Abstract
Across zoonotic pathogens, RNA viruses are responsible for disproportionate levels of human disease, suffering, and death. Neurotropic RNA viruses (e.g. rabies, Japanese and Eastern Equine Encephalitis, Ebola, West Nile, Powassan) infect the brain and spinal cord, causing meningitis, encephalitis, microcephaly, and Guillain-Barre syndrome. Mechanistic data explaining molecular mechanisms of these diseases are lacking, and the enclosure of the central nervous system and associated meninges in bone complicates access for diagnosis, clinical treatment, and research. Here, we discuss new tissue models, imaging methods, and molecular techniques that are changing research aimed at understanding the pathogenesis of neurotropic RNA viruses.
Keywords: pathogenesis, imaging, emerging virus, infection, human brain, organomics
Limitations of model systems
Human tissues, when obtained safely and ethically, are valuable resources for research aimed at defining human disease mechanisms and discovering potential therapeutic targets. However, excepting perhaps blood and placenta, human tissues are generally not easily available, nor are they easily cultured. Two dimensional cell cultures have transformed experimental biology, but transformed cell lines are known to have gene expression patterns and metabolic profiles that are not reflective of human biology. Alternatively, two-dimensional cultures of cells derived from embryonic stem cells or induced pluripotent stem cells closely resemble human cells in their transcriptional profiles; nonetheless, cells in two dimensions fail to replicate the three-dimensional features of tissue organization. Although mouse models have been widely used to gain insights into human disease processes, genetic and physiological differences between humans and mice sometimes limit their value. This has proved to be an issue in studying virus infections, because adult mice (wild-type C57BL/6, BALB/c, or CD-1 mice) are generally not infected by Flaviviruses such as Zika virus (ZIKV) unless the innate immune system is disabled by interrupting STAT2-dependent signaling and interferon responses (reviewed in1). Although important experimental insights have been gained from studying ZIKV infections in mouse models that block innate immune signaling pathways1, the mouse brain does not recapitulate the cellular features or unique transcriptional signatures of the human brain2. Considering these models, 3D organoids derived from human embryonic stem cells or induced pluripotent stem cells offer an important alternative to 2D cell culture and animal models for research aimed at advancing studies on neurotropic viruses.
Enter the Organoids
Organoids--three-dimensional multicellular in vitro tissues that mimic their corresponding in vivo organ--have received recent attention as experimental model systems3. Organoids are grown from embryonic stem cells (ESC) or from induced pluripotent stem cells (iPSC), using specific culture conditions that drive the cells toward defined differentiation pathways. Cerebral organoids mimic the cytoarchitectural and epigenomic signatures of the human brain4,5. These organoids maintain ventricular zones containing proliferating neuronal progenitor cells as well as cortical neurons which, when matured, produce Ca2+ surges with glutamate release and contain functional synapses, spontaneously active neural networks, dendritic spines, and even photosensitive cells that react to light stimulation6. Mature cerebral organoids develop immunocompetent astrocytes, which are functionally analogous to astrocytes isolated from the human brain7. Human cerebral organoids and the human fetal cortex show extensive similarities as they both maintain continually differentiating cell populations. Further, single cell transcriptomics reveals that the transcriptomes of brain neuronal progenitors and cortical neurons parallel cortical cells of cerebral organoids8. These data confirm the stem-cell derived cerebral organoid as an important experimental model of the human brain.
