Abstract
This review provides a distillate of the advances in knowledge about the neurotransmitter functions of acetylcholine over the 50-year period between 1967 and 2017, together with incremental information about the cognate nicotinic and muscarinic acetylcholine receptors, and some brief comments on possible advances in the near future. The text is supplemented by a timelines figure indicating the dates of some key advances in knowledge about acetylcholine receptors and a box-figure providing a snapshot of selected papers about acetylcholine published in the year 1967.
Keywords: Acetylcholine, nicotinic receptors, muscarinic receptors, cholinergic transmission, presynaptic receptors, postsynaptic receptors
Introduction: the research environment 50 years ago
In order to appreciate what was known or not known 50 years ago, and if not, why not, it is important to know what currently used facilities were not available to the lab neuroscientist pre-1967.
Thus, although mainframe computers (accessed by punched cards) were coming in there were no PCs or lab computers. (The best our lab could afford in 1967 was a 64-step programmable calculator costing more than a PC does now.)
Although the structure of DNA was known (in 1953) and the genetic code had been unravelled in 1962, there were no ways of gene-cloning or gene manipulation, and no ‘knock-out’ mice or knock-down siRNA to test what a gene did.
There was also neither chemical nor structural information about membrane proteins such as receptors and ion channels, and no means of seeing their location with antibodies or mRNA hybridisation.
The electrophysiologist was restricted to using microelectrodes (no patch-clamp) for recording and drug application, with no visual aids for seeing neurons like GFP, no calcium indicators for monitoring activity or optogenetics for tracking circuits.
When it came to recording data, this was usually done directly on photographic film (subject to the hazard of dark-room development, when all was lost if someone switched the light on) – neither computer corrections nor enhancement was available.
Papers were written on a typewriter (with carbon paper copies – no word processors or photocopiers), or sometimes just by hand, and submitted for publication by post. There were no e-mails or Internet, and no electronic journals – to read the references meant going to the library with a notebook or a bunch of index cards in hand.
Under the circumstances, one can only be impressed by how much was discovered
What was known by 1967
Acetylcholine as a neurotransmitter
By 1967, acetylcholine (ACh) was firmly accepted as a major neurotransmitter in the peripheral nervous system, including somatic motor nerves and parts of the autonomic nervous system (see, e.g. Goodman and Gilmam, 1965; Krnjevic, 1974). Enzymes for its synthesis ( ‘choline acetylase’ = choline acetyltransferase) and degradation (cholinesterase) had been isolated and studied biochemically. ACh release following nerve stimulation had been detected from vagal parasympathetic nerves, preganglionic sympathetic nerves, cholinergic postganglionic sympathetic nerves and somatic motor nerves. A transmitter function was also supported by inhibition by tubocurarine (motor nerves, preganglionic sympathetic nerves) or atropine (postganglionic parasympathetic nerves).
Transmission at the neuromuscular junction
Comprehensive information regarding the details of somatic nerve-to-muscle transmission had been generated by the work of Bernard Katz and his colleagues (Katz, 1966). Fatt and Katz (1951) used R.W. Gerard’s recently introduced microelectrode technique (Ling and Gerard, 1949: J. cell. comp. Physiol, 34,383 383) to make the first intracellular recordings of the end-plate potential from the frog neuromuscular junction. Using the muscle action potential as a neat way of altering membrane voltage, they deduced that the epp arose from a general increase in ionic conductance (cations and anions) which partially short-circuited the action potential. On the basis of further studies with radioactive tracers (Jenkinson and Nicholls, 1961) and reversal potential measurements under voltage-clamp (Takeuchi and Takeuchi, 1960) the conductance change was adduced to be only to the cations Na+ and K+, not anions. This became the model for other forms of excitatory synaptic transmission (Eccles, 1957; Ginsborg, 1967):
Apart from the facts that they could be activated by acetylcholine and nicotine (and hence was classified as ‘nicotinic’ following Dale’s (1914) nomenclature), and inhibited by tubocurarine and related alkaloids, the physical nature of the muscle end-plate receptor was entirely unknown. [Fatt and Katz (1951) did not even mention a receptor – they only referred to an interaction of acetylcholine with the end-plate membrane.] One approach to the receptor was to use ligand binding to find out more about it. Thus, Peter Waser (1960) used radioactively-labelled tubocurarine to begin to localise the end-plate receptors by autoradiography. However, the resolution was poor and microscopic resolution had to await the later introduction of α-bungarotoxin. Also, Waser and others (e.g., Chothia, 1970) tried to deduce the chemical nature of the acetylcholine binding site from studies comparing chemical congeners.
Transmitter release
Another crucial advance from Katz’ work on the frog neuromuscular junction was the discovery of miniature epps (Fatt and Katz, 1952), which led to the development of the quantal theory of transmitter release (see Katz, 1969). This, coupled with the discovery of synaptic vesicles (de Robertis and Bennett, 1955), provided the foundation stones for nearly all subsequent studies on transmitter release at synapses.
Transmission between neurons
Pre-1967, intracellular microelectrode recordings were also obtained from sympathetic neurons, another prospective site of tubocurarine-sensitive nicotinic cholinergic transmission (Blackman et al., 1963a, 1963b; Eccles, 1955; Nishi and Koketsu, 1960). These revealed a very similar transmission process to that at frog muscle end-plates – a depolarising excitatory postsynaptic potential (epsp), giving rise to a superimposed action potential; and spontaneous mepsps (though at a low frequency unless enhanced by raising (K+)out) forming the quantal components of the epsp:
Notwithstanding, other experiments using extracellular recording methods, were beginning to suggest the presence of slower synaptic processes following repetitive afferent stimulation that were mediated by muscarinic (atropine-sensitive) receptors (see Phillis, 1970). The presence of a slower muscarinic component to the cholinergic excitation of Renshaw cells in the spinal cord (see below) was also emerging (Curtis and Ryall, 1966). Slow muscarinic excitatory effects were subjected to intensive study in subsequent years, generating new concepts of neural information processing and intracellular signalling mechanisms (see Brown, 2010).
Another difference between the motor end-plate and the sympathetic ganglion already apparent by 1967 concerned the nature of the nicotinic receptors. Although both are sensitive to tubocurarine, in an attempt to control essential hypertension a number of selective ganglion-blocking drugs had been developed which had little effect on muscle receptors. These included hexamethonium (Paton and Zaimis, 1949), pentolinium (Mason and Wien, 1955), and mecamylamine (Stone et al., 1956). Much later (following the cloning of the nicotinic receptors it transpired that this difference between nerve and muscle receptors was related to their different subunit compositions (see later).
