Summary
Based on the type-I cannabinoid receptor (CB1) content of hypophysiotropic axons and the involvement of tanycytes in the regulation of the hypothalamic-pituitary-thyroid (HPT) axis, we hypothesized that endocannabinoids are involved in the tanycyte-induced regulation of TRH release in the median eminence (ME). We demonstrated that CB1-immunoreactive TRH axons were associated to DAGLα-immunoreactive tanycyte processes in the external zone of ME and showed that endocannabinoids tonically inhibit the TRH release in this tissue. We showed that glutamate depolarizes the tanycytes, increases their intracellular Ca2+ level and the 2-AG level of the ME via AMPA and kainite receptors and glutamate transport. Using optogenetics, we demonstrated that glutamate released from TRH neurons influences the tanycytes in the ME.
In summary, tanycytes regulate TRH secretion in the ME via endocannabinoid release, whereas TRH axons regulate tanycytes by glutamate, suggesting the existence of a reciprocal microcircuit between tanycytes and TRH terminals that controls TRH release.
Subject Areas: Molecular Physiology, Neuroscience, Neuroanatomy
Graphical Abstract
Highlights
-
•
Tanycytes tonically inhibit the activity of TRH axons via endocannabinoid release
-
•
Glutamate depolarizes the tanycytes and regulates their 2-AG synthesis
-
•
Glutamate released from the hypophysiotropic TRH axons influences tanycytes
-
•
A microcircuit utilizing glutamate and endocannabinoids regulates TRH release
Molecular Physiology; Neuroscience; Neuroanatomy
Introduction
Tanycytes are specialized glial cells lining the floor and lateral walls of the third ventricle behind the optic chiasm (Rodriguez et al., 2005, Prevot et al., 2018). The small cell bodies of these cells form the ventricular wall, whereas their long basal processes project into the median eminence (ME) or into the neuropil of the arcuate, ventromedial, and dorsomedial hypothalamic nuclei (Rodriguez et al., 2005, Prevot et al., 2018).
In the external zone of the ME, tanycyte processes terminate around the fenestrated capillaries of the hypophysial portal circulation and intermingle with the terminals of hypophysiotropic axons, suggesting that the tanycytes (β2 tanycytes) that project to the ME may be involved in the regulation of the neurohypophysial systems (Rodriguez et al., 2005, Prevot et al., 2018). Indeed, a major role of tanycytes has been demonstrated in the regulation of the hypothalamic-pituitary-thyroid (HPT) axis by multiple mechanisms (Lechan and Fekete, 2007, Rodriguez-Rodriguez et al., 2019). As tanycytes are the primary hypothalamic cell types that express the thyroid hormone activating enzyme, type 2 deiodinase (D2), these cells can regulate the activity of hypophysiotropic thyrotropin-releasing hormone (TRH) synthesizing neurons by controlling the hypothalamic availability of T3, the active form of thyroid hormones that can effectively bind to the nuclear receptors and suppresses TRH release (Fekete and Lechan, 2014). For example, endotoxin-induced increase of tanycyte D2 activity has a critical role in the development of central hypothyroidism (nonthyroidal illness syndrome) (Fekete et al., 2005, Freitas et al., 2010). Tanycytes also express the TRH-degrading ectoenzyme (TRH-DE) (Sanchez et al., 2009), providing a mechanism by which they can control the amount of TRH reaching the pituitary by degrading the tripeptide in the extracellular space of the external zone of the ME.
We recently observed that large number of hypophysiotropic axon terminals in the external zone of the ME contain type 1 cannabinoid receptor (CB1) (Wittmann et al., 2007), raising the possibility that endocannabinoids may also regulate the release of hypophysiotropic hormones in the ME. In most cases, endocannabinoids are released from postsynaptic neurons and act on CB1 receptors located on presynaptic axon terminals (Piomelli, 2003, Kano et al., 2009). The external zone of the ME houses very few neuronal perikarya, and endocannabinoids can travel only very short distances in the brain (Regehr et al., 2009) supporting a glial origin of endocannabinoids in this brain region. Therefore, we hypothesized that tanycytes, the most abundant glial cell type of the ME, may control the release of hormones, including TRH, from the hypophysiotropic axon terminals of the ME via the endocannabinoid system. In addition to TRH, hypophysiotropic TRH axons also release glutamate (Hrabovszky et al., 2005). Since tanycytes express glutamate receptors (Eyigor and Jennes, 1998, Kawakami, 2000), and glutamate is known to regulate endocannabinoid synthesis and release (Katona et al., 2006), we further hypothesized that glutamate release from hypophysiotropic axon terminals signal toward the tanycytes, establishing a local regulatory microcircuit between TRH axons and tanycytes in the external zone of the ME.
To test these hypotheses, we determined whether the tanycytes can control the release of TRH from hypophysiotropic terminals in the ME by utilizing the endocannabinoid system and whether glutamate released from axon terminals of hypophysiotropic TRH neurons regulates β-tanycytes of the ME.
Results
Expression of CB1 in Hypophysiotropic TRH Neurons of Mice
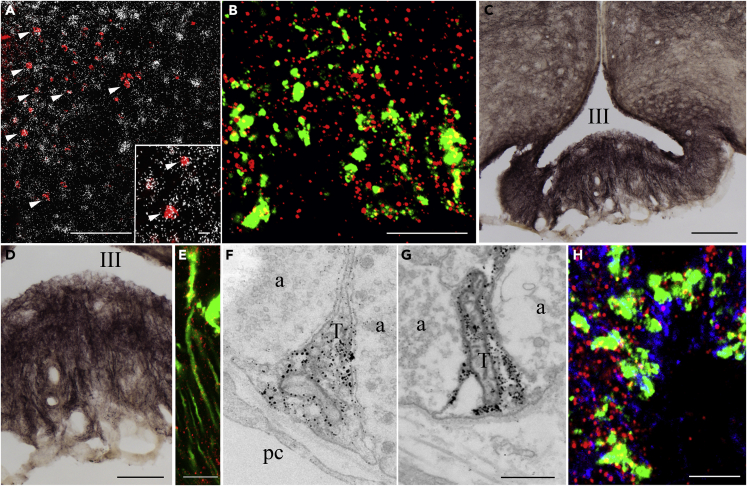
To determine whether the hypophysiotropic TRH neurons express the CB1 receptor, double-labeling in situ hybridization was performed. CB1 mRNA was observed in the majority of neurons in the hypothalamic paraventricular nucleus (PVN) where the perikaryon of the hypophysiotropic TRH neurons reside; however, the intensity of the hybridization signal was much lower than that observed in cortical or hippocampal areas. Analyses of the double-labeled sections showed that silver grains denoting CB1 mRNA were observed above 73.4 ± 1.5% of the TRH neurons in the PVN (Figure 1A). In addition, double-labeling immunofluorescence showed that punctuate CB1-immunoreactive signal was present in the majority of TRH-IR axon varicosities in the external zone of the ME suggesting that the hypophysiotropic TRH axons are sensitive to endocannabinoid signaling (Figure 1B).
Figure 1.
Elements of the Endocannabinoid System Are Present in the Hypophysiotropic TRH Neurons and in the Tanycytes in the External Zone of the ME
(A) Double-labeling in situ hybridization demonstrates that the majority of TRH neurons (red) in the PVN express CB1 mRNA labeled by the presence of silver grains. Arrowheads point to double-labeled neurons expressing both TRH and CB1 mRNAs. Inset illustrates double-labeled neurons at higher magnification (arrowheads).
(B) Double-labeling immunocytochemistry demonstrates the presence of CB1 immunoreactivity (red dots) in TRH-IR hypophysiotropic axon varicosities (green) in the external zone of the ME. The CB1 immunoreactivity within the TRH axons appear yellow owing to the color mixing.
