Abstract
Microfluidic principles have been extensively utilized as powerful tools to fabricate controlled monodisperse cell-laden hydrogel microdroplets for various biological applications, especially tissue engineering. In this review, we report recent advances in microfluidic-based droplet fabrication and provide our rationale to justify the superiority of microfluidics-based techniques over other microtechnology methods in achieving the encapsulation of cells within hydrogels. The three main components of such a system—hydrogels, cells, and device configurations—are examined thoroughly. First, the characteristics of various types of hydrogels including natural and synthetic types, especially concerning cell encapsulation, are examined. This is followed by the elucidation of the reasoning behind choosing specific cells for encapsulation. Next, in addition to a detailed discussion of their respective droplet formation mechanisms, various device configurations including T-junctions, flow-focusing, and co-flowing that aid in achieving cell encapsulation are critically reviewed. We then present an outlook on the current applications of cell-laden hydrogel droplets in tissue engineering such as 3D cell culturing, rapid generation and repair of tissues, and their usage as platforms for studying cell–cell and cell–microenvironment interactions. Finally, we shed some light upon the prospects of microfluidics-based production of cell-laden microgels and propose some directions for forthcoming research that can aid in overcoming challenges currently impeding the translation of the technology into clinical success.
I. INTRODUCTION
Tissue engineering entails the combination of materials, mechanical, chemical, and biological sciences to artificially recreate tissue structures that could repair or even replace lost biological function.1 One of the most successful therapeutic approaches in tissue engineering involves creating scaffolds by encapsulating recompensing cells within a biodegradable material (e.g., a hydrogel). Within these scaffolds, cells can grow under biological conditions that are favorable to their growth and survival.2 Ideally, upon being transplanting in vivo, the biodegradable scaffold will gradually degrade and be replaced by the new desired tissue.1 Cell-laden hydrogels were first transplanted in vivo in 1964 with the objective of minimizing complications resulting from immune rejection that occur post-transplantation.3 Cells encapsulated within hydrogel droplets are not easily accessible to host immune antibodies and are hence protected from their attack. In the duration of their encapsulation, they also attain the opportunity to develop and effectively implement their intended therapeutic effects onto the surrounding environment. These therapies have previously included the regeneration of tissues and the in situ supply of produced proteins.4 In conventional macroscale tissue engineering, cells are cultured within a biodegradable polymeric scaffolding material.5 Over time, as the scaffold degrades, cells grow, produce their own extracellular matrix (ECM), and in the process form the engineered artificial tissue. However, macroscopic approaches often fall short of adequately recreating the intricate microstructure of native tissues, which is an essential requirement for the realization of the successful functionality of an engineered tissue.6,7 Moreover, culturing cells within such macroscopic scaffolds often results in limited cell–cell communication and an inefficient exchange of oxygen, nutrients, and metabolites.6,7 In comparison, the microscale approach aims to create biomimetic engineered microtissues by manipulating the microscale structural features.5 This strategy mainly exploits the benefits of the high surface area-to-volume ratio that is characteristic of the micro-domain, which allows for both the efficient exchange of oxygen and nutrients and enhanced cell–matrix interactions.7 For these reasons, such biomimetic constructs are considered more adept for mimicking the composition and functions of native extracellular matrices.
Common methods for encapsulating cells within microgels, such as emulsification, extrusion, or co-extrusion methods, are limited when it comes to fabricating small sized microdroplets (≤100 μm) with high monodispersity. This ability is necessary for building vascularized, complex tissue structures that are able to function normally.4,6 In order to address these problems, microfabrication techniques including microfluidics, micro-molding, soft lithography, and bioprinting have been developed to fabricate microscale hydrogels that are ready for encapsulating cells. These microscale cell-laden hydrogels have become quite valuable for a wide range of studies, including those seeking to understand cell differentiation, cell–cell interactions, cell–matrix interactions, shear forces imposed on cells,8 and the functioning of 3D tissue structures.5 An in-depth understanding of microscale environmental parameters is especially important for successfully engineering functional tissues. For example, porosity and stiffness of the extracellular matrix (ECM) have been observed to affect cancer cell migration, and so, when fabricating constructs mimicking such a tissue, these parameters and relations must be taken into consideration.9 Cell fate is also influenced by various microenvironmental cues, such as cell–cell interaction, accessibility to growth factors, mechanical stimuli, and shear forces.10
Microfluidic-based cell encapsulation has been widely used in recent years due to its superiority over other microtechnology techniques. Microfluidic techniques have offered the ability to generate high-throughput, well-controlled monodisperse micrometer-sized, spherical or rounded microgel particles with narrow polydispersity.11–13 With microfluidic devices, it is also possible to generate microgels of varying shapes including hollow and cylindrical gel fibers, spheres, disks, and capsules (core–shell),14,15 which cannot be created via other microfabrication techniques. It is also possible to regulate the sizes of the droplets by manipulating parameters such as fluid flow rates, fluid properties such as interfacial tension and viscosity, and the geometry of the device.16–18 In addition, restricting the droplet size (<250 μm) can significantly aid in nutrient and oxygen delivery to the microgel-encapsulated cells.13 Microfluidic technologies offer a high degree of control over the encapsulation process and enable the desired number of cells per particle, cellular spatial organization, cellular position, and morphological and dimensional properties.13,19,20 Achieving these parameters in the majority of microgel particles in a set can greatly aid the studies of cell co-cultures and the effects of confinement and intercellular distance.21 Finally, the use of microfluidic techniques has also demonstrated increased viability of the encapsulated cells, which can be attributed mainly to the presence of microgel shells that protect and shield cells from shear forces and other similarly harmful external stimuli.22,23
In this paper, we review the various facets of microfluidics-based biofabrication of cell-laden microgels. First, we summarize the materials involved in creating microgels. Here, the reader will be introduced to the characteristics of hydrogels as well as the rationale behind the selection of specific cellular components. We then describe the various microfluidic droplet generation mechanisms and device configurations that are used for generating microgels. Next, with a focus on tissue engineering and biofabrication, we discuss the various applications of microgels. Finally, the authors contemplate upon the future of this revolutionary technology and suggest some directions for future research.
II. MATERIALS FOR CELL-LADEN MICRODROPLET GENERATION
A. Hydrogels for microscale cell encapsulation
Hydrogels are hydrophilic polymeric networks that can absorb high volumes of water without dissolving.24 Hydrogels have been used extensively for a wide range of applications in recent decades.25 Their porous nature, coupled with their immense ability to absorb water (up to 90% of their weight), and tissue-like elasticity make them a favorable structure for cells to grow, attach, and adequately receive oxygen and nutrients.26,27 For these reasons, hydrogels have also received considerable attention in the recent years for applications in tissue engineering.27,28
Hydrogels can be broadly classified to two categories: synthetic (initially synthesized in the laboratory) and natural (obtained from natural resources). Both synthetic and natural hydrogels are widely used in tissue engineering because of their porous structure and good biocompatibility.29 Natural hydrogels can either be proteins (e.g., gelatin, collagen, and fibrin) or polysaccharides (e.g., alginate, hyaluronic acid, chitosan, and agarose). Natural hydrogels form networks via physical or ionic interactions; meanwhile, synthetic hydrogels, which include polyethylene glycol (PEG), polyacrylic acid (PAA), and polyvinyl alcohol (PVA), are synthesized via a radical chain or step-growth polymerization, and therefore use covalent bonds as their networking force.30,31 Hydrogels also display varying functional characteristics that depend on their polymerization method and their resulting molecular structure. These in turn influence cellular functions of encapsulated cells, such as their growth, migration, and differentiation. Each hydrogel type possesses its own set of advantages and drawbacks. Natural hydrogels are inherently biocompatible and promote many cellular functions. However, extra processes are required to extract them from biological tissues that only yield limited volumes. In addition, they also tend to lack in variety, and their usage remains limited to specific cells. Synthetic hydrogels, in comparison, are highly reproducible, readily available, and their composition can be tuned as needed for diverse cell lines;32 however, their tendency to absorb protein is limited.33 The most important advantage of synthetic hydrogels is the controllability of their mechanical properties and biochemical cues.24,34
Crosslinking methods, which are hugely dependent on the type of hydrogel used, are essential for the gelation of both synthetic and natural hydrogels. For instance, photosensitive materials such as methacrylated silk fibroin (Sil-MA), polyethylene glycol diacrylate (PEGDA), and gelatin methacrylate (GelMA) are crosslinked via exposure to light (e.g., UV),35–37 thermally sensitive materials (e.g., gelatin and agarose) solidify when the temperature is varied,38 and ion-based materials (e.g., alginate) are crosslinked via divalent ions (e.g., Ca2+).20,39,40 Recently, a high-throughput production of monodisperse bioactive polyethylene glycol microgels applying a parallelized step emulsification technique was reported.41 In this study, crosslinking of the microdroplets was initiated by the dissolved proton acceptors in the oil phase, which ensured the uniform physicochemical properties of the generated microgels. However, such a crosslinking reaction is lengthy and requires overnight incubation. Additionally, in this study, cells were not encapsulated in 3D but seeded in a 2D orientation on the surface of previously crosslinked microgels. The cell-bearing microgels were then covalently interconnected by an enzymatic reaction to construct a microporous scaffold wherein the cells were observed to occupy the interstitial spaces in 3D.
In recent years, the mechanical effect of the matrix on constituent cells has become an increasingly relevant topic of study. It is very well known that mechanical cues from matrix materials can drive the development of cells and are crucial for their proper functioning.42 In fact, the mechanical properties of the matrix have been found to induce changes in phenotype,43 proliferation,44 and mobility.45 For this reason, it is very important that the mechanical properties of hydrogels being used to manufacture cell-laden microgels be well characterized and tuned. The most controllable mechanical characteristic of hydrogels is their stiffness, which can also be understood as their rigidity and ability to resist deformation.46 The stiffness of the hydrogel matrix can be increased by increasing parameters such as polymer concentration and crosslinking density, while the methodology depends on the type of hydrogel used and the intended cell behavior. For instance, in a study by Li et al., chondrocytes that were encapsulated in hydrogels with low stiffness demonstrated an elongated morphology and reduced chondrogenic gene expression in contrast to the rounder morphology and increased chondrogenic gene expression as observed in hydrogels of high stiffness.47
Ideally, in graft transplantations involving engineered tissues, hydrogels degrade over time as cells replace this pre-existing structure with their own extracellular matrix. While natural hydrogels such as collagen, gelatin, fibrin, and chitosan are biodegradable, non-degradable hydrogels such as PVA and PEG can be modified and rendered degradable upon adding hydrolyzable esters48 or peptide functionalities49 in the crosslinking agents. During the degradation process, the hydrophilic backbone of the polymer chains is broken down by enzymatic activity.50,51 As the gel breaks down, again, the crosslinker protein is digested.52 Through the course of this process, the mass of the hydrogel reduces and its mechanical stability decreases. Consequently, the newly desired tissue replaces the transplanted cell-encapsulating hydrogel. Degradability is a particularly important characteristic of hydrogels that must be considered before applying them toward engineering artificial tissues.
B. Cells encapsulated in microgels
Various cell types can be encapsulated within microgels and their selection generally depends on the intended application or study. Some studies aim to carry out fundamental studies concerning cell encapsulation related fabrication techniques and the performance of biomaterials for incorporating specific cells. Some instances of such studies have investigated cellular interactions, the influence of the surrounding environment on cells, the impact of shear forces imposed on cells, and the cellular effects of mechanical stimuli.
The in vivo applications of the whole system including cells as their most important component are also investigated. It is possible to apply incorporated cells for the secretion of a bioactive substance such as a neurotransmitter, a hormone, or a growth factor.53 This secretion could either be a non-regulated direct discharge or be initiated after sensing the internal environment; for instance, the initiation of insulin secretion after sensing an increase of the blood glucose level.54 Such microdroplets with encapsulated cells have also been employed through microtissue injection for repairing damaged tissues.54,55
However, implantation of encapsulated cells has not yet been successful in gaining approvals for clinical trials, primarily due to the consequent immune response that is evoked in the body. If possible, in such cases, the cell choice may shift into the use of autologous cells or even autologous stem cells. The choice of cell types encapsulated within microgels can be said to be governed mainly by the final purpose of the system and is decided upon after considering all the hurdles that such a system is likely to encounter. Table I summarizes the combinations of cells and hydrogels that have been used in microgel fabrication.
