Abstract
Adenosine 5′-triphosphate acts as an extracellular signalling molecule (purinergic signalling), as well as an intracellular energy source. Adenosine 5′-triphosphate receptors have been cloned and characterised. P1 receptors are selective for adenosine, a breakdown product of adenosine 5′-triphosphate after degradation by ectonucleotidases. Four subtypes are recognised, A1, A2A, A2B and A3 receptors. P2 receptors are activated by purine and by pyrimidine nucleotides. P2X receptors are ligand-gated ion channel receptors (seven subunits (P2X1-7)), which form trimers as both homomultimers and heteromultimers. P2Y receptors are G protein-coupled receptors (eight subtypes (P2Y1/2/4/6/11/12/13/14)). There is both purinergic short-term signalling and long-term (trophic) signalling. The cloning of P2X-like receptors in primitive invertebrates suggests that adenosine 5′-triphosphate is an early evolutionary extracellular signalling molecule. Selective purinoceptor agonists and antagonists with therapeutic potential have been developed for a wide range of diseases, including thrombosis and stroke, dry eye, atherosclerosis, kidney failure, osteoporosis, bladder incontinence, colitis, neurodegenerative diseases and cancer.
Keywords: Adenosine 5′-triphosphate, adenosine, P1 receptor, P2X receptor, P2Y receptor
Introduction
The potent actions of adenine compounds were first described by Drury and Szent-Györgyi in 1929. Years later, adenosine 5′-triphosphate (ATP) was proposed as the transmitter responsible for non-adrenergic, non-cholinergic transmission in the gut and bladder, and the term ‘purinergic’ was introduced by Burnstock in 1972. Early resistance to this concept was understandable since ATP was recognised first for its intracellular roles in many biochemical processes and the intuitive feeling was that such a ubiquitous and simple compound was unlikely to be utilised as an extracellular messenger. However, enzymes involved in the breakdown of extracellular ATP had already been described by the mid-1990s (see Yegutkin, 2008).
The concept of purinergic neurotransmission and the potent actions of extracellular ATP on many different cell types implied that there were purinergic membrane receptors. Purinergic receptors were described first in 1976 (Burnstock, 1976). Two years later, a basis for distinguishing two families of purinoceptors was proposed, identifying P1 and P2 receptors (for adenosine and ATP/adenosine 5′-diphosphate (ADP), respectively; Burnstock, 1978). Soon after, two subtypes of the P1 (adenosine) receptor were recognised (Londos et al., 1980; Van Calker et al., 1979). In 1985, a pharmacological basis for distinguishing two subtypes of P2 receptor (P2X and P2Y) was proposed (Burnstock and Kennedy, 1985). In the early 1990s, P1 (adenosine) receptors were cloned and characterised, and four subtypes were recognised (see Fredholm et al., 2001). In 1993, the first G protein-coupled P2Y receptors were cloned (Lustig et al., 1993; Webb et al., 1993) and a year later two P2X ligand-gated ion channel receptors (Brake et al., 1994; Valera et al., 1994). In 1994, it was proposed, on the basis of molecular structure and transduction mechanisms, that purinoceptors should belong to these two major families (Abbracchio and Burnstock, 1994). Currently, seven P2X receptor subtypes and eight P2Y receptor subtypes are recognised, including receptors that are sensitive to pyrimidines as well as purines (Abbracchio et al., 2006; North, 2002; Ralevic and Burnstock, 1998).
Purinergic signalling appears to be a primitive evolutionary system (see Verkhratsky and Burnstock, 2014). Many non-neuronal as well as neuronal mechanisms, including immune responses, exocrine and endocrine secretion, inflammation, pain, platelet aggregation and endothelial-mediated vasodilatation, involve purinergic signalling in mammals (Burnstock, 2006; Burnstock and Knight, 2004). Cell proliferation, differentiation and death that occur in development and regeneration are also mediated by purinergic receptors (Abbracchio and Burnstock, 1998; Burnstock and Verkhratsky, 2010). Reviews describe the history of the development of purinergic signalling and discuss future developments (Burnstock, 2012, 2014).
P1 receptors
Complementary DNAs encoding for two P1 receptor subtypes (A1 and A2) were isolated in 1989 (Libert et al., 1989). Soon after, the A3 subtype was identified (Zhou et al., 1992). Four different P1 receptor subtypes, A1, A2A, A2B and A3, were cloned and characterised in the early 1990s (see Fredholm et al., 2001). Polymorphisms have been observed in the A1 and the A2A receptors (Kumral et al., 2012). P1 receptors couple to adenylate cyclase; A1 and A3 are negatively coupled to adenylate cyclase, while A2A and A2B are positively coupled to adenylate cyclase (Reshkin et al., 2000). P1 subtype-selective agonists and antagonists have been identified (see Table 1). P1 receptor subtypes mediate diverse physiological effects, including modulation of cardiovascular, immune and central nervous system (CNS) activities (Ledent et al., 1997; Sun et al., 2001).
Table 1.
Characteristics of purine-mediated receptors.
| Receptor | Main distribution | Agonists | Antagonists | Transduction mechanisms | |
|---|---|---|---|---|---|
| P1 (Ado) | A1 | Brain, spinal cord, testis, heart, autonomic nerve terminals | CCPA > R-PIA = S-ENBA; CVT-510; GR79236 2′-MeCCPA, SDZ WAG 994, INO-8875, MRS 5474 |
DPCPX, N-0840, MRS1754, WRC-0571, PSB36, SLV320, CGS 16943, PQ-69 | Gi/Go ↓cAMP |
| A2A | Brain, heart, lungs, spleen | HENECA > CGS 21680 = CVT-3146; ATL-146e; Regadenoson | KF17837, SCH58261, ZM241385, KW 6002 | GS ↑cAMP | |
| A2B | Large intestine, bladder | Bay60-6583, NECA | PSB603, MRE-2029-F20, MRS1754, PSB0788 MRS1706, PSB1115, Alloxazine, GS-6201 | GS ↑cAMP | |
| A3 | Lung, liver, brain, testis, heart | IB-MECA > MRS5698 > MRS5168 > 2-Cl-IB-MECA; DBXRM; VT160; HEMADO | MRS1220, L-268605, MRS1191, MRS1523(rat), VUF8504, VUF5574, MRS1334(human), PSB10 | Gi/Go, Gq/G11, ↓cAMP, PLC-β activation | |
| P2X | P2X1 | Smooth muscle, platelets, cerebellum, dorsal horn spinal neurons | BzATP > ATP = 2-MeSATP ⩾ α,β-meATP = L-β,γ-meATP (rapid desensitisation); PAPET-ATP | NF864 > NF449 > IP5I ⩾ TNP-ATP > RO 0437626 > NF279, NF023, RO1, MRS2159 | Intrinsic cation channel (Ca2+ and Na+) |
| P2X2 | Smooth muscle, CNS, retina, chromaffin cells, autonomic and sensory ganglia, pancreas | ATP ⩾ ATPγS⩾2-MeSATP >> α,β-meATP (pH + zinc sensitive); β,γ-CF2ATP | PSB-1011 > RB2, isoPPADS > PPADS > Suramin, NF770, NF778, Aminoglycoside | Intrinsic ion channel (particularly Ca2+) | |
| P2X3 | Sensory neurones, NTS, some sympathetic neurons | 2-MeSATP ⩾ ATP ⩾ Ap4A ⩾ α,β-meATP (rapid desensitisation); PAPET-ATP; BzATP | TNP-ATP, AF353, A317491, RO3, isoPPADS > NF110 > PPADS, Ip5I, phenol red, RN-1838, Spinorphin | Intrinsic cation channel | |
| P2X4 | CNS, testis, colon, endothelial cells, microglia | ATP >> α,β-meATP >> CTP, 2-MeSATP Ivermectin potentiation | 5-BDBD >> TNP-ATP, PPADS > BBG, Paroxetine, phenolphthalein, CO donor (CORM 2), 5MPTP | Intrinsic ion channel (especially Ca2+) | |
