Abstract
Over the last decade, immunotherapy has rapidly changed the therapeutic landscape and prognosis for many hematologic malignancies and adult solid tumors. Despite this success, immunotherapy for pediatric solid tumors remains in the early stages of development, and significant clinical benefit has yet to be realized, with anti-GD2 for neuroblastoma being the exception. The limited neoepitope expression and paucity of T-cell infiltration into the immunosuppressive tumor microenvironment have hampered current established immunotherapies. Emerging approaches to recruit T cells, to convert phenotypically “cold” into “inflamed” tumors, and to vastly improve therapeutic indices hold exceptional promise. Here, we review these approaches, highlighting the role of the tumor microenvironment and novel antibody platforms to maximize the full clinical potential of immunotherapy in pediatric oncology.
Keywords: immunotherapy, childhood cancers, antibody therapy
Introduction
Advances in molecular profiling and next-generation sequencing have jumpstarted successful targeted therapies for adult solid tumors. Such genomics-guided interventions have not been as successful in pediatric solid tumors, largely due to their low tumor mutational burden (TMB) and limited number of actionable or targetable mutations (1). Despite the accelerating pace of immunotherapy in the last decade for adult tumors, low TMB in pediatric cancers also translates into a paucity of neoepitopes and few tumor-infiltrating T cells (TILs), making these “cold” tumors unresponsive to approaches such as immune checkpoint inhibitors (ICIs). As such, survival benefit from immunotherapy has not improved among pediatric cancers.
Current treatment paradigms for pediatric solid tumors consist of chemotherapy and surgery, with or without radiation. For children with high-risk metastatic and/or relapsed disease, survival remains poor, and devastating long-term morbidities are often unavoidable. An obvious unmet need exists for less toxic and more effective approaches. Immunotherapy holds promise not just for refractory disease, but in improving long-term survival without significant additive late toxicities. For example, with the integration of anti-GD2 into standard of care, 50–60% of children with high-risk neuroblastoma, a disease once incurable, are long-term survivors (2,3). Nevertheless, the pain associated with treatment, though manageable, remains a clinical challenge, and the promise of immunotherapy in most other pediatric solid tumors remains elusive. Although non-antibody–based platforms remain encouraging, only two vaccines and two cellular therapies have been approved for cancer therapy in the United States and Europe, compared to over 30 approved monoclonal antibodies. In this review, we overview immunotherapies for pediatric solid tumors, highlighting known immunological hurdles unique in pediatric patients with an emphasis on the tumor microenvironment (TME) and focus on classic antibody-based approaches, as well as novel genetically engineered forms to retarget T cells and precision radiation.
Immunotherapy of pediatric tumors: special hurdles
Aside from the immunosuppressive tumor milieu, special challenges of immunotherapy in pediatric solid tumors include: (i) a young age and immature immune system; (ii) extent of metastatic spread at diagnosis and fast disease progression, with possible privileged sites, such as the brain, not accessible by standard immunotherapies; (iii) the need to use intensive cytotoxic chemotherapy and/or radiation for induction, which deplete immune cells, especially lymphocytes and natural killer (NK) cells; (iv) the resulting comorbidities and infections from such intensive therapies requiring antibiotics, with subsequent alterations in the microbiome; (v) the lack of predictive biomarkers for response to immunotherapy, which is further confounded by the accrual of multiple disease types in phase I/II trials and the small number of eligible patients; (vi) the paucity of mutations at diagnosis, resulting in low T-cell clonal frequencies; and (vii) potential for acute and late toxicities in a young child resulting from overaction of the immune system or on-target, off-tumor effects. With such roadblocks in place, the role of immunotherapy in pediatric solid tumors remains at a crossroads.
The tumor microenvironment in pediatric solid tumors
The interaction between tumor cells and the host immune system evolves over time, from initial elimination, to equilibrium, and finally to escape (4). This latter step occurs as tumor cells evolve under pressure through a variety of mechanisms, including the loss of tumor neoantigens; a decrease in major histocompatibility complex (MHC) class I expression; increased production of immunosuppressive cytokines and immune cells such as regulatory T cells (Tregs), M2 macrophages, and myeloid-derived suppressor cells (MDSCs); and increased expression of inhibitory receptors and inhibitory ligands on T cells and tumor cells, respectively (i.e., PD-1 and PD-L1), eventually leading to a state of T-cell exhaustion.
