Abstract
Caesarean delivery has been linked to a number of inflammatory conditions in childhood and adolescence. Yet the mechanisms underlying these associations and their generalizability across contexts with different postnatal feeding and pathogenic exposures remain unclear. This study tests the association between delivery type and three measures of immune function, inflammation, morbidity, and leukocyte proportions, in Ecuadorian infants and children, aged 6 months to 2 years. Data come from mother-child pairs participating in a nationally-representative health and nutrition survey Encuesta Nacional de Salud y Nutricion (ENSANUT) conducted in 2012. The analytic sample includes 828 mothers and infants with delivery information and measured biomarkers. Logistic regression models were used to examine the association between delivery type and markers of immune function, controlling for maternal and infant characteristics including age, sex, sociodemographic characteristics, and medical indications. 40.8% (n=338) of sample infants and children were delivered by Caesarean. Compared to those born vaginally, infants born by Caesarean were less likely to have elevated CRP (CRP>2mg/L; RR: 0.76, 95% CI: 0.58–1.00) and more likely to have illness symptoms (RR: 1.22, 95%CI: 1.01–1.46) and elevated basophils (RR: 1.83, 95%CI: 1.03–3.25). No other immune cell proportions differed by delivery type. The results suggest that differences in the perinatal exposures accompanying Caesarean delivery may alter immune development and function, particularly in the inflammatory response to infection and in cells involved the allergic response, across infancy and early childhood. Understanding the pathways linking perinatal exposures to immune development is important for preventing the development of inflammatory conditions.
Keywords: Caesarean delivery, inflammation, leukocytes, allergy
The proportion of infants born by Caesarian-section is increasing globally, including in low and middle-income countries like Ecuador. Elective C-sections have been linked to a number of inflammatory conditions in childhood and adolescence, including increased allergy and asthma and elevated risk of overweight or obesity. However, the health implications of delivery mode are not well understood in infancy. We test the impact of C-sections on the development of the immune system in the first year of life. Data come from 828 mother-infant pairs participating in Ecuador’s 2012 ENSANUT study, a nationally representative health and nutrition survey, and an additional 40 mothers and their 41 infants participating in the Birth Practices Study in San Cristobal, Galapagos. Mixed models were used to examine the association between delivery type and markers of immune function, C-reactive protein, hemoglobin, and illness symptoms, controlling for maternal and infant characteristics including age, sex, sociodemographic characteristics, and clustering by community. 40.8% of infants in the national survey and 58.5% in the pilot study were born by C-section. Compared to those born vaginally, infants born by C-section were less likely to have moderate inflammation (CRP 3–10mg/L; RRR: 0.70, 95%CI: 0.50–0.97), more likely to have iron deficiency (hemoglobin <11 g/dL; OR: 1.31, 95%CI: 0.97–1.76), and more likely to have illness symptoms (OR: 1.59; OR: 1.20–2.10). These results suggest that birth practices may be associated with altered immune function in infancy, highlighting the need to understand the pathways linking early life exposures to inflammation and its potential long-term health outcomes.
Increasing evidence links early life exposures to the development of the immune system and long-term risk of immunologic and inflammatory conditions. Birthweight, a marker of maternal nutrition during pregnancy, has been associated with numerous aspects of immune function, including antibody response to vaccines,1 immunoglobulin levels,2,3 elevated C-reactive protein,4 and leukocyte (white blood cell) counts5,6 through childhood into adulthood. Research into the hygiene hypothesis has highlighted the importance of infant and childhood microbial exposures in entraining the immune system to recognize pathogenic vs. benign substances.7–9 Disruptions in exposure to “old friends,” the commensal bacteria and other common pathogens frequently encountered during our evolutionary and historical past, during early life due to increases in hygiene, declining family size, reduced exposure to livestock, and increased antibiotic use have been implicated in the rising prevalence of allergy, atopy and other inflammatory conditions.10 Among these early exposures, birth interventions may also play an important role in the development of the immune system and immune-related diseases in infancy and childhood.11–13
Meta-analyses and analyses of large datasets drawn from national health registries have documented that Caesarean delivery is a risk factor for neonatal respiratory conditions, asthma, atopy, type 1 diabetes, celiac disease, and gastroenteritis.14–18 The associations between delivery and later health outcomes are strengthened when emergency and planned Caesareans are measured separately. Caesarean deliveries occurring prior to the rupture of the membranes carry a greater risk of asthma and inflammatory disorders,19 indicating that the process of labor is important for longer term health. Two inter-related pathways have been proposed to underlie the associations between mode of delivery and later health outcomes.13,20,21 First, the experience of labor serves as stressor in the neonate, upregulating the stress response and activating components of the immune system.21–23 At the same time, passage through the birth canal exposes neonates to their mother’s vaginal and fecal microbiota, seeding the infant’s gut microbiome and beginning the process of entraining the immune system to recognize harmful vs benign exposures.24,25 While the relative importance of these pathways and the mechanisms contributing to the persistence of delivery exposures on later immune function continue to be explored, immunological studies in neonates document that the prevalence of leukocytes, including neutrophils, monocytes, lymphocytes and NK cells, are altered by the process of labor.19,26,27 Levels of inflammatory cytokines produced by these immune cells and of C-reactive protein also tend to be lower in neonates born by planned Caesarean.28,29 Together these functional differences in the immune system may affect morbidity from respiratory and other common infections in the short term and shape immune function and the development of inflammatory conditions in the longer term.11 However, few studies have examined the intervening period of infancy and young childhood to test whether differences in immune cell count and immune function persist beyond the perinatal period.
Another important gap in the research on the perinatal origins of immune disorders is that evidence for the association between Caesarean delivery and later health comes almost entirely from high-income countries.30,31 Yet, rates of Caesarean delivery32 and allergic conditions33 are both increasing dramatically in low and middle income countries (LMIC). The few studies that have examined the association between Caesarean delivery and asthma in LMIC settings have found contradictory results. One comparative study of 8-year old children in India and Vietnam found a stronger association between Caesarean delivery and asthma than that seen in high income countries,34 while two others have found no significant association between delivery mode and asthma in 3–15 year-old children in Malaysia35 and Iraq.36 Such contrasting results may reflect differences in salient environmental risk factors between more and less affluent settings37 and/or differences in immune system development and activation in response to differences in energy availability or pathogenic exposures. Further investigation into the potential impacts of birth practices on immune development in LMICs, where infants and children are exposed to both higher rates of breast feeding and more pathogenic environments,34,38 is needed to establish whether the impacts of early birth exposures persist across a broader range of postnatal contexts.
