Abstract
Succinylation is a post-translational modification of protein lysine residues with succinyl groups derived from succinyl CoA. Succinylation is considered a significant post-translational modification with the potential to impact protein function which is highly conserved across numerous species. The role of succinylation in the heart, especially in heart failure and myofibril mechanics, remains largely unexplored. Mechanical parameters were measured in myofibrils isolated from failing hearts of ischemic cardiomyopathy patients and non-failing donor controls. We employed mass spectrometry to quantify differential protein expression in myofibrils from failing ischemic cardiomyopathy hearts compared to non-failing hearts. In addition, we combined peptide enrichment by immunoprecipitation with liquid chromatography tandem mass spectrometry to quantitatively analyze succinylated lysine residues in these myofibrils. Several key parameters of sarcomeric mechanical interactions were altered in myofibrils isolated from failing ischemic cardiomyopathy hearts, including lower resting tension and a faster rate of activation. Of the 100 differentially expressed proteins, 46 showed increased expression in ischemic heart failure, while 54 demonstrated decreased expression in ischemic heart failure. Our quantitative succinylome analysis identified a total of 572 unique succinylated lysine sites located on 181 proteins, with 307 significantly changed succinylation events. We found that 297 succinyl-Lys demonstrated decreased succinylation on 104 proteins, while 10 residues demonstrated increased succinylation on 4 proteins. Investigating succinyl CoA generation, enzyme activity assays demonstrated that α-ketoglutarate dehydrogenase and succinate dehydrogenase activities were significantly decreased in ischemic heart failure. An activity assay for succinyl CoA synthetase demonstrated a significant increase in ischemic heart failure. Taken together, our findings support the hypothesis that succinyl CoA production is decreased and succinyl CoA turnover is increased in ischemic heart failure, potentially resulting in an overall decrease in the mitochondrial succinyl CoA pool, which may contribute to decreased myofibril protein succinylation in heart failure.
Keywords: Proteomics, heart failure, myofibrils, succinylation, sirtuins, post-translational modification
1. Introduction
Heart failure (HF) is a leading cause of mortality, affecting more than 6 million people in the United States and over 22 million people worldwide [1, 2]. The incidence of HF in the United States is expected to rise by 46 percent by 2030 [3]. Survival rates for patients over 45 years old with HF have not improved since 1998, while many cancer survival rates have doubled over the last four decades [4, 5]. The most important risk factor for HF is ischemic heart disease, which typically leads to ischemic cardiomyopathy (ICM) and HF [6]. Ischemic heart disease is the leading cause of death worldwide, accounting for approximately 18 million, or 31%, of all deaths annually [6]. ICM is the most common cause of HF, affecting more than 2.5 million people in the United States with an annual incidence of 40,000 cases per year and an annual mortality rate of 200,000 [6]. Therefore, revealing underlying biochemical mechanisms contributing to the onset and progression of HF remains a key step toward improving HF treatment and mortality. Known molecular abnormalities associated with HF include post-translational modification (PTM) of sarcomeric proteins, acidosis, reversion of protein isoforms to their fetal phenotype, changes in membrane protein expression, fibrosis, and mitochondrial impairments in oxidative phosphorylation, the TCA cycle, and fatty acid oxidation [7].
Protein PTMs have been shown to play an integral role in a wide variety of human diseases, including HF [8]. However, it is unknown whether these PTMs are a byproduct of HF or a contributing factor to the development of HF. PTMs can regulate protein function through their influence on protein activity, stability, and cellular localization [9, 10]. PTMs are a critical event that can impair the pumping ability of failing hearts and can be caused by fluctuations in energy production and utilization [7, 11]. They can also impact cardiac contractility by affecting both contractile proteins and calcium cycling [7]. It is believed that many membrane proteins contributing to excitation-contraction coupling are subject to modification, yet little to no information exists regarding the acylation status of these proteins in HF [11–14].
Research on PTMs in HF has primarily focused on phosphorylation of proteins involved in cardiac muscle contraction, which have been shown to significantly alter both protein and cardiac function [15–18]. In addition, evidence is building for the impact of SUMOylation and oxidation of cardiac proteins in HF [19, 20]. However, few studies have explored the significance of lysine acylation in HF. Lysine residues are prone to a multitude of acylation events, including acetylation, malonylation, ubiquitination, carbonylation, and succinylation [9, 21–28]. However, because the heart is the organ with the highest concentration of succinyl CoA in the human body, succinylation is thought to function as a key regulatory PTM in the heart [10]. Mitochondrial protein succinylation involves the nonenzymatic, reversible modification of the N-ε-amine of lysine residues with a covalently bound succinyl group provided by succinyl CoA [29, 30]. The dependence upon succinyl-CoA makes mitochondrial protein succinylation a general footprint of metabolic status [10, 12, 31–34]. Lysine acylation is regulated, in part, by the activity of sirtuins, which remove acyl groups from lysine residues [35]. Sirtuins are a highly conserved family of nicotinamide adenine dinucleotide (NAD+)-dependent deacylases with homology to the yeast Sir2 protein [35]. Sirtuin 5 (SIRT5) is the predominant regulator of lysine succinylation, malonylation, and glutarylation within the cytosol and mitochondria [36, 37]. SIRT5 is known to play a role in various pathologies, including diabetes, cancer, neurodegeneration, fatty liver disease, and cardiovascular disease [32, 38–45]. In the heart, SIRT5 has been reported to regulate the TCA cycle, fatty acid metabolism, oxidative phosphorylation, pyruvate metabolism, ketogenesis, branched chain amino acid metabolism, and ATP synthesis [10, 12]. Protein succinylation is known to alter protein activity; including increasing the activity of α-ketoglutarate dehydrogenase (α-KGDH), pyruvate dehydrogenase (PDH), fumarase, and succinate dehydrogenase (SDH) and decreasing the activity of 3-hydroxy-3-methylglutaryl-CoA synthetase (HMGC2) [12, 46–48]. Initial studies have assessed the cardiac acetylome in whole heart tissue from animals under basal conditions and in models of diabetes, fasting, and obesity [13, 49, 50]. While it is known that acetylation and succinylation compete for occupancy on lysine residues of proteins, few studies have investigated the cardiac succinylome [51–53]. The heart has the highest concentration of cellular succinyl-CoA of any organ in the body, which implies that cardiomyocytes are highly dependent on the TCA cycle and branched chain amino acid (BCAA) catabolism for energy production [10, 54–57].
Previous studies on cardiac succinylation have all involved either whole heart tissue or left ventricular tissue from SIRT5 KO mice under basal conditions, chronic pressure overload, or ischemia-reperfusion injury [10, 12, 58]. No cardiac succinylation studies have investigated the myofibril-enriched protein fraction. In this study, we measure cardiac contractile and relaxation parameters of the sarcomeric proteins in myofibril-enriched proteins isolated from the left ventricles of explanted failing ICM human hearts and myofibril-enriched proteins from non-failing human hearts. Within this same myofibril-enriched protein fraction, we quantify both the differences in total protein expression and the succinylome for the first time. A recent study by Fisher-Wellman et al. has reported that protein hyperacylation in cardiac mitochondria has a marginal impact on bioenergetics [59]. While the relevance of acylation to ischemic HF pathology remains unknown, our study reveals significant hyposuccinylation in failing ICM myofibril samples collected from patients demonstrating severe pathology. The results contained herein demonstrate that ICM patients with HF present with hyposuccinylation of cardiac proteins involved in key metabolic and contractile processes.
2. Methods
2.1. Human Samples
Heart samples were obtained from the Adult Cardiac Tissue Bank maintained by the Division of Cardiology at the University of Colorado Anschutz Medical Campus (COMIRB 01–568). All patients with HF had ischemic cardiomyopathy and hearts were collected during orthotopic heart transplantation. Hearts from non-failing donors were hearts that could not be placed for technical reasons such as size or mis-matched blood type. Cardiac tissue was rapidly dissected and flash-frozen in liquid nitrogen at the time of cardiac transplant or donation. All tissue was stored at −80°C until use.
2.2. Isolation of myofibril-enriched proteins
Skinned myofibrils were isolated from the remote region of the left ventricles of explanted hearts of patients with HF due to ICM and non-failing hearts. Myofibrils were isolated from heart tissue using the skinning in suspension method [60]. Briefly, tissue was skinned in Linke’s solution with 0.5% Triton-X [132mM NaCl, 5mM KCl, 1mM MgCl2, 10mM Tris, 5mM EGTA, 1mM NaAzide; pH 7.1, filtered] and protease inhibitors [0.5mM NaAzide, 5μM pepstatin, 0.2mM PMSF, 0.5mM DTT, 10μM E-64, 10μM leupeptin] overnight at 4°C. The samples were then washed with Rigor solution [50mM Tris, 100mM KCl, 2mM MgCl2, 1mM EGTA, pH 7.0] three times for 10 minutes each and placed in Bath solution [100mM Na2EGTA; 1M potassium propionate; 100mM Na2SO4; 1M MOPS; 1M MgCl2; 6.7mM ATP; and 1mM creatine phosphate; pH 7.0] with protease inhibitors and homogenized. The myofibril-enriched proteins isolated include functional sarcomeric proteins and mitochondrial proteins. Skinned myofibrils are demembranated and lose membrane function, however, the sarcomeric proteins retain their function. While this is not representative of a fully functional physiological state, it allows for the assessment of protein succinylation in the same skinned myofibrils in which mechanical function was measured, permitting the mechanical and proteomic analyses to represent equivalent sarcomeric function and overall state.
