Abstract
The name neuroglia is generally translated as nerve glue. In the recent past, this has been used to describe passive structural cells. Presently, this view has been challenged and the true dynamic and multifunctional nature of neuroglia is beginning to be appreciated. In the central nervous system, the main kinds of neuroglia are astrocytes (the primary homeostatic cells that ensure synaptic transmission), oligodendrocytes (which form the myelin that ensures rapid electrical transmission) and microglia (the main immune cells). In the peripheral nervous system, neuroglia comprise Schwann cells, satellite glia and enteric glia. These functionally diverse and specialised cells are fundamental to function at the molecular, cellular, tissue and system levels. Without nerve glue, the body cannot function and the future will begin to unlock their importance in higher cognitive functions that set humans apart from other animals and their true potential as therapeutic targets in neurodegenerative and other diseases.
Keywords: Glia, astrocyte, oligodendrocyte, microglia, Schwann cell, enteric glia, satellite glia
Introduction
The name neuroglia is generally translated as nerve glue. In the recent past, nerve glue has often been used as a derogatory term to describe passive structural cells of little interest or importance compared to electrically excitable neurons. The future will be distinguished by a revalidation of this term – quite literally, the brain falls apart without nerve glue. Neuroglia provide for the homeostasis of the nervous system, its defence and structure, and are central to all neuropathologies.
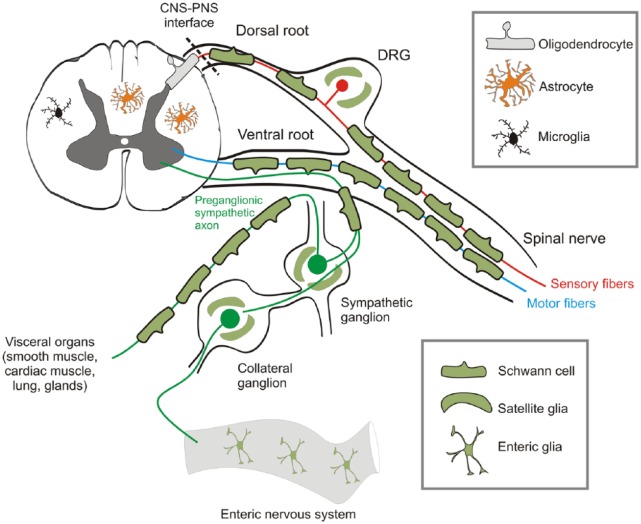
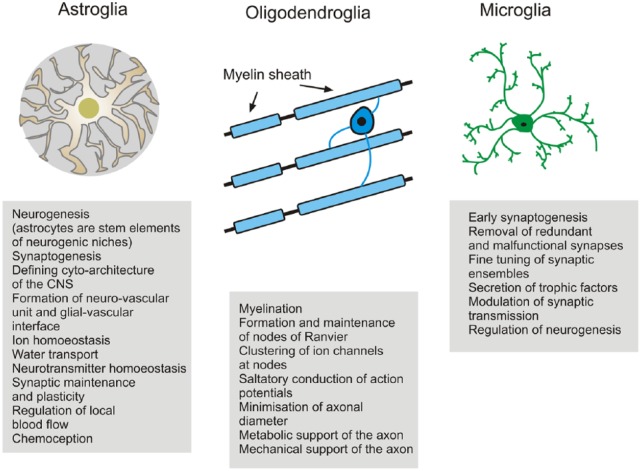
Neuroglia are sub-classified (Verkhratsky and Butt, 2013) into glia of the central nervous system (CNS) and peripheral nervous system (PNS; Figure 1). CNS glia comprise macroglia and microglia. Macroglia are derived from neural stem cells and are represented by astrocytes, oligodendrocytes and NG2-glia (also known as polydendrocytes): astrocytes are the main homeostasis cells; oligodendrocytes myelinate and support axons and NG2-glia act as lifelong oligodendrocyte precursors. Microglia have a myeloid origin and their precursors are foetal macrophages originating from the yolk sac; these precursors enter the CNS in the early embryonic period and disseminate more or less homogeneously through the CNS, where they acquire a microglial phenotype to become the main defensive and immunocompetent cells of the CNS (Kettenmann et al., 2011; Figure 2). The PNS neuroglia include (1) Schwann cells (further subdivided into three: myelinated Schwann cells that cover axons with myelin; non-myelinated Schwann cells that enwrap non-myelinated axons and perisynaptic Schwann cells that cover neuromuscular junctions), (2) satellite glial cells that support neurons in peripheral ganglia; (3) olfactory ensheathing cells and (4) enteric glia. Together, CNS and PNS neuroglia, in the broadest sense, are essential for normal bodily function.
