Abstract
In this article, we describe our involvement in the early days of research into long-term potentiation. We start with a description of the early experiments conducted in Oslo and London where long-term potentiation was first characterised. We discuss the ways in which the molecular pharmacology of glutamate receptors control the induction and expression of long-term potentiation and its counterpart, long-term depression. We then go on to summarise the extraordinary advances in understanding the cellular mechanisms of synaptic plasticity that have taken place in the subsequent half century. Finally, the increasing evidence that impaired long-term potentiation is a core feature of many brain disorders (LToPathies) is addressed by way of a few selected examples.
Keywords: Hippocampus, synaptic plasticity, long-term potentiation (LTP)
In 1968, the year that saw the founding of the Brain Research Association (BRA), later to be reborn as the British Neuroscience Association, one of us (T.V.P.B.) gave a talk at the BRA’s usual venue, the upstairs room of the Black Horse pub, in Rathbone Place, off Oxford St in London, UK. Bliss described his attempts to induce plasticity in surgically isolated cortical pathways of the decerebrate cat. He had recently completed his PhD at McGill University, in Montreal, and had returned to the United Kingdom to take up a job with his PhD supervisor Ben Delisle Burns who had moved from Montreal to become Head of the Division of Neurophysiology at the National Institute for Medical Research (NIMR), in Mill Hill, London. Among the founder members of the BRA present that night were several with an interest in the neural basis of memory, including Steven Rose, Adrian Horridge, Gabriel Horn and John O’Keefe.
The idea that memory can be understood in terms of activity-dependent changes in synaptic strength was as much part of the neuroscientific zeitgeist then as it is now. Donald Hebb’s (1949) book The Organization of Behaviour had been in print for nearly two decades, and although his famous Neurophysiological Postulate, and the proposal that memory is encoded in cell assemblies, had not acquired the status of holy writ that these ideas command today, they were widely if not universally accepted (see Aggleton and Morris, 2018, for a description of some short-lived alternative proposals). In that Hebbian spirit, Bliss’s experiments at McGill University exploited unit recording and electrical stimulation of cortical pathways to ask whether brief periods of intense homosynaptic or heterosynaptic stimulation could alter the probability with which a standard test stimulus was able to evoke an action potential from the recorded neuron (Bliss et al., 1968). The problem with this approach was the difficulty of ascribing any changes to particular synapses in the uncharacterised polysynaptic pathway between stimulating and recording electrodes.
Bliss concluded that a simpler cortical structure was needed. The hippocampus, with its relatively simple and stratified organisation, seemed an obvious place to look, especially given the role of the hippocampus in human episodic memory revealed by the studies of patient HM by the McGill neuropsychologist Brenda Milner. Another advantage of the hippocampus was the opportunity it offered to record synaptically generated responses with an extracellular electrode. This was the technique of field potential recording pioneered by Per Andersen (1959) in Oslo. Andersen showed how the stratified organisation of the hippocampus, with its tightly packed cell fields and afferent projections terminating on restricted regions of the dendritic arborisation of target cells, allows monosynaptic field potentials to be recorded with extracellular electrodes (Figure 1).
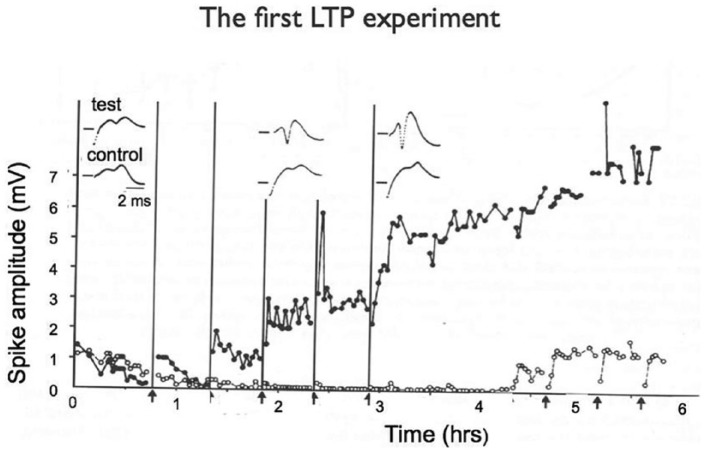
Figure 1.
The first experiment by Bliss and Lømo, performed in the autumn of 1968, on the effects of high-frequency stimulation on synaptic responses in the hippocampus of the anaesthetised rabbit. Because of drifting baselines, this figure was not included in Bliss and Lømo (1973), but the long-lasting potentiation of the responses in the tetanised pathway (filled circles) can be clearly seen. The moribund control pathway was coaxed back to life by repeated trains at the end of the experiment.
Soon after arriving in London, Bliss contacted Andersen and asked whether he could come to his lab to learn about field potential recording in the hippocampus with a view to using the technique to study synaptic plasticity. Andersen told him that his PhD student, Terje Lømo, had discovered something a couple of years before ‘that might interest you’. This something was later to become widely known by its acronym LTP (long-term potentiation). Lømo had been working on the phenomenon of frequency potentiation, a wind-up of monosynaptic field responses in the dentate gyrus to stimulation of the perforant path at 5–20 Hz and had noticed that the amplitude of monosynaptically evoked population spikes remained elevated after these episodes of high-frequency stimulation, sometimes for hours. In an abstract of a talk he gave at a meeting of the Scandanavian Physiological Society in Turku in 1966, he speculated that this might be a form of synaptic memory (Lømo, 1966). Lømo then returned to the main topic of his thesis, until Bliss’ arrival rekindled his interest in what he had discovered 2 years before. So it came about that at roughly weekly intervals during the academic year of 1968–1969, Bliss and Lømo performed the experiments on anaesthetised rabbits that were published in the Journal of Physiology in 1973. They improved the original experimental design by adding a control pathway, and measuring both the field excitatory postsynaptic potentials (EPSP) (the initial, synaptically generated component of the extracellular field response) and the amplitude and the latency of the population spike (the potential generated by the synchronous discharge of granule cells).
The Oslo experiments established that LTP consists of two components, one reflecting a persistent increase in synaptic strength and the other an increase in the excitability of granule cells; in many cases, the increase in the amplitude of the population spike was greater than could be explained by the increase in the field EPSP (this component was later termed EPSP-to-spike or E-S potentiation). LTP could often be enhanced by further episodes of tetanic potentiation, but eventually the effect became saturated. Thus, the effect of a tetanus is dependent on the past history of activity, a property later formalised under the name ‘metaplasticity’ (Abraham and Bear, 1996). Indirect evidence was obtained that potentiation was restricted to tetanised inputs (Bliss et al., 1973) – this is the property of input specificity.
In September 1969, both Bliss and Lømo departed for London, Bliss to return to NIMR, and Lømo to take up a post-doc position in the laboratory of Bernard Katz and Ricardo Miledi at University College London. Over the following few months, Lømo came to NIMR about once a week to continue the experiments that he and Bliss had begun in Oslo. Despite using the same strain of rabbits and the same anaesthetic regime, they found little if any evidence for persistent changes in synaptic efficacy following tetanic stimulation of the perforant path. The reason for this remains mysterious, but may, as Lømo has suggested (Lømo, 2017), reflect greater stress levels in London rabbits, a factor which is now known to impact negatively on the induction of LTP. Meanwhile, Bliss had set up a collaboration with Tony Gardner-Medwin at UCL to examine LTP in the dentate gyrus of awake rabbits, with implanted stimulating and recording electrodes. Greater success attended these experiments and LTP lasting days or weeks was seen in some animals. The Oslo and London experiments were finally published in the Journal of Physiology in 1973 (Bliss and Lømo, 1973; Bliss and Gardner-Medwin, 1973).
