Abstract
This review brings together past and present achievements in memory research, ranging from molecular to psychological discoveries. Despite some false starts, major advances include our growing understanding of learning-related neural plasticity and the characterisation of different classes of memory. One striking example is the ability to reactivate targeted neuronal ensembles so that an animal will seemingly re-experience a particular memory, with the further potential to modify such memories. Meanwhile, human functional imaging studies can distinguish individual episodic memories based on voxel activation patterns. While the hippocampus continues to provide a rich source of information, future progress requires broadening our research to involve other sites. Related challenges include the need to understand better the role of glial–neuron interactions and to look beyond the synapse as the sole site of experience-dependent plasticity. Unmet goals include translating our neuroscientific knowledge in order to optimise learning and memory, especially among disadvantaged populations.
Keywords: Consolidation, hippocampus, plasticity, recall, retrieval, review
Miss Prism: Memory, my dear Cecily, is the diary that we all carry about us.
Cecily: Yes, but it usually chronicles the things that have never happened, and couldn’t possibly have happened. (Oscar Wilde (1895), The Importance of Being Earnest)
This conversation anticipates two complementary themes that run throughout memory research. In the 1960s, when the British Neuroscience Association (BNA - the then Brain Research Asssociation or BRA) was founded, the ‘cognitive revolution’ was underway and the principal goal was to understand how the ‘diary’ of our memories was written and how the correct page became available when looking back to remember past events. A parallel goal was to understand how memory errors occur. Over the intervening period, there has been a growing emphasis on neural circuits, rather than specific brain areas, combined with increasingly detailed analyses of synaptic plasticity. Now, in the 21st century, the traditional representational framework of cognitive neuroscience is under attack from those arguing for ‘embodied cognition’ (Claxton, 2015), a debate that is yet to be resolved. Meanwhile, we can now appreciate that memory not only allows us to look back but can also help us look forward, making the past a platform for future thinking.
Memory: at the birth of the BNA
With the aid of an old textbook (Physiological Psychology by Peter Milner, 1970), it is possible to see how far research in learning and memory has come in 50 years. The answer is a long, long way.
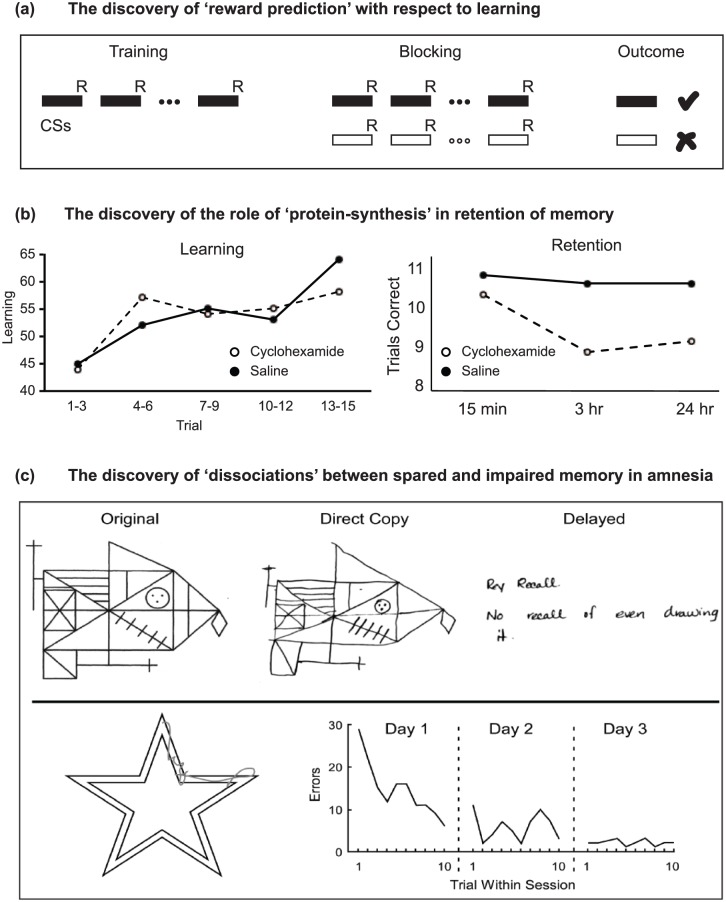
Attempts in the 1960s to understand learning and memory were heavily influenced by animal research. One leading approach for studying memory mechanisms was (and still is) the behavioural analysis of how lesions affect learning, with related research examining the extinction of that learning (Grey, 2000; Milner, 1970). At the time, analyses of animal learning were dominated by ideas and experimental procedures taken from ‘Behaviourism’. ‘Reinforcement’ was regarded as a kind of stamping-in operation for learning, something that increased the probability of a certain class of behaviour, with many ideas about learning inevitably shaped by notions of reinforcement. That said, the pioneers of modern animal learning theory in the 1970s were among the first to recognise that the old ideas of reinforcement and ‘drive reduction’ had to be replaced by the concept of ‘reward-expectancy’. The important discovery of ‘blocking’ (Kamin, 1968) heralded new thinking (Figure 1(a)), and experiments by Robert Rescorla and by Nick Mackintosh led to the idea that learning occurred when expectancies about reward availability were violated. This idea was encapsulated in the famous ‘Rescorla and Wagner’ equation of 1972, which subsequently helped to interpret the actions of dopamine in the brain (Schultz and Dickinson, 2000). The concept of ‘reward-expectancy’ was born and remains a major focus of current thinking.
Figure 1.