Figure 1.
Neuroglial diversity. Neuroglia are sub-classified into CNS and PNS glia, but overall their functions are the same – to provide homeostatic, structural and metabolic support of neurons, to meylinate their axons to ensure rapid signal transmission and to be the main defensive and immunocompetent cells. In the CNS, the main glial cell types are astrocytes, oligodendrocytes and microglia. In the PNS, the main neuroglia are Schwann cells, satellite glial cells within the ganglia and enteric glia, which support neurons within the enteric nervous system of the gastrointestinal tract. The interface between the CNS and the PNS marks a change from an environment of astrocytes, oligodendrocytes and microglia, to one of Schwann cells and satellite glia; there is no change in the axon as it passes from one domain to the other, and PNS and CNS neurons are fundamentally the same. In the broadest sense, CNS and PNS neuroglia are essential for normal bodily function.
Figure 2.
Neuroglia in the CNS. CNS glia are multifunctional cells specialised to enable neurons to perform their function of information transfer. The division of labour between glia and neurons is fundamental to the evolution of the human brain into a supercomputer that fits within the small space of the cranium. It has taken over 100 years to redefine neuroglia as being more than brain glue. Nonetheless, the importance of glue should not be underestimated – the disruption of neuroglia has devastating effects on the neuronal function and is involved in every neuropathology. Quite literally, the brain falls apart without nerve glue and increased knowledge of glial cell biology is beginning to lead to a greater appreciation of the true potential of glial cells as therapeutic targets.
Past
The concept of neuroglia was introduced by Rudolf Virchow in the 1850s and most of the famous neuroanatomists, from Karl Bergmann and Alfred von Kollicker to Camillo Golgi, Ernesto Lugaro and Santiago Ramon-y-Cajal, have contributed to the characterisation of glial cells and conjectured on their functions, ranging from mechanical support of nervous tissue to metabolic support, regulation of sleep and removal of toxic products (for a detailed historical review, see Kettenmann and Verkhratsky, 2008; Verkhratsky and Butt, 2013: 27–49). The main peripheral glial element, the Schwann cell, was named in 1871 by Louis Antoine Ranvier (whom the node of Ranvier is named after) after earlier discoveries by Robert Remak and Theodor Schwann, who first described peripheral myelin and ascribed its formation to specialised cells in 1838–1839; the term myelin was introduced by Rudolf Virchow. Astrocytes were defined as stellate glia by Michael von Lenhossek in 1895 and later Del Rio Hortega is attributed with the identification of microglia and oligodendroglia in 1919. Prior to Río-Hortega, the Scottish pathologist William Ford Robertson made the first remarkably accurate sketches of oligodendrocytes and their precursors in 1899, although NG2-glia were not formally described until the 1980s by William Stallcup and colleagues.
