Abstract
Antibody-mediated central nervous system diseases are a relatively new area of clinical neuroscience with growing impact. Their recognition has challenged the dogma of the blood–brain barrier preventing antibody access into the central nervous system. The antibodies discovered so far are mainly against neurotransmitter receptors (e.g. N-methyl-d-aspartate and glycine receptors) and ion channel–associated proteins (leucine-rich glioma inactivated protein 1 and contactin-associated protein 2) and are expressed on the surface of neuronal synapses and elsewhere. The disorders are reversible with immunotherapies that reduce antibody levels. Although rare, the identification of these disorders in clinical practice has made central nervous system autoimmune diseases a consideration in the differential diagnoses of many clinical presentations. There is still much to learn about the aetiology of the diseases and the mechanisms by which the antibodies act, the neuronal and glial changes that follow antibody-attack, and the compensatory changes that may be required to ensure good recovery.
Keywords: Autoantibody, autoimmune encephalitis, neurodevelopment, NMDA receptor, LGI1, CASPR2
Introduction
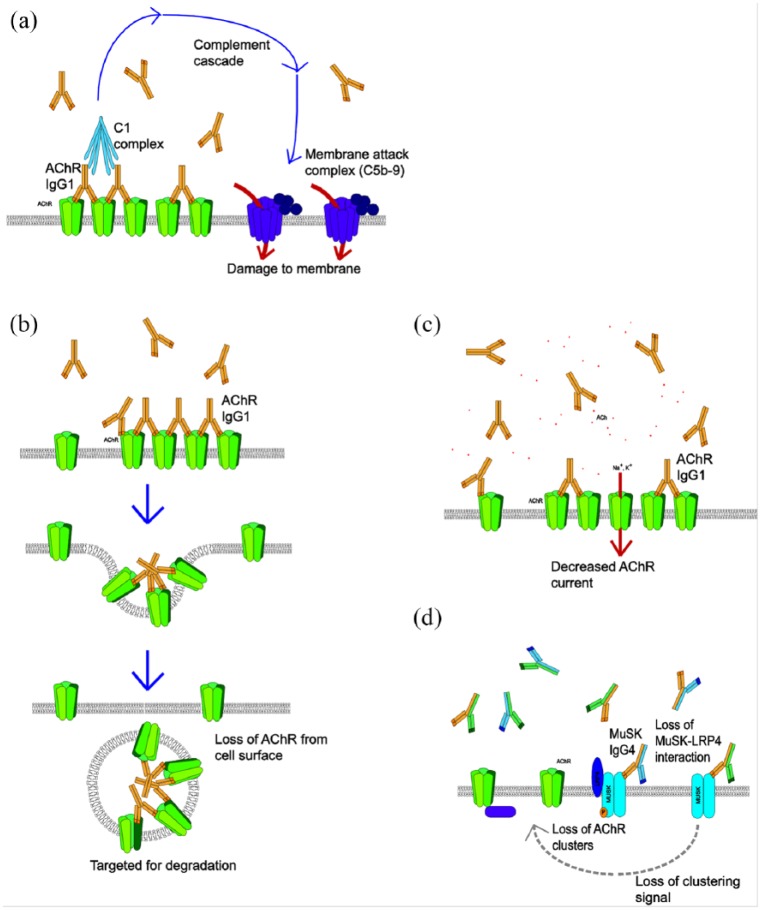
The role of autoantibodies (Abs) in peripheral neurological disorders is well recognised. Myasthenia gravis is a disorder of the neuromuscular junction characterised by fatigable muscle weakness, and it represents the paradigm of an Ab-mediated autoimmune disease. Most patients have subclass IgG1 or IgG3 Abs to the muscle nicotinic acetylcholine receptor (AChR) with loss of AChR demonstrated in patient’s muscle biopsies. The mechanisms of AChR antibodies are well recognised and relevant to the other diseases to be discussed (illustrated in Figure 1(a-c)). Immunisation against purified AChR, or injection of patients’ Abs into experimental animals, can both reproduce features of the human disease. Importantly, the patients respond clinically to approaches such as plasma exchange, which removes the Abs, and steroids, intravenous immunoglobulins and immunosuppressive drugs (e.g. cyclophosphamide) that reduce Abs levels; collectively, these are often referred to as ‘immunotherapies’ a term which will be used here (Gastaldi et al., 2016). Within an individual, the AChR-Abs titres, if measured accurately, correlate well with the clinical severity of the disease. A much smaller proportion of patients (maximum 10%) have antibodies to muscle-specific kinase (MuSK). See Crisp et al. (2016) for a recent review. Interestingly, these antibodies are mainly of the minor human IgG subclass, IgG4, and act by direct block of function (see Figure 1(d)).
Figure 1.
Common mechanisms of antibodies illustrated by AChR and MuSK antibodies at the neuromuscular junction. (a). Immunoglobulin G1 (IgG1) and IgG3 can bind to the AChRs and activate the complement cascade which leads to formation of the membrane attack complex, and local disruption of the postsynaptic membrane. (b) In addition, IgG1 and IgG3 crosslink antigenic targets, leading to internalisation and degradation of the antigen in lysosomes. (c) Less commonly, the antibodies are directed at the acetylcholine binding sites and can directly affect AChR channel function. (d) MuSK antibodies are different as they are mainly of the IgG4 subclass which is monovalent for binding to MuSK, does not activate complement or crosslink receptors. These antibodies inhibit the binding of low-density lipoprotein receptor-related protein 4 (LRP4) to MuSK resulting in impaired AChR clustering.
By contrast, the brain was long considered an immunologically privileged organ due to the presence of the blood–brain barrier (BBB) which prevented the access of Abs and immune cells from the peripheral circulation into the brain parenchyma. However, over the last two decades, it has become apparent that some central nervous system (CNS) diseases are highly associated with Abs to CNS neuronal proteins and that many of these diseases also respond to immunotherapies. Here, we focus on CNS antibody-mediated diseases.
Past
In the late 1960s, inflammatory brain disorders associated with forms of cancer were first recognised, frequently presenting as a ‘limbic’ encephalitis (Brierley et al., 1960; Corsellis et al., 1968), and during that decade, there was the first description of serum antibodies that bound to neurons on healthy brain tissue sections. In the 1980s, it became possible to describe the nuclear or cytoplasmic targets of these antibodies by immunohistology and western blotting, and subsequently to identify, clone and express the proteins. It became clear that the antigens were expressed by the associated tumours and that the antibodies recognised similar or identical proteins expressed by normal mammalian brain tissue. Antibodies to these ‘paraneoplastic’ antigens, however, were almost entirely directed against intracellular epitopes and could not be shown to be causative in animal models. In fact, they are thought to be just part of an aggressive immune response to the tumour, with cytotoxic T-cell mechanisms likely responsible for the irreversible brain pathology that occurs in the patients (Benyahia et al., 1999) who seldom respond to immunotherapies.