The 1973 papers that launched LTP cannot be said to have had an immediately electrifying impact. Graham Goddard, the Canadian neuroscientist who had discovered the phenomenon of kindling (a permanent change in the excitability of cortical tissue induced by repeated episodes of stimulation), was one of the few who made contact with the authors. He was on sabbatical in London in the mid-1970s and called Bliss to ask whether he had tried to obtain LTP in rats. Bliss had not and invited Goddard to come to NIMR where they could do the experiment together. Although that single experiment failed in its objective to elicit LTP, Goddard was impressed by the elegance of the monosynaptic field potentials that could be recorded in the dentate gyrus, and when he returned to the Dalhousie University in Halifax, Nova Scotia, he set up a laboratory to study LTP. In the late 1970s, the Goddard lab, with a trio of exceptional graduate students, Rob Douglas, Bruce McNaughton and Carol Barnes, made a number of fundamental contributions to LTP. Working with anesthetised rats, they established the concepts of cooperativity and associativity (Douglas and Goddard, 1975; McNaughton et al., 1978). They also introduced the first physiological method of blocking the induction of LTP, by combining tetanic stimulation of the perforant path with stimulation of the commissural input to the dentate gyrus which sets up powerful feedforward inhibition (Douglas et al., 1983).
Meanwhile, Bliss had begun to collaborate with Chris Richards who had arrived at NIMR in 1969 from Henry McIlwain’s laboratory (at the Institute of Psychiatry, Denmark Hill in South London), which had pioneered methods of recording electrical activity from isolated slices of cortical tissue. Richards and Bliss looked for LTP in longitudinal slices of the dentate gyrus. They were able to record similar field potentials to those seen in the anesthetised rabbit or rat and to demonstrate many of the same physiological properties such as paired-pulse facilitation and inhibition (Bliss and Richards, 1971), but were disappointed to find no evidence of LTP. The reason for this became clear several years later – granule cells in the dentate gyrus in vitro are hyperpolarised, and a pharmacological block of inhibition is required to obtain LTP. But the experiments led indirectly to the introduction of in vitro methodology that was to result in rapid advances in the mechanistic analysis of synaptic plasticity. In the summer of 1971, Knut Skrede, a graduate student who had worked with Bliss in Oslo, came to NIMR for a few weeks to learn about in vitro recording. Skrede returned to Oslo and had the inspired idea of cutting transverse slices, opening up the trisynaptic hippocampal circuitry to experimental analysis. In 1975, Phil Schwartzkroin and Knut Wester in Andersen’s lab in Oslo published the first account of in vitro LTP in the monosynaptic Schaffer collateral-commissural projection from CA3 to CA1 (pyramidal cells in contrast to granule cells are not hyperpolarised in vitro) (Schwartzkroin and Wester, 1975). It is likely that the first intracellular recordings of LTP were made in Per Andersen’s lab in 1977 (Andersen et al., 1977).
Later, in 1983, Gary Lynch at the University of Irvine in California and his lab made an important breakthrough when they found that LTP in CA1 pyramidal cells could be blocked by the intracellular injection of the Ca2+ chelator EGTA, demonstrating an essential involvement of the postsynaptic cell in the induction of LTP (Lynch et al., 1983). In the same year, Collingridge et al. (1983a) found that the induction (but not the expression) of LTP was blocked by an antagonist of the N-methyl-d-aspartate (NMDA) receptor, 2-amino-5-phosphonopentanoate (APV or AP5 as it is variably called). The subsequent demonstration that the unusual properties of the NMDA receptor (NMDAR), in particular its voltage-dependence and the permeability of its channel to Ca2+, account for many of the characteristics of LTP, including input specificity, cooperativity and associativity, were the crowning achievements of the first 15 years of LTP research. This early history is now retold.
Glutamate receptors and synaptic plasticity
In 1968, the identity of the major excitatory neurotransmitter in the CNS was unknown. David Curtis and Jeff Watkins and their colleagues in Canberra had demonstrated that l-glutamate excited individual central neurons but there were doubts whether such a global excitatory substance was the actual neurotransmitter (Curtis et al., 1960). Definitive evidence followed; first, the discovery that l-glutamate acts on multiple receptor subtypes – now known as NMDA, AMPA, kainate (KA) and metabotropic receptors – and, second, the development of specific glutamate receptor antagonists. Notably Watkins and his colleagues in Bristol and Hugh McLennan and his colleagues in Vancouver discovered the first selective NMDAR antagonists, such as alpha-amino adipate and, more famously, AP5. Of major significance, it was shown by these labs that the slow component of excitation of Renshaw cells in the spinal cord, by activation of dorsal roots, was selectively inhibited by NMDAR antagonists. These groups also found that fast synaptic transmission in the spinal cord was insensitive to NMDAR antagonists but was inhibited by more general glutamate receptor antagonists. This led to the concept that monosynaptic transmission was mediated by a non-NMDARs (potentially AMPA or kainate receptors), whereas polysynaptic excitation involved the activation of NMDARs (Biscoe et al., 1977; McLennan and Lodge, 1979). Subsequent work, performed by Dale and Roberts (1985) in Bristol, provided direct evidence that, in the Xenopus spinal cord, the slow nature of the NMDA response did not necessarily reflect a polysynaptic innervation. They found that they could evoke a unitary monosynaptic NMDAR-mediated EPSP with a relatively slow time-course. This led to the concept of dual-component EPSPs mediated by both non-NMDARs and NMDARs.
In the mid-1970s, the Bristol group made another discovery of fundamental significance. Richard Evans was studying the excitation of the hemisected spinal cord of the rat by glutamate receptor ligands such as NMDA and kainate. Traditionally, frog Ringer lacks Mg2+. Evans added Mg2+ to suppress neurotransmitter release and found, to his great surprise, that there was massive antagonism of the NMDA response but no effect on the kainate response. The discovery that Mg2+ blocks NMDARs was considered to be of insufficient interest for publication in Nature! Unperturbed, over the next few years Evans and his colleagues characterised the Mg2+ block and speculated that its action was likely to involve the ion channel (Ault et al., 1980). Subsequently, it was shown by Philiipe Ascher’s laboratory (Paris, France) and Mayer and Westbrook (National Institutes of Health (NIH), USA) that the Mg2+ block of NMDARs was highly voltage-dependent, diminishing dramatically upon depolarisation (Mayer et al., 1984; Nowak et al., 1984). Subsequently, these and other groups went on to show that the NMDAR has a high permeability to Ca2+ ions.
The kainate receptor was also identified by the Evans and Watkins team as a distinct entity from other non-NMDARs. The non-NMDA, non-kainate class of receptor was originally called the quisqualate receptor but is now known as the AMPA receptor, following the discovery by David Lodge (London, UK) and Povl Krogsgaard-Larsen (Copenhagen, Denmark) that AMPA is a more specific agonist (Krogsgaard-Larsen et al., 1981). David Lodge working with another Danish chemist, Taje Honore, then went on to characterise more selective AMPAR antagonists, the quinoxalinediones (e.g. CNQX, NBQX), which enabled the definitive identification of AMPARs as the mediators of fast synaptic transmission (Andreasen et al., 1989; Davies and Collingridge, 1989).
