Abstract
Objective
To investigate that whether an association between marital status and the female breast cancer risk exists.
Methods
The MEDLINE, EMBASE and PsycINFO databases were searched from their inception to July 2019. The Newcastle-Ottawa Scale was used to rate the methodological quality of included studies. Study data were pooled using random-effects meta-analyses to compare the breast cancer risk between unmarried, widowed, divorced or lifelong single women and married women. This study is registered with PROSPERO (number CRD42018112368).
Results
Forty-nine publications were included in the meta-analysis. Compared with married women, unmarried and lifelong single women had an elevated risk of breast cancer, and the pooled ORs of case-control studies were 1.20 (95% CI: 1.07 to 1.35) and 1.24 (95% CI: 1.05 to 1.45), respectively. In the subgroup analyses under these two comparisons, hospital-based estimates and multivariate-adjusted estimates demonstrated a strong association, while population-based estimates and age-adjusted estimates produced nonsignificant results. The pooled OR of cohort studies examining the effect of being a lifelong single woman was 1.10 (95% CI: 1.04 to 1.16). Heterogeneity was moderate to substantial across case-control studies (I2: 46% to 82%), which may be partially explained by differences in geographic regions, publication years and control types. Possible publication bias was indicated by the funnel plot and Egger’s test (P = 0.03).
Conclusions
Marital status may correlate with the risk of developing female breast cancer. However, suboptimal selection of controls, insufficient exploration of confounding effects, inadequate ascertainment of marital status, and possible publication bias may have limited the quality of the available evidence. Overall, conclusions that marital status is an independent risk factor for breast cancer could not be drawn, and further prospective rigorous cohort studies are warranted.
Introduction
Breast cancer is the most frequently diagnosed cancer and the leading cause of cancer death among women worldwide [1]. Approximately 52.7% of premenopausal breast cancer cases and 54.7% of postmenopausal breast cancer cases can be attributed to physiological, behavioral or genetic risk factors [2]. Feasible changes in risk behaviors, such as alcohol consumption, physical activity and obesity, can contribute to an important reduction in mammary carcinoma risk [3,4]. Under these circumstances, further progress may be achieved by identifying new, modifiable lifestyle-related risk factors.
One factor that may be associated with breast cancer is marital status. Married individuals typically enjoy a higher socioeconomic status than unmarried individuals, which may translate into better access to healthcare [5]. Marriage could also promote healthier lifestyle behaviors, such as regular screenings, healthy diet, and exercise, all of which may be mediating factors preventing breast cancer [6]. The situation is different for the unmarried people. For instance, being widowed or divorced often leaves individuals with a period of intense suffering and induces a series of unhealthy coping approaches that can account for the development of breast cancer [7]. Additionally, lifelong single women tend to have no experience with childbirth or breastfeeding, while parity, age at first full-term birth and the duration of breastfeeding have been proven to have a substantial influence on the incidence of breast cancer [8–10].
Some epidemiological studies have detected higher rates of breast cancer in unmarried people than married people [11,12], while some studies argue that marital status has no influence on this malignant disease [13,14]. Despite the disparity in previous results, no systematic research has been carried out. Thus, we conducted this systematic review and meta-analysis of observational studies to obtain valid knowledge regarding the associations between marital status and the risk of breast cancer in women.
Materials and methods
We conducted a systematic review and meta-analysis in accordance with the MOOSE [15] (S1 Table) and PRISMA guidelines (S2 Table) [16]. The protocol was prospectively registered in the PROSPERO register of systematic reviews (CRD42018112368).
Search strategy
The MEDLINE, EMBASE and PsycINFO databases were searched for electronic journals. The search duration was from the inception of the databases to July 2019. The search strategy included terms related to marital status and breast cancer combined with SIGN filters for observational studies (http://www.sign.ac.uk/search-filters.html). We confined our search to papers published in English. The reference lists of all eligible articles were also checked to identify additional studies. The search strategy is shown in S3 Table.
Inclusion/exclusion criteria
The studies that fulfilled the following criteria were included:
studies employing observational research designs, including cohort, case-control, and cross-sectional designs, using appropriate controls,
studies involving at least two groups of married people and people with another status, including divorced, widowed, and lifelong single, or an aggregated category of all unmarried individuals,
studies presenting the results of analyses adjusted at least for age or studies where the control subjects were matched to cases by age; we contacted the authors of studies reporting unadjusted results and included new adjusted data if provided, and
studies published in English.
The studies that fulfilled the following criteria were excluded:
studies that did not establish a control group comprising participants without breast cancer, and
studies with control subjects matched to cases by marital status.
When two articles reported data from the same study, to avoid duplication, we only used the analysis with the higher methodological quality.
Data extraction
Two reviewers (MLL and ZZL) independently assessed the titles, abstracts and keywords of each record retrieved. The full texts of all potentially relevant articles were investigated. Disagreements were resolved by discussion between the two reviewers and, if necessary, a third reviewer (SQZ).
The data were independently extracted from the included trials by two reviewers (MLL and NZ) and entered into a structured characteristics table. The extracted data included the following: the name of the first author, publication year, study design, features of the study population, strategies used to confirm breast cancer, assessment of marital status, research findings and other required information. We resolved any differences in opinion through consultation with a third person (MH).
Quality assessment
We rated the methodological quality of the included studies using an adapted version of the Newcastle-Ottawa Scale (NOS) [17]. The NOS consists of eight items focusing on the following three domains: selection of study groups, ascertainment of exposure and outcome, and comparability of groups. The ratings are based on a star system with a maximum rating of nine. The assessments were performed by two authors (MLL and JTL), and any disagreements were resolved by discussion with a third author (SQZ).
Statistical analyses
We provided a narrative synthesis of the findings from the included studies and pooled the results if the studies adopted the same methods to categorize marital status. Married women were used as the reference category. In all analyses, we generated inverse-variance weighted random-effects models with the DerSimonian-Laird estimator to account for the high expected heterogeneity across studies resulting from differences in the samples, measures, and designs. Subgroup analyses by control type (population-based studies versus hospital-based studies) and by adjustment level (multivariate-adjusted estimates versus age-adjusted estimates) were performed to determine the association between marital status and the risk of breast cancer.
The odds ratios (ORs) were used to measure the effect. If a study where the control subjects were matched to cases by age did not report the ORs, we calculated the ORs using a 2 × 2 cross tabulation. If a study did not use married people as the reference group, we inverted the ORs based on the method proposed by Greenland and Longnecker [18] with Microsoft Excel software developed by Hamling et al [19]. For studies that provided estimates of the relative risk based on different multivariate models, we prioritized the results from the model with the largest number of covariates.
Heterogeneity was tested using the Cochran Q statistic and quantified with the I-squared (I2) statistic, which describes the variation in the effect size attributable to the heterogeneity across studies. The confidence intervals for I2 were also calculated using the formula proposed by Higgins [20]. Low, moderate, and high degrees of heterogeneity were indicated by I2 values of <25%, 25–75%, and >75%, respectively. The potential sources of heterogeneity were explored if high heterogeneity was detected using a random-effects-weighted meta-regression based on the following variables: (1) adjustment level: multivariate-adjusted estimates versus age-adjusted estimates; (2) control type: population-based studies versus hospital-based studies; (3) geographic region: Asian versus non-Asian populations; and (4) publication year (continuous). Funnel plots and Egger’s test results were generated to detect publication bias for the comparison with the largest number of studies. Review Manager V.5.3 (Cochrane Collaboration) and Stata SE.15 software were used for the statistical analyses.