This study examines the association between delivery type and measures of immune function and morbidity in Ecuadorian infants and young children participating in the nationally-representative Encuesta Nacional de Salud y Nutricion (ENSANUT 2012).39 It tests whether the proportion of five leukocyte types (lymphocytes, monocytes, neutrophils, basophils, and eosinophils), C-reactive protein, and morbidity from respiratory and diarrheal illness differ between infants and young children, aged 6 months to 2 years, delivered vaginally vs. those delivered by Caesarean. These measures permit an examination of the association between delivery mode and several aspects of immune function. Leukocytes serve as the primary mediator of both innate (the rapid, non-specific response to infection) and adaptive (the time-delayed specific cellular and humoral response) immune responses to infection and promote inflammation. The five measured cell types serve different roles in this process. Lymphocytes, which include B cells, T cells, and natural killer (NK) cells, serve as a measure of specific immunity and respond to intra- and extracellular pathogens and viral infections. The other four cells types, monocytes, neutrophils, eosinophils and basophils, participate in the innate immune response. Neutrophils are an indicator of general infection while monocytes are an indicator of chronic infection. Eosinophils and basophils both play a role in inflammation and allergic responses. Eosinophils respond to parasitic infection and serve as marker of inflammatory and allergic responses while basophils indicate asthma and allergic disease. Thus, along with C-reactive protein, an inflammatory marker that plays a role in the acute phase response to infection, these measures provide information on immunity and response to infection in a LMIC context with prevalent Caesarean section, over 40% of births in 201239, and potentially different postnatal feeding and environmental exposures. Based on previous findings in neonates, I hypothesize that, compared to vaginally delivered infants and children, those delivered by Caesarean will show a dampened immune response with lower specific immune cell proportions, lower inflammation, and poorer immune function indicated by higher morbidity. Conversely, due to the association between Caesarean delivery and allergy and asthma, I hypothesize that the leukocytes associated with these conditions, eosinophils and basophils, will be higher in infants and children born by Caesarean.
Methods
Sample:
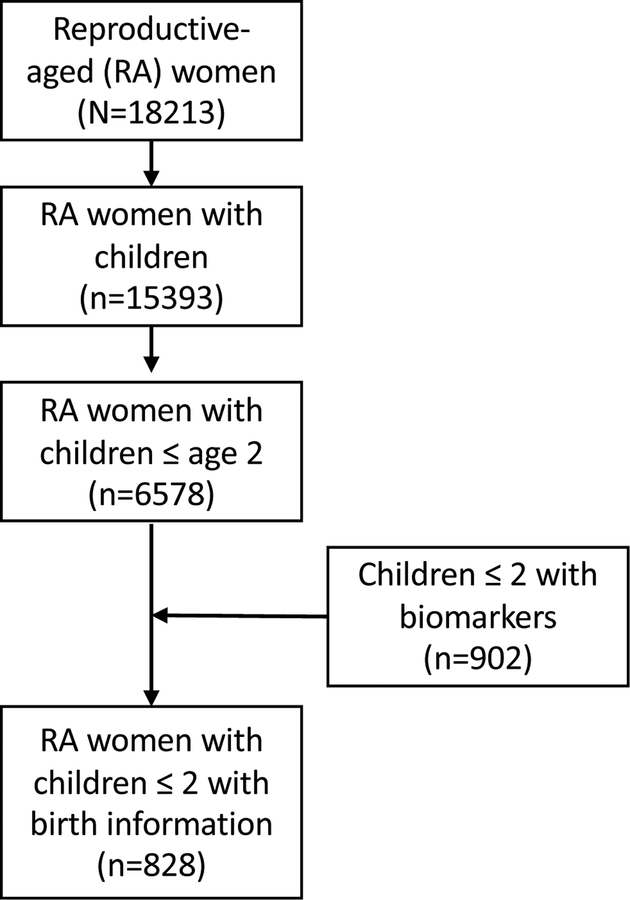
Data come from the Encuesta Nacional de Salud y Nutrición (ENSANUT-ECU) conducted in 201239 and are publicly available at: http://www.ecuadorencifras.gob.ec/category/ensanut/. ENSANUT-ECU collected data from a nationally-representative sample of over 87,000 Ecuadorians (0.6% of the total population), aged 0–59 years, and analyzed biomarkers in a subsample of 21,249 participants, aged 6 months to 59 years. Participants were selected using a multistage, stratified sampling design based on rural/urban residence, region, and province. Twelve households were identified per census tract and, within each household, one individual in each age group of interest (<5, 10–19, and 20–59 years) and one woman of reproductive age were selected to participate. The full sample includes 18,213 women of reproductive age, 15,393 of whom had a child and 6578 with children aged 2 years of younger. 902 children aged two or younger had biomarkers collected and of these 828 could be matched to mothers with complete birth information. Only one child under the age of 5 was measured per household, so the current analytic sample includes 828 mothers and their infants aged 6 to 24 months, with biomarker measures and birth histories (Figure 1). Secondary analyses were approved by the University of North Carolina at Chapel Hill Institutional Review Board.
Figure 1:
Analytic sample of mothers and infants participating in ENSANUT
Birth histories:
Delivery type, vaginal vs. Caesarean, was reported by mothers. No distinction was made between emergency and planned Caesarean, but mothers were asked whether they had contractions prior to delivery. This variable is used in sensitivity testing (described below) to indicate whether the Caesarean delivery was planned.
