Abstract
Non-suicidal self-injury (NSSI) is a serious clinical problem that is common in adolescents. Novel, biologically-informed approaches for treating NSSI in adolescents are needed to prevent negative outcomes such as chronic NSSI and future suicide attempts. N-acetylcysteine (NAC) has been used successfully to address other conditions that involve repetitive maladaptive behaviors and may have utility in addressing NSSI. This study explored neural circuit changes following an open-label, 8-week trial of NAC in female adolescents with NSSI. We measured whole-brain resting-state functional connectivity (RSFC) of the amygdala and the nucleus accumbens before and after treatment using resting-state functional neuroimaging. Usable neuroimaging data from both pre- and post-treatment were available for 18 participants. Reduction in NSSI frequency was associated with a decrease in left amygdala RSFC with right supplementary motor area (SMA), but with an increase in right amygdala RSFC with right inferior frontal cortex. For nucleus accumbens, a reduction in NSSI frequency was associated with a decrease in connectivity between right nucleus accumbens and left superior medial frontal cortex. We also report change in similar circuits accompanying clinical improvement in depression and global psychopathology measures. These preliminary findings suggest amygdala and nucleus accumbens-based circuits as potential treatment targets, and set the stage for future research designed to confirm these neural targets using randomized, placebo-controlled designs to confirm clinical efficacy and mechanisms of effect.
Keywords: non-suicidal self-injury, N-acetylcysteine, amygdala, resting-state functional connectivity
Introduction
Non-suicidal self-injury (NSSI), the act of deliberately harming one’s own body tissue without suicidal intent (Winchel & Stanley, 1991), is a common problem in adolescents, with an international prevalence of approximately 18% (Muehlenkamp, Claes, Havertape, & Plener, 2012). Recurrent NSSI has been associated with negative outcomes such as persistent psychopathology and suicide attempts (Horwitz, Czyz, & King, 2015; Tang et al., 2011; Victor & Klonsky, 2014). Treatments for adolescent NSSI are very limited (Hawton et al., 2015). Research is needed to develop novel treatments that could address NSSI in adolescents and avert deleterious outcomes. Development of new treatments should be rooted in a solid understanding of the biology underlying this behavior. However, the neurobiological underpinnings of NSSI are only now beginning to come to light. Research using a novel medication as a probe along with neuroscience measurements such as brain imaging have the potential to (a) provide insights into the biology of the condition by showing neural changes that are linked to clinical changes, and (b) identify quantifiable treatment targets that can be tested in future studies using an experimental therapeutics approach.
Presently, there are no FDA-approved psychopharmacological treatment options for NSSI. However, in a recent open-label pilot study (Cullen et al., 2017), we provided preliminary evidence for N-acetylcysteine (NAC) as a possible treatment for adolescents with NSSI. NAC is the N-acetyl derivative of the amino acid L-cysteine, and is a precursor in the formation of the antioxidant glutathione in the brain and body. Following 8 weeks of oral NAC treatment, we observed a reduction in NSSI frequency and in associated symptomatology (depression and global psychopathology). The motivation to explore the use of NAC in this population was based on its effectiveness in other psychiatric disorders that involve repetitive, maladaptive behaviors such as addictive disorders and compulsive problems (e.g. hair-pulling, skin picking) (Grant et al., 2016; McClure, Gipson, Malcolm, Kalivas, & Gray, 2014; Rothbart et al., 2013). Furthermore, the low-risk profile associated with this intervention (Zheng et al., 2018) and wide availability of this low cost, over-the-counter nutritional supplement make it an appealing treatment option for adolescent NSSI. To explore the impact of NAC on brain circuitry and to identify potential brain-based mechanisms for NAC’s efficacy in reducing NSSI and related symptoms, we collected neuroimaging data in a subset of adolescents who participated in the open-label pilot study (Cullen et al., 2017) before and after the clinical trial; this is the focus of the current report.
Resting-state functional magnetic resonance imaging (fMRI) can be used to examine resting state functional connectivity (RSFC) within neural networks. This method has appeal for identifying treatment-induced neural changes as it does not involve a task which can suffer from accommodation and/or practice effects with repeated assessments. In selecting the networks for examining NAC’s effects in adolescents with NSSI, we considered neural systems implicated in NSSI as potential targets for treatment. NSSI behavior is maintained both through negative (decrease negative affect) and positive (increase positive affect) reinforcement (Nock & Prinstein, 2004). Since NSSI is a maladaptive coping mechanism that is often used to regulate negative emotion (Klonsky, 2007), brain networks implicating negative affect, such as those involving the amygdala frontal regulatory networks, are implicated in the biology of NSSI (Plener, Bubalo, Fladung, Ludolph, & Lulé, 2012; Westlund Schreiner, Cullen, & Klimes-Dougan, 2017). We recently conducted a study examining baseline amygdala RSFC in an expanded sample (N=45, including 20 healthy controls and 25 adolescents with NSSI, encompassing those included in the current study) (Westlund Schreiner, Klimes-Dougan, et al., 2017). Compared to healthy controls, adolescents with NSSI showed lower amygdala connectivity with frontal pole but greater connectivity with the dorsal cingulate cortex and supplementary motor area (SMA) and dorsal anterior cingulate. Of note, the amygdala-SMA hyperconnectivity finding persisted despite controlling for depressive symptoms, and could possibly suggest excessive influence of negative affect on the planning of motor movements. Furthermore, neural networks underlying positive reinforcement (reward) networks implicated in reward processing (e.g. nucleus accumbens, orbitofrontal cortex) have been implicated in other reward-seeking behaviors such as addiction (Mitchell et al., 2012; Volkow et al., 2007), although these networks have not yet been directly studied in NSSI.