Results
Study selection
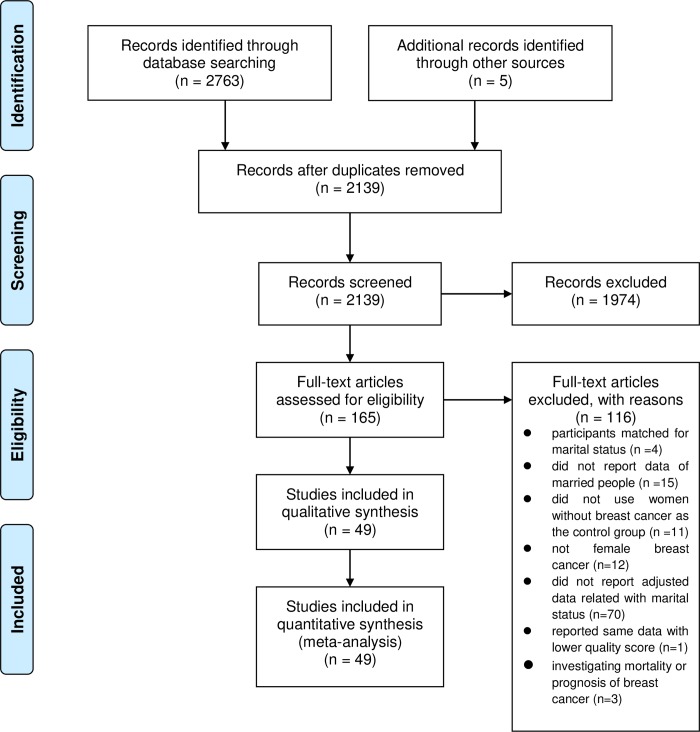
We identified 2763 articles using the search strategies. By reviewing the articles’ reference lists, we found five additional articles. After removing the duplicates and reviewing the titles and abstracts, we retrieved 165 full-text articles. Of these articles, 49 studies [11–14,21–65] fulfilled the inclusion criteria and were included in the meta-analysis (Fig 1).
Fig 1. Flow diagram.
Description of studies
Table 1 describes the key study characteristics. We included two cohort studies [11,13] and 47 case-control studies [12,14,21–65]. The 49 studies included in our analyses involved 1,723,739 women, including 59,992 breast cancer cases. The studies were published between 1978 and 2018 and included participants from Europe, the United States, Australia, Africa and Asia. Overall, 19 studies [14,21,25,29,33,37,38,40–43,46,48,51,52,56,57,60,63] only provided age-adjusted estimates, while 30 studies [11–13,22–24,26–28,30–32,34–36,39,44,45,47,49,50,53–55,58,59,61,62,64,65] controlled for multiple confounding factors, such as demographic characteristics, reproductive factors, behavior and lifestyle factors and several psychological variables (full details are displayed in S4 Table). 20 studies (i.e. population-based studies) [11–14,21,26,29,33,34,36,38,40,41,46,49,51,54,58,62,64] recruited controls from the same community as cancer cases, while 29 studies (i.e. hospital-based studies) [22–25,27,28,30–32,35,37,39,42–45,47,48,50,52,53,55–57,59–61,62,65] selected controls from hospitals or other health care centers. Married people accounted for between 14.3% and 97.9% of the sample (widowed = 2.9% to 31.1%, divorced = 0.6% to 20.2%, and lifelong single = 0.7% to 53.9%).
Table 1. Characteristics of the included studies.
| Study | Sample size | Region | Resource | Matched/adjusted for | Marital status (%) | NOS score | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Cases | Case | Control | Married | Widowed | Divorced | Lifelong single | ||||
| Cohort studies | |||||||||||
| Carlsen et al. [11] | 1,590,000 | 25,855 | Europe | population | population | multiple variables | N/A | N/A | N/A | N/A | 7 |
| Melchior et al. [13] | 5493 | 120 | Europe | employees | employees | multiple variables | N/A | N/A | N/A | N/A | 7 |
| Case-control studies | |||||||||||
| Adami et al. [21] | 358 | 179 | Europe | population | population | age | 60.3 | 24.9 | 4.2 | 10.6 | 6 |
| Balekouzou et al. [22] | 519 | 174 | Africa | hospital | hospital | multiple variables | 14.3 | 85.7 (w/d/lls) | 6 | ||
| Bano et al. [23] | 1246 | 1238 | Asia | hospital | hospital | multiple variables | N/A | N/A | N/A | N/A | 4 |
| Budiningsih et al. [24] | 900 | 300 | Asia | hospital | hospital | multiple variables | N/A | N/A | N/A | N/A | 5 |
| Cho et al. [25] | 705 | 358 | Asia | hospital | screening | age | 81.8 | 13.3 (w/d) | 4.8 | 5 | |
| Dey et al. [26] | 2108 | 900 | Asia | hospital | companion | multiple variables | 83.2 | N/A | 14.9 | 1.9 | 5 |
| Dianatinasab et al. [27] | 1052 | 526 | Asia | hospital | hospital | multiple variables | 92.8 | N/A | N/A | 7.2 | 7 |
| Ebrahimi et al. [28] | 535 | 286 | Asia | hospital | hospital | multiple variables | 77.4 | 16.8 (w/d) | 5.8 | 4 | |
| Ewertz et al. [14] | 3520 | 1782 | Europe | population | population | age | 73.9 | 10.6 | 8.8 | 6.6 | 6 |
| Faheem et al. [29] | 300 | 150 | Asia | hospital | population | age | 92.3 | 7.7 (w/d/lls) | 3 | ||
| Forsen [30] | 174 | 87 | Europe | hospital | hospital | multiple variables | N/A | N/A | N/A | N/A | 4 |
| Gajalakshmi and Shanta [31] | 1062 | 293 | Asia | hospital | hospital | multiple variables | 96.5 | 3.5 (w/d/lls) | 4 | ||
| Ghiasvand et al. [32] | 1042 | 521 | Asia | hospital | hospital | multiple variables | 84.0 | 6.9 (w/d) | 9.1 | 5 | |
| Gilani and Kamal [33] | 1480 | 498 | Asia | hospital | population | age | 96.6 | 3.4 (w/d/lls) | 6 | ||
| Hadjisavvas et al. [34] | 2282 | 1109 | Europe | population | population | multiple variables | 84.8 | 10.8 (w/d) | 4.3 | 7 | |
| Jafari-Mehdiabad et al. [35] | 285 | 98 | Asia | hospital | hospital | multiple variables | 84.2 | 11.9 | 1.8 | 2.1 | 5 |
| Justenhoven et al. [36] | 1997 | 1021 | Europe | population | population | multiple variables | 68.0 | 26.7 (w/d) | 5.3 | 5 | |
| Khalis et al. [37] | 474 | 237 | Africa | hospital | hospital | age | 68.8 | 12.2 | 8.2 | 10.8 | 6 |
| Khan et al. [38] | 196 | 100 | Asia | hospital | population | age | 84.2 | 15.8 (w/d/lls) | 4 | ||
| Kvikstad et al. [12] | 45,685 | 4491 | Europe | population | population | multiple variables | 83.6 | 2.9 | 13.4 | N/A | 7 |
| Laing et al. [39] | 992 | 503 | USA | hospital | hospital | multiple variables | 38.3 | 40.3 (w/d) | 21.4 | 5 | |
| Li et al. [40] | 1982 | 975 | USA | population | population | age | 53.1 | 31.1 | 12.4 | 3.4 | 4 |
| Lotfi and Shobairi [41] | 159 | 80 | Asia | hospital | population | age | 88.1 | 11.9 (w/d/lls) | 5 | ||
| Mahouri et al. [42] | 672 | 168 | Asia | hospital | hospital | age | 82.3 | 13.8 (w/d) | 3.9 | 4 | |
| Marzouk et al. [43] | 351 | 198 | Europe | hospital | hospital | age | 74.6 | 18.8 | 5.1 | 1.4 | 5 |
| Mohite et al. [44] | 434 | 217 | Asia | hospital | hospital | multiple variables | 85.0 | 10.6 | 2.3 | 2.1 | 4 |
| Morales et al. [45] | 1084 | 465 | USA | hospital | hospital | multiple variables | 61.1 | 6.5 | 20.2 | 12.3 | 6 |
| Motie et al. [46] | 254 | 134 | Asia | population | population | age | 97.2 | 2.8 (w/d/lls) | 4 | ||
| Oran et al. [47] | 1244 | 622 | Europe | hospital | hospital | multiple variables | 81.3 | N/A | N/A | 6.0 | 5 |