| P2X5 | Proliferating cells in skin, gut, bladder, thymus, spinal cord, heart, adrenal medulla | ATP = 2-MeSATP = ATPγS >> α,β-meATP > AP4A | BBG > PPADS, Suramin | Intrinsic ion channel | |
| P2X6 | CNS, motor neurons in spinal cord | Only functions as a heteromultimer | – | Intrinsic ion channel | |
| P2X7 | Immune cells including dendritic cells (mast cells, macrophages), pancreas, skin, microglia | BzATP > ATP ⩾ 2-MeSATP >> α,β-meATP (clemastine potentiates) | KN62, BBG, KN04, MRS2427, O-ATP, RN-6189, Perazine, AZ10606120, A740003, A-438079, A-804598, GSK-1370319, Comp 31 (GSK), AZD-9056, CE-224,535, JNJ-47965567, JNJ-42253432 (penetrates BBB), decavanadate, AZ11657312 | Intrinsic cation channel and a large pore with prolonged activation | |
| P2Y | P2Y1 | Epithelial and endothelial cells, platelets, immune cells, osteoclasts, brain | MRS2365 > 2-MeSADP = Ap5(γB) >> ADPβS > ATP > 2-MeSATP = ADP | MRS2500 > MRS2279 > MRS2179, PIT, A3P5P | Gq/G11; PLC-β activation |
| P2Y2 | Immune cells, epithelial and endothelial cells, kidney tubules, osteoblasts | 2-thio-UTP > UTP, MRS2698 ⩾ ATP, INS 365 > INS 37217, UTPγS > Ap4A > MRS 2768, Up4-phenyl ester | AR-C126313 > Suramin > RB2, PSB-716, MRS2576, PSB-0402, AR-C118925 | Gq/G11 and possibly Gi/Go; PLC-β activation | |
| P2Y4 | Endothelial cells, placenta, spleen, thymus | 2′-azido-dUTP > UTPγS, UTP ⩾ ATP ⩾ Ap4A Up4U MRS4062 | ATP (human) > Reactive Blue 2 > Suramin, MRS2577, PPADS | Gq/G11 and possibly Gi; PLC-β activation | |
| P2Y6 | Airway and intestinal epithelial cells, placenta, T cells, thymus, microglia (activated) | MRS2693 > UDPβS, PSB0474 > INS48823, Up3U, 3-phenacyl-UDP >> UDP > UTP >> ATP, α,β-meUDP, MRS2957, MRS4129, 5-OMe-UDP αB | MRS2578 > Reactive Blue 2, PPADS, MRS2567, MRS2575 (human) | Gq/G11; PLC-β activation | |
| P2Y11 | Spleen, intestine, granulocytes | ATPγS > AR-C67085MX > BzATP ⩾ ATP, NF546, NAD+, NAADP+, Sp-2-propylthio-ATP-α-B | NF157 > Suramin > RB2, 5′-AMPS, NF340, AMP-α-5 | Gq/G11 and GS; PLC-β activation | |
| P2Y12 | Platelets, glial cells | 2-MeSADP ⩾ ADP > ATP, ADP-β-S | AR-C69931MX > AZD6140 (Ticagrelor), INS50589 > RB2 > 2-MeSAMP AR-C66096, CT50547, PSB-0413, Carba-nucleosides, MRS2395, AR-C67085, [3H]PSB-0413; clopidogrel, AZD1283; ACT-246475 | GαI; inhibition of adenylate cyclase | |
| P2Y13 | Spleen, brain, lymph nodes, bone marrow, erythrocytes | ADP = 2-MeSADP > 2-MeSATP, ATP | AR-C69931MX > AR-C67085 > MRS2211, 2-MeSAMP | Gi/Go | |
| P2Y14
GPR17 |
Placenta, adipose tissue, stomach, intestine, discrete brain regions, mast cells Oligodendrocytes |
MRS2690 > UDP > UDP glucose ⩾ UDP-galactose, UDP-glucosamine, MRS2905 Uracil nucleotides/cysteinyl-leukotrienes, MDL29,951 |
PPTN PZB01415033 |
Gq/G11
Gi, adenylate cyclase inhibition |
|
Source: Updated from Burnstock (2003), with permission from Elsevier.
A3P5P: adenosine-3′-5′-bisphosphate; ADP: adenosine 5′-diphosphte; ADPβS: adenosine-5′-(β-thio)-diphosphate; 5′-AMPS: 5′-O-thiomnophosphate; Ap4A: diadenosine tetraphosphate; Ap5(γβ): adenosine pentaphosphate (βγ); ATPγS: adenosine-5′-(γ-thio)-triphosphate; ATP: adenosine 5′-triphosphte; ATPγS: adenosine-5′-(γ-thio)-triphosphate; BBG: brilliant blue green; 5-BDBD: 5-(3-bromophenyl)-1,3-dihydro-2H-benzofuro[3,2-e]-1,4-diazepin-2-one; BzATP: 2′(3′)-O-(4-benzoylbenzoyl) adenosine 5′-triphosphate; β,γ-CF2ATP: α,β-difluoromethylene-ATP; cAMP: cyclic AMP; CCPA: chlorocyclopentyl adenosine; 2-Cl-IB-MECA: 2-chloro-N6-(3-iodobenzyl)-9-[5-(methylcarbamoyl)-β-d-ribofuranosyl]adenine; CNS: central nervous system; CORM 2: carbon monoxide donor 2; CTP: cytosine triphosphate; DBXRM: 1,3-dibutylxanthine 7-riboside 5′-N-methylcarboxamide; DPCPX: 1,3-dipropyl-8-cyclopentylxanthine; GTP: guanosine-5′-triphosphate; HEMADO: 2-(1-Hexynyl)-N-methyl adenosine; HENECA: 2-hexynyladenosine-59-N-ethylcarboxamide; IB-MECA: N6-(3-Iodobenzyl)-9-[5-(methylcarbamoyl)-β-d-ribofuranosyl]adenine; IP5I: di-inosine pentaphosphate; isoPPADS: iso-pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid; L-α,β-meATP: L-α,β-methylene ATP; L-β,γ-meATP: L-β,γ-methylene ATP; 2′-MeCCPA: 2-chloro-N-cyclopentyl-2′-methyladenosine; 2-MeSADP: 2-methylthio ADP; 2-MeSAMP: 2-methylthio AMP; 2-MeSATP: 2-methylthio ATP; α,β-meUDP: α,β-methylene UDP; NAADP+: nicotinic acid adenine dinucleotide phosphate; NAD+: nicotinamide adenine dinucleotide; NECA: 5′-N-ethylcarboxamido adenosine; NTS: nucleus tractus solitarius; oATP: oxidised ATP; PAPET: 2-[2-(4-aminophenyl)ethylthio]adenosine-5′-triphosphate; PIT: 2,2′-pyridylisatogen tosylate; PLC: phospholipase C; PPADS: pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid; RB2: reactive blue 2; R-PIA: R-phenylisopropyladenosine; S(–)-ENBA: (2S)-N6-[2-endo-Norbornyl]adenosine; 2-thio-UTP: 2-thio-uridine 5′-triphosphate; TNP-ATP: 2′(3′)-O-(2,4,6-trinitrophenyl) ATP; UDP: uridine 5′-diphosphate; UDPβS: uridine 5′-O-thiodiphosphate; Up3U: diuridine triphosphate; Up4-phenyl ester: uridine tetraphosphate phenyl ester; Up4U: diuridine tetraphosphate; UTP: uridine 5′-triphosphate; UTPγS: uridine 5′-O-3-thiotriphosphate.
P2X receptor subtype agonist potencies are based on rat preparations, while P1 and P2Y receptor subtype agonist potencies are based on human preparations (=, equal potency; >, greater potency; ⩾, greater than or equal potency).
P2X receptors
P2X1-7 receptors show a subunit topology of intracellular N- and C- termini possessing consensus binding motifs for protein kinases and two transmembrane-spanning regions (TM1 and TM2). TM1 is involved with channel gating and TM2 lining the ion pore. There is a large extracellular loop, with 10 conserved cysteine residues (see Alves et al., 2014; North, 2002; Rokic and Stojilkovic, 2013). The stoichiometry of P2X1-7 receptors involves three subunits, which form a stretched trimer or a hexamer of conjoined trimers (Nicke et al., 1998; North, 2002; Stelmashenko et al., 2012). Heteromultimers as well as homomultimers are involved in forming the trimer ion pore (Nicke et al., 1998), including P2X2/3, P2X1/2, P2X1/5, P2X2/6, P2X4/6 and P2X1/4 receptors. Advances have been made by the use of knockout mice for P2X1, P2X2, P2X3, P2X4 and P2X7 receptors and transgenic mice that overexpress the P2X1 receptor. The association of various diseases with P2X receptor polymorphisms has been described (see Caseley et al., 2014).
P2X receptor subtypes
A complementary DNA (cDNA) encoding the P2X1 receptor was made from rat vas deferens (Valera et al., 1994). The agonist actions of ATP by α,β-methylene ATP (α,β-meATP) distinguish P2X1 and P2X3 receptors from the other homomeric forms. Several antagonists have been recognised to be selective for P2X1 receptors (see Table 1). Adenoviral expression of a P2X1 receptor-green fluorescent protein construct shows the receptor to be localised in clusters in vas deferens, with larger clusters apposing nerve varicosities (Burnstock, 2007).
