Abstract
Objective
To assess the frequency and perinatal outcomes of gestational diabetes mellitus (GDM) defined by the criteria according to the International Association of Diabetes in Pregnancy Study Group (IADPSG) and the National Institute for Health and Care Excellence (NICE) diagnostic criteria for GDM.
Design
A retrospective cohort study.
Setting
Six secondary and tertiary delivery hospitals in Finland in 2009.
Population
Pregnant women (N = 4,033) and their offspring.
Methods
We used data on comprehensive screening of pregnant women with a 2-h 75-g oral glucose tolerance test (OGTT), performed between gestational weeks 24 and 40. OGTT glucose concentrations were used to identify women who fulfilled IADPSG and NICE criteria. While cut-offs according to Finnish national criteria partly overlapped with both criteria, a subgroup of IADPSG- or NICE-positive GDM women remained undiagnosed by Finnish criteria and hence non-treated. They were analysed as subgroups and compared to controls who were negative with all cut-offs.
Main outcome measures
GDM prevalence, birth weight SD score (BWSDS), large for gestational age (LGA) and caesarean section (CS) rates.
Results
Among the 4,033 women screened for GDM, 1,249 (31.0%) and 529 (13.1%) had GDM according to the IADPSG and NICE criteria, respectively. The LGA rate was similar in both groups. Regardless of the diagnostic criteria, women with GDM had a higher risk of induced delivery and CSs than controls. In IADPSG-positive non-treated women, offspring’s BWSDS and CS rate were higher than in controls.
Conclusions
GDM prevalence was 2.4-fold higher according to the IADPSG compared with the NICE criteria but the LGA rate did not differ. BWSDS and CS rate were increased already with mild untreated hyperglycaemia.
Introduction
The prevalence of gestational diabetes mellitus (GDM) varies, depending on the screening methods and diagnostic cut-off values applied. For decades, there have been attempts to standardize the definition, but a consensus has yet to be reached. In 2010, the International Association of the Diabetes and Pregnancy Study Group (IADPSG) proposed new diagnostic criteria based on the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study.[1,2] These guidelines recommended universal GDM screening using a 2-h 75-g oral glucose tolerance test (OGTT). The proposed cut-off values represented an odds ratio of 1.75 for birthweight > 90th centile, cord C-peptide > 90th centile (indicating neonatal hyperinsulinemia) and percent body fat > 90th centile. Importantly, for the first time, these diagnostic criteria were based on perinatal outcomes instead of the mother’s subsequent diabetes risk [3].
The diagnostic cut-off values for plasma samples according to the IADPSG criteria are ≥ 5.1 mmol/L at baseline (fasting sample), ≥ 10.0 mmol/L 1 h and ≥ 8.5 mmol/L 2 h after a glucose load. These criteria have been widely adopted and are currently recommended by the World Health Organization and the International Federation of Gynecology and Obstetrics (FIGO) [4,5]. However, the National Institutes of Health in the U.S. and the National Institute for Health and Care Excellence (NICE) in the U.K have not accepted the recommendation or diagnostic cut-offs because of concerns about a low cost-benefit ratio and limited evidence of improvements in maternal and neonatal outcomes [6–8]. Thus, at present, the NICE criteria for the diagnostic cut-offs for fasting and for 2-h postprandial glucose concentrations differ significantly from those of the IADPSG (≥ 5.6 mmol/L whichever ≥ 7.8 mmol/L, respectively), and the 1-h concentration is not included at all [2,7]. Besides these widely adopted diagnostic criteria, in some countries, including Finland, the diagnostic cut-off values for GDM were revised according to American Diabetes Association criteria in 2008 [9,10].
Given the significant differences in the cut-off values for plasma glucose levels, there are also likely differences in the frequency of GDM diagnoses and perinatal outcomes, depending on the guidelines applied. The objective of the present study was to evaluate the impact of two different diagnostic criteria for gestational diabetes mellitus, the IADPSG and the NICE guidelines, on the frequency of GDM and perinatal outcomes.
Methods
The data were obtained from the register-based arm of the Finnish Gestational Diabetes Study, a population-based prospective cohort. [11] The study was initiated in conjunction with the introduction of new nationwide guidelines for GDM screening, diagnosis and treatment in Finland. [9,11]
Cohort
The registry data were obtained from the Medical Birth Register (MBR), which includes data on the course and complications of pregnancy, delivery and perinatal health of the newborn, as well as International Classification of Diseases (ICD) codes for medical diagnoses of the mother and child. All pregnancies resulting in a live born infant or stillbirth at ≥ 22 gestational weeks (gw) or weighting ≥ 500 g are reported in the MBR. These data are linked to the Population Register Centre on live births and Statistics Finland on stillbirths and infant deaths. The coverage of the MBR is practically complete, and the quality of the data is high. [12, 13]
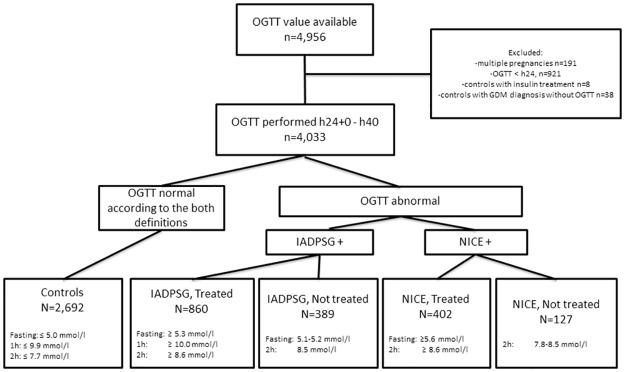
The MBR also includes information on whether an OGTT was performed during pregnancy and whether the result was abnormal, but it does not include data on the actual glucose concentrations. Therefore, we collected numerical OGTT data from all women who delivered during 2009 in six delivery units in Finland: two tertiary-level (Oulu and Tampere) and four secondary-level (Lappeenranta, Seinäjoki, Kajaani and Pori) hospitals, each serving a specific geographical area. These hospitals were chosen as numerical OGTT data were available through the hospitals’ laboratory information systems. The data on OGTTs from 2008 to 2009 from the laboratory information systems were linked to clinical data from the MBR by personnel uninvolved in this study using unique personal identification numbers. After exclusion of women with pre-pregnancy diabetes and multiple pregnancies, the study population consisted of 4,033 women, to whom the OGTTs were performed between 24 and 40 gw. (Fig 1).