(C) High level of DAGLα immunoreactivity is present in tanycyte cell bodies lining the floor of the third ventricle and the wall of the lateral evaginations. Dense DAGLα-IR fiber network is also present in the median eminence.
(D) Higher-magnification image illustrates that the DAGLα-IR fibers run perpendicular to the surface of the median eminence.
(E) DAGLα immunofluorescence (red) in tanycyte processes where the green fluorescent protein labels the tanycytes. The spotted DAGLα immunofluorescence can be observed along the tanycyte processes.
(F and G) Ultrastructural images demonstrate that the DAGLα immunoreactivity (labeled by silver grains) is present in tanycyte endfeet processes terminating around portal capillaries.
(H) The CB1-containing (red) TRH-IR (green) axon varicosities are closely associated to DAGLα-IR (blue) tanycyte processes.
Scale bars, 100 μm in (A) and (C), 10 μm in inset and (B), 50 μm in (D), 10 μm in (E), 0.5 μm in (G) that corresponds to (F) and (G), and 5 μm in (H). Abbreviations: III, third ventricle; a, axon varicosity; pc, portal capillary; T, tanycyte endfeet. See also Figure S1.
DAGLα-Immunoreactivity Is Present in Tanycytes in the ME and Associated with CB1-IR TRH Axons in Mice
To determine the cell type that releases endocannabinoids in the ME, the localization of diacylglycerol lipase α (DAGLα), the synthesizing enzyme of one of the main endocannabinoids, 2-arachinodonoylglycerol (2-AG), was studied. At light microscopic level, DAGLα-immunoreactivity was observed in tanycyte cell bodies lining the floor (β2 tanycytes) and the lateral evagination (β1 tanycytes) of the third ventricle (Figure 1C). In addition, DAGLα-immunoreactivity was also observed in processes running toward the capillary plexus of the external zone of the ME (Figure 1D), reminiscent of the distribution of the β-tanycyte basal processes. Colocalization of DAGLα-immunoreactivity and the ZsGreen fluorescence in Rax/CreERT2//Gt(ROSA)26Sor_CAG/LSL_ZsGreen1 mice where the green fluorescence labels the tanycytes (Pak et al., 2014) demonstrate that DAGLα is indeed present in tanycytes (Figure 1E).
Immunoelectron microscopy also demonstrated DAGLα immunoreactivity to be present in tanycyte cell bodies and processes in the ME. In the external zone of the ME, DAGLα-IR endfeet processes of β2-tanycytes were closely associated with axon terminals of hypophysiotropic neurons (Figures 1F and 1G). Triple-labeling immunofluorescence demonstrated that axon terminals containing both TRH immunoreactivity and punctate immunofluorescence labeling the CB1 immunoreactivity are closely associated to DAGLα-IR tanycyte processes (Figure 1H).
Endocannabinoids Tonically Inhibit TRH Release from the ME in Rats
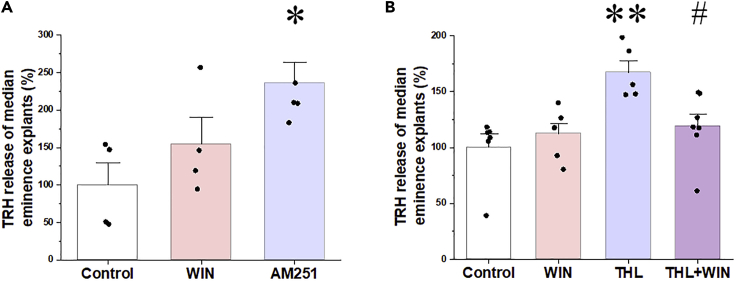
To determine whether endocannabinoids affect basal TRH release, ME explants were treated with the CB1 antagonist, AM251, or CB1 agonist, WIN55,212-2, in the presence of a TRH-degrading enzyme (TRH-DE) inhibitor. AM251 stimulated TRH release causing an approximately 2-fold increase in the TRH concentration of the ME explant supernatant (p < 0.05; Figure 2A). The CB1 agonist, however, had no effect (p = 0.48; Figure 2A). To test the hypothesis that the exogenous CB1 agonist was ineffective because of saturation of CB1 by its endogenous ligand, the effect of WIN55,212-2 was tested in the presence of the DAGLα inhibitor, tetrahydrolipstatin (THL). Treatment of the explants with THL caused an increase in TRH release (p < 0.01), whereas the CB1 agonist decreased THL-induced TRH release (p < 0.05; Figure 2B). These data demonstrate that endocannabinoids inhibit TRH release in the ME and there is a tonic, endocannabinoid-induced inhibition of TRH release in the ME explants.
Figure 2.
Effects of Pharmacological Manipulation of the Endocannabinoid System on TRH Release from Median Eminence Explants
(A) TRH recovered from median eminence explants incubated in ACSF containing the TRH-DE inhibitor, P-TRH (200 nM), was measured by RIA. WIN 55,212-2 (1 μM; N = 4) had no effect on the TRH release of the median eminence explants, whereas AM251 (1 μM; N = 5) induced a 2-fold increase of TRH release (NControl = 4).
(B) To determine whether the absence of CB1 agonist effect is due to saturation of CB1 by endogenous cannabinoids in the explants, the DAGLα inhibitor tetrahydrolipstatin (THL) was used to inhibit endocannabinoid synthesis. THL caused a marked increase of TRH release (N = 5). Although WIN 55,212-2 alone had no effect on TRH release (N = 6), it significantly decreased TRH release when 2-AG synthesis was blocked by THL (N = 7) indicating that a tonic endocannabinoid release inhibits TRH release from axons of the median eminence (NControl = 6). Data are presented as percentage of control group and as mean ± SEM (N = 5). Data were analyzed by one-way ANOVA and Tukey post hoc test. ∗ significantly different from control, ∗p < 0.05, ∗∗p < 0.01; # significantly different from THL treated group (p < 0.05). The amount of TRH released during 2 × 10 min by the control groups was 80 ± 4 pg and residual intracellular TRH was 1860 ± 40 pg/2 ME explants. There was no difference in residual intracellular TRH between control and drug-treated groups. Abbreviations: WIN, WIN55,212-2; THL, tetrahydrolipstatine.
β2-Tanycytes Express Glutamate Receptors and Glutamate Transporters in Mice
To determine whether glutamate and TRH released from the hypophysiotropic TRH axons influence β2-tanycytes, the expression of the glutamate receptor subunits, glutamate transporters, and TRH receptors was studied in β2-tanycyte cell bodies isolated by laser capture microdissection (Table 1). A high level of SLC1A3 (EAAC1/EAAT3) glutamate transporter mRNA and low level of SLC1A1 (GLAST/EAAT1) and SLC1A2 (GLT-1/EAAT2) mRNA was detected in β-tanycytes, whereas SLC1A6 (EAAT4) and SLC1A7 (EAAT5) glutamate transporters were not expressed.
Table 1.