The paradigm shift really began in the 1990s although it did not take off until 2001. In 1994, Rogers et al. (1994) described antibodies to the glutamate receptor subtype 3 (GluR3) in rabbits immunised against recombinant GluR3 protein, which developed seizures and the immunopathology typical of Rasmussen’s syndrome – one of the rarest paediatric epilepsy syndromes. These antibodies were identified in a few patients and plasma exchange had a marked effect on seizure frequency in one child whose serum also contained the GluR3 antibodies. Unfortunately, further work has not found these antibodies frequently in this devastating condition, and most patients do not improve with immunotherapies.
It was more by accident than design that studies on a peripheral nerve hyperexcitability (PNH) disease, neuromyotonia (NMT), helped to identify the presence of antibodies in patients with immunotherapy-responsive CNS disorders. It was hypothesised that PNH might be caused by antibodies to voltage-gated potassium channels (Kv1 VGKCs), which would lead to loss of the VGKCs and subsequent neuronal hyperexcitability; several approaches supported this hypothesis. Some of the patients had mild cognitive problems but much more striking was a 76-year-old man who had presented over weeks with PNH, autonomic disturbance, cognitive impairment and extreme insomnia, a rare syndrome which is usually called Morvan’s syndrome (MoS). The patient was found to have VGKC-Abs by immunoprecipitation from rabbit brain extract and responded markedly to plasma exchange (Liguori et al., 2001). In the same year, two patients with limbic encephalitis (severe amnesia, seizures and psychological disturbance) were found to have similar VGKC-Abs and also responded to immunotherapies (Buckley et al., 2001), and subsequently, a series of 10 patients confirmed that these antibodies were strongly associated with a treatable form of limbic encephalitis (Vincent et al., 2004). It was only some years later that these antibodies were shown not to bind to the VGKC Kv1 subunits themselves but to contactin-associated protein 2 (CASPR2) or leucine-rich glioma inactivated protein 1 (LGI1) that both form part of a VGKC-complex obtained from mammalian brain tissue (Irani et al., 2010a).
Meanwhile in 2005, Abs binding to the neuropil of the hippocampus or cerebellum on rat brain sections were described in patients with subacute limbic encephalitis (Ances et al., 2005) following a psychiatric-dominated disorder presenting in young women with ovarian teratomas (Vitaliani et al., 2005). Further studies demonstrated that some of the patient’s antibodies were directed towards the N-methyl-d-aspartate receptor (NMDAR) (Dalmau et al., 2007). This disorder, termed anti-NMDAR encephalitis, at first appeared to be a severe but treatment-responsive paraneoplastic encephalitis and very rare.
Over the subsequent years however, NMDAR-Abs have turned out to be the most common CNS neuronal surface antibody (NSAb) associated with treatment-responsive disease, followed by LGI1-Abs and CASPR2-Abs. Glycine receptor antibodies (GlyR-Abs) are associated, as expected, with mainly brainstem and spinal disturbance. Other antibodies, as summarised in Table 1, have been reported mostly in patients with forms of autoimmune encephalopathy, and also variable associations with tumours. Here, we will focus on the mechanisms (summarised in Table 2) and many unresolved questions in this relatively recent field of autoimmune CNS diseases.
Table 1.
The main targets of antibodies identified so far.
| Antigen | Main clinical syndromes | Other syndromes | CSF features | Associations | Equivalent genetic syndrome |
|---|---|---|---|---|---|
| Antibodies against synaptic receptors | |||||
| N-methyl-d-aspartate receptor (NMDAR) | NMDAR encephalitis: psychiatric syndrome, seizures, amnesia followed by movement disorders catatonia, autonomic instability | Few cases with purely psychotic features; few in cryptogenic epilepsy syndromes | Lymphocytosis in early stages (70%) and OCBs after (>50%); Abs usually present | Ovarian teratoma in about 50%; post-HSV encephalitis | GRIN1 mutations (encoding for GluN1) associated with severe intellectual disability, seizures, movement disorders and dysmorphic features |
| Glycine receptor (GlyR) | PERM, SPS | LE, brainstem encephalitis; cryptogenic epilepsy | Pleocytosis in half of the cases, OCBs (20%); Abs can be absent in the CSF | Thymoma (<10%) | GLRA1 (encoding α1 subunit) or GLRB (encoding β-subunit) associated with hereditary hyperekplexia |
| α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) | LE | Psychosis | Lymphocytosis; OCBs; abs usually present | Tumour in 50% cases (lung, thymoma, breast) | GRIA2 (subunit 2) and GRIA3 (subunit 3) mutations associated with intellectual disability and autism |
| Gamma-aminobutyric acid A receptor (GABAAR) | LE with prominent seizures | Psychiatric syndromes and catatonia; various presentation including SPS, opsoclonus, ataxia | OCBs; abs can be absent in the CSF | Tumour in 70% cases (thymoma, lung and breast) | GABRA1, GABRB3, GABRG2 and GABRD associated with different idiopathic epilepsy syndromes |
| Gamma-aminobutyric acid B receptor (GABABR) | LE | Ataxia, opsoclonus, status epilepticus | Common pleocytosis; rare OCBs | Tumour in 60% (mainly lung) | No |
| Leucine-rich glioma inactivated 1 (LGI1) | LE with or without FBDS and or hyponatraemia | Cryptogenic epilepsy | Usually normal, rare OCBs; Abs can be absent | Tumour in 10% cases | Mutations associated with autosomal dominant lateral temporal lobe epilepsy (ADLTE) with prominent auditory seizures |
| Contactin-associated protein like 2 (CASPR2) | LE, MoS, NMT | Cerebellar ataxia, movement disorders, cryptogenic epilepsies, Guillain-Barre–like syndrome | Usually normal; rare OCB; abs can be absent | Tumour in 30% cases (mainly thymoma) | Mutations associated with autism, epilepsy and intellectual disability |
| Ig-Like Domain-Containing Protein family member 5 (IgLON5) | NREM sleep disorder, abnormal movement and behaviours with obstructive sleep apnoea and stridor, occasional gait instability and brainstem symptoms | Dementia, movement disorders; isolated dysphagia | Pleocytosis; Abs usually present | Tauopathy at neuropathology | No |
CSF: cerebrospinal fluid; OCB: oligoclonal band; HSV: herpes simplex virus; PERM: progressive encephalitis with rigidity and myoclonus; SPS: stiff-person syndrome; LE: limbic encephalitis; FBDS: faciobrachial dystonic seizures; MoS: Morvan’s syndrome; NMT: neuromyotonia; NREM: non-rapid eye movement.