2.3. Western blot analysis
Myofibril-enriched protein (10 μg) was subjected to standard SDS-PAGE using Criterion TGX Stain-Free 8–16% gradient precast gels (Bio-Rad, Hercules, CA) at 180V for 45 minutes, or Mini-PROTEAN TGX Stain-Free 4–20% gradient precast gels (Bio-Rad, Hercules, CA) at 150V for 1 hour for PTMs, 5% SDS-PAGE at 150V for 1 hour for high molecular weight proteins, and 15% SDS-PAGE at 150 V for 80 minutes for low molecular weight proteins. Protein was transferred to an Immobilon® -PSQ membrane (Merck Millipore, Burlington, MA). Membranes were blocked using 5% (w/v) nonfat dry milk in Tris-buffered saline–0.1% Tween 20 (TBS-T) or Odyssey® Blocking Buffer (PBS) (LI-COR Biotechnology, Lincoln, NE) for 1 hour at room temperature. Membranes were then immunoblotted with primary antibodies directed against succinyllysine (PTM-419, PTM Biolabs, Chicago, IL), SIRT5 (15122–1-AP, Proteintech Group, Rosemont, IL), aspartate aminotransferase (Ab189863, Abcam, Cambridge, MA), trifunctional enzyme subunit alpha (Ab54477), trifunctional enzyme subunit beta (ARP48133_P050, Aviva Systems Biology, San Diego, CA), periostin (Ab14041), isocitrate dehydrogenase 2 (Ab94359), acetyl CoA acetyltransferase 1 (PA5–34676, Thermo Fisher, Rockford, IL), creatine kinase-MM (Ab174672), ATP synthase F1 subunit beta (Ab14730), succinate dehydrogenase complex flavoprotein subunit A (Ab14715), dihydrolipoamide succinyltransferase (E2) component of the 2-oxoglutarate dehydrogenase complex (Ab177934), succinate CoA ligase subunit alpha (Ab97867), acetyllysine (9441, Cell Signaling, Boston, MA), trimethyllysine (PTM-601, PTM Biolabs), mono- and dimethyllysine (PTM-602, PTM Biolabs), glutaryllysine (PTM-1151, PTM Biolabs), malonyllysine (PTM-901, PTM Biolabs), propionyllysine (PTM-201, PTM Biolabs), and crotonyllysine (PTM-502, PTM Biolabs). Following 3 washes with TBS-T, or Phosphate-buffered saline (PBS)-Tween 20 (0 .1% v/v; PBS-T), a horseradish peroxidase conjugated secondary antibody was applied and membranes were developed using Clarity Western ECL Substrate from Bio-Rad. Chemiluminescence was visualized using a Chemidoc® MP (Bio-Rad). Succinyllysine and all other PTM blots were also imaged using the Odyssey imaging system with IRDye® 800CW Secondary Antibodies (#926–32213, LI-COR Biotechnology, Lincoln, NE). Protein band intensities were quantified using Image Lab (Bio-Rad, version 5.0) and normalized to the total protein per lane using stain-free gel imaging on a Chemidoc® MP (Bio-Rad) to ensure equal protein loading.
2.4. LC-MS/MS identification and total protein quantification
20X dilutions of 5 failing ICM and 5 non-failing myofibril-enriched samples were subjected to sodium chloride/acetone precipitation. These samples were processed using a PreOmics sample preparation kit (iST 8x, PreOmics GmbH, Planegg, Germany). Briefly, samples were re-suspended in PreOmics lysis buffer, containing dithiothreitol (DTT) and iodoacetamide (IAA), by mechanical pipetting to break up the cell pellet, heated at 95°C for 10 min to denature, reduce, and alkylate proteins, and bath sonicated for ~1min. All samples were normalized to start with 15 ug of protein using a nano-BCA assay, and brought to a volume of 50 uL with lysis buffer. Samples were digested for 2.5 h at 37°C using trypsin and LysC, then purified using PreOmics cartridges. Eluted peptides were dried in a SpeedVac at 45°C. Dried samples were re-suspended in 35 uL of 80 fmol/uL MassPREP Peptide Mixture (#186002337, Waters, Milford, MA) in 3% ACN, 0.1% formic acid. The nano-BCA assay was repeated and 20 uL of each sample solution was transferred into the appropriate amount of re-suspension buffer to yield a final concentration of 0.15 ug/uL of myofibril-enriched digest. A pooled QC was made by mixing 5 uL of each sample solution. All samples were then aliquoted into duplicate 10 uL autosampler vials (ASVs). ASVs were stored at −80°C with stock solution. Samples were thawed on ice, vortexed and spun-down before being placed in the autosampler for analysis.
225 ng of each myofibril-enriched sample was loaded onto a 2cm PepMap 100, nanoviper trapping column and chromatographically resolved on-line using a 0.075 × 250 mm, 2.0μ Acclaim PepMap RSLC reverse phase nano column (Thermo Scientific) using a 1290 Infinity II LC system equipped with a nanoadapter (Agilent). Mobile phases consisted of water + 0.1% formic acid (A) and 90% aq. acetonitrile + 0.1% formic acid (B). Samples were loaded onto the trapping column at 3.2 μL/min for 2.5 minutes at initial condition before being chromatographically separated at an effective flow rate of 330 nL/min using a gradient of 3–8.5% B over 4 minutes, 8.5–26% B over 48.5 minutes, and 26–40% B over 7.5 minutes for a total 60 minute gradient at 42°C. The gradient method was followed by a column wash at 70% B for 5 minutes. Data was collected on a 6550 Q-TOF equipped with a nano source (Agilent) operated using intensity dependent CID MS/MS to generate peptide identifications and re-injected in MS-only mode for protein quantitation. The capillary voltage, drying gas flow, and drying gas temperature were set to 1300 V, 11.0 L/min and 175 °C, respectively. MS/MS data was collected in positive ion polarity over mass ranges 290–1700 m/z at a scan rate of 10 spectra/second for MS scans and mass ranges 50–1700 m/z at a scan rate of 3 spectra/second for MS/MS scans. All charge states, except singly charged species, were allowed during MS/MS acquisition, and charge states 2 and 3 were given preference. MS-only data was collected in positive ion polarity over mass ranges 290–1700 m/z at a scan rate of 1.5 spectra/second. SpectrumMill software (Agilent) was used to extract, search, and summarize peptide identity results. Spectra were searched against the SwissProt Homo sapiens database allowing up to 2 missed tryptic cleavages with fixed carbamidomethyl (C) and variable deamidated (NQ) and oxidation (M) modifications. The monoisotopic peptide mass tolerance allowed was ± 20.0 ppm and the MS/MS tolerance was ± 50 ppm. A minimum peptide score of 8, scored peak intensity of 50%, and protein score of 10 were used as cut-offs for the generation of an AMRT library.
MS-only quantitative data was extracted and aligned using a recursive workflow with Profinder V.B.08.00 software (Agilent). Samples were initially extracted over retention time 14–73.5 min with an ion count threshold set to two or more ions and 10000 counts and a score threshold of 80 using the molecular feature extractor (MFE) algorithm. Charge states 2–6 were allowed with H+ and Na+ adducts using the peptide isotope model. Retention time window and mass window alignment setting tolerances were set to 0.75 min and 15 ppm, respectively. The extracted compound list was exported to Mass Profiler Professional V.14.8 (Agilent, MPP) and compounds that were found in only 1 of 10 samples were removed before performing a targeted batch feature extraction using the find by ion algorithm in Profinder. Charge states, adducts, isotope model, and mass window alignment settings were set to the same values used with the MFE algorithm. The retention time window was expanded to ±0.5 min. An Absolute height threshold of 10000 counts was used and only compounds that had an overall score greater than 50 were matched based on mass accuracy, isotope abundances and isotope spacing of compounds generated from the MFE algorithm. Final extraction and alignment results were exported to MPP for data analysis. Peptides were annotated using ID Browser software (Agilent) by matching the mass and retention time from aligned experimental data to peptide hits in the AMRT library generated from MS/MS data. 15 ppm mass errors and 0.4 min RT drifts were allowed for initial matching. Annotations that had a score lower than 50 or mass ppm error greater than ±10 ppm were removed and results were imported into MPP for statistical analysis.
Statistical analysis was performed at the protein level. Peptide level data was filtered to only allow annotated peptides found in 80% of samples within a sample condition. Peptide abundances were then summed and rolled up into a total protein abundance for an individual protein within each sample. Proteins were filtered on a volcano plot using a moderated t-test with Benjamini-Hochburg multiple testing corrections. Proteins that had a fold change ≤ −1.5 or ≥ 1.5 and p-value < 0.05 were considered significant.
2.5. Immunoprecipitation of succinylated peptides
Skinned cardiac myofibrils (1mg) were spiked with 50 ng of succinylated BSA as an internal standard and then trypsin-digested overnight, acidified using TFA, purified via Sep-Pak® C18 Classic Cartridges (Waters, #WAT051910), frozen at −80°C for 4 hours, and lyophilized for 48 hours. Samples were incubated 2 hours at 4 °C with immunoaffinity beads conjugated to succinyl-Lys antibody (PTMScan® Succinyl-Lysine Motif [Succ-K] Immunoaffinity Beads #13764, Cell Signaling, Boston, MA). Following incubation, supernatants were removed and the beads were washed twice with IAP buffer (PTM Scan® IAP Buffer (10X) #9993, Cell Signaling, Boston, MA) and 3 times with Burdick and Jackson LC-MS grade water (Honeywell). Peptides were eluted with 0.15% TFA 3 times, pooled, cleaned on Pierce® C18 Spin Columns (Thermo Scientific, Rockford, IL, #89870), evaporated to dryness, and frozen at −80°C. Dried samples were re-suspended in 3% ACN, 0.1% formic acid in water for LC–MS/MS analysis.
2.6. LC-MS/MS identification and quantification of succinylated peptides
Accurate Mass and Retention Time (AMRT) Library Generation.
