a; Ychou 2011)
Primary outcome
Seven trials stated that survival was their primary outcome measure (Ancona 1995; Boonstra 2011; Kelsen 1998; MRC Allum 2009; Stilidi 2006; Wang 2001; Ychou 2011). Reports of the remaining trials did not mention a primary outcome.
Statistical analysis of the primary outcome
Eleven trials used appropriate techniques (eg, Kaplan‐Meier plots, log‐rank tests, Cox regression) to analyze survival data. In one study, which was published as an abstract only (Stilidi 2006), the method of analysis was not clear. Another trial (Wang 2001) did not use survival methods. One trial included in the analysis of survival data participants who had refused randomization (Baba 2000), and unfortunately, we could not selectively extract the information on just the randomized participants.
Incomplete outcome data
Withdrawal and intention‐to‐treat analysis
Five trials appeared to perform a true intention‐to‐treat analysis (Kelsen 1998; Law 1997; MRC Allum 2009; Stilidi 2006; Ychou 2011); for three trials it was not clear whether an intention‐to‐treat analysis had been performed, as information on withdrawals was missing or unclear (Boonstra 2011; Maipang 1994; Wang 2001); from the reports of four of the trials, it was clear that results from participants who withdrew or violated the protocol had been excluded from the analysis (Ancona 2001; Nygaard 1992; Roth 1988; Schlag 1992a); and one trial included nonrandomized participants in the analysis (Baba 2000).
Other potential sources of bias
Assessment of heterogeneity
We observed clinical heterogeneity among the reported trials. All trials evaluated participants with squamous cell carcinoma, except for the largest trials reported by Kelsen and the MRC, as well as the Ychou trial, which also included adenocarcinoma (51%, 66%, and 100%, respectively) (Kelsen 1998; MRC Allum 2009; Ychou 2011). All trials used combination cisplatin chemotherapy and at least one other agent in the treatment arm. Seven trials used cisplatin and 5‐fluorouracil (Ancona 2001; Baba 2000; Kelsen 1998; Law 1997; MRC Allum 2009; Schlag 1992a; Ychou 2011). Three trials used cisplatin in combination with bleomycin (Maipang 1994; Nygaard 1992; Roth 1988). Maipang et al added vinblastine, and Roth et al added vindesine, to the combination. Boonstra et al used cisplatin with etoposide. It is unknown what Wang et al (Wang 2001) added to cisplatin for their Chinese publication. Four trials included additional postoperative chemotherapy (Kelsen 1998; Roth 1988; Stilidi 2006; Ychou 2011).
Dosages of cisplatin varied from 20 mg/m2 to 120 mg/m2 per cycle, and the number of cycles varied from one to three. The most common dose of 5‐fluorouracil was 1000 mg/m2 for five days per cycle, but Law et al used 500 mg/m2 for five days per cycle, Baba et al used 700 mg/m2 for five days per cycle, and Stilidi et al used 425 mg/m2 for three days per cycle (Baba 2000; Law 1997).
The surgical procedure most commonly used in all trials was transthoracic esophagectomy, which incorporated the stomach and rarely the colon for the transposition. Four trials also used a transhiatal approach for lower third and gastroesophageal junction tumors and in participants considered at high risk for a thoracotomy (Boonstra 2011; Kelsen 1998; Law 1997; Ychou 2011). It is unclear which approaches were used in the large MRC trial and in the Chinese trial (MRC Allum 2009; Wang 2001).
The median duration of follow‐up ranged from one year to six years.
Trial quality
When the Jadad scale was used, overall trial quality varied widely (Table 2). Three trials scored 4/5 (Baba 2000; Kelsen 1998; MRC Allum 2009), and all of the others scored 1/5 or 2/5. The mean score was 2.5. All trials were unfairly penalized for lack of blinding. It is clinically impossible to blind participants and clinicians to participants undergoing surgery and/or receiving chemotherapy. This is an unfortunate drawback for quality scores applied to surgical and oncology studies. However, when the primary outcome of interest is survival, blinding becomes less relevant. It must be emphasized that the quality score is based on published information and may not truly reflect methodology.
1. Jadad quality score for each study.
| Study | Randomized study? | Randomization method | Double‐blind? | Method appropriate? | Dropouts described? | Quality score |
| Roth 1988 | yes | yes | no | no | no | 2/5 |
| Nygaard 1992 | yes | no | no | no | yes | 2/5 |
| Schlag 1992a | yes | no | no | no | yes | 2/5 |
| Maipang 1994 | yes | no | no | no | yes | 2/5 |
| Law 1997 | yes | no | no | no | yes | 2/5 |
| Kelsen 1998 | yes | yes | yes | no | yes | 4/5 |
| Baba 2000 | yes | yes | yes | no | yes | 4/5 |
| Wang 2001 | yes | no | no | no | no | 1/5 |
| Ancona 2001 | yes | yes | no | no | yes | 3/5 |
| MRC Allum 2009 | yes | yes | yes | no | yes | 4/5 |
| Stilidi 2006 | yes | no | no | no | no | 1/5 |
| Boonstra 2011 | yes | yes | no | no | yes | 3/5 |
| Ychou 2011 | yes | yes | no | no | yes | 3/5 |
Effects of interventions
See: Table 1
Primary outcome: overall survival
Three studies did not report sufficient information to enable the hazard ratio to be estimated (Baba 2000; Stilidi 2006; Wang 2001). When the remaining 10 trials of 2122 participants were pooled in a random‐effects meta‐analysis, a reduction in risk of mortality was observed for participants given preoperative chemotherapy when compared with those treated with surgery alone (HR 0.88, 95% CI 0.80 to 0.96; P value = 0.003; Analysis 1.1).
1.1. Analysis.
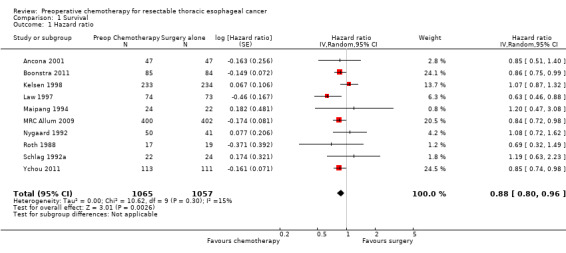
Comparison 1 Survival, Outcome 1 Hazard ratio.
We found no noticeable asymmetry in the funnel plot and, therefore, no strong evidence of publication bias (Figure 4).
4.
Funnel plot of comparison: 1 Survival, outcome: 1.1 Hazard ratio.
Secondary outcomes
Reporting of secondary outcomes varied greatly between trials. Pooling of results proceeded with caution.
Rate of resection
Nine trials of 2157 participants reported data on overall rate of resection, and nine trials of 2135 participants reported data on complete resections. With the exception of Wang et al and Stilidi et al, eight trials reported on both overall and complete resection rates. The overall rate of resection (RR 0.96, 95% CI 0.88 to 1.05; Analysis 2.1) did not suggest a difference between the preoperative chemotherapy arm and the surgery alone arm. However, rates of complete resection (R0) (RR 1.11, 95% CI 1.03 to 1.19; Analysis 2.2) were significantly higher in the preoperative chemotherapy group. Although substantial heterogeneity was evident among trials for the meta‐analysis of overall rate of resection (I2 = 78%; P value < 0.0001), no significant evidence of heterogeneity was found in the meta‐analysis for complete resection rates.
2.1. Analysis.

Comparison 2 Rate of resection, Outcome 1 All resections.
2.2. Analysis.

Comparison 2 Rate of resection, Outcome 2 Complete resections (R0).
Type of resection
Operative approaches were consistent within each trial, with most participants undergoing a transthoracic esophagectomy. All trials used this approach, but in three trials a transhiatal approach was used in some participants (Kelsen 1998; Law 1997; Ychou 2011). In the largest trial (MRC Allum 2009), surgical techniques were selected by the local surgeon according to the site of the tumor and local practice. The extent of lymph node sampling varied within individual trials and among trials, as did the reporting. In three trials, a standard two‐field lymphadenectomy was performed (Ancona 2001; Roth 1988; Schlag 1992a). In one trial, participants underwent at least a two‐field and sometimes a three‐field node resection (Baba 2000). The remaining trials lacked standardization of lymph node resections (Boonstra 2011; Kelsen 1998; Law 1997; Maipang 1994; MRC Allum 2009; Nygaard 1992; Wang 2001; Ychou 2011).
Quality of life
Only one trial of 802 participants reported on quality of life (MRC Allum 2009). The MRC trial used a nonvalidated survey on dysphagia at one year postoperatively. Little difference between the two groups was evident, with 28% of participants in the chemotherapy arm and 27% in the surgery alone arm experiencing improvement in dysphagia one year after randomization.
Response to chemotherapy
The rate of clinical response to chemotherapy was reported for nine trials of 1121 participants and ranged from 30% (Boonstra 2011) to 57% (Baba 2000). Complete pathologic response was reported in eight trials and ranged from 0% (Baba 2000; Maipang 1994) to 13% (Ancona 2001). No single agent or combination of chemotherapeutic agents was found to be superior to the others.