The first electrophysiological recordings from neuroglia in both invertebrates and vertebrates (including mammals) were performed in the 1950s by Walter Hild and colleagues, who defined glia as electrically non-excitable cells. This concept of passive and ‘silent’ glia became the text-book view and was not seriously challenged for decades. Nevertheless, from the outset astrocytes were shown to display slow and long-lasting changes of membrane potential associated with neuronal activity; these were later shown to be due to currents generated by potassium and glutamate uptake, now known to be primary functions of astrocytes and essential for CNS function and integrity. The critical role of astrocytes in potassium homeostasis was proposed by Leif Hertz in 1965 and in 1966 the studies of Steven Kuffler, John Nicolls and Richard Orkand led to the concept of potassium spatial buffering. In 1969, the electron microscopy (EM) studies of Milton Brightman and Tom Reese first identified gap junctions as the substrate for the astroglial syncytium. In the late 1980s and early 1990s, the advent of glial cell cultures, patch-clamp and intracellular Ca2+ probes finally led to the rejection of glia as passive cells. First, the expression of ionotropic glutamate and GABA receptors was found (by Helmut Kettenmann and Harald Kimelberg) in astrocytes and oligodendrocytes in vitro, that is, in the absence of possible neuronal influences. Second, astrocytes were found to generate cytosolic Ca2+ signals in response to mechanical and neurochemical stimulation, which led to the discovery in 1990 by Ann Cornell-Bell of astroglial intercellular Ca2+ waves. The idea of astroglial Ca2+ excitability was formalised in the late 1990s as Ca2+ signals that propagate through the astroglial syncytium via gap junctions and astroglial release of neurotransmitters (Cornell-Bell et al., 1990). In 1995, Vladimir Parpura, Philip Haydon and Maiken Nedergaard found that glial Ca2+ signals trigger the excitation of neurons, thus indicating active glial–neuronal communication. These experiments led to the concept of the tripartite synapse, in which the astroglial compartment is actively involved in neuronal synaptic transmission (Araque et al., 1999). This concept of gliotransmission remains debatable to the modern days (Fiacco and McCarthy, 2018; Savtchouk and Volterra, 2018). One area of controversy was that glial calcium signals were too slow to modulate synaptic activity, appearing tens of seconds after synaptic activity. However, this has now been laid to rest, using novel two-photon imaging and genetically encoded calcium indicators, which shows that astrocyte calcium responses following sensory stimulation are fast enough (~120 ms) to play a role in synaptic modulation and neurovascular coupling (Stobart et al., 2018).
Early studies on oligodendrocytes and Schwann cells focused on their myelinating function, mainly through EM and biochemical analysis of myelin composition (for historical review of myelin, see Boullerne, 2016). In 1884, the founder of neurochemistry Johann Ludwig Thudichum characterised many lipids of myelin, including galactocerebroside, but the composition of myelin only became unravelled after William Norton developed methods for purifying myelin in the 1970s. In the 1930s, Francis Otto Schmitt using X-ray diffraction revealed that the myelin sheath is made up of lipid bilayers intercalated with proteins and Fernández-Morán and Sjöstrand confirmed the lamellar structure of myelin by EM in the 1950s. Remarkably, the g-ratio which defines the relationship between axon diameter and thickness of the myelin sheath was first characterised around the turn of the 20th century, by Henry Donaldson in 1905 and later by Schmitt in 1937. It was not until the advent of EM that the cellular origins of PNS and CNS myelin were confirmed to be Schwann cells and oligodendrocytes. In 1954, Betty Ben Geren showed by EM that the Schwann cell wraps around the axon to form a spiral of compacted myelin. Oligodendrocytes as the cellular origin of CNS myelin was confirmed in the 1960s by the Bunges, Hirano and Peters. The insulating function of myelin was recognised from the earliest times, and the saltatory transmission of nerve impulses, from node of Ranvier to node, was suggested as early as 1925 by Ralph Stayner Lillie; he noted that myelinated nerves transmit impulses 10-fold faster (now known to be up to 100-fold) than unmyelinated fibres. The idea of saltatory conduction was taken forward independently by Ichiji Tasaki and Alan Hodgkin in the late 1930s, and by Andrew Huxley and Robert Stämpfli in the late 1940s, who developed the complex mathematical equations describing how conduction velocity is determined by the resistance and capacitance of myelin. In the 1970s, Norton’s seminal work revealed that myelin contains 70%–85% lipid and 15%–30% protein depending on the source, which remains a bedrock in teaching and launched the modern molecular era of myelin research.
Microglia were largely neglected until the late 1960s, when Geor Kretiuzberg and his colleagues developed the facial nerve lesion to study microglial activation in the CNS (Blinzinger and Kreutzberg, 1968). Subsequently, microglial physiology has been analysed by imaging and electrophysiology in vitro and in isolated tissue, and more recently in vivo, showing that microglial excitability is controlled by a mixture of receptors characteristic for neural cells and for immune cells (for review, see Kettenmann et al., 2011).
