Abstract
Background
This systematic review reports on outcomes and toxicities following stereotactic radiosurgery (SRS) for non-functioning pituitary adenomas (NFAs) and presents consensus opinions regarding appropriate patient management.
Methods
Using the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses, a systematic review was performed from articles of ≥10 patients with NFAs published prior to May 2018 from the Medline database using the key words “radiosurgery” and “pituitary” and/or “adenoma.” Weighted random effects models were used to calculate pooled outcome estimates.
Results
Of the 678 abstracts reviewed, 35 full-text articles were included describing the outcomes of 2671 patients treated between 1971 and 2017 with either single fraction SRS or hypofractionated stereotactic radiotherapy (HSRT). All studies were retrospective (level IV evidence). SRS was used in 27 studies (median dose: 15 Gy, range: 5–35 Gy) and HSRT in 8 studies (median total dose: 21 Gy, range: 12–25 Gy, delivered in 3–5 fractions). The 5-year random effects local control estimate after SRS was 94% (95% CI: 93.0–96.0%) and 97.0% (95% CI: 93.0–98.0%) after HSRT. The 10-year local control random effects estimate after SRS was 83.0% (95% CI: 77.0–88.0%). Post-SRS hypopituitarism was the most common treatment-related toxicity observed, with a random effects estimate of 21.0% (95% CI: 15.0–27.0%), whereas visual dysfunction or other cranial nerve injuries were uncommon (range: 0–7%).
Conclusions
SRS is an effective and safe treatment for patients with NFAs. Encouraging short-term data support HSRT for select patients, and mature outcomes are needed before definitive recommendations can be made. Clinical practice opinions were developed on behalf of the International Stereotactic Radiosurgery Society (ISRS).
Keywords: consensus, ISRS, non-functioning, pituitary adenomas, radiation therapy, radiosurgery
Key Points.
1. SRS is an effective and safe treatment for patients with NFAs.
2. Single fraction SRS is associated with long-term (10-year) disease control for NFAs.
3. HSRT can be used in select patients with NFAs with encouraging short-term results.
Importance of the Study.
SRS is commonly used for patients with non-functioning adenomas. However, the reports on the treatment techniques and clinical outcomes, both efficacy and safety, are retrospective in nature, and therefore it is difficult to make broad conclusions by individually assessing studies. Therefore, on behalf of the ISRS, it was the objective of this report to provide a high-quality critical analysis of the literature to evaluate this treatment approach and provide key opinions for patient management. After an extensive review, we determined that single fraction SRS is associated with high control rates with long-term pooled outcomes, and hypofractionated approaches (3–5 fractions) are also associated with excellent, short-term, outcomes. New-onset hypopituitarism is the most common side effect following treatment, although the time course for its development and clinical sequelae require better reporting. Cranial nerve dysfunction following treatment is rare.
Pituitary adenomas represent 10–20% of all tumors of the central nervous system, of which approximately one-third are non-functioning.1 Multiple options exist for patients with newly diagnosed non-functioning adenomas (NFAs), including conservative management, resection, conventionally fractionated external beam radiotherapy (EBRT), and stereotactic radiosurgery (SRS). Developments in SRS techniques have resulted in this becoming an alternative to resection for medically inoperable patients, an adjuvant treatment for those who have undergone a subtotal resection, or salvage treatment for those who experience growth of residual disease. Multiple retrospective series have demonstrated favorable control rates, often >90% five years following SRS; however, published institutional series often report on cases treated over an extended period of time, along with either functioning pituitary adenomas or with other benign base-of-skull tumors.2 Moreover, the advent of hypofractionated approaches for complex cases near critical structures or larger tumors needs further systematic evaluation.
The purpose of this systematic review of the literature is to describe the demographics, patient characteristics, treatment details, control outcomes, and treatment-related toxicities specifically for patients with NFAs treated with SRS. Based on this review, consensus opinions were made in an effort to provide guidance and more uniform clinical management.
Methods
Selection of Articles
This systematic review of the literature was performed according to criteria of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).3 Initial article selection was performed by searching the MEDLINE (PubMed) and Cochrane electronic bibliographic databases, and additional primary research studies were added based on a review of the bibliographies of the selected articles or other reviews of the literature. Given the reporting of outcomes of patients with NFAs in larger series of base-of-skull tumors or as selected cases in generalized series of pituitary adenomas (including functioning tumors), these generic key words were used: “radiosurgery” and “pituitary” and/or “adenoma.” Full text articles published in the English language up until May 2018 were considered and no publishing date restrictions were used.
The initial query identified 678 articles that were subsequently screened for relevance to the objectives of the present report by thorough review of the article titles, abstracts, and manuscripts, as necessary. Specific inclusion criteria included: retrospective or prospective case series of >10 adult patients, SRS or hypofractionated stereotactic radiotherapy (HSRT; 2–5 fractions), and description of clinical or treatment-related toxicity outcomes specific to NFA patients. Exclusion criteria included: non-clinical reports (ie, dosimetric, physics, or basic science research only); expert opinion, commentary or review studies which did not provide unique data on >10 patients; studies on patients with sellar tumors of different disease entities (ie, craniopharyngiomas, pituitary carcinomas, and metastases), pediatric-only case series, or studies which had fewer than 10 patients with NFAs. As some series included updates on prior reports from the same institution or multi-institutional studies with inclusion of already published patient cohorts, duplicate studies were assessed for any updated data on treatment efficacy or toxicity with the latest report of the largest number of patients included in the final analysis. The search strategy used for this report and the methodology for study inclusion is outlined in Supplementary Fig. 1.
Outcome Measures and Statistical Analysis
The primary outcomes were local control at 5 and 10 years. In addition, key treatment-related toxicities were evaluated, such as new hypopituitarism, optic neuropathy, and other cranial nerve (CN) injury. R Studio v1.1.423 was used for statistical analyses and the R package “metafor” (v2.0-0)4 was used for meta-analyses, tests for heterogeneity, analysis of publication bias, and meta-regressions. Study variances for overall estimate and for meta-regression were calculated using the DerSimonian–Laird method.5,6 Weighted random effects models were used to calculate pooled estimates for 5-year local control, 10-year local control, and new hypopituitarism. Since the studies involved patient treatment decisions, the random effects model was considered superior to the fixed effects model when calculating pooled estimates. In addition, due to the selected studies spanning numerous years, in a number of different populations and in varied geographical locations, the random effects model was considered to be better than the fixed effects model.7,8 Nevertheless, we reported both estimates in the figures. The I 2 statistic was used for identifying heterogeneity: I 2 of 0%, 25%, 50%, and 75% were interpreted as absent, low, moderate, and high heterogeneity, respectively.9 Funnel plots and the Egger test (P < 0.05 indicating presence of bias) were used for identifying publication bias. Finally, meta-regression analyses were performed to identify potential associations between outcomes including 5-year local control, 10-year local control, and new hypopituitarism as a function of tumor volume given the potential for bias in treatment fractionation selection.
Results
After a comprehensive review of the published literature, 35 unique studies met the inclusion and exclusion criteria for this systematic review. All included studies were retrospective in nature and provided low-quality evidence. There was no presence of publication bias (P > 0.05) across the included studies regarding the primary outcomes evaluated in this meta-analysis (Supplementary Fig. 2). A majority of studies (n = 31, 89%) represented single-institution reports, and 4 studies (11%) were multi-institutional collaborations. Eleven reports (31%) were published from institutions in the United States, whereas the majority of reports originated from institutions outside of the United States (n = 24, 69%). The median number of patients evaluated in single institution reports was 31 (range: 10–272 patients).
Basic study details and patient characteristics are presented in Table 1.10–44 Across all studies, 51% of patients were men, and the median age was 53 years (range: 43–69). Few patients were treated definitively with SRS (median 5.5%, range: 0–100%), and only 2 reports (n = 62 and n = 69) described the outcomes of patients who were treated definitively due to medical inoperability or refusal of resection.19,20 The median value across all studies for the proportion of patients who had undergone resection prior to SRS was 95% (range: 0–100%). For these patients, 15 studies (43%) reported the percentage of patients who were treated adjuvantly (immediately after resection for residual disease or within an interval without radiographic evidence of disease growth) or in the salvage setting. For the studies reporting this distinction, the median proportion of patients treated adjuvantly was 47% (range: 17–96%), and the median proportion of patients treated in the salvage setting was 53% (range: 2–83%). For most patients, SRS represented the first course of radiotherapy received; the range of proportions of patients who received prior radiotherapy was 9–17%. Given the substantial number of patients who had undergone resection as first-line treatment for their disease, the overall proportion of patients who had hypopituitarism prior to first SRS was 45% (range: 0–83%).
Table 1.