Expression of Glutamate Receptors and Transporters in β-Tanycytes
| Expression in Beta Tanycytes (CTgeomean housekeeping genes - Ctgene ± SEM) | Short Name of the Gene | Description |
|---|---|---|
| Genes Expressed in Tanycytes | ||
| 5.52 ± 0.92 | SLC1A1 | Solute carrier family 1, member 1 |
| 4.50 ± 0.34 | SLC1A2 | Solute carrier family 1, member 2 |
| −0.15 ± 0.26 | SLC1A3 | Solute carrier family 1, member 3 |
| 2.89 ± 0.17 | GRIA1 | Glutamate receptor, ionotropic, AMPA1 (alpha 1) |
| 4.37 ± 0.51 | GRIA2 | Glutamate receptor, ionotropic, AMPA2 (alpha 2) |
| 5.97 ± 0.68 | GRIK2 | Glutamate receptor, ionotropic, kainate 2 (beta 2) |
| −0.59 ± 0.10 | GRIK3 | Glutamate receptor, ionotropic, kainate 3 |
| 5.81 ± 0.27 | GRIK4 | Glutamate receptor, ionotropic, kainate 4 |
| 2.57 ± 0.35 | GRIK5 | Glutamate receptor, ionotropic, kainate 5 (gamma 2) |
| 3.15 ± 0.09 | GRIN3A | Glutamate receptor ionotropic, NMDA3A |
| 5.29 ± 0.16 | GRM4 | Glutamate receptor, metabotropic 4 |
| 1.57 ± 0.25 | DIO2 | Deiodinase, iodothyronine, type II |
| 5.46 ± 0.31 | DAGLA | Diacylglycerol lipase, alpha |
| Genes Not Expressed in Tanycytes | ||
| 8.07 ± 0.61 | SLC1A6 | Solute carrier family 1, member 6 |
| UD | SLC1A7 | Solute carrier family 1, member 7 |
| 6.83 ± 1.68 | GRIA3 | Glutamate receptor, ionotropic, AMPA3 (alpha 3) |
| 6.85 ± 0.58 | GRIA4 | Glutamate receptor, ionotropic, AMPA4 (alpha 4) |
| 10.24 ± 0.33 | GRIK1 | Glutamate receptor, ionotropic, kainate 1 |
| 6.80 ± 0.75 | GRIN1 | Glutamate receptor, ionotropic, NMDA1 (zeta 1) |
| 6.88 ± 0.30 | GRIN2A | Glutamate receptor, ionotropic, NMDA2A (epsilon 1) |
| 6.54 ± 0.21 | GRIN2B | Glutamate receptor, ionotropic, NMDA2B (epsilon 2) |
| UD | GRIN2C | Glutamate receptor, ionotropic, NMDA2C (epsilon 3) |
| 8.26 ± 0.62 | GRIN2D | Glutamate receptor, ionotropic, NMDA2D (epsilon 4) |
| UD | GRIN3B | Glutamate receptor, ionotropic, NMDA3B |
| 11.77 ± 1.98 | GRM1 | Glutamate receptor, metabotropic 1 |
| UD | GRM2 | Glutamate receptor, metabotropic 2 |
| 6.88 ± 1.21 | GRM3 | Glutamate receptor, metabotropic 3 |
| 6.38 ± 0.73 | GRM5 | Glutamate receptor, metabotropic 5 |
| UD | GRM6 | Glutamate receptor, metabotropic 6 |
| 9.20 ± 0.27 | GRM7 | Glutamate receptor, metabotropic 7 |
| UD | GRM8 | Glutamate receptor, metabotropic 8 |
| 8.08 ± 0.41 | TRHR1 | Thyrotropin-releasing hormone receptor 1 |
| 6.86 ± 1.59 | TRHR2 | Thyrotropin-releasing hormone receptor 2 |
Among the AMPA receptor subunits, mRNA of GRIA1 and GRIA2 was observed. GRIK3 was the kainite subunit with the highest expression, followed by GRIK2,4,5 that, although low, was still detectable. GRIN3A was the only NMDA receptor subunit and GRM4 the only metabotropic glutamate receptor detected in β2-tanycytes, but both had relatively low expression level. Expression of TRH receptors was not detected in the β2-tanycytes.
These data suggest that glutamate effects on β2-tanycytes occur primarily via AMPA and kainate receptors and the glutamate transporter, SLC1A3.
Glutamate Induces Depolarization of the Membrane Potential of β2-Tanycytes in Mice
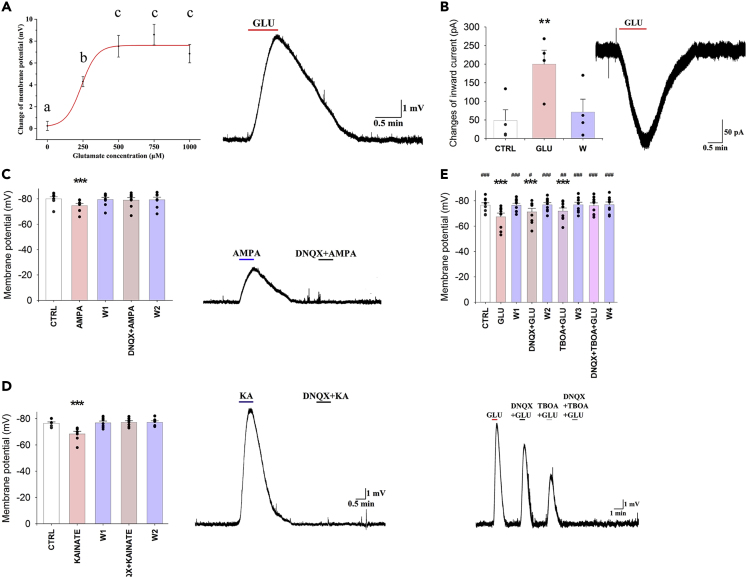
To elucidate the effect of glutamate on the β2-tanycytes, patch clamp electrophysiology was performed. The membrane potential of the β2-tanycytes was −77.52 ± 0.47 mV (n = 83) under control conditions. Four different glutamate concentrations were applied to the cells, each causing dose-dependent depolarization of β2-tanycytes (glutamate treatments induced change of membrane potential: control: 0.25 ± 0.42 mV, n = 6; 250 μM: 4.29 ± 0.46 mV, n = 11, p = 0.038; 500 μM: 7.53 ± 0.99 mV, n = 12, p < 0.001; 750 μM: 8.59 ± 0.94 mV, n = 8, p < 0.001; and 1,000 μM: 6.85 ± 0.83 mV, n = 6, p < 0.001; Figure 3A). Treatment with 250 μM glutamate caused a significant depolarization, but significantly less than the treatment with 500 μM glutamate (p = 0.049). As 500 μM had similar effect as that of 750 and 1,000 μM concentrations, 500 μM was selected for further studies.
Figure 3.
Glutamate Depolarizes the β2-Tanycytes via Activation of AMPA and Kainate Receptors and via Glutamate Transport
Representative traces illustrate the effects of pharmacological treatments on the β2-tanycytes.
(A) Glutamate induced a dose-dependent depolarization of β2-tanycytes (NControl = 6; N250μM = 11; N500μM = 12; N750μM = 8; N1000μM = 6). A representative trace illustrates the change of the membrane potential of a β2-tanycyte in response to 500 μM glutamate.
(B–D) (B) Glutamate (500 μM; N = 4) evoked a large inward current on β2-tanycyte outside-out preparations, demonstrating that glutamate directly influences the tanycytes. Similar to glutamate, both (C) AMPA (100 μM; N = 8) and (D) kainate (125 μM; N = 7) depolarized the β2-tanycytes, and the effects prevented by the administration of the AMPA and kainate receptor antagonist, DNQX (500 μM).
(E) Although DNQX (500 μM; N = 9) and TBOA (1 mM; N = 9) caused significant, but only partial, reduction of the glutamate-induced depolarization, the combination of the two inhibitors completely blocked the effect of glutamate (N = 9), indicating that the effect of glutamate on the membrane potential of β2-tanycytes is mediated via AMPA and kainate receptors and by TBOA-sensitive glutamate transport. Data are expressed as mean ± SEM and were analyzed with repeated measure ANOVA followed by Bonferroni post hoc test.
Data with different letters on (A) are significantly different (p < 0.05). ∗ significantly different from control; # significantly different from glutamate treatment. #p < 0.05; ∗∗ and ##p < 0.01; ∗∗∗ and ###p < 0.001. Abbreviations: CTRL, control; GLU, glutamate; KA, kainate;W, washout. See also Figures S2 and S3.