The primary objective of this study was to explore the utility of amygdala and nucleus accumbens RSFC change as neural correlates of NAC treatment in adolescents with NSSI. We first tested overall changes in amygdala and nucleus accumbens networks, to look for NAC effects regardless of response. We then examined whether clinical improvement (reduction in NSSI frequency, improvement in depression and overall psychopathology) was associated with change in amygdala and nucleus accumbens RSFC. For amygdala connectivity, we predicted that successful treatment would be associated with enhanced connectivity between amygdala and frontal regulatory regions (reflecting enhanced capacity to regulate emotion). Additionally, given our previous work examining the larger sample of adolescents with and without NSSI (Westlund Schreiner, Klimes-Dougan, et al., 2017) which noted hyperconnectivity in the NSSI group between amygdala and SMA, we hypothesized that treatment success might be associated with a reduction in amygdala-SMA connectivity. For nucleus accumbens, we predicted that treatment and/or reduction of symptoms would be associated with altered nucleus accumbens connectivity with regions of the reward network associated with control (e.g. orbitofrontal cortex).
Method
The study was approved by the University of Minnesota Institutional Review Board, was regulated by the FDA (IND# 10345) and was posted on ClinicalTrials.gov ( NCT01111734).
Participants
Recruitment strategies included community postings and referrals from local mental health services. The main inclusion criterion was a history of engaging in NSSI at least 4 times, with at least 1 episode occurring in the last month. Exclusion criteria included a history of bipolar, pervasive developmental, or psychotic disorders, current pregnancy or breastfeeding, unstable medical illnesses, active suicidal intent, presence of MRI-incompatible features, change in psychiatric medication treatment in the past 2 months, a positive urine drug screen, and intelligence quotient (IQ) of less than 80 as measured by the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999). Prior to enrolling in the study, all patients (or their parent/legal guardian if under 18) completed a phone interview to determine initial eligibility. If all inclusion and no exclusion criteria were met, they were invited for a baseline clinical visit. Upon arrival, after completing the informed consent and assent (when applicable) process, participants underwent a diagnostic interview, clinician-administered and self-report assessments, and a health screening and physical exam to assess for current and past medical conditions and medication use. For patients under 18 years old, a diagnostic interview which consisted of the Kiddie Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version (KSADS-PL) (Kaufman et al., 1997) was completed with the subject and the parent/guardian separately. Both interviews were used to establish a consensus diagnosis. Patients 18 and older completed the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (First Michael B. Spitzer Robert L Gibbon Miriam and Williams, 2002). Participants completed the Inventory of Statements About Self-Injury - Lifetime version (ISAS-LV)(Glenn & Klonsky, 2011); after reviewing the results, the research clinician administered the Deliberate Self-Harm Inventory (DSHI) interview (Gratz, 2001), with a focus on the type of self-harm that had been endorsed in the ISAS-LV. In addition, participants completed the: the Beck Depression Inventory (BDI-II)(Beck, Steer, & Brown, 1996), Tanner Staging Questionnaire (Duke, Litt, & Gross, 1980), the Symptom Checklist-90 (SCL-90) (Derogatis & Savitz, 2000), and a demographic questionnaire. Socioeconomic scale (SES) was calculated using the Hollingshead Four-Factor Index (Hollingshead & Others, 1975). Estimated intelligence quotient (IQ) was estimated using the Wechsler Abbreviated Scale of Intelligence (WASI)(Wechsler, 1999).
Treatment and Clinical Assessment at Follow-up Visits
Participants with NSSI underwent an open-labeled, 8-week course of oral NAC: 600 mg twice daily for weeks 1 and 2, 900 mg twice daily for weeks 3 and 4, and 1800 twice mg daily for weeks 5–8. Participants were seen at weeks 2, 4, 6, and 8 for clinical and safety monitoring. At each follow-up visit, NSSI frequency was measured using the ISAS and the DSHI. At the final visit, the participants also repeated the BDI-II and the SCL-90.
Quantification of clinical change
We created consensus NSSI episode frequency scores using both the clinician-administered DSHI and the self-reported ISAS. We subtracted the baseline rate of NSSI (per 4 weeks) from the number of reported NSSI episodes during weeks 4–8 (so that a negative number reflects an improvement). Change in depression and global psychopathology were measured by subtracting baseline from post-treatment respectively for the BDI-II and SCL-90 global symptom index (SCL-90-GSI) scores, and then calculating the percent change.
Neuroimaging Acquisition
Brain imaging took place at the Center for Magnetic Resonance Research at the University of Minnesota on a 3 Tesla Siemens TIM Trio scanner using a 32-channel radio-frequency head coil. Resting-state fMRI data were obtained using the Human Connectome Project multi-band EPI sequence (260 T2*-weighted whole brain volumes, 64 oblique axial slices; 2mm isotropic voxel; TR = 1320 ms; TE = 30 ms; flip angle = 90°, FOV = 212 mm; multiband factor = 4). Patients were instructed to stay awake with their eyes closed and to not think about anything in particular. A five-minute structural scan was acquired using a T1-weighted high-resolution magnetization prepared gradient echo (MPRAGE) sequence: Repetition time (TR) = 2530 ms; echo time (TE) = 3.63 ms; inversion time (TI) = 1100 ms; 1 mm slices, field of view (FOV) = 256, flip angle = 7 degrees.
