Abstract
BACKGROUND
Few biological markers that allow evaluation of the effects of air pollution on human health have been identified. This study evaluated the association of serum C-reactive protein (CRP) concentration in children with their respiratory symptoms and air pollution.
METHODS
Respiratory symptoms and serum concentrations of CRP were examined in 2,094 school children living in 3 communities with different concentrations of air pollutants in Chiba Prefecture, Japan in 2001. The relationships between serum CRP concentration and sex, age, respiratory symptoms, and various environmental factors were analyzed.
RESULTS
Serum CRP concentration decreased with age, and was significantly higher both in children who were bottle-fed in infancy and whose mothers smoked. Children with wheeze had significantly higher serum CRP concentration than those without wheeze. After adjustment for potential confounding factors, increased serum CRP concentrations of the 90th percentile (1.4 mg/L) or above were significantly associated with atmospheric concentration of suspended particulate matter (SPM) (odds ratio [OR] =1.49 for the range of observed concentrations, 95% confidence interval [Cl]: 1.07-2.06) and sulfur dioxide (SO2) (OR =1.45, 95% Cl: 1.04-2.03). In a two-pollutant model including SPM and nitrogen dioxide (NO2) concentrations, increased serum CRP concentrations were also associated with SPM (OR =1.94, 95% Cl: 1.08-3.50), but no such association was found with NO2 (OR =0.62, 95% Cl: 0.26-1.48).
CONCLUSION
Serum CRP concentration is related to wheezing and the degree of air pollution. Because the concentrations of air pollutants are highly correlated, it is difficult to elaborate on which pollutant has a stronger effect on serum CRP concentrations.
Key words: C-reactive protein, Air pollution, Suspended particulate matter (SPM), Asthma, Wheezing
The concentrations of air pollutants, represented by suspended particulate matter (SPM) and nitrogen dioxide (NO2), have increased due to the increase in motor vehicle traffic in urban areas in Japan.1 The effects of such air pollution on human health become of major concern. For children, most epidemiologic studies have focused on the effect of air pollution on respiratory symptoms or disease.2-4 There has long been a need for sensitive biomarkers to objectively evaluate the chronic effects of air pollution on human health as early as possible, but none has yet been identified.5,6 Previously, we reported an association between serum concentrations of acute phase proteins or hyaluronate in children and concentrations of air pollutants,7,8 but assays for these serum factors have not been validated as biomarkers for the effects of air pollution.
C-reactive protein (CRP) in serum has been widely used as a marker for infectious disease.9 A population-based study in Finland reported the association of serum CRP concentration with bronchial asthma.10 Because high-sensitivity tests of CRP are now available, it is possible to detect slight inflammatory conditions or local tissue damage that would have been undetectable by previous methods.11 Serum CRP concentration has been reported to be useful for prediction of cardiovascular disease,12,13 because such concentrations have been associated with atherosclerosis. Furthermore, acute effects of particulate air pollution on serum CRP concentration have been previously documented in adults.14-18 Although the acute effect of air pollution on serum CRP has also been observed in healthy young people,19 there are no reported studies of the association between serum CRP concentration and chronic exposure to air pollution. Therefore, the author conducted a cross-sectional study in 3 communities with different concentrations of air pollutants, and measured serum CRP concentration in school children. The levels of serum CRP were examined for association with sex, age, respiratory symptoms, and various environmental factors.
METHODS
Study Population
The study population consisted of the entire children (2,540 pupils, grades 1-6, aged 6-12 years) attending 6 elementary schools from 3 different communities in Chiba Prefecture, Japan (Figure). Of these schools, three were in an urban district next to the Tokyo metropolitan area (Ichikawa), and located near major roads. The distance between a school and a road ranged from 50 to 700 m. Of the other schools, one was located in the interior of the Boso Peninsula (Obitsu) and two were adjacent to the coast of Tokyo Bay (Kimitsu). There were no major roads near these three schools.
Figure. Location of study schools and major trunk roads in Chiba Prefecture, Japan.
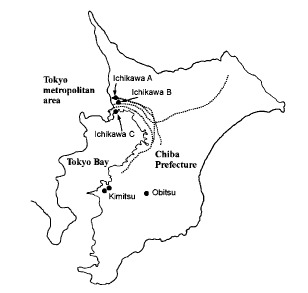
The dotted lines show major trunk roads.
Air Pollution Measurements
Air pollutants are monitored continuously by the local authorities of the Chiba Prefectural Government. In Japan, particulate air pollution is assessed based on the concentration of SPM, which is the fraction of particles with a diameter less than 10 µm. The usual concentration of SPM corresponds approximately to that of particles passing an inlet with 50% cut-off efficacy using an aerodynamic diameter of 7 µm (PM7).20 The average concentrations of SPM, NO2, and sulfur dioxide (SO2) for the 3-year period from 1998 through 2000, measured at monitoring stations proximal to the study schools, are shown in Table 1. The distance between a school and a monitoring station ranged from 0 to 1.4 km. All concentrations measured were lowest in Obitsu. The concentrations in Kimitsu, which is located in an industrial area, were moderately high. All 3 stations in Ichikawa, which has heavy motor vehicle traffic, had the highest concentrations of air pollutants. The concentrations of air pollutants did not change substantially during the study period. The correlation between SPM and NO2 was high (correlation coefficient = 0.86). The concentration of SO2 also showed very strong correlation with SPM and NO2 (correlation coefficients = 0.99 and 0.92, respectively).
