Abstract
Genetically engineered T cells expressing chimeric antigen receptors (CAR) present a new treatment option for patients with cancer. Recent clinical trials of B cell leukemia have demonstrated a response rate of up to 90%. However, CAR cell therapy is frequently accompanied by severe side effects such as cytokine release syndrome and the development of target cell resistance. Consequently, further optimization of CARs to obtain greater long-term efficacy and increased safety is urgently needed. Here we high-light the various efforts of adjusting the intracellular signaling domains of CARs to these major requirements to eventually obtain high-level target cell cytotoxicity paralleled by the establishment of longevity of the CAR expressing cell types to guarantee for extended tumor surveillance over prolonged periods of time. We are convinced that it will be crucial to identify the molecular pathways and signaling requirements utilized by such ‘efficient CARs’ in order to provide a rational basis for their further hypothesis-based improvement. Furthermore, we here discuss timely attempts of how to: i) control ‘on-tumor off-target’ effects; ii) introduce Signal 3 (cytokine responsiveness of CAR cells) as an important building-block into the CAR concept; iii) most efficiently eliminate CAR cells once full remission has been obtained. We also argue that universal systems for the variable and pharmacokinetically-controlled attachment of extracellular ligand recognition domains of choice along with the establishment of ‘off-the-shelf’ cell preparations with suitability for all patients in need of a highly-potent cellular therapy may become future mainstays of CAR cell therapy. Such therapies would have the attraction to work independent of the patients’ histo-compatibility make-up and the availability of functionally intact patient’s cells. Finally, we summarize the evidence that CAR cells may obtain a prominent place in the treatment of non-malignant and auto-reactive T and B lymphocyte expansions in the near future, e.g., for the alleviation of autoimmune diseases and allergies. After the introduction of red blood cell transfusions, which were made possible by the landmark discoveries of the ABO blood groups by Karl Landsteiner, and the establishment of bone marrow transplantation by E. Donnall Thomas to exchange the entire hematopoietic system of a patient suffering from leukemia, the introduction of patient-tailored cytotoxic cellular populations to eradicate malignant cell populations in vivo pioneered by Carl H. June, represents the third major and broadly applicable milestone in the development of human cellular therapies within the rapidly developing field of applied biomedical research of the last one hundred years.
Keywords: Cancer immunotherapy, Chimeric antigen receptor, Adoptive cell therapy, Tumor microenvironment
1. Introduction
The basic concept of chimeric antigen receptor (CAR) therapy aims at redirecting cytotoxic lymphocytes against antigens expressed on tumor cells, eventually leading to tumor elimination. To provide immune cells with the ability to target new antigens, the cells are engineered to express CARs that basically link two functional domains: an extracellular antigen-recognition domain and an intracellular stimulatory domain. The extracellular antigen-recognition domain most commonly consists of a single-chain variable fragment (scFv) derived from a monoclonal antibody and, unlike bona fide T cell receptors (TCR), allows for antigen recognition in an MHC-unrestricted manner [1]. The extracellular domain is linked via a transmembrane region to the intracellular stimulatory domain, most frequently the CD3-ζ chain of the T cell receptor (TCR). To eliminate tumor cells, CARs become introduced into immune cells with cytolytic capacity which upon encounter should attack and lyse the tumor cells. Upon binding of the CAR to the (tumor-) antigen, the intracellular ITAM domains become phosphorylated and downstream signaling proteins activated. This activates the CAR cell, which leads to the release of cytokines, the proliferation of CAR-cells and antigen-specific lysis of tumor cells [2].
Various immune cell types have been evaluated as CAR recipients in the past. The first studies focused on CD8+ T lymphocytes (CTLs) due to their inherently strong cytotoxic activity, but it was soon recognized that the sole use of CTLs does not guarantee durable results and that combination with T helper (Th) lymphocytes could be beneficial [3–5]. In addition to T lymphocytes, natural killer (NK) cells are also known to be highly cytotoxic. Indeed, several recent studies have proven their efficacy in tumor elimination and first clinical studies using NK cells as carriers of CARs are currently being performed [6]. Despite the extensive research on both T and NK cells, a more holistic approach combining different immune cell types might be appropriate. To the best of our knowledge, only one study combining T and NK cells was conducted so far, showing better tumor elimination when both cell types were used concomitantly [7].
In the recent past, CAR T cells have demonstrated great success in the treatment of B cell malignancies revealing up to 90% response rates in clinical trials [8]. Since 2017, two CAR cell therapies approved by the FDA – Kymirah (tisagenlecleucel) and Yescarta (axicabtagene ciloleucel) – are available for clinical use, both targeting the B cell differentiation antigen CD19 [9]. While clinical studies look very promising with remissions observed in more than 70% of patients treated, severe therapy associated side effects such as cytokine release syndrome (CRS) and neurologic events, including encephalopathy and aphasia, were frequently observed [10,11]. Moreover, the encouraging results observed during the treatment of hematological malignancies have yet to be translated to solid tumor treatment [12,13]. To overcome these obstacles, various groups have aimed at improving the design and composition of chimeric receptors. In this review, we first aim to summarize the ‘evolution’ of the design of CAR signaling domains, followed by the description of attempts of how to optimize and potentially universalize ligand recognition domains. In the second part of this article we want to emphasize the importance of the recipient cell type used for CAR expression.
2. CAR signaling domains
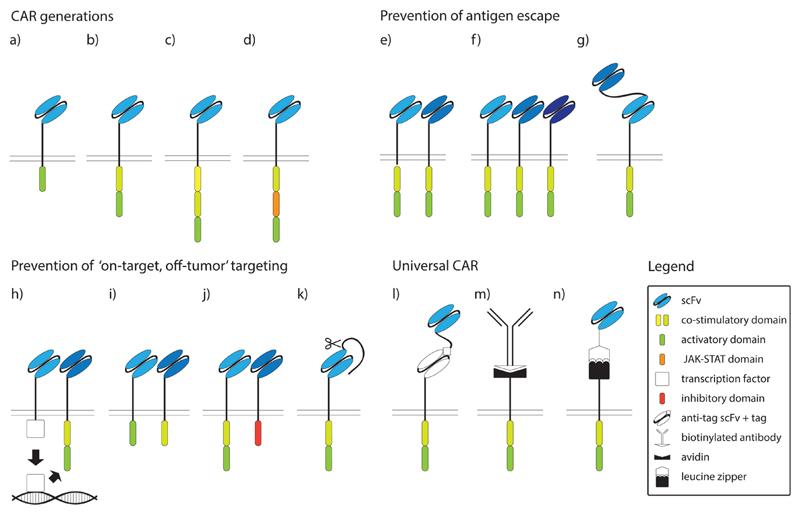
Chimeric antigen receptors (CARs) proven to exert cellular activation and displaying target cell specificity constitute an extended list. Indeed, already in 1993 a paper published by Eshhar et al. demonstrated that the CAR T cells which were directed against 2,4,6-trinitrophenyl (TNP) exhibited potent TNP-specific cell lysis [14]. This 1st-generation CAR contained exclusively CD3-ζ as activation domain, comprising all three ITAMs, and although it generated a cytotoxic effect, it only had limited anti-tumor efficacy due to early T cell exhaustion (Fig. 1a and Table 1) [15]. To circumvent this problem, and to take advantage of other potent T cell activation domains, signaling domains ‘borrowed’ from different costimulatory molecules such as 4-1BB (CD137), CD28, DAP10, OX40 (CD134), or ICOS (CD278) were subsequently fused to the intracellular ζ-chain containing signaling domain in 2nd-generation CARs (Fig. 1b). Multiple studies have documented that these combinations of co-stimulatory and activation domains made 2nd-generation CARs superior to their predecessors, however, optimal combinations with regards to cellular yield and longevity still remain to be determined [15–17]. The most frequently used costimulatory domains are derived from CD28 and 4-1BB and have been compared in several studies, however, results remained controversial as of today. Zhao et al. showed that the introduction of the CD28 signaling domain resulted in faster tumor elimination when compared to 4-1BB CAR T cells, with lower numbers of CAR T cells needed for successful tumor elimination [16]. In contrast, Long et al. demonstrated that 4-1BB CAR T cells not only have superior activity but also persist for longer periods of time in the host [15]. The latter is an important factor in the treatment of cancer patients since longer T cell persistence relates to a superior clinical outcome [5,18,19].
Fig. 1. Intracellular signaling domains and modifications of CARs.
a) First generation CAR, b) second generation CAR, c) third generation CAR, d) JAK-STAT CAR, e) double CAR, f) tripple CAR, g) tandem CAR (tanCAR), h) synNotch CAR, i) CAR with dissociated signaling domains, j) inhibitory CAR (iCAR), k) masked CAR, l) universal CAR (uniCAR), m) biotin-binding immune receptor (BBIR) CAR, n) split universal programmable (SUPRA) CAR. scFv, single chain variable fragment; JAK, Janus kinase; STAT, signal transducers and activators of transcription.
Table 1. Composition and variation of CAR intracellular signalling modules, transmembrane and stalk regions.
| CAR Domain | Component* | References |
|---|---|---|
| Intracellular signalling domain | CD3-zeta§ only | [5,73,74,100,128,142,149,150] |
| CD28 only | [74] | |
| 4-1BB only | [73] | |
| CD28_4-1BB | [74] | |
| 4-1BB_CD3-zeta | [29,67,71,79,117,122,124,129,141,151,152,160,161,166,167,168,169,170,171] | |
| CD28_CD3-zeta | [16,17,22,68,70,75,78,85,92,99,104,108,113,115,116,117,123,127,131,132,138,143,150,161,170,172,173,174,175,176,177,178,179] | |
| CD28_4-1BB_CD3-zeta | [2,7,12,86,101,161,180,181] | |
| 4-1BB_CD28_CD3-zeta | [151] | |
| CD28_OX40_CD3-zeta | [182] | |
| ICOS_CD3_zeta | [135] | |
| Dap10 | [141] | |
| Gal 4 DBD v P64$ | [73] | |
| CTLA-4# | [75] | |
| PD-1# | [75] | |
| CD28_IL2betaR_CD3-zeta_(YXXQ mutation) | [16] | |
| IL4Rbetac | [115,178,179] | |
| Fc epsilon TI gamma | [22] | |
| 2b4 | [142] | |
| 2b4_CD3-zeta | [142] | |
| Transmembrane domain | CD28 | [2,16,17,22,24,29,68,70,71,73,86,92,99,108,113,115,117,122,123,124,131,132,138,143,150,161,166,169,170,172,173,174,175,176,177,178,179,182] |
| CD8 | [7,12,67,73,74,75,79,85,97,101,129,141,151,152,160,167,168,169,170,181] | |
| CD4 | [180] | |
| CD3-zeta | [5,128,142,149,150] | |
| ICOS | [135] | |
| 2b4 | [142] | |
| 4-1BB | [150] | |
| Notch1$ | [73] | |
| IL-4betac | [115,178,179] | |
| IgG1 | [116] | |
| Stalk region | IgG4 | [29,71,122,124,131,166,176] |
| IgG1 | [17,70,104,108,116,127,143,160,161,180,182] | |
| CD8 | [7,12,22,67,73,74,75,79,85,86,101,123,129,141,149,150,151,152,167,168,169,170,172,177,181] | |
| CD28 | [22,24,73,115,173,174,178,179] | |
| Fc spacer | [132] | |
| Notch1$ | [73] | |
| G4S | [117] |
Components shown in N- to C-terminal orientation.
CD3-zeta indicates that all three ITAMs of zeta have been used.
inhibitory CAR.
synNotch CAR.
Although 2nd-generation CARs have shown robust efficacy in the treatment of B-cell leukemias, results of CAR therapy of solid tumors are less promising [12,18]. The tumor microenvironment possesses many immuno-inhibitory elements such as inhibitory cytokines, inhibitory immune cells and a tendency to deplete nutrients essential for immune cell function [19]. To overcome these obstacles, further optimization of CARs was necessary, which led to the development of 3rd-generation CARs, which contain intracellular signaling domains of two different costimulatory molecules paired with constituents of the ζ-chain signaling domain (Fig. 1c). T cells equipped with 3rd generation CARs showed higher proliferation rates and longer in vivo persistence with only modest toxic side effects, however, anti-tumor effects were not greatly enhanced [20,21]. An elegant demonstration of how to identify new and powerful combinations of intracellular signaling domains has been conducted by Duong et al. who generated new and improved CAR constructs by randomly combining intracellular signaling domains from a collection of intracellular signaling domains. They discovered that the construct consisting of the DAP10, CD3-ζ and CD27 signaling domains exhibited the most potent cytolytic activity [22]. Whether such alignment of intracellular domains truly exhibits higher antitumor efficacy in in vivo models remains yet to be determined.
It is well known that T cells require three signals to elicit a productive response: (i) antigen presented by the peptide/MHC complex (ii) a potent costimulus and (iii) growth and differentiation factors in the form of soluble cytokines [23]. CARs of the 2nd and 3rd generation provide the first two signals but fall short with regards to providing the third, cytokine dependent, signal. To actively deliver also the third signal to CAR expressing lymphocytes, Kagoya et al. have linked the cytoplasmic domain of the interleukin (IL)-2 receptor β-chain and a STAT3-binding tyrosine-X-X-glutamine (YXXQ) motif with the CD3-ζ and CD28 signaling domains (Fig. 1d). Notably, the inclusion of the third signaling module resulted in superior in vivo persistence of modified T cells, which delivered a more potent anti-tumor effect [24]. To the best of our knowledge, this is the only study so far combining all three signaling modules for T cell activation in a single CAR construct.
Precise comparison of CAR signaling with bona fide T cell receptor (TCR) signaling might reveal important underlying differences that could form the basis for further optimization steps during future CAR construct development. While low-level tonic TCR signaling enhances T cell responsiveness to foreign antigens, tonic CAR signaling may lead to cellular exhaustion. This could be avoided by using different intracellular signaling domains [25]. CAR signaling also needs to be adapted to the cell type used since it was shown that NK cells exhibit grater cytotoxicity when expressing a CAR with a DAP12 intracellular domain in comparison to T cells expressing CD3-ζ containing CAR [26]. A better understanding of the effects of each individual CAR domain on cell activation will help to further improve CAR cell function. While CD28 augments CAR T cell persistence, its lck-binding domain promotes production of IL-2, which stimulate functional programs in Tregs that in turn may dampen the initially induced anti-tumor effects. Indeed, the deletion of the lck-binding domain of CD28 resulted in enhanced proliferation and better in vivo tumor control [27].
