Key Points
Question
Does the addition of the immune checkpoint inhibitor pembrolizumab to standard neoadjuvant chemotherapy improve efficacy in early-stage, high-risk, ERBB2 (formerly HER2)-negative breast cancer?
Findings
In this analysis of the adaptively randomized phase 2 I-SPY2 trial, including 250 women with early-stage breast cancer, the addition of pembrolizumab to standard neoadjuvant chemotherapy more than doubled complete pathologic response rates compared with chemotherapy alone for both hormone receptor-positive/ERBB2-negative, and triple-negative breast cancer.
Meaning
These results from the I-SPY2 trial suggest that there is a greater than 99% predictive probability that pembrolizumab plus neoadjuvant chemotherapy will be significantly better than chemotherapy alone in a phase 3 randomized clinical trial in ERBB2-negative breast cancer.
This analysis of data from an ongoing open-label adaptively randomized phase 2 platform trial examines the efficacy of adding pembrolizumab to standard neoadjuvant chemotherapy in patients with early-stage, high-risk, ERBB2-negative breast cancer.
Abstract
Importance
Approximately 25% of patients with early-stage breast cancer who receive (neo)adjuvant chemotherapy experience a recurrence within 5 years. Improvements in therapy are greatly needed.
Objective
To determine if pembrolizumab plus neoadjuvant chemotherapy (NACT) in early-stage breast cancer is likely to be successful in a 300-patient, confirmatory randomized phase 3 neoadjuvant clinical trial.
Design, Setting, and Participants
The I-SPY2 study is an ongoing open-label, multicenter, adaptively randomized phase 2 platform trial for high-risk, stage II/III breast cancer, evaluating multiple investigational arms in parallel. Standard NACT serves as the common control arm; investigational agent(s) are added to this backbone. Patients with ERBB2 (formerly HER2)-negative breast cancer were eligible for randomization to pembrolizumab between November 2015 and November 2016.
Interventions
Participants were randomized to receive taxane- and anthracycline-based NACT with or without pembrolizumab, followed by definitive surgery.
Main Outcomes and Measures
The primary end point was pathologic complete response (pCR). Secondary end points were residual cancer burden (RCB) and 3-year event-free and distant recurrence-free survival. Investigational arms graduated when demonstrating an 85% predictive probability of success in a hypothetical confirmatory phase 3 trial.
Results
Of the 250 women included in the final analysis, 181 were randomized to the standard NACT control group (median [range] age, 47 [24.77] years). Sixty-nine women (median [range] age, 50 [27-71] years) were randomized to 4 cycles of pembrolizumab in combination with weekly paclitaxel followed by AC; 40 hormone receptor (HR)-positive and 29 triple-negative. Pembrolizumab graduated in all 3 biomarker signatures studied. Final estimated pCR rates, evaluated in March 2017, were 44% vs 17%, 30% vs 13%, and 60% vs 22% for pembrolizumab vs control in the ERBB2-negative, HR-positive/ERBB2-negative, and triple-negative cohorts, respectively. Pembrolizumab shifted the RCB distribution to a lower disease burden for each cohort evaluated. Adverse events included immune-related endocrinopathies, notably thyroid abnormalities (13.0%) and adrenal insufficiency (8.7%). Achieving a pCR appeared predictive of long-term outcome, where patients with pCR following pembrolizumab plus chemotherapy had high event-free survival rates (93% at 3 years with 2.8 years’ median follow-up).
Conclusions and Relevance
When added to standard neoadjuvant chemotherapy, pembrolizumab more than doubled the estimated pCR rates for both HR-positive/ERBB2-negative and triple-negative breast cancer, indicating that checkpoint blockade in women with early-stage, high-risk, ERBB2-negative breast cancer is highly likely to succeed in a phase 3 trial. Pembrolizumab was the first of 10 agents to graduate in the HR-positive/ERBB2-negative signature.
Trial Registration
ClinicalTrials.gov Identifier: NCT01042379
Introduction
The immune system is regulated by a delicate balance of factors that initiate antitumor immune responses and inhibit excessive inflammation and autoimmunity. Cells from both the innate and adaptive immune systems work to eradicate pathogens and other threats, including cancer. A number of investigations1,2,3 over the past decade suggest that a proportion of breast cancers are immunogenically active and that some breast tumors have a substantial lymphocytic infiltrate. Lymphocyte-predominant breast cancers are characterized by tumor-infiltrating lymphocytes comprising 50% or more of the tumor bed. Primary breast tumors with a robust immune infiltrate are associated with a better response to neoadjuvant chemotherapy.