Fig 1. Flow chart of the study.
The diagnosis of GDM was based on one abnormal value in 75g OGTT. IADPSG: International Association of the Diabetes and Pregnancy Study Group, NICE: The National Institute for Health and Care Excellence, OGTT: a 2-h 75-g oral glucose tolerance test.
GDM screening in Finland
The national guidelines published in 2008 introduced comprehensive screening for GDM and replaced the former risk-factor based screening policy in Finland. According to these guidelines, all women, except those with a very low risk, should be screened for GDM using a 75-g 2-h OGTT at 24–28 gw, with the samples obtained at baseline after an overnight fast and 1 and 2 h after the glucose load. High-risk women (i.e. women with prior GDM, a body mass index [BMI] > 35 kg/m2 or polycystic ovary syndrome with insulin resistance) undergo OGTT screening for the first time between 12 and 16 gw, and the test is repeated between 24 and 28 gw if the results are normal. Accordingly, women diagnosed with GDM in early pregnancy based on the OGTT were not included in the present study.
The OGTT is generally performed after a 12-h overnight fast in a laboratory near the patient’s residence. The samples are drawn from an antecubital vein into fluoride citrate tubes and analysed within 24 h in a local laboratory using commercial enzymatic assays, with the assays used varying between laboratories. The national diagnostic cut-offs were based on recommendation by the American Diabetes Association at the time of the study: ≥ 5.3 mmol/L at baseline (fasting sample), ≥ 10.0 mmol/L 1 h and ≥ 8.6 mmol/L 2 h after the glucose load (S1 Table).9,10 In 2009, which was the first year after the implementation of the new guidelines, 42% of all pregnant women in Finland underwent an OGTT during pregnancy. Thereafter, the coverage increased significantly, reaching 66% in 2017. According to the national guidelines, women with one or more abnormal OGTT values receive individualized dietary and lifestyle counselling and begin glucose self-monitoring. Insulin therapy at the delivery hospital is considered if self-monitored plasma glucose concentrations repeatedly exceed the target levels (< 5.5 mmol/L fasting or < 7.8 mmol/L 1 h postprandial), despite the dietary intervention. The use of oral glycemic agents was occasional and not primarily recommended by the guidelines.
Covariates
Maternal age was defined at the time of delivery, and parity was defined as the number of previous deliveries. The BMI was calculated using self-reported height and weight before pregnancy (kg/m2), both of which were recorded at the first antenatal visit. Socioeconomic status was divided into four categories using the occupation reported in the MBR: upper-level employees, lower-level employees, manual workers and others, such as stay-at-home mothers, students, pensioners and self-employed individuals. Self-reported smoking status was categorized as non-smokers, those who stopped during the first trimester and those who smoked after the first trimester, as registered in the MBR.
Outcomes
The main outcome was the frequency of GDM according to the IADPSG and NICE criteria, and the secondary outcomes were the pregnancy and neonatal outcomes in these groups. Pregnancy outcomes included pregnancy induced hypertension (ICD and Related Health Problems, version 10 [ICD 10] codes O13 and O14 included), induction of labour and delivery mode (vaginal, vacuum extraction or a caesarean section [CS]). Neonatal outcomes included birth weight, birth weight standard deviation (SD) scores, birth weight SD scores over 90%, small for gestational age (SGA) (i.e. birth weight -2 SD percentile), large for gestational age (LGA) (i.e. birth weight SD score +2 SD percentile which is the definition used in clinical practice in Finland; for comparison with other studies, we also report our results with LGA defined as birth weight SD score over 90th percentile) and gestational age at delivery. The birth weight SD score is a sex-specific parameter estimating birth weight and length in singletons born at 23–43 gw to primiparous or multiparous mothers according to Finnish standards [14]. Preterm delivery was defined as a delivery prior to 37+0 gw. Because the diagnostic criteria for GDM according to current Finnish care guidelines overlap with those of the IADPSG and NICE (Table 1), a proportion of women diagnosed with mild GDM by the IADPSG (389 women, 9.6%) or NICE (127 women, 3.1%) criteria remained untreated for GDM during pregnancy. These groups were evaluated in sub-analyses (Fig 1).