Table 1. Average concentrations* of atmospheric air pollutants in the study communities.
| SPM (µg/m3) Mean (SD) |
NO2 (ppb) Mean (SD) |
SO2 (ppb) Mean (SD) |
|
| Ichikawa (School A) | 41.7 (3.2) | 25.3 (2.5) | 6.3 (0.6) |
| Ichikawa (School B) | 42.7 (2.9) | 23.7 (1.5) | 6.3 (0.6) |
| Ichikawa (School C) | 40.3 (6.5) | 29.0 (2.0) | 6.3 (1.5) |
| Kimitsu | 28.0 (1.0) | 19.0 (1.0) | 4.7 (1.2) |
| Obitsu | 26.7 (2.5) | 11.7 (2.1) | 4.3 (0.6) |
*: Data are shown as the mean values (SD) of the annual average concentrations of air pollutants for the 3 year period 1998-2000, measured at ambient air monitoring stations located near the study schools.
SPM: Suspended particulate matter
NO2: Nitrogen dioxide
SO2: Sulfur dioxide
ppb: parts per billion
SD: Standard deviation
Data Collection, Blood Sampling, and Laboratory Measurements
School children were examined in October 2001 using a standard respiratory symptom questionnaire, the modified Japanese version of ATS-DLD-78-C.21 The questionnaires were distributed through their schools and completed by either parents or guardians. Improperly completed questionnaires were returned to the appropriate individuals in order to obtain complete information. Based on replies to the questionnaire, children who had 2 or more past episodes of wheezing accompanied by dyspnea, and who had also experienced asthmatic attacks or the need for any medical treatment for asthma by physicians in the previous 2 years, were classified as having asthma. Children who had 2 or more wheezing episodes in the previous 2 years without a history of asthma were classified as having wheeze. Questions concerning the presence of smokers in the family and feeding methods in infancy were also included in the questionnaire.
Blood samples were collected between October and November 2001 from children for whom written consent had been obtained from their parents or guardians. Children with signs of acute inflammation, such as a cold or fever, were excluded from the present study. The collected blood samples were centrifuged on the same day and concentrations of total IgE and CRP in the serum were determined using latex-enhanced immunonephelometric assays on a BN II analyzer (Dade Behring, Marburg, Germany). The minimum detection level of CRP was 0.2 mg/L.22
Data Analyses
The serum concentrations of CRP were distributed approximately log normally.23 These values were therefore converted to logarithms for analysis, and the results expressed as geometric means with 95% confidence intervals (CIs). The minimum detection limit of the assay (0.2 mg/dL) was used as the value for undetectable CRP level in the calculation of geometric means. Data were compared according to the differences in the following factors: sex, age, feeding methods in infancy, smoking habits of the family, asthma or wheezing. Serum IgE concentration was used as a marker for predisposition to atopy. The serum CRP concentration was compared between children with serum IgE concentrations of 250 IU/mL or greater and those with concentrations below 250 IU/mL, as reported in a previous report.7
Because the serum CRP concentration was undetectable among more than half the children, the concentrations were then dichotomized at the 90th percentile (1.4 mg/L), and the prevalence of a serum CRP concentration of 1.4 mg/L or above was compared in relation to various factors. To evaluate the effects of each factor, multiple logistic regression analyses were performed using the high serum CRP concentrations as a binary outcome. The 3-year average concentrations of air pollutants in the communities where the children lived were included in the models as continuous variables. Statistical analyses were performed using SPSS® software (SPSS Inc., Chicago, IL, USA).
RESULTS
Questionnaires were collected from 2,501 children (98.5%), blood samples were collected from 2,097 children (82.6%), and useable data for both were obtained from 2,094 children (82.4%). The characteristics of the study children are shown according to study communities in Table 2. There were no differences in sex, age, asthma or wheezing, or serum IgE concentrations in the children from the 3 communities. The proportion of bottle-fed children was higher in Obitsu than in other communities, while that of breast-fed children was higher in Ichikawa. However, the proportion of mixed-fed children was similar among the 3 communities. The proportion of smoking mothers in Obitsu was low. In contrast, the proportion of family members who were smokers, other than mothers, was significantly higher in Obitsu than in other communities.
