Abstract
Background:
Venous thromboembolism (VTE) is a common cause of morbidity and mortality among patients with multiple myeloma (MM). The International Myeloma Working Group (IMWG) developed guidelines recommending primary thromboprophylaxis in those identified at high-risk of VTE by the presence of risk factors. The National Comprehensive Cancer Network (NCCN) has adopted these guidelines; however, they lack validation. We sought to develop and validate a risk prediction score for VTE in MM and to evaluate the performance of the current IMWG/NCCN guidelines.
Methods:
Using 4,446 patients within the Veterans Administration Central Cancer Registry, we used time-to-event analyses to develop a risk score for VTE in patients with newly diagnosed MM starting chemotherapy. We externally validated the score using the Surveillance, Epidemiology, End Results (SEER)-Medicare database (N = 4,256).
Results:
After identifying independent predictors of VTE, we combined the variables to develop the IMPEDE VTE score (Immunomodulatory agent; Body Mass Index ≥ 25 kg/m2; Pelvic, hip or femur fracture; Erythropoietin stimulating agent; Dexamethasone/Doxorubicin; Asian Ethnicity/Race; VTE history; Tunneled line/central venous catheter; Existing thromboprophylaxis). The score showed satisfactory discrimination in the derivation cohort, c-statistic = 0.66. Risk of VTE significantly increased as score increased (hazard ratio 1.20, p = <0.0001). Within the external validation cohort, IMPEDE VTE had a c-statistic of 0.64. For comparison, when evaluating the performance of the IMWG/NCCN guidelines, the c-statistic was 0.55.
Conclusion:
In summary, the IMPEDE VTE score outperformed the current IMWG/NCCN guidelines and could be considered as the new standard risk stratification for VTE in MM.
Keywords: Venous Thromboembolism, Multiple Myeloma, Clinical Prediction Rule, Risk, Primary Prevention
Introduction:
Compared to the general population, patients with multiple myeloma (MM) have a 9-fold increased risk of venous thromboembolism (VTE).1 Risk of VTE is even greater in MM patients treated with immunomodulatory (IMID) drugs (e.g., lenalidomide) or additional thrombogenic drugs (e.g., dexamethasone).2–4 With treatment advances transforming MM from a fatal disease to a chronic one5, a common cause of death in this population is VTE.6,7 Despite the overall reduction in MM-related mortality, MM patients with VTE have a 3-fold increased risk of death at one year following MM diagnosis compared to MM patients without VTE.8,9
Thromboprophylaxis is a safe and effective way to prevent VTE. In a recent trial of outpatients with cancer, low-dose apixaban decreased risk of VTE (hazard ratio (HR) 0.41; 95% confidence interval (CI) 0.26–0.65) with a small increase in bleeding compared to placebo.10,11 Two randomized trials12,13 found thromboprophylaxis with aspirin, low-molecular-weight heparin (LMWH), or warfarin to be safe in patients with MM on IMID drugs. However, these trials excluded patients with high risk of VTE (e.g. prior history of VTE) and did not provide guidance on VTE risk stratification.14 Overall, rates of VTE in MM remain >10%.15
The International Myeloma Working Group (IMWG) set forth guidelines, adopted by the National Comprehensive Cancer Network (NCCN), for the prevention of VTE in MM patients on IMID therapy16,17; however, these guidelines lack validation.18 In a recent study, the NCCN/IMWG guidelines identified only 55% of patients who developed VTE as high-risk.15 A validated VTE risk score predicts VTE among patients with solid tumors,11 but not MM.19 A risk score for VTE in MM would allow for the use of thromboprophylaxis in patients at high-risk while avoiding anticoagulant exposure in low-risk patients. Therefore, we sought to develop and validate a risk prediction score for VTE in patients with newly diagnosed MM starting therapy, as well as to evaluate the performance of the NCCN/IMWG guidelines.
Methods:
Assembly of Cohorts
Using International Classification of Diseases (ICD)-O3 codes 9732/3, we identified patients diagnosed with MM between September 1, 1999 and June 30, 2014 within the Veterans Administration Central Cancer Registry (VACCR) (derivation cohort). We excluded patients who did not receive chemotherapy within 6 months of MM diagnosis. We defined chemotherapy start date as the date of administration of the first chemotherapy agent. Selected chemotherapy agents included bendamustine, bortezomib, cisplatin, cyclophosphamide, doxorubicin, etoposide, lenalidomide, melphalan, and thalidomide. Chemotherapy start date for patients receiving dexamethasone monotherapy was the date of first prescription for dexamethasone. We excluded patients who received a transplant within 4 months of chemotherapy start, as these patients likely received treatment outside of the VA. We censored those who underwent transplant between 4 to 6 months at the time of transplant.
Using the VA Informatics and Computing Infrastructure platform, we obtained ICD-9 codes, Pharmacy Benefits Management (PBM) records, and laboratory data. Using ICD-9 codes, we obtained comorbidities present at the time of MM diagnosis by identifying at least two ICD-9 codes within 12 months prior to MM diagnosis. Using ICD-9 procedure codes, we defined recent surgery as cardiovascular, orthopedic, abdominal, urologic, or neurologic surgery occurring within 30 days before MM diagnosis up to start of chemotherapy. Similarly, we identified patients with a fracture of the pelvis, femur, hip within 30 days prior to MM diagnosis up to start of chemotherapy using ICD-9 codes. Using CPT codes, we identified placement of a tunneled line/central venous catheter (CVC). We recorded baseline laboratory data available from 30 days prior to MM diagnosis up to start of chemotherapy. For patients with multiple laboratory values, we selected the values closest to MM diagnosis. We assessed height and weight from 30 days prior to MM diagnosis up to the start date of chemotherapy. Similarly, if multiple height and weight data were available, we selected the value closest to MM diagnosis date. We calculated body mass index (BMI) using height and weight.20 We calculated the estimated glomerular filtration rate (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration formula.21 PBM included dates of administration of all inpatient and outpatient drugs. We defined dexamethasone use as “high-dose” if total monthly dosing was greater than 160 mg; otherwise, we defined it as “low-dose” to conform to current practice patterns. However, for validating current NCCN guidelines, we defined “NCCN high-dose” as ≥ 480 mg monthly; otherwise, we defined it as “NCCN low-dose”. Using standard definitions22,23, we classified LMWH prescriptions as therapeutic or prophylactic dosing. We classified all warfarin prescriptions as therapeutic. We manually abstracted all missing data, if unavailable we excluded those patients.
