Abstract
Background:
Combination diuretic regimens to overcome loop diuretic resistance (DR) are commonly utilized with limited evidence.
Objective:
Compare combination diuretic strategies in acute heart failure (AHF) complicated by DR
Methods:
We conducted a randomized, double-blinded trial in 60 patients hospitalized with AHF and intravenous (IV) loop DR. Patients were randomized to oral metolazone, IV chlorothiazide, or tolvaptan. All patients received concomitant high-dose IV furosemide infusions. The primary outcome was 48-hour weight loss.
Results:
The cohort exhibited DR prior to enrollment, producing 1188 ± 476 ml of urine in 12 hours during high-dose loop diuretics (IV furosemide 612 ± 439 mg/day). All 3 interventions significantly improved diuretic efficacy (p<0.001). Compared to metolazone (4.6 ± 2.7 kg), neither IV chlorothiazide (5.8 ± 2.7 kg), (1.2kg [95% CI −2.9 to 0.6]; p=0.292) or tolvaptan (4.1 ± 3.3 kg), (0.5kg [95% CI −1.5 to 2.4]; p=0.456) resulted in more weight loss at 48-hours. Cumulative urine output increased significantly and did not differ between metolazone (7.78 [6.59, 10.10] L) and chlorothiazide (8.77 [7.37, 10.86] L, p=0.245) or tolvaptan (9.70 [6.36, 13.81] L, p=0.160). Serum sodium decreased less with tolvaptan than metolazone (+4 ± 5 vs −1 ± 3 mEq/L, p =0.001), but 48-hour spot urine sodium was lower with tolvaptan (58 ± 25 mmol/L) than metolazone (104 ± 16 mmol/L, p=0.002) and chlorothiazide (117 ± 14 mmol/L, p<0.001)
Conclusions:
In this moderate-sized diuretic resistance trial, weight loss was excellent with addition of metolazone, IV chlorothiazide or tolvaptan to loop diuretics without a detectable between-group difference.
Keywords: heart failure, diuretic resistance, diuretics, tolvaptan, thiazide, acute heart failure
Introduction
Loop diuretic resistance (DR) in acute heart failure (AHF) is a summative complication resulting from multiple mechanisms and confers a worse prognosis.(1) Distal tubular sodium reabsorption is emerging as a primary cause of DR.(2,3) Current guidelines recommend the addition of a second diuretic such as a thiazide to restore diuretic response, acknowledging the limited data for this recommendation (Class IIa, LOE:B)(4) and (Class IIb, LOE: C)(5).
A paucity of data exists supporting the addition of thiazides in DR. The aggregate body of literature consists of only 300 patients, spread over more than 50 studies with heterogenous patient populations, predominantly observational design, and large variation in both DR definition and diuretic regimens.(6) Intravenous (IV) chlorothiazide is hypothesized to be superior to oral thiazides by eliminating the pharmacokinetic disadvantages of oral thiazides, yet no prospective comparisons exist.(7,8) Addition of thiazides to loop diuretics was associated with worsening electrolyte abnormalities and kidney function in a cohort of AHF hospitalizations after propensity and covariate adjustments.(9)
Tolvaptan did not improve dyspnea in unselected AHF populations but has yet to be compared to other combination nephron blockade regimens on diuretic efficacy outcomes in a DR population receiving high-dose loop diuretics.(10,11) In this population, tolvaptan could theoretically restore diuretic efficacy similar to thiazides but with fewer electrolyte abnormalities.(10,11) Randomized, controlled clinical trials comparing thiazides and other diuretics acting in the distal tubules combined with loop diuretic therapy during DR in AHF are needed. We aimed to investigate the efficacy and safety of diuretic combination strategies employing high-dose loop diuretics plus either oral metolazone, IV chlorothiazide, or oral tolvaptan in a contemporary AHF population exhibiting DR to high-dose loop diuretics.
Methods
Study Design
The 3T study (Comparison of Diuretic Strategies for Combination Nephron Blockade [IV Thiazide vs Oral Thiazide vs Tolvaptan] in Acute Heart Failure Complicated by Loop Diuretic Resistance) was a prospective, randomized, double-blinded, double-dummy trial conducted at Vanderbilt University Medical Center ( NCT 02606253). Patients (n=60) hospitalized with hypervolemic AHF complicated by IV loop DR were enrolled. Hypervolemia was confirmed by the treating cardiologist as either (1) pulmonary artery catheterization with a pulmonary capillary wedge pressure > 19mmHg plus a hypervolemia physical exam finding (peripheral edema, ascites, or rales on auscultation) or (2) in the absence of pulmonary artery catheterization, two of the following signs/symptoms: peripheral edema ascites, jugular venous pressure > 10mmHg, or pulmonary edema on chest x-ray. Loop DR was defined as total urine output < 2 liters in the 12 hours prior to enrollment to an IV furosemide equivalent (FE) dose ≥240mg/day over at least the past 12 hours (40mg IV furosemide = 1mg IV bumetanide).(12) Exclusion criteria included: need of renal replacement therapy or ultrafiltration, estimated glomerular filtration rate < 15 ml/min/1.73m2, systolic blood pressure < 85 mmHg, serum potassium < 3.0 mEq/l, serum sodium < 130 or > 145 mEq/l, advanced liver disease, severe malnutrition, pregnancy or breastfeeding, inability to perform standing weight or collect urine, concomitant strong CYP3A4 inhibitors/inducers, receipt of a thiazide in the previous 24 hours, or concomitant non-study diuretics. The study was approved by the Vanderbilt Institutional Review Board.