Non-functioning pituitary adenoma SRS study details and patient characteristics
| Author | Year | Institution | Location | Years | Study Type | Evidence Quality | N | %Males | Age (y) | %Definitive | Prior Surgery | %Adjuvant | %Salvage | Prior RT | Time from Surgery to RT(mo) | % Hypopituitarism prior to RT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Graffeo | 2018 | Mayo Clinic | Rochester, MN, USA | 2007–2014 | RS | Low | 57 | 45%* | 50* | 7%* | 93% | – | – | None | – | 0% |
| Narayan | 2018 | Louisiana State University Health Sciences Center | Shreveport, LA, USA | 2000–2017 | RS | Low | 87 | 48% | 57 | 7% | 93% | 61% | 32% | None | 12.6 | 34% |
| Pomeraniec | 2017 | Multi-institutional | IGKRF | 1987–2015 | RS | Low | 222 | – | 53 | 0% | 100% | 50% | 50% | None | – | 46% |
| Cohen-Inbar | 2017 | Multi-institutional | IGKRF | 1997–2015 | RS | Low | 357 | – | 55 | 0% | 100% | 0% | 0% | None | – | – |
| Zibar Tomsic | 2017 | University Hospital Centre Zagreb | Zagreb, Croatia | 2003–2014 | RS | Low | 18 | 67% | 63 | – | – | – | – | None | – | 28% |
| McTyre | 2017 | Wake Forest School of Medicine | Winston-Salem, NC, USA | 2013–2015 | RS | Low | 10 | 29%* | 62* | – | – | – | – | – | – | – |
| Sadik | 2017 | Elisabeth-Tweesteden Hospital | Tilburg, Netherlands | 2002–2015 | RS | Low | 50 | 57 | 0% | 100% | 26% | 74% | None | – | ||
| Losa | 2017 | Istituto Scientifico San Raffaele, Vita-Salute University | Milan, Italy | 1994–2014 | RS | Low | 272 | 51% | 52 | 8% | 92% | – | – | 2% | – | – |
| Puataweepong | 2016 | Ramathibodi Hospital, Mahidol University | Bangkok, Thailand | 2009–2012 | RS | Low | 27 | 40%* | 50* | – | 98%* | – | – | 5%* | 24 | 82%* |
| Hasegawa | 2015 | Komaki City Hospital | Komaki, Japan | 1991–2001 | RS | Low | 16 | 50% | 62 | 100% | 0% | 0% | 0% | None | None | 6% |
| Lee | 2014 | Multi-institutional | Multi-institutional | 1988–2012 | RS | Low | 41 | 49% | 69 | 100% | 0% | 0% | 0% | None | None | 37% |
| Liao | 2013 | Chang Gung Memorial Hospital at Linkou | Taoyuan, Taiwan | 2006–2011 | RS | Low | 21 | 35%* | 48* | 0% | 100% | 47%* | 53%* | 6%* | – | – |
| Zeiler | 2013 | University of Manitoba | Winnipeg, Manitoba, Canada | 2003–2011 | RS | Low | 47 | 43%* | 56* | 35%* | 65%* | – | – | – | 59.8* | – |
| Sheehan | 2013 | Multi-institutional | NAGKC | 1988–2011 | RS | Low | 512 | 56% | 53 | 6% | 94% | – | – | 7% | – | – |
| Chen | 2013 | Tri-Service General Hospital, National Defense Medical Center | Taipei, Taiwan | 2007–2011 | RS | Low | 17 | 35% | 58* | 6% | 94% | 82%* | 14%* | None | 58 | – |
| El-Shehaby | 2012 | Gamma Knife Center Cairo, Nasser Institute | Shobra, Egypt | 2002–2008 | RS | Low | 21 | 62% | 48 | 10% | 90% | – | – | None | – | 20% |
| Runge | 2012 | Department of Stereotaxy and Functional Neurosurgery, University Hospital | Cologne, Germany | 1992–2008 | RS | Low | 61 | 59% | 56 | 3% | 97% | 38% | 59% | 3% | – | 43% |
| Wilson | 2012 | Wollongong Hospital | Wollongong, New South Wales, Australia | 1971–2007 | RS | Low | 51 | 61% | 53 | 2% | 98% | 96% | 2% | None | – | – |
| Iwata | 2011 | Nagoya City University Graduate School of Medical Sciences | Nagoya, Japan | 2000–2009 | RS | Low | 100 | 43% | 59 | 6% | 94% | – | – | None | 11 | 26% |
| Park | 2011 | University of Pittsburgh | Pittsburgh, PA, USA | 1987–2009 | RS | Low | 125 | 55% | 54 | 12% | 88% | 20% | 68% | 14% | – | 64% |
| Castro | 2010 | Institute of Neurological Radiosurgery | Sao Paulo, Brazil | 1999–2009 | RS | Low | 14 | 48%* | 43* | 7%* | 93%* | 76%* | 17%* | 5%* | – | – |
| Hayashi | 2010 | Tokyo Women’s Medical University | Tokyo, Japan | 2003–2007 | RS | Low | 43 | 70%* | 50* | 0% | 100% | 95% | 5% | None | – | – |
| Cho | 2009 | St. Mary’s Hospital, The Catholic University of Korea | Seoul, Korea | 2004–2008 | RS | Low | 17 | 47% | 55 | 18% | 82% | – | – | None | – | – |
| Killory | 2008 | Barrow Neurological Institute | Phoenix, AZ, USA | 2004–2006 | RS | Low | 14 | 55% | 47 | 0% | 100% | 45%* | 55%* | 5% | – | – |
| Hoybye | 2009 | Karolinska University Hospital | Stockholm, Sweden | 1994–2004 | RS | Low | 23 | 56% | 49 | 0% | 100% | 17% | 83% | None | 35 | 83% |
| Kobayashi | 2009 | Nagoya Kyoritsu Hospital | Nagoya, Japan | 1996–2009 | RS | Low | 71 | 44% | 50 | 8% | 92% | – | – | 17% | – | – |
| Pollock | 2008 | Mayo Clinic | Rochester, MN, USA | 1992–2004 | RS | Low | 62 | 56% | 53 | 5% | 95% | 24% | 76% | 5% | 28 | 52% |
| Liscak | 2007 | Na Homolce Hospital | Prague, Czech Republic | 1993–2003 | RS | Low | 79 | 56% | 54 | 15% | 85% | – | – | None | – | 62% |
| Kajiwara | 2005 | Yamaguchi University School of Medicine | Ube, Japan | 1999–2002 | RS | Low | 14 | 50% | 68 | 0% | 100% | – | – | 14% | – | 0% |
| Iwai | 2005 | Osaka City General Hospital | Osaka, Japan | 1994–1999 | RS | Low | 31 | 29% | 53 | 0% | 100% | 68% | 32% | 3% | – | 35% |
| Muacevic | 2004 | German Gamma Knife Center Munich, Ludwig-Maximilians University | Munich, Germany | 1994–2004 | RS | Low | 60 | 50 | 0% | 100% | – | – | None | – | – | |
| Petrovich | 2002 | University of Southern California | Los Angeles, CA, USA | 1994–2002 | RS | Low | 56 | 59%* | 53 | 5% | 95% | 17% | 83% | 5% | 61 | 33% |
| Wowra | 2002 | Ludwig- Maximilians- Universität | München, Germany | 1993–2002 | RS | Low | 30 | 47% | 55 | 3% | 97% | – | – | None | – | 70% |
| Mokry | 1999 | University of Graz | Graz, Austria | 1992–1998 | RS | Low | 31 | 49%* | 46* | 3% | 97% | – | – | 10%* | 43* | – |
| Martinez | 1998 | Ruber International Hospital | Madrid, Spain | 1992–1995 | RS | Low | 14 | 46%* | 44* | 50% | 50% | – | – | 7% | – | 43% |
*Entire patient cohort.
Abbreviations: RS, retrospective study; RT, radiotherapy; NAGKC, North American Gamma Knife Consortium; IGKRF, International Gamma Knife Research Foundation.
Most studies reported on the tumor dimensions at the time of SRS, summarized in Supplementary Table 1. For those studies reporting tumor diameter (n = 10, 29%), the median dimension was 2.2 cm (range: 0.1–10.5 cm). For those studies reporting tumor volume (n = 30, 86%), the median was 3.5 cc (range: 0.03–38.7 cc). A total of 27 studies (77%) reported on outcomes of 2451 patients treated with single fraction SRS to a median dose of 15 Gy (range: 5–25 Gy). Eight studies (23%) described the outcomes of patients treated with HSRT, to a median dose of 21 Gy (range: 12 Gy in 3 fractions to 25 Gy in 5 fractions). The radiosurgery delivery technologies included in this review are CyberKnife (Accuray) (n = 6) for HSRT, Gamma Knife (n = 26) (Elekta) for SRS or HSRT treatments, or other linear accelerator–based techniques (n = 3).