Two pooled groups (failing ICM and non-failing) of enriched succinyl-Lys peptides were loaded onto a 2cm PepMap 100, nanoviper trapping column and chromatographically resolved online using a 0.075 × 500 mm, 2.0μ Acclaim PepMap RSLC reverse phase nano column (Thermo Scientific) using a 1290 Infinity II LC system equipped with a nanoadapter (Agilent). These two pooled samples were used to generate an AMRT library, which was used for succinyl-Lys peptide identification and quantification in each individual non-failing and ischemic failing sample. Mobile phases consisted of water + 0.1% formic acid (A) and 90% aq. acetonitrile + 0.1% formic acid (B). 5 uL of sample was loaded onto the trapping column at 3.2 μL/min for 3 minutes at initial condition before being chromatographically separated at an effective flow rate of 330 nL/min using a gradient of 3–8% B over 1 minute and 8–36% B over 59 minutes for a total 60 minute gradient at 60°C. The gradient method was followed by a column wash at 75% B for 5 minutes. Data was collected on a 6550 Q-TOF equipped with a nano source (Agilent) operated using intensity dependent CID MS/MS to generate peptide identifications. The capillary voltage, drying gas flow, and drying gas temperature were set to 1300 V, 11.0 L/min and 175 °C, respectively. MS/MS data was collected in positive ion polarity over mass ranges 260– 1700 m/z at a scan rate of 10 spectra/second for MS scans and mass ranges 50–1700 m/z at a scan rate of 3 spectra/second for MS/MS scans. All charge states, except singly charged species, were allowed during MS/MS acquisition, and charge states 2 and 3 were given preference. SpectrumMill software (Agilent) was used to extract, search, and summarize peptide identity results. Spectra were searched against the SwissProt Homo sapiens database allowing up to 4 missed tryptic cleavages with fixed carbamidomethyl (C) and variable deamidated (NQ), oxidation (M), and succinyl (K) modifications. The monoisotopic peptide mass tolerance allowed was ± 20.0 ppm and the MS/MS tolerance was ± 50 ppm. A minimum peptide score of 8, scored peak intensity of 50%, and protein score of 10 were used as cut-offs for the generation of an AMRT library.
For MS-only quantitation, data was collected in positive ion polarity over mass ranges 260–1700 m/z at a scan rate of 1.5 spectra/second on a 6550 Q-TOF equipped with a nano source (Agilent) operated in MS-only mode. Each individual failing ICM and non-failing enriched succinyl-Lys peptide sample was acquired using the same LC method and source parameters as the pooled samples for AMRT library generation.
Overall, data for MS quantification was extracted and aligned using Profinder V.B.08.00 software (Agilent). Retention times, neutral masses, and chemical formulas generated from identified succinyl peptides in the AMRT library were used to perform a batch targeted feature extraction for each individual sample. Samples were extracted with an ion count threshold set to two or more ions and 12000 counts and a score threshold of 50. The score was based on how the quality of the mass, isotope abundances and isotope spacing of compounds found in each sample matched to a targeted chemical formula within a specified retention time window generated from the AMRT library. Charge states 2–6 were allowed with H+ and Na+ adducts using the peptide isotope model. Retention time window and mass window alignment setting tolerances were set to 0.8 min and 10 ppm, respectively. Succinyl-Lys final extraction and alignment results were exported to Mass Profiler Professional V.14.8 (Agilent) and peak heights were used for quantitation.
Statistical analysis was performed at the peptide level. Compounds were filtered to those found in 100% of 1 of 2 conditions for group-to-group comparisons. Succinyl peptides were filtered on volcano plots using a moderated t-test and peptides that had a fold change ≤ −1.5 or ≥ 1.5 and p-value < 0.05 were considered significant. Benjamini-Hochburg multiple-testing correction was also applied to generate a list of succinyl site candidates that were differentially expressed with high confidence that could be used to probe the effects of succinylation modifications in individual proteins.
2.7. Pathway analysis
Pathway analysis was performed using DAVID Bioinformatics Resources version 6.8 [61]. Lists of UniprotKB IDs for proteins with increased expression, decreased expression, increased succinylation, and decreased succinylation were uploaded to the database. The entire human proteome was used as the reference background. Enrichment analysis was performed using the functional annotation chart function in order to identify the most overrepresented biological terms correlated with our protein list. Significance of enrichment terms was measured by the calculation of a p-value using Fisher’s exact test (EASE score) to determine the probability that a given term was more enriched than random chance. The Benjamini-Hochberg procedure was used to globally correct enrichment p-values of individual term members for functional annotation clustering and to adjust the false discovery rate in both analyses. A threshold of significance for each pathway was set at p < 0.05. Each pathway had to be represented by 3 or more proteins in the list to be considered significant. Pathways represented in figures were chosen from a larger list for relevance to contractile processes and mitochondrial metabolism and for visual simplicity.
2.8. Myofibril mechanics
Mechanical properties were measured using the fast switching solution as described previously [62–64]. In this procedure, a small bundle of myofibrils were mounted between two microtools (one a calibrated cantilevered force probe (between 5.12–11.36μm/μN; frequency response 2–5 KHz) and the other tool attached to a motor to produce length changes (Mad City Labs, Madison, WI)) in bath solution at 15°C. Once mounted, the myofibrils were exposed to solutions of different pCa (pCa 9, 6.25, 6, 5.8, 5.6, 4.5). Customized LabView software (National Instruments, Austin, TX) was used to collect and analyze the data. The myofibrils were set at 5–10% over slack length and mechanical parameters of contraction and relaxation were measured. The maximal tension generation was calculated from the force amplitude at pCa 4.5 and force was normalized using the myofibril cross sectional area. The rate of activation (kACT) was calculated from mono-exponential fits. The rate of the slow phase of relaxation (slow kREL) was calculated using the slope of the regression line fitted to the tension trace and was normalized to the entire amplitude of the tension relaxation trace. The duration of the slow phase (slow tREL) was calculated from the beginning of the linear slope to the leading edge of the shoulder leading to the fast phase of relaxation. The relaxation rate for the fast phase (fast kREL) was calculated by fitting the mono-exponential decay to the data. While skinned myofibrils do not represent a physiological myocyte, this model allows for detailed assessment of differences in sarcomeric protein function by allowing for control of varying calcium concentrations independent of ATP availability. Varying responses by non-failing and ischemic failing myofibrils can be extrapolated to compensatory changes in sarcomeric proteins in response to cellular changes that occur in heart failure. This mechanical model reveals the state of sarcomeric protein function in a controlled setting, and by evaluating succinylation in the same skinned myofibrils, allows for the association of sarcomeric protein interactions with the succinylation state of proteins.
2.9. Spectrophotometric enzyme assays
Succinate dehydrogenase (SDH), alpha-ketoglutarate dehydrogenase (α-KGDH), and succinyl CoA synthetase (SCS) activities were measured using assay kits (BioVision, Inc., Milpitas, CA). Briefly, 10 mg of left ventricular tissue was dounce homogenized in 100 μl of kit assay buffer using a micro tube homogenizer system (SP Scienceware, Wayne, NJ). For the SDH assay, 5 μl of undiluted sample was added to each well and the absorbance at 600 nm was measured every 5 minutes over 30 minutes at 25°C. For the α-KGDH assay, the homogenate was diluted 1:1 with kit assay buffer, then 5 uL of sample was added to each well and the absorbance at 450 nm was measured every 5 minutes over 60 minutes at 37°C. For the SCS assay, 5 uL of sample was added to each well and the absorbance at 450 nm was measured every 5 minutes over 30 minutes at 25°C. SDH activity was expressed as nmol of DCIP/min/μL/g of tissue. α-KGDH activity was expressed as nmol of NADH/min/mL/g of tissue. SCS activity was expressed as nmol of NADH/min/uL/g of tissue. For each assay, left ventricular samples of 3 non-failing and 3 ischemic failing hearts were measured in duplicate.
2.10. Statistics and data processing
Statistical analyses and production of graphs were completed using Prism 7 (GraphPad, La Jolla, CA). Differences between the failing ICM and NF groups were calculated using the unpaired Student’s t-test. Results were considered significant if p < 0.05. For myofibril mechanics data, 8–10 myofibrils were assessed for each heart and averaged to provide one value per patient. Data was assessed for normality using the Shapiro-Wilks test and non-normal data was log-transformed. For Western blot densitometry, graphs represent the average of 3 independent experiments with error bars indicating the SEM.
3. Results
3.1. Patient characteristics
Patient characteristics are summarized in Table S1. Median age of the non-failing donors was 45.5 years and the median age of the patients with ischemic cardiomyopathy was 54.5 years. 50% of ischemic HF patients had ventricular assist devices (VADs) whereas no NF donors had VADs. Medications were more commonly used in ischemic HF patients than in NF donors.
3.2. Myofibril mechanical parameters are distinct between myofibrils isolated from hearts of ischemic cardiomyopathy patients and non-failing donor hearts
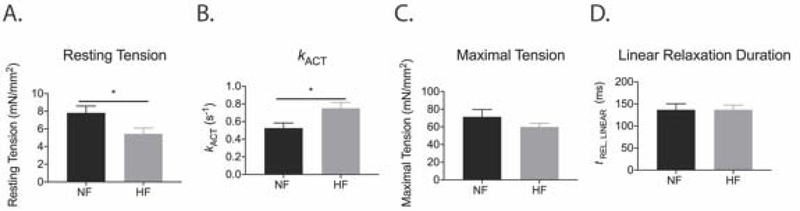
Several key parameters of sarcomeric mechanical interactions were altered in myofibrils isolated from failing ICM hearts (Figure 1, Table S2). Myofibrils from failing ICM hearts had lower resting tension than myofibrils from NF controls (Figure 1A; NF = 7.82 ± 0.77mN/mm2; HF = 5.43 ± 0.66mN/mm2; p < 0.05). While myofibrils from failing ICM hearts generated similar maximal tensions as myofibrils from NF hearts, the rate of activation was faster in myofibrils from failing ICM hearts (Figure 1B-C; NF = 0.53 ± 0.06s−1; HF = 0.75 ± 0.08s−1; p < 0.05). Further, relaxation parameters were not different in the myofibrils isolated from failing ICM hearts compared to myofibrils from NF hearts (Figure 1D). Myofibril mechanics in ischemic heart failure have been previously reported, however, this mechanics data validates that samples are functionally similar to previously published research and presents a targeted assessment of skinned myofibril slurry proteins, which associates the functional state of sarcomeric proteins with protein succinylation.
