Abstract
Psychopharmacology really developed as a discipline from the mid-20th century with the discovery of a number of new classes of psychoactive drugs which could modify behaviour. These drugs were discovered as a consequence of clinical observations of patients, often being treated for other conditions. These serendipitous discoveries were the start of an era of drug development which has led to the antidepressants, antipsychotics, anxiolytics and mood stabilisers used today. Subsequent research focused on understanding why these drugs were effective, and used this information to develop a second generation of drugs that were more selective for their therapeutic targets, and therefore had reduced side effects and improved safety and tolerability. After a period of decline in new discoveries and withdrawal of the majority of the major pharmaceutical companies from active development programmes in psychiatry, new avenues are emerging fuelling renewed interest in this area.
Keywords: Psychiatry, antidepressants, antipsychotics, animal models, behaviour
Introduction
Psychopharmacology has seen huge advances in the last 50 years with the majority of currently licenced psychotropic drugs being developed since the 1950s. The era of contemporary psychopharmacology started with the discovery of a number of novel compounds which could specifically modify human behaviour (Carlsson, 1990; Hyman, 2013; Lopez-Munoz et al., 2012; Shorter, 2008). Throughout human history, there is evidence of people using psychoactive substances to alter their behaviour (Crocq, 2007; Moriarty et al., 1984). These drugs were most commonly used for their mind-altering effects or ability to induce feelings of pleasure. The hypnotic effects of different drugs have been understood by humanity throughout history with drugs such as alcohol and the opiates well known for their anxiolytic and sedative effects. In the late 19th century and into the early 20th century, drugs such as paraldehyde, chloral hydrate and bromides were being used as treatments for anxiety disorders; however, it was the discovery of the barbiturates in the early 20th century which probably saw the start of the development of psychopharmacology.
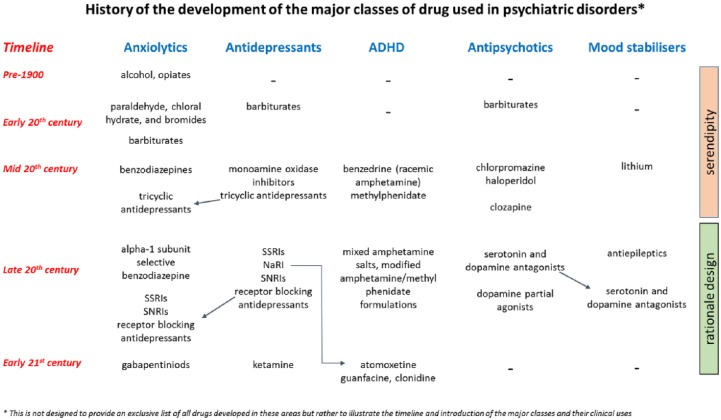
As illustrated in Figure 1, the development of drugs to treat specific psychiatric disorders really began in the 20th century. It is interesting to note, however, that the origins of all the major classes of drugs used to treat psychiatric disorders stem from clinical observations made when investigating treatments for usually unrelated conditions. In 1937, Dr Charles Bradley reported the beneficial effects of benzedrine (racemic amphetamine) in what we now describe as attention deficit hyperactivity disorder (ADHD). The drug was being given to children for severe headaches and Bradley observed that it improved their behaviour and school performance. This was the start of a long history of the use of stimulant drugs to treat ADHD (Mayes and Rafalovich, 2007). In 1949, John Cade worked out that lithium salts could cause sedation in guinea pigs and rapidly moved onto testing lithium in patients with mania. Advances in organic chemistry also facilitated the production of a wide range of novel compounds which were tested for their potential clinical use. The primary interest at that time was in the development of new anti-microbials, anti-parasitics or drugs which could be used to induce sedation or anaesthesia rather than treat psychiatric disorders. Newly synthesised compounds were then tested on animals to assess their effects and safety. The relatively limited legislation compared to today’s standards meant that many of these compounds progressed quickly into tests in human patients. As clinicians observed the effects of these drugs, they noted that some changed mood and behaviour. It is these early serendipitous discoveries which underpin all the major classes of drugs used in psychiatry today. The following chapter considers the role serendipity played in the discovery of the major types of treatment used in psychiatry today. There is a brief overview of all the major drug classes and then a more detailed discussion relating to the anxiolytics, antidepressants and antipsychotics. In relation to these classes of drugs, this article considers how psychopharmacology helped to unravel their biochemical targets and used this knowledge to support the rationale design of improved treatments. The final section looks to the future and considers where the next major developments in the treatment of psychiatric disorders may come from.
Figure 1.
Drugs have been used to modify behaviour throughout humanity; however, the clinical use of drugs for specific conditions really developed from the start of the 20th century. This overview illustrates the timeline associated with the major classes of drugs used to treat psychiatric disorders. Although defined by their therapeutic target, the figure also indicates (arrows) where the same drugs have multiple therapeutic indications. An alternative approach to classification of psychiatric drugs is based on their psychopharmacology. Referred to as ‘Neuroscience-Based Nomenclature’ (http://www.nbn2.com/), this initiative has recategorised these drug treatments based on their pharmacology and mode of action rather than their first therapeutic indication.
Discovery of the major therapeutic classes and their associated pharmacology
The discovery of all the major classes of psychoactive drugs used in psychiatry today preceded a detailed knowledge of their biochemical targets or mechanisms of action. The drugs also came into widespread clinical use at a time when very little was known about the biological basis of these behavioural disorders. In many ways, the discovery of these drugs has also provided the greatest insights into the relationship between chemical processes in the brain and behaviour. However, even today, our understanding of the causes of psychiatric disorders is limited and many patients fail to respond to current treatment. Technological advances over the last 50 years have enabled progress in our knowledge of the biochemical targets of psychoactive drugs, facilitating a rationale design strategy to refine their therapeutic effects while reducing side effects and improving safety and tolerability. The development of methods to characterise the receptor binding profile and intrinsic activity of these compounds, as well as their behavioural effects in rodent models (see Box 1), was critical in this process. In many cases, it is this knowledge that has facilitated the development of the next generation agents, which in themselves have helped further our understanding of their mechanisms of action. Although rational design has played a key role in the design and development of these new treatments, many have ended up with licences for indications in addition to, or instead of, their initial therapeutic target. For example, atomoxetine is a noradrenaline reuptake inhibitor that was initially evaluated as an antidepressant but is now licenced for the treatment of ADHD providing an important alternative to stimulant medications. It should also be noted that psychopharmacology is still broadly divided into drug classes based on their main therapeutic target despite the fact that many of the drugs used in psychiatry may be licenced for and used in different conditions (see Figure 1 and the neuroscience-based nomenclature, http://www.nbn2.com/). This is further illustrated for anxiolytics, antidepressants and antipsychotics in the following sections. Treatments for ADHD and bipolar disorder are summarised in Figure 1 but are not discussed in detail.