Neuroimaging Data Processing and Preliminary Analyses
Pre-processing steps of the fMRI data included motion correction, geometric distortion correction using FSL Topup, brain extraction, high pass temporal filtering, prewhitening, registration to anatomical scan, spatial smoothing (4mm), and registration to standard space. In addition to the motion correction noted above, to address concerns about the potential impact of motion on rs-fMRI analyses, we defined a volume to have “excessive motion” if the framewise dependent value exceeded 0.5 mm, and/or if the DVARS value (i.e. 1000 times the root mean squared fractional signal change from the previous volume, calculated over the whole brain) exceeded 8 (Power, Barnes, Snyder, Schlaggar, & Petersen, 2012). Volumes identified as having “excessive motion” were flagged for de-weighing in RSFC analyses. If a scan had more than 30% of volumes with “excessive motion”, that scan was excluded from analysis. Finally, as an additional cleaning step, we conducted a manual denoising procedure (Kelly et al., 2010); specifically, we conducted an independent components analysis of each participant’s resting-state data using the FSL tool MELODIC (pre-setting the number of components to 40), inspected all components, and removed all components that were determined to reflect motion and physiological noise using the FSL tool fslregfilt.
Mean time series were extracted from anatomical FreeSurfer-defined regions of interest for the amygdala and nucleus accumbens and also from cerebrospinal fluid (CSF) and white matter. Four linear regression analyses were conducted using the each seed region of interest (left amygdala, right amygdala, left nucleus accumbens, right nucleus accumbens) as the main regressor, with the following nuisance regressors: time series from FreeSurfer-defined regions of interest for CSF and white matter, motion parameters from the motion correction step noted above, and finally a regressor that de-weighted volumes flagged by DVARS as excessive motion.
Statistical analysis
To examine overall NAC-related change in amygdala and nucleus accumbens RSFC, we conducted voxel-wise paired t-tests to compare pre-versus-post-treatment amygdala RSFC and pre-versus-post-treatment nucleus accumbens RSFC between baseline and post-8-week treatment using the AFNI program 3dttest+. To examine the links between neural change and clinical improvement, we first subtracted the pre-treatment from the post-treatment amygdala and nucleus accumbens maps for each individual, and then conducted a series of voxel-wise linear regression analyses using the AFNI program 3dRegAna to examine the association between change in clinical variables (NSSI frequency, BDI and SCL-90-GSI scores) and change in amygdala RSFC and change in nucleus accumbens RSFC. For all voxel-wise analyses, given the small sample, to reduce the number of comparisons, we used WFU PickAtlas (Lancaster et al., 2000; Maldjian, Laurienti, & Burdette, 2004; Maldjian, Laurienti, Kraft, & Burdette, 2003) to create masks containing the brain regions to which each of the voxel-wise analyses would be limited (separate masks for amygdala versus nucleus accumbens analyses). For the amygdala RSFC analysis, this mask included the salience and default mode networks (e.g. prefrontal cortex, amygdala, hippocampus, insula, parahippocampal gyrus, anterior and posterior cingulate cortex, precuneus, precentral gyrus, superior and middle temporal gyrus, and lateral parietal cortex) (Buckner, Andrews-Hanna, & Schacter, 2008; Menon, 2015)(Buckner, 2012). For the nucleus accumbens RSFC analyses, the mask that included regions implicated in addiction and reward processing (prefrontal cortex, thalamus, amygdala, hippocampus) (Gipson, Kupchik, & Kalivas, 2014). These masks are shown in Supplementary Figure 1. For all the whole-brain group analyses, we used a Monte Carlo permutations approach as implemented in AFNI’s 3dClustSim, using a voxel-wise p threshold of 0.001 and alpha=0.05.
Results
Participants
Thirty-six adolescents aged 13–21 years with a history of NSSI were recruited and enrolled into the study. Since only one of these was male, we excluded him from analyses and focused on the female patients. Twenty-two participants completed the 8-week NAC clinical trial and post-treatment neuroimaging. Data from 4 subjects were excluded from analysis because of excessive motion (n=2) or acquisition errors (wrong TR used; n=2). Demographic and clinical descriptive data for the participants that were analyzed here is reported in Table 1.
