Abstract
This review examines the role of brain macrophages, i.e. perivascular macrophages and microglia, as a potential viral reservoir in antiretroviral (ART) treated, SIV-infected macaques. The role, if any, of latent viral reservoirs of HIV and SIV in the central nervous system (CNS) during ART suppression is an unresolved issue. HIV and SIV infect both CD4+ lymphocytes and myeloid cells in blood and tissues during acute and chronic infection. HIV spread to the brain occurs during acute infection by the infiltration of activated CD4+ lymphocytes and monocytes from blood and is established in both embryonically derived resident microglia and monocyte-derived perivascular macrophages. ART controls viral replication in peripheral blood and cerebrospinal fluid in HIV-infected individuals but does not directly eliminate infected cells in blood, tissues, or brain. Latently infected resting CD4+ lymphocytes in blood and lymphoid tissues are a well-recognized viral reservoir that can rebound once ART is withdrawn. In contrast, CNS resident microglia and perivascular macrophages in brain have not been examined as potential reservoirs for HIV during suppressive ART. Macrophages in tissues are long-lived cells that are HIV and SIV infected in tissues such as gut, lung, spleen, lymph node and brain and contribute to ongoing inflammation in tissues. However, their potential role in viral persistence and latency or their potential to rebound in the absence ART has not been examined. It has been shown that measurement of HIV latency by HIV DNA PCR in CD4+ lymphocytes overestimate the size of the latent reservoirs of HIV that contribute to rebound i.e. cells containing the genomes of replicative viruses. Thus, the quantitative viral outgrowth assay (QVOA) has been used as a reliable measure of the number of latent cells that harbor infectious viral DNA and, may constitute a functional latent reservoir. Using QVOAs specifically designed to quantitate latently infected CD4+ lymphocytes and myeloid cells in an SIV macaque model, we demonstrated that macrophages in brain harbor SIV genomes that reactivate and produce infectious virus in this assay, demonstrating that these cells have the potential to be a reservoir.
Introduction
During the early AIDS epidemic, HIV was shown to infect both CD4+ lymphocytes and myeloid cells in tissues such as lymph nodes and gut while only myeloid cells and astrocytes were infected in brain, leading to neurologic disease and dementia [1, 2]. A measure of HIV replication in the central nervous system (CNS) could be provided by quantitation of viral particles in cerebrospinal fluid (CSF) [3-5]. HIV in the CSF was shown to be directly associated with the development of neurologic diseases called AIDS Dementia Complex (ADC), which was an important cause of mortality in HIV-infected individuals [1, 2]. Although HIV enters the CNS during acute infection, ADC manifested mainly during later stages of infection, when patients were immunosuppressed [6].
In the era of ART, fully suppressed HIV-infected individuals control virus replication in blood. Consequently, the occurrence of systemic immunosuppression and ADC has been greatly diminished by treatment. However, a significant number of HIV+ suppressed individuals have been diagnosed with HIV-associated neurocognitive disorders (HAND) [7], which is associated with subtle neurocognitive symptoms that may elude diagnosis and are thought to be a consequence of ongoing activation in brain [7-9]. As effectiveness of ART is measured by reduction of systemic viremia, the presence of virus in the CSF in the absence of detectable HIV in plasma suggests that ongoing, low level replication in brain may occur during ART [2 ] [10].
ART prevents the spread of HIV by controlling viral replication, but does not eliminate HIV DNA from tissues. The functional latent reservoir, i.e. the cells that harbor replication competent HIV genomes is recognized as a major barrier to HIV eradication. The primary focus of both HIV latency and cure research has been CD4+ lymphocytes (CD4+T) and the formation of long-lived CD4+ T cell reservoirs [1]. Conversely, the roles of infected monocytes in blood, and macrophages in peripheral tissues and brain as latent reservoirs of HIV have not been addressed. SIV-infected macaques treated with suppressive ART regimens, similar to the ART regimens used in humans, provide the opportunity to determine whether the resident microglia and perivascular macrophages in brain constitute a reservoir that would impact the occurrence of HAND and HIV eradication approaches.
HIV infection is characterized by a dramatic depletion of CD4+ lymphocytes but, despite depletion of these cells, high plasma viral load persists, suggesting that viral replication is occurring in cells other than CD4+ lymphocytes. In the SIV macaque models, experimental depletion of CD4+ lymphocytes results in an increase in viral load and in vivo selection of macrophage-tropic SIV phenotypes and high incidence of SIV encephalitis [11-13], as brain inflammation (encephalitis) is directly associated with the infection of macrophages [14, 15]. In addition, activation of monocytes and macrophages during ART suppression is associated with higher morbidity [16-18] and HIV- and SIV-infected macrophages are not efficiently eliminated by CD8+ lymphocytes, unlike infected CD4+ lymphocytes [19, 20]. It has been shown that resident tissue macrophages infected with HIV or SIV have the potential to divide and expand viral reservoirs in tissues [21]. Resident long-lived tissue macrophages are relatively resistant to the cytopathic effects of HIV infection compared to CD4+ lymphocytes and may serve as stable viral reservoirs. In this review, published studies from our laboratory and others will be used to assess the role of macrophages in brain as potential latent reservoirs.