To exclude the possibility that the effect of glutamate was mediated by tetrodotoxin (TTX; a sodium-channel blocker aimed to inhibit action potential) insensitive release of transmitters from non-tanycyte cell types of the ME, an outside-out patch clamp experiment was performed. Glutamate treatment caused large inward currents (−181.29 ± 28.43 pA, n = 4, p < 0.01) (Figure 3B) even when the tanycyte cell body was displaced into the third ventricle. The effect of glutamate disappeared during the washout period, indicating that the observed effect of glutamate was exerted directly on the β2-tanycytes.
In contrast to glutamate, TRH had no effect on the membrane potential of tanycytes even in a relatively high concentration (1μM; N = 12; Figure S2).
Functional AMPA, Kainate, and GRM4 Receptors Are Present on β2-Tanycytes in Mice
To investigate the receptor types that mediate glutamate-induced depolarization of β2-tanycytes, the effects of glutamate receptor agonists were studied. Similar to glutamate, both AMPA (100 μM; 5.37 ± 1.09 mV; n = 8, p < 0.001; Figure 3C) and kainate (125 μM; 8.04 ± 2.08 mV, n = 7, p < 0.001; Figure 3D) markedly depolarized the β-tanycytes. Bath application of the AMPA and kainate receptor antagonist, DNQX (500 μM), prevented these effects (Figures 3C and 3D).
In contrast to AMPA and kainate, NMDA had no effect on the membrane potential of tanycytes, even at high concentration (0.5 mM: 1.41 ± 0.65 mV, n = 3, p = 0.42 and 4 mM: 2.12 ± 0.3 mV, n = 3, p = 1.00. Figure S3). Administration of the GRM4 agonist VU 0155041 caused a small, but significant, hyperpolarization (1 mM; −1.48 ± 0.54 mV, n = 10, p < 0.01) of the membrane potential of β2-tanycytes (Figure S3F) indicating that it is unlikely that activation of GRM4 contributes to the glutamate-induced depolarization of tanycytes.
Glutamate Depolarized the β2-Tanycytes via Both AMPA and Kainate Receptors and Also by TBOA-Sensitive Glutamate Transport in Mice
To determine whether the effect of glutamate on the membrane potential of β2-tanycytes is mediated exclusively via AMPA and kainate receptors, hypothalamic slices were treated with glutamate in the presence of DNQX. Inhibition of the kainate and AMPA receptors caused a significant but only partial inhibition of the β2-tanycytes (glutamate: 9.18 ± 1.55 mV, n = 9, p < 0.001 versus control; glutamate + DNQX: 5.23 ± 1.24 mV, p < 0.001 versus control and p = 0.019 versus glutamate; Figure 3F). Therefore, we determined whether glutamate transport also contributes to the glutamate-induced depolarization. Similar to DNQX, the glutamate transporter inhibitor TBOA caused a partial inhibition of the glutamate-induced depolarization (glutamate + TBOA: 4.68 ± 0.91 mV, n = 9, p = 0.001 versus control and p = 0.002 vs. glutamate; Figure 3E). However, the combination of DNQX and TBOA completely blocked the effect of glutamate (0.40 ± 0.61 mV, n = 9, p = 1.00 versus control and p < 0.001 vs. glutamate; Figure 3E), demonstrating that glutamate depolarizes the tanycytes via a combination of AMPA and kainate receptor-mediated effects and glutamate transport.
The Effect of TRH Axon Activation on Tanycytes Is Mediated Partially by Glutamate in Mice
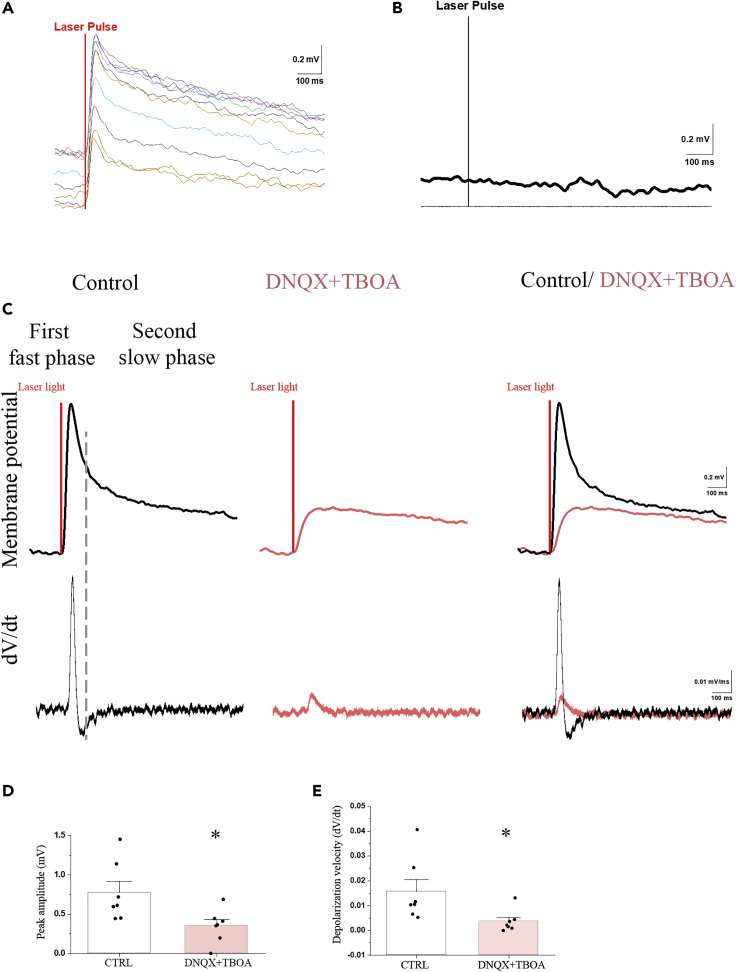
Optogenetic activation of the axon terminals of the TRH neurons around the endfeet processes of β2-tanycytes in the external zone of the median eminence consequently caused a 0.75 ± 0.14 mV (p < 0.001) depolarization of the patched β2-tanycyte cell bodies in the ventricular wall of the ME (Figure 4). The peak of the depolarization was reached 51.74 ± 2.93 ms after the start of the optogenetic activation; the velocity of this depolarization was 0.016 ± 0.005 mV/ms. Examination of the first derivative (dV/dt) of the membrane potential showed two peaks, suggesting that the repolarization has two phases (Figure 4C). The fast depolarization was followed by a fast −0.29 ± 0.09 mV repolarization with 126.66 ± 10.73 ms decay time and then by a very slow repolarization (decay time: 3,127.60 ± 446.29 ms). This suggested that the TRH axons may influence tanycytes by the release of at least two different compounds: a fast-acting transmitter and a transmitter with long-lasting effect. Simultaneous administration of DNQX and TBOA markedly decreased the optogenetic activation-induced depolarization of tanycytes (0.32 ± 0.08 mV; p < 0.01; Figure 4). The velocity of this depolarization was significantly lower than the velocity of depolarization induced by glutamate alone (0.004 ± 0.002; p < 0.05; Figure 4E). The first derivative (dV/dt) of the membrane potential had only one peak (Figure 4C) in the presence of antagonists, indicating that this effect has only one phase. The two antagonists prevented the first fast phase. These data demonstrate that the TRH axons influence the tanycytes with a fast-acting transmitter, glutamate, and also by a currently unknown transmitter(s) with longer-lasting effect.
Figure 4.