Transmission in the CNS
By 1967, there was plenty of evidence suggesting an important role for ACh in the CNS (see Feldberg, 1954; Krnjevic, 1974; Phillis, 1970). It was present therein in high concentrations, as were choline acetyltransferase and cholinesterase (Hebb, 1957). Using a histochemical assay, Shute and Lewis (1963 & elsewhere) describe specific aggregations of neurons and specific neural projection tracts containing a high concentration of acetylcholinesterase, suggesting that they were cholinergic (a designation later supported by co-localization with choline acetyltransferase: Levey et al., 1983). There was also pharmacological evidence for likely transmitter functions (Goodman and Gilman, 1965). Thus, injecting ACh itself into the brain via the cerebral ventricles produced a variety of behavioural effects. CNS-penetrant anti-cholinesterases (including the nerve gases DFP, sarin and tabun, developed during WWII) exerted a variety of central excitatory effects, plausibly caused by enhanced effects of naturally released ACh because they could be diminished by atropine. Nicotine also clearly had central effects, including inhibition of ADH secretion via the hypothalamus (replicated by local ACh injection). The lipophilic muscarinic agonists pilocarpine, muscarine and arecoline produced cortical EEG arousal, whereas hyoscine (scopolamine) desynchronised the EEG and inhibited the arousal effect of photostimulation or reticular formation activation; and scopolamine exerted a well-known amnesic effect (witness its use in obstetrics or pre-anaesthetic medication to produce ‘twilight sleep’). Atropine and scopolamine were also known to be effective in diminishing the tremors of Parkinson’s disease, and, with more lipophilic derivatives thereof such as benztropine, were the mainstay of Parkinson’s disease treatment until the advent of levodopa. Finally, the release of ACh from the surface of the cerebral cortex, and its enhancement by afferent stimulation, could be detected (Mitchell, 1963; see also Box 1).
Box 1.
A snapshot of 1967.
| One way of finding out what topics taxed the cholinergic neuroscientist’s mind 50 years ago is to do a PubMed search for ‘acetylcholine and 1967’. Examples of some pertinent 1967 papers, with explanatory notes, are given below. 1. Collier B, Mitchell JF.(1967). The central release of acetylcholine during consciousness and after brain lesion. J. Physiol. 188(1):83–98. 2. Szerb, JC. (1967) Cortical acetylcholine release and electroencephalographic arousal J Physiol. 192(2):329–43. 3. Krnjevic K. (1967). Chemical transmission and cortical arousal. Anesthesiology. 28(1):100–105. PMID:6017417 Acetylcholine release had been previously detected from the surface of the cerebral cortex (see Mitchell, 1964, and references therein). The first two papers address questions concerning the origin of the acetylcholine and its functional significance. The broad conclusions are that spontaneous release is dependent on the animal’s state of behavioural arousal, and that release is increased by stimulating subcortical structures which generate an EEG arousal. The review by Krnjevic well summarises what was known in 1967 about the relation between the cholinergic system, arousal, attention and consciousness and is still substantially relevant (see Thiele, 2013). 4. Yamamoto KI, Domino EF (1967) Cholinergic agonist-antagonist interactions on neocortical and limbic EEG activation. Int J Neuropharmacol. 6(5):357–373. PMID:6069723 Rinaldi & Himwich (1957. Arch. Neurol. Psychiat. 73: 396–402) invoked a cholinergic component to the midbrain reticular activating system from observing the effects of atropine. This led to a flurry of further studies (e.g. Bradley & Elkes (1957: Brain 88: 77-l 17.). Yamamoto & Domino show that cortical EEG desynchronization is essentially a response to muscarinic receptor stimulation (again see Thiele, 2013). 5. Deutsch JA, Rocklin KW. (1967). Amnesia induced by scopolamine and its temporal variations. Nature. 216:89–90. PMID:4292965. This paper reinforces the point that scopolamine has amnesic properties (Goodman & Gilman, 1965) and hence that the muscarinic cholinergic system is involved in memory encoding and recall (see Hasselmo, 2006: Curr Opin Neurobiol. 16: 710–715.) 6. Phillis JW, Tebĕcis AK, York DH. (1967). A study of cholinoceptive cells in the lateral geniculate nucleus. J Physiol. 192(3):695–713. 7. Yamamoto C, Kawai N. (1967). Presynaptic action of acetylcholine in thin sections from the guinea pig dentate gyrus in vitro. Exp Neurol. 19(2):176–87. PMID:6054723 The paper by Phillis et al. is an example of the numerous careful studies on the responses of individual neurons in the central nervous system of anaesthetised animals to local iontophoretic application of drugs (see Phillis, 1970, for a comprehensive survey). The paper by Yamamoto & Kawai shows the early glimmerings of a new approach to studies on the brain using isolated brain slices maintained in vitro. Yamamoto started recording from brain slices when working with Henry McIlwain, who had previously used them only for biochemical studies (see Yamamoto & McIlwain, 1966: J.Neurochem, 13,1333–1343; see also Richards & McIlwain, 1967: Nature, 215,704–707). In these experiments evoked potentials were recorded with extracellular electrodes, but neurons in such slices later proved amenable to intracellular microelectrode recording (e.g. by Schwartzkroin, 1975: Brain Res., 85, 423–436; Scholfield, 1978: J.Physiol., 275, 535–546). Initially, the use of brain slices was strongly opposed by the Canberra school of physiology and its disciples as being unphysiological, but eventually it became the dominant approach until very recently when improved optical methods such as optogenetics has allowed more precise experiments in vivo. 8. McKinstry DN, Koelle GB (1967). Effects of drugs on acetylcholine release from the cat superior cervical ganglion by carbachol and by preganglionic stimulation. J Pharmacol Exp Ther. 157(2):328-336. PMID:6039824 9. Koketsu K, Nishi S (1967). Acetylcholine depolarization of bullfrog sympathetic preganglionic nerve terminals. Life Sci. 6(11):1169–1177. PMID:4291844 A bit of background. George Koelle did much pioneering work on the localisation of acetylcholinesterase (AChE) in the nervous system and made the interesting observation that in the sympathetic ganglion the highest staining density was on the presynaptic fibres and terminals instead of on the postsynaptic membrane as at the motor end-plate. This, plus other evidence, led him to suggest that the acetylcholine released from the presynaptic endings fed back onto presynaptic acetylcholine receptors to amplify the release process, and that the presynaptic AChE served to limit this positive feedback effect (Koelle, 1962: J.Pharm.Pharmacol., 14, 65–90; see also Chapter 21 in Goodman & Gilman, 1965). One piece of evidence supporting this possibility was that the authors of the 1967 paper had previously shown that carbachol could release acetylcholine from a perfused cat sympathetic ganglion (Mc Kinstry et al., 1963: Canad J Biochem Physiol,41, 2599–2609). This paper shows that the effect of carbachol could be blocked by hexamethonium and tubocurarine so resulted from stimulation of nicotinic receptors. Koelle’s hypothesis of a cholinergic link was paralleled by Burn & Rand’s suggestion of cholinergic link in transmitter release from adrenergic fibres (Burn JH & Rand MJ. Nature. 