SIV Macaque Models of AIDS
AIDS emerged as a new disease in 1981 and the causative agent was identified as identified and called HTLV-III or LAV and the virus was subsequently identified as a lentivirus and re-named the Human Immunodeficiency (HIV) virus in 1983 [22-25] [26-28]. A similar disease was identified in 1983 in a colony of rhesus macaques at the New England Primate Center. The disease was characterized by opportunistic infections similar to those in HIV infected individuals and called “acquired immunodeficiency syndrome” [29, 30]. In 1985 the isolation and characterization of the agent that caused this disease in captive macaques was reported by two groups and the virus, similar to HTLV-III, was initially named STLV-III and later the Simian Immunodeficiency Virus (SIV) [31] [32]. SIVmac239 and SIVmac251, two of the most commonly used SIV strains in current SIV macaque studies, were isolated from individual macaques at the New England Primate Center [33]. The pathologic features of SIV infection of rhesus macaques included CD4+ lymphocytes depletion and immunologic impairment as well as the development of encephalitis in 60% of the infected animals. Encephalitis was characterized by the infiltration of macrophages and multinucleate giant cells that contained SIV particles [34] [35].
The initial isolation of SIVmac239 from macaques led to the characterization of multiple isolates of SIV strains from rhesus macaques at primate centers and variants of SIVmac239 were isolated containing amino acids changes in the envelope gene that conferred different cellular tropism in vitro as well as in their in vivo tropism and pathogenesis [36]. The role of the envelope gene in the cellular tropism of SIV and HIV led to the development of SIV/HIV hybrid viruses (SHIV) containing the envelope of HIV in the genome of SIVmac251 and other SIV isolates. A comprehensive review comparing the pathogenesis of HIV, SIV and SHIV viruses compares the molecular and biologic similarities and differences in these viruses and the role of the SIV and SHIV viruses in models of HIV disease [37].
SIV macaque models, designed using a number of SIV virus strains and molecular clones as well as both rhesus and pigtailed macaques, comprehensively reproduce the immunodeficiency symptoms observed in HIV-infected individuals, i.e. infection of CD4+ lymphocytes and the development of AIDS [38]. In addition, SIVmac251, other SIVmac strains and SIV/HIV hybrid viruses (SHIV) infect monocytes in blood, and macrophages in lymph nodes, lung, and in brain [13, 38-43].
Our laboratory developed and comprehensively evaluated a SIV pigtailed macaque model that caused accelerated AIDS-like disease as well as encephalitis in three months that was highly reproducible; the model was used to examine the viral and immunologic events in vivo that caused systemic immunosuppression and encephalitis [15, 44, 45]. This model used a SIVmac239-based clonal virus (SIV17E-Fr) developed by in vivo passage of SIVmac239 in rhesus macaques [10, 46]. SIV17E-FR is a molecular clone that is macrophage-tropic in vitro and in vivo [15, 47]. In addition to SIV17E-FR, a second virus swarm, SIVdeltaB670 was used to infect pigtail macaques [44, 45]. This combination of SIV viruses caused an accelerated infection leading to an AIDS-like immunosuppression and encephalitis in 3 months of infection of rhesus macaques [45]. Infection of macaques with SIV17E-Fr and the SIV SIVdeltaB670 viral swarm, recapitulated AIDS in humans with rapid depletion of CD4+ lymphocytes in blood, infection in CNS during acute infection, development of encephalitis [44, 45]. Longitudinal virus replication in plasma and CSF demonstrated that the course of infection was similar, albeit accelerated, to that observed in most HIV infected individuals. To investigate infection in peripheral tissues and brain, animals were euthanized during acute, chronic infection, and end-stage disease. Among our findings we demonstrated that infection in tissues, including the brain, occurred as early as 4 days post-inoculation (p.i.) and that CSF viral load reflects the level of virus replication in the brain [44, 48, 49].