Table 1. Diagnostic cut-off values in the 75-g OGTT according to the different diagnostic criteria.
| Diagnostic method | Fasting plasma glucose mmol/L | 1-h plasma glucose mmol/L | 2-h plasma glucose mmol/L |
|---|---|---|---|
| IADPSG | 5.1 | 10.0 | 8.5 |
| NICE | 5.6 | - | 7.8 |
| Finnish guidelines* | 5.3 | 10.0 | 8.6 |
The OGTT test was interpreted as positive for gestational diabetes if one or more values were equal to or exceeded their corresponding cut-offs.
*According to Finnish Guidelines, all pregnant women, except those with a very low risk for GDM (primiparous: age < 25 y, BMI < 25 kg/m2, no family history of diabetes; multiparous: age < 40 y, BMI < 25 kg/m2, no previous history of foetal macrosomia) are screened for GDM.
OGTT: oral glucose tolerance test
IADPSG: International Association of Diabetes in Pregnancy Study Group
NICE: National Institute for Health and Care Excellence
Statistical analyses
All statistical analyses were carried out using the SPSS 21 statistical package. Categorical variables were reported as frequencies (%), and continuous variables were reported using the mean (SD). Pearson’s χ2 test was used to compare the difference in proportions in demographic variables. An independent sample t test was conducted to compare the difference in the means of demographic data. Differences between each GDM group were tested using Fisher’s exact test. Logistic regressions were used to estimate odds ratios (ORs), with their 95% confidence intervals (CIs) and linear regressions mean differences (with 95% CIs) of outcomes associated with GDM, respectively, according to the different diagnostic criteria and treatment status. Logistic and linear regressions were also performed to estimate differences between each GDM group. The models were adjusted for maternal age, parity and pre-pregnancy BMI. A two-sided p value of < 0.05 was considered statistically significant.
The study was approved by the regional ethics committee in Northern Ostrobothnia Hospital District and the National Institute for Health and Welfare. According to Finnish legislation, information consent form is not needed in Finland, when using anonymous register data only.
Results
OGTT was performed between 24+0 and 40+0 gw (mean 27.5, SD 2.5) in 4,033 women who delivered in the study hospitals in 2009. Of these women, 1,249 (31.0%) and 529 (13.1%) had GDM according to the IADPSG and NICE criteria, respectively (Table 2). The control group consisted of 2,692 (66.7%) women who were normoglycaemic according to all criteria. Of all screened women, 860 (21.3%) had GDM according to the prevailing Finnish criteria and were counselled and medically treated for GDM, if needed. As compared with the controls, women who had GDM according to either IADPSG or NICE criteria were older and had a higher pre-pregnancy BMI. Women in the IADPSG GDM group smoked more often and were more often multiparous when compared with women the NICE GDM group. 57/860 women received insulin treatment. This represents 6.6% of women who were diagnosed by Finnish criteria. Of those who met IADPSG criteria, 4.6% received insulin, and of those who met NICE criteria, 7.2% (Table 3).
Table 2. Characteristics of pregnancies with and without GDM, classified according to the different criteria based on the OGTT at 24 to 40 gw.
| Characteristics | No GDM according to all criteria | GDM by IADPSG criteria | GDM by NICE criteria | ||
|---|---|---|---|---|---|
| p-value | p-value | ||||
| N (%) | 2,692 (66.7) | 1,249 (31.0) | 529 (13.1) | ||
| Maternal age, y | 29.4 (5.3) | 30.2 (5.6) | <0.001 | 30.3 (5.8) | <0.001 |
| Pre-pregnancy BMI, kg/m2 | 25.5 (4.3) | 27.2 (5.1) | <0.001 | 27.0 (5.1) | <0.001 |
| Primiparity | 1,333 (49.5) | 560 (44.8) | 0.006 | 259 (49.0) | 0.815 |
| Smoking | |||||
| No | 2,265 (87.5) | 1,017 (84.5) | 0.011 | 435 (85.0) | 0.120 |
| Quit in the first trimester | 109 (4.2) | 59 (4.9) | 0.336 | 26 (5.1) | 0.379 |
| Continued after the first trimester | 215 (8.3) | 128 (10.6) | 0.020 | 51 (10.0) | 0.221 |
| Socioeconomic status | |||||
| Upper-white collar workera | 470 (21.6) | 190 (19.2) | 0.112 | 74 (17.6) | 0.063 |
| Lower-white collar workerb | 889 (40.9) | 421 (42.5) | 0.417 | 181 (43.1) | 0.413 |
| Blue-collar workerc | 358 (16.5) | 184 (18.6) | 0.151 | 83 (19.8) | 0.102 |
| Otherd | 454 (20.9) | 196 (19.8) | 0.464 | 82 (19.5) | 0.520 |
Data are n (%) or mean (SD).
aAdministrative, managerial, professional and related occupations.
bAdministrative and clerical occupations.
cManual labourer.
dStudents, pensioners, self-employed and others.