Tumor recurrence
Eight trials of 1654 participants reported data on local only recurrence. No evidence showed significant reduction in local only recurrence for participants given preoperative chemotherapy (RR 0.86, 95% CI 0.63 to 1.17; P value = 0.3; Analysis 3.1). Evidence suggested heterogeneity for this comparison (I2 = 46%, P value = 0.07), which was due in part to a highly significant result in favor of chemotherapy, as reported by Law 1997. Neither distant only recurrence rates, which were reported for seven trials with 1947 participants (RR 0.94, 95% CI 0.78 to 1.13; P value = 0.5; Analysis 3.2) nor rates of both local and distant recurrence together, as reported in six trials with 1905 participants, were significant (RR 1.00, 95% CI 0.82 to 1.22; P value = 0.99; Analysis 3.3).
3.1. Analysis.
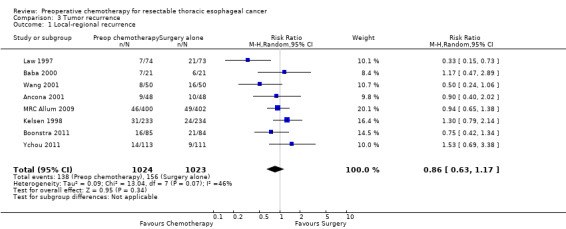
Comparison 3 Tumor recurrence, Outcome 1 Local‐regional recurrence.
3.2. Analysis.

Comparison 3 Tumor recurrence, Outcome 2 Distant recurrence.
3.3. Analysis.

Comparison 3 Tumor recurrence, Outcome 3 Local and distant recurrence.
Treatment morbidity and mortality
Data on the proportion of participants with grade 3 or 4 toxicity as a result of chemotherapy were given for seven trials of 1942 participants; these ranged from 11% (Law 1997) to over 38% (Ychou 2011). Wide variation in the rate of toxicity appeared to be due to differences in the way toxicity was defined; in the Ychou study, all grade 3 or 4 toxicities were reported, but the MRC trial, reported only toxicities that resulted in termination of chemotherapy.
Nine trials of 2194 participants reported the number of preoperative deaths. The proportion of preoperative deaths in the chemotherapy arm ranged from 0% (Law 1997; Nygaard 1992; Wang 2001) to 9% (Schlag 1992a). Only two trials reported any preoperative deaths in the surgery alone arm: one (2%) in the Nygaard study (Nygaard 1992) and two (0.5%) in the MRC study (MRC Allum 2009). It does not make sense to compare preoperative death in chemotherapy versus surgery alone, as immediate surgery does not allow any time for preoperative death, in most cases.
Eight trials with a total of 1501 participants reported data on anastomotic leaks. No evidence was found of a difference between treatment groups in anastomotic leaks (RR 0.92, 95% CI 0.62 to 1.37; P value = 0.69; Analysis 4.1).
4.1. Analysis.

Comparison 4 Treatment morbidity and mortality, Outcome 1 Anastomotic leaks.
Eight trials with a total of 1501 participants reported data on pulmonary complications. No evidence suggested a difference between treatment groups in terms of pulmonary complications (RR 1.10, 95% CI 0.76 to 1.61; P value = 0.61; Analysis 4.2).
4.2. Analysis.
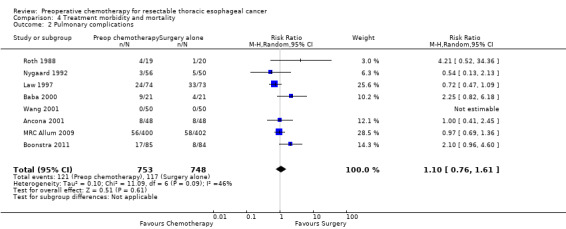
Comparison 4 Treatment morbidity and mortality, Outcome 2 Pulmonary complications.
Only five trials with a total of 1314 participants reported data on cardiac complications. No evidence showed a difference between treatment groups in terms of cardiac complications (RR 1.03, 95% CI 0.69 to 1.55; P value = 0.89; Analysis 4.3).
4.3. Analysis.

Comparison 4 Treatment morbidity and mortality, Outcome 3 Cardiac complications.
Five trials of 1184 participants reported data on infectious complications. No evidence suggested that preoperative chemotherapy reduces the risk of infectious complications (RR 0.65, 95% CI 0.41 to 1.02; P value = 0.06; Analysis 4.4). Only five studies of 1340 participants reported the numbers of participants experiencing 'any complications' (Boonstra 2011; MRC Allum 2009; Nygaard 1992; Roth 1988; Ychou 2011). Other studies listed separate rates for various complications and, as information about the numbers of participants experiencing more than one complication was missing, it was not possible to calculate an overall complication rate for these studies. When the five studies were combined, no evidence showed differences between groups (RR 0.93, 95% CI 0.81 to 1.08; P value = 0.36; Analysis 4.6).
4.4. Analysis.
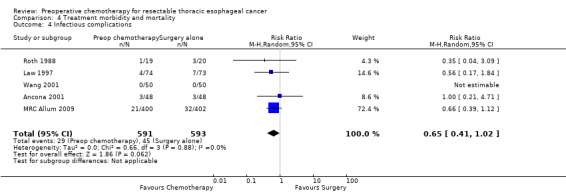
Comparison 4 Treatment morbidity and mortality, Outcome 4 Infectious complications.
4.6. Analysis.
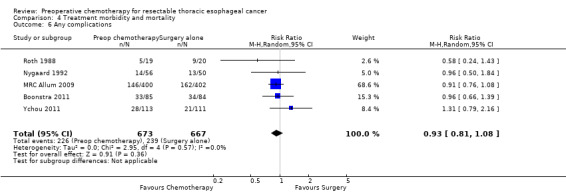
Comparison 4 Treatment morbidity and mortality, Outcome 6 Any complications.
Ten trials with a total of 2196 participants reported data on postoperative death and revealed no evidence of a difference between treatment groups (RR 0.93, 95% CI 0.68 to 1.28; P value = 0.67; Analysis 4.7).
4.7. Analysis.

Comparison 4 Treatment morbidity and mortality, Outcome 7 Postoperative deaths.
Sensitivity analysis
Sensitivity analyses concentrated on the primary outcome of overall survival.
Of the three trials that could not be included in analysis of the primary outcome, one study of 100 participants showed a survival advantage for the chemotherapy arm at five years (46% survival in the chemotherapy arm vs 32% in the surgery only arm ) (Wang 2001); one study of 78 participants reported no difference in overall percent survival at three years (62.9% in the chemotherapy + surgery group vs 27.7% in the surgery alone group; P value = 0.08) (Stilidi 2006); and one study of 42 participants did not compare survival across the two arms of the trial (Baba 2000) but reported little difference between the two groups in terms of time to treatment failure. It seems unlikely that the data missing from these three trials would affect our results dramatically; if anything, it seems that they would strengthen the evidence in favor of chemotherapy.
The random‐effects meta‐analysis was used, as this was originally specified in the protocol. It is worth noting, however, that the result is sensitive to the model selected, and that when a fixed‐effect analysis was performed, the estimate of the hazard ratio was almost unchanged, but the narrower confidence interval strengthens the evidence for a treatment effect (HR 0.87, 95% CI 0.78 to 0.97; P value = 0.01; Analysis 5.1).
5.1. Analysis.
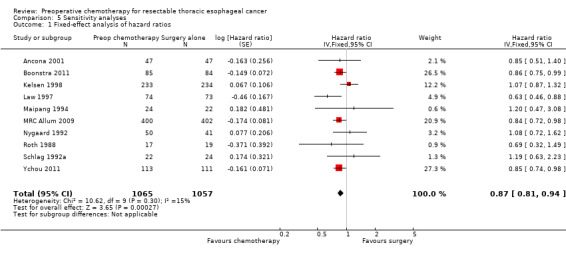
Comparison 5 Sensitivity analyses, Outcome 1 Fixed‐effect analysis of hazard ratios.
Multiple a priori sensitivity analyses were performed to investigate the robustness of results. Just two trials judged to be of high quality used the Jadad scale (Kelsen 1998; MRC Allum 2009). No significant difference in overall survival was seen with preoperative chemotherapy when only high‐quality trials were considered (HR 0.94, 95% CI 0.74 to 1.19; P value = 0.60; Analysis 5.2). The test for subgroup differences was not significant (P value = 0.40). Thus it appears that the lower‐quality studies (according to the Jadad scale) may be driving the treatment effect. This is consistent with the direction of our a priori hypothesis.
