Abstract
Antipsychotic drugs revolutionised psychiatric practice and provided a range of tools for exploring brain function in health and disease. Their development and introduction were largely empirical but based on long and honourable scientific credentials and remarkable powers of clinical observation. The class shares a common core action of attenuating central dopamine transmission, which underlies the major limitation to their use – high liability to disrupt extrapyramidal function – and also the most durable hypothesis of the basis of psychotic disorders, especially schizophrenia. However, the Dopamine Hypothesis, which has driven drug development for almost half a century, has become a straight-jacket, stifling innovation, resulting in a class of compounds that are largely derivative. Recent efforts only cemented this tendency as no clinical evidence supports the notion that newer compounds, modelled on clozapine, share that drug’s unique neurological tolerability and can be considered ‘atypical’. Patients and doctors alike must await a more profound understanding of central dopamine homeostasis and novel methods of maintaining it before they can again experience the intoxicating promise antipsychotics once held.
Keywords: Antipsychotics, atypical antipsychotics, dopamine, extrapyramidal side-effects, clozapine, pharma investment
Antipsychotics have been a major driver of psychiatric practice and neuroscience research for over 60 years. They have altered perceptions of psychiatric disorder and provided the foundation for a social revolution in health care provision. They have become tools not only for exploring mechanisms of disease but also for unlocking the nature of brain function itself. But recent years have witnessed how optimism can be manipulated to produce false hope and how theories that once seemed productive can come to define the limitations of knowledge. The pipeline of new antipsychotics has dried to a trickle and international pharma has moved on. The story of antipsychotics is a sobering rollercoaster.
Unlikely origins
In 1856, 18-year-old William Perkin produced a blue ‘sticky splurge’ trying to create quinine from coal tar, but, despite his youth, immediately saw a commercial application (Swazey, 1974). The colour mauve (‘Perkin’s purple’) soon became the height of fashion. Perkin’s discovery gave birth to the commercial dye industry and the discipline of organic chemistry to feed demand. The structure of one of the most successful dyes, methylene blue, was uncovered by August Bernthsen in 1883 and named ‘thiodiphenylamine’ or phenothiazine (Swazey, 1974).
Many medical applications were sought for methylene blue and phenothiazine, but tolerability issues inhibited their development. However, after many years of neglect, a growing European focus on neurotransmission turned attention back to phenothiazines. In 1937, Daniel Bovet hypothesised that if there were compounds that could block acetylcholine, it might also be possible to produce agents that blocked histamine (Swazey, 1974). French company Rhone-Poulenc began to explore this using the readily synthesisable substituted phenothiazines, the outcome being promethazine, the first synthetic antihistamine.
During the Second World War, substituted phenothiazines were investigated unsuccessfully as antimalarials in both America and France, but French interest in their antihistamine actions led to diphenhydramine. However, the war had a further crucial impact. Major advances in surgical technique were not matched by improvements in outcomes and a new indication arose in the late 1940s when surgeon Henri Laborit postulated (wrongly, as it happened) that surgical hazards, mainly from haemodynamic shock, could be obviated by preoperative administration of a drug cocktail that dampened, or ‘lysed’, the autonomic nervous system. The phenothiazine development programme, instigated at Rhone-Poulenc in October 1950, was first and foremost about preoperative technique and only as an afterthought about psychiatry (Swazey, 1974).
In 1951, psychiatrists who tried the newly synthesised chlorpromazine in their patients thought it merely a sedative, but Laborit noticed something different:
not any loss of consciousness, not any change in the patient’s mentality but a slight tendency to sleep and above all ‘disinterest’ for all that goes on around him. (Laborit et al., 1952)
His patients, although aware of the life-threatening procedures they were facing, seemed curiously unconcerned. What he had noticed was an affective numbing similar, as was later suggested, to a ‘chemical lobotomy’. Laborit thought this would be of interest to psychiatrists, but psychiatry, heavily influenced by psychoanalysis at that time, paid little heed to a surgeon’s exhortations to try this miracle drug. However, psychiatrist Pierre Deniker, alerted by a relative who was a colleague of Laborit’s, and Jean Delay, the pre-eminent French psychiatrist of his generation, were interested and presented their striking results in 38 patients at the prestigious centennial meeting of the Société Médico-Psychologique in May 1952. Within a year, chlorpromazine had spread around the world and the era of ‘anti-psychosis’ had arrived.
