Abstract
Drug discovery, particularly in the field of central nervous system, has had very limited success in the last few decades. A likely contributor is the poor translation between preclinical and clinical phases. The Research Domain Criteria of the National Institutes of Mental Health is a framework which aims to identify new ways of classifying mental illnesses that are based on observable behaviour and neurobiological measures, and to provide a guiding and evolving framework to improve the translation from preclinical to clinical research. At the core of the Research Domain Criteria approach is the assumption that the dimensional constructs described can be assessed across different units of analysis, thus enabling a more precise quantitative understanding of their neurobiological underpinnings, increasing the likelihood of identifying new and effective therapeutic approaches. In the present review, we discuss how the Research Domain Criteria can be applied to drug discovery with the domain Negative Valence, construct Potential Threat (‘Anxiety’) as an example. We will discuss the evidence supporting the utility of the Research Domain Criteria approach and evaluate how close we are to achieving a common thread of translational research from gene to self-report.
Keywords: Drug discovery, neuropsychiatry, research domain criteria, negative valence, anxiety, RDoC
Introduction
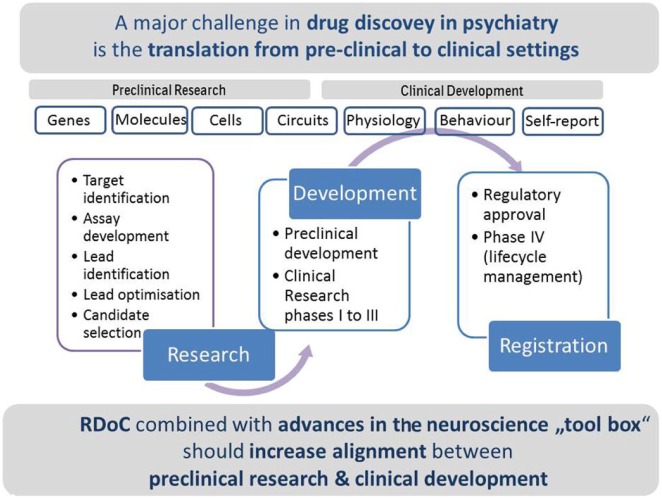
The traditional drug discovery process involves multiple stages and requires significant time and investment. High attrition rates are observed in all disease areas, but particularly so in the field of central nervous system (CNS) diseases (Gribkoff and Kaczmarek, 2017). Consequently, many pharmaceutical companies have moved out of CNS diseases research and development leaving a significant unmet medical need and associated burden on families and society in general (Murray et al., 2012; Roehrig, 2016). The reasons for the poor success have been the topic of many reviews over the last few years (Hyman, 2012; Kaitin and Di Masi, 2011; Paul et al., 2010); many opinions have been aired but with few easy answers. In CNS diseases more than any other area, the gap between preclinical tests and clinical settings appears to be particularly wide, mainly because of lacking bridging quantifiable biological paradigms (Figure 1).
Figure 1.
The traditional drug discovery process: a major challenge in drug discovery is an improvement in translation from preclinical research to clinical development. RDoC combined with advances in the neuroscience ‘tool box’ should increase alignment between preclinical research and clinical development.
The research domain criteria (RDoC) project was initiated by National Institutes of Mental Health (NIMH) in response to the 2008 NIMH Strategic Plans’ call for ‘new ways of classifying mental illnesses that are based on observable behaviour and neurobiological measures’; this, in an attempt to provide a guiding and evolving framework to improve the translation from preclinical to clinical research (Insel et al., 2010). RDoC postulates that psychiatric conditions are disorders of brain circuits, and it emphasises the study of neurobiological mechanisms that cut across psychiatric disorders as defined by current diagnostic classification systems (Morris and Cuthbert, 2012).
In contrast, the established Diagnostic and Statistical Manual of Mental Disorders (5th ed.; DSM-5) defines and classifies mental disorders based on observable symptoms, but fails to take into consideration the underlying neurobiology. Indeed, many of the symptoms described in DSM-5 are overlapping across diagnoses, and heterogeneity within any particular patient group is large. In drug discovery, a clear and precise understanding of the pathophysiology of disease is the starting point for any new therapeutic concept, which forms the basis for new research projects. The breakdown of a mental syndrome into smaller units – individual symptoms and even sub-symptoms – with an increased understanding of the underlying neurobiology at multiple levels of analysis will put decisions on project transitions on a new, data-driven level and ultimately lead to less late-stage attritions in the field of psychiatry. Furthermore, the improved ability to align the most appropriate drug with the individual needs of the patient towards a more personalised medicine approach will be made possible. Essential will be the technologies and methods that are available and sufficient to provide informative and meaningful data that should ultimately translate into the objective measurement of a clinically meaningful effect. Recent advances in technologies and methods available to neuroscientists have improved the feasibility of working in this field, and these combined with the RDoC approach might enable innovative ideas to be realised, thus making it an opportune time to be investing in this area. In this review, we focus on the RDoC domain Negative Valence and construct Potential Threat (‘Anxiety’) and discuss how data from different units of analysis can be integrated and combined in the context of drug discovery.
The RDoC framework
In its present form, the RDoC framework structures research around five major domains:
Negative valence systems: primarily responsible for responses to aversive situations such as fear, anxiety and loss.
Positive valence systems: primarily responsible for positive motivational situations or contexts such as reward-seeking, consummatory behaviour and reward/habit learning.
Cognitive systems: these include various mental processes relating to cognition such as attention, perception, declarative memory, language, cognitive control and working memory.
Systems for social processes: the mediators in interpersonal settings of various types including perception and interpretation of others’ actions.
Arousal and regulatory systems: these systems are responsible for generating activation of neural systems as appropriate for various contexts and providing appropriate homeostatic regulation of such systems as energy balance and sleep.
RDoC-based research on these systems and processes is organised around a dimensional approach incorporating different levels of analysis ranging from genes, molecules, cells, circuits, physiology, behaviour and finally self-report. By re-orienting research away from DSM-5 categories and towards a multimodal dimensional framework based on empirically validated constructs, the long-term goal is to develop a scientific base that can inform future neuroscience-based diagnostic systems for mental illness (Cuthbert, 2014).
