Abstract
The use of cognitive-enhancing drugs by healthy individuals has been a feature for much of recorded history. Cocaine and amphetamine are modern cases of drugs initially enthusiastically acclaimed for enhancing cognition and mood. Today, an increasing number of healthy people are reported to use cognitive-enhancing drugs, as well as other interventions, such as non-invasive brain stimulation, to maintain or improve work performance. Cognitive-enhancing drugs, such as methylphenidate and modafinil, which were developed as treatments, are increasingly being used by healthy people. Modafinil not only affects ‘cold’ cognition, but also improves ‘hot’ cognition, such as emotion recognition and task-related motivation. The lifestyle use of ‘smart drugs’ raises both safety concerns as well as ethical issues, including coercion and increasing disparity in society. As a society, we need to consider which forms of cognitive enhancement (e.g. pharmacological, exercise, lifelong learning) are acceptable and for which groups under what conditions and by what methods we would wish to improve and flourish.
Keywords: Cognitive-enhancing drugs, workplace enhancement, modafinil, hot and cold cognition, improved task-related motivation, neuroethics
Ancient legends described humans striving for self-improvement in areas such as immortality, excellence in sport or in rhetorical competitions (Gordijn, 2015). Today, self-enhancement can be observed in many domains: the use of cosmetics or cosmetic surgery to improve appearance, the use of interventions aiming at improving brain function and cognitive performance, also called neuroenhancement, and using doping for improving physical performance in athletics. This chapter aims at giving an overview of the recent history of neuroenhancement with a particular focus on ethical questions of neuroenhancement.
Historically, tonics, herbs and other substances have in many cultures been used to improve general strength and endurance. Some examples are ma huang (Ephedra vulgaris) in China, Khat (Catha edulis) in Northern Africa, and coca (Erythroxylon coca) in South America (Angrist and Sudilovsky, 1978).
Cocaine and amphetamine are modern cases of drugs initially enthusiastically acclaimed for enhancing cognition and mood. But in both cases, these initial Golden Periods were followed by a painful sobering, with the social and health–economic consequences still visible today. We want to portray these two, surprisingly parallel, stories shortly and then discuss the lessons learned (and further to be learned) from this from a regulatory and ethical perspective on the discussion on cognitive- (and mood-) enhancing drugs today.
At least since the 6th century AD, coca leaves have been chewed in South America (Petersen and Stillman, 1977), initially for spiritual purposes and then as a resource to improve work performance in arduous circumstances (Petersen and Stillman, 1977). For various reasons (limited cocaine concentration in leaves, bitter taste), ‘overindulgence’, overdosing, or clear abuse and dependency in that population are rare (Carroll, 1977). In the mid-19th century (1870s and 1880s), researchers in North America and Europe isolated the main active ingredient, cocaine, and tested its effects in animals and humans, including in soldiers in Bavaria (Freud, 1885; Petersen and Stillman, 1977). In the influential essay ‘On Coca’, Sigmund Freud (1885: 298–304) praised cocaine as having a stimulating and enhancing effect, in part from his own experiences, and generally downplayed or even negated side effects, stating a desire to take higher and repeated doses. Freud recommended cocaine as a stimulant to enhance and maintain physical and mental performance. He also recommended it for various mental problems, including hypochondria, depression, as an aid for withdrawal from morphine and alcohol, as an aphrodisiac, as well as for multiple other ailments (Freud, 1885). In the late 19th century, to a greater extent in America than in Europe, cocaine consumption quickly increased (Coca Cola as ‘brain tonic’, patented combinations such as red wine and coca marketed as ‘Vin Mariani’; Musto, 1989; Petersen and Stillman, 1977), in particular by athletes to enhance performance (Grinspoon and Bakalar, 1981). Initially, some cases of chronic cocaine abuse were reported, leading to early laws regulating cocaine and other drugs (Musto, 1989). Around 1900, the problem of cocaine abuse became increasingly apparent, resulting in reports about an ‘epidemic’, such that at the beginning of the 20th century, national and even international laws regulating the access to opium and cocaine were established (Goldstein et al., 2009).
In the case of amphetamines, the story is very similar (Rasmussen, 2011): in the late 1920s, amphetamine’s anti-asthmatic effect was discovered, quickly followed by its mood enhancing and stimulating effect. In the 1930s, students in the United States used it as a ‘pep pill’, while it was used as a ‘confidence drug’ in the United Kingdom. Cyclists replaced cocaine with amphetamine, leading to amphetamine’s way as a performance enhancer. Around the start of the Second World War, military leaders were looking for a drug to reduce the effects of fatigue, to improve alertness and to enhance performance, although caffeine was available. Amphetamine improved the decline in performance normally seen over time, especially on repetitive or tedious tasks, possibly including improvements in motivation. In parallel, soldiers in the British Royal Air Force (RAF) were already unofficially using amphetamine (Benzedrine®), and officers were recommending its use particularly due to the increase in morale and aggression. In early 1942, the RAF recommended a dose of 10 mg Benzedrine for each mission. The British Army also ordered Benzedrine for the troops fighting in El Alamein, recommending a dose of 20 mg/day for five consecutive days (November 1942). Although a US Army report in 1941 found no superiority of amphetamine over caffeine and warned of the risk of habit formation, amphetamines were endorsed in 1942 as performance enhancers for aviators and officially approved in 1943. The US Army and US Air Force were at that point already buying amphetamines in bulk and supplying them to ground forces and aviators by the beginning of 1943, with the US Navy following a few months later. There were no official restrictions and recommendations about the maximal dosage in the United States. Military purchases in the United States continued during both the Korean (1950–1953) and the Vietnam War (1955–1975), and some authors claim that the US military still uses amphetamines (Bower, 2003). The Japanese military uses it ‘to inspire the fighting spirits’ (Rasmussen, 2008). However, the German military, which had already been using methamphetamine (Pervitin®) in 1939, stopped military consumption by the end of 1940 and classified amphetamine and methamphetamine as dangerously addictive narcotics in 1941/1942. In the United States, the misuse of amphetamine pills, as well as the contents of amphetamine inhalers, started to come to the attention of researchers, doctors, and authorities at the end of the 1940s, with the main groups being World War II (WWII) veterans and some subcultures (beatniks etc.; Rasmussen, 2008). In the 1950s, the use of amphetamine increased steadily, also enhanced by the effort of pharmaceutical companies to widen the ‘medical’ indications from narcolepsy, depression, and Parkinson’s disease to conditions such as anhedonia and obesity, although with rather loose regulatory oversight (Rasmussen, 2008). In parallel, the reports on negative effects such as addiction and psychotic effects increased (Rasmussen, 2008). But it took until the late 1960s for regulations on amphetamine distribution to be introduced in both the United States (Comprehensive Drug Abuse Prevention and Control Act, 1970; Drug Abuse Control Amendments, 1965; Rasmussen, 2008) and the United Kingdom (Medicines Act, 1968; Misuse of Drugs Act, 1971).