5.2. Analysis.
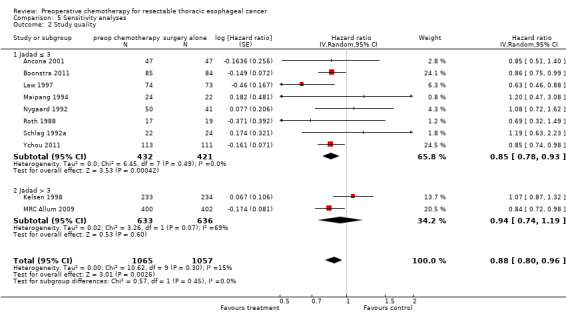
Comparison 5 Sensitivity analyses, Outcome 2 Study quality.
Ychou et al included only adenocarcinoma. No significant difference in overall survival was observed with preoperative chemotherapy when only trials that included adenocarcinoma were considered (HR 0.90, 95% CI 0.79 to 1.03; P value = 0.12; Analysis 5.5). The test for subgroup differences was not significant (P value = 0.58). Thus it appears that trials of squamous cell cancer may be driving the treatment effect. However, this subgroup effect might reflect the fact that two of the three trials that included adenocarcinoma (Kelsen 1998; MRC Allum 2009) were the same two trials that had a Jadad score indicating high quality.
5.5. Analysis.

Comparison 5 Sensitivity analyses, Outcome 5 Adenocarcinoma vs squamous cell.
Four trials gave some postoperative chemotherapy in addition to preoperative treatment (Ancona 2001; Kelsen 1998; Roth 1988; Ychou 2011). No significant difference in overall survival was noted with preoperative chemotherapy when only trials that also gave postoperative chemotherapy were considered (HR 0.91, 95% CI 0.79 to 1.06; P value = 0.22; Analysis 5.3). When these trials are excluded and only trials that gave preoperative chemotherapy alone were combined, very little change in the results was observed. The test for subgroup differences was not significant (P value = 0.40).
5.3. Analysis.
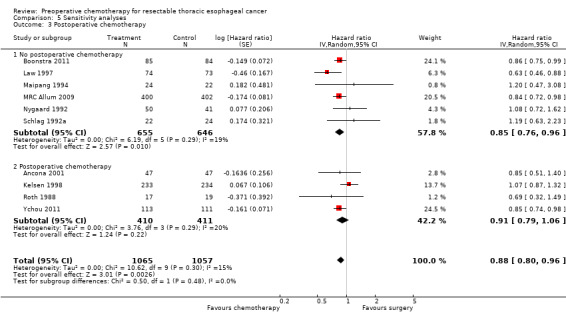
Comparison 5 Sensitivity analyses, Outcome 3 Postoperative chemotherapy.
The more recent (1990 or later) trials (Ancona 2001; Boonstra 2011; Kelsen 1998; Law 1997; MRC Allum 2009; Wang 2001; Ychou 2011) showed a survival advantage for chemotherapy (HR 0.86, 95% CI 0.78 to 0.95; P value = 0.004; Analysis 5.4). Earlier trials (before 1990) (Maipang 1994; Nygaard 1992; Roth 1988; Schlag 1992a) showed no evidence of a survival advantage for chemotherapy when combined (HR 1.04, 95% CI 0.78 to 1.40; P value = 0.78; Analysis 5.4). Note that the recent trials were the ones that used cisplatin and 5‐fluorouracil‐based chemotherapy (Ancona 2001; Boonstra 2011; Kelsen 1998; Law 1997; MRC Allum 2009; Wang 2001; Ychou 2011).
5.4. Analysis.
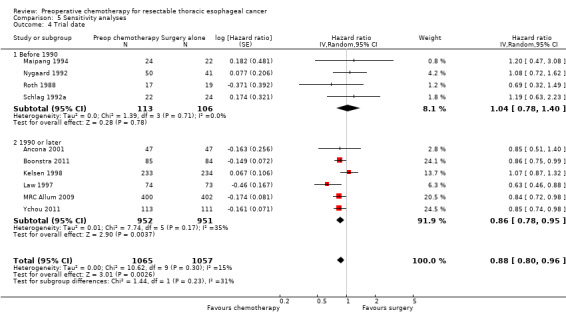
Comparison 5 Sensitivity analyses, Outcome 4 Trial date.
Discussion
Summary of main results
This review is based on 13 randomized trials with 2362 participants comparing preoperative chemotherapy versus surgery alone for resectable esophageal cancer. A survival advantage seems to be associated with chemotherapy. Sensitivity analyses suggest that low‐quality studies as well as studies of squamous cell cancer as opposed to adenocarcinoma may be driving the survival advantage observed with preoperative chemotherapy. Sensitvity analyses also suggest that only trials performed after 1990 show a survival advantage with preoperative chemotherapy. These later trials used predominantly cisplatin and 5‐fluorouracil‐based regimens, in contrast to earlier trials. Although this is an interesting observation, we cannot infer on the basis of our findings alone that this chemotherapy regimen was the causal mechanism for the survival advantage. Sensitivity analyses indicate that perhaps the timing of chemotherapy does not matter, as long as it is given. That being said, this finding was based on study‐level data; thus we cannot draw these conclusions without individual‐participant meta‐analysis. Depending on the outcome, between three and ten trials were available for each analysis. Some of the trials included in the analysis of survival are not included in the other analyses, and some of the trials included in the analysis of additional outcomes are not included in the analysis of survival. Nevertheless, results show a small benefit of chemotherapy, and inclusion of trials without survival data might lend greater weight to this. Preoperative chemotherapy was associated with significantly higher rates of complete (R0) resection, and this may be the mechanism by which preoperative chemotherapy confers a survival advantage. However, little from the recurrence data can support this mechanism for the apparent improvement in survival. Ultimately, data of better quality are needed. Morbidity data are also very limited, but an increase in toxicity is suggested with certain chemotherapeutic agents, so again data of better quality are needed. It is not possible with the current published data to speculate whether any patient subgroup defined by, for example, histology or stage might benefit more or less from chemotherapy. Individual patient data are needed to address this.
Neoadjuvant chemotherapy did not appear to alter the rate of resection, local or distant tumor recurrence, or postoperative complications. However, some preoperative toxicities and preoperative mortalities were reported. No strong conclusions can be made about effects of treatment on quality of life.
Overall completeness and applicability of evidence
Although clinical heterogeneity among the trials was apparent, the most discordant results came from the two largest trials (Kelsen 1998; MRC Allum 2009). Much speculation has been documented in the literature as to why these two well‐designed and high‐quality trials produced such different outcomes (Bosset 2002; MRC Allum 2009). Both trials used cisplatin and 5‐fluorouracil preoperatively, but the North American protocol (Kelsen 1998) gave a higher dose of cisplatin (100 mg/m2 vs 80 mg/m2), gave three cycles instead of two cycles, and administered chemotherapy postoperatively. Perhaps the higher total dose of chemotherapy was detrimental to patients who then underwent esophagectomy. It is also possible that the high perioperative mortality (10%) and the low median survival (13 months) reported in the surgery alone arm in the MRC trial may explain the difference. The median survival in the North American trial in the surgery alone arm was 16.1 months ‐ almost equivalent to the 16.8 months reported in the chemotherapy arm of the MRC trial (Kelsen 1998; MRC Allum 2009). It is difficult to postulate why this might be, as the surgical techniques were decided on by each local surgeon and were not described in the published report.
Some participants (9%) in the MRC trial received preoperative radiation in addition to chemotherapy, and in the North American trial, an unknown number of participants received postoperative radiation when resection margins were positive. Although the MRC investigators claim that the number of participants who received preoperative radiation was insignificant, it certainly could contribute to the higher postoperative mortality seen in the surgery alone group. What effect postoperative radiation had in the North American trial remains speculative.
The trial completed by Cunningham et al compared preoperative and postoperative chemotherapy plus surgery versus surgery alone in participants with operable gastric, gastroesophageal, or lower esophageal cancer (Cunningham 2006). Results of this trial show a significant survival benefit among participants who received chemotherapy, and the study authors concluded that among participants with operable gastric or lower esophageal cancer, perioperative chemotherapy significantly improved survival. This trial was excluded from our analysis, as we were unable to identify outcomes for participants with esophageal cancer alone. If included, this trial would strengthen our conclusion of a survival advantage with preoperative chemotherapy; however inclusion of results based primarily on participants with gastric cancer (74%) was believed to introduce too much clinical heterogeneity.
Quality of the evidence
As planned in the protocol, we used the Jadad scale in assessing the quality of included trials (Jadad 1996). Newer methods for assessment of risk of bias have been presented by the Cochrane Group; however, in this limited update of our previous review (only one additional study), it was believed that retaining the protocol methods was appropriate. When a risk of bias assessment was completed for the included studies, the two studies judged to be of high quality according to the Jadad score (Kelsen 1998; MRC Allum 2009) were judged to have low risk of bias, and all other studies were judged to have unclear or high risk of bias. This confirms that our previous assessments based on study quality are likely accurate.
Potential biases in the review process
None known.