The construct potential threat (‘anxiety’) of the RDoC negative valence domain is present as the primary disturbance in multiple DSM-5 categorised disorders including social and generalised anxiety disorders, phobia, panic, and post-traumatic stress disorder. It also presents as a comorbidity in other indications, for example, schizophrenia (Braga et al., 2004), major depressive disorder (Zbozinek et al., 2012), substance use disorders (Merikangas et al., 1998) and autism spectrum disorders (Bitsika et al., 2016; Zaboski and Storch, 2018). In the following section, we will discuss the literature that has investigated Negative Valence, Potential Threat (Anxiety) using the RDoC units of analysis.
Applying the RDoC units of analysis to drug discovery for negative valence, potential threat (‘anxiety’)
Genes
Genetic evidence for the involvement of a particular protein in a disease state can be a compelling starting point for drug discovery. Considerable evidence exists suggesting that anxiety and related disorders are moderately heritable and influenced by multiple genes, combined with environmental influences (McGregor et al., 2018; Meyer-Lindenberg and Weinberger, 2006). The genetics of negative valence system traits has been elegantly reviewed by Savage et al. (2017), where a large number of candidate genes have been described based on genetic epidemiological data (twin studies, heritability) and molecular genetic association findings. Some of the genes described are well known and have been replicated and studied already at multiple different levels of analysis, for example, FKBP5 (Zannas et al., 2016). For others, the association with neuropsychiatric disease is less clear but could provide a starting point for further exploration.
Gene expression and changes thereof can be measured in post-mortem tissue (Balestri et al., 2017), assuming sufficient quality, and can be used to build confidence in a novel drug target if it is expressed in brain nuclei and circuitry considered to be relevant to a particular psychiatric domain. Changes in gene expression are, however, often more difficult to interpret than expression per se since factors such as drug treatment history and duration of disease can be confounding. Nonetheless, confirmation that a particular gene is expressed in the human brain is essential for drug discovery projects and knowledge that the system is conserved across species is important for feasibility. In addition, the influence of gene variants on target expression can be evaluated in brain regions such as the anterior cingulate cortex (Sommer et al., 2010), a region which plays a key role in the limbic-cortical modulation of emotional behaviour (Drevets et al., 2008). A more recent study has investigated brain-specific gene co-expression in a brain region known to be involved in schizophrenia, that is, the dorsolateral prefrontal cortex, to explore how different genes are associated and thus to detect molecular pathways of risk genes. The authors describe the development a novel data-driven strategy for characterising clusters of strongly interacting genes, which have been linked to DRD2, a target gene for schizophrenia (Monaco et al., 2018). This approach could be applied to the evaluation of gene expression patterns in tissue from other psychiatric populations, including those with negative valence, and could be used as a starting point for biological validation of gene communities and further exploration of their relevance to disease. Exploration in preclinical species would also be feasible to further increase understanding of the associated molecular mechanisms and function, as well as exploration of the effect of particular challenge conditions (e.g. stress) on expression patterns.
Advances in gene editing technologies have led to increased speed and feasibility for exploring the effect of manipulation of genes in preclinical species (Jennings et al., 2016; Zhuo et al., 2017) and for exploring the functional consequences of genetic changes such as variations in copy number which are associated with numerous psychiatric disorders (Hiroi, 2018). The traditional gene knockout technology has contributed to the validation of novel genes such as transient receptor potential channels 4/5 (TRPC4/5) (Just et al., 2018; Riccio et al., 2014) as being important for negative valence. TRPC4/5 channels are non-selective cation channels which are expressed in the brain in areas involved in anxiety including the amygdala. In the study of Riccio et al. (2014), genetic ablation of TRPCs resulted in a reduced firing pattern of neurones of the lateral amygdala challenged with cholecystokinin tetrapeptide (CCK-4) and decreased anxiety-like behaviour in knockout mice. These findings have been confirmed pharmacologically by Just et al. (2018) following the systemic administration of the selective TRPC4/5 inhibitor HC-070, thus confirming the therapeutic potential of TRPC4/5 inhibitors for the treatment of anxiety-related disorders.
Molecules
Assessment of soluble molecules or biomarkers in the cerebrospinal fluid (CSF) or plasma could be an ideal way to stratify patients for clinical trials and for determination of clinical proof of pharmacological principle. However, despite large efforts, such predictive biomarkers are largely non-existent and, even if they were, drawing inferences from the neurochemical composition of plasma and CSF on the processes of the brain is not always considered necessarily straightforward (Bandelow et al., 2017). One area that has been extensively investigated in this regard, however, has been the hypothalamic-pituitary-adrenal (HPA) axis. Disruption of the HPA axis is common across numerous psychiatric disorders including those associated with negative valence including anxiety (Dedic et al., 2018). Consequently, measurement of molecules such as cortisol could be used as biomarkers for patient stratification or treatment response and indeed for the establishment of animal tests using these molecules as read-outs. In drug discovery, however, the track record here is not good; the failure of the corticotropin-releasing factor receptor type 1 (CRF1) antagonists in clinical trials for depression and anxiety (Binneman et al., 2008; Zorrilla and Koob, 2010) suggests that the system is complex and that the optimal point for therapeutic intervention has not yet been identified. However, more recent findings have identified polymorphisms in the FKBP5 gene as vulnerability factors to anxiety (and depression) and when combined with environmental insults such as early life stress can disrupt the equilibrium of HPA axis functioning and lead to psychiatric disorders (Gross and Hen, 2004; Hovens et al., 2012). Other examples of systems involved in anxiety with the potential for measurement of blood or CSF-based biomarkers include the kynurenine system (Schwarcz et al., 2012), serine racemase system (Basu et al., 2009) and neuropeptides such as orexin (Flores et al., 2015). However, while the correlative link to human pathophysiology is there, these have so far only been explored at the preclinical level in drug discovery projects addressing negative valence.