One historical case in which a politician has admitted to using pharmacological ‘helpers’ such as barbiturates and amphetamines to be able to fall asleep and to be fit the next day again is the former British prime minister Anthony Eden, and this was shown to have affected his decision-making.
These two stories of cocaine and amphetamines may already evoke some feelings of déjà vu: A substance aimed at improving human mental and physical performance was used widely before the negative long-term effects and risks became apparent. Following a number of serious incidents due to a lack of or insufficient safety testing, in particular after the thalidomide disaster, strict drug regulations procedures have been developed to ensure safety standards before approval and to monitor adverse effects after approval (Rägo and Santoso, 2008). However, these regulations apply primarily to drugs intended for the medical treatment of disorders rather than for cognitive enhancement in healthy people.
External means to enhance oneself
Today, the use of external means to enhance oneself can be observed in many domains, such as in the use of cosmetics or even cosmetic surgery to improve appearance or the use of interventions aimed at improving brain function and cognitive performance, also known as ‘neuroenhancement’ or doping in athletics. At a basic level, interventions to enhance cognition include education, good sleep (quality and quantity), physical exercise, meditation, and nutritional methods (nicotine, coffee, tea, vitamins and food constituents, and supplements; Lieberman, 2003). At a more specific level, neuroenhancement includes primarily pharmacological interventions, also called ‘smart drugs’, but also transcranial electrical stimulation methods which repeatedly make it into the headlines of the media (e.g. ‘Electrical brain stimulation beats caffeine – and the effect lasts longer’, Sample (2014), or ‘Can Electric ‘Brain Training’ Devices Make You Smarter?’ Alsever (2015)), and app-based brain training (Sahakian et al., 2015). Historically, research into the effects of pharmacological substances on mental functions began in the early 19th century (Badiani, 2015), although systematic research only began around the beginning of the 20th century (Badiani, 2015). With the discovery of the performance-enhancing effects of amphetamine in the 1930s came their use in the military in WWII (Bower and Phelan, 2003; Rasmussen, 2011; Tracey and Flower, 2014). After the negative effects of these stimulants, particularly addiction/dependency and psychoses, became apparent in the 1960s (Bell et al., 2012), the next big wave of pharmacological agents aimed at improving cognitive performance targeted the cholinergic system with a focus on memory (Drachman and Sahakian, 1980; Muramoto et al., 1979), resulting in tacrine as the first acetylcholinesterase inhibitor (ACHEI) approved for use in Alzheimer’s and dementia (Eagger et al., 1991). In recent years, the range of interventions aimed at or used for improving cognitive function has broadened considerably, now including methylphenidate, modafinil, and other drugs acting on pathways involving dopamine, noradrenaline, acetylcholine, glutamate, histamine, melatonin as well as glucocorticoids, and immune-modulating agents (Fond et al., 2015). Furthermore, some authors include so-called ‘soft enhancers’ such as caffeine, nicotine, food supplements as well as over-the-counter drugs within the field of neuroenhancement (if it is taken to enhance cognitive performance, e.g. Maier et al., 2015b). In addition, as mentioned above, methods to directly modulate brain activity, as well as app-based training, are increasingly investigated, marketed, and used to improve cognition.
Nowadays, the exact numbers of healthy people, that is, people not diagnosed with a measurable disorder or deficit, using prescription drugs to enhance cognitive functions are unknown. However, a recent survey of 15 countries reported that the use of cognitive-enhancing drugs was on the increase (Maier et al., 2018). The United States had the highest use of stimulant drugs, such as methylphenidate, while the United Kingdom had the highest use of modafinil (Maier et al., 2018). Surveys indicate smaller percentages in the general population as compared with university surveys. This likely reflects the fact that people wish to enhance features that they value or which will lead to success, for example, knowledge in a knowledge-based economy. Estimates in the general population are typically between 3% and 5% (Dietz et al., 2013; Emanuel et al., 2013; Maier et al., 2013; Singh et al., 2014; Smith and Farah, 2011; Webb et al., 2013, but see also Low and Gendaszek, 2002), but there is a considerable degree of uncertainty. Two recent studies in students at secondary schools and university students in Switzerland found higher rates of lifetime use of prescription drugs of 9% and 7%, respectively, for cognitive enhancement (Liakoni et al., 2015; Maier et al., 2015a). A recent report issued by one of the big German health insurers reported findings from a representative population survey (5017 people, aged 20–50 years, Kordt, 2015): 6.7% of the respondents reported the life-time use of pharmacological neuroenhancement (previous report (2009): 4.7%), of which 3.3% reported improving work-related performance as their motivation and 4.7% aimed at improving mood and anxiety. However, the authors estimate the true use to probably be considerably higher than reported and may be as high as 12%, but underreported due to response bias. The estimated 1 year prevalence was 5.8% with 63% of users reporting regular, more than once a month intake. Work environments with high prevalence were characterized by high pressure (serious consequences due to small mistakes), a requirement not to show emotions and working at the limit of capabilities. In summary, this report showed an increasing frequency of the use of pharmacological substances with the aim of improving work-related performance, which amounts to up to 5 million people in Germany (population: 82.6 million). In parallel, prescription rates of stimulants (including methylphenidate, dexamfetamine, atomoxetine, and modafinil) in England (population 54 million) have been rising steadily from about 220.000 in 1998 to 460.000 in 2004 to 1.160.000 in 2014 (The Health Social Care Information Centre, 2015), corresponding to an increase of 150% (methylphenidate alone increased from 360.000 to 922.000, i.e. + 156%), of which modafinil (+ 212%) and atomoxetine (+ 668%) showed the strongest proportional increases. For modafinil, there are estimates of around 90% ‘off-label’ use (Vastag, 2004). According to a UK report from the Academy of Medial Sciences, a small percentage increase in cognitive performance can be associated with significant improvements in functional outcome in school, university or in a workplace environment (Academy of Medical Sciences, 2012). This might be the reason for Duke University’s prohibition of the use of prescription drugs by students without authorized prescription (Duke University, 2014), classifying it as ‘cheating’ in the category ‘academic dishonesty’. Healthy people are also using drugs to enhance creativity at work. An increasingly popular phenomenon reported in the media is ‘microdosing’ – taking minute quantities of psychedelic drugs such as d-lysergic acid diethylamide (LSD), psilocybin, or mescaline every few days to enhance cognitive function, perception and creativity (https://theconversation.com/lsd-microdosing-is-trending-in-silicon-valley-but-can-it-actually-make-you-more-creative-72747). Anecdotal evidence suggests that under pressure to perform, professionals are microdosing psychedelics to enhance performance at work, gain a competitive advantage, stay focused, and manage stress. Some find that microdosing psychedelics alongside certain prescribed medications, such as stimulants for attention deficit hyperactivity disorder (ADHD), has allowed them to reduce the dose and associated unpleasant side effects of their prescribed medications. Other people report general positive health effects, such as managing anxiety, sleeping better, eating more healthily, and exercising more (Sahakian et al., 2017). However, without any laboratory tests into the effects of microdosing as of yet, the evidence is purely anecdotal and the effects – short and long term – remain unknown. Furthermore, since purchasing LSD is illegal, determining what is being purchased and at what dose is problematic. Those who microdose incorrectly risk having unwanted, full-blown trips or even experience unpleasant trips. There are some reports of psychosis-like symptoms in certain vulnerable individuals who use LSD recreationally. The use of cognition-enhancing drugs by healthy people also raises safety concerns, given that users often report purchasing drugs from the Internet. This is alarming because manufacture and supply may not be subject to the same regulatory controls and some of the smart drugs advertised over the Internet have not been tested in humans.