Box 1.
Role of animal models in psychopharmacology.
Anxiolytics
Probably the disorder for which humans have longest sought ‘treatment’ is anxiety with alcohol and opiates used to induce feelings of relaxation and calm. The origin of anxiolytic drugs was the barbiturates which were first synthesised in the early 20th century with diethyl-barbituric acid introduced for clinical use in 1904. The drug was the start of a new era of treatment for a wide range of psychiatric conditions and was also the first effective treatment for epilepsy. Acting through the GABAA (gamma-aminobutyric acid) receptor, this positive allosteric modulator could potentiate the effects of endogenous GABA to induce sedative and hypnotic effects making it an effective treatment for a range of psychiatric disorders. Patients with severe neuroses or psychoses were sometimes administered intravenous barbiturates which enabled them to benefit from psychotherapy (Lambert and Rees, 1944). It is interesting to consider how this may relate to the recent use of intravenous ketamine in treatment-resistant depression and the revisiting of the use of psychedelics in the treatment of a number of the more severe psychiatric disorders. The barbiturates enjoyed a long period of use with little competition, but the development of the safer benzodiazepines saw their use for anxiety and sleep disorders rapidly decline. The first benzodiazepine chlordiazepoxide was discovered serendipitously, but its potential to provide a safer alternative to the barbiturates saw Hoffmann-La Roche rapidly bringing it to market with structural analogues such as diazepam following closely behind (Wick, 2013). The rapid onset of action of their anxiolytic effects makes the benzodiazepines very useful for short-term management of anxiety-related disorders but they are also associated with dependence and tolerance and so their long-term use is restricted.
Both the barbiturates and benzodiazepines act through enhancing inhibitory transmission via the GABAA receptor but these receptors are widespread throughout the brain and are involved in many different functional roles. Attempts to develop more selective anxiolytics have principally followed two avenues. The first sought to apply a rationale design approach to identify and target GABAA receptor subunits which were involved in the modulation of anxiety but not sedation, muscle relaxation, or tolerance and dependence. The second was to identify novel mechanisms linked to the pathophysiology and target specific receptors which were identified as playing a role in anxiety-related behaviour.
The efficacy of the barbiturates and benzodiazepines and a more detailed understanding of the complexity of the GABAA receptor fuelled studies to try to develop more selective compounds. As molecular methods improved, evidence for many different subunits and subunit combinations associated with the GABA receptor complex became apparent. Extensive characterisation of these is still ongoing and beyond the scope of this discussion but may well deliver better anxiolytics in the future. Alpha1 subunit selective compounds have been developed on the basis that they would have lower abuse liability and drugs such as zolpidem show some preference in binding for the alpha1 subunit. They also have a short half-life and are generally only used as hypnotics although they do have anxiolytic properties.
Among the other targets, the serotonin system has been the most successful in terms of new treatments for anxiety and much of this has occurred alongside the development of the second-generation antidepressants (discussed in the next section). Serotonin is implicated in anxiety as well as major depression (Andrews et al., 2015; Deakin, 1998) although the exact role of this system in the aetiology of these conditions is not fully understood. Two of the many serotonin receptor subtypes found in the brain seem to be particularly important in affective processing: the 5-HT1A and 5-HT2A receptors (Carhart-Harris and Nutt, 2017). Two major groups of serotonergic anxiolytics have been developed: the azapirones which are 5-HT1A partial agonists and also enhance dopaminergic and noradrenergic function (Eison and Temple, 1986), and the serotonin-specific reuptake inhibitors (Nutt et al., 1999). Both of these drug classes offer a non-sedating anxiolytic without the risk for dependence and abuse. The azapirones have shown efficacy for generalised anxiety and buspirone has been used as an adjunct therapy in major depression although their clinical success has been limited and they are not widely prescribed today. As a detailed understanding of how 5-HT and its complex family of receptors interact with anxiety disorders is still not fully understood, the reasons why these drugs have failed to deliver is still not understood. One theory relates to the location of the 5-HT1A receptor and the fact that it is found in a post-synaptic location where agonism has a therapeutic effect but it is also a pre-synaptic autoreceptor where activation leads to reduced endogenous serotonin release. Although the azapirones are not widely used in the clinic and have largely been superseded by the selective serotonin reuptake inhibitors (SSRIs), it is interesting to note that the antipsychotic clozapine and two new antidepressants, vilazodone and vortioxetine, include partial 5-HT1A receptor agonism alongside their other pharmacological effects (Köhler et al., 2016). The SSRIs in contrast have rapidly become the first-line treatment for generalised anxiety disorder (Baldwin et al., 2014). Acting through inhibition of the serotonin transporter, these drugs alongside the mixed serotonin and noradrenaline reuptake inhibitors have shown efficacy in a variety of anxiety disorders. Unlike the drugs acting through the GABAergic system, their effects are non-sedating but also improve with time and clinical benefits are often delayed with a period of time needed between initially taking the medication and subjective changes in the patient’s perception of their symptoms (see further discussion below). The reasons for this are not yet fully understood with different hypotheses proposed including receptor adaptation, specifically with regard to the 5-HT1A and 5-HT2A receptor subtypes.
The final group of drugs which has recently become more widely used in the treatment of anxiety is the calcium channel modulating gabapentinoids of which pregabalin is the most commonly prescribed treatment for anxiety (Baldwin et al., 2013, 2014). Originally developed for the treatment of epilepsy and thought to be acting through a GABAergic mechanism, gabapentin and pregabalin are known to be anxiolytic with reduced adverse side effects compared to the SSRIs, for example, sexual dysfunction. However, evidence of their abuse liability and risks associated with co-administration with other drugs of abuse are a concern (Baldwin et al., 2013; Lyndon et al., 2017).