Randomization and Treatments
Patients were randomized in a 1:1:1 ratio to 1) oral metolazone 5mg twice daily, 2) IV chlorothiazide 500mg twice daily, or 3) oral tolvaptan 30mg once daily in addition to loop diuretic therapy. Metolazone and chlorothiazide doses were chosen to be equipotent.(7,13) Oral chlorothiazide was not used because of poor bioavailability.(7) The investigational pharmacy performed randomization using a random number generated table without blocks or stratification. Blinding was maintained by administering one identical capsule and one IV dose every 12 hours, with dummy capsules or dummy IV bolus doses administered based upon treatment arm.
Loop diuretic infusion was initiated at a FE of 100mg IV bolus and 20mg/h (580mg/day) in those resistant to a total IV furosemide dose of 240-479mg/day and 100mg IV bolus and 30mg/h (820mg/day) in those resistant to ≥ 480mg/day in the 24 hours prior to enrollment. A diuretic titration algorithm similar to the CARRESS-HF trial was employed.(14) (Supplemental Figure 1) The goal total urine output after 24 hours of study diuretic therapy was 3-5 liters. At 24 hours, the loop diuretic infusion could be increased or decreased according to the total urine output. IV inotropes and vasodilators could be initiated according to the algorithm at 24 hours if the patient was not on these therapies at baseline.
All patients received a 2,000ml fluid restriction and 2,000mg/day sodium diet. All patients received twice daily laboratory and continuous telemetry monitoring. Strict fluid intake and urine output were measured. Management of non-study HF medications and electrolyte replacement were directed by the treating cardiologist. The study treatment was continued up to 48 hours. After 48 hours, all treatment was open-label at the discretion of the treating cardiologist.
Study Endpoints
The primary outcome was the change in weight from baseline to 48 hours, measured on the same standing scale by study personnel.(14,15) Secondary outcomes included 48-hour total and net urine output, change in patient-reported congestion on a visual analog scale, diuretic efficiency, potassium and magnesium replacement cumulative doses, change in serum electrolytes from baseline or new abnormalities, new arrhythmias, need for loop diuretic escalation/ de-escalation or inotrope/vasopressor initiation at 24-h, and treatment failures. Treatment failures were defined as requiring non-study diuretics, ultrafiltration, or renal replacement therapies during the study period. Diuretic efficiency was cumulative over 48-hours expressed as 48-h urine output in ml per 40mg of IV furosemide.(1) Weight-based diuretic efficiency was calculated using the change in weight in place of urine output. We defined patients as “poor responders” if their 48-h weight loss was below the 25th percentile for the total population (2.2kg). For patient-reported congestion scores, patients were provided with a 10cm vertical line with 0.5cm increments and instructed to score their global congestive symptoms from worst (zero) to best (ten) congestion symptoms ever.(12) 30-day outcomes were assessed via phone call.
Urine substudy
The last 16 patients sequentially enrolled underwent two sequential 24-hour urine collections, spot urine samples, and timed blood sample collections to evaluate the diuretic’s pharmacokinetic profiles and resultant urine composition, including urine electrolytes and fractional excretion of sodium (FENa). Blood and plasma were collected at baseline, 24, and 48 hours. Spot urine samples were collected at baseline, 1, 4, 25, and 28 hours to provide spot urine samples 1 hour and 4 hours after study drug administration. Urine samples were also collected at 24 and 48 hours from the 24-hour cumulative urine collections to evaluate baseline to 24-hour and 24-hour to 48-hour urine.
Urine electrolytes were measured via indirect ion sensitive electrodes on the Randox Imola clinical chemistry analyzer (Randox Laboratories, Ireland, UK). If urine sodium was below the limit of detection, samples were diluted 1:1 with normal saline and reanalyzed with the final result calculated after adjustment for the dilution. Urine creatinine was determined using a modified Jaffe method.
Statistical Analysis
Primary analysis was performed on an intent-to-treat basis. The primary outcome was additionally analyzed on a per protocol basis in those missing a study diuretic dose. In previous studies, the standard deviation of weight changes between diuretic treatments was 1.6kg.(15-17) A total sample size of 60 patients (n=20 in each arm) was determined to provide 82% power to detect a meaningful difference of 1.5kg weight loss at 48 hours with metolazone as the standard of comparison for chlorothiazide and tolvaptan. The Type I error rate was 0.05.