Rat P2X2 receptor cDNA was isolated from PC12 cells (Brake et al., 1994) and human receptor cDNA from pituitary gland (Lynch et al., 1999). No agonists are currently known at present that are selective for P2X2 receptors. However, protons and low concentrations of zinc and copper potentiate P2X2 receptors. Antagonists for P2X2 receptors are shown in Table 1. The P2X2 receptor is non-desensitising, compared with the P2X1 and P2X3 receptors.
P2X1/2 heteromultimer receptors have been described in de-folliculated Xenopus oocytes (Brown et al., 2002; Calvert and Evans, 2004). pH sensitivity is characteristic of heteromeric P2X1/2 ion channels.
P2X3 receptor subunit cDNAs were taken from rat dorsal root ganglion cDNA libraries (Chen et al., 1995; Lewis et al., 1995), from a human heart cDNA library (Garcia-Guzman et al., 1997) and a zebrafish library (Egan et al., 2000). The antagonist NF023 is about 20 times less effective at P2X3 compared to P2X1 receptors (see Table 1). P2X3 receptors are prominently expressed on sensory neurons, including nociceptive nerve endings (Burnstock, 2003).
P2X2/3 heteromer receptors have been described (Lewis et al., 1995; Spelta et al., 2003). They exhibit a sustained current elicited by α,β-meATP. However, like homomeric P2X2 receptors, they are potentiated by low pH and, in common with the homomeric P2X3 receptor, they are very sensitive to block by 2′(3′)-O-(2,4,6-trinitrophenyl) ATP. P2X2/3 receptors are expressed by subpopulations of sensory neurons, sympathetic ganglion cells and brain neurons.
cDNAs for rat P2X4 receptors were isolated from both superior cervical ganglion (SCG) and brain (Bo et al., 1995; Buell et al., 1996). cDNAs for human, mouse, chick and Xenopus have also been isolated. P2X4 receptors are activated by ATP, but not by α,β-meATP. A useful distinguishing feature of ATP-evoked currents at P2X4 receptors is their potentiation by ivermectin. Unusual among the P2X receptors, the rat P2X4 receptor shows relative insensitivity to blockade by the conventional non-selective antagonists suramin and pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS). Carbamazepine derivatives have been claimed to be potent P2X4 receptor antagonists (Tian et al., 2014; see Table 1). Currents evoked by ATP at the mouse P2X4 receptor are increased by PPADS and suramin, probably because they are also ectonucleotidase inhibitors (Burnstock, 2003).
P2X1/4 heteromeric receptors were shown to have kinetic properties resembling homomeric P2X4 receptors and a pharmacological profile similar to homomeric P2X1 receptors (Nicke et al., 2005). An important advance was made when the crystal structure of the P2X4 receptor was described (Kawate et al., 2009; Figure 1).
Figure 1.
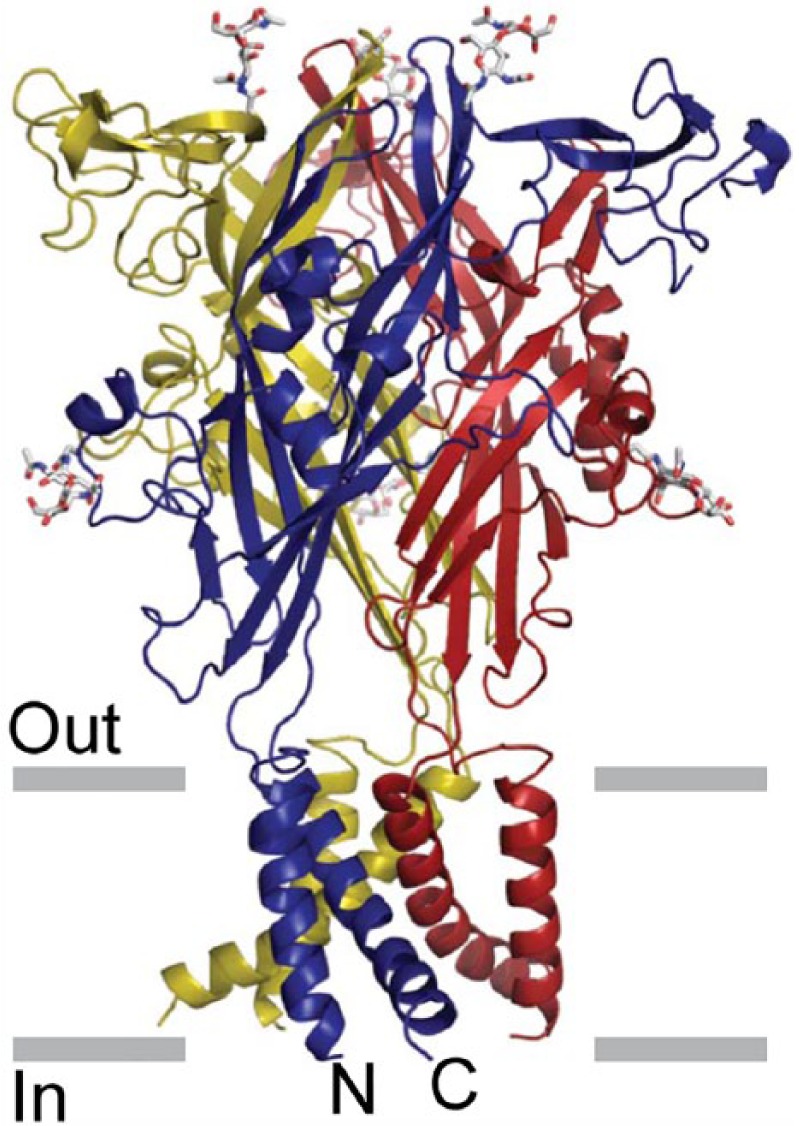
The architecture of the P2X4 receptor. Stereoview of the homotrimeric ΔzfP2X4 structure viewed parallel to the membrane. Each subunit is depicted in a different colour. N-acetylglucosamine (NAG) and glycosylated asparagine residues are shown in stick representation. The grey bars suggest the boundaries of the outer (out) and inner (in) leaflets of the membrane bilayer.
Source: Reproduced from Kawate et al. (2009), with permission from the Nature Publishing Group.
P2X5 receptor cDNA was first isolated from rat coeliac ganglion and heart (Burnstock, 2003; Garcia-Guzman et al., 1996). It was also cloned from embryonic chick skeletal muscle (Bo et al., 2000) and a bullfrog P2X5 receptor from larval skin. Human P2X5 receptor cDNAs are missing exon 10 (hP2X5a) or exons 3 and 10 (P2X5b) (Roberts et al., 2006; Stojilkovic et al., 2005). Currents elicited by ATP in cells expressing the rat P2X5 receptor are of small amplitude, compared with the currents observed with P2X1, P2X2, P2X3 or P2X4 receptors. P2X5 mRNA is highly expressed in developing skeletal muscle (Burnstock, 2003).
The P2X1/5 heteromer is characterised by a sustained current evoked by α,β-meATP, which does not occur for either of the homomers when expressed separately (Burnstock, 2007; Surprenant et al., 2000). Cells expressing P2X1/5 receptors are more sensitive to ATP than those with homomeric receptors. P2X2/5 heteromeric receptors have also been recognised (Compan et al., 2012).
The rat P2X6 receptor was cloned from SCG (Burnstock, 2007) and rat brain (Soto et al., 1996). The human equivalent was isolated from peripheral lymphocytes and was abundantly expressed in human and mouse skeletal muscle. The P2X6 subunit is only functionally expressed as a heteromultimer.
Heteromeric P2X2/6 receptors were expressed in HEK293 cells (Torres et al., 1999). The most convincing difference in oocytes expressing P2X2/6 receptors compared to those expressing only P2X2 receptors is that at pH 6.5 the inhibition of the current by suramin is biphasic (King et al., 2000). P2X2/6 receptors are expressed particularly by respiratory neurons in the brain stem.
P2X4/6 heteromeric receptors were described in oocytes (Khakh et al., 1999). The P2X4/6 heteromer differs only in minor respects from that of P2X4 homomers. They are prominently expressed in adult trigeminal mesencephalic nucleus and in hippocampal CA1 neurons (North, 2002).