Higher TMB correlates with better response to ICIs across many adult cancer types (5). With the exception of pediatric patients with tumors having biallelic mismatch repair deficiency, TMB and consequently immunogenicity and antigenicity for immunotherapy are both low (1,6). Most pediatric solid tumors carry just one or a few driver mutations, which is typical for embryonal tumors due to insufficient time or exposure to accumulate genotoxic environmental insults. With the absence of TILs, the expression of PD-1, PD-L1, and PD-L2 is also low across pediatric solid tumors (7). MDSCs, tumor-associated macrophages, tumor-associated fibroblasts, and Tregs can also inhibit lymphocyte infiltration or derail TIL function (8). Strategies to increase T-cell clonal frequency and drive them into tumors are a major unmet need in immunotherapy, and reversing the immunosuppressive tumor milieu should enhance T cell–based immunotherapy to its full potential (8).
Current immunotherapeutic approaches for pediatric solid tumors
Non-antibody–based platforms for pediatric solid tumors
As is exemplified by the large discrepancy in number of FDA approvals for antibody-based versus non-antibody–based immunotherapies for adult tumors, the efficacy of the latter in pediatric solid tumors has been so far elusive (Supplementary Table S1). Despite the success of CAR-T therapy in hematologic malignancies, these therapies in solid tumors have largely been unsuccessful, due to multiple hurdles: failure of T cells to survive and expand, T-cell exhaustion, activation-induced cell death (AICD), trogocytosis and fatricide, antigen loss, and inadvertent gene transduction of tumor cells (9,10). Novel CAR-T receptor constructs to confront these limitations include alternative costimulatory domains, weaponizing with “armors” to enhance cytokine secretion, improving in vivo persistence and expansion, overcoming antigen heterogeneity or antigen loss, and limiting cytokine release syndrome and neurotoxicity (9–11). Because certain treatments can persist in the body, its on-target, off-tumor toxicity could be life-long (e.g. B-cell aplasia) and devastating (e.g. GD2-related neurotoxicity)(12). Hence, finding the ideal target, although daunting, is much needed.
Novel T-cell platforms targeting multiple antigens highlight the potential to overcome antigen loss in pediatric solid tumors. The first-in-human trial evaluating tumor-specific T cells targeting Wilms tumor gene 1and survivin showed safe administration and a 73% response rate in 15 pediatric patients with relapsed/refractory solid tumors (13). Gamma-delta (γδ) T cells constitute another viable cell product given their T cell-like properties, lack of MHC restriction, presence of CD16, and ability to mediate ADCC. Their expansion was made possible with the combination of zomedronate plus IL2, and their clinical investigation in pediatric cancer has accelerated, although their in vivo survival and persistence are uncertain (14).
In view of the paucity of neoepitopes in pediatric solid tumors, the innate ability of NK cells to recognize activating ligands on tumors is a distinct advantage. Their possession of CD16 (FcγRIII) that mediates NK-ADCC in the presence of tumor-selective IgGs adds a critical dimension. A small pilot study utilizing haploidentical stem cell transplant with haploidentical NK cell infusion showed some responses in refractory pediatric solid tumors (15), and many studies evaluating the role of NK cells in pediatric solid tumors are ongoing (NCT03420963, NCT02573896, NCT02650648). Although these studies have shown feasibility, NK cell antitumor responses have been modest. With improved ex vivo proliferation of NK cells seen with expression of membrane-bound IL21 (16), as well as novel platforms that can enhance target specificity and redirection of NK cells to tumor cells (e.g., NK CAR cells and bispecific NK cell engagers)(17), NK cell–based therapy for pediatric solid tumors remains encouraging.
Vaccines using whole tumor cells, peptides, lysates, and proteins have all been developed and tested in various settings for pediatric tumors, without clear clinical benefit. In a large clinical trial in patients with high-risk neuroblastoma in remission, subcutaneous GD2- and GD3-lactone-keyhole limpet hemocyanin (KLH) in the presence of the subcutaneous adjuvant QS21 plus oral beta-glucan reported no significant toxicities, and excellent survival rates were achieved. Both progression-free survival (PFS) and overall survival (OS) correlated with higher anti-GD2 titer, which was s boosted by oral glucan (18). This strong correlation of immune response with clinical outcome is uncommon given the disconnect between laboratory evidence of immune responses and clinical benefit in most vaccine trials, where biomarkers chosen might be irrelevant, the response insufficient, or the tumor bulk too advanced.
Lastly, checkpoint blockade using ICIs is now accepted as the standard immunotherapy modality for many adult solid tumors, as well as Hodgkin’s lymphoma (5,19). However, their performance in pediatric solid tumors has not been efficacious. Combination studies (e.g. nivolumab and ipilimumab) are ongoing, but outcomes are uncertain.