1959;184:163–165.) Both hypotheses aroused much discussion and argumentation at the time but have since died a natural death. (A telling blow against Koelle’s idea was that acetylcholine itself did not itself release radiolabelled acetylcholine: Brown et al., 1970: Nature, 226, 878–879). More importantly, however, Koelle’s observations, plus those of Koketsu & Nishi on bullfrog sympathetic ganglia, represent some of the first studies suggesting the presence of presynaptic nicotinic receptors on nerve endings; these were subsequently recognized to be a dominant (perhaps predominant) form of physiologically functional nicotinic receptor in the brain, where they facilitate neurotransmitter release (see McGehee & Role, 1996). |
| 10. Douglas WW, Kanno T, Sampson SR (1967). Influence of the ionic environment on the membrane potential of adrenal chromaffin cells and on the depolarizing effect of acetylcholine. J Physiol. 191:107–121. By 1967, it was known that acetylcholine stimulated the secretion of catecholamines from the adrenal medulla and that this depended on the presence of extracellular calcium, but the reason was unknown. In these 1967 experiments Douglas et al. made the first substantial microelectrode recordings from dissociated gerbil adrenal medullary cells. By recording membrane potential responses to acetylcholine in solutions containing different cation concentrations they concluded that ‘depolarization is not, in itself, a key event in stimulus-secretion coupling. The evidence is held to favour the view that movement of calcium into the chromaffin cells on exposure to acetylcholine is responsible for evoking secretion’. This concept of the role of calcium in stimulus-secretion coupling was new at that time and was extended by Douglas to some other exocrine glands in his Gaddum Memorial Lecture to the British Pharmacological Society in 1968 (Douglas WW, 1968: Br J Pharmacol. 34(3):451–474.). In essence, this put vesicular secretion from gland cells into the same category of calcium-dependent processes as transmitter secretion from nerve endings. 11. Dodge FA Jr, Rahamimoff R. (1967). Co-operative action of calcium ions in transmitter release at the neuromuscular junction. J Physiol. 193(2):419–432. Thanks to the work of Bernard Katz and his colleagues, knowledge regarding the role of calcium in transmitter release was more advanced than that in glandular secretion, though how it worked once inside the nerve terminal was still unknown. In this paper (from Bernard Katz’ laboratory) the authors deduced that the number of quanta of acetylcholine released by a nerve impulse was a very steep and highly non-linear function of external calcium concentration, as though it depended on a reaction that proceeded according to the fourth power of the increase in intracellular calcium concentration. This paper is much cited and its conclusions frequently incorporated into studies and discussions on transmitter release to the present day. 12. A. M. Brown, ‘Cardiac sympathetic adrenergic pathways in which synaptic transmission is blocked by atropine sulfate’, J. Physiol., 191, 271–288 (1967). As indicated above, information was emerging that sympathetic neurons possessed muscarinic receptors, as well as nicotinic receptors, and Takeshige & Volle (1962: J.Pharmacol. Exper. Ther., 138, 66–73) reported a delayed, asynchronous atropine-sensitive discharge from postganglionic sympathetic nerves following repetitive stimulation of the preganglionic nerves. In this 1967 paper, Buzz Brown made an important advance in suggesting a potential physiological role for these muscarinic receptors. He showed that the rise in blood pressure and heart rate in cats and dogs produced by stimulating the preganglionic thoracic sympathetic nerves could only be partially reduced by blocking nicotinic ganglionic transmission, and that the residual response, accompanied by an asynchronous postganglionic discharge, could be obtunded by atropine. 13. Larrabee MG.(1967). The influence of neural activity on neural metabolism of glucose and phospholipid. Res Publ Assoc Res Nerv Ment Dis. 45:64–85. PMID:4295650. Hokin & Hokin (1958; J.Biol.Chem., 233,818–821) reported that application of acetylcholine to cortical slices strongly accelerated the synthesis of a monophosphoinositide. In this 1967 paper and preceding ones (Larrabee et al, 1963: J.Neurochem, 10, 549–570; 1965,J.Neurochem, 12,1–13) Martin Larrabee extended these studies to show that repetitive preganglionic nerve stimulation could also accelerate the incorporation of radiolabelled inositol into phosphatidylinositol monophosphate in isolated rat sympathetic ganglia. Neither showed that these effects were specifically mediated by muscarinic receptor activation, but Larrabee later surmised that they probably were because of the lack of desensitization expected for nicotinic effects (Burt & Larrabee, 1976: J.Neurochem., 27,753–763). It is also now known (see Hille et al., 2014) that the effect of muscarinic receptor stimulation is to stimulate the hydrolysis of a higher phosphoinositide, phosphatidylinositol-4,5-bisphosphate ( ‘PIP2’), and that accelerated synthesis of the monophosphate is a secondary consequence of this. Notwithstanding, these were remarkably prescient experiments, leading the way to the discovery of a completely new form of cell signalling. As Martin Larrabee said at the end of a University of London lecture in the early sixties ‘Phosphatidyl – he knows it all’. Looking back, I only wish I had taken more notice of it at the time. |