We utilized student’s t-test for the primary outcome and continuous data with parametric distributions and the Kruskal-Wallis test for outcomes that were continuous data with nonparametric distributions. If differences existed between groups, pairwise comparisons were performed, reported as metolazone as the comparison group for both intravenous chlorothiazide and oral tolvaptan. For secondary outcomes, chi-square was used for secondary outcomes that were nominal data. Continuous data is reported as mean ± SD for parametric distributions and as the median with interquartile ranges for nonparametric distributions. All statistical comparisons were performed with IBM SPSS statistical software for Macintosh, version 25.0. (Armok, NY: IBM Corp.)
Results
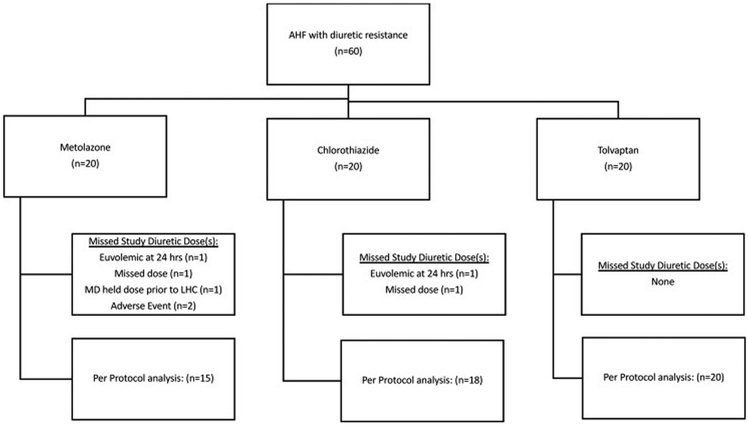
A total of 60 patients were enrolled. (Figure 1) Baseline characteristics are presented in Table 1. Treatment groups were balanced at baseline. The mean age was 62 ± 14 years, and the majority of patients (77%) had HF with reduced ejection fraction. The cohort displayed a high prevalence of diabetes (70%), hypertension (83%), and chronic kidney disease (eGFR 41 ± 20 ml/min/1.73m2). The cohort displayed severe loop DR, producing 1188 ± 476 ml of urine in the 12 hours prior to randomization despite receiving 612 ± 439 mg/day of IV furosemide equivalents.
Figure 1: Patient flow diagram.
While the primary analysis was performed on an intention-to-treat basis, a supplemental per protocol analysis was performed only in patients receiving all study diuretic doses.
AHF= acute heart failure; LHC = left heart catheterization; MD = treating physician”
Table 1:
Baseline Characteristics
| Characteristic | Total Population (n=60) |
Metolazone (n=20) |
Chlorothiazide (n=20) |
Tolvaptan (n=20) |
|---|---|---|---|---|
| Ages (yrs) | 62 ± 14 | 61 ± 15 | 67 ± 11 | 58 ± 14 |
| Male Gender | 43 (71%) | 15 (75%) | 16 (80%) | 12 (60%) |
| Ethnicity | ||||
| White | 40 (67%) | 13 (65%) | 12 (60%) | 15 (75%) |
| Black | 19 (32%) | 6 (30%) | 8 (40%) | 5 (25%) |
| Body-Mass Index (kg/m2) | 38.6 ± 11.2 | 38.7 ± 12.8 | 36.8 ± 7.5 | 40.5 ± 12.7 |
| Weight (kg) | 116.2 ± 39.3 | 119 ± 44 | 110 ± 25 | 119 ± 47 |
| HFpEF | 14 (23%) | 8 (40%) | 4 (20%) | 2 (10%) |
| HFrEF | 46 (77%) | 12 (60%) | 16 (80%) | 18 (90%)* |
| Ischemic cardiomyopathy | 23 (50%) | 6 (50%) | 9 (56%) | 8 (44%) |
| LVEF | 30% ± 16% | 35% ± 19% | 29% ± 17% | 27% ± 13% |
| ICD | 24 (40%) | 5 (25%) | 9 (45%) | 10 (50%) |
| CRT | 17 (28%) | 4 (20%) | 6 (30%) | 7 (35%) |
| Comorbidities | ||||
| Coronary artery disease | 35 (58%) | 10 (50%) | 12 (60%) | 13 (65%) |
| Hypertension | 49 (82%) | 18 (90%) | 18 (90%) | 13 (65%) |