Memory: At the birth of the BNA. (a) Reward predictability: A major trigger for changes in the way learning was studied was the discovery that reinforcement was only effective for learning, even in motivated animals, if it was unexpected. One phenomenon that led to this discovery is that of ‘blocking’, first reported by Leon Kamin. A conditional stimulus (white box, CS) paired with reinforcement (R) fails to be conditioned (X) if the reinforcement is already predicted by another stimulus (black box, CS). (b) Protein synthesis and memory retention: The role of protein synthesis in memory followed the discovery that post-training administration of blockers of protein synthesis (e.g. cyclohexamide) selectively interrupted long-term memory retention (after Davis and Squire, 1984). (c) Memory dissociations in amnesia: The apparently selective nature of ‘global amnesia’ followed observations by Brenda Milner that patient H.M. could successfully copy but was unable to remember the Rey–Ostereith figure for any length of time (top), but could learn the motor task of mirror drawing (bottom). Similar dissociations in other patients soon followed.
Students of animal learning went on to develop numerous training regimes, sometimes of such esoteric precision that their advances risked becoming impenetrable to others. Happily, many influential ideas did emerge from these analyses, including Tony Dickinson’s distinction between learned actions and learned habits, a distinction that now stimulates research on the striatum and provides a framework for understanding aspects of drug addiction (Everitt and Robbins, 2005). There remained, however, a clear gap between those who sought to carry on working with laboratory rats and others who believed that to understand the human brain it was necessary to conduct invasive work in nonhuman primates. In addition, the separation between disciplines, such as that between physiology and psychology, was also deeply rooted. The interdisciplinary nature of contemporary neuroscience remained far in the future.
Beyond primates and rats, a different approach was to examine learning in simpler organisms. In St Andrews, Graham Horridge published studies of learning in arthropods. Although not influential at the time, it is salutary to reflect on his prescience in writing a book in the 1960s on ‘interneurons’ (Horridge, 1968) – long before Peter Somogyi’s anatomical studies revealed both their complexity and importance (Somogyi and Klausberger, 2005). Also, just as the BRA/BNA was being formed, Eric Kandel first described his work in France on the conditioning of single neurons in the sea slug (Aplysia depilans; Kandel and Tauc, 1965), followed by the programme of research on the neural basis of habituation and sensitisation in Aplysia californica that was to win him the Nobel Prize in 2000. A key feature of this research was the detailed understanding of functional circuitry, leading the way to investigate molecular mechanisms of plasticity (Kandel, 2001).
Another focus was on memory consolidation – what made memory traces last – with protein synthesis emerging as a key idea. However, this concept was not without curious precursors. One idea was that DNA, or at least RNA, might be modified by the patterns of electrical activity affecting a neuron (Milner, 1970), an idea made fashionable by the dramatic breakthroughs in genetic coding and transcription. Certain infamous cannibalism experiments with flatworms (Planaria) suggested that memories could be transferred by the process of feeding minced but ‘educated’ flatworms to ‘naïve’ flatworms. Closely related work involved the transfer of RNA from trained to naïve rats. The textbook Physiological Psychology stated that ‘the most popular analogy for memory at present … is the mechanism of genetic storage’ (Milner, 1970: 426) before, wisely, questioning this conclusion. The memory transfer experiments did, nonetheless, stimulate research that revealed a role for protein synthesis in long-term retention (Davis and Squire, 1984). Consequently, Milner (1970) could conclude that the search for molecular changes in learning should be directed to proteins and lipids, a sentiment that has stood the test of time (Figure 1(b)). It is, however, worth adding that the emerging field of epigenetics now examines how environmental factors can have long-term effects on gene expression.
But what of humans, or, as they were then described, of ‘man’ [sic]? During that same period, new information emerged from neuropsychological studies of patients suffering from memory loss. It was known that damage in one of two regions, the medial diencephalon and the medial temporal lobe, was consistently linked with the failure to retain new information, that is, with anterograde amnesia (Mair et al., 1979; Scoville and Milner, 1957). More novel was the realisation that anterograde amnesia was not as ‘global’ as first thought, as perceptual-motor learning is preserved (Milner et al., 1968; Figure 1(c)). The full significance of this dissociation between memory types (subsequently termed explicit vs implicit, or declarative vs nondeclarative) would not emerge for another decade or more (Squire and Zola-Morgan, 1991; Weiskrantz and Warrington, 1979). These same neuropsychological studies were, however, severely limited in one key respect: it was impossible to visualise the particular patterns of brain injury in those individuals being studied. Researchers had to make educated guesses based on separate postmortem findings. Remarkably, the first study to combine detailed neuropsychological investigations of memory loss in amnesia along with subsequent postmortem data from the same patient would not be published for another decade (Mair et al., 1979), with the studies of Squire’s group later setting an exacting standing in postmortem neuroanatomical analyses (Zola-Morgan et al., 1986). Even now, such studies remain rare, though they include a postmortem description of the brain of the famous amnesic patient H.M. (Annese et al., 2014).
Finally, in a fascinating section in Physiological Psychology, Milner (1970) considered hippocampal lesion studies in animals. He described the perplexing finding that bilateral medial temporal lesions in monkeys did not produce the expected anterograde amnesic syndrome (Orbach et al., 1960), an apparent anomaly that has taken decades to resolve (Murray and Wise, 2010). Milner (1970) also briefly described the outcome of hippocampal lesions in rats in just a single sentence, which mentioned that spatial learning is usually impaired (Kaada et al., 1961; Kimble, 1963). With hindsight, it seems incredible that there was so much uncertainty about the importance of the hippocampus for memory in animals other than humans. In 1970, however, Peter Milner felt forced to infer that there must be an ‘evolutionary discontinuity’, leaving the hippocampus important for only human memory. We now know that this is incorrect.