We retrospectively followed patients for 180 days after start of chemotherapy. The VA Vital Status File provided date of death from multiple sources: VA Beneficiary Identification and Records Locator Subsystem Death File, the Social Security Administration Death Master File, the Medicare Vital Status File, and the Medical SAS® Inpatient Datasets. Using ICD-9 codes, we identified cases of VTE prior to MM diagnosis and within 6 months of starting chemotherapy for MM with the requirement of the presence of at least two ICD-9 codes. We confirmed all cases of VTE with manual chart abstraction.
Using the linked Surveillance, Epidemiology, End Results (SEER)-Medicare cohort, we assembled an external validation cohort of patients with MM. Using ICD-O3 code 9732, we identified patients diagnosed with MM from January 1, 2007 to December 31, 2013. For inclusion, patients had to be Medicare eligible (≥ age 65) and continuously enrolled on Medicare parts A, B, and D starting one year prior to MM diagnosis. As with the derivation cohort, we excluded patients without treatment with chemotherapy within 6-months after MM diagnosis.
Definitions for all variables within the SEER-Medicare cohort were consistent with those used for the VACCR cohort with the following alterations. However, we did not exclude patients who received a transplant within 4 months of chemotherapy start, as unlike the VA data, this occurrence was unlikely to suggest care outside of the Medicare healthcare system. We censored those who underwent transplant between chemotherapy start date and 6 months at the time of transplant. We identified placement of a tunneled line/CVC using a combination of ICD-9, CPT and HCPCS codes. Using the National Claims History, Outpatient Medicare claims, and Part D medication files, we identified administration of all prescription medications including chemotherapy. Aspirin use was not available within this cohort. The SEER-Medicare data lacks vital sign information, thus we used ICD-9 codes to identify patients with a BMI of overweight, obese and morbidly obese. We identified VTE through use of a previously validated algorithm that used the combination of VTE-related treatment and ICD-9 code to identify VTE as manual abstraction was not possible.24
For baseline patient characteristics within the derivation and validation cohorts, we used the χ2 to compare proportions, the Cochran-Mantel-Haenszel and Wilcoxon tests for categorical variables, and unpaired Student’s t tests for continuous variables. Prior to cohort assembly, the St. Louis VA Medical Center and Washington University institutional review boards approved the study.
IMPEDE VTE Score Derivation
We identified candidate risk factors in myeloma through literature review and univariate analyses (Supplemental Table 1). We considered candidate predictors for entry into the multivariate model, if on univariate analysis they had a p < 0.05 or with a p < 0.5 with findings consistent with prior literature, as recommended.25 We used the methods of Fine and Gray to model time to VTE while accounting for the competing risk of non-VTE death.26 We entered intermittent treatments as time-varying variables, minimizing risk of immortal time bias and loss of information from variables that change over time.25,27 We adjusted the model for year of diagnosis. Using a backward, stepwise approach, we retained variables in the multivariate model with a p < 0.05, or with a p < 0.4 with findings consistent with prior literature as above.25 We derived the risk score by multiplying the parameter estimate for each variable by a common number, and rounding to the nearest integer. The risk score for each patient was the sum of integers for all predictor variables. Using risk scores, we calculated incidence rates for VTE.
We assessed discrimination by calculating Harrell’s c-statistic.28 We assessed the association of the prediction score and development of VTE using competing risk analysis. Using the D’Agostino modification of the Hosmer-Lemeshow test, we assessed model calibration defined as the agreement between the observed and predicted probability of VTE by IMPEDE VTE.29 We internally validated the IMPEDE VTE score using a bootstrap procedure whereby we generated a new sample equal to the size of the cohort by randomly drawing subjects, with replacement, from the original cohort. For each of the 500 bootstrapped samples, we calculated the risk score and Harrell’s c-statistic. Using the 500 samples, we calculated the average c-statistic.
IMPEDE VTE External Validation
We externally validated the IMPEDE VTE risk score using the SEER-Medicare cohort. We used the c-statistic to assess discrimination and competing risk analysis to quantify the association of the risk score and development of VTE.26
Sensitivity Analyses
In both the validation cohort, we excluded patients on therapeutic or prophylactic dose anticoagulation and then assessed the score discrimination through calculation of Harrell’s c-statistic.
IMWG/NCCN Validation
Within the VACCR cohort, we validated the NCCN/IMWG VTE guidelines (Supplemental Table 2) for MM using a subgroup of patients receiving IMID therapy after excluding those receiving anticoagulation. As suggested by the guidelines we defined a score of 0–1 points as low-risk and ≥2 points as high-risk. In addition, we defined all patients receiving NCCN high-dose dexamethasone, doxorubicin, or multiagent chemotherapy (excluding bortezomib) in combination with IMID therapy as high-risk. To assess discrimination, we calculated Harrell’s c-statistic.28 We assessed the association of a high-risk score with development of VTE using competing risk analysis.26 We carried out statistical analyses using SAS version 9.4 (SAS Institute, Cary, NC).