Table 2.
Mechanisms of action of antibodies.
| Target | Main epitope | Other epitopes | IgG subclasses and other Ig classes | Mechanism of Abs | Functional consequences | Animal models |
|---|---|---|---|---|---|---|
| NMDAR | GluN1 | GluN2a-2b | IgG1; IgA, IgM | Cross-linking and internalisation with reversible reduction in cluster density; surface receptors laterally displaced out of synapse, mostly in vitro | Reduced NMDAR currents, reduced LTP and hyper-glutamatergic state; reduction in strength of interaction between NMDAR and ephrin-B2 receptors | Yes |
| GlyR | α1 subunit | IgG1 | Cross-linking and internalisation in HEK cells | Unknown | No | |
| AMPAR | GluA1, GluA2 | NA | Internalisation and degradation with reduction of surface synaptic AMPAR | Decreased AMPAR- mediated currents; changes in the pattern of action potential firing in neurons | No | |
| GABAAR | α1 subunit | β3/γ2 | IgG | Cross-linking and internalisation with selective reduction of GABAA receptor clusters at synapses | Unknown | No |
| GABABR | R1 subunit | Mainly IgG1 | Unknown, not internalisation | Unknown | No | |
| LGI1 | LRR and EPTP repeat domains | IgG4, less IgG1 | Unknown but disruption of the interaction with ADAM22 and reduction of AMPAR with changes in AMPAR distribution and KV1 channel activity; possible complement activation | Increased spontaneous depolarisations in hippocampal CA3; enhanced hippocampal mossy fibre to CA3 pyramidal cell transmission | No but cats with epilepsy and LGI1 abs have inflammatory infiltrates and develop hippocampal atrophy | |
| CASPR2 | Discoidin domain | Lam1, Lam2, Egf1 domains | IgG4 but also IgG1 | Unknown but possible complement activation | Reduction of hippocampal synaptic gephyrin clusters/disruption of inhibitory synaptic contacts of GABAergic neurons | Passive transfer to adult mice has peripheral and central effects Maternal-foetal transfer has long-term effects on behaviour and neuropathology |
| IgLON5 | Immunoglobulin-like domain 2 | IgG4 but also IgG1 | Irreversible internalisation with reduction of IgLon5 expression | Unknown | No |
NMDAR: N-methyl-d-aspartate receptor; GlyR: glycine receptor; AMPAR: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; NA: not applicable; GABAAR: gamma-aminobutyric acid A receptor; GABABR: gamma-aminobutyric acid B receptor; LGI1: leucine-rich glioma inactivated 1; CASPR2: contactin-associated protein like 2; IgLON5: Ig-like domain-containing protein family member 5. LTP, long-term potentiation LRR, leucine-rich repeat ETPT, Epitempin.
Present
Anti-NMDAR encephalitis
Clinical features
‘Anti-NMDAR encephalitis’ (here termed NMDAR-Ab encephalitis) is the most commonly recognised autoimmune encephalitis and has been the main focus of experimental studies. The patients usually present with prominent psychiatric symptoms, seizures, memory deficits and subsequently develop movement disorders (classically choreoathetosis but also catatonia), autonomic instability and decreased level of consciousness. The serum antibodies are immunoglobulin G (IgG), mainly IgG1, and relatively high levels in cerebrospinal fluid (CSF; but still lower than those in serum) indicate intrathecal synthesis of NMDAR-Abs by resident plasma cells (Dalmau et al., 2008; Irani et al., 2010b).
Mechanisms
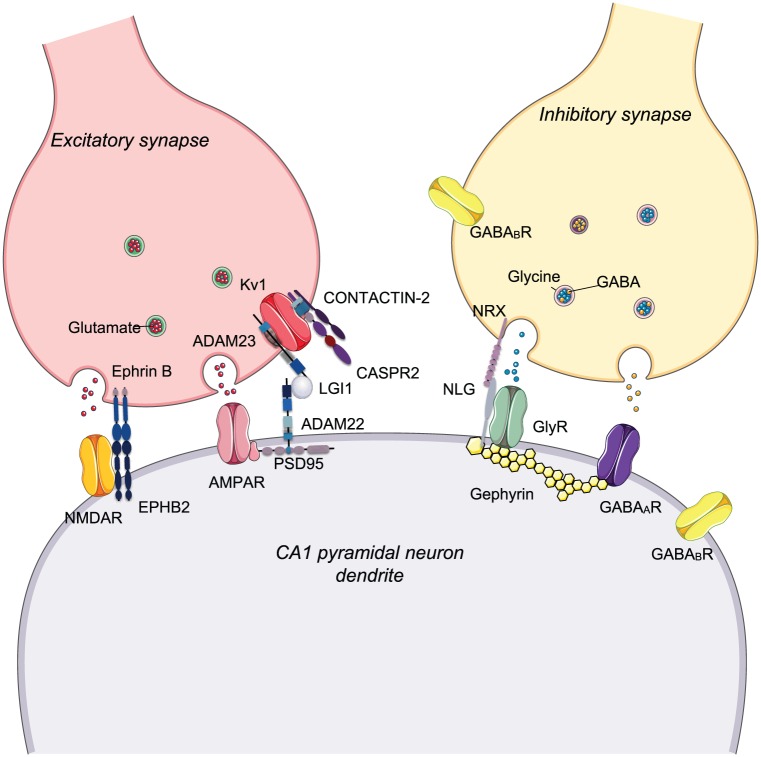
The NMDAR is a subtype of glutamate receptor composed of two GluN1 and two GluN2/3 (also called NR1, NR2 and NR3, respectively) subunits that form a central ion channel. Initially described as antibodies to NR2A or NR2B, patient’s antibodies were subsequently shown to react against the NR1 subunit and, when applied at 37°C for hours to days, they decreased the expression of NMDARs on dendrites of neurons in culture. The reduction of NMDAR expression was mediated by the IgG antibodies that cross-link adjacent NMDARs resulting in their internalisation (Hughes et al., 2010). This occurred in a titre- and time-dependent fashion but without damage to the neurons; when the antibodies were washed away, the NMDAR surface expression recovered. In another study, using high-resolution single nanoparticle imaging, the antibodies altered NMDAR trafficking, dispersing NMDARs from the synapse by blockade of the interaction between the extracellular domains of GluN1/2 subunits and ephrin-B2 receptors (EPHB2R) (see Figure 2). In that study, internalisation appeared to occur only at extrasynaptic sites (Mikasova et al., 2012). Altogether these changes reduce synaptic NMDAR currents and impair long-term potentiation (Zhang et al., 2012), explaining some of the cognitive symptoms observed in patients.
