Abstract
Objective
This study aimed to evaluate the association between clinical and examination features at admission and late preterm birth.
Study Design
The present study is a secondary analysis of a randomized trial of singleton pregnancies at 340/7 to 365/7 weeks’ gestation. We included women in spontaneous preterm labor with intact membranes and compared them by gestational age at delivery (preterm vs. term). We calculated a statistical cut-point optimizing the sensitivity and specificity of initial cervical dilation and effacement at predicting preterm birth and used multivariable regression to identify factors associated with late preterm delivery.
Results
A total of 431 out of 732 (59%) women delivered preterm. Cervical dilation ≥ 4 cm was 60% sensitive and 68% specific for late preterm birth. Cervical effacement ≥ 75% was 59% sensitive and 65% specific for late preterm birth. Earlier gestational age at randomization, nulliparity, and fetal malpresentation were associated with late preterm birth. The final regression model including clinical and examination features significantly improved late preterm birth prediction (81% sensitivity, 48% specificity, area under the curve = 0.72, 95% confidence interval [CI]: 0.68–0.75, and p-value < 0.01).
Conclusion
Four in 10 women in late-preterm labor subsequently delivered at term. Combination of examination and clinical features (including parity and gestational age) improved late-preterm birth prediction.
Keywords: ALPS, late preterm birth, spontaneous labor, prediction, preterm labor
Preterm birth, defined as birth before 37 weeks’ gestation, remains a major public health problem.1 After a 7-year decline, the United States preterm delivery rate has risen from 9.57% in 2014 to 9.85% in 2016, and this increase is largely in part to an increase in late preterm birth to 7.09%.2 Late preterm neonates, born at 34 to 36 weeks of gestation, are at greater risk of short- and long-term complications compared with their term counterparts, including worse cognitive, language, and motor development.3–5
The ability to accurately predict preterm birth remains elusive.6,7 Even among women with cervical dilation and/or effacement in the presence of symptoms, some women will have arrested preterm labor and deliver at term, whereas others will progress to a preterm delivery. Women admitted with preterm labor in the late-preterm period (340/7 through 366/7 weeks) are typically managed expectantly. Provided that there are no fetal or maternal contraindications to pregnancy continuation (e.g., chorioamnionitis or nonreassuring fetal status), the woman is carefully observed but no attempts are made to either augment or stop preterm labor. This period of “watchful waiting” and heightened monitoring can lead to significant costs and maternal anxiety, as observation is frequently performed in the hospital.
Hence, identification of women with late-preterm labor that results in late preterm versus term delivery would prove valuable in the management of women presenting with symptoms of spontaneous late-preterm labor, as it could reduce unnecessary interventions and hospitalization. Therefore, the objective of this study was to identify clinical risk factors (such as demographics, pregnancy characteristics, and previous pregnancy history) and examination findings present at admission that might be associated with late-preterm delivery in women admitted with spontaneous late-preterm labor. We hypothesized that use of clinical factors would improve upon prediction of late-pre-term birth made by examination findings alone.
Materials and Methods
This was a secondary analysis of the trial Antenatal Betamethasone for Women at Risk for Late Preterm Delivery (ALPS), a multicenter randomized controlled trial that enrolled women with singleton pregnancies at 340/7 to 365/7 weeks of gestation at high risk for late-preterm delivery and assigned them to receive betamethasone or placebo. The randomized trial was conducted from October 2010 to February 2015 at 17 participating clinical centers in the Eunice Kennedy Shriver, National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Trained research nurses obtained all clinical data and outcomes prospectively at the time of the original study. Details of the parent trial were previously published.8
Our analysis included women with singleton pregnancies at 340/7 to 365/7 weeks of gestations, who participated in the original trial admitted in spontaneous preterm labor with intact membranes, and who were deemed to be at high probability of delivery. Pregnancies were dated using a combination of the last menstrual period (if known) and obstetric ultrasound. The parent trial defined spontaneous preterm labor and high probability of delivery as at least six contractions in 1 hour and either cervical dilation of at least 3 cm (but less than 8 cm) or cervical effacement greater than or equal to 75% on admission to labor and delivery. Women with unknown gestational age at delivery and with missing cervical dilation or cervical effacement at randomization were excluded from this analysis. We also excluded women who had preeclampsia with severe features, nonreassuring fetal status by fetal heart rate tracing, or ultrasound at the time of randomization, and neonates who were postnatally diagnosed with a major fetal anomaly, from this study.