Memory: what happened next (1970s, 1980s and 1990s)?
These decades brought a series of remarkable discoveries that form the bedrock of much current thinking. At the beginning of the period, our understanding of brain circuitry was rudimentary – a problem exacerbated by how older ‘lesion’ techniques did not distinguish between damage to cells of origin and to fibres of passage. A crucial first step was the introduction of axonal tracing techniques such as autoradiography and horseradish peroxidase (Cowan et al., 1972; LaVail and LaVail, 1972), which heralded a quiet revolution that continues to this day with new single-cell connectional techniques (Figure 2(a)).
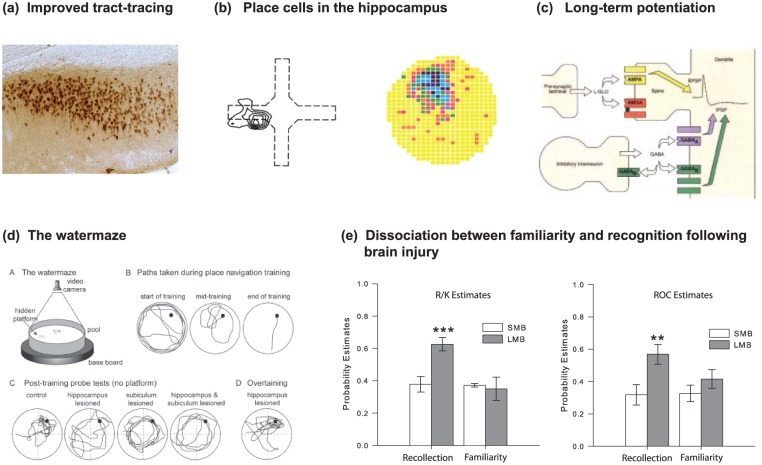
Figure 2.
Memory: What happened next (1970s, 1980s and 1990s)? (a) Neuroanatomy: The development of axonal tract-tracing techniques such as HRP and autoradiography replaced older degeneration-based lesion techniques that were unable to distinguish cell–cell connections from damage to fibres of passage. Image shows evidence of dense projections from the monkey hippocampus (subiculum) to the mammillary bodies, a pattern that helped make sense of patients being studied at the National Hospital, Queen Square, by Elizabeth Warrington. (b) Electrophysiology: The presence of hippocampus place-cells was first revealed by John O’Keefe and Jonathan Dostrovsky through single-cell recording of neurons in area CA1 of freely moving rats in simple T- and X-mazes. Later work by Robert Muller and John Kubie used open-arenas and automated tracking of the animal and recording of cell-firing to realise much greater objectivity. (c) Electrophysiology: The discovery of long-term potentiation by Bliss and Lomo in 1973 was rapidly followed by a developing understanding of the interaction of glutamatergic and GABAergic transmission in mediating and regulating synaptic plasticity. This was summarised by Tim Bliss and Graham Collingridge in 1993 in one of the most cited papers in neuroscience. (d) Behaviour: The water maze was one of a variety of new behavioural paradigms designed to test the ostensibly spatial functions of the hippocampus realised through the discovery of place-cells (a, b). The water maze was developed at the Gatty Marine Laboratory of the University of St Andrews by Richard Morris. Hippocampal lesions caused severe deficits in learning (c). (e) Neuropsychological dissociations: Studies of brain injured patients helped to dissociate separate processes associated with full recall of an event and its context (‘who, what and where’) from the mere sense of familiarity of prior occurrence (‘I’ve seen this before’). Building on a body of experimental work, John Aggleton and Malcolm Brown developed a theoretical framework implicating different brain regions in these distinct forms of memory retrieval (Aggleton & Brown, 1999, 2006). Consistent with this dissociation, patients with greater pathology in the mammillary bodies and fornix (SMB) show a loss of recollective-based recognition but preservation of familiarity-based recognition (LMB, matched patient controls) (Vann et al., 2009).
Meanwhile, some of the most influential breakthroughs involved research on the hippocampus, with two electrophysiological discoveries taking centre stage. The first was the description of place-cells in the rat hippocampus (O’Keefe and Dostrovsky, 1971), research that led to a Nobel Prize for John O’Keefe (Figure 2(b)). The activity of a single place cell is tuned to a particular location, as defined by distal landmarks (i.e. by allocentric space), with different cells firing in different locations. The significance of this discovery was further enhanced by publication of The Hippocampus as a Cognitive Map (O’Keefe and Nadel, 1978), which provided a comprehensive spatial theory of hippocampal function. The second discovery was long-term potentiation (LTP), first described in the rabbit dentate gyrus (Bliss and Collingridge, 1993; Bliss and Lomo, 1973; Figure 2(c)). While models of learning based on prolonged changes in synaptic strength had been postulated, the discovery of LTP revealed direct evidence that lasting changes in synaptic strength could be observed in mammals. Critical issues included the physiological and pharmacological basis of LTP (and its companion, long-term depression) as well as the need to determine whether LTP was, in fact, necessary for hippocampal-based learning.
