Abstract
The claustrum is a highly conserved but enigmatic structure, with connections to the entire cortical mantle, as well as to an extended and extensive range of heterogeneous subcortical structures. Indeed, the human claustrum is thought to have the highest number of connections per millimetre cubed of any other brain region. While there have been relatively few functional investigations of the claustrum, many theoretical suggestions have been put forward, including speculation that it plays a key role in the generation of consciousness in the mammalian brain. Other claims have been more circumspect, suggesting that the claustrum has a particular role in, for example, orchestrating cortical activity, spatial information processing or decision making. Here, we selectively review certain key recent anatomical, electrophysiological and behavioural experimental advances in claustral research and present evidence that calls for a reassessment of its anatomical boundaries in the rodent. We conclude with some open questions for future research.
Keywords: Claustrum, subcortical, function, consciousness, anatomical connections
Introduction
The claustrum of the mammalian brain is an anatomically substantial and complex but functionally under-explored structure (Edelstein and Denaro, 2004). In the rat, the claustrum comprises a paired subcortical sheet of grey matter that largely spans the extent of the rostral half of the telencephalon. It is surrounded in its rostral aspect (anterior to the striatum) by the forceps minor of the corpus callosum and the orbital cortex; in its central and caudal levels, it is bordered by the putamen and the caudate nuclei of the striatum medially and by the insular cortex laterally. As a result, the claustrum possesses a complex three-dimensional morphology across both its longitudinal and dorso-ventral axes. Indeed, its proximity to both cortical and subcortical regions has presented a considerable spatial challenge for voxel-based imaging and, similarly, its longitudinal extent and dorso-ventral complexity have made lesion analyses difficult. Despite these difficulties, however, there have been a reasonable number of anatomical studies, and to a lesser degree physiological investigations, the majority of which have been in anaesthetised preparations.
Anatomical boundaries
The anatomical delineation of the claustrum and the nomenclature used in the literature is varied and this has been discussed at length (see Mathur, 2014; Mathur et al., 2009; Johnson and Fenske, 2014). Claustral subdivisions have been defined according to regions (termed ‘puddles’) that share common cortical connectivity; according to proximity to cortical regions, for example, insular claustrum and endopiriform claustrum; or according to combinations of anatomical position, connectivity, genomics and/or proteomics.
A degree of confusion and difficulty continues to be present in the literature regarding the delineation of the rodent claustral boundary, predominantly because lisencephalic species, unlike gyrencephalic species, for example, cats and primates, do not have a well-defined extreme capsule such that the claustrum and neighbouring cortex are continuous. While there is now consensus on the nuclear boundary of the main body of the claustrum (i.e. at striatal levels; Mathur et al., 2009; Wang et al., 2017), a degree of contention exists within the literature regarding whether, or not, the claustrum boundary extends rostral to the anterior apex of the striatum, ventral to the forceps minor of the corpus callosum (Mathur et al., 2009; Smith and Alloway, 2010; White et al., 2017), or if this region is instead possibly a deep layer of insular cortex. For example, the expression distribution of numerous genes, for example, Gng2, parvalbumin (Mathur et al., 2009), Gnb4 (Wang et al., 2017), among others, have been identified and verified as clear delineating markers for the claustrum while reports of their apparent lack of expression in the region of rostral claustrum delineated by various brain atlases (e.g. Paxinos and Watson, 2005) have been reported as a basis for its exclusion. Unlike Gng2 and Gnb4, in which claustral expression is enriched (Mathur et al., 2009; Wang et al., 2017), expression of crystallin mu (Crym; Mathur et al., 2009; Wang et al., 2017), a cortical marker, has been shown to delineate the border of the claustrum through its absence. Indeed, its expression was critical in demonstrating that the claustrum is not juxtaposed to the external capsule but, instead, is bordered, on its medial face, by a layer of cortical neurons (Mathur et al., 2009).
Here, using a freely accessible data source (Allen Mouse Brain Atlas; http://www.brain-map.org), we have revisited the expression profiles of 49 genes that have, thus far, been identified as being either enriched or attenuated in the claustrum (predominantly through the studies of Mathur et al., 2009; Wang et al., 2017), with findings that we feel warrant another reassessment of the anatomical boundaries of the rodent claustrum.
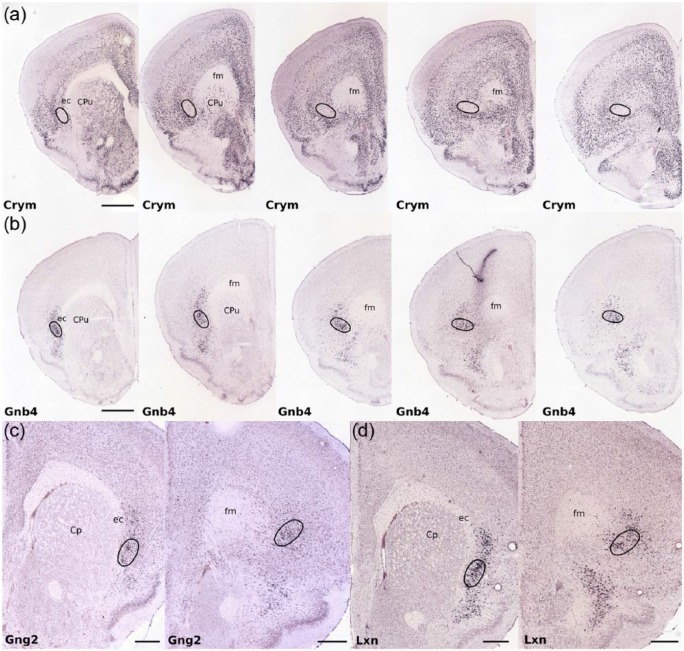
Our findings are as follows: as mentioned above, Crystallin mu expression is a cortical marker that has been shown to clearly delineate the anatomical limits of the claustrum through a combination of its paucity of expression within the claustrum itself and its dense surrounding cortical expression. On inspection of Crym expression in the mouse brain, it is apparent that the Crym-based delineation of the claustral boundary extends considerably beyond the anterior apex of the striatum (Figure 1). Unlike numerous atlases, however, the border of the claustrum in the region does not elongate beneath the arch of the forceps minor of the corpus callosum but instead maintains its oval cross-section at the ventrolateral aspect of the forceps minor within the deep layers of the insular cortex (Figure 1). Given this template, we reassessed the expression distribution of those genes identified by Wang et al., and in the vast majority of cases (all except one), those genes that exhibited differential expression in ‘striatal’ claustrum showed an equivalent profile in the ‘rostral’ claustrum (Figure 1; Table 1).