We grouped our cohort by timing of birth (preterm vs. term). We defined late-preterm birth as women who ultimately delivered spontaneously in the late preterm period of 340/7 to 366/7 weeks of gestation. Women who were admitted with spontaneous preterm labor but then delivered at term (at or beyond 370/7 weeks of gestation) constituted the term-birth group. We assessed demographic and pregnancy characteristics of the groups, including maternal age, race/ethnicity, nulliparity, history of prior preterm delivery (previous delivery < 37 weeks’ gestation among multiparous women with data available), smoking any time during pregnancy, abruption or significant bleeding during pregnancy, and any infections diagnosed during the current pregnancy (including chlamydia, gonorrhea, syphilis, trichomonas, bacterial vaginosis, herpes, group-B streptococcus (GBS) urinary tract infection, rubella, hepatitis B, human immunodeficiency virus, pyelonephritis, urinary tract infection, pneumonia, or cholecystitis). Similarly, we evaluated admission features among the groups, including gestational age at randomization, number of contractions per hour at screening, cervical dilation at randomization, cervical effacement at randomization, and fetal malpresentation (breech, transverse, and compound). In addition, we assessed the proportion of women who delivered less than 48 hours after randomization, and greater than or equal to 48 hours but less than 168 hours (1 week) after randomization.
We compared groups using Chi-square test, Fisher’s exact test, Student’s t-test, and Wilcoxon’s rank-sum test, as appropriate. We used multivariable logistic regression modeling to identify risk factors independently associated with late spontaneous preterm birth. We performed stepwise regression with backward elimination to select an adjusted model and retained variables in the model if p-values were <0.20. We calculated a statistical cut-point optimizing the sensitivity and specificity of admission cervical dilation and effacement at predicting late-preterm birth using the method by Liu et al.9 Next, we performed receiver operating characteristics (ROC) curve analyses using the final logistic regression model and the previously calculated cut points. We graphed the area under the curve (AUC) for cervical dilation at admission and cervical effacement at admission, separately, to estimate the predictive ability of these examination findings. We compared statistically the AUC for the final model combining clinical characteristics with cervical examination findings versus the AUC for cervical dilation alone or cervical effacement alone at admission. Lastly, we compared the AUC for the final model versus the AUC for combined cervical dilation and effacement.
We performed all statistical analyses using STATA/SE, Version 14.1 (StataCorp, Inc., College Station, TX). p-Value < 0.05 was considered statistically significant. All women provided written, informed consent for the original trial at their local center. We performed this secondary analysis using a deidentified dataset under a waiver of informed consent approved by the Institutional Review Board at the University of North Carolina at Chapel Hill. This study was supported by grants (HL098554 and HL098354) from the National Heart, Lung, and Blood Institute (NHLBI), by grants (HD21410, HD27915, HD27917, HD27869, HD34116, HD34208, HD40485, HD40500, HD40512, HD40544, HD40545, HD40560, HD53097, HD53118, HD68268, HD68258, HD68282, and HD36801) from the National Institute of Child Health and Human Development (NICHD), and by a grant (UL1 TR000040) from the National Center for Advancing Translational Sciences, National Institutes of Health.