Present
Astroglia
Technological advances, such as high-resolution imaging, optogenetics and designer drugs, have allowed the study of astroglia in vivo in the brain, in both anaesthetised and awake animals. Astroglia are now defined as cells responsible for CNS homeostasis at all levels of organisation – molecular, cellular, network, organ and system levels (Verkhratsky and Nedergaard, 2018). Astroglia possess a specific form of excitability mediated by changes in cytoplasmic concentrations of Ca2+ and Na+, the former interacting with Ca2+-dependent enzymes (or ‘Ca2+ sensors’), while the latter determines the activity (through the transmembrane concentration gradient) of multiple plasmalemmal transporters that are essential for controlling the molecular composition of the CNS interstitium, which in turn determines neuronal excitability and information transfer (Bazargani and Attwell, 2016). Astrocytes are intimate elements of CNS synapses, regulating their genesis, maturation, maintenance and extinction, as well as releasing neurotransmitters directly to regulate synaptic activity (Araque et al., 2014). This integrative role of astrocytes at synapses has given rise to the concept of the astroglial ‘synaptic cradle’ (Verkhratsky and Nedergaard, 2014). Through multiple Na+-dependent transporters expressed in their perisynaptic membranes, astrocytes regulate the ionic composition of the synaptic cleft, control the removal and catabolism of major neurotransmitters (including glutamate, GABA, glycine and adenosine) and maintain synaptic transmission by providing neurons with glutamine, which is an obligatory precursor for glutamate and GABA. Astrocytes are bona fide secretory cells in the CNS and release ions, neurotransmitters, neurohormones, trophic factors, immunoactive molecules and amino acids, being (together with microglia) a part of the gliocrine system (Verkhratsky et al., 2016). In the grey matter, astrocytes divide the tissue into relatively independent neuro-glio-vascular units, coupled to the vasculature through astroglial endfeet. The same endfeet plaster the CNS blood vessels and form the basis of the glymphatic system, which by utilising convection flow of cerebrospinal fluid removes waste products from the CNS parenchyma. In sleep, the efficacy of the glymphatic system greatly increases, thus facilitating the purge of potentially toxic molecules from the nerve tissue (Iliff et al., 2013). Astroglia are also critical for controlling global ionic composition in the brain and spinal cord interstitium, thus being the main element of ion homeostasis of the CNS. Dynamic changes in ion homeostatic control in turn affect overall excitability and functional status of the nervous tissue, which is well recognised in pathology, where the disruption of astroglial potassium and glutamate regulation results in a loss of neuronal function and integrity, as occurs in epilepsy, ischemia and amyotrophic lateral sclerosis (ALS). Astroglial functions also manifestly regulate behaviour, for example, astrocytes are fundamental for adenosine metabolism in the brain and hence contribute to the regulation of adenosine-dependent sleep homeostatis. Finally, astrocytes are chemosensing elements of the brain involved in glucose, oxygen, CO2 sensing and contributing to systemic homeostasis of ions, metabolites and energy.
Astrocytes of higher primates and humans are substantially larger and more complex compared to other species studied so far. In addition, the humanoid brain contains several types of astroglia (interlaminar astrocytes, polarised astrocytes and varicose projection astrocytes) that do not appear in other species such as rodents (Colombo, 2018; Verkhratsky et al., 2018). Notably, human astrocytes grafted into the brains of mice retained their specific morphological appearance and conferred enhanced memory and the ability to learn. Whether this reflects higher supportive abilities of human astroglia or indeed indicates their contribution to higher cognitive abilities remains to be addressed.
Unsurprisingly, in light of their essential homeostatic functions, astrocytes are an important element of virtually all neurological disorders (Pekny et al., 2016). Pathological changes in astrocytes can be generally classified into reactive responses, degeneration with morphological atrophy and loss of function and pathological remodelling. Reactive astrogliosis represents a complex remodelling of astrocytes, accompanied by changes in gene expression and functional changes; astrogliosis is a genuinely defensive and evolutionarily conserved response, the inhibition of which, as a rule, exacerbates neuropathological development and jeopardises post lesion regeneration (Burda and Sofroniew, 2014). Hence, astrocytes are therapeutic targets for promoting neuroprotection, for example, in stroke and Parkinson’s disease (Song et al., 2018; Vermehren et al., 2018). The astroglial component of neuropathology is highly variable and is often disease specific. Astroglial atrophy with loss of function is observed in major psychiatric diseases (schizophrenia, temporal lobe epilepsy and major depressive disorder), whereas astrodegeneration and compromised astroglial glutamate uptake plays a leading role in excitotoxicity in ALS, in Korsakoff–Wernicke syndrome and in toxic encephalopathies. Atrophic changes in astroglia are also observed in several types of neurodegeneration including Alzheimer’s disease (AD; Rodríguez et al., 2016). Astroglial expression of abnormal tau proteins seems to be the key element in astrogliopathy that underlies several types of age-related dementia.