Figure 1.
Photomicrographs showing the nucleotide sequence expression (in situ hybridisation) of four example genes that are enriched or attenuated in the claustrum as identified by Wang et al. (2017). In each example claustrum-specific label extends rostral to the anterior apex of the striatum. (a) Crystallin mu (Crym) expression is attenuated in the claustrum but enriched in surrounding cortex delineating a claustral boundary that extends anterior to the claustrum, maintaining an ovoid cross-section at the ventro-lateral apex of the forceps minor of the corpus callosum (fm). (b) Gnb4 (Guanine nucleotide binding protein (G protein), beta 4) expression is enriched in the claustrum throughout the claustrum (Wang et al., 2017), including its rostral extent as defined by the Cyrm template in (a). (c) Gng2 (guanine nucleotide binding protein (G protein), gamma 2) expression is enriched in the claustrum (Mathur et al., 2009), again extending rostral to the anterior apex of the striatum. (d) Lxn (Latexin) expression is also enriched throughout the claustrum including its rostral extent. Of the remaining 45 genes identified by Wang et al. (2017), 44 showed equivalent differential expression in the rostral claustrum when compared to the ‘claustrum proper’. Black oval outlines delineate the claustral border in each case. The images were taken from the Allen Institute for Brain Science Mouse Brain Atlas, Allen Institute for Brain Science, Allen Mouse Brain Atlas. Available at: http://mouse.brain-map.org/
Table 1.
Showing the continuity of the differential expression of 49 claustrum-delineating genes into the rostral aspect of the nucleus.
| Abbreviation | Enriched in ‘striatal’ claustrum (+)/(−) | Enriched in ‘rostral’ claustrum (+)/(−) | Full name |
|---|---|---|---|
| Adamtsl2 | (+)a | (+) | ADAMTS-like 2 |
| Bace1 | (+)a | (+) | Beta-site APP cleaving enzyme 1 |
| B3gat2 | (+)a | (+) | Beta-1,3-glucuronyltransferase 2 |
| BC100451 | (+)a | (+) | cDNA sequence BC100451 |
| Btg1 | (+)a | (+) | B-cell translocation gene 1, antiproliferative |
| Cadps2 | (+)a | (+) | Ca21-dependent activator protein for secretion 2 |
| Car12 | (+)a | (+) | Carbonic anhydrase 12 |
| Cbln2 | (+)a | (+) | Cerebellin 2 precursor protein |
| Chst11 | (+)a | (+) | Carbohydrate sulfotransferase 11 |
| Cntnap3 | (+)a | (+) | Contactin-associated protein-like 3 |
| Col11a1 | (+)a | (+) | Collagen, type XI, alpha 1 |
| Cux2 | (+)a | (+) | Cut-like homeobox 2 |
| Gadd45g | (+)a | X | Growth arrest and DNA damage-inducible 45 gamma |
| Galnt14 | (+)a | (+) | UDP-N-acetyl-alpha-d-galactosamine:polypeptide N-Acetylgalactosaminyltransferase 14 |
| Gfra1 | (+)a | (+) | Glial cell line–derived neurotrophic factor family receptor alpha 1 |
| Gnb4 | (+)a | (+) | Guanine nucleotide binding protein (G protein), beta 4 |
| Gng2 | (+)a,b | (+) | Guanine nucleotide binding protein (G protein), gamma 2 |
| Gnao1 | (+)a | (+) | Guanine nucleotide binding protein, alpha O |
| Gpd2 | (+)a | (+) | Glycerol phosphate dehydrogenase 2, mitochondrial |
| Gucy1a3 | (+)a | (+) | Guanylate cyclase 1, soluble, alpha 3 |
| Id2 | (+)a | (+) | Inhibitor of DNA binding 2 |
| Inpp5a | (+)a | (+) | Inositol polyphosphate-5-phosphatase A |
| Itga7 | (+)a | (+) | Integrin alpha 7 |
| Laptm4b | (+)a | (+) | Lysosomal-associated protein transmembrane 4B |
| LOC433093 | (+)a | (+) | Similar to MAM domain-containing glycosylphosphatidylinositol anchor 1; glycosyl-phosphatidyl-inositol-MAM |
| Lypd6b | (+)a | (+) | LY6/PLAUR domain containing 6B |
| Lxn | (+)a | (+) | Latexin |
| Mt3 | (+)a | (+) | Metallothionein 3 |
| Nfxl1 | (+)a | (+) | Nuclear transcription factor, X-box binding-like 1 |
| Nmb | (+)a | (+) | Neuromedin B |
| Nr4a2 | (+)a | (+) | Nuclear receptor subfamily 4, group A, member 2 |
| Nsdhl | (+)a | (+) | NAD(P)-dependent steroid dehydrogenase-like |
| Ntng2 | (+)a | (+) | Netrin G2 |
| Oprk1 | (+)a | (+) | Opioid receptor, kappa 1 |
| Pdia5 | (+)a | (+) | Protein disulphide isomerase associated 5 |
| Plcl1 | (+)a | (+) | Phospholipase C-like 1 |
| Ppp1r1a | (+)a | (+) | Protein phosphatase 1, regulatory (inhibitor) subunit 1A |
| Rab3c | (+)a | (+) | RAB3C, member RAS oncogene family |
| Rtn4rl2 | (+)a | (+) | Reticulon 4 receptor-like 2 |
| SStr2 | (+)a | (+) | Somatostatin receptor 2 |
| Tmem163 | (+)a | (+) | Transmembrane protein 163 |
| Tox | (+)a | (+) | Thymocyte selection-associated high mobility group box |
| Zfp804a | (+)a | (+) | Zinc finger protein 804A |
| Col23a1 | (+)a | (−) | Collagen, type XXIII, alpha 1 |
| Crym | (−)a,b | (−) | Crystallin, mu |
| Ctgf | (−)a | (−) | Connective tissue growth factor |
| Nxph3 | (−)a | (−) | Neurexophilin 3 |
| Slit1 | (−)a | (−) | Slit homolog 1 (Drosophila) |
| Rasal1 | (−)a | (−) | RAS protein activator like 1 (GAP1 like) |
Source: Modified from Wang et al. (2017).
cDNA: complementary DNA.