Antibody therapy for pediatric solid tumors
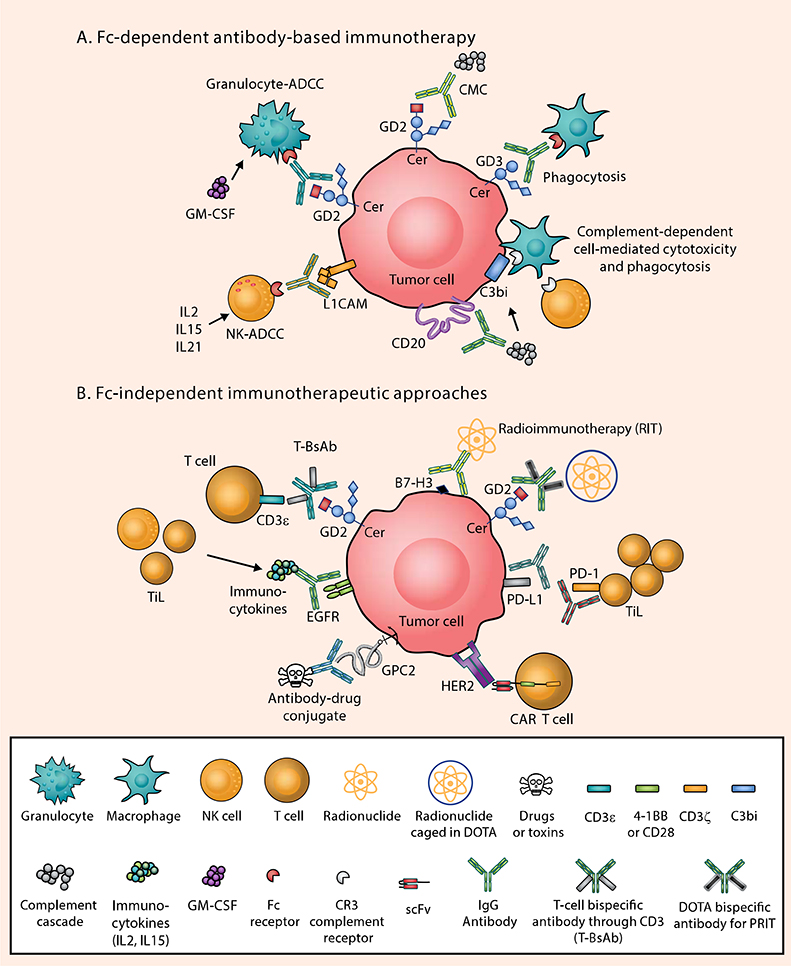
Monoclonal antibodies (mAbs) target tumor-specific surface antigens, resulting in the activation of Fc-mediated killing including complement-mediated cytotoxicity (CMC), NK cell antibody-dependent cellular cytotoxicity (NK-ADCC), neutrophil-ADCC, complement-dependent cellular cytotoxicity (CDCC), and antibody-dependent cell-mediated phagocytosis (ADCP)(Fig. 1). The first use of anti-CD20 and the later clinical development of rituximab in non-Hodgkin lymphoma have benefited pediatric patients and provided impetus to parallel efforts for solid tumors. The clinical benefit using mAbs to target GD2 in neuroblastoma has since transformed the treatment paradigm and prognosis for patients with high-risk neuroblastoma, where the murine IgG3 anti-GD2, 3F8, was used alone and in combination with granulocyte-macrophage colony-stimulating factor (GM-CSF)(3,20). The landmark phase III Children’s Oncology Group (COG) randomized trial confirmed the survival benefit of the chimeric GD2 antibody ch14.18 (dinutuximab), combined with GM-CSF and IL2 for neuroblastoma patients in first remission (2), although the role of IL2 was questioned in a subsequent phase III trial (21). To decrease human anti-mouse antibody formation, humanized versions, hu3F8 (naxitamab) and hu14.18-K322A, were developed and show excellent antitumor activity with less toxicities, with the latter carrying the K322A mutation to eliminate complement activation in the hope of reducing pain side-effects (22,23).
Figure 1. Antibody-based Immunotherapy in Pediatric Solid Tumors: Fc-dependent and Fc-independent Mechanisms.