Results
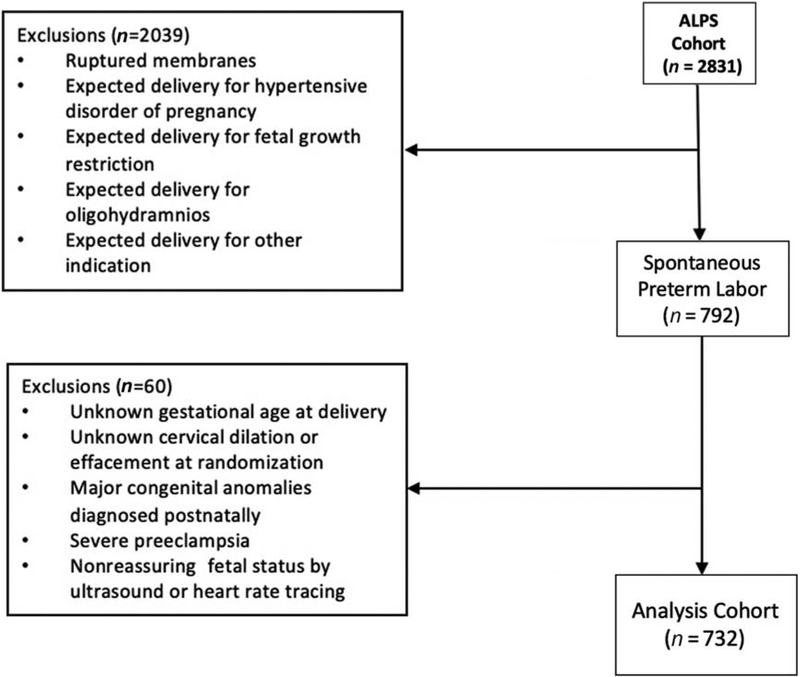
Overall, 2,831 women were randomized in the main trial, including 792 (28%) women enrolled due to spontaneous preterm labor. Of these, 732 (26% overall) met inclusion criteria for our analysis (Fig. 1). A total of 60 women were excluded due to unknown gestational age at delivery, unknown cervical dilation or effacement at randomization, major congenital anomalies diagnosed postnatally, preeclampsia with severe features as documented in the medical record, or nonreassuring fetal status by antenatal testing (ultrasound or heart rate tracing). On admission, 661/732 (90%) women presented with cervical dilation of at least 3 cm (but less than 8 cm), and 359/732 (49%) presented with a cervical effacement of at least 75%. A total of 292 women (40%) were admitted with both cervical dilation of at least 3 cm and cervical effacement of at least 75%.
Fig. 1.
Flow diagram of eligibility criteria for inclusion into analysis. ALPS, Antenatal Betamethasone for Women at Risk for Late Preterm Delivery.
Regarding timing of delivery, a total of 431/732 (59%) women delivered preterm (median 360/7 weeks, interquartile range [IQR]: 35.1–36.4); the remainder delivered at a median of 38 0/7 weeks’ gestation (IQR: 37.4–38.7). A total of 101/732 (14%) women delivered between 48 hours and 1 week after randomization, the optimal time period for antenatal corticosteroid effect (median = 100 hours, IQR: 76.1–130.3 hours). The majority of women delivered outside of this optimal time period, with 293/732 (40%) women delivered <48 hours after randomization (median = 11.0 hours, IQR: 5.5–21.4 hours), and 338/732 (46%) women delivered more than 1 week after randomization (median = 417.4 hours, IQR: 299.5–568.8 hours).
Maternal demographic and pregnancy characteristics by timing of birth are shown in Table 1. In univariable analysis, there were no statistically significant differences among the groups with respect to maternal age, race, prepregnancy body mass index (BMI), history of a prior preterm delivery < 37 weeks’ gestation (among multiparous women), or tobacco, alcohol, or street drug use during pregnancy. Bleeding or abruption at any time during pregnancy was also similar between the groups. Notably, late-preterm birth occurred more commonly in nulliparous women (26.2 vs. 16.3%, p = 0.001). Lastly, mean gestational age at randomization (35.5 vs. 35.4 weeks, p = 0.09), and median number of contractions per hour at screening were also similar among the groups.
Table 1.
Demographic and pregnancy characteristics of women admitted in spontaneous late preterm labor, stratified by timing of birth (n = 732)
| Characteristic/risk factor | Term birth (n = 301) | Late preterm birth (n = 431) | p-Value |
|---|---|---|---|
| Maternal age | 26.1 ± 5.5 | 26.4 ± 5.7 | 0.49 |
| Maternal race | 0.77 | ||
| Black or African American | 82 (27.2) | 110 (25.5) | |
| White | 166 (55.1) | 236 (54.8) | |
| Asian | 8 (2.7) | 17 (3.9) | |
| Other, unknown, or more than one race | 45 (15.0) | 68 (15.8) | |
| Nulliparity | 49 (16.3) | 113 (26.2) | <0.01 |
| Prepregnancy BMI (kg/m2) | 24.6 (21.6–28.3) | 24.1 (21.2–28.6) | 0.29 |
| Prior preterm birtha | 91/239 (38.1) | 99/283 (35.0) | 0.47 |
| Smoking any time during pregnancy | 41 (13.6) | 64 (14.9) | 0.64 |
| Use of “street drugs” any time during pregnancy | 15 (5.0) | 14 (3.3) | 0.24 |
| Alcohol use at any time during pregnancy | 9 (3.0) | 17 (3.9) | 0.50 |
| Abruption or significant bleeding during pregnancy | 8 (2.7) | 17 (3.9) | 0.35 |
| Any infections diagnosed this pregnancyb | 111 (36.9) | 128 (29.7) | 0.04 |
| Gestational age at randomization (weeks) | 35.5 ± 0.8 | 35.4 ± 0.8 | 0.09 |
| Contractions per hour at screening | 12 (8–16) | 12 (9–15) | 0.44 |
| Cervical dilation at randomization (cm) | 3 (3–4) | 4 (3–4.5) | <0.01 |
| Cervical effacement (%) at randomization | 60 (50–75) | 75 (50–90) | <0.01 |
| Fetal malpresentation | 1 (0.3) | 9 (2.1) | 0.05 |
| Mother’s length of hospital stay at delivery (days) | 2 (2–3) | 3 (2–3) | <0.01 |
Abbreviation: BMI, body mass index.