Wang et al. (2017) and bMathur et al. (2009); ‘X’ in column 3 highlights when differential expression in the rostral claustrum does not follow the same pattern as the main body of the claustrum; (+) and (−) indicate enrichment or attenuation of expression, respectively.
In light of this assessment and given the anatomical similarities of the mouse and rat brain, it is clear that the anatomical boundaries of the rostral extent of the claustrum need to be assessed through determination of the expression distribution of these genes in the rat brain.
Anatomical connections
Cortical connectivity
It is well established that extensive projections exist between the claustrum and the cortical areas with which it interacts (in particular, prefrontal, cingulate, auditory, visual and somatosensory cortices; Figure 2; Atlan et al., 2017; Crick and Koch, 2005; Edelstein and Denaro, 2004; Smythies et al., 2012a; Wang et al., 2017; White et al., 2017). In recent years, several excellent and comprehensive anatomical studies in the rodent have outlined the afferent and efferent cortical connectivity of the claustrum (see Wang et al., 2017), for example, parahippocampal (Agster et al., 2016; Kitanishi and Matsuo, 2016; Tomás Pereira et al., 2016; White et al., 2017), somatosensory-motor (Smith and Alloway, 2010) and visuomotor cortices (Smith et al., 2014), prefrontal and entorhinal cortices (Watson et al., 2017). There is a consistent pattern of bilateral cortico-claustral projections, which are weaker (but nonetheless extant), to the ipsilateral hemisphere. Reciprocal claustro-cortical connectivity is characterised by dense projections, largely confined to the ipsilateral hemisphere.
Figure 2.
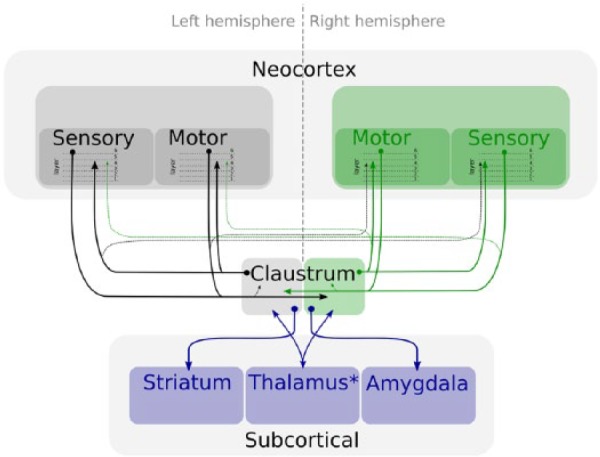
Schematic diagram showing the neural basis of how the claustrum acts as a mediator of cortical and subcortical activity. Like cortico-thalamic inputs, cortico-claustral inputs are predominantly derived from cortical layer 6, while claustro-cortical inputs are predominantly to cortical layer 4, suggesting that they may comprise a driver input rather than a modulatory one. Further thalamic comparisons are evident in that the claustrum receives bilateral (mainly contralateral) inputs but its reciprocal projections are largely unilateral and ipsilateral.
Earlier anatomical studies indicated an antero-posterior gradient of cortical connectivity in the claustrum, such that frontal cortical regions connect with anterior claustrum and sensory areas of cortex connect with posterior claustrum (Fernández-Miranda et al., 2008; Neal et al., 1986; Olson and Graybiel, 1980). In the cat/primate claustrum, a dorsal-ventral compartmentalisation of visual and auditory cortical connectivity, respectively, exists with no apparent interconnectivity (Edelstein and Denaro, 2004). Conversely, in the rat, in which this dorsal-ventral compartmentalisation is not apparent, significant long-range intra-claustral interconnectivity was found (Smith and Alloway, 2010).
Subcortical connectivity
In addition to its extensive cortical connectivity, the claustrum is densely interconnected with an array of subcortical structures (Figure 2), for example, thalamus (Figure 3; Herkenham, 1978; McKenna and Vertes, 2004; Vertes et al., 2006; Vertes and Hoover, 2008), striatum (Andersen, 1968; Wang et al., 2017), hippocampus (Amaral and Cowan, 1980; Zhang et al., 2013), amygdala (Majak et al., 2002; Wang et al., 2017) and hypothalamus (Vertes, 1992). Of its thalamic interactions, claustro-reuniens and claustro-rhomboid connectivity is particularly striking (Figure 3; Herkenham, 1978; McKenna and Vertes, 2004; Vertes et al., 2006). Nucleus reuniens itself has a strong excitatory influence on the hippocampus and medial prefrontal cortex (McKenna and Vertes, 2004; Vertes et al., 2006), two structures within which it acts as a critical hub for interconnectivity (Figure 3; Roy et al., 2017), playing key roles in memory consolidation (in spatial memory (Ito et al., 2015)) and the generalisation of classically conditioned fear memories (Eleore et al., 2011; Loureiro et al., 2012; Wheeler et al., 2013; Xu and Sudhof, 2013). Nucleus reuniens also contains an unexpected and unpredicted population of head direction cells (Jankowski et al., 2014) as well as cells with spatial-response properties (Jankowski et al., 2015), possibly providing a route for spatial modulation of claustral neuronal responses. Vertes et al. (2006) and McKenna and Vertes (2004) have shown that the connections between nucleus reuniens and claustrum are reciprocal and remarkably dense within the rostral claustrum. It is interesting to note that clear similarities exist in the hodological properties of the claustrum and the anterior thalamus: both receive extensive, predominantly contralateral cortical projections from layer 6 with contrasting unilateral and ipsilateral cortical outputs (Mathiasen et al., 2017). Claustro-cortical projections, however, appear to differ from those of the anterior thalamo-cortical projections in that they are thought to act as higher order ‘driver’ inputs (through particularly dense layer 4 projections, for example, LeVay and Sherk, 1981; Wang et al., 2017) rather than relay ‘modulatory’ inputs (layer 1/2 inputs; Sherman and Guillery, 1998).