TABLE I.
Summary of the types of cells and hydrogels that have been used in microgel droplet fabrication.
| Material | Cell types | Gelation method | Cell density | Cell viability | Reference |
|---|---|---|---|---|---|
| Alginate | Human kidney 293 cells | Ionic crosslinking (CaCl2) | Not reported | 70% | 109 |
| Chlamydomonas | Ionic crosslinking (CaCO3) | Not reported | 70% | 165 | |
| Chondrocytes | Ionic crosslinking (CaCl2) | 3.5 cells/microgel | 96% | 166 | |
| Mammalian cells | Ionic crosslinking (CaCO3) | Not reported | 74.3% | 77 | |
| Yeast cells | Ionic crosslinking (CaCl2) | 2 cells/microgel | Not reported | 85 | |
| HepG2 | Ionic crosslinking (CaCl2) | Not reported | Not reported | 86 | |
| HeLa cells | No crosslinking | 1 cell/microgel | Not reported | 100 | |
| P19 EC | Ionic crosslinking (CaCO3) | Not reported | 90% | 99 | |
| P19 EC, MCF7, HepG2 | Ionic crosslinking (Calcified oleic acid) | Not reported | 95% | 98 | |
| Agarose | R1 and YC5–YFP–NEO mES cells | Physical | Not reported | 79.6% (R1) and 80% (YC5–YFP–NEO) | 59 |
| Escherichia coli cells | Temperature variation | 1 cell/microgel | Not reported | 104 | |
| PFPE (perfluoropolyether)-PEG | 2C6 hybridoma cells | Not reported | 1–3 cells/microgel | 85% | 103 |
| PEG | C2C12(p7), Placenta-derived human MSC/ESC | Physical | 2–10 cells/microgel | Not reported | 102 |
| Alginate, agarose | Eukaryotic cells, Sertoli cells | Chemical (Alginate) and physical (agarose) | Not reported | Not reported | 101 |
| Polystyrene | HL60 | N/A | 1 cell/microgel | Not reported | 97 |
| GelMA | CSP (cardiac side population) cells | Photopolymerization | Not reported | ∼90% | 167 |
| NIH-3T3 | Photopolymerization | Not reported | 85.2% | 116 | |
| Bone marrow stem cells | Photopolymerization | Not reported | >60% | 53 |
C. Characterization of cell-laden microgels
In order to ensure monodispersity of microgels and deterministic cell encapsulation, it is necessary to characterize and sort microgels. Since the size of the microenvironment has a tremendous impact on cell behavior, it is especially important to regulate the size of the microgels. An approach that is frequently taken to optimize droplet diameter is to tweak the flow rate ratio between the continuous and dispersed phases. Usually, the higher the ratio, the larger the observed diameters will be.56 Since a 200 μm limit57 exists for oxygen diffusion, the droplet must be limited to a size smaller than that to prevent hypoxia and larger than the cell diameter to prevent cell escape.
In applications requiring deterministic cell encapsulation, for instance, single-cell encapsulation, it is important to sort microgels based on whether they consist of single cells, multiple cells, or no cells. As this process is governed by the Poisson distribution, approximately 37% of microgels manufactured will have the desired density of a single cell. It is, therefore, important to perform a post-process enrichment of the sample to sort the desired microgels. One method that has gained prominence for cell sorting is fluorescence-activated cell sorting (FACS).58 In this method, cells are fluorescently tagged using biochemical biomarkers to have a specific signal, using an optical detector; cells with different fluorescence signals are sorted. This method can also be used to sort microgels based on whether cells are present and their quantity.
III. MICROFLUIDIC GENERATION OF CELL-LADEN MICROGELS
A. Droplet formation mechanisms
Most aqueous monodisperse droplets are formed via continuous pressure, driven by passive methods such as T-junction,59 flow-focusing,60 or coaxial capillaries.12 In these methods, the sources of pressure exist remotely to the droplet generation mechanism 61 (for instance, the force required for fluid flow can be achieved using a syringe pump outside the geometry of the device), while the droplet formation occurs due to the interaction between the dispersed phase and the continuous phase. However, active methods require an additional energy source for droplet manipulation and may utilize piezoelectric actuators62 and electric field methods63 for on-demand droplet generation. These methods offer a higher degree of controllability and faster response times and are especially important for manufacturing microgels with deterministic cell encapsulation.64 While active methods are important for microgel production applications, in this review, we focus mainly on passive methods for droplet formation. Passive methods for generating droplets offer a high throughput and the ability to co-fabricate two or three emulsion droplets by adding parallel channels to the device.65,66 Based on the required droplet frequency, variability in droplet size, and droplet monodispersity, various microfluidic devices with different geometries have been utilized for droplet generation.67
Microfluidic devices used for droplet generation are categorized into two major classes according to their fabrication material as either being poly (dimethylsiloxane) (PDMS)-based or glass-based. PDMS-based devices were first introduced by the Whitesides research group68–70 and are reusable, cost-effective, easy to fabricate,68 and, most importantly, transparent. Despite the fact that PDMS-based devices are compatible with aqueous solvents, the swelling of PDMS in nonpolar solvents is a significant drawback.71 The second class of droplet generating devices are glass-based and originate from the Utada research group.72 Glass is transparent and does not face the same issue of swelling as observed in PDMS. However, fabricating glass-based microfluidic devices requires a high level of expertise.71 Other materials such as polyether-ether-ketone (PEEK) and poly-methyl-methacrylate (PMMA) have also been used to fabricate microfluidic devices, but their usage is limited due to the high costs associated with handling them and their laborious fabrication process.73–75
Hydrogel-based pre-polymer solution droplets are generated in a microfluidic device through the formation of laminar flow of the aqueous solution (disperse phase) by an immiscible phase (continuous phase). Typically, the hydrogel containing the cells is immersed through one inlet while, in order to surround the hydrogel solution, the continuous phase is entered through another. Usually, a nonpolar liquid such as mineral oil, fluorinated oil, or vegetable oil is used as the continuous phase.21,76,77 In flow-focusing and co-flowing devices, droplets break up when the viscous force of the continuous phase fluid overcomes the interfacial force that keeps droplets connected to the disperse phase fluid. The mechanism of droplet generation at a high oil flow rate in the T-junction design is like that of flow-focusing and co-flowing. The droplets created via this mechanism are supported by the surface-induced instability (Rayleigh instability) theory. Based on this theory, surface tension energy tends to minimize with reducing interfacial area, while viscosity acts to lengthen and drag the interface downstream. Once the viscous force of the continuous phase overcomes surface tension, droplets are created. At low oil flow rates in T-junction devices, droplets are created when the disperse phase fluid blocks the channel. This is followed by creation of the buildup pressure, which leads to the pinching off of the droplets.17 To reduce the surface energy, prevent the coalescence, and increase the monodispersity, a surfactant can be added to the continuous phase fluid.67,78
B. Microfluidic devices
1. T-junction devices
T-junction microfluidic devices are widely used for droplet generation due to the ease at which they can be fabricated and operated.79–82 In the T-junction configuration, the disperse phase is entered through an inlet while the continuous phase is entered through the main channel. The continuous phase that intersects the disperse phase at the junction leads the tip of the dispersed phase flow to enter the main channel. The shear force of the continuous phase and the pressure gradient make a thin neck of disperse phase at the junction and finally create a droplet.83 The size of these droplets depends on the flow rates and viscosities of the disperse and continuous phases as well as the geometry of the channel.83,84
In a pioneering study where T-junction microfluidics was applied for cell-laden droplet generation, Choi et al. described the fabrication of alginate microgels.85 As alginate-based droplets came into contact with calcium, they were polymerized via chaotic mixing in the microfluidic device [Figs. 1(a)–1(e)]. The effects of continuous flow rate and non-dimensional capillary number on droplet size and droplet distribution were discussed in this study.85 In a similar study, Tan et al. also demonstrated the formation of alginate particles in microfluidic devices.77 They fabricated alginate particles with a narrow size distribution using a new method that combined internal gelation with T-junction within a microfluidic device. Calcium carbonate nanoparticles were mixed in the alginate solution, inducing the internal crosslinking process. Droplets were formed at the T-junction and were sheared off to the channel by corn oil fluid. The corn oil along with lectin and acetic acid was diffused into the channel toward the crosslinked alginate droplet that was mixed with calcium carbonate. The size of the droplet was controlled by tuning the flow rate.77 Um et al. described the formation of cell-laden hydrogel droplets by applying a double T-junction device.86 This device consisted of three inlets, the pre-gel solution was entered into the first inlet and the oil phase (mineral oil with span 80) was entered into the second inlet in order to cut off the pre-gel solution and fabricate the droplets. A HepG2-containing medium was then introduced into the third inlet downstream in the channel to mix with the droplets. The microgel beads were polymerized by ions in the cell medium.
FIG. 1.
Applied T-junction devices. (a) A diagram of a T-junction microfluidic device with two injection lines for microdroplets generation. (b) A diagram of the same device with an extra line for cell encapsulation. (c)–(e) Three microscopy images of the GFP (Green Fluorescent Protein)–yeast encapsulated microbeads: (c) optical image and (d) fluorescence image. (e) Combined image of the optical and fluorescence photos. Images (a)–(e) are reproduced with permission from Choi et al., Biomed. Microdevices 9(6), 855 (2007). Copyright 2007 Springer Nature. (f) A diagram of the T-junction-based microfluidic device for the generation and analysis of 3D lymphoma spheroids. (g) Illustration of the spheroid docking microarray unit of the device. (h)–(k) Representative micrographic pictures of the T-junction for generating cell-laden hydrogel microdroplets (h), droplets docking in the microarray (i), and the crosslinked spheroids microgels at day 1 (j) and day 5 (k). (l) Tracking a 3D immunogenic spheroid cell proliferation for 5 days. Scale bars = 50 μm. Images (f)–(l) are reproduced with permission from Sabhachandani et al., J. Control. Release 295, 21–30 (2019). Copyright 2019 Elsevier.
Several studies have used T-junction microfluidic devices to encapsulate cells within microgels and to investigate the behavior of the encapsulated cells. Kumachev et al. described the influence of the elasticity of the cellular microenvironment on cell fate by fabricating high-throughput 3D microgels with varying elasticities using a T-junction microfluidic device.59 Most recently, Sabhachandani et al. reported the development of a T-junction-based integrated microfluidic platform for the high-throughput anti-cancer therapeutic screening with hydrogel-based immunogenic tumor spheroids [Figs. 1(f)–1(l)].87 These spheroids consisted of cancer cells, fibroblasts, and lymphocytes in a hydrogel combination of alginate and puramatrix. The pre-gel microdroplets with encapsulated cells were generated at the T-junction and were driven into an integrated docking array, which could house up to 250 cell-laden micro-spheroids. Then, calcium chloride in a complete growth media was perfused to crosslink the hydrogel within the droplets, which allowed for in situ microgel polymerization while maintaining their spherical structure.