When considering the ethical aspects of neuroenhancing interventions, there are a couple of important questions:
First, we need to know whether and to what extent these interventions work, and what their side effects may be, that is, the question of safety and efficacy or benefit versus risk. This question is not only of individual and societal interest, but also has particular implications for regulatory and policy making decisions. Until now, the evidence for the benefits of cognition-enhancing interventions is mixed, with many studies having relatively small sample sizes and results showing different effect sizes across cognitive domains (e.g. meta-analysis on modafinil by Battleday and Brem (2015), on methylphenidate by Ilieva et al. (2015) and Linssen et al. (2014)). Furthermore, the tests used in many of the studies are not designed and validated to measure differences in healthy subjects but instead are designed to measure deficits in patients with neuropsychiatric disorders compared to a normal population. Therefore, some of the studies suffer from ceiling effects and might underestimate the effects of cognitive enhancers. Furthermore, there are no studies investigating the long-term effects of cognitive enhancers in healthy people, where effects such as habituation or even a potentiation of the beneficial effects, as well as different side effects (mental, somatic), compared to a single dose could be detected. Surveys or other long-term follow-up observational studies, similar to the monitoring of side effects in place for pharmacological agents used as treatments, would be suited to assess long-term side effects. In addition to pure cognitive effects, studies should also investigate affective, motivational, and social–cognitive processes such as the greater task-related motivation reported with modafinil (Muller et al., 2013). The same holds true for the electrical stimulation methods such as transcranial direct current stimulation (tDCS), in which there is uncertainty about the mechanisms and effects on cognition (e.g. Coffman et al., 2014; Parkin et al., 2015). Although there are some positive reports and even higher hopes (Coffman et al., 2014; Shin et al., 2015), there are also very critical reports showing, if any, only a weak effect in healthy people (Parkin et al. (2015), potential negative effect (Steenbergen et al., 2016), and meta-analysis (Horvath et al., 2015), but see criticism in Price et al. (2015).
A particular safety concern is the use of cognitive enhancers in healthy children and adolescents, whose brains are still in development. Improving cognitive abilities could help to reduce the impact of, for instance, poverty or low socioeconomic status or other adverse childhood events on the development of cognition and academic achievement (Dadvand et al., 2015; Hackman et al., 2010, 2014), which would in turn enable children subjected to these influences to buffer them or to compensate for them, thus allowing for a better educational and social outcome at school. Furthermore, cognitive improvements in childhood could increase the educational outcome and improve socioeconomic situations later in life as a small percentage increment in performance can considerably improve functional outcome, such as a better grade at school (Academy of Medical Sciences, 2012). Due to the higher plasticity of the child and adolescent brain, any cognitive-enhancing interventions can be expected to have stronger effects than in adult brains, as seen in the beneficial effects of enriched environments and exercise (Hamilton et al., 2014). From an ethical perspective, however, this higher plasticity also reflects a higher vulnerability to potential side effects, particularly as there is much less research on cognitive enhancement in healthy children (but cf. experience in ADHD, e.g., Coghill et al. (2014), discussion about side effects by Ptacek et al. (2014)). For instance, methylphenidate treatment in children with ADHD may negatively impact on growth in height (Graham et al., 2011). Other even more relevant ethical considerations include the limited capacity to consent, the developing autonomy and personhood in children (Graf et al., 2013), and the potential for even higher peer, academic and parental pressure in childhood and adolescence. In this situation, parents can have multiple roles, such as the child’s advocate and surrogate decision-maker, the overprotective parent with a tendency to medicalize deviations from a putative ‘norm’, but also the child’s academic driver, setting high expectations and defining standards (Graf et al., 2013). Due to their still developing autonomy and capacity to understand and weigh risks and benefits, children are typically more sensitive to implicit and explicit coercion such as peer pressure and competition, as well as due to parental expectations (Greely et al., 2008; O’Leary, 1993). In reality, however, the prevalence of the use of neuroenhancing drugs in adolescents (grade 8–12) is rather similar to the prevalence in adults (5%–10% use in the last 12 months), with sources mainly consisting of friends and relatives (Johnston et al., 2015). Nonetheless, research should be done very cautiously, and regulatory authorities should protect children and adolescents from interventions to which they cannot legally consent and whose consequences no one can accurately estimate without sufficient scientific evidence.