Immune measures:
Venous blood samples were collected in the participants’ homes by trained phlebotomists. 5.5 ml of blood was collected from infants aged 6 months or older and young children and divided between EDTA coated tubes (for hematological analysis) and tubes with lithium heparin (for C-reactive protein and other biochemical markers). Samples were analyzed at a central, internationally-accredited laboratory, Netlab S.A., in Quito, Ecuador (International Organization for Standardization: 15189). Immune cell counts were measured using automated fluorescent flow cytometry (Sysmex XE-2100). The proportion of five leukocyte types (neutrophils, lymphocytes, eosinophils, monocytes and basophils) were assessed to measure various aspects of immune function. Standard pediatric cut-points were used to dichotomize counts into normal vs. elevated.40 High levels were defined as: neutrophils >45%, lymphocytes > 76%, monocytes> 6%, eosinophils> 3% and basophils >1%. Serum was analyzed for C-reactive protein (CRP) after being spun at 3500 rpm for 15 minutes using automated nephelometry (Roche/Hitachi Modular EvoP-800 system). For this analysis, CRP over 2mg/L was considered elevated for infants and young children following previous studies.41,42
Morbidity:
Infant morbidity was assessed through maternal report. Infants were considered ill if they had exhibited symptoms of diarrhea or respiratory infection (cough, runny nose, difficulty breathing, sore throat or flu) in the past 2 weeks.
Covariates:
In addition to the birth and health measures, ENSANUT collected numerous household, maternal and infant characteristics during the household surveys.39 Birthweight was recorded from the participating infants’ child health card. Mothers were asked whether they had ever breastfed their infant and the age at which they stopped breast feeding. For this analysis, children are classified as still breastfed if they received any breastmilk at the time of the interview. Mothers were asked the number of children they had given birth to including the index child. Economic status was measured at the household level from an index summarizing household characteristics and assets. The score was normally distributed and divided into quintiles for analysis.
Statistical methods
Descriptive statistics (t-tests and chi-square tests) were used to test for differences in infant, maternal and household characteristics by delivery type, vaginal vs Caesarean. Because of concerns with the validity of odds ratios for assessing risk in cross-sectional studies with outcomes of varying prevalence,43,44 multilevel Poisson regression with robust variance was used to assess prevalence risk ratios in immune measures (CRP, morbidity and elevated immune cell types) by delivery mode. All models controlled for infant age, current breastfeeding, infant sex, economic quintile, and region (urban vs. rural) and clustering by province. Education and ethnicity were highly correlated with income and region and were not included in the final model. The immune biomarker models (elevated CRP and immune cell types) adjust for illness in the past two weeks to control for elevations due to recent illness. To provide a proxy for planned Caesarean delivery, which may carry greater risk for altered immune development, two sensitivity analyses were conducted. First, I tested the final adjusted models in the subsample of women who did not experience contractions prior to giving birth by Caesarean. Next, I tested the final model in the subsample of multiparous women. Vaginal birth after Caesarean (VBAC) is not a common practice in Ecuador so Caesarean delivery in multiparous women may be more likely to represent a planned Caesarean delivery.45 All analyses were conducted by using Stata 14 (StataCorp, College Station, TX). Statistical significance was set at p<0.05.
Results
Nearly 41% of the sample infants were born by Caesarean section. Mothers delivering vaginally or by Caesarean differed in a number of sociodemographic characteristics (Table 1). Those delivering by Caesarean were slightly older, more highly educated, non-indigenous, more urban, and of a higher income quintile. Fewer differences were seen in infant characteristics by delivery type. No differences were seen in birthweight, the prevalence of low birth weight, or breastfeeding initiation. However, infants born by Caesarean were more likely to be preterm (defined as birth before 9 months) and have a shorter duration of breastfeeding.
Table 1:
Sample Characteristics by Delivery Type
| Sample (N=828) |
Vaginal (N=490) |
Caesarean (N=338) |
|
|---|---|---|---|
| Mean(SD)/%(n) | |||
| Maternal characteristics | |||
| Maternal age, years | 26.6 (6.7) | 26.0 (6.6) | 27.1 (6.7)* |
| Maternal education, %secondary | 68.5 (614) | 66.1 (324) | 76.3 (258)** |
| Maternal ethnicity, %mestizo | 80.3 (719) | 79.8 (391) | 86.1 (291)*** |
| % indigenous | 11.3 (101) | 12.5 (61) | 3.9 (13)*** |
| Income, % bottom quintile | 30.0 (269) | 29.4 (144) | 24.0 (81)*** |
| % top quintile | 11.6 (104) | 8.0 (39) | 18.1 (61)*** |
| Region, %urban | 60.7 (544) | 58.6 (287) | 70.7 (239)*** |
| Marital status, %unmarried | 22.8 (204) | 21.6 (106) | 25.7 (87) |
| Parity, %primiparous | 37.9 (312) | 37.1 (125) | 38.4 (187) |
| Infant characteristics | |||
| Infant sex, %male | 51.2 (424) | 49.4 (242) | 53.9 (182) |
| Infant age at survey, mo | 15.7 (6.0) | 16.0 (6.1) | 15.3 (5.8) |
| Preterm, % yesa | 10.5 (94) | 6.5 (32) | 17.8 (60)*** |
| Birthweight, gm (n=572) | 3186 (532) | 3160 (551) | 3185 (491) |
| Low birthweight (n=572)b | 6 (35) | 6.7 (22) | 5.7 (13) |
| Breast feeding initiation, % yes | 98.4 (811) | 98.8 (481) | 97.9 (330) |
| Breast feeding duration, mo | 11.3 (5.4) | 11.8 (5.4) | 10.8 (5.4)** |
p<0.05,
p<0.01,
p<0.001 for difference between vaginally and Caesarean deliveries from chi-square for categorical variables or t-tests for continuous variables.
maternal report of birth prior to 9 months
birthweight <2500gm
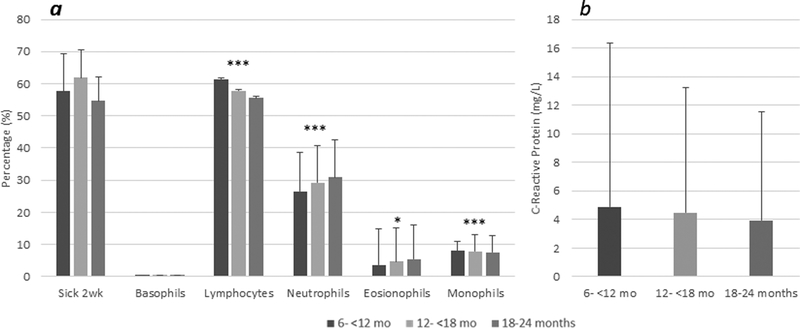
Across all age groups, the majority of infants and young children experienced infectious symptoms in the two weeks prior to the survey (Figure 2a). Leukocyte prevalence did vary significantly with age for four of the immune cell types: the percentage of lymphocytes decreased across age groups, the percentage of neutrophils and eosinophils increased across age groups, and the percentage of monocytes was highest in the middle age group (toddlers aged 12–18 months) compared to younger or older infants. Neither the mean level of CRP (Figure 2b) nor the prevalence of elevated CRP differed across the age groups. Similar age patterns were seen for vaginally and Caesarean delivered infants (data not shown).