Programmed cell death ligand-1 (PD-L1) is expressed on the surface of multiple types of cells, including tumor and infiltrating immune cells. Programmed cell death protein-1 (PD-1) is expressed primarily on T cells. Programmed cell death protein-1 interacts with its ligands (PD-L1 and PD-L2) and directly inhibits apoptosis of tumor cells, and promotes peripheral T effector cell exhaustion and the conversion of T effector cells to immunosuppressive T regulatory cells. Pembrolizumab (MK-3475, Keytruda, Merck) a highly selective, humanized IgG4 monoclonal antibody specific for PD-1, is US Food and Drug Administration (FDA)-approved for use in a number of advanced malignant diseases.4
Pembrolizumab has been investigated as monotherapy for the treatment of advanced triple-negative breast cancer (TNBC) and hormone receptor (HR)-positive breast cancer. Initial studies have reported low response rates in previously treated, advanced HR-positive and TNBC (12% and 4.8%-18.5%, respectively).5,6,7 KEYNOTE 086-cohort B reported a monotherapy response rate of 23% in previously untreated, PD-L1–positive, advanced TNBC. Adams and colleagues8 reported that anthracycline- or taxane-based chemotherapy in this setting had a rate of 23%. Although the response rates are similar in the frontline setting, the duration of response with immunotherapy is longer.9
Based on a 2018 meta-analysis,10 24.9% of those with early-stage breast cancer who receive (neo)adjuvant therapy had a distant recurrence by 5 years. The promising efficacy observed with single-agent checkpoint blockade for advanced ERBB2 (formerly HER2)-negative breast cancer, and the considerable benefits observed with PD-1 inhibitors combined with chemotherapy for lung cancer and other malignant diseases, led us to evaluate the efficacy of adding pembrolizumab to standard neoadjuvant chemotherapy in the I-SPY2 trial, with the hypothesis that immune-targeted agents would be more effective in the early-stage setting when the immune system is less likely to be compromised.
Methods
Study Design
The I-SPY2 study is an ongoing, multicenter, open-label, adaptively randomized phase 2 multicenter trial of neoadjuvant chemotherapy (NACT) for early-stage breast cancer at high risk of recurrence (NCT01042379).11,12 It is a platform trial evaluating multiple investigational arms in parallel, each consisting of standard NACT (serving as the common control arm) plus an investigational agent/combination. The primary end point is pathologic complete response (pCR), defined as the absence of invasive tumor in breast and regional nodes at the time of surgery. The primary analysis is modified intention to treat, where participants receiving allocated therapy are considered evaluable; participants who switch to nonprotocol assigned therapy, forgo surgery, or withdraw from the trial are considered “non-pCR” during analysis. Secondary end points include residual cancer burden (RCB), 3-year event-free survival (EFS), and distant relapse-free survival (DRFS). All patients are followed up for long-term outcome and safety.
Biomarker assessments performed at baseline are used to classify patients into 1 of 8 subtypes based on HR, ERBB2-receptor, and MammaPrint status.11 Adaptive randomization in I-SPY2 preferentially assigns patients to agents according to Bayesian probabilities of rates of pCR for each subtype; 20% of patients are randomly assigned to control.
Agents graduate from I-SPY2 by reaching, in any of the 10 clinically relevant signatures, a predefined efficacy threshold of 85% probability of success in a subtype-specific, hypothetical 300-patient, 1-to-1 confirmatory phase 3 trial.13,14 Agents may be dropped for futility if the predicted probability of success in phase 3 is less than 10% for all signatures or the maximum enrollment threshold is reached for that arm. Graduation probabilities for each actively enrolling arm are continually updated using a longitudinal model based on change in tumor volume by magnetic resonance imaging (MRI, for those still undergoing treatment) and pathologic response for those that have completed surgical therapy. Additional details on the study design have been published previously.11,12,15 The trial protocol and protocol amendments are available in Supplement 1.
Eligibility
Patients eligible for I-SPY2 are women aged 18 years or older, with stage II or III breast cancer and primary tumors larger than 2.5 cm by clinical examination or larger than 2.0 cm by imaging, and Eastern Cooperative Oncology Group performance status of 0 or 1.16 Patients with MammaPrint low-risk HR-positive, ERBB2-negative disease are excluded from I-SPY2 because their lower risk of recurrence does not justify escalation of therapy.17 All patients provide written informed consent prior to screening and again after randomization. Only ERBB2-negative patients were eligible for randomization to the pembrolizumab arm.
Treatment
Participants in the control arm received standard NACT: 80 mg/m2 intravenous paclitaxel weekly for 12 weeks, followed by 4 cycles of 60 mg/m2 doxorubicin plus 600 mg/m2 intravenous cyclophosphamide every 2 to 3 weeks (AC). Participants in the pembrolizumab arm received standard NACT plus 200 mg intravenous pembrolizumab every 3 weeks for 4 cycles (weeks 1, 4, 7, and 10) concurrently with paclitaxel. Steroid premedication was standardized across sites. For the first infusion, pembrolizumab was given first, followed by 20 mg of dexamethasone received orally after a 30-minute waiting period, followed by paclitaxel 30 minutes later. If no infusion reaction occurred, dexamethasone was reduced to 10 mg for week 2. If participants did not experience an infusion reaction with the first 2 doses of paclitaxel, dexamethasone was discontinued. If patients had an infusion reaction despite corticosteroid premedications, switching to nab-paclitaxel was allowed. If infusion reactions were manageable with dexamethasone, the premedications remained unchanged.