Agreements and disagreements with other studies or reviews
Our conclusions differ slightly from those presented in previous systematic reviews (Malthaner 2001; Malthaner 2003; Urschel 2002). The conclusions provided by Urschel et al (Urschel 2002) were similar to those of our initial review (Malthaner 2001). Our current review includes the updated results from two trials (Boonstra 2011; MRC Allum 2009) and one new trial (Ychou 2011). As in our most recent update, this update reports a summary hazard ratio for overall survival. The methods of Parmar et al (Parmar 1998) that have been used in this update assume a constant hazard, which seems a biologically plausible assumption. The findings of this review corroborate both qualitatively and quantitatively the findings reported by Ronellenfitsch et al in their recent meta‐analysis combining aggregate and individual participant data (Ronellenfitsch 2013).
The individual participant data meta‐analysis published in abstract form by Thirion and colleagues (Thirion 2007) reported a significant benefit in overall survival with chemotherapy plus surgery when compared with surgery alone (HR 0.87, 95% CI 0.79 to 0.95; P value = 0.003). As the study authors identify, this likely represents the highest level of evidence. Trial quality, however, is not reported in this abstract; therefore it is not possible yet to determine whether this represents data from high‐quality trials, which would be considered the highest level of evidence, or from lower‐quality trials, which may call into question these results. We are anticipating publication of this individual participant data meta‐analysis so these results can be further evaluated in relation to the results reported here.
Authors' conclusions
Implications for practice.
In summary, preoperative chemotherapy plus surgery appears to offer a survival advantage compared with surgery alone for resectable thoracic esophageal cancer of any histologic type, but we require further research. Preoperative chemotherapy was associated with significantly higher rates of complete (R0) resection. Based on the sometimes limited available data, no evidence suggests a difference in rate of resection, tumor recurrence, or postoperative morbidity. Any survival advantage with chemotherapy may be tempered by risks of toxicity and preoperative mortality. The most beneficial chemotherapy combination appears to be cisplatin and 5‐fluorouracil‐based; however this meta‐analysis did not look at trials that compared chemotherapy combinations or dosing schedules.
Just as no clinical trial can provide a 'prescription' for how to treat individual cases, neither can a meta‐analysis do this. Ultimately, decisions on the use of chemotherapy need to be made by the clinician and the patient together and will depend on many factors, including survival, toxicity, quality of life, and economic costs of treatment.
If survival is the principal endpoint, preoperative chemotherapy should be considered in patients with resectable thoracic esophageal cancer. A combination of cisplatin and 5‐fluorouracil is probably most appropriate. No strong recommendation can be made about dosing or the number of cycles.
Implications for research.
A meta‐analysis of individual participant data comparing preoperative chemotherapy and surgery versus surgery alone has been performed and needs to be reported in the peer‐reviewed literature (Thirion 2007). As newer chemotherapeutic agents continue to be developed, they will need to be evaluated. Chemotherapy with radiation in conjunction with surgery should be examined both preoperatively and postoperatively. Future trials should include validated measures of quality of life and should report complications more fully. Hazard ratio should remain the standard measure for future time‐to‐event meta‐analyses. The addition of radiation therapy to preoperative chemotherapy needs to be summarized. Future randomized trials should compare preoperative chemotherapy versus preoperative chemoradiation.
What's new
| Date | Event | Description |
|---|---|---|
| 1 October 2013 | New citation required but conclusions have not changed | One new study has been incorporated, and data updated for three existing studies. Results remain unchanged |
| 1 October 2013 | New search has been performed | Searches were rerun and new results incorporated |
History
Protocol first published: Issue 3, 2000 Review first published: Issue 1, 2001
| Date | Event | Description |
|---|---|---|
| 22 May 2010 | Amended | Order of authorship corrected |
| 23 February 2010 | New search has been performed | Updated |
| 30 October 2008 | Amended | Converted to new review format |
| 18 May 2006 | New search has been performed | Minor update made |
| 7 April 2006 | New citation required and conclusions have changed | Conclusions changed |
| 12 August 2003 | Amended | New studies found but not yet included or excluded |
| 12 August 2003 | Feedback has been incorporated | Feedback added and response to feedback added |
| 23 March 2003 | Amended | Reformatted |
| 23 March 2003 | Amended | New studies found and included or excluded |
Notes
For the update written on 23 February 2010, the following text was incorporated:
One new trial and two updates on on previously included trials have been added. Siginificant changes have been made to the results and thus also to the text.
Acknowledgements
We thank Ms Janet Lilleyman, Ms Iris Gordon, and Professor David Forman from the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group for their support, suggestions, and encouragement on the initial review . Further thanks to the reviewers, especially Jayne Tierney, for their insightful comments and criticisms.
Appendices
Appendix 1. CENTRAL search strategy
exp esophageal neoplasms/
exp esophagus/
esophag$.tw.
(esophag$ adj3 neoplas$).tw.
(oesophag$ adj3 neoplas$).tw.
(esophag$ adj3 cancer).tw.
(oesophag$ adj3 cancer).tw.
(esophag$ adj3 carcinoma$).tw.
(oesophag$ adj3 carcinoma$).tw.
or/1‐9
exp Drug Therapy/
chemothera$.tw.
exp chemotherapy adjuvant/
exp drug therapy combination/
(chemotherap$ adj5 adjuvant).tw.
(preop$ adj5 chemotherap$).tw.
or/11‐16
10 and 17
preoperative.tw.
surg$.tw.
19 or 20
18 and 21
lung$.ti.
22 not 23
Appendix 2. MEDLINE search strategy
exp esophageal neoplasms/
exp esophagus/
esophag$.tw.
(esophag$ adj3 neoplas$).tw.
(oesophag$ adj3 neoplas$).tw.
(esophag$ adj3 cancer).tw.
(oesophag$ adj3 cancer).tw.
(esophag$ adj3 carcinoma$).tw.
(oesophag$ adj3 carcinoma$).tw.
or/1‐9
exp Drug Therapy/
chemothera$.tw.
exp chemotherapy adjuvant/
exp drug therapy combination/
(chemotherap$ adj5 adjuvant).tw.
(preop$ adj5 chemotherap$).tw.
or/11‐16
10 and 17
preoperative.tw.
surg$.tw.
19 or 20
18 and 21
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
or/23‐30
exp animals/ not humans.sh.
31 not 32
22 and 33
lung$.ti.
34 not 35
Appendix 3. EMBASE search strategy
exp esophagus tumor/
exp esophagus/
esophag$.tw.
(esophag$ adj3 neoplas$).tw.
(oesophag$ adj3 neoplas$).tw.
(esophag$ adj3 carcin$).tw.
(oesophag$ adj3 carcin$).tw.
(esophag$ adj3 cancer$).tw.
(oesophag$ adj3 cancer$).tw.
or/1‐9
exp chemotherapy/
chemothera$.tw.
Adjuvant chemotherapy/ or Adjuvant therapy/
Cancer chemotherapy/ or Cancer therapy/
or/11‐14
10 and 15
surg$.tw.
16 and 17
random:.tw. or placebo:.mp. or double‐blind:.tw.
18 and 19
Data and analyses
Comparison 1. Survival.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hazard ratio | 10 | 2122 | Hazard ratio (Random, 95% CI) | 0.88 [0.80, 0.96] |
Comparison 2. Rate of resection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All resections | 9 | 2157 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.05] |
| 2 Complete resections (R0) | 9 | 2135 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.03, 1.19] |
Comparison 3. Tumor recurrence.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Local‐regional recurrence | 8 | 2047 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.63, 1.17] |
| 2 Distant recurrence | 7 | 1947 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.78, 1.13] |
| 3 Local and distant recurrence | 6 | 1905 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.82, 1.22] |
Comparison 4. Treatment morbidity and mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Anastomotic leaks | 8 | 1501 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.62, 1.37] |
| 2 Pulmonary complications | 8 | 1501 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.76, 1.61] |
| 3 Cardiac complications | 5 | 1314 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.69, 1.55] |
| 4 Infectious complications | 5 | 1184 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.41, 1.02] |
| 5 Gastrointestinal complications | 2 | 902 | Risk Ratio (M‐H, Random, 95% CI) | 7.77 [0.02, 3360.76] |
| 6 Any complications | 5 | 1340 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.81, 1.08] |
| 7 Postoperative deaths | 10 | 2196 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.68, 1.28] |
4.5. Analysis.
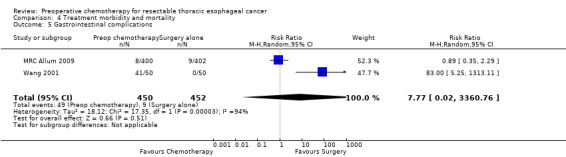
Comparison 4 Treatment morbidity and mortality, Outcome 5 Gastrointestinal complications.