At the same time, new behavioural tests of spatial memory, most notably those using the radial-arm maze (Olton and Samuelson, 1976), the Barnes Maze (Barnes, 1979) and the water maze (Morris, 1981), provided more sophisticated ways of analysing behaviour. These advances then coalesced when studies using the water maze not only confirmed the importance of the rat hippocampus for allocentric spatial learning (Morris et al., 1982) but also offered the opportunity to study the link between hippocampal LTP and spatial memory (Morris et al., 1986; Figure 2(d)). Successive studies revealed that blocking LTP impaired learning, as demonstrated by specific drugs that act as antagonists of the N-methyl-d-aspartate receptor, by the physiological saturation of LTP and later by gene targeting that affected LTP induction and expression. Interestingly, disruption of LTP can occur without affecting memory retrieval – an important dissociation. Despite such findings, there has long been debate over the true relevance of LTP for memory formation (Stevens, 1998), while work with genetically modified mice (such as Grin1ΔDGCA1 developed by Peter Seeburg in Germany) points to a more complex picture than often appreciated (Bannerman et al., 2014). Specifically, a distinction must be made between the likely necessity for N-methyl-D-aspartate (NMDA) receptor-dependent LTP in the neocortex to store lasting ‘engrams’ and the sometimes lack of its necessity in the hippocampus during learning (e.g. for straightforward spatial tasks that lack ambiguity). Meanwhile, recent advances in using optogenetics to study fear conditioning have arguably provided some of the most compelling evidence of a causal link between LTP and memory (Nabavi et al., 2014). It should also be remembered that this extensive body of work on neural plasticity stands on the shoulders of neural models of memory developed by the British mathematician David Marr (1971), followed by later work on distributed associative memory (Dayan and Abbott, 2001; McNaughton and Morris, 1987; Rolls, 2016).
Novel ideas in cognitive psychology also transformed the landscape. In 1972, Endel Tulving introduced the concept of ‘episodic memory’, which together with the companion notion of semantic memory make up explicit (or declarative) memory. Episodic memory concerns the recollection of autobiographic events that are set in a particular time and place (Tulving, 1972). In contrast, semantic memory concerns factual knowledge, highlighting fundamental differences between ‘knowing’ (semantic) and ‘remembering’ (episodic). As already noted, there was prior evidence from amnesics that explicit memory and implicit memory rely on different neural substrates. Evidence accumulated showing how phenomena such as priming, perceptual-motor learning and classical conditioning should all be considered as examples of implicit learning, that is, memory without conscious remembering (Schacter, 1992; Schacter et al., 1993).
One of many consequences was a reappraisal of the cognitive demands in memory tests given to humans and other animals. In particular, if the animal task taxes implicit memory, while the corresponding human task taxes explicit memory, then cross-species generalisations are confounded. A related consequence was the protracted debate over how recognition memory, which can readily be tested in both humans and other animals, should be categorised. One group argued that recognition and recall are two sides of the same coin (Squire et al., 2007), while others argued that recognition memory comprises two independent elements – one based on recall processes, the other on a sense of familiarity (Yonelinas, 2002). A key first step was the discovery of electrophysiological responses in the rhinal cortices that distinguish whether a stimulus is novel or familiar (Brown et al., 1987), revealing that activity in areas such as perirhinal cortex might be sufficient to guide recognition judgements based on familiarity (Aggleton and Brown, 1999, 2006; Eichenbaum et al., 1994). Both clinical and monkey lesion evidence further supported the notion that the hippocampus is needed for recollective-based recognition while extra-hippocampal processes support familiarity-based recognition (Aggleton et al., 2000; Murray and Mishkin, 1998). The gradual resolution of this debate about the nature of recognition memory (Figure 2(e)) had important implications for animal models of amnesia and for how we consider hippocampal function (Murray and Wise, 2010).
In these same decades, Loftus and Palmer (1974) highlighted the malleability of memory by first showing that the words you use when interrogating someone’s memory can seemingly change that memory. Numerous subsequent studies confirmed the unreliability of eye-witness testimony (Loftus, 1979). These findings not only have enormous practical importance but are also of theoretical significance. Some recall errors reveal that specific memories, even when ostensibly ‘consolidated’, can change after their initial formation (Wright and Loftus, 1998). This simple but powerful idea has fundamentally altered the concept of memory consolidation. Rather than it being a fixing process, whereby memory is written down and later read from a ‘diary’, it appears that accessing a memory can lift it from the pages of the notional diary, creating an unstable state in which the memory representation can be altered. This rewriting or updating process is called ‘reconsolidation’. How consolidation and reconsolidation work at circuit and molecular levels is now an active area of current research (Lee, 2009; Lee et al., 2017).
Memory: the contemporary scene
Three approaches have dominated memory research over the last quarter century. The first relates to the search for plastic mechanisms at the synapse, where a key focus has been on glutamate receptors and the downstream biochemical pathways that regulate their expression at the synapse. The second arises from the ability to genetically modify brain cells and organisms in order to test the importance of specific molecules for learning and memory. The third reflects the extraordinary advances in noninvasive imaging, which now make it possible to reveal structural detail (magnetic resonance imaging (MRI)), measure markers of neuronal and neurochemical activity (positron emission tomography, PET; functional MRI, fMRI; magnetoncephalography, MEG) and reconstruct white matter (e.g. diffusion tensor imaging (DTI)).
The focus on plasticity began, as mentioned above, with work on LTP (Figure 2(c)). The discovery that glutamate is the principal excitatory neurotransmitter of the brain stimulated numerous efforts to understand the fundamental mechanisms by which ionotropic and metabotropic glutamate receptors work together to regulate synaptic efficacy. The basic idea is that there may be alterations in the release of glutamate on the presynaptic side, alongside changes in the trafficking of a sub-type of glutamate receptor, AMPA receptors, on the post-synaptic side. Coupled to this concept have been advances in molecular-genetic technology, enabling specific genes of interest to be deleted or expressed in transgenic mice. Within the field of memory research, this work began with a global deletion of the alpha subunit of calcium-calmodulin kinase II (aCaMKII) that is greatly enriched in the post-synaptic density of glutamatergic synapses (Silva et al., 1992). Later work used Cre–Lox technology to restrict the gene deletion of interest to specific neural circuits and brain regions, which when coupled to temporally inducible manipulations created opportunities for imaginative interventions, as typified by the research of Nobel Laureate Susumu Tonegawa at MIT.