Table 1:
Description of the Sample
| Demographic Characteristics | Baseline Mean ± Standard Deviation | Post-Treatment Mean ± Standard Deviation |
|---|---|---|
| Age | 17.11 ± 2.07 | |
| Minority | 0% | |
| Hispanic | 5.6% | |
| Parent SES | 46.50 ± 11.80 | |
| Intelligence Quotient | 104.44 ± 10.55 | |
| Handedness (Right) | 88.9 | |
| Compliance to NAC treatment protocol | 89.63% ± 7.32% | |
| Clinical Characteristics | ||
| Taking other medications | 22.2% | |
| Beck Depression Inventory-II | 27.17 ± 13.70 | 22.56 ± 15.16 |
| Symptom Checklist 90-Revised Global Symptom Index | 1.42 ± 0.60 (n = 17) | 1.07 ± 0.73 (n = 18) |
| Weekly Cutting Episodes | 0.85 ± 1.34 | 0.19 ± 0.30 |
Clinical Outcome Results
Similar to our previously reported results from the larger sample (total N=35, number of participants completing NAC treatment=24)(Cullen et al., 2017), results with this sample who participated in the NAC treatment and also had usable pre-post RSFC data (N=18), showed a treatment associated decrease in NSSI frequency (t=2.18, p=0.049), BDI-II scores (t=2.36, p=0.03) and SCL-90-GSI scores (t=4.52, p<0.001). While change in depression scores were correlated with change in SCL-90-GSI scores (r=0.441, p=.038), neither of these measures was correlated with change in NSSI frequency (r=−0.107, p=.336 for change in BDI-II; r=−0.099, p=.352 for change in SCL-90-GSI scores).
Overall RSFC Changes After NAC Treatment
After treatment, there were no significant overall changes in either amygdala or nucleus accumbens RSFC that survived statistical corrections.
Amygdala RSFC Change Correlates of Clinical Improvement
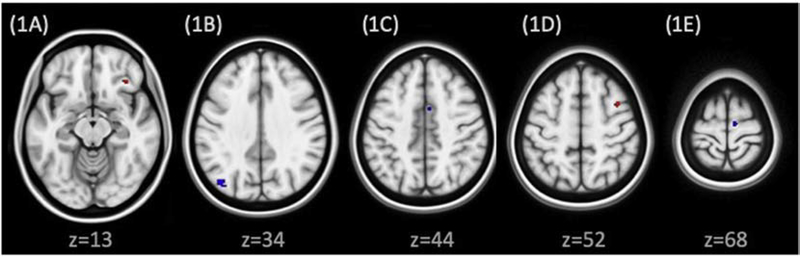
All amygdala RSFC change correlates of clinical improvement are shown in Figure 1. Decrease in the frequency of NSSI episodes was associated with an increase in right amygdala RSFC with right inferior frontal cortex (Brodmann Area 47; Figure 1A) and with a decrease in left amygdala RSFC with right medial frontal cortex / supplemental motor area (SMA) (Brodmann Area 32; Figure 1C). Decrease in BDI-II score was associated with an increase in left amygdala RSFC with the right middle frontal cortex (Brodmann Area 6; Figure 1D) and a decrease in right amygdala RSFC with right medial frontal cortex / SMA (Brodmann Area 6; Figure 1E). Decrease in Global Severity Index scores on the SCL-90 was associated with a decrease in RSFC between left amygdala and left precuneus (Brodmann Area 19; Figure 1B).
Figure 1:
Association between change in amygdala RSFC and clinical improvement. Clusters overlayed on MNI brain (0.5 mm), neurological orientation. Red clusters signify regions where increase in amygdala connectivity was associated with clinical improvement; blue clusters signify regions where decrease in amygdala connectivity was associated with clinical improvement. 1A: Right amygdala to right inferior frontal cortex (Brodmann area 47): Increased RSFC correlates with decreased NSSI frequency. 1B: Left amygdala to left precuneus (Brodmann area 19): decreased RSFC correlates with decreased SCL-90 scores. 1C: Left amygdala to right medial frontal gyrus or supplementary motor area (SMA) (Brodmann area 32): decreased RSFC is associated with decreased NSSI frequency. 1D: Left amygdala to right middle frontal gyrus (Brodmann area 6): Increased RSFC is associated with decreased BDI scores. 1E: Right amygdala to right medial frontal gyrus / SMA (Brodmann area 6): Decreased RSFC is associated with decreased BDI scores.
Nucleus accumbens RSFC Change Correlates of Clinical Improvement
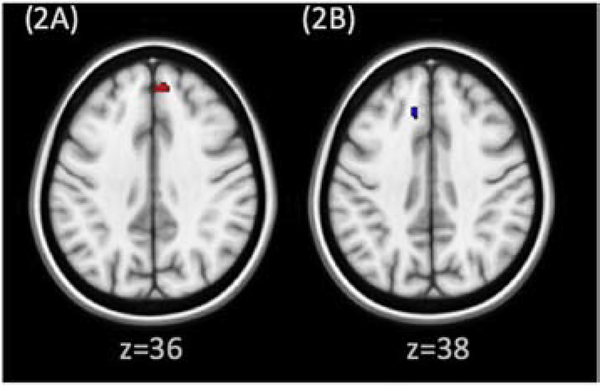
All nucleus accumbens change correlates of clinical improvement are shown in Figure 2. Decrease in NSSI frequency was associated with a decrease in right nucleus accumbens RSFC with left superior medial frontal cortex (Brodmann Area 8; Figure 2B). Decrease in Global Severity Index scores on the SCL-90 was associated with an increase in both right and left nucleus accumbens with right superior medial cortex (Brodmann Area 9; figure 2A).
Figure 2:
Association between change in nucleus accumbens RSFC and clinical improvement. Clusters overlayed on MNI brain (0.5 mm), neurological orientation. Red clusters signify regions where increase in nucleus accumbens connectivity was associated with clinical improvement; blue clusters signify regions where decrease in nucleus accumbens connectivity was associated with clinical improvement. 2A: Left nucleus accumbens to right middle frontal cortex (Brodmann area 9): increased RSFC correlates with decreased SCL-90 scores. 2B: Right nucleus accumbens to left medial frontal gyrus (Brodmann area 8): decreased RSFC correlates with decreased NSSI frequency.