2. Flow-focusing devices
Flow-focusing devices are another class of microfluidic devices used for droplet generation.13,88–90 Although the fabrication of flow-focusing devices is slightly complex as compared to T-junctions, they make it possible to obtain better monodispersity and a higher droplet generation frequency.91 Flow-focusing devices offer droplet generation frequencies up to about 1000 Hz. For encapsulating delicate objects such as cells in microgels, flow-focusing devices and co-flow devices are most commonly used.67 By varying the fluid flow rates in flow-focusing devices, it is possible to yield a wider range of droplet sizes.67,92,93 It is also possible to achieve a jetting regime using a flow-focusing device wherein the size of these jets can be regulated by modifying the flow rates.94
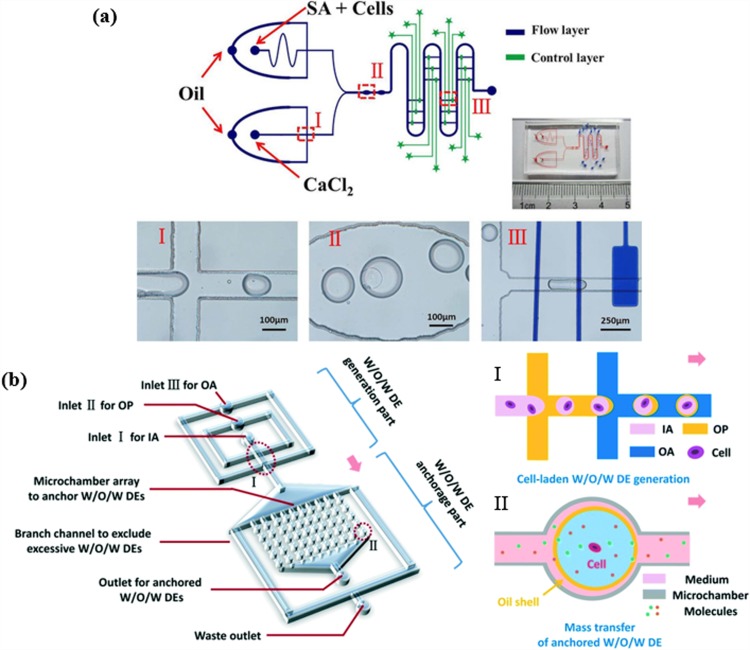
Many studies have been conducted on cell-encapsulated microgels created by a flow-focusing microfluidic geometry. As shown in Fig. 2(a), a valve-regulated device with a double flow-focusing junctions was fabricated to integrate on-chip cell immobilization and treatment with the generation and gelation of single cell-laden alginate microdroplets.95 In another study, water-in-oil-in-water (W/O/W) double emulsions (DEs) encapsulating single cells were generated in two sequential flow-focusing junctions [Fig. 2(b)].96 These microdroplets were subsequently anchored in an array of microchambers located downstream for at least one week. This microfluidic setup has allowed for long-term treatment and monitoring of the behavior of non-adherent cells in situ and in real-time. Edd et al. proposed the encapsulation of a single cell into a monodisperse picoliter drop using the same category of microfluidic devices.97 A new type of flow-focusing device was introduced by Kim et al. that enhances the cell viability via the rapid exchange of oil phase. When cell-laden alginate was crosslinked with calcified oleic acid, the toxic oleic acid was transformed into harmless mineral oil and then flushed out.98 A few years later, the same group introduced a three-dimensional flow-focusing device to generate a core–shell microcapsule for the efficient formation of a cell spheroid, which was achieved by adding a hillock to the device.99 Wu et al. established an integrated device with fluorescence-activated sorting to increase the rate of single-cell encapsulation.100 This device consisted of two segments: a flow-focusing component for generating droplets and a cross shaped hydrodynamic gating for sorting droplets. Hydrogel droplets were created in the flow-focusing part, after which droplets carrying single cells were detected at the hydrodynamic gating part and were isolated in the reservoir.100 Capretto et al. described the formation and characterization of alginate/agarose microdroplets for the encapsulation of Sertoli cells within a flow-focusing device.101 The cell viability and functional capability of Sertoli cells encapsulated in alginate microdroplets was high and demonstrated the effectiveness of flow-focusing device in cell encapsulation. Allazetta et al. used a microfluidic flow-focusing device to synthesize PEG microgels via Michael-type addition. Using the microfluidic flow-focusing device, different chemicals were added to the reactive PEG to modify it with tethered biomolecules in order to adjust their bioactive properties and their application with different stem cell types.102 Köster et al. fabricated a new microfluidic device that consolidates all functions that are to be performed by the chip including the encapsulation, incubation, and manipulation of cells in a picodroplet. The development and integration of microfluidic cell cytometers and sorters on the chip can further increase its functionality.103
FIG. 2.
Targeted modifications of flow-focusing microfluidic devices for cell-laden microdroplets generation and manipulation. (a) Schematic of the two-layer valve-based microfluidic platform and a photograph of the microfabricated device. This device comprises three functional parts—two flow-focusing junctions for microdroplets generation (I), on-chip hydrogel crosslinking chambers (II), and the control part made of independently regulated valve-based trap-release units along the serpentine main channel (III). Images in (a) are reproduced with permission from Sun et al., Electrophoresis 40, 961–968 (2019). Copyright 2019 Wiley-VCH. (b) Schematics of the microfluidic platform for the generation, treatment, and monitoring of water-in-oil-in-water (W/O/W) cell-laden double emulsions (DEs) (“IA” refers to the inner aqueous phase, “OA” refers to the outer aqueous phase, and “OP” refers to the oil phase). The platform consists of two adjacent flow-focusing junctions for DE generation (I) and a DE anchorage microarray (II). Images in (b) are reproduced with permission from Cai et al., Lab Chip 19, 422–431 (2019). Copyright 2019 Royal Society of Chemistry.
Eun et al. described the analysis and isolation of bacterial cells encapsulated in microgels using fluorescence-activated cell sorting. Small microgels that were about 30 μm in size were fabricated by the flow-focusing device, facilitating bacterial screening and isolation.104 Rossow et al. succeeded in achieving the formation of PEG microgel using a double emulsion flow-focusing device.32 In this study, microfluidic particle patterning was combined with bio-orthogonal thiol–ene chemistry to produce microgel droplets with high cell viability. Bio-orthogonal click reactions were used with the objective of carrying out the crosslinking process without the formation of harmful free radicals that reduce cell viability. Dithiolated PEG cross-linkers, acrylated hyperbranched polyglycerol, and cells were entered into the device via different inlets and were emulsified at the first junction. Mineral oil was used as the continuous phase and surrounded the emulsified solution to create droplets. Subsequently, the gelation process took place and formed monodisperse microdroplets.32 Matsunaga et al. used a flow-focusing microfluidic device to encapsulate different types of cells in collagen microgels with sizes in the range of 50–300 μm. These cells included NIH-3T3 mouse fibroblast cells, primary neurons, MIN6 pancreatic cells, primary rat hepatocytes, human umbilical vein endothelial cells (HUVECs), and HepG2 cells. Results indicated the attachment of the cells to the microgels in less than 2 h. For studying the proliferation of cells and their ability to create 3D tissue constructs, NIH-3T3-encapsulated microparticles were placed in a PDMS mold with the goal of building a 3D tissue structure, which was realized after 17 h.105 Meanwhile, Tirandazi and Hidrovo described an approach that utilized liquid droplets within gaseous flow. Rather than using a second liquid carrier, a continuous gas could be used as alternative approach in droplet microfluidics.106
3. Co-flow glass capillary devices and others
These devices consist of two concentric glass capillaries where the inner capillary is assembled into the outer one. Conventionally, two fluid phases flow in the same direction in the capillaries. The disperse phase (cells-containing hydrogel) flows into the inner glass capillary, while the continuous phase fluid (oil phase) flows in the same direction between the inner and outer capillaries.53,107 The size of droplets created in coaxial geometries is larger than those generated in flow-focusing device with similar geometries. The hydrogel droplets are created at the orifice of the inner round capillary. Glass capillary devices are especially advantageous due to their inherent wettability, chemical resistivity, and their three-dimensional geometry. However, the fabrication of the glass capillary devices is more laborious and less reproducible. In addition, these devices display a limited fabrication throughput. For these reasons, the widespread use of this design has been hindered in comparison to other configurations discussed above.13
In an attempt to simplify the design, a coaxial airflow–induced microdroplet generator was used to fabricate human mesenchymal stem cell (MSC)-laden dual-crosslinkable alginate microgels consisting of methacrylated and oxidized alginates.108 The cell–pregel solution mixture was pumped at a rate of 0.5 ml/s with an outer air flow rate of 15 l/min, and the generated droplets were collected into a CaCl2-containing bath for crosslinking. These cell-laden microgels could then be directly assembled into complicated 3D shapes via photopolymerization under UV light. The cell-laden microgels were also found to be cryo-preservable for on-demand applications. Furthermore, thawed microgels with encapsulated cells exhibited similar cell-viabilities to those that were freshly encapsulated. In a similar approach, Sugiura et al. fabricated a “micro-airflow-nozzle” device to generate alginate droplets with a narrow droplet size distribution. The device consists of a nozzle for the alginate solution channel and an airflow channel beside the nozzle. The alginate droplets were sheared off by the airflow and dropped into a calcium chloride solution, thus yielding calcium alginate droplets. The results indicated that the cells encapsulated in these calcium alginate droplets showed a higher growth rate in comparison to alginate droplets generated by other older approaches due to the large diffusion efficiency.55 The same group later restricted the size of the calcium alginate droplets, restricting them to a size smaller than 300 μm by fabricating and employing a micro-nozzle array.109 Most recently, an oil-free production of cell-laden core–shell microgels in an all-aqueous-phase coaxial microfluidics system was reported. This was done in order to prevent oil caused cytotoxicity and protein denaturation.110 The microfluidic device consisted of three concentric capillaries with varying diameters. The dispersed phase and immiscible continuous phase comprised of different concentration gradients of aqueous solutions. At the water–water interface, an oscillating solenoid valve controlled the generation of the microgels. The high viability of the encapsulated cells in the 3D culture validated the biocompatibility of the system.
IV. APPLICATIONS
A. Cell encapsulation and 3D microscale tissue culture
3D cell culturing is one of the most extensive applications of hydrogel droplets. As illustrated by many previous studies111,112 and review papers,113–115 a 3D microenvironment can mimic in vivo native tissues more accurately and is more suitable for upregulating cell functionality. There have been numerous examples in the literature dedicated to using hydrogel droplets as platforms for 3D cell culturing. These have included the encapsulation of 3T3 fibroblasts with gelatin methacrylate,116 collagen-gelatin with controllable mechanical properties,117 murine embryonic stem cells with alginate,59 mesenchymal stem cells with PEG,118 and many others as summarized in Table I. The droplet-based method has become a well-accepted 3D cell culturing method for research studies in both engineering119 and biotechnology.120 Utech et al. presented a method to crosslink the alginate-based droplet homogeneously. This provides for a more uniform and highly controllable method for polymerizing droplets without any unintended gelation.121 Experimental results showed the cell viability of mesenchymal stem cells as being around 83% right after encapsulation.
Due to the ease of droplet generation, cell-laden hydrogel droplets have become one of the most popular building blocks for the rapid construction of desired tissues.122 Recently, microfluidics-assisted annealable 20 wt. % GelMA beads (B-GelMA) were fabricated to develop a microporous environment to couple optimum porosity and stiffness, for promoting 3D cell adhesion, spreading, and proliferation [Figs. 3(a)–3(d)].123 First, the microfluidically generated GelMA microgels were physically crosslinked at 4 °C in cold water, after which they were streamed to shape a scaffold structure, and chemically annealed. This innovative approach reaps the advantage of the orthogonal thermo-chemical responsivity of GelMA to decouple the effects of porosity and stiffness in 3D tissue engineering applications. B-GelMA, as opposed to bulk GelMA of the same concentration, exhibited a remarkably high cell viability and fast 3D seeding. In a similar study, jammed microgels were used as ink for extrusion printing with the aim of demonstrating an alternate approach for regular hydrogel-based 3D bioprinting [Figs. 3(e)–3(g)].88 This innovative approach to bioinks could be designed to exhibit a range of properties through the design of individual microgels (e.g., composition and size) and the mixing of such microgels. Microgels composed of different materials (e.g., thermo-sensitive agarose, photo-crosslinked PEG, and thiol–ene crosslinked hyaluronic acid) were used. More importantly, the jamming process and printing have not been observed to affect the viability of encapsulated cells. Secondary crosslinking (wherein an additional crosslinker and photoinitiator were included and exposed to UV light) was applied when further stabilization was needed for interpenetrate crosslinking. Previously, Matsunaga et al.105 developed a quicker way to build millimeter scale tissues via molding cell-laden droplets. The droplets were then placed into a PDMS mold where cells were either mono-cultured or co-cultured on the gel beads for several days. After 17 h, the cells had spread out, filled the space between the droplets, and formed a 3D tissue structure. However, this method is not suitable for long-term culturing since the cells ultimately fill the space between droplets, which results in a lack of room for nutrients and oxygen to propagate inside the tissue. Due to the consequent insufficiency of oxygen and nutrients, cells positioned within the inner parts of the tissue begin to die.124 A possible solution to this problem is to integrate a microvascular network,125,126 with the droplets during the molding process. This can be achieved by building microchannels to aid with nutrition propagation after the formation of the microscale tissues. In addition to tissue generation, the cell-laden hydrogel droplets can be utilized for repairing damaged tissues.127–129 After generating and culturing cell-laden hydrogel droplets, they were injected to fill the space of defect. The droplets ultimately fused with nearby tissues and repaired the defect. Injectable cell-based therapy offers the advantages of being both a patient-friendly procedure129 and displaying a high viability for the formation of large-scale tissues.128 A study by Chung et al. demonstrated that mouse preadipocytes that were encapsulated in porous microspheres and injected exhibit histological features to native adipose tissues within four weeks.128
FIG. 3.