An array of substituted phenothiazines followed before, in 1958, Paul Janssen applied heat to pethidine (meperidine), producing norpethidine, which when heated further produced the basis of the first butyrophenone, haloperidol, for many years the class ‘market leader’, while the first thioxanthene, a slight chemical and pharmacological modification of the phenothiazines, emerged in Denmark. Also in the late 1950s, French researchers, seeking to increase the antiarrhythmic properties of procainamide, produced metoclopramide, whose subsequent refinement led to sulpiride, the first substituted benzamide (Owens, 2014).
With the exception of cognitive enhancers, every class of medication that now comprises the heart of psychopharmacology had its birth in this ‘golden age’ of the 1950s. The associated optimism spread beyond clinicians and their patients as, for the first time, science was presented with a broad range of safe, clinically usable compounds with which to explore not only disease mechanisms but also the workings of the central nervous system. Neuroscience was coming of age.
By definition, ‘golden ages’ are limited and while the years that followed have produced an exponential increase in knowledge of brain mechanisms, the story of antipsychotics illustrates how elusive understanding remains.
The 1970s and 1980s: impact, theories and growing alarm
Psychiatric NHS bed numbers have fallen markedly, from a high of 150,000 in the 1950s to <30,000 currently (Tyrer and Johnson, 2011). Traditionally, most were occupied by long-stay patients with schizophrenia, and while a number of factors contributed to the shift to community care, the role of antipsychotics was crucial. However, the first randomised controlled trial (RCT) to demonstrate the efficacy of chlorpromazine illustrated an important fact–antipsychotics do not ‘cure’ schizophrenia (Elkes and Elkes, 1954), a conclusion replicated in the classic American study of Cole (1964), whose results remain ‘state of the art’:
Antipsychotics are of equal efficacy (this includes clozapine, where advantage probably lies solely with tolerability – see Owens, 2014 for discussion).
Efficacy is limited (in this study, to approximately half of patients whose mental states improved).
Beneficial effects relate specifically to what became known as ‘positive’ symptoms.
An early period of (probably non-specific) accelerated improvement is followed by more gradual, but probably specific, symptom improvement.
In RCTs in schizophrenia, some patients on placebo improve.
The limitations were clear from the start. Nonetheless, the benefits prompted two obvious questions:
How do they work?
What underlying disruption to brain function do they address?
The first report of parkinsonism with chlorpromazine (by Steck) reached the literature in 1954 but Delay noted this from the start, including it as one of the five criteria for a ‘neurone-seizing’, or ‘neuroleptic’, drug (Delay and Deniker, 1957). This began a debate as to whether parkinsonism was necessary for ‘anti-psychosis’. The eventual consensus was negative but the discussion cemented extrapyramidal actions as ‘side-effects’ (EPS), distracting from an alternative view that although unwanted, they may nonetheless be inherent: the ‘conditio sine qua non’ of anti-psychosis (Haase, 1961).
In the early 1960s, Carlsson and Lindquist (1963) explored why chlorpromazine and haloperidol possessed reserpine-like properties but not its monoamine-depleting action and found that these ‘neuroleptics’ accelerated metabolite formation of dopamine and noradrenaline, while leaving neurotransmitter levels unchanged. This enhanced ‘turnover’ pointed to a mode of action based on post-synaptic dopamine receptor antagonism. When receptor function came into vogue some years later, this formed the backbone of ‘the Dopamine Hypothesis’ (Matthysse, 1973; Snyder, 1976).