These fundamental properties of glutamate receptors are central to their roles in synaptic plasticity, as revealed over the ensuing decade.
Adding excitement to LTP
In 1980, one of us (G.L.C.) arrived in Vancouver (Canada) as a postdoc in the McLennan laboratory. When asked what the project was to be, the reply was anything that involves glutamate receptors (the topic of the lab’s funding). In the lab, David West, a visiting scientist from the United Kingdom, had started to study LTP in rat hippocampal slices. Collingridge remembers seeing LTP demonstrated for the first time and being totally transfixed – this was the phenomenon that he wanted to work on. Assuming l-glutamate to be the neurotransmitter, he decided to investigate the roles of the three ionotropic glutamate receptors subtypes in synaptic transmission and LTP at the monosynaptic connection between CA3 and CA1 pyramidal neurons – the Schaffer collateral-commissural pathway (SCCP). Together with a graduate student, Stephen Kehl, he applied selective agonists and antagonists and found that activation of NMDARs was the trigger for LTP, while non-NMDARs (presumed AMPARs) were the mediators of the fast, modifiable synaptic response. These findings were initially presented to the Physiological Society meeting in Aberdeen in 1981 and then published in the Journal of Physiology (Collingridge et al., 1983a; Collingridge et al., 1983b). An example of one of the original experiments is presented in Figure 2.
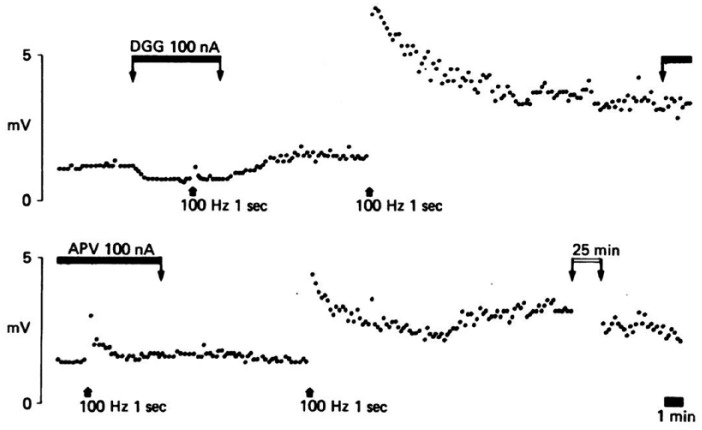
Figure 2.
An experiment from the study that first investigated the effects of glutamate receptor subtypes on the induction and expression of LTP (Collingridge et al., 1983b). Synaptic transmission was recorded from the CA1 cell body region of a rat hippocampal slice in response to stimulation of Schaffer collateral/commissural fibres and drugs applied ionophoretically into the dendritic region. γ-d-glutamlyglycine (DGG), which is an AMPAR and NMDAR antagonist, inhibited synaptic transmission and the induction of LTP, in response to a single tetanus (100 Hz, 1s; marked by arrow). A subsequent tetanus, delivered after washout of DGG, induced a substantial LTP. 2-amino-5-phosphonopentanoate (APV), a selective NMDAR antagonist, had no effect on this LTP. When the stimulus was reduced and a third tetanus delivered little synaptic plasticity was induced. A fourth tetanus delivered after washout of APV induced additional LTP, which was followed for a further 45 min.
In 1983, Collingridge established his laboratory at the University of Bristol (UK) and set out to ascertain the mechanisms by which NMDARs trigger the induction of LTP. By studying synaptic transmission at the SCCP and applying the new information regarding the properties of glutamate receptors described above, his team made a number of relevant observations.
First, they omitted Mg2+ from the perfusing medium, given its potent ability to block NMDARs. What they observed was a massive increase in the synaptic response that was due mainly to the unblocking of NMDARs. This showed that NMDARs could contribute to the evoked response elicited by a single volley, but were largely prevented from doing so by the Mg2+ present extracellularly (Herron et al., 1985a). Critically they showed how this Mg2+ block could be alleviated physiologically by the delivery of a high-frequency synaptic train (Collingridge et al., 1988).
In addition, they depolarised the membrane and recorded evoked synaptic currents. This revealed, for the first time, both the slow activation and deactivation kinetics of the EPSC (Collingridge et al., 1988). During the course of this work, they observed that depolarisation of the neuron paired with baseline stimulation could lead to LTP and so took care to maintain the neurons in a hyperpolarised state when not directly recording the NMDAR-mediated synaptic current. This property was also observed by several other groups and used to show how LTP could be induced by pairing low-frequency stimulation with depolarisation (e.g. Gustafsson et al., 1987).
They also showed how the synaptic activation of NMDARs was powerfully reduced by GABA-mediated synaptic inhibition (Herron et al., 1985b). It was proposed that the hyperpolarising effect of the inhibitory postsynaptic potentials (IPSP) was critical for limiting the synaptic activation of NMDARs during low-frequency activation.
Significantly, they found that GABA inhibition could be transiently suppressed by a GABAB-receptor auto receptor mechanism (Davies et al., 1990) and that this enables small numbers of appropriately timed stimuli to activate NMDARs sufficiently to trigger LTP (Davies et al., 1991). The best timing corresponds to the theta rhythm, a pattern of activity that is optimal for the induction of LTP (Larson et al., 1986). In other words, the physiological induction of LTP involves GABAB receptors to depress GABA inhibition and thereby enable the appropriate level of activation of NMDARs during these repeated brief high-frequency discharges (see Bliss and Collingridge, 1993).
Finally, they found that the synaptic activation of NMDARs resulted in a highly localised and transient increase in cytosolic Ca2+ (Alford et al., 1993)
These results, and related findings made by others, could explain the hallmark properties of LTP: input specificity, co-operativity and associativity. These properties also endowed synapses with Hebbian-like characteristics. This directly led to the widely accepted model for the induction of NMDAR-dependent LTP (Bliss and Collingridge, 1993).
Storing up excitement
By the start of the 1990s, the mechanism of induction of NMDAR-LTP was essentially established. So the Collingridge lab, and many other labs worldwide, turned their attention to what changes in LTP. In other words, what is the mechanism of expression of LTP? For many years, a raging debate ensued – the postsynaptic side claiming that expression was exclusively postsynaptic (in contrast to NMDAR-independent mossy fibre LTP, where expression was agreed by most researchers to be presynaptic), while the presynaptic camp was less doctrinal in claiming only that the expression of NMDAR-LTP was at least in part presynaptic. In reality, NMDAR-LTP can involve changes at both sides of the synapse (see reviews by Bliss and Collingridge, 2013; Padamsey and Emptage, 2014). Collingridge has long held the view that, at CA3-CA1 synapses, short-term potentiation (STP) is expressed presynaptically, while LTP has a major postsynaptic component (Davies et al., 1989). It has long been postulated that a presynaptic mechanism of expression requires the existence of a retrograde messenger to convey information from the postsynaptic site of induction to the presynaptic terminal (Bliss et al., 1986). The gaseous signalling molecule nitric oxide remains the most promising candidate for the retrograde messenger (Pigott and Garthwaite, 2016), though conclusive evidence is still lacking. The existence of presynaptic NMDARs (McGuinness et al., 2010) adds another twist to the situation.