Antidepressants
Antidepressant effects of drugs were first seen unexpectedly in patients being treated for very different, non-psychiatric conditions. Among these, the first mood-improving drug was iproniazid, which was first used to treat tuberculosis (Berger and Barchas, 1977). The tricyclic antidepressants (TCAs) originate from the initial observations that chlorpromazine was an effective treatment for psychiatric patients, inducing a distinct type of sedation. Chemists seeking to develop related compounds synthesised the first antidepressant, imipramine, which was trialled in 1955 (Kuhn, 1958). Interestingly, its effects were observed after only a few days which appear somewhat in contradiction with our current view of the delayed onset of action of conventional antidepressants (Kuhn, 1958). Subsequent pharmacological studies revealed that both iproniazid and imipramine were able to increase monoamine transmitter levels in the brain through distinct mechanisms; iproniazid acting via inhibition of monoamine oxidase and imipramine acting via monoamine (noradrenaline and serotonin) reuptake inhibition (Axelrod, 1972; López-Muñoz and Alamo, 2009; Slattery et al., 2004). Over a similar era, the pharmacology of other drugs which affected mood was also pointing towards a central role for the monoamine transmitters – particularly noradrenaline and serotonin. For example, the antihypertensive drug, reserpine, was found to cause depression in patients which was attributed to its ability to deplete monoamine levels in the brain (Freis, 1954). This evidence was central to the monoamine hypothesis of depression, which proposes that depression arises due to a deficit in monoamine function and triggered a surge in interest in developing a second generation of antidepressant drugs (Heninger et al., 1996; Nutt, 2008; Schildkraut, 1965). The aim for chemists and pharmacologists was to develop novel drugs which could mimic the monoamine transmission-enhancing effects of first-generation antidepressants, but reduce the side-effect burden which arose from the extensive receptor-binding profiles of these early antidepressants. Perhaps, the most important development needed was an improvement in safety. The TCAs have a low therapeutic index meaning overdose is a major risk in this vulnerable population. The early irreversible monoamine oxidase inhibitors also suffered from drug and food interactions. In the 1990s, the rationally designed selective serotonin and/or noradrenaline reuptake inhibitors reached the market and have subsequently become some of the most widely prescribed drugs in the world (Cleare et al., 2015). Targeting the reuptake transporter selectively had achieved the initial objective of maintaining the effectiveness of the TCAs but with reduced side effects and improved safety and tolerability. As discussed previously, these drugs are now also the most common treatment for many anxiety disorders (Nutt et al., 1999).
The SSRIs and subsequent family of second-generation antidepressants have certainly improved the treatment of depression but they are not without side effects. Initial increases in anxiety, emotional blunting, gastrointestinal (GI) disturbance and sexual dysfunction are all linked to their effects on serotonin levels in the central nervous system (CNS) and periphery and while some do improve with time, there is still room for further improvements. Attempts to reduce these side effects, improve effectiveness and address concerns over the delayed onset of action have also led to a small number of newer antidepressants reaching the market (Carvalho et al., 2016). These have largely been drugs where multiple sites of action have been attributed to their clinical benefit, for example, multiple therapeutic mechanisms. For example, mirtazapine is a receptor-blocking antidepressant which blocks alpha-2 adrenoceptors, 5-HT2 and 5-HT3 receptors (Stimmel et al., 1997). This combination of pharmacological targets produces a more sophisticated neurochemical alteration. Alpha-2 adrenoceptor antagonism results in increased synaptic levels of both noradrenaline and serotonin, while the blockade of the 5-HT2 and 3 receptors helps to moderate some of the known 5-HT-mediated side effects and has the potential to enhance efficacy (Nutt, 1998; Stimmel et al., 1997). Agomelatine deviates from the more typical monoamine targets but is a 5-HT2 C antagonist alongside agonism at the melatonin receptors and therefore has less of the serotonergic side effects and is reported to have reduced emotional blunting (Carvalho et al., 2016; De Berardis et al., 2011). Other new antidepressants with mixed receptor and reuptake actions are vilazodone and vortioxetine (Köhler et al., 2016) which include 5-HT1A partial agonism.
The latest development in antidepressant therapy has been the use of intravenous infusions of low, sub-anaesthetic doses of ketamine (Machado-Vieira et al., 2009; Zarate et al., 2006). Exhibiting a very rapid and relatively long-lasting antidepressant effect, this non-competitive N-methyl-D-aspartate (NMDA) antagonist has further complicated the picture in terms of how antidepressants work but also sets the challenge to researchers to find similarly effective treatments but lacking the adverse effects associated with ketamine. There is also renewed interest in the use of psychedelics in the treatment of mood disorders (Carhart-Harris and Nutt, 2017) including new evidence about the effects of drugs such as the 5-HT2A agonist, psilocybin on brain activity in relevant regions (Carhart-Harris et al., 2017) and on emotional processing (Stroud et al., 2018). The serotonin releasing agent, 3,4-methylenedioxymethamphetamine (MDMA), has also been shown to modify emotional processing and affect brain activity in regions linked to major depressive disorder (MDD) suggesting it may be effective as an antidepressant (Carhart-Harris et al., 2014).
Many attempts to explain the actions of antidepressant drugs have been made but these still remain hypotheses, with basic research and clinical evidence both for and against. A lack of good biomarkers, or robust evidence for causality in humans, coupled with the limitations of animal models (see Box 1), makes the challenges of understanding the neurobiology of depression all the more difficult. Another key question comes in the mismatch between the rapid onset of biochemical changes induced by antidepressants and the much slower rate of onset of clinical efficacy. Attempts to explain this temporal inconsistency and to use this knowledge to develop new treatments have so far been largely unsuccessful. Hypotheses relating to receptor adaptation (Artigas et al., 1996; Blier, 2001; Charney et al., 1981), neurotrophic effects (Duman and Li, 2012; Duman et al., 1999; Sahay and Hen, 2007) and neuropsychological mechanisms (Harmer et al., 2017; Robinson and Roiser, 2016) have all been proposed and significant bodies of both clinical and preclinical evidence have been reported in their support. However, it remains unclear why people get depressed or how antidepressant drugs work to alleviate these symptoms making the generation of new antidepressants a challenge.
Antipsychotics
The first antipsychotic discovered was chlorpromazine which was noted for its ability to induce a calming effect that was distinct from the sedation seen with the barbiturate drugs (Courvoisier, 1956; López-Muñoz et al., 2005). Soon after the discovery of chlorpromazine, Paul Janssen and colleagues observed that a butyrophenone compound they had synthesised had similar effects in animal studies to chlorpromazine but was less potent (Granger and Albu, 2005; Janssen et al., 1960). Clinical trials of this new drug, haloperidol, found it to be very effective against the hallucinations and delusions in psychosis (Divry et al., 1958). These drugs were the foundation for the typical antipsychotics, with around 40 different compounds licenced between 1950 and 1975 (Shen, 1999). Similar to the story for the antidepressants, little was known about the mechanism of action of these drugs or why they were able to alleviate positive symptoms. Later studies, however, suggested that the efficacy of antipsychotics was related to their binding at D2 receptors (Seeman et al., 1976). Perhaps, one of the most redrawn figures in psychopharmacology textbooks, the correlation between affinity for the D2 receptor and clinical efficacy, has been a major factor in support for the dopamine hypothesis in schizophrenia (Seeman et al., 1976). Despite the ability of these drugs to provide powerful effects on behaviour and to modify psychotic symptoms, their actions were not specific. Side effects associated with dopamine receptor blockade in the nigrostriatal pathway, hormone effects and binding at other receptor sites led to a heavy side-effect burden.