| Diabetes mellitus | 42 (70%) | 12 (60%) | 19 (95%)* | 11 (55%) |
| Chronic kidney disease | 44 (73%) | 16 (80%) | 16 (80%) | 12 (60%) |
| Atrial fibrillation | 32 (53%) | 13 (65%) | 10 (50%) | 9 (45%) |
| Home Medications | ||||
| Beta blocker | 48 (80%) | 17 (855) | 14 (70%) | 17 (85%) |
| ACEI/ARB/ARNI | 30 (50%) | 9 (45%) | 11 (55%) | 10 (50%) |
| Hydralazine | 13 (22%) | 7 (35%) | 4 (20%) | 2 (10%) |
| Aldosterone antagonist | 18 (30%) | 8 (40%) | 4 (20%) | 6 (30%) |
| Inotrope | 2 (3%) | 1 (5%) | 0 (0%) | 1 (5%) |
| Loop diuretic | 56 (93%) | 19 (95%) | 19 (95%) | 18 (90%) |
| Loop diuretic dose in | 176 ± 119 | 165 ± 90 | 184 ± 151 | 179 ± 112 |
| Furosemide Equivalents (mg/day) | ||||
| Thiazide | 6 (10%) | 2 (10%) | 2 (10%) | 2 (10%) |
| Potassium supplement | 21 (35%) | 10 (50%) | 4 (20%)* | 7 (35%) |
| Potassium dose (mEq/day) | 42 ± 40 | 37 ± 25 | 25 ± 10 | 60 ± 61 |
| Magnesium supplement | 8 (13%) | 5 (25%) | 2 (10%) | 1 (5%) |
| Pre-Study Loop Diuretic Regimen | ||||
| Cumulative IV Loop diuretic dose in Furosemide Equivalents for previous 24 hours (mg/24 hrs) | 612 ± 439 | 680 ± 517 | 611 ± 464 | 546 ± 324 |
| Total urine output in past 12 hours (mls) | 1188 ± 476 | 1170 ± 412 | 1372 ± 500 | 1022 ± 465 |
| Diuretic Efficiency | 209 ± 134 | 199 ± 129 | 254 ± 161 | 174 ± 96 |
| Serum Laboratory Values | ||||
| Sodium (mEq/L) | 138 ± 3 | 139 ± 2 | 138 ± 4 | 137 ± 3 |
| Potassium (mEq/L) | 3.9 ± 0.5 | 3.9 ± 0.5 | 4.0 ± 0.4 | 4.0 ± 0.5 |
| Chloride (mEq/L) | 100 ± 5 | 100 ± 6 | 100 ± 4 | 100 ± 5 |
| Bicarbonate (mEq/L) | 25 ± 5 | 26 ± 5 | 25 ± 4 | 25 ± 5 |
| BUN (mg/dl) | 45 ± 25 | 46 ± 19 | 48 ± 31 | 41 ± 23 |
| Serum Creatinine (mg/dl) | 1.9 ± 0.7 | 2.0 ± 0.9 | 2.1 ± 0.7 | 1.8 ± 0.7 |
| eGFR (ml/min/m2) | 41 ± 20 | 41 ± 19 | 36 ± 15 | 46 ± 24 |
| Albumin (g/dl) | 3.5 ± 0.4 | 3.5 ± 0.3 | 3.6 ± 0.4 | 3.6 ± 0.4 |
| Vital Signs | ||||
| Heart rate (bpm) | 83 ± 16 | 82 ± 17 | 83 ± 13 | 85 ± 17 |
| Systolic blood pressure (mmHg) | 115 ± 15 | 114 ± 13 | 119 ± 20 | 114 ± 12 |
| Patient-Reported Congestion Score | 3.4 ± 2.3 | 2.5 ± 2.2 | 4.1 ± 2.2* | 3.75 ± 2.3 |
| Medications at baseline | ||||
| Beta blocker | 46 (77%) | 16 (80%) | 13 (65%) | 17 (85%) |
| ACEI/ARB/ARNI | 24 (40%) | 6 (30%) | 9 (45%) | 9 (45%) |
| Aldosterone antagonist | 27 (45%) | 10 (50%) | 6 (30%) | 11 (55%) |
| Inotrope | 6 (10%) | 2 (10%) | 2 (10%) | 2 (10%) |
| Loop diuretic infusion starting dose | ||||
| 100mg IV bolus + 20mg/hr | 29 (48%) | 10 (50%) | 10 (50%) | 9 (45%) |
| 100mg IV bolus + 30mg/hr | 31 (52%) | 10 (50%) | 10 (50%) | 11 (55%) |
Continuous variables are presented as Mean ± SD and categorical variables are presented as n (%).