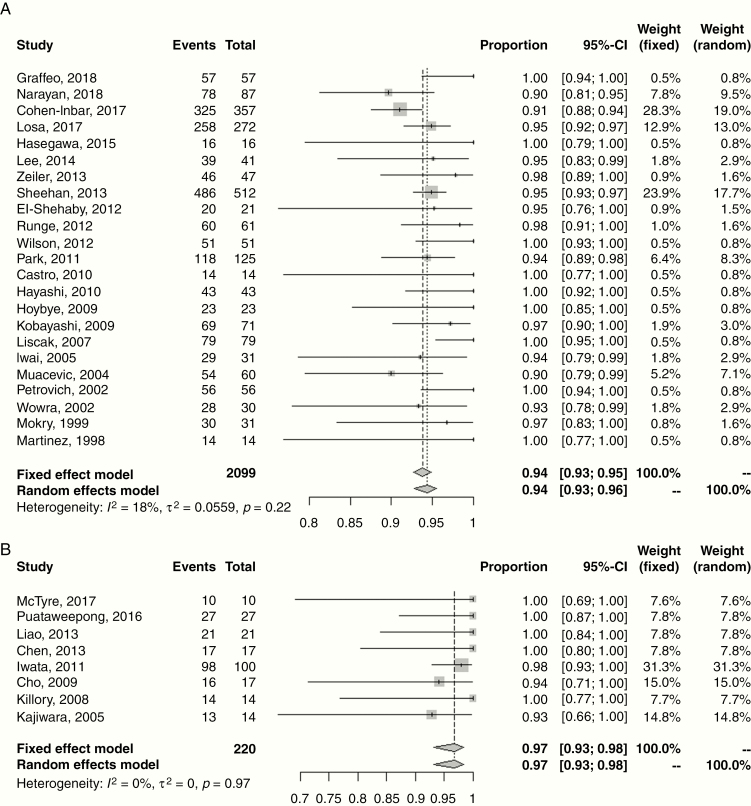
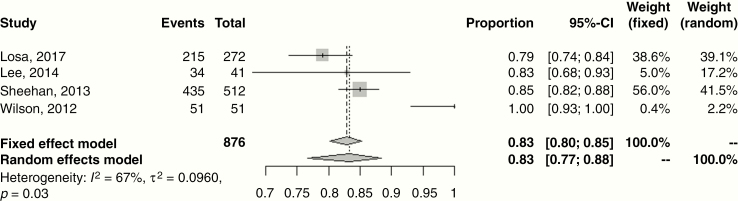
The disease control and treatment-related toxicity outcomes are presented in Table 2. The median follow-up was 42 months (range: 21–86 mo). The majority of studies (n = 21, 60%) reported crude, rather than actuarial, rates of local control ranging 90–100%. This is a possible confounder in terms of overestimating effect size. However, for the 10 studies reporting actuarial outcomes, the 5-year rate of progression-free survival (PFS) was 95% (range: 90–100%), identical to the studies utilizing non-actuarial reporting methodology. Random effects meta-analyses for 5-year local control for single fraction SRS and HSRT are shown in Fig. 1, with estimates of 94.0% (95% CI: 93.0–96.0%) and 97.0% (95% CI: 93.0–98.0%), respectively. Four studies reported 10-year rates of PFS ranging 79–100%. Fig. 2 shows the meta-analysis for 10-year local control after SRS with a random effects estimate of 83.0% (95% CI: 77.0–88.0%); HSRT outcomes were limited to <5 years. Reported salvage treatment included repeat SRS, EBRT, chemotherapy, or resection (Table 2).
Table 2.
Non-functioning pituitary adenoma SRS treatment outcomes and toxicities
| Author | Year | N | Median Dose (Gy) | Median Fx | Median Follow-up (m) | Local Control (5 y) | Local Control (10 y) | Salvage Treatment | New Hypopituitarism | Maximum Dose to Optic Pathway (Gy) | Optic Neuropathy | CN Injury |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Graffeo | 2018 | 57 | 15 | 1 | 48* | 100% | NR | Surgery + EBRT | 31% at 5 years* | 12 Gy | None | None |
| Narayan | 2018 | 87 | 15 | 1 | 48 | 90% (crude)* | NR | GK or surgery | 23% | 10 Gy | None | 3% |
| Pomeraniec | 2017 | 222 | 15 | 1 | 69 | NR | NR | NR | NR | 13.7 Gy | NR | NR |
| Cohen-Inbar | 2017 | 357 | 14 | 1 | 40 | 91% (crude) | NR | SRS, RT | 10% | NR | NR | NR |
| Zibar Tomsic | 2017 | 18 | 20 | 1 | 71 | NR | NR | NR | 28% | NR | NR | NR |
| McTyre | 2017 | 10 | 20 | 4 | NR | 100% (crude) | NR | NR | NR | NR | NR | NR |
| Sadik | 2017 | 50 | 15 | 1 | 40 | 95% at 40 months | NR | Surgery ± EBRT | 22% | 9Gy | None | None |
| Losa | 2017 | 272 | 15 | 1 | 79 | 95% | 79% | Surgery, EBRT, GK, chemo | NR | 10 Gy | NR | NR |
| Puataweepong | 2016 | 27 | 25 | 5 | 39* | 100% (crude) | NR | None | 0% | 32Gy/5 fx | None | None |
| Hasegawa | 2015 | 16 | 15 | 1 | 86 | 100% (crude) | NR | None | 0% | 12.3 Gy | None | none |
| Lee | 2014 | 41 | 12 | 1 | 48 | 94% | 83% | Surgery | 25% | 11 Gy | None | 2% |
| Liao | 2013 | 21 | 21 | 3 | 37* | 100% (crude) | NR | None | 0% | 21 Gy/3 fx | None | None |
| Zeiler | 2013 | 47 | 14 | 1 | 35 | 98% (crude) | NR | NR | 13%* | 12.2 Gy | 2% | 2% |
| Sheehan | 2013 | 512 | 16 | 1 | 36 | 95% | 85% | Surgery and/or EBRT | 21% | NR | 7% | 3% |
| Chen | 2013 | 17 | 25 | 5 | 31 | 100% | NR | None | 0% | 17 Gy/5 fx | None | None |
| El-Shehaby | 2012 | 21 | 12 | 1 | 44 | 95% (crude) | NR | None | 0% | 12.5 Gy | None | None |
| Runge | 2012 | 61 | 13 | 1 | 83 | 98% (crude) | NR | NR | 10% | 9 Gy | None | None |
| Wilson | 2012 | 51 | 14 | 1 | 50 | 100% | 100% | None | NR | NR | None | None |
| Iwata | 2011 | 100 | 21/25 | 3–5 | 33 | 98% | NR | NR | 3% | 25 Gy/5 fx | None | None |
| Park | 2011 | 125 | 13 | 1 | 62 | 94% | NR | NR | 23% at 5 years | 11 Gy | 2% | 3% |
| Castro | 2010 | 14 | 12.5 | 1 | 42* | 100% (crude) | NR | None | 3% | 9 Gy | None | None |
| Hayashi | 2010 | 43 | 18 | 1 | 36* | 100% (crude) | NR | None | 0% | 10 Gy | None | None |
| Cho | 2009 | 17 | 19 | 3 | 27 | 93% (crude) | NR | None | 0% | NR | None | None |
| Killory | 2008 | 14 | 25 | 5 | 27* | 100% (crude) | NR | None | 5% | 25 Gy/5 fx | None | 5% |
| Hoybye | 2009 | 23 | 20 | 1 | 78 | 100% (crude) | NR | None | 0% | 11 Gy | None | 4% |
| Kobayashi | 2009 | 71 | 14 | 1 | NR | 97% (crude) | NR | None | 8% | NR | NR | NR |
| Pollock | 2008 | 62 | 16 | 1 | 64 | 95% at 7 years | NR | EBRT or SRS | 32% | 12 Gy | None | 2% |
| Liscak | 2007 | 79 | 20 | 1 | 60 | 100% (crude) | NR | None | 14% | 8 Gy | None | None |
| Kajiwara | 2005 | 14 | 13 | 3 | 35 | 93% (crude) | NR | NR | 7% | NR | None | None |
| Iwai | 2005 | 31 | 14 | 1 | 60 | 93% | NR | Surgery | 7% | 11 Gy | None | None |
| Muacevic | 2004 | 60 | 17 | 1 | 22 | 90% | NR | GK | 4% | NR | None | None |
| Petrovich | 2002 | 56 | 15 | 1 | 36 | 100% (crude) | NR | None | 4% | 9 Gy | None | 4% |
| Wowra | 2002 | 30 | 16 | 1 | 58 | 93% (crude) | NR | GK | 14% at 6 years | NR | None | None |
| Mokry | 1999 | 31 | 14 | 1 | 21 | 98% (crude) | NR | Surgery | 20% | 9 Gy | None | None |
| Martinez | 1998 | 14 | 14 | 1 | 36 | 100% (crude) | NR | None | 0% | NR | None | 7% |
*Entire patient cohort.