The side-effect burden faced by patients treated with these antipsychotics was a concern, primarily arising from hormonal and extrapyramidal side effects, and there was a clear need for more effective treatments. However, it was not until 1990 that a new atypical antipsychotic was introduced. Clozapine had first been discovered in the 1950s when a researcher was synthesising derivatives of the antidepressant imipramine (Schmutz and Eichenberger, 1982). Early trials suggested an antipsychotic action but clozapine also caused agranulocytosis and the death of some patients, leading to its withdrawal from use (Griffith and Saameli, 1975; Gross and Langner, 1966). Clozapine was reintroduced in the 1990s following studies which showed that it had lower incidence of extrapyramidal side effects and was effective in some treatment-resistant populations (Kane et al., 1988). This was the start of a new era in antipsychotic treatment with a number of new drugs coming to the market which all shared a reduced propensity for extrapyramidal side effects. The range of pharmacological effects of the atypical antipsychotics is diverse although the majority exhibit high affinity for the 5-HT2A receptor and are sometimes referred to as dopamine and serotonin antagonists (Kim et al., 2009; Seeman, 1990). There are also newer agents such as aripiprazole which are partial agonists at D2 receptors as well as 5-HT2 receptor antagonists. Although sharing some pharmacological characteristics, the atypical antipsychotics are a relatively diverse class of agents and linking their specific biochemical targets to their behavioural effects remains a challenge (Kim et al., 2009). The main difference between the typical and atypical drugs is their likelihood of inducing extrapyramidal side effects. Research suggests that this may involve the combined actions at serotonin and dopamine receptors. It may also relate to more favourable pharmacokinetic profiles, making it easier to maintain an antipsychotic dose while avoiding motor side effects.
While these atypical antipsychotics have in the same way as the second-generation antidepressants led to improvements in patient care through reduced side effects, the overall efficacy of these compounds is not really improved (except perhaps clozapine). Meta-analyses have overall failed to find good evidence supporting improved efficacy for alleviation of negative or cognitive symptoms although a lower side-effect burden has reduced the adverse effects of treatment of these other symptoms in schizophrenia. Attempts to develop drugs to target the negative symptoms have not as yet delivered new treatments, and patient’s cognitive symptoms remain unresolved. Emotional aspects of the disease can be treated with additional therapies such as antidepressants but essentially, antipsychotic drugs are primarily a method for managing psychotic symptoms and neither address the whole spectrum of the disease nor treat the underlying cause.
As with the antidepressants, many attempts to explain the relationship between effective treatments and underlying disease biology have been made; however, the cause of schizophrenia remains unknown. Knowledge of the receptor-binding profiles and neurochemical effects of the antipsychotics supported a key role for dopamine receptors (particularly D2) and the hypothesis that schizophrenia is a disease of hyperdopaminergic function developed (Horacek et al., 2006; Moncrieff, 2009). Both animal studies and observations in patients have shown that drugs which elevate dopamine levels in the brain can induce, in normal subjects, the positive symptoms of schizophrenia (Lieberman et al., 1990). Similarly, drugs acting at the 5-HT2A or NMDA receptor have a similar propensity to induce positive symptoms which in animals can be attenuated by pre-treatment with antipsychotics (Halberstadt and Geyer, 2013; Jones et al., 2011; Steinpreis, 1996). Despite this large body of pharmacological evidence, studies in patients are less clear with few studies finding any robust evidence of neurochemical deficits which could explain why these drugs are effective. In recent years, there has been a shift towards the idea that schizophrenia is a developmental disorder which manifests in adolescence when the ‘damage’ starts to have profound effects on the patient’s behaviour. Birth cohort studies have found that there are early signs of impairment in patients who go on to develop schizophrenia, and these can be detected as early as the 1-year developmental milestones (Isohanni et al., 2001). There is also increasing evidence that early cognitive and emotional impairments develop before the onset of psychoses and these represent an important new area to try to target in terms of alleviating symptoms and potentially preventing or delaying the onset of positive symptoms (Jahshan et al., 2010). Although some trials have been completed, to date, no successful drug treatment for the cognitive impairments in schizophrenia has reached the market.
Where next?
The last 50 years have seen huge advances in the field of psychopharmacology; however, there remain a lot of unknowns relating to the cause and treatment of psychiatric disorders. As discussed in the examples above, the refinement of the pharmacological targets has aided the development of more specific and thus better tolerated drug treatments and the benefit of this in terms of patient care should not be underestimated. Animal models have also played a major role in the clinical progression of these treatments though there are notable caveats to this research strategy (see discussion Box 1). The challenge for the next 50 years is to more precisely define the relationships between these biochemical targets and effects on behaviour. Moreover, the underlying pathological processes which lead to the disease and how current therapies interact with these processes remain to be fully elucidated. Because of this lack of knowledge, we do not know whether current treatments intervene with the underlying neurobiological mechanisms of psychiatric disorders directly, indirectly or actually target more downstream processes that are merely linked to these abnormal behaviours. New treatments are clearly needed as many patients fail to respond to current drugs or retain residual symptoms. Side effects of these drugs, even the newer second-generation ones, are still an issue and for most patients, long-term medications are needed with relapse common. For many patients, the conditions are lifelong with current treatments providing a means to manage symptoms but are not a cure.
So, what does the future for psychopharmacology look like? Box 2 provides a summary of some of the areas I consider may have the biggest influence on the future of psychopharmacology, how we consider psychiatric disorders and approaches to their treatment.
Box 2.
Examples of some of the new methods and areas of development relevant to the future of psychopharmacology.
One area which offered great promise and potentially a route to new treatments was the role of genetics in psychiatry. However, despite the huge volume of research in this area, it has proved to be a complex field with many genes linked to vulnerability but with varying degrees of penetrance. Knowledge around genetics and the ability to generate genetically modified animals are now widely used to generate disease models and evaluate novel drug targets, but how well these recapitulate the human condition is difficult to quantify considering the limitations associated with methods to quantify the arising behavioural phenotypes (see Box 1). There is also increasing evidence of the complexity of understanding how these genes interact with environmental factors and the role epigenetic mechanisms play in the relationship between genetic risk and manifestation of disease (Mahgoub and Monteggia, 2013). These epigenetic mechanisms may in themselves provide new targets for intervention. Although yet to deliver new targets, the identification of mutations which are associated with increased risk could also yield new opportunities for research and development as a greater understanding of the associated pathways and relationship to pathophysiology develop. For example, stem cells derived from patients could be used to generate cell lines to study the specific impact of the mutation on neurobiology (Wen, 2017). Using genetic information to define subpopulations within the core diagnoses may also provide a route to better diagnosis and targeting of the most suitable medication. Studying the neurobiology of psychiatric disorders has also extended beyond the brain with evidence for a central role for the immune system (Dantzer et al., 2008; Horowitz et al., 2013; Khandaker et al., 2014; Mondelli et al., 2017) and most recently ideas relating to the microbiome and how the gut–brain interactions may contribute to mental health (Clapp et al., 2017; Rea et al., 2016).