The ‘Mark 1’ version of this hypothesis – relating to the mode of action of antipsychotics as post-synaptic dopamine (D2) antagonists – was supported early by a wealth of preclinical evidence, such as blockade of amphetamine-induced stereotyped and other behaviours (Randrup and Munkvad, 1974) and especially the strong correlation between D2 affinity and clinical potency (Creese et al., 1976; Seeman et al., 1975). Furthermore, clinically, it was shown that only the D2 antagonist isomer of flupenthixol (then called the alpha-isomer) was effective in reducing psychotic symptomatology, the beta-isomer, with little D2 antagonist action, being no better than placebo (Johnstone et al., 1978). Attenuation of dopamine transmission by antagonism (or partial agonism) is the action common to all compounds licensed/approved as antipsychotics.
The ‘Mark 2’ version, extrapolating from this that schizophrenia is due to excess central dopamine activity, has been difficult to validate, though recent evidence has shifted an initial focus on post-synaptic dopamine receptor supersensitivity (Owen et al., 1978; Wong et al., 1986) to hyperdopaminergia mediated presynaptically (Howes and Kapur, 2009).
During this period of theorising, new drug development entered a lull, not least because the market seemed well-provisioned. What had been created, however, was a catalogue of largely derivative (‘me-too’) compounds which, by sharing a core pharmacology, shared its problems. While antipsychotics provided invaluable tools for increasingly sophisticated neuroscience, clinically concern was rising that parkinsonism may be the least of the neurological problems patients might experience. The literature came to be dominated by tardive dyskinesia, a syndrome of involuntary movements that could be disfiguring and irreversible. With a point prevalence of 20% and an annual incidence rate of 5% (Owens, 2014), the concern was legitimate. Lurking in the doldrums was a potential saviour.
1990 to the present: clozapine and its impact
Clozapine emerged from a wish for more antidepressants. Synthesised in 1959 as an iminodibenzyl derivative related to imipramine, it was not an effective antidepressant and pre-clinically was ‘defective’ on standard neuroleptic screening tests (Hippius, 1999). Nonetheless, clinically, it did have antipsychotic activity and development continued. In 1974, 13 cases of agranulocytosis were reported from Finland, 8 of which were fatal (Idanpaan-Heikklia et al., 1975), a blow that would have consigned it to the ‘experimental’ category but for a lingering impression that this was something different. While the evidence of inherently greater efficacy was – and remains – weak (Owens, 2014), its diminished proclivity to produce extrapyramidal side-effects (EPS) was consistent. With rising alarm about neurological class effects, there was every reason to assess its clinical place systematically.
The Clozaril Collaborative Group study (Kane et al., 1988) was highly influential, but because of the known haematological risks of clozapine, was targeted on ‘treatment-resistant schizophrenia’ only. Although it was established that many patients did not do well on antipsychotics, for the first time ‘treatment resistance’ was operationally defined. The study found the following in those on clozapine:
In all, 30% were ‘responders’ versus 4% on comparator (chlorpromazine + the antimuscarinic, benztropine), a highly significant difference.
Negative symptoms improved significantly.
Extrapyramidal rating scale totals significantly declined.
These results electrified a discipline disappointed by drugs that seemed to confound the expectation they originally offered, and within 2 years, clozapine received licensing approval in the United States and Europe. However, while responder criteria present striking headlines, they obfuscate the fact that the study set an exceptionally low hurdle for improvement, corresponding to just ‘minimally better’ (Leucht et al., 2008). Critiquing findings (2) and (3) are aided by knowledge of the relative doses utilised. A clozapine:chlorpromazine ratio of 1:2 was chosen, resulting in chlorpromazine-treated patients receiving exceptionally high average doses (up to 1800 mg/day). It is unsurprising that extrapyramidal tolerability on clozapine was significantly better, while ‘negative’ symptoms improved.