Definitive surgery followed AC, with lumpectomy or mastectomy at the discretion of the treating surgeon. Sentinel node dissection was allowed in patients with node-negative disease, with axillary node dissection in patients with node-positive disease according to National Comprehensive Cancer Network (NCCN) and local practice guidelines.18 Adjuvant treatment was not mandated by the trial, but was at the discretion of the treating oncologist. However, standard-of-care adjuvant therapy per NCCN guidelines was recommended.
Assessments
Core biopsies and breast MRIs were performed at baseline and following 3 weeks of therapy. Additional MRIs were performed between paclitaxel and AC and again following AC, as previously described.11,12 Surgical specimens were analyzed for response by local pathologists trained to assess residual cancer burden (RCB).19 Biomarkers assessed include the 70-gene MammaPrint17,20 and TargetPrint ERBB2 gene expression assays21 using the 44K full-genome microarray (Agendia).
Trial Oversight
The trial was designed by the I-SPY2 study investigators. Merck provided study drug but played no role in the study design, collection/analysis of data or manuscript preparation. All participating sites received institutional review board approval, and patients provided written informed consent. The I-SPY2 DSMB meets monthly to review patient safety and study progress.
Statistical Analysis
In the standard I-SPY2 Bayesian approach, probability distributions of pCR rate for each regimen in each subtype are updated continuously via a covariate analysis with HR, ERBB2, and MP status as covariates, adjusting for time trends to allow comparisons against all enrolled I-SPY2 controls prior to the date randomization was stopped for the investigational arm (eMethods in Supplement 2). Adaptive randomization probabilities and the Bayesian probability that each regimen is superior to control are derived from these distributions. Graduation of a treatment arm occurs if the predicted pCR rate in any signature meets the prespecified threshold of 85% probability of success in a hypothetical 300-patient, 1-to-1 randomized, phase 3 trial.13,14 Final end point analysis was completed after all participants completed surgery, in March 2017.
Kaplan-Meier survival curves for each arm were generated, with hazard ratios (HRs) by Cox proportional hazard modeling. Twenty-six of the 172 controls included in this exploratory analysis were concurrently randomized with pembrolizumab and no adjustments for time trends were made. Statistics regarding this exploratory EFS analysis, assessed in March 2019, are descriptive only because sample sizes were small and I-SPY2 is not powered for EFS or other survival end points.
Results
Patient Population
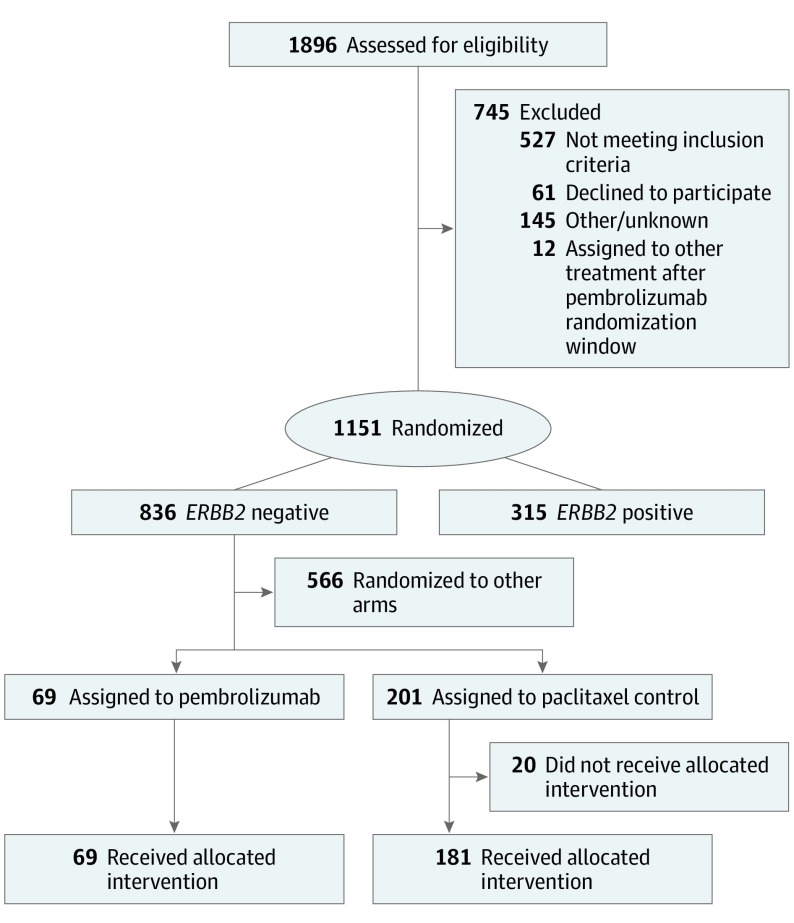
Patients with ERBB2-negative breast cancer who enrolled in I-SPY2 between November 26, 2015, and November 5, 2016, were eligible for randomization to pembrolizumab. A total of 69 patients were adaptively randomized to pembrolizumab and were evaluable for the primary end point (40 HR-positive/ERBB2-negative, 29 TNBC). The contemporary control population for the primary efficacy analysis consisted of 181 patients randomized to the control arm who received their allocated treatment from the opening of I-SPY2 enrollment on March 30, 2010 until November 5, 2016 (Figure 1). Baseline characteristics were similar between the pembrolizumab and control arms (eTable in Supplement 2). Twenty patients in the control arm did not proceed with their allocated assignment. Two patients on pembrolizumab and 7 in the control arm did not proceed to surgery; all 9 were counted as non-pCR.