Note: Data are presented as n (%), mean ± standard deviation, or median (interquartile range), unless otherwise specified.
Previous delivery < 37 weeks’ gestation among multiparous women with gestational age data available.
Includes chlamydia, gonorrhea, syphilis, trichomonas, bacterial vaginosis, herpes, GBS urinary tract infection, rubella, hepatitis B, HIV, pyelonephritis, urinary tract infection, pneumonia, or cholecystitis.
Women who delivered preterm were less likely to have a history of infection during pregnancy (29.7 vs. 36.9%, p = 0.04). At the time of randomization, women who delivered preterm had a median cervical dilation of 4 cm (IQR: 3–4.5)and a median cervical effacement of 75% (IQR: 50–90) compared with 3-cm dilation (IQR: 3–4) and 60% effacement (IQR: 50–75) for women who delivered at term (p-value < 0.001 for both). Among women who were randomized at 3- or 3.5-cm dilation (n = 309), 47.2% (146) delivered preterm. Of those randomized at 4-cm dilation (n = 213), 67.1% (143) delivered preterm. Among women randomized with a cervical examination ≥ 5 cm (n = 128), 80.5% (103) delivered preterm. Fetuses in the nonvertex presentation were nominally more likely to be delivered preterm (p = 0.05). Women who delivered preterm had slightly longer lengths of hospital stay for delivery (median = 3 vs. 2 days, p-value < 0.01) than women who delivered at term.
In calculation of statistical cut points, a cervical dilation cut-off ≥ 4 cm was 60% sensitive and 68% specific for predicting late-preterm birth (AUC = 0.64, 95% confidence interval [CI] 0.61–0.68). A cervical effacement cut-off ≥ 75% was 59% sensitive and 65% specific for predicting late-preterm birth (AUC = 0.62, 95% CI: 0.58–0.65). Multivariable regression modeling initially controlled for gestational age at randomization, nulliparity, history of a prior preterm birth, antepartum infections, cervical dilation ≥ 4 cm at randomization, cervical effacement ≥ 75% at randomization, and fetal malpresentation. In the final adjusted model, cervical dilation ≥ 4 cm dilation at randomization, ≥ 75% effacement at randomization, earlier gestational age at randomization, nulliparity, and fetal malpresentation remained associated with preterm delivery (Table 2).
Table 2.
Multivariable logistic regression of factors associated with preterm birth <37 weeks at the time of hospitalization for spontaneous late preterm labor
| Characteristic/risk factor | Crude odds ratioa | Adjusted odds ratiob | 95% confidence intervalc | p-Value |
|---|---|---|---|---|
| ≥4-cm dilation at randomization | 3.5 | 3.5 | 2.5–4.8 | <0.001 |
| ≥75% effacement at randomization | 2.1 | 2.2 | 1.6–3.0 | <0.001 |
| Gestational age at randomization (per completed week) | 0.8 | 0.8 | 0.6–1.0 | 0.02 |
| Nulliparity | 1.6 | 1.7 | 1.1 −2.6 | 0.01 |
| Fetal malpresentation | 6.1 | 6.5 | 0.8–53.6 | 0.08 |
Initial multivariable model controlled for gestational age at randomization (per completed week), nulliparity, history of a prior preterm birth, antepartum infections, ≥4-cm dilation at randomization, ≥75% effacement at randomization, and fetal malpresentation.
Final model adjusted for ≥4-cm dilation at randomization, ≥75% effacement at randomization, gestational age at randomization (per completed week), nulliparity, and fetal malpresentation.
Confidence interval for the adjusted odds ratio.