Comparison 5. Sensitivity analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fixed‐effect analysis of hazard ratios | 10 | 2122 | Hazard ratio (Fixed, 95% CI) | 0.87 [0.81, 0.94] |
| 2 Study quality | 10 | 2122 | Hazard ratio (Random, 95% CI) | 0.88 [0.80, 0.96] |
| 2.1 Jadad ≤ 3 | 8 | 853 | Hazard ratio (Random, 95% CI) | 0.85 [0.78, 0.93] |
| 2.2 Jadad > 3 | 2 | 1269 | Hazard ratio (Random, 95% CI) | 0.94 [0.74, 1.19] |
| 3 Postoperative chemotherapy | 10 | 2122 | Hazard ratio (Random, 95% CI) | 0.88 [0.80, 0.96] |
| 3.1 No postoperative chemotherapy | 6 | 1301 | Hazard ratio (Random, 95% CI) | 0.85 [0.76, 0.96] |
| 3.2 Postoperative chemotherapy | 4 | 821 | Hazard ratio (Random, 95% CI) | 0.91 [0.79, 1.06] |
| 4 Trial date | 10 | 2122 | Hazard ratio (Random, 95% CI) | 0.88 [0.80, 0.96] |
| 4.1 Before 1990 | 4 | 219 | Hazard ratio (Random, 95% CI) | 1.04 [0.78, 1.40] |
| 4.2 1990 or later | 6 | 1903 | Hazard ratio (Random, 95% CI) | 0.86 [0.78, 0.95] |
| 5 Adenocarcinoma vs squamous cell | 10 | Hazard ratio (Random, 95% CI) | 0.88 [0.80, 0.96] | |
| 5.1 Jadad ≤ 3 | 7 | Hazard ratio (Random, 95% CI) | 0.85 [0.74, 0.98] | |
| 5.2 Jadad > 3 | 3 | Hazard ratio (Random, 95% CI) | 0.90 [0.79, 1.03] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ancona 2001.
| Methods | RCT, 1992‐1997 | |
| Participants | 96 participants Italy, single center 100% squamous cell Resectable T2,3 N0,1 18‐70 years No other cancers No metastases | |
| Interventions | Cisplatin 100 mg/m2 × 1 day × 2‐3 cycles 5‐Fluorouracil 1000 mg/m2 × 5 days × 2‐3 cycles + esophagectomy + postop chemotherapy and radiation for residual disease vs esophagectomy (right thoracotomy, abdomen, left neck with gastric transposition, 2‐field lymph nodes + postop chemotherapy and radiation for residual disease) | |
| Outcomes | Survival Rate of resection Response to chemotherapy Tumor recurrence Treatment morbidity and mortality | |
| Notes | No difference in survival.
Study stopped after 96/240 because of slow accrual. Only complete responders to chemotherapy had increased survival Ancona 1995 is a preliminary report (in Italian) on response to chemotherapy and morbidity and mortality with no survival data Ancona 1998 reports (in English) on a single institution subset of a larger multicenter trial. No significant survival advantage was seen with preoperative chemotherapy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | For primary outcome of interest |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not stated |
Baba 2000.
| Methods | RCT, 1993‐1995 | |
| Participants | 42 participants, Japan, single center 100% squamous cell < 75 years, Karnofsky > 90 Upper, middle, and lower third esophageal tumors No metastases, no previous cancer, no TE fistulas | |
| Interventions | Cisplatin 70 mg/m2 × 1 day × 2 cycles 5‐Fluorouracil 700 mg/m2 × 5 days × 2 cycles Leucovorin 20 mg/m2 × 5 days × 2 cycles + esophagectomy vs esophagectomy (right thoracotomy, laparotomy, neck incision, gastric or colon interposition with 2‐field or 3‐field node dissections) | |
| Outcomes | Chemotherapy response Complications | |
| Notes | T3 tumors did worse with chemotherapy No survival data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | For primary outcome of interest |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
Boonstra 2011.
| Methods | Parallel RCT (1989‐1996) Randomization stratified by age (< 50; 51‐60; > 60), gender (male; female), weight loss (kg) in the past 4 months (0‐5; 6‐10; > 10), and length of the tumor (cm) as measured by esophagogastroscopy (1‐3; 4‐6; 7‐10; > 10) |
|
| Participants | 169 participants, Netherlands, multicenter (6 centers), but 122/169 were from 1 center
100% squamous cell cancer of thoracic esophagus T1‐3, any N, M0 (M1a eligible if distal esophageal cancer AND suspected celiac nodes) < 80 years of age Karnofsky > 70 Upper, middle, and lower third esophageal tumors "Patients with previous malignancies were eligible if more than 5 years had elapsed from diagnosis without evidence of tumour recurrence; exceptions were made for adequately treated basal cell cancer of the skin or carcinoma in situ of the cervix" |
|
| Interventions |
Preoperative chemo Cycle 1 Cisplatin (80 mg/m2 IV over 4 hours on day 1 of each cycle) Etoposide (100 mg/m2 IV over 2 hours on days 1 and 2 of each cycle) Etoposide (200 mg/m2 PO on days 3 and 5 of each cycle) Cycle 2 (as above, repeated on week 4) Participants with complete or partial responses received 2 additional cycles of chemotherapy on weeks 8 and 11, whereas nonresponding participants (stable disease or progressive disease) OR those with severe toxic side effects were referred for immediate surgery Surgery: esophagectomy (see details below) vs Esophagectomy • For upper half cancers, a right‐sided thoracotomy was performed • For lower half cancers, a transhiatal esophagectomy was done • En bloc resection of tumor and adjacent lymph nodes • "Left gastric artery was transected at its origin, with resection of local lymph nodes" • Gastric tube reconstruction or colonic interposition with a cervical anastomosis |
|
| Outcomes | • Overall survival • Disease‐free survival • 30‐Day postoperative mortality • Complications |
|
| Notes | Full report of KOK 1997 trial abstract Better median overall survival and disease‐free survival in preop chemo group. More pulmonary complications in preop chemo group, but no difference in other morbidity or mortality |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not stated |
| Allocation concealment (selection bias) | Low risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | For primary outcome of interest |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
Kelsen 1998.
| Methods | RCT, 1990‐1995 | |
| Participants | 467 participants North American multicenter 44% squamous cell and 51% adenocarcinoma Stage I, II, III Operable |
|
| Interventions | Cisplatin 100 mg/m2 × 1 day × 3 cycles 5‐Fluorouracil 1000 mg/m2 × 5 days × 3 cycles + esophagectomy + postop cisplatin 75 mg/m2 + 5‐fluorouracil 1000 mg/m2 × 2 cycles if responder + radiation if positive margins vs esophagectomy with all 'accessible' nodes (abdominothoracic or thoracoabdominocervical or transhiatal with gastric or colon interposition) + radiation if positive margins |
|
| Outcomes | Survival Rate of resection Response to chemotherapy Tumor recurrence Treatment morbidity and mortality |
|
| Notes | No difference in survival Preoperative and postoperative (52%) chemotherapy given. No difference in local recurrence Kelsen 2007 paper is an English update on RTOG trial (Kelsen 1998) but presents analysis for fewer participants (433 vs 467) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not stated |
| Allocation concealment (selection bias) | Low risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | For primary outcome of interest |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
Law 1997.
| Methods | RCT 1989‐1995 | |
| Participants | 147 participants Hong Kong, single center 100% squamous cell, resectable, no metastases, no previous cancer, no TE fistulas | |
| Interventions | Cisplatin 100 mg/m2 × 1 day × 2 cycles + 5‐fluorouracil 500 mg/m2 × 5 days × 2 cycles + esophagectomy vs esophagectomy and adjacent nodes (abdominothoracic or transhiatal with gastric interposition) | |
| Outcomes | Survival Rate of resection Response to chemotherapy Tumor recurrence Treatment morbidity and mortality | |
| Notes | No difference in survival. Responders lived longer but nonresponders did worse | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | For primary outcome of interest |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
Maipang 1994.
| Methods | RCT 1988‐1990 | |
| Participants | 46 participants Thailand, single center 100% squamous cell < 75 years of age ECOG 0, 1, 2 Stage I, II, III Distal 2/3 esophagus, no other cancer, no TE fistulas, no cervical lesions | |
| Interventions | Cisplatin 100 mg/m2 × 1 day × 2 cycles + vinblastine 3 mg/m2 × 4 days × 2 cycles + bleomycin 10 mg/m2 × 5 days × 2 cycles + esophagectomy vs esophagectomy (laparotomy, right thoracotomy with gastric or colon interposition) | |
| Outcomes | Survival Rate of resection Response to chemotherapy Treatment morbidity and mortality | |
| Notes | No difference in survival Early survival better in surgery alone group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | For primary outcome of interest |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
MRC Allum 2009.