The low mutational burden in pediatric solid tumors, the immaturity of the immune system in children, downregulation of tumor HLA molecules, and the use of intensive chemotherapy all combine to make the tumor microenvironment of pediatric solid tumors poorly immunogenic (8). Examples of surface antigen–antibody pairs include: B7-H3 [Omburtamab, 8H9], CD20 [Rituximab], EGFR [cetuximab], GD2 [Naxitamab or Dinutuximab], GD3 [R24], HER2 [trastuzumab], PD-1 [Pembrolizumab or Nivolumab], PD-L1 [Atezolizumab]. A) In the presence of IgG monoclonal antibodies targeting one of these antigens, tumor cells succumb to Fc-dependent cytotoxic killing: antibody-dependent NK cell–mediated cytotoxicity (NK-ADCC); granulocyte-mediated ADCC (granulocyte-ADCC); complement-mediated cytotoxicity (CMC) by binding to C1q (thereby activating the complement cascade); antibody-dependent macrophage-mediated phagocytosis; and CR3-dependent cell-mediated cytotoxicity through recognition of complement breakdown products (e.g. C3bi). B) Through genetic engineering, IgG antibodies can now be reformatted to engage T cells, which have no Fc receptor functions, by exploiting chimeric antigen receptors (CARs) or bispecific antibodies (anti-GD2 × anti-CD3), where polyclonal T cells can be driven into cold tumors as tumor-infiltrating lymphocytes (TILs) to initiate the tumor killing process. Multistep pre-targeted radioimmunotherapy (PRIT) and external-beam radiation therapy can also be utilized to increase neoantigen presentation and TIL infiltration. Immune checkpoint inhibitors (ICIs) as a monotherapy have been largely unsuccessful in pediatric solid tumors due to the paucity of neoepitopes and low number of TILs, but combinatorial approaches with radiotherapy, bispecifics, or CAR T cells that can attract TILs are promising for enhanced functioning of ICIs. Cytokines such as IL2, IL15, and IL21 can also combat the “cold” phenotype by attracting T cells and NK cells to the tumor. CR3, complement receptor 3; EGFR, epidermal growth factor receptor; GD2, disialoganglioside GD2; GD3, disialoganglioside GD3; GPC2, glypican-2; HER2, human epidermal growth factor receptor 2; L1CAM, L1 cell-adhesion molecule.
Given the efficacy and relative lack of long-term toxicities of anti-GD2, humanized or chimeric anti-GD2 are being explored in other GD2+ pediatric solid tumors including osteosarcoma and soft tissue sarcomas (NCT02484443, NCT01419834, NCT01662804, NCT02159443, and NCT00743496). Beyond GD2, another promising target for mAb therapy is B7-H3, a cell surface immunomodulatory glycoprotein overexpressed in a broad spectrum of adult and pediatric tumors. Compartmental radioimmunotherapy using 8H9 (a mouse mAb targeting B7-H3) has shown promising safety and efficacy in phase I studies, including patients with desmoplastic small round–cell tumors, neuroblastoma, and diffuse intrinsic pontine glioma (24–26). Enoblituzmab, another mAb targeting B7-H3, is undergoing a phase I trial (NCT02982941).
Despite these successes, other mAbs for pediatric solids tumors have been less impactful, despite their theoretical potential and encouraging preclinical data (Supplementary Table S1). The success of GD2 antibodies derives in part from the unique sensitivity of neuroblastoma to CMC and both neutrophil-ADCC and NK-ADCC, uncommon among most solid tumors. The probable underlying reason for this is the downregulation or absence of HLA, hence missing ligands for inhibitory KIR (killer cell immunoglobulin-like receptor) during NK-ADCC (27) and for inhibitory LILRB (leukocyte immunoglobulin-like receptor subfamily B receptor) during neutrophil-ADCC (28).
However, these Fc-dependent tumoricidal mechanisms and tissue-selective trafficking of innate effectors have limitations. For example, whereas metastatic neuroblastoma in the bone marrow is effectively eliminated by anti-GD2, soft tissue and CNS diseases are poorly controlled. Here, the accessibility of antibodies and effectors to tumors in the bone marrow is key. Other IgG antibodies have encountered unexpected biodistribution issues, such as anti-CD99 MAB-O13 for Ewing Sarcoma (NCT00582608). As drug carriers, IgGs are hampered by their unfavorable pharmacokinetics and suboptimal therapeutic indices (see below). By overcoming these limitations, novel antibody forms to redirect T cells and to deliver payloads such as cytotoxic drugs, toxins, or radionuclides could offer new opportunities.
In cancer immunotherapy, cell-engaging bispecific antibodies (BsAbs) are tumor-binding antibodies reformatted with additional non-Fc specificity for effector cells (Fig. 1). The bispecific T-cell engagers (BiTEs) consist of two binding domains: (i) a single-chain variable fragment (scFv) that binds to tumor, and (ii) a second scFv to engage an activating receptor on T cells (e.g. CD3 of the T-cell receptor complex). More than sixty different T cell–based BsAb platforms are in preclinical and clinical testing (29). By directing T cells to tumor cells, BsAbs have the potential to steer T cells deep into tumors and activate TILs for tumor ablation. Blinatumomab, a BiTE specific for CD3 and CD19, has shown clinical benefit in pediatric patients with B-cell acute lymphoblastic leukemia (30). Using the IgG(L)-scFv platform, polyclonal T cells can be quantitatively driven into solid tumors and can overcome PD-1/PD-L1 inhibition to ablate GD2+ human xenografts in mice (31). Phase I/II studies are underway treating patients with neuroblastoma, osteosarcoma, and other GD2+ tumors with anti-(GD2 × CD3) BsAbs (NCT02173093, NCT03860207).