Imaging technologies such as positron emission tomography (PET) and single-photon emission computerised tomography (SPECT) offer the potential to measure binding of radioligands to target proteins in the brain and changes thereof in healthy volunteers or in patients and can be used to give an indirect indication of differences in transmitter systems (Maron and Nutt, 2017; Martin et al., 2009; Suhara et al., 2017). In a study by Van der Wee et al. (2008), the dopamine and serotonin systems have been assessed in patients with generalised social anxiety disorder using SPECT and compared with healthy controls. Significantly higher binding potentials were found for serotonin in the thalamus and for dopamine in the striatum indicating dysregulation of these neurotransmitter systems in this patient group. Magnetic resonance spectroscopy (MRS) can be used as a direct measure of chemical composition of the brain and changes in neurotransmitter levels in specific brain regions (Novotny et al., 2003) and has been used to assess gamma-aminobutyric acid (GABA) levels in patients with panic disorder (Goddard et al., 2001). In this study, patients with panic disorder had significantly reduced GABA levels in the occipital cortex, although a significant correlation between GABA levels and measures of illness or state anxiety was not observed (Goddard et al., 2001). A recent review has focused on metabolic alterations in patients with generalised anxiety disorder as assessed using MRS. Alterations in metabolites such as N-acetyl-aspartate, phosphocreatine and creatinine, and glycerophosphocholine were highlighted in brain regions including the dorsolateral prefrontal cortex and hippocampus (Delvecchio et al., 2017). Such imaging technologies can also be applied preclinically enabling close alignment between these two settings and could enable the identification of specific biomarkers for patient selection and treatment response.
Cells
The potential of cellular systems has advanced significantly over the last decades. There has been an increasing recognition of the limitations of target-centric drug screening in recombinant systems and the need for cellular systems that engage signalling pathways and processes that are relevant to the pathophysiology of the disease. Primary neuronal cultures are increasingly being used, as well as phenotypic screens, which aim to identify new drug targets based on disease-relevant cellular signalling. Biased signalling is also increasingly recognised and for the purpose of drug screening is sometimes also considered (Kenakin, 2015).
Cellular systems based on human patient material are developing at a fast pace and could offer significant advantages over the recombinant and animal-derived cellular systems described above. The generation of induced pluripotent stem cells from adult human fibroblasts was first described in 2007 (Takahashi et al., 2007) – a considerable feat at the time since no one had been able to convert adult skin cells into stem cells; and thereafter by varying the chemical environment the cells could be differentiated into neurones or any other cell type in vitro. Since then, a wealth of possibilities has opened up for increasing understanding of human disease and modelling it in vitro. iPSC technology provides the possibility to develop distinct cell types that are central to multiple psychiatric disorders (Borsini and Zunszain, 2016; Madison et al., 2015; O’Shea and McInnis, 2016; Vaccarino et al., 2011; Wen et al., 2016). Furthermore, in a recent study, Stern et al. (2018) described the identification of a division of intrinsically different sub-populations of patient-derived neurones, which could be used to predict the patient’s responsiveness to lithium. Application of such know-how could help understanding of why some patients respond to certain drugs and others not; it is indeed estimated that over one-third of patients suffering from anxiety-related disorders are treatment resistant (Bystritsky, 2006).
Circuits
Understanding brain circuitry is central to the RDoC concept. Non-invasive neuroimaging methods such as functional magnetic resonance imaging (fMRI) can be applied at every phase of drug discovery, across species, and have led to significant advancement of our understanding of brain circuitry underlying anxiety and related disorders (Borsook et al., 2006). fMRI can determine changes in brain blood oxygenation levels in the microcirculation, thus providing an indirect measure of neural activity. It can be performed in awake animals and humans in resting state or, for example, during performance of a task or challenge intervention. In terms of negative valence, numerous reports have demonstrated using fMRI that the amygdala (in particular the basolateral amygdala (BLA)) is over-activated in some patients with major depressive disorder (MDD)/anxiety both at baseline and in response to stimuli such as sad faces or negative words, possibly reflecting the increased negative emotional and cognitive state associated with these conditions (Drevets and Raichle, 1992; Sheline et al., 2001). Furthermore, the degree of amygdala activation has been shown to be positively correlated with symptom severity in disorders such as phobia and social anxiety disorder and a measurable reduction in amygdala activation following treatment with pharmaco- and psychotherapy has also been described (Duval et al., 2015; Labuschagne et al., 2010; Phan et al., 2013). By extension, stratification of patients for clinical trials using fMRI could lead to a form of precision medicine in psychiatry, in which treatments are delivered to particular patients based on the degree to which the treatment targets a particular form of circuitry dysfunction (Le Doux and Pine, 2016) and thus the effect of a novel pharmacological intervention might be better evinced.
There have been numerous initiatives to further advance imaging technologies. For example, the Human Connectome Project was initiated in 2010 following support from National Institutes of Health (NIH) for the establishment of two consortia to develop improved neuroimaging methods and to acquire a data set of unprecedented size and quality for mapping the healthy human macroscale connectome (Glasser et al., 2016). This and similar initiatives, such as the ENIGMA consortium, a large global consortium aiming to compile genetic and imaging data from numerous patient populations (Thompson et al., 2014), are essential to the progression of our understanding of neural circuitry in health and disease.
A critical question to the RDoC approach is whether psychiatric symptoms are truly transdiagnostic. Since anxiety is present in numerous disorders, one report has used fMRI to determine if the same mechanisms contribute to anxiety in individuals with and without autism spectrum disorder (ASD). The study found that the pattern of activation of the amygdala circuitry observed in ASD patients is the same as that described for patients suffering from anxiety without ASD. This finding supports the transdiagnostic nature of the negative valence domain and suggests that anxiety in ASD should be responsive to interventions targeting maladaptive responses to negative information (Herrington et al., 2017). It is anticipated that additional data from similar studies will be become available soon to shed further light on this important aspect of the RDoC concept.
In preclinical phases, fMRI can be used to define relevant CNS animal tests, which can be used to explore the validity of a new therapeutic concept. These should both engage the brain circuitry considered to be aberrant in the patient and in-line with the site of action of the novel therapeutic approach. The demonstration of a drug-induced change in a particular circuit (circuit engagement) using imaging can increase understanding of the brain pathways involved in the mechanism of action of a drug and build confidence in a novel therapeutic approach, thus enabling its advancement through the drug discovery process. An important consideration, however, is that unlike in the clinical setting, preclinical fMRI measures often require the use of anaesthesia to minimise stress and reduce motion artefacts during the scans. It is, therefore, important to have a good understanding of the effects of the anaesthetic used on neuronal activity (Jonckers et al., 2015).