In the era of ART in HIV infected individual, our laboratory published the first suppressive combination antiretroviral regimen to fully suppress virus replication in the plasma and CSF in this SIV macaque model of AIDS and CNS infection and encephalitis the development of fully suppressive ART regimens for SIV infected macaques [50]. ART suppression of SIV-infected macaques was reported in 2009-2010 in our SIV dual-infected pigtail macaque model and in other SIVmac239 rhesus macaque models [50-52]. The SIVmac239 and also SIVmac251 models demonstrated decline of virus in the plasma and the dual-infected model demonstrated decay of virus in both plasma and CSF [50-52]. We have also published multiple ART regimens that fully suppressed viral load to undetectable levels in the plasma and CSF in the SIV dual-inoculated macaque model suppressed. Further, the kinetics of viral decay for both plasma and CSF were similar to those observed in HIV-infected individuals treated with ART [50]. There exists an extensive literature addressing the frequency of HIV infection and latency in CD4+ lymphocytes in ART suppressed individuals, but a rigorous analysis using similar sensitive assays had not been applied to ART suppressed SIV macaque models. To address this discrepancy between HIV and SIV latency research, our group developed an SIV resting CD4+ lymphocytes quantitative viral outgrowth assay (QVOA) analogous to that applied in HIV research [50]. Using this assay, we measured the frequency of resting CD4+ lymphocytes harboring replication competent SIV latent genomes in blood and multiple lymph nodes and demonstrated that the frequencies of latently infected resting CD4+ lymphocytes in these compartments were similar to those in ART suppressed HIV-infected patients [50]. These studies provided validation for the SIV dual-inoculated macaque model despite the rapid development of AIDS and CNS disease, ART reduced viral replication with the same kinetics measured in HIV individuals, and the frequency of CD4+ lymphocytes functionally latent reservoirs was similar to ART-treated HIV infected individuals (one cell per million CD4+ lymphocytes) [50].
In the era of ART and HIV eradication, measuring reservoirs should include a comprehensive evaluation of all latently infected cells that may contribute to viral rebound after cessation of ART. Initial trials evaluating HIV eradication strategies have focused on viral load in plasma and levels of viral RNA and DNA in PBMCs as indication of HIV reactivation or change in the latent reservoir. However, there is evidence from the “Boston Patients” that virus rebound occurred not only in the blood but also in the brain [53]. In the SIV dual-inoculated model, SIV DNA persists in the CNS despite undetectable levels of cellular SIV RNA in brain or CSF [52], suggesting that transcriptionally silent, latently infected macrophages persist in the brain during ART suppression. Additionally, recent evidence suggests that latent SIV genomes located in brain may be modulated differently in response to latency reversing agents (LRA) [54]. Because the mechanisms that drive latency in macrophages appear to be distinct from those in CD4+ lymphocytes [55-58], we used our SIV dual-inoculated model to address the role of infection and potential latency in tissue macrophages in ART suppressed macaques.
Reactivation of SIV in ART Suppressed Macaques: Evidence of a Functional Viral Reservoir in Brain Macrophages
In this review article, we will focus on studies in the SIV dual-inoculated macaque model, which recapitulates both AIDS and CNS infection, for evidence of SIV latency of SIV in microglia and perivascular macrophages. In previous articles, we assessed the contribution of brain macrophages in SIV latency and the potential for reactivation during HIV eradication interventions [54]. SIV-infected macaques were suppressed with ART for over 18 months and two synergistic LRAs, the protein kinase C activator ingenol-B and the histone deacetylase inhibitor vorinostat, were tested for the ability to activate latent reservoirs in vivo. We had shown that ingenol-B reactivated HIV genomes in two different in vitro HIV- latency models as well as in ex vivo CD4+ lymphocytes isolated from HIV infected individuals [59]. In our in vivo study, one of the LRA-treated macaques had a significant increase in viral load in the CSF that was 10-fold higher than the levels measured in the plasma. Phylogenetic analyses demonstrated that genetically distinct viruses were found in the plasma and CSF, suggesting that reactivation occurred independently in the CNS and peripheral tissues [54]. Since only one out of two macaques treated with the LRA demonstrated viral rebound in plasma or CSF, the direct cause of viral rebound is unclear. However, the data does support other studies in HIV and SIV that there are genetically distinct viruses in the CSF and blood. Further, the data support the role of a functional latent reservoir of SIV, and potentially HIV, in the CNS despite long-term ART suppression, and shows that these reservoirs can reactivate. This study is the first in vivo demonstration that brain macrophages have the potential to be another viral reservoir [54].
Development of a Mancrophage Quantitative Viral Outgrowth Assay to Measure Latently Infected Brain Macrophages in ART Suppressed SIV Macaques
HIV infection in macrophages in the CNS has been examined in a number of studies by quantitating viral DNA in brain of ART-treated patients by PCR and in situ hybridization [60-65] demonstrating that HIV persists in the brain despite ART. However, the presence of HIV DNA indicates the persistence of viral genomes but does not provide a measure of how much of the DNA reflects intact genomes that can reactivate and produce replication competent viruses. In HIV Art suppressed individuals CD4+ lymphocytes quantitation of viral DNA overestimates the number of productively infected CD4+ lymphocytes by as much as 500-fold due to the presence of a large proportion of defective proviruses in vivo [66]. To understand whether macrophages in tissue, particularly the brain, harbor DNA that is replication competent and to quantify the frequency of replication competent virus in infected macrophages compared to CD4+ lymphocytes, our laboratory developed a QVOA for myeloid cells (MΦ-QVOA), i.e. monocytes and resident macrophages [60, 67],
Development of the MΦ-QVOA required additional steps to isolate cells from tissues, and specific cell isolation methods were needed for each of the studies organs [60, 67]. Microglia and perivascular macrophages (referred to as brain macrophages) were isolated through enzymatic digestion with trypsin and then enriched using anti-CD11b magnetic bead-tagged antibodies [60, 67]. After adhering to the plates, macrophages were lightly treated with a trypsin solution to dislodge and eliminate CD4+ lymphocytes that could potential compromise the assay specificity. To evaluate the potential contribution of lymphocytes to virus detected in macrophage cultures, the number of CD3+ lymphocytes was assessed by measuring TCRβ RNA in control wells for each MΦ-QVOA assay [60, 67]. Based on these published studies, the probability of having infected CD4+ lymphocytes in a MΦ-QVOA well ranged from 0 to 2% of the input cells [60].