Figure 2: Mean Morbidity Prevalence, C-Reactive Protein and Leukocyte Proportion by Age in Infants and Young Children.
Bars represent mean prevalence of morbidity and leukocyte proportions with standard deviation (A); Bars represent mean CRP level (mg/L) with standard deviation (B); *p<0.05, **p<0.01, ***p<0.001for trend by age group.
Several immune measures differed by delivery type in both crude and adjusted models (Table 2). Infants born by Caesarean were less likely to have elevated CRP but more likely to have experienced illness symptoms in the previous two weeks. Of the leukocyte types, the risk of having elevated basophils differed significantly by delivery mode with Caesarean delivered infants more likely to have elevated basophils. Among the covariates, few showed a consistent independent association with immune markers. Older infant and child age was associated with a greater risk of having high neutrophils but a lower risk of having high lymphocytes. Higher income was associated with a greater risk of having elevated basophils, which was significantly higher for infants and children in the middle quintile compared to the lowest quintile. Conversely, higher income, in the 4th and 5th quintile, was associated with a decreased risk for elevated eosinophils. As expected, experiencing illness symptoms in the past two weeks was an independent risk factor for having elevated CRP.
Table 2:
Unadjusted and adjusted associations between delivery mode and immune biomarkers and morbidity
| Elevated CRPa | Morbidityb | High lymphocytesc | High monocytes | High neutrophils | High basophils | High eosinophils | |
|---|---|---|---|---|---|---|---|
| RR [95% CI] | RR [95% CI] | RR [95% CI] | RR [95% CI] | RR [95% CI] | RR [95% CI] | RR [95% CI] | |
| Unadjusted model | |||||||
| Caesarean delivery | 0.77 | 1.43* | 1.07 | 1.19 | 0.94 | 1.99* | 0.88 |
| [0.59,1.01] | [1.05,1.94] | [0.55,2.08] | [0.84,1.68] | [0.55,1.60] | [1.12,3.53] | [0.65,1.20] | |
| Adjusted model | |||||||
| Caesarean delivery | 0.76* | 1.22* | 1 | 1.01 | 1.11 | 1.83* | 1 |
| [0.58,1.00] | [1.01,1.46] | [0.51,1.92] | [0.86,1.18] | [0.66,1.86] | [1.03,3.25] | [0.82,1.22] | |
| Sick past 2 weeksb | 1.50** | -- | 0.66 | 1.06 | 1.18 | 1.02 | 1.11 |
| [1.14,1.98] | -- | [0.35,1.21] | [0.90,1.24] | [0.71,1.95] | [0.57,1.80] | [0.91,1.34] | |
| Infant age, months | 0.98 | 0.99 | 0.90** | 0.99 | 1.07* | 0.99 | 1.02 |
| [0.96,1.01] | [0.97,1.01] | [0.84,0.97] | [0.98,1.01] | [1.01,1.12] | [0.94,1.05] | [1.00,1.04] | |
| Current breastfeeding, yes | 0.98 | 0.81 | 1.3 | 0.93 | 1.15 | 0.9 | 0.98 |
| [0.70,1.37] | [0.64,1.03] | [0.61,2.78] | [0.76,1.15] | [0.59,2.26] | [0.44,1.86] | [0.76,1.26] | |
| Infant sex, female | 0.8 | 0.98 | 0.87 | 0.9 | 0.84 | 1.03 | 0.89 |
| [0.68,1.13] | [0.82,1.17] | [0.47,1.60] | [0.77,1.05] | [0.52,1.36] | [0.60,1.79] | [0.74,1.07] | |
| Income <20th%ile | reference | reference | reference | reference | reference | reference | reference |
| 20–40th %ile | 1.05 | 0.95 | 1.19 | 1.04 | 1.67 | 1.3 | 0.92 |
| [0.74,1.48] | [0.74,1.23] | [0.43,3.31] | [0.83,1.30] | [0.91,3.09] | [0.50,3.38] | [0.71,1.18] | |
| 40–60th %ile | 0.87 | 0.90 | 1.92 | 1.03 | 0.92 | 2.64* | 0.94 |
| [0.60,1.27] | [0.69,1.17] | [0.76,4.84] | [0.82,1.29] | [0.44,1.91] | [1.13,6.18] | [0.72,1.22] | |
| 60–80th %ile | 0.91 | 0.81 | 1.63 | 1.1 | 0.43 | 1.32 | 0.72* |
| [0.60,1.39] | [0.61,1.09] | [0.55,4.82] | [0.86,1.41] | [0.16,1.20] | [0.48,3.61] | [0.53,0.99] | |
| >80th %ile | 0.80 | 0.79 | 2.56 | 1.07 | 0.67 | 1.42 | 0.63* |
| [0.57,1.48] | [0.57,1.10] | [0.90,7.34] | [0.81,1.42] | [0.25,1.78] | [0.49,4.12] | [0.43,0.92] | |
| Area, rural | 1.06 | 0.90 | 1.09 | 0.98 | 0.94 | 0.67 | 0.89 |
| [0.80, 1.40] | [0.74,1.10] | [0.54,2.20] | [0.82,1.16] |
p<0.05,
p<0.01,
p<0.001 from multilevel, adjusted (prevalence) risk ratio regression models with Poisson specification and clustering by province
defined as CRP >2mg/L
symptoms of diarrhea or respiratory infection (cough, runny nose, difficulty breathing, sore throat or flu) in the past 2 weeks
defined based on standard pediatric cut-points16 as: basophils >1%, lymphocytes >76%, neutrophils >45%, eosinophils>3% and monocytes >6%.