Figure 3.
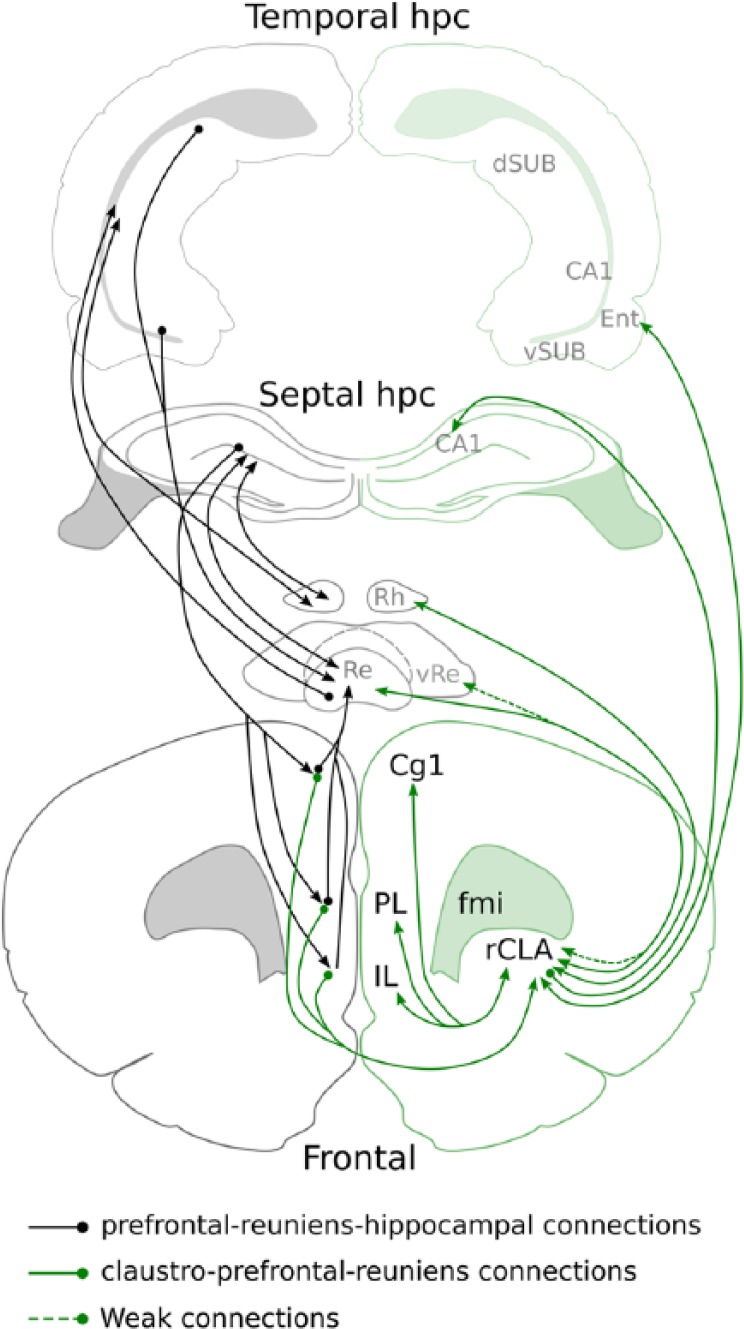
Semi-schematic diagram showing how claustral connectivity fits into an extended cortico-thalamo-hippocampal circuit. Nucleus reuniens (Re) and the rhomboid nuclei (Rh) of the midline thalamus act as a critical hub for medial prefrontal-hippocampal interactions that are known to underlie mnemonic processes. The claustrum shares many of these connections, through dense reciprocal thalamic, prefrontal and parahippocampal connections.
CA1: field CA1 of the hippocampal formation; Cg1: cingulate cortex, area 1; dSUB: dorsal subiculum; Ent: entorhinal cortices; fmi: forceps minor of the corpus callosum; IL: infralimbic cortex; PL: prelimbic cortex; rCLA: rostral claustrum; vRE: ventral reuniens thalamic nucleus.