What about cholinergic synapses in the CNS? The introduction of a technique for the electrophoretic ejection of charged substances such as ACh from glass micropipettes ( ‘iontophoresis’) led to a vast plethora of experiments in which ACh was applied directly onto individual neurons in the CNS, and changes in their activity levels recorded – excitation or acceleration or inhibition of ongoing discharges (see Phillis, 1970 for a detailed survey). Notwithstanding, in only one case was a truly cholinergic synaptic pathway established. This involved the activation of a group of inhibitory interneurons in the spinal cord ( ‘Renshaw cells’) by intraspinal recurrent collateral branches of motor axons, which were already known to be cholinergic at their peripheral ending onto skeletal muscle. Although direct intracellular recordings from these cells were not possible at the time, focal extracellular recordings from within the spinal cord in anaesthetised revealed a burst of action potentials following antidromic motor nerve stimulation that were enhanced and prolonged by anticholinesterase drug and suppressed by dihydro-β-erythroidine, an analogue of the nicotinic blocker d-tubocurarine; these drugs produced concomitant effects on the simultaneous recurrent inhibitory postsynaptic potential (ipsp) recorded intracellularly from the motor neurons that resulted from Renshaw cell activation (see Eccles, 1957):
Although analogous to cholinergic transmission at the neuromuscular junction and at autonomic ganglia, other and subsequent studies revealed some differences. First, like sympathetic neurons, Renshaw cells also possess excitatory muscarinic receptors (Phillis, 1970), though how far they contribute to cholinergic synaptic excitation seems unclear. Second, the co-release of glutamate with the acetylcholine also contributes to transmission between motor axon collaterals and Renshaw cells (Lamotte d’Incamps and Ascher, 2008) though not apparently to synaptic transmission at the peripheral end of the motor fibres onto skeletal muscle (Nishimaru et al, 2005) (Co-release of two transmitters was unheard of in 1967, but co-release of glutamate with acetylcholine from other “cholinergic” neurons in the CNS such as basal forebrain neurons (Allen et al., 2006: J Neurosci 26: 1588–1595) has since been reported (see also Lamotte d’Incamps and Ascher, 2008, for some more examples).. Contrary to common belief, it does not contradict “Dale’s Principal “ (that the same chemical should be released from all processes of the same neuron; Dale, 1935: Proc.Roy.Soc.Med., 28: 319–332) since Dale did not specify only one transmitter. However, the apparent selective release of glutamate from only the collateral terminals would seem to do so.)
Fast excitatory cholinergic transmission has been identified at a few other synapses in the brain (see Lamotte d’Incamps and Ascher, 2008, for examples) but these are rare. Most nicotinic receptors in the brain seem to be presynaptic and most postsynaptic cholinergic effects are mediated by muscarinic receptors. (see Brown, 2010)
Advances 1967–2017
Nicotinic receptors
Individual receptor currents
Membrane ‘noise’ was recorded during ACh depolarisation of frog muscle end-plates using focal extracellular recording (Katz and Miledi, 1972): ‘… the orders of magnitude of the calculated “shot effect”… provide a basis for discussing certain questions which seemed previously not to be open to experimental attack. Among these are: the number of ionic gates involved in the production of a miniature e.p.p.; the absolute conductance of a single ion gate opened by ACh molecules; the duration of the gating action and the total transfer of charge through the ion channel; the relation between the time course of the elementary current and the kinetics of drug/receptor action; the probability of single or repeated action of individual ACh molecules during normal transmission, etc’. ACh-induced current fluctuations were subsequently recorded under voltage-clamp by Anderson and Stevens (1973: J.Physiol., 235: 655–691).
Single channel currents of ACh receptors were recorded from denervated skeletal muscle membranes (Neher and Sakmann, 1976): ‘Recordings of single-channel currents finally resolves the third level of quantitation in the process of neuromuscular transmission after the discovery of endplate currents and miniature endplate currents’. Resolution was improved with the introduction of the gigaseal patch (Hamill et al., 1981: Pflug.Arch., 391, 85–100). This allowed the molecular interaction of ACh molecules with single nicotinic receptors to be examined at high temporal resolution to obtain realistic rate constants for suggested kinetic schemes of agonist–receptor interaction (Colquhoun and Sakmann, 1985) and the basis for such mysterious concepts as ‘partial agonism’ to be determined (Lape et al., 2008: Nature, 454, 722–727; Figure 1).
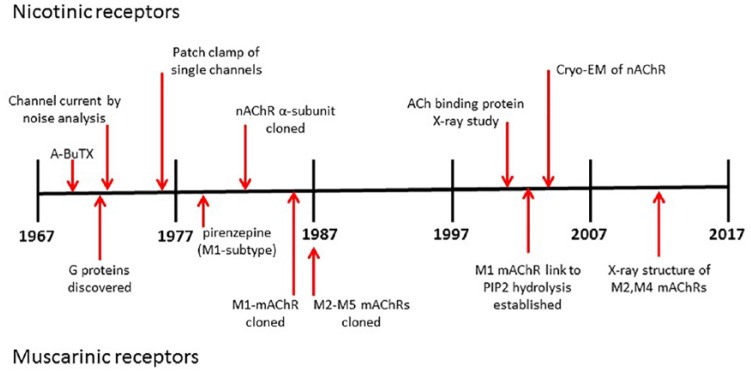
Figure 1.
Cholinergic receptors: discovery timeline 1967–2017.
Clones and genes
Using the electric organ (electroplax) of the electric eel Torpedo as a rich source of muscle-type nicotinic receptors, by 1980 the overall structure of the receptor had been determined by protein chemistry to comprise a pentamer containing four subunits designated α2βγ∂ (e.g. Raftery et al, 1980).
In 1982, using DNA probes derived from a partial amino acid sequence of the Torpedo receptor, Shosaku Numa and his colleagues cloned the full-length cDNA and deduced the complete amino acid sequence for the α-subunit of the Torpedo receptor (Noda et al., 1982); and in subsequent papers reported cDNAs and sequences for the other three subunits (Noda et al., 1983: Nature, 301, 251–255, and 302, 538–542).
Knowledge of the muscle receptor composition allowed the neural nicotinic receptors cDNAs to be isolated by homology screening from neural tissue (e.g. Boulter et al., 1986, see Dani, 2015; McGehee and Role, 1995 for others). Like muscle channels, neuronal channels are pentamers, but composed only of α and β subunits, or sometimes homomeric α-subunits. In mammalian neurons eight α-subunits (α1-α7, α9, and α10) and three β-subunits (β2-β4) have been identified. In the mammalian CNS, the most common combinations are α42β23, with two ACh binding sites at the α-β interfaces, or α43 β22, with potentially three binding sites, or five homomeric α7 subunits with up to five binding sites (Dani, 2015). Uniquely among neural receptors, the latter are blocked by bungarotoxins. They also have a fivefold higher calcium permeability than the α-β heteromers (and 10 times more than muscle receptors): this probably contributes to the presynaptic action of ACh (and nicotine) in enhancing transmitter release (McGehee and Role, 1996). The most prominent receptor in the peripheral nervous system (including sympathetic ganglia) is an α3β4 heteromer, though this is also present in the medial habenular and interpeduncular nuclei.