| Pakseresht et al. [48] | 332 | 115 | Asia | hospital | hospital | age | 80.1 | 19.9 (w/d/lls) | 5 | ||
| Parameshwari et al. [49] | 100 | 20 | Asia | population | population | multiple variables | 95.0 | 5.0 (w/d/lls) | 4 | ||
| Peled et al. [50] | 622 | 255 | Europe | hospital | hospital | multiple variables | 72.7 | 20.9 (w/d/lls) | 4 | ||
| Pimhanam et al. [51] | 888 | 444 | Asia | hospital | population | age | 67.5 | 10.0 (w/d) | 22.5 | 4 | |
| Price et al. [52] | 504 | 239 | Australia | suspicion | suspicion | age | 68.5 | 12.5 | 12.1 | 6.9 | 4 |
| Rao et al. [53] | 1400 | 689 | Asia | hospital | hospital | multiple variables | 76.4 | 20.6 | 0.6 | 2.4 | 4 |
| Rookus and van Leeuwen [54] | 1836 | 918 | Europe | population | population | multiple variables | 88.1 | 11.9 (w/d/lls) | 5 | ||
| Shamsi et al. [55] | 883 | 297 | Asia | hospital | hospital | multiple variables | N/A | N/A | N/A | N/A | 6 |
| Shaukat et al. [56] | 94 | 42 | Asia | hospital | hospital | age | 97.9 | 2.1 (w/d/lls) | 4 | ||
| Sufian et al. [57] | 216 | 108 | Asia | hospital | hospital | age | 93.5 | 6.5 (w/d/lls) | 4 | ||
| Tazhibi et al. [58] | 257 | 216 | Asia | relatives | relatives | multiple variables | 94.2 | 5.8 (w/d/lls) | 5 | ||
| Tehranian et al. [59] | 624 | 312 | Asia | hospital | hospital | multiple variables | 83.8 | N/A | 1.6 | 14.6 | 4 |
| Thompson et al. [60] | 1076 | 541 | USA | hospital | screening | age | 60.9 | 39.1 (w/d/lls) | 5 | ||
| Wakai et al. [61] | 678 | 226 | Asia | hospital | hospital | multiple variables | 78.3 | 17.3 | 3.7 | 0.7 | 4 |
| White et al. [62] | 1607 | 747 | USA | population | population | multiple variables | 73.0 | 15.8 (w/d) | 11.2 | 7 | |
| Yan et al. [63] | 1042 | 521 | Asia | hospital | hospital | age | 93.5 | 6.5 (w/d/lls) | 6 | ||
| Eaker et al. [64] | 28566 | 4761 | Europe | population | population | multiple variables | 62.9 | 2.9 | 17.8 | 16.8 | 8 |
| Randi et al. [65] | 14429 | 5856 | Europe | hospital | hospital | multiple variables | N/A | N/A | N/A | N/A | 7 |
N/A: not applicable.
Methodological quality
In our review, the diagnosis of breast cancer was considered definitive if confirmed by pathological records. Guided by this criterion, all studies were deemed qualified and rated one star, except for three studies [40,49,54]. For the item regarding the ascertainment of exposure, studies using a secure record or structured questionnaire with details regarding the timing of potential changes in marital status were rated one star. Almost all studies failed to meet this standard, except for three studies that used regularly updated marriage registry records [11,14,64]. The studies were rated two stars in the domain of comparability if they adjusted for at least three of the following known breast cancer risk factors: family history of breast cancer, parity, usage of hormone replacement therapy, usage of oral contraceptives, age at first and final birth, number of live births/abortions/miscarriages, birth interval, history of breastfeeding, lifelong menstrual pattern, menopausal status, age at menarche/menopause, education level, body mass index (BMI), alcohol intake and smoking. All studies controlled for age, but only 12 studies [13,22,27,32,34,36,39,45,47,55,58,65] were considered to have adequate comparability between the cases and controls.
Among the cohort studies, the studies selecting participants from a population or community with an initial response rate over 70% were considered adequately representative. Thus, one study [11] comprising all eligible residents in a country was rated one star, while the other study [13] in which only 45% accepted and completed a baseline survey received no stars. Regarding the second selection item, both studies obtained the non exposed cohort from the same source as the exposed cohort. In addition, both studies demonstrated that the outcome of interest was not present at the start of the study. Concerning follow-up, one study [11] followed up for less than 10 years with a dropout rate of 5.6%, while the other study [13] followed up for an average of 10.6 years with a dropout rate of 1%.
Among the case-control studies, 19 studies [12,21,24,27,30,32,33,35,37,41,43,44,46,48,54,55,62,64,65] were rated one star for the item of representativeness of the cases since they selected consecutive eligible cases in a defined area over a defined period. However, selection bias was still possible as only 12 studies recruited controls from the same community as the cases, while the remaining studies either used a hospital-based design [22–24,27,28,30–32,35,39,42–45,47,48,50,52,53,55–57,59,60,63,65] or provided no description of the source [25,26,29,37,38,46,51,58,60]. Eight studies [14,29,30,36,40,41,44,46] did not receive a star in the domain of control definition since these studies did not clearly demonstrate that the controls had no history of breast cancer. 18 studies reported a nonresponse rate with details, including 14 studies [12,14,22,25–27,34,37,40,45,60,62,63,65] with the same rate (difference less than 10%) between the cases and controls, which were rated one star.
The mean methodological quality score of the cohort studies and case-control studies was 7/9 and 5/9, respectively. Overall, both designs failed to demonstrate a satisfying performance in the domain of ascertainment of exposure and comparability of participants. The case-control studies also scored poorly on the item related to the representativeness of the cases and selection of controls. The full details of the methodological assessment are shown in S5 Table.