Yet another area of neuroenhancement is the improvement of mood and affect on one hand Schermer, 2015) and of social(-cognitive) skills on the other hand (Patin and Hurlemann, 2015). Both consist of areas in which the borderline between treatment of possibly unrecognized problems and enhancement of healthy and well-functioning people might be more blurred than in the field of cognition (Farah, 2012). The ability to change one’s feelings and moral and social behaviour might sound even more profound and intrusive than improving one’s memory or being more attentive for longer periods. The reason for this is that mood, affect, and social-cognitive skills are typically understood as components defining a person’s identity and authenticity (Schermer, 2015). However, the reality of what is currently possible and what enhancers such as antidepressants or drugs such as the so-called trust-hormone oxytocin can actually do is much less colourful and clear. In healthy people, a single application of oxytocin not only improves the recognition of happy faces but also that of fearful and angry faces (Shahrestani et al., 2013). Moreover, in addition to increasing pro-social behaviour, oxytocin can also increase aggressive behaviour (Mitchell et al., 2015). In the area of antidepressants, there was initially historical hope of a ‘happiness pill’, but in general, the mood-enhancing effects of chronic antidepressant intake in healthy people are at best weak, although there are relatively few studies investigating this. The best evidence shows a possible effect in decreasing negative affect but not clearly increasing positive affect (De Jongh et al., 2008; Knorr and Kessing, 2010; Repantis et al., 2009). All these meta-analyses and reviews, however, support the general safety of these drugs with respect to side effects and adverse events. But overall, the evidence in this field is much weaker than in the field of cognitive enhancement, emphasizing the need for more well-designed studies to enable an evaluation of the use of pharmaceutical enhancers of mood and affect regarding safety, efficacy, and general ethical, moral, and societal questions.
Finally, one general ethical question relates to the definition of neuroenhancement and whether it differs to treatment in a medical sense. This definitional aspect has multiple implications (e.g. regulatory, policy, funding). In the case of neuropsychiatric disorders, it is generally accepted to use drugs or other interventions in order to cure or ameliorate symptoms, such as cognitive deficits, to enable affected people, which are usually defined as patients (as they fulfil or fulfilled criteria for a medically defined diagnosis) to participate in society, for example, education or work. Enhancement, however, is usually understood as aimed at improving cognition in otherwise healthy people. The definition of improvement is in this case already a rather difficult one, because of the problem of defining normal compared to the usually better accepted definition of deficit in neuropsychiatric disorders (although this definition usually relies on a statistically defined normal performance, termed norms). These two definitions are often not clearly differentiated. Returning to the definition of neuroenhancement: At present, it is not clear how and by how much neuroenhancing interventions can improve cognition in relation to an individual’s normal or optimal performance. When is a generally healthy person performing at their best? From an empirical perspective, there are a couple of general ‘lifestyle’ methods that can contribute to reaching optimal cognitive performance by improving general wellbeing, such as sufficient and good quality sleep, physical activity, healthy lifestyle and nutrition, positive social interaction and enrichment (Kent et al., 2015; Llewellyn et al., 2008; The Government Office for Science, 2008). Cognitive-enhancing drugs, particularly modafinil, tend to show stronger and more robust effects when they are used to mitigate negative effects such as sleep deprivation (e.g. Flindall et al., 2016; Gurtman et al., 2008; Killgore et al., 2009; Sugden et al., 2011, review in Repantis et al., 2010) or when they are used to maintain performance for longer than normal duration in suboptimal circumstances or in participants with lower baseline performance (e.g. Agay et al., 2014). The effect size in healthy people who are not sleep-deprived is moderate (Nicholson et al., 2015) and indeed task-related motivation can be improved. Besides these questions about the safety and efficacy of neuroenhancers and other methods to restore and improve cognitive performance, an ethically more relevant question relates to when it would be ethically acceptable to use neuroenhancers: For instance, to overcome tiredness or jet-lag or to be able to perform well at the end of a night shift, so to restore or maintain his or her ‘normal’ performance? However, might someone in an environment where a single error could have serious consequences such as the death of people be asked to or feel compelled to exhaust all available options to prevent such errors? Could employers or regulators offer cognitive enhancers instead of improving working conditions to mitigate the effects of stress or long hours at work? What if someone uses cognitive enhancers to get an advantage over a competitor, for instance, in a test at school or university? There are situations in which a single exam result could potentially have important consequences for one’s future career options, which could make the use of neuroenhancers a rather tempting choice. How does this compare to the use of performance-enhancing substances in sport, which is usually referred to as doping and is prohibited (see above, Duke University’s regulation on this issue)? How could the use of such methods change the competition and the expected performance levels in such tests? In many test situations, it is not only the absolute performance but also the comparison to class mates, which are both similarly relevant as outcome measures. The use of cognitive enhancers could then shift the whole class to higher levels of performance, making it rather normal to use them and ultimately have potentially coercive effects due to peer pressure.
However, what is the difference between cognitive-enhancing drugs compared with tutoring, computer- or app-based training or other non-pharmacological training methods? From a cognitive perspective, the effect size of training can be comparable to pharmacological enhancers (Anguera and Gazzaley, 2015; Green and Bavelier, 2015), although there could be a trade-off between extended times spent in front of a computer and less time spent doing physical exercise and social interactions. Intuitively, one might say that compared to training, pharmacological enhancers count as ‘cheating’. This might be due to the wider availability and the concept that achieving something by training is considered deserved and therefore more authentic than the intake of a drug, which is perceived as easier and therefore cheating (Buyx, 2015). However, one could also argue that the availability of training, tutoring, high-quality and well-balanced nutrition, and other cultural and social enrichment (or the lack thereof) is one of the main reasons for the well-known cognitive and emotional developmental deficits due to low socioeconomic status (Hackman et al., 2010, 2015; Larson et al., 2015; Noble et al., 2015). Although these factors are often seen as circumstances, rather than as interventions, the effects are widespread, affecting cognition, mental health, brain development, and academic achievement (Hackman et al., 2010). So, from an empirical descriptive perspective, one could say that low and negative socioeconomic circumstances are toxic for cognitive development or performance and that the beneficial effects of high socioeconomic status are comparable to neuroenhancement (see also the extensive literature of the effects of enrichment on cognition (Clemenson et al., 2015; Kelly, 2015) and brain volume in mice (Garthe et al., 2016) and humans (Erickson et al., 2011; however, see also Young et al., 2015). This thought experiment (supported by neuroscientific evidence) is considered here to stimulate the discussion about ethical issues in cognitive enhancement which sometimes seem to make categorical differentiations between interventions that are seen as ‘artificial’ and unauthentic and interventions which might not even be considered cognitive enhancing, but do have these effects. From a public health or preventive medicine perspective, one could also consider cognitive enhancers as empowering people to perform well, particularly in stressful environments, and therefore protect them against the negative effects of stress on mental and physical health. However, a very liberal use of these methods could increase expectations and demands and as a consequence increase stress and the implicit or even explicit need for cognitive enhancement, resulting in a spiral of performance enhancement and demands and an exploitation by employers. In comparison, the improvement or enhancement achieved with training methods such as brain training apps might be more sustainable, more balanced, and therefore potentially less prone to spiralling out of control as they are more influenced by individual motivation and time spent training as well as a general healthy and balanced lifestyle.