BsAbs can also be used for ex vivo arming of expanded T cells before reinfusion into patients (NCT02173093)(31), avoiding cytokine storm or neurotoxicity. Detailed structural studies have shown that cis-configuration and inter-binding domain distance are critical parameters for building BsAbs, and the IgG(L)-scFv format could outperform BiTE, BiTE-Fc, heterodimeric constructs, and chemical conjugates (32). Fc-silencing is also critical for optimal function of BsAbs (33). Other BsAbs with relevance for pediatric solid cancers are being explored including those targeting HER-2 (34), polysialic acid, L1CAM, ROR2, STEAP-1, and those directed against B7-H3 (MGD009) and glypican-3 (ERY-974)(Fig. 1). An approach using combinations of target-specific BsAbs for ex vivo arming could theoretically broaden target coverage, thereby, overcoming tumor heterogeneity while modulating multiple activators or inhibitors on the same effector cell (31).
Future strategies of antibody-based therapies: a reassessment
Given the many challenges, discovery and preclinical validation of novel immune targets with limited expression on normal human tissues is only the first step, even when utilizing modern omics techniques. Specific antibody clones can be rapidly identified using human IgG transgenic animals, human phage libraries, or humanized single VH domain libraries (35). These antibody binding domains can then be reshaped into chimeric receptors, multivalent and/or multi-specific formats for diagnostic and therapeutic applications. Nonetheless, one should not lose sight of the established targets (e.g. NCI priority list (36)), many of which have already been proven safe in humans, although ineffective in their classic IgG forms. Inhibitory cells in the TME such as fibroblasts, macrophages, Tregs, MDSCs, and type-2 innate lymphoid cells are all potential candidates for immune modulation (8). Vasculature and hypoxia are also viable areas for immune interventions (Supplementary Table S2).
Beyond target discovery and the TME, overhauling pharmacokinetics and amplifying antibody effector functions are major unmet needs. Radioimmunotherapy (RIT) has not materialized because of the inability of single-step whole IgG or its fragments to deliver curative doses. Despite tumor to tissue ratios appearing favorable with time delay, low therapeutic indices are typical. Multistep pre-targeted RIT was a major breakthrough (37), and issues regarding immunogenicity and renal retention, steric interference, protocol complexity, and more versatile reagents have been resolved (38). With these improvements, curative radiation doses of are now possible in several target systems and animal models without chemical, clinical, or histologic toxicities. Better precision, dose, and dose-rate have also improved radiation oncology and could improve delivery of biologics and drugs. The coupling of radiotherapy with immunotherapy to enhance immunogenic cell death could be further exploited for neoantigen induction, T-cell infiltration, and modulation of Tregs and the TME (Fig. 1).
Despite the many immune strategies beyond ICIs (Supplementary Table S2), classic IgG-based strategies have been saddled with limitations, e.g. lack of specificity and low potency. Beyond CAR-T therapies, T-cell BsAbs represent a different approach, and among the many different platforms, the minimal and optimal structural requirements need to be elucidated (32). Already, some of these antibody platforms, such as BiTEs, heterodimeric or IgG(L)-scFv formats, have yielded promising preclinical and clinical data. Although phase I/II studies of these single agents will soon weed out less-effective candidates, combinatorial principles in pediatric solid tumors are needed to advance long-term cures with low toxicities. One old combinatorial strategy that has proven successful in neuroblastoma is the addition of cytokines to anti-GD2. The use of IL15 (with or without its receptor, IL-15Rα) (39) and IL21 (40), which can enhance both NK cells and CD8+ T cells, is timely (Fig. 1). Combining ICIs with CAR-T therapy or BsAbs is logical. Similarly, interfering with key immune inhibitory molecules such as TGFβ (41) is now possible, with enhanced ADCC seen with TGFβR1 blockade in combination with ex vivo–activated NK cells and anti-GD2 (42).