BMI: body mass index
Table 3. Outcomes of pregnancies with GDM classified according to the different criteria based on the OGTT at 24 to 40 gw.
| Characteristics | No GDM according to all criteria | IADPSG | NICE | ||
|---|---|---|---|---|---|
| N (%/SD) | p-value* | N (%/SD) | p-value* | ||
| N (%) | 2,692 (66.7) | 1,249 (31.0) | 529 (13.1) | ||
| Gestational age at delivery, wk | 39.9 (1.6) | 39.6 (1.8) | <0.001 | 39.4 (2.0) | <0.001 |
| Birth weight, g (SD) | 3,571 (523.7) | 3,558 (557.5) | 0.511 | 3490 (620.0) | 0.002 |
| Birth weight, SD score | -0.05 (1.0) | 0.04 (1.0) | 0.012 | 0.01 (1.1) | 0.293 |
| Small for gestational age, <-2 SD | 76 (2.8) | 32 (2.6) | 0.640 | 15 (2.8) | 0.987 |
| Large for gestational age, >+2SD | 72 (2.7) | 36 (2.9) | 0.710 | 21 (4.0) | 0.104 |
| Large for gestational age, >90% | 250 (9.3) | 141 (11.3) | 0.050 | 65 (12.3) | 0.034 |
| Induced delivery | 414 (15.4) | 259 (20.7) | <0.001 | 121 (22.9) | <0.001 |
| Pre-term birth | 22 (0.8) | 18 (1.4) | 0.069 | 11 (2.1) | 0.008 |
| Insulin treatment | 0 | 57 (4.6) | <0.001 | 38 (7.2) | <0.001 |
| Pregnancy induced hypertension* | 165 (6.1) | 100 (8.0) | 0.029 | 48 (9.1) | 0.013 |
| Type of delivery | |||||
| Vaginal non-instrumental | 2,048 (76.1) | 894 (71.6) | 0.003 | 372 (70.3) | 0.005 |
| Instrumental | 242 (9.0) | 95 (7.6) | 0.148 | 40 (7.6) | 0.288 |
| Caesarean section | 402 (14.9) | 260 (20.8) | <0.001 | 117 (22.1) | <0.001 |
| Hospital stay of mother in days | 3.1 (1.4) | 3.2 (1.4) | 0.001 | 3.4 (1.5) | <0.001 |
| Hospital stay of offspring in days | 3.1 (2.7) | 3.3 (1.9) | 0.025 | 3.4 (2.5) | 0.017 |
Data are n (%) or mean (SD)
*p-value between GDM-group and controls.
**International classification of diseases ICD-10: O13, O14
When the pregnancy outcomes of the GDM groups were compared with those of the controls, the rates of labour induction, pregnancy induced hypertension was more common and CSs were higher in both GDM groups, and the gestational age at delivery was lower. In the NICE group, the proportion of pre-term deliveries was higher than that in the IADPSG group and in the controls (Table 3). The LGA rate in the two GDM groups did not differ from that in the controls.
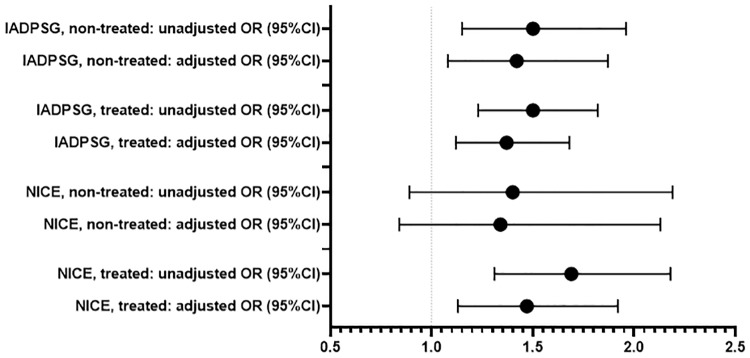
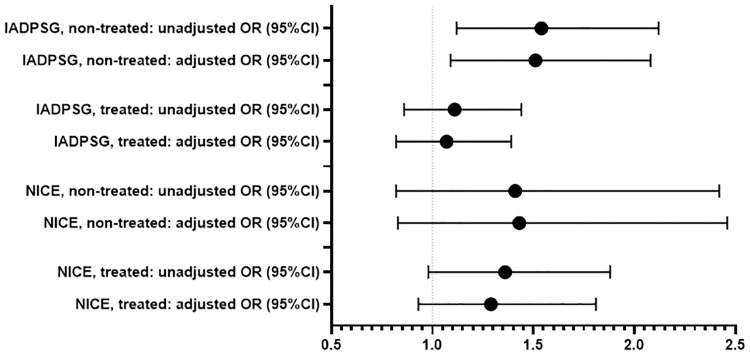
In the IADPSG and NICE groups, 68.9 and 76.0% of women, respectively, fulfilled national Finnish diagnostic criteria and thus received counselling and treatment. The characteristics of these pregnancies are presented in the Table 4. Proportion of insulin treated women was higher in the treated NICE group than in the treated IADPSG group, 9.5% and 6.6%, respectively. The delivery induction and CS rates in both treated GDM groups were higher than those in the controls. The CS rate and birth weight SD score and large for gestational age as defined at >90%, were higher in the non-treated IADPSG group than in controls (Table 5, Figs 2 and 3).