Figure 1. Mechanical parameters of myofibrils isolated from ischemic HF patients are distinct from myofibrils isolated from NF hearts.
(A) Resting tension was decreased in ischemic HF myofibrils compared to NF myofibrils (p < 0.05). (B) Ischemic HF myofibrils had faster activation of contraction (kACT) than NF myofibrils (p < 0.05). (C and D) There were no differences in maximal tension or the duration of linear phase relaxation between ischemic HF and NF myofibrils. Statistical analyses of normal data for A-D were assessed by Student’s t-test with Welch’s correction [N = 8 NF patients (6–13 myofibril measured per patient), 9 ischemic HF patients (5–16 myofibrils measured per patient)]. See raw data in Supplemental Table S2.
3.3. LC-MS/MS total protein quantification in ischemic failing and non-failing human cardiac myofibrils
A total of 548 proteins were identified in ischemic HF samples, and a total of 542 proteins were identified in NF samples. The number of unique proteins identified in ischemic HF samples was 8, while the number of unique proteins identified in NF samples was 2. The number of shared proteins between ischemic HF and NF samples was 540. A total of 147 proteins met the differential expression criteria of FC ≥ 1.5 or ≤ −1.5, p < 0.05, which was reduced to 100 proteins by removing those represented by only 1 peptide (Table S3). Of these 100 differentially expressed proteins, 46 showed increased expression in failing ICM hearts, while 54 demonstrated decreased expression in failing ICM hearts (Figure 2A).
Figure 2. Differential protein expression in ischemic failing and non-failing human cardiac myofibrils.
(A) LC-MS/MS total protein quantification for ischemic failing vs non-failing human cardiac myofibrils demonstrating the number of differentially expressed proteins in ischemic HF vs NF samples (n = 5, log2(fold change) ≥ 1.5 or ≤ −1.5, p < 0.05). (B) Western blot confirmation of increased periostin expression in ischemic HF samples using Student’s t-test (mean ± SEM, n = 4, p < 0.005). (C) Western blot confirmation of decreased IDH2 expression in ischemic HF samples (mean ± SEM, n = 5, p < 0.05). (D) Western blot confirmation of increased ACAT1 expression in ischemic HF samples (mean ± SEM, n = 3, p < 0.005). (E) Pathway analysis of proteins with increased expression in ischemic HF. (F) Pathway analysis of proteins with decreased expression in ischemic HF. (G) Comparison of differentially expressed proteins in human ischemic HF identified by LC-MS/MS with differentially expressed proteins and genes from previous human heart failure studies. *For E and F, the first number after each bar on the graph represents the number of proteins identified per pathway. The second number represents the fold enrichment for that pathway.
Western blot analysis verified protein differences between ischemic HF and NF samples detected by MS/MS for a variety of proteins of interest. Western analysis of periostin confirmed the ~17-fold increase (p < 0.005) detected in ischemic HF samples by MS/MS (Figure 2B). Immunoblotting also confirmed the ~1.7-fold decrease in IDH2 expression (p < 0.05) in ischemic HF samples (Figure 2C). Western analysis of ACAT1 validated the ~2.0-fold increase (p < 0.005) in ischemic HF samples reported by MS/MS (Figure 2D) [65–67].
Pathway analysis of proteins increased in ischemic HF compared to proteins decreased in ischemic HF revealed striking differences in biological processes. Proteins demonstrating increased expression in ischemic HF were highly represented in both cardiac function and HF-related pathways (Figure 2E). Proteins demonstrating decreased expression in ischemic HF were highly represented in cardiac function and metabolism (Figure 2F).
Many proteins identified as having increased expression in ischemic HF samples compared to NF samples corresponded to previously reported increases in gene and/or protein expression for end-stage ischemic heart failure, including elevated ECM proteins (COL1A1, POSTN, PRELP, OGN, LUM, ASPN, FBN1, FMOD), HBA1, HBB, PDLIM1, and EFEMP1 (Figure 2G). [68–71]. Similarly, proteins identified with decreased expression in ischemic HF samples also corresponded to previously reported decreases in gene and/or protein expression for end-stage ischemic heart failure, including decreased MYOM2, ATP2A2, SRL, NDUFA6, UQCRH, COX7A1, ATP5PB, IDH2, and FHL2 (Figure 2G). [68–71]. Importantly, proteomics analysis revealed a number of proteins that were increased or decreased in ischemic HF samples that were previously unknown, including TNXB, DCN, BGN, NES, PRPH, LAMB1, SLC25A3, MYH15, MYH4, ATP2A3, NNT, NADH dehydrogenase [ubiquinone] 1 alpha and beta subcomplex subunits, cytochrome b-c1 complex subunits, cytochrome c oxidase subunits, and ATP synthase subunits (Figure 2G).
3.4. Decreased lysine succinylation of myofibril and mitochondrial proteins in the failing human ICM heart
Immunoblot analysis with an anti-succinyllysine antibody of extracts from ischemic HF and NF human cardiac myofibrils revealed that protein succinylation was decreased in ischemic HF samples (Figure 3B–C). This decrease in protein succinylation was shown to occur independent of SIRT5 expression, which was also found to be decreased in ischemic HF samples (Figure 3D–E). Overall, ischemic HF samples demonstrated an 81% decrease (p < 0.005) in myofibril protein succinylation (Figure 3B–C). Our analysis reveals that decreases in succinylation in ischemic HF samples are not due to changes in SIRT5 expression, since SIRT5 protein levels decreased in ischemic HF samples by 65% (p < 0.05) (Figure 3D–E).
Figure 3. Myofibril and mitochondrial protein succinylation are altered in ischemic heart failure.
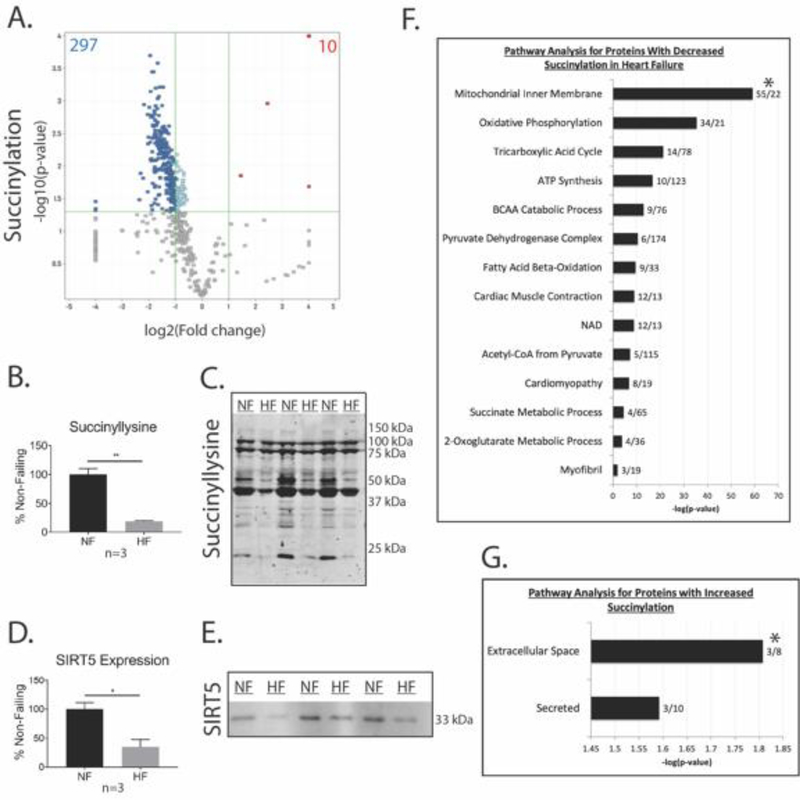
(A) Volcano plot showing the number of peptides with significant changes in succinylation for ischemic HF vs NF samples (n = 4, log2(fold change) ≥ 1.5 or ≤ −1.5, p < 0.05). (B) Densitometry for anti-succinyllysine western blot using Student’s t-test (mean ± SEM, n = 3, **p < 0.005). (C) Representative anti-succinyllysine western blot. (D) Densitometry for anti-SIRT5 western blot (mean ± SEM, n = 3, *p < 0.05). (E) Representative SIRT5 Western blot. (F) Pathway analysis of myofibril proteins with decreased succinylation in ischemic HF samples. (G) Pathway analysis of myofibril proteins with increased succinylation in ischemic HF samples. *For F and G, the first number after each bar on the graph represents the number of succinylated proteins identified per pathway. The second number represents the fold enrichment for that pathway.
The myofibril succinylome of the human heart was characterized in samples prepared from skinned myofibrils of 4 cardiac transplant recipients with end-stage ischemic cardiomyopathy and 4 NF samples from non-failing donors with normal LV function. LC-MS/MS analysis identified a total of 572 unique succinylated lysine sites located on 181 proteins (Tables S4 and S5). A total of 52% of the succinylated proteins contained more than one succinylation site (Tables S4 and S5). Interestingly, 307 protein succinylation sites were differentially expressed on 60% of proteins in ischemic HF samples. We found that 297 residues demonstrated decreased succinylation on 104 proteins, while 10 residues demonstrated increased succinylation on 4 proteins (Figure 3A). All of the 10 residues displaying increased succinylation were on extracellular matrix proteins known to be significantly increased in ischemic HF.