A significant advance for the preclinical evaluation of brain circuits has been the development of optogenetics – the combination of genetic and optical methods to cause or inhibit well-defined events in specific cells of living tissues and behaving animals (Fenno et al., 2011). The technology involves the use of genetically encoded light-sensitive proteins (ion channels or pumps) called opsins, which can be expressed in specific cell types of the brain. The application of light into the brain region of interest can precisely activate or inhibit the opsins, thus enabling fast and focused manipulation of neural activity. Since the discovery of optogenetics over 10 years ago, optogenetic techniques have become standard tools to understand how cell types, circuits and systems operate in normal and pathological states (Steinberg et al., 2015). Furthermore, coupling of optogenetics with complementary technologies including fMRI (Lee et al., 2017), electrophysiology and behaviour is now routine in many labs, thus enabling a more integrated approach to the investigation of brain circuitry (Kim et al., 2013).
The neural circuits underlying negative valence including anxiety have been explored using optogenetics (Tye et al., 2011). In this study, channel rhodopsin was expressed in the pyramidal neurones of the BLA and an optical fibre was implanted over the central nucleus of the amygdala (CeA). Photostimulation of the BLA terminals in the CeA resulted in an acute and reversible decrease in anxiety-like behaviour as measured in the elevated plus maze and open-field paradigms. The opposite was observed when an inhibitory opsin NpHR was used. This type of approach can be applied in preclinical drug discovery where the efficacy of novel pharmacological approaches can be investigated for the validation of new therapeutic concepts. To date, optogenetics has been employed in preclinical settings only. It is conceivable, however, that the same technology could be applied as a therapeutic modality of the future (Delbeke et al., 2017).
Physiology
The potentiated startle test as suggested by RDoC as a physiological read-out for the negative valence, construct potential threat is considered to have substantial translational validity and relevance for anxiety disorders (Davis et al., 2010; Grillon and Baas, 2003; Schmitz and Grillon, 2012). It can be performed in humans (the NPU-threat test) as well as in preclinical species as an objective measurement of anxiety (Lissek et al., 2008). The test involves classical fear conditioning, which has long been implicated in development of pathological anxiety. Thus, the task employs the learning process by which a neutral unconditioned stimulus comes to evoke fear following its pairing with an aversive unconditioned stimulus (Mineka and Zinbarg, 2006). However, the lack of effect of benzodiazepines has been reported under certain experimental conditions indicating an increased need for further understanding of the paradigm and its relation to fear as well as anxiety. The neuropeptide and hormone oxytocin, which is known to have anxiolytic effects in humans as well as preclinical species (see Neumann and Slattery (2016) for review), have been reported to suppress acoustic startle following fear conditioning compared with startle before conditioning (background anxiety) but did not have an effect on cue-specific fear-potentiated startle. These findings suggest that oxytocin reduces background anxiety – an anxious state not directly related to cue-specific fear, but sustained beyond the immediate threat (Missig et al., 2010).
Other directly translational physiological paradigms that might be considered include administration of CCK-4 CO2 caffeine, sodium lactate and yohimbine challenge. All of these agents have been used both preclinically and clinically as pharmacological tools to activate brain circuitry involved in anxiety and could be coupled with several physiological read-outs such as skin conductance, measurement of cortisol or cardiovascular parameters (Siepmann and Joraschky, 2007). Testing of a novel drug in such a paradigm in healthy volunteers in the clinic could enable proof of pharmacological principle and an early decision-making for a drug discovery project.
Behaviour
The complexity of the human brain and behaviour poses a distinct challenge to drug discovery in the field of neuropsychiatric diseases. Psychiatric symptoms are often uniquely human, making the development of an animal model that reproduces a whole psychiatric symptom virtually impossible (Figure 2). Therefore, it follows that animal behavioural tests are best employed to investigate distinct components of more complex phenomena, such as mechanistic tests, which engage circuitry considered relevant for a particular clinical symptom and predict clinically needed drug exposures. Numerous back-translated behavioural tasks have been developed, for example, for cognitive systems (Robbins, 2017), where the circuitry and associated mechanisms involved have been explored and validated, thus increasing confidence that findings obtained with such tests will translate. For the domain negative valence and construct potential threat (anxiety), such behavioural read-outs have so far not been described.
Figure 2.
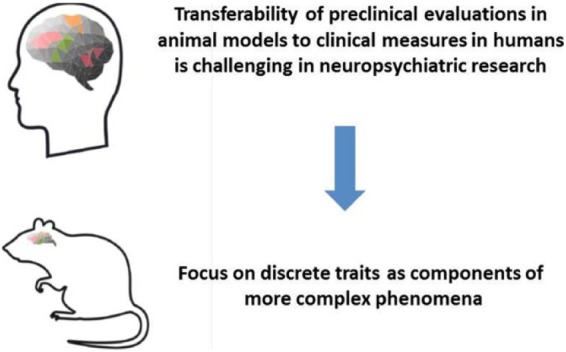
The complexity of the human brain and behaviour poses a challenge to neuropsychiatric drug development. Focus on discrete traits as components of more complex phenomena.
In the future, information on human behaviour collected using smartphone technologies could be used and would be extremely informative for the field of psychiatry as a whole. Numerous smartphone apps have already become commercially available; however, the majority lacked involvement of health care professionals in their development (Sucala et al., 2017). If collected appropriately, such human data could be extremely powerful for increasing understanding of altered patterns of behaviour in patients suffering from anxiety and related disorders. However, linking such behavioural data to associated brain circuitry could prove difficult.
Preclinically, classical pharmacological animal tests such as the elevated plus maze and open field are still widely used to explore the potential of new therapeutic concepts for anxiety and related disorders. Their predictive validity might be enhanced by applying pharmacological challenges such as with CCK or yohimbine, which have been shown to induce anxiety in healthy volunteers and where the associated brain circuitry is somewhat understood. Chronic social stress models are considered relevant due to the activation of anxiety-related circuitry (Bath et al., 2017) and where a number of novel therapeutic approaches have been shown to be effective in rodents (Fuertig et al., 2016; Just et al., 2018).