What can and should neuroscience contribute to the discussion about the neuroethics of cognitive enhancement?
Neuroscience should deliver the scientific evidence in regard to effects and side effects of interventions aimed at enhancing cognition by rigorously testing them. This also includes the development of tasks suitable to detect even small changes in normal to high performing healthy individuals as well as of methods to measure the transfer of learned skills into other domains as well as effects of enhancement on everyday functioning. Tests of ‘cold’ and ‘hot’ cognition as well as creativity and motivation (Roiser and Sahakian, 2013; Sahakian and Labuzetta, 2013) should be assessed (see Figures 1 and 2). This rigorous research should then result in balanced evidence-based information regarding the safety and efficacy of a given intervention, which then informs potential ‘users’, the media, policy makers, and regulatory authorities. One requirement for this is that neuroscientists have access to substances and other methods aimed at enhancing cognition and are able to test their effects without facing restrictive regulatory hurdles, while keeping the ethics of human research in mind. Furthermore, it is important to assess whether drugs such as modafinil with relatively low side effects and no demonstrable abuse potential are safe and effective for healthy people to use in the long term. Neuroscientists should, however, also acknowledge the limitations of knowledge and methods and proceed with caution in regard to neuroenhancement methods used, particularly in the healthy. From this perspective, neuroscience should engage in ethical and societal discussions, with an overall focus on benefitting society, reducing harm, and unwanted consequences in order to help society flourish and individuals realize their potential.
Figure 1.

Normal ‘cold’ cognition in non-depressed individuals. ‘Cold’ (emotion-independent) cognition is instantiated via a complex set of circuits, including interactions (blue arrows) between the dorsolateral prefrontal cortex (DLPFC), the dorsal anterior cingulate cortex (ACC), and the hippocampus (H). Limbic structures connected to DLPFC, ACC, and H, such as the amygdala (A), the nucleus accumbens (NAcc), and the subgenual portion of the ACC (sgACC) may also be activated during cold cognition, but are more strongly engaged during ‘hot’ (emotion-laden) cognition (Figure 2). Monoamine neurotransmitter (NT) projections (purple arrows) emanating from the brainstem, including serotonin (5-HT), norepinephrine (NE), and dopamine (DA), may influence cold cognition via modulatory actions in cortical and subcortical regions. Note that most circuit nodes and connections are excluded in this and later figures for clarity, and that some connections may be indirect.
Figure 2.
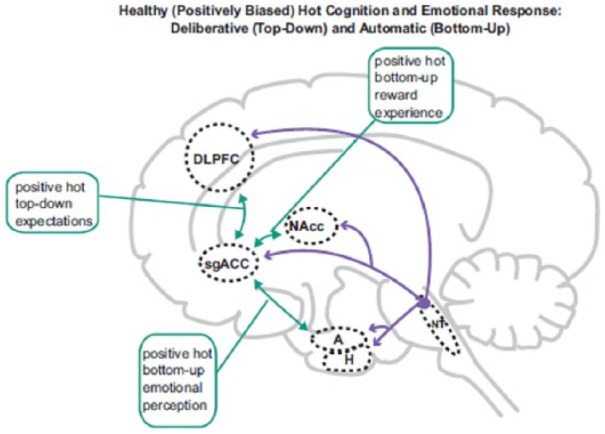
Normal positively biased ‘hot’ (emotion-laden) cognition in non-depressed individuals. Hot cognition is instantiated by circuits including interactions (green arrows) between limbic regions including A, NAcc, and sgACC, the activity which is profoundly modulated by 5-HT, NE, and DA. These regions share reciprocal connections with DLPFC, ACC, and H, and consequently hot and ‘cold’ (emotion-independent) cognition necessarily interact (for example, motivation alters ostensibly cold cognitive test performance). Non-depressed individuals exhibit positive (green arrows) bottom-up (perceptions/experience) and top-down (expectation) biases, providing resilience to adverse events. Abbreviations and indications to colour as in Figure 1.
Acknowledgments
We thank Alicja Malinowska for editorial assistance. Figures 1 and 2 from Roiser and Sahakian (2013) ©Cambridge University Press. The authors gratefully acknowledge the assistance of Stephen Stahl and Nancy Muntner in preparing these figures.
Footnotes
Declaration of conflicting interests: Barbara J Sahakian consults for Cambridge Cognition and PEAK.
Funding: This work was supported by the NIHR Cambridge Biodmedical Research Centre (BRC) (Mental Health Theme and Neurodegeneration Theme) and the NIHR MedTech and in vitro Diagnostic Co-operative. Annette Bruhl was funded by grants from Eton College and The Wallitt Foundation.