Oligodendrocytes
The modern era has seen the recognition that without myelin the human brain simply could not have evolved. Besides speeding up electrical transmission up to 100-fold, which is essential for the massive computing power of the human brain, myelin also provides for the miniaturisation of this supercomputer – it is calculated that without myelin each human optic nerve would need to be 0.75 m in diameter to conduct information as rapidly as it does. The importance of myelin is profoundly demonstrated in demyelinating diseases, such as multiple sclerosis (MS), where the specific loss of myelin has devastating effects on the CNS function and neurodegeneration. The development of transgenic mice has dramatically increased our knowledge of the mechanisms regulating oligodendrogenesis and myelination. Fate-map studies in transgenic mice by Akiko Nishiyama, William Richardson and others have demonstrated that new oligodendrocytes are generated throughout life from a pool of adult precursor cells (NG2-glia; Nishiyama et al., 2016). The lifelong generation of new oligodendrocytes in the adult is essential in pathology and for continued growth and replacement of myelin loss through natural ‘wear and tear’. A key finding by Richardson and colleagues is that the generation of oligodendrocytes in the adult is essential for the myelination of new connections developed in response to new life experiences and, what is more, the learning of new tasks is severely hampered without adaptive myelination (Xiao et al., 2016). Notably, in humans, white matter volume steadily increases until the age of 50, but declines thereafter, and this is accompanied by cognitive decline, which is accelerated in dementia; this has led to a myelinocentric view of cognitive decline and neurodegeneration (Rivera et al., 2016). This argument is strengthened by studies in transgenic mice targeting specific myelin genes, such as proteolipid protein (PLP) and 2′,3′-cyclic nucleotide-3′-phosphodiesterase (CNPase), which when ablated resulted in axonal degeneration. Studies by Klaus-Armin Nave and others have shown that oligodendrocytes via their myelin sheaths provide substrates and signals for axons that are essential for axonal function and long-term integrity (Simons and Nave, 2015). One such mechanism is that myelinating oligodendrocytes release lactate which is then utilised by axons for mitochondrial adenosine triphosphate (ATP) generation. These studies reaffirm that axons and oligodendrocytes are functionally interdependent units and that myelin is essential for axonal function and long-term integrity.
NG2-glia (polydendrocytes)
NG2-glia are defined by their expression of the chondroitin sulphate proteoglycan NG2 (cspg4) and are identified using antibodies against NG2, which was discovered in the 1980s and characterised in the 1990s by William Stallcup, Joel Levine and Akiko Nishiyama. NG2-glia are oligodendroglial in lineage and act as a pool of oligodendrocyte progenitor cells, which were first described in the early 1980s by Martin Raff, Charles ffrench-Constant, Mark Noble and colleagues. A major advance in recent years has been the use of genetic fate-mapping in Cre-loxP mice, which demonstrated that NG2-glia generate oligodendrocytes in both grey and white matter of the developing and adult brain (Nishiyama et al., 2016). Notably, up to 30% of oligodendrocytes in the adult are generated from NG2-glia after the age of 2–3 months in mice. In 2000, Dwight Bergles showed that NG2-glia are unique among glia in that they form direct synapses with neurons and display spontaneous and evoked synaptic currents ((Bergles et al., 2000). The sensing of neuronal activity by neurotransmitter receptors and ion channels in NG2-glia is likely to play an important role in driving oligodendrocyte generation, which in turn is essential for the learning of new tasks and higher cognitive function (Bergles and Richardson, 2015). Hence, NG2-glia are essential for the normal brain function and are inextricably involved in neuropathology.
Microglia
Microglial cells are of myeloid origin; they originate from foetal macrophages derived form the yolk sac; these macrophages invade the CNS at the very early developmental stages (Ginhoux et al., 2013). After entering the CNS, microglial precursors disseminate through the parenchyma and undergo complex morphological and functional metamorphosis. In the healthy brain, microglial cells appear in the ramified form with small cell body and long and much elaborated thin processes with multiple branches. Every microglial cell occupies its own territory, about 15–30 µm wide, with a little overlap between neighbouring territories. The processes of ramified microglial cells are constantly moving through its territory. This movement occurs at a speed of about 1.5 µm/min and thus microglial processes represent the fastest moving structures in the brain. At the same time, microglial processes also constantly send out and retract small protrusions, which can grow and shrink by 2–3 µm/min. Due to their highly motile processes, microglia scan through their domains, with particular attention to synaptic sites, where microglial processes rest for periods of minutes. Considering the velocity of this movement, the brain parenchyma can be completely scanned by microglial processes every several hours. Microglial cells express a full complement of receptors to neurotransmitters and neurohormones that allows them to follow the activity of neuronal networks (Kettenmann et al., 2011). Microglial cells secrete numerous signalling molecules that can interfere with synaptic transmission. In embryonic development, microglial cells contribute to phagocytotic removal of apoptotic neurons and to synaptic pruning, thus shaping neuronal networks.