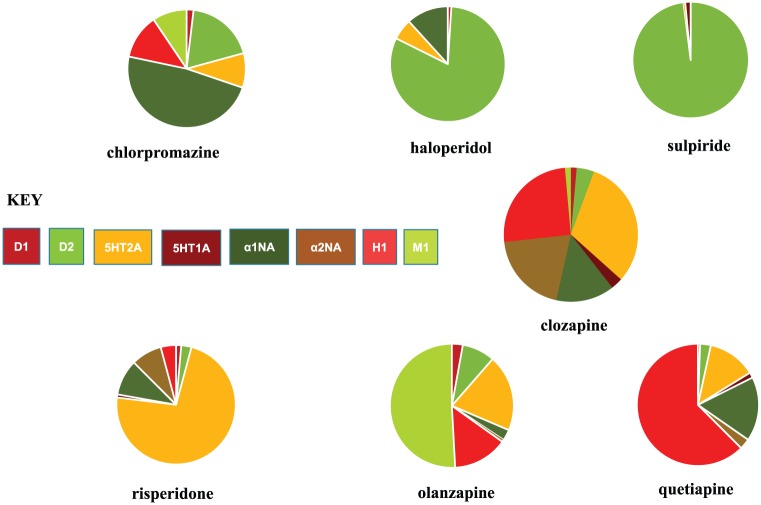
Despite these criticisms, clozapine’s unique neurological tolerability and consequent therapeutic value are undoubted, but with rigorous debate about the Collaborative Study, a more sober path for antipsychotic development might have been charted. Its findings were, however, sufficient to justify pharma’s pursuit of a ‘safe’ clozapine, already advanced. The method, in line with the times, was based on similarity of receptor-binding profiles. If clozapine had broad interactions, it was time to abandon the ‘highly selective’ D2 antagonist approach, increasingly advocated until then, and widen our horizons again (Figure 1).
Figure 1.
Antipsychotic drugs: schematic representation of some receptor-binding profiles (percentages of total binding: for method of calculation, see Hyttel et al., 1984 and Goldstein, 2000).
One potential problem was that clozapine’s receptor-binding profile is not dissimilar to chlorpromazine’s. However, clozapine’s greater affinity for 5HT2A receptors resonated with the laboratory observation that central dopamine systems are modulated via a two-pronged, serotonergic mechanism based on 5HT1A agonism and, more importantly, 5HT2A antagonism. A ‘serotonin-dopamine hypothesis’, where benefit accrues from greater serotonergic than dopaminergic actions (Huttunen, 1995), could be postulated to support the flurry of new antipsychotics – drugs, that being based on clozapine, must also, like clozapine, be ‘atypical’.
The concept of an ‘atypical’ antipsychotic long antedated interest in clozapine, but by the 1980s had become an aspiration rather than an achievement (Owens, 2008). To a profession dazzled by the over-burnished lustre of clozapine, however, ‘atypical’ was ready for a re-launch and, powered by modern marketing, took hold.
In this ‘second’ incarnation, ‘atypical’ has a particular meaning, extrapolated from clozapine – reduced liability to promote not just EPS in general but specifically parkinsonism (Owens, 2014). Leaving aside the inadequacies of assessment techniques, such a judgement would only allow an inference of pharmacological difference if comparator studies minimised any differences in the way trial drug and standard comparator were used. All phase III studies for new antipsychotics launched post-clozapine failed this test. Put simply, they did not compare ‘like-with-like’. The comparator choice was driven by the US market, where haloperidol exemplified ‘common practice’. This high potency, relatively D2-selective drug (Figure 1) with a particular proclivity to cause motor disturbance (Owens, 2012), was never an appropriate choice to set against generally intermediate or low-potency drugs with, overall, broad receptor-binding profiles. Many phenothiazines, the most widely utilised antipsychotics internationally and drugs of generally broader binding profiles, would have made for more valid comparisons. Furthermore, because of its wide therapeutic index, haloperidol was often subject to high/ultra-high dose use (Dencker, 1976; Donlon et al., 1980; McCreadie and MacDonald, 1977). It was from this environment that haloperidol comparator doses were chosen. Pharmacokinetically derived minimum effective doses of new drugs were evaluated against ‘common practice’ doses of haloperidol that in reality were four to five times those established from clinical (Oosthuizen et al., 2001; Rosebush and Mazurek, 1999; Rosenheck et al., 2003) and imaging data (Kapur et al., 1997) as ‘minimally effective’ (<5 mg/day).