References
- Academy of Medical Sciences (2012) Human enhancement and the future of work. Report from a joint workshop hosted by the Academy of Medical Sciences, The British Academy, The Royal Academy of Engineering and The Royal Society London: Academy of Medical Sciences. [Google Scholar]
- Agay N, Yechiam E, Carmel Z, et al. (2014) Methylphenidate enhances cognitive performance in adults with poor baseline capacities regardless of attention-deficit/hyperactivity disorder diagnosis. Journal of Clinical Psychopharmacology 34(2): 261–265. [DOI] [PubMed] [Google Scholar]
- Alsever J. (2015) Can electric ‘brain training’ devices make you smarter? Fortune. Available at: http://fortune.com/2015/11/17/electric-brain-training-devices-cognitive-enhancement/
- Angrist B, Sudilovsky A. (1978) Central nervous system stimulants: Historical aspects and clinical effects. In: Iversen LL, Iversen SD, Snyder SH. (eds) Stimulants. Boston, MA: Springer US, pp. 99–165. [Google Scholar]
- Anguera JA, Gazzaley A. (2015) Video games, cognitive exercises, and the enhancement of cognitive abilities. Current Opinion in Behavioral Sciences 4: 160–165. [Google Scholar]
- Badiani A. (2015) History of psychopharmacology. In: Stolerman IP, Price LH. (eds) Encyclopedia of Psychopharmacology. Berlin; Heidelberg: Springer, pp. 752–764. [Google Scholar]
- Battleday RM, Brem AK. (2015) Modafinil for cognitive neuroenhancement in healthy non-sleep-deprived subjects: A systematic review. European Neuropsychopharmacology 25(11): 1865–1881. [DOI] [PubMed] [Google Scholar]
- Bell SK, Lucke JC, Hall WD. (2012) Lessons for enhancement from the history of cocaine and amphetamine use. AJOB Neuroscience 3(2): 24–29. [Google Scholar]
- Bower EA, Phelan JR. (2003) Use of amphetamines in the military environment. The Lancet 362(Suppl.): s18–s19. [DOI] [PubMed] [Google Scholar]
- Buyx A. (2015) Smart drugs: Ethical issues. In: Clausen J, Levy N. (eds) Handbook of Neuroethics. Dordrecht: Springer Netherlands, pp. 1191–1206. [Google Scholar]
- Carroll E. (1977) Coca: The plant and its use. Cocaine 13: 35–45. [PubMed] [Google Scholar]
- Clemenson GD, Deng W, Gage FH. (2015) Environmental enrichment and neurogenesis: From mice to humans. Current Opinion in Behavioral Sciences 4: 56–62. [Google Scholar]
- Coffman BA, Clark VP, Parasuraman R. (2014) Battery powered thought: Enhancement of attention, learning, and memory in healthy adults using transcranial direct current stimulation. Neuroimage 85(Pt 3): 895–908. [DOI] [PubMed] [Google Scholar]
- Coghill DR, Seth S, Pedroso S, et al. (2014) Effects of methylphenidate on cognitive functions in children and adolescents with attention-deficit/hyperactivity disorder: Evidence from a systematic review and a meta-analysis. Biological Psychiatry 76(8): 603–615. [DOI] [PubMed] [Google Scholar]
- Dadvand P, Nieuwenhuijsen MJ, Esnaola M, et al. (2015) Green spaces and cognitive development in primary schoolchildren. Proceedings of the National Academy of Sciences 112(26): 7937–7942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jongh R, Bolt I, Schermer M, et al. (2008) Botox for the brain: Enhancement of cognition, mood and pro-social behavior and blunting of unwanted memories. Neuroscience & Biobehavioral Reviews 32(4): 760–776. [DOI] [PubMed] [Google Scholar]
- Dietz P, Striegel H, Franke AG, et al. (2013) Randomized response estimates for the 12-month prevalence of cognitive-enhancing drug use in university students. Pharmacotherapy 33(1): 44–50. [DOI] [PubMed] [Google Scholar]
- Drachman DA, Sahakian BJ. (1980) Memory and cognitive function in the elderly: A preliminary trial of physostigmine. Archives of Neurology 37(10): 674–675. [DOI] [PubMed] [Google Scholar]
- Drug Abuse Control Amendments (1965) https://catalog.archives.gov/id/299906 (accessed 20 November 2018).
- Duke University (2014) Policy on academic dishonesty. Available at: http://studentaffairs.duke.edu/conduct/z-policies/academic-dishonesty
- Eagger SA, Levy R, Sahakian BJ. (1991) Tacrine in Alzheimer’s disease. The Lancet 337(8748): 989–992. [DOI] [PubMed] [Google Scholar]
- Emanuel RM, Frellsen SL, Kashima KJSM, et al. (2013) Cognitive enhancement drug use among future physicians: Findings from a multi-institutional census of medical students. Journal of General Internal Medicine 28(8): 1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, et al. (2011) Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America 108(7): 3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah MJ. (2012) Neuroethics: The ethical, legal, and societal impact of neuroscience. Annual Review of Psychology 63: 571–591. [DOI] [PubMed] [Google Scholar]
- Flindall I, Leff DR, Goodship J, et al. (2016) Structured cues or modafinil for fatigue amelioration in clinicians? A double-blind, randomized controlled trial of critical clinical information recall in fatigued clinicians. Surgery 159(4): 1181–1190. [DOI] [PubMed] [Google Scholar]
- Fond G, Micoulaud-Franchi J-A, Brunel L, et al. (2015) Innovative mechanisms of action for pharmaceutical cognitive enhancement: A systematic review. Psychiatry Research 229(1–2): 12–20. [DOI] [PubMed] [Google Scholar]
- Freud S. (1885) Über coca. Centralblatt für die ges. therapie 2: 289–314. [Google Scholar]
- Garthe A, Roeder I, Kempermann G. (2016) Mice in an enriched environment learn more flexibly because of adult hippocampal neurogenesis. Hippocampus 26(2): 261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RA, DesLauriers C, Burda A, et al. (2009) Cocaine: History, social implications, and toxicity: A review. Seminars in Diagnostic Pathology 26(1): 10–17. [DOI] [PubMed] [Google Scholar]
- Gordijn B. (2015) Neuroenhancement. In: Clausen J, Levy N. (eds) Handbook of Neuroethics. Dordrecht: Springer Netherlands, pp. 1169–1175. [Google Scholar]
- Graf WD, Nagel SK, Epstein LG, et al. (2013) Pediatric neuroenhancement: Ethical, legal, social, and neurodevelopmental implications. Neurology 80(13): 1251–1260. [DOI] [PubMed] [Google Scholar]