The application of this molecular technology has reinvigorated systems neuroscience. Already, ‘gene knock-out’ is becoming a dated technology with new viral techniques for monitoring and selectively activating the brain in a cell-type and regionally specific manner becoming the new ‘state-of-the-art’. Viral approaches have made optogenetics more viable as an approach, with the potential to allow physiology and behaviour to move from correlation to causation (Figure 3(b)). However, for all the excitement about these new approaches, they come at a price. Much of the work has been conducted with mice, which offer a more limited repertoire of behavioural analyses than rats, although comparable genetic and viral methodologies for rats are gradually emerging. One consequence is an ever-greater focus on rodents, such that many with comparative interests, or loyalty to the idea that the primate brain is the one we should always have in mind, are becoming concerned. Nonhuman primate work has simultaneously become more restricted.
Figure 3.
Memory: The contemporary scene. (a) Use of multi-voxel pattern recognition in fMRI to distinguish distinct episodic memories. fMRI has traditionally been used to reveal that a brain area is ‘activated’ by distinct forms of experience. The introduction of high-field imaging coupled to analysis of individual voxels reveals the capacity to distinguish the ‘hot-spot’ representations of different memories. This advance takes human imaging to a new level that, while not yet capable of decoding the nature of the memory, can nonetheless distinguish one memory trace from another within a brain area devoted to memory (after Chadwick et al., 2010). (b) Use of molecular engineering tools to address systems issues in memory research. Gene knock-out techniques are gradually being replaced by viral expression techniques in which, for example, an adenovirus expressing the optogenetic construct channelrhodopsin (ChR2), with a red marker called mCherry, is introduced into specific brain areas (here the dentate gyrus (DG) of the hippocampus and the basolateral amygdala (BLA)) in such a way it is only expressed when a specific immediate early gene (e.g. c-fos) is expressed. Administering doxycycline to the drinking water prevents the virus from being expressed, but when this is removed, brain cells that are active during a specific learning experience can be ‘tagged’ with ChR2 at the time of learning. Later application of blue light via miniature light guides implanted above these brain areas enables only those specific neurons that participated in a learning experience to be activated. By doing this in hippocampus and amygdala, and combining their activation with different forms of positive or negative reinforcement, Susumu Tonegawa et al. were able to investigate the neural mechanisms by which the valence of a memory (good or bad) could be transformed (after Redondo et al., 2014). (c) Cognition and deprivation: Vocabulary scores of Ecuadorian children aged 36 to 72 divided by wealth, showing the divergent performances of children associated with poverty (after Grantham-McGregor et al., 2007).
Turning back to humans, cognitive neuroscience in the 1960s and 1970s was severely hampered by the lack of imaging methods. The first clinical computerised tomography (CT) scan took place in 1971, with the first MRI scan of the human body published in 1977 by Raymond Damadian. Subsequently, Paul Lauterbur and Peter Mansfield shared a Nobel Prize for further developing the MRI technique. The resulting proliferation of noninvasive imaging techniques reinvigorated cognitive neuroscience, beginning with PET studies by Marcus Raichle et al. (Posner and Raichle, 1994). Perhaps the most salutary lesson is how classical neuropsychology, based on patients with brain injury, had only highlighted the limited number of brain structures necessary for a particular form of memory, while PET and MRI techniques could reveal the multiple brain sites and pathways whose activity is associated with the same classes of memory. An example of the latter concerns the many parietal, frontal and parahippocampal areas now implicated in episodic memory (Cabeza et al., 2008; Ranganath and Ritchey, 2012). These imaging findings, some using multi-voxel pattern-recognition techniques for analysis (Figure 3(a)), promote an integrated, systems-based approach to memory that not only considers encoding but also the control processes involved in memory formation and retrieval.
New ideas also emerged about how memory processes are used. One insight was that episodic memory not only provides access to past events but also aids imagining future events (Ingvar, 1984; Schacter et al., 2012). Evidence includes the finding that amnesics, who lack episodic memory, have an impoverished ability to imagine fictitious events (Hassabis et al., 2007), that is, to imagine ‘things that couldn’t possible have happened’ (to quote Cecily from the ‘Importance of Being Earnest’). One possibility is that their inability to construct past scenes in episodic memory reflects a more general failure of scene representation, so affecting imagination (Zeidman and Maguire, 2016).
Memory: current needs and future challenges
The big challenges appear to lie at polar ends of the research spectrum. At one end are studies into the molecular basis of memory, closely combined with unravelling the precise details of how ensembles of neurons interact. At the other end of the spectrum are unmet translational needs concerned with how to apply our understanding of neural mechanisms of learning to medical and societal issues.
Research into neuronal mechanisms of plasticity faces numerous obstacles. One example stems from how different forms of memory (e.g. explicit vs implicit) depend on different anatomical substrates, creating the challenge of determining the generality of any given plastic mechanism. Even within one form of memory, the situation is incredibly complex. To take the case of episodic memory, it is not all about the hippocampus, as other structures (e.g. within the medial diencephalon and frontal cortex) are also vital – but we do not know why. Added complexities stem from the need to decipher the role of local and more global oscillations as conveyors of episodic information, as well as the assimilation of newly learned information into existing semantic networks.