Cell-laden hydrogel droplets as building blocks for the rapid construction of macroscale 3D tissue structures. (a) Schematic of GelMA microbead generation in a flow-focusing microfluidic junction, followed by physical crosslinking at 4 °C. (b) B-GelMA scaffolds pore characterization. (c) Side view fluorescence images showing on top three-dimensionally seeded HUVECs readily penetration through the micropores of B-GelMA (20% w/v). Image dimensions ∼1550 × 1550 × 254 μm3. (d) Representative fluorescent images of live/dead assay of 3D-encapsulated NIH/3T3 cells in 20% w/v B-GelMA scaffolds. Scale bar = 500 μm. Images (a)–(d) are reproduced with permission from Sheikhi et al., Biomaterials 192, 560–568 (2019). Copyright 2019 Elsevier. (e) Jammed microgel ink fabrication: (i) Microdroplets were generated on a flow-focusing microfluidic device then crosslinked through either light [norbornene-modified hyaluronic acid (NorHA) and poly(ethylene glycol) diacrylate] or cooling (agarose). (ii) Suspended microgels (left) that were then jammed through vacuum filtration into an extrudable solid ink (right). Scale bars = 200 μm. (f) Extrusion-based printing of lattice structures with jammed microgel ink. (g) Representative fluorescence viability images of the 3D cell-laden microgels (i) before and (ii) after jamming and extrusion. Scale bars = 200 μm. Images (e)–(g) are reproduced with permission from Highley et al., Adv. Sci. 6, 1801076 (2019). Copyright 2019 Wiley-VCH.
Studies have also reported the ability of hydrogel microdroplets to maintain and accommodate the differentiation of several stem cell types.130 For instance, neuronal differentiation from cancer stem cells in alginate beads131 and neural stem cells in matrigel core–alginate shells132 have been successfully demonstrated. Mesenchymal stem cells (MSCs) are the most widely utilized stem cell type to study differentiation within microgels.133,134 Their differentiation into osteogenic, adipogenic, myogenic, and chondrogenic pathways while being encapsulated within various types of hydrogels including collagen core–alginate shells, hyaluronic acid, modified alginate beads, and alginate beads has been well documented. Moreover, MSCs have also been used to promote osteogenic differentiation by the inclusion and release of BMP-2 osteogenic growth factor (Fig. 4).53 Similarly, embryonic stem cells have also been employed for a wider range of differentiation studies due to their inherently pluripotential properties.135–138 ESCs (Embryonic Stem Cells) have also been shown to differentiate into hepatocytes, pancreatic cells, cardiomyocytes, and endoderm while being encapsulated within various hydrogel materials such as liquid core–alginate shell, alginate bead, and PEG beads.
FIG. 4.
Stem cell-laden microspheres for osteogenic tissue engineering. (a) Schematic diagram of GelMA microspheres fabrication. (b) Bone marrow stem cells osteogenesis in GelMA microgels. (c) The coaxial flow microfluidic device. (d) Droplet generation process. (e) The generated microdroplets in oil. (f) Crosslinked microparticles. Reproduced from Zhao et al., Adv. Funct. Mater. 26, 2809–2819 (2016). Copyright 2016 Wiley-VCH.
B. Platforms for studying cell–cell and cell–microenvironment interactions
One of the most important advantages of microfluidic droplet generation is the high degree of microenvironmental control.122 For this reason, hydrogel droplets are also widely used in cell co-culturing systems.139As shown in Figs. 5(a)–5(e), 3D bioprinting of versatile spiral microarchitectures within microgels with an airflow-assisted microfluidic nozzle has been reported.140 With this method, it could be possible to produce heterogeneous microgels that carry different cell types in asymmetrically separated compartments of controlled micro-tissues. Due to the laminar flow in microfluidics, different adjacent solutions can flow while having distinct boundaries.141 Taking advantage of this property, alginate pre-gel mixtures either with or without cells, were extruded while an accurately set small airflow rotated the droplets so that the originally parallel jets could have different tunable arrangements inside the microdroplet as it grew. These microdroplets were then immediately gelled in a CaCl2 solution. By controlling the fluidic parameters, the microarchitecture inside and outside the microgels could be arranged on demand. Furthermore, in the study, mesenchymal stem cells and human umbilical vein endothelial cells were co-cultured in a complicated microenvironment within the microgels, subsequent to which, spatially controlled osteogenesis and angiogenesis were realized. In their study, Matsunaga et al. cultured different types of cells on collagen-based droplets and screened their attachments and interactions.105 The various cell types cultured included NIH-3T3 fibroblasts, primary neurons, human umbilical vein endothelial cells (HUVECs), primary rat hepatocytes, and MIN6 pancreatic cells. Each cell type was encapsulated with the collagen flow before the formation of the droplet followed by the seeding of the other type of cells on the surface of the droplet. Toward the same objective, Tumarkin et al. presented a comprehensive study of cell–cell and cell–biochemical interactions using a T-junction.21 In this study, factor-dependent (MBA2) cells and reactive blood progenitor cell lines (M07e cells) were encapsulated together at different ratios. The viability of the M07e cells was attributed to being regulated by the presence of paracrine signals in proportion to the MBA2 cell population. The M07e cell line is interleukin-3 (IL-3)-dependent, and IL-3 is generated by MBA-2 cells. Experimental results showed that as the M07e:MBA2 ratio decreased, the cell viability of M07e increased significantly.
FIG. 5.
Platforms for studying micro-environmental control. (a) Diagram of the setup for the 3D fabrication of versatile spiral microarchitectures within microgels with an airflow-assisted microfluidic nozzle. (b) Schematic illustrating the proportional laminar extrusion of alginate solutions out from a microfluidic nozzle and the applied tunable airflow for microdroplets designed rotation. (c) Spherical microgel. (d) Rose-like microgel. (e) Fluorescence image of co-cultured HUVECs and HMSCs (Human Mesenchymal Stem Cells) in a spatially controlled spiral-based microspheroid microenvironment. Images (a)–(e) are reproduced with permission from Zhao et al., Small 14, 1802630 (2018). Copyright 2018 Wiley-VCH. (f) Schematic of the microfluidic-based one-step fabrication of methyl cellulose core–GelMA shell microgels. (g) A photograph of the fabricated chip. (h) Bright-field and fluorescence microscopic images of the cocultured encapsulated HUVECs and HepG2 cells at day 1. Scale bar = 100 μm. (i) Evaluative comparison of the effect of co-culturing and mono-culturing conditions on the HepG2 cells’ urea synthesis at days 1, 3, 5, and 7. Images (f)–(i) are reproduced with permission from Wang et al., Adv. Mater. Technol. 4, 1800632 (2019). Copyright 2019 Wiley-VCH.
It is also possible to fabricate droplets containing various chemicals through a simple method presented by Shepherd et al. who in their sheath-flow device focused laminar flow of different chemical compositions within droplets, with the occurrence of negligible mixing between the co-flowing chemicals.142 This is achieved primarily due to the use of low flow rates and small channel dimensions. This laminar stream was then interrupted by oil dispersed flow, to form Janus-like droplets (hemispherically distinct) with multiple-chemicals within. These Janus droplets can be used to study cells response to certain chemicals since such droplets simulate the in vivo chemical and mechanical anisotropies more accurately.122,142,143 More recently, Wang et al. demonstrated the use of a flow-focusing based microfluidic device for the one-step generation of methyl cellulose core–GelMA shell microgels [Figs. 5(f) and 5(g)].89 The encapsulated liver cells exhibited high viability for 15 days, thus demonstrating the good biocompatibility of the system. The microgels were then used to evaluate the co-culturing of HepG2 liver cells and HUVECs vascular endothelial cells within the cores of the microgels. As shown in Figs. 5(h) and 5(i), the level of urea production from hepatic cells in the co-culture system was found to be significantly higher than from the same number of HepG2 cells in monoculture, which expounds the role of cell–cell interactions.89
C. Other applications
In addition to the purely tissue engineering-based uses that have been discussed in Secs. IV A and IV B, microfluidics-based cell-laden droplets have also been employed as high-throughput tools in other interdisciplinary studies and applications. These have included cell sorting,144 combinatorial studies of tumor microenvironment using microarrays and droplets,145 and immunoassays of single cells.146,147 In one example, a high-throughput study and the sorting of single cells was carried out for a million cells in 2–6 h.144 This was achieved by co-compartmentalizing a mouse hybridoma cell, an anti-mouse IgG antibody-coated bead, and a fluorescent probe, all within a single microdroplet. The fluorescence probe made it possible to sort microdroplet encapsulated cells secreting antibodies from those that did not. Similarly, Aeby et al. studied the long-term 3D culturing of various cell-laden microdroplets on a versatile time-lapse high-resolution confocal microscopy-compatible microfluidic platform.148 This microfluidic chip enabled the stable and precise immobilization of the microtissue in continuously perfused hanging microgels. Such a microfluidic platform could aid in the visualization of sub-cellular level processes that take place within organotypic 3D microtissues all the way down to the single-cell level. In another model, Li et al. developed a microfluidic-based platform for the high-throughput fabrication of tunable microtissues for investigating the response of tumor cells to different microenvironmental signals such as soluble factors, ECM, and stromal cells in 3D.145 It was also possible to sort out specific microtissue populations according to their proliferation and homotypic or heterotypic cell density. Furthermore, the enhancement of lung adenocarcinoma cells upon being encapsulated within fibronectin and the reduction of their proliferation when placed in collagen-1 containing microtissues were successfully demonstrated. It is noteworthy that these 3D-encapsulated cells had reduced proliferation when exposed to TGF-β, the opposite of what is observed in a 2D culture. This was found to be mediated via TGF-βR2 inhibition, only in the context of a 3D environment, further elaborating the importance of using 3D microenvironmental niches for conducting cell behavior and drug testing related studies.
Single activated T-cells have also been encapsulated microfluidically in agarose microdroplets with functionalized cytokine beads for detecting cytokines secretion.146 Similarly, encapsulation of antibody secreting cells in alginate microgels enabled the screening of anti-TNF-alpha-secreting cells from cells secreting other antibodies.147 These studies have allowed for high-throughput heterogeneous immunoassays that can detect antigen-specific antibody secreting cells, map cell sub-populations in order to accelerate monoclonal antibody detection, and isolate them for therapeutic applications.
Cell-laden microgels have been particularly useful in cell-screening and have been used to create an antibiogram through the exposure of monoclonal bacterial colonies to different gradients of antibiotics. Using this platform, it was also possible to select and melt particular microgels for further off-chip genetic analysis.149 Recently, digital microfluidic platforms have been modified to enable the production of single-cell-laden microgels. While these processes are governed by Poisson's statistics, meaning that most microgels would be crosslinked empty, while approximately 37% would be fabricated containing single cells, sorting protocols like FACS (fluorescence-activated cell sorting) have made it possible to yield high-throughput batches of single-cell microgels by eliminating empty microgels.61 Ever since the advent of research into single-cell heterogeneity, that has especially been relevant to studies probing cancer tumors and therapeutics, a wide range of single-cell “omics” studies have sought to demystify and genetically analyze cells at the single-cell level. These platforms have primarily been powered by droplet microfluidics and have included protocols like “in drop”150 “drop seq”151 and “cyto seq.”152 However, in these methods, cells are suspended in liquid solutions, which has hampered long-term studies. Microgel platforms have offered a more versatile platform for genetic analysis by enabling their existence in a more realistic, 3D microenvironment. This versatility of applications further solidifies the potential of microfluidics-based cell-laden hydrogel droplets as useful tools for fundamental research of biology, pharmacology, clinical diagnosis, and therapeutics.
V. CONCLUDING REMARKS AND FUTURE PROSPECTS
Microfluidics-based cell-laden microgels have become a highly tunable and cost-effective technique for tissue engineering. Droplets have served as an excellent platform for 3D cell culturing, the rapid generation of macrotissues, and for the investigation of the cell–cell and cell–microenvironment interactions. Also, due to the simple and accurate manipulation of the cellular microenvironment, hydrogel droplets have been combined with other microengineered platforms, such as microfluidic devices and microarrays, to enable a more comprehensive study of cell behavior.