In pathology, both in acute or chronic, microglial cells undergo activation that changes their phenotype and function. Activation of microglia is a multicomponent and complex process that results in multiple activated phenotypes that are often disease specific. Furthermore, it can be modified in the course of pathological evolution. In certain conditions, it may produce neurotoxic forms that exacerbate the disease, becoming a pathological component per se.
Future
The most intriguing question facing glial biologists is to unravel the role glial cells play in higher nervous function – cognition, emotions and consciousness – the things that distinguish Homo sapiens from other living forms. Recent studies in rodents have provided direct evidence that astrocytes, oligodendrocytes and NG2-glia play important roles in the learning of new tasks and behaviour. However, it remains difficult to extrapolate from mouse to man. Human astrocytes are much larger and much more complex than astroglia in other mammals. On average, the volume of human protoplasmic and fibrous astrocytes is 15–20 times larger than that of rodent astroglial cells, and human astrocytes have 10 times more primary processes. As a result, a single human protoplasmic astrocyte covers ~2 millions synapses, providing each astroglial cell with (potentially) exceptional integrative capacity. Furthermore, brains of humans and higher primates contain several types of specific astrocytes (such as interlaminar astrocytes, polarised astrocytes and varicose projection astrocytes), which all send long processes possibly involved in the interlayer integration. Most intriguingly, implantation of human glial cell progenitors into the mouse brain increased learning abilities of the rodents; the human progenitor cells retained their specific morphology and slowly replaced host astroglia (Han et al., 2013). However exciting these data are, they do not answer the ultimate question of the direct contribution of glia to intelligence; the increased performance of ‘astroglia-humanised’ mice may reflect higher homeostatic capabilities of human cells or remodelling of the host neuronal circuitry in response to some factors released by human grafted astrocytes. Similarly, microglial cells are the most ‘receptive’ cells in the CNS and their physiological role remains to be explored. The definitive experiments on the physiological importance of glial cells in higher brain functions lie ahead.
Perhaps, the major advance in the immediate future will be the reconsideration of the importance of glia in neuropathology. The neurocentric view has been challenged and the understudy of neuroglia is beginning to be appreciated. However, it remains that glial research is just a drop in the ocean compared to that on neurons. Gliopathology is diverse and complex and is generally represented by degeneration, a loss of essential homeostatic functions and pathological remodelling, often in combination and in a disease-specific manner. Degenerative changes in astrocytes and oligodendrocytes are observed at the earliest stages of neurodegenerative diseases and are the leading morphological changes in major psychiatric diseases and in the ageing brain. The role of microglia in these events is in its infancy, but their activity determines the overall neuroprotective or neurodestructive status in the CNS, and changes in the brain’s immune system are associated with ageing of the human brain. The true potential of glial cells as therapeutic targets is largely untapped. It may be an overstatement to suggest that the failure to cure neurodegenerative diseases in the last 100 years is because most researchers have been looking in the wrong direction, but for sure we cannot afford to neglect nerve glue for another 100 years.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
References
- Araque A, Carmignoto G, Haydon PG, et al. (2014) Gliotransmitters travel in time and space. Neuron 81(4): 728–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, et al. (1999) Tripartite synapses: Glia, the unacknowledged partner. Trends in Neuroscience 22(5): 208–215. [DOI] [PubMed] [Google Scholar]
- Bazargani N, Attwell D. (2016) Astrocyte calcium signaling: The third wave. Nature Neuroscience 19(2): 182–189. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Richardson WD. (2015) Oligodendrocyte development and plasticity. Cold Spring Harbor Perspectives in Biology 8(2): a020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Roberts JD, Somogyi P, et al. (2000) Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 405(6783): 187–191. [DOI] [PubMed] [Google Scholar]