Results:
Patient Characteristics
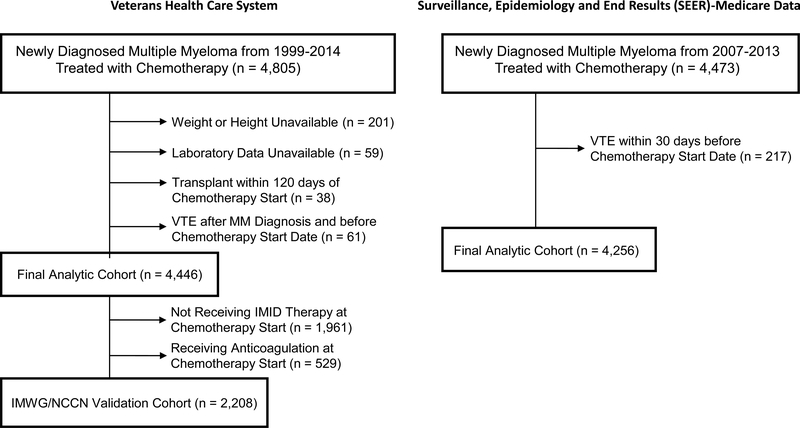
The VACCR cohort contained 4,446 patients (Figure 1). The average follow up for the cohort was 5.1 months. Of the patients in the cohort, 2,837 were diagnosed after 7/½006 and thus after approval of lenalidomide. Within 6-months of starting chemotherapy, 259 patients (5.8%) developed VTE (Supplemental Table 3). Baseline characteristics of patients with VTE versus those without are in Table 1. In addition to the 110 patients who underwent transplant during the study period, an additional 463 proceeded to one after the study period. The SEER-Medicare cohort contained 4,256 patients. Two hundred and twenty-one patients (5.2%) developed VTE within 6-months of starting chemotherapy. Baseline patient characteristics are presented in Supplemental Table 4. A comparison of the baseline characteristics between the VACCR and SEER-Medicare cohorts is listed in Supplemental Table 5.
Figure 1.
Flow diagram showing selection process of patients with multiple myeloma.
Table 1:
Demographic and clinical characteristics at chemotherapy start stratified by VTE in 6 months (Yes vs. No) among US veterans diagnosed with MM from 1999 to2014 (N=4446)
| Demographic clinical characteristics | VTE in 6 months after chemo |
P-value | |
|---|---|---|---|
| Yes (n=259) | No (n=4187) | ||
| Age (mean years) | 67.2 | 68.5 | 0.048 |
| Male (%) | 253 (97.7) | 4099 (97.9) | 0.82 |
| Race (%) | 0.33 | ||
| White | 188 (72.6) | 2872 (68.6) | |
| Black | 69 (26.6) | 1257 (30.0) | |
| Asian/Pacific Islander | 2 (0.8) | 58 (1.4) | |
| Body Mass Index (BMI) (%) | 0.048 | ||
| BMI < 18.5 | 2 (0.8) | 105 (2.5) | |
| 18.5 <= BMI < 25 | 70 (27) | 1302 (31.1) | |
| 25 <= BMI < 30 | 107 (41.3) | 1612 (38.5) | |
| BMI >= 30 | 80 (30.9) | 1168 (27.9) | |
| Hemi- or Paraplegia (%) | 6 (2.3) | 71 (1.7) | 0.50 |
| History of Venous Thromboembolism | 13 (5.0) | 97 (2.3) | <0.001 |
| Diabetes (%) | 59 (22.8) | 1139 (27.2) | 0.12 |
| Diagnostic year (median) | 2007 | 2008 | 0.16 |
| Central Venous Catheter (%) | 11 (4.3) | 180 (4.3) | 0.95 |
| Transplant (%) | 13 (5.0) | 96 (2.3) | <0.001 |
| Immunomodulatory Drug (%) | 148 (57.1) | 1813 (43.3) | <0.001 |
| Dexamethasone (%) | <0.001 | ||
| No use | 54 (20.9) | 1405 (33.6) | |
| Low dose | 113 (43.6) | 1830 (43.7) | |
| High dose | 92 (35.5) | 952 (22.7) | |
| Bortezomib (%) | 70 (27) | 1252 (29.9) | 0.32 |
| Doxorubicin (%) | 37 (14.3) | 301 (7.2) | <0.001 |
| Erythropoietin (%) | 78 (30.1) | 1080 (25.8) | 0.13 |
| Warfarin (%) | 26 (10) | 578 (13.8) | 0.09 |
| Low Molecular Weight Heparin (%) | <0.001 | ||
| Prophylactic | 16 (6) | 121 (2.9) | |
| Therapeutic | 9 (3.5) | 50 (1.2) | |
| Aspirin (%) | 69 (26.6) | 1357 (32.4) | 0.05 |
| Fracture (%) | 14 (5.4) | 117 (2.8) | 0.02 |
| Surgery (%) | 10 (3.9) | 230 (5.5) | 0.25 |
| Hemoglobin < 10g/dL OR Hematocrit < 30g/dL (%) | 103 (39.8) | 1670 (40.6) | 0.80 |
| Platelet ≥ 350 × 109/L (%) | 19 (7.3) | 251 (6.0) | 0.39 |
| Estimated Glomerular Filtration Rate < 30 mL/min (%) | 56 (21.6) | 942 (22.5) | 0.73 |
| White Blood Cell ≥ 10 × 109/L (%) | 15 (5.8) | 389 (9.3) | 0.06 |
IMPEDE VTE Score Derivation
Supplemental Table 1 shows results of all univariate analyses. Using VACCR cohort, the final multivariate model included the following predictors after elimination: use of IMIDs; BMI ≥ 25 kg/m2; pelvic, hip or femur fracture; use of ESAs, doxorubicin, or dexamethasone; history of VTE; presence of a tunneled line/CVC, while Asian/Pacific Islander ethnicity/race and use of thromboprophylaxis (therapeutic anticoagulation or prophylactic anticoagulation/aspirin) were protective for VTE (Table 2).