Knowing the genes allows the construction of knock-in or knock-out subunits. Cordero-Erausquin et al. (2000) summarise some of the effects of individual neuronal receptor subunit knock-outs in mice. As expected, sympathetic and autonomic functions are disrupted in α3 or β4 null mice. Deletion of α4 or β2 reduced high-affinity nicotine binding and some presynaptic transmitter release enhancing nicotinic receptors were non-functional in the β2 knockout mice. Furthermore, β2 subunits appeared to have a role in learning and in protection against ageing.
Looking at nicotinic receptors
An important early post-1967 advance was the discovery by C.Y. Lee of the snake venom toxin α-bungarotoxin, which binds irreversibly to muscle nicotinic receptors (Miledi and Potter, 1971). This not only facilitated the isolation and identification of the receptor but its tight binding allowed its use as a probe for localising the receptor at a much higher resolution than that that obtained with radiolabelled curare (Fertuck and Salpeter, 1974). The persistent binding of bungarotoxin also permitted experiments on end-plate receptor turnover and regulation (e.g. Levitt et al., 1980: Science, 210, 550–551).
The detailed atomic structure of the nicotinic ACh receptor has not yet been determined by X-ray crystallography, but the discovery of a secreted water-soluble ACh binding protein (Smit et al., 2001: Nature, 411, 261–268 has allowed the crystal structure of the homologous binding domain in the nicotinic receptor to be determined (Brejc et al., 2001).
On the other hand, the exceptional density and organisation of the receptors in Torpedo electroplax has been brilliantly exploited to provide images of the intact receptor down to 4Å resolution by cryo-electron microscopy (Unwin, 2013) – now much-favoured molecular imaging technique (see Fernandez-Leiro and Scheres, 2016: Nature, 537, 339–346).
What do neural nicotinic receptors do?
Other than the pre-1967 Renshaw cells, only a few functional cholinergic synapses with postsynaptic nicotinic receptors have yet been identified in the mammalian CNS (Jones et al., 1999). Instead, the majority of nicotinic receptors are presynaptic, and serve to enhance the release of other neurotransmitters such as glutamate (McGehee and Role, 1996) – very much as predicated from the experiments of Koelle and Nishi on sympathetic ganglia (see Box 1) (In some cases, this seemed truly surprising. Thus, as pointed out to us by Dr M.J.Brownstein (National Institutes of Mental Health (NIMH) one of the most striking “cholinergic” tracts in the brain (neurochemically speaking is the fasciculus retroflexus of Meynert (FRM), between the medial habenula and the interpeduncular nucleus (IPN). When we saw this gleaming white tract in dissected brain slices just begging to be stimulated, we thought this must be the perfect central homologue of the sympathetic ganglion synapse. But not so: although ACh and nicotinic agonists readily excited IPN neurons, the IPN response to FRM stimulation was not at all diminished by nicotinic antagonists; instead it was inhibited by glutamate antagonists, suggesting that transmission was glutamatergic (Brown et al., 1983: J.Physiol., 341, 655–670)!!. The most prominent effect of ACh or nicotinic agonists on FRM stimulation was to reduce the amplitude of the action potential recorded from the FRM terminals within the IPN and slow its conduction (Brown et al., 1984: J.Physiol. 353, 101–109; we suggested that this was due to a depolarization of the unmyelinated fibre terminals, as seen on peripheral C-fibres. In some elegant experiments, McGehee et al (1995: Science, 260, 1692–1696) later confirmed this presynaptic action, and showed that it led to an entry of Ca2+ through the nicotinic channels, and consequent enhancement of the glutamatergic epsc. More recently, Pen et al (2010: Neuron, 69, 445–452) have found that selective stimulation of choline acetyltransferase-expressing fibres in the FRM release both glutamate and ACh: glutamate drives the individual fast epscs in the IPN neurons while ACh released by repetitive 20–50 Hz FRM stimulation induces a lower amplitude slow nicotinic epsc in the IPN neurons.). Most of these presynaptic receptors are α4β2, occasionally α3β2 or β4 (e.g. medial habenula and interpeduncular nucleus, see footnote), or α7 homomers. Some clues regarding their overall functional significance may be gleaned from the effects of subunit knock-outs noted above – for example, disruption of some forms of learning, or loss of responses to nicotine such as antinociception (Cordero-Erausquin et al., 2000). Notwithstanding, there are very few (if any) examples of true cholinergic axo-axonal synapses, so presumably these presynaptic receptors are activated by more remotely released ACh–the ‘soup’ theory of transmission (Sivilotti and Colqhuoun, 1995: Science, 269, 1681–1682).
Muscarinic receptors (mAChRs)
The nature of the receptor
As with the nicotinic receptor, the physical nature of the muscarinic receptor was unknown in 1967. The first muscarinic receptor was cloned from a pig brain cDNA library by Kubo et al. (1986). The predicted amino acid sequence showed a clear homology to the β-adrenergic receptor (Dixon et al., 1986: Nature, 321, 75–79) and to the visual pigment rhodopsin (Ovchinnikov, 1982: FEBS Lett, 148 179–191) and hence it joined the family of heptahelical (7 transmembrane domain=7TM) signalling proteins.
Previous and ongoing pharmacological studies (e.g. Hammer et al., 1980) indicated that there may be more than one subtype of muscarinic receptor. Eventually, five genetic subtypes designated M1 through M5, were cloned (Bonner et al., 1987: Science, 237, 527–532; Fukuda et al., 1987: Nature, 327, 623–625; summarised in Bonner, 1989). Interestingly, each receptor is encoded by a separate intronless gene. The original pig brain receptor corresponded to the pharmacological M1 subtype described by Hammer et al (1980) – the most abundant muscarinic receptor expressed in the brain.
The structures of the M2 (Haga et al., 2012: Nature, 482 547–551) and M3 (Kruse et al., 2012: Nature, 482 552–556) receptors in their resting state have now been determined by X-ray crystallography, so that it is now possible to envisage the ligand-binding and G protein-binding domains, and the possible conformational changes accompanying ligand and G protein binding, in some detail (Hulme, 2013).