Figure 2.
Synaptic proteins targeted by antibodies in the CNS. Schematic diagram showing simplified excitatory and inhibitory synapses, each of which expresses elements targeted by antibodies associated with neurological diseases. These mechanisms are mainly based on in vitro observations. LGI1 modulates AMPAR trafficking by interacting with ADAM22. Antibodies against LGI1 (mainly IgG4) disrupts its interaction with ADAM22 causing a reduction of AMPAR expression postsynaptically and likely modulate (reduce) Kv1 function presynaptically causing neuronal hyperexcitability. In NMDAR encephalitis, the excitatory glutamatergic transmission may be disrupted by autoantibody-mediated cross-linking of GluN1 subunits or by disruption of the interaction between NMDARs and EPHB2, with consequent lateral displacement of the receptors out of synapse. At inhibitory synapses, antibodies directed against the GlyR (IgG1 predominantly) cause cross-linking and internalisation with loss of GlyRs.
ADAM22: disintegrin and metalloproteinase domain-containing protein 22; ADAM23: disintegrin and metalloproteinase domain-containing protein 23; EPHB2: ephrin type-B receptor 2; NLG: neuroligin; NRX: neurexin; PSD95: postsynaptic density protein 95.
To establish in vivo pathogenicity, however, in vivo experiments are essential. Mice were continuously infused bilaterally into the lateral ventricles, for 14 days, with NMDAR-Ab-positive CSFs; the animals displayed some memory deficits, anhedonia and depressive-like behaviours associated with IgG deposition and reduced NMDAR clusters in the hippocampal neuropil. Both the IgG deposition and the behavioural changes reversed within a few days of discontinuing the infusion (Planagumà et al., 2015). However, there were no seizures or movement disorders observed. A different approach used a single larger intraventricular injection of IgG purified from individuals with NMDAR-Abs. This did not cause seizures directly, but intraperitoneal injection of the chemoconvulsant, pentylenetetrazol (PTZ), demonstrated more frequent and more severe seizures in NMDAR-IgG-injected mice compared with healthy control IgG-injected animals (Wright et al., 2015). Convincingly, the seizure scores correlated with the intensity of IgG bound to the hippocampi; but curiously, there was no apparent loss of surface NMDARs overall, raising the possibility that some antibodies may directly inhibit NMDAR function without leading to NMDAR internalisation.
These two animal models support the pathogenicity of the antibodies, which postmortem studies suggest do not involve complement-mediated damage, thus explaining why patients often improve substantially following the immunotherapies. However, further studies show that many patients have long-term cognitive deficits and brain magnetic resonance imaging (MRI) demonstrated structural hippocampal damage correlating with disease duration and delayed or insufficient treatment (Finke et al., 2016). This suggests that, while the acute effects of NMDAR-Abs are functional and reversible, their persistence can impact on neuronal function in a way that is not yet understood.
One possibility is that the long-term changes are related to the accumulation of excitatory mediators such as glutamate within the synaptic regions. Indeed, injections of patients’ CSF and purified IgGs resulted in an acute increase both in glutamate levels and in the excitability of the motor cortex in a rat model (Manto et al., 2010). In drug-induced models, with NMDAR antagonists, glutamate levels also increase and can cause both reversible and irreversible effects depending on the dosage and duration (Newcomer et al., 2000).
LGI1-Ab-related syndromes
Clinical features
Autoantibodies to LGI1 (LGI1-Abs) are the most common autoantibody in patients with limbic encephalitis. Temporal lobe seizures are common at presentation and retrograde and anterograde amnesia can be severe. A specific epilepsy type, characterised by contractions of homolateral face, arm and sometimes leg, known as faciobrachial dystonic seizures (FBDS), has been described often preceding the limbic encephalitis (Irani et al., 2011) and responding very well to corticosteroids. In those patients where these seizures are the first sign of LGI1-Abs, early treatment may prevent future cognitive involvement. Once the patients have developed limbic encephalitis, however, recovery although considerable is not complete with only 35% of patients returning to their baseline cognitive function and many showing hippocampal atrophy (Ariño et al., 2016). Interestingly, LGI1 mutations have been associated with an autosomal dominant lateral temporal lobe epilepsy manifesting often with auditory features (Kalachikov et al., 2002).
Mechanisms
LGI1 is a protein secreted by neurons, which binds to membrane proteins ADAM22 and ADAM23, two proteins located postsynaptically and presynaptically, respectively, and involved in cell–cell adhesion. Thus, via these two proteins, LGI1 forms a bridge between the pre- and the post-synapse (see Figure 2) and is believed to play an important role in synaptic maturation (Fukata et al., 2006; Owuor et al., 2009). By binding to ADAM22, LGI1 regulates AMPA receptor-mediated synaptic currents in the hippocampus. Although the details are not clear, it appears that LGI1, via a cytoplasmic regulatory protein, Kvβ, selectively prevents inactivation of the presynaptic VGKC Kv1.1 (Schulte et al., 2006). This would be responsible for the neuronal hyperexcitability found when the antibodies are applied to rodent brain slices (Lalic et al., 2011; Petit-Pedrol et al 2018).
LGI1-Abs have been shown to disrupt the interaction of LGI1 with ADAM22, resulting in reversible reduction in synaptic AMPARs in cultured hippocampal neurons (Ohkawa et al., 2013; Petit-Pedrol et al 2018). This observation, along with the good response to immunotherapy, supports a pathogenic role of the antibodies although their pathogenicity has not yet been confirmed using animal models. Intriguingly, LGI1-Abs have been detected in the sera of cats with complex partial seizures with orofacial involvement. Postmortem analysis in these cases showed the presence of complement deposition with neuronal loss (Klang et al., 2014), as also found in the limited number of human postmortem studies (Bien et al., 2012). The finding of complement deposition is surprising as LGI1-Abs belong mainly to the IgG4 subclass, which is not complement-activating but it suggests that even the small proportion of IgG1, 2 or 3 antibodies could be contributing to the pathogenicity.