Table 2:
Time-Varying Multivariate Prediction Model Derivation
| Predictor |
Backward Elimination |
|
|---|---|---|
| β Coefficient | P - Value | |
| Immunomodulatory Drug | 0.76 | <0.001 |
| Body Mass Index ≥ 25 kg/m2 | 0.22 | 0.11 |
| Pelvic, Hip or Femur Fracture | 0.86 | <0.001 |
| Erythropoiesis-Stimulating Agent | 0.21 | 0.22 |
| Doxorubicin | 0.50 | 0.04 |
| Dexamethasone | ||
| High-Dose | 0.86 | <0.001 |
| Low-Dose | 0.48 | 0.01 |
| Asian/Pacific Islander | −0.63 | 0.37 |
| History of Venous Thromboembolism before MM | 1.05 | <0.001 |
| Central Venous Catheter | 0.46 | 0.04 |
| Therapeutic Low Molecular Weight Heparin or Warfarin | −0.72 | <0.001 |
| Prophylactic Low Molecular Weight Heparin or Aspirin | −0.59 | <0.001 |
We assigned points for each variable by multiplying the parameter estimate from the Fine and Gray model by 5 and rounding the product to the nearest integer. This resulted in the acyronym and the final point assignment as listed in Table 3 (IMPEDE VTE score). Using the point assignments, the IMPEDE VTE score had a c-statistic of 0.66. When using the beta coefficients instead of point scores, the c-statistic was unchanged at 0.66.
Table 3:
IMPEDE VTE Score
| Predictor | Acronym | Score |
|---|---|---|
| Immunomodulatory Drug | I | 4 |
| Body Mass Index ≥ 25 kg/m2 | M | 1 |
| Pelvic, Hip or Femur Fracture | P | 4 |
| Erythropoiesis-Stimulating Agent | E | 1 |
| Doxorubicin | D | 3 |
| Dexamethasone | ||
| High-Dose | 4 | |
| Low-Dose | 2 | |
| Ethnicity/Race = Asian/Pacific Islander | E | −3 |
| History of Venous Thromboembolism before MM | V | 5 |
| Tunneled Line/Central Venous Catheter | T | 2 |
| Existing Thromboprophylaxis: Therapeutic LMWH or Warfarin | E | −4 |
| Existing Thromboprophylaxis: Prophylactic LMWH or Aspirin | −3 | |
| LWMH = Low Molecular Weight Heparin | ||
Validation and Risk of VTE according to the Clinical Prediction Score
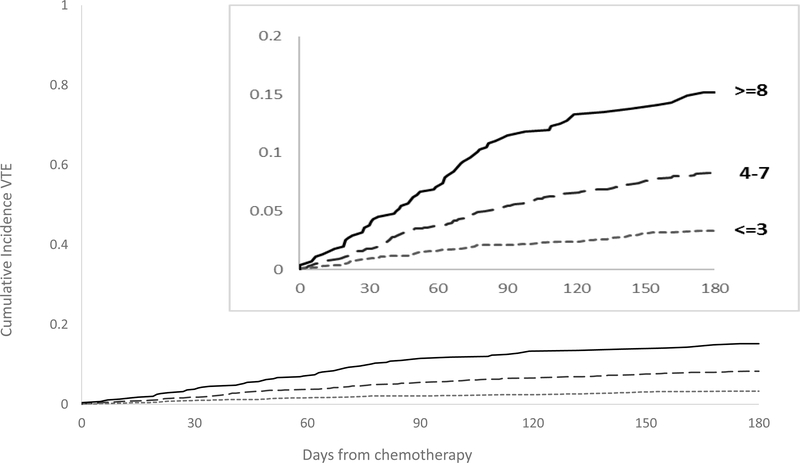
Using the bootstrap method, we internally validated our score. The average Harrell’s c-statistic of the samples was 0.66 (95% CI: 0.63 – 0.70). The D’Agostino modification of the Hosmer-Lemeshow test indicated good agreement between the observed and predicted probability of VTE (p = 0.41). Using the point assignments, Table 4 and Figure 2 show the 6-month cumulative incidence of VTE. The HR for VTE increased by 1.20 per point (95% CI: 1.15–1.24, p = <0.0001). Patients in the lowest risk group, IMPEDE VTE score ≤ 3, have a 6-month cumulative incidence of VTE after start of chemotherapy of 3.3% (95% CI: 2.6–4.1) while those with a score of ≥ 8 had an incidence of > 15% (95% CI: 12.1–19).
Table 4:
6-Month Cumulative Incidence of VTE based on IMPEDE VTE Score
| Score | Derivation Cohort (VACCR) |
|
|---|---|---|
| VTE/No VTE in 6 months | 6-Month Cumulative Incidence % (95% CI) | |
| ≤ 3 | 67/2245 | 3.3 (2.6–4.1) |
| 4–7 | 129/1553 | 8.3 (7.1–9.8) |
| ≥8 | 63/389 | 15.2 (12.1–19) |
Figure 2.
6-Month Cumulative Incidence Curves of VTE according to IMPEDE VTE Score.
IMPEDE VTE External Validation
Within the SEER-Medicare cohort, IMPEDE VTE had a c-statistic of 0.64. In the validation cohort, the HR for VTE increased by 1.16 per point (95% CI: 1.11 – 1.21, p = <0.0001).
Sensitivity Analyses
At the time of chemotherapy start, 389 patients within the VACCR cohort was taking therapeutic or prophylactic anticoagulation. After exclusion of these patients, the IMPEDE VTE had a c-statistic of 0.65. Within the validation cohort, after exclusion of patients on anticoagulation, the c-statistic = 0.62.
NCCN/IMWG Validation
In the VACCR cohort, 2,208 patients initiated lenalidomide or thalidomide and were not receiving anticoagulation at the start of therapy. In this population, Harrell’s c-statistic for the NCCN/IMWG guidelines was 0.55. Risk of VTE in patients defined as high-risk (≥2 points) versus low risk (0–1 points) was 1.39 (95% CI: 1.00–1.92, p-value 0.05).