Despite the great importance of the intracellular domains of CARs for the function and longevity of lymphocytes expressing them, the contributions of other elements to CAR function, such as the transmembrane and linker domains connecting the scFv ligand binding domains and the respective transmembrane regions, should not be underestimated. Since some antigens are localized closer to the plasma membrane of the tumor cell surface, a longer linker might be needed for their efficient recognition [28]. While a longer linker might enhance the chances for antigen recognition, it also increases the chances for generating an immunogenic molecule, which might become recognized by other immune cells, potentially leading to the deletion of the respective CAR T cells in vivo [29]. CARs are powerful receptors to target cell types of choice. Nevertheless, it is noteworthy that as of today no optimized design for the composition of the signaling modules has been agreed upon on. Although 1st generation CAR constructs, exclusively consisting of a ζ-chain intracellular domain, have ‘evolved’ into constructs consisting of various domains derived from different signaling molecules, such as the recently described CAR construct endowed for instance with a JAK-STAT signaling domain, only little knowledge exists when it comes to deciding which type of combination would be optimal for a given purpose. Studies exploring the T cell activating potential of different chimeric constructs may guide us towards the creation of improved signaling modules. For example, simple clustering of chimeric constructs harboring the Syk kinase domain led to increased Ca2+ flux and cytolysis [30]. Interestingly, clustering of ZAP70 chimeric constructs failed to induce similar effector programs while co-clustering of ZAP70 with the Scr-kinases Lck or Fyn could overcome this impairment [30]. Thus, it may be reasonable to establish constructs containing signaling modules consisting of an active Src kinase or Syk domain together with the critical ζ-chain or ZAP70 phosphorylation sites. Such constructs could trans-phosphorylate each other upon antigen encounter and dimerization may enhance signal propagation and thus, more robust T cell effector responses. Moreover, signaling domains of other, non-cytotoxic immune cell types, e.g., B cells, could be adopted to enhance T/NK cell function. Our increasing understanding of the physiologically triggered TCR and associated co-stimulatory and cytokine-mediated signaling pathways will improve and certainly also expand the scope of application of CAR technology.
3. Targeting
Effective CAR cell activation not only depends on the appropriate function of the intracellular activation domains but also on proper recognition of the targeted antigen. For CAR cell activation to efficiently occur, a sufficient quantity of the antigen needs to be present on target cells [25]. While the paucity of CAR-specific antigens might reduce the outcome of CAR treatment, ‘on-target, off-tumor’ effects, in which CAR cells recognize normal, non-malignant cells and tissues that may express the targeted antigen, might occur with higher frequency [31,32]. The selection of appropriately targeted antigens is therefore of crucial importance.
3.1. Extracellular ligand recognition
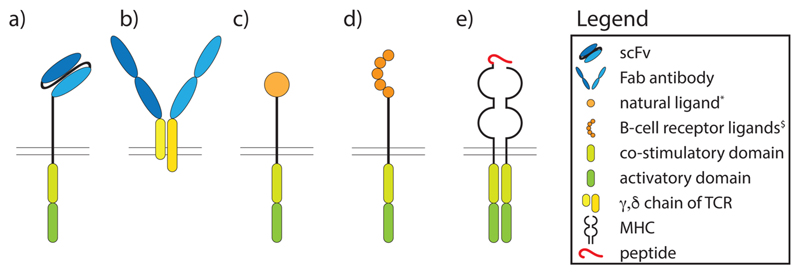
This chapter will summarize our current knowledge on ligand recognition domains that have been used to generate CARs. The following four classes will be discussed: (i) single-chain variable fragments (scFvs) derived from antibodies, (ii) fragments antigen-binding (Fab), (iii) natural ligands that engage their cognate receptor independently of MHC, and (iv) bona fide TCR ligands (Fig. 2).
Fig. 2. Extracellular ligand recognition domains of CARs.
a) Single domain variable antibody fragments, b) Fab, c) natural ligands like cytokines and receptors, d) B cell receptor ligands, e) TCR ligands. scFv, single chain variable fragment; Fab, fragment antigen binding; TCR, T cell receptor; MHC, major histocompatibility complex. * IL-13, FcεRI, Ly49H, $ Egg lysozyme, Desmoglein-3, factor VIII.
3.1.1. Single chain variable antibody fragments
ScFvs consist of the antibody variable light-chain (VL) tethered to the variable heavy-chain (VH) of the same antibody by virtue of a peptide linker (Fig. 2a). They comprise the predominant type of extracellular ligand recognition domains (ELRD) used for the creation of CARs [33,34]. ScFv fused to T cell receptor subunits were first described by Eshhar et al. in 1993 and many different scFvs for CAR T-cell therapy have been developed since then [14]. While several CARs have been evaluated in preclinical studies, some have entered clinical trials such as CD20 (NCT03576807) and a few have already been FDA/EMA approved such as those targeting CD19 used in Kymriah and Yescarta [35,36]. Other targets of scFvs currently being investigated clinically or pre-clinically are mesothelin, an antigen expressed on many different malignant tumors such as ovarian and pancreatic cancers, the prostate specific membrane antigen (PSMA) as well as epidermal growth factor receptor (EGFR) vIII, representing a mutated form of EGFRIII present in glioblastoma which is commonly expressed also on other tumors [37–39]. B cell maturation antigen (BCMA, CD269) represents another important differentiation molecule targeted by a CAR. BCMA is a cell surface receptor of the TNF-receptor superfamily 17 (TNFRSF17), which is highly expressed on malignant myeloma cells and specifically binds B cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL) [40–42]. Ligand-induced signaling via BCMA promotes the growth of myeloma cells [43]. An scFv-based CAR directed against BCMA (CART-BCMA) has been tested in a Phase I clinical trial with patients suffering from relapsed multiple myeloma recently where it showed a toxicity profile similar to that of CD19-specific CAR T cells with excellent clinical response rates ranging from 20 to 64% [44].
One interesting general question in that context is the selection of scFvs with optimal affinity for the target antigen. Does high affinity binding of the scFv necessarily translate into better CAR cell efficacy? For example, choosing an scFv with too high a affinity might result in profound T cell activation, however, at the expense of reduced mobility of the CAR T cell as it might get stuck to the cell expressing the target antigen and thus preventing serial killing [45,46]. On the other, too low affinity might increase the number of non-engaged CARs and thus lead to inappropriate cellular activation.
3.1.2. Fabs
Although scFvs are the most frequently used extracellular ligand recognition domains nowadays, the first chimeric receptors targeting antigen and inducing antigen-specific signaling were antibody variable regions fused to T cell receptor constant regions (Fig. 2b) as described by Kuwana et al. as early as in the year 1987 [47]. However, this concept has by far not been as intensively pursued as the more widely used scFvs. Nevertheless, an adaptation of this Fab concept has experienced a recent revival in a study describing the fusion of CD19-specific antibody Fab fragments to TCR γ and δ constant regions. This design allows for the assembly of so-called ‘antibody TCRs’ which efficiently associate with the CD3 signaling complex of molecules endowing them with several advantageous features over classical CARs, like reduced cytokine release associated with less T cell exhaustion [48]. Moreover, compared to the classical concept applying Fab fragments fused to αβTCR chains this new concept avoids mispairings with the endogenously expressed αβTCR chains because usually these two chain types do not mispair [49]. The refinement of TCR constant domain-based CARs might prove to be successful, since this CAR generation relies on the interplay of the individual components of the primary TCR signaling machinery, instead of a non-physiologic, chimeric intracellular domain, possibly permitting improvement of cellular activation.
3.1.3. Natural ligands, natural receptors and B cell receptor ligands
CAR cells expressing the cytokine IL-13 (Fig. 2c) and intended to target tumor cell-associated IL13 receptor(R)α2 have been designed and underwent clinical testing in patients with glioblastoma in which the IL-13Rα2 is overexpressed on the malignant but not in the normal brain tissue of the majority of patients [50]. An elegant example of natural receptors used for targeting virus infected cells with CARs is Ly49H, the activating natural killer cell receptor, which has been shown in one study in mice to protect from lethal CMV infection [51]. Notably, CD8+ T cells expressing the chimeric antigen receptor comprised of Ly49H as the ligand recognition domain and fused to the ζ-chain intracytoplasmic signaling domain effectively protected mice by clearing cells, which expressed viral antigen (Fig. 2c) [51].
In principle, the majority of CARs known as of today have been designed for the treatment of malignant diseases in which, ideally, the targeted antigen is densely expressed on the surface of malignant but not normal cells. However, recently the exploitation of CARs for the treatment of auto-immune diseases was increasingly moving into the scientific focus. Herein, the selective targeting of subsets of autoantigen-specific immune cells becomes the primary goal to alleviate or even cure disease. In initial studies targeting autoantibody-producing B cells, Nguyen et al. equipped CAR T cells with hen egg lysozyme linked to intracellular signaling domains (Fig. 2d). Such T cells showed cytolytic potential in vitro and in vivo against antigen-specific B cells even in the presence of secreted, CAR-specific antibodies. However, when transferred into mice, these CAR T cells were found to be immunogenic because they induced an antibody response against the extracellular ligand recognition domain, i.e., HEL, showing that despite their ability to selectively kill antigen specific B cells, some B cells may escape elimination resulting in persistent antibody responses [52]. In another study relying on similar technology and conducted by Ellebrecht et al. the target antigen desmoglein-3 was used as ligand recognition domain of a CAR (Fig. 2d) to efficiently and specifically kill anti-desmoglein-3 antibody producing hybridoma cells in vivo [53]. Desmoglein-3 is one of the key target antigens in pemphigus vulgaris, an auto-immune disease characterized by the aberrant production of auto-antibodies against this important adhesion protein expressed by keratinocytes, who’s functional blockage results in the loosening of the interconnections between the lower layers of keratinocytes within the dermis resulting in the formation of skin blisters and detachment of the epidermis [54]. A further elegant approach established along those lines was the use of blood clotting factor VIII CAR expressing cytotoxic T cells, to kill anti-blood clotting factor VIII producing hybridomas in vitro and in vivo recently [55]. Moreover, blood clotting factor VIII -/- mice treated with blood clotting factor VIII CAR expressing cytotoxic T cells in the same study were able to protect from a factor VIII immunization-related increase in anti-factor VIII antibodies in vivo [55]. Hemophilia patients requiring substitution with recombinant blood clotting factor VIII to avoid bleeding, frequently mount neutralizing antibody responses against factor VIII, which represents a severe impairment for long-term treatment. In a very recent approach, one group aimed at targeting IgE-producing B cells with a CAR consisting of the high affinity FcεRI on its extracellular ligand recognition domain [56]. These CARs were able to target IgE producing cells but not effector cells (basophils) binding free IgE via their FcεRs [56]. The major disadvantage of this approach is that high levels of IgE would inhibit such CAR T cells by interfering with their binding to IgE+ B cells. However, mutated forms of the CARs’ extracellular FcεRI domain were generated which are supposed to circumvent this problem of interception by free IgE due to reduced affinity [56]. The last examples underscore, how elegantly and precisely wellknown target antigens in auto-immune and auto-inflammatory diseases can be adopted to specifically target antigen-specific B cells/plasma cells, which may allow to ameliorate or even cure a variety of autoimmune/allergic diseases with almost no off-target effects in the future. Especially in the field of allergies this concept could be highly interesting, since many allergens are nowadays characterized on the molecular level and humanized mouse models are available [57–59]. One could imagine the use of “Allergen-CARs” in the future where the major allergen towards which the patient reacts could be used for the construction of a chimeric antigen receptor. This would allow for the selective elimination of allergen-specific B cells and specific Ig bearing mast cells and basophils thus also inhibiting the type I hypersensitivity reaction associated with allergen-specific IgE. However, the risk of such a therapy potentially leading to effector cell activation is hard to predict. Nevertheless, future developments in the field of CAR cell therapy could make the therapy safe even for this application. This would be especially interesting for food allergens which are supposed to be one of the most common causes of anaphylaxis [60].
3.1.4. Bona fide TCR ligands Bona fide TCR ligands
One of the clear benefits of CAR cell therapy is its independence of MHC restriction. However, since auto-reactive T cells commonly recognize peptide antigens only when they are presented by the respective MHC, some groups have resorted to the application of peptide/MHC (pMHC) complexes as antigen-recognition domains of CARs (Fig. 2e). Such pMHC expressing CAR T cells eliminate auto-reactive T cells expressing pMHC-specific T cells which express pMHC-specific TCRs of different clonality [61]. This opens the possibility for the treatment of autoimmune diseases that are preferentially driven by auto-reactive T cells.
For instance, in type I diabetes, CTLs are implicated to be important for the development of the disease. To specifically target diabetogenic CD8+ T cells, a CAR consisting of the insulin peptide InsB15-23 linked to β-2 microglobulin, the monomorphic binding partner of HLA Class I alpha chains, was fused to the intracellular CD3ζ signaling domain [62]. Notably, receptor modified T cells (RMTCs), which expressed this CAR, efficiently eliminated insulin-reactive CD8+ T cells which resulted in a lower incidence and a delayed onset of diabetes in a preclinical model of diabetes [62].
In experimental autoimmune encephalomyelitis (EAE), which is used as a preclinical model for multiple sclerosis in humans (MS), CD4+ lymphocytes play an important role for disease initiation and propagation. In fact, they represent the major cell type found in the inflammatory infiltrates in the brain [63]. Because these cells recognize their cognate peptide presented by MHC class II molecules, Mekala et al. have used a combination of myelin basic protein (MBP) and MHC class II molecules to build the extracellular antigen-recognition domain of that 1st-generation CAR [64,65]. In vivo cytolysis of target cells by such RMTCs was found to be efficient, with CAR cells undergoing vigorous proliferation. Moreover, the prevention of EAE by such CARs was significant but more importantly it was also able to alleviate established disease, showing lower disease severity and increased survival of animals already suffering from EAE [64,65]. CAR systems relying on HLA molecules as extracellular ligand recognition domain could also be beneficial to remove unwanted clonal T cell populations as observed in allergic diseases and their potency could be easily evaluated in preclinical humanised allergy models [57,66].
3.2. Prevention of antigen escape
Despite the high efficacy of CAR treatment of B-cell malignancies, antigen escape remains an important cause of relapse. In CD19 CAR trials, relapse rates varied between 29% and 57%, with up to 75% of relapsed patients presenting with CD19− B cell blasts [31]. While such leukemia cells lack the initially targeted CAR antigen, they continue to express other B cell specific differentiation antigens. Therefore, initial targeting of multiple antigens by CAR cells might help to prevent the selection of antigen escape variants.
CAR T cells expressing two different receptors (Fig. 1e), each recognizing a B cell-specific antigen, e.g., CD19 and CD20, have shown to efficiently prevent relapse due to antigen escape [22,67,68]. Along those lines, Hegde et al. demonstrated that lymphocytes concomitantly transduced with both CAR constructs showed greater efficacy than co-administration of two CAR T cell lines expressing the respective CARs on two separate cellular entities [68]. Bielamowicz et al. have developed this concept even further by transducing T cells with a tri-cistronic expression vector coding for three chimeric receptors recognizing three distinct tumor antigens (Fig. 1f) and treatment with such CAR cells was shown to be efficient in an in vivo mouse model of glioblastoma [69]. Increasing the number of targeted antigens may be useful for the treatment of tumors, which express various degrees of tumour antigens, however, this maneuver potentially also increases the frequencies of ‘on-target, off-tumor’ effects, which must be taken into consideration.
Instead of expressing two (or three) separate chimeric receptors, Hegde et al. have created ‘tandem CARs’ (tanCAR) that consecutively connect two antigen-binding domains – HER2-binding scFv and IL13R-α2 – in a single CAR (Fig. 1g). Such ‘OR-gate’ CARs are activated upon binding of either of the two antigens and it was shown that this prevents antigen escape and improves survival in animal tumor models [70]. A similar concept was put forward by Zah et al. targeting both CD19 and CD20 antigens. Besides their importance for the prevention of antigen escape, CARs with dual specificity have also demonstrated that the length of the linkers between two scFv domains and those linking VH and VL chains of an individual CAR greatly affect T cell activation, pointing to the importance of a thoughtful CAR design with well-specified linker lengths [71].