| Methods | RCT 1992‐1998 | |
| Participants | 802 participants
United Kingdom
31% squamous cell
66% adenocarcinoma 3% undifferentiated Upper, middle, lower third esophageal tumors Undifferentiated |
|
| Interventions | Cisplatin 80 mg/m2 × 1 day × 2 cycles 5‐Fluorouracil 1000 mg/m2 × 4 days × 2 cycles + preoperative radiation 25‐32.5 Gy in 10 fractions + esophagectomy vs preoperative radiation 25‐32.5 Gy in 10 fractions + esophagectomy | |
| Outcomes | Survival Rate of resection Quality of life Response to chemotherapy Tumor recurrence Treatment morbidity and mortality | |
| Notes | Type of surgery not reported
9% received preoperative radiation
Preoperative chemotherapy survival was better (HR 0.84, 95% CI 0.72 to 0.98) Long‐term follow‐up: median follow‐up of 6 years (Clark 2000) English abstract with only 2‐year survival data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated in manuscript |
| Allocation concealment (selection bias) | Low risk | Stated in manuscript |
| Blinding (performance bias and detection bias) All outcomes | Low risk | For primary outcome of interest |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates clearly reported in manuscript |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
Nygaard 1992.
| Methods | RCT, 1983‐1988 Randomly assigned to 4 groups: • Surgery • Preop chemotherapy + surgery • Preop radiation + surgery • Preop chemotherapy + radiation + surgery | |
| Participants | 106 participants Scandinavia, multicenter 100% squamous cell < 75 years of age Karnofsky score > 50 T1, T2, Nx, M0 > 21 cm from incisors, no metastases | |
| Interventions | Cisplatin 20 mg/m2 × 5 days × 2 cycles + bleomycin 10 mg/m2 × 5 days × 2 cycles + esophagectomy vs esophagectomy (laparotomy and right thoracotomy with stomach interposition) | |
| Outcomes | Survival Rate of resection Treatment morbidity and mortality | |
| Notes | No difference in survival Only chemotherapy + surgery vs surgery alone data were compared in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | For primary outcome of interest |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
Roth 1988.
| Methods | RCT 1982‐1986 | |
| Participants | 39 participants USA, multicenter 95% squamous cell, 5% undifferentiated Stage I, II, III No metastases, no previous radiation | |
| Interventions | Cisplatin 120 mg/m2 × 1 day × 1 cycle + vindesine 3 mg/m2 × 4 days × 2 cycles + bleomycin 10 U/m2 × 4 days × 2 cycles + esophagectomy + cisplatin 120 mg/m2 q 6 weeks × 6 months + vindesine 3 mg/m2 q 2 weeks × 6 months vs esophagectomy with 2‐field lymph node resection (transthoracic with cervical or thoracic anastomosis) | |
| Outcomes | Survival Response to chemotherapy Treatment morbidity and mortality | |
| Notes | Preoperative and postoperative chemotherapy given No difference in survival. Survival advantage in responders and in those with < 10% weight loss | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | For primary outcome of interest |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
Schlag 1992a.
| Methods | RCT ? dates | |
| Participants | 46 participants Germany, single center 100% squamous cell < 68 years of age Karnofsky > 70 Stage I, II, III No metastases, no TE fistulas, no previous chemotherapy or radiation | |
| Interventions | Cisplatin 20 mg/m2 × 5 days × 3 cycles + 5‐fluorouracil 1000 mg/m2 × 5 days × 3 cycles if responder after 1st cycle + esophagectomy vs esophagectomy (abdominothoracic or thoracoabdominocervical with gastric or colon interposition + 2‐field lymph node resection) | |
| Outcomes | Survival Rate of resection Response to chemotherapy Treatment morbidity and mortality | |
| Notes | No difference in survival More complications with chemotherapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | For primary outcome of interest |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
Stilidi 2006.
| Methods | RCT, no dates reported | |
| Participants | 78 participants 99% squamous cell, 1% histology not reported Resectable thoracic esophageal cancer |
|
| Interventions | Cisplatin 80 mg/m2 × 1 day × 2 cycles Etoposide 80 mg/m2 × 3 days × 2 cycles Leucovorin 20 mg/m2 × 2 cycles 5‐Fluorouracil 425 mg/m2 × 3 days × 2 cycles + esophagectomy 4 weeks after completion of chemotherapy vs esophagectomy (Ivor Lewis approach) |
|
| Outcomes | Suruvial Rate of R0 resection Response to chemotherapy Toxicity of chemotherapy |
|
| Notes | Abstract only Survival data reported as parentage survival at 3 years only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | For primary outcome of interest |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
Wang 2001.
| Methods | RCT 1991‐1994 | |
| Participants | 100 participants China, single center 97% squamous cell, 3% adenocarcinoma Stage II, III | |
| Interventions | Cisplatin 30 mg/d × 5 days × 1 cycle ? PCM for squamous cell ? "Me‐PMF" for adenocarcinoma + esophagectomy vs esophagectomy | |
| Outcomes | Survival Rate of resection Treatment morbidity and mortality | |
| Notes | Translated from Chinese. Details of chemotherapy not clearly reported. Preoperative chemotherapy improved survival (Wang 1986) Chinese paper with no survival data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
Ychou 2011.
| Methods | Parallel RCT (1995‐2003) Randomization stratified by center, WHO performance status (0 vs 1), and site of tumor (non‐GEJ stomach, GEJ, esophagus) via minimization procedure |
|
| Participants | 169 participants, France, multicenter (28 centers) but 122/169 were from 1 center
Resectable adenocarcinoma of the lower third of the esophagus or GEJ or stomach (only 25% & 24% in each arm had non‐GEJ stomach cancer) "original trial design included patients with adenocarcinoma of the lower third of the esophagus or the GEJ, but eligibility criteria were extended in 1998 to include adenocarcinoma of the stomach" 18 to 75 years of age WHO performance status = 0 or 1; adequate renal (Cr < 120 mol/L) and hematologic functions Excluded: in situ carcinoma or prior chemotherapy or radiotherapy |
|
| Interventions |
Preoperative chemo 2‐3 cycles of FU 800 mg/m2/d as IV infusion for 5 consecutive days (days 1 to 5) and cisplatin 100 mg/m2 as a 1‐hour infusion, every 28 days Surgery: esophagectomy (see details below) Note: 3‐4 postoperative cycles were administered if good tolerance and no evidence of progressive disease after preoperative chemotherapy vs Esophagectomy •En bloc resection of tumour and extended lymphadenectomy (D2 recommended), "Local surgeon decided the surgical procedure in accordance with the site of the tumor and local practice." •Transthoracic esophagectomy (40% in both arms) •Transhiatal esophagectomy (10 % in both arms) •Extended gastrectomy (9% in preop chemo group vs 4% in surgery group) •Total gastrectomy (23% in preop chemo group vs 26% in surgery group) •Distal gastrectomy (15% in preop chemo group vs 14% in surgery group) |
|
| Outcomes | Primary: overall survival Secondary: disease‐free survival (DFS), R0 resection rate, and safety |
|
| Notes | Better overall survival and disease‐free survival in preop chemo group. Higher R0 resection rate in preop chemo group. No difference in morbidity or mortality | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | For primary outcome of interest |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
CI = confidence interval, FU = fluorouracil. DFS = disease‐free survival. GEJ = gastroesophageal junction.
HR = hazard ratio. PCM = preoperative chemotherapy. PMF = undefined. RCT = randomized controlled trial. TE = thoracic esophagus. vs = versus. WHO = World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bhansali 1996 | English meta‐analysis Included chemotherapy alone, chemotherapy and radiotherapy, radiation alone, preoperatively and postoperatively. Trials are clinically too heterogeneous to be combined |
| Boige 2007 | English abstract Reports on outcomes combined for gastric and lower esophageal cancer |
| Cunningham 2006 | English RCT Reports on results for adenocarcinoma of the stomach, esophagogastric junction, and lower esophagus Only 14% of participants with esophageal cancer, unable to separate results based on tumor location |
| EORTC40954 2010 | Excluded, as looked at gastric cancer |
| Fietkau 1999 | German abstract Reports on Kelsen et al paper No new data |
| Hohenberger 2003 | German abstract Reports on MRC Allum 2009 paper No new data |
| Roth 1992 | German paper Re‐publication of previous data |
| Schlag 1992b | German paper Re‐publication of previous data |
| Thirion 2007 | English abstract Individual participant meta‐analysis, not a trial |
| Urschel 2002 | Meta‐analysis, not a trial |
Differences between protocol and review
In the original protocol, analysis of survival was planned using risk ratios at five time points at yearly intervals. The protocol was amended to use hazard ratios to compare survival, so the complete survival experience can be summarized in a single statistic.