A chimeric cDNA encoding the rat P2X7 receptor was first isolated from SCG and medial habenula. Full-length cDNAs were later isolated from a rat brain cDNA library (Surprenant et al., 1996). The unique feature of the P2X7 receptor is that in addition to the usual rapid opening of the cation-selective ion channel, with prolonged exposure to high concentrations of ATP it undergoes a channel to pore conversion to allow the passage of large dye molecules such as ethidium and YO-PRO-1. This often leads to apoptotic cell death. 2′,3′-O-(benzoyl-4-benzoyl)-ATP (BzATP) is a potent, although not selective, agonist at the P2X7 receptor. After continuous application of BzATP (30 µM) for about 30 s, the plasma membrane develops large blebs. Blebs are usually preceded by the shedding of smaller vesicles (<1 µm diameter) that release inflammatory cytokines. A number of potent antagonists have been developed (see Table 1).
P2Y receptors
P2Y1 and P2Y2 receptors were cloned in 1993 (Lustig et al., 1993; Webb et al., 1993). Since then several other subtypes have been isolated by homology cloning. The Gi-coupled ADP receptor (P2Y12) of platelets was isolated by expression cloning in 2001 (Hollopeter et al., 2001). P2Y13 and P2Y14 receptors were characterised during a study of orphan receptors (Chambers et al., 2000; Communi et al., 2001a). At present, there are eight recognised human P2Y receptors: P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13 and P2Y14 (Abbracchio et al., 2003, 2006; see Table 1). The missing numbers represent either non-mammalian orthologs or receptors which have no functional evidence of responsiveness to nucleotides. A p2y8 receptor was cloned from the frog embryo, which was shown to be involved in the development of the neural plate (Bogdanov et al., 1997). A P2Y-like receptor, GPR17, has been described (Parravicini et al., 2008). Two distinct P2Y receptor subgroups characterised by a relatively high level of sequence divergence have been identified (Abbracchio et al., 2006). The first subgroup includes P2Y1,2,4,6,11 and the second subgroup includes the P2Y12,13,14 subtypes (see Figure 2). For some of the P2Y receptor subtypes, potent and selective synthetic agonists and antagonists have been identified (see Table 1). Activation of several P2Y receptors is associated with the stimulation of mitogen-activated protein kinase (MAPK; Abbracchio et al., 2006). Ion channel couplings of P2Y receptors are primarily of importance in neurons, but they have been detected in various other tissues, including cardiac muscle cells (Abbracchio et al., 2006; Vassort, 2001). Polymorphisms of P2Y1, P2Y2 and P2Y12 receptors have been identified associated with various diseases (Läer et al., 2013; Lev et al., 2007; Wesselius et al., 2013). There is dimerisation involving P2Y receptors with non-P2Y receptors, for example, rat P2Y1 and adenosine A1 receptors in neurons, in rat cortex, hippocampus and cerebellum (Yoshioka et al., 2002). Functional P2Y1 and P2Y2 receptors, co-localised at the neuromuscular junctions with nicotinic acetylcholine (ACh) receptors, have been reported in mammalian, chicken and amphibian muscles.
Figure 2.
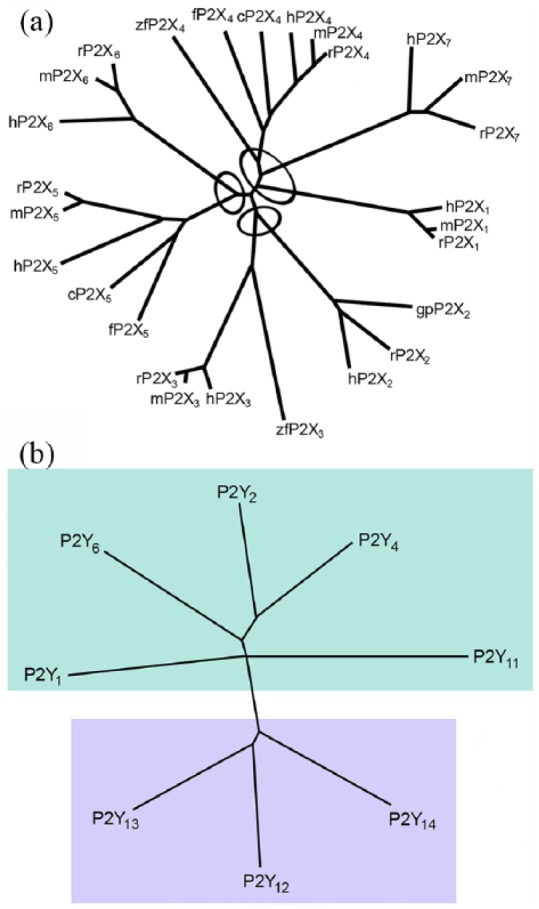
(a) Dendrogram to show relatedness of 29 P2X receptor subunits. Full-length amino acid sequences were aligned with Clustal W using default parameters. The dendrogram was constructed with TreeView. h, human (Homo sapiens); r, rat (Rattus norvegicus); m, mouse (Mus musculus); gp, guinea pig (Cavia porcellus); c, chicken (Gallus gallus); zf, zebrafish (Danio rerio); bf, bullfrog (Rana catesbeiana); x, claw-toed frog (Xenopus laevis); f, fugu (Takifugu rubripes). The ellipses indicate the apparent clustering by relatedness into subfamilies. Source: Reproduced from North (2002), with permission from the American Physiological Society. (b) A phylogenetic tree (dendrogram) showing the relationships among the current members of the P2Y receptor family (human P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12 and P2Y13 receptors) and the human UDP-glucose receptor (here indicated as the P2Y14 receptor). The P2Y receptors can be divided into two subgroups shown with green and lilac backgrounds. Sequences were aligned using CLUSTALX and the tree was built using the TREEVIEW software. Source: Reproduced from Abbracchio et al. (2003), with permission from Elsevier.
P2Y receptor subtypes
P2Y1 receptors have been cloned and characterised in human, rat, mouse, cow, chick, turkey and Xenopus tissues. ADP is a more potent agonist than ATP and 2-methylthio ADP is even more potent. The most effective antagonists to display selectivity for the P2Y1 receptor at present are MRS2179, MRS2279 and MRS2500 (see Table 1). P2Y1 mRNA is high in different regions of the human brain, prostate gland and placenta (Burnstock and Knight, 2004; Léon et al., 1996). In post-mortem brain sections from Alzheimer’s disease patients, the P2Y1-like immunoreactivity in the hippocampus and entorhinal cortex was localised to neurofibrillary tangles, neuritic plaques and neuropil threads (Moore et al., 2000).
P2Y2 receptors have been cloned and characterised from human, rat, mouse, canine and porcine cells or tissues (Shen et al., 2004). P2Y2 receptors are activated by equivalent concentrations of ATP and UTP. The γ-thiophosphate, UTPγS and INS 37217 are potent hydrolysis-resistant agonists of P2Y2 receptors (Abbracchio et al., 2006). Current P2Y2 receptor antagonists are shown in Table 1. Expression of P2Y2 receptor mRNA has been described in many tissues (Burnstock and Knight, 2004). P2Y2 receptor expression in smooth muscle cells is up-regulated by inflammatory agents, including interleukin-1β, interferon-γ and tumour necrosis factor-α (Hou et al., 2000). In epithelial cells, P2Y2 receptor activation increases Cl− secretion and inhibits Na+ absorption (Kellerman et al., 2002). A P2Y2 receptor knockout mouse has been described that is defective in nucleotide-stimulated ion secretion in airway epithelial cells (Cressman et al., 1999). P2Y2 receptors inhibit bone formation by osteoblasts (Hoebertz et al., 2002) and N-type calcium currents in neurons (Brown et al., 2000).
P2Y4 receptors have been cloned and characterised from human, rat and mouse. UTP is the most potent activator of the human P2Y4 receptor (Communi et al., 2004). However, the recombinant rat and mouse P2Y4 receptors are activated equipotently by ATP and UTP (Bogdanov et al., 1998). Reactive Blue 2 blocks rat P2Y4 receptors, but only partially blocks human P2Y4 receptors. P2Y4 mRNA and protein was most abundant in the intestine of both mouse and humans, but has also been described in other organs (Burnstock and Knight, 2004). P2Y4-null mice show normal behaviour, growth and reproduction; however, the chloride secretory response to apical UTP and ATP of the jejunal epithelium was abolished (Robaye et al., 2003).
P2Y6 receptors are selective for UDP in mouse, rat and human (Lazarowski et al., 2001). Selective agonists and antagonists are described in Table 1. A wide tissue distribution of P2Y6 mRNA and protein has been reported, and the highest expression was in spleen, intestine, liver, brain and pituitary (Burnstock and Knight, 2004).
The human P2Y11 receptor has a unique profile (Abbracchio et al., 2006). The hP2Y11 receptor gene differs from other P2Y receptor subtype genes having the presence of a 1.9-kb intron in the coding sequence that separates an exon encoding the first 6 amino acid residues from a second exon encoding the remaining part of the protein (Communi et al., 2001b).