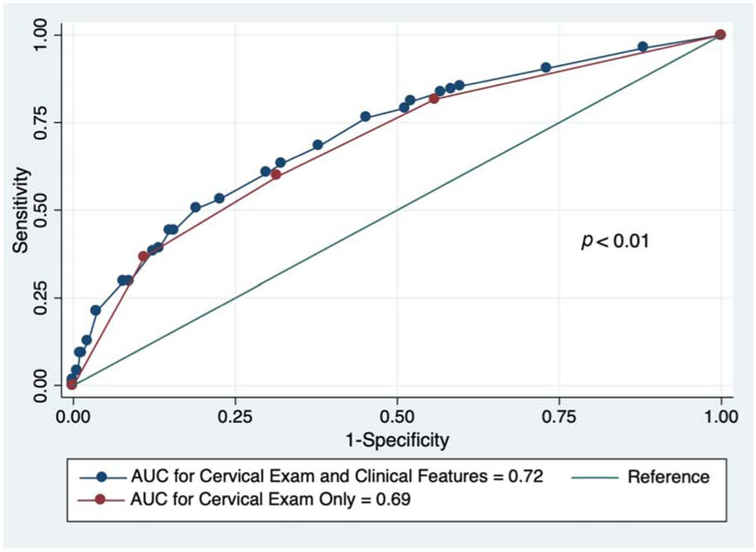
We then compared the area under the ROC curves for the models using the examination features of cervical effacement cut-off greater than or equal to 75% and cervical dilation cut-off ≥ 4 cm versus cervical examination cut-off findings combined with clinical characteristics. Combining examination findings of dilation ≥ 4 cm and effacement ≥ 75% at randomization yielded 82% sensitivity and 44% specificity at predicting late preterm birth (AUC = 0.69, 95% CI: 0.65–0.73). The final adjusted model combining cervical examination cut-off findings with clinical characteristics associated with late-preterm delivery had improved statistical performance in the prediction of late preterm birth (sensitivity 81%, specificity, 48%, AUC = 0.72, and 95% CI: 0.68–0.75) as compared with cervical dilation or effacement-only models (Fig. 2). This difference was statistically significant (p-value <0.01, Table 3).
Fig. 2.
Receiver operating characteristic curves for prediction of late preterm birth at the time of hospitalization for spontaneous late preterm labor. AUC, area under curve.
Table 3.
Sensitivity, specificity, area under the receiver operating characteristic curve, and 95% confidence intervals for predictive models by features on admission
| Feature | Sensitivity (%) | Specificity (%) | Area under the curve | 95% confidence interval |
|---|---|---|---|---|
| ≥4-cm dilation at randomization | 60 | 68 | 0.64 | 0.61–0.68 |
| ≥ 75% effacement at randomization | 59 | 65 | 0.62 | 0.58–0.65 |
| Combined dilation ≥ 4 cm and effacement ≥ 75% at randomization | 82 | 44 | 0.69 | 0.65–0.73 |
| Combined cervical exam features and clinical characteristicsa | 81 | 48 | 0.72 | 0.68–0.75 |
Statistically significant difference (p-value < 0.01) between cervical examination-only model and combined model including cervical examination and clinical characteristics on admission.
Discussion
Main Findings
In this secondary analysis of the ALPS trial, we found that 60% of women admitted in spontaneous late-preterm labor delivered in the late-preterm period. The use of clinical characteristics (e.g., nulliparity, earlier gestational age at admission, and fetal malpresentation) in conjunction with cervical examination parameters at admission significantly improved the prediction of which women are most likely to have a late-preterm birth after presenting in spontaneous late-preterm labor. This finding highlights the complexity of preterm birth prediction, even at gestational ages near term and advanced cervical examination parameters that heighten clinical suspicion for delivery.
All statistical models, including those with cervical examination findings alone and cervical examination plus clinical factors, remained only modestly predictive of late-preterm birth. This is common in tests of heterogeneous conditions, such as preterm birth.10 Given that our model was only modestly predictive, we are unable to confidently identify women who are unlikely to benefit from late-preterm antenatal corticosteroids. Rather, our findings reinforce current recommendations to administer antenatal corticosteroids to eligible women, as the majority of this high-risk cohort delivered in the late-preterm period.
Interestingly, we found in univariable analysis that almost one-third of women in our cohort who proceeded to deliver in the late preterm period were nulliparous, whereas only 16% who delivered at term were nulliparous. We hypothesize that multiparous women may develop physiologic cervical dilation and/or effacement as they approach 37 weeks’ gestation which might then be attributed to preterm labor by obstetric providers when in conjunction with uterine contractions. Additionally, it is worth noting that our cohort’s median BMI was normal at 24 kg/m2, thereby limiting our findings to normal-sized women.