How do the receptors work?
Unlike nicotinic receptors, muscarinic receptors are not ion channels. Instead, they are members of the G protein-coupled receptor (GPCR) superfamily, that is, when activated by ACh, their usual (I say ‘usual’ because there is accumulating evidence that GPCRs can sometimes alternatively route through other associated proteins such as β-arrestins (DeFea, 2008: Br J Pharmacol, 153, 5298–5309). Primary response is to dock onto, and activate, a trimeric guanine nucleotide-binding protein called a G protein (Oldham and Hamm, 2008). G proteins were discovered in the 1970s through a requirement for guanosine triphosphate (GTP) in the solution when studying GPCR activity in broken cell preparations (Rodbell et al., 1971: J.Biol.Chem., 246, 1877–1882).
The G protein comprises α, β, and γ subunits. The α-subunit contains the guanine nucleotide binding domain, and also a GTPase catalytic domain. At rest the α-subunit binds guanosine diphosphate (GDP). The activated GPCR induces a conformational change in the G-protein leading to (a) dissociation of the trimer into α- and coupled βγ-subunits and (b) dissociation of GDP and its replacement by GTP. Hydrolysis of GTP by the GTPase activity of the α-subunit leads to replacement of GTP by GDP and reassembly of the αβγ trimer. The GTPase activity determines the rate of recovery, and may be accelerated by ancillary GTPase activating proteins (GAPs). The GPCR-induced reduction in the binding affinity of GDP to the α-subunit is matched by a reciprocal reduction in the apparent binding affinity of the agonist for the GPCR (shown for muscarinic receptors by Berrie et al. (1979: Biochem. Biophys. Res. Comm., 87 1000–1005).
There are a number of different G proteins, differentiated in terms of the structures and downstream targets of their α-subunit. The individual muscarinic receptors show a general pattern of G protein ‘preferences’ as follows (Bonner, 1989; Caulfield, 1993):
| Receptor subtype | G protein | Main targets |
|---|---|---|
| M1, M3, M5 | Gq, G11 | Phospholipase C (PLC) activation → Phosphatidylinositol-4,5-bisphosphate (PIP2) hydrolysis |
| M2, M4 | Gi | α-subunit: adenylate cyclase inhibition |
| Go | βγ-subunits: Kir3 activation CaV2 inhibition |
What do they do to a neuron?
In the short term, activation of mAChRs modifies the signalling properties of neurons by altering the activity of selected membrane ion channels using the apposite G protein (or one of its downstream biochemical effectors) as the receptor – ion channel transducer (see Brown, 2010; Caulfield, 1993 for reviews.) Thus, in brief, and with considerable simplification, activation of M1/M3/M5 receptors tends to increases neuronal excitability by inhibiting one or more of several potassium channels and/or by activating cation channels; whereas activation of M2 or M4 receptors produces postsynaptic inhibtion by activating Kir potassium channels, or presynaptic inhibition by inhibiting CaV2 calcium channels. However, the indirect nature of the pathway connecting receptor to ion channel mitigates against any hard and fast rules linking the receptor to the response, for the following reasons.
The ion channel only ‘sees’ the final transducer, not the receptor, so in principle cannot tell whether (say) Go has been activated by a muscarinic receptor or a metabotropic glutamate receptor; or, if the former, by the M2 or M4 receptor. Dissecting or predicting the final response and its mechanistic pathway when (say) ACh is applied to a neuron or a mixture of ACh and glutamate are released onto a neuron then becomes a question of anatomy: that is, which receptor/G protein/intermediary transducer/ion channel is/are present in that neuron, and whereabouts are they located in the neuron. For the latter, one might think first of which compartment of the neuron houses the receptors and channels. Thus, the CaV2 calcium channels closed by stimulating M2 or M4 receptors (and thence by Goβγ-subunits) are heavily concentrated in the presynaptic terminals of central and peripheral neurons, where they drive action potential-evoked transmitter release; so the principal effect of M2 or M4 receptor stimulation is to inhibit transmitter release – either of ACh itself (feedback auto-inhibition, for example, from basal forebrain axons: Allen and Brown, 1996: J.Physiol., 492 453–466), or of other transmitters (hetero-inhibition). On the other hand, the Kir3 channels opened by M2 or M4 receptors are primarily postsynaptic and generate a form of postsynaptic inhibition. As an example of subcellular compartmentation: in hippocampal neurons the Kv7 channels inhibited by M1 receptor activation are localised to the axon initial segment where they bind to ankyrinG and control the action potential threshold; hence, their inhibition by ACh increases excitability by facilitating local spike generation (Martinello et al., 2015; Shah et al., 2008: Proc.Natl.Acad.Sci.,USA, 105, 7869–7874). In other neurons where the Kv7 channels are somatic, their inhibition by mAChR receptors can also increase excitability more generally, by depolarising the cell and increasing input resistance. Further micro-anatomical association and segregation of muscarinic receptors with their cognate G proteins and ion channels may be achieved by association with ancillary scaffolding proteins such as A-kinase Anchoring Proteins (AKAPs; Kosenko et al., 2012: EMBO J., 31, 3147–3156).
Unlike nicotinic receptors, or other transmitter-gated ionotropic receptors, the response to stimulating muscarinic receptors is indirect and takes time, from about 30–50 ms for the activation of a G protein-gated inward rectifier Kir3 potassium channel by an M2 receptor, to ⩾200 ms for the closure of an M-type Kv7 potassium channel by an M1 receptor (via Gq and consequent fall in membrane PIP2 concentration: Hille et al., 2014), seconds or minutes for responses involving downstream phosphorylation or dephosphorylation, and several hours for transcription changes (e.g. a change in the number of M-type channels induced by a calcium-dependent transcriptional response to neural excitation by M1-mAChRs (Zhang and Shapiro, 2012: Neuron. 76 1133–1146).
Muscarinic receptors and global nervous system function
Thiele (2013) provides a contemporary update on the contributions of muscarinic receptors to central nervous system physiology. Wess (2004) summarises the roles of the individual muscarinic receptors to nervous system function in mice as revealed by genetic subtype deletions. A few points of interest from these surveys:
As predicated by Krnjevic (1967, 1974 (see Box 1) a major contribution of muscarinic excitation of cortical and hippocampal neurons to cortical arousal and cognition has been established. This is mediated substantially by M1 receptors, but probably with additional input from M5 receptors, and with a negative feedback effect from presynaptic M2 autoreceptors onto ascending cholinergic afferents.