Discussion
Here we report preliminary evidence of neural correlates for clinical improvement in response to NAC in adolescents with NSSI. In this pilot study, although we did not show overall treatment-induced differences in amygdala and nucleus accumbens RSFC, we found that previously-reported improvements in NSSI frequency, depression and overall psychopathology could be mapped to specific circuitry changes in amygdala and nucleus accumbens networks.
Consistent with our hypothesis that clinical improvement would be associated with an increase in amygdala-frontal connectivity, we found that greater improvements in NSSI were associated with greater increases in RSFC between right amygdala and right frontal pole, and that greater reductions in depression scores were associated with increases in left amygdala RSFC with left middle frontal cortex. Cortico-limbic circuits are well-established in the pathophysiology of depression (Mayberg, 1997; Phillips, Drevets, Rauch, & Lane, 2003) and more recently have begun to be implicated in NSSI (Plener et al., 2012; Westlund Schreiner, Cullen, et al., 2017). The fact that the increased amygdala RSFC with frontal regions was not observed in the results of the overall pre-post treatment analysis suggests that not all participants showed this change, but that the adolescents that did show the change were the ones whose depression and/or NSSI (depending on the specific area amygdala-frontal circuit) improved. Findings like these may help explain the variability in prior research examining NAC as an antidepressant (Berk et al., 2008, 2011; Caddy et al., 2015; Fernandes, Dean, Dodd, Malhi, & Berk, 2016), and it provides an in-road for future research designed to confirm whether this change in turn leads to improved symptoms such as depression and NSSI and to test interventions that may optimize engagement of the treatment target (increasing amygdala-frontal RSFC).
A second key finding in this study was that improvement in both NSSI and in depression symptoms was associated with a decrease in positive connectivity (and in some cases, increases in negative connectivity) with the supplementary motor area (SMA). The SMA is involved in planning complex movements as well as in switching from automatic to controlled behavior (Cunnington, Windischberger, & Moser, 2005). Because the decision to engage in NSSI is highly influenced by negative affect, the SMA-amygdala circuit is implicated in NSSI. It is possible that hyperconnectivity between limbic and the SMA region could underlie the entrenchment of NSSI behaviors (i.e., chronically heightened amygdala-SMA RSFC reflects or contributes to persistent and excessive influence of negative affect on behavior planning). We have previously reported results comparing this sample of adolescents with NSSI compared at baseline to a matched sample of healthy controls showing that they had greater amygdala RSFC with the dorsal cingulate and SMA than controls (Westlund Schreiner, Klimes-Dougan, et al., 2017). Therefore, at least for the adolescents with NSSI that showed a change in this hyperconnectivity after NAC treatment, the decrease in RSFC after NAC treatment represents a normalization. These findings suggest that the amygdala-SMA circuit may have promise as a treatment target that could be utilized in future studies.
The third amygdala RSFC finding in this study was that a decrease in Global Severity Index scores on the SCL-90 was associated with a decrease in connectivity (or for most of the subjects who improved this can be viewed as an increase in negative connectivity) between left amygdala RSFC with left precuneus. A prior study showed that in healthy adults, RSFC between amygdala and this region of the occipital is negative (Roy et al., 2009). Furthermore, we previously showed that adolescents with major depression have greater amygdala-precuneus connectivity than healthy controls (Cullen et al., 2014). Thus, the change shown here by those who showed a decrease in psychopathology as measured by the SCL-90 could reflect a normalization of amygdala-precuneus connectivity.
With respect to nucleus accumbens RSFC correlates of clinical response, connections to the frontal cortex were also implicated. In a similar pattern to our amygdala RSFC findings, a reduction in global symptomatology as measured by the SCL-90 was associated with increased RSFC between both right and left nucleus accumbens to the right superior medial gyrus. This adds to previous research in adolescents with NSSI showing that during self-administered painful stimulus, adolescents with NSSI exhibited greater activation but decreased functional connectivity within reward circuitry (Osuch, Ford, Wrath, Bartha, & Neufeld, 2014). However, surprisingly, we found that reduction in NSSI was associated with a decrease in left nucleus accumbens connectivity with the left superior medial frontal gyrus. These lateralized findings suggest a possible dissociation of nucleus accumbens-frontal circuit changes in response to NAC. Future research is needed to replicate and further explore the implications of the asymmetry in these correlates of NSSI and global symptom reduction.