Effect estimates
Table 2 displays a summary of the effect estimates of the association between marital status and risk of breast cancer. Since the method used to categorize marital status varied across studies, we performed meta-analyses using the following comparisons:
Table 2. Summary of effect estimates.
| Subgroups | No. of studies | Pooled estimates | P-value | heterogeneity | ||
|---|---|---|---|---|---|---|
| I2 | P-value | |||||
| Unmarried | ||||||
| Cohort | 0 | / | / | / | / | |
| case-control | total | 41 | 1.20 [1.07, 1.35] | 0.002 | 82% [77%, 87%] | < 0.00001 |
| population-based | 17 | 1.13 [0.99, 1.29] | 0.07 | 80% [68%, 87%] | < 0.00001 | |
| hospital-based | 24 | 1.23 [1.01, 1.50] | 0.04 | 81% [72%, 87%] | < 0.00001 | |
| subgroup differences | 0.49 | |||||
| Widowed | ||||||
| cohort | (population-based) | 1 | 0.98 [0.93, 1.03] | 0.44 | / | / |
| case-control | total | 13 | 0.96 [0.86, 1.08] | 0.51 | 46% [0%, 72%] | 0.03 |
| population-based | 5 | 0.99 [0.87, 1.13] | 0.89 | 44% [0%, 80%] | 0.13 | |
| hospital-based | 8 | 0.94 [0.75, 1.18] | 0.60 | 47% [0%, 77%] | 0.06 | |
| subgroup differences | 0.70 | |||||
| Divorced | ||||||
| cohort | (population-based) | 1 | 1.04 [0.99, 1.09] | 0.08 | / | / |
| case-control | total | 15 | 1.16 [0.96, 1.39] | 0.12 | 80% [67%, 87%] | < 0.00001 |
| population-based | 6 | 1.03 [0.84, 1.26] | 0.79 | 85% [70%, 93%] | < 0.00001 | |
| hospital-based | 9 | 1.66 [1.02, 2.70] | 0.04 | 77% [55%, 88%] | < 0.0001 | |
| subgroup differences | 0.08 | |||||
| Lifelong single | ||||||
| cohort | (population-based) | 2 | 1.10 [1.04, 1.16] | 0.0004 | 0% [0%, 23%] | 0.33 |
| case-control | total | 25 | 1.24 [1.05, 1.45] | 0.01 | 69% [54%, 80%] | < 0.00001 |
| population-based | 9 | 1.00 [0.93, 1.08] | 0.97 | 0% [0%, 99%] | 0.50 | |
| hospital-based | 16 | 1.51 [1.07, 2.13] | 0.02 | 79% [66%, 87%] | < 0.00001 | |
| subgroup differences | 0.02 | |||||
| Unmarried | ||||||
| Cohort | 0 | / | / | / | / | |
| case-control | total | 41 | 1.20 [1.07, 1.35] | 0.002 | 82% [77%, 87%] | < 0.00001 |
| multi-adjusted | 22 | 1.23 [1.05, 1.45] | 0.01 | 88% [83%, 91%] | < 0.00001 | |
| age-adjusted | 19 | 1.16 [0.99, 1.37] | 0.07 | 65% [43%, 78%] | < 0.0001 | |
| subgroup differences | 0.61 | |||||
| Widowed | ||||||
| cohort | (multi-adjusted) | 1 | 0.98 [0.93, 1.03] | 0.44 | / | / |
| case-control | total | 13 | 0.96 [0.86, 1.08] | 0.51 | 46% [0%, 72%] | 0.03 |
| multi-adjusted | 7 | 1.02 [0.84, 1.23] | 0.85 | 67% [28%, 85%] | 0.005 | |
| age-adjusted | 6 | 0.89 [0.79, 1.02] | 0.09 | 0% [0%, 64%] | 0.80 | |
| subgroup differences | 0.27 | |||||
| Divorced | ||||||
| cohort | (multi-adjusted) | 1 | 1.04 [0.99, 1.09] | 0.08 | / | / |
| case-control | total | 15 | 1.16 [0.96, 1.39] | 0.12 | 80% [67%, 87%] | < 0.00001 |
| Multi-adjusted | 9 | 1.25 [0.97, 1.61] | 0.08 | 87% [78%, 93%] | < 0.00001 | |
| age-adjusted | 6 | 1.02 [0.83, 1.27] | 0.82 | 20% [0%, 64%] | 0.28 | |
| subgroup differences | 0.23 | |||||
| Lifelong single | ||||||
| cohort | (multi-adjusted) | 2 | 1.10 [1.04, 1.16] | 0.0004 | 0% [0%, 23%] | 0.33 |
| case-control | total | 25 | 1.24 [1.05, 1.45] | 0.01 | 69% [54%, 80%] | < 0.00001 |
| multi-adjusted | 16 | 1.28 [1.04, 1.58] | 0.02 | 76% [62%, 85%] | < 0.00001 | |
| age-adjusted | 9 | 1.17 [0.91, 1.49] | 0.22 | 39% [0%, 72%] | 0.11 | |
| subgroup differences | 0.56 | |||||
unmarried (an aggregated category including widowed, divorced and lifelong single) versus married people (n = 41);
widowed versus married people (n = 14);
divorced versus married people (n = 16); and
lifelong single (i.e., never married) versus married people (n = 27).