Pathway analysis demonstrated that proteins with decreased lysine succinylation in ischemic HF samples were highly represented in pathways involved in myofibril function, catabolic pathways and ATP synthesis pathways, including cardiac muscle contraction, cardiomyopathy, muscle filament sliding, sarcomere organization, BCAA catabolism, ketone body catabolism, fatty acid β-oxidation, ETC pathways, TCA cycle, succinate metabolism, and alpha-ketoglutarate metabolism (Figure 3F, Figure 5). Proteins with increased succinylation in ischemic HF samples were predominantly represented in the extracellular matrix and extracellular space, reflecting the uniquely increased expression of ECM proteins in ischemic HF samples compared to NF samples (Figure 3G).
Figure 5. Proteomic map of decreased succinylation in ischemic HF samples.
Map includes proteins with succinylation changes ≤ −1.5, p < 0.05, which demonstrate decreased succinylation independent of changes in protein expression.
Taken together, our proteomic and succinylomic analyses reveal that the observed decreases in lysine succinylation represent a change in PTM, rather than protein expression (Figure 4A–F, Tables S3–S5). As a result, 56% of the proteins with decreased succinylation were shown to either increase (8%) or not change (48%) in terms of protein expression quantified by MS and 17% of proteins with decreased succinylation displayed decreased protein expression by quantitative MS (Tables S3–S5). The remaining 27% of proteins identified as having decreased succinylation did not overlap with the MS total protein quantification list.
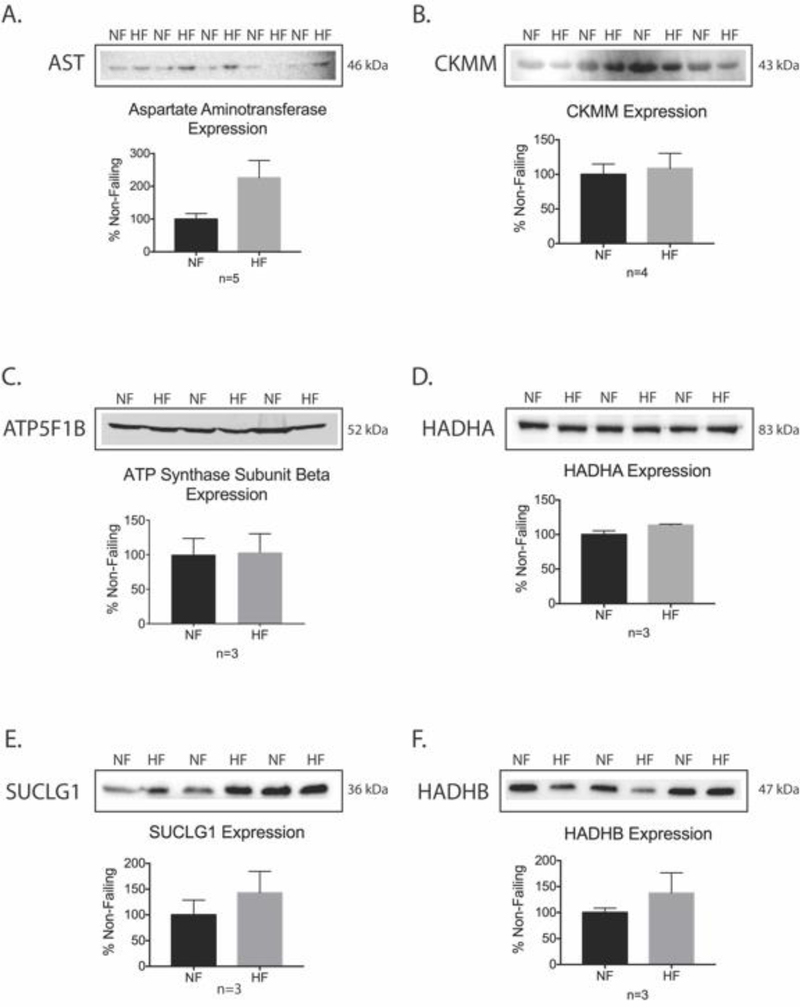
Figure 4. Western blot validation of specific HPLC-MS/MS total protein results for proteins with decreased succinylation.
(A-F) Representative western blot images and corresponding densitometry quantification using Student’s t-test for aspartate aminotransferase, creatine kinase M-type (CKMM), ATP synthase subunit beta (ATP5F1B), trifunctional enzyme subunit alpha (HADHA), succinyl CoA ligase [GDP forming] subunit alpha (SUCLG1), trifunctional enzyme subunit beta (HADHB) (mean ± SEM, *p < 0.05)
Many of the succinylation sites we identified in myofibrils from the left ventricles of ischemic failing human hearts corresponded to previously reported succinylation sites from the analysis of left atrial appendage tissue in human valvular heart disease and atrial fibrillation (Bai, 2019), as well as from the analysis of mitochondrial extracts of SIRT5 KO mouse hearts subjected to ischemia/reperfusion injury (Boylston, 2015). The Bai study identified 3,785 succinylation sites on 874 proteins, and quantified 3,019 succinylation sites on 666 proteins. Of the quantified succinylation sites, 246 sites had increased succinylation on 132 proteins, while 117 sites had decreased succinylation on 45 proteins. Given that this study analyzed whole atrial appendage tissue, it makes sense that it found almost 7 times more succinylation sites compared to our analysis. The Bai study identified approximately 75% of the total succinylation sites that we identified. With regard to statistically significant quantified succinylation sites, the Bai study identified almost 84% of our decreased succinylation sites, however, they identified 14% of these as increased, 68 % as unchanged, and 2% as decreased. The Bai study identified 80% of our succinylation sites found to be increased, however, their quantification found the expression of these sites to be unchanged. The Boylston study identified 887 unique succinylation sites on 184 proteins. They found 16 specific sites with increased succinylation on 9 proteins and no sites with decreased succinylation, since they used SIRT5 knockout hearts which have a tendency toward hypersuccinylation. Our analysis revealed succinylation sites on 8 of these proteins, with 3 of the exact same succinylation sites on SLC25A4, ATP5F1A, and ATP5PO. Unfortunately, the 16 differentially expressed peptides are the only peptides with specific lysine succinylation sites identified, so it is not possible to assess any further overlap between our analyses.
3.5. Western blot analysis demonstrates a general decrease in lysine acylation and an increase in lysine trimethylation in ischemic failing hearts
Lysine residues are prone to a wide variety of PTMs. These PTMs may compete with each other for binding to lysine sites on proteins [51]. To determine whether a competing PTM was preferentially binding to lysine residues with greater affinity than succinyl groups, we probed a wide variety of PTMs in failing ICM and non-failing myofibril samples using western blot analysis. Western blot analysis demonstrated no change in lysine acetylation and glutarylation, decreased lysine malonylation (p < 0.05), crotonylation (p < 0.05), propionylation (p < 0.005), and mono and di methylation (p < 0.05), and increased lysine trimethylation (p < 0.05) (Supplemental Figure S1).
3.6. Enzyme activity assays demonstrate decreased TCA cycle function, decreased succinyl-CoA production, and increased succinyl CoA turnover in the failing ICM heart
To explore the functional aspect of the quantitative changes observed in protein succinylation, biochemical analyses were undertaken to evaluate the activity and expression of several TCA cycle and respiratory chain enzymes, some of which are involved in succinyl CoA metabolism. These included the activity of α-KGDH, SCS, and SDH. Results demonstrated that α-KGDH and SDH activities were significantly decreased by 75% ± 7% (p < 0.001) and 61% ± 20% (p < 0.05) in ischemic HF, respectively (Figure 6A,C). Immunoblot analysis of SDHA, and MS analysis of SDHA and SDHB revealed that protein expression did not change significantly for these SDH subunits, however, succinylation was decreased (Figure 6A,D,E, Tables S3–S5). Additionally, immunoblot analysis of OGDH (not shown) and DLST, and MS analysis of OGDH, DLST, and DLD revealed that protein expression did not change significantly for any of these α-KGDH subunits, yet succinylation of these proteins was decreased (Figure 6C–E, Tables S3–S5). An activity assay for SCS demonstrated a significant increase of 306% ± 75% (p < 0.05) in ischemic HF (Figure 6B). Immunoblot analysis of SCS revealed no change in protein expression of subunit SUCLG1, while MS analysis displayed no change in protein expression of the SUCLG1 and SUCLA2 subunits, however, succinylation of SUCLG1 was decreased (Figure 6B,D,E, Tables S3–S5). Taken together, the results of these three enzyme assays support the hypothesis that succinyl CoA production is decreased and succinyl CoA turnover is increased in ischemic HF, potentially resulting in an overall decrease in the mitochondrial succinyl CoA pool, which may contribute to decreased myofibril protein succinylation in ischemic HF.
Figure 6. Enzyme activity assays demonstrate decreased TCA cycle function, decreased succinyl-CoA production, and increased succinyl CoA turnover in the ischemic failing heart.
(A) SDH activity was significantly decreased, but expression of the SDHA subunit was unchanged in failing ICM hearts compared to NF hearts (mean ± SEM, n = 3, *p < 0.05). (B) SCS activity was significantly increased, but expression of the SUCLG1 subunit was unchanged in failing ICM hearts compared to NF hearts (mean ± SEM, n = 3, *p < 0.05). (C) α-KGDH activity was significantly decreased, but expression of the DLST subunit was unchanged in failing ICM hearts (mean ± SEM, n = 3, *p < 0.001). Statistical significance for A-C was assessed using Student’s t-test. (D) LC-MS/MS total protein quantification demonstrated no change in expression for the SDHA subunit of SDH, the SUCLG1 subunit of SCS, and the DLST subunit of α-KGDH. (E) LC-MS/MS protein succinylation quantification revealed that succinylation of SDH, SCS, and α-KGDH subunits were decreased.