Methods to study the brain in relation to mental health have also seen major advances. For example, optogenetics and chemogenetics provide research tools to selectively target distinct neuronal populations and pathways to enable investigations into their role in behaviour (Kim et al., 2017; Sjulson et al., 2016; Steinberg et al., 2015; Urban and Roth, 2015). While currently a research tool, there is the potential for future treatments to build on these methods whereby the restrictions associated with current drug treatments, that is, they hit all receptors expressed within both target and non-target regions, could be addressed (Whittle et al., 2014). Could the future of psychopharmacology include the use of viral-mediated gene transfer of novel, designer receptors or optically activated receptors which can then be used to manipulate the activity of a specific, dysfunctional pathway? Both fundamental biology research and clinical studies have benefitted from advances in how the physiology of the living brain can be measured particularly using imaging or electrophysiological methods. Studies recorded from populations of neurones and network activity quantified using electroencephalogram (EEG) or imaging methods are revealing how local and brain-wide networks interact and the dysfunctional activity which is associated with psychiatric disorders. One important aspect on the interpretation of these data is how they relate to deficits at a functional, particularly behavioural, level. These methods are most useful when integrated with behavioural methods but equally, particularly in animal studies, can be limited where the behavioural methods and models have poor construct validity.
Perhaps one of the most important areas for development is the need for robust translational biomarkers which can provide objective methods for diagnosis and to assess response to treatment (Jentsch et al., 2015; Slaney et al., 2018; Strawbridge et al., 2017). Biomarkers could also help with the current challenge of diagnosis and patient stratification. While psychiatric disorders are categorised based on symptom clusters, the current methods, as discussed in the Diagnostic and Statistical Manual of Mental Disorders – 5th Edition (DSM-V), do not allow for the heterogeneous nature of many of the conditions. To date, a traditional approach to a biomarker for any psychiatric disorder has failed to provide anything suitable (Jentsch et al., 2015; Slaney et al., 2018; Strawbridge et al., 2017). Perhaps an alternative approach would be to utilise a behavioural approach to these disorders which predominantly manifest as dysfunctional cognitive, emotional and social functioning (Slaney et al., 2018). The Research Domain Criteria (RDoC; Cuthbert and Insel, 2013; Insel, 2014) provides a useful starting point for this approach whereby the different behavioural domains relevant to psychiatric disorders have been described within a framework which includes specific neuropsychological tests and animal paradigms which can provide a quantitative approach to measuring deficits. Extending this to a more objective, phenotype assessment of how these domains are specifically altered in different conditions has the potential to provide an objective and translatable approach for future psychopharmacological studies. Whether future diagnoses of psychiatric disorder could be made on the basis of such cognitive biomarkers remains to be seen. However, if such developments could be made, then future clinical trials could benefit from a more symptom-based objective assessment. Our own research has attempted to develop a translational and objective approach to studying MDD based on these ideas (Robinson, 2018; Slaney et al., 2018).
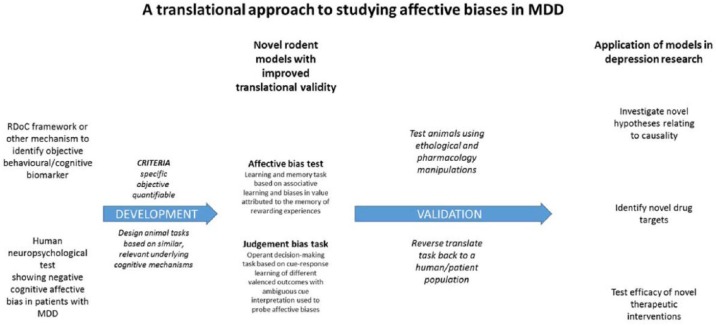
Studying emotional disorders in animals has proved very challenging and the ability to relate subjective symptoms of low mood, suicidal ideation and even loss of interest in pleasurable activities to methods used in animals is impossible. However, neuropsychological studies have revealed that patients with depression exhibit changes in emotional processing which can be measured using objective tasks (Robinson, 2018; Robinson and Roiser, 2016). These deficits can be linked to relevant neuronal pathways and show robust sensitivity to antidepressant treatment (Harmer et al., 2017; Pringle et al., 2011). Although not directly translatable, the underlying concept that emotional state can bias cognitive processes has the potential to be translated between species if task can be developed in the appropriate domain (Robinson, 2018). The first example of this was reported in Nature in 2004 by Harding et al. and demonstrated that rodents’ behavioural responses in a decision-making task were influenced by their affective state. There is now a large body of literature demonstrating that similar affective biases can be observed in a variety of species from invertebrates to humans (Hales et al., 2014; Robinson, 2018). Our lab has also developed a task which can detect biases in learning and memory in a simple task of associative learning. In our affective bias test, animals are presented with two independent learning experiences of equal absolute value, but their affective state is modulated before one of the learning sessions (Stuart et al., 2013). This leads to a robust bias in the subsequent recall of the value of reward associated with that experience which is consistent with the direction of affective state change, that is, a positive affective state leads to a positive bias and a negative affective state causes a negative bias (Stuart et al., 2013, 2014, 2017). In this task, we observe effects following pharmacological and psychosocial manipulations of affective state as well as effects following acute treatment with antidepressant or pro-depressant treatments which are predicative of their subsequent effects on mood in humans. Furthermore, in the affective bias test, and a modified version of Harding’s original decision-making task, we have been able to observe differential effects with conventional delayed onset antidepressants versus the rapid onset antidepressant, ketamine (Hales et al., 2017; Stuart et al., 2015). The actions of these drugs are also linked to relevant neural circuits (Stuart et al., 2015). These data demonstrate how this approach can be used to achieve a more translational approach to studying the psychopharmacology of depression. Could a similar strategy (Figure 2) also be employed for other disorders and behavioural deficits?
Figure 2.
Overview of an approach to develop better translational behavioural methods to study relevant neuropsychological characteristics of psychiatric disorders and improve integration of fundamental biology, preclinical drug discovery and development, and clinical studies. See also discussion in Robinson, 2018 in relation to major depressive disorder.
In summary, psychopharmacology has seen major advances from the early developments of the major treatment classes used today. Knowledge about the biochemical effects of these serendipitously discovered drugs has been critical to the development of the second generation of treatments which are commonly used today. However, the complexity of psychiatric disorders is becoming more apparent as knowledge about the role of genes and the environment are better understood and there are still a lot of unknowns. While development in understanding of pathophysiology will undoubtably develop from this knowledge, the challenge for psychopharmacology remains in terms of translating this knowledge into new pharmacological treatments. As such, there may also be a case for not forgetting the past. Novel insights are often gained from very different contexts (i.e. the serendipitous discoveries of 1950s) and perhaps a more blue-skies strategy could bring about novel theories and progress in our understanding. Better translational methods, including improving animal models, are critical for testing hypotheses from population health, clinical studies and fundamental biology and taking these from the bench, through preclinical development and to the patient.