On the whole, the timing of immunotherapy in relation to other cytotoxic therapies must be carefully optimized. Until chemotherapy and radiotherapy are reduced or eliminated, lymphocyte depletion and immunosuppression that occur after such therapies can hamper the immune system. (43). However, passive monoclonal antibody therapy, even after intensive chemotherapy, can be very effective in the consolidative setting, as shown with anti-GD2, because ADCC and cell-mediated cytotoxicity are dependent on complement and myeloid cells such as neutrophils, which recover faster than lymphocytes. Concurrent administration of antibodies with induction chemotherapy also may be beneficial, with response rates of close to 80% and encouraging survival outcomes seen in the phase II setting with anti-GD2 (44). For T-cell based therapies, regeneration of lymphocytes via cytokines or harvesting T cells prior administering cytotoxic therapy are possible strategies to jumpstart lymphocytes. Arming rejuvenated T cells with CARs or BsAb ex vivo is another passive immunotherapy approach. Once the immune system is recovered, a vaccine approach could also be introduced, whether antibody-based (e.g., GD2 vaccine) or cell-based. Collaboration among major cancer centers and harmonization of immune monitoring (45) should facilitate data sharing, dissemination of novel discoveries, and transformation from clinical trials to standards of care.
Concluding remarks
Immunotherapy is an established cancer treatment modality, proven effective in some subtypes, with potential to be applicable to all pediatric cancers. Although chemotherapy has a proven benefit on survival, the quality of survival can be improved as immunotherapy is further optimized. Although childhood cancers share biology and issues confronting adult patients, their development and drivers can be different. In a rapidly evolving landscape in pediatric immunotherapy, where large clinical trials are not possible, a multicenter approach needs to be developed so that we move beyond the crossroads.
Supplementary Material
Acknowledgments
This was supported in part by the NCI Cancer Center Support Grant P30 CA008748 and U01 CA232491.
Grant Support: NIH grant P30 CA 008748, U01 CA232491
Potential Conflicts of Interest:
NKC reports receiving commercial research grants from Y-mabs Therapeutics and Abpro-Labs Inc.; holding ownership interest/equity in Y-Mabs Therapeutics Inc., holding ownership interest/equity in Abpro-Labs, and owning stock options in Eureka Therapeutics. NKC is the inventor and owner of issued patents licensed by MSK to Ymabs Therapeutics, Biotec Pharmacon, and Abpro-labs. Hu3F8 and 8H9 were licensed by MSK to Y-mabs Therapeutics. Both MSK and NKC have financial interest in Y-mabs. NKC is an advisory board member for Abpro-Labs and Eureka Therapeutics. DC has no disclosures to report.
REFERENCES
- 1.Downing JR, Wilson RK, Zhang J, Mardis ER, Pui CH, Ding L, et al. The Pediatric Cancer Genome Project. Nat Genet 2012;44(6):619–22 doi 10.1038/ng.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 2010;363(14):1324–34 doi 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheung NK, Cheung IY, Kushner BH, Ostrovnaya I, Chamberlain E, Kramer K, et al. Murine Anti-GD2 Monoclonal Antibody 3F8 Combined With Granulocyte-Macrophage Colony-Stimulating Factor and 13-Cis-Retinoic Acid in High-Risk Patients With Stage 4 Neuroblastoma in First Remission. J Clin Oncol 2012;30(26):3264–70 doi 10.1200/JCO.2011.41.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 2011;331(6024):1565–70 doi 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 5.Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 2019;51(2):202–6 doi 10.1038/s41588-018-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shlien A, Campbell BB, de Borja R, Alexandrov LB, Merico D, Wedge D, et al. Combined hereditary and somatic mutations of replication error repair genes result in rapid onset of ultra-hypermutated cancers. Nat Genet 2015;47(3):257–62 doi 10.1038/ng.3202. [DOI] [PubMed] [Google Scholar]
- 7.Pinto N, Park JR, Murphy E, Yearley J, McClanahan T, Annamalai L, et al. Patterns of PD-1, PD-L1, and PD-L2 expression in pediatric solid tumors. Pediatr Blood Cancer 2017;64(11) doi 10.1002/pbc.26613. [DOI] [PubMed] [Google Scholar]
- 8.Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nature medicine 2018;24(5):541–50 doi 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown CE, Mackall CL. CAR T cell therapy: inroads to response and resistance. Nat Rev Immunol 2019;19(2):73–4 doi 10.1038/s41577-018-0119-y. [DOI] [PubMed] [Google Scholar]