Abbreviations: Fx, fraction; m, months; y, years; GK, GammaKnife; RT, radiotherapy; NR, not reported.
Fig. 1.
Forest plot of 5-year local control following treatment of non-functioning pituitary adenomas with (A) single fraction stereotactic radiosurgery (SRS) and (B) hypofractionated stereotactic radiotherapy (HSRT). Squares indicate the proportions from individual studies and horizontal lines indicate the 95% confidence interval. The size of the data marker corresponds to the relative weight assigned in the pooled analysis using the random effects model. Diamond indicates the pooled proportion with 95% CI. Both the fixed effect and random effects models pooled estimates are presented and heterogeneity analysis is included.
Fig. 2.
Forest plot of 10-year local control following treatment of non-functioning pituitary adenomas with single fraction SRS. Squares indicate the proportions from individual studies and horizontal lines indicate the 95% CI. The size of the data marker corresponds to the relative weight assigned in the pooled analysis using the random effects model. Diamond indicates the pooled proportion with 95% CI. Both the fixed effect and random effects models pooled estimates are presented and heterogeneity analysis is included.
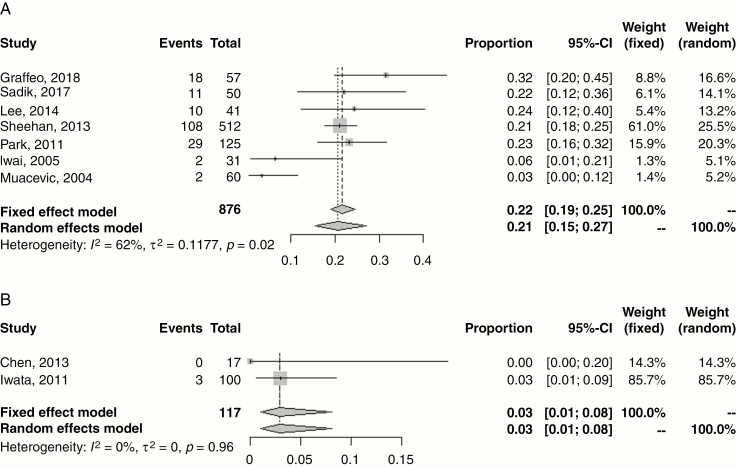
Treatment-related toxicities that were evaluated in each of the selected articles included cerebrovascular accident (CVA) (n = 0), new-onset hypopituitarism (n = 31), optic pathway dysfunction (n = 29), other CN injury (n = 29), and secondary malignancy (n = 0) (Table 2). New-onset hypopituitarism ranged 0–32%. Random effects meta-analyses for new hypopituitarism following single fraction SRS and HSRT are shown in Fig. 3, with estimates of 21.0% (95% CI: 15.0–27.0%) and 3.0% (95% CI: 1.0%–8.0%), respectively. Twenty-four studies described dose constraints to the adjacent optic nerve or chiasm, with doses ranging 8–13.7 Gy in 1 fraction, and typically 25 Gy in 5 fractions. Declining visual function occurred 0–7% of the time as a result of radiotherapy, and in the absence of reported tumor progression. Similarly, the incidence of other cranial neuropathies ranged 0–7% and were most often temporary in nature. CN injuries frequently involved CN III, but were also seen in CNs IV, V, and VI.
Fig. 3.
Forest plot of new hypopituitarism following treatment of non-functioning pituitary adenomas with (A) single fraction SRS and (B) HSRT. Squares indicate the proportions from individual studies and horizontal lines indicate the 95% CI. The size of the data marker corresponds to the relative weight assigned in the pooled analysis using the random effects model. Diamond indicates the pooled proportion with 95% CI. Both the fixed effect and random effects models pooled estimates are presented and heterogeneity analysis is included.
Given the potential for SRS fractionation selection, local control, and treatment-related toxicity to be related to tumor volume, we investigated this correlation. Supplementary Fig. 3 shows meta-regression plots of median lesion volume versus 5- and 10-year local control and new hypopituitarism by SRS and HSRT. Meta-regression analysis did not identify a statistically significant relationship for 5- and 10-year local control or new hypopituitarism as a function of tumor volume.
Discussion
SRS for a pituitary adenoma was first described anecdotally in 1968 and since then this technique has been utilized for patients in the definitive setting, for residual disease after resection, and as salvage therapy in the setting of disease recurrence.45 Several case series and institutional reports have described outcomes of patients with NFAs treated with SRS; however, there are clear limitations to the current literature. All of the studies, single or multi-institutional, are effectively retrospective case series providing low level IV evidence. Furthermore, a broad selection of patients within a case series were observed, including those with NFAs, functioning pituitary adenomas, and other base-of-skull tumors. This limited a clear identification of the key patient selection criteria and treatment outcomes relevant for this specific analysis.
Interestingly, of the 678 published reports screened for this systematic review, only 35 unique studies (5.2%) were included after review, 20 duplicate series or prior reports from the same institution were identified and excluded, and 142 articles in the medical literature provided only a summary, commentary, or other type of review without reporting new patient data, thereby creating an anomalous ratio of data versus opinion of 35:142 (~1:4). The objective of this systematic review was to provide a summary of the current data regarding the role of SRS for NFA and to provide key opinions for appropriate patient management.
Treatment Efficacy
SRS was observed to be an effective treatment for patients with NFAs. The majority of patients identified in this review were treated in the adjuvant or recurrent setting. Treatment of patients definitively with SRS was often due to medical inoperability or patient refusal. It is important to note that a small proportion of patients were treated in the recurrent setting after prior conventional EBRT (0–17%), and treatment response and toxicity concerns may differ in this subset of patients. Although all studies were retrospective in nature and a minority reported actuarial outcomes, the pooled estimate of local control after 5 years following single fraction SRS was favorable at 94% (and similar to the value of non-actuarial studies). Since tumor control declines with longer follow-up, it is important to observe patients following treatment; in this meta-analysis, the pooled local control estimate at 10 years was 83%. Unfortunately, long-term tumor control was rarely reported, with only 4 studies in this meta-analysis describing 10-year clinical outcomes. It is important to note, however, that the few studies with long-term follow-up demonstrate interesting findings on recurrence patterns. For example, an Italian institutional series reported by Losa et al observed recurrences at a median of 8–9 years following treatment.17 After examining these late recurrences, the authors surmised that there are 2 different pathophysiological mechanisms as well as prognostic significance to “in-field” and “out-of-field” late recurrences. This is especially important to identify, as practice trends are now changing to include the resection bed, and not only residual disease, in the treatment volume.17,25
In addition to the lack of time-dependent endpoint reporting, the lack of standardization of the definition of tumor control is clearly evident and may present a challenge to understanding late recurrence patterns of failure. Most institutions report tumor control either as a percentage of tumors which reduce in size or are stable on imaging or with a volumetric cutoff, such as 15% or 25%, for tumor response and tumor progression. For a benign tumor, stabilization is typically the goal of treatment, but given that patients do experience reduction of tumor volume, this is also an important endpoint to describe, although its clinical value could be debatable in small NFAs. Reduction in tumor volume may be important in terms of improving CN dysfunction from tumor compression or other neurologic symptomatology, for larger tumors. Losa and colleagues described different dose thresholds for tumor volume reduction (~17 Gy) versus tumor volume stabilization (~15 Gy)46; however, other reports demonstrated similar rates of tumor volume reduction with doses as low as 12 Gy.25 Standardized response criteria for solid tumors such as the Response Evaluation Criteria in Solid Tumors (RECIST 1.1)47 should be included in future studies.
Dose and Fractionation Schedules
A majority of patients reported herein were treated with a median peripheral dose of 15 Gy. Prescription doses at each institution varied considerably and are typically based on the maximum dose delivered to the optic apparatus—in other words, the practice of “restriction-guided dosing.” The largest multi-institutional study reporting this described 512 patients with NFAs, 94% of whom had undergone prior resection and 7% of whom had received prior EBRT; the median SRS peripheral dose of 16 Gy resulted in excellent tumor control rates of 95% at 5 years and 85% at 10 years.23 However, the minimally effective dose remains controversial given the wide dose variation utilized in practice. Mingione and colleagues reported a minimal effective SRS dose of 12 Gy and suggested that doses greater than 20 Gy did not provide increased tumor control.48 El-Shehaby and colleagues reviewed the outcomes of 21 patients (median tumor volume 4.8 cc; 44 mo follow-up) treated with 12 Gy, with tumor reduction observed in 52% and disease stabilization in another 9 patients (43%).25 Additional long-term studies with clear objective response criteria are needed to determine a potential dose threshold to balance the rate of tumor control with toxicities such as new hypopituitarism, a risk which appears to increase significantly beyond a marginal dose of 18 Gy.20 Dose escalation may be considered for patients at higher risk for local recurrence, such as those with larger adenomas or those with more aggressive subtypes, such as silent corticotroph staining pituitary adenomas13,49; however, firm data to make recommendations are lacking. Based on the available literature, we recommend a dose of 14–16 Gy for single fraction SRS.