The Optogenetic Activation of TRH Axons in the ME Induces Depolarization of β2-Tanyctes Which Effect Is Partially Mediated by Glutamate
(A and B) The membrane potential changes of a representative tanycyte in response to 10 consecutive optic activation of TRH axons (A). The membrane potential of tanycytes is not influenced by the light impulse if the TRH axons do not express channelrhodopsin (B).
(C-E) (C) The mean response of the tanycyte membrane potential in response to 10 sweeps of optic stimulation of TRH axons. The upper traces show the membrane potential changes of the tanycyte under control conditions (black line), when the same cell was treated with a combination of DNQX (0.5 μM) and TBOA (1 mM) (red) and the overlay of the two traces. The lower graphs illustrate the first derivative (dV/dt) of the membrane potential changes. The two peaks of the dV/dt of the control trace suggest that the optic stimulation-induced membrane potential change has two phases under control conditions: an initial fast phase including a fast depolarization and a fast repolarization followed by a long-lasting phase of slow repolarization. When inhibitors are applied, the membrane potential change has only a single phase and markedly decreased amplitude. The overlay of the two traces indicates that the speed of depolarization is markedly decreased in the presence of DNQX and TBOA. Bar graphs summarize the effects of activation of TRH axons on the peak amplitude of membrane potential (D) and the depolarization velocity (E) under control condition and when the cells are treated with DNQX + TBOA.
Data are expressed as mean ± SEM and were analyzed with paired Student's t test. ∗ significantly different (p < 0.05).
Optic stimulation of slices from TRH-IRES-tdTomto mice where the TRH axons expressed tdTomato (but did not express channelrhodopsin) had no effect on the membrane potential of tanycytes (Figure 4B).
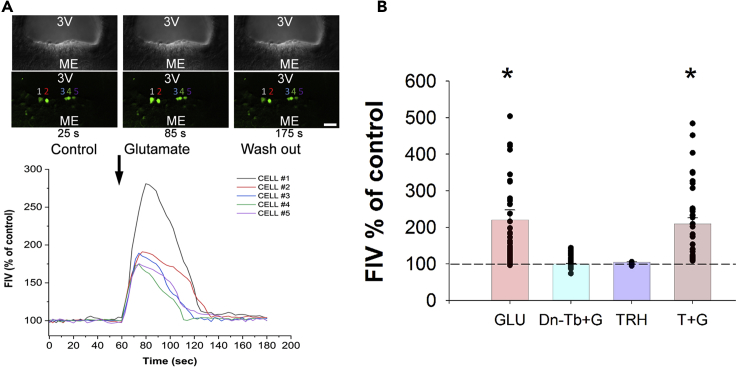
Effect of Glutamate and TRH on the Intracellular Ca2+ Levels of Tanycytes in Mice
As increase of intracellular Ca2+ levels is a crucial signal to increase DAGlα activity (Piomelli, 2003), the effect of glutamate was studied on the intracellular Ca2+ level of β2-tanycytes. Glutamate treatment (500 μM) caused a robust increase of fluorescent intensity values (FIV) of β2-tanycytes (baseline: 189.19 ± 26.52 treatment: 416.13 ± 31.28; p < 0.001; Ncell = 56) increasing it to 219.95 ± 27.66% of the baseline values (Figure 5). To determine whether similar to the effect of glutamate on the membrane potential of tanycytes, the effect of glutamate on the intracellular Ca2+ level can also be blocked by inhibition of AMPA and kainite receptors and glutamate transport, we administered DNQX (0.5 mM) and TBOA (1 mM) during the glutamate treatment. Application of these inhibitors completely prevented the increase of FIV (baseline: 242.13 ± 28.06 treatment: 250.27 ± 28.67; Ncell = 79; p = 0.99) (Figure 5).
Figure 5.
Effects of Glutamate and TRH Treatment on the Intracellular Ca2+ Level of β2-Tanycytes
The changes of fluorescent intensity values (FIV) of the Ca2+-sensitive dye Fluo-4 AM was measured as a marker of the changes of intracellular Ca2+ level of β2-tanycytes after different treatments in vitro.
(A) Representative recording shows that glutamate treatment (500 μM) increases FIVs in the measured β2-tanycyte perikarya. Black arrow represents the beginning of the glutamate treatment.
(B) Bar graph summarizes effects of different treatments. GLU, glutamate (500 μM, N = 56); Dn-Tb + G, DNQX + TBOA + GLU (DNQX + TBOA, 0.5 mM and 1 mM, respectively; N = 79); TRH (5 μM, N = 34); T + G, TRH + glutamate (5 and 500 μM, respectively; N = 37). Only glutamate and TRH + glutamate treatment increased FIV% values significantly (p < 0.001 and p = 0.002, respectively). TRH alone could not initiate any significant changes. The effects of Glutamate alone and the combined Glutamate + TRH treatment did not differ from each other (p = 0.999). Treatment of sections with DNQX + TBOA prevented the increase of intracellular Ca2+ level induced by glutamate treatment (DNQX + TBOA + GLU versus control: p = 0.999). Data are shown as mean ± SEM, for statistical comparison one-way ANOVA (F (4, 235) = 15.849; p < 0.0001) was used followed by Bonferroni post hoc test. ∗ significantly different from control p < 0.05. Scale bar, 50 μm.
To test whether the main transmitter of the hypophysiotropic TRH neurons can influence the intracellular Ca2+ level of β2 tanycytes, the effects of TRH and its combination with glutamate were tested (Figure 5). TRH treatment (5 μM) caused no significant changes either in the FIV of β2-tanycytes (control: 187.46 ± 30.58 treatment: 186.71 ± 30.42) or in the percentage values of FIV (99.79 ± 0.30%) when compared with the control, baseline values (p = 0.9; Ncell = 34).
Treatment of β2-tanycytes with the combination of TRH + Glutamate (5 and 500 μM, respectively) caused a robust increase of FIV (control: 154.70 ± 26.09, treatment: 323.85 ± 34.68) increasing it to 209.34 ± 16.88% of the baseline values (p = 0.002; Ncell = 37). The effect of combined TRH + glutamate treatment, however, did not differ from the effect of treatment with glutamate alone (p = 0.99).
A positive allosteric modulator of GRM4 receptor VU0155041 (1.5 mM) caused no significant changes either in the FIV of β2-tanycytes (control: 76.01 ± 16.56, treatment: 77.13 ± 16.43) or the percentage values (102.90 ± 1.39%) when compared with the control, baseline values (p = 0.9; Ncell = 21).
Effect of the Inhibition of Glutamate Action on the 2-AG Content of the Rat Median Eminence Explants
To determine whether endogenous glutamate can stimulate the 2-AG synthesis of tanycytes, the effect of glutamate receptor and transporter inhibitors was studied on the 2-AG content of ME explants. The 2-AG content of control ME explants was readily detected (0.54 ± 0.08 ng/mg tissue). Simultaneous inhibition of AMPA and kainate receptors and the TBOA-sensitive glutamate transporters caused an approximately 50% decrease of the 2-AG content of the ME explants (0.29 ± 0.03 ng/mg tissue; p = 0.01) suggesting that endogenous glutamate stimulates 2-AG synthesis in tanycytes.
Discussion
Endocannabinoids are well known as retrograde transmitters used by neurons to control their own neuronal inputs (Mayer et al., 1984, Ohno-Shosaku and Kano, 2014). In the current manuscript, however, we demonstrate that the β-tanycytes of the ME also utilize endocannabinoids to regulate TRH release from hypophysiotropic neurons.