Discussion:
We developed a risk prediction score to quantify risk of VTE in patients with MM starting chemotherapy. Using a nationwide sample, we developed the IMPEDE VTE score, which comprises the following nine variables: IMIDs; BMI; Pelvic, hip or femur fracture; use of ESAs, use of Dexamethasone/Doxorubicin; Ethnicity/Race; VTE history; Tunneled line/CVC; and Existing thromboprophylaxis. The rate of VTE significantly increased as IMPEDE VTE risk score increased (HR 1.20 per point, p = <0.0001). The 6-month cumulative incidence of VTE for scores of ≤ 3, 4–7 and ≥8 was 3.3, 8.3 and 15.2, respectively in the derivation cohort (Figure 2, Table 4).
The IMPEDE VTE score discrimination was adequate in both the derivation cohort (c-statistic = 0.66) and the validation cohort (c-statistic = 0.64). Given availability of data in SEER-Medicare, we were unable to account for aspirin use in the validation cohort. Based off characteristics in the VACCR, as well as that observed in the United States, we estimate 19–50% of patients in the SEER-Medicare cohort were likely using aspirin.30–32 In addition, vital signs are not reported in SEER-Medicare data, preventing calculation of BMI for identification of obesity.33 Prior studies have shown that use of ICD-9 codes for obesity have low sensitivity.34 We identified 479 patients (11.3%) within SEER-Medicare with an ICD-9 code for overweight, obesity or morbid obesity. Prevalence of BMI ≥ 25 kg/m2 within the VACCR was 66.8%. In a sensitivity analysis, when we eliminated points for aspirin or BMI within the derivation cohort, the c-statistic decreased to 0.65. Thus, it is possible the lower c-statistic in the validation cohort reflects the unknown aspirin use and BMI in the SEER-Medicare cohort.
The discrimination (c–statistic) of our prediction model is in line with the performance of alternate prediction models for VTE, including in the cancer and non-cancer population.35,36 In addition, the discrimination of the IMPEDE VTE score outperforms the current IMWG/NCCN guidelines, which had a c-statistic 0.55 in the VACCR cohort. These guidelines use VTE risk estimates based on expert opinion as the only available option for risk stratification.37 Current guidelines are poorly adopted in clinical practice, with selection of thromboprophylaxis unrelated to VTE risk category.38 Rates of VTE in MM patients starting chemotherapy remain high, exceeding 10%.15. Of patients who develop thrombosis, only 55% are identified as high-risk by current guidelines.16 Hence, we developed an evidence-based risk prediction score with external validation, IMPEDE VTE score. While our score offers a significant improvement on current guidelines, given the risk of the MM population, further improvements in risk prediction would likely require incorporation of biomarkers into a model (e.g. d-dimer).
Thromboprophylaxis is safe and effective in patients with cancer at high-risk of VTE. In the AVERT trial, the rate of major bleeding was 4.2% with no bleeding in to critical organs noted.10 In AVERT, 6-month rates of VTE in patients with a Khorana score ≥ 2 was >10%. Patients with an IMPEDE VTE score ≥8 had a similar 6-month incidence. In the CASSINI trial, the rate of major bleeding during prophylaxis with rivaroxaban in high-risk cancer patients was low at 2.0%.39 When considered together, there was a significant reduction in risk of symptomatic VTE in patients with cancer and high-risk of VTE receiving prophylaxis versus placebo (relative risk (RR) 0.58; 95% CI 0.35–0.94).40 In addition, there was no significant increase in risk of major bleeding compared to placebo (RR 1.96; 95% CI 0.88–4.33). Similarly, prescribing prophylaxis in MM who have a high-risk of VTE risk could reduce morbidity and mortality. Two prior trials assessed thromboprophylaxis in MM with major bleeding rates < 1%.12,13 However, these trials excluded patients with history of VTE, and lacked power to quantify the benefit of thromboprophylaxis. No formal VTE risk assessment was conducted in either trial but they demonstrated that thromboprophylaxis was safe in this population. Our risk score allows provider-based VTE risk assessment. Given the continued high rate of VTE in MM,15 future thromboprophylaxis trials should incorporate a formal risk assessment score for VTE such as IMPEDE VTE.
In our model, patients with a score of ≤ 3 had a 6-month cumulative incidence of VTE of 3.3% (upper limit of confidence bounds = 4.1%). Based on risk-benefit, avoidance of thromboprophylaxis in this population might be an acceptable strategy. Conversely, patients with scores of ≥ 8 had 6-month cumulative rates of VTE that exceeded 15% and could be considered for thromboprophylaxis (lower limit of confidence bounds = 12.1%). For example, low-dose apixaban reduced the rate of VTE from 10.2% to 4.2% in a recent trial of high-risk cancer patients.10
Several variables associated with VTE-risk in alternate populations, were not significant predictors in our analysis. Age and male gender, in contrast to prior studies41, were not associated with risk of VTE in our population. This finding was similar to prior MM studies.13,14,42 In addition, surgery has been associated with a high risk of post-operative VTE.41 However, we found no increased risk of VTE in after recent surgery. We suspect that the variable for recent fracture fully adjusted for the increase risk of VTE after hip fracture repair. Of the 89 patients who had a fracture, 56 subsequently underwent surgery within 30 days. Our model includes treatments that may have decreased in utilization since the start of the study period (e.g. high-dose dexamethasone and doxorubicin). However, the NCCN guidelines list the option to use doxorubicin in combination therapy for upfront treatment of MM. In addition, while the derivation cohort includes patients diagnosed back to 1999, the validation cohort demonstrates performance in a modern patient population diagnosed after approval of modern therapy (e.g. lenalidomide). Last, some studies have suggested a possible protective effect of bortezomib for VTE in MM.43,44 However, several large, phase III randomized trials found no significant association between bortezomib assignment and VTE.45–47 Thus, given the inconsistent association of bortezomib with VTE in MM and its insignificant association in the derivation dataset, we did not force bortezomib into the model.