Efficacy studies for antipsychotics launched in the wake of clozapine had such profound design weakness that the results could be predicted by examination of the methods. But psychiatry was only interested in the bottom line. Now, we had ‘two dichotomous groups’ of antipsychotics (Kinon and Lieberman, 1996): one reflecting old technology, typical and typically flawed; the other, new and sophisticated – ‘atypical’ – like clozapine. Between 1993 and 2006, antipsychotic psychopharmacology was the most fertile therapeutic area for new drug launches, with international pharma vying for prominence. In the literature and in the clinic, ‘atypical’ became shorthand for ‘better’.
Trying to find a unifying pharmacological theory to explain ‘atypicality’ was, however, proving difficult. The serotonin–dopamine hypothesis did not stand up well to scrutiny and was completely irrelevant to some compounds, such as amisulpride. The uncomfortable fact was that no specific pharmacological theory could be envisaged that underpinned dichotomous classification of antipsychotics – unless that was ‘clozapine’ versus ‘the rest’.
The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) was one of the seminal studies in psychopharmacology, and compared an older antipsychotic (perphenazine), chosen on clinical and binding characteristics, with the four newer drugs then available in the United States (Lieberman et al., 2005). This large pragmatic trial, funded independently of industry, failed to demonstrate any EPS advantage to the new drugs. The Cost Utility of the Latest Antipsychotic drugs in Schizophrenia Study (CUtLASS; Jones et al., 2006) came to a similar conclusion by a different route, as did others (Fischer-Barnicol et al., 2008; Geddes et al., 2000; Kahn et al., 2008). Although published a year after CATIE, CUtLASS was completed first, yet only deemed publishable by editors after CATIE! Science is most rigid when in the grip of an orthodoxy.
Many attempts were made to discredit these and similar findings (Naber and Lambert, 2009), but as no independent, quality studies have been able to validate the concept of ‘atypical’, the evidence is irrefutable – excepting clozapine, any EPS advantage attributable to newer antipsychotics can be explained entirely by the fact that new drugs are utilised more prudently than older ones (Geddes et al., 2000; Owens, 2014). Yet despite pleas that the concept of ‘atypical’ be again consigned to the ranks of aspiration (Fischer-Barnicol et al., 2008; Leucht et al., 2009; Owens, 2008), it lingers, its hegemony secured by powerful marketing.
This is deeply concerning. Clinically, psychiatry has shifted practice almost exclusively to newer antipsychotics, with a generation of practitioners unaware of, far less skilled in, the use of older compounds, which are increasingly falling to ‘sourcing’ problems. Some, for commercial reasons, are already lost (the latest, fluphenazine decanoate discontinued in 2018). Having been offered the prospect of doubling our therapeutic repertoire, we have ended up halving it. This is to ignore that the greater part of the 22% annual increase in prescribing costs for antipsychotics in recent years (Ilyas and Moncrieff, 2012) is attributable to the switch from inexpensive, generic compounds to branded products under exclusive licence.
Concern goes deeper. The uniqueness of clozapine most likely relates less to what it does than how it does it (Seeman and Tallerico, 1998) – to its rapid dissociation from dopaminergic sites rather than just the breadth of its binding profile. Yet literature searches reveal that the basic scientists on whom we depend for products of the future are also influenced by what marketing departments have sold us. The term ‘atypical’ still receives regular outings there, too. Will lines of research continue on the basis of old science that simply repeats the complacency of half a century ago, generating more ‘me-too’ drugs under the guise of progress?
50 years and beyond
So, what is the future for antipsychotics? Looking at the current commercial landscape, it is hard to be optimistic. And the commercial landscape matters for, with available psychotropics, we can look to academia for primary development of only one – lithium. All the major companies that so dominated antipsychotic development in the second ‘golden age’ have either withdrawn or down-graded neuroscience investment substantially. The torch has passed from large multinationals with deep pockets to small-/medium-sized organisations on one-off gambles. Such a major shift is not because of perceived lack of need – quite the contrary. It is commercial – economic – reality. The Dopamine Hypothesis did not create antipsychotic development but has been its driver for almost half a century. To those willing to invest, it is looking old and stale. More compounds to directly attenuate central D2 transmission, be it by post-synaptic antagonism or partial or inverse agonism, seem uninspiring. There may be room to exploit current evidence of disrupted pre-synaptic mechanisms but there are clinicians who still recall drugs of the past which did just that (e.g. tetrabenazine) but were abandoned on ‘weak’ and ineffective reputations and poor tolerability profiles.