To establish the importance of the endocannabinoid system in regulating hypophysiotropic TRH neurons, we demonstrated that the majority of TRH neurons in the PVN expresses CB1 mRNA and that CB1 protein is present on the axon varicosities of the hypophysiotropic TRH neurons that terminate around the portal capillaries in the external zone of the ME. This observation is especially intriguing as only very few neuronal perikarya are present in the ME and the majority are located in the subependymal zone (Rethelyi, 1975). Thus, neurons are virtually absent from the external zone of the ME. As endocannabinoids can travel only extremely short distances in the neuropil before degradation (Regehr et al., 2009), the lack of neurons in the external zone of the ME excludes a neuronal origin of endocannabinoids to act on CB1 on hypophysiotropic axon terminals.
The main endocannabinoid that acts on CB1 is 2-AG (Tanimura et al., 2010) and synthesized by DAGLα (Tanimura et al., 2010). DAGLα-immunoreactivity was observed in cell bodies and basal processes of β-tanycytes in the ME. Immunoelectron microscopy further established the presence of DAGLα in β-tanycytes and demonstrated its location not only in tanycyte cell bodies, but also in their basal processes with high concentrations in the endfeet processes around portal capillaries. This is in contrast to the data of Suarez et al. (2010), who observed DAGLα immunoreactivity only in the apical part of tanycyte cell bodies. To determine whether the reason of this discrepancy may be species difference, DAGLα immunostaining was performed in the ME of rats. Similar to the observation of Suarez et al. (2010), we observed strong DAGLα immunoreactivity in the cell bodies of tanycytes in the ME, and we also observed DAGLα immunoreactivity in the basal processes of tanycytes, indicating that DAGLα is present in tanycyte processes in both species. The absence of DAGLα immunoreactivity in the ME of DAGLα knockout (KO) mice clearly demonstrates the specificity of our findings. We observed CB1-IR varicosities closely associated to DAGLα-IR tanycyte processes in the external zone of the ME and, specifically, CB1-IR TRH varicosities in juxtaposition to DAGLα-IR tanycyte processes. Thus, tanycytes are in position to regulate TRH release from hypophysiotropic axon terminals in the ME.
In agreement with the morphological findings, the CB1 antagonist, AM251, increased the amount of TRH released from ME explants indicating a tonic inhibitory effect of endocannabinoids on the release of TRH. Surprisingly, incubation with the CB1 agonist WIN55,212-2 had no effect on TRH release from ME explants. However, when endocannabinoid synthesis was inhibited by THL, TRH release from the explants increased and this was prevented by WIN55,212-2. We presume that tanycytes may tonically release high concentrations of endocannabinoids in ME explants that are sufficient to saturate CB1 receptors in hypophysiotropic TRH terminals; therefore, a further increase in agonist activity by WIN55,212-2 would not be expected to influence TRH release. This hypothesis is supported by the observation that, when the synthesis of endocannabinoids is blocked in the ME explants, the lower occupancy of CB1 receptors permits CB1 agonist-induced inhibition of TRH release, further demonstrating the key and dynamic role of tanycytes in the regulation of the HPT axis.
In contrast to the inhibitory effect of endocannabinoids on the TRH release, PGE2 has been shown to cause retraction of tanycyte processes allowing access of hypophysiotropic axons to capillaries, thus facilitating hormone release (de Seranno et al., 2010). Intriguingly, the synthesizing enzymes of PGE2, cyclooxygenase (COX)-1 and COX-2, are synthesized in tanycytes (de Seranno et al., 2010), and arachidonic acid (AA) is similarly necessary for the synthesis of endocannabinoids and PGE2 (Malcher-Lopes and Buzzi, 2009). In the immune system, humoral signals can shift the balance between the endocannabinoid and prostaglandin system that plays, for example important role in the mediation of the effects of glucocorticoids (Malcher-Lopes and Buzzi, 2009). Thus, it is feasible to hypothesize that humoral signals may also regulate the balance of these two antagonistic signaling systems in the tanycytes to cause simultaneous regulation of hormone release and capillary access of hypophysiotropic terminals. However, this hypothesis requires further studies.
In neuronal circuits, glutamate is an important driving force of endocannabinoid synthesis (Piomelli, 2003). Presynaptically released glutamate binds to Grm1 and Grm5, and the activation of these metabotropic glutamate receptors stimulate the DAGLα via an increase in intracellular Ca2+ levels (Katona et al., 2006, Piomelli, 2003). In the external zone of the ME, there are no synapses between TRH axons and tanycyte processes, but glutamate that is released from the hypophysiotropic TRH axons (Hrabovszky et al., 2005) into the extracellular space is well positioned to influence tanycytes due to the close juxtaposition of the two cells. Our in situ hybridization data indicated that Grm1 and Grm5 are not present in tanycytes, but kainate and AMPA receptor subunits have been previously described in β-tanycytes (Kawakami, 2000, Eyigor and Jennes, 1998). In agreement with these findings, we detected the GRIA1 and GRIA2 AMPA receptor subunits and the GRIK3 kainate receptor subunit as the glutamate receptor subunits expressed in β-tanycytes. The GRIK2, GRIK4, and GRIK5 kainate receptor subunits and mGLU4 are also expressed, but at a much lower level. In contrast, expression of NMDA and the other metabotropic glutamate receptor subunits was barely detectable.
The presence of glutamate receptor subunits in β-tanycytes strongly suggested that glutamate influences β-tanycytes. Indeed, patch clamp electrophysiology experiments showed that the administration of glutamate dose-dependently depolarizes β-tanycytes. Similarly, kainate and AMPA also depolarized β-tanycytes, suggesting the importance of AMPA and/or kainate receptors in the mediation of the glutamate-induced regulation of tanycytes. Inhibition of these receptors, however, only partially inhibited glutamate-induced depolarization. Administration of GRM4 agonist caused a small hyperpolarization of β-tanycytes excluding the possibility that this receptor could be involved in the mediation of glutamate-induced depolarization of tanycytes. Since in addition to the receptor-mediated effect, glutamate also causes depolarization by uptake of glutamate via specific glutamate transporters (Kim et al., 2008), we tested the presence of glutamate transporters in β-tanycytes. A high level of expression of the glutamate transporter SLC1A3 was observed in β-tanycytes. Accordingly, simultaneous inhibition of AMPA and kainate receptors and glutamate transporters completely blocked the glutamate-induced depolarization of β-tanycytes. Thus, the two ionotropic glutamate receptor types as well as the glutamate transporter are critical for the glutamate-induced regulation of this cell type.
To determine whether hypophysiotropic TRH axons can influence the β-tanycytes by glutamate release, an optogenetic experiment was performed. Using combined optogenetic activation of the hypophysiotropic TRH axons and the patch clamp recording of β2-tanycyte cell bodies, we showed that TRH axon activation causes a biphasic depolarization of tanycytes. The fast component of this depolarization can be prevented by administration of DNQX and TBOA demonstrating that the fast component of this depolarization is caused by glutamate release of the TRH axons. The slower and more prolonged component of the tanycyte depolarization was not influenced by glutamate inhibitors suggesting that the TRH axons also use other transmitters to influence tanycytes.
As peptides could have longer-lasting effect compared with glutamate and the work by Muller-Fielitz et al. (2017) showed that TRH increases the intracellular Ca2+level of β-tanycytes via TRHR1, we hypothesized that this long-lasting effect could be due to the TRH release of hypophysiotropic axons. Owing to the lack of TRH antagonists, we tested the effect of TRH on the membrane potential of tanycytes. Administration of a relatively high TRH dose (1 μM), however, had no effect on the membrane potential of tanycytes. TRH also did not have effect on the intracellular Ca2+ level of tanycytes, and we could not detect expression of any TRH receptor in the transcriptome of tanycytes isolated by laser capture microdissection. Explanation for the discrepancy between these two observations is uncertain, but we note that Muller-Fielitz et al. (2017) used an extremely high, pharmacological dose of TRH (30 μM) in their studies. Our data, however, exclude the possibility that activation of TRH axons causes the long-lasting depolarization of tanycytes via release of TRH. Further studies are needed to determine the mechanism of this effect.