However, current technologies to produce cell-laden microgels production still have many restrictions that prevent their widespread use. One significant challenge is realizing the precise control of cell number and cell escapement.90 For instance, it has not been possible to uniformly distribute cell in microgels, which is an important problem for many applications.116,153,154Also, microfluidic cellular encapsulation results in a highly disturbed polydisperse microdroplet generation process.116,153–155 Meanwhile, double emulsions like water-in-oil-in-water structures have provided insights into using a physical and/or chemical method to prevent cell escapement.134
For applications in biofabrication and cancer tumor study, it is important to consider the heterogeneity of the tissue. Microgels used for such applications require a co-culture of multiple cell types. As mentioned earlier, different cells require different microenvironments for their growth, and it is important, therefore, to consider these parameters when co-culturing cells. Recently, Husman et al. demonstrated a microgel in gel model, which included a particular cell type in one shell and another in the surrounding gel.156 The shells accommodated for the varied mechanical properties of the microenvironments and spatial heterogeneity. Other challenges that exist with such models as compared to microgels with single-cell types is the administration of a cell medium, which is rendered more difficult since the choice of medium varies with cell type.
Another challenge is the relatively low cellular viability of the encapsulated cells comparing to conventional tissue engineering approaches.157–160 The droplet generation and gelation steps are relatively lengthy processes that affect the viability of encapsulated cells. This is also partially due to the shear stress that is applied to separate microgel droplets by centrifugation.161,162 Strategies to eliminate the centrifugation step also require further study.
The microfluidic generation of cell-laden microgels has a comparatively low throughput and is not yet feasible, especially when considering applications in tissue engineering. The flow rates of the dispersed phase containing suspended cells are in the range of 0.1 ml/h137,163 A higher throughput coupled with higher efficiency is essential for future research and commercialization. Therefore, any off-chip handling of the produced microgels, such as crosslinking or centrifugation for extracting droplets from the continuous phase, should be eliminated to allow efficient automation. This includes integrating all the production steps effectively and working synchronously in the same chip. In addition, scaling up and mass production via parallelization of a network of multiple devices can mitigate the current challenges of low throughput and facilitate the scalable fabrication of microgels using microfluidics. However, this parallelization of cell-laden microgels may result in the complexity of the structure composed of multiple channels and layers, which can cause a significant challenge for fabrication and operation.164 Hence, future work must also be geared toward developing new designs and fabrication materials that are more robust. Poly (methyl methacrylate) (PMMA) is a potential candidate for improving the mechanical stability and reusability of the devices for post prototyping devices using PDMS.
Innovative hydrogel-based materials and preparation methods are also necessary to facilitate the coupling of the innate biomimetic nature of the natural hydrogels with the low-cost mass production of synthetic hydrogels. In summary, the cell-laden hydrogel droplet is a well-accepted building block and a high-throughput tool for the accurate control of cellular microenvironments. It can also be said that these tiny droplets could aid researchers in tapping groundbreaking discoveries and progress in efforts to advance tissue engineering and regenerative medicine.
ACKNOWLEDGMENTS
This work was supported by Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grants (No. RGPIN-2014-04010) and the Canadian Foundation for Innovation John R. Evans Leaders Opportunity Fund.
REFERENCES
- 1.Tissue Engineering, edited by Pallua N. and Suscheck C. V. (Springer, Berlin, 2011). 10.1007/978-3-642-02824-3 [DOI] [Google Scholar]
- 2.Hong J., Shin Y., Kim S., Lee J., and Cha C., “Complex tuning of physical properties of hyperbranched polyglycerol-based bioink for microfabrication of cell-laden hydrogels,” Adv. Funct. Mater. 29(13), 1808750 (2019). 10.1002/adfm.201808750 [DOI] [Google Scholar]
- 3.Chang T. M., “Semipermeable microcapsules,” Science 146(3643), 524–525 (1964). 10.1126/science.146.3643.524 [DOI] [PubMed] [Google Scholar]
- 4.Rabanel J. M., Banquy X., Zouaoui H., Mokhtar M., and Hildgen P., “Progress technology in microencapsulation methods for cell therapy,” Biotechnol. Prog. 25(4), 946–963 (2009). 10.1002/btpr.226 [DOI] [PubMed] [Google Scholar]
- 5.Nichol J. W. and Khademhosseini A., “Modular tissue engineering: Engineering biological tissues from the bottom up,” Soft Matter 5(7), 1312 (2009). 10.1039/b814285h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikada Y., “Challenges in tissue engineering,” J. R. Soc. Interface 3(10), 589–601 (2006). 10.1098/rsif.2006.0124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khademhosseini A., Langer R., Borenstein J., and Vacanti J. P., “Microscale technologies for tissue engineering and biology,” Proc. Natl. Acad. Sci. U.S.A. 103(8), 2480–2487 (2006). 10.1073/pnas.0507681102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGuigan A. P. and Sefton M. V., “Design and fabrication of sub-mm-sized modules containing encapsulated cells for modular tissue engineering,” Tissue Eng. 13(5), 1069–1078 (2007). 10.1089/ten.2006.0253 [DOI] [PubMed] [Google Scholar]
- 9.Pathak A. and Kumar S., “Biophysical regulation of tumor cell invasion: Moving beyond matrix stiffness,” Integr. Biol. 3(4), 267–278 (2011). 10.1039/c0ib00095g [DOI] [PubMed] [Google Scholar]
- 10.Atala A., Lanza R., Thomson J. A., and Nerem R., Principles of Regenerative Medicine (Academic Press, 2010). [Google Scholar]
- 11.Tumarkin E. and Kumacheva E., “Microfluidic generation of microgels from synthetic and natural polymers,” Chem. Soc. Rev. 38(8), 2161–2168 (2009). 10.1039/b809915b [DOI] [PubMed] [Google Scholar]
- 12.Kumacheva E. and Garstecki P., Microfluidic Reactors for Polymer Particles (John Wiley & Sons, 2011). [Google Scholar]
- 13.Mohamed M. G. A., Kheiri S., Islam S., Kumar H., Yang A., and Kim K., “An integrated microfluidic flow-focusing platform for on-chip fabrication and filtration of cell-laden microgels,” Lab Chip 19(9), 1621–1632 (2019). 10.1039/C9LC00073A [DOI] [PubMed] [Google Scholar]
- 14.Selimović Š, Oh J., Bae H., Dokmeci M., and Khademhosseini A., “Microscale strategies for generating cell-encapsulating hydrogels,” Polymers 4(3), 1554–1579 (2012). 10.3390/polym4031554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H., Tumarkin E., Peerani R. et al. , “Microfluidic production of biopolymer microcapsules with controlled morphology,” J. Am. Chem. Soc. 128(37), 12205–12210 (2006). 10.1021/ja0635682 [DOI] [PubMed] [Google Scholar]
- 16.Nie Z., Seo M., Xu S. et al. , “Emulsification in a microfluidic flow-focusing device: Effect of the viscosities of the liquids,” Microfluid. Nanofluidics 5, 585–594 (2008). 10.1007/s10404-008-0271-y [DOI] [Google Scholar]
- 17.van Steijn V., Korczyk P. M., Derzsi L., Abate A. R., Weitz D. A., and Garstecki P., “Block-and-break generation of microdroplets with fixed volume,” Biomicrofluidics 7(2), 24108 (2013). 10.1063/1.4801637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahimi M., Shams Khorrami A., and Rezai P., “Effect of device geometry on droplet size in co-axial flow-focusing microfluidic droplet generation devices,” Colloids Surf. A Physicochem. Eng. Asp 570, 510–517 (2019). 10.1016/j.colsurfa.2019.03.067 [DOI] [Google Scholar]
- 19.Engel E., Michiardi A., Navarro M., Lacroix D., and Planell J. A., “Nanotechnology in regenerative medicine: The materials side,” Trends Biotechnol. 26(1), 39–47 (2008). 10.1016/j.tibtech.2007.10.005 [DOI] [PubMed] [Google Scholar]
- 20.Kang A., Park J., Ju J., Jeong G. S., and Lee S. H., “Cell encapsulation via microtechnologies,” Biomaterials 35(9), 2651–2663 (2014). 10.1016/j.biomaterials.2013.12.073 [DOI] [PubMed] [Google Scholar]
- 21.Tumarkin E., Tzadu L., Csaszar E. et al. , “High-throughput combinatorial cell co-culture using microfluidics,” Integr. Biol. 3, 653–662 (2011). 10.1039/c1ib00002k [DOI] [PubMed] [Google Scholar]
- 22.Breguet V., Gugerli R., Pernetti M., Von Stockar U., and Marison I. W., “Formation of microcapsules from polyelectrolyte and covalent interactions,” Langmuir 21(21), 9764–9772 (2005). 10.1021/la0512796 [DOI] [PubMed] [Google Scholar]
- 23.Dove A., “Cell-based therapies go live,” Nat. Biotechnol. 20(4), 339–343 (2002). 10.1038/nbt0402-339 [DOI] [PubMed] [Google Scholar]
- 24.Wan J., “Microfluidic-based synthesis of hydrogel particles for cell microencapsulation and cell-based drug delivery,” Polymers 4(2), 1084–1108 (2012). 10.3390/polym4021084 [DOI] [Google Scholar]
- 25.Brannon-Peppas L. and Harland R. S., Absorbent Polymer Technology (Elsevier, 2012). [Google Scholar]
- 26.Orive G., Hernández R. M., Rodríguez Gascón A. et al. , “History, challenges and perspectives of cell microencapsulation,” Trends Biotechnol. 22(2), 87–92 (2004). 10.1016/j.tibtech.2003.11.004 [DOI] [PubMed] [Google Scholar]
- 27.Balakrishnan B. and Banerjee R., “Biopolymer-based hydrogels for cartilage tissue engineering,” Chem. Rev. 111(8), 4453–4474 (2011). 10.1021/cr100123h [DOI] [PubMed] [Google Scholar]
- 28.Zimmermann H., Ehrhart F., Zimmermann D. et al. , “Hydrogel-based encapsulation of biological, functional tissue: Fundamentals, technologies and applications,” Appl. Phys. A 89(4), 909–922 (2007). 10.1007/s00339-007-4270-8 [DOI] [Google Scholar]
- 29.Drury J. L. and Mooney D. J., “Hydrogels for tissue engineering: Scaffold design variables and applications,” Biomaterials 24(24), 4337–4351 (2003). 10.1016/S0142-9612(03)00340-5 [DOI] [PubMed] [Google Scholar]
- 30.Slaughter B. V., Khurshid S. S., Fisher O. Z., Khademhosseini A., and Peppas N. A., “Hydrogels in regenerative medicine,” Adv. Mater. 21(32–33), 3307–3329 (2009). 10.1002/adma.200802106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watanabe T., Motohiro I., and Ono T., “Microfluidic formation of hydrogel microcapsules with a single aqueous core by spontaneous cross-linking in aqueous two-phase system droplets,” Langmuir 35(6), 2358–2367 (2019). 10.1021/acs.langmuir.8b04169 [DOI] [PubMed] [Google Scholar]
- 32.Rossow T., Heyman J. A., Ehrlicher A. J. et al. , “Controlled synthesis of cell-laden microgels by radical-free gelation in droplet microfluidics,” J. Am. Chem. Soc. 134(10), 4983–4989 (2012). 10.1021/ja300460p [DOI] [PubMed] [Google Scholar]