We took several steps to improve the validity of the IMPEDE VTE score. We used a large, nationwide cohort with individual patient data. We manually confirmed all VTE events. Third, we validated it externally. Fourth, we studied two real-world populations. Fifth, we used readily available clinical variables to assess VTE risk. Sixth, we avoided developing a model based exclusively on stepwise selection, which can lead to a loss of predictive information.48,49 Instead, we used a liberal p value (p = 0.4) to include variables that predicted VTE in previous studies, as recommended25,50. We included tunneled line/CVC in our model as presence significantly increased risk of VTE (p = 0.04). Of the events that occurred in our cohort, 6.2% (n=16) were classified as line-associated upper extremity deep vein thrombosis. A recent post-hoc analysis of the CASSINI trial suggested a reduction in risk of line-associated VTE with thromboprophylaxis.51 Lastly, to increase generalizability, we included patients already taking thromboprophylaxis. Half of American adults use aspirin regularly.30 Accounting for the potential protective effect52 of aspirin improves VTE prediction. In addition, patients may be on anticoagulation at the start of chemotherapy for alternate indications (e.g. atrial fibrillation, prior VTE). Given our model, providers will still be able to risk assess this population and provide patient education regarding risk. A subgroup analysis excluding this population in the validation cohort found the model still discriminated risk with a c-statisic of 0.62.
The study has some limitations. Given the retrospective study design, we were not able to assess whether biomarkers (e.g. D-dimer) could improve score discrimination. Future research should focus on addition of these variables to IMPEDE VTE. Second, while the derivation cohort was a nationwide sample, it contained relatively few women, Asians or Pacific islanders, which may have resulted in reduced power to quantify risk of VTE in these populations. However, as with prior literature,53 Asian ethnicity protected against VTE. In addition, a recent study looking at risk of VTE in MM found a reduction in risk associated with Asian ethnicity.54 Aspirin is available as an over-the-counter medication, thus it is possible some Veterans received aspirin outside of the VA pharmacy and aspirin use was not known in the validation cohort. Last, in the time after the study period, the FDA has approved additional chemotherapy agents for MM (e.g. carfilzomib, ixazomib, daratumumab). However, in upfront clinical trials, these agents have not been associated with high rates of VTE.55–57
Summary:
In summary, we developed and validated the IMPEDE VTE score, which outperformed the risk stratification in the IMWG/NCCN guidelines. Risk assessment can help clinicians select thromboprophylaxis in high-risk patients, and avoid anticoagulants in those at low VTE risk. These data suggest that the IMPEDE VTE score could replace the risk stratification within the current guidelines for identification of patients with MM at high risk of VTE.
Supplementary Material
Essentials.
Venous thromboembolism (VTE) is a cause of morbidity and mortality in multiple myeloma (MM)
Guidelines recommend VTE prophylaxis in MM at high-risk by a risk-assessment model (NCCN/IMWG)
The NCCN/IMWG model discriminates VTE risk poorly, c-statistic = 0.55
IMPEDE VTE improves VTE risk-discrimination in MM and could be considered the new standard model
Acknowledgements:
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute at the National Institutes of Health; the Office of Research, Development and Information, Centers for Medicare & Medicaid Services; Information Management Services Inc; and the SEER program tumor registries in the creation of the SEER-Medicare database.
Funding: This work was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health (1K01HL136893–01 and 5K12 HL087107–95 to K.M.S.); the National Center for Advancing Translational Sciences at the National Institutes of Health (1UL1TR002345–01 to B.A.E.); and the National Cancer Institute at the National Institutes of Health (K12CA167540 to T.M.W.).
The SEER-Medicare data analysis is supported by the Center for Administrative Data Research at Washington University. Center for Administrative Data Research is supported in part by the Washington University Institute of Clinical and Translational Sciences grant from the National Center for Advancing Translational Sciences at the National Institutes of Health (UL1 TR000448); Agency for Healthcare Research and Quality (R24 HS19455); and the National Cancer Institute at the National Institutes of Health (KM1CA156708).
Footnotes
Disclosure of Potential Conflicts of Interest:
K.M.S. was on the Bristol-Myers Squibb speaker bureau, served on an advisory board for Pfizer and Bayer, received travel expenses from AstraZeneca and received research funding from NHLBI. T-F. W. received travel expenses from Daiichi Sankyo. T.M.W. received research funding from Janssen. J.M. received research funding from Onyx, Celgene, Sanofi, and AbbVie. N.M.K. received consultancy fees from Janssen, Halozyme, Pfizer, Myriad Genetics, Agendia, and Celldex; research funding from Celldex; and travel expenses from Janssen, Halozyme, Pfizer, and Agendia. K.R.C. received consultancy fees from Roche. All other authors have no conflicts to disclose.