Virus Infections, Organoids, and Technologies
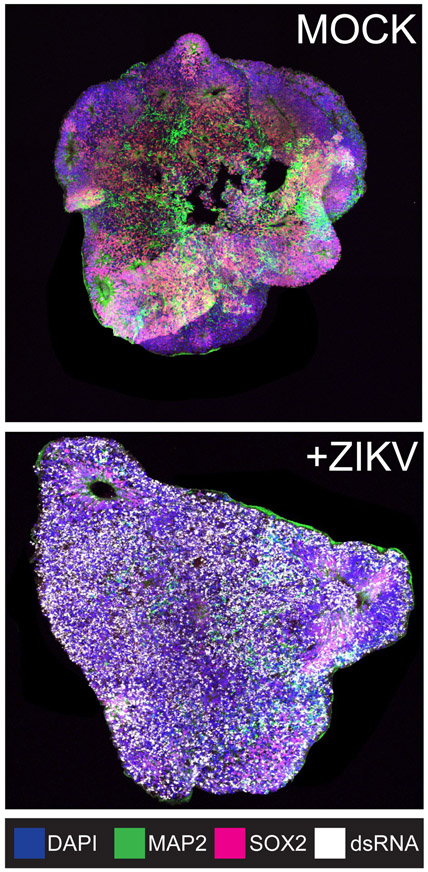
Virus infections cause “autonomous” or “non-autonomous” cellular effects, depending on whether the virus has entered the cell and replicated the viral genome (autonomous effects), or if uninfected cells are affected indirectly by cytokines or metabolites released by the infected cell (non-autonomous effects)9. This distinction is important because, although some cells escape virus infection, they may nonetheless contribute to the infection environment. The environment, in an in vivo infection, is multicellular and three-dimensional; therefore, the use of 3D cell culture models of interacting cell types is optimal for understanding mechanisms of disease-causing viral infection in the brain. Cerebral organoids are being used to elucidate the causes of Zika virus-associated microcephaly (reviewed in10). The effects of ZIKV infection on cytoarchitecture is notable in the cerebral organoid model. Mock-infected cerebral organoids are characterized by SOX2-staining neuronal progenitor cells and ventricular zones, as well as MAP2-stained neurons (Figure 1, mock). Alternatively, ZIKV-infected organoids are disorganized (few ventricles are present), there is greatly diminished progenitor cell staining (SOX2) accompanied by clear double stranded RNA signal (Figure 1, +ZIKV). ZIKV infected organoids show a growth defect that is observable by measuring infected and uninfected organoids, suggesting a size phenotype similar to ZIKV-induced microcephaly11. Further study is necessary to define the mechanisms underlying ZIKV-induced microcephaly and disease, and cerebral organoids represent a promising technology for these analyses.
Figure 1:
ESC-derived cerebral organoids model ZIKV infection. Cytoarchitectural changes, including loss of ventricles, are observed upon infection with ZIKV Cambodia. Neuronal progenitor cells, neurons, and replicating ZIKV RNA are identified by SOX2, MAP2, and double stranded RNA staining, respectively.
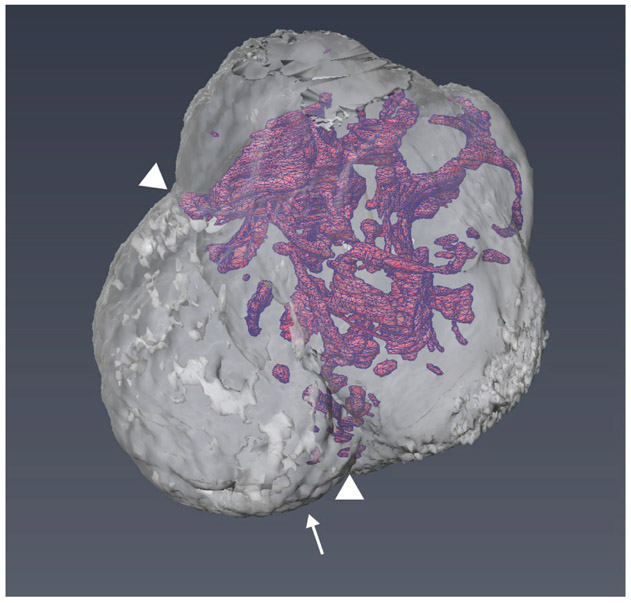
Other emerging technologies are being adapted to study virus infections in three dimensional organoids. Single cell RNA sequencing is being applied to organoids as an approach for understanding autonomous and non-autonomous effects of virus infection12. Single cell sequence analysis is defining cell-specific transcriptional profiles12, making it possible to characterize the effect of cell type on virus infection. Lipid clearing methods (“Clarity”) have yielded remarkable images of the brain and the networks of neurons and glia13. The same approaches are being applied to organoids14 to yield images that can be analyzed as slices, or computationally assembled to create three-dimensional views of complex ventricle networks (Figure 2). The combination of single cell sequencing, immunostaining, lipid clearing methods, and 3D image assembly will provide new insights into understanding the differential pathogenesis of RNA viruses.