The activation of TRH axons resulted in less depolarization of tanycytes than the administration of glutamate. We have to consider, however, that exogenous glutamate can act on the entire surface of tanycytes including their cell body, whereas transmitters released from the TRH axons can act only on the endfeet processes of tanycytes that are located far, approximately 80–100 μm, from the tanycyte cell bodies where the membrane potential was measured.
How depolarization regulates glial endocannabinoid synthesis is yet unknown, but an increase in intracellular Ca2+ level is a well-known activator of DAGLα (Katona et al., 2006). Using Ca2+ imaging, we demonstrated that glutamate not only depolarizes β-tanycytes, but also increases the intracellular Ca2+ level of these cells. This effect of glutamate was also blocked by inhibition of the AMPA and kainite receptors and glutamate transport indicating that glutamate influences the membrane potential and the intracellular Ca2+ level of tanycytes via similar mechanisms. The effect of glutamate on the intracellular Ca2+ level of tanycytes suggests that glutamate can stimulate the endocannabinoid synthesis of tanycytes. Indeed, inhibition of the AMPA and kainite receptors together with inhibition of glutamate transport markedly decreased the 2-AG content of the ME explants demonstrating that endogenous glutamate has a stimulatory effect on the 2-AG synthesis in the ME.
Regulation of TRH release by this novel neuroglial microcircuit utilizing endocannabinoids and glutamate may be important for regulating pulsatile TRH release by synchronizing the activity of hypophysiotropic axon terminals. However, in addition to the TRH-containing CB1-IR terminals, CB1 immunoreactivity is also present in the external zone of the ME in hypophysiotropic terminals other than the axons of hypophysiotropic TRH neurons, suggesting that regulation of hypophysiotropic terminals by tanycyte-derived endocannabinoids may play a more generalized role in the control of the neuroendocrine systems.
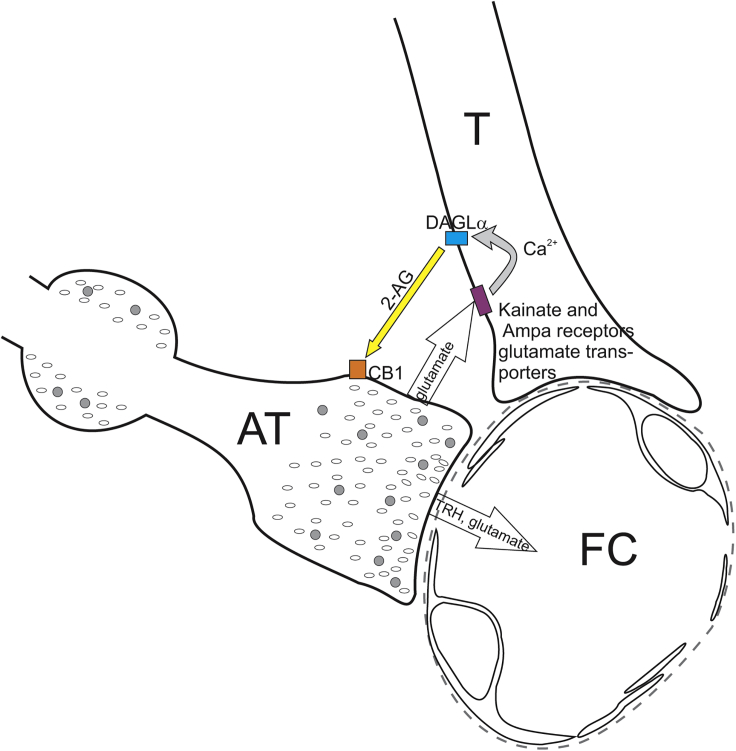
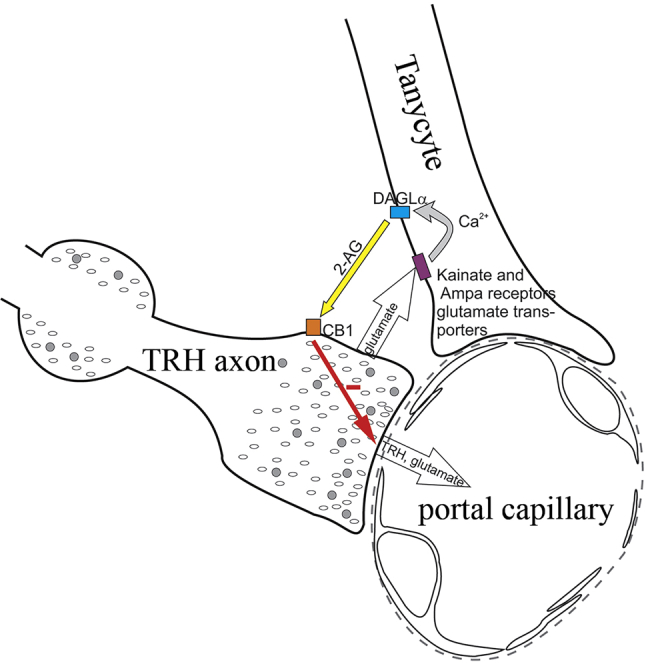
In summary, these data demonstrate that a regulatory microcircuit exists between β-tanycytes and hypophysiotropic TRH axons, utilizing the release of endocannabinoids and glutamate (Figure 6). This circuit may contribute to controlling the release of TRH into the ME and may be an important mechanism to synchronize the activity of hypophysiotropic terminals.
Figure 6.
Schematic Illustration of the Neuro-glial Microcircuit in the External Zone of the ME
Axon terminals (AT) of the hypophysiotropic TRH neurons are closely associated to the endfeet processes of tanycytes (T) in the vicinity of fenestrated capillaries (FC) of the hypophyseal portal circulation. The TRH axons release glutamate that stimulates the DAGLα activity of tanycytes, and therefore the 2-AG synthesis of these cells, by acting through kainite and AMPA receptors and glutamate transport and by the resulting increase of intracellular Ca2+ level. The released endocannabinoids bind to the CB1 of the hypophysiotropic TRH axons and inhibit the amount of TRH released into the portal capillary.
Limitations of the Study
Although the morphological and electrophysiological experiments were done in mice, the explant experiments had to be performed in a different species, in rats, because of the very small volume of the mouse median eminence.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgment
This work was supported by Grants from the Hungarian Science Foundation (OTKA K109710), the Hungarian National Brain Research Program (2017-1.2.1-NKP-2017-00002), EU H2020 THYRAGE no. 666869, CONACYT-Mexico (CB2015/254960), ERC682426, KFI-2016-0177, GINOP-2016-00979, NVKP-2016-0043 and by the BME-Biotechnology FIKP grant of EMMI (BME FIKP-BIO). The authors express their gratitude to Zsófia László for her help during the preparing of some of the confocal microscopic images.
Author Contributions
E.F. performed the immunofluorescent and ultrastructural studies. E.V. and B.K. designed and performed the patch clamp experiments. B.K. performed the optogenetic experiments. Z.P. deigned and performed the calcium imaging experiments. A.C.-V., J.-L.C., P.J.-B., A.S.-S., and Y.R. designed and performed the ME explant experiments and analyzed these data. M.M. synthesized, purified, and chemically characterized P-TRH. B.T. performed the 2-AG measurement. M.T. performed and analyzed the in situ hybridization experiments. F.E., Z.M., G. S., and B.G. were involved in the generation of TRH-IRES-Cre mice. A.S.-S. and D.K. isolated the tanycytes and performed the gene expression analysis. D.Z. performed the stereotaxic AAV injections. A.K. performed immunostaining. Zs.M. and B.R. helped to design and perform the calcium imaging experiments and analyzed data. M.W. provided CB1 antibody. M.K. provided tissues of DAGLα KO mice. K.M. provided DAGLα antibody. R.M.L. analyzed data and wrote the manuscript. C.F. acquired the funding, conceptualized and interpreted the studies, and wrote the manuscript.