Table 2. Demographics and health characteristics (%) of the study subjects, by community.
| Ichikawa (n = 1009) |
Kimitsu (n = 746) |
Obitsu (n = 339) |
p value | |
| Sex | ||||
| Male | 50.0 | 52.5 | 51.6 | 0.577 |
| Female | 50.0 | 47.5 | 48.4 | |
| Age (year) | ||||
| 6-7 | 22.2 | 26.1 | 22.1 | 0.227 |
| 8-10 | 49.1 | 47.7 | 46.9 | |
| 11-12 | 28.7 | 26.1 | 31.0 | |
| Mean age (years) | 9.19 | 9.02 | 9.24 | 0.068 |
| (SD) | (1.79) | (1.83) | (1.79) | |
| Feeding method in infancy | ||||
| Bottle | 18.2 | 26.5 | 36.6 | <0.001 |
| Breast | 34.9 | 25.7 | 18.3 | |
| Mixed | 46.9 | 47.7 | 45.1 | |
| Familial smoking habits | ||||
| Mother smokes | 18.3 | 21.7 | 11.8 | <0.001 |
| Others smoke | 40.1 | 44.4 | 56.9 | |
| No one smokes | 41.5 | 33.9 | 31.3 | |
| Respiratory symptoms | ||||
| Wheezing | 6.0 | 7.1 | 4.4 | 0.292 |
| Asthma | 8.4 | 8.0 | 6.2 | |
| No symptoms | 85.5 | 84.9 | 89.4 | |
| Serum IgE concentration | ||||
| 0-249 IU/mL | 69.8 | 69.4 | 65.8 | 0.37 |
| ≥ 250 IU/mL | 30.2 | 30.6 | 34.2 |
SD : Standard deviation
Table 3 shows the results of univariate analysis of serum CRP concentration and the prevalence of a serum CRP concentration of the 90th percentile (1.4 mg/L) or above, according to sex, age, feeding method in infancy, familial smoking habits, respiratory symptoms, serum IgE concentrations, and study communities. The serum CRP concentration was highest in the youngest children (6-7 years), and decreased with age. The prevalence of a serum CRP concentration of the 90th percentile or above was highest in children aged 6-7 years. Children who were bottle-fed in infancy had significantly higher serum CRP concentrations than children who were either breast- or mixed-fed. The serum CRP concentration in children whose mothers were smokers was significantly higher than in children without smokers in the family. The smoking habits of family members other than mothers were not significantly related to serum CRP concentration. The serum CRP concentration in children with wheeze was higher than in children without wheeze. The prevalence of a serum CRP concentration of the 90th percentile or above was also higher in children with wheeze. Children with asthma had higher serum CRP concentrations than those without, but the difference was not statistically significant. Analysis of serum CRP concentration according to study community revealed that the concentrations were highest in Ichikawa among the three communities, followed by Kimitsu, and then Obitsu, however these differences were not statistically significant. The differences in serum CRP concentration according to sex and serum IgE concentrations were not statistically significant.
Table 3. Univariate analysis of serum C-reactive protein (CRP) concentration (mg/L) and prevalence (%) of CRP ≥ the 90th percentile (1.4 mg/L) in children relative to various factors.
| n | Geometric mean (mg/L) | CRP ≥ 1.4 mg/L | ||||
| (95% CI) | p value | % | p value | |||
| Sex | ||||||
| Male | 1072 | 0.36 | (0.34-0.38) | 0.349 | 10.5 | 0.619 |
| Female | 1022 | 0.35 | (0.33-0.37) | 9.9 | ||
| Age (years) | ||||||
| 6-7 | 494 | 0.46 | (0.42-0.51) | <0.001 | 16.4 | <0.001 |
| 8-10 | 1011 | 0.34* | (0.32-0.36) | 9.7 | ||
| 11-12 | 589 | 0.29*,† | (0.27-0.31) | 5.9 | ||
| Feeding method in infancy | ||||||
| Bottle | 506 | 0.4 | (0.36-0.44) | 0.002 | 13.8 | 0.009 |
| Breast | 606 | 0.34‡ | (0.32-0.37) | 8.9 | ||
| Mixed | 982 | 0.34§ | (0.32-0.35) | 9.2 | ||
| Familial smoking habits | ||||||
| Mother smokes | 387 | 0.41 | (0.37-0.46) | <0.001 | 14.2 | 0.014 |
| Others smoke | 929 | 0.35|| | (0.33-0.37) | 9.7 | ||
| No one smokes | 778 | 0.33|| | (0.31-0.35) | 8.9 | ||
| Respiratory symptoms | ||||||
| Wheezing | 129 | 0.47 | (0.38-0.57) | <0.001 | 17.8 | 0.002 |
| Asthma | 166 | 0.41 | (0.35-0.47) | 13.9 | ||
| No symptoms | 1799 | 0.34¶ | (0.33-0.36) | 9.3 | ||
| Serum IgE concentration | ||||||
| 0-249 IU/mL | 1445 | 0.35 | (0.33-0.37) | 0.475 | 10.1 | 0.794 |
| ≥ 250 IU/mL | 649 | 0.36 | (0.33-0.39) | 10.5 | ||
| Study community | ||||||
| Ichikawa | 1009 | 0.36 | (0.34-0.38) | 0.483 | 11.3 | 0.259 |
| Kimitsu | 746 | 0.35 | (0.33-0.37) | 9.5 | ||
| Obitsu | 339 | 0.34 | (0.31-0.37) | 8.6 | ||
The significance of the differences in serum CRP concentration among the groups was evaluated by Tukey's method.