- Graham J, Banaschewski T, Buitelaar J, et al. (2011) European guidelines on managing adverse effects of medication for ADHD. European Child & Adolescent Psychiatry 20(1): 17–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greely H, Sahakian B, Harris J, et al. (2008) Towards responsible use of cognitive-enhancing drugs by the healthy. Nature 456(7223): 702–705. [DOI] [PubMed] [Google Scholar]
- Green CS, Bavelier D. (2015) Action video game training for cognitive enhancement. Current Opinion in Behavioral Sciences 4: 103–108. [Google Scholar]
- Grinspoon L, Bakalar JB. (1981) Coca and cocaine as medicines: An historical review. Journal of Ethnopharmacology 3(2–3): 149–159. [DOI] [PubMed] [Google Scholar]
- Gurtman CG, Broadbear JH, Redman JR. (2008) Effects of modafinil on simulator driving and self-assessment of driving following sleep deprivation. Human Psychopharmacology: Clinical and Experimental 23(8): 681–692. [DOI] [PubMed] [Google Scholar]
- Hackman DA, Betancourt LM, Gallop R, et al. (2014) Mapping the trajectory of socioeconomic disparity in working memory: Parental and neighborhood factors. Child Development 85(4): 1433–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ, Meaney MJ. (2010) Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nature Reviews Neuroscience 11(9): 651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Gallop R, Evans GW, et al. (2015) Socioeconomic status and executive function: Developmental trajectories and mediation. Developmental Science 18(5): 686–702. [DOI] [PubMed] [Google Scholar]
- Hamilton GF, Jablonski SA, Schiffino FL, et al. (2014) Exercise and environment as an intervention for neonatal alcohol effects on hippocampal adult neurogenesis and learning. Neuroscience 265: 274–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath JC, Forte JD, Carter O. (2015) Quantitative review finds no evidence of cognitive effects in healthy populations from single-session transcranial Direct Current Stimulation (tDCS). Brain Stimulation 8(3): 535–550. [DOI] [PubMed] [Google Scholar]
- Ilieva IP, Hook CJ, Farah MJ. (2015) Prescription stimulants’ effects on healthy inhibitory control, working memory, and episodic memory: A Meta-analysis. Journal of Cognitive Neuroscience 27(6): 1069–1089. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, et al. (2015) Monitoring the Future National Survey Results on Drug Use: 1975–2014: Overview, Key Findings on Adolescent Drug Use. Ann Arbor, MI: Institute for Social Research, The University of Michigan. [Google Scholar]
- Kelly AM. (2015) Non-pharmacological approaches to cognitive enhancement. Handbook of Experimental Pharmacology 228: 417–439. [DOI] [PubMed] [Google Scholar]
- Kent BA, Oomen CA, Bekinschtein P, et al. (2015) Cognitive enhancing effects of voluntary exercise, caloric restriction and environmental enrichment: A role for adult hippocampal neurogenesis and pattern separation? Current Opinion in Behavioral Sciences 4: 179–185. [Google Scholar]
- Killgore WD, Kahn-Greene ET, Grugle NL, et al. (2009) Sustaining executive functions during sleep deprivation: A comparison of caffeine, dextroamphetamine, and modafinil. Sleep 32(2): 205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knorr U, Kessing LV. (2010) The effect of selective serotonin reuptake inhibitors in healthy subjects: A systematic review. Nordic Journal of Psychiatry 64(3): 153–163. [DOI] [PubMed] [Google Scholar]
- Kordt M. (2015) DAK-Gesundheitsreport 2015. Available at: http://www.dak.de/dak/download/Vollstaendiger_bundesweiter_Gesundheitsreport_2015–1585948.pdf (accessed 29 September 2015).
- Larson K, Russ SA, Nelson BB, et al. (2015) Cognitive ability at kindergarten entry and socioeconomic status. Pediatrics 135(2): e440–e448. [DOI] [PubMed] [Google Scholar]
- Liakoni E, Schaub MP, Maier LJ, et al. (2015) The use of prescription drugs, recreational drugs, and ‘soft enhancers’ for cognitive enhancement among Swiss secondary school students. PLoS ONE 10(10): e0141289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman HR. (2003) Nutrition, brain function and cognitive performance. Appetite 40(3): 245–254. [DOI] [PubMed] [Google Scholar]
- Linssen AM, Sambeth A, Vuurman EF, et al. (2014) Cognitive effects of methylphenidate in healthy volunteers: A review of single dose studies. International Journal of Neuropsychopharmacology 17(6): 961–977. [DOI] [PubMed] [Google Scholar]
- Llewellyn DJ, Lang IA, Langa KM, et al. (2008) Cognitive function and psychological well-being: Findings from a population-based cohort. Age and Ageing 37(6): 685–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low KG, Gendaszek AE. (2002) Illicit use of psychostimulants among college students: A preliminary study. Psychology, Health & Medicine 7(3): 283–287. [Google Scholar]
- Maier LJ, Ferris JA, Winstock AR. (2018) Pharmacological cognitive enhancement among non-ADHD individuals – A cross-sectional study in 15 countries. International Journal of Drug Policy 58: 104–112. [DOI] [PubMed] [Google Scholar]
- Maier LJ, Liakoni E, Schildmann J, et al. (2015. a) Swiss university students’ attitudes toward pharmacological cognitive enhancement. PLoS ONE 10(12): e0144402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier LJ, Liechti ME, Herzig F, et al. (2013) To dope or not to dope: Neuroenhancement with prescription drugs and drugs of abuse among Swiss university students. PLoS ONE 8(11): e77967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier LJ, Wunderli MD, Vonmoos M, et al. (2015. b) Pharmacological cognitive enhancement in healthy individuals: A compensation for cognitive deficits or a question of personality? PLoS ONE 10(6): e0129805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misuse of Drugs Act (1971) https://www.legislation.gov.uk/ukpga/1971/38/contents (accessed 20 November 2018).