Hypofractionated schedules identified in this analysis included 21 Gy in 3 fractions,21 20 Gy in 4 fractions,15 and 25 Gy in 5 fractions,28 with a pooled 5-year tumor control estimate of 97%. Each of these schedules yields a biologically equivalent single fraction dose of approximately 11–13 Gy (assuming an α/β ratio of 3 Gy), although in vitro studies suggest that these may translate to a higher effective single fraction dose.50 Although the median follow-up periods for these reports are typically shorter than 5 years, the reported local control rates are high, with low rates of treatment-related toxicity. Given the heterogeneity in treatment selection, however, there was no observed difference in local control between fractionation schedules. Given the lack of association between tumor control and dose as a function of tumor volume in our meta-regression analyses, it is likely that dose and fractionation are most important for reducing the risk of toxicity, such as optic pathway dysfunction, rather than tumor control. Therefore, hypofractionated schedules can be considered for tumors that are in close proximity to the optic pathways, or in previously irradiated situations, but limited long-term data prevent this from becoming a routine fractionation schedule. Given the lack of long-term (>10 y) tumor control data, patients should be consented appropriately.
Subgroups at Increased Risk for Local Recurrence
A number of factors have been evaluated for increased risk of tumor recurrence following SRS. Given that the articles in this meta-analysis were restricted to an SRS cohort, there is an initial bias in the types of tumors treated (typically well-delineated targets in the upfront or recurrent setting), without inclusion of the resection cavity, and smaller treatment volumes. Moreover, the small sample sizes and relatively short follow-up of single institution reports prevent detailed analysis of patient, disease, and treatment-related factors associated with clinical outcome. Across the studies, the median tumor volume was 3.5 cc (effectively the volumetric rendition of an 18.8 mm diameter spheroid), and the majority of patients were treated with single fraction SRS. There have been a number of variables evaluated for increased risk of tumor recurrence, including age, sex, tumor volume, presence of suprasellar extension, cavernous sinus extension, timing of radiosurgery (intact vs postoperative and adjuvant vs salvage), and dose. When evaluating most SRS series individually, tumor size itself is not often a factor associated with treatment outcome, but that may be biased by the cases preselected for SRS as well as sample sizes (median of 31 patients per report). For example, in one of the largest studies with the longest follow-up included in this review, tumor volume was not associated with disease control.17 However, the median tumor volume in that series was only 1.5 cc (effectively the volumetric rendition of a 14.2 mm diameter spheroid) with a maximum of 2.6 cc—well below the median in this meta-analysis. Tumor volume >5 cc (effectively the volumetric rendition of a 21.3 mm diameter spheroid) was an important factor associated with disease control reported in a study by Narayan et al of 58 NFAs (median PFS 98.4 mo > 5 cc compared with 136.5 mo < 5 cc, P = 0.05),11 and a similar volume threshold of 4.5 cc (5-year PFS 86% vs 97%) by Park and colleagues.51 A similar finding was not observed in this meta-analysis as volumes and tumor control rates were summarized by abstracting only the medians and a larger patient-level study would be needed to investigate this further.
Upfront vs Delayed Radiosurgery
The rate of tumor recurrence after subtotal resection of NFAs varies widely in the literature from approximately 20–80% by 10 years following initial resection. In this meta-analysis, only 35% of studies reported on the proportion of patients treated adjuvantly versus those treated in the salvage setting with SRS—with half of patients treated in either setting. Multiple studies in the literature support the role of immediate fractionated EBRT compared with observation and salvage therapy in patients with residual NFAs.51,52 However, there is little consensus in the literature on the appropriate timing of SRS in patients who have undergone resection and have residual disease, because although upfront SRS might yield superior local control, it remains unclear whether close follow-up and salvage as necessary might yield similar outcomes. More recently, Pomeraniec et al reported on the clinical outcomes of 64 patients (32 treated in the early setting defined as ≤6 mo from resection, and 32 treated in the late setting defined as >6 mo from resection) treated with SRS for NFAs.53 The authors observed a reduction in risk of radiographic and symptomatic progression in patients treated in the early group. This finding was later corroborated by a 9-center multi-institutional study of an expanded cohort of 222 patients, suggesting that earlier treatment results in more favorable outcomes than expectant management. Caveats to this management strategy include lack of clear data on early salvage as described above (radiological progression) versus delayed salvage (symptomatic progression), as other reports stratifying patients by adjuvant treatment (within 6 mo) or delayed salvage (radiological progression) have not demonstrated benefit with early treatment.16 Selection of patients with high risk for tumor recurrence after resection is complex as prediction models remain limited in scope at this time and further studies are clearly needed.
Toxicities Associated with Treatment
SRS was associated with low rates of permanent treatment-related toxicities. However, transient side effects do occur and it is important to counsel patients about these risks. For example, in the report by Zeiler et al, 31 of the 76 patients (34%) in their series experienced transient side effects as a result of treatment; however, approximately 75% of these toxicities were related to the stereotactic frame placement (including pin site swelling, infection, dysesthesias). Other acute toxicities included visual blurring, short-term memory loss, and ataxia.22
Cranial neuropathies, injury to the carotid artery or subsequent risk of CVA as a result of irradiation, and radiation necrosis were rarely encountered in this analysis. Narayan et al observed visual field deficits (n = 9, 8%), isolated CN palsies (n = 9, 8%), hydrocephalus (n = 3, 3%), and radiation necrosis (n = 2, 2%) in a series of patients treated over a 17-year period without specific dosimetric or patient-related variables associated with development of toxicity.11 In an experience of 89 patients treated with SRS for pituitary adenomas invading the cavernous sinus, Hayashi and colleagues reported on 2 patients who developed transient abducens nerve palsy and oculomotor nerve palsy; however, the latter patients received less than the prescription dose of 24 Gy. Therefore, peritumoral edema and resulting compression of an adjacent nerve may also be a factor to consider. With the lower dosing for NFAs, cranial neuropathies are rarely reported; however, in the setting of cavernous sinus invasion or extrasellar extension, it is important to carefully delineate the adjacent cranial nerves to ensure they do not fall in a high-dose region.31,41 Fractionated EBRT is associated with a 1.5- to 2-fold increased risk of CVA incidence and mortality, but once again whether this is technique or patient/disease specific remains unclear.54 Fewer than 5 cases in this literature reported on CVAs after SRS,55–57 of which 2 of the 3 series reported on patients with functioning adenomas treated to higher doses than NFAs. Currently no dose threshold exists for carotid artery stenosis, but for patients with significant baseline risk factors and comorbidities, this should be considered at the time of SRS planning.
Given the smaller treatment volumes and stereotactic techniques, the risk is likely to be lower than that of secondary tumors following EBRT for patients with pituitary tumors, which was approximately 2% at 10 years and 2.4% at 20 years, translating to a relative risk of secondary brain tumor development of 10.5 compared with the incidence in the normal population.58 This risk is substantially lower in the SRS population,59 and no studies evaluated in this meta-analysis reported secondary cancer events.
Visual Pathway Tolerance
Optimal consideration for single fraction SRS (vs HSRT or EBRT) is the location of the tumor in relation to the optic pathways. In patients with NFAs, a dose of 10–12 Gy to the anterior visual pathway is usually achievable and is in line with the dose tolerance reported by many institutional series (Table 2).60 Leavitt and colleagues reported a retrospective review of 222 patients treated with single fraction SRS to benign tumors in close proximity to the visual pathways and reported a neuropathy risk of 9% with maximum doses ≤12 Gy and 10% for those >12 Gy.61 However, it is important to note the tolerance of the visual pathways in the setting of prior radiotherapy, as cases of vision loss in the re-irradiation setting have been observed.22 Nevertheless, cases of visual deterioration have been observed even with doses of less than 8 Gy.29 Although the risk for visual dysfunction remains low, formal visual acuity and visual field testing should be performed at initial diagnosis, following resection, and at the time of SRS to ensure accurate assessment of pretreatment visual function.