Table 4. Characteristics of pregnancies with GDM classified according to the different GDM diagnostic criteria, with or without treatment.
| Characteristics | Control group | IADPSG | p-value** | NICE | p-value** | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treated | Non-treated | Treated | Non-treated | ||||||||
| p-value* | p-value* | p-value* | p-value* | ||||||||
| N | 2,692 (66.7) | 860 (21.3) | 389 (9.6) | 402 (10.0) | 127 (3.1) | ||||||
| Maternal age, y | 29.4 (5.3) | 30.2 (5.6) | <0.001 | 30.0 (5.7) | 0.033 | 0.462 | 30.4 (5.9) | 0.001 | 30.0 (5.5) | 0.223 | 0.498 |
| Pre-pregnancy BMI, kg/m2 | 25.5 (4.3) | 27.4 (5.2) | <0.001 | 26.9 (4.7) | <0.001 | 0.150 | 27.4 (5.1) | <0.001 | 25.4 (4.6) | 0.799 | <0.001 |
| Primiparity | 1,333 (49.5) | 378 (44.0) | 0.004 | 182 (46.8) | 0.314 | 0.351 | 193 (48.0) | 0.573 | 66 (52.0) | 0.589 | 0.437 |
| Smoking | |||||||||||
| No | 2,265 (87.5) | 691 (83.9) | 0.008 | 326 (85.8) | 0.354 | 0.390 | 326 (83.8) | 0.044 | 109 (88.6) | 0.710 | 0.193 |
| Quit in the first trimester | 109 (4.2) | 39 (4.7) | 0.521 | 20 (5.3) | 0.347 | 0.692 | 19 (4.9) | 0.541 | 7 (5.7) | 0.428 | 0.722 |
| Continued after the first trimester | 215 (8.3) | 94 (11.4) | 0.007 | 34 (8.9) | 0.673 | 0.198 | 44 (11.3) | 0.050 | 7 (5.7) | 0.302 | 0.070 |
| Socioeconomic status | |||||||||||
| Upper-white collar workera | 470 (21.6) | 125 (18.4) | 0.067 | 65 (20.9) | 0.764 | 0.350 | 62 (19.5) | 0.382 | 12 (11.8) | 0.017 | 0.074 |
| Lower-white collar workerb | 889 (40.9) | 294 (43.2) | 0.291 | 127 (40.8) | 0.970 | 0.478 | 141 (44.3) | 0.252 | 40 (39.2) | 0.728 | 0.363 |
| Blue-collar workerc | 358 (16.5) | 136 (20.0) | 0.035 | 48 (15.4) | 0.638 | 0.086 | 56 (17.6) | 0.616 | 27 (26.5) | 0.009 | 0.051 |
| Otherd | 454 (20.9) | 125 (18.4) | 0.152 | 71 (22.8) | 0.439 | 0.103 | 59 (18.6) | 0.332 | 23 (22.5) | 0.692 | 0.376 |
Data are n (%) or mean (SD).
*p-value between GDM-group and controls.
**p-value between treated/non-treated.
aAdministrative, managerial, professional and related occupations.
b Administrative and clerical occupations.
cManual labourer.
dStudents, pensioners, self-employed and others.
BMI: body mass index
Table 5. Outcomes of pregnancies with GDM classified according to the different GDM diagnostic criteria, with or without treatment.
| Characteristics | No GDM | IADPSG | p-value** | NICE | p-value** | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treated | Non-treated | Treated | Non-treated | ||||||||
| p-value* | p-value* | p-value* | p-value* | ||||||||
| N | 2,692 (66.7) | 860 (21.3) | 389 (9.6) | 402 (10.0) | 127 (3.1) | ||||||
| Gestational age at delivery, wk | 39.9 (1.6) | 39.5 (1.9) | <0.001 | 39.9 (1.6) | 0.377 | 0.001 | 39.3 (2.0) | <0.001 | 39.5 (2.0) | 0.002 | 0.339 |
| Birth weight, g | 3,571 (524) | 3,530 (558) | 0.061 | 3,620 (552) | 0.095 | 0.008 | 3,499 (624) | 0.013 | 3,463 (610) | 0.025 | 0.565 |
| Birth weight, SD score | -0.046 (1.0) | 0.017 (1.0) | 0.120 | 0.099 (1.1) | 0.012 | 0.208 | 0.037 (1.2) | 0.176 | -0.074 (1.1) | 0.768 | 0.317 |
| Small for gestational age, <-2 SD | 76 (2.8) | 20 (2.3) | 0.433 | 12 (3.1) | 0.772 | 0.432 | 10 (2.5) | 0.703 | 5 (3.9) | 0.463 | 0.391 |
| Large for gestational age, >+2 SD | 72 (2.7) | 23 (2.7) | 1.000 | 13 (3.3) | 0.453 | 0.514 | 17 (4.2) | 0.082 | 4 (3.1) | 0.747 | 0.587 |
| Large for gestational age, >90% | 250 (9.3) | 88 (10.2) | 0.411 | 53 (13.6) | 0.007 | 0.079 | 49 (12.2) | 0.066 | 16 (12.6) | 0.212 | 0.903 |
| Induced delivery | 414 (15.4) | 192 (22.3) | <0.001 | 67 (17.2) | 0.349 | 0.039 | 94 (23.4) | <0.001 | 27 (21.3) | 0.075 | 0.619 |
| Preterm birth | 22 (0.8) | 13 (1.5) | 0.073 | 5 (1.3) | 0.354 | 0.756 | 7 (1.7) | 0.073 | 4 (3.1) | 0.007 | 0.332 |
| Insulin treatment | 0 | 57 (6.6) | <0.001 | 0 | <0.001 | 38 (9.5) | <0.001 | 0 | <0.001 | ||