Similar to the CD4+ lymphocyte QVOA, the MΦ-QVOA involves serial dilution of the selected cells, in this case CD11b+ myeloid cells and the frequency of infectious virus per million (IUPM) was calculated using limiting dilution statistical analyses [68]. In contrast to CD4+ lymphocytes, however, macrophages do not divide exponentially in culture and the release of viral particles throughout the experiment could at times be less robust than that observed in the wells of CD4+ lymphocyte QVOAs. Therefore, in MΦ-QVOA, supernatants from each well were collected and assessed by qPCR at multiple time points from 4-14 days of cultivation [67].
To validate this assay, we first examined the number of SIV-infected myeloid and CD4+ lymphocytes in blood and tissues of untreated SIV dual-inoculated macaques [60]. We demonstrated that a significant number of monocytes in blood and macrophages in spleen harbored SIV DNA, with values comparable to the levels of infected CD4+ lymphocytes in the same compartments. Infected tissue resident macrophages were quantified in spleen, lung and brain (424, 32, 231 IUPM, respectively). Monocytes in blood had a frequency of infection of 31 infected cells per million compared to 206 CD4+ lymphocytes per million [60]. These studies demonstrated that, during SIV infection, monocytes and macrophages harbored infectious virus that had the potential to become latent during ART.
In another published study, we evaluated whether ART suppressed macaques harbored a functional latent viral reservoir in brain macrophages capable of producing infectious virus [67]. Using dual-inoculated SIV macaque model with ART suppression, plasma and CSF were analyzed longitudinally. In one group of macaques (G1), virus was suppressed in both compartments for 100-400 days before animals were euthanized for post mortem evaluation. Another group of macaques (G2) was suppressed for more than 500 days and were used for the in vivo assessment of the LRA combination described above [67]. Viral DNA was detected in the brain tissue of all the macaques, but viral RNA was undetectable in fully suppressed macaques, except for the animal that was treated with LRA and presented virus in the CSF [67]. The results from the MΦ-QVOA demonstrated that all but one of the suppressed macaques had latently infected brain macrophages [67], with a frequency of latently infected cells varying from 0.11 to 7.36 IUPM (Figure 1). The frequency of productively infected brain macrophages measured by QVOA in the SIV infected ART-treated macaques was significantly lower than those from untreated SIV-infected animals (IUPM median of 0.268 vs. 231, p<0.0001) (Figure 1), and no significant difference was observed in the frequency of latent brain macrophages due to the duration of ART treatment. This demonstrated that brain macrophages were productively infected in vivo prior to ART and that post-ART suppression latent SIV that can be reactivated persisted in these cells that can be reactivated.
Figure 1.
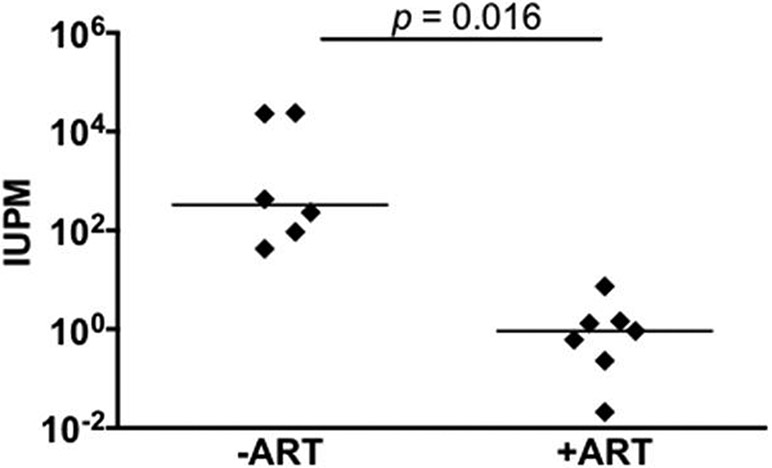
Quantitation of infected and latently infected brain macrophages in SIV infected macaques with and without ART by MΦ-QVOA. Quantitation of infected brain macrophages from ART-treated macaques (32, 36). The horizontal black line represents the median IUPM values. The MΦ QVOA results from SIV-infected animals with and without ART have been reported (32, 36). Significance was determined by Mann-Whitney nonparametric t test; a P of <0.05 was considered significant. This figure was reprinted from Avalos et al.,mBio.01186-17, 2017 according to ASM open access policies.