* : p<0.01 compared with children 6-7 years old.
† : p<0.01 compared with children 8-10 years old.
‡ : p<0.05, § : p<0.01 compared with children who were bottle-fed in infancy.
|| : p<0.01 compared with children whose mothers smoke.
¶ : p<0.01 compared with children with wheeze.
CI : confidence interval
The effects of various factors on serum CRP concentration of the 90th percentile or above were analyzed using logistic regression analysis (Table 4). The adjusted odds ratio (OR) of high serum CRP concentration for age was significantly below one. High serum CRP concentration was also significantly associated with bottle-feeding in infancy, maternal smoking habit and wheezing. The association of high serum CRP concentration with SPM concentration in the communities where the children lived was significant (OR = 1.49 for the range of observed concentrations, 95% CI: 1.07-2.06). High serum CRP concentration was also associated with SO2 concentration (OR = 1.45 for the range of observed concentrations, 95% CI: 1.04-2.03), but the association with NO2 concentration was not statistically significant (OR = 1.41 for the range of observed concentrations, 95% CI: 0.87-2.28). In a two-pollutant model including SPM and NO2 concentrations, high serum CRP concentration was also significantly associated with SPM (OR = 1.94 for the range of observed concentrations, 95% CI: 1.08-3.50), but no such association was found with NO2 (OR = 0.62 for the range of observed concentrations, 95% CI: 0.26-1.48). Because SO2 concentration strongly correlated with SPM and NO2, two-pollutant models including SO2 concentration were not analyzed.
Table 4. Adjusted odds ratios (ORs)* and 95% confidence intervals (CIs) for various factors on C-reactive protein (CRP) ≥ the 90th percentile (1.4 mg/L).
| A single-pollutant model including SPM concentration |
A single-pollutant model including NO2 concentration |
A single-pollutant model including SO2 concentration |
A two-pollutant model including SPM and NO2 concentrations |
|||||
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Age (1 year increase) | 0.83 (0.76-0.90) | <0.001 | 0.83 (0.77-0.90) | <0.001 | 0.83 (0.76-0.90) | <0.001 | 0.83 (0.76-0.90) | <0.001 |
| Sex (females vs. males) | 0.92 (0.69-1.23) | 0.581 | 0.93 (0.70-1.24) | 0.621 | 0.92 (0.69-1.24) | 0.594 | 0.92 (0.69-1.23) | 0.562 |
| Bottle-fed | 1.67 (1.22-2.28) | 0.001 | 1.63 (1.19-2.23) | 0.002 | 1.66 (1.22-2.27) | 0.001 | 1.65 (1.21-2.26) | 0.002 |
| Maternal smoking | 1.48 (1.06-2.07) | 0.023 | 1.47 (1.05-2.06) | 0.024 | 1.48 (1.06-2.07) | 0.023 | 1.49 (1.06-2.08) | 0.021 |
| Serum IgE concentration (≥ 250 IU/mL vs. 0-249 IU/mL) |
1.09 (0.77-1.53) | 0.624 | 1.09 (0.78-1.53) | 0.619 | 1.09 (0.77-1.53) | 0.621 | 1.09 (0.77-1.53) | 0.628 |
| Respiratory symptoms | ||||||||
| Wheezing | 1.85 (1.11-3.08) | 0.018 | 1.84 (1.11-3.06) | 0.018 | 1.85 (1.11-3.07) | 0.018 | 1.86 (1.12-3.10) | 0.017 |
| Asthma | 1.58 (0.95-2.63) | 0.078 | 1.59 (0.95-2.64) | 0.076 | 1.58 (0.95-2.63) | 0.077 | 1.58 (0.95-2.63) | 0.078 |
| No symptoms | 1.00 (referene) | 1.00 (referene) | 1.00 (referene) | 1.00 (referene) | ||||
| SPM (range: 26.7-42.7 µg/m3) | 1.49 (1.07-2.06) | 0.017 | - | - | 1.94 (1.08-3.50) | 0.027 | ||
| NO2 (range: 11.7-29.0 ppb) | - | 1.41 (0.87-2.28) | 0.164 | - | 0.62 (0.26-1.48) | 0.287 | ||
| SO2 (range: 4.3-6.3 ppb) | - | - | 1.45 (1.04-2.03) | 0.031 | - | |||
Two-pollutant models including SO2 concentration were not analyzed, because the SO2 concentration very strongly correlated with SPM and NO2 (correlation coefficients = 0.99 and 0.92, respectively).