Not surprisingly, we found that fetal malpresentation predicted late-preterm birth in the setting of threatened late-preterm labor in unadjusted analysis. In contrast to mothers whose fetuses are cephalic, when there is fetal malpresentation, the clinician must continuously assess whether expectant management is appropriate or whether to proceed with prompt delivery by Cesarean section. We thus theorize that since delivery timing is dependent on the clinician’s decision to proceed with Cesarean, deliveries may occur earlier in the preterm labor process and, therefore, at earlier gestational ages due to concern for expeditious cervical change leading to emergent cesarean.
The unadjusted association between antepartum infections and delivery at term was unexpected, as previously published studies have demonstrated associations between maternal infection and risk for preterm birth.11–14 This variable included multiple infectious complications at any time during the pregnancy, such as history of sexually transmitted infections, bacterial vaginosis, GBS urinary tract infection, rubella, pyelonephritis, non-GBS urinary tract infections, pneumonia, or cholecystitis. Given this extensive inclusion of infectious morbidities, unmeasurable confounding may be present to account for our results. Moreover, we lack information on whether these women underwent treatment of their antepartum infections and to what extent, further adding to our inability to account for this finding.
Few prediction models of spontaneous late-preterm birth based on clinical risk factors and symptoms at the time of presentation to labor and delivery at 34 to 36 weeks’ gestation currently exist. Bastek et al15 performed a secondary analysis of a prospective cohort study of singleton pregnancies at 22 to 336/7 weeks of gestation with preterm labor, with the goal of developing prediction rules to identify women at greatest risk of delivery within 10 days of admission and before 37 weeks’ gestation. Their models included tobacco use during pregnancy, no prenatal care, cervical dilation at initial assessment, and obstetric history. The authors also demonstrated modest predictive ability for spontaneous preterm birth within 10 days of admission (AUC = 0.75) and before 37 weeks’ gestation (AUC = 0.73). However, they included women presenting at a wide range of gestational ages but did not include women presenting in the late-preterm period. Other studies have evaluated the role of cervical examination assessed by Bishop score at 22 to 24 weeks’ gestation to predict spontaneous preterm delivery among asymptomatic low-risk women.16,17 Though these authors demonstrated that a Bishop’s score ≥ 4 in the midtrimester is associated with spontaneous preterm delivery, they also described that the Bishop score has low sensitivity and positive predictive value for prediction of preterm birth less than 35 weeks’ gestation. Recently, Kokanali et al18 evaluated the role of transvaginal ultrasonographic cervical length assessment at 34 weeks in predicting late-preterm delivery. They demonstrated that a cervical length of 25.5 mm or less at 34 weeks’ gestation had a sensitivity of 80%, specificity of 93.9%, and positive predictive value of 52.6% at predicting late-preterm birth. However, the majority of their cohort delivered at term or late term, and only asymptomatic women were included.
Our study is not without limitations. Women presenting to labor and delivery in the late-preterm period and meeting a prespecified definition of preterm labor at presentation were enrolled in the original randomized trial. As such, we do not have data regarding prior antenatal evaluations for cervical change or preterm contractions, nor do we have information on women who presented in the late preterm period and were not at least 3-cm dilated or at least 75% effaced. Similarly, we were unable to calculate Bishop’s score and its ability to predict late-preterm birth, as we do not have data for our cohort on cervical consistency, position, or fetal station at the time of randomization. Our findings may reflect selection bias and not truly reflect the overall population in spontaneous late-preterm labor, as only women who (1) were approached for trial enrollment and (2) agreed to participate in the trial could be included in this secondary analysis. Additionally, our prediction model is not yet validated, as we do not currently have access to a similar large cohort of women admitted in spontaneous late-preterm labor with similar cervical examination findings.
Despite these limitations, our study has several strengths. We used prospectively collected data from women enrolled in the original randomized trial who met a prespecified definition of spontaneous preterm labor which included both physical examination findings and frequent contractions. By using this uniform definition, our findings can be applied to many women at risk of late-preterm birth, thereby making our findings more generalizable to the late-preterm obstetric population. In addition, aside from the randomization assignment, the main study was a multicenter pragmatic randomized controlled trial—obstetric management was at the discretion of the primary clinician and not proscribed by the study. Thus, these findings are likely to be generalizable to women not enrolled in clinical trials.