Unmarried versus married women
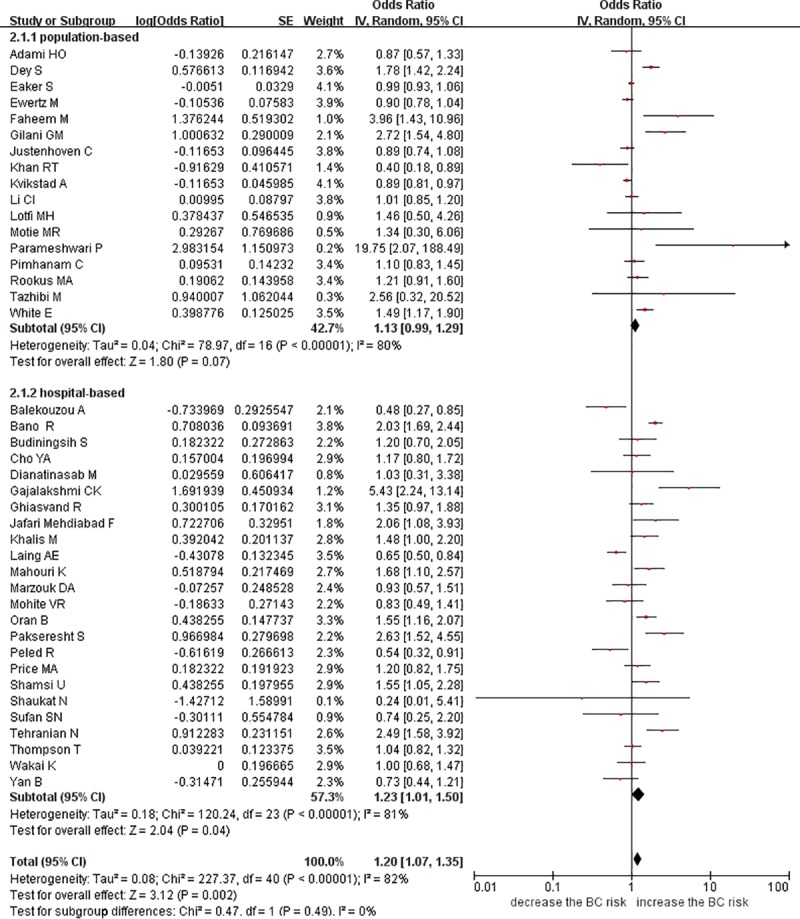
No eligible cohort study was included in this comparison. In the 41 case-control studies [12,14,21–27,29,31–33,35–44,46–52,54–64], a 20% risk increase (OR = 1.20; 95% confidence interval (95% CI): 1.07 to 1.35) for breast cancer was detected in the unmarried women versus the married women, with considerable heterogeneity (I2 = 82%; 95% CI: 77% to 87%). The meta-regression identified geographic region as a potential source of heterogeneity (P = 0.004) (S2 File). When we stratified our analysis by control type, we found a persistent positive association among the hospital-based studies (OR = 1.23, 95% CI: 1.01 to 1.50; I2 = 81%, 95% CI: 72% to 87%; n = 24), but no significant association was observed among the population-based studies (OR = 1.13, 95% CI: 0.99 to 1.29; I2 = 80%, 95% CI: 68% to 87%; n = 17). However, the subgroup difference was not significant (P = 0.49) (Fig 2). According to another subgroup analysis, the studies that adjusted for multiple variables resulted in an OR of 1.23 (95% CI: 1.05 to 1.45; I2 = 88%, 95% CI: 83% to 91%), whereas the studies that adjusted for age produced a nonsignificant OR of 1.16 (95% CI: 0.99 to 1.37; I2 = 65%, 95%CI: 43% to 78%). However, a subgroup difference was not detected (P = 0.61) (S3 File).
Fig 2. Forest plot of breast cancer risk among unmarried women versus married women stratified by control type.
Widowed versus married women
According to the results of the cohort study [11], the breast cancer risk in widowed people did not differ from that in married people (relative risk (RR) = 0.98; 95% CI: 0.93 to 1.03). The analysis of the 13 case-control studies [12,14,21,35,37,40,43–45,52,61,64,65] yielded a similar combined OR of 0.96 (95% CI: 0.86 to 1.08) with moderate between-study heterogeneity (I2 = 46%, 95% CI: 0% to 72%). Subgroup analyses did not identify any statistically significant association between being widowed and the breast cancer risk. The population-based studies produced an OR of 0.99 (95% CI: 0.87 to 1.13; I2 = 44%, 95% CI: 0% to 80%; n = 5) while the hospital-based studies produced an OR of 0.94 (95% CI: 0.75 to 1.18; I2 = 47%, 95% CI: 0% to 77%; n = 8). The studies with maximal adjustment produced an OR of 1.02 (95% CI: 0.84 to 1.23; I2 = 67%, 95% CI: 28% to 85%; n = 7) while the studies that adjusted for age produced an OR of 0.89 (95% CI: 0.79 to 1.02; I2 = 0%, 95% CI: 0% to 64%; n = 6). A significant subgroup difference was not observed in both stratified analyses (P = 0.70; P = 0.27) (S3 File).
Divorced versus married women
One cohort study [11] investigated the association between divorced status and the breast cancer risk and provided an RR of 1.04 (95% CI: 0.99 to 1.09), while 15 case-control studies [12,14,21,26,35,37,40,43–45,52,59,61,64,65] yielded a nonsignificant pooled OR of 1.16 (95% CI: 0.96 to 1.39; I2 = 80%, 95% CI: 67% to 87%). The meta-regression showed that geographic region (P = 0.017) and publication year (P = 0.024) were significantly associated with the effect estimates and may have been the causes of heterogeneity (S2 File). The hospital-based subgroup analysis produced an OR of 1.66 (95% CI: 1.02 to 2.70; I2 = 77%, 95% CI: 55% to 88%; n = 9), while the population-based analysis produced a nonsignificant OR of 1.03 (95% CI: 0.84 to 1.26; I2 = 85%, 95% CI: 70% to 93%; n = 6). The multivariate-adjusted subgroup analysis resulted in an OR of 1.25 (95% CI: 0.97 to 1.61; I2 = 87%, 95% CI: 78% to 93%; n = 9), whereas the age-adjusted analysis produced a nonsignificant OR of 1.02 (95% CI: 0.83 to 1.27; I2 = 20%, 95% CI: 0% to 64%; n = 6). A significant subgroup difference was not observed in both stratified analyses (P = 0.08; P = 0.23) (S3 File).
Lifelong single versus married women
Two cohort studies [11,13] investigated the association between lifelong single status and the breast cancer risk and provided an OR of 1.10 (95% CI: 1.04 to 1.16; I2 = 0%, 95% CI: 0% to 23%), while 25 case-control studies [14,21,25,26,28,30,32,34–37,39,40,42–45,47,51,52,59,61,62,64,65] yielded an OR of 1.24 (95% CI: 1.05 to 1.45; I2 = 69%, 95% CI: 54% to 80%). The subgroup analysis according to control type detected a significant subgroup difference (P = 0.02), but the subgroup analysis according to adjustment level failed to detect any difference (P = 0.56). The hospital-based studies produced a combined OR of 1.51 (95% CI: 1.07 to 2.13; I2 = 79%, 95% CI: 66% to 87%; n = 16), while the population-based studies yielded a nonsignificant OR of 1.00 (95% CI: 0.93 to 1.08; I2 = 0%, 95% CI: 0% to 99%; n = 9). The multivariate-adjusted estimates increased the overall OR to 1.28 (95% CI: 1.04 to 1.58; I2 = 76%, 95% CI: 62% to 85%; n = 16), while the age-adjusted estimates produced a nonsignificant combined OR of 1.17 (95% CI: 0.91 to 1.49; I2 = 39%, 95% CI: 0% to 72%; n = 9) (S3 File).
Publication bias
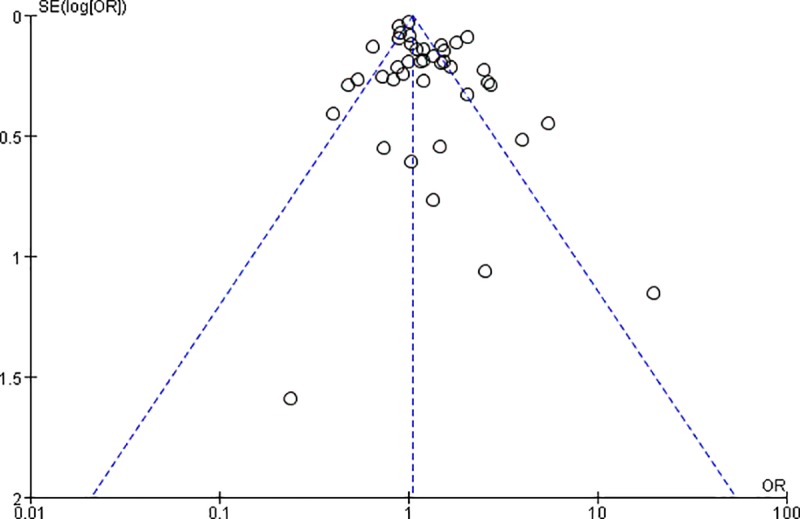
There was evidence of significant funnel plot asymmetry (Egger’s test: P = 0.03), suggesting publication bias among the studies that investigated the breast cancer risk in unmarried women (n = 41) (Fig 3).