Figure 2:
Reconstructed three-dimensional image of a human cerebral organoid, including a network of ventricle structures. Arrowheads point to choroid plexus. Reprinted with permission from Renner M, Lancaster MA, Bian S, Choi H, Ku T, Peer A, Chung K, Knoblich JA. EMBO J [Internet]. 2017 May 15; 36(10):1316-1329. Copyright 2017; John Wiley & Sons.
High Containment Viruses and Organoids
The group of emerging or reemerging neurotropic viruses includes pathogens whose safe handling requires enhanced biocontainment. West Nile virus has been a threat in the United States for a number of years15, while other examples of arthropod-transmitted neurotropic viruses are Eastern Equine Encephalitis, Japanese Encephalitis, Tick Borne Encephalitis, Deer Tick, and Powassan. Outside of West Nile virus, little is known about the molecular mechanisms of pathogenesis, and human cerebral organoids offer important advantages for study in a physiologically relevant model system. Organoids can be prepared outside of the containment facility, and brought in for virus infection. Although sequence analysis core facilities are often reluctant to process and analyze pathogen-containing samples, the SeqWell method for single cell sequence has been applied to study tuberculosis16, and could likely be used for BSL3 viruses as well. Although live-cell imaging would require dedicated instrumentation inside a BSL3 containment facility, tissue fixatives (formalin, paraformaldehyde) inactivate viruses such that fixed samples could be removed from the containment facility for further processing and analysis. Experimental analysis of viruses requiring higher biocontainment levels will continue to be challenging, but not necessarily excluded from technical advances.
Challenges and Opportunities
While cerebral organoids are physiologically relevant models of the human brain, there are limitations and challenges for improvement. The absence of organoid vascularization leads to progressive necrosis in the core during long-term culturing (months). If cell death is observed, it is important to distinguish virus-related effects from cell death resulting from organoid growth. Although new technologies for incorporating vasculature are appearing17, no standardized protocol has been universally adopted. The blood-brain barrier (BBB) is another challenge area. The BBB is formed by specialized capillary endothelial cells that have tight intercellular junctions. The BBB is a physical barrier that neurotropic viruses must pass in order to infect the brain. Current approaches for infecting organoids with viruses focus on inoculating organoid culture media with virus. Although this model allows the investigator to examine infection kinetics from the outside surface of the organoid, it is not clear if the infection accurately models the BBB or virus infection in vivo. Current trans-well models (Reviewed in18) or organ-on-chip models19 are useful to study the general effects of infection on BBB function; however, the lack of physical interaction between BBB cells and the cerebral organoid poses a barrier to fully understanding the relationship between these complex cell types. Additionally, the neuroimmune system, which is distinct from the peripheral immune system, is an important area of consideration for studying brain pathogens. Upon virus infection, a neuroimmune response is mounted and certain peripheral immune cells cross the BBB to respond to invading pathogens20. Although astrocytes and microglia, cells often referred to as the immune system of the brain, differentiate naturally in cerebral organoids21, further research is needed to understand the interactions among brain cell types and neurotropic viruses. Additionally, co-culture of organoids with other cell types (including pre-infected microglia) may provide new insights into pathogen infections in the brain and the accompanying immune responses.