CASPR2-Ab-related syndromes
Clinical features
CASPR2 is the other main target of VGKC-complex antibodies. CASPR2-Abs can be associated with cognitive impairment, memory loss, hallucinations, delusions, cerebellar symptoms and epilepsy as well as with peripheral nerve involvement with pain, neuropathy and NMT. The combination of central, autonomic and peripheral nerve symptoms defines the Morvan’s syndrome described above, but many patients have limbic encephalitis or more restricted symptoms. Neuropathic-type pain is an interesting feature.
CASPR2-Abs are described with a broad spectrum of clinical presentations including cerebellar ataxia (Bien et al., 2017). These could be related to differences in epitope specificity, antibody titres and/or the sites of antibody production. Very high titres of antibodies can be found in the serum, but CSF levels are more varied and one study found CASPR2-Abs in both serum and CSF in patients with autoimmune encephalitis, whereas they were restricted to the serum in patients with NMT or MoS (Joubert et al., 2016). Interestingly, mutations in the CNTNAP2 gene, encoding for CASPR2, are associated with focal epilepsy, schizophrenia and autism spectrum disorders (Friedman et al., 2008).
Mechanisms
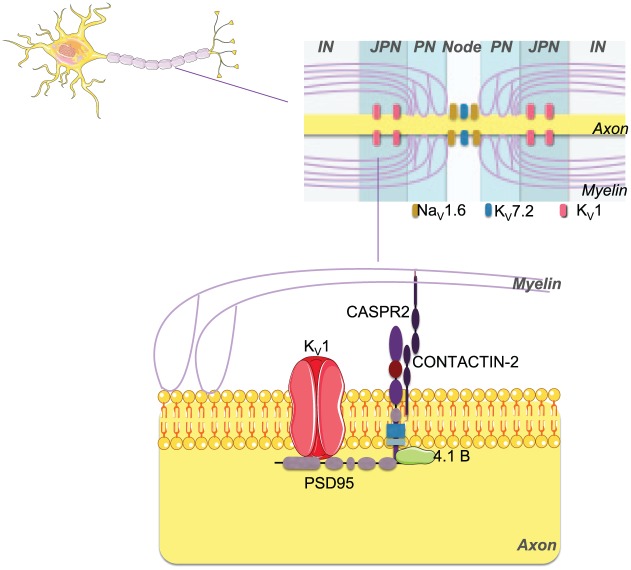
CASPR2 is a neurexin-related cell adhesion molecule expressed in the central and peripheral nervous system and CASPR2-Abs react with both the brain and peripheral nerve tissues (Irani et al., 2010b). CASPR2 is essential for clustering Kv1.1 and Kv1.2 at the juxtaparanodes of myelinated axons (see Figure 3). These channels are important for repolarisation of the nerve axon, avoiding repetitive firing and helping to maintain the internodal resting potential.
Figure 3.
Proteins targeted by antibodies in the peripheral nervous system. In the peripheral nerve, CASPR2 protein is essential for clustering Kv1 channels at the juxtaparanodes of myelinated axons. Antibodies against CASPR2 (both IgG1 and IgG4) can down-regulate Kv expression by internalisation with consequent impairment of action potential repolarisation leading to excessive or prolonged release of acetylcholine and peripheral nerve hyperexcitability (PNH). It is not clear yet to what extent complement activation plays a role in the peripheral disease, if any.
IN: internode; JPN: juxtaparnode; KV: voltage-gated potassium channel; NaV: voltage-gated sodium channel; 4.1B: cytoskeletal adaptor protein band 4.1B; PN: paranode; PSD95: postsynaptic density protein 95.
For CASPR2-Abs, the mechanisms of action are still largely unclear. CASPR2-Abs are predominantly IgG4, although often in association with IgG1; the most likely common mechanism of their action is a disruption of CASPR2 interaction with associated molecules, rather than internalisation and complement activation. Accordingly, a recent study showed that CASPR2-Abs do not reduce CASPR2 expression on the surface of cultured hippocampal neurons, but they reduce CASPR2 interaction with contactin-2 (Patterson et al., 2018). However, this is in contrast to a recently reported pathological case showing reduced CASPR2 expression in the brain (Sundal et al., 2017) and with two pathological cases associated with the presence of complement deposition (Körtvelyessy et al., 2015; Liguori et al., 2001). These discrepancies might be related to several factors, including different proportions of IgG1 and 4 in different patients and the intrinsic limitations of in vitro studies, which only partially reproduce the mechanisms involved in vivo.
How CASPR2-Abs produce the CNS symptoms is still largely unknown. A study, using cultured hippocampal neurons, showed that serum IgGs targeted inhibitory interneurons where they reduced the number of synaptic gephyrin clusters (Pinatel et al., 2015) which anchor GABAA receptors. More recently, however, treatment of cultured dorsal root ganglia (DRG) neurons with CASPR2-Abs caused a reduction in Kv1 channel surface expression and consequent neuronal hyperexcitability (Dawes et al., 2018).
The same study produced the first passive transfer model of CASPR2-Abs by intraperitoneal (ip) injections showing that these antibodies are able to cause mechanical-pain hypersensitivity in the exposed mice. The effects on the CNS were not investigated in that study. In order to explore this aspect, in a similar study, we used lipopolysaccharide (LPS) to open the BBB and allow the antibody to reach the CNS. We showed that, after LPS, CASPR2-Abs were able to access and bind to the brain parenchyma causing working memory defects in the exposed animals along with neuropathological changes (M.P.G. and A.V., in preparation). Finally, two recent studies using a maternal-foetal transfer model showed that the offspring of dams injected with CASPR2-Abs demonstrated behavioural disorders and neuropathological features (Brimberg et al., 2016; Coutinho et al., 2017) raising interesting questions about the role of these antibodies in neurodevelopment disorders.
GlyR-Ab-related syndromes
Clinical features
These antibodies are associated with progressive encephalitis with rigidity and myoclonus (PERM), a rare neurological condition characterised by muscle stiffness and painful spasms, brainstem dysfunction, tactile and auditory stimulus-triggered exaggerated startle responses, and autonomic crises or failure. Some patients present with a similar disorder without brainstem symptoms called stiff-person syndrome (SPS; Carvajal-González et al., 2014).