In the studies discussed in this review, suppressed macaques had fewer than 10 copies of SIV DNA per million brain cells, measured by digital droplet PCR. In most other assays or studies, which use real time qPCR, this level of SIV DNA containing cells would be considered undetectable or below the limit of quantification. These data support the hypothesis that low levels of SIV DNA in brain of ART suppressed SIV macaques reflect a functional latent viral reservoir that can produce virus when reactivated. Thus, the quantitation of SIV DNA or RNA by qPCR in brain tissue does not fully reflect the size of the latent functional reservoir that is the main target in eradication strategies.
The brain of most animals in this study harbored latently infected macrophages in regions that contained no detectable or quantifiable viral RNA. Three long-termed suppressed macaques showed no viral RNA in basal ganglia and parietal cortex but had replication competent virus produced in the isolated brain macrophages isolated during the QVOAs. In addition to studying the dual-inoculated SIV macaque model, unpublished studies from our laboratory in ART suppressed SIVmac251-infected rhesus macaques demonstrate that animals without measurable SIV RNA in brain also had latently infected brain macrophages that produced virus at a frequency of 0.35 to 13.4 per million cells (under review), providing evidence that brain macrophages harbor functional latent SIV in two different macaque models.
This review highlights studies that demonstrate that brain macrophages in ART suppressed macaques harbor replication competent virus at a frequency similar to CD4+ lymphocytes and indicates that the highly debated question of SIV latency in macrophages, at least in brain, has been addressed in ART suppressed SIV-infected macaques [67]. The presence of latent replication-competent virus in the brain of SIV infected ART suppressed macaques could explain the occurrence of ongoing macrophage activation in both SIV-infected macaques and HIV-infected individuals suppressed on ART. Recent studies have suggested that, while virus does not spread during ART suppression, there is ongoing stochastic activation of virus genomes in latently infected cells [69, 70]. Reactivation of virus in the macrophage without spread in the macrophage is likely to induce innate immune responses and cellular activation. Thus, productively infected latent macrophages in brain provide a mechanism for continuous inflammation during HIV infection in a fully suppressed individual. Additionally, it has been recently demonstrated that defective provirus expressed in rCD4 lymphocytes is recognized by adaptive immune responses, shaping the proviral landscape {Pollack, 2017 #103}. It is possible that similar responses might occur with viral proteins generated from defective proviruses in brain macrophages.
Discussion
The presence of a long-term functional reservoir in the brain of ART suppressed SIV-infected macaques, which parallels the biologic and pathologic features of infected individuals with HAND, suggests that HIV in the brain is a barrier for strategies designed to eliminate latent reservoirs. Such strategies should consider whether: a) ART regimens used in each study population sufficiently penetrate the CNS to protect the brain during virus reactivation with LRAs; b) the eradication approaches also penetrate or act in the CNS; and c) release of LRA-induced viral proteins or particles in the brain will trigger harmful inflammatory responses. In addition, the current focus on the “deep reservoir,” i.e. using approaches that further suppress HIV latency, should consider whether such strategies include the cells in brain. The presence of low levels of replication competent viral DNA in brain of ART suppressed macaques can contribute to virus spread in the CNS and potentially in the periphery during cessation of ART or in the presence of ineffective ART with eradication regimens. Since the CNS is protected from peripheral effects by the blood brain barrier, many therapeutic approaches to suppress or decrease the latent reservoir in the periphery may not be efficacious in the brain.
Strategies that include activation of virus in brain may have the adverse effects and lead to an increase in inflammation and neuronal toxicity due to increased macrophage activation and production of cytokines, as we observed in a suppressed macaque treated with two cycles of LRAs [54]. Our studies demonstrating the presence of a functional latent reservoir in brain macrophages have major implications for SIV latency studies used to model treatment for HIV-infected individuals. Since most current studies in humans only measure rebound of virus in plasma and not CSF, these studies are likely to overlook a source of virus that significantly contributes to treatment outcomes, potentially leading to additional CNS damage and reinfection of cells in both the brain and the peripheral blood.
Acknowledgements
These studies were funded by NIH awards R01NS089482, R01NS077869, U2OD0131117, R01NS055651, R56AI118753, R01AI127142, P01MH070306, P01AI131306, U42OD13117 and the Johns Hopkins University Center for AIDS Research P30AI094189.
Anti-retroviral compounds for these studies were kindly donated by Gilead, ViiV Healthcare, Bristol-Meyers Squibb, Merck, Abbvie, Janssen, and Roche. These studies were supported by the excellent technical staff in the Retrovirus Lab at Johns Hopkins.