*: ORs were adjusted for all variables using each logistic regression model
SPM: Suspended particulate matter
NO2: Nitrogen dioxide
SO2: Sulfur dioxide
ppb: parts per billion
In models without respiratory symptoms and /or serum IgE concentration, the results were essentially the same (data not shown).
DISCUSSION
CRP is one of the major acute-phase proteins in humans. It is produced mainly by the liver, and its production is regulated by cytokines, including interleukin-6.9 The production of CRP is enhanced when the complement system is activated by inflammatory reactions that accompany bacterial infections or tissue injury in vivo.24 Therefore, serum CRP concentration has frequently been used as a marker for inflammation.9
The author and coworkers previously reported that the serum concentrations of the acute-phase proteins C3c and C4 in children reflect their exposure level to air pollutants.8 In addition, the serum C3c concentration in boys is significantly increased by exposure to environmental tobacco smoke.25 In these previous studies, children with symptoms of a cold or fever were excluded to eliminate the effects of acute inflammation. Therefore, the serum CRP concentration in most children was below the detection level of 3.0 mg/L. A newly available assay for measuring CRP concentration has a detection level of 0.2 mg/dL, and has been found to be useful for early detection of infectious disease in newborns and immature infants.9 This serum CRP assay is considered to be particularly useful for evaluation of mild chronic inflammation in apparently healthy individuals.11,24
In the present study, the author conducted a cross-sectional study in 3 communities with different concentrations of air pollutants, and examined the serum CRP concentration in children. Serum CRP concentration differed significantly according to age, feeding method in infancy, maternal smoking habit, and wheezing. There were differences in feeding method in infancy and maternal smoking habit among the study communities. Multiple logistic regression analyses adjusted for these potential confounding factors showed that serum CRP concentrations of the 90th percentile or above were significantly associated with SPM concentration in each community. These findings suggest that air pollution affects serum CRP concentration in children, and are consistent with the results of previous reports showing an association between air pollution and concentrations of serum acute-phase proteins.8 Children with signs of acute inflammation were excluded from the present study, eliminating the possibility that reported differences were due to an epidemic of inflammatory diseases. In multivariate models without respiratory symptoms and/or serum IgE concentration, the associations between air pollutants and serum CRP concentration were essentially the same.
Serum CRP concentration is associated with factors such as age, obesity, and smoking habit.24,26 In chronic inflammation, a slight elevation in serum CRP concentration can persist.9 Therefore, serum CRP has been considered a useful marker for prediction of cardiac infarction or for evaluation of inflammation of blood vessels due to atherosclerosis in adults.12 A study in elderly people showed that serum CRP concentration increases with age, because older people are subject to the effects of low grade inflammation due to chronic disease.26 Adults with serum CRP concentrations of 3 mg/L or above are considered at high risk for cardiovascular diseases.27 The serum CRP concentration is reportedly lower in children than adults.28 In the present study, the concentrations were then dichotomized at the 90th percentile (1.4 mg/L). Serum CRP concentration was highest in the youngest children, and decreased with age among school children under 12 years old. Because younger children easily contract respiratory infections,29 low grade inflammation due to subclinical respiratory conditions may influence serum CRP concentration in young children.
The serum CRP concentration in children with wheeze was significantly higher than in children without wheeze. A population-based study in Finland reported that the prevalence of asthma increased gradually with increasing serum CRP concentration.10 Inflammation in the respiratory tract is thought to play an important role in the pathological conditions of wheezing and asthma.30 Wheezing is often caused by a viral infection.31 In the present study, the serum CRP concentration was slightly higher in children with asthma than those without, but the difference was not significant, possibly because most asthmatic children were asymptomatic when examined. An allergic predisposition, accompanied by increases in serum IgE concentration, is thought to be important for the development of the pathological states of childhood asthma. However, the author believes that no direct relationship exists between allergies and serum CRP concentration, because no association was found between serum IgE and CRP concentration.
The serum CRP concentration in children with mothers who smoked was significantly higher than those in children with non-smoking mothers. The serum CRP concentration in smokers is higher than in non-smokers.32 The author previously reported that concentrations of acute-phase proteins in children were elevated upon exposure to environmental tobacco smoke, and suggested the possibility that passive smoking may lead to slight inflammation in children.25 In the present study, the effects of smoking by family members other than mothers, i.e., mainly fathers, were not detected. This may reflect the lack of opportunity for fathers to smoke when with their children.
The complement activities of children who were bottle-fed in infancy have been reported to be high.33 Children who were both bottle- and breast-fed have been reported to have higher CRP concentrations than those who were only breast-fed.34 In the present study, children who were only bottle-fed had higher serum CRP concentrations than those who were only breast-fed or those who received mixed-feeding. The reason for this is not clear, but might be related to the protective effects of breast-feeding against infection during infancy.35
No significant difference in serum CRP concentration according to sex was observed. A study based on 3605 subjects found no differences in serum CRP concentration by sex between 5-39 years of age.26 Cook et al34 determined a relationship between serum CRP concentration in children and adiposity and suggested an association between CRP and cardiovascular disease. The involvement of serum CRP concentration with various factors such as sex, adiposity, body constitution, and symptoms other than respiratory symptoms should be further evaluated.