Contributions of authors
Literature review: Richard Malthaner, Kelly Vogt, Biniam Kidane, Shaun Coughlin Literature search: Richard Malthaner, Iris Gordon, Kelly Vogt, Biniam Kidane, Shaun Coughlin Screening of trials: Richard Malthaner, Kelly Vogt, Biniam Kidane, Shaun Coughlin Data extraction: Richard Malthaner, Kelly Vogt, Biniam Kidane, Shaun Coughlin Analysis of data: Richard Malthaner, Kelly Vogt, Biniam Kidane Writing of the manuscript: Richard Malthaner, Kelly Vogt, Biniam Kidane Review of the manuscript: Richard Malthaner, Kelly Vogt, Biniam Kidane, Shaun Coughlin Update of previous review: Biniam Kidane, Shaun Coughlin, Kelly Vogt, Richard Malthaner
Sources of support
Internal sources
Western University, Canada.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Ancona 2001 {published data only}
- Ancona E, Ruol A, Chiarion‐Sileni V, Santi S, Baldan N, Buin F, et al. Prospective randomized trial of preoperative chemotherapy versus surgery alone in operable squamous cell carcinoma of the esophagus. Preliminary report on response to chemotherapy, pre‐ and postoperative morbidity and mortality [Studio prospettico randomizzato di chemioterapia neoadiuvante versus sola chirurgia nel cancro operabile (T2‐3, ogni N, M0) dell'esofago. Valutazione della risposta alla chemioterapia e della morbilita e mortalita pre e postoperatorie]. Acta Chirurgica Italica 1995;51(4):308‐17. [95277909] [Google Scholar]
- Ancona E, Ruol A, Santi S, Chiarion V, Merigliano S, Petrin GF, et al. Prognostic role of downstaging after neoadjuvant chemotherapy in potentially resectable (T2‐3 Nx M0) esophageal squamous cell carcinoma. Canadian Journal of Gastroenterology 1998;12(Suppl B):82. [Google Scholar]
- Ancona E, Ruol A, Santi S, Merigliano S, Sileni VC, Koussis H, et al. Only pathologic complete response to neoadjuvant chemotherapy improves significantly the long term survival of patients with resectable esophageal squamous cell carcinoma: final report of a randomized, controlled trial of preoperative chemotherapy versus surgery alone. Cancer 2001;91(11):2165‐74. [MEDLINE: ; PUBMED: 11391598] [PubMed] [Google Scholar]
Baba 2000 {published data only (unpublished sought but not used)}
- Baba M, Natsugoe S, Shimada M, Nakano S, Kusano C, Fukumoto T, et al. Prospective evaluation of preoperative chemotherapy in resectable squamous cell carcinoma of the thoracic esophagus. Diseases of the Esophagus 2000;13(2):136‐41. [2000408961; PUBMED: 14601905] [DOI] [PubMed] [Google Scholar]
- Baba M, Natsugoe S, Shimada M, Nakano S, Shirao K, Kusano C, et al. Does preoperative chemotherapy cause adverse effects on the perioperative course of patients undergoing esophagectomy for carcinoma?. Japanese Journal of Thoracic and Cardiovascular Surgery 1999;47(5):199‐203. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Boonstra 2011 {published data only}
- Boonstra JJ, Kok TC, Wijnhoven PL, Heijl M, Berge Henegouwen MI, Kate FJW, et al. Chemotherapy followed by surgery versus surgery alone in patients with resectable oesophageal squamous cell carcinoma: Longterm results of a randomized controlled trial. BMC Cancer 2011;11:181. [DOI: 10.1186/1471-2407-11-181; PUBMED: 21595951] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok TC, Lanschot J, Siersema PD, Overhagen H, Tilanus HW. Neoadjuvant chemotherapy in operable esophageal squamous cell cancer: final report of a phase III multicenter randomized controlled trial. Proceedings of the Annual Meeting of the American Society of Clinical Oncology 1997;17:A984. [98642984] [Google Scholar]
Kelsen 1998 {published data only}
- Kelsen DP, Ginsberg R, Pajak TF, Sheahan DG, Gunderson L, Mortimer J, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. New England Journal of Medicine 1998;339(27):1979‐84. [PUBMED: 9869669] [DOI] [PubMed] [Google Scholar]
- Kelsen DP, Winter KA, Gunderson LI, Mortimer J, Estes NC, Haller DG, et al. Long‐term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer [Long‐term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer]. Journal of Clinical Oncology 2007;25:3719‐25. [DOI] [PubMed] [Google Scholar]
Law 1997 {published data only}
- Law S, Fok M, Chow S, Chu KM, Wong J. Preoperative chemotherapy versus surgical therapy alone for squamous cell carcinoma of the esophagus: a prospective randomized trial. Journal of Thoracic and Cardiovascular Surgery 1997;114(2):210‐7. [MEDLINE: ; PUBMED: 9270638] [DOI] [PubMed] [Google Scholar]
Maipang 1994 {published data only}
- Maipang T, Vasinanukorn P, Petpichetchian C, Chamroonkul S, Geater A, Chansawwaang S, et al. Induction chemotherapy in the treatment of patients with carcinoma of the esophagus. Journal of Surgical Oncology 1994;56(3):191‐7. [MEDLINE: ; PUBMED: 7518020] [DOI] [PubMed] [Google Scholar]
MRC Allum 2009 {published data only}
- Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long‐term results of a randomized trial of surgery with or without pre‐operative chemotherapy in esophageal cancer. Journal of Clinical Oncology 2009;27(30):5062‐7. [DOI: 10.1200/JCO.2009.22.2083; MEDLINE: ; 12049861; PUBMED: 19770374] [DOI] [PubMed] [Google Scholar]
- Clark PI. Medical Research Council randomised phase III trial of surgery with or without pre‐operative chemotherapy in resectable cancer of the oesophagus. British Journal of Cancer 2000;83 (Supplement 1):1, Y. [Google Scholar]
- Medical Research Council Oesophageal Cancer Working Party. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 2002;359:1727‐33. [DOI] [PubMed] [Google Scholar]
Nygaard 1992 {published data only}
- Nygaard K, Hagen S, Hansen HS, Hatlevoll R, Hultborn R, Jakobsen A, et al. Pre‐operative radiotherapy prolongs survival in operable esophageal carcinoma: a randomized, multicenter study of pre‐operative radiotherapy and chemotherapy. The second Scandinavian trial in esophageal cancer. World Journal of Surgery 1992;16(6):1104‐9. [MEDLINE: ; PUBMED: 1455880] [DOI] [PubMed] [Google Scholar]
Roth 1988 {published data only}
- Roth JA, Pass HI, Flanagan MM, Graeber GM, Rosenberg JC, Steinberg S. Randomized clinical trial of preoperative and postoperative adjuvant chemotherapy with cisplatin, vindesine, and bleomycin for carcinoma of the esophagus. Journal of Thoracic Cardiovascular Surgery 1988;96(2):242‐8. [MEDLINE: ; PUBMED: 2456424] [PubMed] [Google Scholar]
Schlag 1992a {published data only}
- Schlag PM. Randomized trial of preoperative chemotherapy for squamous cell cancer of the esophagus. The Chirurgische Arbeitsgemeinschaft Fuer Onkologie der Deutschen Gesellschaft Fuer Chirurgie Study Group. Archives of Surgery 1992;127(12):1446‐50. [MEDLINE: ; PUBMED: 1365692] [DOI] [PubMed] [Google Scholar]
Stilidi 2006 {published data only (unpublished sought but not used)}
- Stilidi I, Bokhyan V, Tryakin A, Suleymanov E, Kononets P, Malikhova O, et al. Preoperative chemotherapy followed by resection vs. surgery alone for locally advanced esophageal carcinoma: interim analysis of a randomized study. Journal of Clinical Oncology 2006;24(18S):4055. [Google Scholar]
Wang 2001 {published data only (unpublished sought but not used)}
- Wang C, Ding T, Chang L. A randomized clinical study of preoperative chemotherapy for esophageal carcinoma. Zhonghua Zhong Liu Za Zhi 2001;23(3):254‐5. [MEDLINE: ; PUBMED: 11783101] [PubMed] [Google Scholar]
- Wang ZY. Preoperative chemotherapy for esophageal cancer. Chung‐Hua‐Chung‐Liu‐Tsa‐Chih 1986;8(1):61‐63. [MEDLINE: ] [PubMed] [Google Scholar]
Ychou 2011 {published data only}
- Ychou M, Boige V, Pignon JP, Conroy T, Bouche O, Lebreton G, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. Journal of Clinical Oncology 2011;29(13):1715‐21. [PUBMED: 21444866] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bhansali 1996 {published data only}
- Bhansali MS, Vaidya JS, Bhatt RG, Patil PK, Badwe RA, Desai PB. Chemotherapy for carcinoma of the esophagus: a comparison of evidence from meta‐analyses of randomized trials and of historical control studies. Annals of Oncology 1996;7(4):355‐9. [DOI] [PubMed] [Google Scholar]
Boige 2007 {published data only}
- Boige V, Pignon J, Saint‐Aubert B, Lasser P, Conroy T, Bouche O, et al. Final results of a randomized trial comparing preoperative 5‐fluorouracil (F)/cisplatin (P) to surgery alone in adenocarcinoma of the stomach and lower esophagus (ASLE): FNLCC ACCORD07‐FFCD 9703 trial. Journal of Clinical Oncology 2007;25(18S):4510. [Google Scholar]
Cunningham 2006 {published data only}
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Velde CJH, Nicoldon M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. The New England Journal of Medicine 2006;355(1):11‐20. [DOI] [PubMed] [Google Scholar]