Mechanisms
Glycine receptors (GlyRs) are pentameric proteins belonging to the superfamily of ligand-gated ion channels. Under physiological conditions, glycine-mediated activation of the GlyR leads to an influx of chloride into the neurons and results in hyperpolarisation of the membrane potential with consequent reduced excitation (Figure 2). Incubation of HEK293 cells expressing GlyRs with IgG derived from individuals with GlyR-Abs resulted in internalisation and targeting of the receptors to lysosomes. Therefore, if a similar response occurs in vivo, the expected reduced glycinergic neurotransmission may explain the symptoms seen in patients with PERM.
These receptors can exist as homomers of α1-4 subunits or as heteromers consisting of α and β subunits. The different α subunits are differentially expressed in the CNS and the binding of autoantibodies to these different subunits could explain the range of spinal cord and brainstem symptoms observed in individuals with GlyR-Abs. However, a recent study showed that the majority of sera from GlyR-Abs-positive patients bind to α1β heteromers, and no correlation between different subunit binding affinities and clinical phenotype was found (Carvajal-González et al., 2014).
Future
Despite their relative rarity (incidence individually likely about 2/million per year), antibody-mediated CNS diseases, with their substantial treatment responses, have become widely recognised. However, many questions remain unanswered and we end by considering some areas for future exploration.
To cause symptoms, the antibodies must access the brain. Serum levels of total IgG are usually 200–400 times those of the CSF. Serum levels of the specific IgG antibodies we describe here are almost always higher than CSF levels strongly suggesting that they are initially produced in the periphery. However, the serum:CSF ratios are often less than 200–400 which indicates either that there is some leakiness of the BBB or that there is ‘intrathecal synthesis’ of the specific antibody. The latter depends on access of B-cells into the brain where they can undergo restimulation (by local antigen), clonal expansion and differentiation into antibody producing plasma cells (Hauser et al., 2008). This intrathecal synthesis is often substantial with NMDAR-Abs, but is more variable with LGI1, CASPR2 and GlyR-Abs. There is, however, recent evidence that absorption of specific antibodies from the CSF by the brain tissue may complicate interpretation of data (Castillo-Gómez et al., 2016a).
Nevertheless, most workers agree that the initial stimulus for the antibody formation takes place in the periphery and that subsequently, perhaps following some temporary breach of the BBB, the antibodies and B cells gain access to the brain parenchyma. Thus, one could posit that the BBB damage is the essential initiating cause of the disease in a patient who already has circulating antibodies to neuronal proteins. These seem likely in those cases where the patient has high levels to the NMDAR due to expression of this antigen in the ovarian teratoma tissue, but is not likely the case in all patients. In one of the few informative cases where preceding sera were available, the antibodies rose from negative values to strongly positive shortly before the clinical syndrome was identified (Buckley et al., 2001); thus, the existence of the disease was related to the presence of the antibody. In addition, the target specificity of the antibodies determine the clinical features (e.g. movement disorders with NMDAR-Abs; startle responses, spasms and rigidity with GlyR-Abs).
Whether the specific clinical features of, for instance, memory loss, epilepsy or movement disorder are only caused by intrathecally produced antibodies or also by antibodies that cross the BBB is also of interest. In mouse models, peripherally administered CASPR2 antibodies can access the brain if LPS is given to ‘open the BBB’ and produce some behavioural changes (M.P.G. and A.V., in preparation), but to date there has not been a systematic comparison of the changes in this peripheral model with those found when antibodies are injected intraventricularly as described above (Planagumà et al., 2015; Wright et al., 2015). This needs to be done.
One aspect is where do the antibodies act? Most of the proteins are widely expressed in the CNS and yet the clinical features (e.g. memory defects) strongly suggest a focal pathology. Is this because there are different epitopes expressed in different regions, related to access of the antibodies to brain parenchyma through a leaky BBB or the site of intrathecal antibody production, or because different neurons and their pathways are more susceptible to their effects?
Are the antibodies alone pathogenic? The binding of antibodies to proteins expressed on the surface of neurons is an essential prerequisite, and in vitro assays have shown that the antibodies can affect surface expression and function of their targets. Passive transfer of NMDAR-Abs-positive IgG or CSFs (intraventricular or systemic injection) demonstrated some effects in mice, but notably the full range of clinical features was not obtained. For the other antigens, evidence is even less compelling, but (unpublished) failed attempts may reflect the lack of sensitive read-outs for neuronal dysfunction at the level of the whole animal; looking at hippocampal slices following such experiments needs to be done, but will not easily address the role of other immune factors such as complement or immune cells, or the multiple pathways that may be secondarily involved.
Very little is known about the origin of these antibodies except in cases where there is a tumour. We know that ovarian teratomas often express neuronal antigens explaining the development of NMDAR-Abs in patients with these otherwise benign tumours. One recent observation that helps to explain disease in non-tumour patients is that NMDAR-Ab-associated ‘relapses’ can arise following herpes simplex virus encephalitis (e.g. Hacohen et al., 2014a, 2014b). This interesting situation could be due to molecular mimicry between the virus and NMDAR NR1 or due to a secondary immune response to inflamed and damaged brain tissue resulting from the viral infection. Even in this situation when the stimulus for antibody production is assumed to be the inflamed brain tissue, the serum levels of the antibodies that arise are clearly higher than those in the CSF (Hacohen et al., 2014a), suggesting that most of the antigen stimulus takes place following drainage of lymph directly into the circulation and via draining lymph nodes.
An area that is potentially exciting is the possibility that some of these antibodies are present in patients with psychiatric disorders, which might then be responsive to immunotherapies. Although there are reports of individual cases of patients presenting with restricted features, and only later or not at all developing the full spectrum of limbic encephalitis or NMDAR-Ab encephalitis, the results of large cohorts of patients with recent onset psychosis or seizures are highly variable. Some studies suggest raised IgG NMDAR-Abs, but others find no difference from controls and a relatively high frequency of IgM and IgA (Doss et al., 2014; Pollak et al., 2016). NMDAR-Abs of IgG, M or A have been shown to be active in vitro (Castillo-Gómez et al., 2016b), but their pathogenicity in vivo is still unclear. Nevertheless, new forms of disease can be found, and sometimes in disorders that would not normally be considered ‘autoimmune’. In 2014, Sabater et al. described a few patients with an unusual sleep disorder and a variable combination of bulbar and cerebellar signs associated with high titres of an antibody directed against the IgLON5 protein. These patients had a chronic disease, which did not improve with immunotherapy and two patients died. Intriguingly, tau protein deposition was found at postmortem. Is this an autoimmune syndrome that causes neurodegenerative changes or are the antibodies secondary to the tauopathy? These observations raise the possibility of other unexplored links between neurodegeneration and autoimmunity, and the need to recognise that even ‘pathogenic’ antibodies may sometimes be secondary to other pathologies.