Electrophysiological recordings in the claustrum
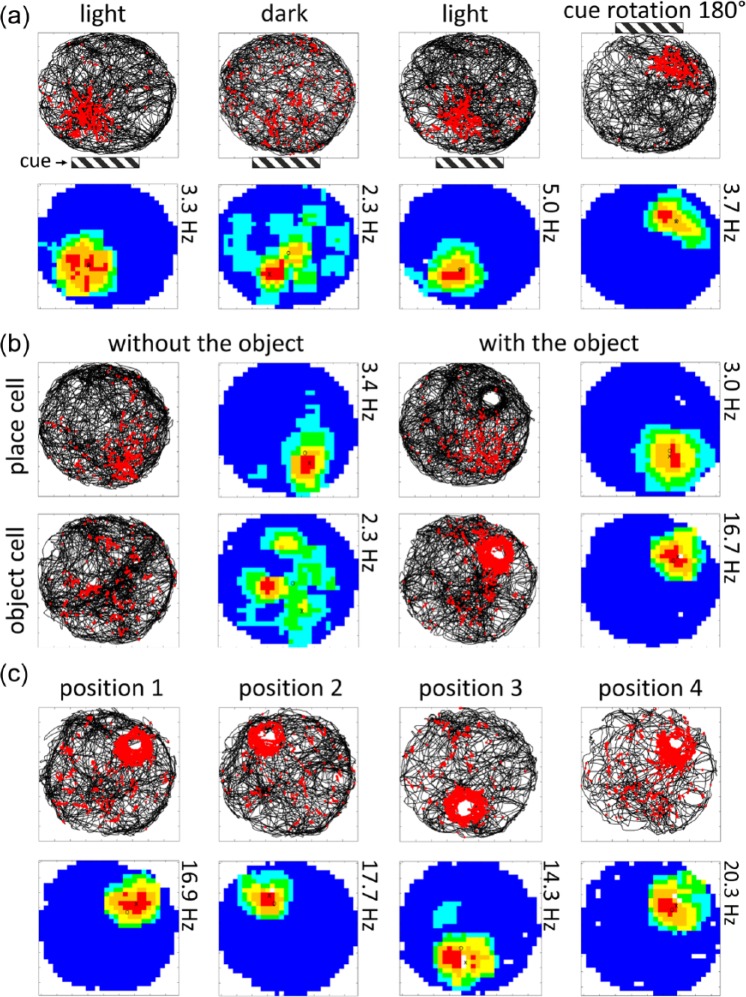
The vast majority of electrophysiological recordings in the claustrum have been conducted in anaesthetised preparations (disclosing the existence of a claustral somatotopic map; Olson and Graybiel, 1980) or in the head-fixed and immobilised non-human primate (disclosing neurons representing a variety of naturalistic sensory stimuli, especially audition and vision; Remedios et al., 2010). Early electrophysiological experiments by Gabor and Peele (1964) showed that electrical stimulation of the awake behaving cat claustrum resulted in an apparently relaxed state and the subsequent onset of sleep. Local field potential recordings during these recording epochs showed distinct spike-and-wave oscillations. In recent and ongoing experiments carried out in our lab, for the first time, claustral neurons of awake behaving rats were recorded, revealing quite unexpected and remarkable findings (Figure 4; Jankowski and O’Mara, 2015). With electrodes implanted in the rostral claustrum, one subpopulation of recorded units was found to resemble the classic place cells in the hippocampal formation. These cells were active while the animal was freely moving and showed diminished activity during recordings in the dark and responded to the movement of environmental cues (Figure 4). In addition to place cells, other claustral neurons were responsive to the presence of objects in the environment, resembling, in certain respects, the object-responsive cells found in subiculum (Anderson and O’Mara, 2004), lateral entorhinal cortex (Deshmukh and Knierim, 2011; Tsao et al., 2013) and anterior cingulate cortex (Weible et al., 2009, 2012). These claustral ‘object-responsive’ neurons signalled the presence of objects or landmarks, but their activity differed from object-responsive neurons in other region because their activity dynamically tracked the repositioning of the object (Figure 4). These data, in the context of established and substantial thalamo-claustral circuitry, suggest an interesting and previously unexplored novel role for the reuniens-claustral axis in the network integration of memory, decision-making and action (specifically, spatial decision-making and navigation). One possibility therefore is that the claustrum dynamically represents both extended space without the body and incorporates landmark information from the environment, creating in effect an ‘action space’ within which behavioural decision selection occurs. Related to this idea are the findings of Ryan Remedios, who at the 2012 First Annual Francis Crick Memorial Conference. Remedios reported that when aclaustral rats were placed in the centre of an eight-arm radial arm maze, they were found to freeze, seemingly for the most part unable or unwilling to explore any of the arms. These findings were subsequently commented upon by Smythies et al. (2012a) in the context of their oscillatory synchrony hypothesis, which is outlined below. It should be noted that while these reported finding are of great interest, they should be treated with a degree of caution given that they have not been subject to the peer-review process.
Figure 4.
Spatially responsive neurons in the rostral claustrum. (a) The response pattern of claustral place cells is visually driven, as shown by both a decrease in spatial coherence in the dark and place fields shifting in response to visual cue rotation. (b) Claustral place cells show a typical place field that is unchanged in the presence of an object, while object cells show no spatial tuning when no object is present but an increased firing rate around the perimeter of an object when one is present. (c) Claustral object cells show increased firing activity in and around the location of the object. When the position of the object is changed, the firing field of the object relocates to the novel position (taken from Jankowski et al., 2015).
Claustral functions
As we have alluded to already, and perhaps understandably given the elaborate claustral connectome, the possible functions of the claustrum have received considerable attention, with many hypotheses posed (Goll et al., 2015; Mathur, 2014; Remedios et al., 2010, 2014; Smythies et al., 2012b). Most recently, two related articles (Smythies et al., 2012a; Yin et al., 2016) suggest that complex connectivity and organisation of the claustrum is best understood in terms of underlying mechanisms of information transfer, rather than many of those previous that have proposed explanations for specific features, for example, visual, auditory or somatosensory in the context of particular ethological behaviours, for example, multi-sensory integration, attention. The first (Smythies et al., 2012a) proposes that the claustrum processes an array of incoming parallel signals generated by a primary signal, that is, a salient or unexpected stimulus, before amplifying oscillations of a particular frequency through ‘reverberating claustro-cortical loops’, thus potentiating selected, previously weak, cortical oscillations. A second hypothesis (Yin et al., 2016) elaborated upon this ‘oscillation-synchrony theory’ with one of time perception and consciousness, a topic that will be revisited in more detail later in the review.
Somewhat in accordance with the oscillatory-synchrony theory of Smythies et al., and the claustrum stimulation experiments in the cat of Gabor and Peele (described above), the claustrum has been implicated as having a role in regulating the subcortical-cortical synchrony of frequency band oscillations associated with rapid eye movement (REM) sleep. Through a combination of immediate early gene expression and neuronal pathway tracing, Renouard et al. (2015) identified both the claustrum – anterior cingulate cortex axis and the claustrum-retrosplenial cortex axis as likely candidates for the regulation of REM sleep.