Air pollution due to automobile exhaust is a serious problem in the urban areas of Japan. The effect of fine particles in diesel exhaust on human health has become a matter of concern.3,36 Recently, air quality standards for atmospheric concentration of particulate matter less than 2.5 µm in diameter (PM2.5) have been established in the United States and European countries.37 Because atmospheric concentrations of PM2.5 have been rarely measured in Japan,38 the effects of SPM, NO2, and SO2 were examined in the present study. When various confounding factors were adjusted for, increased serum CRP concentrations of the 90th percentile or above were significantly associated with SPM and SO2 concentration. The adjusted OR of high serum CRP concentrations for the range of observed NO2 concentrations was similar to that for SPM and SO2, but this was not significant. In a two-pollutant model including both SPM and NO2 concentrations, high serum CRP concentration was associated only with SPM. These results suggest that SPM in the atmosphere may induce an inflammatory response in children more readily than NO2. However, the concentrations of SPM and NO2 are correlated, and the concentration of SO2 showed stronger correlation with these air pollutants. Therefore, it is difficult to elaborate on which pollutant has a stronger effect on serum CRP concentration. In addition, the effects of specific characteristics of the communities where the children lived cannot be completely eliminated.
A slight elevation in serum CRP concentration is thought to reflect chronic inflammation in vivo.39 Acute effects of particulate air pollution on serum CRP concentration have been reported in middle-aged or elderly subjects,16,18,40 and in individuals with diabetes, obesity, hypertension, and coronary heart disease.15,17 Recently, an acute effect of particulate air pollution on serum CRP was also observed in healthy young people.19 A weak positive association between CRP and 60-day mean exposure to PM2.5 has been also reported.14 Because the concentrations of air pollutants did not change greatly during the study period, the chronic effect of air pollution on serum CRP concentration was examined using 3-year average concentrations of air pollutants. Induction of proinflammatory cytokines and CRP has also been reported in macrophage cell lines exposed to ambient air particulates.41,42 Particulate air pollution may cause slight chronic inflammation in the respiratory tract in children,4 and may induce an inflammatory response in humans more readily than NO2. The pathophysiological significance of changes in serum CRP concentration is unknown, but is a topic worthy of additional study.
In conclusion, high serum CRP concentrations of the 90th percentile or above are associated with atmospheric concentrations of SPM. These findings suggest that air pollutants such as SPM may cause a slight inflammation in vivo. Slight elevations in serum CRP concentration, such as those observed in the present study, are thought to be a risk factor for cardiovascular disease and have been associated with particulate air pollution.
ACKNOWLEDGMENTS
The author thanks the school children, their parents, teachers, and the principals of the participating schools.
REFERENCES
- 1.Tamura K, Ando M, Sagai M, Matsumoto Y.. Estimation of levels of personal exposure to suspended particulate matter and nitrogen dioxide in Tokyo. Environ Sci 1996; 4: 37-51. [Google Scholar]
- 2.Brauer M, Hoek G, Van Vliet P, Meliefste K, Fischer PH, Wijga A, et al. . Air pollution from traffic and the development of respiratory infections and asthmatic and allergic symptoms in children. Am J Respir Crit Care Med 2002; 166: 1092-8. 10.1164/rccm.200108-007OC [DOI] [PubMed] [Google Scholar]
- 3.Shima M, Nitta Y, Adachi M.. Traffic-related air pollution and respiratory symptoms in children living along trunk roads in Chiba Prefecture, Japan. J Epidemiol 2003; 13: 108-19. 10.2188/jea.13.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Vliet P, Knape M, de Hartog J, Janssen N, Harssema H, Brunekreef B.. Motor vehicle exhaust and chronic respiratory symptoms in children living near freeways. Environ Res 1997; 74: 122-32. 10.1006/enrs.1997.3757 [DOI] [PubMed] [Google Scholar]