Almost 30 years ago, the amphetamine-based model that supported pursuit of direct dopaminergic antagonism acquired a rival. Clinically, psychoses associated with the dissociative anaesthetics, ketamine and phencyclidine are more convincingly ‘schizophrenic’ than the circumscribed, predominantly delusional states associated with amphetamine (Javitt and Zukin, 1991; Krystal et al., 1994), face-validity that has supported intense interest in modulation of excitatory glutamatergic mechanisms as the ‘next-generation’ approach to antipsychotic development. But the secrets of these complex systems remain hidden. Looking at pipeline developments for the foreseeable future, it is ironic that despite the research emphasis of past decades, it seems that drugs modifying glutamatergic mechanisms will make their impact on the antidepressant, rather than the antipsychotic, market.
Ways forward might come from widening our repertoire of non-dopaminergic targets beyond glutamate to nicotinic, peptidogenic, hormonal, histaminergic and pro-inflammatory mechanisms among others, but the evidence so far is not encouraging that new avenues in this direction have hitherto gone undetected (Girgis et al., 2018). Alternatively, clearer understanding of the structure and binding mechanics of the D2 receptor itself (Wang et al., 2018), particularly with use of different antipsychotic ligands, may allow for better pharmacological targeting on efficacy, though this work is still in its infancy.
The immediate future does not look rosy and the shift of investment remains a concern. As the British Neuroscience Association moves into its second half-century, it is to be hoped that a younger generation of researchers will break out of the confines of traditional theorising that started a process but left the path to its conclusion obscure.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
References
- Carlsson A, Lindquist M. (1963) The effect of chlorpromazine or haloperidol on formation of 3-methoxytyramine and noradrenaline in mouse brain. Acta Pharmacologica et Toxicologica 20(2): 140–144. [DOI] [PubMed] [Google Scholar]
- Cole JO. and the NIMH-psychopharmacology service center. (1964) NIMH-psychopharmacology service center collaborative study group. Phenothiazine treatment in acute schizophrenia. Archives of General Psychiatry 10: 246–261. [PubMed] [Google Scholar]
- Creese I, Burt DR, Snyder SH. (1976) Dopamine receptor binding predicts clinical and pharmacological potencies or antischizophrenic drugs. Science 192(4238): 481–483. [DOI] [PubMed] [Google Scholar]
- Delay J, Deniker P. (1957) Characteristique neurophysiologique des medicaments neuroleptiques. Rapport symposium internationale medicaments neuroleptiques psychotropies. In: Garattini S, Ghetti V. (eds) Psychotropic Drugs. Amsterdam: Elsevier. [Google Scholar]
- Dencker SJ. (1976) High-dose treatment with neuroleptics in the acute phase of mental disease. Proceedings of the Royal Society of Medicine 69(Suppl. 1): 32–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlon PT, Hopkin JT, Tupin JP, et al. (1980) Haloperidol for acute schizophrenic patients: An evaluation of three oral regimes. Archives of General Psychiatry 37(6): 691–695. [DOI] [PubMed] [Google Scholar]
- Elkes J, Elkes C. (1954) The effects of chlorpromazine on the behaviour of chronically overactive psychotic patients. British Medical Journal 29(4887): 560–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Barnicol D, Lanquillon S, Haen E, et al. (2008) Typical and atypical antipsychotics–The misleading dichotomy. Neuropsychobiology 57(1–2): 80–87. [DOI] [PubMed] [Google Scholar]