So how does the activation of NMDARs lead to persistent changes in l-glutamate release and AMPARs? Although some details are still lacking, considerable progress has been made in cracking this problem. It is known that NMDAR activation results in turn in the activation of several different protein kinases. The first to be implicated in LTP was protein kinase A (PKA) (Frey et al., 1993; Matthies and Reymann, 1993). This was followed shortly after by the demonstration of crucial, yet distinct, roles for calcium/calmodulin-dependent protein kinase II (CaMKII), protein kinase C (PKC), phosphatidylinositiol-3-kinase (PI3K), extracellular signal–regulated kinase (ERK) and certain protein-tyrosine kinases (PTKs) (see Bliss et al., 2007). A kinase of particular interest is PKMzeta, an unusual PKC isoform that is involved in the storage of LTP (Pastalkova et al., 2006; Tsokas et al., 2016). One idea is that PKMzeta maintains more AMPARs at the synapse by stabilising the interaction between the GluA2 subunit of AMPARs and N-ethylmaleimide-sensitive factor (NSF) (Nishimune et al., 1998; Yao et al., 2008), thereby restricting the AP2-dependent endocytosis of AMPARs (see Collingridge et al., 2004, for a detailed description of the regulation of AMPAR trafficking).
In addition to purely post-translational mechanisms, it is evident that both de novo protein synthesis and gene transcription are important for the full expression of LTP. The idea that rapid protein synthesis may be required for LTP originated from the Magdeburg laboratory and has since become a widely studied phenomenon (Frey et al., 1988). Originally, it was thought that a weak stimulus (tetanus or theta burst train) induced a decaying form of LTP, lasting no more than a couple of hours, that did not require protein synthesis and was termed Early-LTP (E-LTP). In contrast, multiple trains induced a longer lasting form of LTP that required protein synthesis and possibly gene transcription, so-called Late LTP (L-LTP). However, it is now clear that the situation is not so straightforward. Both protein synthesis dependent and independent forms of LTP can persist for long periods of time (several hours at least) (Park et al., 2014). The protein synthesis–dependent form requires multiple spaced stimuli (at least two), with a separation interval in the order of minutes. The mechanism has recently been identified at CA1 synapses. The first train induces the transient insertion of calcium permeable (GluA2-lacking) AMPARs (CP-AMPARs) into the plasma membrane close to the synapse. Subsequent trains drive CP-AMPARs into the synapse where they are activated by low frequency stimulation and help trigger the protein synthesis component of LTP (Park et al., 2016). The timing requirements are presumed to reflect the dwell time of CP-AMPARs on the plasma membrane.
In addition to these two forms of NMDAR-LTP, a tetanus or theta burst triggers an STP that decays at a rate dependent on the post train stimulation frequency. If stimulation is halted, then STP does not decay further until stimulation is resumed (Volianskis and Jensen, 2003). This use-dependent potentiation is therefore another potentially long-lasting form of LTP with an extreme activity dependence. Intriguingly, different NMDAR subtypes mediate STP and LTP (Volianskis et al., 2013), explaining why STP is a little more resistant than LTP to the actions of AP5 (see Fig. 2).
In summary, several NMDAR-dependent forms of synaptic potentiation can co-exist at CA1 synapses. As we have posited previously, the co-existence of these multiple forms of LTP can go a long way to explaining many of the controversies that have for too long plagued the LTP field (Bliss and Collingridge, 2013). It is now clear that at CA1 synapses, LTP is a highly complex family of processes, the functional relevance of which has still to be fully determined. In a recent review, we have described three forms of NMDAR-dependent LTP, termed STP, LTP1 and LTP2, in more detail and have noted the existence of transcriptional-dependent form of LTP–LTP3 (Bliss et al., 2018). But we are still left with the crucial question of how long these forms of LTP last, a question that can only be properly answered by in vivo experiments. LTP – presumably LTP3 in the family of LTPs outlined here – in the dentate gyrus can persist for at least a year (Abraham, 2003). Further work is required to establish the time courses of STP, LTP1, LTP2 and LTP3 in vivo.
The depressing side of LTP
It is now widely accepted that information is stored at synapses by increasing synaptic weights using LTP-like mechanisms. But if synapses only became stronger, then the system would eventually become saturated and potentially hyperexcitable. Passive decay of potentiated synapses could, eventually, return the synapse back to a resting state, but given how long LTP can last this would be a slow and inefficient mechanism. Rather, there has evolved a variety of ways in which synapses can be weakened via a generic process known as long-term depression (LTD). LTD may occur homosynaptically at stimulated synapses or at neighbouring non-activated inputs (heterosynaptic LTD). LTD can occur from a resting state (sometimes referred to as de novo LTD) or from a potentiated state (where it is termed depotentiation). LTD requires longer periods of stimulation than LTP for its induction (typically, several hundred trains at 1 Hz). Interestingly, activation of NMDARs can induce LTD; accordingly, NMDARs confer plasticity but not necessarily the direction of the change. Other factors such as the duration of the activity-induced Ca2+-transient can control whether NMDAR activation leads to LTP or LTD. It should be noted that whereas NMDARs are common triggers for LTD, they are not the only ones. The G-protein-coupled mGluRs, and indeed other G-protein-linked receptors, can also trigger LTD via mechanisms that only partially overlap with those induced by NMDARs. Considerable progress has been made in uncovering the mechanisms of NMDAR-LTD. A major mechanism of expression is the AP2-dependent endocytosis of AMPARs. NMDAR activation triggers a protein phosphatase cascade, the activation of protein kinases, such as GSK-3, and alterations in the actin cytoskeleton. See Collingridge et al. (2010) for a more detailed discussion of the various types of LTD and their underlying mechanisms.
There is also a homeostatic mechanism that prevents LTP from increasing synaptic strength without bound (Turrigiano, 2012). The synaptic homeostasis hypothesis (Tononi and Cirelli, 2003) proposes that synapses that are potentiated during the waking state are scaled down during sleep to preserve the overall excitability of the mnemonic network. Up-down state changes in membrane potential, which occur during slow wave sleep, may mediate this effect (Gonzalez-Rueda et al., 2018).
LToPathies
There is increasing evidence that deficits in LTP and LTD contribute to major neurological and psychiatric conditions. Here, we summarise some of the conditions where NMDAR-dependent plasticity is compromised.