Current theories (e.g. Buzsáki and Moser, 2013) of the means by which the brain represents space (and memory) rely on an anatomical model revolving around the existence of cells representing position (place cells) and heading (head-direction cells). The key structures implicated are the hippocampal formation (principally concerned with representing spatial position), parahippocampal and related anterior thalamic areas (principally concerned with representing heading information) and the entorhinal cortices (principally concerned with representing metric information). This overall structural model is very influential and predominates theoretical views of spatial navigation and representation of the brain. Biologically inspired models of spatial navigation rely, similarly, on core ideas concerning the dissociation of place information from heading information, and their subsequent integration in other downstream areas to facilitate goal-directed navigation (e.g. Barrera and Weitzenfeld, 2009). Here, we suggest that there are important components of this overall theoretical picture missing, in particular relating to evolutionarily conserved brain regions (Buchanan and Johnson, 2011) that, to this point, have been under-explored. Furthermore, we suggest that significant revision of current models of how the brain controls behavioural choices in a spatial context may be warranted. Unexpectedly, there are populations of place and (dynamic) object/landmark cells in claustrum (Figure 4; Jankowski et al., 2015b). Most contemporary models place their greatest emphasis on the integrated functioning of the entorhinal-hippocampal system and considerations of either extending the entorhinal-hippocampal system to include thalamus or other structures are made perhaps less often than they could or should be. Thus, our data suggest that spatially active claustral neurons extends contemporary models of spatial navigation and representation, and perhaps episodic memory (e.g. Buzsáki and Moser, 2013).
The claustrum, consciousness and giant neurons
Crick and Koch (2005) proposed that the claustrum possessed many of the key anatomical features that could be necessary for a brain area to form the neural underpinnings of conscious perception, prompting a resurgence of interest in the structure. More recently, the claustrum returned to the forefront of this line of thinking, again through the work of Christof Koch, with the finding in the mouse that projections of ‘giant neurons’, whose cell bodies lay within the claustrum, wrapped around the entire brain like a ‘crown of thorns’ (published communication with Reardon, 2017). One interesting indication of a potential relationship between claustral activity and consciousness is the case study of an epileptic patient implanted with deep-brain stimulating electrodes targeting among other structures, a portion of the claustrum (Koubeissi et al., 2014), specifically left claustrum and anterior-dorsal insula. This patient had previously undergone left hippocampectomy. The authors summarise their findings as follows: ‘[s]timulation of the claustral electrode reproducibly resulted in a complete arrest of volitional behaviour, unresponsiveness, and amnesia without negative motor symptoms or mere aphasia’. These are dramatic findings, with interesting implications. They suggest that claustral activation is key to the loss of consciousness, or alternatively, claustral activation followed by substantial neurotransmitter rundown from pre-synaptic terminals, results in loss of consciousness. The short duration of stimulation may rule out the latter idea. Furthermore, such claustral activation would affect a wide range of other cortical and subcortical structures, either directly or through passive conduction. In the current case, high stimulation intensities of up to 14 mA were used, opening a range of other electrophysiological possibilities in the claustrum as well as adjacent and connected structures (such as neuronal afterhyperpolarisation, transient changes in threshold potentials, disfacilitation, inhibition and the like). There are also clear experimental implications: deep-brain stimulation of this target in an animal model should also result in immediate loss of consciousness (cf. Gabor and Peele, 1964).
The functions of claustrum remain obscure, despite its extensive connectivity with the whole cortical mantle and extensive subcortical connectivity. Recent data (Cascella et al., 2011) indicate that schizophrenic patients with delusions may have an atrophic left claustrum. Intriguingly, Ishii et al. (2011) describe a patient with symmetric claustral lesions arising from viral encephalitis who suffered, as a result, from delusions and hallucinations, but which resolved after post-infectious demyelination and focal oedema subsided. There are other indications that the claustrum, given its pivotal cortico-cortical connectivity, may be involved in the spread of epilepsy (Zhang et al., 2001).
Conclusion and open questions
Although the claustrum is a well-known structure, compared with other brain regions it has been comparatively neglected. Perhaps the reason for this is that the neuroscientific tools required to study a structure with such complex topographies and far reaching and diverse connections have simply not been available. It has not been feasible to study or perturb specific connections (e.g. visual and limbic) or populations with conventional (e.g. lesion) approaches. Now, however, with techniques such as DREADDs, optogenetics and gene-based lesion approaches, for example, saporin conjugates, this is now possible. If indeed the claustrum is multi-functional, it will be important to ascertain whether claustro-cortical modulation is region-specific, that is, does claustro-cortical input uniformly drive/modulate all of its cortical targets? Furthermore, along the lines of the ‘conductor of a cortical orchestra’ hypothesis, it will be important to understand whether ‘puddles’ of common claustral connectivity and neurochemistry affect cortical activity either synchronously or independently, a question that may be especially suited to investigation in the context of the cortical disinhibition that occurs during paradoxical sleep (cf. Renouard et al., 2015). Given its extensive and heterogeneous cortical and subcortical connections, the claustrum is likely to have a plurality of task- and state-dependent roles, which depend on behavioural state. Our own data suggest that the rostral claustrum both dynamically represents extended space without the body and incorporates landmark information from the environment, possibly affecting behavioural decision-making within this action space.
Given the suggestion that the claustrum may have roles in the network integration of memory, decision-making and action (specifically, spatial decision-making and navigation), some of the following questions and comments arise:
The boundary of the claustrum in rodents has long been a subject of contention (e.g. Mathur et al., 2009). After re-examining the expression of the claustral marker genes identified by Wang et al. (2017), we suggest that the previously suggested anterior claustrum boundary be re-extended in the mouse brain to incorporate its rostral extent. The boundaries of the claustrum in the rat could be profitably re-examined with these tools.
What is the origin of the place signal in the claustrum? Is the place signal dependent upon primary, somatosensory, visual and motoric influences or is it dependent upon a pre-integrated input derived from hippocampus/parahippocampal regions? Presumably if the latter were true, given the uniformity of hippocampal/entorhinal input throughout the claustrum, one would expect these putative claustral place cells to exist throughout the anterior-posterior extent of the nucleus. Conversely, should the putative place signal in the claustrum be dependent upon indirect hippocampal input via the midline thalamus, or indeed the head direction cell population of nucleus reuniens, given the weak midline thalamic input to posterior claustrum, one would expect the rostral claustrum to exhibit these properties in isolation. Temporal inhibition or lesion of nucleus reuniens in combination with electrophysiological recording in the rostral claustral would be particularly informative in this respect.
Are there behavioural goal-related or action selection influences on claustral activity?
What changes result from wake–sleep transitions? Are these changes reliably associated with changes in conscious, behavioural states (or at least REM and non-REM sleep states, and the transitions into and out of sleep)?