Key limitations of the current study include the small sample size, the lack of a placebo control arm, and some potential limitations of external validity (e.g., the focus only on females, limited socioeconomic and racial diversity of the sample). Additionally, while this study points to possible neural correlates of NAC’s effects on NSSI, we acknowledge that this tool falls short of quantifying neural changes at the cellular level; the exact mechanisms of how NAC leads to changes in RSFC are not yet understood. For example, a key biological mechanism thought to underlie NAC’s positive impact on mental health is via enhanced production of glutathione, the primary antioxidant in the brain that reduces free radicals, providing a neuroprotective effect against the toxicity associated with stress. Research in humans has shown that NAC preserves mitochondrial bioenergetics and normalizes glutathione levels following spinal cord injury (Patel et al., 2014) and following brain injury (Pandya et al., 2014). A recent study of children with autism showed that NAC led to increased glutathione levels compared to placebo (Wink et al., 2016). In a developmental animal model of schizophrenia (early hippocampal lesions in rats), NAC prevented lesion-induced brain and behavioral alterations through diminishing oxidative stress (Cabungcal et al., 2014). Research in rodents has suggested that NAC leads to increased redox ratios in the brain that persist after NAC is out of circulation (Zhou et al., 2015). Oxidative stress mechanisms have generally been implicated in affective disorders (Siwek et al., 2013); conceptualizing NSSI as an ingrained, maladaptive habitual behavior to regulate negative affect, NAC may act on RSFC as a downstream product of the reduction of brain toxicity from oxidative stress induced by NAC. While we propose that the RSFC changes here may reflect remodeling that occurs as a result of NAC’s antioxidant effects, there may be other mechanisms at play. In our study we did not collect blood or brain measures of glutathione; future research is needed to explore whether brain-based correlates of oxidative stress (e.g. glutathione levels) might track with RSFC changes in the course of NAC treatment.
In conclusion, we report on amygdala and nucleus accumbens RSFC neural correlates of clinical improvement during a trial of NAC for adolescents with NSSI. These findings suggest amygdala and nucleus accumbens-based circuits as potential treatment targets, and set the stage for future research designed to confirm these neural targets using randomized, placebo-controlled designs to confirm clinical efficacy and mechanisms of effect.
Supplementary Material
SUPPLEMENTARY FIGURE. Masks used to limit number of comparisons in voxel-wise analyses. 2A: This mask was used for the amygdala RSFC analyses, and includes the salience and default mode networks (prefrontal cortex, amygdala, hippocampus, insula, parahippocampal gyrus, anterior and posterior cingulate cortex, precuneus, precentral gyrus, superior and middle temporal gyrus, and lateral parietal cortex) (Buckner, Andrews-Hanna, & Schacter, 2008; Menon, 2015)(Buckner, 2012). 2B. This mask was used for the nucleus accumbens RSFC analyses, and includes regions implicated in addiction and reward processing (prefrontal cortex, thalamus, amygdala, hippocampus) (Gipson, Kupchik, & Kalivas, 2014).
Highlights.
This study explored neural circuit changes following an open-label, 8-week trial of N-acetylcysteine in female adolescents with NSSI.
We examined how change in clinical functioning tracked with change in resting-state functional connectivity (RSFC) stemming from the amygdala and the nucleus accumbens.
Reduction in NSSI frequency was associated with a decrease in left amygdala RSFC with right supplementary motor area (SMA), but with an increase in right amygdala RSFC with right inferior frontal cortex.
For nucleus accumbens, a reduction in NSSI frequency was associated with a decrease in connectivity between right nucleus accumbens and left frontal cortex.
These preliminary findings suggest amygdala and nucleus accumbens-based circuits as potential treatment targets and set the stage for future research designed to confirm these neural targets using randomized, placebo-controlled designs to confirm clinical efficacy and mechanisms of effect.
Acknowledgements
The authors would like to express our sincere gratitude to the adolescents and their families who participated in this study. This study was funded by the National Institute of Mental Health (R21MH094558; Cullen) and the Academic Health Center at the University of Minnesota (Faculty Research Development Grant; Cullen & Eberly). This work was carried out in part using computing resources at the University of Minnesota Supercomputing Institute.
Footnotes
Conflicts of Interests
The authors have no conflicts of interest to disclose.
Ethical Statement
The study was approved by the University of Minnesota Institutional Review Board, was regulated by the FDA (IND# 10345) and was posted on ClinicalTrials.gov ( NCT01111734). The study was conducted according to internationally-accepted standards for ethical guidelines. All authors have contributed meaningfully to the study and the manuscript, and have approved of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beck AT, Steer RA, & Brown GK (1996). Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Berk M, Copolov DL, Dean O, Lu K, Jeavons S, Schapkaitz I, … Bush AI (2008). N-acetyl cysteine for depressive symptoms in bipolar disorder--a double-blind randomized placebo-controlled trial. Biological Psychiatry, 64(6), 468–475. [DOI] [PubMed] [Google Scholar]