New Onset Hypopituitarism
The most common delayed effects of radiotherapy for NFAs is the risk of new onset hypopituitarism. There are multiple factors associated with this risk, including patient age, pituitary function prior to resection, prior surgery and extent of disease resected, duration of follow-up, adenoma size and disease extension, and radiotherapy technique. This is a concern given the long natural history of patients with NFAs and the increased risk of death in those who develop secondary hypopituitarism compared with otherwise healthy patients.62 EBRT has been associated with a 30% risk of clinical hypopituitarism at 10 years, and 50% at 20 years.63 The pooled estimate of new hypopituitarism was 21% following single fraction SRS in this meta-analysis; however, a clear time point to compare this could not be established. Tumor volume has been associated with hypopituitarism in some studies,29,64,65 whereas others have not observed this relationship,65 as was the finding in this study.
There are multiple factors associated with post-SRS hypopituitarism, including visualization of the normal pituitary gland at the time of SRS target volume delineation, tumor extension outside of the cavernous sinus and sella, marginal prescription dose, and dose to the pituitary gland or infundibulum. Marek and colleagues reported the effect of marginal dose, with a 2% risk of hypopituitarism in 45 patients treated to a mean pituitary dose <15 Gy compared with 73% in those with a dose >15 Gy.66 In this series, the dose to the distal infundibulum was also associated with hypopituitarism with a maximum recommended dose of 17 Gy. Graffeo et al specifically evaluated dosimetric factors on 97 patients (57 of whom were diagnosed with NFAs) treated with single fraction SRS, and of the 27 patients (28%) in their series who developed posttreatment endocrine deficits, multivariable analysis found that male sex (hazard ratio [HR]: 2.38, P = 0.04), a smaller pituitary gland volume (HR: 0.99, P = 0.01), and increased mean gland dose (HR: 1.31, P < 0.001) were associated with posttreatment hypopituitarism.10 Given the various factors described in the literature, it is likely that the mean dose most closely approximates the dose received by most of the organ and, therefore, correlates best with long-term risk of hypopituitarism. On the other hand, the maximum dose to the infundibulum, either because of its function as a serial structure connecting hormonal secretion pathways between the hypothalamus and the pituitary or because of its small size, may be the most important variable to monitor.67 Suprasellar extension, which has been observed in some series to correlate with posttreatment hypopituitarism,68 likely leads to higher doses to the infundibulum and hypothalamus, and these structures should be delineated and monitored at the time of SRS planning. Baseline hormone levels should be obtained prior to surgery and prior to radiotherapy for comparison to post-SRS values.
One of the key limitations of the present review is that it comprises primarily retrospective cohort studies spanning long time intervals with limited long-term follow-up beyond 10 years. As patients were not treated on prospective observational study protocols, length of follow-up and posttreatment imaging and neuroendocrine evaluation was performed at varying intervals across studies. This limits evaluation of key variables, especially with lack of time-dependent analyses. Also, every effort was made to prevent overlap of data across studies, and if multi-institutional patient level data were available, these were given preference over individual studies as they would incorporate data from centers which did not always publish individual outcomes. However, given the multi-institutional nature of the data collection and analysis, fewer variables were reported on. Also, different multi-institutional reports had overlapping time periods of data collection and therefore were included in order to evaluate the maximum number of patients, with partial but unknown quantity of overlap with subsequent studies.
Given the key interest of the neuro-oncology community in the management of patients with NFAs, as highlighted by the vast number of commentaries, summaries, and reviews of the literature, it was felt timely and necessary to perform a meta-analysis and high-level overview of the role of SRS in the management of these tumors. Despite the aforementioned limitations of this study, key differences are important to highlight from prior works. First, approximately 25% of the reports included in this meta-analysis (9/35 manuscripts) were newly published since the last guidelines.69 Second, this report focused on SRS, instead of all types of radiation therapy, and provided a more detailed analysis of key variables regarding SRS-specific practice, study details, procedure details (dose/fractionation), and outcome variables. For example, in this report, we specifically reviewed each study in great detail to describe key variables not addressed in prior reports, such as the proportions of patients treated in the definitive versus adjuvant versus salvage setting, incidence of prior radiotherapy, description of individual technique details (dose/fractionation), tumor volume ranges, etc, which are important to consider in evaluating patients for SRS and are different for patients receiving conventionally fractionated radiotherapy. Third, the current study provides pooled estimates for both local control and hypopituitarism and, new to published literature, separated these outcomes by type of SRS, a specific and unique focus. Finally, this study analyzed SRS-specific toxicities and described them in detail throughout the report. Together, these key features of this study make this report the most recent study on the role of SRS for patients with NFAs with the most updated data on published outcomes.
Conclusion
From this meta-analysis and detailed review of the literature for NFAs, the ISRS provides key management and key treatment opinions for patients with NFAs (Table 3). To recapitulate, SRS is an effective treatment with 10-year outcome results for patients treated to doses of 14–16 Gy and promising 5-year results for radiobiologically equivalent HSRT schedules, with the long-term risks of new hypopituitarism and rare risk of CN or vascular injury and extremely low risk for radiation-induced malignancy.
Table 3.
Key opinions for treatment and management of non-functioning pituitary adenomas
| Recommendations for Treatment and Management of Non-Functioning Pituitary Adenomas (NFAs) |
| Patient Selection |
| 1. Patients with NFAs who are medically inoperable or refuse resection can be considered for SRS as the primary definitive treatment. |
| 2. After resection, patients with residual disease should be presented in a multidisciplinary setting where the risks and benefits of immediate adjuvant SRS, or observation with salvage SRS, should be reviewed in light of patient characteristics, disease extent, pathology for high-risk features, and imaging findings. |
| 3. Prior to SRS, patients should undergo comprehensive neurological, neuro-ophthalmologic, and neuroendocrine evaluations. |
| 4. For patients who have received prior external beam radiotherapy, a thorough review of the prior treatment records and doses received to nearby critical structures at risk should be evaluated by the treatment team. |
| Treatment |
| 1. A high-resolution volumetric treatment planning MRI, with at least a T1 post-gadolinium and axial T2 sequence, should be performed at the time of SRS to ensure accurate target volume delineation. |
| 2. Key at-risk structures important for consideration at the time of treatment include the hypothalamus, infundibulum, residual pituitary, optic pathway, and brainstem. |
| 3. Single fraction SRS is preferred to HSRT if constraints to nearby structures at risk can be met given the long-term control and toxicity data. |
| a. A prescription dose of 14–16 Gy is recommended for patients treated in the definitive setting. |
| b. A prescription dose of 14–16 Gy is recommended for patients with residual or recurrent disease. |
| c. HSRT (21 Gy in 3 fractions, 20 Gy in 4 fractions, or 25 Gy in 5 fractions) can be considered for patients with larger adenomas (>2–3 cm) or close to the optic apparatus; however, the lack of long-term (>10 year) tumor control data is acknowledged and patients must be consented appropriately in this context. |
| Treatment Outcomes |
| 1. Patients treated with SRS should undergo routine clinical follow-up, including neuro-ophthalmology and neuroendocrine visits, and imaging surveillance for at least 5 years. A schedule of every 6 months for the first year, annually for up to 5 years, and every 2 years thereafter is reasonable. Earlier follow-up can be considered based upon clinical events. |
| a. Tumor dimensions or volumetric assessments should be performed at each follow-up imaging time point using standardized response criteria. |
| b. Recurrent disease following SRS should be categorized as “in-field” or “out-of-field” recurrences and subsequent salvage treatments should be comprehensively recorded in the shared medical record. |
| 2. Treatment-related toxicities should be recorded and graded using standardized reporting criteria. |
| a. The development of new or worsening hypopituitarism should be defined as “biochemical” or “clinically significant.” |
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Conflict of interest statement. R. Kotecha: Elsevier, Elekta AB, Accuray Inc, Novocure. A. Sahgal: advisor/consultant with Abbvie, Merck, Roche, Varian (Medical Advisory Group), Elekta (Gamma Knife Icon), BrainLAB, and VieCure (Medical Advisory Board); Ex officio Board Member: International Stereotactic Radiosurgery Society (ISRS); Past educational seminars with Elekta AB, Accuray Inc, Varian (CNS Teaching Faculty), BrainLAB, Medtronic Kyphon; Research grant with Elekta AB; Travel accommodations/expenses by Elekta, Varian, BrainLAB; Dr. Sahgal also belongs to the Elekta MR Linac Research Consortium, Elekta Spine, Oligometastases and Linac Based SRS Consortia. I. Paddick: Elekta AB. No other authors have relevant disclosures.
Authorship statement. Conception and design: RK, AS, JS, SY, JS. Analysis: RK, MR, JS, SY. Critical review of manuscript: RK, AS, MR, ADS, LF, BP, ML, LM, IP, JR, JS, SY, JS.
Note: Presented in part at 14th International Stereotactic Radiosurgery Society Congress in Rio de Janeiro, Brazil, June 9–13, 2019.