Finally, the potential for personalized medicine approaches; that is, the use of patient-derived iPSC to generate patient and disease-specific organoids, is very exciting, technically feasible5, and has recently been used to screen for anti-ZIKV drugs22. It is also possible to apply genome editing methods to generate knock-out or knock-in organoids23 to confirm candidate pro-viral and virus restriction factor genes. Cerebral organoids, as well as the cutting-edge sequencing and imaging technologies available to analyze them, are promising tools for experimental analysis of neurotropic virus infections.
Acknowledgments:
Supported by award U19AI131135 from the U.S. Public Health Service. We thank the members of the Gehrke laboratory, as well as Kwanghun Chung’s laboratory (MIT), Rudolf Jaenisch’s laboratory (Whitehead Institute), and David Sabatini’s laboratory (Whitehead Institute), Cambridge Massachusetts, for creating the research environment for this work.
Abbreviations:
- ZIKV
Zika Virus
- ESC
embryonic stem cell
- iPSC
induced pluripotent stem cell
Footnotes
Conflict of interest: Dr. Gehrke is a co-founder of E25Bio. There are no conflicts with the work described in this article.
References Cited:
- (1).Morrison TE; Diamond MS Animal Models of Zika Virus Infection, Pathogenesis, and Immunity. J. Virol 2017, 91 (8). 10.1128/JVI.00009-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Hodge RD; Bakken TE; Miller JA; Smith KA; Barkan ER; Graybuck LT; Close JL; Long B; Johansen N; Penn O; et al. Conserved Cell Types with Divergent Features in Human versus Mouse Cortex. Nature 2019 10.1038/s41586-019-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Wang H Modeling Neurological Diseases With Human Brain Organoids. Front. Synaptic Neurosci 2018, 10, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Luo C; Lancaster MA; Castanon R; Nery JR; Knoblich JA; Ecker JR Cerebral Organoids Recapitulate Epigenomic Signatures of the Human Fetal Brain. Cell Rep. 2016, 17 (12), 3369–3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Lancaster MA; Renner M; Martin C-A; Wenzel D; Bicknell LS; Hurles ME; Homfray T; Penninger JM; Jackson AP; Knoblich JA Cerebral Organoids Model Human Brain Development and Microcephaly. Nature 2013, 501 (7467), 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Quadrato G; Nguyen T; Macosko EZ; Sherwood JL; Min Yang S; Berger DR; Maria N; Scholvin J; Goldman M; Kinney JP; et al. Cell Diversity and Network Dynamics in Photosensitive Human Brain Organoids. Nature 2017, 545 (7652), 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Dezonne RS; Sartore RC; Nascimento JM; Saia-Cereda VM; Romao LF; Alves-Leon SV; de Souza JM; Martins-de-Souza D; Rehen SK; Gomes FC A. Derivation of Functional Human Astrocytes from Cerebral Organoids. Scientific Reports. 2017. 10.1038/srep45091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Camp JG; Badsha F; Florio M; Kanton S; Gerber T; Wilsch-Bräuninger M; Lewitus E; Sykes A; Hevers W; Lancaster M; et al. Human Cerebral Organoids Recapitulate Gene Expression Programs of Fetal Neocortex Development. Proc. Natl. Acad. Sci. U. S. A 2015, 112 (51), 15672–15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Olmo IG; Carvalho TG; Costa VV; Alves-Silva J; Ferrari CZ; Izidoro-Toledo TC; da Silva JF; Teixeira AL; Souza DG; Marques JT; et al. Zika Virus Promotes Neuronal Cell Death in a Non-Cell Autonomous Manner by Triggering the Release of Neurotoxic Factors. Front. Immunol 2017, 8, 1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Qian X; Nguyen HN; Song