- Mitchell IJ, Gillespie SM, Abu-Akel A. (2015) Similar effects of intranasal oxytocin administration and acute alcohol consumption on socio-cognitions, emotions and behaviour: Implications for the mechanisms of action. Neuroscience & Biobehavioral Reviews 55: 98–106. [DOI] [PubMed] [Google Scholar]
- Muller U, Rowe JB, Rittman T, et al. (2013) Effects of modafinil on non-verbal cognition, task enjoyment and creative thinking in healthy volunteers. Neuropharmacology 64: 490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramoto O, Sugishita M, Sugita H, et al. (1979) Effect of physostigmine on constructional and memory tasks in Alzheimer’s disease. Archives of Neurology 36(8): 501–503. [DOI] [PubMed] [Google Scholar]
- Musto DF. (1989) America’s first cocaine epidemic. The Wilson Quarterly (1976-) 13(3): 59–64. [PubMed] [Google Scholar]
- Nicholson PJ, Mayho G, Sharp C. (2015) Cognitive Enhancing Drugs and the Workplace. London: BMA. [Google Scholar]
- Noble KG, Engelhardt LE, Brito NH, et al. (2015) Socioeconomic disparities in neurocognitive development in the first two years of life. Developmental Psychobiology 57(5): 535–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary JC. (1993) An analysis of the legal issue surrounding the forced use of Ritalin: Protecting a child’s right to ‘just say no’. New England Law Review 27(4): 1173–1209. [PubMed] [Google Scholar]
- Parkin BL, Ekhtiari H, Walsh VF. (2015) Non-invasive human brain stimulation in cognitive neuroscience: A primer. Neuron 87(5): 932–945. [DOI] [PubMed] [Google Scholar]
- Patin A, Hurlemann R. (2015) Social cognition. In: Kantak KM, Wettstein JG. (eds) Cognitive Enhancement, vol. 228 Cham: Springer International Publishing, pp. 271–303. [Google Scholar]
- Petersen RC, Stillman RC. (1977) Cocaine, vol. 77, no. 471. Rockville, Maryland: Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration. [Google Scholar]
- Price AR, McAdams H, Grossman M, et al. (2015) A meta-analysis of transcranial direct current stimulation studies examining the reliability of effects on language measures. Brain Stimulation 8(6): 1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptacek R, Kuzelova H, Stefano GB, et al. (2014) ADHD and growth: Questions still unanswered. Neuroendocrinology Letters 35(1): 1–6. [PubMed] [Google Scholar]
- PUBLIC LAW 91-513-OCT. 27,1970; https://www.gpo.gov/fdsys/pkg/STATUTE-84/pdf/STATUTE-84-Pg1236.pdf (accessed 20 November 2018).
- Rägo L, Santoso B. (2008) Drug regulation: History, present and future. In: van Boxtel CJ, Santosa B, Edwads IR. (eds) Drug Benefits and Risks: International Textbook of Clinical Pharmacology (revised 2nd edn). Amsterdam: IOS Press, pp. 65–78. [Google Scholar]
- Rasmussen N. (2008) On Speed: The Many Lives of Amphetamine. New York: New York University Press. [Google Scholar]
- Rasmussen N. (2011) Medical science and the military: The Allies’ use of amphetamine during World War II. Journal of Interdisciplinary History 42(2): 205–233. [DOI] [PubMed] [Google Scholar]
- Repantis D, Schlattmann P, Laisney O, et al. (2009) Antidepressants for neuroenhancement in healthy individuals: A systematic review. Poiesis & Praxis 6(3–4): 139–174. [Google Scholar]
- Repantis D, Schlattmann P, Laisney O, et al. (2010) Modafinil and methylphenidate for neuroenhancement in healthy individuals: A systematic review. Pharmacological Research 62(3): 187–206. [DOI] [PubMed] [Google Scholar]
- Roiser J, Sahakian BJ. (2013) Hot and cold cognition in depression. CNS Spectrums 18(3): 139–149. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Labuzetta JN. (2013) Bad Moves: How Decision Making Goes Wrong and the Ethics of Smart Drugs. Oxford, UK: Oxford University Press. [Google Scholar]
- Sahakian BJ, Brühl AB, Cook J, et al. (2015) The impact of neuroscience on society: Cognitive enhancement in neuropsychiatric disorders and in healthy people. Philosophical Transactions of the Royal Society B: Biological Sciences 370(1677): 20140214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahakian, et al. (2017). https://theconversation.com/lsd-microdosing-is-trending-in-silicon-valley-but-can-it-actually-make-you-more-creative-72747 (accessed 20 November 2018).
- Sample I. (2014) Electrical brain stimulation beats caffeine – And the effect lasts longer. The Guardian. Available at: https://www.theguardian.com/science/2014/nov/19/electrical-brain-stimulation-caffeine
- Schermer M. (2015) Ethics of pharmacological mood enhancement. In: Clausen J, Levy N. (eds) Handbook of Neuroethics. Dordrecht: Springer Netherlands, pp. 1177–1190. [Google Scholar]
- Shahrestani S, Kemp AH, Guastella AJ. (2013) The impact of a single administration of intranasal oxytocin on the recognition of basic emotions in humans: A meta-analysis. Neuropsychopharmacology 38(10): 1929–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y-I, Foerster Ã, Nitsche MA. (2015) Transcranial direct current stimulation (tDCS) – Application in neuropsychology. Neuropsychologia 69: 154–175. [DOI] [PubMed] [Google Scholar]
- Singh I, Bard I, Jackson J. (2014) Robust resilience and substantial interest: A survey of pharmacological cognitive enhancement among university students in the UK and Ireland. PLoS ONE 9(10): e105969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ME, Farah MJ. (2011) Are prescription stimulants ‘smart pills’? The epidemiology and cognitive neuroscience of prescription stimulant use by normal healthy individuals. Psychological Bulletin 137(5): 717–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbergen L, Sellaro R, Hommel B, et al. (2016) ‘Unfocus’ on foc.us: Commercial tDCS headset impairs working memory. Experimental Brain Research 234(3): 637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden C, Housden CR, Aggarwal R, et al. (2011) Effect of pharmacological enhancement on the cognitive and clinical psychomotor performance of sleep-deprived doctors: A randomized controlled trial. Ann Surg 255(2): 222–227. [DOI] [PubMed] [Google Scholar]
- The Government Office for Science (2008) Foresight Mental Capital and Wellbeing Project – Final Report. London: The Government Office for Science, p. 1. [Google Scholar]
- The Health Social Care Information Centre (2015) Prescriptions dispensed in the community, July 7 Available at: http://www.hscic.gov.uk/catalogue/PUB17644 (accessed 30 November 2015).
- Tracey I, Flower R. (2014) The warrior in the machine: Neuroscience goes to war. Nature Reviews Neuroscience 15(12): 825–834. [DOI] [PubMed] [Google Scholar]
- Vastag B. (2004) Poised to challenge need for sleep, ‘wakefulness enhancer’ rouses concerns. Journal of the American Medical Association 291(2): 167–170. [DOI] [PubMed] [Google Scholar]
- Webb JR, Valasek MA, North CS. (2013) Prevalence of stimulant use in a sample of US medical students. Annals of Clinical Psychiatry 25(1): 27–32. [PubMed] [Google Scholar]
- Young J, Angevaren M, Rusted J, et al. (2015) Aerobic exercise to improve cognitive function in older people without known cognitive impairment. The Cochrane Database of Systematic Reviews 2015(4): CD005381. [DOI] [PMC free article] [PubMed] [Google Scholar]