P value < 0.05 using metolazone as the comparator group
Abbreviations: , ACEI = angiotensin converting enzyme inhibitor, ARB = angiotensin receptor blocker, ARNI = angiotensin receptor and Neprilysin inhibitor, CRT = cardiac resynchronization therapy, HFrEF = heart failure reduced ejection fraction, HFpEF = heart failure preserved ejection fraction, ICD= implantable cardiac defibrillator, LVEF = left ventricular ejection fraction, LV= left ventricle, RV = right ventricle
48-hour endpoints
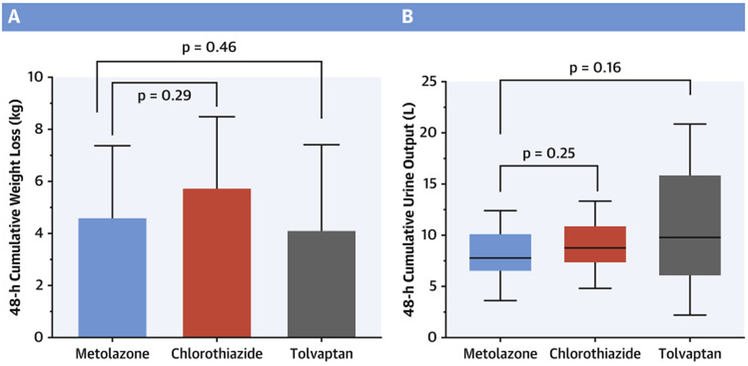
Chlorothiazide did not result in greater 48-hour weight loss than metolazone (5.8 ± 2.7 kg vs 4.6 ± 2.7 kg) (1.2kg [95% CI −2.9 to 0.6]; p=0.292). (Central Illustration) Likewise, tolvaptan (4.1 ± 3.3 kg) did not result in greater 48-hour weight loss than metolazone (0.5kg [95% CI −1.5 to 2.4]; p=0.456). The results were similar when restricted to a per-protocol analysis. (Table 2) Cumulative urine output at 48-hours did not differ between metolazone (7.78 [6.59, 10.10] L), chlorothiazide (8.77 [7.37, 10.86] L), or tolvaptan (9.79 [6.36, 13.81] L). Compared to the thiazide arms, tolvaptan trended toward less cumulative weight loss despite more cumulative urine output. The correlation between weight loss and total urine output was higher with metolazone (r=0.739, P< 0.01) and chlorothiazide (r=0.651, P=0.02) than with tolvaptan (r=0.559, P=0.01); suggesting, the discrepancy between urine output and weight loss with tolvaptan may be due to increased thirst and resultant fluid intake. Patients treated with chlorothiazide (n=1) were less likely to be categorized as “poor responders” compared to patients treated with metolazone (n=5) and tolvaptan (n=7) (p=0.043 vs pooled metolazone-tolvaptan). The metolazone arm had a higher cumulative loop diuretic requirement and lower diuretic efficiency than chlorothiazide or tolvaptan, but this did not reach statistical significance. (Table 2) While all treatments increased diuretic efficiency, tolvaptan was associated with an improved urine output-based diuretic efficiency from baseline (151 ± 207) compared to metolazone (18 ± 133) (p=0.035). Neither tolvaptan nor chlorothiazide produced better weight-based diuretic efficiency than metolazone, although chlorothiazide trended toward significance (p=0.055).
Central Illustration. 48-hour diuretic efficacy.
Part A: 48-hour cumulative weight loss – Mean ± SD Weight loss over 48 hours. Part B: 48-hour cumulative urine output – Median (IQR) urine output over 48 hours
Table 2:
Endpoints at 48 hours
| Characteristic | Metolazone (n=20) |
Chlorothiazide (n=20) |
Tolvaptan (n=20) |
p value Chlorothiazide vs Metolazone |
p value Tolvaptan vs Metolazone |
|---|---|---|---|---|---|
| Primary outcome: Intention-to-treat analysis | |||||
| Cumulative Weight loss (kg) | 4.6 ± 2.7 | 5.8 ± 2.7 | 4.1 ± 3.3 | 0.228 | 0.606 |
| Primary Outcome: Per-protocol analysis | |||||
| Cumulative Weight loss (kg) | 4.7 ± 2.8 | 6.1 ± 2.7 | 4.1 ± 3.3 | 0.188 | 0.579 |