P2Y12 receptors have been identified and characterised in human, rat and mouse (Abbracchio et al., 2006). ADP is the agonist of this receptor. The P2Y12 receptor is highly expressed in platelets where it is the molecular target of antiplatelet drugs, clopidogrel and ticagrelor (Savi and Herbert, 2005). The P2Y12 receptor is also expressed in sub-regions of the brain, glial cells, brain capillary endothelial cells, smooth muscle cells and chromaffin cells (Burnstock and Knight, 2004). P2Y13 receptors have been identified and characterised in human, mouse and rat (Abbracchio et al., 2006). Naturally occurring agonists of the P2Y13 receptor are ADP and di-adenosine triphosphate. Selective antagonists of the human P2Y13 receptor are shown in Table 1. The P2Y13 receptor is expressed in spleen, placenta, liver, heart, bone marrow, monocytes, T cells, lung and various regions of the brain (Fumagalli et al., 2004).
The P2Y14 receptor is 47% identical to the P2Y12 and P2Y13 receptors. The gene for this receptor is in human chromosome 3q24-3q25 (Abbracchio et al., 2003). The P2Y14 receptor is activated by UDP-glucose and is coupled to the Gi/o family of G proteins (Harden, 2004). P2Y14 mRNA is distributed in many systems in the human body. Chemoattractant and neuroimmune functions have been claimed for the P2Y14 receptor.
Physiology of purinergic signalling
Early studies were focused largely on short-term purinergic signalling in neurotransmission, neuromodulation, secretion, chemoattraction and acute inflammation, but there is increasing interest in long-term (trophic) signalling involving cell proliferation, differentiation, motility and death in development, regeneration, wound healing, restenosis, epithelial cell turnover, cancer and ageing (Abbracchio and Burnstock, 1998; Burnstock and Verkhratsky, 2010). In blood vessels, there is dual purinergic short-term control of vascular tone by ATP (Burnstock, 2002): ATP released as a cotransmitter from perivascular sympathetic nerves activate P2X receptors resulting in contraction of smooth muscle; ATP released from endothelial cells during changes in blood flow (shear stress) and hypoxia acts on P2X and P2Y receptors on endothelial cells, resulting in production of nitric oxide and relaxation. There is also long-term control of cell proliferation and differentiation, migration and death involved neovascularisation, restenosis following angioplasty and atherosclerosis (Erlinge and Burnstock, 2008). Purinergic signalling is involved in development, ageing and regeneration (Burnstock, 2007). Purinergic receptors are expressed on stem cells (Burnstock and Ulrich, 2011; Delic and Zimmermann, 2010; Trujillo et al., 2009). Many cell types release ATP physiologically in response to gentle mechanical distortion, hypoxia or some agents (Bodin and Burnstock, 2001). The mechanism of ATP transport includes, in addition to vesicular release, ABC transporters, connexin or pannexin hemi-channels, maxi-ion channels and even P2X7 receptors (Burnstock, 2007; Lazarowski et al., 2011). Extracellular breakdown of released ATP is by ectonucleotidases, including E-NTPDases, E-NPPS, alkaline phosphatase and ecto-5′-nucleotidose (Yegutkin, 2008; Zimmermann et al., 2007). P1 and P2 receptors are involved in neurotransmission and neuromodulation in the CNS, and in normal behaviour, including memory, feeding, locomotion and cognition (Burnstock et al., 2011).
Purinergic pathophysiology and therapeutic potential
ATP was shown early to be a major cotransmitter with ACh in parasympathetic nerves mediating contraction of the urinary bladder of rodents (Burnstock et al., 1978). However, in healthy human bladder, the role of ATP as a cotransmitter is minor, but under pathological conditions, such as interstitial cystitis, outflow obstruction and most types of neurogenic bladder, the purinergic component is increased to about 40% (Burnstock, 2001, 2006). There is also a significantly greater cotransmitter role for ATP in sympathetic nerves in spontaneously hypertensive rats (Vidal et al., 1986). P2X3 receptors are located on small nociceptive sensory nerves and are involved in the initiation of pain (Bradbury et al., 1998; Burnstock, 1996, 2013; Chen et al., 1995). There are peripheral extensions of the sensory nerves in skin, tongue and visceral organs and central projections to inner lamina 2 of the spinal cord. A hypothesis describing purinergic mechanosensory transduction and pain in visceral organs was published in 1999 (Burnstock, 1999). It was proposed that ATP, released from urothelial/epithelial cells during distension, acts on P2X3 and P2X2/3 receptors on subepithelial sensory nerve endings to send nociceptive messages via sensory ganglia to the pain centres in the brain (Burnstock, 1999). Supporting evidence has been reported in the bladder (Vlaskovska et al., 2001), ureter (Rong and Burnstock, 2004) and gut (Wynn and Burnstock, 2006). Purinergic mechanosensory transduction via low threshold fibres is also involved in urine voiding (Cockayne et al., 2000). Antagonist to P2X4, P2X7 and P2Y12 receptors on microglia have been shown to reduce neuropathic and inflammatory pain (Burnstock, 2009; Inoue, 2007). There is increasing attention to the potential roles of purinergic signalling in trauma, ischaemia and neurodegenerative conditions, including Alzheimer’s, Parkinson’s and Huntington’s diseases, multiple sclerosis and amyotrophic lateral sclerosis (Burnstock, 2007; Sperlágh and Illes, 2014). The involvement of purinergic signalling in epilepsy, neuropsychiatric diseases and mood disorders has also been reported (Boisson and Burnstock, 2010; Burnstock, 2008b).
Purinergic agents are being investigated for the treatment of disorders of the urinary tract (Burnstock, 2011), skeletal muscle (Ryten et al., 2004), gut (Burnstock, 2008a), bone (Burnstock and Arnett, 2006; Orriss et al., 2010), the cardiovascular system (Erlinge and Burnstock, 2008), kidney (Bailey et al., 2007; Taylor et al., 2009) and the reproductive system (Calvert et al., 2008; Gür et al., 2009). There are also reports that extracellular ATP acts on P2Y2 receptors to facilitate HIV-1 infection (e.g. Séror et al., 2011). The therapeutic potential of purinergic compounds for the treatment of cancer is being explored (Burnstock and Di Virgilio, 2013; Shabbir and Burnstock, 2009; Shabbir et al., 2008; Stagg and Smyth, 2010; White and Burnstock, 2006; White et al., 2009). Selective purinoceptor agonists and antagonists with therapeutic potential are also being developed for thrombosis and stroke, atherosclerosis, kidney failure, osteoporosis, bladder incontinence and colitis (Bartlett et al., 2014; Burnstock, 2006, 2013). This would be facilitated by the discovery of selective purinoceptor agonists and antagonists that are orally bioavailable and stable in vivo.
Footnotes
Declaration of conflicting interests: The author declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author received no financial support for the research, authorship and/or publication of this article.