Alzheimer’s disease
Perhaps the brain disorder with the strongest association with a synaptic plasticity deficit is Alzheimer’s disease (AD) (Chapman et al., 1999; Rowan et al., 2003). The earliest symptoms of AD are usually an impairment in the learning and remembering of new information. AD is characterised by plaques and tangles that represent predominantly aggregates of Abeta(1-42) peptide and hyper-phosphorylation of tau, respectively. Early work established that mouse models with human disease mutations in genes associated with early onset AD, such as APP and PS1, could result in impairments in LTP and/or synaptic transmission. Furthermore, it was found that acutely applied Abeta oligomers inhibit LTP and facilitate LTD (Rowan et al., 2003). It has also been shown that molecules closely associated with AD, such as GSK-3 (also known as tau kinase) and tau itself, play integral roles in the physiological LTD process (Kimura et al., 2014; Peineau et al., 2007). A working model for the early stages of dementia runs as follows. LTD is a physiological process used to prune superfluous synapses. Ordinarily, LTD is kept in check by LTP, via the inhibition of GSK-3. If this regulation is perturbed, then excessive GSK-3 activation drives aberrant synapse elimination via phosphorylation of tau. Many factors, genetic, epigenetic and environmental, could shift the balance in favour of LTD and hence synapse elimination. Synapse elimination is associated with hyper-phosphorylation of tau. Consistent with the view that AD is a disorder of synaptic plasticity, the available treatments that provide temporary symptomatic relief for AD probably work by regulating synaptic plasticity, and, specifically, by shifting the LTP/LTD balance in favour of LTP. Thus, AChE inhibitors, by boosting cholinergic function, can augment NMDAR function and LTP (Segal and Auerbach, 1997) and memantine, a weak NMDAR channel blocker, can, like Mg2+ (Coan et al., 1989), normalise aberrant NMDAR-mediated synaptic plasticity (Danysz and Parsons, 2003).
Epilepsy
The first condition in which an LTP-like synaptic plasticity was noted was epilepsy. Repeated stimulation can lead to seizures via a process known as kindling (Goddard et al., 1969). Subsequent work demonstrated that NMDAR antagonists could effectively reduce seizures in experimental animals (Croucher et al., 1982). These findings raised the possibility that some forms of epilepsy may involve NMDAR-mediated synaptic plasticity. However, the relationship between epilepsy and LTP has not been firmly established and is likely to be complex.
Down’s syndrome
In a range of Down’s syndrome models, a reduction in NMDAR-dependent LTP and/or an enhancement in NMDAR-dependent LTD have been demonstrated (O’Doherty et al., 2005)
Huntington’s disease
Similarly in Huntington’s disease, alterations in NMDAR-mediated synaptic plasticity have been demonstrated. Such alteration could underlie the cognitive deficits that may precede movement disorders (Murphy et al., 2000)
Multiple sclerosis
Another condition in which cognitive deficits are often an early symptom is multiple sclerosis (MS). Here again alterations in NMDAR-dependent synaptic plasticity in the hippocampus have been observed (Nistico et al., 2014).
Acute neurodegenerative conditions
Several acute neurodegenerative conditions, including stroke-induced and trauma-induced brain damage, seem to engage similar mechanisms to the chronic neurodegenerative conditions discussed above. Indeed, it is established that NMDAR antagonists are effective against various CNS insults, such as ischemic damage (Simon et al., 1984). Accordingly, NMDAR antagonists have, and continue to be, in clinical trials for neuroprotection against acute brain insults.
Chronic pain
Another disorder where there is strong evidence that dysregulated synaptic plasticity is an integral part of the condition is chronic pain. This probably occurs at both spinal and supra-spinal levels. In the latter case, there is strong evidence that chronic pain results in LTP in areas such as the anterior cingulate cortex (ACC). More specifically, chronic pain induces NMDAR-dependent LTP and treatment with a PKMzeta inhibitor, ZIP, that reverses LTP can lead to analgesia. Furthermore, activation of pyramidal neurons in the ACC is both sufficient and necessary for hyperalgesia. Finally, chronic pain may lead to inhibition of LTD. Together, these findings show that very similar synaptic mechanisms that are employed by the hippocampus for spatial learning and memory are used in ACC and related brain areas as well as in the spinal cord for the storing and amplification of pain (reviewed in Bliss et al., 2016).
Anxiety
There is recent evidence to implicate synaptic plasticity in the genesis of anxiety, at least with respect to the anxiety that is associated with chronic pain. It was found that in the ACC, pain-associated anxiety involves a presynaptic form of LTP. This LTP resembles mossy fibre LTP in the hippocampus in that it involves GluK1 kainate receptors, adenylyl cyclase and inhibition of ZD7288-sensive channels (putatively HCN channels). Inhibition of this target with ZD7288 reduced both the expression of LTP and this form of anxiety (Koga et al., 2015). These findings suggest that presynaptic NMDAR-independent LTP can augment postsynaptic NMDAR-dependent LTP at synapses, and in this way anxiety can exacerbate the subjective feeling associated with a chronic pain state (Bliss et al., 2016).
Chronic stress
It is well established that chronic stress leads to impaired NMDAR-LTP in the hippocampus and that it also promotes NMDAR-LTD (Xu et al., 1997). Interestingly, acute stress may enhance LTP, by leading to the insertion of CP-AMPARs, which results in an additive form of LTP (Whitehead et al., 2013). Therefore, stress exerts a bimodal effect on LTP depending on its duration/intensity. Presumably, acute stress leads to transient insertion of CP-AMPARs to enhance plasticity and hence to an increase cognitive potential when required. However, chronic stress leads to reduced LTP by a neurotoxic insult resulting from excessive activation of NMDARs and or CP-AMPARs.
Depression
Two fundamental discoveries are that ketamine is an NMDAR antagonist (Anis et al., 1983) and that a single administration of ketamine can induce a rapid and long-lasting antidepressant effect (Berman et al., 2000). Furthermore, ketamine is remarkably effective in patients who are refractory to classic antidepressants and significantly reduces suicidal tendencies. Whether this is due to an effect on NMDAR-mediated synaptic plasticity is uncertain, but the duration of the effect makes this seem likely. One idea is that depression involves the NMDAR-dependent reconsolidation of a negative mood state by reactivating LTP in negative reward pathways and/or reactivating LTD in positive reward pathways (Collingridge et al., 2017). Ketamine breaks the negative mood cycle by blocking reconsolidation. Alternative explanations involve NMDAR-independent actions of ketamine that affect AMPAR-mediated synaptic transmission and thereby, presumably, tap into the expression mechanisms of synaptic plasticity or mechanisms that are independent of glutamate receptors. Given the promise of ketamine-like compounds for the treatment of depression, resolving the mechanism of its therapeutic action is a high priority.
Fragile-X
Although Fragile-X is commonly associated with enhanced mGluR-induced LTD, there is also evidence that NMDAR-dependent LTP is reduced. Interestingly, both the LTP deficit and the enhanced mGluR-LTD can be reversed by selective inhibition of GluN2A-containing NMDARs (Lundbye et al., 2018). Subtype-selective modulation of NMDARs therefore may be a promising therapeutic angle for Fragile-X.
Autism
Several studies have shown that synaptic plasticity is impaired in genetic models of autism. For example, deletion of various exons of the scaffolding protein SHANK2 produces autistic-like features in mice and can lead to either potentiation or inhibition of NMDAR-mediated synaptic plasticity (Schmeisser et al., 2012; Won et al., 2012). Significantly, normalisation of NMDAR function, using for example d-cycloserine, can reverse some of these autism-like features in mice. These data suggest that impaired NMDAR-mediated synaptic plasticity may underlie some forms of autism. Given that autism involves an impairment of social learning, the implication is that NMDAR-triggered synaptic plasticity is crucial for this type of learning and memory.