Figure 1. CONSORT Diagram for the Pembrolizumab Arm and Control Arm Populations.
I-SPY2 utilizes contemporary controls for analysis purposes, in which the comparator control arm consists of all patients enrolled to the control arm from the start of I-SPY2 until the close of the specified investigational arm.
Efficacy
After 69 patients were randomized to the intervention arm, pembrolizumab achieved the prespecified graduation threshold (≥85% likelihood of predictive probability of success in a phase 3 trial) for all 3 signatures studied. When pembrolizumab reached the graduation threshold based on MRI models in the 3 signatures, randomization to the arm was halted. Once patients completed surgery and RCB was assessed, the final predictive probabilities were generated, as reported in Table 1. Probability distributions for achieving pCR are shown in eFigure 1A in Supplement 2, and the distribution of patients by RCB are shown in eFigure 1B in Supplement 2. Estimated pCR rates for ERBB2-negative, HR-positive/ERBB2-negative, and TNBC signatures in the pembrolizumab arm were 44%, 30%, and 60%, compared with 17%, 13%, and 22% in the control populations, respectively. A lower percentage of patients in the pembrolizumab arm had RCB-III at the time of surgery compared with control; no patients with TNBC in the pembrolizumab arm had RCB-III (eFigure 1B in Supplement 2). Nine patients (2 in the pembrolizumab and 7 in the control arm) did not proceed to surgery and are not included in the RCB analysis (eFigure 1B in Supplement 2).
Table 1. Final Predictive Probabilities of Success of 4 Cycles of Pembrolizumab With Paclitaxel Followed by Anthracyclines in Phase 3 Testing in the 3 ERBB2 Biomarker Signaturesa.
| Biomarker Signature | Estimated Rate of Pathologic Complete Response (95% Probability Interval) | Probability, % | ||
|---|---|---|---|---|
| Pembrolizumab (n = 69) | Control (n = 181) | Probability Superior to Control | Predictive Probability of Success in Phase 3 Trial | |
| ERBB2 negative | 44 (33-55) | 17 (11-23) | >99.9 | 98.5 |
| HR positive/ERBB2 negative | 30 (17-43) | 13 (7-19) | >99.9 | 99.6 |
| TNBC | 60 (44-75) | 22 (13-30) | 99.6 | 83.4 |
Abbreviations: HR, hormone receptor; TNBC, triple-negative breast cancer.
The pembrolizumab regimen graduated in all 3 signatures, based on the predefined efficacy threshold for graduation (85% probability of success in a 300-patient phase 3 trial). As enrollment to an investigational arm progresses, the probability of success in a phase 3 trial is continually updated based on longitudinal magnetic resonance imaging assessments and pathologic responses in patients who have had surgery. Agents graduate and accrual to the investigational arm closes when the predictive probability of success is 85% or more. The final predicted probability of success is updated after all patients in the investigational arm have completed surgery, thus graduates can have a final predictive probability of less than 85%, but are still considered graduates.
Six patients in the pembrolizumab arm either progressed or did not respond to treatment; pembrolizumab was discontinued and carboplatin was administered to 3 of these 6. These patients were counted as non-pCR to protocol-directed therapy regardless of their final pathologic response.
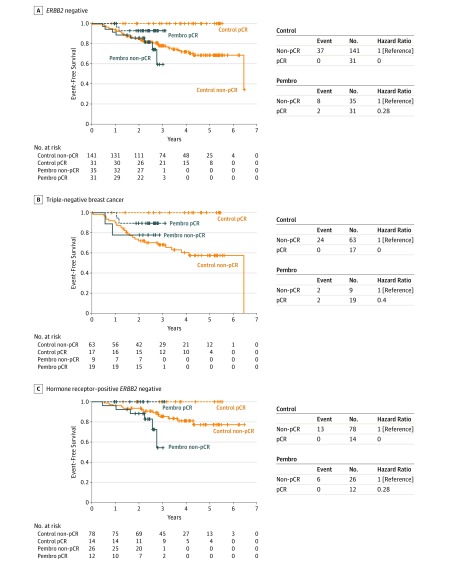
Participants in the pembrolizumab (n = 66) and control (n = 172) arms who had follow-up data as of February 26, 2019, were included in an exploratory EFS analysis (Figure 2) (eFigure 2 in Supplement 2). Median follow-up times for patients in the pembrolizumab and control arms were 2.8 and 3.5 years, respectively; only 4 of 69 patients randomized to pembrolizumab had 3 or more years of follow-up. Qualitatively similar EFS was observed between the pembrolizumab and control arms for the overall cohort (Figure 2), although caution must be emphasized in drawing conclusions owing to the small number of patients. Patients who achieved pCR had excellent outcomes regardless of arm.
Figure 2. Event-Free Survival by Signature and Pathologic Complete Response (pCR).