3.3. Prevention of ‘on-target, off-tumor’ activity
A clinical case of a patient, treated with an ErbB2-specific CAR therapy who experienced a detrimental cytokine storm five days after CAR T cell infusion, because of the preferential trafficking of the CAR T cells to the healthy lungs of the patient, possibly due to the low level expression of ErbB2 in the lungs under physiological conditions, clearly highlights the importance of the specificity of CAR treatment [32].
A possible strategy to increase target cell specificity would be to target two tumor antigens that are simultaneously expressed in tumor but not healthy tissues. ‘AND-gate’ CARs are specific for two different antigens but unlike ‘OR-gate’ CARs, that are activated in the presence of any of the two antigens, ‘AND-gate’ CARs exhibit cytotoxic effects only in the concomitant presence of both antigens (preferably on the same cell). This technology is used by the ‘synNotch’ receptor, which consists of an antigen receptor linked to the intracellular transcription factor TetR-VP64 or Gal4.VP64. Upon ligation of the synNotch receptor the transcription factor becomes cleaved from the receptor and subsequently binds to its respective tetracycline-controlled trans-activator (tTA), which in turn directs the expression of the CAR that recognizes a second tumor antigen (Fig. 1h). It was shown that such receptors successfully eliminated GFP+CD19+ tumors while sparing those expressing only a single antigen. In theory, also ‘AND-gate’ CARs could become primed at one site in the body, express the CAR gene and then get translocated to another site where they may cause undesired ‘on-target, off-tumor’ effects. However, this was not observed in animal models carrying two distantly-located tumors, one expressing both antigens and the other only expressing a single antigen. One of the mechanisms that prevented the translocation of the T cells is possibly brought about by the rapid downregulation of CAR expression upon cessation of the first synNotch-related stimulus [72].
Since for efficient tumor eradication, CAR constructs need to express both an activation domain (usually provided by CD3-ζ) and a costimulatory domain (most often provided by CD28 or 4-1BB), the separation of the two on distinct antigen recognition molecules may increase overall specificity. Indeed, Kloss et al. have shown in a prostate cancer specific model that the dissociation of the costimulatory CD28–4-1BB domain expressed along with an PSMA-scFv from a CD3-ζ signaling domain linked to the PSCA-scFv efficiently eradicated prostate tumors expressing both prostate-specific antigens but showed only limited activity against tumors expressing a single tumor antigen (Fig. 1i) [73]. Nevertheless, when selecting CD19 and PSMA as a model for two unrelated targeting antigens, CAR T cells still showed strong residual cytotoxic activity against cells exclusively expressing the CD19 antigen, pointing to the importance of appropriate antigen selection [73]. Lanitis et al. made similar observations with such CARs, showing preferential targeting of tissues expressing two tumor antigens, while lower numbers of CAR T cells accumulated in tumors expressing either one of the tumor antigens [74]. Whether diminished but not abolished activity against tissues expressing only one targeted tumor antigen would be sufficient to avoid serious side effects against normal tissues remains unanswered so far.
An alternative approach was followed by Fedorov and colleagues, who established inhibitory CARs (iCARs) (Fig. 1j). Inhibitory CARs accommodate inhibitory intracellular domains instead of activating ones and thus inhibit cellular activation upon recognition of the respective antigen. Co-expression of the activating CD19::CD28::ζ CARs with an inhibitory PSMA::CTLA-4 or PSMA::PD-1 CAR resulted in strong CAR T cell inhibition upon recognition of both antigens. However, such inhibition could be overruled in cases in which the primary tumor antigen was recognized exclusively [75]. While the co-introduction into CAR T cells of inhibitory receptors proved to be efficient in preventing ‘on-target, off-tumor’ effects, the identification of appropriate antigens for the activation of such inhibitory receptors might prove to be difficult due to the variability of antigens expressed on the many non-tumor tissues.
To circumvent the need for the selection of two different antigens, ‘masked CARs’ have been developed which take advantage of the presence of proteases in the tumor microenvironment for their unmasking (Fig. 1k). These CARs are based on pro-bodies, and consist of a scFv domain protected by a linker that can be cleaved-off by proteases which are typically secreted by the tumors of interest. Han et al. showed that the masking linkers were efficiently cleaved off once CAR T cells entered into the tumor and their resulting effects on tumor elimination in vivo were comparable to the respective conventional CAR constructs. However, it was also observed that some tumors tended to secrete lower amounts of proteases resulting in incomplete activation of CAR T cells in such environments [12].
3.4. Elimination of CAR T cells
While the prevention of ‘on-target, off-tumor’ activity avoids undesired targeting effects, tumor targeting may still evoke severe side effects such as neurotoxicity and cytokine release syndrome (CRS). Although CRS can be effectively treated with the anti-IL-6 monoclonal antibody tocilizumab, additional immunosuppressive treatment with corticosteroids may be needed [76]. Of note, long-term persistence of CAR T cells may lead to the permanent elimination of cells carrying the targeted antigen which can have undesired side effects, such as those observed in patients undergoing CD19 CAR therapy and presenting with B-cell aplasia [76].
To alleviate such side effects, various groups have investigated the possibility to eliminate CAR cells by the activation of suicide genes once full remission has been achieved. Inducible caspase 9 (iCasp9) represents such a suicide gene that connects the intracellular domain of the pro-apoptotic protein caspase 9 to the extracellular FK506 binding protein (FKBP). Upon administration of the chemical inducer for dimerization (CID) iCasp9 dimerizes and leads to programmed cell death [77]. Several studies have demonstrated that this mechanism can be efficiently applied in vivo and several clinical studies introduced iCasp9 into their model as a safety switch (e.g., NCT03016377, NCT03696784) [78–80].
Co-expression on CAR lymphocytes of neo-antigens that can be deliberately targeted with antibodies and allow for their elimination via antibody-dependent cellular cytotoxicity (ADCC) represent further possibilities for the targeted elimination of CAR cells. Accordingly, a truncated version of human epidermal growth factor receptor (huEGFRt) that can be targeted by the anti-EGFR monoclonal antibody cetuximab was shown to efficiently eliminate CAR T cells in vivo. Additionally, huEGFRt was shown to be useful for the purification of CAR T cells during the production process as well as for tracking purposes (e.g., NCT03085173, NCT03618381) [81].
3.5. Universal CAR systems
Commonly, CAR T cells target their specific antigens with the help of pre-defined cell-surface expressed scFvs, which are linked to potent intracellular T cell signaling domains, endowing the transduced lymphocytes with a monospecific reaction potential. Thus, the creation of CAR systems that could switch their target antigen specificity would represent certain advantages over conventional CAR expression systems. For instance, UniCARs express an extracellular “anti-Tag” scFv that specifically binds to the tagging sequence, which had been attached to the respective targeting-antibodies and which become co-applied with the CAR T cells to opsonize the tumor cells of choice (Fig. 1l). The tagging sequence is specifically recognized by the UniCAR “anti-Tag” scFv and such recognition leads to specific lysis of target cells to which the tagged antibody has bound [82].
An even more straightforward system was proposed by Urbanska et al. who used a biotin-binding immune receptor (BBIR) containing a dimeric form of avidin that binds to biotinylated tumor targeting antibodies with high affinity (Fig. 1m) [83,84]. When such CAR cells are incubated with two different antibodies this allows for the targeting of two different antigens, although sequential targeting is also possible by sequential administration of antibodies. Although biotin is physiologically present in all bodily fluids, even concentrations 20-times higher than the physiological levels were found to be unable to block the recognition of the biotinylated antibodies by the avidin expressing CARs [85].
The Split Universal Programmable (SUPRA) CAR system allows for even more sophisticated fine-tuning of the targeting and killing process. It consists of an RR zipCAR that contains a leucine zipper instead of an antigen-recognizing scFv and recognizes EE zipFv-tagged antibodies (Fig. 1n) [86]. Upon binding of RR zipCar to the EE zipFv tag the CAR cells become activated, which leads to the elimination of the target cells. Additionally, this system allows to fine-tune antibody affinity via the modification of the leucine zipper, which helps to regulate killing efficiency. However, the addition of the competitive RR zipFv leads to the binding of EE zipFv to RR zipFv which results in neutralization of the EE zipFv tag and thus works as an “OFF switch”. Since zipCARs can be linked to different intracellular signaling domains, distinct signaling pathways may be activated upon binding of receptors to different antigens, including the possibility of specific transduction of different cell types. Indeed, such systems offer a multitude of possibilities for variation and optimization, from “OFF switch” to affinity modulation and targeting of antigen combinations, however, its efficacy and applicability in the human system still needs to be thoroughly evaluated.
Another interesting concept was described by Clémenceau et al. in 2006 which made use of a fusion construct consisting of the FcγRIIIa extracellular domain and FcεRγ1 intracellular part and allowed T cells to kill an autologous B-lymphoblastoid cell line upon coating with anti-CD20 mAb [87]. The principal idea of this concept was to combine such transduced T cells with a monoclonal antibody for targeting malignant cells which had been opsonized with a monoclonal antibody. Although interesting, we believe this concept will face many disadvantages in the real-life clinical setting such as the potential binding of autoantibodies to the T cell expressed FcR eventually generating self-reactive T cells. Moreover, occupation of the T cell-expressed FcR by other Ig present in the patient serum might lower the efficiency of the binding of the specific antibody even when administered in excess.
With the discovery of the new disease-related antigens the applicability of CAR therapy is broadening and could be further extended and clinically implemented to non-neoplastic diseases such as autoimmune and infectious diseases, or allergies. To be able to target the pathognomonic cell types in such diseases the appropriate antigens need to be identified. That gave rise to various antigen-finding platforms, such as MCR-TCR chimeric receptors, that could help further broaden the applicability of CAR therapy [88]. Although the identification of novel antigens is important, the affinity to these antigens might also play an important part since a too high affinity is known to potentially lower the killing efficiency due to interference with serial killing [45,46]. Universal CAR systems that enable the modification of the CAR affinity could help determine optimal affinities in that respect.
Concepts such as the UniCAR systems promise certain fascinating advantages such as flexibility and adaptability. Moreover, since the activity of the CAR T cell is supposed to be dependent on the presence of the respective tagged antibody/scFv from here on referred to as “module” also the pharmacological properties of the CAR T cell activity get linked to this module. This linking might also allow for a better control and monitoring of the therapy. We believe that such platforms offer certain highly advantageous features since they are more flexible as the target antigen can be switched easily. One major disadvantage of the UniCAR concept compared to the traditional CAR concept could be, that in the case of malignant diseases the UniCAR would lose its immune-surveillance function in the absence of regular application of the respective module. On the other hand, UniCAR cells can easily be switched off which, in the case of using CD19 as the targeting structure, would allow for reconstitution of B cells in the further presence of UniCAR cells. Nevertheless, similar effects could be achieved by applying the classical CAR concept together with a kill-switch, e.g., dimerizable caspases. The implementation of such a safety switch which even allows dose dependent CAR T cells reduction has already been described in preclinical studies [80].
4. Enhancing the activity of CAR cells
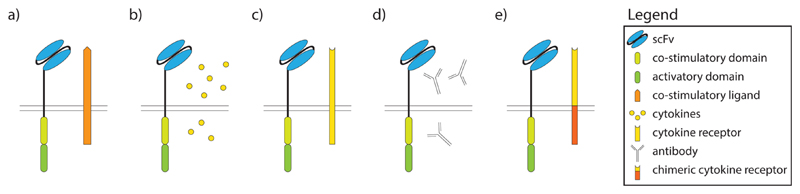
Lately the focus of CAR cell improvement has expanded to the coexpression of cytokines and/or additional stimulatory receptors in CAR cells. In this chapter we focus first on the co-expression of stimulatory ligands or cytokines and their receptors. While the cell activity can be enhanced by direct stimulation (e.g., with cytokines (Fig. 3b)), the interference with inhibitory signals may have a similar effect. Upon CAR ligation, such interference can be achieved by the expression of inhibitory antibodies or chimeric antigen receptors that convert an inhibitory into a stimulatory signal (Fig. 3d and e). In addition to the direct anti-tumor effect, such modulated CAR cells may have a beneficial impact also on the tumor microenvironment and other immune cells for instance by stimulating the innate immune cells and directing them towards cancer cells [89].
Fig. 3. The strategies to enhance the activity of CAR cells.
a) co-expression of cytokine receptor, b) co-transduction with cytokines, c) expression of co-stimulatory ligands, d) co-transduction with inhibitory antibodies, e) expression of chimeric cytokine receptors.
4.1. Co-expression of stimulatory ligands
Co-stimulatory proteins have proven to be effective for tumor elimination. One such protein is the CD40 ligand (CD154) which becomes expressed on activated T cells. When it binds to its receptor CD40, expressed on tumor cells, direct tumor cell apoptosis is induced, while engagement of the same receptor on dendritic cells increases the recruitment of T cells with anti-tumor activity [90]. Antibodies targeting the CD40L/CD40 axis have shown promising clinical results indicating that the co-expression of CD40L along with a chimeric TCR receptor on cytotoxic lymphocytes may lead to augmented anti-tumor activity [91]. Indeed, Curran et al. have shown enhanced cytotoxicity of CD19-CAR T cells against CD40+ cancer cells leading to prolonged survival of tumor-bearing mice [92].
Another protein of interest is 4-1BB (CD137), a stimulatory receptor belonging to the TNF surface receptor family that is expressed on T cells and other immune cells. 4-1BB increases survival and induces cytolytic cell activity upon ligation with its ligand, i.e., 4-1BBL that is expressed on antigen presenting cells (APCs) [93]. To benefit from 4-1BBL-driven signals, Zhao et al. have expressed full-length 4-1BBL on the surface of T cells together with a CD19-CD28::ζ CAR (Fig. 3a). This combination engaged both the CD28 and 4-1BB signaling pathways in CAR T cells and, in turn, reduced PD-1-, LAG-3- and TIM-3-driven inhibitory signals. The in vivo application of these CAR T cells resulted in higher survival rates, stronger and more rapid T cell proliferation, longer T cell persistence and faster tumour eradication [16].
4.2. Secretion of cytokines
Cytokines are small proteins which are important for the differentiation and function of immune cells. Accordingly, many cytokines (e.g., IL-2, -12, -18 and -21) have been tested in clinical trials for their potential to treat cancer [94]. However, systemic administration of e.g., IL-12 for the treatment of multiple myeloma resulted in serious side effects [95]. To reduce the toxic side effects, intratumoral administration of IL-12 was tested in head and neck squamous cell carcinoma patients, which attracted and activated CTL and NK cells, however, still at the expense of serious toxicity [95,96].