Finally, an emerging field is the role of the antibodies in neurodevelopment disorders. IgG can cross the placenta during pregnancy, and it was shown in the 1990s that antibodies to the foetal AChR could paralyse the baby leading to arthrogryposis multiplex congenital (multiple fixed joints) due to lack of movement (see Crisp et al., 2016). Both NMDAR and CASPR2 are known to be involved in brain development from human genetic disorders and transgenic mouse models (see Coutinho et al., 2017), and further work on the roles of these and other antibodies in neurodevelopmental disorders needs to be performed.
Conclusion
The discovery of antibody-associated brain disorders has helped to identify patients amenable to immunotherapies. The field has improved clinical outcomes but raised many questions that need to be addressed by both the immunological and neuroscience communities. Answering these questions will help to develop novel therapies tailored to patient needs, leading to more rapid recovery and improved outcomes. In addition, a better understanding of the cellular targets and networks involved in these diseases may provide insights into the mechanisms of other neurological and psychiatric disorders.
Footnotes
Declaration of conflicting interests: A.V. and the University of Oxford hold a patent for LGI1 and CASPR2 antibody assays, licenced to Euroimmun AG, Luebeck, Germany, and A.V. receives a proportion of royalties. M.P.G. and S.J.C. report no conflict of interest.
Funding: There was no specific funding for this review. M.P.G. is supported by the University of Bologna.
References
- Ances BM, Vitaliani R, Taylor RA, et al. (2005) Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain 128(Pt. 8): 1764–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariño H, Armangué T, Petit-Pedrol M, et al. (2016) Anti-LGI1-associated cognitive impairment: Presentation and long-term outcome. Neurology 87(8): 759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyahia B, Liblau R, Merle-Béral H, et al. (1999) Cell-mediated autoimmunity in paraneoplastic neurological syndromes with anti-Hu antibodies. Annals of Neurology 45(2): 162–167. [DOI] [PubMed] [Google Scholar]
- Bien CG, Mirzadjanova Z, Baumgartner C, et al. (2017) Anti-contactin-associated protein-2 encephalitis: Relevance of antibody titres, presentation and outcome. European Journal of Neurology 24(1): 175–186. [DOI] [PubMed] [Google Scholar]
- Bien CG, Vincent A, Barnett MH, et al. (2012) Immunopathology of autoantibody-associated encephalitides: Clues for pathogenesis. Brain 135(Pt. 5): 1622–1638. [DOI] [PubMed] [Google Scholar]
- Brierley JB, Corsellis JA, Hierons R, et al. (1960) Subacute encephalitis of later adult life. Mainly affecting the limbic areas. Brain 83(3): 357–368. [Google Scholar]
- Brimberg L, Mader S, Jeganathan V, et al. (2016) Caspr2-reactive antibody cloned from a mother of an ASD child mediates an ASD-like phenotype in mice. Molecular Psychiatry 21(12): 1663–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley C, Oger J, Clover L, et al. (2001) Potassium channel antibodies in two patients with reversible limbic encephalitis. Annals of Neurology 50(1): 73–78. [DOI] [PubMed] [Google Scholar]
- Carvajal-González A, Leite MI, Waters P, et al. (2014) Glycine receptor antibodies in PERM and related syndromes: Characteristics, clinical features and outcomes. Brain 137(Pt. 8): 2178–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Gómez E, Kästner A, Steiner J, et al. (2016. a) The brain as immunoprecipitator of serum autoantibodies against N-Methyl-D-aspartate receptor subunit NR1. Annals of Neurology 79(1): 144–151. [DOI] [PubMed] [Google Scholar]
- Castillo-Gómez E, Oliveira B, Tapken D, et al. (2016. b) All naturally occurring autoantibodies against the NMDA receptor subunit NR1 have pathogenic potential irrespective of epitope and immunoglobulin class. Molecular Psychiatry 22(12): 1776–1784. [DOI] [PubMed] [Google Scholar]
- Corsellis JA, Goldberg GJ, Norton AR. (1968) ‘Limbic encephalitis’ and its association with carcinoma. Brain 91(3): 481–496. [DOI] [PubMed] [Google Scholar]
- Coutinho E, Menassa DA, Jacobson L, et al. (2017) Persistent microglial activation and synaptic loss with behavioral abnormalities in mouse offspring exposed to CASPR2-antibodies in utero. Acta Neuropathologica 134(4): 567–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisp SJ, Kullmann DM, Vincent A. (2016) Autoimmune synaptopathies. Nature Reviews Neuroscience 17(2): 103–117. [DOI] [PubMed] [Google Scholar]
- Dalmau J, Gleichman AJ, Hughes EG, et al. (2008) Anti-NMDA-receptor encephalitis: Case series and analysis of the effects of antibodies. The Lancet Neurology 7(12): 1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmau J, Tüzün E, Wu HY, et al. (2007) Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Annals of Neurology 61(1): 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes JM, Weir GA, Middleton SJ, et al. (2018) Immune or genetic-mediated disruption of CASPR2 causes pain hypersensitivity due to enhanced primary afferent excitability. Neuron 97(4): 806.e10–822.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doss S, Wandinger KP, Hyman BT, et al. (2014) High prevalence of NMDA receptor IgA/IgM antibodies in different dementia types. Annals of Clinical and Translational Neurology 1(10): 822–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finke C, Kopp UA, Pajkert A, et al. (2016) Structural hippocampal damage following anti-N-methyl-D-aspartate receptor encephalitis. Biological Psychiatry 79(9): 727–734. [DOI] [PubMed] [Google Scholar]
- Friedman JI, Vrijenhoek T, Markx S, et al. (2008) CNTNAP2 gene dosage variation is associated with schizophrenia and epilepsy. Molecular Psychiatry 13(3): 261–266. [DOI] [PubMed] [Google Scholar]
- Fukata Y, Adesnik H, Iwanaga T, et al. (2006) Epilepsy-related ligand/receptor complex LGI1 and ADAM22 regulate synaptic transmission. Science 313(5794): 1792–1795. [DOI] [PubMed] [Google Scholar]
- Gastaldi M, Thouin A, Vincent A. (2016) Antibody-mediated autoimmune encephalopathies and immunotherapies. Neurotherapeutics 13(1): 147–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacohen Y, Absoud M, Hemingway C, et al. (2014. a) NMDA receptor antibodies associated with distinct white matter syndromes. NeurologyNeuroimmunology & Neuroinflammation 1(1): e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacohen Y, Deiva K, Pettingill P, et al. (2014. b) N-methyl-D-aspartate receptor antibodies in post-herpes simplex virus encephalitis neurological relapse. Movement Disorders 29(1): 90–96. [DOI] [PubMed] [Google Scholar]