| Pregnancy induced hypertension*** | 165 (6.1) | 75 (8.7) | 0.008 | 25 (6.4) | 0.820 | 0.167 | 38 (9.5) | 0.012 | 10 (7.9) | 0.426 | 0.589 |
| Type of delivery | |||||||||||
| Vaginal | 2,048 (76.1) | 616 (71.6) | 0.009 | 278 (71.5) | 0.048 | 0.953 | 278 (69.2) | 0.003 | 94 (74.0) | 0.595 | 0.296 |
| Instrumental | 242 (9.0) | 65 (7.6) | 0.193 | 30 (7.7) | 0.406 | 0.924 | 32 (8.0) | 0.498 | 8 (6.3) | 0.297 | 0.537 |
| Caesarean section | 402 (14.9) | 179 (20.8) | <0.001 | 81 (20.8) | 0.003 | 0.997 | 92 (22.9) | <0.001 | 25 (19.7) | 0.144 | 0.447 |
| Hospital stay of mother in days | 3.1 (1.4) | 3.2 (1.4) | 0.001 | 3.2 (1.3) | 0.196 | 0.237 | 3.4 (1.5) | <0.001 | 3.3 (1.3) | 0.033 | 0.615 |
| Hospital stay of offspring in days | 3.1 (2.7) | 3.4 (2.1) | 0.021 | 3.2 (1.3) | 0.253 | 0.157 | 3.5 (2.8) | 0.008 | 3.2 (1.3) | 0.718 | 0.058 |
Data are n (%) or mean (SD).
*p-value between GDM-group and controls.
**p-value between treated/non-treated.
***International classification of diseases ICD-10: O13, O14
Fig 2. Association between caesarean section and GDM groups identified by different diagnostic criteria.
Logistic regression analyses were used to estimate unadjusted and adjusted (for maternal age, parity and pre-pregnancy body mass index) odds ratios (OR) with 95% CI, whiskers expressing the 5th and 95th percentiles. IADPSG: International Association of the Diabetes and Pregnancy Study Group, NICE: The National Institute for Health and Care Excellence.
Fig 3. Association between large for gestational age, defined as birth weight >90th percentile, in GDM groups identified by different diagnostic criteria.
Logistic regression analyses were used to estimate unadjusted and adjusted (for maternal age, parity and pre-pregnancy body mass index) odds ratios (OR) with 95% CI, whiskers expressing the 5th and 95th percentiles. IADPSG: International Association of the Diabetes and Pregnancy Study Group, NICE: The National Institute for Health and Care Excellence.
The associations we found were also present after adjustment for maternal age, parity and pre-pregnancy BMI, although most were slightly attenuated (Table 6).
Table 6. Multivariate logistic and linear regression analysis of perinatal and neonatal outcomes in patients according to the application of the different GDM diagnostic methods.
| Non-treated IADPSG | Treated IADPSG | Non-treated NICE | Treated NICE | |||||
|---|---|---|---|---|---|---|---|---|
| Outcome | OR | aOR | OR | aOR | OR | aOR | OR | aOR |
| Induction of labour | 1.15 (0.86–1.52) |
1.10 (0.83–1.47) |
1.58 (1.31–1.92) |
1.46 (1.20–1.78) |
1.49 (0.96–2.30) |
1.48 (0.95–2.30) |
1.68 (1.30–2.16) |
1.53 (1.18–1.99) |
| Caesarean section | 1.50 (1.15–1.96) |
1.42 (1.08–1.87) |
1.50 (1.23–1.82) |
1.37 (1.12–1.68) |
1.40 (0.89–2.19) |
1.34 (0.84–2.13) |
1.69 (1.31–2.18) |
1.47 (1.13–1.92) |
| Birth weight SD score | 0.14 (0.04–0.25) |
0.14 (0.03–0.25) |
0.06 (-0.02–0.14) |
0.05 (-0.03–0.13) |
-0.03 (-0.21–0.15) |
-0.02 (-0.20–0.16) |
0.08 (-0.03–0.19) |
0.07 (-0.04–0.18) |
| Small for gestational age, <2SD | 1.10 (0.59–2.03) |
1.18 (0.63–2.20) |
0.82 (0.50–1.35) |
0.82 (0.49–1.37) |
1.41 (0.56–3.55) |
1.45 (0.58–3.68) |
0.88 (0.45–1.71) |
0.83 (0.41–1.68) |
| Large for gestational age, >2SD | 1.26 (0.69–2.29) |
1.17 (0.64–2.14) |
1.00 (0.62–1.61) |
0.87 (0.53–1.43) |
1.18 (0.43–3.29) |
1.18 (0.42–3.30) |
1.61 (0.94–2.76) |
1.43 (0.82–2.50) |
| Large for gestational age, >90% | 1.54 (1.12–2.12) |
1.51 (1.09–2.08) |
1.11 (0.86–1.44) |
1.07 (0.82–1.39) |
1.41 (0.82–2.42) |
1.43 (0.83–2.46) |
1.36 (0.98–1.88) |
1.29 (0.93–1.81) |
Data are OR (95% CI), aOR = OR adjusted for maternal age, parity and pre-pregnancy BMI. Exception: Mean difference (95% CI = confidential interval) for birth weight SD score.