References
- Abbracchio MP, Burnstock G. (1994) Purinoceptors: Are there families of P2X and P2Y purinoceptors? Pharmacology & Therapeutics 64(3): 445–475. [DOI] [PubMed] [Google Scholar]
- Abbracchio MP, Burnstock G. (1998) Purinergic signalling: Pathophysiological roles. Japanese Journal of Pharmacology 78(2): 113–145. [DOI] [PubMed] [Google Scholar]
- Abbracchio MP, Boeynaems J-M, Barnard EA, et al. (2003) Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends in Pharmacological Sciences 24(2): 52–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbracchio MP, Burnstock G, Boeynaems J-M, et al. (2006) International Union of Pharmacology: Update on the P2Y G protein-coupled nucleotide receptors: From molecular mechanisms and pathophysiology to therapy. Pharmacological Reviews 58(3): 281–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves LA, da Silva JH, Ferreira DN, et al. (2014) Structural and molecular modeling features of P2X receptors. International Journal of Molecular Sciences 15(3): 4531–4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MA, Shirley DG, King BF, et al. (2007) Extracellular nucleotides and renal function. In: Alpern RJ and Herbert SCG (eds) The Kidney: Physiology and Pathophysiology (4th edn). San Diego, CA: Elsevier, pp. 425–443. [Google Scholar]
- Bartlett R, Stokes L, Sluyter R. (2014) The P2X7 receptor channel: Recent developments and the use of P2X7 antagonists in models of disease. Pharmacological Reviews 66(3): 638–675. [DOI] [PubMed] [Google Scholar]
- Bo X, Schoepfer R, Burnstock G. (2000) Molecular cloning and characterization of a novel ATP P2X receptor subtype from embryonic chick skeletal muscle. Journal of Biological Chemistry 275(19): 14401–14407. [DOI] [PubMed] [Google Scholar]
- Bo X, Zhang Y, Nassar M, et al. (1995) A P2X purinoceptor cDNA conferring a novel pharmacological profile. FEBS Letters 375: 129–133. [DOI] [PubMed] [Google Scholar]
- Bodin P, Burnstock G. (2001) Purinergic signalling: ATP release. Neurochemical Research 26(1-2): 959–969. [DOI] [PubMed] [Google Scholar]
- Bogdanov YD, Dale L, King BF, et al. (1997) Early expression of a novel nucleotide receptor in the neural plate of Xenopus Embryos. Journal of Biological Chemistry 272(19): 12583–12590. [DOI] [PubMed] [Google Scholar]
- Bogdanov YD, Wildman SS, Clements MP, et al. (1998) Molecular cloning and characterization of rat P2Y4 nucleotide receptor (Special report). British Journal of Pharmacology 124(3): 428–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson D, Burnstock G. (eds) (2010) Purinergic signalling in epilepsy. The Open Neuroscience Journal 4: 23–113. [Google Scholar]
- Bradbury EJ, Burnstock G, McMahon SB. (1998) The expression of P2X3 purinoceptors in sensory neurons: Effects of axotomy and glial-derived neurotrophic factor. Molecular and Cellular Neurosciences 12(4-5): 256–268. [DOI] [PubMed] [Google Scholar]
- Brake AJ, Wagenbach MJ, Julius D. (1994) New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature 371(6497): 519–523. [DOI] [PubMed] [Google Scholar]
- Brown DA, Filippov AK, Barnard EA. (2000) Inhibition of potassium and calcium currents in neurones by molecularly-defined P2Y receptors. Journal of the Autonomic Nervous System 81: 31–36. [DOI] [PubMed] [Google Scholar]
- Brown SG, Townsend-Nicholson A, Jacobson KA, et al. (2002) Heteromultimeric P2X1/2 receptors show a novel sensitivity to extracellular pH. Journal of Pharmacology and Experimental Therapeutics 300(2): 673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buell G, Collo G, Rassendren F. (1996) P2X receptors: An emerging channel family. European Journal of Neuroscience 8(10): 2221–2228. [DOI] [PubMed] [Google Scholar]
- Burnstock G. (1972) Purinergic nerves. Pharmacological Reviews 24(3): 509–581. [PubMed] [Google Scholar]
- Burnstock G. (1976) Purinergic receptors. Journal of Theoretical Biology 62(2): 491–503. [DOI] [PubMed] [Google Scholar]
- Burnstock G. (1978) A basis for distinguishing two types of purinergic receptor. In: Straub RW, Bolis L. (eds) Cell Membrane Receptors for Drugs and Hormones: A Multidisciplinary Approach. New York: Raven Press, pp. 107–118. [Google Scholar]
- Burnstock G. (1996) A unifying purinergic hypothesis for the initiation of pain. Lancet 347(9015): 1604–1605. [DOI] [PubMed] [Google Scholar]
- Burnstock G. (1999) Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. Journal of Anatomy 194(3): 335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. (2001) Purinergic signalling in lower urinary tract. In: Abbracchio MP, Williams M. (eds) Handbook of Experimental Pharmacology: Purinergic and Pyrimidinergic Signalling I – Molecular, Nervous and Urinogenitary System Function, vol. 151/I Berlin: Springer-Verlag, pp. 423–515. [Google Scholar]
- Burnstock G. (2002) Purinergic signalling and vascular cell proliferation and death. Arteriosclerosis, Thrombosis, and Vascular Biology 22(3): 364–373. [DOI] [PubMed] [Google Scholar]
- Burnstock G. (2003) Introduction: ATP and its metabolites as potent extracellular agonists. In: Schwiebert EM. (ed.) Current Topics in Membranes: Purinergic Receptors and Signalling, vol. 54 San Diego, CA: Academic Press, pp. 1–27. [Google Scholar]
- Burnstock G. (2006) Pathophysiology and therapeutic potential of purinergic signaling. Pharmacological Reviews 58(1): 58–86. [DOI] [PubMed] [Google Scholar]
- Burnstock G. (2007) Physiology and pathophysiology of purinergic neurotransmission. Physiological Reviews 87(2): 659–797. [DOI] [PubMed] [Google Scholar]
- Burnstock G. (2008. a) Commentary: Purinergic receptors as future targets for treatment of functional GI disorders. Gut 57(9): 1193–1194. [DOI] [PubMed] [Google Scholar]
- Burnstock G. (2008. b) Purinergic signalling and disorders of the central nervous system. Nature Reviews Drug Discovery 7(7): 575–590. [DOI] [PubMed] [Google Scholar]
- Burnstock G. (2009) Purinergic receptors and pain. Current Pharmaceutical Design 15(15): 1717–1735. [DOI] [PubMed] [Google Scholar]
- Burnstock G. (2011) Therapeutic potential of purinergic signalling for diseases of the urinary tract. BJU International 107(2): 192–204. [DOI] [PubMed] [Google Scholar]
- Burnstock G. (2012) Purinergic signalling: Its unpopular beginning, its acceptance and its exciting future. Bioessays 34(3): 218–225. [DOI] [PubMed] [Google Scholar]
- Burnstock G. (2013) Purinergic mechanisms and pain – An update. European Journal of Pharmacology 716(1-3): 24–40. [DOI] [PubMed] [Google Scholar]
- Burnstock G. (2014) Cotransmission. In: Caplan M. (ed.). Reference Module in Biomedical Sciences. Oxford: Elsevier, pp. 1–8. [Google Scholar]
- Burnstock G, Arnett TR. (2006) Edited Monograph: Nucleotides and Regulation of Bone Cell Function. Boca Raton, FL: Taylor & Francis Group. [Google Scholar]
- Burnstock G, Di Virgilio F. (2013) Purinergic signalling in cancer. Purinergic Signalling 9(4): 491–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Kennedy C. (1985) Is there a basis for distinguishing two types of P2-purinoceptor? General Pharmacology 16(5): 433–440. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Knight GE. (2004) Cellular distribution and functions of P2 receptor subtypes in different systems. International Review of Cytology 240: 31–304. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Ulrich H. (2011) Purinergic signalling in embryonic and stem cell development. Cellular and Molecular Life Sciences 68(8): 1369–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Verkhratsky A. (2010) Long-term (trophic) purinergic signalling: Purinoceptors control cell proliferation, differentiation and death. Cell Death & Disease 1: e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Cocks T, Kasakov L, et al. (1978) Direct evidence for ATP release from non-adrenergic, non-cholinergic (‘purinergic’) nerves in the guinea-pig taenia coli and bladder. European Journal of Pharmacology 49(2): 145–149. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Krügel U, Abbracchio MP, et al. (2011) Purinergic signalling: From normal behaviour to pathological brain function. Progress in Neurobiology 95(2): 229–274. [DOI] [PubMed] [Google Scholar]
- Calvert JA, Evans RJ. (2004) Heterogeneity of P2X receptors in sympathetic neurons: Contribution of neuronal P2X1 receptors revealed using knockout mice. Molecular Pharmacology 65(1): 139–148. [DOI] [PubMed] [Google Scholar]
- Calvert RC, Khan MA, Thompson CS, et al. (2008) A functional study of purinergic signalling in the normal and pathological rabbit corpus cavernosum. BJU International 101(8): 1043–1047. [DOI] [PubMed] [Google Scholar]