Substantially more information about the cellular mechanisms of muscarinic excitation of cortical and hippocampal neurons and their consequences for network behaviour has been obtained over the past 50 years (see also Brown, 2010; Martinello et al., 2015: 346–353).
Also, as predicated from old pharmacological knowledge, activation of muscarinic receptors affects basal ganglion locomotor function, probably through release of ACh from striatal interneurons and indirect inhibition of dopamine release by M4 receptors. An additional role for M1 receptors is indicated by an increase in striatal dopamine levels in M 1R k-o mice.
A cholinergic input also enhances dopamine release in the ventral tegmental ‘reward centre’ through an action on M5 receptors. This is subject to M4 auto-inhibition of ACh release, so dopamine levels in the nucleus accumbens are raised in M4 k-o mice.
Muscarinic agonists induce an analgesic effect of supraspinal origin when injected intra-thecally. This is mediated by a combination of M2 and M4 receptors, offering interesting prospects for drug development.
Future prospects
-
With increasingly precise information about the structure of the receptors, we might expect the development of increasingly selective drugs targetting different subtypes of muscarinic receptors and subtype combinations of nicotinic receptors.
For the muscarinic receptors, subtype selectivity is likely to be best achieved by targetting allosteric sites (see Conn et al., 2009: Trends Pharmacol Sci., 30: 148–155). Apart from greater selectivity, positive alllosteric modulators (PAMS) have the advantage over direct agonists that they only affect ongoing cholinergic activity. Thus, M1-PAMS show promise for treatment of cognitive disorders while M4-PAMS may be appropriate for treatment of schizophrenia.
In the past, much work on nicotinic receptor pharmacology has been driven by the need to control nicotine addiction, but this will be a declining market in the future. Nevertheless, drugs interacting with nicotinic receptors may have beneficial effects independently of nicotine actions. For example, PAMs for α7 nicotinic receptors have beneficial effects on cognition and pain in animal studies (Bagdas et al., 2017: Br. J. Pharmacol., 173(16): 2506–2520; Potasiewicz et al., 2017: Neuropharmacol, 113: 188–197).
Optogenetic techniques coupled with refined recording of neuronal responses in vivo should allow a much more precise description of the cholinergic circuits underlying the behavioural responses to cholinergic stimulation in the brain and of their effect on neural coding, to the extent that they might be simulated in virtual reality and the effects of pathological lesions in (e.g.) Alzheimer’s Disease and their pharmacological amelioration fully understood.
Acknowledgments
I thank Dr Susan Jones (University of Cambridge) for helpful comments.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Blackman JG, Ginsborg BL, Ray C. (1963. a) On the quantal release of the transmitter at a sympathetic synapse. Journal of Physiology 167(2): 402–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman JG, Ginsborg BL, Ray C. (1963. b) Spontaneous synaptic activity in sympathetic ganglion cells of the frog. Journal of Physiology 167(2): 389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman JG, Ginsborg BL, Ray C. (1963. c) Synaptic transmission in the sympathetic ganglion of the frog. Journal of Physiology 167(2): 355–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner TI. (1989) The molecular basis of muscarinic receptor diversity. Trends in Neurosciences 12(4): 148–151. [DOI] [PubMed] [Google Scholar]
- Boulter J, Evans K, Goldman D, et al. (1986) Isolation of a cDNA clone coding for a possible neural nicotinic acetylcholine receptor alpha-subunit. Nature 319(6052): 368–374. [DOI] [PubMed] [Google Scholar]
- Brejc K, van Dijk WJ, Klaassen RV, et al. (2001) Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature 411(6835): 269–276. [DOI] [PubMed] [Google Scholar]
- Brown DA. (2010) Muscarinic acetylcholine receptors (mAChRs) in the nervous system: Some functions and mechanisms. Journal of Molecular Neuroscience 41(3): 340–346. [DOI] [PubMed] [Google Scholar]
- Caulfield MP. (1993) Muscarinic receptors – Characterization, coupling and function. Pharmacology & Therapeutics 58(3): 319–379. [DOI] [PubMed] [Google Scholar]
- Chothia C. (1970) Interaction of acetylcholine with different cholinergic nerve receptors. Nature 225(5227): 36–38. [DOI] [PubMed] [Google Scholar]
- Colquhoun D, Sakmann B. (1985) Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. Journal of Physiology 369: 501–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero-Erausquin M, Marubio LM, Klink R, et al. (2000) Nicotinic receptor function: New perspectives from knockout mice. Trends in Pharmacological Sciences 21(6): 211–217. [DOI] [PubMed] [Google Scholar]
- Curtis DR, Ryall RW. (1966) The excitation of Renshaw cells by cholinomimetics. Experimental Brain Research 2(1): 49–65. [DOI] [PubMed] [Google Scholar]
- Dani JA. (2015) Neuronal nicotinic acetylcholine receptor structure and function and response to nicotine. International Review of Neurobiology 124: 3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EDP, Bennett HS. (1955) Some features of the submicroscopic morphology of synapse in frog and earthworm. Journal of Biophysical and Biochemical Cytology 1(1): 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC. (1957) The Physiology of Nerve Cells. London: Oxford University Press. [Google Scholar]
- Eccles RM. (1955) Intracellular potentials recorded from a mammalian sympathetic ganglion. Journal of Physiology 130(3): 572–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatt P, Katz B. (1951) An analysis of the end-plate potential recorded with an intra-cellular electrode. Journal of Physiology 115(3): 320–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatt P, Katz B. (1952) Spontaneous subthreshold activity at motor nerve endings. Journal of Physiology 117(1): 119–128. [PMC free article] [PubMed] [Google Scholar]
- Feldberg W. (1954) Transmission in the central nervous system and sensory transmission. Pharmacological Reviews 6: 85–93. [PubMed] [Google Scholar]