Acknowledgments
The author would like to thank Charles Clarke Williams and Dr Sarah Stuart for their helpful suggestions about how this manuscript could be improved.
Footnotes
Declaration of conflicting interests: The author currently holds research grant funding from the MRC, BBSRC and Wellcome Trust. She has also received research funding from Boehringer Ingelheim, Eli Lilly, MSD, Pfizer and SmallPharma although these companies have not had any influence on the content of this article.
Funding: Work which has contributed to this article was funded by BBSRC, MRC and Wellcome Trust. E.S.J.R. has also received research funding from industry collaborators, Boehringer Ingelheim, Eli Lilly, MSD, Pfizer and SmallPharma, although they have not had any direct influence on the context of this article.
References
- Andrews PW, Bharwani A, Lee KR, et al. (2015) Is serotonin an upper or a downer? The evolution of the serotonergic system and its role in depression and the antidepressant response. Neuroscience & Biobehavioral Reviews 51: 164–188. [DOI] [PubMed] [Google Scholar]
- Artigas F, Romero L, de Montigny C, et al. (1996) Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends in Neurosciences 19(9): 378–383. [DOI] [PubMed] [Google Scholar]
- Axelrod J. (1972) Biogenic amines and their impact in psychiatry. Seminars in Psychiatry 4(3): 199–210. [PubMed] [Google Scholar]
- Baldwin DS, Ajel K, Masdrakis VG, et al. (2013) Pregabalin for the treatment of generalized anxiety disorder: An update. Neuropsychiatric Disease and Treatment 9: 883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin DS, Anderson IM, Nutt DJ, et al. (2014) Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: A revision of the 2005 guidelines from the British Association for Psychopharmacology. Journal of Psychopharmacology 28(5): 403–439. [DOI] [PubMed] [Google Scholar]
- Berger P, Barchas JD. (1977) Monoamine oxidase inhibitors. In: Usdin E, Forrest IS. (eds) Psychotherapeutic Drugs. New York: Marcel Dekker, pp. 1173–1216. [Google Scholar]
- Blier P. (2001) Pharmacology of rapid-onset antidepressant treatment strategies. Journal of Clinical Psychiatry 62(Suppl. 15): 12–17. [PubMed] [Google Scholar]
- Bradley C. (1937) The behavior of children receiving Benzedrine. American Journal of Psychiatry 94(3): 577–585. [Google Scholar]
- Cade JF. (1949) Lithium salts in the treatment of psychotic excitement. Medical Journal of Australia 2(36): 349–352. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Nutt DJ. (2017) Serotonin and brain function: A tale of two receptors. Journal of Psychopharmacology 31(9): 1091–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Roseman L, Bolstridge M, et al. (2017) Psilocybin for treatment-resistant depression: fMRI-measured brain mechanisms. Scientific Reports 7(1): 13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Wall MB, Erritzoe D, et al. (2014) The effect of acutely administered MDMA on subjective and BOLD-fMRI responses to favourite and worst autobiographical memories. International Journal of Neuropsychopharmacology 17(4): 527–540. [DOI] [PubMed] [Google Scholar]
- Carlsson A. (1990) Early psychopharmacology and the rise of modern brain research. Journal of Psychopharmacology 4(3): 120–126. [DOI] [PubMed] [Google Scholar]
- Carvalho AF, Sharma MS, Brunoni AR, et al. (2016) The safety, tolerability and risks associated with the use of newer generation antidepressant drugs: A critical review of the literature. Psychotherapy and Psychosomatics 85(5): 270–288. [DOI] [PubMed] [Google Scholar]
- Charney DS, Menkes DB, Heninger GR. (1981) Receptor sensitivity and the mechanism of action of antidepressant treatment. Implications for the etiology and therapy of depression. Archives of General Psychiatry 38(10): 1160–1180. [DOI] [PubMed] [Google Scholar]
- Clapp M, Aurora N, Herrera L, et al. (2017) Gut microbiota’s effect on mental health: The gut-brain axis. Clinical Practice 7(4): 987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleare A, Pariante CM, Young AH. (2015) Evidence-based guidelines for treating depressive disorders with antidepressants: A revision of the 2008 British Association for Psychopharmacology guidelines. Journal of Psychopharmacology 29(5): 459–525. [DOI] [PubMed] [Google Scholar]
- Courvoisier S. (1956) Pharmacodynamic basis for the use of chlorpromazine in psychiatry. Journal of Clinical and Experimental Psychopathology 17(1): 25–37. [PubMed] [Google Scholar]
- Crocq M-A. (2007) Historical and cultural aspects of man’s relationship with addictive drugs. Dialogues in Clinical Neuroscience 9(4): 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. (2013) Toward the future of psychiatric diagnosis: The seven pillars of RDoC. BMC Medicine 11(14): 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, et al. (2008) From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Reviews Neuroscience 9(1): 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Berardis D, Di Iorio G, Acciavatti T, et al. (2011) The emerging role of melatonin agonists in the treatment of major depression: Focus on agomelatine. CNS Neurol Disord Drug Targets 10(1):119–132. [DOI] [PubMed] [Google Scholar]
- Deakin J. (1998) The role of serotonin in depression and anxiety. European Psychiatry 13(Suppl. 2): 57s–63s. [DOI] [PubMed] [Google Scholar]
- Divry P, Bobon J, Collard J, et al. (1958) 1625: Nouvelle thérapeutique symptomatique de l’agitation psychomotrice. Acta neurologica et psychiatrica Belgica 58(10): 878–888. [PubMed] [Google Scholar]
- Duman RS, Li N. (2012) A neurotrophic hypothesis of depression: Role of synaptogenesis in the actions of NMDA receptor antagonists. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences 367(1601): 2475–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Thome J. (1999) Neural plasticity to stress and antidepressant treatment. Biological Psychiatry 46(9): 1181–1191. [DOI] [PubMed] [Google Scholar]
- Eison AS, Temple DL. (1986) Buspirone: Review of its pharmacology and current perspectives on its mechanism of action. The American Journal of Medicine 80(3 Suppl. 2): 1–19. [DOI] [PubMed] [Google Scholar]
- Freis ED. (1954) Mental depression in hypertensive patients treated for long periods with large doses of reserpine. New England Journal of Medicine 251(25): 1006–1008. [DOI] [PubMed] [Google Scholar]
- Granger B, Albu S. (2005) The haloperidol story. Annals of Clinical Psychiatry 17(3): 137–140. [DOI] [PubMed] [Google Scholar]
- Griffith RW, Saameli K. (1975) Clozapine and agranulocytosis. The Lancet 306(7936): 657. [DOI] [PubMed] [Google Scholar]