Schizophrenia
Early evidence that alterations in synaptic plasticity may underlie schizophrenia were the three independent discoveries that (a) PCP, administration of which induces schizophrenic-like symptoms, is able to inhibit the induction of LTP at CA1 synapses (Stringer and Guyenet, 1983); (b) that LTP at these synapses requires activation of NMDARs (Collingridge et al., 1983); and (c) that PCP is a potent NMDAR antagonist (Anis et al., 1983). This, and other evidence, led to the glutamate hypo function theory of schizophrenia (Olney and Farber, 1995). This, in turn, stimulated efforts to target schizophrenia by boosting glutamatergic function, in particular using compounds such as d-cycloserine to augment NMDAR function. Subsequent to the pharmacological evidence for a link between synaptic plasticity and schizophrenia, there has been a wealth of genetic data that has substantiated this hypothesis and revealed new targets for therapeutic intervention (Fromer et al., 2014). Perhaps the most direct evidence for a role of NMDARs, and presumably synaptic plasticity, in schizophrenia is the finding that autoimmune encephalopathy, which presents as a schizophrenic-like syndrome, is often triggered by antibodies to the GluN1 subunit of NMDARs (Dalmau et al., 2007).
These, and other acute and chronic neurodegenerative conditions, point to a common mechanism of action, namely a dysregulation in NMDAR-mediated synaptic plasticity. Although the mechanism is broadly the same, often involving a shift from LTP to LTD, the driving factors vary considerably from condition to condition. In some cases, the underlying cause may be entirely or largely genetic (e.g. Huntington’s disease), while in others, it may be entirely or largely environmental (e.g. head injury). In many cases it will involve an interplay between genetic, epigenetic and environmental conditions, with the brain region(s) affected dictating the manifestation of the condition.
Looking ahead
Interest in synaptic plasticity has grown hand in hand with the spectacular advances in molecular biological and optical techniques available to study it. Induction of LTP drives the expression of a number of immediate early genes (IEGs), such as cfos, zif268 and arc, and the use of the promoters of these IEGs to drive the conditional expression of light-sensitive channel proteins such as channelrhodopsin and halorhodopsin has ushered in the era of optogenetics. It is now possible to excite or suppress activity in a subpopulation of cells which can be regarded as the engram of a particular learnt response. In the first of an on-going series of experiments on fear conditioning from Susumo Tonegawa’s laboratory, light-induced activation of an IEG-expressing subpopulation of granule cells resulted in the mice emitting the learned response (freezing) without any specific external stimulus (Tonegawa et al., 2015). In another approach, the induction of LTD in an auditory conditioned stimulus (CS) pathway resulted in abolition of fear learning, while the induction of LTP restored the learned response (Nabavi et al., 2014). These results reflect the progress that is now being made in linking synaptic plasticity and learning at the network level. A complete account of an individual memory will include a description of the synaptic engram by labelling the synapses whose strength has been altered to encode that memory. Recent papers have begun to apply optogenetic techniques to labelling and manipulating the engram at a synaptic level (Abdou et al., 2018; Choi et al., 2018; Hayashi-Takagi et al., 2015).
These approaches allow us to be reasonably confident that a complete description of the engram, at both the cellular and synaptic levels, will ultimately be achievable. The challenge for the future will be to exploit these techniques to throw light on the various stages of memory processing: acquisition, consolidation, retrieval, extinction and reconsolidation. The most elusive and mysterious of these processes is retrieval; indeed, it remains unclear to what extent memory failure is due to faulty consolidation or inadequate retrieval. Given the enormous progress in understanding the cellular mechanisms underlying memory during the BNA’s first 50 years, we can be reasonably confident that these problems will be well on the way to a resolution before its centenary year.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Abdou K, Shehata M, Choko K, et al. (2018) Synapse-specific representation of the identity of overlapping memory engrams. Science 360(6394): 1227–1231. [DOI] [PubMed] [Google Scholar]
- Abraham WC. (2003) How long will long-term potentiation last? Philosophical Transactions of the Royal Society of London: Series B, Biological Sciences 358(1432): 735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham WC, Bear MF. (1996) Metaplasticity: The plasticity of synaptic plasticity. Trends in Neurosciences 19(4): 126–130. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Morris RGM. (2018) Memory: Looking back and looking forward. Brain and Neuroscience Advances. Epub ahead of print 1 January DOI: 10.1177/2398212818794830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford S, Frenguelli BG, Schofield JG, et al. (1993) Characterization of Ca2+ signals induced in hippocampal CA1 neurones by the synaptic activation of NMDA receptors. Journal of Physiology 469(1): 693–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P. (1959) Interhippocampal impulses I. Origin, course and distribution in cat, rabbit and rat. Acta Physiologica Scandinavica 47(1): 63–90. [DOI] [PubMed] [Google Scholar]
- Andersen P, Sundberg SH, Sveen O, et al. (1977) Specific long-lasting potentiation of synaptic transmission in hippocampal slices. Nature 266(5604): 736–737. [DOI] [PubMed] [Google Scholar]
- Andreasen M, Lambert JD, Jensen MS. (1989) Effects of new non-N-methyl-D-aspartate antagonists on synaptic transmission in the in vitro rat hippocampus. Journal of Physiology 414(1): 317–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anis NA, Berry SC, Burton NR, et al. (1983) The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. British Journal of Pharmacology 79(2): 565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ault B, Evans RH, Francis AA, et al. (1980) Selective depression of excitatory amino acid induced depolarizations by magnesium ions in isolated spinal cord preparations. Journal of Physiology 307(1): 413–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, et al. (2000) Antidepressant effects of ketamine in depressed patients. Biological Psychiatry 47(4): 351–354. [DOI] [PubMed] [Google Scholar]
- Biscoe TJ, Evans RH, Francis AA, et al. (1977) D-alpha-Aminoadipate as a selective antagonist of amino acid-induced and synaptic excitation of mammalian spinal neurones. Nature 270(5639): 743–745. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. (1993) A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 361(6407): 31–39. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. (2013) Expression of NMDA receptor-dependent LTP in the hippocampus: Bridging the divide. Molecular Brain 6(1): 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TVP, Gardner-Medwin AR. (1973) Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. Journal of Physiology 232(2): 357–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TVP, Lømo T. (1973) Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. Journal of Physiology 232(2): 331–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TVP, Richards CD. (1971) Some experiments with in vitro hippocampal slices. Journal of Physiology 214(1): 7P–9P. [PubMed] [Google Scholar]