Supplementary Material
References
- 1. Ezzat S, Asa SL, Couldwell WT, et al. The prevalence of pituitary adenomas: a systematic review. Cancer. 2004;101(3):613–619. [DOI] [PubMed] [Google Scholar]
- 2. Lau SKM, Patel K, Kim T, et al. Clinical efficacy and safety of surface imaging guided radiosurgery (SIG-RS) in the treatment of benign skull base tumors. J Neurooncol. 2017;132(2):307–312. [DOI] [PubMed] [Google Scholar]
- 3. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010;36(3):48. [Google Scholar]
- 5. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. [DOI] [PubMed] [Google Scholar]
- 6. Raudenbush SW. Analyzing effect sizes: random-effects models. In: Handbook of Research Synthesis and Meta-analysis. 2nd ed New York, NY: Russell Sage Foundation; 2009:295–315. [Google Scholar]
- 7. Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making. 2005;25(6):646–654. [DOI] [PubMed] [Google Scholar]
- 8. Fleiss JL, Gross AJ. Meta-analysis in epidemiology, with special reference to studies of the association between exposure to environmental tobacco smoke and lung cancer: a critique. J Clin Epidemiol. 1991;44(2):127–139. [DOI] [PubMed] [Google Scholar]
- 9. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- 10. Graffeo CS, Link MJ, Brown PD, Young WF Jr, Pollock BE. Hypopituitarism after single-fraction pituitary adenoma radiosurgery: dosimetric analysis based on patients treated using contemporary techniques. Int J Radiat Oncol Biol Phys. 2018;101(3):618–623. [DOI] [PubMed] [Google Scholar]
- 11. Narayan V, Mohammed N, Bir SC, et al. Long-term outcome of nonfunctioning and hormonal active pituitary adenoma after Gamma Knife radiosurgery. World Neurosurg. 2018;114:e824–e832. [DOI] [PubMed] [Google Scholar]
- 12. Pomeraniec IJ, Kano H, Xu Z, et al. Early versus late Gamma Knife radiosurgery following transsphenoidal surgery for nonfunctioning pituitary macroadenomas: a multicenter matched-cohort study. J Neurosurg. 2018;129(3):648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cohen-Inbar O, Xu Z, Lee CC, et al. Prognostic significance of corticotroph staining in radiosurgery for non-functioning pituitary adenomas: a multicenter study. J Neurooncol. 2017;135(1):67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zibar Tomšić K, Dušek T, Kraljević I, et al. Hypopituitarism after Gamma Knife radiosurgery for pituitary adenoma. Endocr Res. 2017;42(4):318–324. [DOI] [PubMed] [Google Scholar]
- 15. McTyre E, Helis CA, Farris M, et al. Emerging indications for fractionated Gamma Knife radiosurgery. Neurosurgery. 2017;80(2):210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sadik ZHA, Voormolen EHJ, Depauw PRAM, et al. Treatment of nonfunctional pituitary adenoma postoperative remnants: adjuvant or delayed Gamma Knife radiosurgery? World Neurosurg. 2017;100:361–368. [DOI] [PubMed] [Google Scholar]
- 17. Losa M, Spatola G, Albano L, et al. Frequency, pattern, and outcome of recurrences after Gamma Knife radiosurgery for pituitary adenomas. Endocrine. 2017;56(3):595–602. [DOI] [PubMed] [Google Scholar]
- 18. Puataweepong P, Dhanachai M, Hansasuta A, et al. The clinical outcome of hypofractionated stereotactic radiotherapy with cyberknife robotic radiosurgery for perioptic pituitary adenoma. Technol Cancer Res Treat. 2016;15(6):NP10–NP15. [DOI] [PubMed] [Google Scholar]
- 19. Hasegawa T, Shintai K, Kato T, Iizuka H. Stereotactic radiosurgery as the initial treatment for patients with nonfunctioning pituitary adenomas. World Neurosurg. 2015;83(6):1173–1179. [DOI] [PubMed] [Google Scholar]
- 20. Lee CC, Kano H, Yang HC, et al. Initial Gamma Knife radiosurgery for nonfunctioning pituitary adenomas. J Neurosurg. 2014;120(3):647–654. [DOI] [PubMed] [Google Scholar]
- 21. Liao HI, Wang CC, Wei KC, et al. Fractionated stereotactic radiosurgery using the Novalis system for the management of pituitary adenomas close to the optic apparatus. J Clin Neurosci. 2014;21(1):111–115. [DOI] [PubMed] [Google Scholar]
- 22. Zeiler FA, Bigder M, Kaufmann A, et al. Gamma Knife in the treatment of pituitary adenomas: results of a single center. Can J Neurol Sci. 2013;40(4):546–552. [DOI] [PubMed] [Google Scholar]
- 23. Sheehan JP, Starke RM, Mathieu D, et al. Gamma Knife radiosurgery for the management of nonfunctioning pituitary adenomas: a multicenter study. J Neurosurg. 2013;119(2):446–456. [DOI] [PubMed] [Google Scholar]
- 24. Chen YH, Chang SD, Ma HI, et al. Multisession CyberKnife radiosurgery for post-surgical residual and recurrent pituitary adenoma: preliminary result from one center. J Radiosurg SBRT. 2013;2(2):105–117. [PMC free article] [PubMed] [Google Scholar]
- 25. El-Shehaby AM, Reda WA, Tawadros SR, Abdel Karim KM. Low-dose Gamma Knife surgery for nonfunctioning pituitary adenomas. J Neurosurg. 2012;117(Suppl):84–88. [DOI] [PubMed] [Google Scholar]
- 26. Runge MJ, Maarouf M, Hunsche S, et al. LINAC-radiosurgery for nonsecreting pituitary adenomas. Long-term results. Strahlenther Onkol. 2012;188(4):319–325. [DOI] [PubMed] [Google Scholar]
- 27. Wilson PJ, De-Loyde KJ, Williams JR, Smee RI. A single centre’s experience of stereotactic radiosurgery and radiotherapy for non-functioning pituitary adenomas with the linear accelerator (Linac). J Clin Neurosci. 2012;19(3):370–374. [DOI] [PubMed] [Google Scholar]
- 28. Iwata H, Sato K, Tatewaki K, et al. Hypofractionated stereotactic radiotherapy with CyberKnife for nonfunctioning pituitary adenoma: high local control with low toxicity. Neuro Oncol. 2011;13(8):916–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Park KJ, Kano H, Parry PV, et al. Long-term outcomes after Gamma Knife stereotactic radiosurgery for nonfunctional pituitary adenomas. Neurosurgery. 2011;69(6):1188–1199. [DOI] [PubMed] [Google Scholar]
- 30. Castro DG, Cecílio SA, Canteras MM. Radiosurgery for pituitary adenomas: evaluation of its efficacy and safety. Radiat Oncol. 2010;5:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hayashi M, Chernov M, Tamura N, et al. Gamma Knife robotic microradiosurgery of pituitary adenomas invading the cavernous sinus: treatment concept and results in 89 cases. J Neurooncol. 2010;98(2):185–194. [DOI] [PubMed] [Google Scholar]
- 32. Cho CB, Park HK, Joo WI, Chough CK, Lee KJ, Rha HK. Stereotactic radiosurgery with the cyberknife for pituitary adenomas. J Korean Neurosurg Soc. 2009;45(3):157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Killory BD, Kresl JJ, Wait SD, Ponce FA, Porter R, White WL. Hypofractionated CyberKnife radiosurgery for perichiasmatic pituitary adenomas: early results. Neurosurgery. 2009;64(2 Suppl):A19–A25. [DOI] [PubMed] [Google Scholar]
- 34. Höybye C, Rähn T. Adjuvant Gamma Knife radiosurgery in non-functioning pituitary adenomas: low risk of long-term complications in selected patients. Pituitary. 2009;12(3):211–216. [DOI] [PubMed] [Google Scholar]
- 35. Kobayashi T. Long-term results of stereotactic Gamma Knife radiosurgery for pituitary adenomas. Specific strategies for different types of adenoma. Prog Neurol Surg. 2009;22:77–95. [DOI] [PubMed] [Google Scholar]
- 36. Pollock BE, Cochran J, Natt N, et al. Gamma Knife radiosurgery for patients with nonfunctioning pituitary adenomas: results from a 15-year experience. Int J Radiat Oncol Biol Phys. 2008;70(5):1325–1329. [DOI] [PubMed] [Google Scholar]
- 37. Liscak R, Vladyka V, Marek J, Simonova G, Vymazal J. Gamma Knife radiosurgery for endocrine-inactive pituitary adenomas. Acta Neurochir (Wien). 2007;149(10):999–1006; discussion 1006. [DOI] [PubMed] [Google Scholar]