The results of the sensitivity analyses revealed no differences in the magnitude or direction of the association between delivery type and the immune or morbidity measures. Not all results remained significant, however, likely due to the smaller size of the subsamples (n=511 for primiparous mothers and n=585 for infants of mothers giving birth by Caesarean without experiencing contractions compared to vaginally-delivered infants).
Discussion
The results of the present study show that measures of morbidity and immune function differ by delivery type and that these differences are measurable across the first two years of life in Ecuadorian infants and young children. Such findings are important since over 40% of Ecuadorian infants are born by Caesarean39, a figure comparable to other Latin American countries32 and four to eight times higher than the WHO recommendation that 5–15% of infants be delivered by Caesarean section46. In the current study, infants born by Caesarean were less likely to have elevated CRP and were more likely to have been sick in the previous two weeks and to have elevated basophils. These differences were little attenuated by the inclusion of maternal and infant covariates or indicators of “emergency” vs. scheduled Caesarean. The findings support the importance of birth exposures in shaping the development and function of the immune system even in a context with prevalent breastfeeding and differing postnatal pathogenic exposures. Further, the finding that Caesarean delivery increases the risk of elevated basophils in infants and young children provides a potential mechanism linking delivery mode to the risk of allergy and asthma in infants and children born by Caesarean, further supporting a developmental origin for these inflammatory conditions.
Contrary to the hypotheses and previous studies focusing on cord blood or immune measures in neonates, few significant differences in leukocyte proportions were seen between vaginally vs. Caesarean delivered infants at 6 to 24 months of age. Previous research has documented that neonates born by Caesarean have lower proportions of neutrophils, monocytes, natural killer cells, T-cells, B-cells, and granulocytes compared to vaginally delivered neonates.19,26 These differences in cell types are enhanced or even exclusively seen in infants born by pre-labor Caesarean, suggesting that labor is an important process for immune activation. Research into the mechanisms linking delivery to immune development have suggested that contractions of the uterus and hypoxia during passage through the birth canal stimulate a stress response in the fetus that leads to catecholamine and cortisol production.21,22 In turn, these stress hormones redistribute and activate immune cells,23,26 serving as an adaptation to protect neonates against immediate infection in the external environment.13,20 This experience of labor and immune activation has been proposed to be “stored” in the immune system through memory T-cells and/or epigenetic modifications.13,21 However, few studies have examined whether these early differences in immune cell proportions persist after the first days of life. The current results suggest that, for the most part, these differences do not persist into infancy and early childhood among this sample of Ecuadorian children. However, other research has found persistent differences in the number of IgA and IgG-secreting cells in one-year old infants by delivery type47 and other research has found persistent effects of birthweight on immune cell counts in childhood through young adulthood,5,6 suggesting that further investigation of the impact of delivery practices on immune cell function across infancy and early childhood is warranted.
As hypothesized, Caesarean delivery was associated with an increased risk of having elevated basophils, a leukocyte that plays a role in acute and chronic allergic diseases, including asthma and atopic dermatitis, and plays a role in the inflammatory response.48 Infants delivered by Caesarean were almost twice as likely to have elevated basophils even after adjustment for maternal and infant characteristics. Previous research into early life risk factors for asthma and allergic disease has documented that infants and young children with a higher number of eosinophil/basophil progenitor cells in their cord blood have a greater Th2 response (an elevation in the T-helper cells that produce interleukins that, in turn, promote immunoglobulin-E (IgE) and eosinophil response to atopy) and increased risk of respiratory symptoms, wheezing and bronchitis, in the first two years of life.49 While the study did not look at delivery directly, its results support the importance of perinatal exposures on the development of inflammatory conditions. This current finding that basophils are elevated in Caesarean delivered infants across early life provides a potential mechanism for the documented association between Caesarean delivery and the later development of allergic diseases.50
Rates of Caesarean delivery and allergy and asthma are increasing concurrently in LMICs, leading some researchers to propose that changing delivery practices in part underlie the increasing prevalence of atopic disease.20,34,47 Linked to the “hygiene hypothesis,” the lack of exposure to maternal vaginal and fecal microbiota that accompany Caesarean delivery has been proposed to lead to a more pro-inflammatory composition of the gut and a dysregulation in immune cell development.25,51 However, much of the work linking Caesarean delivery and later health comes from high income countries, where postnatal exposures differ considerably from those of LMIC where infants and children may experience significant microbial exposures beyond those transmitted by their mothers during birth.35 In contrast to higher income countries where Caesarean delivery is associated with lower rates of breastfeeding,38 Caesarean delivery in this study was not associated with lower initiation of breastfeeding. While Caesarean delivered infants were breastfed for a significantly shorter duration, breastfeeding duration was over 10 months for both vaginally and Caesarean delivered infants in the current sample. This exposure to breastmilk in both vaginally and Caesarean delivered infants may have attenuated some of the expected differences in specific and innate immune cell proportions. Given the long duration of breastfeeding in this sample, the one-month average difference in breastfeeding duration between vaginally and Caesarean delivered infants is unlikely to have a significant biological effect. Previous studies suggest that early exposure to breastmilk in the first month to six months of life may be the most important in shaping leukocyte development52,53. Exclusive breastfeeding in early infancy is known to modify immune cell proportions, with lower monocytes, neutrophils, and cytokine production seen in exclusively breastfed infants.53 These differences, which may stem from the passive immune protection infants receive from their mothers during breastfeeding and/or the reduced pathogen exposure from contaminated non-breastmilk substances during breastfeeding, may mitigate some of the impacts of Caesarean delivery on immune development.