To overcome that problem but still taking advantage of the cytokines’ action, Pegram et al. have transduced T cells with a bicistronic vector coding for a CD19-CAR and IL-12, resulting in constitutive expression of IL-12 from CAR T cells (Fig. 3b). These CAR T cells were preferentially homing to tumor sites where they secreted IL-12 which contributed to the efficient eradication of the tumor [97]. CD19-IL-12 CAR T cells were also resistant to the inhibitory effects of the regulatory T (Treg) cells, which gets important when T cells move to the tumor and become exposed to its microenvironment since it is known that Treg cells within the tumor dampen the anti-tumor response [97,98]. Additionally, the T cells’ persistence at the tumor site was prolonged along with clear signs for the activation of macrophages [99,100]. Nevertheless, even under these circumstances, IL-12 secretion levels needed to be tightly regulated since toxic effects of constitutively expressed IL-12 in CAR T cells were observed when T cells were administered in large numbers. However, exuberant toxicity could be avoided when IL-12 expression was induced exclusively upon antigen stimulation [101]. In addition to the aforementioned benefits of IL-12, Pegram et al. also demonstrated that preconditioning with cyclophosphamide was not strictly required to establish CAR T cells upon adoptive transfer, while other studies obtained different results [97,101].
Another cytokine that potently stimulates NK and CD8+ T cells is IL-15. Although administration of IL-15 seems to be less toxic, its activity is limited due to a very short in vivo half-life. While mono-therapy with IL-15 showed only limited effects on tumor regression, it revealed great potential when combined with other immunotherapeutic agents [102]. For instance, constitutive expression on one cistron of IL-15 together with a 1st generation CD19-CAR was shown to significantly improve the antitumor activity of T cells in vivo leading to greater expansion and longer-lasting persistence of CAR T cells [78].
4.3. Co-expression of cytokine receptors
Another aspect which has to be taken into account is the fact that only cells that carry the appropriate receptor for a respective cytokine will also sense the presence of that very cytokine. IL-2 is known as a potent activation and differentiation factor of T cells, however, the IL-2 receptor (IL2R) is expressed on all T cells in different configurations (high-affinity, low affinity), and it can be especially well sensed by Treg cells due to their expression of the high-affinity IL-2R CD25 [103]. The secretion of IL-2 by activated T cells was shown to lead to Treg cell accumulation within the tumor and thus lower efficacy of infiltrating CAR T cells [104]. While the IL2R is present on all T cell subsets in different configurations including Treg cells, IL-7 preferentially stimulates naive and memory T cells without having an effect on Treg cells that characteristically down-regulate the respective high-affinity IL-7 receptor-α chain (IL7R-α, CD127) [105,106]. The lack of expression of the IL7R-α on cytotoxic T cells limits its use for the expansion of CAR T cells [107]. To overcome this shortfall, Perna et al. have transduced virus-specific CTLs with a GD2-CAR and an expressible IL7R-α chain (Fig. 3c). This resulted in proliferation of these CAR T cells upon administration of IL-7 but had no effects on Treg cells. Of note, when such CAR- and IL-7Rα-expressing T cells were administered together with IL-7 growth of xenografted neuroblastoma cells was controlled even in the presence of Treg cells and supplementation with IL-2 [108].
4.4. Secretion of antibodies – Interference with inhibitory signals
In general, the tumor microenvironment is well-known for its expression of several inhibitory factors [109]. While stimulation of T cells may protect them from the inhibitory cues derived from the inhibitory tumor microenvironment, specific interference with tumor-derived inhibitors could also be actively used for enhancing T cell activity.
Programmed cell death protein 1 (PD-1) is an immunomodulatory receptor expressed on T cells [110]. PD-L1, its immunosuppressive ligand is known to be highly expressed in many tumors, and it is responsible for the inhibition of immune responses within the tumor. PD-1 antibodies inhibit the interaction between PD-1 and PD-L1 resulting in enhanced T cell function [111]. In fact, systemic administration of PD-1 inhibitors has led to a clinically significant antitumor response in various types of tumors [112]. To combine the PD-1/PD-L1-based inhibition with CAR therapy, Rafiq et al. have co-expressed a CD19–CAR together with a secreted form of an anti-PD-1-specific scFv able to inhibit PD-1/PD-L1 interaction (Fig. 3d). This resulted in constitutive production and secretion of the PD-1-specific scFv in vivo at the site(s) CAR cells re-located to. Secreted PD-1 antibodies bound to PD-1 and protected not only CAR T cells but also bystander T cells present within the tumor, while the cells outside of the tumor were not affected. This resulted in the generation of a more vigorous anti-tumor response and CD19-PD-1-CAR T therapy was shown to be superior to the combination therapy of CAR T cells and PD-1 inhibitors [113].
4.5. Chimeric cytokine receptors
A similar concept was followed using cytokine receptors, combining receptors that express inhibitory, cytokine-specific extracellular domains linked to the intracellular domain of an immunostimulatory cytokine receptor, thus converting any inhibitory stimulus into a stimulatory one (Fig. 3e) [114].
To take advantage of such conversion, Stegen et al. have ligated the extracellular domain of the IL4R-α chain to the intracellular, signaling domain of the IL2R-β chain, thereby creating a receptor termed 4αβ. The co-expression of 4αβ with an ErbB-CAR allowed for a selective expansion of CAR T cells upon addition of IL-4 to the cell culture medium [115]. Intra-tumoral application of such CAR T cells generated with the help of exogenously added IL-4 is now being tested in a clinical study of advanced head and neck cancer (NCT01818323). A similar approach was adopted by Mohammed et al. in which they have applied a PSCA-28::ζ CAR with a chimeric receptor linking the extracellular part of the IL-4 receptor to the intracellular IL-7Rα signaling domain. They have shown that addition of IL-4 leads to selective expansion of CAR T cells even in the absence of added IL-2 [116]. This might also help avoiding undesired expansion of Treg cells as mentioned in chapter 4.2. Moreover, it has been shown that the inhibitory PD-1 signal can be directly converted into an activation signal, by fusion of the extracellular domain of PD-1 to the intracellular domain of CD28 and co-expressed together with a 2nd generation CAR using a 4-1BB::ζ intracellular domain. This prevented the tumor-induced inhibition of T cell function and mice treated with the combination of PD1::CD28 CAR T cells had a longer survival, which was associated with significantly increased tumor regression [117].
The advances in the field of the CAR cell activity enhancement are shifting the focus from the mere optimization of the CAR construct to more holistic approaches in which the overall cellular activation of the CAR cells is being addressed. The introduction of additional cytokine stimuli puts the need for costimulatory domains within the CAR construct in question. Additionally, such enhanced cell therapies could lower the need for multiple drug application (e.g., co-application of PD-1 antibodies along with CAR cells) or chemotherapeutic pre-conditioning.
5. Cells used in CAR therapy
Immune cells represent the pivotal backbone of CAR therapy and the therapeutic power of the treatment not only depends on CAR design but also on the phenotype, further differentiation and longevity of the cells that become equipped with CARs and are transferred back into the patients [118]. For the production of CAR cells, usually peripheral blood mononuclear cells (PBMCs) are collected from a patient which then become expanded in the presence of different stimuli (e.g., anti-CD3 antibodies, interleukins and/or feeder cells) (Table 2). CAR fusion genes are then introduced into their genome either by electroporation or viral transduction (Table 3) and cells become further expanded. Once adequate numbers of cells are obtained the cells are re-infused into the patient [119]. Although research initially centered on the use of autologous cytotoxic T cells, other cell types such as NK cells but also NK cell lines have gained increased attention recently. This chapter focuses on T cells and NK cells as cellular backbones for the expression of therapeutic CARs as well as genetically modified cell lines designed for universal application.
Table 2. Cell types and corresponding expansion protocols frequently used to express CARs.
| Cell type | Culture conditions for expansion of CARs |
References | ||||
|---|---|---|---|---|---|---|
| Antibodies | Interleukins | Feeder cells | Sorting | Others | ||
| PBMC | CD3, CD28 | IL-2, IL-15, Il-17, IL-4 | NIH-3T3-CD19-CD80 aAPCs, K562-PSCA | Gentamicin resistance, magnetic bead based isolation of CD4+CD25+ T cells | PHA, PMA, Th17 polarizing conditions, anti-ICOS beads | [12,16,17,68,69,70,75,92,100,104,115,116,123,127,129,135,167,169,170,172,173,174,180,182] |
| Splenocytes, CD3+ splenocytes | CD3, CD28 | IL-2, IL-7 | Concanavalin A, PHA | [101,113,132,175,181] | ||
| CD8+ and CD4+ PBMC | CD3, CD28 | IL-2, IL-7, IL-15 | CD19+ LCL | Negative or positive selection | With or without defined CD4+/CD8+ ratio, grown separately or together | [85,86,122,166,1,72,74,79,117,168] |
| CD8 + TCM and CD4+ PBMC | CD3, CD28 | IL-2 | CD19+ EBV LCL | [124] | ||
| CD8 + TCMs form PBMC | CD3, CD28 | IL-2 | CD19+ LCL | [29] | ||
| CD3+ and CD8+ PBMC | IL2, IL-15 | K562, NALM-6, aAPC7mOKT3 expressing OKT3, CD80 and CD83 | [24] | |||
| CD3+ cells from PBMC | CD3, CD28 | IL-2 | PMA | [2,78,99] | ||
| EBV-specific CTL | CD3 | IL-2, IL-7 | EBV-LBL | [5,108,128] | ||
| Gamma-delta T cells | IL-2, IL-21 | Non-specified feeder cells | Negative selection w/magnetic beads | Zoledronic acid | [97,132] | |
| Treg | CD3 | IL-2 | Non-specified feeder cells | Sorting for CD4+ CD25ROlow CD24RAhigh CD25high | [138] | |
| NK-92, NK-92 MI cell lines | IL-2 | Horse sera, human sera, X-VIVO medium | [71,150,151,152,177] | |||
| PB NK cells | IL-2 | K562-mb15-41BBL | [141,142,143,171] | |||
| NK and gamma-delta T cells | CD3 | IL-2 | K562-mb15-41bbL | Zometa, IFN gamma | [7] | |
PBMC peripheral blood mononuclear cells, CD cluster of differentiation, PHA phytohemagglutinin, PMA phorbol myristate acetate, CTL cytotoxic T lymphocyte, EBV Epstein-Barr virus, Treg regulatory T cells, NK natural killer, PB peripheral blood, IFN interferon, aAPC artificial antigen presenting cell, ICOS inducible T-cell costimulator, PSCA prostate stem cell antigen, LBL lymphoblastic lymphoma.
Table 3. Expression and packaging vector systems and virus producer cells lines used to deliver CAR constructs to effector cells.
| References | ||
|---|---|---|
| Retroviral vectors | SFG | [2,5,16,17,68,69,70,75,78,79,92,97,99,108,115,116,127,128,142,182] |
| pMX | [24] | |
| pSAMEN | [172] | |
| pMP71 | [12,175] | |
| pLXSN | [123] | |
| pMSCV | [68,141,143,171] | |
| pMSGV | [101,173,174] | |
| Lentiviral vectors | epHIV7 | [29,117,122,124,166] |
| pLenO | [149] | |
| pSIN | [89] | |
| pSIEW | [150,177] | |
| pELPS | [67,168] | |
| pELNS | [1,74,85] | |
| pHR | [72,86] | |
| Packaging constructs | psPAX2 | [122,124,166] |
| pRDF | [2,69,70,78,141,160,171,182] | |
| pEQ-PAM3(-E) | [141,171] | |
| pVSVg | [1,74,85,150,152,168,177] | |
| pCL | [143,152] | |
| pMEVSVg | [68] | |
| pMD2G | [72,86,122,124,149,166] | |
| pMDLg/p.RRE | [1,74,85,149,168] | |
| pRSV.REV | [1,74,85,149] | |
| pTSV.rev | [168] | |
| pCHGP | [29,117] | |
| pCMV-g, pCMV-Rev2, pCMVdR8.91 | [29,72,117] | |
| pGALV | [12] | |
| Virus producer cell lines | pG13 cell line | [5,22,115,128,172,173,174] |
| GP + e86 cell line | [101,152,181] | |
| Phoenix Eco cell line | [5,97,113,128,142] | |
| HEK 293T cell line | [1,2,12,17,29,74,78,79,100,104,108,116,117,124,138,141,143,149,150,160,166,168,171,175] | |
| GP 293T cell line | [123,175] | |
| 293-Glv9 cell line | [92,99,113] | |
| Lenti-X 293T | [72] | |
| NIH 293T cells | [182] | |
| FLYRD18 cell line | [142] | |
| H92 cell line | [75,113] |
5.1. T cells and their subtypes
T lymphocytes are well-known for their central role in cell-mediated immunity [34]. They consist of several subsets, each one able to execute very different effector functions [120].
5.1.1. CD4+ and CD8+ T cells
Due to their cytotoxic potential, CD8+ T cells were the cell type of choice in the first CAR study [14]. Although cytotoxic effects were observed, it was recognized only later that the sole infusion of CD8+ T cells does not produce long-lasting effects in patients. Instead, the co-application of CD4+ lymphocytes was found to increase T cell longevity [121]. Because T cells are usually obtained from the peripheral blood of patients, the inter-individual cellular composition may vary considerably. Even more so in cancer patients who may have received one or more cycles of chemotherapy, resulting in skewed composition of peripheral T cell subsets [122]. To avoid such inter-individual differences, Moeller et al. have used a predefined one-to-one ratio between CD4+ and CD8+ T cells as the basis for the generation of CARs, which upon transfusion induced complete tumor eradication in preclinical models, which was confirmed by others [16,123,124]. Of importance, clinical trials applying standardized ratios of CD4+ to CD8+ CAR T cells have shown high complete response rates in lymphoma patients pointing to the superiority of such cellular compositions [125].
CD4+ and CD8+ T cells can be further subdivided into functionally different subtypes, some accounting for increased treatment efficacy. Along those lines, many studies have recognized the importance of the CD8+ central memory T (TCM) cell subset, which guarantees for enhanced engraftment and thus prolonged persistence, while effector memory CD8+ T cells were found to be clearly inferior in that respect [5,126]. Although TCM cells engraft well, their combination with CD4+ T helper cells is still of utmost importance to ensure their long-term persistence, which is not only crucial for complete tumor cell elimination but also for continuous tumor immune surveillance [122].
Some T cell subsets, such as central memory T cells, have a low concentration in peripheral blood which renders their isolation and use for the generation of CARs difficult. Nonetheless, optimized cell culture conditions might improve their enrichment. For instance, the use of IL-7 and IL-15 instead of IL-2 for the expansion of T cells to be transduced with CARs was shown to yield larger amounts of CD8+ T memory stem cells, which, upon transfusion, resulted in increased in vivo persistence and higher anti-tumor activity [127]. Thus, improved production protocols may greatly influence the efficacy of the final product.
5.1.2. Virus-specific cytotoxic T cells
Because tumors lack many of the costimulatory factors required to fully activate T cells, CAR T cell might not undergo sustained activation upon antigen recognition. In contrast, virus-specific CTL continuously receive stimulation from viral antigens and costimulatory ligands expressed on antigen presenting cells (APCs). To benefit from such a natural stimulatory environment, virus-specific CTL were used for the expression of CARs in some studies. While such cells could lyse both virus infected and tumor targets they did not show a superior long-term survival in the host [5,128].