- Gross VH, Langner E. (1966) Das Wirkungsprofil eines chemisch neuartigen Breitbandneuroleptikums der Dibenzodiazepingruppe. Wiener Medizinische Wochenschrift 116(40): 814–816. [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. (2013) Characterization of the head-twitch response induced by hallucinogens in mice: Detection of the behavior based on the dynamics of head movement. Psychopharmacology 227(4): 727–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales CA, Houghton CJ, Robinson ESJ. (2017) Behavioural and computational methods reveal differential effects for how delayed and rapid onset antidepressants effect decision making in rats. European Neuropsychopharmacology 27(12): 1268–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales CA, Stuart SA, Anderson MH, et al. (2014) Modelling cognitive affective biases in major depressive disorder using rodents. British Journal of Pharmacology 171(20): 4524–4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding EJ, Paul ES, Mendl M. (2004) Animal behaviour: Cognitive bias and affective state. Nature 427(6972): 312. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Duman RS, Cowen PJ. (2017) How do antidepressants work? New perspectives for refining future treatment approaches. Lancet Psychiatry 4(5): 409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heninger GR, Delgado PL, Charney DS. (1996) The revised monoamine theory of depression: A modulatory role for monoamines, based on new findings from monoamine depletion experiments in humans. Pharmacopsychiatry 29(1): 2–11. [DOI] [PubMed] [Google Scholar]
- Horacek J, Bubenikova-Valesova V, Kopecek M, et al. (2006) Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs 20(5): 389–409. [DOI] [PubMed] [Google Scholar]
- Horowitz MA, Zunszain PA, Anacker C, et al. (2013) Glucocorticoids and inflammation: A double-headed sword in depression? How do neuroendocrine and inflammatory pathways interact during stress to contribute to the pathogenesis of depression? Modern Trends in Pharmacopsychiatry 28: 127–143. [DOI] [PubMed] [Google Scholar]
- Hyman SE. (2013) Psychiatric drug development: Diagnosing a crisis. Cerebrum 5 Available at: http://www.dana.org/Cerebrum/Default.aspx?id=39489 [PMC free article] [PubMed] [Google Scholar]
- Insel TR. (2014) The NIMH Research Domain Criteria (RDoC) Project: Precision medicine for psychiatry. American Journal of Psychiatry 171(4): 395–397. [DOI] [PubMed] [Google Scholar]
- Isohanni M, Jones PB, Moilanen K, et al. (2001) Early developmental milestones in adult schizophrenia and other psychoses: A 31-year follow-up of the Northern Finland 1966 Birth Cohort. Schizophrenia Research 52(1–2): 1–19. [DOI] [PubMed] [Google Scholar]
- Jahshan C, Heaton RK, Golshan S, et al. (2010) Course of neurocognitive deficits in the prodrome and first episode of schizophrenia. Neuropsychology 24(1): 109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen PAJ, Jageneau AHM, Schellekens KHL. (1960) Chemistry and pharmacology of compounds related to 4-(4-hydroxy-4-phenyl-piperidino)-butyrophenone. Part IV. Influence of haloperidol (R 1625) and of chlorpromazine on the behaviour of rats in an unfamiliar ‘open field’ situation. Psychopharmacologia 1(5): 389–392. [DOI] [PubMed] [Google Scholar]
- Jentsch MC, Van Buel EM, Bosker FJ, et al. (2015) Biomarker approaches in major depressive disorder evaluated in the context of current hypotheses. Biomarkers in Medicine 9(3): 277–297. [DOI] [PubMed] [Google Scholar]
- Jones CA, Watson DJ, Fone KC. (2011) Animal models of schizophrenia. British Journal of Pharmacology 164(4): 1162–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane JM, Honigfeld G, Singer J, et al. (1988) Clozapine for the treatment resistant schizophrenic: A double-blind comparison with chlorpromazine. Archives of General Psychiatry 45(9): 789–797. [DOI] [PubMed] [Google Scholar]
- Khandaker GML, Cousins J, Deakin BR, et al. (2014) Inflammation and immunity in schizophrenia: Implications for pathophysiology and treatment. Lancet Psychiatry 2(3): 258–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CK, Adhikari A, Deisseroth K. (2017) Integration of optogenetics with complementary methodologies in systems neuroscience. Nature Reviews Neuroscience 18(4): 222–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Maneen MJ, Stahl SM. (2009) Building a better antipsychotic: Receptor targets for the treatment of multiple symptom dimensions of schizophrenia. Neurotherapeutics 6(1): 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler S, Cierpinsky K, Kronenberg G, et al. (2016) The serotonergic system in the neurobiology of depression: Relevance for novel antidepressants. Journal of Psychopharmacology 30(1): 13–22. [DOI] [PubMed] [Google Scholar]
- Kuhn R. (1958) The treatment of depressive states with G 22355 (imipramine hydrochloride). American Journal of Psychiatry 115(5): 459–464. [DOI] [PubMed] [Google Scholar]
- Lambert C, Rees WC. (1944) Intravenous barbiturates in treatment of hysteria. British Medical Journal 2(4358): 70–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA, Kinon BJ, Loebel AD. (1990) Dopaminergic mechanisms in idiopathic and drug-induced psychoses. Schizophrenia Bulletin 16(1): 97–110. [DOI] [PubMed] [Google Scholar]
- López-Muñoz F, Alamo C, Cuenca E, et al. (2005) History of the discovery and clinical introduction of chlorpromazine. Annals of Clinical Psychiatry 17(3): 113–135. [DOI] [PubMed] [Google Scholar]
- López-Muñoz F, Alamo C. (2009) Monoaminergic neurotransmission: The history of the discovery of antidepressants from 1950s until today. Current Pharmaceutical Design 15(14): 1563–1586. [DOI] [PubMed] [Google Scholar]
- López-Muñoz F, Baumeister AA, Hawkins MF, et al. (2012) The role of serendipity in the discovery of the clinical effects of psychotropic drugs: Beyond of the myth. Actas Españolas de Psiquiatría 40(1): 34–42. [PubMed] [Google Scholar]
- Lyndon A, Audrey S, Wells C, et al. (2017) Risk to heroin users of polydrug use of pregabalin or gabapentin. Addiction 112(9): 1580–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Salvadore G, Diazgranados N, et al. (2009) Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacology & Therapeutics 123(2): 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahgoub M, Monteggia LM. (2013) Epigenetics and psychiatry. Neurotherapeutics 10(4): 734–741. DOI: 10.1007/s13311-013-0213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes R, Rafalovich A. (2007) Suffer the restless children: The evolution of ADHD and pediatric stimulant use, 1900-80. History of Psychiatry 18(72 Pt. 4): 435–457. [DOI] [PubMed] [Google Scholar]