- Geddes J, Freemantle N, Harrison P, et al. (2000) Atypical antipsychotics in the treatment of schizophrenia: Systematic overview and meta-regression analysis. British Medical Journal 321(7273): 1371–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis RR, Zoghbi AW, Javitt DC, et al. (2018) The past and future of novel, non-dopamine-2 receptor therapeutics for schizophrenia: A critical and comprehensive review. Journal of Psychiatric Research. Epub ahead of print 21 July. DOI: 10.1016/j.jpsychires.2018.07.006. [DOI] [PubMed] [Google Scholar]
- Goldstein JM. (2000) The new generation of antipsychotic drugs: How atypical are they? International Journal of Neuropsychopharmacology 3(4): 339–349. [DOI] [PubMed] [Google Scholar]
- Haase J-H. (1961) Extrapyramidal modification of fine movements: A ‘conditio sine qua non’ of the fundamental therapeutic action of neuroleptic drugs. Revue canadienne de biologie 20: 425–449. [PubMed] [Google Scholar]
- Hippius H. (1999) A historical perspective of clozapine. The Journal of Clinical Psychiatry 60(Suppl. 12): 22–23. [PubMed] [Google Scholar]
- Howes OD, Kapur S. (2009) The dopamine hypothesis of schizophrenia: Version III – the final common pathway. Schizophrenia Bulletin 35(3): 549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen M. (1995) The evolution of the serotonin-dopamine antagonist concept. Journal of Clinical Psychopharmacology 15(Suppl. 1): 4S–10S. [DOI] [PubMed] [Google Scholar]
- Hyttel J, Larsen J-J, Christensen AV, et al. (1984) Receptor-binding profiles of neuroleptics. In: Casey DE, Chase TN, Christensen AV, et al. (eds) Dyskinesia: Research and Treatment. Berlin; Heidelberg; New York; Tokyo: Springer-Verlag, pp. 9–18. [Google Scholar]
- Idanpaan-Heikklia J, Alhava E, Olkinuora M, et al. (1975) Clozapine and agranulocytosis. The Lancet 2(7935): 611. [DOI] [PubMed] [Google Scholar]
- Ilyas S, Moncrieff J. (2012) Trends in prescriptions and costs of drugs for mental disorders in England, 1998 – 2010. British Journal of Psychiatry 200(5): 393–398. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. (1991) Recent advances in the phencyclidine model of schizophrenia. The American Journal of Psychiatry 148(10): 1301–1308. [DOI] [PubMed] [Google Scholar]
- Johnstone EC, Crow TJ, Frith CD, et al. (1978) Mechanism of the antipsychotic effect in the treatment of acute schizophrenia. The Lancet 1(8069): 848–851. [DOI] [PubMed] [Google Scholar]
- Jones PB, Barnes TR, Davies L, et al. (2006) Randomised controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Archives of General Psychiatry 63(10): 1079–1087. [DOI] [PubMed] [Google Scholar]
- Kahn RS, Fleischhacker WW, Boter H, et al. (2008) Effectiveness of antipsychotic drugs in first episode schizophrenia and schizophreniform disorder: An open, randomised clinical trial. The Lancet 371(9618): 1085–1097. [DOI] [PubMed] [Google Scholar]
- Kane J, Honigfeld G, Singer J, et al. (1988) Clozapine for treatment-resistant schizophrenia. Archives of General Psychiatry 45(9): 789–796. [DOI] [PubMed] [Google Scholar]
- Kapur S, Zipursky R, Roy P, et al. (1997) The relationship between D2 receptor occupancy and plasma levels on low dose oral haloperidol: A PET study. Psychopharmacology 131(2): 148–152. [DOI] [PubMed] [Google Scholar]
- Kinon B, Lieberman JA. (1996) Mechanisms of action of atypical antipsychotic drugs: A critical analysis. Psychopharmacology 124(1–2): 2–34. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, et al. (1994) Subanaesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychometric, perceptual, cognitive, and neuroendocrine responses. Archives of General Psychiatry 51(3): 199–214. [DOI] [PubMed] [Google Scholar]
- Laborit H, Huguenard P, Alluaume R. (1952) Un nouveau stabilisateur vegetatif. Press Medicale 60(10): 206–208. [PubMed] [Google Scholar]