5.1.3. γδ T cells
Infusion of genetically engineered T cells may be associated with serious side effects and toxicities and may cause graft-versus-host disease (GVHD) in the case of application of allogenic CAR T cells due to HLA mismatching [129]. Since γδ T cells are usually unable to recognize allogeneic HLA molecules but are still endowed with strong cytotoxic capability, such cells may be obtained from healthy allogeneic donors and would present an ideal option for ‘off-the-shelf’ cellular therapies. In addition, a meta-analysis of expression signatures from ~18.000 human tumors across 39 malignancies has shown that the intra-tumoral γδ T cell signature is among the most favorable prognostic parameters for survival [130]. This makes γδ T cells a very attractive cellular backbone for CAR therapy and, in fact, various studies have already shown that γδ CAR T cells are perfectly capable of conducting target cell-specific tumor lysis [131,132].
5.1.4. Th17 T cells
Th17 cells are a subset of T helper cells defined by their production of the pro-inflammatory cytokine IL-17 [133]. When appropriately targeted, they can induce significant tumor regression and are superior in tumor eradication when compared to Th1 or Th0 T cells [134]. Guedan et al. have used a CAR containing the inducible co-stimulator (ICOS) intracellular domain to generate tumour-specific IL-17-producing effector T cells. Although this study showed an increased secretion of Th17 signature cytokines and enhanced T cell persistence in mouse tumor models, more severe forms of GVHD were observed as a side effect [135].
5.1.5. T regulatory cells
Treg cells have a negative impact on cancer therapy due to their multiple inhibitory effects on T effector cells [136]. Although inhibition of immune responses is undesired during cancer therapy, such inhibition may be useful when it comes to the treatment of autoimmune diseases or allergies. Notably, previous studies have shown that Treg represent an interesting agent for the treatment of autoimmune diseases, GVHD and allergies [137] (ref. Ward2018). In fact, MacDonald et al. have used a CD28::ζ CAR targeting HLA-A2 and have introduced it into Treg cells. These cells maintained their suppressive function and were superior at preventing GVHD when compared to Treg cells expressing an irrelevant CAR [138]. CAR Treg cells may impact also on bystander T cells by changing their cytokine profile from IFN-γ producing Th1 cells towards IL-10 producing Tr1 cells, thereby creating a more immunotolerant environment [139].
5.2. Natural killer cells, NKT cells and NK-92 cell lines
NK cells represent another interesting backbone for CAR expression. NK cells are able to target cancer cells in various ways, via (i) CAR-dependent antigen-specific mechanisms, (ii) spontaneous cytotoxic activity or (iii) indirectly by activation of adaptive and innate immune cells [140]. Another advantage is the possibility to use different sources for obtaining NK cells, such as umbilical cord blood, human embryonic stem cells and induced pluripotent stem cells or even allogenic NK cells or cell lines since they do not require HLA matching [6].
5.2.1. NK cells
CAR expressing NK cells produce larger amounts of cytokines upon target recognition, which leads to tumor cell lysis with various in vivo tumor models confirming their efficacy [141–144]. Although NK cells exhibit strong cytotoxic activity their short lifespan limits their successful application. This drawback can be overcome by the use of IL-12, IL-15, and IL-18, respectively, all cytokines which differentiate NK cells towards a memory-like cell phenotype with improved in vivo persistence [145]. Two clinical studies (NCT0095137, NCT01974479) evaluating the effects of CD19-CAR transduced NK cells are currently being performed.
5.2.2. NK-92 based cell lines
The NK-92 cell line was established from a male patient suffering from rapidly progressive non-Hodgkin lymphoma in 1994 [146]. Because of the need for a standardized ‘off-the-shelf’ CAR backbone, the NK-92 cell line represents an interesting cell type. The transfusion of high doses of irradiated NK-92 cells were well-tolerated in clinical studies and resulted in only mild toxic side effects, while irradiation of the cells did not change their efficacy [147–150]. Additionally, transduction of NK-92 cells with CARs greatly enhanced their cytolytic activity and concomitantly reduced the tumor burden in pre-clinical models [150–152]. Currently, the first patients are being treated in a first clinical study, evaluating anti-HER2 CAR expressing NK-92 cells applied intracranially into glioblastoma tumors (NCT02892695).
5.2.3. NKT cells
NK T cells are a subset of T cells that join the properties of both T lymphocytes and NK cells [153]. Higher levels of tumor-infiltrating NKT cells have been related to a favorable outcome in cancer and the infusion of NKT cells was shown to be well-tolerated in clinical studies [154–158]. Low numbers of these cells in peripheral blood makes them difficult to isolate in sufficient numbers and purity, however, their restriction to monomorphic rather than polymorphic HLA molecules makes the application to the patients of allogeneic donor cells possible [159]. Upon application of CAR expressing NKT cells they produced large amounts of Th1-type cytokines, which resulted in potent antitumor activity [160,161]. Currently, one clinical study evaluating NKT GD2-CAR transduced cells for the treatment of neuroblastoma in pediatric patients is being performed with results still undisclosed (NCT03294954).
5.3. Combination of different cell types
While the majority of studies focusses only on one cell type, the combined treatment with different cell types might provide better results. Du et al. have developed a protocol for expansion of T lymphocytes, γδ T cells and NKT cells. Using zoledronic acid, interferon-gamma (IFN-γ), interleukin 2 (IL-2), anti-CD3 antibody and engineered K562 feeder cells PBMCs were greatly expanded with γδ T cells constituting over 20% of the population. The infusion of such cellular combination exhibited superior tumor cell lysis when compared to more ‘conventional’ CAR T cells [7].
5.4. Genetically modified cells for universal application
Due to the great potential of CAR therapy, the interest for ‘off-the-shelf’ CAR cell products is increasing constantly, but the need for the use of autologous lymphocytes considerably limits the large scale application of CAR therapies. To prevent the development of GVHD when using an allogenic cell source, the disruption of the endogenous TCR and HLA class loci would be necessary. A first advancement towards that direction was made with the establishment of the FT819 cell line. This CD19-CAR expressing T cell line was created from an induced pluripotent stem cell (iPSC) line by bi-allelic disruption of the TRAC locus [162,163]. A step further was made by Ren et al. who combined lentiviral CD19 CAR transduction with the use of the CRISPR/Cas9 system for the simultaneous disruption of three genes, TCR (TRAC and TRBC), β-2 microglobulin and PD1 genes. This maneuver (TCR and β2-m disruption) resulted in reduced alloreactivity and enhanced in vivo activity [164]. Currently, two clinical studies (NCT03166878, NCT03229876) are evaluating the use of such universal CAR cells.
In addition, CAR activity monitoring and tracing currently relies on the detection of CAR cells in peripheral blood, however, the importance of their activity at the tumor site remains only poorly appreciated. In that respect, Keu et al. have developed a reporter gene system for noninvasive detection of CAR cells by positron emission tomography (PET) scanning [165]. Such innovative in vivo cell monitoring approaches would greatly improve open questions regarding optimal dosing of CAR cells (quantity, frequency) and help to better monitor the longevity of CAR cells in the body.
6. Summary and outlook
CAR cells have become a very powerful and versatile tool for the targeting of malignant and autoreactive cell types of choice. With prototypic CAR treatments for acute lymphocytic leukemia already available and in clinical use, it is foreseeable that the scope of applications of this technology will continuously grow during the next decade. The further success of CAR cell technology will strongly depend on the (i) development of CAR constructs optimally suited for individual applications, (ii) identification of the most appropriate cell types to express CARs and to execute desired effector programs, and (iii) the possibility to safely and completely remove CAR cells upon achievement of full remission of the respective disease. Once solved, adoptive CAR cell therapy may become an affordable, efficient, highly selective and safe treatment modality able to fight apart from leukemias also solid malignancies but also autoimmune and allergic diseases in which (poly)clonally expanded cell types drive pathology.
Acknowledgement
This project was supported by the Austrian Science Fund (FWF) projects DK 1248-B30 and SFB F4609.
Abbreviations
- ADCC
antibody-dependent cellular cytotoxicity
- APC
antigen presenting cell
- APRIL
A proliferation-inducing ligand
- BAFF
B-cell activating factor
- BBIR
biotin-binding immune receptor
- BCMA
B cell maturation antigen
- CAR
chimeric antigen receptor
- Cas9
caspase 9
- CD
cluster of differentiation
- CID
chemical inducer of dimerization
- CMV
cytomegalovirus
- CRISPR
clustered regularly interspaced short palindromic repeats
- CRS
cytokine release syndrome
- CTL
cytotoxic T lymphocyte
- CTLA-4
cytotoxic T-lymphocyte-associated protein 4
- EAE
experimental autoimmune encephalomyelitis
- EGFR
Epidermal growth factor receptor
- ELRD
extracellular ligand recognition domain
- EMA
European Medicines Agency
- Fab
Fragment antigen binding
- Fc
Fragment crystallizable
- FcεRI
Fc epsilon receptor I
- FDA
Food and Drug Administration
- FKBP
FK506-binding protein
- GVHD
graft versus host disease
- HEL
hen egg lysozyme
- HER2
human epidermal growth factor receptor 2
- HLA
human leukocyte antigen
- huEGFRTt
human epidermal growth factor receptor truncated
- iCAR
inhibitory CAR
- iCasp9
inducible caspase 9
- ICOS
inducible T-cell costimulator
- IFN
interferon
- Ig
Immunoglobulin
- IL
interleukin
- IL13Rα2
IL-13 receptor α 2
- IL2R
IL-2 receptor
- IL7R
IL-7 receptor
- iPSC
induced pluripotent stem cell
- ITAM
immunoreceptor tyrosine-based motif
- LAG-3
lymphocyte-activation gene 3
- MBP
myelin basic protein
- MHC
major histocompatibility complex
- NK cell
natural killer cell
- NKT cell
natural killer T cell
- PBMCs
peripheral blood mononuclear cells
- PD-1
programmed cell death protein 1
- PD-L1
programmed death ligand
- PSCA
prostate stem cell antigen
- PSMA
Prostate specific membrane antigen
- RMTC
receptor modified T cell
- scFV
single-chain variable fragment
- STAT3
signal transducer and activator of transcription 3
- SUPRA CAR
Split Universal Programmable CAR
- tanCAR
tandem CAR
- TCM cell
central memory T cell
- TCR
T cell receptor
- Th cell
T helper cell
- TIM-3
T-cell immunoglobulin and mucin-domain containing 3
- TNP
2,4,6-trinitrophenyl
- TRAC locus
T-cell receptor alpha constant locus
- TRBC locus
T-cell receptor beta constant locus
- Treg cell
T regulatory cell
- VH
variable heavy chain
- VL
variable light chain
References
- [1].Lanitis E, Poussin M, Hagemann IS, Coukos G, Sandaltzopoulos R, Scholler N, Powell DJ. Redirected antitumor activity of primary human lymphocytes transduced with a fully human anti-mesothelin chimeric receptor. Mol Ther. 2012;20:633–643. doi: 10.1038/mt.2011.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Karlsson H, Svensson E, Gigg C, Jarvius M, Olsson-Strömberg U, Savoldo B, Dotti G, Loskog A. Evaluation of intracellular signaling downstream chimeric antigen receptors. PLoS One. 2015;10:e0144787. doi: 10.1371/journal.pone.0144787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hwu P, Sharer GE, Treisman J, Schindler DG, Gross G, Cowherd R, Rosenberg SA, Eshhar Z. Lysis of ovarian cancer cells by human lymphocytes redirected with a chimeric gene composed of an antibody variable region and the Fc receptor gamma chain. J Exp Med. 1993;178:361–366. doi: 10.1084/jem.178.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jensen MC, Popplewell L, Cooper LJ, DiGiusto D, Kalos M, Ostberg JR, Forman SJ. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant. 2010;16(9):1245–1256. doi: 10.1016/j.bbmt.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, Rossig C, Russell HV, Diouf O, Liu E, Liu H, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hu Y, Tian Z-g, Zhang C. Chimeric antigen receptor (CAR)-transduced natural killer cells in tumor immunotherapy. Acta Pharmacol Sin. 2018;39:167–176. doi: 10.1038/aps.2017.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Du SH, Li Z, Chen C, Tan WK, Chi Z, Kwang TW, Xu XH, Wang S. Co-expansion of cytokine-induced killer cells and Vγ9Vδ2 T cells for CAR T-cell therapy. PLoS One. 2016;11:e0161820. doi: 10.1371/journal.pone.0161820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Oluwole OO, Davila ML. At the bedside: clinical review of chimeric antigen receptor (CAR) T cell therapy for B cell malignancies. J Leukoc Biol. 2016;100:1265–1272. doi: 10.1189/jlb.5BT1115-524R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].FDA approves second CAR T-cell therapy. Cancer Discov. 2018;8(1):5–6. doi: 10.1158/2159-8290.CD-NB2017-155. [DOI] [PubMed] [Google Scholar]
- [10].Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, Timmerman JM, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, Qayed M, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Han S, Latchoumanin O, Wu G, Zhou G, Hebbard L, George J, Qiao L. Recent clinical trials utilizing chimeric antigen receptor T cells therapies against solid tumors. Cancer Lett. 2017;390:188–200. doi: 10.1016/j.canlet.2016.12.037. [DOI] [PubMed] [Google Scholar]
- [13].Pettitt D, Arshad Z, Smith J, Stanic T, Holländer G, Brindley D. CAR-T cells: a systematic review and mixed methods analysis of the clinical trial landscape. Mol Ther. 2018;26:342–353. doi: 10.1016/j.ymthe.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90(2):720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, Smith JP, Walker AJ, Kohler ME, Venkateshwara VR, Kaplan RN, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015;21:581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhao Z, Condomines M, van der Stegen SJC, Perna F, Kloss CC, Gunset G, Plotkin J, Sadelain M. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR t cells. Cancer Cell. 2015;28(4):415–428. doi: 10.1016/j.ccell.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, Kamble RT, Bollard CM, Gee AP, Mei Z, Liu H, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121(5):1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gill S, Maus MV, Porter DL. Chimeric antigen receptor T cell therapy: 25years in the making. Blood Rev. 2016;30:157–167. doi: 10.1016/j.blre.2015.10.003. [DOI] [PubMed] [Google Scholar]