4. Discussion
We investigated a critical and unique patient population composed of patients with heart failure due to ischemic cardiomyopathy and non-failing donors, and uncovered novel and noteworthy changes in PTMs associated with changes in myofibril mechanics. Our myofibril mechanics analysis of ischemic failing and non-failing hearts identified key differences in ischemic HF samples. Myofibrils from ischemic cardiomyopathy hearts have lower resting tension than myofibrils from non-failing controls. While myofibrils from ischemic failing hearts may generate slightly lower maximal tensions compared to myofibrils from non-failing hearts, this finding was not significant. In addition, the rate of activation is faster in myofibrils from ischemic failing hearts. Further, we found there were no significant changes in relaxation in this patient population which has never been reported before. Importantly, our observed mechanical function in the myofibrils are similar to previous findings in this patient population [72, 73]. Interestingly, while the lack of significant changes in maximal tension between myofibrils from NF and ischemic HF patients is in line with the study by Bollen et al, another study by Lee et al demonstrated a significant decrease in the tension generated by skinned myocytes [73, 74]. There may be several reasons for these differences, including differences between the techniques (we are presenting the first study in skinned myofibrils) and differences in patient populations. To better understand the intersection between sarcomeric function and the succinylome of the myofibril enriched proteins, we took a proteomics approach to investigate alterations in protein expression between failing ICM and non-failing hearts, which identified differential expression of protein that agrees with previous studies looking at both protein and gene expression changes in whole heart and left ventricular tissue in ischemic HF [68–71]. Pathway analysis of these differentially expressed proteins revealed acetylation as a significantly impacted pathway (Figure 2F). Because we have previously shown that acetylation and succinylation compete for lysine residues on proteins in the liver, and the heart possesses the highest concentration of succinyl CoA of any organ in the body, we decided to further our proteomics investigation by quantifying differences in protein acetylation and succinylation between failing ICM and non-failing hearts. While our results demonstrated no significant changes in acetylation, a striking decrease in protein succinylation was revealed in failing ICM hearts compared to non-failing hearts using both LC-MS/MS and western blot analysis (Supplemental Figure 1). This substantial decrease in protein succinylation in ischemic heart failure led us to probe succinyl CoA metabolism, which we also found to be significantly altered in ischemic HF.
The data presented in this study shows a global decrease in protein succinylation in myofibrils isolated from failing ICM hearts, and raises a question about whether this decrease in succinylation is due to decreased succinyl CoA concentrations in the ischemic failing heart, especially since SIRT5 expression appears to be decreased and is most likely not responsible for decreased protein succinylation. However, it is very difficult to measure mitochondrial succinyl CoA levels directly, because it is a transient intermediate in the TCA cycle that has a rapid turnover rate and exists in very low concentrations relative to cardiac tissue mass [75]. Instead, we looked at the activity and expression of proteins in pathways that produce succinyl CoA to see if the production of succinyl CoA is inhibited. We also investigated the activity and expression of proteins in pathways that use succinyl CoA as a substrate to assess whether succinyl CoA is being depleted through its consumption in other processes. Succinyl CoA levels could be decreased in ischemic HF due to impaired production as a result of decreased α-KGDH or IDH2 activity or expression. IDH2 was shown to have decreased expression in ischemic HF samples in our LC-MS/MS total protein quantification experiment. Results from α-KGDH and SDH enzyme assays support the idea that succinyl CoA production is decreased, while succinyl CoA utilization is increased in ischemic failing human cardiac myofibrils. Succinyl CoA levels could also be decreased in ischemic HF due to increased SCS activity or expression, which would increase succinyl CoA turnover to succinate. Results from the SCS enzyme assay suggest that succinyl CoA turnover to succinate is increased, possibly resulting in lower mitochondrial succinyl CoA concentrations and less protein succinylation. The progression to ischemic heart failure is characterized by impaired cardiac energy metabolism, including impaired TCA cycle function involving reduced IDH2 activity [76]. In addition, succinate accumulation resulting from TCA cycle activity has been reported in cardiac ischemia [77]. Therefore, our data supports the idea that cardiac succinyl CoA levels may be decreased in ischemic HF patients. Given that succinyl CoA concentrations are so high in the heart, it is possible that cardiac protein succinylation is a normal process supportive of protein function, resulting in a gain of function, rather than a loss of function. Research in mouse livers has demonstrated that succinylation of SDHA and pyruvate dehydrogenase E1 component subunit alpha (PDHA) are most likely PTMs that activate these proteins [46]. Additionally, research in mouse neurons has shown that α-KGDH regulates itself, as well as fumarase and the pyruvate dehydrogenase complex (PDHC) through its function as a succinyltransferase, and the activity of all three enzymes is increased by succinylation [48]. Interestingly, both mitochondrial and cytosolic protein succinylation can be decreased through inhibition of α-KGDH activity [48]. An overall decrease in mitochondrial succinyl CoA would explain why we observed a decrease in SDH and α-KGDH activity, but no change in SDHA, SDHB, OGDH, DLST, and DLD subunit expression. The observed decrease in α-KGDH activity could possibly explain the overall decrease in protein succinylation observed in ischemic HF samples, as well as the decrease in α-KGDH activity itself.
Succinyl CoA levels could also be decreased in ischemic HF samples due to increased shuttling of succinyl CoA away from the TCA cycle to collateral metabolic pathways. This phenomenon has been explored for succinyl CoA in the TCA cycle, which can either be converted to succinate by succinyl CoA synthetase to promote ATP production, or be consumed during heme synthesis [75]. Our data provides evidence that succinyl CoA synthetase activity and expression are increased in ischemic HF samples and both hemoglobin subunit alpha and beta are increased in ischemic HF samples, all of which are supported by what is known in the HF literature; ATP production is impaired due to mitochondrial dysfunction in ischemic HF and HF patients can have high hemoglobin levels in response to low blood oxygen levels resulting from poor heart function, or can require more hemoglobin synthesis in response to anemia [65–67, 78, 79]. In response to succinyl CoA being shuttled away from the TCA cycle, other metabolic substrates can be diverted from their primary pathways to collateral pathways that help replenish succinyl CoA levels in the TCA cycle. This process of intermediate replenishment via collateral metabolic pathways is known as anaplerosis [75]. Replenishment of succinyl CoA in the TCA cycle can be achieved through the synthesis of succinyl CoA from propionyl CoA. The extent of anaplerosis in the generation of succinyl CoA in ischemic HF has not yet been determined [75].
This study presents novel data relevant to ischemic cardiomyopathy with the potential to lead to innovative therapeutic strategies for a disease condition lacking effective long-term treatments. Our recent findings demonstrated a role for altered myofibril acetylation in the control of diastolic function [80]. Now, we describe the first quantification of succinylation in human ischemic heart failure samples and provide a stepping stone in understanding the potential impact of metabolically driven PTMs on myofibril mechanics in ischemic HF. While it is unclear whether differences in myofibril mechanics are driven by a decrease in protein succinylation, this is an idea worth exploring further given that ischemic HF samples demonstrate a significant decrease in protein succinylation, a tendency toward an overall decrease in cellular succinyl CoA production, as well as an increase in succinyl CoA turnover. Given that changes in protein succinylation often result in altered activity, it is imperative to explore whether this trend extends to sarcomeric proteins in the decreased succinylation state observed in ischemic HF [81–84]. The discovery of decreased succinylation in ischemic HF also supports the further investigation of other acyl PTMs, as well as mono, di, and trimethyllysine in an attempt to identify and quantify PTMs that are increased in ischemic heart failure samples, and whether they have any impact on myofibril mechanics.
5. Limitations
When assessing the field of proteomics and clinical samples it is vital to understand the source of the data generated. The process of preparing skinned cardiac myofibrils from left ventricular tissue clearly results in the isolation of both mitochondria and myofibrils, since cardiac mitochondria are dispersed among myofibrils [85]. Two distinct cardiac mitochondrial subpopulations have been identified in the hearts of many mammalian species including human hearts [86, 87]. These mitochondrial subpopulations are known as subsarcolemmal mitochondria [SSM] and interfibrillar mitochondria (IFM). Early research on these subpopulations concluded that IFM have significantly higher ADP dependent state 3 respiration rates compared to SSM in human hearts [87]. The remainder of the research in this field was performed in animals. However, current research in mice has cast doubt on the ability to truly separate and classify SSM and IFM subpopulations [88]. Based on cardiac myofibril skinning literature, the skinning procedure used in the current study most likely results in the isolation of all cardiac mitochondrial subpopulations, which explains the abundance of mitochondrial proteins detected by LC-MS/MS analysis in both the total protein quantification and the lysine succinylation quantification [85]. This skinned myofibril isolation is well characterized and provides a novel platform for myofibril mechanical analysis coupled with proteomic investigation to provide insights into mechanisms underlying altered cardiac function [60, 80]. Importantly, the isolation procedure utilized was identical for both ischemic HF and non-failing samples.
Supplementary Material
Highlights:
A succinylomic analysis of human ischemic failing cardiac myofibrils was performed
Quantitative LC-MS/MS revealed decreased protein succinylation in failing hearts
Decreased succinylation was found to occur independent of SIRT5 expression
Succinyl CoA metabolism was significantly altered in ischemic failing hearts
Protein trimethylation was found to be increased in ischemic failing hearts
Acknowledgements
This study was supported, in part, by the Skaggs School of Pharmacy and Pharmaceutical Sciences ADR Grant program, University of Colorado Anschutz Medical Campus. T.A.M. received support from the NIH [HL147558, HL116848, HL127240 and DK119594] and American Heart Association [16SFRN31400013]. Y.H.L. was supported by a fellowship from the American Heart Association [16POST30960017]. KCW received support from the NIH (2K12HD057022) and Lorna G. Moore Award (University of Colorado Anschutz Medical Campus). This work was also supported by Colorado CTSA Grant UL1 TR002535.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no conflict of interest to declare.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.yjmcc.