- 33.Tan J., Gemeinhart R. A., Ma M., and Saltzman W. M., “Improved cell adhesion and proliferation on synthetic phosphonic acid-containing hydrogels,” Biomaterials 26(17), 3663–3671 (2005). 10.1016/j.biomaterials.2004.09.053 [DOI] [PubMed] [Google Scholar]
- 34.Liu X., Toprakcioglu Z., Dear A. J. et al. , “Fabrication and characterization of reconstituted silk microgels for the storage and release of small molecules,” Macromol. Rapid Commun. 40, 1800898 (2019). 10.1002/marc.201800898 [DOI] [PubMed] [Google Scholar]
- 35.Daniele M. A., Adams A. A., Naciri J., North S. H., and Ligler F. S., “Interpenetrating networks based on gelatin methacrylamide and PEG formed using concurrent thiol click chemistries for hydrogel tissue engineering scaffolds,” Biomaterials 35(6), 1845–1856 (2014). 10.1016/j.biomaterials.2013.11.009 [DOI] [PubMed] [Google Scholar]
- 36.Kim S. H., Yeon Y. K., Lee J. M. et al. , “Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing,” Nat. Commun. 9(1), 1620 (2018). 10.1038/s41467-018-03759-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie M., Gao Q., Zhao H. et al. , “Electro-assisted bioprinting of low-concentration GelMA microdroplets,” Small 15(4), 1804216 (2019). 10.1002/smll.201804216 [DOI] [PubMed] [Google Scholar]
- 38.Tan H. and Marra K. G., “ Injectable, biodegradable hydrogels for tissue engineering applications,” Materials 3(3), 1746–1767 (2010). 10.3390/ma3031746 [DOI] [Google Scholar]
- 39.Huang R. Y. M., Pal R., and Moon G. Y., “Characteristics of sodium alginate membranes for the pervaporation dehydration of ethanol–water and isopropanol–water mixtures,” J. Memb. Sci. 160(1), 101–113 (1999). 10.1016/S0376-7388(99)00071-X [DOI] [Google Scholar]
- 40.Bajpai S. K. and Sharma S., “Investigation of swelling/degradation behaviour of alginate beads crosslinked with Ca2+ and Ba2+ ions,” React. Funct. Polym. 59(2), 129–140 (2004). 10.1016/j.reactfunctpolym.2004.01.002 [DOI] [Google Scholar]
- 41.de Rutte J. M., Koh J., and Di Carlo D., “Scalable high-throughput production of modular microgels for in situ assembly of microporous tissue scaffolds,” Adv. Funct. Mater. 29, 1900071 (2019). 10.1002/adfm.201900071 [DOI] [Google Scholar]
- 42.Ahearne M., “Introduction to cell-hydrogel mechanosensing,” Interface Focus 4(2), 20130038 (2014). 10.1098/rsfs.2013.0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Engler A. J., Sen S., Sweeney H. L., and Discher D. E., “Matrix elasticity directs stem cell lineage specification,” Cell 126(4), 677–689 (2006). 10.1016/j.cell.2006.06.044 [DOI] [PubMed] [Google Scholar]
- 44.Bott K., Upton Z., Schrobback K. et al. , “The effect of matrix characteristics on fibroblast proliferation in 3D gels,” Biomaterials 31(32), 8454–8464 (2010). 10.1016/j.biomaterials.2010.07.046 [DOI] [PubMed] [Google Scholar]
- 45.Peyton S. R. and Putnam A. J., “Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion,” J. Cell Physiol. 204(1), 198–209 (2005). 10.1002/jcp.20274 [DOI] [PubMed] [Google Scholar]
- 46.Vining K. H. and Mooney D. J., “Mechanical forces direct stem cell behaviour in development and regeneration,” Nat. Rev. Mol. Cell Biol. 18(12), 728–742 (2017). 10.1038/nrm.2017.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li X., Chen Y., Kawazoe N., and Chen G., “Influence of microporous gelatin hydrogels on chondrocyte functions,” J. Mater. Chem. B 5(29), 5753–5762 (2017). 10.1039/C7TB01350G [DOI] [PubMed] [Google Scholar]
- 48.Ferreira L., Gil M. H., and Dordick J. S., “Enzymatic synthesis of dextran-containing hydrogels,” Biomaterials 23(19), 3957–3967 (2002). 10.1016/S0142-9612(02)00132-1 [DOI] [PubMed] [Google Scholar]
- 49.Patterson J. and Hubbell J. A., “Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2,” Biomaterials 31(30), 7836–7845 (2010). 10.1016/j.biomaterials.2010.06.061 [DOI] [PubMed] [Google Scholar]
- 50.Li S., Molina I., Bueno Martinez M., and Vert M., “Hydrolytic and enzymatic degradations of physically crosslinked hydrogels prepared from PLA/PEO/PLA triblock copolymers,” J. Mater. Sci. Mater. Med. 13(1), 81–86 (2002). 10.1023/A:1013651022431 [DOI] [PubMed] [Google Scholar]
- 51.Lévesque S. G. and Shoichet M. S., “Synthesis of enzyme-degradable, peptide-cross-linked dextran hydrogels,” Bioconjugate Chem. 18(3), 874–885 (2007). 10.1021/bc0602127 [DOI] [PubMed] [Google Scholar]
- 52.Lee K. Y., Bouhadir K. H., and Mooney D. J., “Controlled degradation of hydrogels using multi-functional cross-linking molecules,” Biomaterials 25(13), 2461–2466 (2004). 10.1016/j.biomaterials.2003.09.030 [DOI] [PubMed] [Google Scholar]
- 53.Zhao X., Liu S., Yildirimer L. et al. , “Injectable stem cell-laden photocrosslinkable microspheres fabricated using microfluidics for rapid generation of osteogenic tissue constructs,” Adv. Funct. Mater. 26(17), 2809–2819 (2016). 10.1002/adfm.201504943 [DOI] [Google Scholar]
- 54.Hernández R. M., Orive G., Murua A., and Pedraz J. L., “Microcapsules and microcarriers for in situ cell delivery,” Adv. Drug. Deliv. Rev. 62(7–8), 711–730 (2010). 10.1016/j.addr.2010.02.004 [DOI] [PubMed] [Google Scholar]
- 55.Sugiura S., Oda T., Aoyagi Y. et al. , “Microfabricated airflow nozzle for microencapsulation of living cells into 150 micrometer microcapsules,” Biomed. Microdevices 9(1), 91–99 (2007). 10.1007/s10544-006-9011-9 [DOI] [PubMed] [Google Scholar]
- 56.Jiang W., Li M., Chen Z., and Leong K. W., “Cell-laden microfluidic microgels for tissue regeneration,” Lab Chip 16(23), 4482–4506 (2016). 10.1039/C6LC01193D [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rouwkema J., Koopman B. F. J. M., Van Blitterswijk C. A., Dhert W. J., and Malda J., “Supply of nutrients to cells in engineered tissues,” Biotechnol. Genet. Eng. Rev. 26(1), 163–178 (2009). 10.5661/bger-26-163 [DOI] [PubMed] [Google Scholar]
- 58.Sutermaster B. A. and Darling E. M., “Considerations for high-yield, high-throughput cell enrichment: Fluorescence versus magnetic sorting,” Sci. Rep. 9(1), 229 (2019). 10.1038/s41598-018-36698-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumachev A., Greener J., Tumarkin E., Eiser E., Zandstra P. W., and Kumacheva E., “High-throughput generation of hydrogel microbeads with varying elasticity for cell encapsulation,” Biomaterials 32(6), 1477–1483 (2011). 10.1016/j.biomaterials.2010.10.033 [DOI] [PubMed] [Google Scholar]
- 60.Anna S. L., Bontoux N., and Stone H. A., “Formation of dispersions using ‘flow focusing’ in microchannels,” Appl. Phys. Lett. 82, 364–366 (2003). 10.1063/1.1537519 [DOI] [Google Scholar]
- 61.Collins D. J., Neild A., deMello A., Liu A.-Q., and Ai Y., “The Poisson distribution and beyond: Methods for microfluidic droplet production and single cell encapsulation,” Lab Chip 15(17), 3439–3459 (2015). 10.1039/C5LC00614G [DOI] [PubMed] [Google Scholar]
- 62.Xu J. and Attinger D., “Drop on demand in a microfluidic chip,” J. Micromech. Microeng. 18, 065020 (2009). 10.1088/0960-1317/18/6/065020 [DOI] [Google Scholar]
- 63.Kim H., Luo D., Link D., Weitz D A., Marquez M., and Cheng Z., “Controlled production of emulsion drops using an electric field in a flow-focusing microfluidic device,” Appl. Phys. Lett. 91(13), 2005–2008 (2007). 10.1063/1.2790785 [DOI] [Google Scholar]
- 64.Zhu P. and Wang L., “Passive and active droplet generation with microfluidics: A review,” Lab Chip 17(1), 34–75 (2017). 10.1039/C6LC01018K [DOI] [PubMed] [Google Scholar]
- 65.Chu L. Y., Utada A. S., Shah R. K., Kim J. W., and Weitz D. A., “Controllable monodisperse multiple emulsions,” Angew. Chem. Int. Ed. 46(47), 8970–8974 (2007). 10.1002/anie.200701358 [DOI] [PubMed] [Google Scholar]
- 66.Okushima S., Nisisako T., Torii T., and Higuchi T., “Controlled production of monodisperse double emulsions by two-step droplet breakup in microfluidic devices,” Langmuir 20(23), 9905–9908 (2004). 10.1021/la0480336 [DOI] [PubMed] [Google Scholar]
- 67.Seemann R., Brinkmann M., Pfohl T., and Herminghaus S., “Droplet based microfluidics,” Rep. Prog. Phys. 75(1), 016601 (2011). 10.1088/0034-4885/75/1/016601 [DOI] [PubMed] [Google Scholar]
- 68.McDonald J. C. and Whitesides G. M., “Poly (dimethylsiloxane) as a material for fabricating microfluidic devices,” Acc. Chem. Res. 35(7), 491–499 (2002). 10.1021/ar010110q [DOI] [PubMed] [Google Scholar]
- 69.Qin D., Xia Y., and Whitesides G. M., “Soft lithography for micro- and nanoscale patterning,” Nat. Protoc. 5(3), 491–502 (2010). 10.1038/nprot.2009.234 [DOI] [PubMed] [Google Scholar]
- 70.Xia Y., Rogers J. A., Paul K. E., and Whitesides G. M., “Unconventional methods for fabricating and patterning nanostructures,” Chem. Rev. 99(7), 1823–1848 (1999). 10.1021/cr980002q [DOI] [PubMed] [Google Scholar]
- 71.Baah D. and Floyd-Smith T., “Microfluidics for particle synthesis from photocrosslinkable materials,” Microfluid. Nanofluid. 17, 431–455 (2014). 10.1007/s10404-014-1333-y [DOI] [Google Scholar]
- 72.Utada A. S., Chu L.-Y., Fernandez-Nieves A., Link D. R., Holtze C., and Weitz D. A., “Dripping, jetting, drops, and wetting: The magic of microfluidics,” MRS Bull. 32(09), 702–708 (2007). 10.1557/mrs2007.145 [DOI] [Google Scholar]
- 73.Kosloh J., Sackmann J., and Schomburg W. K., “Ultrasonic fabrication of micro fluidic channels from polyether ether ketone (PEEK),” Microsyst. Technol. 23(12), 5505–5513 (2017). 10.1007/s00542-017-3284-1 [DOI] [Google Scholar]
- 74.Bressan L. P., Adamo C. B., Quero R. F., de Jesus D. P., and da Silva J. A. F., “A simple procedure to produce FDM-based 3D-printed microfluidic devices with an integrated PMMA optical window,” Anal. Methods 11(8), 1014–1020 (2019). 10.1039/C8AY02092B [DOI] [Google Scholar]
- 75.Riley A., Green V., Cheah R. et al. , “A novel microfluidic device capable of maintaining functional thyroid carcinoma specimens ex vivo provides a new drug screening platform,” BMC Cancer 19(1), 259 (2019). 10.1186/s12885-019-5465-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clausell-Tormos J., Lieber D., Baret J. C. et al. , “Droplet-based microfluidic platforms for the encapsulation and screening of mammalian cells and multicellular organisms,” Chem. Biol. 15(5), 427–437 (2008). 10.1016/j.chembiol.2008.04.004 [DOI] [PubMed] [Google Scholar]
- 77.Tan W. H. and Takeuchi S., “Monodisperse alginate hydrogel microbeads for cell encapsulation,” Adv. Mater. 19(18), 2696–2701 (2007). 10.1002/adma.200700433 [DOI] [Google Scholar]
- 78.Xu J. H., Dong P. F., Zhao H., Tostado C. P., and Luo G. S., “The dynamic effects of surfactants on droplet formation in coaxial microfluidic devices,” Langmuir 28, 9250 (2012). 10.1021/la301363d [DOI] [PubMed] [Google Scholar]