- 10.Shah NN, Fry TJ. Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol 2019;16(6):372–85 doi 10.1038/s41571-019-0184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.June CH, Sadelain M. Chimeric Antigen Receptor Therapy. N Engl J Med 2018;379(1):64–73 doi 10.1056/NEJMra1706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mount CW, Majzner RG, Sundaresh S, Arnold EP, Kadapakkam M, Haile S, et al. Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M(+) diffuse midline gliomas. Nat Med 2018;24(5):572–9 doi 10.1038/s41591-018-0006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hont AB, Cruz CR, Ulrey R, O’Brien B, Stanojevic M, Datar A, et al. Immunotherapy of Relapsed and Refractory Solid Tumors With Ex Vivo Expanded Multi-Tumor Associated Antigen Specific Cytotoxic T Lymphocytes: A Phase I Study. J Clin Oncol 2019;37(26):2349–59 doi 10.1200/JCO.19.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silva-Santos B, Mensurado S, Coffelt SB. gammadelta T cells: pleiotropic immune effectors with therapeutic potential in cancer. Nat Rev Cancer 2019;19(7):392–404 doi 10.1038/s41568-019-0153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perez-Martinez A, de Prada Vicente I, Fernandez L, Gonzalez-Vicent M, Valentin J, Martin R, et al. Natural killer cells can exert a graft-vs-tumor effect in haploidentical stem cell transplantation for pediatric solid tumors. Exp Hematol 2012;40(11):882–91 e1 doi 10.1016/j.exphem.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Denman CJ, Senyukov VV, Somanchi SS, Phatarpekar PV, Kopp LM, Johnson JL, et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS One 2012;7(1):e30264 doi 10.1371/journal.pone.0030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nayyar G, Chu Y, Cairo MS. Overcoming Resistance to Natural Killer Cell Based Immunotherapies for Solid Tumors. Front Oncol 2019;9:51 doi 10.3389/fonc.2019.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kushner BH, Cheung IY, Modak S, Kramer K, Ragupathi G, Cheung NK. Phase I trial of a bivalent gangliosides vaccine in combination with beta-glucan for high-risk neuroblastoma in second or later remission. Clin Cancer Res 2014;20(5):1375–82 doi 10.1158/1078-0432.CCR-13-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer discovery 2018;8(9):1069–86 doi 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 20.Cheung NK, Cheung IY, Kramer K, Modak S, Kuk D, Pandit-Taskar N, et al. Key role for myeloid cells: phase II results of anti-G(D2) antibody 3F8 plus granulocyte-macrophage colony-stimulating factor for chemoresistant osteomedullary neuroblastoma. Int J Cancer 2014;135(9):2199–205 doi 10.1002/ijc.28851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ladenstein R, Potschger U, Valteau-Couanet D, Luksch R, Castel V, Yaniv I, et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): a multicentre, randomised, phase 3 trial. Lancet Oncol 2018;19(12):1617–29 doi S1470–2045(18)30578–3 [pii] 10.1016/S1470-2045(18)30578-3. [DOI] [PubMed] [Google Scholar]
- 22.Navid F, Sondel PM, Barfield R, Shulkin BL, Kaufman RA, Allay JA, et al. Phase I Trial of a Novel Anti-GD2 Monoclonal Antibody, Hu14.18K322A, Designed to Decrease Toxicity in Children With Refractory or Recurrent Neuroblastoma. J Clin Oncol 2014;32(14):1445–52 doi 10.1200/JCO.2013.50.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kushner BH, Cheung IY, Modak S, Basu EM, Roberts SS, Cheung NK. Humanized 3F8 Anti-GD2 Monoclonal Antibody Dosing With Granulocyte-Macrophage Colony-Stimulating Factor in Patients With Resistant Neuroblastoma: A Phase 1 Clinical Trial. JAMA Oncol 2018;4(12):1729–35 doi 10.1001/jamaoncol.2018.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kramer K, Kushner BH, Modak S, Pandit-Taskar N, Smith-Jones P, Zanzonico P, et al. Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. J Neurooncol 2010;97(3):409–18 doi 10.1007/s11060-009-0038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kramer K, Smith M, Souweidane MM. Safety profile of long-term intraventricular access devices in pediatric patients receiving radioimmunotherapy for central nervous system malignancies. Pediatr Blood Cancer 2014;61(9):1590–2 doi 10.1002/pbc.25080. [DOI] [PubMed] [Google Scholar]