In recent years, novel techniques, such as those using designed receptors exclusively activated by designer drugs (DREADDS) and optogenetics, have provided new levels of specificity with which to manipulate neuronal assemblies in vivo. It already appears possible to identify neurons in rodents that are activated by a particular experience and then subsequently reactivate those same neurons to re-create a representation of the original experience (Liu et al., 2014; Tonegawa et al., 2015). This work is presented in the context of searching for the ‘engram’, such that the goal of visualising learning in complex organisms in real time at the cellular level is now becoming a reality (Figure 3(b)). This ability is clearly just the beginning of research into the encoding, storage, consolidation and retrieval of memory at levels of temporal and anatomical specificity not previously realised. A challenge is to ensure appropriate levels of sophistication in the behavioural analyses that are intrinsic to interpreting these emerging methods. Clinical applications could include the removal (or at least lowered levels of expression) of specific distressing memories, notwithstanding the concomitant ethical issues (Parsons and Ressler, 2013). At the same time, the search for mechanisms of plasticity will extend beyond the synapse. It is already known that white matter changes following experience may contribute to cognition (Zatorre et al., 2012). One priority is to know just how little experience is required to initiate alterations in axonal properties, with current evidence suggesting that changes might occur after just a few learning trials (Hofstetter et al., 2013). Related issues centre on the possible role of glia in memory. The last few years have seen extraordinary interest in glial–neuronal interactions that might influence CNS plasticity.
The study of individual differences will become a major focus. This task is aided by improved, noninvasive imaging, set alongside comprehensive genetic analyses. These techniques will be informed by the identification of numerous gene variants that can affect different aspects of learning and memory. Epigenetics will also increasingly aid our understanding of individual differences. These same advances will, however, also raise new ethical issues. At present, individual episodic memories can be detected and distinguished from their pattern of fMRI signals across different voxels (Figure 3(a)) (Bonnici et al., 2013; Rissman et al., 2016). Further advances at the individual level will create even greater concerns about mind reading and lie detection (Evers and Sigman, 2013).
One major obstruction to progress is our woeful lack of knowledge about human brain connectivity (Rockland, 2015). This long-standing problem arises principally from the need to inject axonal tracers in vivo and to visualise postmortem in order to confirm neuronal connectivity, that is, methods that can only be used in other animals. Although MRI is helping to reduce the species gulf, there are major limitations. Initiatives such as the Human Connectome Project will undoubtedly help, but the methods still fall short on anatomical resolution. Furthermore, while DTI can visualise white matter, it cannot distinguish between afferent or efferent connections, nor identify unmyelinated pathways. There also remains the problem of transposing and validating findings from other animals to the human brain. Despite the many genetic and molecular discoveries showing how conservative neural mechanisms often remain, it is also worth remembering that the human brain is unique. It is, for example, three times the size it should be for a primate of our body size.
A major challenge concerns how we apply our knowledge of memory mechanisms to improve quality of life. Naturally, there is great interest in drugs that enhance cognitive performance (Greely et al., 2008; Sahakian and Morein-Zamir, 2015), but, at present, these are typically of relatively modest impact in those with a healthy CNS. More realistically, findings from neuroscience should be informing education in schools, yet progress in this area is still in its infancy. For this to happen, memory researchers need to collaborate more with those in other disciplines, as well as to understand the demands on teachers and others in educational settings. There also remains the ever-growing challenge of how to prevent and treat memory loss in an ageing world population, and how to counteract the consequences of chronic disease and deprivation on cognition, including memory, in developing countries. In 2007, it was estimated that 200 million children under 5 years of age are not fulfilling their cognitive development potential (Grantham-McGregor et al., 2007). Strategies to reduce these inequalities (Engle et al., 2011) will require neuroscientists to adopt completely different approaches, with these challenges representing some of the most important goals for the discipline.
Acknowledgments
Richard G. M. Morris thanks John O’Keefe and Lynn Nadel for inspiration and keeping me on track in the early years, and the many students and post-docs with whom he has been privileged to have worked over 30+ years, including colleagues in Edinburgh Neuroscience. Thanks also to the Medical Research Council who provided many years of Programme Grant support, and latterly the European Research Council. John P. Aggleton thanks Mort Mishkin and Malcolm Brown for their wisdom and guidance, along with numerous colleagues at Cardiff University, Durham University and NIH. The Wellcome Trust and Medical Research Council have provided invaluable support.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Aggleton JP, Brown MW. (1999) Episodic memory, amnesia, and the hippocampal–anterior thalamic axis. Behavioral and Brain Sciences 22: 425–444. [PubMed] [Google Scholar]
- Aggleton JP, Brown MW. (2006) Interleaving brain systems for episodic and recognition memory. Trends in Cognitive Sciences 10: 455–463. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, McMackin D, Carpenter K, et al. (2000) Differential cognitive effects of colloid cysts in the third ventricle that spare or compromise the fornix. Brain 123: 800–815. [DOI] [PubMed] [Google Scholar]
- Annese J, Schenker-Ahmed NM, Bartsch H, et al. (2014) Postmortem examination of patient H.M.’s brain based on histological sectioning and digital 3D reconstruction. Nature Communications 5: 3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Sprengel R, Sanderson DJ, et al. (2014) Hippocampal synaptic plasticity, spatial memory and anxiety. Nature Reviews Neuroscience 15: 181–192. [DOI] [PubMed] [Google Scholar]