- [19].Newick K, Brien SO, Moon E, Albelda SM. CAR T-cell therapy for solid tumors? Cancer Discov. 2018;8:1341. doi: 10.1158/2159-8290.CD-ND2018-008. [DOI] [PubMed] [Google Scholar]
- [20].Ramos CA, Rouce R, Robertson CS, Reyna A, Narala N, Vyas G, Mehta B, Zhang H, Dakhova O, Carrum G, Kamble RT, et al. In vivo fate and activity of second- versus third-generation CD19-Specific CAR-T cells in B cell non-Hodgkin’s lymphomas. Mol Ther. 2018;26:2727–2737. doi: 10.1016/j.ymthe.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Enblad G, Karlsson H, Gammelgård G, Wenthe J, Lövgren T, Amini RM, Wikstrom KI, Essand M, Savoldo B, Hallböök H, Höglund M, et al. A phase I/IIa trial using CD19-targeted third-generation CAR t cells for lymphoma and leukemia. Clin Cancer Res. 2018;24:6185–6194. doi: 10.1158/1078-0432.CCR-18-0426. [DOI] [PubMed] [Google Scholar]
- [22].Duong CPM, Westwood JA, Yong CSM, Murphy A, Devaud C, John LB, Darcy PK, Kershaw MH. Engineering T cell function using chimeric antigen receptors identified using a DNA library approach. PLoS One. 2013;8:e63037. doi: 10.1371/journal.pone.0063037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Curtsinger JM, Mescher MF. Inflammatory cytokines as a third signal for T cell activation. Curr Opin Immunol. 2010;22:333–340. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kagoya Y, Tanaka S, Guo T, Anczurowski M, Wang CH, Saso K, Butler MO, Minden MD, Hirano N. A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nat Med. 2018;24:352–359. doi: 10.1038/nm.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ajina A, Maher J. Strategies to address chimeric antigen receptor tonic signaling. Mol Cancer Ther. 2018;17:1795–1815. doi: 10.1158/1535-7163.MCT-17-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Topfer K, Cartellieri M, Michen S, Wiedemuth R, Muller N, Lindemann D, Bachmann M, Fussel M, Schackert G, Temme A. DAP12-based activating chimeric antigen receptor for NK cell tumor immunotherapy. J Immunol. 2015;194(7):3201–3212. doi: 10.4049/jimmunol.1400330. [DOI] [PubMed] [Google Scholar]
- [27].Gulati P, Ruhl J, Kannan A, Pircher M, Schuberth P, Nytko KJ, Pruschy M, Sulser S, Haefner M, Jensen S, Soltermann A, et al. Aberrant Lck signal via CD28 costimulation augments antigen-specific functionality and tumor control by redirected T cells with PD-1 blockade in humanized mice. Clin Cancer Res. 2018;24:3981–3993. doi: 10.1158/1078-0432.CCR-17-1788. [DOI] [PubMed] [Google Scholar]
- [28].Guest RD, Hawkins RE, Kirillova N, Cheadle EJ, Arnold J, O’Neill A, Irlam J, Chester KA, Kemshead JT, Shaw DM, Embleton MJ, et al. The role of extracellular spacer regions in the optimal design of chimeric immune receptors. J Immunother. 2005;28:203–211. doi: 10.1097/01.cji.0000161397.96582.59. [DOI] [PubMed] [Google Scholar]
- [29].Hudecek M, Sommermeyer D, Kosasih PL, Silva-Benedict A, Liu L, Rader C, Jensen MC, Riddell SR. The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol Res. 2015;3(2):125–135. doi: 10.1158/2326-6066.CIR-14-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kolanus W, Romeo C, Seed B. T cell activation by clustered tyrosine kinases. Cell. 1993;74(1):171–183. doi: 10.1016/0092-8674(93)90304-9. [DOI] [PubMed] [Google Scholar]
- [31].Majzner RG, Mackall CL. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 2018;8:1219–1226. doi: 10.1158/2159-8290.CD-18-0442. [DOI] [PubMed] [Google Scholar]
- [32].Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of t cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bird R, Hardman K, Jacobson J, Johnson S, Kaufman B, Lee S, Lee T, Pope S, Riordan G, Whitlow M. Single-chain antigen-binding proteins. Science. 1988;242:423–426. doi: 10.1126/science.3140379. [DOI] [PubMed] [Google Scholar]
- [34].Sadelain M, Rivière I, Riddell S. Therapeutic T cell engineering. Nature. 2017;545:423–431. doi: 10.1038/nature22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fernández CR. The FDA Approves a Second CAR-T Therapy, Cheaper Than Novartis’. 2017 [Google Scholar]
- [36].Salmikangas P, Kinsella N, Chamberlain P. Chimeric antigen receptor T-cells (CAR T-cells) for cancer immunotherapy – moving target for industry? Pharm Res. 2018;35:152. doi: 10.1007/s11095-018-2436-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Moon EK, Carpenito C, Sun J, Wang L-CS, Kapoor V, Predina J, Powell DJ, Riley JL, June CH, Albelda SM. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin Cancer Res. 2011;17:4719–4730. doi: 10.1158/1078-0432.CCR-11-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Junghans RP, Ma Q, Rathore R, Gomes EM, Bais AJ, Lo ASY, Abedi M, Davies RA, Cabral HJ, Al-Homsi AS, Cohen SI. Phase I trial of Anti-PSMA designer CAR-T cells in prostate cancer: possible role for interacting interleukin 2-T cell pharmacodynamics as a determinant of clinical response. Prostate. 2016;76:1257–1270. doi: 10.1002/pros.23214. [DOI] [PubMed] [Google Scholar]
- [39].O’Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, Martinez-Lage M, Brem S, Maloney E, Shen A, Isaacs R, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9:399. doi: 10.1126/scitranslmed.aaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Novak AJ, Darce JR, Arendt BK, Harder B, Henderson K, Kindsvogel W, Gross JA, Greipp PR, Jelinek DF. Expression of BCMA, TACI, and BAFF-R in multiple myeloma: a mechanism for growth and survival. Blood. 2004;103(2):689–694. doi: 10.1182/blood-2003-06-2043. [DOI] [PubMed] [Google Scholar]
- [41].Thompson JS, Schneider P, Kalled SL, Wang L, Lefevre EA, Cachero TG, MacKay F, Bixler SA, Zafari M, Liu ZY, Woodcock SA, et al. BAFF binds to the tumor necrosis factor receptor-like molecule B cell maturation antigen and is important for maintaining the peripheral B cell population. J Exp Med. 2000;192(1):129–135. doi: 10.1084/jem.192.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yu G, Boone T, Delaney J, Hawkins N, Kelley M, Ramakrishnan M, McCabe S, Qiu WR, Kornuc M, Xia XZ, Guo J, et al. APRIL and TALL-I and receptors BCMA and TACI: system for regulating humoral immunity. Nat Immunol. 2000;1(3):252–256. doi: 10.1038/79802. [DOI] [PubMed] [Google Scholar]
- [43].Tai YT, Acharya C, An G, Moschetta M, Zhong MY, Feng X, Cea M, Cagnetta A, Wen K, van Eenennaam H, van Elsas A, et al. APRIL and BCMA promote human multiple myeloma growth and immunosuppression in the bone marrow microenvironment. Blood. 2016;127(25):3225–3236. doi: 10.1182/blood-2016-01-691162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Cohen AD, Garfall AL, Stadtmauer EA, Melenhorst JJ, Lacey SF, Lancaster E, Vogl DT, Weiss BM, Dengel K, Nelson A, Plesa G, et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J Clin Invest. 2019;130:2210–2221. doi: 10.1172/JCI126397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].McMahan RH, McWilliams JA, Jordan KR, Dow SW, Wilson DB, Slansky JE. Relating TCR-peptide-MHC affinity to immunogenicity for the design of tumor vaccines. J Clin Invest. 2006;116(9):2543–2551. doi: 10.1172/JCI26936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kalergis AM, Boucheron N, Doucey MA, Palmieri E, Goyarts EC, Vegh Z, Luescher IF, Nathenson SG. Efficient T cell activation requires an optimal dwell-time of interaction between the TCR and the pMHC complex. Nat Immunol. 2001;2(3):229–234. doi: 10.1038/85286. [DOI] [PubMed] [Google Scholar]
- [47].Kuwana Y, Asakura Y, Utsunomiya N, Nakanishi M, Arata Y, Itoh S, Nagase F, Kurosawa Y. Expression of chimeric receptor composed of immunoglobulin-derived V resions and T-cell receptor-derived C regions. Biochem Biophys Res Commun. 1987;149:960–968. doi: 10.1016/0006-291x(87)90502-x. [DOI] [PubMed] [Google Scholar]
- [48].Xu Y, Yang Z, Horan LH, Zhang P, Liu L, Zimdahl B, Green S, Lu J, Morales JF, Barrett DM, Grupp SA, et al. A novel antibody-TCR (AbTCR) platform combines Fab-based antigen recognition with gamma/delta-TCR signaling to facilitate T-cell cytotoxicity with low cytokine release. Cell Discov. 2018;4:62. doi: 10.1038/s41421-018-0066-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Saito T, Hochstenbach F, Marusic-Galesic S, Kruisbeek AM, Brenner M, Germain RN. Surface expression of only gamma delta and/or alpha beta T cell receptor heterodimers by cells with four (alpha, beta, gamma, delta) functional receptor chains. J Exp Med. 1988;168(3):1003–1020. doi: 10.1084/jem.168.3.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Brown CE, Aguilar B, Starr R, Yang X, Chang WC, Weng L, Chang B, Sarkissian A, Brito A, Sanchez JF, Ostberg JR, et al. Optimization of IL13Ralpha2-targeted chimeric antigen receptor t cells for improved anti-tumor efficacy against glioblastoma. Mol Ther. 2018;26(1):31–44. doi: 10.1016/j.ymthe.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Iizuka K, Nakajima C, Iizuka YM, Takase M, Kato T, Noda S, Tanaka K, Kanagawa O. Protection from lethal infection by adoptive transfer of CD8 T cells genetically engineered to express virus-specific innate immune receptor. J Immunol. 2007;179(2):1122–1128. doi: 10.4049/jimmunol.179.2.1122. [DOI] [PubMed] [Google Scholar]
- [52].Nguyen P, Duthoit CT, Geiger TL. Induction of tolerance and immunity by redirected B cell-specific cytolytic T lymphocytes. Gene Ther. 2007;14:1739–1749. doi: 10.1038/sj.gt.3303045. [DOI] [PubMed] [Google Scholar]
- [53].Ellebrecht CT, Bhoj VG, Nace A, Choi EJ, Mao X, Cho MJ, Di Zenzo G, Lanzavecchia A, Seykora JT, Cotsarelis G, Milone MC, et al. Reengineering chimeric antigen receptor T cells for targeted therapy of auto-immune disease. Science. 2016;353:179–184. doi: 10.1126/science.aaf6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Pollmann R, Schmidt T, Eming R, Hertl M. Pemphigus: a comprehensive review on pathogenesis, clinical presentation and novel therapeutic approaches. Clin Rev Allergy Immunol. 2018;54:1–25. doi: 10.1007/s12016-017-8662-z. [DOI] [PubMed] [Google Scholar]
- [55].Parvathaneni K, Scott DW. Engineered FVIII-expressing cytotoxic T cells target and kill FVIII-specific B cells in vitro and in vivo. Blood Adv. 2018;2:2332–2340. doi: 10.1182/bloodadvances.2018018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ward DE, Fay BL, Adejuwon A, Han H, Ma Z. Chimeric antigen receptors based on low affinity mutants of FcepsilonRI Re-direct T cell specificity to cells expressing membrane IgE. Front Immunol. 2018;9:2231. doi: 10.3389/fimmu.2018.02231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Neunkirchner A, Kratzer B, Kohler C, Smole U, Mager LF, Schmetterer KG, Trapin D, Leb-Reichl V, Rosloniec E, Naumann R, Kenner L, et al. Genetic restriction of antigen-presentation dictates allergic sensitization and disease in humanized mice. EBioMedicine. 2018;31:66–78. doi: 10.1016/j.ebiom.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Valenta R, Karaulov A, Niederberger V, Gattinger P, van Hage M, Flicker S, Linhart B, Campana R, Focke-Tejkl M, Curin M, Eckl-Dorna J, et al. Molecular aspects of allergens and allergy. Adv Immunol. 2018;138:195–256. doi: 10.1016/bs.ai.2018.03.002. [DOI] [PubMed] [Google Scholar]
- [59].Matricardi PM, Kleine-Tebbe J, Hoffmann HJ, Valenta R, Hilger C, Hofmaier S, Aalberse RC, Agache I, Asero R, Ballmer-Weber B, Barber D, et al. EAACI molecular allergology user’s guide. Pediatr Allergy Immunol. 2016;27(Suppl. 23):1–250. doi: 10.1111/pai.12563. [DOI] [PubMed] [Google Scholar]
- [60].Yocum MW, Butterfield JH, Klein JS, Volcheck GW, Schroeder DR, Silverstein MD. Epidemiology of anaphylaxis in Olmsted County: a population-based study. J Allergy Clin Immunol. 1999;104:452–456. doi: 10.1016/s0091-6749(99)70392-1. [DOI] [PubMed] [Google Scholar]
- [61].Scott GS, Fishman S, Khai Siew L, Margalit A, Chapman S, Chervonsky AV, Wen L, Gross G, Wong FS. Immunotargeting of insulin reactive CD8 T cells to prevent diabetes. J Autoimmun. 2010;35(4):390–397. doi: 10.1016/j.jaut.2010.08.005. [DOI] [PubMed] [Google Scholar]
- [62].Fishman S, Lewis MD, Siew LK, De Leenheer E, Kakabadse D, Davies J, Ziv D, Margalit A, Karin N, Gross G, Wong FS. Adoptive transfer of mRNA-transfected T cells redirected against diabetogenic CD8 T cells can prevent diabetes. Mol Ther. 2017;25(2):456–464. doi: 10.1016/j.ymthe.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Constantinescu CS, Farooqi N, O’Brien K, Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS) Br J Pharmacol. 2011;164:1079–1106. doi: 10.1111/j.1476-5381.2011.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Jyothi MD, Flavell RA, Geiger TL. Targeting autoantigen-specific T cells and suppression of autoimmune encephalomyelitis with receptor-modified T lymphocytes. Nat Biotechnol. 2002;20(12):1215–1220. doi: 10.1038/nbt758. [DOI] [PubMed] [Google Scholar]
- [65].Moisini I, Nguyen P, Fugger L, Geiger TL. Redirecting therapeutic T cells against myelin-specific T lymphocytes using a humanized myelin basic protein-HLA-DR2-zeta chimeric receptor. J Immunol. 2008;180(5):3601–3611. doi: 10.4049/jimmunol.180.5.3601. [DOI] [PubMed] [Google Scholar]
- [66].Akdis CA. Allergy and hypersensitivity: mechanisms of allergic disease. Curr Opin Immunol. 2006;18(6):718–726. doi: 10.1016/j.coi.2006.09.016. [DOI] [PubMed] [Google Scholar]