Fig 3. Funnel plot of the effect of being unmarried on the risk of breast cancer.
Discussion
Main findings
Our study summarized all accessible published evidence and demonstrated that unmarried women, especially lifelong single women, had a higher risk of developing breast cancer than married women. Even though positive results were obtained, we could not draw the conclusion that marital status is an independent factor associated with breast cancer.
As indicated by the subgroup analysis where only hospital-based studies showed positive associations between marital status and the breast cancer risk, control type may affect the effect estimates. According to another subgroup analysis, the multivariate-adjusted estimates demonstrated a significant association in several comparisons, but the age-adjusted estimates revealed such associations in no comparisons. We speculate that these results might be attributed to interactions among the independent variables analyzed in the multivariable-adjusted models. Unfortunately, we could not investigate whether there are interactions since none of the original studies provided enough details.
Heterogeneity was moderate to substantial across the included case-control studies, which may be partly explained by geographic region, control type and publication year according to the subgroup analyses and meta-regression analyses. The results were consistent with those reported in previous studies. For instance, individuals of different races may experience different relationships between marriage and health [66], and the impact of marital status on the cancer incidence may also vary across cultures and change over time [67]. Moreover, the proportion of suspicious cases and the distribution of marital status were different between hospital-based studies and population-based studies, which supported the role of control type in the generation of between-study heterogeneity. Although several other factors, such as age and cancer subtypes, should also be considered, further analyses were not employed due to the limited data in the original studies.
We also identified possible publication bias, suggesting that some studies that failed to show a significant association between unmarried status and the breast cancer risk may have not been published.
Strengths and limitations
Thus far, no formal attempts have been made to systematically review data regarding the potential relationship between marital status and the risk of breast cancer. Therefore, the data included in our analysis represent the only available evidence for the enrichment of clinical screening and prevention decisions. However, considerable caution is warranted when interpreting these results due to the limitations of this review.
First, most case-control studies used a hospital-based design, resulting in controls that often fail to reflect the distribution of key characteristics of the population from which the cases were drawn. Another concern is that retrospective studies are believed to be subject to recall bias caused by memory distortion. However, major life events, including divorce, bereavement and getting married [68], show minimal change in recall over time and are, therefore, associated with greater reporting reliability [69]. Moreover, few included studies have recorded details of the changes in and duration of the marital status; thus, whether the current status or previous status produced the effect observed in the studies could not be determined in this review. Consequently, our review could not confirm the temporal relationship between the recorded marital status and the initiation of breast cancer.
Implications for practice and further research
Prospective cohort studies with sufficient details regarding the marital status and adequate adjustment for confounding factors should be carried out to explore the dose-response effect of different marital status categories and provide support for a causal relationship between marital status and breast cancer incidence. The moderating and mediating mechanisms of this relationship deserve substantial consideration to develop tailored interventions and strategies to detect breast cancer at an early stage.
Conclusions
This review detects a higher rate of breast cancer in unmarried women, especially lifelong single women, than married women. However, the quality of the available data is limited by possible publication bias and several methodological drawbacks, including suboptimal selection of controls, insufficient exploration of confounding effects, and inadequate ascertainment of marital status. Overall, conclusions that marital status is an independent risk factor for breast cancer could not be drawn, and fully adjusted prospective cohort studies with sufficient details regarding marital status should be conducted in the future.
Supporting information
(DOC)
(DOC)
(DOCX)
(DOCX)
(DOC)
(PDF)
(DOCX)
(DOCX)
Acknowledgments
We specifically thank Dr. Zheng Li for his kind assistance during the process of downloading the literature and American Journal Experts (AJE) for the English language editing.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68: 394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Engmann NJ, Golmakani MK, Miglioretti DL, Sprague BL, Kerlikowske K. Population-Attributable Risk Proportion of Clinical Risk Factors for Breast Cancer. JAMA Oncol. 2017;3:1228–1236. 10.1001/jamaoncol.2016.6326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Key TJ, Verkasalo PK, Banks E. Epidemiology of breast cancer. Lancet Oncol. 2001;2: 133–140. 10.1016/S1470-2045(00)00254-0 [DOI] [PubMed] [Google Scholar]
- 4.Barnes BB, Steindorf K, Hein R, Flesch-Janys D, Chang-Claude J. Population attributable risk of invasive postmenopausal breast cancer and breast cancer subtypes for modifiable and non-modifiable risk factors. Cancer Epidemiol. 2011;35:345–352. 10.1016/j.canep.2010.11.003 [DOI] [PubMed] [Google Scholar]
- 5.Williams K. Has the future of marriage arrived? A contemporary examination of gender, marriage, and psychological well-being. J Health Soc Behav. 2003; 44: 470–487. [PMC free article] [PubMed] [Google Scholar]