Event-free survival of the (A) entire ERBB2 (formerly HER2)-negative cohort, (B) triple-negative cohort, and (C) HR-positive/ERBB2-negative cohort. The blue curves represent patients treated with pembrolizumab and the orange those treated with control; the dashed lines represent those who achieved a pCR and the solid line those who did not. Only those individuals who had follow-up information as of the cutoff date of February 26, 2019, are included in this analysis (66 and 172, in the pembrolizumab [Pembro] and control arms, respectively).
Safety and Toxic Effects
All patients who received at least 1 dose of study-assigned therapy were evaluable for safety and toxic effects. Selected clinically relevant adverse events reported within 180 days of the last investigational agent dose (pembrolizumab for investigational arm and paclitaxel for control arm) are summarized in Table 2. The most notable differences in adverse events between the arms were in the incidence of immune-related adverse events (irAEs), most of which were grade 1 to 2 and treated per protocol with dose interruption or steroid therapy. Adverse events observed in the pembrolizumab plus chemotherapy arm were consistent with the known safety profile of each component; no new safety concerns were identified. The most common irAEs reported were endocrinopathies, with thyroid dysfunction being the most common (hypothyroidism and hyperthyroidism), occurring in 9 of the 69 patients who received pembrolizumab (13.0%). Adrenal insufficiency (AI) was observed in 6 of the 69 patients who received pembrolizumab (8.7%), with 5 of these irAE occurring more than 30 days after last dose of pembrolizumab. Three of the cases of AI were classified as hypophysitis and 1 as primary adrenal insufficiency; in the remaining 2 cases determination of primary vs secondary AI was not possible owing to initiation of steroid therapy prior to completion of workup. A number of irAEs, with the exception of pruritus, occurred more than 30 days after the final dose of pembrolizumab. These irAEs were successfully treated per protocol with dose interruption or steroids, and no irAE fatalities were observed.
Table 2. Selected Adverse Events Reported Within 180 Days of Last Investigational Treatment (Pembrolizumab for the Investigational Arm and Paclitaxel for the Control Arm)a.
| Event | No. (%) | ||||||
|---|---|---|---|---|---|---|---|
| Pembrolizumab (n = 69) | Control (n = 181) | ||||||
| Days | Total | Days | Total | ||||
| <30 | 30-180 | <30 | 30-180 | ||||
| Selected Adverse Events | |||||||
| All grades | |||||||
| Febrile neutropenia | 1 (1.4) | 5 (7.2) | 6 (8.7) | 7 (3.9) | 6 (3.3) | 13 (7.2) | |
| Neutropenia | 5 (7.2) | 2 (2.9) | 7 (10.1) | 2 (1.1) | 2 (1.1) | 4 (2.2) | |
| Anemia | 14 (20.3) | 9 (13.0) | 23 (33.3) | 17 (9.4) | 18 (9.9) | 35 (19.3) | |
| Fatigue | 51 (73.9) | 9 (13.0) | 60 (87) | 133 (73.5) | 19 (10.5) | 152 (84) | |
| Nausea | 44 (63.8) | 11 (15.9) | 55 (79.7) | 117 (64.6) | 14 (7.7) | 131 (72.4) | |
| Vomiting | 18 (26.1) | 8 (11.6) | 26 (37.7) | 30 (16.6) | 6 (3.3) | 36 (19.9) | |
| Diarrhea | 29 (42) | 10 (14.5) | 39 (56.5) | 60 (33.1) | 10 (5.5) | 70 (38.7) | |
| Peripheral motor neuropathy | 6 (8.7) | 3 (4.3) | 9 (13) | 7 (3.9) | 2 (1.1) | 9 (5) | |
| Peripheral sensory neuropathy | 36 (52.2) | 3 (4.3) | 39 (56.5) | 101 (55.8) | 14 (7.7) | 115 (63.5) | |
| Grade 3-4 | |||||||
| Febrile neutropenia | 1 (1.4) | 5 (7.2) | 6 (8.7) | 7 (3.9) | 6 (3.3) | 13 (7.2) | |
| Neutropenia | 1 (1.4) | 0 | 1 (1.4) | 1 (0.6) | 1 (0.6) | 2 (1.1) | |
| Anemia | 1 (1.4) | 4 (5.8) | 5 (7.2) | 2 (1.1) | 8 (4.4) | 10 (5.5) | |
| Fatigue | 4 (5.8) | 1 (1.4) | 5 (7.2) | 1 (0.6) | 0 | 1 (0.6) | |
| Nausea | 3 (4.3) | 1 (1.4) | 4 (5.8) | 0 | 0 | 0 | |
| Vomiting | 1 (1.4) | 0 | 1 (1.4) | 0 | 0 | 0 | |
| Diarrhea | 3 (4.3) | 2 (2.9) | 5 (7.2) | 3 (1.7) | 1 (0.6) | 4 (2.2) | |
| Peripheral motor neuropathy | 1 (1.4) | 0 | 1 (1.4) | 0 | 0 | 0 | |
| Peripheral sensory neuropathy | 1 (1.4) | 0 | 1 (1.4) | 3 (1.7) | 0 | 3 (1.7) | |
| Adverse Events of Special Interest (Including Immune-Related Toxic Effects) | |||||||
| All grades | |||||||
| Hypothyroidismb | 3 (4.3) | 4 (5.8) | 7 (10.1) | 0 | 0 | 0 | |