- 38. Kajiwara K, Saito K, Yoshikawa K, et al. Image-guided stereotactic radiosurgery with the CyberKnife for pituitary adenomas. Minim Invasive Neurosurg. 2005;48(2):91–96. [DOI] [PubMed] [Google Scholar]
- 39. Iwai Y, Yamanaka K, Yoshioka K. Radiosurgery for nonfunctioning pituitary adenomas. Neurosurgery. 2005;56(4):699–705; discussion 699. [DOI] [PubMed] [Google Scholar]
- 40. Muacevic A, Uhl E, Wowra B. Gamma Knife radiosurgery for nonfunctioning pituitary adenomas. Acta Neurochir Suppl. 2004;91:51–54. [DOI] [PubMed] [Google Scholar]
- 41. Petrovich Z, Yu C, Giannotta SL, Zee CS, Apuzzo ML. Gamma Knife radiosurgery for pituitary adenoma: early results. Neurosurgery. 2003;53(1):51–59; discussion 59–61. [DOI] [PubMed] [Google Scholar]
- 42. Wowra B, Stummer W. Efficacy of Gamma Knife radiosurgery for nonfunctioning pituitary adenomas: a quantitative follow up with magnetic resonance imaging-based volumetric analysis. J Neurosurg. 2002;97(5 Suppl):429–432. [DOI] [PubMed] [Google Scholar]
- 43. Mokry M, Ramschak-Schwarzer S, Simbrunner J, Ganz JC, Pendl G. A six year experience with the postoperative radiosurgical management of pituitary adenomas. Stereotact Funct Neurosurg. 1999;72(Suppl 1):88–100. [DOI] [PubMed] [Google Scholar]
- 44. Martinez R, Bravo G, Burzaco J, Rey G. Pituitary tumors and Gamma Knife surgery. Clinical experience with more than two years of follow-up. Stereotact Funct Neurosurg. 1998;70(Suppl 1):110–118. [DOI] [PubMed] [Google Scholar]
- 45. Laws ER, Sheehan JP, Sheehan JM, Jagnathan J, Jane JA Jr, Oskouian R. Stereotactic radiosurgery for pituitary adenomas: a review of the literature. J Neurooncol. 2004;69(1-3):257–272. [DOI] [PubMed] [Google Scholar]
- 46. Losa M, Valle M, Mortini P, et al. Gamma Knife surgery for treatment of residual nonfunctioning pituitary adenomas after surgical debulking. J Neurosurg. 2004;100(3):438–444. [DOI] [PubMed] [Google Scholar]
- 47. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 48. Mingione V, Yen CP, Vance ML, et al. Gamma surgery in the treatment of nonsecretory pituitary macroadenoma. J Neurosurg. 2006;104(6):876–883. [DOI] [PubMed] [Google Scholar]
- 49. Xu Z, Ellis S, Lee CC, et al. Silent corticotroph adenomas after stereotactic radiosurgery: a case-control study. Int J Radiat Oncol Biol Phys. 2014;90(4):903–910. [DOI] [PubMed] [Google Scholar]
- 50. Iwata H, Shibamoto Y, Murata R, et al. Estimation of errors associated with use of linear-quadratic formalism for evaluation of biologic equivalence between single and hypofractionated radiation doses: an in vitro study. Int J Radiat Oncol Biol Phys. 2009;75(2):482–488. [DOI] [PubMed] [Google Scholar]
- 51. Park P, Chandler WF, Barkan AL, et al. The role of radiation therapy after surgical resection of nonfunctional pituitary macroadenomas. Neurosurgery. 2004;55(1):100–106; discussion 106–107. [DOI] [PubMed] [Google Scholar]
- 52. van den Bergh AC, van den Berg G, Schoorl MA, et al. Immediate postoperative radiotherapy in residual nonfunctioning pituitary adenoma: beneficial effect on local control without additional negative impact on pituitary function and life expectancy. Int J Radiat Oncol Biol Phys. 2007;67(3):863–869. [DOI] [PubMed] [Google Scholar]
- 53. Pomeraniec IJ, Dallapiazza RF, Xu Z, Jane JA Jr, Sheehan JP. Early versus late Gamma Knife radiosurgery following transsphenoidal resection for nonfunctioning pituitary macroadenomas: a matched cohort study. J Neurosurg. 2016;125(1):202–212. [DOI] [PubMed] [Google Scholar]
- 54. Brada M, Ashley S, Ford D, Traish D, Burchell L, Rajan B. Cerebrovascular mortality in patients with pituitary adenoma. Clin Endocrinol (Oxf). 2002;57(6):713–717. [DOI] [PubMed] [Google Scholar]
- 55. Lim YL, Leem W, Kim TS, Rhee BA, Kim GK. Four years’ experiences in the treatment of pituitary adenomas with Gamma Knife radiosurgery. Stereotact Funct Neurosurg. 1998;70(Suppl 1):95–109. [DOI] [PubMed] [Google Scholar]
- 56. Muramatsu J, Yoshida M, Shioura H, et al. Clinical results of LINAC-based stereotactic radiosurgery for pituitary adenoma. Nihon Igaku Hoshasen Gakkai Zasshi. 2003;63(5):225–230. [PubMed] [Google Scholar]
- 57. Pollock BE, Nippoldt TB, Stafford SL, Foote RL, Abboud CF. Results of stereotactic radiosurgery in patients with hormone-producing pituitary adenomas: factors associated with endocrine normalization. J Neurosurg. 2002;97(3):525–530. [DOI] [PubMed] [Google Scholar]
- 58. Minniti G, Traish D, Ashley S, Gonsalves A, Brada M. Risk of second brain tumor after conservative surgery and radiotherapy for pituitary adenoma: update after an additional 10 years. J Clin Endocrinol Metab. 2005;90(2):800–804. [DOI] [PubMed] [Google Scholar]
- 59. Wolf A, Naylor K, Tam M, et al. Risk of radiation-associated intracranial malignancy after stereotactic radiosurgery: a retrospective, multicentre, cohort study. Lancet Oncol. 2019;20(1):159–164. [DOI] [PubMed] [Google Scholar]
- 60. Milano MT, Grimm J, Soltys SG, et al. Single- and multi-fraction stereotactic radiosurgery dose tolerances of the optic pathways. [published online ahead of print Jan 31, 2018]. Int J Radiat Oncol Biol Phys. pii:S0360-3016(18)30125-1. 2018. doi:10.1016/j.ijrobp.2018.01.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Leavitt JA, Stafford SL, Link MJ, Pollock BE. Long-term evaluation of radiation-induced optic neuropathy after single-fraction stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2013;87(3):524–527. [DOI] [PubMed] [Google Scholar]
- 62. Tomlinson JW, Holden N, Hills RK, et al. Association between premature mortality and hypopituitarism. West Midlands Prospective Hypopituitary Study Group. Lancet. 2001;357(9254):425–431. [DOI] [PubMed] [Google Scholar]
- 63. Brada M, Rajan B, Traish D, et al. The long-term efficacy of conservative surgery and radiotherapy in the control of pituitary adenomas. Clin Endocrinol (Oxf). 1993;38(6):571–578. [DOI] [PubMed] [Google Scholar]
- 64. Starke RM, Williams BJ, Jane JA Jr, Sheehan JP. Gamma Knife surgery for patients with nonfunctioning pituitary macroadenomas: predictors of tumor control, neurological deficits, and hypopituitarism. J Neurosurg. 2012;117(1):129–135. [DOI] [PubMed] [Google Scholar]
- 65. Gopalan R, Schlesinger D, Vance ML, Laws E, Sheehan J. Long-term outcomes after Gamma Knife radiosurgery for patients with a nonfunctioning pituitary adenoma. Neurosurgery. 2011;69(2):284–293. [DOI] [PubMed] [Google Scholar]
- 66. Marek J, Jezková J, Hána V, et al. Is it possible to avoid hypopituitarism after irradiation of pituitary adenomas by the Leksell Gamma Knife? Eur J Endocrinol. 2011;164(2):169–178. [DOI] [PubMed] [Google Scholar]
- 67. Vladyka V, Liscak R, Novotny J Jr, Marek J, Jezkova J. Radiation tolerance of functioning pituitary tissue in Gamma Knife surgery for pituitary adenomas. Neurosurgery. 2003;52(2):309–316; discussion 316-307. [DOI] [PubMed] [Google Scholar]
- 68. Xu Z, Lee Vance M, Schlesinger D, Sheehan JP. Hypopituitarism after stereotactic radiosurgery for pituitary adenomas. Neurosurgery. 2013;72(4):630–637; 636–637. [DOI] [PubMed] [Google Scholar]
- 69. Sheehan J, Lee CC, Bodach ME, et al. Congress of neurological surgeons systematic review and evidence-based guideline for the management of patients with residual or recurrent nonfunctioning pituitary adenomas. Neurosurgery. 2016;79(4):E539–E540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.