- 6.Williams K, Umberson D. Marital status, marital transitions, and health: a gendered life course perspective. J Health Soc Behav. 2004; 45:81–98. 10.1177/002214650404500106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee S, Cho E, Grodstein F, Kawachi I, Hu FB, Colditz GA. Effects of marital transitions on changes in dietary and other health behaviours in US women.Int J Epidemiol. 2005; 34:69–78. 10.1093/ije/dyh258 [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi S, Sugiura H, Ando Y, Shiraki N, Yanagi T, Yamashita H, et al. Reproductive history and breast cancer risk. Breast Cancer. 2012;19: 302–308. 10.1007/s12282-012-0384-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vishwakarma G, Ndetan H, Das DN, Gupta G, Suryavanshi M, Mehta A, et al. Reproductive factors and breast cancer risk: A meta-analysis of case-control studies in Indian women. South Asian J Cancer. 2019;8:80–84. 10.4103/sajc.sajc_317_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brouckaert O, Rudolph A, Laenen A, Keeman R, Bolla MK, Wang Q, et al. Reproductive profiles and risk of breast cancer subtypes: a multi-center case-only study. Breast Cancer Res. 2017;19:119 10.1186/s13058-017-0909-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlsen K, Hoybye MT, Dalton SO, Tjonneland A. Social inequality and incidence of and survival from breast cancer in a population-based study in Denmark, 1994–2003. Eur J Cancer. 2008;44: 1996–2002. 10.1016/j.ejca.2008.06.027 [DOI] [PubMed] [Google Scholar]
- 12.Kvikstad A, Vatten LJ, Tretli S, Kvinnsland S. Death of a husband or marital divorce related to risk of breast cancer in middle-aged women. A nested case-control study among Norwegian women born 1935–1954. Eur J Cancer. 1994;30: 473–477. [DOI] [PubMed] [Google Scholar]
- 13.Melchior M, Goldberg M, Krieger N, Kawachi I, Menvielle G, Zins M, et al. Occupational class, occupational mobility and cancer incidence among middle-aged men and women: a prospective study of the French GAZEL Cohort. Cancer Causes Control. 2005;16: 515–524. 10.1007/s10552-004-7116-0 [DOI] [PubMed] [Google Scholar]
- 14.Ewertz. Bereavement and breast cancer. Br J Cancer, 1986, 53:701–703. 10.1038/bjc.1986.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283: 2008–2012. 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med. 2009;18: e123. [PMC free article] [PubMed] [Google Scholar]
- 17.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2016. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Cited 8 October 2018. [Google Scholar]
- 18.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135: 1301–1309. 10.1093/oxfordjournals.aje.a116237 [DOI] [PubMed] [Google Scholar]
- 19.Hamling J, Lee P, Weitkunat R, Ambühl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med. 2008;27: 954–970. 10.1002/sim.3013 [DOI] [PubMed] [Google Scholar]
- 20.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002; 21:1539–1558. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 21.Adami HO, Rimsten A, Stenkvist B, Vegelius J. Reproductive history and risk of breast cancer: a case-control study in an unselected Swedish population. Cancer. 1978;41: 747–757. [DOI] [PubMed] [Google Scholar]
- 22.Balekouzou A, Yin P, Pamatika CM, Bekolo CE, Nambei SW, Djeintote M, et al. Reproductive risk factors associated with breast cancer in women in Bangui: a case-control study. BMC Womens Health. 2017;17: 14 10.1186/s12905-017-0368-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bano R, Ismail M, Nadeem A, Khan MH, Rashid H. Potential risk factors for breast cancer in Pakistani women. Asian Pac J Cancer Prev. 2016;17: 4307–4312. [PubMed] [Google Scholar]
- 24.Budiningsih S, Ohno YO, Prihartono J, Dillon DS, Tjahjadi G, Soetrisno E, et al. Breast cancer risk factors among Sundanese and other ethnic groups in Indonesia. Med J Indonesia. 1999;8: 128–132. [Google Scholar]
- 25.Cho YA, Kim J, Park KS, Lim SY, Shin A, Sung MK, et al. Effect of dietary soy intake on breast cancer risk according to menopause and hormone receptor status. Eur J Clin Nutr. 2010;64: 924–932. 10.1038/ejcn.2010.95 [DOI] [PubMed] [Google Scholar]
- 26.Dey S, Boffetta P, Mathews A, Brennan P, Soliman A, Mathew A. Risk factors according to estrogen receptor status of breast cancer patients in Trivandrum, South India. Int J Cancer. 2009;125: 1663–1670. 10.1002/ijc.24460 [DOI] [PubMed] [Google Scholar]
- 27.Dianatinasab M, Fararouei M, Mohammadianpanah M, Zare-Bandamiri M, Rezaianzadeh A. Hair coloring, stress, and smoking increase the risk of breast cancer: a case-control study. Clin Breast Cancer. 2017;17: 650–659. 10.1016/j.clbc.2017.04.012 [DOI] [PubMed] [Google Scholar]
- 28.Ebrahimi M, Vahdaninia M, Montazeri A. Risk factors for breast cancer in Iran: a case-control study. Breast Cancer Res. 2002;4: R10 10.1186/bcr454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faheem M, Khurram M, Jafri IA, Mehmood H, Hasan Z, Iqbal GS, et al. Risk factors for breast cancer in patients treated at NORI hospital, Islamabad. J Pak Med Assoc. 2007;57: 242–245. [PubMed] [Google Scholar]
- 30.Forsen A. Psychosocial stress as a risk for breast cancer. Psychother Psychosom. 1991;55: 176–185. 10.1159/000288427 [DOI] [PubMed] [Google Scholar]
- 31.Gajalakshmi CK, Shanta V. Risk factors for female breast cancer a hospital-based case-control study in Madras, India. Acta Oncol. 1991;30: 569–574. 10.3109/02841869109092419 [DOI] [PubMed] [Google Scholar]
- 32.Ghiasvand R, Maram ES, Tahmasebi S, Tabatabaee SH. Risk factors for breast cancer among young women in southern Iran. Int J Cancer. 2010;129: 1443–1449. 10.1002/ijc.25748 [DOI] [PubMed] [Google Scholar]
- 33.Gilani GM, Kamal S. Risk factors for breast cancer in Pakistani women aged less than 45 years. Ann Hum Biol. 2004;31: 398–407. 10.1080/0301446042000226763 [DOI] [PubMed] [Google Scholar]
- 34.Hadjisavvas A, Loizidou MA, Middleton N, Michael T, Papachristoforou R, Kakouri E, et al. An investigation of breast cancer risk factors in Cyprus: a case control study. BMC Cancer. 2010;10: 447–447. 10.1186/1471-2407-10-447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jafari-Mehdiabad F, Savabi-Esfahani M, Mokaryan F, Kazemi A. Relationship between breastfeeding factors and breast cancer in women referred to Seyed Al-Shohada hospital in Isfahan, Iran. Iran J Nurs Midwifery Res. 2016;21: 622–627. 10.4103/1735-9066.197670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Justenhoven C, Winter S, Dunnebier T, Hamann U, Baisch C, Rabstein S, et al. Combined UGT1A1 and UGT1A6 genotypes together with a stressful life event increase breast cancer risk. Breast Cancer Res Treat. 2010;124: 289–292. 10.1007/s10549-010-1093-7 [DOI] [PubMed] [Google Scholar]
- 37.Khalis M, Charbotel B, Chajes V, Rinaldi S, Moskal A, Biessy C, et al. Menstrual and reproductive factors and risk of breast cancer: a case-control study in the Fez region, Morocco. PLoS One. 2018;13: e0191333 10.1371/journal.pone.0191333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan RT, Siddique A, Shahid N, Khokher S, Fatima W. Breast cancer risk associated with genes encoding DNA repair MRN complex: a study from Punjab, Pakistan. Breast Cancer. 2018;25: 350–355. 10.1007/s12282-018-0837-9 [DOI] [PubMed] [Google Scholar]
- 39.Laing AE, Demenais FM, Williams R, Kissling G, Chen VW, Bonney GE. Breast cancer risk factors in African-American women: the Howard university tumor registry experience. J Natl Med Assoc. 1993;85: 931–939. [PMC free article] [PubMed] [Google Scholar]
- 40.Li CI, Malone KE, Weiss NS, Boudreau DM, Cushing-Haugen KL, Daling JR. Relation between use of antihypertensive medications and risk of breast carcinoma among women ages 65–79 years. Cancer. 2003;98: 1504–1513. 10.1002/cncr.11663 [DOI] [PubMed] [Google Scholar]