- 5.Autrup H, Daneshvar B, Dragsted LO, Gamborg M, Hansen M, Loft S, et al. . Biomarkers for exposure to ambient air pollution — comparison of carcinogen-DNA adduct levels with other exposure markers and markers for oxidative stress. Environ Health Perspect 1999; 107: 233-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leonardi GS, Houthuijs D, Steerenberg PA, Fletcher T, Armstrong B, Antova T, et al. . Immune biomarkers in relation to exposure to particulate matter: a cross-sectional survey in 17 cities of central Europe. Inhal Toxicol 2000; 12 (suppl 4): 1-14. 10.1080/08958370050164833 [DOI] [PubMed] [Google Scholar]
- 7.Fuji Y, Shima M, Ando M, Adachi M, Tsunetoshi Y.. Effect of air pollution and environmental tobacco smoke on serum hyaluronate concentrations in school children. Occup Environ Med 2002; 59: 124-128. 10.1136/oem.59.2.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shima M, Adachi M, Tanaka T, Tsunetoshi Y.. Serum complement levels in children in communities with different levels of air pollution in Japan. Arch Environ Health 1999; 54: 264-70. 10.1080/00039899909602484 [DOI] [PubMed] [Google Scholar]
- 9.Gabay C, Kushner I.. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 1999; 340: 448-54. 10.1056/NEJM199902113400607 [DOI] [PubMed] [Google Scholar]
- 10.Jousilahti P, Salomaa V, Hakala K, Rasi V, Vahtera E, Palosuo T.. The association of sensitive systemic inflammation markers with bronchial asthma. Ann Allergy Asthma Immunol 2002; 89: 381-5. 10.1016/S1081-1206(10)62039-X [DOI] [PubMed] [Google Scholar]
- 11.Roberts WL, Moulton L, Law TC, Farrow G, Cooper-Anderson M, Savory J, et al. . Evaluation of nine automated high-sensitivity C-reactive protein methods: implications for clinical and epidemiological applications. Part 2. Clin Chem 2001; 47: 418-25. [PubMed] [Google Scholar]
- 12.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000; 342: 836-43. 10.1056/NEJM200003233421202 [DOI] [PubMed] [Google Scholar]
- 13.Sandhu RS, Petroni DH, George WJ. Ambient particulate matter, C-reactive protein, and coronary artery disease. Inhal Toxicol 2005; 17: 409-13. 10.1080/08958370590929538 [DOI] [PubMed] [Google Scholar]
- 14.Diez Roux AV, Auchincloss AH, Astor B, Barr RG, Cushman M, Dvonch T, et al. . Recent exposure to particulate matter and C-reactive protein concentration in the multi-ethnic study of atherosclerosis. Am J Epidemiol 2006; 164: 437-48. 10.1093/aje/kwj186 [DOI] [PubMed] [Google Scholar]
- 15.Dubowsky SD, Suh H, Schwartz J, Coull BA, Gold DR. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environ Health Perspect 2006; 114: 992-8. 10.1289/ehp.8469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pope CA 3rd, Hansen ML, Long RW, Nielsen KR, Eatough NL, Wilson WE, et al. . Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ Health Perspect 2004; 112: 339-45. 10.1289/ehp.6588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rückerl R, Ibald-Mulli A, Koenig W, Schneider A, Woelke G, Cyrys J, et al. . Air pollution and markers of inflammation and coagulation in patients with coronary heart disease. Am J Respir Crit Care Med 2006; 173: 432-41. 10.1164/rccm.200507-1123OC [DOI] [PubMed] [Google Scholar]
- 18.Seaton A, Soutar A, Crawford V, Elton R, McNerlan S, Cherrie J, et al. . Particulate air pollution and the blood. Thorax 1999; 54: 1027-32. 10.1136/thx.54.11.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riediker M, Cascio WE, Griggs TR, Herbst MC, Bromberg PA, Neas L, et al. . Particulate matter exposure in cars is associated with cardiovascular effects in healthy young men. Am J Respir Crit Care Med 2004; 169: 934-40. 10.1164/rccm.200310-1463OC [DOI] [PubMed] [Google Scholar]
- 20.Special Committee on National Air Quality Standard of Standard Particulate Matter Data for decision on the national air quality standard of suspended particulate matter in Japan. Journal of Japan Society of Air Pollution 1973; 8: 1-57. (in Japanese) [Google Scholar]
- 21.Ferris BG. Epidemiology standardization project. II. Recommended respiratory disease questionnaires for use with adults and children in epidemiological research. Am Rev Respir Dis 1978; 118 (suppl 6): 7-53. [PubMed] [Google Scholar]
- 22.Rifai N, Tracy RP, Ridker PM. Clinical efficacy of an automated high-sensitivity C-reactive protein assay. Clin Chem 1999; 45: 2136-41. [PubMed] [Google Scholar]
- 23.Yamada S, Gotoh T, Nakashima Y, Kayaba K, Ishikawa S, Nago N, et al. . Distribution of serum C-reactive protein and its association with atherosclerotic risk factors in a Japanese population: Jichi Medical School Cohort Study. Am J Epidemiol 2001; 153: 1183-90. 10.1093/aje/153.12.1183 [DOI] [PubMed] [Google Scholar]