Discussion
Main findings
Among all the women who underwent OGTT, the proportion of GDM was 2.4-fold higher when diagnosed by the IADPSG (31.0%) criteria as compared with when diagnosed by the NICE criteria (13.1%). The proportion of LGA infants was similar in both the GDM groups and controls, which may reflect successful counselling and treatment. While the diagnostic cut-offs partly overlapped, we had also a possibility to evaluate a subgroup without treatment. Mild untreated hyperglycaemia was associated with an increased CS rate and higher birth weights, as found in the HAPO study and some recent studies [15,16].
The main short-term goal of GDM management is to prevent macrosomia. Large randomized studies have demonstrated that achievement of euglycaemia leads to better perinatal outcomes such as lower birth weight and lower macrosomia rate already in mild cases of GDM. [17, 18] In our, and also some previous studies, GDM treatment can be considered successful, as neither birth weight nor perinatal outcomes differed between the controls and GDM groups [19]. The success of GDM treatment may also be attributed to the widespread adoption of national guidelines in Finland and the existence of good co-operation between primary and special health care services. The rates of induced deliveries and CSs were higher in both the treated GDM groups, irrespective of which diagnostic criteria were applied. It may be supposed that the diagnosis of GDM itself at some extent predispose women to those interventions. On the other hand, the birth weight SD score and CS rate showed an increasing tendency in cases with mild hyperglycaemia, without a diagnosis of GDM. This finding is in accordance with the results of the HAPO study, which reported a linear increase in adverse perinatal outcomes without specific cut-offs. [1]
Different diagnostic cut-offs in GDM screening might be expected to result in different maternal profiles: The IADPSG criteria emphasize the importance of fasting glucose, whereas the NICE guidelines focus on low 2-h postprandial glucose concentration. As compared with the control group, women in the IADPSG group with lower fasting glucose concentrations were more often multiparous and smokers. In the NICE GDM group, the proportion of insulin-treated mothers (7.2%) was higher than that in the IADPSG GDM group (4.6%), which may partly indicate the different grades of severity of GDM in the two groups. Between the treated groups, the difference between NICE and IADPSG groups was even higher, 9.5% vs 6.6%. Pre-term birth was most common in the NICE groups, regardless of treatment. The reason remained unclear.
In multivariate logistic and linear regression analyses the results were adjusted with maternal age, parity and pre-pregnancy BMI, which are independent risk factors for adverse pregnancy outcome. In our study, the significance of the results remained despite adjustment with these factors.
Strengths and limitations
The strengths of this study are the large cohort and ability to evaluate the significance of numerical OGTT values. The quality and completeness of the MBR are high, and the current data cover geographically diverse regions of Finland. As a limitation, during the study period the coverage of new nationwide GDM screening was rather low (42%) while the new screening protocol had been launched just a year earlier and therefore not fully implemented. Control population consisted of those with known OGTT results. Thus, non-screened women with the lowest risk of GDM were not included in this study, but this is not a bias because we classified women with known results of OGTT, not with the risk of GDM. We speculate that those who did not undergo OGTT and the undiagnosed GDM mothers would on average represent a milder end of the GDM spectrum and the present study may underestimate their proportion. In order to exclude possible overt or pre-pregnancy diabetes, only women with OGTT performed after recommended screening time point (> 24 gestational weeks) were included. The study also had limited power to assess rare perinatal outcomes.
Conclusion
In conclusion, there was a significant difference in the prevalence of GDM diagnosed by the different criteria, but the pregnancy outcomes were similar using the two diagnostic methods: the use of NICE criteria only would have identified less GDM women than the use of IADPSG criteria, but would also likely to have left unidentified a group of GDM women who had a similar proportion of these pregnancy outcomes and might have benefited from GDM treatment. The main aim of GDM treatment, prevention of macrosomia, was attained by both criteria. The similarity in pregnancy outcomes in GDM mothers and normoglycaemic controls may reflect successful, uniform counselling and treatment and well-organized maternal health care. Mild untreated hyperglycaemia was associated with an increased CS rate and higher birth weights, as reported by the HAPO and some recent studies. [1,20] In the future, studies are needed to determine how these different diagnostic methods will predict a woman´s subsequent risk for type 2 diabetes and other long-term outcomes.
Supporting information
(DOCX)
Data Availability
Permission to use the confidential and sensitive register data of this study was approved by the National Institute for Health and Welfare (THL) in Finland. No additional data are available. The current data can only be used for this study and the data cannot be shared. A similar dataset can be applied from THL: https://thl.fi/en/web/thlfi-en/statistics/informations-for-reseachers/authorisation-application. We assure that the data available from this URL is sufficient to replicate the findings of our study.