- 79.Caprini D., Sinibaldi G., Marino L., and Casciola C. M., “A T-junction device allowing for two simultaneous orthogonal views: Application to bubble formation and break-up,” Microfluid. Nanofluid. 22(8), 85 (2018). 10.1007/s10404-018-2101-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li X., He L., Gu H., Sun F., and Liu M., “Numerical study of droplet formation in the T-junction microchannel with wall velocity slip,” Energy Procedia 158, 5459–5464 (2019). 10.1016/j.egypro.2019.01.601 [DOI] [Google Scholar]
- 81.Ma P., Fu T., Zhu C., and Ma Y., “Asymmetrical breakup and size distribution of droplets in a branching microfluidic T-junction,” Korean J. Chem. Eng. 36(1), 21–29 (2019). 10.1007/s11814-018-0165-y [DOI] [Google Scholar]
- 82.Liang D., Ma R., Fu T. et al. , “Dynamics and formation of alternating droplets under magnetic field at a T-junction,” Chem. Eng. Sci. 200, 248–256 (2019). 10.1016/j.ces.2019.01.053 [DOI] [Google Scholar]
- 83.Chakraborty I., Ricouvier J., Yazhgur P., Tabeling P., and Leshansky A. M., “Droplet generation at Hele-Shaw microfluidic T-junction,” Phys. Fluids 31(2), 022010 (2019). 10.1063/1.5086808 [DOI] [Google Scholar]
- 84.Sun L., Fan M., Li P. et al. , “Microbubble characteristic in a T-junction microchannel in microfluidic chip,” Mol. Phys. 117, 2535–2545 (2019). 10.1080/00268976.2019.1572926 [DOI] [Google Scholar]
- 85.Choi C. H., Jung J. H., Rhee Y. W., Kim D. P., Shim S. E., and Lee C. S., “Generation of monodisperse alginate microbeads and in situ encapsulation of cell in microfluidic device,” Biomed. Microdevices 9(6), 855–862 (2007). 10.1007/s10544-007-9098-7 [DOI] [PubMed] [Google Scholar]
- 86.Um E., Lee D.-S., Pyo H.-B., and Park J.-K., “Continuous generation of hydrogel beads and encapsulation of biological materials using a microfluidic droplet-merging channel,” Microfluid. Nanofluid. 5(4), 541–549 (2008). 10.1007/s10404-008-0268-6 [DOI] [Google Scholar]
- 87.Sabhachandani P., Sarkar S., Mckenney S., Ravi D., Evens A. M., and Konry T., “Microfluidic assembly of hydrogel-based immunogenic tumor spheroids for evaluation of anticancer therapies and biomarker release,” J. Control Release 295, 21–30 (2019). 10.1016/j.jconrel.2018.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Highley C. B., Song K. H., Daly A. C., and Burdick J. A., “Jammed microgel inks for 3D printing applications,” Adv. Sci. 6(1), 1801076 (2019). 10.1002/advs.201801076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang H., Liu H., Liu H., Su W., Chen W., and Qin J., “One-step generation of core-shell gelatin methacrylate (GelMA) microgels using a droplet microfluidic system,” Adv. Mater. Technol. 4, 1800632 (2019). 10.1002/admt.201800632 [DOI] [Google Scholar]
- 90.Benavente-Babace A., Haase K., Stewart D. J., and Godin M., “Strategies for controlling egress of therapeutic cells from hydrogel microcapsules,” J. Tissue Eng. Regen. Med. 13, 612 (2019). 10.1002/term.2818 [DOI] [PubMed] [Google Scholar]
- 91.Gañán-Calvo A., “Generation of steady liquid microthreads and micron-sized monodisperse sprays in gas streams,” Phys. Rev. Lett. 80(2), 285–288 (1998). 10.1103/PhysRevLett.80.285 [DOI] [Google Scholar]
- 92.Wang Z., Samanipour R., Gamaleldin M., Sakthivel K., and Kim K., “An automated system for high-throughput generation and optimization of microdroplets,” Biomicrofluidics 10, 54110 (2016). 10.1063/1.4963666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mohamed M., Kumar H., Wang Z. et al. , “Rapid and inexpensive fabrication of multi-depth microfluidic device using high-resolution LCD stereolithographic 3D printing,” J. Manuf. Mater. Process. 3(1), 26 (2019). 10.3390/jmmp3010026 [DOI] [Google Scholar]
- 94.Samanipour R., Wang Z., Ahmadi A., and Kim K., “Experimental and computational study of microfluidic flow-focusing generation of gelatin methacrylate hydrogel droplets,” J. Appl. Polym. Sci. 133, 43701 (2016). 10.1002/app.43701 [DOI] [Google Scholar]
- 95.Sun Y., Cai B., Wei X. et al. , “A valve-based microfluidic device for on-chip single cell treatments,” Electrophoresis 40(6), 961–968 (2019). 10.1002/elps.201800213 [DOI] [PubMed] [Google Scholar]
- 96.Cai B., Ji T.-T., Wang N. et al. , “A microfluidic platform utilizing anchored water-in-oil-in-water double emulsions to create a niche for analyzing single non-adherent cells,” Lab Chip 19(3), 422–431 (2019). 10.1039/C8LC01130C [DOI] [PubMed] [Google Scholar]
- 97.Edd J. F., Di Carlo D., Humphry K. J. et al. , “Controlled encapsulation of single-cells into monodisperse picolitre drops,” Lab Chip 8(8), 1262–1264 (2008). 10.1039/b805456h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim C., Lee K. S., Kim Y. E. et al. , “Rapid exchange of oil-phase in microencapsulation chip to enhance cell viability,” Lab Chip 9(9), 1294–1297 (2009). 10.1039/b819044e [DOI] [PubMed] [Google Scholar]
- 99.Kim C., Chung S., Kim Y. E. et al. , “Generation of core-shell microcapsules with three-dimensional focusing device for efficient formation of cell spheroid,” Lab Chip 11(2), 246–252 (2011). 10.1039/C0LC00036A [DOI] [PubMed] [Google Scholar]
- 100.Wu L., Chen P., Dong Y., Feng X., and Liu B. F., “Encapsulation of single cells on a microfluidic device integrating droplet generation with fluorescence-activated droplet sorting,” Biomed. Microdevices 15(3), 553–560 (2013). 10.1007/s10544-013-9754-z [DOI] [PubMed] [Google Scholar]
- 101.Capretto L., Mazzitelli S., Luca G., and Nastruzzi C., “Preparation and characterization of polysaccharidic microbeads by a microfluidic technique: Application to the encapsulation of Sertoli cells,” Acta Biomater. 6(2), 429–435 (2010). 10.1016/j.actbio.2009.08.023 [DOI] [PubMed] [Google Scholar]
- 102.Allazetta S., Hausherr T. C., and Lutolf M. P., “Microfluidic synthesis of cell-type-specific artificial extracellular matrix hydrogels,” Biomacromolecules 14(4), 1122–1131 (2013). 10.1021/bm4000162 [DOI] [PubMed] [Google Scholar]
- 103.Köster S., Angilè F. E., Duan H. et al. , “Drop-based microfluidic devices for encapsulation of single cells,” Lab Chip 8(7), 1110 (2008). 10.1039/b802941e [DOI] [PubMed] [Google Scholar]
- 104.Eun Y. J., Utada A. S., Copeland M. F., Takeuchi S., and Weibel D. B., “Encapsulating bacteria in agarose microparticles using microfluidics for high-throughput cell analysis and isolation,” ACS Chem. Biol. 6(3), 260–266 (2011). 10.1021/cb100336p [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Matsunaga Y. T., Morimoto Y., and Takeuchi S., “Molding cell beads for rapid construction of macroscopic 3D tissue architecture,” Adv Mater. 23(12), 90–94 (2011). 10.1002/adma.201004375 [DOI] [PubMed] [Google Scholar]
- 106.Tirandazi P. and Hidrovo C. H., “Liquid-in-gas droplet microfluidics; experimental characterization of droplet morphology, generation frequency, and monodispersity in a flow-focusing microfluidic device,” J. Micromech. Microeng. 27(7), 075020 (2017). 10.1088/1361-6439/aa7595 [DOI] [Google Scholar]
- 107.Choi C.-H., Wang H., Lee H. et al. , “One-step generation of cell-laden microgels using double emulsion drops with a sacrificial ultra-thin oil shell,” Lab Chip 16(9), 1549–1555 (2016). 10.1039/C6LC00261G [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jeon O., Lee Y. B., Hinton T. J., Feinberg A. W., and Alsberg E., “Cryopreserved cell-laden alginate microgel bioink for 3D bioprinting of living tissues,” Mater. Today Chem. 12, 61–70 (2019). 10.1016/j.mtchem.2018.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sugiura S., Oda T., Izumida Y. et al. , “Size control of calcium alginate beads containing living cells using micro-nozzle array,” Biomaterials 26(16), 3327–3331 (2005). 10.1016/j.biomaterials.2004.08.029 [DOI] [PubMed] [Google Scholar]
- 110.Zhu K., Yu Y., Cheng Y., Tian C., Zhao G., and Zhao Y., “All-aqueous-phase microfluidics for cell encapsulation,” ACS Appl. Mater. Interfaces 11(5), 4826–4832 (2019). 10.1021/acsami.8b19234 [DOI] [PubMed] [Google Scholar]
- 111.Baharvand H., Hashemi S. M., Kazemi Ashtiani S., and Farrokhi A., “Differentiation of human embryonic stem cells into hepatocytes in 2D and 3D culture systems in vitro,” Int. J. Dev. Biol. 50(7), 645–652 (2006). 10.1387/ijdb.052072hb [DOI] [PubMed] [Google Scholar]
- 112.Baker B. M. and Chen C. S., “Deconstructing the third dimension—How 3D culture microenvironments alter cellular cues,” J. Cell Sci. 125(13), 3015–3024 (2012). 10.1242/jcs.079509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ronaldson-Bouchard K. and Vunjak-Novakovic G., “Organs-on-a-chip: A fast track for engineered human tissues in drug development,” Cell Stem Cell 22(3), 310–324 (2018). 10.1016/j.stem.2018.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang Z., Samanipour R., and Kim K., “Organ-on-a-chip platforms for drug screening and tissue engineering,” in Biomedical Engineering Frontier Research and Converging Technologies (Springer International Publishing, 2015), pp. 209–233. [Google Scholar]
- 115.Zhang Q., Sito L., Mao M., He J., Zhang Y. S., and Zhao X., “Current advances in skin-on-a-chip models for drug testing,” Microphysiol. Syst. 2, 4 (2018). 10.21037/mps.2018.08.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jung J. and Oh J., “Cell-induced flow-focusing instability in gelatin methacrylate microdroplet generation,” Biomicrofluidics 8(3), 036503 (2014). 10.1063/1.4880375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ma S., Natoli M., Liu X. et al. , “Monodisperse collagen–gelatin beads as potential platforms for 3D cell culturing,” J. Mater. Chem. B 1(38), 5128 (2013). 10.1039/c3tb20851f [DOI] [PubMed] [Google Scholar]
- 118.Headen D. M., Aubry G., Lu H., and García A. J., “Microfluidic-based generation of size-controlled, biofunctionalized synthetic polymer microgels for cell encapsulation,” Adv. Mater. 26(19), 3003–3008 (2014). 10.1002/adma.201304880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bajaj P., Schweller R. M., Khademhosseini A., West J. L., and Bashir R., “3D biofabrication strategies for tissue engineering and regenerative medicine,” Annu. Rev. Biomed. Eng. 16, 247–276 (2014). 10.1146/annurev-bioeng-071813-105155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.van Duinen V., Trietsch S. J., Joore J., Vulto P., and Hankemeier T., “Microfluidic 3D cell culture: From tools to tissue models,” Curr. Opin. Biotechnol. 35, 118–126 (2015). 10.1016/j.copbio.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 121.Utech S., Prodanovic R., Mao A. S., Ostafe R., Mooney D. J., and Weitz D. A., “Microfluidic generation of monodisperse, structurally homogeneous alginate microgels for cell encapsulation and 3D cell culture,” Adv. Healthcare Mater. 4(11), 1628–1633 (2015). 10.1002/adhm.201500021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Annabi N., Tamayol A., Uquillas J. A. et al. , “25th anniversary article: Rational design and applications of hydrogels in regenerative medicine,” Adv. Mater. 26(1), 85–124 (2014). 10.1002/adma.201303233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sheikhi A., de Rutte J., Haghniaz R. et al. , “Microfluidic-enabled bottom-up hydrogels from annealable naturally-derived protein microbeads,” Biomaterials 192, 560–568 (2019). 10.1016/j.biomaterials.2018.10.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kato-Negishi M., Morimoto Y., Onoe H., and Takeuchi S., “Millimeter-sized neural building blocks for 3D heterogeneous neural network assembly,” Adv. Healthc. Mater. 2(12), 1564–1570 (2013). 10.1002/adhm.201300052 [DOI] [PubMed] [Google Scholar]