- Fertuck HC, Salpeter MM. (1974) Localization of acetylcholine receptor by 125I-labeled alpha-bungarotoxin binding at mouse motor endplates. Proceedings of the National Academy of Sciences of the United States of America 71(4): 1376–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsborg BL. (1967) Ion movements in junctional transmission. Pharmacological Reviews 19(3): 289–316. [PubMed] [Google Scholar]
- Goodman LS, Gilman A. (1965) The Pharmacological Basis of Therapeutics (3rd ed). New York: Macmillan. [Google Scholar]
- Hammer R, Berrie CP, Birdsall NJ, et al. (1980) Pirenzepine distinguishes between different subclasses of muscarinic receptors. Nature 283: 90–92. [DOI] [PubMed] [Google Scholar]
- Hebb CO. (1957) Biochemical evidence for the neural function of acetylcholine. Physiological Reviews 37(2): 196–220. [DOI] [PubMed] [Google Scholar]
- Hille B, Dickson E, Kruse M, et al. (2014) Dynamic metabolic control of an ion channel. Progress in Molecular Biology and Translational Science 123: 219–247. [DOI] [PubMed] [Google Scholar]
- Hulme EC. (2013) GPCR activation: A mutagenic spotlight on crystal structures. Trends Pharmacol Sci 34(1): 67–84.. [DOI] [PubMed] [Google Scholar]
- Jenkinson DH, Nicholls JG. (1961) Contractures and permeability changes produced by acetylcoline in denervated depolarized muscle. Journal of Physiology 159: 111–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Sudweeks S, Yakel JL. (1999) Nicotinic receptors in the brain: Correlating physiology with function. Trends in Neurosciences 22(12): 555–561. [DOI] [PubMed] [Google Scholar]
- Katz B. (1966) Nerve, Muscle and Synapse. New York: McGraw-Hill. [Google Scholar]
- Katz B. (1969) The Release of Neural Transmitter Substances. Liverpool: Liverpool University Press. [Google Scholar]
- Katz B, Miledi R. (1972) The statistical nature of the acetylcholine potential and its molecular components. Journal of Physiology 224(3): 665–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjevic K. (1974) Chemical nature of synaptic transmission in vertebrates. Physiological Reviews 54(2): 419–540. [Google Scholar]
- Kubo T, Fukuda K, Mikami A, et al. (1986) Cloning, sequencing and expression of complementary DNA encoding the muscarinic acetylcholine receptor. Nature 323(6087): 411–416. [DOI] [PubMed] [Google Scholar]
- Lamotte d’Incamps B, Ascher P. (2008) Four excitatory postsynaptic ionotropic receptors coactivated at the motoneuron-Renshaw cell synapse. Journal of Neuroscience 28(52): 14121–14131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AI, Wainer BH, Mufson EJ, et al. (1983) Co-localization of acetylcholinesterase and choline acetyltransferase in the rat cerebrum. Neuroscience 9(1): 9–22. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Role LW. (1995) Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annual Review of Physiology 57: 521–546. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Role LW. (1996) Presynaptic ionotropic receptors. Current Opinion in Neurobiology 6(3): 342–349. [DOI] [PubMed] [Google Scholar]
- Martinello K, Huang Z, Lujan R, et al. (2015) Cholinergic afferent stimulation induces axonal function plasticity in adult hippocampal granule cells. Neuron 85(2): 346–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason DF, Wien R. (1955) The actions of heterocyclic bisquaternary compounds, especially of a pyrrolidinium series. British Journal of Pharmacology and Chemotherapy 10(1): 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R, Potter LT. (1971) Acetylcholine receptors in muscle fibres. Nature 233(5322): 599–603. [DOI] [PubMed] [Google Scholar]
- Mitchell JF. (1963) The spontaneous and evoked release of acetylcholine from the cerebral cortex. Journal of Physiology 165(1): 98–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E, Sakmann B. (1976) Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature 260(5554): 799–802. [DOI] [PubMed] [Google Scholar]
- Nishi S, Koketsu K. (1960) Electrical properties and activities of single sympathetic neurons in frogs. Journal of Cellular and Comparative Physiology 55(1): 15–30. [DOI] [PubMed] [Google Scholar]
- Nishimaru H, Restrepo CE, Ryge J, et al. (2005) Mammalian motor neurons corelease glutamate and acetylcholine at central synapses. Proceedings of the National Academy of Sciences of the United States of America 102(14): 5245–5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M, Takahashi H, Tanabe T, et al. (1982) Primary structure of alpha-subunit precursor of Torpedo californica acetylcholine receptor deduced from cDNA sequence. Nature 299(5886): 793–797. [DOI] [PubMed] [Google Scholar]
- Oldham WM, Hamm HE. (2008) Heterotrimeric G protein activation by G-protein-coupled receptors. Nature Reviews Molecular Cell Biology 9(1): 60–71. [DOI] [PubMed] [Google Scholar]
- Paton WD, Zaimis EJ. (1949) The pharmacological actions of polymethylene bistrimethyl-ammonium salts. British Journal of Pharmacology and Chemotherapy 4(4): 381–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillis JW. (1970) The Pharmacology of Synapses. Oxford: Pergamon Press. [Google Scholar]
- Raftery MA, Hunkapiller MW, Strader CD, et al. (1980) Acetylcholine receptor: Complex of homologous subunits. Science 208(4451): 1454–1456. [DOI] [PubMed] [Google Scholar]
- Shute CC, Lewis PR. (1963) Cholinesterase-containing systems of the brain of the rat. Nature 199: 1160–1164. [DOI] [PubMed] [Google Scholar]
- Stone CA, Torchiana MI, Navarro A, et al. (1956) Ganglionic blocking properties of 3-methylaminoisocamphane hydrochloride (mecamylamine): A secondary amine. Journal of Pharmacology and Experimental Therapeutics 117(2): 169–183. [PubMed] [Google Scholar]
- Takeuchi A, Takeuchi N. (1960) On the permeability of end-plate membrane during the action of transmitter. Journal of Physiology 154(1): 52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele A. (2013) Muscarinic signaling in the brain. Annual Review of Neuroscience 36: 271–294. [DOI] [PubMed] [Google Scholar]
- Unwin N. (2013) Nicotinic acetylcholine receptor and the structural basis of neuromuscular transmission: Insights from Torpedo postsynaptic membranes. Quarterly Reviews of Biophysics 46(4): 283–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waser PG. (1960) The cholinergic receptor. Journal of Pharmacy and Pharmacology 12: 577–594. [DOI] [PubMed] [Google Scholar]
- Wess J. (2004) Muscarinic acetylcholine receptor knockout mice: Novel phenotypes and clinical implications. Annual Review of Pharmacology and Toxicology 44: 423–250. [DOI] [PubMed] [Google Scholar]