- Caseley EA, Muench SP, Roger S, et al. (2014) Non-synonymous single nucleotide polymorphisms in the P2X receptor genes: Association with diseases, impact on receptor functions and potential use as diagnosis biomarkers. International Journal of Molecular Sciences 15(8): 13344–13371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers JK, Macdonald LE, Sarau HM, et al. (2000) A G protein-coupled receptor for UDP-glucose. Journal of Biological Chemistry 275(15): 10767–10771. [DOI] [PubMed] [Google Scholar]
- Chen CC, Akopian AN, Sivilotti L, et al. (1995) A P2X purinoceptor expressed by a subset of sensory neurons. Nature 377(6548): 428–431. [DOI] [PubMed] [Google Scholar]
- Cockayne DA, Hamilton SG, Zhu Q-M, et al. (2000) Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 407(6807): 1011–1015. [DOI] [PubMed] [Google Scholar]
- Communi D, Gonzalez NS, Detheux M, et al. (2001. a) Identification of a novel human ADP receptor coupled to Gi. Journal of Biological Chemistry 276(44): 41479–41485. [DOI] [PubMed] [Google Scholar]
- Communi D, Robaye B, Boeynaems J-M. (2004) Nucleotide receptor P2Y4. Afcs-Nature Molecule Pages, ID A 001691. [Google Scholar]
- Communi D, Suarez-Huerta N, Dussossoy D, et al. (2001. b) Cotranscription and intergenic splicing of human P2Y11 and SSF1 genes. Journal of Biological Chemistry 276(19): 16561–16566. [DOI] [PubMed] [Google Scholar]
- Compan V, Ulmann L, Stelmashenko O, et al. (2012) P2X2 and P2X5 subunits define a new heteromeric receptor with P2X7-like properties. Journal of Neuroscience 32(12): 4284–4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressman VL, Lazarowski E, Homolya L, et al. (1999) Effect of loss of P2Y2 receptor gene expression on nucleotide regulation of murine epithelial Cl-transport. Journal of Biological Chemistry 274(37): 26461–26468. [DOI] [PubMed] [Google Scholar]
- Delic J, Zimmermann H. (2010) Nucleotides affect neurogenesis and dopaminergic differentiation of mouse fetal midbrain-derived neural precursor cells. Purinergic Signalling 6(4): 417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury AN, Szent-Györgyi A. (1929) The physiological activity of adenine compounds with special reference to their action upon the mammalian heart. Journal of Physiology 68(3): 213–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan TM, Cox JA, Voigt MM. (2000) Molecular cloning and functional characterization of the zebrafish ATP-gated ionotropic receptor P2X3 subunit. FEBS Letters 475(3): 287–290. [DOI] [PubMed] [Google Scholar]
- Erlinge D, Burnstock G. (2008) P2 receptors in cardiovascular physiology and disease. Purinergic Signalling 4(1): 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, IJzerman AP, Jacobson KA, et al. (2001) International union of pharmacology. XXV: Nomenclature and classification of adenosine receptors. Pharmacological Reviews 53(4): 527–552. [PMC free article] [PubMed] [Google Scholar]
- Fumagalli M, Trincavelli L, Lecca D, et al. (2004) Cloning, pharmacological characterisation and distribution of the rat G-protein-coupled P2Y13 receptor. Biochemical Pharmacology 68(1): 113–124. [DOI] [PubMed] [Google Scholar]
- Garcia-Guzman M, Soto F, Laube B, et al. (1996) Molecular cloning and functional expression of a novel rat heart P2X purinoceptor. FEBS Letters 388(2-3): 123–127. [DOI] [PubMed] [Google Scholar]
- Garcia-Guzman M, Stühmer W, Soto F. (1997) Molecular characterization and pharmacological properties of the human P2X3 purinoceptor. Brain Research, Molecular Brain Research 47(1-2): 59–66. [DOI] [PubMed] [Google Scholar]
- Gür S, Kadowitz PJ, Abdel-Mageed AS, et al. (2009) Management of erectile function by purinergic P2 receptors in diabetic rat. Journal of Urology 181(2374): 2375–2382. [DOI] [PubMed] [Google Scholar]
- Harden TK. (2004) Nucleotide receptor P2Y14. Afcs-Nature Molecule Pages ID A 002814. [Google Scholar]
- Hoebertz A, Mahendran S, Burnstock G, et al. (2002) ATP and UTP at low concentrations strongly inhibit bone formation by osteoblasts: A novel role for the P2Y2 receptor in bone remodelling. Journal of Cellular Biochemistry 86(3): 413–419. [DOI] [PubMed] [Google Scholar]
- Hollopeter G, Jantzen H-M, Vincent D, et al. (2001) Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature 409: 202–207. [DOI] [PubMed] [Google Scholar]
- Hou M, Moller S, Edvinsson L, et al. (2000) Cytokines induce upregulation of vascular P2Y2 receptors and increased mitogenic responses to UTP and ATP. Arteriosclerosis, Thrombosis and Vascular Biology 20(9): 2064–2069. [DOI] [PubMed] [Google Scholar]
- Inoue K. (2007) P2 receptors and chronic pain. Purinergic Signalling 3(1-2): 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawate T, Michel JC, Birdsong WT, et al. (2009) Crystal structure of the ATP-gated P2X4 ion channel in the closed state. Nature 460(7255): 592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellerman D, Evans R, Mathews D, et al. (2002) Inhaled P2Y2 receptor agonists as a treatment for patients with cystic fibrosis lung disease. Advanced Drug Delivery Reviews 54(11): 1463–1474. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Proctor WR, Dunwiddie TV, et al. (1999) Allosteric control of gating and kinetics at P2X4 receptor channels. Journal of Neuroscience 19(17): 7289–7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BF, Townsend-Nicholson A, Wildman SS, et al. (2000) Coexpression of rat P2X2 and P2X6 subunits in Xenopus oocytes. Journal of Neuroscience 20(13): 4871–4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumral A, Tuzun F, Yesilirmak DC, et al. (2012) Genetic basis of apnoea of prematurity and caffeine treatment response: Role of adenosine receptor polymorphisms: Genetic basis of apnoea of prematurity. Acta Paediatrica 101(7): e299–e303. [DOI] [PubMed] [Google Scholar]
- Läer K, Vennemann M, Rothämel T, et al. (2013) Association between polymorphisms in the P2RY1 and SSTR2 genes and sudden infant death syndrome. International Journal of Legal Medicine 127(6): 1087–1091. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Rochelle LG, O’Neal WK, et al. (2001) Cloning and functional characterization of two murine uridine nucleotide receptors reveal a potential target for correcting ion transport deficiency in cystic fibrosis gallbladder. Journal of Pharmacology and Experimental Therapeutics 297(1): 43–49. [PubMed] [Google Scholar]
- Lazarowski ER, Sesma JI, Seminario-Vidal L, et al. (2011) Molecular mechanisms of purine and pyrimidine nucleotide release. Advances in Pharmacology 61: 221–261. [DOI] [PubMed] [Google Scholar]
- Ledent C, Vaugeois JM, Schiffmann SN, et al. (1997) Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature 388(6643): 674–678. [DOI] [PubMed] [Google Scholar]
- Léon C, Vial C, Cazenave JP, et al. (1996) Cloning and sequencing of a human cDNA encoding endothelial P2Y1 purinoceptor. Gene 171(2): 295–297. [DOI] [PubMed] [Google Scholar]
- Lev EI, Patel RT, Guthikonda S, et al. (2007) Genetic polymorphisms of the platelet receptors P2Y12, P2Y1 and GP IIIa and response to aspirin and clopidogrel. Thrombosis Research 119(3): 355–360. [DOI] [PubMed] [Google Scholar]
- Lewis C, Neidhart S, Holy C, et al. (1995) Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature 377(6548): 432–435. [DOI] [PubMed] [Google Scholar]
- Libert F, Parmentier M, Lefort A, et al. (1989) Selective amplification and cloning of four new members of the G protein-coupled receptor family. Science 244(4904): 569–572. [DOI] [PubMed] [Google Scholar]
- Londos C, Cooper DM, Wolff J. (1980) Subclasses of external adenosine receptors. Proceedings of the National Academy of Sciences of the United States of America 77(5): 2551–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig KD, Shiau AK, Brake AJ, et al. (1993) Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proceedings of the National Academy of Sciences of the United States of America 90(11): 5113–5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch KJ, Touma E, Niforatos W, et al. (1999) Molecular and functional characterization of human P2X2 receptors. Molecular Pharmacology 56(6): 1171–1181. [DOI] [PubMed] [Google Scholar]
- Moore D, Iritani S, Chambers J, et al. (2000) Immunohistochemical localization of the P2Y1 purinergic receptor in Alzheimer’s disease. Neuroreport 11(17): 3799–3803. [DOI] [PubMed] [Google Scholar]
- Nicke A, Baumert HG, Rettinger J, et al. (1998) P2X1 and P2X3 receptors form stable trimers: A novel structural motif of ligand-gated ion channels. EMBO Journal 17(11): 3016–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicke A, Kerschensteiner D, Soto F. (2005) Biochemical and functional evidence for heteromeric assembly of P2X1 and P2X4 subunits. Journal of Neurochemistry 92(4): 925–933. [DOI] [PubMed] [Google Scholar]