References:
- 1.Kristinsson SYP RM: Bjorkholm M: Goldin LR: Schulman S: Blimark C: Mellqvist UH: Wahlin A: Turesson I: Landgren O. Arterial and venous thrombosis in monoclonal gammopathy of undetermined significance and multiple myeloma: a population-based study. Blood. 2010;115(24):4991–4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zonder JA, Crowley J, Hussein MA, et al. Lenalidomide and high-dose dexamethasone compared with dexamethasone as initial therapy for multiple myeloma: a randomized Southwest Oncology Group trial (S0232). Blood. 2010;116(26):5838–5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li W, Garcia D, Cornell RF, et al. Cardiovascular and Thrombotic Complications of Novel Multiple Myeloma Therapies: A Review. JAMA Oncol. 2017;3(7):980–988. [DOI] [PubMed] [Google Scholar]
- 4.Carrier MLG G: Tay J: Wu C: Lee AY Rates of venous thromboembolism in multiple myeloma patients undergoing immunomodulatory therapy with thalidomide or lenalidomide: a systematic review and meta-analysis. Journal of thrombosis and haemostasis : JTH. 2011;9(4):653–663. [DOI] [PubMed] [Google Scholar]
- 5.Cowan AJ, Allen C, Barac A, et al. Global Burden of Multiple Myeloma: A Systematic Analysis for the Global Burden of Disease Study 2016. JAMA Oncol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mai EK, Haas E-M, Lücke S, et al. A systematic classification of death causes in multiple myeloma. Blood Cancer Journal. 2018;8(3):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoen M, Luo S, Gage B, Carson K, Sanfilippo K. Association of venous thromboembolism with increased mortality in patients with multiple myeloma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018;36(suppl):abstr 8051. [Google Scholar]
- 8.Kristinsson SYP RM: Bjorkholm M: Schulman S: Landgren O. Thrombosis is associated with inferior survival in multiple myeloma. Haematologica. 2012;97(10):1603–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137(12):e67–e492. [DOI] [PubMed] [Google Scholar]
- 10.Carrier M, Abou-Nassar K, Mallick R, et al. Apixaban to Prevent Venous Thromboembolism in Patients with Cancer. The New England journal of medicine. 2018. [DOI] [PubMed] [Google Scholar]
- 11.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larocca A, Cavallo F, Bringhen S, et al. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma treated with lenalidomide. Blood. 2012;119(4):933–939. [DOI] [PubMed] [Google Scholar]
- 13.Palumbo A, Cavo M, Bringhen S, et al. Aspirin, warfarin, or enoxaparin thromboprophylaxis in patients with multiple myeloma treated with thalidomide: a phase III, open-label, randomized trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(8):986–993. [DOI] [PubMed] [Google Scholar]
- 14.Crowley MP, Eustace JA, O’Shea SI, Gilligan OM. Venous Thromboembolism in Patients With Myeloma: Incidence and Risk Factors in a “Real-World” Population. Clinical and applied thrombosis/hemostasis : official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis. 2014. [DOI] [PubMed] [Google Scholar]
- 15.Bradbury CA, Jenner MW, Striha A, et al. Thrombotic Events in Patients with Myeloma Treated with Immunomodulatory Drugs; Results of the Myeloma XI Study. Blood. 2017;130(Suppl 1):553–553. [Google Scholar]
- 16.IMWG Guidelines for the Prevention of Thalidomide- and Lenalidomide-Associated Thrombosis in Myeloma. 2010. [DOI] [PubMed]
- 17.Streiff MB, Holmstrom B, Ashrani A, et al. Cancer-Associated Venous Thromboembolic Disease, Version 1.2015. Journal of the National Comprehensive Cancer Network : JNCCN. 2015;13(9):1079–1095. [DOI] [PubMed] [Google Scholar]
- 18.Palumbo A, Rajkumar SV, Dimopoulos MA, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. 2008;22(2):414–423. [DOI] [PubMed] [Google Scholar]
- 19.Sanfilippo K, Wang TF, Luo S, et al. Predictive ability of the khorana score for venous thromboembolism (VTE) in multiple myeloma (MM). Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018;36(suppl):abstr e18733. [Google Scholar]
- 20.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 Suppl 2):S102–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enoxaparin [package insert]. In. Bridgewater, NJ: Sanofi-Aventis U.S. LLC; 2017. [Google Scholar]
- 23.Dalteparin [package insert] In. New York, NY: Pfizer Health AB; 2017. [Google Scholar]
- 24.Sanfilippo KM, Wang TF, Gage BF, Liu W, Carson KR. Improving accuracy of International Classification of Diseases codes for venous thromboembolism in administrative data. Thrombosis research. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steyerberg EW, Eijkemans MJ, Harrell FE Jr., Habbema JD Prognostic modeling with logistic regression analysis: in search of a sensible strategy in small data sets. Medical decision making : an international journal of the Society for Medical Decision Making. 2001;21(1):45–56. [DOI] [PubMed] [Google Scholar]
- 26.Fine GG R. A proportional hazards model for the subdistribution of a competing risk. J Am Statistical Association 1999;94(446):496–509. [Google Scholar]
- 27.Chaiteerakij R, Petersen GM, Bamlet WR, et al. Metformin Use and Survival of Patients With Pancreatic Cancer: A Cautionary Lesson. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(16):1898–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrell FE Jr., THe PHGLM Procedure. In: Cary, NC: SAS Institute Inc; 1986. [Google Scholar]
- 29.D’Agostino RBB-H N. . Evaluation of the performance of survival analysis models: discrimination and calibration measures. Handbook of statistics. 2004;23:1–25. [Google Scholar]
- 30.Williams CD, Chan AT, Elman MR, et al. Aspirin use among adults in the U.S.: results of a national survey. Am J Prev Med. 2015;48(5):501–508. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Y, Boudreau DM, Freedman AN. Trends in the use of aspirin and nonsteroidal anti-inflammatory drugs in the general U.S. population. Pharmacoepidemiology and drug safety. 2014;23(1):43–50. [DOI] [PubMed] [Google Scholar]
- 32.Pignone M, Anderson GK, Binns K, Tilson HH, Weisman SM. Aspirin use among adults aged 40 and older in the United States: results of a national survey. Am J Prev Med. 2007;32(5):403–407. [DOI] [PubMed] [Google Scholar]
- 33.Division of Cancer Control & Population Sciences NCI. SEER-Medicare Linked Database: Measures that are Limited or not Available in the Data. Retrieved from https://healthcaredeliverycancergov/seermedicare/considerations/measureshtml. 2018.