| Hyperthyroidismb | 2 (2.9) | 2 (2.9) | 4 (5.8) | 0 | 0 | 0 | |
| Adrenal insufficiencyc | 1 (1.4) | 5 (7.2) | 6 (8.7) | 0 | 0 | 0 | |
| Hepatitisd | 1 (1.4) | 1 (1.4) | 2 (2.9) | 0 | 0 | 0 | |
| Pneumonitis | 2 (2.9) | 1 (1.4) | 3 (4.3) | 1 (0.6) | 1 (0.6) | 2 (1.1) | |
| Colitis | 0 | 1 (1.4) | 1 (1.4) | 0 | 1 (0.6) | 1 (0.6) | |
| Pruritus | 22 (31.9) | 0 | 22 (31.9) | 19 (10.5) | 3 (1.7) | 22 (12.2) | |
| Grade 3-4 | |||||||
| Hypothyroidism | 0 | 1 (1.4) | 1 (1.4) | 0 | 0 | 0 | |
| Hyperthyroidism | 0 | 0 | 0 | 0 | 0 | 0 | |
| Adrenal insufficiencyc | 1 (1.4) | 4 (5.8) | 5 (7.2) | 0 | 0 | 0 | |
| Hepatitisd | 1 (1.4) | 1 (1.4) | 2 (2.9) | 0 | 0 | 0 | |
| Pneumonitis | 0 | 0 | 0 | 0 | 1 (0.6) | 1 (0.6) | |
| Colitis | 0 | 1 (1.4) | 1 (1.4) | 0 | 1 (0.6) | 1 (0.6) | |
| Pruritus | 0 | 0 | 0 | 1 (0.6) | 0 | 1 (0.6) | |
Abbreviation: AEs, adverse events.
The number of AEs occurring within 30 days and those occurring between 30-180 days of last dose are reported.
Two patients had both hypothyroidism and hyperthyroidism over the course of their treatment.
Includes primary and secondary causes of adrenal insufficiency (eg, hypophysitis and hypopituitarism).
Includes autoimmune hepatitis and hepatitis.
Discussion
We describe results from the I-SPY2 study arm examining immune checkpoint blockade in combination with chemotherapy for high-risk, early-stage ERBB2-negative breast cancer. The addition of 4 cycles of pembrolizumab to standard-of-care NACT more than doubled estimated pCR rates in all biomarker signatures studied. Notably, pembrolizumab was the first agent of 10 studied to graduate in the HR-positive/ERBB2-negative signature since I-SPY2 opened in 2010.
The pCR rates in the I-SPY2 control population have been stable over time, but are lower than reported in other neoadjuvant studies.22 I-SPY2 is an unblinded study where both patients and physicians are aware of treatment assignment; analysis is intention to treat. Treating physicians concerned about poor response may modify the treatment regimen (eg, add carboplatin to the paclitaxel portion of treatment); these cases are counted as non-pCR regardless of response, due to nonadherence to study assignment. Three patients in the pembrolizumab arm and 11 patients in the control arm who received neoadjuvant carboplatin were classified as non-pCR. It should be emphasized that the estimated pCR rates that are the reporting standard in I-SPY2 are different than pCR rates reported from the studies using a typical randomized clinical trial design. The estimated pCR rate represents the mean of the final posterior probability distribution for pCR in a given subtype, using a model adjusting for subtype and time trend. In this study, actual (raw) rates of pCR, (the standard for reporting in a typical randomized clinical trial), were higher than the estimated rates, but may be biased owing to the adaptive randomization procedure that favors regimens and subtypes exhibiting better response. The comparative estimated pCR rates between the experimental and control arms provide perspective on the potential impact of an individual therapy in a given subtype. However, it is important to bear in mind that I-SPY2 is a phase 2 study designed to rapidly screen for agents that are likely to succeed in phase 3 trials. In this respect, preliminary reports from the phase 3 KEYNOTE 522 study provide strong validation of this approach.23,24
Initial concerns that steroid premedications required for paclitaxel might interfere with the efficacy of pembrolizumab appear unfounded. Although steroid premedications were discontinued after 2 doses of (weekly) paclitaxel if no infusion reactions were observed, they were routinely used per physician discretion for infusion reactions and management of irAEs. Because median time to response with pembrolizumab monotherapy in advanced breast cancer was reportedly 18 weeks,5 there was initial concern that the 20- to 24-week duration of NACT would be too short to see the benefit of neoadjuvant pembrolizumab. Although we observed no complete responses on the week 3 MRI, 16 of 31 (52%) of those who eventually achieved a pCR showed greater than 95% reduction in tumor volume by week 12, with the rest achieving a pCR by 24 weeks.