References
- 1.Pierson T, McArthur J, and Siliciano RF, Reservoirs for HIV-1: mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy. Annu Rev Immunol, 2000. 18: p. 665–708. [DOI] [PubMed] [Google Scholar]
- 2.Spudich SS, et al. , Cerebrospinal fluid HIV infection and pleocytosis: relation to systemic infection and antiretroviral treatment. BMC Infect Dis, 2005. 5: p. 98. PMC1299327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw GM, et al. , HTLV-III infection in brains of children and adults with AIDS encephalopathy. Science, 1985. 227(4683): p. 177–82. [DOI] [PubMed] [Google Scholar]
- 4.Ho DD, et al. , Isolation of HTLV-III from cerebrospinal fluid and neural tissues of patients with neurologic syndromes related to the acquired immunodeficiency syndrome. N Engl J Med, 1985. 313(24): p. 1493–7. [DOI] [PubMed] [Google Scholar]
- 5.Chiodi F, et al. , Isolation frequency of human immunodeficiency virus from cerebrospinal fluid and blood of patients with varying severity of HIV infection. AIDS Res Hum Retroviruses, 1988. 4(5): p. 351–8. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy PG, Neurological complications of human immunodeficiency virus infection. Postgrad Med J, 1988. 64(749): p. 180–7. PMC2428834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolson D, Neurologic Complications in Persons With HIV Infection in the Era of Antiretroviral Therapy. Top Antivir Med, 2017. 25(3): p. 97–101. PMC5935214. [PMC free article] [PubMed] [Google Scholar]
- 8.Nath A, Neurologic Complications of Human Immunodeficiency Virus Infection. Continuum (Minneap Minn), 2015. 21(6 Neuroinfectious Disease): p. 1557–76. [DOI] [PubMed] [Google Scholar]
- 9.Valcour V, et al. , Pathogenesis of HIV in the central nervous system. Curr HIV/AIDS Rep, 2011. 8(1): p. 54–61. PMC3035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson MG, et al. , Analysis of envelope changes acquired by SIVmac239 during neuroadaption in rhesus macaques. Virology, 1993. 195(2): p. 616–26. [DOI] [PubMed] [Google Scholar]
- 11.Ortiz AM, et al. , Depletion of CD4(+) T cells abrogates post-peak decline of viremia in SIV-infected rhesus macaques. J Clin Invest, 2011. 121(11): p. 4433–45. PMC3204830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francella N, et al. , Decreased plasticity of coreceptor use by CD4-independent SIV Envs that emerge in vivo. Retrovirology, 2013. 10: p. 133. PMC3833851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Igarashi T, et al. , The emergence and characterization of macrophage-tropic SIV/HIV chimeric viruses (SHIVs) present in CD4+ T cell-depleted rhesus monkeys. J Leukoc Biol, 2003. 74(5): p. 772–80. [DOI] [PubMed] [Google Scholar]
- 14.Mankowski JL, et al. , Pathogenesis of simian immunodeficiency virus pneumonia: an immunopathological response to virus. Am J Pathol, 1998. 153(4): p. 1123–30. PMC1853060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mankowski JL, et al. , Pathogenesis of simian immunodeficiency virus encephalitis: viral determinants of neurovirulence. J Virol, 1997. 71(8): p. 6055–60. PMC191864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hearps AC, et al. , Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell, 2012. 11(5): p. 867–75. [DOI] [PubMed] [Google Scholar]
- 17.McArthur JC, et al. , Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol, 2010. 67(6): p. 699–714. [DOI] [PubMed] [Google Scholar]
- 18.Burdo TH, Lackner A, and Williams KC, Monocyte/macrophages and their role in HIV neuropathogenesis. Immunol Rev, 2013. 254(1): p. 102–13. PMC3704190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rainho JN, et al. , Nef Is Dispensable for Resistance of Simian Immunodeficiency Virus-Infected Macrophages to CD8+ T Cell Killing. J Virol, 2015. 89(20): p. 10625–36. PMC4580207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vojnov L, et al. , The majority of freshly sorted simian immunodeficiency virus (SIV)-specific CD8(+) T cells cannot suppress viral replication in SIV-infected macrophages. J Virol, 2012. 86(8): p. 4682–7. PMC3318662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenkins SJ, et al. , Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science, 2011. 332(6035): p. 1284–8. PMC3128495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottlieb MS, et al. , Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med, 1981. 305(24): p. 1425–31. [DOI] [PubMed] [Google Scholar]
- 23.Siegal FP, et al. , Severe acquired immunodeficiency in male homosexuals, manifested by chronic perianal ulcerative herpes simplex lesions. N Engl J Med, 1981. 305(24): p. 1439–44. [DOI] [PubMed] [Google Scholar]
- 24.Barre-Sinoussi F, et al. , Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science, 1983. 220(4599): p. 868–71. [DOI] [PubMed] [Google Scholar]
- 25.Coffin J, et al. , What to call the AIDS virus? Nature, 1986. 321(6065): p. 10. [DOI] [PubMed] [Google Scholar]