- Bliss TVP, Burns BD, Uttley AM. (1968) Factors affecting the conductivity of pathways in the cerebral cortex. Journal of Physiology 195(2): 339–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TVP, Douglas RM, Errington ML, Lynch MA. (1986) Correlation between long-term potentiation and release of endogenous amino acids from dentate gyrus of anaesthetized rats. Journal of Physiology 377: 391–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL, Morris RG. (2007) Synaptic plasticity in the hippocampus. In: Andersen P, Morris R, Amaral A, et al. (eds) The Hippocampus Book. New York: Oxford University Press, pp. 343–474. [Google Scholar]
- Bliss TVP, Collingridge GL, Kaang BK, et al. (2016) Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nature Reviews Neuroscience 17(8): 485–496. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL, Morris RGM, Reymann KG. (2018) Long-term potentiation in the hippocampus: discovery, mechanisms and function. Neuroforum 24(3): A103–120. 10.1515/nf-2017-A059. [DOI] [Google Scholar]
- Bliss TVP, Gardner-Medwin AR, Lømo T. (1973) Synaptic plasticity in the hippocampal formation. In: Ansell GB, Bradley PB. (eds) Macromolecules and Behavior. Baltimore, MD: University Park Press, pp. 193–203. [Google Scholar]
- Chapman PF, White GL, Jones MW, et al. (1990) Impaired synaptic plasticity and learning in amyloid precursor protein transgenic mice. Nature Neuroscience 2(3): 271–276. [DOI] [PubMed] [Google Scholar]
- Choi JH, Sim SE, Kim JI, et al. (2018) Interregional synaptic maps among engram cells underlie memory formation. Science 360(6387): 430–435. [DOI] [PubMed] [Google Scholar]
- Coan EJ, Irving AJ, Collingridge GL. (1989) Low-frequency activation of the NMDA receptor system can prevent the induction of LTP. Neuroscience Letters 105(1–2): 205–210. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Herron C, Lester RAJ. (1988) Synaptic activation of N-methyl-D-aspartate receptors in the Schaffer collateral-commissural pathway of rat hippocampus. Journal of Physiology 399(1): 283–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Isaac JT, Wang YT. (2004) Receptor trafficking and synaptic plasticity. Nature Reviews Neuroscience 5(12): 952–962. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Kehl SJ, McLennan H. (1983. a) Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. Journal of Physiology 334(1): 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Kehl SJ, McLennan H. (1983. b) The antagonism of amino acid-induced excitations of rat hippocampal CA1 neurones in vitro. Journal of Physiology 334(1): 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Lee Y, Bortolotto ZA, et al. (2017) Antidepressant actions of ketamine versus hydroxynorketamine. Biological Psychiatry 81(8): e65–e67. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Peineau S, Howland JG, et al. (2010) Long-term depression in the CNS. Nature Reviews Neuroscience 11(7): 459–473. [DOI] [PubMed] [Google Scholar]
- Croucher MJ, Collins JF, Meldrum BS. (1982) Anticonvulsant action of excitatory amino acid antagonists. Science 216(4548): 899–901. [DOI] [PubMed] [Google Scholar]
- Curtis DR, Phillis JW, Watkins JC. (1960) The chemical excitation of spinal neurones by certain acidic amino acids. Journal of Physiology 150(3): 656–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N, Roberts A. (1985) Dual-component amino-acid-mediated synaptic potentials: Excitatory drive for swimming in Xenopus embryos. Journal of Physiology 363(1): 35–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmau J, Tüzün E, Wu HY, et al. (2007) Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Annals of Neurology 61(1): 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danysz W, Parsons CG. (2003) The NMDA receptor antagonist memantine as a symptomatological and neuroprotective treatment for Alzheimer’s disease: Preclinical evidence. International Journal of Geriatric Psychiatry 18(1): S23–S32. [DOI] [PubMed] [Google Scholar]
- Davies CH, Davies SN, Collingridge GL. (1990) Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. Journal of Physiology 424(1): 513–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CH, Starkey SJ, Pozza MF, et al. (1991) GABA autoreceptors regulate the induction of LTP. Nature 349(6310): 609–611. [DOI] [PubMed] [Google Scholar]
- Davies SN, Collingridge GL. (1989) Role of excitatory amino acid receptors in synaptic transmission in area CA1 of rat hippocampus. Proceedings of the Royal Society of London: Series B, Biological Sciences 236(1285): 373–384. [DOI] [PubMed] [Google Scholar]
- Davies SN, Lester RA, Reymann KG, et al. (1989) Temporally distinct pre- and post-synaptic mechanisms maintain long-term potentiation. Nature 338(6215): 500–503. [DOI] [PubMed] [Google Scholar]
- Douglas RM, Goddard GV. (1975) Long-term potentiation of the perforant path-granule cell synapse in the rat hippocampus. Brain Research 86(2): 205–215. [DOI] [PubMed] [Google Scholar]
- Douglas RM, McNaughton BL, Goddard GV. (1983) Commissural inhibition and facilitation of granule cell discharge in fascia dentata. Journal of Comparative Neurology 219(3): 285–294. [DOI] [PubMed] [Google Scholar]
- Frey U, Huang Y-Y, Kandel ER. (1993) Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science 260(5114): 1661–1664. [DOI] [PubMed] [Google Scholar]
- Frey U, Krug M, Reymann KG, et al. (1988) Anisomycin, an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro. Brain Research 452(1–2): 57–65. [DOI] [PubMed] [Google Scholar]
- Fromer M, Pocklington AJ, Kavanagh DH, et al. (2014) De novo mutations in schizophrenia implicate synaptic networks. Nature 506(7487): 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard GV, McIntyre DC, Leech CK. (1969) A permanent change in brain function resulting from daily electrical stimulation. Experimental Neurology 25(3): 295–330. [DOI] [PubMed] [Google Scholar]
- Gonzalez- Rueda A, Pedrosa V, Feord RC, et al. (2018) Activity-dependent downscaling of subthreshold synaptic inputs during slow-wave-sleep-like activity in vivo. Neuron 97(6): 1244–1252.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B, Wigström H, Abraham WC, et al. (1987) Long-term potentiation in the hippocampus using depolarizing current pulses as the conditioning stimulus to single volley synaptic potentials. Journal of Neuroscience 7(3): 774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi-Takagi A, Yagishita S, Nakamura M, et al. (2015) Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature 525(7569): 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. (1949) The Organization of Behavior. New York: John Wiley & Sons. [Google Scholar]
- Herron CE, Lester RAJ, Coan EJ, et al. (1985. a) Intracellular demonstration of an N-methyl-D-aspartate receptor mediated component of synaptic transmission in the rat hippocampus. Neuroscience Letters 60(1): 19–23. [DOI] [PubMed] [Google Scholar]
- Herron CE, Williamson R, Collingridge GL. (1985. b) A selective N-methyl-D-aspartate antagonist depresses epileptiform activity in rat hippocampal slices. Neuroscience Letters 61(3): 255–260. [DOI] [PubMed] [Google Scholar]
- Kimura T, Whitcomb DJ, Jo J, et al. (2014) Microtubule-associated protein tau is essential for long-term depression in the hippocampus. Philosophical Transactions of the Royal Society of London: Series B, Biological Sciences 369(1633): 20130144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga K, Descalzi G, Chen T, et al. (2015) Coexistence of two forms of LTP in ACC provides a synaptic mechanism for the interactions between anxiety and chronic pain. Neuron 85(2): 377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P, Honoré T, Hansen JJ, et al. (1981) Structure-activity studies on ibotenic acid and related muscimol analogues. Advances in Biochemical Psychopharmacology 27: 285–294. [PubMed] [Google Scholar]
- Lømo T. (1966) Frequency potentiation of excitatory synaptic activity in the dentate area of the hippocampal formation. Acta Physiologica Scandinavica 68(Suppl. 277): 128. [Google Scholar]