As mentioned above, data collected from a combination of complementary methods and technologies are increasingly being used and arguably essential for ensuring that the circuits and mechanisms considered relevant to the clinical disorder – and hypothesis for its treatment – are indeed being engaged in behavioural tests. Face, construct and predictive validity will always be a challenge in the field of psychiatry, and therefore, the use of, and interpretation of findings from, behavioural tests simply as mechanistic read-outs might prove beneficial.
Self-reports
In drug discovery, all knowledge and understanding underlying new drug target identification and validation culminates in the nomination of a new molecular entity for testing in humans. This final stage in the process enables the determination of whether the drug leads to a clinically meaningful improvement in particular symptoms and overall disease severity. For this, self-reports are needed, and RDoC specifies several self-reports considered suitable for the evaluation of negative valence, potential threat (anxiety): the Intolerance of Uncertainty Scale (12-item version; Carleton et al., 2007), Behavioural Inhibition Scale (BIS; Carver and White, 1994), Fear of Negative Evaluation Scale (Watson and Friend, 1969), Anxiety Sensitivity Index (Taylor et al., 2007), and Life Events and Difficulties Schedule (LEDS; Brown and Harris, 1978). The Intolerance of Uncertainty Scale is perhaps the most commonly used measure and has been used to evaluate anxiety in numerous psychiatric disorders including generalised anxiety disorder (GAD) and obsessive-compulsive disorder (OCD; Gillett et al., 2018), ASD (Rodgers et al., 2018), attention-deficit hyperactivity disorder (ADHD) (Gramszlo et al., 2018) and in individuals with co-occurring post-traumatic stress disorder (PTSD) and substance use disorders (Banducci et al., 2016). Furthermore, a recent study has demonstrated the contribution of improvement in intolerance of uncertainty to overall treatment gains in patients undergoing transdiagnostic cognitive behavioural therapy (Talkovsky and Norton, 2018).
Perspective
In the current review, we have described evidence for Negative Valence with a focus on the construct Potential Threat (‘Anxiety’) at each level of analysis in the context of drug discovery. Much of the literature described has been performed at one or more levels of analysis, but relatively few studies to date have systematically or directly explored the potential of RDoC as a primary objective, or interpreted their results in a manner consistent with the RDoC framework (Carcone and Ruocco, 2017). Moreover, much of the literature cited herein is based on studies stemming from DSM-based criteria. This is perhaps not surprising in view of the relative newness of the RDoC approach, but should be borne in mind. Nonetheless, in terms of building a comprehensive understanding in neuropsychiatry, studies using integrated methodological approaches where scientific evidence is compiled from studies spanning the units of analysis would surely improve the likelihood of identifying drug targets that are clinically effective.
In general, the literature appears in support of RDoC, although some reports are highly critical and express concerns (Hershenberg, 2015; Patrick and Hajcak, 2016; Zoellner and Foa, 2016). Certainly, there will be challenges in the practical implementation of the framework which will likely be greater for some of the RDoC domains than others. Precise alignment across all the units of analysis could be difficult and is dependent on the tools available. Furthermore, the RDoC project is a living project and is still at an early stage. The need for further elucidation and more precise definition of the terms used could help avoid variable interpretation of the RDoC constructs that seems to be emerging (Patrick and Hajcak, 2016). More fundamental concerns such as the emphasis on neurobiological dysfunction as the root of clinical disorders and the reductionist approach of RDoC have also been aired (Hershenberg and Goldfried, 2015). Thus, some controversy in the field exists highlighting the need for continued dialogue in order that progress can be made, and the full potential of RDoC to be realised.
The pace of technological development in neuroscience has accelerated in the recent past, providing researchers with powerful and precise new tools and making it an exciting time to work in this field. Feasibility is better than it has ever been, to increase our understanding of neuropsychiatric disorders and to develop effective therapies for their treatment. Increased understanding of the role of genes in neuropsychiatric disorders offers the possibility of selecting homogeneous groups of patients, and those most likely to respond to treatment. Patient-derived induced pluripotent stem cells offer a revolutionary tool for disease modelling and for drug discovery. The generation of tissue-relevant cell types exhibiting a patient’s genetic and molecular background offers the ability to develop personalised therapies. Imaging technologies span both ends of the drug discovery spectrum – enabling understanding of the malfunctioning circuitry in patients as well as the objective selection of those patients most likely to respond to treatment and ultimately to proof of clinical principle. Circuit disturbances can be modelled and precisely explored preclinically with the use of optogenetics. With the integration of methods and tools across levels of analysis, physiological and behavioural tests and self-reports can be more closely aligned between preclinical and clinical settings to improve the likelihood of translation of a new therapeutic concept to a clinically meaningful outcome in the patient (Figure 3).
Figure 3.
Tools and methods available at each level of analysis at different stages of the drug discovery process. Integration of data from multiple units of analysis should enable a more complete picture of neuropsychiatric syndromes, which should lead to improved target identification, validation and ultimately proof of clinical principle (PoCP).
The primary aim of RDoC is to improve the classification system of psychiatric disorders, building on the premise that an improved diagnostic system will improve treatment outcomes. The approach is a move towards a more tailored or personalised approach to diagnosis and consequent treatment (Insel and Cuthbert, 2015) which holds significant promise for the future success of drug discovery. However, much work is still to be done: regulatory systems will need to be adapted, and standardised measures and protocols need to be implemented along with common data acquisition and handling standards (Brady and Insel, 2012). However, despite the challenges, RDoC coupled with advances in the neuroscience tool box makes it an exciting and promising time to be working in the field of drug discovery for neuropsychiatric diseases. It is still early days and further advancements are clearly needed; but with the use of this common framework, we have the opportunity to increase our understanding of neuropsychiatric disorders and work towards achieving a common thread of translational research from gene to self-report.