- Leucht S, Heres S, Hamann J, et al. (2008) Methodological issues in current antipsychotic drug trials. Schizophrenia Bulletin 34(2): 275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht S, Kissling W, Davis JM. (2009) Second-generation antipsychotics for schizophrenia: Can we resolve the conflict? Psychological Medicine 39(10): 1591–1602. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, et al. (2005) Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. The New England Journal of Medicine 353(12): 1209–1223. [DOI] [PubMed] [Google Scholar]
- McCreadie RG, MacDonald IM. (1977) High dosage haloperidol in chronic schizophrenia. British Journal of Psychiatry 131(3): 310–316. [DOI] [PubMed] [Google Scholar]
- Matthysse S. (1973) Antipsychotic drug actions–A clue to the neuropathology of schizophrenia? Federation Proceedings 32(2): 200–205. [PubMed] [Google Scholar]
- Naber D, Lambert M. (2009) The CATIE and CUtLASS studies in schizophrenia: Results and implications for clinicians. CNS Drugs 23(8): 649–659. [DOI] [PubMed] [Google Scholar]
- Oosthuizen P, Emsley RA, Turner J, et al. (2001) Determining the optimal dose of haloperidol for first-episode psychosis. Journal of Psychopharmacology 15(4): 251–255. [DOI] [PubMed] [Google Scholar]
- Owen F, Cross AJ, Crow TJ, et al. (1978) Increased dopamine-receptor sensitivity in schizophrenia. The Lancet 2(8083): 223–226. [DOI] [PubMed] [Google Scholar]
- Owens DGC. (2008) How CATIE brought us back to Kansas: A critical re-evaluation of the concept of atypical antipsychotics and their place in the treatment of schizophrenia. Advances in Psychiatric Treatment 14(1): 17–28. [Google Scholar]
- Owens DGC. (2012) Meet the relatives: A reintroduction to the clinical pharmacology of ‘typical’ antipsychotics (Part 2). Advances in Psychiatric Treatment 18(5): 337–350. [Google Scholar]
- Owens DGC. (2014) A Guide to the Extrapyramidal Side-effects of Antipsychotic Drugs (2th edn). Cambridge: Cambridge University Press. [Google Scholar]
- Randrup A, Munkvad I. (1974) Pharmacology and physiology of stereotyped behaviour. Journal of Psychiatric Research 11: 1–10. [DOI] [PubMed] [Google Scholar]
- Rosebush PI, Mazurek MF. (1999) Neurologic side-effects in neuroleptic-naïve patients treated with haloperidol and risperidone. Neurology 52(4): 782–785. [DOI] [PubMed] [Google Scholar]
- Rosenheck RA, Perlick D, Bingham S, et al. (2003) Effectiveness and cost of olanzapine and haloperidol in the treatment of schizophrenia: A randomised controlled trial. Journal of the American Medical Association 290(20): 2693–2702. [DOI] [PubMed] [Google Scholar]
- Seeman P, Chau-Wong M, Tadesco J, et al. (1975) Brain receptors for antipsychotic drugs and dopamine: direct binding assays. Proceedings of the National Academy of Sciences (USA) 72(11): 4376–4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P, Tallerico T. (1998) Antipsychotic drugs which elicit little or no parkinsonism bind more loosely than dopamine to brain D2| receptors yet occupy high levels of these receptors. Molecular Psychiatry 3(2): 123–134. [DOI] [PubMed] [Google Scholar]
- Snyder SH. (1976) The dopamine hypothesis of schizophrenia: Focus on the dopamine receptor. The American Journal of Psychiatry 133(2): 197–202. [DOI] [PubMed] [Google Scholar]
- Swazey JP. (1974) Chlorpromazine in Psychiatry: A Study in Therapeutic Innovation. Boston, MA: The MIT Press. [Google Scholar]
- Tyrer P, Johnson S. (2011) Has the closure of psychiatric beds gone too far? British Medical Journal 343(7833): 1088–1089. [Google Scholar]
- Wang S, Che T, Levit A, et al. (2018) Structure of the D2 dopamine receptor bound to the atypical antipsychotic drug risperidone. Nature 555(7695): 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D, Wagner H, Tune L, et al. (1986) Positron emission tomography reveals elevated D2 dopamine receptors in drug-naïve schizophrenics. Science 234(4783): 1588–1563. [DOI] [PubMed] [Google Scholar]