- 26.Souweidane MM, Kramer K, Pandit-Taskar N, Zhou Z, Haque S, Zanzonico P, et al. Convection-enhanced delivery for diffuse intrinsic pontine glioma: a single-centre, dose-escalation, phase 1 trial. The Lancet Oncology 2018;19(8):1040–50 doi 10.1016/S1470-2045(18)30322-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boudreau JE, Giglio F, Gooley TA, Stevenson PA, Le Luduec JB, Shaffer BC, et al. KIR3DL1/ HL A-B Subtypes Govern Acute Myelogenous Leukemia Relapse After Hematopoietic Cell Transplantation. J Clin Oncol 2017;35(20):2268–78 doi 10.1200/JCO.2016.70.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Touw W, Chen HM, Pan PY, Chen SH. LILRB receptor-mediated regulation of myeloid cell maturation and function. Cancer Immunol Immunother 2017;66(8):1079–87 doi 10.1007/s00262-017-2023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Z, Cheung NV. T cell engaging bispecific antibody (T-BsAb): From technology to therapeutics. Pharmacol Ther 2018;182:161–75 doi 10.1016/j.pharmthera.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Topp MS, Gokbuget N, Stein AS, Zugmaier G, O’Brien S, Bargou RC, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. The Lancet Oncology 2015;16(1):57–66 doi 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 31.Park JA, Xu H, Santich BH, Cheung NK. Exceptionally Potent Cytotherapy Using T Cells Armed with Novel Tetravalent Recombinant Bispecific Antibodies Specific for GD2 and HER2. 2018; San Diego, CA, USA Blood. p 4536. [Google Scholar]
- 32.Santich BH, Park A, Tran H, Huse M, Cheung NK. Valency and spatial configuration drive the potency of T-cell bispecific antibodies. Sci Transl Med. 2019. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang LL, Hoseini SS, Xu H, Ponomarev V, Cheung NK. Silencing Fc in T cell engaging bispecific antibodies is critical for T cell trafficking and anti-tumor potency. Cancer Immunology Research 2019; 7 :2013–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez-Albaitero A, Xu H, Guo H, Wang L, Wu Z, Tran H, et al. Overcoming resistance to HER2-targeted therapy with a novel HER2/CD3 bispecific antibody. Oncoimmunology 2017;6(3):e1267891 doi 10.1080/2162402X.2016.1267891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arbabi-Ghahroudi M Camelid Single-Domain Antibodies: Historical Perspective and Future Outlook. Front Immunol 2017;8:1589 doi 10.3389/fimmu.2017.01589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res 2009;15(17):5323–37 doi 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheung NK, Modak S, Lin Y, Guo H, Zanzonico P, Chung J, et al. Single-chain Fv-streptavidin substantially improved therapeutic index in multistep targeting directed at disialoganglioside GD2. J Nucl Med 2004;45(5):867–77. [PubMed] [Google Scholar]
- 38.Cheal SM, Xu H, Guo HF, Zanzonico PB, Larson SM, Cheung NK. Preclinical evaluation of multistep targeting of diasialoganglioside GD2 using an IgG-scFv bispecific antibody with high affinity for GD2 and DOTA metal complex. Mol Cancer Ther 2014;13(7):1803–12 doi 10.1158/1535-7163.MCT-13-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen R, Moustaki A, Norrie JL, Brown S, Akers WJ, Shirinifard A, et al. Interleukin-15 enhances anti-GD2 antibody-mediated cytotoxicity in an orthotopic PDX model of neuroblastoma. Clin Cancer Res 2019;25:7554–7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spolski R, Leonard WJ. Interleukin-21: a double-edged sword with therapeutic potential. Nat Rev Drug Discov 2014;13(5):379–95 doi 10.1038/nrd4296. [DOI] [PubMed] [Google Scholar]
- 41.Ganesh K, Massague J. TGF-β Inhibition and Immunotherapy: Checkmate. Immunity. 2018;48:626–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tran HC, Wan Z, Sheard MA, Sun J, Jackson JR, Malvar J, et al. TGFbetaR1 Blockade with Galunisertib (LY2157299) Enhances Anti-Neuroblastoma Activity of the Anti-GD2 Antibody Dinutuximab (ch14.18) with Natural Killer Cells. Clin Cancer Res 2017;23(3):804–13 doi 10.1158/1078-0432.CCR-16-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mackall CL. T-cell immunodeficiency following cytotoxic antineoplastic therapy: a review. Oncologist 1999;4(5):370–8. [PubMed] [Google Scholar]
- 44.Furman WL, Federico SM, McCarville MB, Shulkin BL, Davidoff AM, Krasin MJ, et al. A Phase II Trial of Hu14.18K322A in Combination with Induction Chemotherapy in Children with Newly Diagnosed High-Risk Neuroblastoma. Clin Cancer Res 2019. doi 10.1158/1078-0432.CCR-19-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nierkens S, Lankester AC, Egeler RM, Bader P, Locatelli F, Pulsipher MA, et al. Challenges in the harmonization of immune monitoring studies and trial design for cell-based therapies in the context of hematopoietic cell transplantation for pediatric cancer patients. Cytotherapy 2015;17(12):1667–74 doi 10.1016/j.jcyt.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.