Footnotes
Declaration of conflicting interests: J.R.N. and B.S. disclose that they are full-time employees of Boehringer Ingelheim Pharma GmbH & Co. KG.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
References
- Balestri M, Crisafulli C, Donato L, et al. (2017) Nine differentially expressed genes from a post mortem study and their association with suicidal status in a sample of suicide completers, attempters and controls. Journal of Psychiatric Research 91(1): 98–104. [DOI] [PubMed] [Google Scholar]
- Bandelow B, Baldwin D, Abelli M, et al. (2017) Biological markers for anxiety disorders, OCD and PTSD: A consensus statement. Part II: Neurochemistry, neurophysiology and neurocognition. World Journal of Biological Psychiatry 18(3): 162–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banducci AN, Bujarski SJ, Bonn-Miller MO, et al. (2016) The impact of intolerance of emotional distress and uncertainty on veterans with co-occurring PTSD and substance use disorders. Journal of Anxiety Disorders 41: 73–81. [DOI] [PubMed] [Google Scholar]
- Basu AC, Tsai GE, Ma CL, et al. (2009) Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Molecular Psychiatry 14(7): 719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath KG, Russo SJ, Pleil KE, et al. (2017) Circuit and synaptic mechanisms of repeated stress: Perspectives from differing contexts, duration, and development. Neurobiology of Stress 7: 137–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binneman B, Feltner D, Kolluri S, et al. (2008) A 6-week randomized, placebo-controlled trial of CP-316,311 (a selective CRH1 antagonist) in the treatment of major depression. American Journal of Psychiatry 165(5): 617–620. [DOI] [PubMed] [Google Scholar]
- Bitsika V, Sharpley CF, Andronicos NM, et al. (2016) Prevalence, structure and correlates of anxiety-depression in boys with an autism spectrum disorder. Research in Developmental Disabilities 49–50: 302–311. [DOI] [PubMed] [Google Scholar]
- Borsini A, Zunszain PA. (2016) Advances in stem cells biology: New approaches to understand depression. In: Pfaff D, Christen Y. (eds) Stem Cells in Neuroendocrinology. Cham: Springer, pp. 123–133. [PubMed] [Google Scholar]
- Borsook D, Becerra L, Hargreaves R. (2006) A role for fMRI in optimizing CNS drug development. Nature Reviews Drug Discovery 5(5): 411–424. [DOI] [PubMed] [Google Scholar]
- Brady LS, Insel TR. (2012) Translating discoveries into medicine: Psychiatric drug development in 2011. Neuropsychopharmacology 37(1): 281–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga RJ, Petrides G, Figueira I. (2004) Anxiety disorders in schizophrenia. Comprehensive Psychiatry 45(6): 460–468. [DOI] [PubMed] [Google Scholar]
- Brown GW, Harris T. (1978) Social origins of depression: A reply. Psychological Medicine 8(4): 577–588. [DOI] [PubMed] [Google Scholar]
- Bystritsky A. (2006) Treatment-resistant anxiety disorders. Molecular Psychiatry 11(9): 805–814. [DOI] [PubMed] [Google Scholar]
- Carcone D, Ruocco AC. (2017) Six years of research on the National Institute of Mental Health’s Research Domain Criteria (RDoC) Initiative: A systematic review. Frontiers in Cellular Neuroscience 11: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carleton RN, Norton MA, Asmundson GJ. (2007) Fearing the unknown: A short version of the Intolerance of Uncertainty Scale. Journal of Anxiety Disorders 21(1): 105–117. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. (1994) Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology 67(2): 319–333. [Google Scholar]
- Cuthbert BN. (2014) The RDoC framework: Facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry 13(1): 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, et al. (2010) Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology 35(1): 105–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedic N, Chen A, Deussing JM. (2018) The CRF family of neuropeptides and their receptors – Mediators of the central stress response. Current Molecular Pharmacology 11(1): 4–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbeke J, Hoffman L, Mols K, et al. (2017) And then there was light: Perspectives of optogenetics for deep brain stimulation and neuromodulation. Frontiers in Neuroscience 11: 663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delvecchio G, Stanley JA, Altamura AC, et al. (2017) Metabolic alterations in generalised anxiety disorder: A review of proton magnetic resonance spectroscopic studies. Epidemiology and Psychiatric Sciences 26(6): 587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME. (1992) Neuroanatomical circuits in depression: Implications for treatment mechanisms. Psychopharmacology Bulletin 28(3): 261–274. [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. (2008) The subgenual anterior cingulate cortex in mood disorders. CNS Spectrums 13(8): 663–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval ER, Javanbakht A, Liberzon I. (2015) Neural circuits in anxiety and stress disorders: A focused review. Therapeutics and Clinical Risk Management 11: 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenno L, Yizhar O, Deisseroth K. (2011) The development and application of optogenetics. Annual Review of Neuroscience 34(1): 389–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores A, Saravia R, Maldonado R, et al. (2015) Orexins and fear: Implications for the treatment of anxiety disorders. Trends in Neurosciences 38(9): 550–559. [DOI] [PubMed] [Google Scholar]
- Fuertig R, Azzinnari D, Bergamini G, et al. (2016) Mouse chronic social stress increases blood and brain kynurenine pathway activity and fear behaviour: Both effects are reversed by inhibition of indoleamine 2,3-dioxygenase. Brain, Behavior, and Immunity 54: 59–72. [DOI] [PubMed] [Google Scholar]
- Gillett CB, Bilek EL, Hanna GL, et al. (2018) Intolerance of uncertainty in youth with obsessive-compulsive disorder and generalized anxiety disorder: A transdiagnostic construct with implications for phenomenology and treatment. Clinical Psychology Review 60: 100–108. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Smith SM, Marcus DS, et al. (2016) The Human Connectome Project’s neuroimaging approach. Nature Neuroscience 19(9): 1175–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard AW, Mason GF, Almai A, et al. (2001) Reductions in occipital cortex GABA levels in panic disorder detected with 1h-magnetic resonance spectroscopy. Archives of General Psychiatry 58(6): 556–561. [DOI] [PubMed] [Google Scholar]
- Gramszlo C, Fogleman ND, Rosen PJ, et al. (2018) Intolerance of uncertainty in children with attention-deficit/hyperactivity disorder. Attention Deficit and Hyperactivity Disorders 10(3): 189–197. [DOI] [PubMed] [Google Scholar]