- Barnes CA. (1979) Memory deficits associated with senescence: A neurophysiological and behavioral study in the rat. Journal of Comparative and Physiological Psychology 93: 74–104. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. (1993) A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 361: 31–39. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. (1973) Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. The Journal of Physiology 232: 331–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnici HM, Chadwick MJ, Maguire EA. (2013) Representations of recent and remote autobiographical memories in hippocampal subfields. Hippocampus 23: 849–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MW, Wilson FAW, Riches IP. (1987) Neuronal evidence that inferomedial temporal cortex is more important than hippocampus in certain processes underlying recognition memory. Brain Research 409: 158–162. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, et al. (2008) The parietal cortex and episodic memory: An attentional account. Nature Reviews Neuroscience 9: 613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick MJ, Hassabis D, Weiskopf N, et al. (2010) Decoding individual episodic memory traces in the human hippocampus. Current Biology 20: 544–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claxton G. (2015) Intelligence in the Flesh: Why Your Mind Needs Your Body Much More Than It Thinks. New Haven, CT: Yale University Press. [Google Scholar]
- Cowan WM, Gottlieb DI, Hendrickson AE, et al. (1972) The autoradiographic demonstration of axonal connections in the central nervous system. Brain Research 37: 21–51. [DOI] [PubMed] [Google Scholar]
- Davis HP, Squire LR. (1984) Protein synthesis and memory: A review. Psychological Bulletin 96: 518–559. [PubMed] [Google Scholar]
- Dayan P, Abbott LF. (2001) Theoretical Neuroscience, vol. 10 Cambridge, MA: The MIT Press, pp. S0306–S4522. [Google Scholar]
- Eichenbaum H, Otto T, Cohen NJ. (1994) Two functional components of the hippocampal memory system. Behavioral and Brain Sciences 17: 449–472. [Google Scholar]
- Engle PL, Fernald LC, Alderman H, et al. (2011) Strategies for reducing inequalities and improving developmental outcomes for young children in low-income and middle-income countries. The Lancet 378: 1339–1353. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. (2005) Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nature Neuroscience 8: 1481–1489. [DOI] [PubMed] [Google Scholar]
- Evers K, Sigman M. (2013) Possibilities and limits of mind-reading: A neurophilosophical perspective. Consciousness and Cognition 22: 887–897. [DOI] [PubMed] [Google Scholar]
- Grantham-McGregor S, Cheung YB, Cueto S, et al. (2007) Developmental potential in the first 5 years for children in developing countries. The Lancet 369: 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA. (2000) The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo-Hippocampal System. Oxford: Oxford University Press. [Google Scholar]
- Greely H, Sahakian B, Harris J, et al. (2008) Towards responsible use of cognitive-enhancing drugs by the healthy. Nature 456: 702–705. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Vann SD, et al. (2007) Patients with hippocampal amnesia cannot imagine new experiences. Proceedings of the National Academy of Sciences 104: 1726–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstetter S, Tavor I, Moryosef ST, et al. (2013) Short-term learning induces white matter plasticity in the fornix. Journal of Neuroscience 33: 12844–12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horridge GA. (1968) Interneurons. Dallas, TX: Freeman. [Google Scholar]
- Ingvar DH. (1984) ‘Memory of the future’: An essay on the temporal organization of conscious awareness. Human Neurobiology 4: 127–136. [PubMed] [Google Scholar]
- Kaada BR, Rasmussen EW, Kveim O. (1961) Effects of hippocampal lesions on maze learning and retention in rats. Experimental Neurology 3: 333–355. [DOI] [PubMed] [Google Scholar]
- Kamin LJ. (1968) ‘Attention-like’ processes in classical conditioning. In: Jones MR. (ed.) Miami Symposium on the Prediction of Behavior: Aversive Stimulation. Miami, FL: University of Miami Press, pp. 9–31. [Google Scholar]
- Kandel ER. (2001) The molecular biology of memory storage: A dialogue between genes and synapses. Science 294: 1030–1038. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Tauc L. (1965) Heterosynaptic facilitation in neurones of the abdominal ganglion of Aplysia depilans. The Journal of Physiology 181: 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble DP. (1963) The effects of bilateral hippocampal lesions in rats. Journal of Comparative and Physiological Psychology 56: 273–283. [DOI] [PubMed] [Google Scholar]
- LaVail JH, LaVail MM. (1972) Retrograde axonal transport in the central nervous system. Science 176: 1416–1417. [DOI] [PubMed] [Google Scholar]
- Lee JL. (2009) Reconsolidation: Maintaining memory relevance. Trends in Neurosciences 32: 413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JLC, Nader K, Schiller D. (2017) An update on memory reconsolidation updating. Trends in Cognitive Sciences 21: 531–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ramirez S, Tonegawa S. (2014) Inception of a false memory by optogenetic manipulation of a hippocampal memory engram. Philosophical Transactions of the Royal Society B 369: 20130142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus EF. (1979) Eyewitness Testimony. Cambridge, MA: Harvard University Press. [Google Scholar]
- Loftus EF, Palmer JC. (1974) Reconstruction of automobile destruction: An example of the interaction between language and memory. Journal of Verbal Learning and Verbal Behavior 13: 585–589. [Google Scholar]
- McNaughton BL, Morris RGM. (1987) Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends in Neuroscience 10: 408–415. [Google Scholar]
- Mair WG, Warrington EK, Weiskrantz L. (1979) Memory disorder in Korsakoff’s psychosis: A neuropathological and neuropsychological investigation of two cases. Brain 102: 749–783. [DOI] [PubMed] [Google Scholar]
- Marr D. (1971) Simple memory: A theory for archicortex. Philosophical Transactions of the Royal Society B 262: 23–28. [DOI] [PubMed] [Google Scholar]