- Blinzinger K, Kreutzberg G. (1968) Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Zeitschrift für Zellforschung und mikroskopische Anatomie 85(2): 145–157. [DOI] [PubMed] [Google Scholar]
- Boullerne AI. (2016) The history of myelin. Experimental Neurology 283(Pt B): 431–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda JE, Sofroniew MV. (2014) Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81(2): 229–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo JA. (2018) Interlaminar glia and other glial themes revisited: Pending answers following three decades of glial research. Neuroglia 1(1): 7–20. [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, et al. (1990) Glutamate induces calcium waves in cultured astrocytes: Long-range glial signaling. Science 247(4941): 470–473. [DOI] [PubMed] [Google Scholar]
- Fiacco TA, McCarthy KD. (2018) Multiple lines of evidence indicate that gliotransmission does not occur under physiological conditions. Journal of Neuroscience 38(1): 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Lim S, Hoeffel G, et al. (2013) Origin and differentiation of microglia. Frontiers in Cellular Neuroscience 7 Available online: 10.3389/fncel.2013.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Chen M, Wang F, et al. (2013) Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell 12(3): 342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Lee H, Yu M, et al. (2013) Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. Journal of Clinical Investigation 123(3): 1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Verkhratsky A. (2008) Neuroglia: The 150 years after. Trends in Neuroscience 31(12): 653–659. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, et al. (2011) Physiology of microglia. Physiological Reviews 91(2): 461–553. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Boshans L, Goncalves CM, et al. (2016) Lineage, fate, and fate potential of NG2-glia. Brain Research 1638(Pt B): 116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M, Pekna M, Messing A, et al. (2016) Astrocytes: A central element in neurological diseases. Acta Neuropathologica 131(3): 323–345. [DOI] [PubMed] [Google Scholar]
- Rivera A, Vanzuli I, Arellano JJ, et al. (2016) Decreased regenerative capacity of oligodendrocyte progenitor cells (NG2-Glia) in the ageing brain: A vicious cycle of synaptic dysfunction, myelin loss and neuronal disruption? Current Alzheimer’s Research 13(4): 413–418. [DOI] [PubMed] [Google Scholar]
- Rodríguez JJ, Butt AM, Gardenal E, et al. (2016) Complex and differential glial responses in Alzheimer’s disease and ageing. Current Alzheimer’s Research 13(4): 343–358. [DOI] [PubMed] [Google Scholar]
- Savtchouk I, Volterra A. (2018) Gliotransmission: Beyond black-and-white. Journal of Neuroscience 38(1): 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Nave KA. (2015) Oligodendrocytes: Myelination and axonal support. Cold Spring Harbor Perspectives in Biology 8(1): a020479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D, Ma K, Verkhratsky A, et al. (2018) L-Dopa and fluoxetine upregulate astroglial 5-HT2B receptors and ameliorate depression in Parkinson’s disease mice. Neuroglia 1(1): 48–62. [Google Scholar]
- Stobart JL, Ferrari KD, Barrett MJP, et al. (2018) Cortical circuit activity evokes rapid astrocyte calcium signals on a similar timescale to neurons. Neuron 98(4): 726.e4–735.e4. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Butt AM. (2013) Glial Physiology and Pathophysiology. Chichester: Wiley-Blackwell. [Google Scholar]
- Verkhratsky A, Nedergaard M. (2014) Astroglial cradle in the life of the synapse. Philosophical Transactions of the Royal Society London B Biological Science 369(1654): 20130595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Nedergaard M. (2018) Physiology of astroglia. Physiological Reviews 98(1): 239–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Matteoli M, Parpura V, et al. (2016) Astrocytes as secretory cells of the central nervous system: Idiosyncrasies of vesicular secretion. EMBO Journal 35(3): 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Oberheim Bush NA, Nedergaard M, et al. (2018) The special case of human astrocytes. Neuroglia 1(1): 21–29. [Google Scholar]
- Vermehren P, Trotman-Lucas M, Hechler B, et al. (2018) Cooperation between NMDA-Type glutamate and P2 receptors for neuroprotection during stroke: Combining astrocyte and neuronal protection. Neuroglia 1(1): 30–47. [Google Scholar]
- Xiao L, Ohayon D, McKenzie IA, et al. (2016) Rapid production of new oligodendrocytes is required in the earliest stages of motor skill learning. Nature Neuroscience 19(9): 1210–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]