- Gribkoff VK, Kaczmarek LK. (2017) The need for new approaches in CNS drug discovery: Why drugs have failed, and what can be done to improve outcomes. Neuropharmacology 120: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Baas J. (2003) A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clinical Neurophysiology 114(9): 1557–1579. [DOI] [PubMed] [Google Scholar]
- Gross C, Hen R. (2004) The developmental origins of anxiety. Nature Reviews Neuroscience 5(7): 545–552. [DOI] [PubMed] [Google Scholar]
- Herrington JD, Maddox BB, McVey AJ, et al. (2017) Negative valence in autism spectrum disorder: The relationship between amygdala activity, selective attention, and co-occurring anxiety. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 2(6): 510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershenberg R, Goldfried MR. (2015) Implications of RDoC for the research and practice of psychotherapy. Behavior Therapy 46(2): 156–165. [DOI] [PubMed] [Google Scholar]
- Hiroi N. (2018) Critical reappraisal of mechanistic links of copy number variants to dimensional constructs of neuropsychiatric disorders in mouse models. Psychiatry and Clinical Neurosciences 72(5): 301–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovens JG, Giltay EJ, Wiersma JE, et al. (2012) Impact of childhood life events and trauma on the course of depressive and anxiety disorders. Acta Psychiatrica Scandinavica 126(3): 198–207. [DOI] [PubMed] [Google Scholar]
- Hyman SE. (2012) Revolution stalled. Science Translational Medicine 4(155): 155cm111. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, et al. (2010) Research Domain Criteria (RDoC): Toward a new classification framework for research on mental disorders. American Journal of Psychiatry 167(7): 748–751. [DOI] [PubMed] [Google Scholar]
- Insel TR, Cuthbert BN. (2015) Medicine. Brain disorders? Precisely. Science 348(6234): 499–500. [DOI] [PubMed] [Google Scholar]
- Jennings CG, Landman R, Zhou Y, et al. (2016) Opportunities and challenges in modeling human brain disorders in transgenic primates. Nature Neuroscience 19(9): 1123–1130. [DOI] [PubMed] [Google Scholar]
- Jonckers E, Shah D, Hamaide J, et al. (2015) The power of using functional fMRI on small rodents to study brain pharmacology and disease. Frontiers in Pharmacology 6: 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just S, Chenard BL, Ceci A, et al. (2018) Treatment with HC-070, a potent inhibitor of TRPC4 and TRPC5, leads to anxiolytic and antidepressant effects in mice. PLoS ONE 13(1): e0191225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaitin KI, Di Masi JA. (2011) Pharmaceutical innovation in the 21st century: New drug approvals in the first decade, 2000–2009. Clinical Pharmacology & Therapeutics 89(2): 183–188. [DOI] [PubMed] [Google Scholar]
- Kenakin T. (2015) Gaddum Memorial Lecture 2014: Receptors as an evolving concept: From switches to biased microprocessors. British Journal of Pharmacology 172(17): 4238–4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Adhikari A, Lee SY, et al. (2013) Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature 496(7444): 219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuschagne I, Phan KL, Wood A, et al. (2010) Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology 35(12): 2403–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Doux JE, Pine DS. (2016) Using neuroscience to help understand fear and anxiety: A two-system framework. American Journal of Psychiatry 173(11): 1083–1093. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kreitzer AC, Singer AC, et al. (2017) Illuminating neural circuits: From molecules to MRI. Journal of Neuroscience 37(45): 10817–10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Biggs AL, Rabin SJ, et al. (2008) Generalization of conditioned fear-potentiated startle in humans: Experimental validation and clinical relevance. Behaviour Research and Therapy 46(5): 678–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor NW, Dimatelis JJ, Van Zyl PJ, et al. (2018) A translational approach to the genetics of anxiety disorders. Behavioural Brain Research 341: 91–97. [DOI] [PubMed] [Google Scholar]
- Madison JM, Zhou F, Nigam A, et al. (2015) Characterization of bipolar disorder patient-specific induced pluripotent stem cells from a family reveals neurodevelopmental and mRNA expression abnormalities. Molecular Psychiatry 20(6): 703–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron E, Nutt D. (2017) Biological markers of generalized anxiety disorder. Dialogues in Clinical Neuroscience 19(2): 147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EI, Ressler KJ, Binder E, et al. (2009) The neurobiology of anxiety disorders: Brain imaging, genetics, and psychoneuroendocrinology. Psychiatric Clinics of North America 32(3): 549–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Mehta RL, Molnar BE, et al. (1998) Comorbidity of substance use disorders with mood and anxiety disorders: Results of the International Consortium in Psychiatric Epidemiology. Addictive Behaviors 23(6): 893–907. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. (2006) Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nature Reviews Neuroscience 7(10): 818–827. [DOI] [PubMed] [Google Scholar]
- Mineka S, Zinbarg R. (2006) A contemporary learning theory perspective on the etiology of anxiety disorders: It’s not what you thought it was. American Psychologist 61(1): 10–26. [DOI] [PubMed] [Google Scholar]
- Missig G, Ayers LW, Schulkin J, et al. (2010) Oxytocin reduces background anxiety in a fear-potentiated startle paradigm. Neuropsychopharmacology 35(13): 2607–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco A, Monda A, Amoroso N, et al. (2018) A complex network approach reveals a pivotal substructure of genes linked to schizophrenia. PLoS ONE 13(1): e0190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SE, Cuthbert BN. (2012) Research Domain Criteria: Cognitive systems, neural circuits, and dimensions of behavior. Dialogues in Clinical Neuroscience 14(1): 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJ, Vos T, Lozano R, et al. (2012) Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. The Lancet 380(9859): 2197–2223. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Slattery DA. (2016) Oxytocin in general anxiety and social fear: A translational approach. Biological Psychiatry 79(3): 213–221. [DOI] [PubMed] [Google Scholar]
- Novotny EJ, Jr, Fulbright RK, Pearl PL, et al. (2003) Magnetic resonance spectroscopy of neurotransmitters in human brain. Annals of Neurology 54(Suppl. 6): S25–S31. [DOI] [PubMed] [Google Scholar]