Along with these differences in breastfeeding after Caesarean delivery, postnatal pathogenic exposures also differ in LMIC.35,54 In the present study, rates of morbidity were high; over half of infants experienced at least one respiratory or gastrointestinal symptom in the two weeks prior to measurement. Caesarean-born infants were significantly more likely to have illness symptoms even after adjustment for maternal and infant characteristics associated with greater exposure or vulnerability to illness. Interestingly, while Caesarean delivery was associated with higher morbidity, infants born by Caesarean had significantly lower risk of inflammation measured by CRP. This contrasting result suggests that the immune response of Caesarean delivered infants may be dampened. Previous research has shown that inflammatory cytokines, including IL-I, TNF-a, IL-6 and IL-12,11,19,27 and CRP are lower in neonates born by planned Caesareans compared to those born by emergency Caesarean delivery or vaginal delivery,29 though other studies have found no difference when all Caesarean deliveries are compared to vaginal deliveries.28 Such altered cytokine production could negatively impact the inflammatory response to infectious diseases.11 While the persistence of these effects beyond the first days of life is not well-described, the results of this analysis provide preliminary evidence that Caesarean delivery may influence the inflammatory response to illness across the first two years of life. Importantly, these findings suggest that Caesarean delivery may dampen the immune response to infection even in an environment with persistent pathogenic exposures. This study is among the first to examine the association between delivery mode and immune development and function across infancy and early childhood in a large, population-based study in a LMIC. Several cell types assessed through flow cytometry, a biomarker of inflammation, and maternal reports of morbidity were examined, while controlling for a number of maternal and infant characteristics that could confound the relationship between delivery type and later immune function. Given the high prevalence of Caesarean delivery and very low prevalence of VBAC in Latin American countries like Ecuador,45 this sample likely has less selectivity in Caesareans (i.e. due to maternal obesity, pre-existing maternal health conditions, etc.) than samples from countries with lower rates of planned Caesarean delivery. The sensitivity analysis found few differences in the association between immune markers and delivery in primiparous vs. multiparous mothers, lending support to a lack of selectivity in Caesarean delivery.
Despite these strengths, this study has several important limitations. Delivery type was collected by maternal recall and does not distinguish planned vs. emergency Caesareans. Sensitivity analysis limiting the sample to women delivering by Caesarean who did not report feeling contractions showed few differences from the full model but this sample size is quite small (n=405). Further, since the data were collected retrospectively 6 months to 2 years after birth, the current analysis is not able to fully control for the factors that may contribute to selection into vaginal vs. Caesarean delivery or other factors that may confound the association between birth characteristics and immune measurement at ages 6–24 months. While our results may be influenced by residual confounding, the associations between delivery mode and immune markers showed little attenuation when the available maternal or infant characteristics were included in the model. Indeed, Caesareans were more likely in women who were wealthier, more urban, or non-indigenous. Since these mothers may have infants with fewer pathogenic exposures, the results may underestimate the contribution of Caesarean delivery to altered immune function. Nonetheless, adjustment for these factors had little effect on the magnitude of the associations in the analyses. Additionally, concerns have been raised with the analyzer used in this study for measuring basophilia in pediatric populations.55 The “flag” function for elevated levels overestimates the percentage of infants with high basophils. However, validation studies find good agreement between the measured cell counts and those done manually in children older than 3 months.56 This analysis used only the measured cell counts, not the “flags” and created variables based on standard pediatric cut-points for elevation. Importantly, these cut-points have been derived from high-income, healthy pediatric populations, and further work is needed to establish their appropriateness for use in other ecological or epidemiological contexts. While study results should be considered preliminary, the wide range of “normal” values for leukocyte counts, the use of proportions vs. absolute counts in the current study, which show fewer differences across populations,57,58 and the similar patterns of leukocyte change with age seen in the current study compared to previous research,59 lend support to the current findings.
Conclusion
The findings of the current study suggest that differences in the very early exposures that accompany Caesarean and vaginal delivery may be associated with altered immune function, particularly in the inflammatory response to infection and in the proportion of cells involved in the allergic response. Further, these differences persist across the first 6 to 24 months of life in infants and young children growing up in an environment with different postnatal exposures than those that generally characterize research into the association between delivery and later health outcomes. The higher morbidity, lower inflammation, and elevated basophils seen in Ecuadorian infants and young children born by Caesarean highlight the need to understand the pathways linking early life exposures to immune development and the potential long-term health effects of birth interventions. Future research should focus on identifying the environmental factors that may interact with delivery mode to shape immune development and activation across the life cycle. With rates of Caesarean deliveries and inflammatory conditions increasing in LMIC, additional work is needed to fully elucidate these pathways and to understand the exposures that influence immune development in a broader range of contexts. These findings contribute a potential mechanism, differences in leukocyte counts and inflammatory response across infancy and early childhood, to the growing body of literature documenting the importance of both perinatal factors and postnatal environmental exposures for shaping immune development and risk of immune-related disease. Thus, further investigation into the causes and consequences of variation in leukocyte populations and inflammatory markers may be an important avenue of research for understanding the developmental origins of health and disease.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Footnotes
Potential Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose.
Ethical Standards
The author asserts that all procedures contributing to this work comply with the ethical standards of the Belmont Report and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional review committee of the University of North Carolina at Chapel Hill.