EORTC40954 2010 {published data only}
- Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. Journal of Clinical Oncology 2010;28:5210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fietkau 1999 {published data only}
- Fietkau R. No improvement of prognosis by neoadjuvant chemotherapy alone in operable esophageal carcinoma [Keine Verbesserung der Prognose des operablen Osophaguskarzinoms durch eine alleinige neoadjuvante Chemotherapie]. Strahlentherapie und Onkologie 1999;175(5):251‐2. [99285106] [DOI] [PubMed] [Google Scholar]
Hohenberger 2003 {published data only}
- Hohenberger W. Surgical resection with or without preoperative chemotherapy in esophageal cancer: a randomized controlled trial [German]. Strahlentherapie und Onkologie 2003;179(4):271‐2. [MEDLINE: ] [Google Scholar]
Roth 1992 {published data only}
- Roth JA. Squamous cell cancer of the esophagus. Treatment concept at the Houston M.D. Anderson Cancer Center. Chirurg 1992;63(9):683‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Schlag 1992b {published data only}
- Schlag P. Randomized study of preoperative chemotherapy in squamous cell cancer of the esophagus. CAO Esophageal Cancer Study Group. Chirurg 1992;63(9):709‐14. [MEDLINE: ] [PubMed] [Google Scholar]
Thirion 2007 {published data only}
- Thirion PG, Michiels S, Maitre A, Tierney J. Individual patient data‐based meta‐analysis assessing pre‐operative chemotherapy in resectable oesophageal carcinoma. Journal of Clinical Oncology 2007;45(18S):4512. [Google Scholar]
Urschel 2002 {published data only}
- Urschel JD, Vasan H, Blewett CJ. A meta‐analysis of randomized controlled trials that compared neoadjuvant chemotherapy and surgery to surgery alone for resectable esophageal cancer. American Journal of Surgery 2002;183(3):274‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
Ancona 1995
- Ancona E, Ruol A, Chiarion‐Sileni V, Santi S, Baldan N, Buin F, et al. Prospective randomized trial of preoperative chemotherapy versus surgery alone in operable squamous cell carcinoma of the esophagus. Preliminary report on response to chemotherapy, pre‐ and postoperative morbidity and mortality [Studio prospettico randomizzato di chemioterapia neoadiuvante versus sola chirurgia nel cancro operabile (T2‐3, ogni N, M0) dell'esofago. Valutazione della risposta alla chemioterapia e della morbilita e mortalita pre e postoperatorie]. Acta Chirurgica Italica 1995;51(4):308‐17. [95277909] [Google Scholar]
Ancona 1998
- Ancona E, Ruol A, Santi S, Chiarion V, Merigliano S, Petrin GF, et al. Prognostic role of downstaging after neoadjuvant chemotherapy in potentially resectable (T2‐3 Nx M0) esophageal squamous cell carcinoma. Canadian Journal of Gastroenterology 1998;12(Suppl B):82. [Google Scholar]
Baba 1999
- Baba M, Natsugoe S, Shimada M, Nakano S, Shirao K, Kusano C, et al. Does preoperative chemotherapy cause adverse effects on the perioperative course of patients undergoing esophagectomy for carcinoma?. Japanese Journal of Thoracic and Cardiovascular Surgery 1999;47(5):199‐203. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Blot 1991
- Blot WJ, Devesa SS, Kneller RW. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA 1991;265:1287‐9. [PubMed] [Google Scholar]
Bosset 2002
- Bosset JF, Mercier M, Triboulet JP, Conroy T, Seitz JF. Surgical resection with and without chemotherapy in oesophageal cancer. Lancet 2002;360(9340):1173‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cancer Care 2009
- Cancer Care Ontario. Incidence and mortality in Ontario, 2009. Internet Communication. https://www.cancercare.on.ca/ocs/csurv/stats/ontario/ (accessed 24 April 2015).
Clark 2000
- Clark PI. Medical Research Council randomised phase III trial of surgery with or without pre‐operative chemotherapy in resectable cancer of the oesophagus. British Journal of Cancer 2000;83 (Supplement 1):1, Y.. [Google Scholar]
DeMeester 1988
- DeMeester TR, Barlow AP. Surgery and current management for cancer of the esophagus. Current Problems in Surgery 1988;25:535‐605. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gebski 2007
- Gebski V, Burmeister B, Smithers BM, Foo K, Zalcberg J, Simes J. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta analysis. Lancet Oncology 2007;8:226‐34. [DOI] [PubMed] [Google Scholar]
Goldie 1984
- Goldie JH, Coldman AJ. The genetic origin of drug resistance in neoplasms: implication for systemic therapy. Cancer Research 1984;44:3643. [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Jadad 1996
- Jadad AR. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Kaklamanos 2003
- Kaklamanos IG, Walker GR, Ferry K, Franceschi D, Livingstone AS. Neoadjuvant treatment for resectable cancer of the esophagus and the gastroesophageal junction: a meta‐analysis of randomized clinical trials. Annals of Surgical Oncology 2003;10(7):754‐61. [DOI] [PubMed] [Google Scholar]
Kelsen 2007
- Kelsen DP, Winter KA, Gunderson LI, Mortimer J, Estes NC, Haller DG, et al. Long‐term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer [Long‐term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer]. Journal of Clinical Oncology 2007;25:3719‐25. [DOI] [PubMed] [Google Scholar]
Kok 1997
- Kok TC, Lanschot J, Siersema PD, Overhagen H, Tilanus HW. Neoadjuvant chemotherapy in operable esophageal squamous cell cancer: final report of a phase III multicenter randomized controlled trial. Proceedings of the Annual Meeting of the American Society of Clinical Oncology 1997;17:A984. [98642984] [Google Scholar]
L'Abbe 1987
- L'Abbe KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107:224‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Law 1992
- Law SY, Fok M, Cheng SW, Wong J. A comparison of outcome after resection for squamous cell carcinomas and adenocarcinomas of the esophagus and cardia. Surgery Gynecology & Obstetrics 1992;175(2):107‐12. [PubMed] [Google Scholar]
Lerut 1992
- Lerut T, Leyn P, Coosemans W, Raemdonck D, Scheys I, LeSaffre E. Surgical strategies in esophageal carcinoma with emphasis on radical lymphadenectomy [see comments]. Annals of Surgery 1992;216(5):583‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lerut 1998
- Lerut T. Esophageal surgery at the end of the millennium. Journal of Thoracic and Cardiovascular Surgery 1998;116(1):1‐20. [DOI] [PubMed] [Google Scholar]
Lieberman 1995
- Lieberman MD, Shriver CD, Bleckner S, Burt M. Carcinoma of the esophagus. Prognostic significance of histologic type. Journal of Thoracic and Cardiovascular Surgery 1995;109(1):130‐8. [DOI] [PubMed] [Google Scholar]
MRC 2002
- Medical Research Council Oesophageal Cancer Working Party. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: Aa randomised controlled trial. Lancet 2002;359:1727‐33. [DOI] [PubMed] [Google Scholar]
Orringer 1993a
- Orringer MB. Multimodality therapy for esophageal carcinoma ‐ update. Chest 1993;103:406S‐9S. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta‐analysis of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Petitti 1994
- Petitti DB. In: Kelsey JL, Marmot MG, Stolley PD, Vessey MP editor(s). Meta‐analysis, Decision Analysis, and Cost‐Effectiveness Analysis. 1st Edition. Vol. 24, New York: Oxford University Press, 1994. [Google Scholar]
Pisani 1999
- Pisani P, Parkin DM, Bray F, Ferlay J. Estimates of the worldwide mortality from 25 cancers in 1990. International Journal of Cancer 1999;83(1):18‐29. [0020‐7136] [DOI] [PubMed] [Google Scholar]
Ronellenfitsch 2013
- Ronellenfitsch U, Schwarzbach M, Hofheinz R, Kienle P, Kieser M, Slanger TE, et al. Preoperative chemo(radio)therapy versus primary surgery for gastroesophageal adenocarcinoma: systematic review with meta‐analysis combining individual patient and aggregate data. European Journal of Cancer 2013;49:3149‐58. [DOI] [PubMed] [Google Scholar]
Wang 1986
- Wang ZY. Preoperative chemotherapy for esophageal cancer. Chung‐Hua‐Chung‐Liu‐Tsa‐Chih 1986;8(1):61‐3. [MEDLINE: ] [PubMed] [Google Scholar]
References to other published versions of this review
Malthaner 2001
- Malthaner R, Fenlon D. Preoperative chemotherapy for resectable thoracic esophageal cancer. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD001556.pub2] [DOI] [PubMed] [Google Scholar]
Malthaner 2003
- Malthaner R, Fenlon D. Preoperative chemotherapy for resectable thoracic esophageal cancer. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD001556.pub2] [DOI] [PubMed] [Google Scholar]
Malthaner 2006
- Malthaner R, Collin S, Fenlon D. Preoperative chemotherapy for resectable thoracic esophageal cancer. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD001556.pub2] [DOI] [PubMed] [Google Scholar]