Conclusion
In conclusion, preterm birth remains difficult to predict, regardless of gestational age, even for women presenting with spontaneous preterm labor in the late preterm period at cervical dilation ≥ 3 cm or effacement ≥ 75% and regular contractions. Though the combination of clinical factors with cervical examination findings significantly improved upon the prediction of spontaneous late-preterm birth achieved by cervical dilation or effacement alone, overall models remained only modestly predictive. Future research must focus on studying other cost-conscious strategies to improve upon the identification of women in late-preterm labor who will go on to have a late-preterm delivery, thereby aiding clinicians in deciding optimal candidates for corticosteroid administration.
Acknowledgments
The authors thank Felecia Ortiz, RN, BSN and Sabine Bousleiman, RNC, MSN, MPH for protocol development and coordination between clinical research centers, Kathleen Jablonski, PhD for protocol and data management, and Ronald Wapner, MD, Elizabeth A. Thom, PhD, Carol Blaisdell, MD, and Catherine Spong, MD for protocol development and oversight.
Funding
This study was supported by grants (HL098554 and HL098354) from the NHLBI, by grants (HD21410, HD27915, HD27917, HD27869, HD34116, HD34208, HD40485, HD40500, HD40512, HD40544, HD40545, HD40560, HD53097, HD53118, HD68268, HD68258, HD68282, and HD36801) from the NICHD, and by a grant (UL1 TR000040) from the National Center for Advancing Translational Sciences, National Institutes of Health. The views expressed in this article are those of the authors and do not necessarily represent the views of the National Institutes of Health.
Appendix A
In addition to the authors, other members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network are as follows:
Columbia University, New York, NY—S Bousleiman, R Wapner, M DiVito, M Talucci, L Plante (Drexel University), C Tocci (Drexel University), M Hoffman (Christiana Care Health Systems), S Lynch (Christiana Care Health Systems), A Ranzini (St. Peter’s University Hospital), M Lake (St. Peter’s University Hospital), J Smulian (Lehigh Valley Health Network), D Skupski (New York Hospital Queens). UT Health- University of Texas Medical School at Houston–Children’s Memorial Hermann Hospital, Houston, TX—F Ortiz, B Sibai, M. Hutchinson, P Givens, L Garcia (LBJ General Hospital).
University of Alabama at Birmingham, Birmingham, AL—S Harris, J Biggio, A Todd, L Merin, G Adams M. Tew, J. Grant. University of Texas Medical Branch, Galveston, TX—A Salazar, L McCoy, B Aguillon, M Wilson, J Sikes, G Hankins, G Olson, H Harirah.
Brown University, Providence, Rhode Island—D Allard, L Beati, B Wallin, J Rousseau, B Hughes.
The Ohio State University, Columbus, OH—F Johnson, M Prasad, D McKenna, R Ozug, T Dible, K Snow, K Fennig, S Webster, M Donohue.
University of Utah Health Sciences Center, Salt Lake City, UT—K Hill, A Sowles, S Timothy, P Reed (deceased; Inter-mountain Healthcare), M Varner.
University of North Carolina at Chapel Hill, Chapel Hill, NC—K Clark, J Thorp, S Timlin, R Bass, K Dorman, S Brody (WakeMed Health & Hospitals), J Warren (Mission Health System).
MetroHealth Medical Center-Case Western Reserve University, Cleveland, OH—M Duchon, W Dalton, C Milluzzi, L Wolfe, K Kushner, B Mercer.
Northwestern University, Chicago, IL—G Mallett, W Grobman, L Stein, M Dinsmoor (NorthShore University HealthSystem), K Paychek (NorthShore University HealthSystem).
University of Colorado, Denver, CO—K Hale, M Hoffman, JC Carey, H Galan, K Heyborne, T Metz, A Rosenberg.
Duke University, Durham, NC—T Bishop, A Murtha, R Heine, C Grotegut, L Brancazio.
Stanford University, Stanford, CA—K Kushniruk, Y El-Sayed, D Lyell, A Sit, C Willson, A Monk, E Kogut, R Knapp.
University of Texas Southwestern Medical Center, Dallas, TX—L Moseley, J Price, M Santillan, J Gerald, A Sias, K Gonzales.
University of Pittsburgh, Pittsburgh, PA—H Simhan, H Birk-land, P Cotroneo.