- O’Shea KS, McInnis MG. (2016) Neurodevelopmental origins of bipolar disorder: iPSC models. Molecular and Cellular Neuroscience 73: 63–83. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Hajcak G. (2016) RDoC: Translating promise into progress. Psychophysiology 53(3): 415–424. [DOI] [PubMed] [Google Scholar]
- Paul SM, Mytelka DS, Dunwiddie CT, et al. (2010) How to improve R&D productivity: The pharmaceutical industry’s grand challenge. Nature Reviews Drug Discovery 9(3): 203–214. [DOI] [PubMed] [Google Scholar]
- Phan KL, Coccaro EF, Angstadt M, et al. (2013) Corticolimbic brain reactivity to social signals of threat before and after sertraline treatment in generalized social phobia. Biological Psychiatry 73(4): 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio A, Li Y, Tsvetkov E, et al. (2014) Decreased anxiety-like behavior and Gαq/11-dependent responses in the amygdala of mice lacking TRPC4 channels. Journal of Neuroscience 34(10): 3653–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. (2017) Cross-species studies of cognition relevant to drug discovery: A translational approach. British Journal of Pharmacology 174(19): 3191–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers J, Herrema R, Honey E, et al. (2018) Towards a treatment for intolerance of uncertainty for autistic adults: A single case experimental design study. Journal of Autism and Developmental Disorders 48(8): 2832–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrig C. (2016) Mental disorders top the list of the most costly conditions in the United States: $201 billion. Health Affairs 35(6): 1130–1135. [DOI] [PubMed] [Google Scholar]
- Savage JE, Sawyers C, Roberson-Nay R, et al. (2017) The genetics of anxiety-related negative valence system traits. American Journal of Medical Genetics, Part B: Neuropsychiatric Genetics 174(2): 156–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz A, Grillon C. (2012) Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test). Nature Protocols 7(3): 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Bruno JP, Muchowski PJ, et al. (2012) Kynurenines in the mammalian brain: When physiology meets pathology. Nature Reviews Neuroscience 13(7): 465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, et al. (2001) Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: An fMRI study. Biological Psychiatry 50(9): 651–658. [DOI] [PubMed] [Google Scholar]
- Siepmann M, Joraschky P. (2007) Modelling anxiety in humans for drug development. Current Neuropharmacology 5(1): 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer WH, Lidstrom J, Sun H, et al. (2010) Human NPY promoter variation rs16147: T>C as a moderator of prefrontal NPY gene expression and negative affect. Human Mutation 31(8): E1594–E1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg EE, Christoffel DJ, Deisseroth K, et al. (2015) Illuminating circuitry relevant to psychiatric disorders with optogenetics. Current Opinion in Neurology 30: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern S, Santos R, Marchetto MC, et al. (2018) Neurons derived from patients with bipolar disorder divide into intrinsically different sub-populations of neurons, predicting the patients’ responsiveness to lithium. Molecular Psychiatry 23(6): 1453–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucala M, Cuijpers P, Muench F, et al. (2017) Anxiety: There is an app for that. A systematic review of anxiety apps. Depression and Anxiety 34(6): 518–525. [DOI] [PubMed] [Google Scholar]
- Suhara T, Chaki S, Kimura H, et al. (2017) Strategies for utilizing neuroimaging biomarkers in CNS drug discovery and development: CINP/JSNP Working Group report. International Journal of Neuropsychopharmacology 20(4): 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, et al. (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5): 861–872. [DOI] [PubMed] [Google Scholar]
- Talkovsky AM, Norton PJ. (2018) Negative affect and intolerance of uncertainty as potential mediators of change in comorbid depression in transdiagnostic CBT for anxiety. Journal of Affective Disorders 236: 259–265. [DOI] [PubMed] [Google Scholar]
- Taylor S, Zvolensky MJ, Cox BJ, et al. (2007) Robust dimensions of anxiety sensitivity: Development and initial validation of the Anxiety Sensitivity Index-3. Psychological Assessment 19(2): 176–188. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Stein JL, Medland SE, et al. (2014) The ENIGMA Consortium: Large-scale collaborative analyses of neuroimaging and genetic data. Brain Imaging and Behavior 8(2): 153–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, et al. (2011) Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471(7338): 358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino FM, Stevens HE, Kocabas A, et al. (2011) Induced pluripotent stem cells: A new tool to confront the challenge of neuropsychiatric disorders. Neuropharmacology 60(7–8): 1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Wee NJ, van Veen JF, Stevens H, et al. (2008) Increased serotonin and dopamine transporter binding in psychotropic medication-naive patients with generalized social anxiety disorder shown by 123I-beta-(4-iodophenyl)-tropane SPECT. Journal of Nuclear Medicine 49(5): 757–763. [DOI] [PubMed] [Google Scholar]
- Watson D, Friend R. (1969) Measurement of social-evaluative anxiety. Journal of Consulting and Clinical Psychology 33(4): 448–457. [DOI] [PubMed] [Google Scholar]
- Wen Z, Christian KM, Song H, et al. (2016) Modeling psychiatric disorders with patient-derived iPSCs. Current Opinion in Neurology 36: 118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaboski BA, Storch EA. (2018) Comorbid autism spectrum disorder and anxiety disorders: A brief review. Future Neurology 13(1): 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannas AS, Wiechmann T, Gassen NC, et al. (2016) Gene-stress-epigenetic regulation of FKBP5: Clinical and translational implications. Neuropsychopharmacology 41(1): 261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbozinek TD, Rose RD, Wolitzky-Taylor KB, et al. (2012) Diagnostic overlap of generalized anxiety disorder and major depressive disorder in a primary care sample. Depression and Anxiety 29(12): 1065–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo C, Hou W, Hu L, et al. (2017) Genomic editing of non-coding RNA genes with CRISPR/Cas9 ushers in a potential novel approach to study and treat schizophrenia. Frontiers in Molecular Neuroscience 10: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoellner LA, Foa EB. (2016) Applying Research Domain Criteria (RDoC) to the study of fear and anxiety: A critical comment. Psychophysiology 53(3): 332–335. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. (2010) Progress in corticotropin-releasing factor-1 antagonist development. Drug Discovery Today 15(9–10): 371–383. [DOI] [PMC free article] [PubMed] [Google Scholar]