- 34.Ammann EM, Kalsekar I, Yoo A, Johnston SS. Validation of body mass index (BMI)-related ICD-9-CM and ICD-10-CM administrative diagnosis codes recorded in US claims data. Pharmacoepidemiology and drug safety. 2018. [DOI] [PubMed] [Google Scholar]
- 35.van Es N, Di Nisio M, Cesarman G, et al. Comparison of risk prediction scores for venous thromboembolism in cancer patients: a prospective cohort study. Haematologica. 2017;102(9):1494–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tosetto A, Testa S, Martinelli I, et al. External validation of the DASH prediction rule: a retrospective cohort study. Journal of thrombosis and haemostasis : JTH. 2017;15(10):1963–1970. [DOI] [PubMed] [Google Scholar]
- 37.Palumbo AR SV: Dimopoulos MA: Richardson PG: San Miguel J: Barlogie B: Harousseau J: Zonder JA: Cavo M: Zangari M: Attal M: Belch A: Knop S: Joshua D: Sezer O: Ludwig H: Vesole D: Blade J: Kyle R: Westin J: Weber D: Bringhen S: Niesvizky R: Waage A: von Lilienfeld-Toal M: Lonial S: Morgan GJ: Orlowski RZ: Shimizu K: Anderson KC: Boccadoro M: Durie BG: Sonneveld P: Hussein MA Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. 2008;22(2):414–423. [DOI] [PubMed] [Google Scholar]
- 38.Lipe B, Baker H, Weckbaugh B, et al. Validation of a thrombosis risk assessment model in patients with newly diagnosed multiple myeloma. Journal of Clinical Oncology. 2016;34(15_suppl):8055–8055. [Google Scholar]
- 39.Khorana AA, Soff GA, Kakkar AK, et al. Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer. The New England journal of medicine. 2019;380(8):720–728. [DOI] [PubMed] [Google Scholar]
- 40.Agnelli G. Direct Oral Anticoagulants for Thromboprophylaxis in Ambulatory Patients with Cancer. The New England journal of medicine. 2019;380(8):781–783. [DOI] [PubMed] [Google Scholar]
- 41.Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis. 2016;41(1):3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leleu XR P: Hulin C: Daley L: Dauriac C: Hacini M: Decaux O: Eisemann JC: Fitoussi O: Lioure B: Voillat L: Slama B: Al Jijakli A: Benramdane R: Chaleteix C: Costello R: Thyss A: Mathiot C: Boyle E: Maloisel F: Stoppa AM: Kolb B: Michallet M: Lamblin A: Natta P: Facon T: Elalamy I: Fermand JP: Moreau P. MELISSE, a large multicentric observational study to determine risk factors of venous thromboembolism in patients with multiple myeloma treated with immunomodulatory drugs. Thrombosis and haemostasis. 2013;110(4):844–851. [DOI] [PubMed] [Google Scholar]
- 43.Zangari M, Guerrero J, Cavallo F, Prasad HK, Esseltine D, Fink L. Hemostatic effects of bortezomib treatment in patients with relapsed or refractory multiple myeloma. Haematologica. 2008;93(6):953–954. [DOI] [PubMed] [Google Scholar]
- 44.Rupa-Matysek J, Gil L, Wojtasinska E, et al. Inhibitory effects of bortezomib on platelet aggregation in patients with multiple myeloma. Thrombosis research. 2014;134(2):404–411. [DOI] [PubMed] [Google Scholar]
- 45.Rosinol L, Oriol A, Teruel AI, et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood. 2012;120(8):1589–1596. [DOI] [PubMed] [Google Scholar]
- 46.Durie BG, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389(10068):519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cavo M, Pantani L, Petrucci MT, et al. Bortezomib-thalidomide-dexamethasone is superior to thalidomide-dexamethasone as consolidation therapy after autologous hematopoietic stem cell transplantation in patients with newly diagnosed multiple myeloma. Blood. 2012;120(1):9–19. [DOI] [PubMed] [Google Scholar]
- 48.Efron B. Estimating the error rate of a prediction rule: improvement on cross-validation. J Am Stat Assoc. 1983;78:316–331. [Google Scholar]
- 49.Chatfield C. Model uncertainty, data mining and statistical inference. J R Stat Soc A. 1995;158:419–466. [Google Scholar]
- 50.Steyerberg EW, Eijkemans MJ, Van Houwelingen JC, Lee KL, Habbema JD. Prognostic models based on literature and individual patient data in logistic regression analysis. Stat Med. 2000;19(2):141–160. [DOI] [PubMed] [Google Scholar]
- 51.Khorana AA, Mones J, Soff GA. Preventing Venous Thromboembolism in Patients with Cancer. Reply. The New England journal of medicine. 2019;380(22):2181. [DOI] [PubMed] [Google Scholar]
- 52.Simes J, Becattini C, Agnelli G, et al. Aspirin for the prevention of recurrent venous thromboembolism: the INSPIRE collaboration. Circulation. 2014;130(13):1062–1071. [DOI] [PubMed] [Google Scholar]
- 53.White RH, Zhou H, Romano PS. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thrombosis and haemostasis. 2003;90(3):446–455. [DOI] [PubMed] [Google Scholar]
- 54.Li A, Wu Q, Luo S, et al. Derivation and Validation of a Risk Assessment Model for Immunomodulatory Drug-Associated Thrombosis Among Patients With Multiple Myeloma. Journal of the National Comprehensive Cancer Network : JNCCN. 2019;17(7):840–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.San-Miguel JF, Echeveste Gutierrez MA, Spicka I, et al. A phase I/II dose-escalation study investigating all-oral ixazomib-melphalan-prednisone induction followed by single-agent ixazomib maintenance in transplant-ineligible newly diagnosed multiple myeloma. Haematologica. 2018;103(9):1518–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Facon T, Lee JH, Moreau P, et al. Carfilzomib or bortezomib with melphalan-prednisone for transplant-ineligible patients with newly diagnosed multiple myeloma. Blood. 2019;133(18):1953–1963. [DOI] [PubMed] [Google Scholar]
- 57.Mateos MV, Dimopoulos MA, Cavo M, et al. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. The New England journal of medicine. 2018;378(6):518–528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.