To what extent is neuronal signalling in the claustrum intrinsic (arising from claustral collaterals) or extrinsic (arising from inputs from other regions)?
What is the functional significance of the giant ‘crown of thorns’ neurons for claustral signalling and cortical signalling more generally?
Are there units present in claustrum signalling mnemonic duration, similar to those found in the adjacent dorsolateral prefrontal cortices in the non-human primate?
Does the claustrum have conserved functions across species?
The claustrum presents a considerable challenge, but there is a rich body of theory and data to be discovered within its complexities.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: This work was supported by Science Foundation Ireland grant SFI 13/IA/2014.
References
- Agster KL, Tomás Pereira I, Saddoris MP, et al. (2016) Subcortical connections of the perirhinal, postrhinal, and entorhinal cortices of the rat, II: Efferents. Hippocampus 26(9): 1213–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Cowan WM. (1980) Subcortical afferents to the hippocampal formation in the monkey. The Journal of Comparative Neurology 189(4): 573–591. [DOI] [PubMed] [Google Scholar]
- Andersen D. (1968) Some striatal connections to the claustrum. Experimental Neurology 20(2): 261–267. [DOI] [PubMed] [Google Scholar]
- Anderson MI, O’Mara SM. (2004) Responses of dorsal subicular neurons of rats during object exploration in an extended environment. Experimental Brain Research 159(4): 519–529. [DOI] [PubMed] [Google Scholar]
- Atlan G, Terem A, Peretz-Rivlin N, et al. (2017) Mapping synaptic cortico-claustral connectivity in the mouse. The Journal of Comparative Neurology 525(6): 1381–1402. [DOI] [PubMed] [Google Scholar]
- Barrera A, Weitzenfeld A. (2009) Behavioral match evaluation of spatial cognition in rats and robots. In: Conference proceedings : Annual international conference of the IEEE engineering in medicine and biology society, Minneanapolis, MN, 3–6 September 2009, pp. 7188–7191. New York: IEEE. [DOI] [PubMed] [Google Scholar]
- Buchanan KJ, Johnson JI. (2011) Diversity of spatial relationships of the claustrum and insula in branches of the mammalian radiation. Annals of the New York Academy of Sciences 1225(S1): E30–E63. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Moser EI. (2013) Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nature Neuroscience 16(2): 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella NG, Gerner GJ, Fieldstone SC, et al. (2011) The insula–claustrum region and delusions in schizophrenia. Schizophrenia Research 133(1–3): 77–81. [DOI] [PubMed] [Google Scholar]
- Crick FC, Koch C. (2005) What is the function of the claustrum? Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences 360(1458): 1271–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh SS, Knierim JJ. (2011) Representation of non-spatial and spatial information in the lateral entorhinal cortex. Frontiers in Behavioral Neuroscience 5: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein LR, Denaro FJ. (2004) The claustrum: A historical review of its anatomy, physiology, cytochemistry and functional significance. Cellular and Molecular Biology 50(6): 675–702. [PubMed] [Google Scholar]
- Eleore L, López-Ramos JC, Guerra-Narbona R, et al. (2011) Role of reuniens nucleus projections to the medial prefrontal cortex and to the hippocampal pyramidal CA1 area in associative learning (ed I Izquierdo). PLoS ONE 6(8): e23538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Miranda JC, Rhoton AL, Kakizawa Y, et al. (2008) The claustrum and its projection system in the human brain: A microsurgical and tractographic anatomical study. Journal of Neurosurgery 108(4): 764–774. [DOI] [PubMed] [Google Scholar]
- Gabor AJ, Peele TL. (1964) Alterations of behavior following stimulation of the claustrum of the cat. Electroencephalography and Clinical Neurophysiology 17(5): 513–519. [DOI] [PubMed] [Google Scholar]
- Goll Y, Atlan G, Citri A. (2015) Attention: The claustrum. Trends in Neurosciences 38(8): 486–495. [DOI] [PubMed] [Google Scholar]
- Herkenham M. (1978) The connections of the nucleus reuniens thalami: Evidence for a direct thalamo-hippocampal pathway in the rat. The Journal of Comparative Neurology 177(4): 589–609. [DOI] [PubMed] [Google Scholar]
- Ishii K, Tsuji H, Tamaoka A. (2011) Mumps virus encephalitis with symmetric claustrum lesions. American Journal of Neuroradiology 32(7): E139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito HT, Zhang S-J, Witter MP, et al. (2015) A prefrontal–thalamo–hippocampal circuit for goal-directed spatial navigation. Nature 522(7554): 50–55. [DOI] [PubMed] [Google Scholar]
- Jankowski MM, O’Mara SM. (2015) Dynamics of place, boundary and object encoding in rat anterior claustrum. Frontiers in Behavioral Neuroscience 9: 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MM, Islam MN, Wright NF, et al. (2014) Nucleus reuniens of the thalamus contains head direction cells. eLife 3: E03075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MM, Passecker J, Islam MN, et al. (2015) Evidence for spatially-responsive neurons in the rostral thalamus. Frontiers in Behavioral Neuroscience 9: 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JI, Fenske BA. (2014) History of the study and nomenclature of the claustrum. In: Smythies J, Edelstein L, Ramachandran VS. (eds) The Claustrum. USA: Elsevier, pp. 1–27. [Google Scholar]
- Kitanishi T, Matsuo N. (2016) Organization of the claustrum-to-entorhinal cortical connection in mice. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. Epub ahead of print 30 November DOI: 10.1523/JNEUROSCI.1360-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koubeissi MZ, Bartolomei F, Beltagy A, et al. (2014) Electrical stimulation of a small brain area reversibly disrupts consciousness. Epilepsy and Behavior 37: 32–35. [DOI] [PubMed] [Google Scholar]
- LeVay S, Sherk H. (1981) The visual claustrum of the cat, I: Structure and connections. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 1(9): 956–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loureiro M, Cholvin T, Lopez J, et al. (2012) The ventral midline thalamus (reuniens and rhomboid nuclei) contributes to the persistence of spatial memory in rats. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 32(29): 9947–9959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JT, Vertes RP. (2004) Afferent projections to nucleus reuniens of the thalamus. The Journal of Comparative Neurology 480(2): 115–142. [DOI] [PubMed] [Google Scholar]