- 41.Lotfi M, Shobairi SCS. Breast cancer risk factors in an urban area of Yazd City-Iran, 2006. Acta Med Iran. 2008;46: 258–264. [Google Scholar]
- 42.Mahouri K, Zahedani MD, Zare S. Breast cancer risk factors in south of Islamic Republic of Iran: a case-control study. East Mediterr Health J. 2007;13: 1265–1273. 10.26719/2007.13.6.1265 [DOI] [PubMed] [Google Scholar]
- 43.Marzouk DA, El Gaafary MM, El Damaty SI, Sabbour SM, Mecky FAS, Saker M, et al. Breast cancer and hormonal intake among Egyptian females. Eur J Oncol. 2009;14: 37–52. [Google Scholar]
- 44.Mohite VR, Pratinidhi AK, Mohite RV. Reproductive risk factors and breast cancer: a case control study from rural India. Bangladesh J Med Sci. 2015;14: 258–264. [Google Scholar]
- 45.Morales L, Alvarez-Garriga C, Matta J, Ortiz C, Vergne Y, Vargas W, et al. Factors associated with breast cancer in Puerto Rican women. J Epidemiol Glob Health. 2013;3: 205–215. 10.1016/j.jegh.2013.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Motie MR, Besharat S, Torkjazi R, Shojaa M, Besharat M, Keshtkar A, et al. Modifiable risk of breast cancer in Northeast Iran: hope for the future. A case-control study. Breast Care (Basel). 2011;6: 453–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oran B, Celik I, Erman M, Baltali E, Zengin N, Demirkazik F, et al. Analysis of menstrual, reproductive, and life-style factors for breast cancer risk in Turkish women: a case-control study. Med Oncol. 2004;21: 31–40. 10.1385/MO:21:1:31 [DOI] [PubMed] [Google Scholar]
- 48.Pakseresht S, Ingle GK, Bahadur AK, Ramteke VK, Singh MM, Garg S, et al. Risk factors with breast cancer among women in Delhi. Indian J Cancer. 2009;46: 132–138. 10.4103/0019-509x.49151 [DOI] [PubMed] [Google Scholar]
- 49.Parameshwari P, Muthukumar K, Jennifer HG. A population based case control study on breast cancer and the associated risk factors in a rural setting in Kerala, Southern India. J Clin Diagn Res. 2013;7: 1913–1916. 10.7860/JCDR/2013/5830.3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peled R, Carmil D, Siboni-Samocha O, Shoham-Vardi I. Breast cancer, psychological distress and life events among young women. BMC Cancer. 2008;8: 245 10.1186/1471-2407-8-245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pimhanam C, Sangrajrang S, Ekpanyaskul C. Tobacco smoke exposure and breast cancer risk in Thai Urban females. Asian Pac J Cancer Prev. 2014;15: 7407–7411. 10.7314/apjcp.2014.15.17.7407 [DOI] [PubMed] [Google Scholar]
- 52.Price MA, Tennant CC, Smith RC, Butow PN, Kennedy SJ, Kossoff MB, et al. The role of psychosocial factors in the development of breast carcinoma: part I. The cancer prone personality. Cancer. 2001;91: 686–697. [PubMed] [Google Scholar]
- 53.Rao DN, Ganesh B, Desai PB. Role of reproductive factors in breast cancer in a low-risk area: a case-control study. Br J Cancer. 1994;70: 129–132. 10.1038/bjc.1994.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rookus MA, van Leeuwen FE. Oral contraceptives and risk of breast cancer in women aged 20–54 years. Netherlands oral contraceptives and breast cancer study group. Lancet. 1994;344: 844–851. 10.1016/s0140-6736(94)92826-6 [DOI] [PubMed] [Google Scholar]
- 55.Shamsi U, Khan S, Usman S, Soomro S, Azam I. A multicenter matched case control study of breast cancer risk factors among women in Karachi, Pakistan. Asian Pac J Cancer Prev. 2013;14: 183–188. 10.7314/apjcp.2013.14.1.183 [DOI] [PubMed] [Google Scholar]
- 56.Shaukat N, Jaleel F, Moosa FA, Qureshi NA. Association between Vitamin D deficiency and breast cancer. Pak J Med Sci. 2017;33: 645–649. 10.12669/pjms.333.11753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sufian SN, Masroor I, Mirza W, Butt S, Afzal S, Sajjad Z. Evaluation of common risk factors for breast carcinoma in females: a hospital based study in Karachi, Pakistan. Asian Pac J Cancer Prev. 2015;16: 6347–6352. 10.7314/apjcp.2015.16.15.6347 [DOI] [PubMed] [Google Scholar]
- 58.Tazhibi M, Dehghani M, Babazadeh S, Makkarian F, Tabatabaeian M, Sadeghi M, et al. Hormonal and reproductive risk factors associated with breast cancer in Isfahan patients. J Educ Health Promot. 2014;3: 69 10.4103/2277-9531.134818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tehranian N, Shobeiri F, Pour FH, Hagizadeh E. Risk factors for breast cancer in Iranian women aged less than 40 years. Asian Pac J Cancer Prev. 2010;11: 1723–1725. [PubMed] [Google Scholar]
- 60.Thompson T, Rodebaugh TL, Perez M, Schootman M, Jeffe DB. Perceived social support change in patients with early stage breast cancer and controls. Health Psychol. 2013;32: 886–895. 10.1037/a0031894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wakai K, Dillon DS, Ohno Y, Prihartono J, Budiningsih S, Ramli M, et al. Fat intake and breast cancer risk in an area where fat intake is low: a case-control study in Indonesia. Int J Epidemiol. 2000;29: 20–28. 10.1093/ije/29.1.20 [DOI] [PubMed] [Google Scholar]
- 62.White E, Malone KE, Weiss NS, Daling JR. Breast cancer among young U.S. women in relation to oral contraceptive use. J Natl Cancer Inst. 1994;86: 505–514. 10.1093/jnci/86.7.505 [DOI] [PubMed] [Google Scholar]
- 63.Yan B, Lu MS, Wang L, Mo XF, Luo WP, Du YF, et al. Specific serum carotenoids are inversely associated with breast cancer risk among Chinese women: a case-control study. Br J Nutr. 2016;115: 129–137. 10.1017/S000711451500416X [DOI] [PubMed] [Google Scholar]
- 64.Eaker S, Wigertz A, Lambert PC, Bergkvist L, Ahlgren J, Lambe M. Breast Cancer, Sickness Absence, Income and Marital Status. A Study on Life Situation 1 Year Prior Diagnosis Compared to 3 and 5 Years after Diagnosis. PLoS One. 2011;6: e18040 10.1371/journal.pone.0018040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Randi G, Altieri A, Gallus S, Chatenoud L, Montella M, Franceschi S, et al. Marital status and cancer risk in Italy. Prev Med. 2004;38: 523–528. 10.1016/j.ypmed.2003.12.004 [DOI] [PubMed] [Google Scholar]
- 66.Williams DR, Takeuchi DT, Adair RK. Marital Status and Psychiatric Disorders Among Blacks and Whites. J Health Soc Behav. 1992; 33:140–157. [PubMed] [Google Scholar]
- 67.Newton NJ, Ryan LH, King RT, Smith J. Cohort differences in the marriage-health relationship for midlife. Soc Sci Med. 2014; 116: 64–72. 10.1016/j.socscimed.2014.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holmes TH, Rahe RH. The social readjustment rating scale. J Psychosom Res. 1967;11: 213–218. 10.1016/0022-3999(67)90010-4 [DOI] [PubMed] [Google Scholar]
- 69.Kruk J. Self-reported psychological stress and the risk of breast cancer: a case-control study. Stress. 2012;15: 162–171. 10.3109/10253890.2011.606340 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(DOCX)
(DOCX)
(DOC)
(PDF)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.