| Secondary Outcomes | |||||
| Total Urine output (L) | 7.78 (6.59, 10.10) | 8.77 (7.37, 10.86) | 9.79 (6.36, 13.81) | 0.245 | 0.160 |
| Total Net Input and Output (L) | −4.60 (3.73, 6.94) | (−6.28 (4.36, 7.91) | −6.43 (4.04, 9.22) | 0.130 | 0.168 |
| Cumulative loop diuretic dose (FE in mg) | 1540 (1060, 2580) | 1350 (1060, 1540) | 1540 (1060, 1540) | 0.057 | 0.198 |
| Cumulative Diuretic Efficiency | 217 ± 107 | 294 ± 123 | 326 ± 213 | 0.066 | 0.105 |
| Change in Diuretic Efficiency from baseline | 18 ± 133 | 40 ± 176 | 151 ± 207 | 0.745 | 0.035 |
| Weight-based Diuretic Efficiency | 0.12 ± 0.09 | 0.19 ± 0.12 | 0.12 ± 0.10 | 0.055 | 0.994 |
| Change in Patient Congestion Score | 4.0 (2.5, 7.0) | 3.0 (2.0, 4.6) | 3.0 (2.0, 5.0) | 0.317 | 0.351 |
| Clinical decongestion achieved | 6 (30%) | 14 (70%) | 9 (45%) | 0.026 | 0.514 |
| Change in Serum Sodium from Baseline (mEq/L) | −1 ± 3 | −1 ± 3 | +4 ± 5 | 0.364 | 0.001 |
| Change in Serum Potassium from Baseline (mEq/L) | −0.1 ± 0.7 | −0.2 ± 0.5 | +0.1 ± 0.5 | 0.303 | 0.489 |
| Change in Serum Chloride from Baseline (mEq/L) | −7 ± 4 | −7 ± 2 | +2 ± 3 | 0.831 | <0.001 |
| Change in Serum Bicarbonate from Baseline (mEq/L) | 5 ± 6 | 3 ± 4 | 2 ± 4 | 0.204 | 0.059 |
| Change in Serum Creatinine from Baseline (mEq/L) | 0.3 ± 0.3 | 0.5 ± 0.5 | 0.03 ± 0.3 | 0.103 | 0.006 |
| Change in eGFR from Baseline (mEq/L) | −6 ± 7 | −9 ± 9 | 2 ± 11 | 0.422 | 0.061 |
| Change in BUN from Baseline (mEq/L) | 9 ± 12 | 12 ± 13 | 2 ± 9 | 0.361 | 0.02 |
| Serum Sodium < 135mEq/L | 2 (10%) | 9 (45%) | 2 (10%) | 0.031 | 1.00 |
| Serum Potassium < 3.5mEq/L | 3 (15%) | 2 (10%) | 2 (10%) | 0.661 | 0.661 |
| Supplemental Potassium administered | 16 (80%) | 15 (75%) | 13 (65%) | 1.0 | 0.48 |
| Cumulative Supplemental Potassium dose (mEq) | 103 ± 131 | 63 ± 60 | 58 ± 56 | 0.446 | 0.411 |
| Supplemental Magnesium administered | 9 (45%) | 10 (50%) | 7 (35%) | 1.0 | 0.748 |
| Cumulative Supplemental Magnesium dose (mg) | 868 ± 1294 | 1015 ± 1198 | 1015 ± 1721 | 0.649 | 0.783 |
Continuous variables are presented as Mean ± SD or Median (IQR) and categorical variables are presented as n (%).
FE = furosemide equivalents
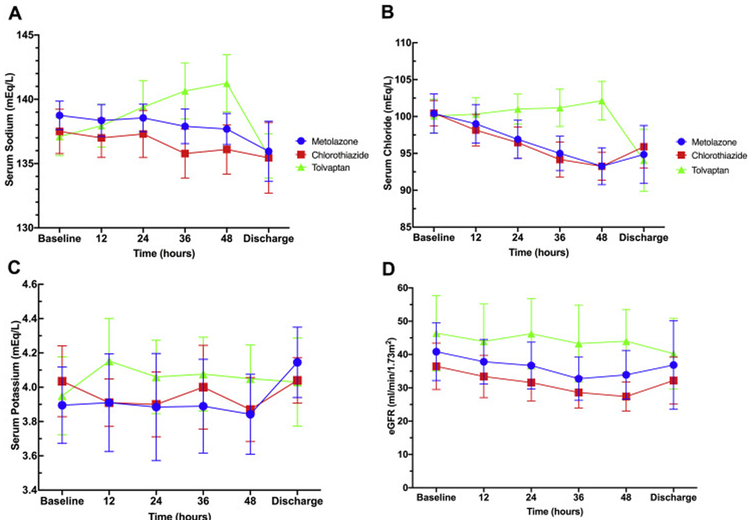
Tolvaptan causes smaller decreases in serum sodium (Figure 2A) and serum chloride (Figure 2B) compared to metolazone, while no differences between chlorothiazide and metolazone were observed. Neither chlorothiazide nor tolvaptan differed from metolazone in metrics of potassium depletion including serum potassium (Figure 2C), incidence of hypokalemia, or incidence of potassium repletion. Metolazone required greater cumulative potassium repletion doses than either chlorothiazide or tolvaptan, but these differences did not reach statistical significance. (Table 2) No treatment group differed in eGFR at any timepoint (Figure 2D), although tolvaptan had a smaller change from baseline in BUN than metolazone (2 ± 9 mg/dl vs 9 ± 12 mg/dl, p=0.002).
Figure 2A: Laboratory value trends.