Oregon Health & Science University, Portland, OR—L Pereira, C McEvoy, M Rincon, J Snyder.
Wayne State University, Detroit, MI—N Hauff.
The George Washington University Biostatistics Center, Washington, DC—E Thom, K Jablonski, V Momirova, G Heinrich, T Billingsley, T Spangler.
National Heart, Lung, and Blood Institute, Bethesda, MD—C Blaisdell.
Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD—C Spong, S Tolivaisa.
MFMU Network Steering Committee Chair (Medical University of South Carolina, Charleston, SC)—JP VanDorsten, MD.
Footnotes
Additional members of this network are listed in the Appendix A.
This study was presented as a poster at the 38th Annual Pregnancy Meeting, Society for Maternal-Fetal Medicine, February 1 to 3, 2018, Dallas, TX.
Conflict of Interest
None declared.
References
- 1.Frey HA, Klebanoff MA. The epidemiology, etiology, and costs of preterm birth. Semin Fetal Neonatal Med 2016;21(02):68–73 [DOI] [PubMed] [Google Scholar]
- 2.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: final data for 2016. Natl Vital Stat Rep 2018;67(01):1–55 [PubMed] [Google Scholar]
- 3.Hibbard JU, Wilkins I, Sun L, et al. ; Consortium on Safe Labor. Respiratory morbidity in late preterm births. JAMA 2010;304(04):419–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol 2008;111(01):35–41 [DOI] [PubMed] [Google Scholar]
- 5.Cheong JL, Doyle LW, Burnett AC, et al. Association between moderate and late preterm birth and neurodevelopment and social-emotional development at age 2 years. JAMA Pediatr 2017;171(04):e164805. [DOI] [PubMed] [Google Scholar]
- 6.Esplin MS, Elovitz MA, Iams JD, et al. ; nuMoM2b Network. Predictive accuracy of serial transvaginal cervical lengths and quantitative vaginal fetal fibronectin levels for spontaneous preterm birth among nulliparous women. JAMA 2017;317(10):1047–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berghella V, Baxter JK, Hendrix NW. Cervical assessment by ultrasound for preventing preterm delivery. Cochrane Database Syst Rev 2013;((01):CD007235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al. ; NICHD Maternal–Fetal Medicine Units Network. Antenatal Betamethasone for Women at Risk for Late Preterm Delivery. N Engl J Med 2016;374(14):1311–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu A, Wu C, Schisterman EF. Nonparametric sequential evaluation of diagnostic biomarkers. Stat Med 2008;27(10):1667–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science 2014;345(6198):760–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donders GG, Van Calsteren K, Bellen G, et al. Predictive value for preterm birth of abnormal vaginal flora, bacterial vaginosis and aerobic vaginitis during the first trimester of pregnancy. BJOG 2009;116(10):1315–1324 [DOI] [PubMed] [Google Scholar]
- 12.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med 2000;342(20):1500–1507 [DOI] [PubMed] [Google Scholar]
- 13.Lockwood CJ, Kuczynski E. Markers of risk for preterm delivery. J Perinat Med 1999;27(01):5–20 [DOI] [PubMed] [Google Scholar]
- 14.Sheiner E, Mazor-Drey E, Levy A. Asymptomatic bacteriuria during pregnancy. J Matern Fetal Neonatal Med 2009;22(05):423–427 [DOI] [PubMed] [Google Scholar]
- 15.Bastek JA, Sammel MD, Srinivas SK, et al. Clinical prediction rules for preterm birth in patients presenting with preterm labor. Obstet Gynecol 2012;119(06):1119–1128 [DOI] [PubMed] [Google Scholar]
- 16.Iams JD, Goldenberg RL, Mercer BM, et al. ; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. The preterm prediction study: can low-risk women destined for spontaneous preterm birth be identified? Am J Obstet Gynecol 2001;184(04):652–655 [DOI] [PubMed] [Google Scholar]
- 17.Newman RB,Goldenberg RL, Iams JD, et al. ; National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU). Preterm prediction study: comparison of the cervical score and Bishop score for prediction of spontaneous preterm delivery. Obstet Gynecol 2008;112(03):508–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kokanali MK, Çelik H, Kokanali D, Taşçi Y. Predictive role of transvaginal ultrasonographic measurement of cervical length at 34 weeks for late pre-term and late-term deliveries in nulliparous women. J Matern Fetal Neonatal Med 2016;29(11):1789–1794 [DOI] [PubMed] [Google Scholar]