- North RA. (2002) Molecular physiology of P2X receptors. Physiological Reviews 82(4): 1013–1067. [DOI] [PubMed] [Google Scholar]
- Orriss IR, Burnstock G, Arnett TR. (2010) Purinergic signalling and bone remodelling. Current Opinion in Pharmacology 10(3): 322–330. [DOI] [PubMed] [Google Scholar]
- Parravicini C, Ranghino G, Abbracchio MP, et al. (2008) GPR17: Molecular modeling and dynamics studies of the 3-D structure and purinergic ligand binding features in comparison with P2Y receptors. BMC Bioinformatics 9: 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. (1998) Receptors for purines and pyrimidines. Pharmacological Reviews 50(3): 413–492. [PubMed] [Google Scholar]
- Reshkin SJ, Guerra L, Bagorda A, et al. (2000) Activation of A3 adenosine receptor induces calcium entry and chloride secretion in A6 cells. Journal of Membrane Biology 178(2): 103–113. [DOI] [PubMed] [Google Scholar]
- Robaye B, Ghanem E, Wilkin F, et al. (2003) Loss of nucleotide regulation of epithelial chloride transport in the jejunum of P2Y4-null mice. Molecular Pharmacology 63(4): 777–783. [DOI] [PubMed] [Google Scholar]
- Roberts JA, Vial C, Digby HR, et al. (2006) Molecular properties of P2X receptors. Pflugers Arch: European Journal of Physiology 452(5): 486–500. [DOI] [PubMed] [Google Scholar]
- Rokic MB, Stojilkovic SS. (2013) Two open states of P2X receptor channels. Frontiers in Cellular Neuroscience 7: 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong W, Burnstock G. (2004) Activation of ureter nociceptors by exogenous and endogenous ATP in guinea pig. Neuropharmacology 47(7): 1093–1101. [DOI] [PubMed] [Google Scholar]
- Ryten M, Yang SY, Dunn PM, et al. (2004) Purinoceptor expression in regenerating skeletal muscle in the mdx mouse model of muscular dystrophy and in satellite cell cultures. FASEB Journal 18(12):1404–1406. [DOI] [PubMed] [Google Scholar]
- Savi P, Herbert JM. (2005) Clopidogrel and ticlopidine: P2Y12 adenosine diphosphate-receptor antagonists for the prevention of atherothrombosis. Seminars in Thrombosis and Hemostasis 31(2): 174–183. [DOI] [PubMed] [Google Scholar]
- Séror C, Melki MT, Subra F, et al. (2011) Extracellular ATP acts on P2Y2 purinergic receptors to facilitate HIV-1 infection. Journal of Experimental Medicine 208(9): 1823–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabbir M, Burnstock G. (2009) Purinergic receptor-mediated effects of adenosine 5’-triphosphate in urological malignant diseases. International Journal of Urology 16(2): 143–150. [DOI] [PubMed] [Google Scholar]
- Shabbir M, Thompson CS, Jarmulowicz M, et al. (2008) Effect of extracellular ATP on the growth of hormone refractory prostate cancer in vivo. BJU International 102(1): 108–112. [DOI] [PubMed] [Google Scholar]
- Shen J, Seye CI, Wang M, et al. (2004) Cloning, up-regulation, and mitogenic role of porcine P2Y2 receptor in coronary artery smooth muscle cells. Molecular Pharmacology 66(5): 1265–1274. [DOI] [PubMed] [Google Scholar]
- Soto F, Garcia-Guzman M, Karschin C, et al. (1996) Cloning and tissue distribution of a novel P2X receptor from rat brain. Biochemical and Biophysical Research Communication 223(2): 456–460. [DOI] [PubMed] [Google Scholar]
- Spelta V, Mekhalfia A, Rejman D, et al. (2003) ATP analogues with modified phosphate chains and their selectivity for rat P2X2 and P2X2/3 receptors. British Journal of Pharmacology 140(6): 1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperlágh B, Illes P. (2014) P2X7 receptor: An emerging target in central nervous system diseases. Trends in Pharmacological Sciences 35(10): 537–547. [DOI] [PubMed] [Google Scholar]
- Stagg J, Smyth MJ. (2010) Extracellular adenosine triphosphate and adenosine in cancer. Oncogene 29(39): 5346–5358. [DOI] [PubMed] [Google Scholar]
- Stelmashenko O, Lalo U, Yang Y, et al. (2012) Activation of trimeric P2X2 receptors by fewer than three ATP molecules. Molecular Pharmacology 82(4): 760–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojilkovic S, Tomic M, He ML, et al. (2005) Molecular dissection of purinergic P2X receptor channels. Annals of the New York Academy of Sciences 1048(1): 116–130. [DOI] [PubMed] [Google Scholar]
- Sun D, Samuelson LC, Yang T, et al. (2001) Mediation of tubuloglomerular feedback by adenosine: Evidence from mice lacking adenosine 1 receptors. Proceedings of the National Academy of Sciences of the United States of America 98(17): 9983–9988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A, Rassendren F, Kawashima E, et al. (1996) The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 272(5262): 735–738. [DOI] [PubMed] [Google Scholar]
- Surprenant A, Schneider DA, Wilson HL, et al. (2000) Functional properties of heteromeric P2X1/5 receptors expressed in HEK cells and excitatory junction potentials in guinea-pig submucosal arterioles. Journal of the Autonomic Nervous System 81(1-3): 249–263. [DOI] [PubMed] [Google Scholar]
- Taylor SRJ, Turner CM, Elliott JI, et al. (2009) P2X7-deficiency ameliorates accelerated nephrotoxic nephritis in mice. Journal of the American Society of Nephrology 20(6): 1275–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M, Abdelrahman A, Weinhausen S, et al. (2014) Carbamazepine derivatives with P2X4 receptor-blocking activity. Bioorganic & Medicinal Chemistry 22(3): 1077–1088. [DOI] [PubMed] [Google Scholar]
- Torres GE, Egan TM, Voigt MM. (1999) Hetero-oligomeric assembly of P2X receptor subunits. Specificities exist with regard to possible partners. Journal of Biological Chemistry 274(10): 6653–6659. [DOI] [PubMed] [Google Scholar]
- Trujillo CA, Schwindt TT, Martins AH, et al. (2009) Novel perspectives of neural stem cell differentiation: From neurotransmitters to therapeutics. Cytometry A 75(1): 38–53. [DOI] [PubMed] [Google Scholar]
- Valera S, Hussy N, Evans RJ, et al. (1994) A new class of ligand-gated ion channel defined by P2X receptor for extra-cellular ATP. Nature 371(6497): 516–519. [DOI] [PubMed] [Google Scholar]
- Van Calker D, Müller M, Hamprecht B. (1979) Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. Journal of Neurochemistry 33(5): 999–1005. [DOI] [PubMed] [Google Scholar]
- Vassort G. (2001) Adenosine 5’-triphosphate: A P2-purinergic agonist in the myocardium. Physiological Reviews 81(2): 767–806. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Burnstock G. (2014) Biology of purinergic signalling: Its ancient evolutionary roots, its omnipresence and its multiple functional significance. BioEssays 36 (7): 697–705. [DOI] [PubMed] [Google Scholar]
- Vidal M, Hicks PE, Langer SZ. (1986) Differential effects of alpha-beta-methylene ATP on responses to nerve stimulation in SHR and WKY tail arteries. Naunyn-Schmiedebergs Archives of Pharmacology 332(4): 384–390. [DOI] [PubMed] [Google Scholar]
- Vlaskovska M, Kasakov L, Rong W, et al. (2001) P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. Journal of Neuroscience 21(15): 5670–5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb TE, Simon J, Krishek BJ, et al. (1993) Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Letters 324(2): 219–225. [DOI] [PubMed] [Google Scholar]
- Wesselius A, Bours MJ, Henriksen Z, et al. (2013) Association of P2Y2 receptor SNPs with bone mineral density and osteoporosis risk in a cohort of Dutch fracture patients. Purinergic Signalling 9(1): 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N, Burnstock G. (2006) P2 receptors and cancer. Trends in Pharmacological Sciences 27(4): 211–217. [DOI] [PubMed] [Google Scholar]
- White N, Knight GE, Butler PEM, et al. (2009). An in vivo model of melanoma: Treatment with ATP. Purinergic Signalling 5(3): 327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn G, Burnstock G. (2006) Adenosine 5’-triphosphate and its relationship with other mediators that activate pelvic afferent neurons in the rat colorectum. Purinergic Signalling 2(3): 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegutkin GG. (2008) Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochimica Etbiophysica Acta 1783(5): 673–694. [DOI] [PubMed] [Google Scholar]
- Yoshioka K, Hosoda R, Kuroda Y, et al. (2002) Hetero-oligomerization of adenosine A1 receptors with P2Y1 receptors in rat brains. FEBS Letters 531(2): 299–303. [DOI] [PubMed] [Google Scholar]
- Zhou QY, Li C, Olah ME, et al. (1992) Molecular cloning and characterization of an adenosine receptor: The A3 adenosine receptor. Proceedings of the National Academy of Sciences of the United States of America 89(16): 7432–7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H, Mishra SK, Shukla V, et al. (2007) Ecto-nucleotidases, molecular properties and functional impact. Anales de la Real Academia Nacional de Farmacia 73: 537–566. [Google Scholar]