Trend of serum sodium (mEq/L) - (Metolazone = blue circles; Chlorothiazide = red squares; Tolvaptan = green triangles). 2B: Trend of serum chloride (mEq/L). 2C: Trend of serum potassium (mEq/L). 2D: Trend of eGFR
24-hour endpoints
All 3 interventions significantly improved diuretic efficacy based on urine output at 24 hours compared to the 24-hour urine output prior to randomization (p<0.001 for all arms). Compared to metolazone, 24-hour weight loss was not greater with chlorothiazide (3.4 ± 1.7kg vs 3.1 ± 1.9kg, p=0.515) or tolvaptan (2.0 ± 1.3kg vs 3.1 ± 1.9kg, p=0.050). For 24-hour median urine output, neither chlorothiazide (4.85 L (4.32, 5.81), p=0.066) nor tolvaptan (4.18 L(2.69, 7.56), p=0.617) differed from metolazone (4.19 L (2.64, 5.38)). Similarly, no group required more increases in loop diuretic infusion rate for urine output < 3 liters at 24 hours (Metolazone: 20%, Chlorothiazide: 20%, Tolvaptan: 10%).
Urine and Plasma analyses data
Spot and cumulative urine samples were collected in a subgroup of metolazone (n=5), chlorothiazide (n=5) and, tolvaptan (n=6) patients (Table 3). Chlorothiazide resulted in a greater 24-hour FENa than metolazone or tolvaptan. Compared to metolazone, chlorothiazide no longer produced greater natriuresis at 48-hours, indicating an early benefit that dissipated with continued therapy. Tolvaptan resulted in less natriuresis across all metrics when compared to chlorothiazide. Natriuretic differences were less pronounced between metolazone and tolvaptan. Both chlorothiazide and tolvaptan caused less urinary potassium loss than metolazone.
Table 3:
Urine Electrolytes
| Urine Electrolyte |
Metolazone (n=5) |
Chlorothiazide (n=5) |
Tolvaptan (n=6) |
P value M vs C |
P value M vs T |
P value C vs T |
P value Pooled thiazides vs T |
|---|---|---|---|---|---|---|---|
| Spot Urine Sodium (mmol/L) | |||||||
| Baseline | 85 ± 17 | 79 ± 32 | 69 ± 30 | 0.736 | 0.407 | 0.595 | 0.425 |
| 4-h | 101 ± 19 | 111 ± 18 | 52 ± 21 | 0.471 | 0.001 | 0.001 | < 0.001 |
| 24-h | 104 ± 15 | 120 ± 9 | 55 ± 16 | 0.087 | <0.001 | <0.001 | <0.001 |
| 48-h | 104 ± 16 | 117 ± 14 | 58 ± 25 | 0.308 | 0.002 | <0.001 | <0.001 |
| FENa (%) | |||||||
| Baseline | 4.4 ± 2.9 | 3.5 ± 3.2 | 1.6 ± 1.5 | 0.642 | 0.146 | 0.274 | 0.140 |
| 4-h | 5.9 ± 5.1 | 6.4 ± 4.3 | 2.5 ± 2.2 | 0.848 | 0.180 | 0.146 | 0.103 |
| 24-h | 5.9 ± 2.6 | 10.0 ± 3.0 | 3.2 ± 2.7 | 0.037 | 0.127 | 0.001 | 0.005 |
| 48-h | 7.1 ± 3.5 | 9.7 ± 3.5 | 4.0 ± 4.8 | 0.327 | 0.231 | 0.037 | 0.056 |
| Total Sodium Output | |||||||
| 48-h Cumulative Na+ output (mmol) | 976 ± 341 | 1,195 ± 330 | 638 ± 316 | 0.311 | 0.112 | 0.015 | 0.020 |
| 48-h Cumulative Na+ output (mmol/40mg FE) | 25 ± 11 | 39 ± 14 | 22 ± 14 | 0.108 | 0.725 | 0.050 | 0.163 |
| Urine Potassium | |||||||
| 48-h Delta Spot K+ (mmol/L) | 1 ± 8 | −10 ± 7 | −20 ± 12 | 0.102 | 0.006 | 0.112 | 0.012 |
| 48-h Cumulative K+ output (mmol) | 231 ± 139 | 146 ± 36 | 169 ± 49 | 0.135 | 0.250 | 0.654 | 0.669 |
| Cumulative Na+/K+ ratio | 5.5 ± 4.0 | 8.6 ± 2.9 | 4.1 ± 2.6 | 0.149 | 0.479 | 0.036 | 0.096 |
All values are presented as Mean ± SD.
Delta values are calculated as the spot urine value minus the baseline value.
Pooled thiazides = combination of metolazone and chlorothiazide
C = Chlorothiazide; FE = IV furosemide equivalents; FENa = Fractional excretion of sodium; K+ = potassium; M= Metolazone; Na+ = sodium; T= Tolvaptan
Adverse Events
Total adverse event rates were similar for metolazone (n=7), chlorothiazide (n=6), and tolvaptan (n=10). No new arrhythmias or treatment failures occurred. Only 2 adverse events required study drug discontinuation. Both were symptomatic hypotension in the metolazone arm. Two patients in the tolvaptan arm had asymptomatic increases in serum sodium ≥12 mEq/L in 24 hours. One patient increased 12mEq/L (135 to 147 mEq/L) that stabilized despite continuing therapy. The second patient increased 14 mEq/L (134 to 148 mEq/L) at the end of study and returned to 144mEq/L in 24 hours with continued open-label diuresis. Common adverse events are detailed in Table 4.