The addition of pembrolizumab increased irAEs, particularly endocrinopathies. Rates of thyroid abnormalities (including both hypothyroidism and hyperthyroidism) were similar to published reports with pembrolizumab, whereas rates of AI were higher.25 Adrenal insufficiency (primary and secondary) onset was typically observed after completion of pembrolizumab, with 5 of 6 patients diagnosed postoperatively, more than 12 weeks following the last dose of pembrolizumab. Presentation included symptoms of extreme fatigue, nausea, and emesis. Five patients with AI were hospitalized for evaluation and treatment. As of this writing, all 6 cases of AI observed in this arm are ongoing; patients are doing well on replacement therapy. Serial cortisol assessments (not routinely performed at the outset) were instituted on January 13, 2017, to detect subclinical cases of AI prior to surgery. Half of those who developed AI did so prior to routine screening, and the other 3 of 6 had presurgical cortisol testing.
The reason for elevated rates of AI in this study is unclear. Of the 6 patients who developed AI, there was no discernable correlation with clinicopathologic features, including age, stage, HR status, and pCR/RCB (3 achieved pCR, 3 did not), with the latter equally distributed among RCB-I, RCB-II, and RCB-III (the patient who achieved RCB-I also received carboplatin owing to suboptimal response). In KEYNOTE 522, 4.5% of patients developed AI (1.8% and 2.7% had primary and secondary AI, respectively). The higher rates observed in our trial could be related to difference in chemotherapy backbone, aggressive tapering of steroid premedications, or simply an artifact of small sample size. Regardless, future work to characterize the risk factors for developing irAEs is warranted, to improve the therapeutic index of these agents.
Limitations
In an exploratory analysis, no significant differences in 3-year EFS were found between the pembrolizumab and control arms. However, follow-up for the pembrolizumab arm was shorter (median, 2.8 years vs 3.5 years for control), many of the nonresponders received additional therapy, and the study was underpowered for this end point. This is a limitation of the I-SPY2 approach, where the adaptive randomization results in fewer patients being required to reach statistical significance compared with standard randomized clinical trials. Patients with HR-positive/ERBB2-negative disease who failed to achieve a pCR seemed to do particularly poorly. It is possible that pembrolizumab moves patients to pCR who would have otherwise had a good EFS outcome despite residual disease and thus the failure to achieve a pCR in HR-positive/ERBB2-negative disease with the addition of pembrolizumab identifies a particularly bad prognosis group. It is also possible that pembrolizumab worsens outcome for a subset of patients in this subtype; the ongoing randomized phase 3 KEYNOTE 756 will further clarify the outcome of these patients. Most importantly, though, those patients who achieved a pCR regardless of signature had good outcomes.
Conclusions
The immune checkpoint inhibitor pembrolizumab, when added to standard NACT, was associated with improvement in pCR rates over chemotherapy alone in women with high-risk, early-stage, ERBB2-negative breast cancer. Randomized phase 3 registrational trials evaluating pembrolizumab in combination with standard NACT for TNBC (KEYNOTE 522) and high-risk, HR-positive/ERBB2-negative breast cancer (KEYNOTE 756) are ongoing. Preliminary reports that the addition of pembrolizumab to standard NACT in TNBC is associated with improved pCR rates in the KEYNOTE 522 randomized phase 3 trial provides validation of the I-SPY2 concept, which aims to accelerate drug development by efficiently identifying effective agents and the signatures in which they are most effective. Future I-SPY2 arms will continue to build on the promise of checkpoint blockade for women with high-risk, early-stage breast cancer, and biomarker work to better select those who will benefit from immune checkpoint inhibition is ongoing.23
Trial Protocol.
eAppendix 1. Search strategy for Medline (using PubMed)
eMethods. Using Contemporary Controls and Adjusting for Time Trends in I-SPY2: The “Time Machine”
eTable 1. Baseline characteristics of participants in the pembrolizumab and control arms.
eFigure 1. Final probability distributions and RCB classifications.
eFigure 2. Event-free survival (EFS) by signature.
eTable 2. All Adverse events reported within 180 days of last treatment.
Data Sharing Statement.