- [67].Ruella M, Barrett DM, Kenderian SS, Shestova O, Hofmann TJ, Perazzelli J, Klichinsky M, Aikawa V, Nazimuddin F, Kozlowski M, Scholler J, et al. Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J Clin Invest. 2016;126:3814–3826. doi: 10.1172/JCI87366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Hegde M, Corder A, Chow KK, Mukherjee M, Ashoori A, Kew Y, Zhang YJ, Baskin DS, Merchant FA, Brawley VS, Byrd TT, et al. Combinational Targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma. Mol Ther. 2013;21:2087–2101. doi: 10.1038/mt.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Bielamowicz K, Fousek K, Byrd TT, Samaha H, Mukherjee M, Aware N, Wu MF, Orange JS, Sumazin P, Man TK, Joseph SK, et al. Trivalent CAR T cells overcome interpatient antigenic variability in glioblastoma. Neuro Oncol. 2018;20:506–518. doi: 10.1093/neuonc/nox182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hegde M, Mukherjee M, Grada Z, Pignata A, Landi D, Navai SA, Wakefield A, Fousek K, Bielamowicz K, Chow KKH, Brawley VS, et al. Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. J Clin Invest. 2016;126:3036–3052. doi: 10.1172/JCI83416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Zah E, Lin MY, Silva-Benedict A, Jensen MC, Chen YY. T cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunol Res. 2016;4(6):498–508. doi: 10.1158/2326-6066.CIR-15-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Roybal KT, Rupp LJ, Morsut L, Walker WJ, McNally KA, Park JS, Lim WA. Precision tumor recognition by T cells with combinatorial antigen-sensing circuits. Cell. 2016;164:770–779. doi: 10.1016/j.cell.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol. 2013;31:71–75. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Lanitis E, Poussin M, Klattenhoff AW, Song D, Sandaltzopoulos R, June CH, Powell DJ., Jr Chimeric antigen receptor T Cells with dissociated signaling domains exhibit focused antitumor activity with reduced potential for toxicity in vivo. Cancer Immunol Res. 2013;1(1):43–53. doi: 10.1158/2326-6066.CIR-13-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med. 2013;5(215) doi: 10.1126/scitranslmed.3006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev. 2019;34:45–55. doi: 10.1016/j.blre.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Straathof KC, Pulè MA, Yotnda P, Dotti G, Vanin EF, Brenner MK, Heslop HE, Spencer DM, Rooney CM. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105:4247–4254. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Hoyos V, Savoldo B, Quintarelli C, Mahendravada A, Zhang M, Vera J, Heslop HE, Rooney CM, Brenner MK, Dotti G. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24:1160–1170. doi: 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Shum T, Omer B, Tashiro H, Kruse RL, Wagner DL, Parikh K, Yi Z, Sauer T, Liu D, Parihar R, Castillo P, et al. Constitutive signaling from an engineered IL7 receptor promotes durable tumor elimination by tumor-redirected T cells. Cancer Discov. 2017;7:1238–1247. doi: 10.1158/2159-8290.CD-17-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Diaconu I, Ballard B, Zhang M, Chen Y, West J, Dotti G, Savoldo B. Inducible Caspase-9 selectively modulates the toxicities of CD19-specific chimeric antigen receptor-modified T cells. Mol Ther. 2017;25(3):580–592. doi: 10.1016/j.ymthe.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wang X, Chang WC, Wong CLW, Colcher D, Sherman M, Ostberg JR, Forman SJ, Riddell SR, Jensen MC. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood. 2011;118:1255–1263. doi: 10.1182/blood-2011-02-337360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Cartellieri M, Feldmann A, Koristka S, Arndt C, Loff S, Ehninger A, von Bonin M, Bejestani EP, Ehninger G, Bachmann MP. Switching CAR T cells on and off: a novel modular platform for retargeting of T cells to AML blasts. Blood Cancer J. 2016;6(8):e458. doi: 10.1038/bcj.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Bayer EA, Wilchek M, Skutelsky E. Affinity cytochemistry: the localization of lectin and antibody receptors on erythrocytes via the avidin-biotin complex. FEBS Lett. 1976;68(2):240–244. doi: 10.1016/0014-5793(76)80445-0. [DOI] [PubMed] [Google Scholar]
- [84].Wilchek M, Bayer EA. The avidin-biotin complex in immunology. Immunol Today. 1984;5(2):39–43. doi: 10.1016/0167-5699(84)90027-6. [DOI] [PubMed] [Google Scholar]
- [85].Urbanska K, Lanitis E, Poussin M, Lynn RC, Gavin BP, Kelderman S, Yu J, Scholler N, Powell DJ. A universal strategy for adoptive immunotherapy of cancer through use of a novel T-cell antigen receptor. Cancer Res. 2012;72:1844–1852. doi: 10.1158/0008-5472.CAN-11-3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Cho JH, Collins JJ, Wong WW. Universal chimeric antigen receptors for multiplexed and logical control of T cell responses. Cell. 2018;173:1426–1438 e11. doi: 10.1016/j.cell.2018.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Clemenceau B, Congy-Jolivet N, Gallot G, Vivien R, Gaschet J, Thibault G, Vie H. Antibody-dependent cellular cytotoxicity (ADCC) is mediated by genetically modified antigen-specific human T lymphocytes. Blood. 2006;107(12):4669–4677. doi: 10.1182/blood-2005-09-3775. [DOI] [PubMed] [Google Scholar]
- [88].Kisielow J, Obermair FJ, Kopf M. Deciphering CD4(+) T cell specificity using novel MHC-TCR chimeric receptors. Nat Immunol. 2019;20(5):652–662. doi: 10.1038/s41590-019-0335-z. [DOI] [PubMed] [Google Scholar]
- [89].Chmielewski M, Abken H. CAR T cells transform to trucks: chimeric antigen receptor-redirected T cells engineered to deliver inducible IL-12 modulate the tumour stroma to combat cancer. Cancer Immunol Immunother. 2012;61(8):1269–1277. doi: 10.1007/s00262-012-1202-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Khong A, Nelson DJ, Nowak AK, Lake RA, Robinson BWS. The use of agonistic anti-CD40 therapy in treatments for cancer. Int Rev Immunol. 2012;31:246–266. doi: 10.3109/08830185.2012.698338. [DOI] [PubMed] [Google Scholar]
- [91].Vonderheide RH, Glennie MJ. Agonistic CD40 antibodies and cancer therapy. Clin Cancer Res. 2013;19:1035–1043. doi: 10.1158/1078-0432.CCR-12-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Curran KJ, Seinstra BA, Nikhamin Y, Yeh R, Usachenko Y, Van Leeuwen DG, Purdon T, Pegram HJ, Brentjens RJ. Enhancing antitumor efficacy of chimeric antigen receptor T cells through constitutive CD40L expression. Mol Ther. 2015;23:769–778. doi: 10.1038/mt.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Wang C, Lin GHY, McPherson AJ, Watts TH. Immunol Rev. John Wiley & Sons, Ltd; 2009. Immune regulation by 4-1BB and 4-1BBL: complexities and challenges; pp. 192–215. (10.1111) [DOI] [PubMed] [Google Scholar]
- [94].Anestakis D, Petanidis S, Kalyvas S, Nday CM, Tsave O, Kioseoglou E, Salifoglou A. Mechanisms and applications of interleukins in cancer immunotherapy. Int J Mol Sci. 2015;16(1):1691–1710. doi: 10.3390/ijms16011691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Lacy MQ, Jacobus S, Blood EA, Kay NE, Rajkumar SV, Greipp PR. Phase II study of interleukin-12 for treatment of plateau phase multiple myeloma (E1A96): a trial of the Eastern Cooperative Oncology Group. Leuk Res. 2009;33:1485–1489. doi: 10.1016/j.leukres.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].van Herpen CM. Intratumoral administration of recombinant human interleukin 12 in head and neck squamous cell carcinoma patients elicits a T-Helper 1 profile in the Locoregional Lymph Nodes. Clin Cancer Res. 2004;10:2626–2635. doi: 10.1158/1078-0432.ccr-03-0304. [DOI] [PubMed] [Google Scholar]
- [97].Pegram HJ, Lee JC, Hayman EG, Imperato GH, Tedder TF, Sadelain M, Brentjens RJ. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119:4133–4141. doi: 10.1182/blood-2011-12-400044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6(4):295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- [99].Koneru M, Purdon TJ, Spriggs D, Koneru S, Brentjens RJ. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. Oncoimmunology. 2015;4(3):e994446. doi: 10.4161/2162402X.2014.994446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Chmielewski M, Kopecky C, Hombach AA, Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 2011;71:5697–5706. doi: 10.1158/0008-5472.CAN-11-0103. [DOI] [PubMed] [Google Scholar]
- [101].Chinnasamy D, Yu Z, Kerkar SP, Zhang L, Morgan RA, Restifo NP, Rosenberg SA. Local delivery of lnterleukin-12 using T cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice. Clin Cancer Res. 2012;18:1672–1683. doi: 10.1158/1078-0432.CCR-11-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Robinson TO, Schluns KS. The potential and promise of IL-15 in immuno-oncogenic therapies. Immunol Lett. 2017;190:159–168. doi: 10.1016/j.imlet.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–1164. [PubMed] [Google Scholar]
- [104].Kofler DM, Chmielewski M, Rappl G, Hombach A, Riet T, Schmidt A, Hombach AA, Wendtner CM, Abken H. CD28 costimulation impairs the efficacy of a redirected T-cell antitumor attack in the presence of regulatory T cells which can be overcome by preventing lck activation. Mol Ther. 2011;19:760–767. doi: 10.1038/mt.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203(7):1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203(7):1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Vera JF, Hoyos V, Savoldo B, Quintarelli C, Giordano Attianese GMP, Leen AM, Liu H, Foster AE, Heslop HE, Rooney CM, Brenner MK, et al. Genetic manipulation of tumor-specific cytotoxic T lymphocytes to restore responsiveness to IL-7. Mol Ther. 2009;17:880–888. doi: 10.1038/mt.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Perna SK, Pagliara D, Mahendravada A, Liu H, Brenner MK, Savoldo B, Dotti G. Interleukin-7 mediates selective expansion of tumor-redirected cytotoxic T lymphocytes (CTLs) without enhancement of regulatory T-cell inhibition. Clin Cancer Res. 2014;20(1):131–139. doi: 10.1158/1078-0432.CCR-13-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Nishimura H, Honjo T. PD-1: an inhibitory immunoreceptor involved in peripheral tolerance. Trends Immunol. 2001;22(5):265–268. doi: 10.1016/s1471-4906(01)01888-9. [DOI] [PubMed] [Google Scholar]
- [111].Wang Y, Wu L, Tian C, Zhang Y. PD-1-PD-L1 immune-checkpoint blockade in malignant lymphomas. Ann Hematol. 2018;97:229–237. doi: 10.1007/s00277-017-3176-6. [DOI] [PubMed] [Google Scholar]
- [112].Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, Iyer AK. PD-1 and PD-L1 checkpoint signaling inhibition for Cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:1–15. doi: 10.3389/fphar.2017.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Rafiq S, Yeku OO, Jackson HJ, Purdon TJ, van Leeuwen DG, Drakes DJ, Song M, Miele MM, Li Z, Wang P, Yan S, et al. Targeted delivery of a PD-1-blocking scFV by CAR-T cells enhances anti-tumor efficacy in vivo. Nat Biotechnol. 2018;36:847–858. doi: 10.1038/nbt.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Leen AM, Sukumaran S, Watanabe N, Mohammed S, Keirnan J, Yanagisawa R, Anurathapan U, Rendon D, Heslop HE, Rooney CM, Brenner MK, et al. Reversal of tumor immune inhibition using a chimeric cytokine receptor. Mol Ther. 2014;22:1211–1220. doi: 10.1038/mt.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].van der Stegen SJ, Davies DM, Wilkie S, Foster J, Sosabowski JK, Burnet J, Whilding LM, Petrovic RM, Ghaem-Maghami S, Mather S, Jeannon JP, et al. Preclinical in vivo modeling of cytokine release syndrome induced by ErbB-retargeted human T cells: identifying a window of therapeutic opportunity? J Immunol. 2013;191(9):4589–4598. doi: 10.4049/jimmunol.1301523. [DOI] [PubMed] [Google Scholar]
- [116].Mohammed S, Sukumaran S, Bajgain P, Watanabe N, Heslop HE, Rooney CM, Brenner MK, Fisher WE, Leen AM, Vera JF. Improving chimeric antigen receptor-modified T cell function by reversing the immunosuppressive tumor microenvironment of pancreatic cancer. Mol Ther. 2017;25:249–258. doi: 10.1016/j.ymthe.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Liu X, Ranganathan R, Jiang S, Fang C, Sun J, Kim S, Newick K, Lo A, June CH, Zhao Y, Moon EK. A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors. Cancer Res. 2016;76:1578–1590. doi: 10.1158/0008-5472.CAN-15-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Gattinoni L, Klebanoff CA, Restifo NP. Paths to stemness: building the ultimate antitumour T cell. Nat Rev Cancer. 2012;12(10):671–684. doi: 10.1038/nrc3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Gomes-Silva D, Ramos CA. Cancer immunotherapy using CAR-T cells: from the research bench to the assembly line. Biotechnol J. 2018;13(2) doi: 10.1002/biot.201700097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Zhu J, Paul WE. Heterogeneity and plasticity of T helper cells. Cell Res. 2010;20(1):4–12. doi: 10.1038/cr.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Marzo AL, Kinnear BF, Lake RA, Frelinger JJ, Collins EJ, Robinson BW, Scott B. Tumor-specific CD4+ T cells have a major "post-licensing" role in CTL mediated anti-tumor immunity. J Immunol. 2000;165(11):6047–6055. doi: 10.4049/jimmunol.165.11.6047. [DOI] [PubMed] [Google Scholar]
- [122].Sommermeyer D, Hudecek M, Kosasih PL, Gogishvili T, Maloney DG, Turtle CJ, Riddell SR. Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia. 2016;30:492–500. doi: 10.1038/leu.2015.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Moeller M, Haynes NM, Kershaw MH, Jackson JT, Teng MWL, Street SE, Cerutti L, Jane SM, Trapani JA, Smyth MJ, Darcy PK. Adoptive transfer of gene-engineered CD4+ helper T cells induces potent primary and secondary tumor rejection. Blood. 2005;106:2995–3003. doi: 10.1182/blood-2004-12-4906. [DOI] [PubMed] [Google Scholar]
- [124].Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, Sommermeyer D, Melville K, Pender B, Budiarto TM, Robinson E, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126(6):2123–2138. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Abramson JS, Palomba L, Gordon LI, Lunning M, Arnason J, Forero-Torres A, Albertson TM, Exton VS, Sutherland C, Xie B, Snodgrass S, et al. Transcend NHL. 001: immunotherapy with the CD19-directed CAR T-Cell product JCAR017 results in high complete response rates in relapsed or refractory B-cell non-hodgkin lymphoma. Blood. 2016;128(22) 4192. [Google Scholar]