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose
References
- 1.McDade TW, Beck MA, Kuzawa C, Adair LS. Prenatal undernutrition, postnatal environments, and antibody response to vaccination in adolescence. Am J Clin Nutr. 2001;74(4):543–548. [DOI] [PubMed] [Google Scholar]
- 2.McDade TW, Beck MA, Kuzawa CW, Adair LS. Prenatal undernutrition and postnatal growth are associated with adolescent thymic function. J Nutr. 2001;131(4):1225–1231. [DOI] [PubMed] [Google Scholar]
- 3.McDade TW, Kuzawa CW, Adair LS, Beck MA. Prenatal and early postnatal environments are significant predictors of total immunoglobulin E concentration in Filipino adolescents. Clin Exp Allergy. 2004;34(1):44–50. [DOI] [PubMed] [Google Scholar]
- 4.McDade TW, Rutherford J, Adair L, Kuzawa CW. Early origins of inflammation: microbial exposures in infancy predict lower levels of C-reactive protein in adulthood. Proc Biol Sci. 2010;277(1684):1129–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen W, Srinivasan SR, Berenson GS. Influence of birth weight on white blood cell count in biracial (black-white) children, adolescents, and young adults: the Bogalusa Heart Study. Am J Epidemiol. 2009;169(2):214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDade TW, Jones MJ, Miller G, Borja J, Kobor MS, Kuzawa CW. Birth weight and postnatal microbial exposures predict the distribution of peripheral blood leukocyte subsets in young adults in the Philippines. J Dev Orig Health Dis. 2018;9(2):198–207. [DOI] [PubMed] [Google Scholar]
- 7.Rautava S, Ruuskanen O, Ouwehand A, Salminen S, Isolauri E. The hygiene hypothesis of atopic disease - An extended version. J Pediatr Gastr Nutr. 2004;38(4):378–388. [DOI] [PubMed] [Google Scholar]
- 8.Rook GA, Lowry CA, Raison CL. Microbial ‘Old Friends’, immunoregulation and stress resilience. Evol Med Public Health. 2013;2013(1):46–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299(6710):1259–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol. 2009;7(12):887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gollwitzer ES, Marsland BJ. Impact of Early-Life Exposures on Immune Maturation and Susceptibility to Disease. Trends Immunol. 2015;36(11):684–696. [DOI] [PubMed] [Google Scholar]
- 12.Kristensen K, Henriksen L. Cesarean section and disease associated with immune function. J Allergy Clin Immunol. 2016;137(2):587–590. [DOI] [PubMed] [Google Scholar]
- 13.Romero R, Korzeniewski SJ. Are infants born by elective cesarean delivery without labor at risk for developing immune disorders later in life? Am J Obstet Gynecol. 2013;208(4):243–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bager P, Simonsen J, Nielsen NM, Frisch M. Cesarean section and offspring’s risk of inflammatory bowel disease: a national cohort study. Inflamm Bowel Dis. 2012;18(5):857–862. [DOI] [PubMed] [Google Scholar]
- 15.Cardwell CR, Stene LC, Joner G, et al. Caesarean section is associated with an increased risk of childhood-onset type 1 diabetes mellitus: a meta-analysis of observational studies. Diabetologia. 2008;51(5):726–735. [DOI] [PubMed] [Google Scholar]
- 16.Leung JY, Li AM, Leung GM, Schooling CM. Mode of delivery and childhood hospitalizations for asthma and other wheezing disorders. Clin Exp Allergy. 2015;45(6):1109–1117. [DOI] [PubMed] [Google Scholar]
- 17.Sevelsted A, Stokholm J, Bonnelykke K, Bisgaard H. Cesarean section and chronic immune disorders. Pediatrics. 2015;135(1):e92–98. [DOI] [PubMed] [Google Scholar]
- 18.Thavagnanam S, Fleming J, Bromley A, Shields MD, Cardwell CR. A meta-analysis of the association between Caesarean section and childhood asthma. Clin Exp Allergy. 2008;38(4):629–633. [DOI] [PubMed] [Google Scholar]
- 19.Thysen AH, Larsen JM, Rasmussen MA, et al. Prelabor cesarean section bypasses natural immune cell maturation. J Allergy Clin Immunol. 2015;136(4):1123–1125 e1126. [DOI] [PubMed] [Google Scholar]
- 20.Cho CE, Norman M. Cesarean section and development of the immune system in the offspring. Am J Obstet Gynecol. 2013;208(4):249–254. [DOI] [PubMed] [Google Scholar]
- 21.Dahlen HG, Downe S, Wright ML, Kennedy HP, Taylor JY. Childbirth and consequent atopic disease: emerging evidence on epigenetic effects based on the hygiene and EPIIC hypotheses. BMC Pregnancy Childbirth. 2016;16:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisler G, Hjertberg R, Lagercrantz H. Mimicking the stress of being naturally born improves the neonatal outcome after elective Caesarean section. Pediatric Research. 1998;44:442. [Google Scholar]
- 23.Duijts L, Bakker-Jonges LE, Labout JA, et al. Perinatal stress influences lymphocyte subset counts in neonates. The generation R study. Pediatr Res. 2008;63(3):292–298. [DOI] [PubMed] [Google Scholar]
- 24.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107(26):11971–11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neu J, Rushing J. Cesarean versus vaginal delivery: long-term infant outcomes and the hygiene hypothesis. Clin Perinatol. 2011;38(2):321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Almanzar G, Schonlaub J, Hammerer-Lercher A, Koppelstaetter C, Bernhard D, Prelog M. Influence of the delivery modus on subpopulations and replication of lymphocytes in mothers and newborns. Early Hum Dev. 2015;91(12):663–670. [DOI] [PubMed] [Google Scholar]
- 27.Weinberger B, Vetrano AM, Syed K, et al. Influence of labor on neonatal neutrophil apoptosis, and inflammatory activity. Pediatr Res. 2007;61(5 Pt 1):572–577. [DOI] [PubMed] [Google Scholar]
- 28.Kaapa P, Koistinen E. Maternal and neonatal C-reactive protein after interventions during delivery. Acta Obstet Gynecol Scand. 1993;72(7):543–546. [DOI] [PubMed] [Google Scholar]
- 29.Logan CA, Thiel L, Bornemann R, et al. Delivery Mode, Duration of Labor, and Cord Blood Adiponectin, Leptin, and C-Reactive Protein: Results of the Population-Based Ulm Birth Cohort Studies. PLoS One. 2016;11(2):e0149918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carrillo-Larco RM, Miranda JJ, Bernabé-Ortiz A. Delivery by caesarean section and risk of childhood obesity: analysis of a Peruvian prospective cohort. PeerJ. 2015;3:e1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veile A, Kramer KL. Childhood body mass is positively associated with cesarean birth in Yucatec Maya subsistence farmers. Am J Hum Biol. 2017;29(2). [DOI] [PubMed] [Google Scholar]