- 125.Hoch E., Tovar G. E. M., and Borchers K., “Bioprinting of artificial blood vessels: Current approaches towards a demanding goal,” Eur. J. Cardiothorac. Surg. 46(5), 767–778 (2014). 10.1093/ejcts/ezu242 [DOI] [PubMed] [Google Scholar]
- 126.Bertassoni L. E., Cecconi M., Manoharan V. et al. , “Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs,” Lab Chip 14, 2202 (2014). 10.1039/C4LC00030G [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Choi S.-W., Zhang Y., Yeh Y.-C., Lake Wooten A., and Xia Y., “Biodegradable porous beads and their potential applications in regenerative medicine,” J. Mater. Chem. 22(23), 11442 (2012). 10.1039/c2jm16019f [DOI] [Google Scholar]
- 128.Chung H. J. and Park T. G., “Injectable cellular aggregates prepared from biodegradable porous microspheres for adipose tissue engineering,” Tissue Eng. Part A 15(6), 1391 (2009). 10.1089/ten.tea.2008.0344 [DOI] [PubMed] [Google Scholar]
- 129.Chung H. J., Kim I. K., Kim T. G., and Park T. G., “Highly open porous biodegradable microcarriers: In vitro cultivation of chondrocytes for injectable delivery,” Tissue Eng. Part A 14(5), 607 (2008). 10.1089/tea.2007.0263 [DOI] [PubMed] [Google Scholar]
- 130.Huang H., Yu Y., Hu Y. et al. , “Generation and manipulation of hydrogel microcapsules by droplet-based microfluidics for mammalian cell culture,” Lab Chip 2, 1–11 (2017). 10.1039/C7LC00262A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kim C., Bang J. H., Kim Y. E., Lee J. H., and Kang J. Y., “Stable hydrodynamic trapping of hydrogel beads for on-chip differentiation analysis of encapsulated stem cells,” Sens. Actuators B Chem. 166–167, 859–869 (2012). 10.1016/j.snb.2012.02.008 [DOI] [Google Scholar]
- 132.Alessandri K., Feyeux M., Gurchenkov B. et al. , “A 3D printed microfluidic device for production of functionalized hydrogel microcapsules for culture and differentiation of human neuronal stem cells (hNSC),” Lab Chip 16(9), 1593–1604 (2016). 10.1039/C6LC00133E [DOI] [PubMed] [Google Scholar]
- 133.Ma Y., Neubauer M. P., Thiele J., Fery A., and Huck W. T. S., “Artificial microniches for probing mesenchymal stem cell fate in 3D,” Biomater. Sci. 2(11), 1661–1671 (2014). 10.1039/C4BM00104D [DOI] [PubMed] [Google Scholar]
- 134.Chan H. F., Zhang Y., Ho Y.-P., Chiu Y.-L., Jung Y., and Leong K. W., “Rapid formation of multicellular spheroids in double-emulsion droplets with controllable microenvironment,” Sci. Rep. 3(1), 3462 (2013). 10.1038/srep03462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Maguire T., Novik E., Schloss R., and Yarmush M., “Alginate-PLL microencapsulation: Effect on the differentiation of embryonic stem cells into hepatocytes,” Biotechnol. Bioeng. 93(3), 581–591 (2006). 10.1002/bit.20748 [DOI] [PubMed] [Google Scholar]
- 136.Richardson T., Kumta P. N., and Banerjee I., “Alginate encapsulation of human embryonic stem cells to enhance directed differentiation to pancreatic islet-like cells,” Tissue Eng. Part A. 20(23–24), 3198–3211 (2014). 10.1089/ten.tea.2013.0659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Agarwal P., Zhao S., Bielecki P. et al. , “One-step microfluidic generation of pre-hatching embryo-like core-shell microcapsules for miniaturized 3D culture of pluripotent stem cells,” Lab Chip 13(23), 4525–4533 (2013). 10.1039/c3lc50678a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Siltanen C., Yaghoobi M., Haque A. et al. , “Microfluidic fabrication of bioactive microgels for rapid formation and enhanced differentiation of stem cell spheroids,” Acta Biomater. 34, 125–132 (2016). 10.1016/j.actbio.2016.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Allazetta S. and Lutolf M. P., “Stem cell niche engineering through droplet microfluidics,” Curr. Opin. Biotechnol. 35, 86–93 (2015). 10.1016/j.copbio.2015.05.003 [DOI] [PubMed] [Google Scholar]
- 140.Zhao H., Chen Y., Shao L. et al. , “Airflow-assisted 3D bioprinting of human heterogeneous microspheroidal organoids with microfluidic nozzle,” Small 14(39), 1802630 (2018). 10.1002/smll.201802630 [DOI] [PubMed] [Google Scholar]
- 141.Colosi C., Shin S. R., Manoharan V. et al. , “Microfluidic bioprinting of heterogeneous 3D tissue constructs using low-viscosity bioink,” Adv. Mater. 28(4), 677–684 (2016). 10.1002/adma.201503310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shepherd R. F., Conrad J. C., Rhodes S. K. et al. , “Microfluidic assembly of homogeneous and Janus colloid-filled hydrogel granules,” Langmuir 22(21), 8618–8622 (2006). 10.1021/la060759+ [DOI] [PubMed] [Google Scholar]
- 143.Chung B. G., Lee K.-H., Khademhosseini A., and Lee S.-H., “Microfluidic fabrication of microengineered hydrogels and their application in tissue engineering,” Lab Chip 12(1), 45 (2012). 10.1039/C1LC20859D [DOI] [PubMed] [Google Scholar]
- 144.Mazutis L., Gilbert J., Ung W. L., Weitz D. A., Griffiths A. D., and Heyman J. A., “Single-cell analysis and sorting using droplet-based microfluidics,” Nat. Protoc. 8(5), 870–891 (2013). 10.1038/nprot.2013.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li C. Y., Wood D. K., Huang J. H., and Bhatia S. N., “Flow-based pipeline for systematic modulation and analysis of 3D tumor microenvironments,” Lab Chip 13(10), 1969–1978 (2013). 10.1039/c3lc41300d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chokkalingam V., Tel J., Wimmers F. et al. , “Probing cellular heterogeneity in cytokine-secreting immune cells using droplet-based microfluidics,” Lab Chip 13(24), 4740–4744 (2013). 10.1039/c3lc50945a [DOI] [PubMed] [Google Scholar]
- 147.Akbari S. and Pirbodaghi T., “A droplet-based heterogeneous immunoassay for screening single cells secreting antigen-specific antibodies,” Lab Chip 14(17), 3275–3280 (2014). 10.1039/C4LC00082J [DOI] [PubMed] [Google Scholar]
- 148.Aeby E. A., Misun P. M., Hierlemann A., and Frey O., “Microfluidic hydrogel hanging-drop network for long-term culturing of 3D microtissues and simultaneous high-resolution imaging,” Adv. Biosyst. 2(7), 1800054 (2018). 10.1002/adbi.201800054 [DOI] [Google Scholar]
- 149.Amselem G., Guermonprez C., Drogue B., Michelin S., and Baroud C. N., “Universal microfluidic platform for bioassays in anchored droplets,” Lab Chip 16(21), 4200–4211 (2016). 10.1039/C6LC00968A [DOI] [PubMed] [Google Scholar]
- 150.Klein A. M., Weitz D. A., Kirschner M. W. et al. , “Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells,” Cell 161, 1187 (2015). 10.1016/j.cell.2015.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Macosko E. Z. et al. , “Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets,” Cell 161(5), 1202–1214 (2015). 10.1016/j.cell.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fan H. C., Fu G. K., and Fodor S. P. A., “Combinatorial labeling of single cells for gene expression cytometry,” Science 347(6222), 1258367 (2015). 10.1126/science.1258367 [DOI] [PubMed] [Google Scholar]
- 153.Ceyhan E., Xu F., Gurkan U. A. et al. , “Prediction and control of number of cells in microdroplets by stochastic modeling,” Lab Chip 12(22), 4884 (2012). 10.1039/c2lc40523g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Demirci U. and Montesano G., “Single cell epitaxy by acoustic picolitre droplets,” Lab Chip 7(9), 1139–1145 (2007). 10.1039/b704965j [DOI] [PubMed] [Google Scholar]
- 155.Cheng E., Yu H., Ahmadi A., and Cheung K. C., “Investigation of the hydrodynamic response of cells in drop on demand piezoelectric inkjet nozzles,” Biofabrication 8(1), 015008 (2016). 10.1088/1758-5090/8/1/015008 [DOI] [PubMed] [Google Scholar]
- 156.Husman D., Welzel P. B., Vogler S. et al. , “Multiphasic microgel-in-gel materials to recapitulate cellular mesoenvironments in vitro,” Biomater. Sci. 8(1), 101–108 (2020). 10.1039/C9BM01009B [DOI] [PubMed] [Google Scholar]
- 157.Murphy S V. and Atala A., “3D bioprinting of tissues and organs,” Nat. Biotechnol. 32(8), 773–785 (2014). 10.1038/nbt.2958 [DOI] [PubMed] [Google Scholar]
- 158.Grogan S. P., Chung P. H., Soman P. et al. , “Digital micromirror device projection printing system for meniscus tissue engineering,” Acta Biomater. 9(7), 7218–7226 (2013). 10.1016/j.actbio.2013.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gauvin R., Chen Y.-C., Lee J. W. et al. , “Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography,” Biomaterials 33(15), 3824–3834 (2012). 10.1016/j.biomaterials.2012.01.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lin H., Zhang D., Alexander P. G. et al. , “Application of visible light-based projection stereolithography for live cell-scaffold fabrication with designed architecture,” Biomaterials 34(2), 331–339 (2013). 10.1016/j.biomaterials.2012.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Deng Y., Zhang N., Zhao L. et al. , “Rapid purification of cell encapsulated hydrogel beads from oil phase to aqueous phase in a microfluidic device,” Lab Chip 11(23), 4117 (2011). 10.1039/c1lc20494g [DOI] [PubMed] [Google Scholar]
- 162.Hong S., Hsu H.-J., Kaunas R., and Kameoka J., “Collagen microsphere production on a chip,” Lab Chip 12(18), 3277 (2012). 10.1039/c2lc40558j [DOI] [PubMed] [Google Scholar]
- 163.Stucky E. C., Schloss R. S., Yarmush M. L., and Shreiber D. I., “Alginate micro-encapsulation of mesenchymal stromal cells enhances modulation of the neuro-inflammatory response,” Cytotherapy 17(10), 1353–1364 (2015). 10.1016/j.jcyt.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jeong H.-H., Issadore D., and Lee D., “Recent developments in scale-up of microfluidic emulsion generation via parallelization,” Korean J. Chem. Eng. 33(6), 1757–1766 (2016). 10.1007/s11814-016-0041-6 [DOI] [Google Scholar]
- 165.Morimoto Y., Tan W., Tsuda Y., and Takeuchi S., “Monodisperse semi-permeable microcapsules for continuous observation of cells,” Lab Chip 9(15), 2217–2223 (2009). 10.1039/b900035f [DOI] [PubMed] [Google Scholar]
- 166.Wu M. H. and Pan W. C., “Development of microfluidic alginate microbead generator tunable by pulsed airflow injection for the microencapsulation of cells,” Microfluid. Nanofluidics 8(6), 823–835 (2010). 10.1007/s10404-009-0522-6 [DOI] [Google Scholar]
- 167.Wang X., Camci-unal G., Wan K., Liao R., and Khademhosseini A., “Microfluidics-assisted fabrication of gelatin-silica core−shell microgels for injectable tissue constructs,” Biomacromolecules 15, 283−290 (2014). 10.1021/bm401533y [DOI] [PMC free article] [PubMed] [Google Scholar]