- Lømo T. (2017) Discovering long-term potentiation (LTP) - recollections and reflections on what came after. Acta Physiol (Oxf). 222(2). doi.org:10.1111/apha.12921. [DOI] [PubMed] [Google Scholar]
- Larson J, Wong D, Lynch G. (1986) Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Research 368(2): 347–350. [DOI] [PubMed] [Google Scholar]
- Lundbye CJ, Toft AKH, Banke TG. (2018) Inhibition of GluN2A NMDA receptors ameliorates synaptic plasticity deficits in the Fmr1−/y mouse model. Journal of Physiology 596(20): 5017–5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G, Larson J, Kelso S, et al. (1983) Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature 305(5936): 719–721. [DOI] [PubMed] [Google Scholar]
- McGuinness L, Taylor C, Taylor RD, et al. (2010) Presynaptic NMDARs in the hippocampus facilitate transmitter release at theta frequency. Neuron 68(6): 1109–1127. [DOI] [PubMed] [Google Scholar]
- McLennan H, Lodge D. (1979) The antagonism of amino acid-induced excitation of spinal neurones in the cat. Brain Research 169(1): 83–90. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Douglas RM, Goddard GV. (1978) Synaptic enhancement in fascia dentata: Cooperativity among coactive afferents. Brain Research 157(2): 277–293. [DOI] [PubMed] [Google Scholar]
- Matthies H, Reymann KG. (1993) Protein kinase A inhibitors prevent the maintenance of hippocampal long-term potentiation. Neuroreport 4(6): 712–714. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. (1984) Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature 309(5965): 261–263. [DOI] [PubMed] [Google Scholar]
- Murphy KP, Carter RJ, Lione LA, et al. (2000) Abnormal synaptic plasticity and impaired spatial cognition in mice transgenic for exon 1 of the human Huntington’s disease mutation. Journal of Neuroscience 20(13): 5115–5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi S, Fox R, Proulx CD, et al. (2014) Engineering a memory with LTD and LTP. Nature 511(7509): 348–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimune A, Isaac JT, Molnar E, et al. (1998) NSF binding to GluR2 regulates synaptic transmission. Neuron 21(1): 87–97. [DOI] [PubMed] [Google Scholar]
- Nistico R, Mori F, Feligioni M, et al. (2014) Synaptic plasticity in multiple sclerosis and in experimental autoimmune encephalomyelitis. Philosophical Transactions of the Royal Society of London: Series B, Biological Sciences 369(1633): 20130162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, et al. (1984) Magnesium gates glutamate-activated channels in mouse central neurones. Nature 307(5950): 462–465. [DOI] [PubMed] [Google Scholar]
- O’Doherty A, Ruf S, Mulligan C, et al. (2005) An aneuploid mouse strain carrying human chromosome 21 with Down syndrome phenotypes. Science 309(5743): 2033–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW, Farber NB. (1995) Glutamate receptor dysfunction and schizophrenia. Archives of General Psychiatry 52(12): 998–1007. [DOI] [PubMed] [Google Scholar]
- Padamsey Z, Emptage N. (2014) Two sides to long-term potentiation: A view towards reconciliation. Philosophical Transactions of the Royal Society of London: Series B, Biological Sciences 369(1633): 20130154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park P, Sanderson TM, Amici M, et al. (2016) Calcium-permeable AMPA receptors mediate the induction of the protein kinase A-dependent component of long-term potentiation in the hippocampus. Journal of Neuroscience 36(2): 622–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park P, Volianskis A, Sanderson TM, et al. (2014) NMDA receptor-dependent long-term potentiation comprises a family of temporally overlapping forms of synaptic plasticity that are induced by different patterns of stimulation. Philosophical Transactions of the Royal Society of London: Series B, Biological Sciences 369(1633): 20130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, et al. (2006) Storage of spatial information by the maintenance mechanism of LTP. Science 313(5790): 1141–1144. [DOI] [PubMed] [Google Scholar]
- Peineau S, Taghibiglou C, Bradley C, et al. (2007) LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron 53(5): 703–717. [DOI] [PubMed] [Google Scholar]
- Pigott BM, Garthwaite J. (2016) Nitric oxide is required for L-type Ca(2+) channel-dependent long-term potentiation in the hippocampus. Frontiers in Synaptic Neuroscience 8: 17. DOI: 10.3389/fnsyn.2016.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan MJ, Klyubin I, Cullen WK, et al. (2003) Synaptic plasticity in animal models of early Alzheimer’s disease. Philosophical Transactions of the Royal Society of London: Series B, Biological Sciences 358(1432): 821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeisser MJ, Ey E, Wegener S, et al. (2012) Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature 486(7402): 256–260. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA, Wester K. (1975) Long-lasting facilitation of a synaptic potential following tetanization in the in vitro hippocampal slice. Brain Research 89(1): 107–119. [DOI] [PubMed] [Google Scholar]
- Segal M, Auerbach JM. (1997) Muscarinic receptors involved in hippocampal plasticity. Life Sciences 60(13–14): 1085–1091. [DOI] [PubMed] [Google Scholar]
- Simon RP, Swan JH, Griffiths T, et al. (1984) Blockade of N-methyl-D-aspartate receptors may protect against ischemic damage in the brain. Science 226(4676): 850–852. [DOI] [PubMed] [Google Scholar]
- Stringer JL, Guyenet PG. (1983) Elimination of long-term potentiation in the hippocampus by phencyclidine and ketamine. Brain Research 258(1): 159–164. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. (2003) Sleep and synaptic homeostasis: a hypothesis. Brain Research Bulletin 62(2): 143–150. [DOI] [PubMed] [Google Scholar]
- Tonegawa S, Liu X, Ramirez S, et al. (2015) Memory engram cells have come of age. Neuron 87(5): 918–931. [DOI] [PubMed] [Google Scholar]
- Tsokas P, Hsieh C, Yao Y, et al. (2016) Compensation for PKMzeta in long-term potentiation and spatial long-term memory in mutant mice. DOI: 10.7554/eLife.14846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G. (2012) Homeostatic synaptic plasticity: Local and global mechanisms for stabilizing neuronal function. Cold Spring Harbor Perspectives in Biology 4(1): a005736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volianskis A, Jensen MS. (2003) Transient and sustained types of long-term potentiation in the CA1 area of the rat hippocampus. Journal of Physiology 550(Pt. 2): 459–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volianskis A, Bannister N, Collett VJ, et al. (2013) Different NMDA receptor subtypes mediate induction of long-term potentiation and two forms of short-term potentiation at CA1 synapses in rat hippocampus in vitro. Journal of Physiology 591(4): 955–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead G, Jo J, Hogg EL, et al. (2013) Acute stress causes rapid synaptic insertion of Ca2+ -permeable AMPA receptors to facilitate long-term potentiation in the hippocampus. Brain 136(Pt. 12): 3753–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won H, Lee HR, Gee HY, et al. (2012) Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature 486(7402): 261–265. [DOI] [PubMed] [Google Scholar]
- Xu L, Anwyl R, Rowan MJ. (1997) Behavioural stress facilitates the induction of long-term depression in the hippocampus. Nature 387(6632): 497–500. [DOI] [PubMed] [Google Scholar]
- Yao Y, Kelly MT, Sajikumar S, et al. (2008) PKM zeta maintains late long-term potentiation by N-ethylmaleimide-sensitive factor/GluR2-dependent trafficking of postsynaptic AMPA receptors. Journal of Neuroscience 28(31): 7820–7827. [DOI] [PMC free article] [PubMed] [Google Scholar]