- 26.Gonda MA, et al. , Heteroduplex mapping in the molecular analysis of the human T-cell leukemia (lymphotropic) viruses. Cancer Res, 1985. 45(9 Suppl): p. 4553s–4558s. [PubMed] [Google Scholar]
- 27.Gonda MA, et al. , Sequence homology and morphologic similarity of HTLV-III and visna virus, a pathogenic lentivirus. Science, 1985. 227(4683): p. 173–7. [DOI] [PubMed] [Google Scholar]
- 28.Gonda MA, et al. , Human T-cell lymphotropic virus type III shares sequence homology with a family of pathogenic lentiviruses. Proc Natl Acad Sci U S A, 1986. 83(11): p. 4007–11. PMC323654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Letvin NL, et al. , Acquired immunodeficiency syndrome in a colony of macaque monkeys. Proc Natl Acad Sci U S A, 1983. 80(9): p. 2718–22. PMC393899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunt RD, et al. , Transmission of naturally occurring lymphoma in macaque monkeys. Proc Natl Acad Sci U S A, 1983. 80(16): p. 5085–9. PMC384193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daniel MD, et al. , Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science, 1985. 228(4704): p. 1201–4. [DOI] [PubMed] [Google Scholar]
- 32.Letvin NL, et al. , Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science, 1985. 230(4721): p. 71–3. [DOI] [PubMed] [Google Scholar]
- 33.Kestler HW 3rd, et al. , Comparison of simian immunodeficiency virus isolates. Nature, 1988. 331(6157): p. 619–22. [DOI] [PubMed] [Google Scholar]
- 34.Ringler DJ, et al. , Simian immunodeficiency virus-induced meningoencephalitis: natural history and retrospective study. Ann Neurol, 1988. 23 Suppl: p. S101–7. [DOI] [PubMed] [Google Scholar]
- 35.Sharer LR, et al. , Comparison of simian immunodeficiency virus and human immunodeficiency virus encephalitides in the immature host. Ann Neurol, 1988. 23 Suppl: p. S108–12. [DOI] [PubMed] [Google Scholar]
- 36.Borda JT, et al. , Cell tropism of simian immunodeficiency virus in culture is not predictive of in vivo tropism or pathogenesis. Am J Pathol, 2004. 165(6): p. 2111–22. PMC1618703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lackner AA and Veazey RS, Current concepts in AIDS pathogenesis: insights from the SIV/macaque model. Annu Rev Med, 2007. 58: p. 461–76. [DOI] [PubMed] [Google Scholar]
- 38.Kumar N, Chahroudi A, and Silvestri G, Animal models to achieve an HIV cure. Curr Opin HIV AIDS, 2016. 11(4): p. 432–41. PMC4922307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams K, Lackner A, and Mallard J, Non-human primate models of SIV infection and CNS neuropathology. Curr Opin Virol, 2016. 19: p. 92–8. PMC5021597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Igarashi T, et al. , Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): Implications for HIV-1 infections of humans. Proc Natl Acad Sci U S A, 2001. 98(2): p. 658–63. PMC14644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luciw PA, et al. , Persistent infection of rhesus macaques with T-cell-line-tropic and macrophage-tropic clones of simian/human immunodeficiency viruses (SHIV). Proc Natl Acad Sci U S A, 1995. 92(16): p. 7490–4. PMC41365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sui Y, et al. , Microarray analysis of cytokine and chemokine genes in the brains of macaques with SHIV-encephalitis. J Med Primatol, 2003. 32(4–5): p. 229–39. [DOI] [PubMed] [Google Scholar]
- 43.Miller CJ, et al. , In vivo replication capacity rather than in vitro macrophage tropism predicts efficiency of vaginal transmission of simian immunodeficiency virus or simian/human immunodeficiency virus in rhesus macaques. J Virol, 1998. 72(4): p. 3248–58. PMC109796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zink MC, et al. , High viral load in the cerebrospinal fluid and brain correlates with severity of simian immunodeficiency virus encephalitis. J Virol, 1999. 73(12): p. 10480–8. PMC113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clements JE, et al. , The central nervous system as a reservoir for simian immunodeficiency virus (SIV): steady-state levels of SIV DNA in brain from acute through asymptomatic infection. J Infect Dis, 2002. 186(7): p. 905–13. [DOI] [PubMed] [Google Scholar]
- 46.Sharma DP, et al. , Derivation of neurotropic simian immunodeficiency virus from exclusively lymphocytetropic parental virus: pathogenesis of infection in macaques. J Virol, 1992. 66(6): p. 3550–6. PMC241136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flaherty MT, et al. , Molecular and biological characterization of a neurovirulent molecular clone of simian immunodeficiency virus. J Virol, 1997. 71(8): p. 5790–8. PMC191833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Witwer KW, et al. , Coordinated regulation of SIV replication and immune responses in the CNS. PLoS One, 2009. 4(12): p. e8129. PMC2790080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eden A, et al. , HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis, 2010. 