- Berk M, Dean O, Cotton SM, Gama CS, Kapczinski F, Fernandes BS, … Malhi GS (2011). The efficacy of N-acetylcysteine as an adjunctive treatment in bipolar depression: an open label trial. Journal of Affective Disorders, 135(1–3), 389–394. [DOI] [PubMed] [Google Scholar]
- Buckner RL (2012). The serendipitous discovery of the brain’s default network. NeuroImage, 62(2), 1137–1145. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, & Schacter DL (2008). The Brain’s Default Network. Annals of the New York Academy of Sciences, 1124(1), 1–38. [DOI] [PubMed] [Google Scholar]
- Cabungcal J-H, Counotte DS, Lewis EM, Tejeda HA, Piantadosi P, Pollock C, … O’Donnell P (2014). Juvenile antioxidant treatment prevents adult deficits in a developmental model of schizophrenia. Neuron, 83(5), 1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caddy C, Amit BH, McCloud TL, Rendell JM, Furukawa TA, McShane R, … Cipriani A (2015). Ketamine and other glutamate receptor modulators for depression in adults. Cochrane Database of Systematic Reviews, 9, CD011612. [DOI] [PubMed] [Google Scholar]
- Cullen KR, Klimes-Dougan B, Westlund Schreiner M, Carstedt P, Marka N, Nelson K, … Eberly LE (2017). N-Acetylcysteine for Nonsuicidal Self-Injurious Behavior in Adolescents: An Open-Label Pilot Study. Journal of Child and Adolescent Psychopharmacology. 10.1089/cap.2017.0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KR1, Westlund MK2, Klimes-Dougan B2, Mueller BA1, Houri A1, Eberly LE3, Lim KO1. Abnormal amygdala resting-state functional connectivity in adolescent depression. JAMA Psychiatry. 2014. October;71(10):1138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, & Moser E (2005). Premovement activity of the presupplementary motor area and the readiness for action: studies of time-resolved eventrelated functional MRI. Human Movement Science, 24(5–6), 644–656. [DOI] [PubMed] [Google Scholar]
- Derogatis LR, & Savitz KL (2000). The SCL-90-R and the Brief Symptom Inventory (BSI) in Primary Care In Maruish ME (Ed.), Handbook of psychological assessment in primary care settings (pp. 297–334). Mahwah, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Duke PM, Litt IF, & Gross RT (1980). Adolescents’ self-assessment of sexual maturation. Pediatrics, 66(6), 918–920. [PubMed] [Google Scholar]
- Fernandes BS, Dean OM, Dodd S, Malhi GS, & Berk M (2016). N-Acetylcysteine in depressive symptoms and functionality: a systematic review and meta-analysis. The Journal of Clinical Psychiatry, 77(4), e457–e466. [DOI] [PubMed] [Google Scholar]
- First Michael B Gibbon Miriam Spitzer Robert L and Williams JBW (2002). Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen CID-I/P W/ PSY SCREEN). New York: New York: Biometrics Research Institute. [Google Scholar]
- Gipson CD, Kupchik YM, & Kalivas PW (2014). Rapid, transient synaptic plasticity in addiction. Neuropharmacology, 76 Pt B, 276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn CR, & Klonsky ED (2011). One-year test-retest reliability of the Inventory of Statements about Self-Injury (ISAS). Assessment, 18(3), 375–378. [DOI] [PubMed] [Google Scholar]
- Grant JE, Chamberlain SR, Redden SA, Leppink EW, Odlaug BL, & Kim SW (2016). N-Acetylcysteine in the Treatment of Excoriation Disorder: A Randomized Clinical Trial. JAMA Psychiatry, 73(5), 490–496. [DOI] [PubMed] [Google Scholar]
- Gratz KL (2001). Measurement of Deliberate Self-Harm: Preliminary Data on the Deliberate Self-Harm Inventory. Journal of Psychopathology and Behavioral Assessment, 23(4), 253–263. [Google Scholar]
- Hawton K, Witt KG, Taylor Salisbury TL, Arensman E, Gunnell D, Townsend E, … Hazell P (2015). Interventions for self-harm in children and adolescents. Cochrane Database of Systematic Reviews, 12, CD012013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB, & Others. (1975). Four factor index of social status. Retrieved from http://www.academia.edu/download/30754699/yjs_fall_2011.pdf#page=21
- Horwitz AG, Czyz EK, & King CA (2015). Predicting Future Suicide Attempts Among Adolescent and Emerging Adult Psychiatric Emergency Patients. Journal of Clinical Child and Adolescent Psychology: The Official Journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53, 44(5), 751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, … Ryan N (1997). Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry, 36(7), 980–988. [DOI] [PubMed] [Google Scholar]
- Kelly RE, Alexopoulos GS, Wang Z, Gunning FM, Murphy CF, Morimoto SS, … Hoptman MJ (2010). Visual inspection of independent components: defining a procedure for artifact removal from fMRI data. Journal of Neuroscience Methods, 189(2), 233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonsky ED (2007). The functions of deliberate self-injury: a review of the evidence. Clinical Psychology Review, 27(2), 226–239. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, … Fox PT (2000). Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping, 10(3), 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, & Burdette JH (2004). Precentral gyrus discrepancy in electronic versions of the Talairach atlas. NeuroImage, 21(1), 450–455. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, & Burdette JH (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19(3), 1233–1239. [DOI] [PubMed] [Google Scholar]
- Mayberg HS (1997). Limb ic-Cortical Dysregulation : Depression. Journal of Neuropsychiatry, 9, 471–481. [DOI] [PubMed] [Google Scholar]
- McClure EA, Gipson CD, Malcolm RJ, Kalivas PW, & Gray KM (2014). Potential role of N-acetylcysteine in the management of substance use disorders. CNS Drugs, 28(2), 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V (2015). Salience Network. In Brain Mapping (pp. 597–611). [Google Scholar]