- [126].Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Xu Y, Zhang M, Ramos CA, Durett A, Liu E, Dakhova O, Liu H, Creighton CJ, Gee AP, Heslop HE, Rooney CM, et al. Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood. 2014;123:3750–3759. doi: 10.1182/blood-2014-01-552174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, Huls MH, Liu E, Gee AP, Mei Z, Yvon E, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Dai H, Zhang W, Li X, Han Q, Guo Y, Zhang Y, Wang Y, Wang C, Shi F, Zhang Y, Chen M, et al. Tolerance and efficacy of autologous or donor-derived T cells expressing CD19 chimeric antigen receptors in adult B-ALL with extramedullary leukemia. Oncoimmunology. 2015;4(11):e1027469. doi: 10.1080/2162402X.2015.1027469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, Nair VS, Xu Y, Khuong A, Hoang CD, Diehn M, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21:938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Deniger DC, Switzer K, Mi T, Maiti S, Hurton L, Singh H, Huls H, Olivares S, Lee DA, Champlin RE, Cooper LJ. Bispecific T-cells expressing polyclonal repertoire of endogenous γδ T-cell receptors and introduced CD19-specific chimeric antigen receptor. Mol Ther. 2013;21:638–647. doi: 10.1038/mt.2012.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Harrer DC, Simon B, Fujii Si, Shimizu K, Uslu U, Schuler G, Gerer KF, Hoyer S, Dörrie J, Schaft N. RNA-transfection of γ/δ T cells with a chimeric antigen receptor or an α/β T-cell receptor: a safer alternative to genetically engineered α/β T cells for the immunotherapy of melanoma. BMC Cancer. 2017;17:551. doi: 10.1186/s12885-017-3539-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Guery L, Hugues S. Th17 cell plasticity and functions in cancer immunity. Biomed Res Int. 2015;2015 doi: 10.1155/2015/314620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, Gattinoni L, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Guedan S, Chen X, Madar A, Carpenito C, McGettigan SE, Frigault MJ, Lee J, Posey AD, Scholler J, Scholler N, Bonneau R, et al. ICOS-based chimeric antigen receptors program bipolar TH17/ TH1 cells. Blood. 2014;124:1070–1080. doi: 10.1182/blood-2013-10-535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression - implications for anticancer therapy. Nat Rev Clin Oncol. 2019 doi: 10.1038/s41571-019-0175-7. [DOI] [PubMed] [Google Scholar]
- [137].Yao X, Ahmadzadeh M, Lu YC, Liewehr DJ, Dudley ME, Liu F, Schrump DS, Steinberg SM, Rosenberg SA, Robbins PF. Levels of peripheral CD4 +FoxP3 + regulatory T cells are negatively associated with clinical response to adoptive immunotherapy of human cancer. Blood. 2012;119:5688–5696. doi: 10.1182/blood-2011-10-386482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].MacDonald KG, Hoeppli RE, Huang Q, Gillies J, Luciani DS, Orban PC, Broady R, Levings MK. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J Clin Invest. 2016;126:1413–1424. doi: 10.1172/JCI82771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Mekala DJ, Alli RS, Geiger TL. IL-10-dependent infectious tolerance after the treatment of experimental allergic encephalomyelitis with redirected CD4+CD25+ T lymphocytes. Proc Natl Acad Sci U S A. 2005;102:11817–11822. doi: 10.1073/pnas.0505445102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Kohlgruber AC, Donado CA, LaMarche NM, Brenner MB, Brennan PJ. Activation strategies for invariant natural killer T cells. Immunogenetics. 2016;8(68):649–663. doi: 10.1007/s00251-016-0944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376–383. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Altvater B, Landmeier S, Pscherer S, Temme J, Schweer K, Kailayangiri S, Campana D, Juergens H, Pule M, Rossig C. 2B4 (CD244) signaling by recombinant antigen-specific chimeric receptors costimulates natural killer cell activation to leukemia and neuroblastoma cells. Clin Cancer Res. 2009;15:4857–4866. doi: 10.1158/1078-0432.CCR-08-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Kruschinski A, Moosmann A, Poschke I, Norell H, Chmielewski M, Seliger B, Kiessling R, Blankenstein T, Abken H, Charo J. Engineering antigen-specific primary human NK cells against HER-2 positive carcinomas. Proc Natl Acad Sci U S A. 2008;45(105):17481–17486. doi: 10.1073/pnas.0804788105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Chang YH, Connolly J, Shimasaki N, Mimura K, Kono K, Campana D. A chimeric receptor with NKG2D specificity enhances natural killer cell activation and killing of tumor cells. Cancer Res. 2013;73:1777–1786. doi: 10.1158/0008-5472.CAN-12-3558. [DOI] [PubMed] [Google Scholar]
- [145].Romee R, Schneider SE, Leong JW, Chase JM, Keppel CR, Sullivan RP, Cooper MA, Fehniger TA. Cytokine activation induces human memory-like NK cells. Blood. 2012;120:4751–4761. doi: 10.1182/blood-2012-04-419283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Gong JH, Maki G, Klingemann HG. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. 1994;4(8):652–658. [PubMed] [Google Scholar]
- [147].Tonn T, Schwabe D, Klingemann HG, Becker S, Esser R, Koehl U, Suttorp M, Seifried E, Ottmann OG, Bug G. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy. 2013;15(12):1563–1570. doi: 10.1016/j.jcyt.2013.06.017. [DOI] [PubMed] [Google Scholar]
- [148].Arai S, Meagher R, Swearingen M, Myint H, Rich E, Martinson J, Klingemann H. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy. 2008;10(6):625–632. doi: 10.1080/14653240802301872. [DOI] [PubMed] [Google Scholar]
- [149].Jiang H, Zhang W, Shang P, Zhang H, Fu W, Ye F, Zeng T, Huang H, Zhang X, Sun W, Man-Yuen Sze D, et al. Transfection of chimeric anti-CD138 gene enhances natural killer cell activation and killing of multiple myeloma cells. Mol Oncol. 2014;8:297–310. doi: 10.1016/j.molonc.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Schönfeld K, Sahm C, Zhang C, Naundorf S, Brendel C, Odendahl M, Nowakowska P, Bönig H, Köhl U, Kloess S, Köhler S, et al. Selective inhibition of tumor growth by clonal NK cells expressing an ErbB2/HER2-specific chimeric antigen receptor. Mol Ther. 2015;23:330–338. doi: 10.1038/mt.2014.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Chen KH, Wada M, Pinz KG, Liu H, Lin KW, Jares A, Firor AE, Shuai X, Salman H, Golightly M, Lan F, et al. Preclinical targeting of aggressive T-cell malignancies using anti-CD5 chimeric antigen receptor. Leukemia. 2017;31:2151–2160. doi: 10.1038/leu.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Tassev DV, Cheng M, Cheung NK. Retargeting NK92 cells using an HLA-A2-restricted, EBNA3C-specific chimeric antigen receptor. Cancer Gene Ther. 2012;19:84–100. doi: 10.1038/cgt.2011.66. [DOI] [PubMed] [Google Scholar]
- [153].Lai LJ, Shen J, Ran ZH. Natural killer T cells and ulcerative colitis. Cell Immunol. 2018;335:1–5. doi: 10.1016/j.cellimm.2018.08.010. [DOI] [PubMed] [Google Scholar]
- [154].Tachibana T, Onodera H, Tsuruyama T, Mori A, Nagayama S, Hiai H, Imamura M. Increased intratumor Vα24-positive natural killer T cells: a prognostic factor for primary colorectal carcinomas. Clin Cancer Res. 2005;11:7322–7327. doi: 10.1158/1078-0432.CCR-05-0877. [DOI] [PubMed] [Google Scholar]
- [155].de Lalla C, Rinaldi A, Montagna D, Azzimonti L, Bernardo ME, Sangalli LM, Paganoni AM, Maccario R, Di Cesare-Merlone A, Zecca M, Locatelli F, et al. Invariant NKT cell reconstitution in pediatric leukemia patients given HLA-haploidentical stem cell transplantation defines distinct CD4+ and CD4- subset dynamics and correlates with remission state. J Immunol. 2011;186(7):4490–4499. doi: 10.4049/jimmunol.1003748. [DOI] [PubMed] [Google Scholar]
- [156].Kawamura T, Takeda K, Mendiratta SK, Kawamura H, Van Kaer L, Yagita H, Abo T, Okumura K. Cutting edge: critical role of NK1(+) T cells in IL-12-induced immune responses in vivo. J Immunol. 1998;160(1):16–19. [PubMed] [Google Scholar]
- [157].Exley MA, Friedlander P, Alatrakchi N, Vriend L, Yue S, Sasada T, Zeng W, Mizukami Y, Clark J, Nemer D, LeClair K, et al. Adoptive transfer of invariant NKT cells as immunotherapy for advanced melanoma: a phase I clinical trial. Clin Cancer Res. 2017;23:3510–3519. doi: 10.1158/1078-0432.CCR-16-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Motohashi S, Ishikawa A, Ishikawa E, Otsuji M, Iizasa T, Hanaoka H, Shimizu N, Horiguchi S, Okamoto Y, Fujii S-i, Taniguchi M, et al. A phase I study of in vitro expanded natural killer T cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2006;12:6079–6086. doi: 10.1158/1078-0432.CCR-06-0114. [DOI] [PubMed] [Google Scholar]
- [159].Kriegsmann K, Kriegsmann M, von Bergwelt-Baildon M, Cremer M, Witzens-Harig M. NKT cells - New Players in CAR Cell Immunotherapy? Eur J Haematol. 2018;101:750–757. doi: 10.1111/ejh.13170. [DOI] [PubMed] [Google Scholar]
- [160].Tian G, Courtney AN, Jena B, Heczey A, Liu D, Marinova E, Guo L, Xu X, Torikai H, Mo Q, Dotti G, et al. CD62L+NKT cells have prolonged persistence and antitumor activity in vivo. J Clin Invest. 2016;126:2341–2355. doi: 10.1172/JCI83476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Heczey A, Liu D, Tian G, Courtney AN, Wei J, Marinova E, Gao X, Guo L, Yvon E, Hicks J, Liu H, et al. Invariant NKT cells with chimeric antigen receptor provide a novel platform for safe and effective cancer immunotherapy. Blood. 2014;124:2824–2833. doi: 10.1182/blood-2013-11-541235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Clarke RL, Stegen Svd, Lee T, Mansilla-Soto J, Chang C-w, Sasaki J, Husain M, Peralta E, Hardy I, Ortiz E, Riviere I, et al. Abstract LB-108: generation of off-the-shelf TCR-less CAR-targeted cytotoxic T cells from renewable pluripotent cells for cancer immunotherapy. Cancer Res. 2018;78:LB-108–LB-108. [Google Scholar]
- [163].Zhao J, Lin Q, Song Y, Liu D. Universal CARs, universal T cells, and universal CAR T cells. J Hematol Oncol. 2018;11:132. doi: 10.1186/s13045-018-0677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Ren J, Liu X, Fang C, Jiang S, June CH, Zhao Y. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin Cancer Res. 2017;23:2255–2266. doi: 10.1158/1078-0432.CCR-16-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Keu KV, Witney TH, Yaghoubi S, Rosenberg J, Kurien A, Magnusson R, Williams J, Habte F, Wagner JR, Forman S, Brown C, et al. Reporter gene imaging of targeted T cell immunotherapy in recurrent glioma. Sci Transl Med. 2017;9(373) doi: 10.1126/scitranslmed.aag2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Gardner RA, Finney O, Annesley C, Brakke H, Summers C, Leger K, Bleakley M, Brown C, Mgebroff S, Spratt K, Hoglund V, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129(25):3322–3331. doi: 10.1182/blood-2017-02-769208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Wang CM, Wu ZQ, Wang Y, Guo YL, Dai HR, Wang XH, Li X, Zhang YJ, Zhang WY, Chen MX, Zhang Y, et al. Autologous T cells expressing CD30 chimeric antigen receptors for relapsed or refractory hodgkin lymphoma: an open-label phase I trial. Clin Cancer Res. 2017;23(5):1156–1166. doi: 10.1158/1078-0432.CCR-16-1365. [DOI] [PubMed] [Google Scholar]
- [168].Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, Samanta M, Lakhal M, Gloss B, Danet-Desnoyers G, Campana D, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17(8):1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Zhang WY, Wang Y, Guo YL, Dai HR, Yang QM, Zhang YJ, Zhang Y, Chen MX, Wang CM, Feng KC, Li SX, et al. Treatment of CD20-directed chimeric antigen receptor-modified T cells in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: an early phase IIa trial report. Signal Transduct Target Ther. 2016;1 doi: 10.1038/sigtrans.2016.2. 16002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Kawalekar OU, O’Connor RS, Fraietta JA, Guo L, McGettigan SE, Posey AD, Jr, Patel PR, Guedan S, Scholler J, Keith B, Snyder NW, et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity. 2016;44(2):380–390. doi: 10.1016/j.immuni.2016.01.021. [DOI] [PubMed] [Google Scholar]
- [171].Chu Y, Hochberg J, Yahr A, Ayello J, van de Ven C, Barth M, Czuczman M, Cairo MS. Targeting CD20+ aggressive B-cell non-hodgkin lymphoma by anti-CD20 CAR mRNA-Modified expanded natural killer cells in vitro and in NSG mice. Cancer Immunol Res. 2015;3(4):333–344. doi: 10.1158/2326-6066.CIR-14-0114. [DOI] [PubMed] [Google Scholar]
- [172].Ritchie DS, Neeson PJ, Khot A, Peinert S, Tai T, Tainton K, Chen K, Shin M, Wall DM, Hönemann D, Gambell P, et al. Persistence and efficacy of second generation CAR T cell against the LeY antigen in acute myeloid leukemia. Mol Ther. 2013;11(21):2122–2129. doi: 10.1038/mt.2013.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Locke FL, Neelapu SS, Bartlett NL, Siddiqi T, Chavez JC, Hosing CM, Ghobadi A, Budde LE, Bot A, Rossi JM, Jiang Y, et al. Phase 1 results of ZUMA-1: a multicenter study of KTE-C19 anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther. 2017;25(1):285–295. doi: 10.1016/j.ymthe.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Kochenderfer JN, Feldman SA, Zhao Y, Xu H, Black MA, Morgan RA, Wilson WH, Rosenberg SA. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J Immunother. 2009;32(7):689–702. doi: 10.1097/CJI.0b013e3181ac6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Cheadle EJ, Sheard V, Rothwell DG, Bridgeman JS, Ashton G, Hanson V, Mansoor AW, Hawkins RE, Gilham DE. Differential role of Th1 and Th2 cytokines in autotoxicity driven by CD19-specific second-generation chimeric antigen receptor T cells in a mouse model. J Immunol. 2014;192(8):3654–3665. doi: 10.4049/jimmunol.1302148. [DOI] [PubMed] [Google Scholar]
- [176].Kowolik CM, Topp MS, Gonzalez S, Pfeiffer T, Olivares S, Gonzalez N, Smith DD, Forman SJ, Jensen MC, Cooper LJ. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 2006;66(22):10995–11004. doi: 10.1158/0008-5472.CAN-06-0160. [DOI] [PubMed] [Google Scholar]
- [177].Sahm C, Schonfeld K, Wels WS. Expression of IL-15 in NK cells results in rapid enrichment and selective cytotoxicity of gene-modified effectors that carry a tumor-specific antigen receptor. Cancer Immunol Immunother. 2012;61(9):1451–1461. doi: 10.1007/s00262-012-1212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Wilkie S, van Schalkwyk MC, Hobbs S, Davies DM, van der Stegen SJ, Pereira AC, Burbridge SE, Box C, Eccles SA, Maher J. Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling. J Clin Immunol. 2012;32(5):1059–1070. doi: 10.1007/s10875-012-9689-9. [DOI] [PubMed] [Google Scholar]
- [179].Davies DM, Foster J, Van Der Stegen SJ, Parente-Pereira AC, Chiapero-Stanke L, Delinassios GJ, Burbridge SE, Kao V, Liu Z, Bosshard-Carter L, Van Schalkwyk MC, et al. Flexible targeting of ErbB dimers that drive tumorigenesis by using genetically engineered T cells. Mol Med. 2012;18:565–576. doi: 10.2119/molmed.2011.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Till BG, Jensen MC, Wang J, Qian X, Gopal AK, Maloney DG, Lindgren CG, Lin Y, Pagel JM, Budde LE, Raubitschek A, et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood. 2012;119(17):3940–3950. doi: 10.1182/blood-2011-10-387969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Chinnasamy D, Yu Z, Theoret MR, Zhao Y, Shrimali RK, Morgan RA, Feldman SA, Restifo NP, Rosenberg SA. Gene therapy using genetically modified lymphocytes targeting VEGFR-2 inhibits the growth of vascularized syngenic tumors in mice. J Clin Invest. 2010;120(11):3953–3968. doi: 10.1172/JCI43490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Pulè MA, Straathof KC, Dotti G, Heslop HE, Rooney CM, Brenner MK. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005;12(5):933–941. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]