References
- [1].Klabunde RE. Cardiovascular physiology concepts. 2nd ed Philadelphis, PA: Lippincott Williams &Wilkins/Wolters Kluwer; 2011. 2011. [Google Scholar]
- [2].Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart Disease and Stroke Statistics—2015 Update: A Report From the American Heart Association. Circulation. 2015;131(4):e29–e322. [DOI] [PubMed] [Google Scholar]
- [3].Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135(10):e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mamas MA, Sperrin M, Watson MC, Coutts A, Wilde K, Burton C, et al. Do patients have worse outcomes in heart failure than in cancer? A primary care-based cohort study with 10-year follow-up in Scotland. European journal of heart failure. 2017;19(9):1095–104. [DOI] [PubMed] [Google Scholar]
- [5].Taylor CJ, Ryan R, Nichols L, Gale N, Hobbs FR, Marshall T. Survival following a diagnosis of heart failure in primary care. Family practice. 2017;34(2):161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Benjamin Emelia J, Muntner P, Alonso A, Bittencourt Marcio S, Callaway Clifton W, Carson April P, et al. Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association. Circulation. 2019;139(10):e56–e528. [DOI] [PubMed] [Google Scholar]
- [7].Katz A, Konstam M. Heart Failure: Pathophysiology, Molecular Biology, and Clinical Management. Second Edition ed. Philadelphia: Lippincott Williams & Wilkins; 2009. [Google Scholar]
- [8].Xu H, Wang Y, Lin S, Deng W, Peng D, Cui Q, et al. PTMD: A Database of Human Disease-associated Post-translational Modifications. Genomics, proteomics & bioinformatics. 2018;16(4):244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nishida Y, Rardin MJ, Carrico C, He W, Sahu AK, Gut P, et al. SIRT5 Regulates both Cytosolic and Mitochondrial Protein Malonylation with Glycolysis as a Major Target. Molecular cell. 2015;59(2):321–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sadhukhan S, Liu X, Ryu D, Nelson OD, Stupinski JA, Li Z, et al. Metabolomics-assisted proteomics identifies succinylation and SIRT5 as important regulators of cardiac function. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(16):4320–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Horton JL, Martin OJ, Lai L, Riley NM, Richards AL, Vega RB, et al. Mitochondrial protein hyperacetylation in the failing heart. JCI insight. 2016;2(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Boylston JA, Sun J, Chen Y, Gucek M, Sack MN, Murphy E. Characterization of the cardiac succinylome and its role in ischemia-reperfusion injury. Journal of molecular and cellular cardiology. 2015;88:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Foster DB, Liu T, Rucker J, O’Meally RN, Devine LR, Cole RN, et al. The cardiac acetyl-lysine proteome. PloS one. 2013;8(7):e67513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Grillon JM, Johnson KR, Kotlo K, Danziger RS. Non-histone lysine acetylated proteins in heart failure. Biochimica et biophysica acta. 2012;1822(4):607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rapundalo ST. Cardiac protein phosphorylation: functional and pathophysiological correlates. Cardiovascular research. 1998;38(3):559–88. [DOI] [PubMed] [Google Scholar]
- [16].Wijnker PJ, Murphy AM, Stienen GJ, van der Velden J. Troponin I phosphorylation in human myocardium in health and disease. Netherlands heart journal : monthly journal of the Netherlands Society of Cardiology and the Netherlands Heart Foundation. 2014;22(10):463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rosas PC, Liu Y, Abdalla MI, Thomas CM, Kidwell DT, Dusio GF, et al. Phosphorylation of cardiac Myosin-binding protein-C is a critical mediator of diastolic function. Circulation Heart failure. 2015;8(3):582–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Park WJ, Oh JG. SERCA2a: a prime target for modulation of cardiac contractility during heart failure. BMB reports. 2013;46(5):237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lee A, Oh JG, Gorski PA, Hajjar RJ, Kho C. Post-translational Modifications in Heart Failure: Small Changes, Big Impact. Heart, lung & circulation. 2016;25(4):319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Canton M, Menazza S, Sheeran FL, Polverino de Laureto P, Di Lisa F, Pepe S. Oxidation of myofibrillar proteins in human heart failure. Journal of the American College of Cardiology. 2011;57(3):300–9. [DOI] [PubMed] [Google Scholar]
- [21].Chen Y, Sprung R, Tang Y, Ball H, Sangras B, Kim SC, et al. Lysine propionylation and butyrylation are novel post-translational modifications in histones. Molecular & cellular proteomics : MCP. 2007;6(5):812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Du J, Zhou Y, Su X, Yu JJ, Khan S, Jiang H, et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science. 2011;334(6057):806–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Galligan JJ, Smathers RL, Fritz KS, Epperson LE, Hunter LE, Petersen DR. Protein carbonylation in a murine model for early alcoholic liver disease. Chemical research in toxicology. 2012;25(5):1012–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Galligan JJ, Smathers RL, Shearn CT, Fritz KS, Backos DS, Jiang H, et al. Oxidative Stress and the ER Stress Response in a Murine Model for Early-Stage Alcoholic Liver Disease. Journal of toxicology. 2012;2012:207594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Peng C, Lu Z, Xie Z, Cheng Z, Chen Y, Tan M, et al. The first identification of lysine malonylation substrates and its regulatory enzyme. Molecular & cellular proteomics : MCP. 2011;10(12):M111 012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shearn CT, Pulliam CF, Pedersen K, Meredith K, Mercer KE, Saba LM, et al. Knockout of the Gsta4 Gene in Male Mice Leads to an Altered Pattern of Hepatic Protein Carbonylation and Enhanced Inflammation Following Chronic Consumption of an Ethanol Diet. Alcoholism, clinical and experimental research. 2018;42(7):1192–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tan M, Peng C, Anderson KA, Chhoy P, Xie Z, Dai L, et al. Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell metabolism. 2014;19(4):605–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhang Z, Tan M, Xie Z, Dai L, Chen Y, Zhao Y. Identification of lysine succinylation as a new post-translational modification. Nature chemical biology. 2011;7(1):58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wagner GR, Hirschey MD. Nonenzymatic protein acylation as a carbon stress regulated by sirtuin deacylases. Molecular cell. 2014;54(1):5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wagner GR, Payne RM. Widespread and enzyme-independent Nepsilon-acetylation and Nepsilon-succinylation of proteins in the chemical conditions of the mitochondrial matrix. The Journal of biological chemistry. 2013;288(40):29036–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hebert AS, Dittenhafer-Reed KE, Yu W, Bailey DJ, Selen ES, Boersma MD, et al. Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Molecular cell. 2013;49(1):186–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kendrick AA, Choudhury M, Rahman SM, McCurdy CE, Friederich M, Van Hove JL, et al. Fatty liver is associated with reduced SIRT3 activity and mitochondrial protein hyperacetylation. The Biochemical journal. 2011;433(3):505–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Rardin MJ, Newman JC, Held JM, Cusack MP, Sorensen DJ, Li B, et al. Label-free quantitative proteomics of the lysine acetylome in mitochondria identifies substrates of SIRT3 in metabolic pathways. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(16):6601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Still AJ, Floyd BJ, Hebert AS, Bingman CA, Carson JJ, Gunderson DR, et al. Quantification of mitochondrial acetylation dynamics highlights prominent sites of metabolic regulation. The Journal of biological chemistry. 2013;288(36):26209–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Nakagawa T, Guarente L. Sirtuins at a glance. Journal of Cell Science. 2011;124(6):833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Molecular and cellular biology. 2007;27(24):8807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Schwer B, North BJ, Frye RA, Ott M, Verdin E. The human silent information regulator (Sir)2 homologue hSIRT3 is a mitochondrial nicotinamide adenine dinucleotide-dependent deacetylase. The Journal of cell biology. 2002;158(4):647–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Alhazzazi TY, Kamarajan P, Verdin E, Kapila YL. SIRT3 and cancer: tumor promoter or suppressor? Biochimica et biophysica acta. 2011;1816(1):80–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bellizzi D, Rose G, Cavalcante P, Covello G, Dato S, De Rango F, et al. A novel VNTR enhancer within the SIRT3 gene, a human homologue of SIR2, is associated with survival at oldest ages. Genomics. 2005;85(2):258–63. [DOI] [PubMed] [Google Scholar]
- [40].Cho EH. SIRT3 as a Regulator of Non-alcoholic Fatty Liver Disease. Journal of lifestyle medicine. 2014;4(2):80–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fritz KS, Galligan JJ, Hirschey MD, Verdin E, Petersen DR. Mitochondrial acetylome analysis in a mouse model of alcohol-induced liver injury utilizing SIRT3 knockout mice. Journal of proteome research. 2012;11(3):1633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Fritz KS, Green MF, Petersen DR, Hirschey MD. Ethanol metabolism modifies hepatic protein acylation in mice. PloS one. 2013;8(9):e75868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kong X, Wang R, Xue Y, Liu X, Zhang H, Chen Y, et al. Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PloS one. 2010;5(7):e11707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Shi T, Wang F, Stieren E, Tong Q. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. The Journal of biological chemistry. 2005;280(14):13560–7. [DOI] [PubMed] [Google Scholar]