Funding Statement
This work was supported by the Academy of Finland (Grants 127437, 129306, 130326, 134791, 263924 and 315690 to Eero Kajantie); the European Commission (Horizon 2020 award 733280) RECAP Research on Children and Adults born preterm to EK); the Finnish Foundation for Pediatric Research (to EK); the Juho Vainio Foundation (to EK and Marja Vääräsmäki); the Novo Nordisk Foundation (to EK and MV); The Signe and Ane Gyllenberg Foundation (to EK); the Sigrid Juselius Foundation (to EK); and the Yrjö Jahnsson Foundation (to EK and MV), Medical Research Center Oulu (Sanna Koivunen); National Graduate School of Clinical Investigation (SK); Pohjois-Suomen terveydenhuollon Tukisäätiö (SK) and The Finnish Medical Foundation (SK). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. 10.1056/NEJMoa0707943 [DOI] [PubMed] [Google Scholar]
- 2.International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33:676–82. 10.2337/dc09-1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373:1773–9. 10.1016/S0140-6736(09)60731-5 [DOI] [PubMed] [Google Scholar]
- 4.Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization Guideline. Diabetes Research Clin Pract. 2014;103:341–63. [DOI] [PubMed] [Google Scholar]
- 5.The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: A pragmatic guide for diagnosis, management, and care. Hod M, Kapur A, Sacks DA, Hadar E, Agarwal M, Di Renzo GC, Cabero Roura L, et al. Int J Gynaecol Obstet. 2015;131 Suppl 3:S173–211. [DOI] [PubMed] [Google Scholar]
- 6.Vandorsten JP, Dodson WC, Espeland MA, Grobman WA, Guise JM, Mercer BM, et al. NIH Consensus Development Conference: Diagnosing Gestational Diabetes Mellitus. NIH Consensus and State-of-the-Science Statements. 2013;29:1–31. [PubMed] [Google Scholar]
- 7.National Institute for Health and Care Excellence. Diabetes in pregnancy: management from preconception to the postnatal period. NICE guideline 3. https://www.nice.org.uk/guidance/ng3. Published 2015. Accessed November 12, 2015. [PubMed]
- 8.Liao L, Xu Y, Zhuang X, Hong S, Wang Z, Dobs A, et al. Evaluation of guidelines on the screening and diagnosis of gestational diabetes mellitus: systematic review. BMJ Open. 2019;6:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finnish Medical Society Duodecim. Gestational diabetes. Helsinki, Finland: The Finnish Medical Society Duodecim; 2008. www.kaypahoito.fi. [Google Scholar]
- 10.American Diabetes Association. Standards of medical care in diabetes -2008. Diabetes Care. 2008;31(Suppl 1):S12–S54. [DOI] [PubMed] [Google Scholar]
- 11.Koivunen S, Kajantie E, Torkki A, Bloigu A, Gissler M, Pouta A, et al. The changing face of gestational diabetes: the effect of the shift from risk factor-based to comprehensive screening. Eur J Endocrinol. 2015;173:623–32. 10.1530/EJE-15-0294 [DOI] [PubMed] [Google Scholar]
- 12.Gissler M, Teperi J, Hemminki E, Merilainen J. Data quality after restructuring a national medical registry. Scand J Soc Med. 1995;23:75–80. 10.1177/140349489502300113 [DOI] [PubMed] [Google Scholar]
- 13.Gissler M, Shelley J. Quality of data on subsequent events in a routine medical birth register. Medical Inform Internet Med. 2002;27:33–8. [DOI] [PubMed] [Google Scholar]
- 14.Sankilampi U, Hannila ML, Saari A, Gissler M, Dunkel L. New population-based references for birth weight, length, and head circumference in singletons and twins from 23 to 43 gestation weeks. Ann Med. 2013;45:446–54. 10.3109/07853890.2013.803739 [DOI] [PubMed] [Google Scholar]
- 15.Black M, Sacks D, Xiang A, Lawrence J. Clinical outcomes of pregnancies complicated by mild gestational diabetes mellitus differ by combinations of abnormal oral glucose tolerance test values. Diabetes Care. 2010;33(12):2524–30. 10.2337/dc10-1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Disse E, Graeppi-Dulac J, Joncour-Mills G, Dupuis O, Thivolet C. Heterogeneity of pregnancy outcomes and risk of LGA neonates in Caucasian females according to IADPSG criteria for gestational diabetes mellitus. Diabetes Metab. 2013;39(2):132–8. 10.1016/j.diabet.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 17.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477–86. 10.1056/NEJMoa042973 [DOI] [PubMed] [Google Scholar]
- 18.Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009:361:1339–48. 10.1056/NEJMoa0902430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatia M, Mackillop L, Bartlett K, Loerup L, Kenworthy Y, Levy J, et al. Clinical Implications of the NICE 2015 Criteria for Gestational Diabetes Mellitus. J Clin Med. 2018;7(10):E376 10.3390/jcm7100376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ethridge J, Catalano P, Waters T. Perinatal outcomes associated with the diagnosis of gestational diabetes made by the international association of the diabetes and pregnancy study groups criteria. Obstet Gynecol. 2014;124(3):571–8. 10.1097/AOG.0000000000000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
Permission to use the confidential and sensitive register data of this study was approved by the National Institute for Health and Welfare (THL) in Finland. No additional data are available. The current data can only be used for this study and the data cannot be shared. A similar dataset can be applied from THL: https://thl.fi/en/web/thlfi-en/statistics/informations-for-reseachers/authorisation-application. We assure that the data available from this URL is sufficient to replicate the findings of our study.