- Mitchell JM, O’Neil JP, Janabi M, Marks SM, Jagust WJ, & Fields HL (2012). Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Science Translational Medicine, 4(116), 116ra6. [DOI] [PubMed] [Google Scholar]
- Muehlenkamp JJ, Claes L, Havertape L, & Plener PL (2012). International prevalence of adolescent non-suicidal self-injury and deliberate self-harm. Child and Adolescent Psychiatry and Mental Health, 6(1), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, & Prinstein MJ (2004). A functional approach to the assessment of selfmutilative behavior. Journal of Consulting and Clinical Psychology, 72(5), 885–890. [DOI] [PubMed] [Google Scholar]
- Osuch E, Ford K, Wrath A, Bartha R, & Neufeld R (2014). Functional MRI of pain application in youth who engaged in repetitive non-suicidal self-injury vs. psychiatric controls. Psychiatry Research, 223(2), 104–112. [DOI] [PubMed] [Google Scholar]
- Pandya JD, Readnower RD, Patel SP, Yonutas HM, Pauly JR, Goldstein GA, … Sullivan PG (2014). N-acetylcysteine amide confers neuroprotection, improves bioenergetics and behavioral outcome following TBI. Experimental Neurology, 257, 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SP, Sullivan PG, Pandya JD, Goldstein GA, VanRooyen JL, Yonutas HM, … Rabchevsky AG (2014). N-acetylcysteine amide preserves mitochondrial bioenergetics and improves functional recovery following spinal trauma. Experimental Neurology, 257, 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, & Lane R (2003). Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biological Psychiatry, 54(5), 504–514. [DOI] [PubMed] [Google Scholar]
- Plener PL, Bubalo N, Fladung AK, Ludolph AG, & Lulé D (2012). Prone to excitement: adolescent females with Non-suicidal self-injury (NSSI) show altered cortical pattern to emotional and NSS-related material. Psychiatry Research, 203(2–3), 146–152. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes K. a., Snyder AZ, Schlaggar BL, & Petersen SE (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59(3), 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart R, Amos T, Siegfried N, Ipser JC, Fineberg N, Chamberlain SR, & Stein DJ (2013). Pharmacotherapy for trichotillomania. Cochrane Database of Systematic Reviews, 11, CD007662. [DOI] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AMC, Uddin LQ, Gotimer K, … Milham MP (2009). Functional connectivity of the human amygdala using resting state fMRI. NeuroImage, 45(2), 614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwek M, Sowa-Kućma M, Dudek D, Styczeń K, Szewczyk B, Kotarska K, … Nowak G (2013). Oxidative stress markers in affective disorders. Pharmacological Reports: PR, 65(6), 1558–1571. [DOI] [PubMed] [Google Scholar]
- Tang J, Yu Y, Wu Y, Du Y, Ma Y, Zhu H, … Liu Z (2011). Association between non-suicidal self-injuries and suicide attempts in Chinese adolescents and college students: a cross-section study. PloS One, 6(4), e17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor SE, & Klonsky ED (2014). Correlates of suicide attempts among self-injurers: a meta-analysis. Clinical Psychology Review, 34(4), 282–297. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Jayne M, … Wong C (2007). Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 27(46), 12700–12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1999). Wechsler Abbreviated Scale of Intelligence. New York, NY: The Psychological Corporation: Harcourt Brace & Company. [Google Scholar]
- Westlund Schreiner M, Cullen KR, & Klimes-Dougan B (2017). Multi-level Analysis of the Functioning of the Neurobiological Threat System in Adolescents: Implications for Suicide and Nonsuicidal Self-Injury. Current Behavioral Neuroscience Reports, 4(2), 79–86. [Google Scholar]
- Westlund Schreiner M, Klimes-Dougan B, Mueller BA, Eberly LE, Reigstad KM, Carstedt PA, … Cullen KR (2017). Multi-modal neuroimaging of adolescents with non-suicidal self-injury: Amygdala functional connectivity. Journal of Affective Disorders, 221, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winchel RM, & Stanley M (1991). Self-injurious behavior: a review of the behavior and biology of self-mutilation. The American Journal of Psychiatry, 148(3), 306–317. [DOI] [PubMed] [Google Scholar]
- Wink LK, Adams R, Wang Z, Klaunig JE, Plawecki MH, Posey DJ, … Erickson CA (2016). A randomized placebo-controlled pilot study of N-acetylcysteine in youth with autism spectrum disorder. Molecular Autism, 7, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Zhang Q-E, Cai D-B, Yang X-H, Qiu Y, Ungvari GS, … Xiang Y-T (2018). N-acetylcysteine for major mental disorders: a systematic review and meta-analysis of randomized controlled trials. Acta Psychiatrica Scandinavica, 137(5), 391–400. [DOI] [PubMed] [Google Scholar]
- Zhou J, Coles LD, Kartha RV, Nash N, Mishra U, Lund TC, & Cloyd JC (2015). Intravenous Administration of Stable-Labeled N-Acetylcysteine Demonstrates an Indirect Mechanism for Boosting Glutathione and Improving Redox Status. Journal of Pharmaceutical Sciences, 104(8), 2619–2626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTARY FIGURE. Masks used to limit number of comparisons in voxel-wise analyses. 2A: This mask was used for the amygdala RSFC analyses, and includes the salience and default mode networks (prefrontal cortex, amygdala, hippocampus, insula, parahippocampal gyrus, anterior and posterior cingulate cortex, precuneus, precentral gyrus, superior and middle temporal gyrus, and lateral parietal cortex) (Buckner, Andrews-Hanna, & Schacter, 2008; Menon, 2015)(Buckner, 2012). 2B. This mask was used for the nucleus accumbens RSFC analyses, and includes regions implicated in addiction and reward processing (prefrontal cortex, thalamus, amygdala, hippocampus) (Gipson, Kupchik, & Kalivas, 2014).