- 32.Betran AP, Ye J, Moller AB, Zhang J, Gulmezoglu AM, Torloni MR. The Increasing Trend in Caesarean Section Rates: Global, Regional and National Estimates: 1990–2014. PLoS One. 2016;11(2):e0148343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pearce N, Ait-Khaled N, Beasley R, et al. Worldwide trends in the prevalence of asthma symptoms: phase III of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax. 2007;62(9):758–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lavin T, Franklin P, Preen DB. Association between Caesarean Delivery and Childhood Asthma in India and Vietnam. Paediatr Perinat Epidemiol. 2017;31(1):47–54. [DOI] [PubMed] [Google Scholar]
- 35.Nathan AM, de Bruyne J, Khalid F, Arumugam K. Caesarean section and asthma in Malaysian children: a case-control study. Asian Pac J Allergy Immunol. 2012;30(3):204–208. [PubMed] [Google Scholar]
- 36.Al-Kubaisy W, Ali SH, Al-Thamiri D. Risk factors for asthma among primary school children in Baghdad, Iraq. Saudi Med J. 2005;26(3):460–466. [PubMed] [Google Scholar]
- 37.Garcia-Marcos L, Mallol J, Sole D, Brand PL, Group ES. International study of wheezing in infants: risk factors in affluent and non-affluent countries during the first year of life. Pediatr Allergy Immunol. 2010;21(5):878–888. [DOI] [PubMed] [Google Scholar]
- 38.Prior E, Santhakumaran S, Gale C, Philipps LH, Modi N, Hyde MJ. Breastfeeding after cesarean delivery: a systematic review and meta-analysis of world literature. Am J Clin Nutr. 2012;95(5):1113–1135. [DOI] [PubMed] [Google Scholar]
- 39.Freire WB, Belmont P, Rivas-Marino G, et al. TOMO ii Encuesta Nacional de Salud y Nutricion. Salud Sexual y Reproductiva. Quito, Ecuador: Ministerio de Salud Publica/Instituto Nacional de Estadistica y Censos;2015. [Google Scholar]
- 40.Orkin S, Nathan D, Ginsburg D, Look A, Fisher D, Lux S. Nathan and Oski’s Hematology of Infancy and Childhood. 7th ed Philadelphoa: Saunders Elsevier; 2009. [Google Scholar]
- 41.Thompson AL, Houck KM, Adair L, et al. Pathogenic and obesogenic factors associated with inflammation in Chinese children, adolescents and adults. Am J Hum Biol. 2014;26(1):18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wander K, Brindle E, O’Connor KA. Sensitivity and specificity of C-reactive protein and alpha(1) -acid glycoprotein for episodes of acute infection among children in Kilimanjaro, Tanzania. Am J Hum Biol. 2012;24(4):565–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greenland S Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case-control studies. Am J Epidemiol. 2004;160(4):301–305. [DOI] [PubMed] [Google Scholar]
- 44.Spiegelman D, Hertzmark E. Easy SAS Calculations for RIsk or Prevalence Ratios and Differences. Am J Epidemiol. 2005;162:199–200. [DOI] [PubMed] [Google Scholar]
- 45.Magne F, Puchi Silva A, Carvajal B, Gotteland M. The Elevated Rate of Cesarean Section and Its Contribution to Non-Communicable Chronic Diseases in Latin America: The Growing Involvement of the Microbiota. Front Pediatr. 2017;5:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.(WHO) WHO. WHO Statement on Caesarean Section Rates. 2015. [Google Scholar]
- 47.Huurre A, Kalliomaki M, Rautava S, Rinne M, Salminen S, Isolauri E. Mode of delivery - effects on gut microbiota and humoral immunity. Neonatology. 2008;93(4):236–240. [DOI] [PubMed] [Google Scholar]
- 48.Mukai K, Galli S. Basophils In: eLS. Chichester: John Wiley and Sons, Ltd; 2013. [Google Scholar]
- 49.Junge KM, Hornig F, Herberth G, et al. The LINA cohort: Cord blood eosinophil/basophil progenitors predict respiratory outcomes in early infancy. Clin Immunol. 2014;152(1–2):68–76. [DOI] [PubMed] [Google Scholar]
- 50.Almqvist C, Oberg AS. The association between caesarean section and asthma or allergic disease continues to challenge. Acta Paediatr. 2014;103(4):349–351. [DOI] [PubMed] [Google Scholar]
- 51.Collado MC, Cernada M, Bauerl C, Vento M, Perez-Martinez G. Microbial ecology and host-microbiota interactions during early life stages. Gut Microbes. 2012;3(4):352–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andersson Y, Hammarstrom ML, Lonnerdal B, Graverholt G, Falt H, Hernell O. Formula feeding skews immune cell composition toward adaptive immunity compared to breastfeeding. J Immunol. 2009;183(7):4322–4328. [DOI] [PubMed] [Google Scholar]
- 53.Belderbos ME, Houben ML, van Bleek GM, et al. Breastfeeding modulates neonatal innate immune responses: a prospective birth cohort study. Pediatr Allergy Immunol. 2012;23(1):65–74. [DOI] [PubMed] [Google Scholar]
- 54.Bueso A, Figueroa M, Cousin L, et al. Poverty-associated risk factors for wheezing in the first year of life in Honduras and El Salvador. Allergol Immunopathol (Madr). 2010;38(4):203–212. [DOI] [PubMed] [Google Scholar]
- 55.Jacomo RH, Lozano VF, da Cunha Neto JG, Costa SS. What’s the meaning of basophilia in Sysmex XE-2100? Arch Pathol Lab Med. 2011;135(4):415. [DOI] [PubMed] [Google Scholar]
- 56.Becker PH, Fenneteau O, Da Costa L. Performance evaluation of the Sysmex XN-1000 hematology analyzer in assessment of the white blood cell count differential in pediatric specimens. Int J Lab Hematol. 2016;38(1):54–63. [DOI] [PubMed] [Google Scholar]
- 57.Lee BW, Yap HK, Chew FT, et al. Age- and sex-related changes in lymphocyte subpopulations of healthy Asian subjects: from birth to adulthood. Cytometry. 1996;26(1):8–15. [DOI] [PubMed] [Google Scholar]
- 58.Lugada ES, Mermin J, Kaharuza F, et al. Population-based hematologic and immunologic reference values for a healthy Ugandan population. Clin Diagn Lab Immunol. 2004;11(1):29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Georgountzou A, Papadopoulos NG. Postnatal Innate Immune Development: From Birth to Adulthood. Front Immunol. 2017;8:957. [DOI] [PMC free article] [PubMed] [Google Scholar]