202(12): p. 1819–25. PMC3052942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dinoso JB, et al. , A simian immunodeficiency virus-infected macaque model to study viral reservoirs that persist during highly active antiretroviral therapy. J Virol, 2009. 83(18): p. 9247–57. PMC2738256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.North TW, et al. , Viral sanctuaries during highly active antiretroviral therapy in a nonhuman primate model for AIDS. J Virol, 2010. 84(6): p. 2913–22. PMC2826073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zink MC, et al. , Simian immunodeficiency virus-infected macaques treated with highly active antiretroviral therapy have reduced central nervous system viral replication and inflammation but persistence of viral DNA. J Infect Dis, 2010. 202(1): p. 161–70. PMC2880623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Henrich TJ, et al. , Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann Intern Med, 2014. 161(5): p. 319–27. PMC4236912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gama L, et al. , Reactivation of simian immunodeficiency virus reservoirs in the brain of virally suppressed macaques. AIDS, 2017. 31(1): p. 5–14. PMC5131686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barber SA, et al. , Mechanism for the establishment of transcriptional HIV latency in the brain in a simian immunodeficiency virus-macaque model. J Infect Dis, 2006. 193(7): p. 963–70. [DOI] [PubMed] [Google Scholar]
- 56.Barber SA, et al. , Longitudinal analysis of simian immunodeficiency virus (SIV) replication in the lungs: compartmentalized regulation of SIV. J Infect Dis, 2006. 194(7): p. 931–8. [DOI] [PubMed] [Google Scholar]
- 57.Barber SA, et al. , Innate immune responses and control of acute simian immunodeficiency virus replication in the central nervous system. J Neurovirol, 2004. 10 Suppl 1: p. 15–20. [DOI] [PubMed] [Google Scholar]
- 58.Ravimohan S, et al. , Regulation of SIV mac 239 basal long terminal repeat activity and viral replication in macrophages: functional roles of two CCAAT/enhancer-binding protein beta sites in activation and interferon beta-mediated suppression. J Biol Chem, 2010. 285(4): p. 2258–73. PMC2807283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abreu CM, et al. , Dual role of novel ingenol derivatives from Euphorbia tirucalli in HIV replication: inhibition of de novo infection and activation of viral LTR. PLoS One, 2014. 9(5): p. e97257. PMC4020785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Avalos CR, et al. , Quantitation of Productively Infected Monocytes and Macrophages of Simian Immunodeficiency Virus-Infected Macaques. J Virol, 2016. 90(12): p. 5643–56. PMC4886778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gelman BB, et al. , Neurovirological correlation with HIV-associated neurocognitive disorders and encephalitis in a HAART-era cohort. J Acquir Immune Defic Syndr, 2013. 62(5): p. 487–95. PMC3664102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gray LR, et al. , Is the central nervous system a reservoir of HIV-1? Curr Opin HIV AIDS, 2014. 9(6): p. 552–8. PMC4215931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lamers SL, et al. , HIV DNA Is Frequently Present within Pathologic Tissues Evaluated at Autopsy from Combined Antiretroviral Therapy-Treated Patients with Undetectable Viral Loads. J Virol, 2016. 90(20): p. 8968–83. PMC5044815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ko A, et al. , Macrophages but not Astrocytes Harbor HIV DNA in the Brains of HIV-1-Infected Aviremic Individuals on Suppressive Antiretroviral Therapy. J Neuroimmune Pharmacol, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gelman BB, Endsley J, and Kolson D, When do models of NeuroAIDS faithfully imitate “the real thing”? J Neurovirol, 2018. 24(2): p. 146–155. PMC5910470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pollack RA, et al. , Defective HIV-1 Proviruses Are Expressed and Can Be Recognized by Cytotoxic T Lymphocytes, which Shape the Proviral Landscape. Cell Host Microbe, 2017. 21(4): p. 494–506 e4. PMC5433942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Avalos CR, et al. , Brain Macrophages in Simian Immunodeficiency Virus-Infected, Antiretroviral-Suppressed Macaques: a Functional Latent Reservoir. MBio, 2017. 8(4)PMC5559639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosenbloom DI, et al. , Designing and Interpreting Limiting Dilution Assays: General Principles and Applications to the Latent Reservoir for Human Immunodeficiency Virus-1. Open Forum Infect Dis, 2015. 2(4): p. ofv123. PMC4602119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weinberger LS, et al. , Stochastic gene expression in a lentiviral positive-feedback loop: HIV-1 Tat fluctuations drive phenotypic diversity. Cell, 2005. 122(2): p. 169–82. [DOI] [PubMed] [Google Scholar]
- 70.Dar RD, et al. , Screening for noise in gene expression identifies drug synergies. Science, 2014. 344(6190): p. 1392–6. PMC4122234. [DOI] [PMC free article] [PubMed] [Google Scholar]