References
- 1.Mao Y, Qu Q, Chen X, Huang O, Wu J, Shen K. The prognostic value of tumor-infiltrating lymphocytes in breast cancer: a systematic review and meta-analysis. PLoS One. 2016;11(4):e0152500. doi: 10.1371/journal.pone.0152500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravelli A, Roviello G, Cretella D, et al. Tumor-infiltrating lymphocytes and breast cancer: beyond the prognostic and predictive utility. Tumour Biol. 2017;39(4):1010428317695023. doi: 10.1177/1010428317695023 [DOI] [PubMed] [Google Scholar]
- 3.Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40-50. doi: 10.1016/S1470-2045(17)30904-X [DOI] [PubMed] [Google Scholar]
- 4.Kwok G, Yau TCC, Chiu JW, Tse E, Kwong Y-L. Pembrolizumab (Keytruda). Hum Vaccin Immunother. 2016;12(11):2777-2789. doi: 10.1080/21645515.2016.1199310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nanda R, Chow LQM, Dees EC, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34(21):2460-2467. doi: 10.1200/JCO.2015.64.8931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rugo HS, Delord J-P, Im SA, et al. Safety and antitumor activity of pembrolizumab in patients with estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer. Clin Cancer Res. 2018;24(12):2804-2811. doi: 10.1158/1078-0432.CCR-17-3452 [DOI] [PubMed] [Google Scholar]
- 7.Adams S, Schmid P, Rugo HS, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30(3):397-404. doi: 10.1093/annonc/mdy517 [DOI] [PubMed] [Google Scholar]
- 8.Adams S, Loi S, Toppmeyer D, et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30(3):405-411. doi: 10.1093/annonc/mdy518 [DOI] [PubMed] [Google Scholar]
- 9.Gradishar WJ, Krasnojon D, Cheporov S, et al. Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancer. J Clin Oncol. 2009;27(22):3611-3619. doi: 10.1200/JCO.2008.18.5397 [DOI] [PubMed] [Google Scholar]
- 10.Asselain B, Barlow W, Bartlett J, et al. ; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) . Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19(1):27-39. doi: 10.1016/S1470-2045(17)30777-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JW, Liu MC, Yee D, et al. ; I-SPY 2 Investigators . Adaptive randomization of neratinib in early breast cancer. N Engl J Med. 2016;375(1):11-22. doi: 10.1056/NEJMoa1513750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rugo HS, Olopade OI, DeMichele A, et al. ; I-SPY 2 Investigators . Adaptive randomization of veliparib-carboplatin treatment in breast cancer. N Engl J Med. 2016;375(1):23-34. doi: 10.1056/NEJMoa1513749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berry DA. Adaptive clinical trials in oncology. Nat Rev Clin Oncol. 2011;9(4):199-207. doi: 10.1038/nrclinonc.2011.165 [DOI] [PubMed] [Google Scholar]
- 14.Berry DA. The brave new world of clinical cancer research: adaptive biomarker-driven trials integrating clinical practice with clinical research. Mol Oncol. 2015;9(5):951-959. doi: 10.1016/j.molonc.2015.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barker AD, Sigman CC, Kelloff GJ, Hylton NM, Berry DA, Esserman LJ. I-SPY 2: an adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin Pharmacol Ther. 2009;86(1):97-100. doi: 10.1038/clpt.2009.68 [DOI] [PubMed] [Google Scholar]
- 16.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649-655. doi: 10.1097/00000421-198212000-00014 [DOI] [PubMed] [Google Scholar]
- 17.Cardoso F, van’t Veer LJ, Bogaerts J, et al. ; MINDACT Investigators . 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016;375(8):717-729. doi: 10.1056/NEJMoa1602253 [DOI] [PubMed] [Google Scholar]
- 18.Gradishar WJ, Anderson BO, Balassanian R, et al. NCCN guidelines insights: breast cancer, version 1.2017. J Natl Compr Canc Netw. 2017;15(4):433-451. doi: 10.6004/jnccn.2017.0044 [DOI] [PubMed] [Google Scholar]
- 19.Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25(28):4414-4422. doi: 10.1200/JCO.2007.10.6823 [DOI] [PubMed] [Google Scholar]
- 20.Glas AM, Floore A, Delahaye LJ, et al. Converting a breast cancer microarray signature into a high-throughput diagnostic test. BMC Genomics. 2006;7(1):278. doi: 10.1186/1471-2164-7-278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roepman P, Horlings HM, Krijgsman O, et al. Microarray-based determination of estrogen receptor, progesterone receptor, and HER2 receptor status in breast cancer. Clin Cancer Res. 2009;15(22):7003-7011. doi: 10.1158/1078-0432.CCR-09-0449 [DOI] [PubMed] [Google Scholar]
- 22.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164-172. doi: 10.1016/S0140-6736(13)62422-8 [DOI] [PubMed] [Google Scholar]
- 23.Campbell M, Yau C, Borowsky A, et al. Analysis of immune infiltrates (assessed via multiplex fluorescence immunohistochemistry) and immune gene expression signatures as predictors of response to the checkpoint inhibitor pembrolizumab in the neoadjuvant I-SPY 2 trial (Abstract PD6-08). Cancer Res. 2018;78(4)(suppl). doi: 10.1158/1538-7445.SABCS17-PD6-08 [DOI] [Google Scholar]
- 24.Schmid P, Cortes Castan J, Dent R, et al. Phase III study of pembrolizumab (pembro) + chemotherapy (chemo) vs placebo (pbo) + chemo as neoadjuvant treatment, followed by pembro vs pbo as adjuvant treatment for early triple-negative breast cancer (TNBC). Annals Oncol. 2019;30(suppl 5). doi: 10.1093/annonc/mdz394.003 [DOI] [Google Scholar]
- 25.Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol. 2018;4(2):173-182. doi: 10.1001/jamaoncol.2017.3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
eAppendix 1. Search strategy for Medline (using PubMed)
eMethods. Using Contemporary Controls and Adjusting for Time Trends in I-SPY2: The “Time Machine”
eTable 1. Baseline characteristics of participants in the pembrolizumab and control arms.
eFigure 1. Final probability distributions and RCB classifications.
eFigure 2. Event-free survival (EFS) by signature.
eTable 2. All Adverse events reported within 180 days of last treatment.
Data Sharing Statement.