- Milner B, Corkin S, Teuber H-L. (1968) Further analysis of the hippocampal amnesic syndrome: 14-year follow-up study of H.M. Neuropsychologia 6: 215–234. [Google Scholar]
- Milner PM. (1970) Physiological Psychology. New York: Holt, Rinehart & Winston. [Google Scholar]
- Morris RG. (1981) Spatial localization does not require the presence of local cues. Learning and Motivation 12: 239–260. [Google Scholar]
- Morris RG, Anderson E, Lynch GS, et al. (1986) Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature 319: 774–776. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, et al. (1982) Place navigation impaired in rats with hippocampal lesions. Nature 297: 681–683. [DOI] [PubMed] [Google Scholar]
- Murray EA, Mishkin M. (1998) Object recognition and location memory in monkeys with excitotoxic lesions of the amygdala and hippocampus. Journal of Neuroscience 18: 6568–6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Wise SP. (2010) What, if anything, can monkeys tell us about human amnesia when they can’t say anything at all? Neuropsychologia 48: 2385–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi S, Fox R, Proulx CD, et al. (2014) Engineering a memory with LTD and LTP. Nature 511: 348–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. (1971) The hippocampus as a spatial map: Preliminary evidence from unit activity in the freely-moving rat. Brain Research 34: 171–175. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. (1978) The Hippocampus as a Cognitive Map. Oxford: Oxford University Press. [Google Scholar]
- Olton DS, Samuelson RJ. (1976) Remembrance of places passed: Spatial memory in rats. Journal of Experimental Psychology: Animal Behavior Processes 2: 97–116. [DOI] [PubMed] [Google Scholar]
- Orbach J, Milner B, Rasmussen T. (1960) Learning and retention in monkeys after amygdala-hippocampus resection. Archives of Neurology 3: 230–251. [DOI] [PubMed] [Google Scholar]
- Parsons RG, Ressler KJ. (2013) Implications of memory modulation for post-traumatic stress and fear disorders. Nature Neuroscience 16: 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Raichle ME. (1994) Images of Mind. New York: Scientific American Library/Scientific American Books. [Google Scholar]
- Ranganath C, Ritchey M. (2012) Two cortical systems for memory-guided behavior. Nature Reviews Neuroscience 13: 713–726. [DOI] [PubMed] [Google Scholar]
- Redondo RL, Kim J, Arons AL, et al. (2014) Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature 513: 426–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman J, Chow TE, Reggente N, et al. (2016) Decoding fMRI signatures of real-world autobiographical memory retrieval. Journal of Cognitive Neuroscience 28: 604–620. [DOI] [PubMed] [Google Scholar]
- Rockland KS. (2015) About connections. Frontiers in Neuroanatomy 9: 61 DOI: 10.3389/fnana.2015.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. (2016) Cerebral Cortex: Principles of Operation. Oxford: Oxford University Press. [Google Scholar]
- Sahakian BJ, Morein-Zamir S. (2015) Pharmacological cognitive enhancement: Treatment of neuropsychiatric disorders and lifestyle use by healthy people. The Lancet Psychiatry 2: 357–362. [DOI] [PubMed] [Google Scholar]
- Schacter DL. (1992) Priming and multiple memory systems: Perceptual mechanisms of implicit memory. Journal of Cognitive Neuroscience 4: 244–256. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Hassabis D, et al. (2012) The future of memory: Remembering, imagining, and the brain. Neuron 76: 677–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Chiu CYP, Ochsner KN. (1993) Implicit memory: A selective review. Annual Review of Neuroscience 16: 159–182. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. (2000) Neuronal coding of prediction errors. Annual Review Neuroscience 23: 473–500. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. (1957) Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery, and Psychiatry 20: 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Stevens CF, Tonegawa S, et al. (1992) Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science 257: 201–206. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Klausberger T. (2005) Defined types of cortical interneurone structure space and spike timing in the hippocampus. Journal of Physiology 562: 9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. (1991) The medial temporal lobe memory system. Science 253: 1380–1386. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. (2007) Recognition memory and the medial temporal lobe: A new perspective. Nature Reviews Neuroscience 8: 872–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CF. (1998) A million dollar question: Does LTP = memory? Neuron 20: 1–2. [DOI] [PubMed] [Google Scholar]
- Tonegawa S, Liu X, Ramirez S, et al. (2015) Memory engram cells have come of age. Neuron 87: 918–931. [DOI] [PubMed] [Google Scholar]
- Tulving E. (1972) Episodic and semantic memory. In: Tulving E, Donaldson W. (eds) Organization of Memory. New York: Academic Press, pp. 381–403. [Google Scholar]
- Vann SD, Tsivilis D, Denby CE, et al. (2009) Impaired recollection but spared familiarity in patients with extended hippocampal system damage revealed by 3 convergent methods. Proceedings of the National Academy of Sciences 106: 5442–5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskrantz L, Warrington EK. (1979) Conditioning in amnesic patients. Neuropsychologia 17: 187–194. [DOI] [PubMed] [Google Scholar]
- Wilde O. (1895) The Importance of Being Earnest, a Trivial Comedy for Serious People. [Google Scholar]
- Wright DB, Loftus EF. (1998) How misinformation alters memories. Journal of Experimental Child Psychology 71: 155–164. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. (2002) The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language 46: 441–517. [Google Scholar]
- Zatorre RJ, Fields RD, Johansen-Berg H. (2012) Plasticity in gray and white: Neuroimaging changes in brain structure during learning. Nature Neuroscience 15: 528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidman P, Maguire EA. (2016) Anterior hippocampus: The anatomy of perception, imagination and episodic memory. Nature Reviews Neuroscience 17: 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG. (1986) Human amnesia and the medial temporal region: Enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. Journal of Neuroscience 6: 2950–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]