MM; Hadiono C; Ogden SC; Hammack C; Yao B; Hamersky GR; Jacob F; Zhong C; et al. Brain-Region-Specific Organoids Using Mini-Bioreactors for Modeling ZIKV Exposure. Cell 2016, 165 (5), 1238–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Qian X; Nguyen HN; Jacob F; Song H; Ming G-L Using Brain Organoids to Understand Zika Virus-Induced Microcephaly. Development 2017, 144 (6), 952–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).O’Neal JT; Upadhyay AA; Wolabaugh A.; Patel NB; Bosinger SE; Suthar MS. West Nile Virus-Inclusive Single-Cell RNA Sequencing Reveals Heterogeneity in the Type I Interferon Response within Single Cells. J. Virol 2019, 93 (6). 10.1128/JVI.01778-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Chung K; Deisseroth K CLARITY for Mapping the Nervous System. Nat. Methods 2013, 10(6), 508–513. [DOI] [PubMed] [Google Scholar]
- (14).Renner M; Lancaster MA; Bian S; Choi H; Ku T; Peer A; Chung K; Knoblich JA Self-organized Developmental Patterning and Differentiation in Cerebral Organoids. EMBO J. 2017, 36 (10), 1316–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Brinton MA The Molecular Biology of West Nile Virus: A New Invader of the Western Hemisphere. Annu. Rev. Microbiol 2002, 56, 371–402. [DOI] [PubMed] [Google Scholar]
- (16).Gierahn TM; Marc H Wadsworth II, Travis K Hughes, Bryan D Bryson, Andrew Butler, Rahul Satija, Sarah Fortune, J Christopher Love, and Alex K Shalek. Seq-Well: Portable, Low-Cost Rna Sequencing of Single Cells at High Throughput. Nat. Methods 2017, 14(A), 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Grebenyuk S; Ranga A Engineering Organoid Vascularization. Frontiers in Bioengineering and Biotechnology. 2019. 10.3389/fbioe.2019.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Helms HC; Abbott NJ; Burek M; Cecchelli R; Couraud P-O; Deli MA; Förster C; Galla HJ; Romero IA; Shusta EV; et al. In Vitro Models of the Blood-brain Barrier: An Overview of Commonly Used Brain Endothelial Cell Culture Models and Guidelines for Their Use. J. Cereb. Blood Flow Metab 2016, 36 (5), 862–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Maoz BM; Herland A; FitzGerald EA; Grevesse T; Vidoudez C; Pacheco AR; Sheehy SP; Park T-E; Dauth S; Mannix R; et al. A Linked Organ-on-Chip Model of the Human Neurovascular Unit Reveals the Metabolic Coupling of Endothelial and Neuronal Cells. Nat. Biotechnol 2018. 10.1038/nbt.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Dantzer R Neuroimmune Interactions: From the Brain to the Immune System and Vice Versa. Physiol. Rev 2018, 98 (1), 477–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Ormel PR; Vieira de Sá R; van Bodegraven EJ; Karst H; Harschnitz O; Sneeboer MAM; Johansen LE; van Dijk RE; Scheefhals N; Berdenis van Berlekom A; et al. Microglia Innately Develop within Cerebral Organoids. Nat. Commun 2018, 9(1), 4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Watanabe M; Buth JE; Vishlaghi N; de la Torre-Ubieta L; Taxidis J; Khakh BS; Coppola G; Pearson CA; Yamauchi K; Gong D; et al. Self-Organized Cerebral Organoids with Human-Specific Features Predict Effective Drugs to Combat Zika Virus Infection. Cell Rep. 2017, 21 (2), 517–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Li Y; Muffat J; Javed AO; Keys HR; Lungjangwa T; Bosch I; Khan M; Virgilio MC; Gehrke L; Sabatini DM; et al. Genome-Wide CRISPR Screen for Zika Virus Resistance in Human Neural Cells. Proceedings of the National Academy of Sciences. 2019, p 201900867 10.1073/pnas.1900867116. [DOI] [PMC free article] [PubMed] [Google Scholar]