Declaration of Interests
B.R. is one of the founders of Femtonics and is a member of its scientific advisory board. The other authors have declared that no conflict of interest exists.
Published: March 27, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.100921.
Supplemental Information
References
- de Seranno S., D'anglemont De Tassigny X., Estrella C., Loyens A., Kasparov S., Leroy D., Ojeda S.R., Beauvillain J.C., Prevot V. Role of estradiol in the dynamic control of tanycyte plasticity mediated by vascular endothelial cells in the median eminence. Endocrinology. 2010;151:1760–1772. doi: 10.1210/en.2009-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyigor O., Jennes L. Identification of kainate-preferring glutamate receptor subunit GluR7 mRNA and protein in the rat median eminence. Brain Res. 1998;814:231–235. doi: 10.1016/s0006-8993(98)01056-7. [DOI] [PubMed] [Google Scholar]
- Fekete C., Lechan R.M. Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr. Rev. 2014;35:159–194. doi: 10.1210/er.2013-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete C., Sarkar S., Christoffolete M.A., Emerson C.H., Bianco A.C., Lechan R.M. Bacterial lipopolysaccharide (LPS)-induced type 2 iodothyronine deiodinase (D2) activation in the mediobasal hypothalamus (MBH) is independent of the LPS-induced fall in serum thyroid hormone levels. Brain Res. 2005;1056:97–99. doi: 10.1016/j.brainres.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Freitas B.C., Gereben B., Castillo M., Kallo I., Zeold A., Egri P., Liposits Z., Zavacki A.M., Maciel R.M., Jo S. Paracrine signaling by glial cell-derived triiodothyronine activates neuronal gene expression in the rodent brain and human cells. J. Clin. Invest. 2010;120:2206–2217. doi: 10.1172/JCI41977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabovszky E., Wittmann G., Turi G.F., Liposits Z., Fekete C. Hypophysiotropic thyrotropin-releasing hormone and corticotropin-releasing hormone neurons of the rat contain vesicular glutamate transporter-2. Endocrinology. 2005;146:341–347. doi: 10.1210/en.2004-0856. [DOI] [PubMed] [Google Scholar]
- Kano M., Ohno-Shosaku T., Hashimotodani Y., Uchigashima M., Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Katona I., Urban G.M., Wallace M., Ledent C., Jung K.M., Piomelli D., Mackie K., Freund T.F. Molecular composition of the endocannabinoid system at glutamatergic synapses. J. Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami S. Glial and neuronal localization of ionotropic glutamate receptor subunit-immunoreactivities in the median eminence of female rats: GluR2/3 and GluR6/7 colocalize with vimentin, not with glial fibrillary acidic protein (GFAP) Brain Res. 2000;858:198–204. doi: 10.1016/s0006-8993(00)01980-6. [DOI] [PubMed] [Google Scholar]
- Kim M.H., Uehara S., Muroyama A., Hille B., Moriyama Y., Koh D.S. Glutamate transporter-mediated glutamate secretion in the mammalian pineal gland. J. Neurosci. 2008;28:10852–10863. doi: 10.1523/JNEUROSCI.0894-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechan R.M., Fekete C. Infundibular tanycytes as modulators of neuroendocrine function: hypothetical role in the regulation of the thyroid and gonadal axis. Acta Biomed. 2007;78(Suppl 1):84–98. [PubMed] [Google Scholar]
- Malcher-Lopes R., Buzzi M. Glucocorticoid-regulated crosstalk between arachidonic acid and endocannabinoid biochemical pathways coordinates cognitive-, neuroimmune-, and energy homeostasis-related adaptations to stress. Vitam Horm. 2009;81:263–313. doi: 10.1016/S0083-6729(09)81011-X. [DOI] [PubMed] [Google Scholar]
- Mayer M.L., Westbrook G.L., Guthrie P.B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Muller-Fielitz H., Stahr M., Bernau M., Richter M., Abele S., Krajka V., Benzin A., Wenzel J., Kalies K., Mittag J. Tanycytes control the hormonal output of the hypothalamic-pituitary-thyroid axis. Nat. Commun. 2017;8:484. doi: 10.1038/s41467-017-00604-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T., Kano M. Endocannabinoid-mediated retrograde modulation of synaptic transmission. Curr. Opin. Neurobiol. 2014;29:1–8. doi: 10.1016/j.conb.2014.03.017. [DOI] [PubMed] [Google Scholar]
- Pak T., Yoo S., Miranda-Angulo A.L., Wang H., Blackshaw S. Rax-CreERT2 knock-in mice: a tool for selective and conditional gene deletion in progenitor cells and radial glia of the retina and hypothalamus. PLoS One. 2014;9:e90381. doi: 10.1371/journal.pone.0090381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat. Rev. Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Prevot V., Dehouck B., Sharif A., Ciofi P., Giacobini P., Clasadonte J. The versatile tanycyte: a hypothalamic integrator of reproduction and energy metabolism. Endocr. Rev. 2018;39:333–368. doi: 10.1210/er.2017-00235. [DOI] [PubMed] [Google Scholar]
- Regehr W.G., Carey M.R., Best A.R. Activity-dependent regulation of synapses by retrograde messengers. Neuron. 2009;63:154–170. doi: 10.1016/j.neuron.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rethelyi M. Neurons in the subependymal layer of the rat median eminence. Neuroendocrinology. 1975;17:330–339. doi: 10.1159/000122371. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Rodriguez A., Lazcano I., Sanchez-Jaramillo E., Uribe R.M., Jaimes-Hoy L., Joseph-Bravo P., Charli J.L. Tanycytes and the control of thyrotropin-releasing hormone flux into portal capillaries. Front. Endocrinol. (Lausanne) 2019;10:401. doi: 10.3389/fendo.2019.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez E.M., Blazquez J.L., Pastor F.E., Pelaez B., Pena P., Peruzzo B., Amat P. Hypothalamic tanycytes: a key component of brain-endocrine interaction. Int. Rev. Cytol. 2005;247:89–164. doi: 10.1016/S0074-7696(05)47003-5. [DOI] [PubMed] [Google Scholar]
- Sanchez E., Vargas M.A., Singru P.S., Pascual I., Romero F., Fekete C., Charli J.L., Lechan R.M. Tanycyte pyroglutamyl peptidase II contributes to regulation of the hypothalamic-pituitary-thyroid axis through glial-axonal associations in the median eminence. Endocrinology. 2009;150:2283–2291. doi: 10.1210/en.2008-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez J., Romero-Zerbo S.Y., Rivera P., Bermudez-Silva F.J., Perez J., De Fonseca F.R., Fernandez-Llebrez P. Endocannabinoid system in the adult rat circumventricular areas: an immunohistochemical study. J. Comp. Neurol. 2010;518:3065–3085. doi: 10.1002/cne.22382. [DOI] [PubMed] [Google Scholar]
- Tanimura A., Yamazaki M., Hashimotodani Y., Uchigashima M., Kawata S., Abe M., Kita Y., Hashimoto K., Shimizu T., Watanabe M. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron. 2010;65:320–327. doi: 10.1016/j.neuron.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Wittmann G., Deli L., Kallo I., Hrabovszky E., Watanabe M., Liposits Z., Fekete C. Distribution of type 1 cannabinoid receptor (CB1)-immunoreactive axons in the mouse hypothalamus. J. Comp. Neurol. 2007;503:270–279. doi: 10.1002/cne.21383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.