- Moncrieff J. (2009) A critique of the dopamine hypothesis of schizophrenia and psychosis. Harvard Review of Psychiatry 17(3): 214–510. [DOI] [PubMed] [Google Scholar]
- Mondelli V, Vernon AC, Turkheimer F, et al. (2017) Brain microglia in psychiatric disorders. Lancet Psychiatry 4(7): 563–572. [DOI] [PubMed] [Google Scholar]
- Moriarty KM, Alagna SW, Lake CR. (1984) Psychopharmacology. An historical perspective. Psychiatric Clinics of North America 7(3): 411–433. [PubMed] [Google Scholar]
- Nutt DJ. (1998) Efficacy of mirtazapine in clinically relevant subgroups of depressed patients. Depression and Anxiety 7(Suppl. 1): 7–10. [PubMed] [Google Scholar]
- Nutt DJ. (2008) Relationship of neurotransmitters to the symptoms of major depressive disorder. Journal of Clinical Psychiatry 69(Suppl. E1): 4–7. [PubMed] [Google Scholar]
- Nutt DJ, Forshall S, Bell C, et al. (1999) Mechanisms of action of selective serotonin reuptake inhibitors in the treatment of psychiatric disorders. European Neuropsychopharmacology 9(Suppl. 3): S81–S86. [DOI] [PubMed] [Google Scholar]
- Pringle A, Browning M, Cowen PJ, et al. (2011) A cognitive neuropsychological model of antidepressant drug action. Progress in Neuro-Psychopharmacology & Biological Psychiatry 35(7): 1586–1592. [DOI] [PubMed] [Google Scholar]
- Rea K, Dinan TG, Cryan JF. (2016) The microbiome: A key regulator of stress and neuroinflammation. Neurobiology of Stress 4: 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ESJ. (2018) Translational new approaches for investigating mood disorders in rodents and what they may reveal about the underlying neurobiology of major depressive disorder. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences 373(1742). pii: 20170036. doi: 10.1098/rstb.2017.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ESJ, Roiser JP. (2016) Affective biases in humans and animals. Current Topics in Behavioral Neurosciences 28: 263–286. [DOI] [PubMed] [Google Scholar]
- Sahay A, Hen R. (2007) Adult hippocampal neurogenesis in depression. Nature Neuroscience 10(9): 1110–1115. [DOI] [PubMed] [Google Scholar]
- Schildkraut JJ. (1965) The catecholamine hypothesis of affective disorders: A review of supporting evidence. American Journal of Psychiatry 122(5): 509–522. [DOI] [PubMed] [Google Scholar]
- Schmutz J, Eichenberger E. (1982) Clozapine. In: Bindra JS, Lednicer D. (eds) Chronicles of Drug Discovery, vol. 1 New York: John Wiley & Sons, pp. 39–59. [Google Scholar]
- Seeman P. (1990) Atypical neuroleptics: Role of multiple receptors, endogenous dopamine, and receptor linkage. Acta Psychiatrica Scandinavica 82(S358): 14–20. [DOI] [PubMed] [Google Scholar]
- Seeman P, Lee T, Chau-Wong M, et al. (1976) Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature 261(5562): 717–719. [DOI] [PubMed] [Google Scholar]
- Shen WW. (1999) A history of antipsychotic drug development. Comprehensive Psychiatry 40(6): 407–414. [DOI] [PubMed] [Google Scholar]
- Shorter E. (2008) History of psychiatry. Current Opinion in Psychiatry 21(6): 593–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjulson L, Cassataro D, DasGupta S, et al. (2016) Cell-specific targeting of genetically encoded tools for neuroscience. Annual Review of Genetics 50: 571–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaney C, Hinchcliffe JK, Robinson ESJ. (2018) Translational shifts in preclinical models of depression: Implications for biomarkers for improved treatments. Current Topics in Behavioral Neurosciences. Epub ahead of print 26 April. DOI: 10.1007/7854_2018_44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery DA, Hudson AL, Nutt DJ. (2004) Invited review: The evolution of antidepressant mechanisms. Fundamental & Clinical Pharmacology 18(1): 1–21. [DOI] [PubMed] [Google Scholar]
- Steinberg EE, Christoffel DJ, Deisseroth K, et al. (2015) Illuminating circuitry relevant to psychiatric disorders with optogenetics. Current Opinion in Neurobiology 30: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinpreis RE. (1996) The behavioral and neurochemical effects of phencyclidine in humans and animals: Some implications for modeling psychosis. Behavioural Brain Research 74(1–2): 45–55. [DOI] [PubMed] [Google Scholar]
- Stimmel GL, Dopheide JA, Stahl SM. (1997) Mirtazapine: An antidepressant with noradrenergic and specific serotonergic effects. Pharmacotherapy 17(1): 10–21. [PubMed] [Google Scholar]
- Strawbridge R, Young AH, Cleare AJ. (2017) Biomarkers for depression: Recent insights, current challenges and future prospects. Neuropsychiatric Disease and Treatment 13: 1245–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud JB, Freeman TP, Leech R, et al. (2018) Psilocybin with psychological support improves emotional face recognition in treatment-resistant depression. Psychopharmacology 235(2): 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart SA, Butler P, Robinson ESJ. (2014) Animal Models of Risk Factors for Suicidal Ideation and Behaviour. Basel: Springer. [Google Scholar]
- Stuart SA, Butler P, Munafo MR, et al. (2013) A translational rodent assay of affective biases in depression and antidepressant therapy. Neuropsychopharmacology 38(9): 1625–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart SA, Butler P, Munafò MR, et al. (2015) Distinct neuropsychological mechanisms may explain delayed- versus rapid-onset antidepressant efficacy. Neuropsychopharmacology 40(9): 2165–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart SA, Wood CM, Robinson ESJ. (2017) Using the affective bias test to predict drug-induced negative affect: Implications for drug safety. British Journal of Pharmacology 174(19): 3200–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban DJ, Roth BL. (2015) DREADDs (designer receptors exclusively activated by designer drugs): Chemogenetic tools with therapeutic utility. Annual Review of Pharmacology and Toxicology 55: 399–417. [DOI] [PubMed] [Google Scholar]
- Wen Z. (2017) Modeling neurodevelopmental and psychiatric diseases with human iPSCs. Journal of Neuroscience Research 95(5): 1097–1109. DOI: 10.1002/jnr.24031. [DOI] [PubMed] [Google Scholar]
- Whittle AJ, Walsh J, de Lecea L. (2014) Light and chemical control of neuronal circuits: Possible applications in neurotherapy. Expert Review of Neurotherapeutics 14(9): 1007–1017. [DOI] [PubMed] [Google Scholar]
- Wick JY. (2013) The history of benzodiazepines. The Consultant Pharmacist 28(9): 538–548. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, et al. (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Archives of General Psychiatry 63(8): 856–864. [DOI] [PubMed] [Google Scholar]