- Hughes EG, Peng X, Gleichman AJ, et al. (2010) Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. Journal of Neuroscience 30(17): 5866–5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani SR, Alexander S, Waters P, et al. (2010. a) Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain 133(9): 2734–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani SR, Bera K, Waters P, et al. (2010. b) N-methyl-D-aspartate antibody encephalitis: Temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain 133(Pt. 6): 1655–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani SR, Michell AW, Lang B, et al. (2011) Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Annals of Neurology 69(5): 892–900. [DOI] [PubMed] [Google Scholar]
- Joubert B, Saint-Martin M, Noraz N, et al. (2016) Characterization of a subtype of autoimmune encephalitis with anti-contactin-associated protein-like 2 antibodies in the cerebrospinal fluid, prominent limbic symptoms, and seizures. JAMA Neurology 73(9): 1115–1124. [DOI] [PubMed] [Google Scholar]
- Kalachikov S, Evgrafov O, Ross B, et al. (2002) Mutations in LGI1 cause autosomal-dominant partial epilepsy with auditory features. Nature Genetics 30(3): 335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klang A, Schmidt P, Kneissl S, et al. (2014) IgG and complement deposition and neuronal loss in cats and humans with epilepsy and voltage-gated potassium channel complex antibodies. Journal of Neuropathology and Experimental Neurology 73(5): 403–413. [DOI] [PubMed] [Google Scholar]
- Körtvelyessy P, Bauer J, Stoppel CM, et al. (2015) Complement-associated neuronal loss in a patient with CASPR2 antibody-associated encephalitis. Neurology Neuroimmunology & Neuroinflammation 2(2): e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalic T, Pettingill P, Vincent A, et al. (2011) Human limbic encephalitis serum enhances hippocampal mossy fiber-CA3 pyramidal cell synaptic transmission. Epilepsia 52(1): 121–131. [DOI] [PubMed] [Google Scholar]
- Liguori R, Vincent A, Clover L, et al. (2001) Morvan’s syndrome: Peripheral and central nervous system and cardiac involvement with antibodies to voltage-gated potassium channels. Brain 124(Pt. 12): 2417–2426. [DOI] [PubMed] [Google Scholar]
- Manto M, Dalmau J, Didelot A, et al. (2010) In vivo effects of antibodies from patients with anti-NMDA receptor encephalitis: Further evidence of synaptic glutamatergic dysfunction. Orphanet Journal of Rare Diseases 5: November 26, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikasova L, De Rossi P, Bouchet D, et al. (2012) Disrupted surface cross-talk between NMDA and Ephrin-B2 receptors in anti-NMDA encephalitis. Brain 135(Pt. 5): 1606–1621. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Farber NB, Olney JW, et al. (2000) NMDA receptor function, memory, and brain aging. Dialogues in Clinical Neuroscience 2(3): 219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa T, Fukata Y, Yamasaki M, et al. (2013) Autoantibodies to epilepsy-related LGI1 in limbic encephalitis neutralize LGI1-ADAM22 interaction and reduce synaptic AMPA receptors. Journal of Neuroscience 33(46): 18161–18174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owuor K, Harel NY, Englot DJ, et al. (2009) LGI1-associated epilepsy through altered ADAM23-dependent neuronal morphology. Molecular and Cellular Neurosciences 42(4): 448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson KR, Dalmau J, Lancaster E. (2018) Mechanisms of Caspr2 antibodies in autoimmune encephalitis and neuromyotonia. Annals of Neurology 83(1): 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit-Pedrol M, Sell J, Planagumà J, et al. (2018)LGI1 antibodies alter Kv1.1 and AMPA receptors changing synaptic excitability, plasticity and memory. Brain 141(11): 3144–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinatel D, Hivert B, Boucraut J, et al. (2015) Inhibitory axons are targeted in hippocampal cell culture by anti-Caspr2 autoantibodies associated with limbic encephalitis. Frontiers in Cellular Neuroscience 9: 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planagumà J, Leypoldt F, Mannara F, et al. (2015) Human N-methyl D-aspartate receptor antibodies alter memory and behaviour in mice. Brain 138(Pt. 1): 94–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak TA, Beck K, Irani SR, et al. (2016) Autoantibodies to central nervous system neuronal surface antigens: Psychiatric symptoms and psychopharmacological implications. Psychopharmacology 233(9): 1605–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SW, Andrews PI, Gahring LC, et al. (1994) Autoantibodies to glutamate receptor GluR3 in Rasmussen’s encephalitis. Science 265(5172): 648–651. [DOI] [PubMed] [Google Scholar]
- Sabater L, Gaig C, Gelpi E, et al. (2014) A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: A case series, characterisation of the antigen, and post-mortem study. The Lancet Neurology 13(6): 575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte U, Thumfart JO, Klöcker N, et al. (2006) The epilepsy-linked Lgi1 protein assembles into presynaptic Kv1 channels and inhibits inactivation by Kvbeta1. Neuron 49(5): 697–706. [DOI] [PubMed] [Google Scholar]
- Sundal C, Vedeler C, Miletic H, et al. (2017) Morvan syndrome with Caspr2 antibodies. Clinical and autopsy report. Journal of the Neurological Sciences 372: 453–455. [DOI] [PubMed] [Google Scholar]
- Vincent A, Buckley C, Schott JM, et al. (2004) Potassium channel antibody-associated encephalopathy: A potentially immunotherapy-responsive form of limbic encephalitis. Brain 127(Pt. 3): 701–712. [DOI] [PubMed] [Google Scholar]
- Vitaliani R, Mason W, Ances B, et al. (2005) Paraneoplastic encephalitis, psychiatric symptoms, and hypoventilation in ovarian teratoma. Annals of Neurology 58(4): 594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S, Hashemi K, Stasiak L, et al. (2015) Epileptogenic effects of NMDAR antibodies in a passive transfer mouse model. Brain 138(Pt. 11): 3159–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Tanaka K, Sun P, et al. (2012) Suppression of synaptic plasticity by cerebrospinal fluid from anti-NMDA receptor encephalitis patients. Neurobiology of Disease 45(1): 610–615. [DOI] [PubMed] [Google Scholar]