- 24.Muscari A, Bastagli L, Poggiopollini G, Tomassetti V, Massarelli G, Cappelletti O, et al. . Different associations of C-reactive protein, fibrinogen and C3 with traditional risk factors in middle-aged men. Int J Cardiol 2002; 83: 63-71. 10.1016/S0167-5273(02)00017-7 [DOI] [PubMed] [Google Scholar]
- 25.Shima M, Adachi M.. Effects of environmental tobacco smoke on serum levels of acute phase proteins in schoolchildren. Prev Med 1996; 25: 617-24. 10.1006/pmed.1996.0097 [DOI] [PubMed] [Google Scholar]
- 26.Herbeth B, Siest G, Henny J.. High sensitivity C-reactive protein (CRP) reference intervals in the elderly. Clin Chem Lab Med 2001; 39: 1169-70. 10.1515/CCLM.2001.184 [DOI] [PubMed] [Google Scholar]
- 27.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 2003; 107: 363-9. 10.1161/01.CIR.0000053730.47739.3C [DOI] [PubMed] [Google Scholar]
- 28.Chenillot O, Henny J, Steinmetz J, Herbeth B, Wagner C, Siest G.. High sensitivity C-reactive protein: biological variations and reference limits. Clin Chem Lab Med 2000; 38: 1003-11. 10.1515/CCLM.2000.149 [DOI] [PubMed] [Google Scholar]
- 29.Duff AL, Pomeranz ES, Gelber LE, Price GW, Farris H, Hayden FG, et al. . Risk factors for acute wheezing in infants and children: viruses, passive smoke, and IgE antibodies to inhalant allergens. Pediatrics 1993; 92: 535-40. [PubMed] [Google Scholar]
- 30.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma from bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 2000; 161: 1720-45. 10.1164/ajrccm.161.5.9903102 [DOI] [PubMed] [Google Scholar]
- 31.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, et al. . Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 1999; 354: 541-5. 10.1016/S0140-6736(98)10321-5 [DOI] [PubMed] [Google Scholar]
- 32.Hayaishi-Okano R, Yamasaki Y, Katakami N, Ohtoshi K, Gorogawa S, Kuroda A, et al. . Elevated C-reactive protein associates with early-stage carotid atherosclerosis in young subjects with type 1 diabetes. Diabetes Care 2002; 25: 1432-8. 10.2337/diacare.25.8.1432 [DOI] [PubMed] [Google Scholar]
- 33.Chandra RK. Prospective studies of the effect of breast feeding on incidence of infection and allergy. Acta Paediatr Scand 1979; 68: 691-4. 10.1111/j.1651-2227.1979.tb18439.x [DOI] [PubMed] [Google Scholar]
- 34.Cook DG, Mendall MA, Whincup PH, Carey IM, Ballam L, Morris JE, et al. . C-reactive protein concentration in children: relationship to adiposity and other cardiovascular risk factors. Atherosclerosis 2000; 149: 139-50. 10.1016/S0021-9150(99)00312-3 [DOI] [PubMed] [Google Scholar]
- 35.Infante-Rivard C, Amre D, Gautrin D, Malo JL. Family size, day-care attendance, and breastfeeding in relation to the incidence of childhood asthma. Am J Epidemiol 2001; 153: 653-8. 10.1093/aje/153.7.653 [DOI] [PubMed] [Google Scholar]
- 36.Schwartz J, Neas LM. Fine particles are more strongly associated than coarse particles with acute respiratory health effects in schoolchildren. Epidemiology 2000; 11: 6-10. 10.1097/00001648-200001000-00004 [DOI] [PubMed] [Google Scholar]
- 37.Environmental Protection Agency National Ambient Air Quality Standards for Particulate Matter; Final Rule. Federal Register 2006; 71: 61143-233. [Google Scholar]
- 38.Omori T, Fujimoto G, Yoshimura I, Nitta H, Ono M.. Effects of particulate matter on daily mortality in 13 Japanese cities. J Epidemiol 2003; 13: 314-22. 10.2188/jea.13.314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donaldson K, Stone V, Seaton A, MacNee W.. Ambient air particle inhalation and the cardiovascular system: potential mechanisms. Environ Health Perspect 2001; 109 (suppl 4): 523-7. 10.1289/ehp.01109s4523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peters A, Fröhlich M, Döring A, Immervoll T, Wichmann HE, Hutchinson WL, et al. . Particulate air pollution is associated with an acute phase response in men results from the MONICA-Augsburg study. Eur Heart J 2001; 22: 1198-204. 10.1053/euhj.2000.2483 [DOI] [PubMed] [Google Scholar]
- 41.Monn C, Naef R, Koller T.. Reactions of macrophages exposed to particles <10 µm. Environ Res 2003; 91: 35-44. 10.1016/S0013-9351(02)00021-X [DOI] [PubMed] [Google Scholar]
- 42.Vogel CF, Sciullo E, Wong P, Kuzmicky P, Kado N, Matsumura F. Induction of proinflammatory cytokines and C-reactive protein in human macrophage cell line U937 exposed to air pollution particulates. Environ Health Perspect 2005; 113: 1536-41. 10.1289/ehp.8094 [DOI] [PMC free article] [PubMed] [Google Scholar]