- [45].Sun W, Liu C, Chen Q, Liu N, Yan Y, Liu B. SIRT3: A New Regulator of Cardiovascular Diseases. Oxidative medicine and cellular longevity. 2018;2018:7293861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Park J, Chen Y, Tishkoff DX, Peng C, Tan M, Dai L, et al. SIRT5-Mediated Lysine Desuccinylation Impacts Diverse Metabolic Pathways. Molecular cell. 2013;50(6):919–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rardin MJ, He W, Nishida Y, Newman JC, Carrico C, Danielson SR, et al. SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell metabolism. 2013;18(6):920–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gibson GE, Xu H, Chen HL, Chen W, Denton TT, Zhang S. Alpha-ketoglutarate dehydrogenase complex-dependent succinylation of proteins in neurons and neuronal cell lines. J Neurochem. 2015;134(1):86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Holper S, Nolte H, Bober E, Braun T, Kruger M. Dissection of metabolic pathways in the Db/Db mouse model by integrative proteome and acetylome analysis. Molecular bioSystems. 2015;11(3):908–22. [DOI] [PubMed] [Google Scholar]
- [50].Romanick SS, Ulrich C, Schlauch K, Hostler A, Payne J, Woolsey R, et al. Obesity-mediated regulation of cardiac protein acetylation: parallel analysis of total and acetylated proteins via TMT-tagged mass spectrometry. Bioscience reports. 2018;38(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ali HR, Assiri MA, Harris PS, Michel CR, Yun Y, Marentette JO, et al. Quantifying Competition among Mitochondrial Protein Acylation Events Induced by Ethanol Metabolism. Journal of proteome research. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Meyer JG, D’Souza AK, Sorensen DJ, Rardin MJ, Wolfe AJ, Gibson BW, et al. Quantification of Lysine Acetylation and Succinylation Stoichiometry in Proteins Using Mass Spectrometric Data-Independent Acquisitions (SWATH). Journal of the American Society for Mass Spectrometry. 2016;27(11):1758–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Weinert BT, Scholz C, Wagner SA, Iesmantavicius V, Su D, Daniel JA, et al. Lysine succinylation is a frequently occurring modification in prokaryotes and eukaryotes and extensively overlaps with acetylation. Cell reports. 2013;4(4):842–51. [DOI] [PubMed] [Google Scholar]
- [54].Alleyn M, Breitzig M, Lockey R, Kolliputi N. The dawn of succinylation: a posttranslational modification. American journal of physiology Cell physiology. 2018;314(2):C228–C32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Li T, Zhang Z, Kolwicz SC, Jr., Abell L, Roe ND, Kim M, et al. Defective Branched-Chain Amino Acid Catabolism Disrupts Glucose Metabolism and Sensitizes the Heart to Ischemia-Reperfusion Injury. Cell metabolism. 2017;25(2):374–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sun H, Olson KC, Gao C, Prosdocimo DA, Zhou M, Wang Z, et al. Catabolic Defect of Branched-Chain Amino Acids Promotes Heart Failure. Circulation. 2016;133(21):2038–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wang W, Zhang F, Xia Y, Zhao S, Yan W, Wang H, et al. Defective branched chain amino acid catabolism contributes to cardiac dysfunction and remodeling following myocardial infarction. American journal of physiology Heart and circulatory physiology. 2016;311(5):H1160–H9. [DOI] [PubMed] [Google Scholar]
- [58].Hershberger KA, Abraham DM, Liu J, Locasale JW, Grimsrud PA, Hirschey MD. Ablation of Sirtuin5 in the postnatal mouse heart results in protein succinylation and normal survival in response to chronic pressure overload. The Journal of biological chemistry. 2018;293(27):10630–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Fisher-Wellman KH, Draper JA, Davidson MT, Williams AS, Narowski TM, Slentz DH, et al. Respiratory Phenomics across Multiple Models of Protein Hyperacylation in Cardiac Mitochondria Reveals a Marginal Impact on Bioenergetics. Cell reports. 2019;26(6):1557–72 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Woulfe KC, Ferrara C, Pioner JM, Mahaffey JH, Coppini R, Scellini B, et al. A Novel Method of Isolating Myofibrils From Primary Cardiomyocyte Culture Suitable for Myofibril Mechanical Study. Front Cardiovasc Med. 2019;6:12-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic acids research. 2009;37(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Brandt PW, Colomo F, Piroddi N, Poggesi C, Tesi C. Force regulation by Ca2+ in skinned single cardiac myocytes of frog. Biophysical journal. 1998;74(4):1994–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Moussavi-Harami F, Razumova MV, Racca AW, Cheng Y, Stempien-Otero A, Regnier M. 2-Deoxy adenosine triphosphate improves contraction in human end-stage heart failure. Journal of molecular and cellular cardiology. 2015;79:256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Racca AW, Klaiman JM, Pioner JM, Cheng Y, Beck AE, Moussavi-Harami F, et al. Contractile properties of developing human fetal cardiac muscle. The Journal of physiology. 2016;594(2):437–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, et al. The Failing Heart Relies on Ketone Bodies as a Fuel. Circulation. 2016;133(8):698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Janardhan A, Chen J, Crawford PA. Altered systemic ketone body metabolism in advanced heart failure. Texas Heart Institute journal. 2011;38(5):533–8. [PMC free article] [PubMed] [Google Scholar]
- [67].Kolwicz SC, Jr., Airhart S, Tian R. Ketones Step to the Plate: A Game Changer for Metabolic Remodeling in Heart Failure? Circulation. 2016;133(8):689–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kim EH, Galchev VI, Kim JY, Misek SA, Stevenson TK, Campbell MD, et al. Differential protein expression and basal lamina remodeling in human heart failure. Proteomics Clinical applications. 2016;10(5):585–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kittleson MM, Minhas KM, Irizarry RA, Ye SQ, Edness G, Breton E, et al. Gene expression analysis of ischemic and nonischemic cardiomyopathy: shared and distinct genes in the development of heart failure. Physiological genomics. 2005;21(3):299–307. [DOI] [PubMed] [Google Scholar]
- [70].Li W, Rong R, Zhao S, Zhu X, Zhang K, Xiong X, et al. Proteomic analysis of metabolic, cytoskeletal and stress response proteins in human heart failure. Journal of cellular and molecular medicine. 2012;16(1):59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Tan FL, Moravec CS, Li J, Apperson-Hansen C, McCarthy PM, Young JB, et al. The gene expression fingerprint of human heart failure. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(17):11387–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Neagoe C, Kulke M, Monte Fd, Gwathmey JK, Tombe PPd, Hajjar RJ, et al. Titin Isoform Switch in Ischemic Human Heart Disease. Circulation. 2002;106(11):1333–41. [DOI] [PubMed] [Google Scholar]
- [73].Bollen IAE, Ehler E, Fleischanderl K, Bouwman F, Kempers L, Ricke-Hoch M, et al. Myofilament Remodeling and Function Is More Impaired in Peripartum Cardiomyopathy Compared with Dilated Cardiomyopathy and Ischemic Heart Disease. The American Journal of Pathology. 2017;187(12):2645–58. [DOI] [PubMed] [Google Scholar]
- [74].Lee S, Lu R, Müller-Ehmsen J, Schwinger RH, Brixius K. Increased Ca2+ sensitivity of myofibrillar tension in ischaemic vs dilated cardiomyopathy. Clinical and Experimental Pharmacology and Physiology. 2010;37(12):1134–8. [DOI] [PubMed] [Google Scholar]
- [75].Des Rosiers C, Labarthe F, Lloyd SG, Chatham JC. Cardiac anaplerosis in health and disease: food for thought. Cardiovascular research. 2011;90(2):210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Sheeran FL, Pepe S. Energy deficiency in the failing heart: linking increased reactive oxygen species and disruption of oxidative phosphorylation rate. Biochimica et biophysica acta. 2006;1757(5–6):543–52. [DOI] [PubMed] [Google Scholar]
- [77].Zhang J, Wang YT, Miller JH, Day MM, Munger JC, Brookes PS. Accumulation of Succinate in Cardiac Ischemia Primarily Occurs via Canonical Krebs Cycle Activity. Cell reports. 2018;23(9):2617–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Desai AS. Hemoglobin concentration in acute decompensated heart failure: a marker of volume status? Journal of the American College of Cardiology. 2013;61(19):1982–4. [DOI] [PubMed] [Google Scholar]
- [79].Wende AR, Brahma MK, McGinnis GR, Young ME. Metabolic Origins of Heart Failure. JACC Basic to translational science. 2017;2(3):297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Jeong MY, Lin YH, Wennersten SA, Demos-Davies KM, Cavasin MA, Mahaffey JH, et al. Histone deacetylase activity governs diastolic dysfunction through a nongenomic mechanism. Science Translational Medicine. 2018;10(427):eaao0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Choudhary C, Weinert BT, Nishida Y, Verdin E, Mann M. The growing landscape of lysine acetylation links metabolism and cell signalling. Nat Rev Mol Cell Biol. 2014;15(8):536–50. [DOI] [PubMed] [Google Scholar]
- [82].He W, Newman JC, Wang MZ, Ho L, Verdin E. Mitochondrial sirtuins: regulators of protein acylation and metabolism. Trends Endocrinol Metab. 2012;23(9):467–76. [DOI] [PubMed] [Google Scholar]
- [83].Nandi SK, Rakete S, Nahomi RB, Michel C, Dunbar A, Fritz KS, et al. Succinylation Is a Gain-of-Function Modification in Human Lens alphaB-Crystallin. Biochemistry. 2019;58(9):1260–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Park J, Chen Y, Tishkoff DX, Peng C, Tan M, Dai L, et al. SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Molecular cell. 2013;50(6):919–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Andrienko T, Kuznetsov AV, Kaambre T, Usson Y, Orosco A, Appaix F, et al. Metabolic consequences of functional complexes of mitochondria, myofibrils and sarcoplasmic reticulum in muscle cells. The Journal of experimental biology. 2003;206(Pt 12):2059–72. [DOI] [PubMed] [Google Scholar]
- [86].Hollander JM, Thapa D, Shepherd DL. Physiological and structural differences in spatially distinct subpopulations of cardiac mitochondria: influence of cardiac pathologies. American journal of physiology Heart and circulatory physiology. 2014;307(1):H1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Weinstein ES, Benson DW, Fry DE. Subpopulations of human heart mitochondria. The Journal of surgical research. 1986;40(5):495–8. [DOI] [PubMed] [Google Scholar]
- [88].Hendgen-Cotta UB, Esfeld S, Jastrow H, Totzeck M, Altschmied J, Goy C, et al. Mouse cardiac mitochondria do not separate in subsarcolemmal and interfibrillar subpopulations. Mitochondrion. 2018;38:1–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.