- Majak K, Pikkarainen M, Kemppainen S, et al. (2002) Projections from the amygdaloid complex to the claustrum and the endopiriform nucleus: A Phaseolus vulgaris leucoagglutinin study in the rat. The Journal of Comparative Neurology 451(3): 236–249. [DOI] [PubMed] [Google Scholar]
- Mathiasen ML, Dillingham CM, Kinnavane L, et al. (2017) Asymmetric cross-hemispheric connections link the rat anterior thalamic nuclei with the cortex and hippocampal formation. Neuroscience 349: 128–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur BN. (2014) The claustrum in review. Frontiers in Systems Neuroscience 8: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur BN, Caprioli RM, Deutch AY. (2009) Proteomic analysis illuminates a novel structural definition of the claustrum and insula. Cerebral Cortex 19(10): 2372–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal JW, Pearson RC, Powell TP. (1986) The relationship between the auditory cortex and the claustrum in the cat. Brain Research 366(1–2): 145–151. [DOI] [PubMed] [Google Scholar]
- Olson CR, Graybiel AM. (1980) Sensory maps in the claustrum of the cat. Nature 288(5790): 479–481. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. (2005) The Rat Brain in Stereotaxic Coordinates (5th edn). Burlington, MA: Elsevier Academic Press. [Google Scholar]
- Reardon S. (2017) A giant neuron found wrapped around entire mouse brain. Nature 543(7643): 14–15. [DOI] [PubMed] [Google Scholar]
- Remedios R. (2012) The claustrum and the orchestra of cognitive control. In: Francis Crick Memorial conference, Cambridge Available at: http://fcmconference.org/#program [Google Scholar]
- Remedios R, Logothetis NK, Kayser C. (2010) Unimodal responses prevail within the multisensory claustrum. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 30(39): 12902–12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remedios R, Logothetis NK, Kayser C. (2014) A role of the claustrum in auditory scene analysis by reflecting sensory change. Frontiers in Systems Neuroscience 8: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renouard L, Billwiller F, Ogawa K, et al. (2015) The supramammillary nucleus and the claustrum activate the cortex during REM sleep. Science Advances 1(3): E1400177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Svensson FP, Mazeh A, et al. (2017) Prefrontal-hippocampal coupling by theta rhythm and by 2–5 Hz oscillation in the delta band: The role of the nucleus reuniens of the thalamus. Brain Structure and Function. Epub ahead of print 16 February DOI: 10.1007/s00429-017-1374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. (1998) On the actions that one nerve cell can have on another: Distinguishing ‘drivers’ from ‘modulators’. Proceedings of the National Academy of Sciences of the United States of America 95(12): 7121–7126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JB, Alloway KD. (2010) Functional specificity of claustrum connections in the rat: Interhemispheric communication between specific parts of motor cortex. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 30(50): 16832–16844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JB, Alloway KD, Mathur BN, et al. (2014) Interhemispheric claustral circuits coordinate sensory and motor cortical areas that regulate exploratory behaviors. Frontiers in Systems Neuroscience 8: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythies J, Edelstein L, Ramachandran V. (2012. a) Hypotheses relating to the function of the claustrum. Frontiers in Integrative Neuroscience 6: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythies J, Edelstein L, Ramachandran V. (2012. b) The functional anatomy of the claustrum: The net that binds. Neurosciences 3(3): WMC003182. [Google Scholar]
- Tomás Pereira I, Agster KL, Burwell RD. (2016) Subcortical connections of the perirhinal, postrhinal, and entorhinal cortices of the rat, I: afferents. Hippocampus 26(9): 1189–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao A, Moser M-B, Moser EI. (2013) Traces of experience in the lateral entorhinal cortex. Current Biology 23(5): 399–405. [DOI] [PubMed] [Google Scholar]
- Vertes RP. (1992) PHA-L analysis of projections from the supramammillary nucleus in the rat. The Journal of Comparative Neurology 326(4): 595–622. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB. (2008) Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. The Journal of Comparative Neurology 508(2): 212–237. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Do Valle AC, et al. (2006) Efferent projections of reuniens and rhomboid nuclei of the thalamus in the rat. The Journal of Comparative Neurology 499(5): 768–796. [DOI] [PubMed] [Google Scholar]
- Wang Q, Ng L, Harris JA, et al. (2017) Organization of the connections between claustrum and cortex in the mouse. The Journal of Comparative Neurology 525(6): 1317–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson GDR, Smith JB, Alloway KD. (2017) Interhemispheric connections between the infralimbic and entorhinal cortices: The endopiriform nucleus has limbic connections that parallel the sensory and motor connections of the claustrum. The Journal of Comparative Neurology 525(6): 1363–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weible AP, Rowland DC, Pang R, et al. (2009) Neural correlates of novel object and novel location recognition behavior in the mouse anterior cingulate cortex. Journal of Neurophysiology 102(4): 2055–2068. [DOI] [PubMed] [Google Scholar]
- Weible AP, Rowland DC, Monaghan CK, et al. (2012) Neural correlates of long-term object memory in the mouse anterior cingulate cortex. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 32(16): 5598–5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler AL, Teixeira CM, Wang AH, et al. (2013) Identification of a functional connectome for long-term fear memory in mice (ed O Sporns). PLoS Computational Biology 9(1): e1002853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Sudhof TC. (2013) A neural circuit for memory specificity and generalization. Science 339(6125): 1290–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin B, Terhune DB, Smythies J. (2016) Claustrum, consciousness, and time perception. Current Opinion in Behavioral Sciences 8: 258–267. [Google Scholar]
- Zhang S-J, Ye J, Miao C, et al. (2013) Optogenetic dissection of entorhinal-hippocampal functional connectivity. Science 340(6128): 1232627. [DOI] [PubMed] [Google Scholar]
- Zhang X, Hannesson DK, Saucier DM, et al. (2001) Susceptibility to kindling and neuronal connections of the anterior claustrum. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 21(10): 3674–3687. [DOI] [PMC free article] [PubMed] [Google Scholar]