Table 4:
Adverse events
| Adverse Event | Total Population (n=60) |
Metolazone (n=20) |
Chlorothiazide (n=20) |
Tolvaptan (n=20) |
|---|---|---|---|---|
| SCr increase > 1mg/dl | 4 | 1 | 3 | 0 |
| Hypotension (SBP < 85mmHg) | 4 | 2 | 0 | 2 |
| Symptomatic Gout flare | 3 | 0 | 0 | 3 |
| Severe hypokalemia (Serum K+ < 3.0 mEq/L) | 1 | 1 | 0 | 0 |
| Myalgia/muscle cramping | 5 | 2 | 2 | 1 |
K+= serum potassium, SBP = systolic blood pressure, SCr = serum creatinine
Discharge Data and 30-day follow up
Forty-eight percent of patients reached clinical decongestion and changed to oral diuretics at 48-h. The median additional length of stay after 48-h study completion was 5 (IQR 2, 8)) days, during which the majority of patients underwent additional, non-study IV diuresis and lost 4.4 ± 6.5 kg (p> 0.05 between groups). As a result, the change in serum chloride from baseline was no longer significant at discharge between metolazone (−6 ± 10 mEq/l), chlorothiazide (−4 ± 6 mEq/L), and tolvaptan (−6 ± 8 mEq/L). Likewise, the change in serum sodium was no longer significant at discharge in metolazone (−3 ± 5 mEq/L), chlorothiazide (−2 ± 6 mEq/L), or tolvaptan (−2 ± 4 mEq/L). The change in eGFR from baseline to discharge did not differ between metolazone (−2 ± 19 ml/min/1.73m2), chlorothiazide (−2 ± 13 ml/min/1.73m2), or tolvaptan (−6 ± 10 ml/min/1.73m2). All patients were discharged alive. At 30 days, 1 patient’s mortality status and 4 patient’s rehospitalization status were unknown. One patient in each of the chlorothiazide and tolvaptan arms died during 30-day follow up. The 30-day readmission rate did not differ for chlorothiazide (35%) or tolvaptan (30%) compared to metolazone (25%).
Discussion
We studied 3 combination diuretic strategies in patients hospitalized with hypervolemic AHF complicated by resistance to high-dose IV loop diuretics. All diuretic adjuvant strategies increased diuretic responsiveness. Compared to metolazone, tolvaptan did not result in greater weight loss or urine output but restored diuretic efficacy while causing smaller decreases in serum sodium and chloride. However, tolvaptan resulted in substantially less sodium excretion. Compared to metolazone, IV chlorothiazide did not result in greater weight loss or urine output but did increase early urine sodium output.
The prespecified analyses of weight loss and urine output failed to find a difference between treatment arms, but the power to detect a difference was lower than anticipated due to larger than predicted standard deviations. Nevertheless, this study has several important findings with direct application to patient care. All three diuretic combinations improved diuretic efficacy, producing a weight loss of approximately 4 kg and urine output of 8.8 liters over 48 hours. Failure to achieve clinical decongestion by discharge occurs in 30% of AHF hospitalizations and is associated with higher rates of 1-year mortality and AHF rehospitalizations.(18,19) While it is unknown how often DR contributes to residual congestion, establishing the safety and efficacy of combination diuretic therapy regimens could improve the rates of decongestion. Despite high-dose combination diuretic therapy with robust response, only 4 patients experienced significant changes in kidney function, which were transient. Our findings correlate with the transient kidney function changes observed in DOSE-HF and ROSE-HF, which appeared not to be driven by true renal injury.(12,20) Electrolyte adverse events were infrequent, with only twice daily monitoring and reasonable potassium repletion. The diuretic combinations studied could be safely employed to most patients hospitalized with AHF without extensive changes in monitoring.
These data add significantly to the limited DR literature. The majority of previous combination diuretic literature did not: quantify baseline DR, require standardized, concomitant high-dose IV loop diuretics, or employ prospective design, randomization or blinding.(6) The largest study included 40 patients resistant to only IV furosemide 160mg/day.(17) We studied a population with baseline DR equivalent to previous low diuretic efficiency cohorts associated with increased mortality.(1,21) The initial study IV furosemide dose (Mean FE ~700mg/day) exceeded the doses in high-dose diuretic arm of DOSE-AHF (Median FE 258 mg/day) and CARRESS-HF and ROSE-HF (Median FE ~200mg/day).(12,21,22) The 48-h weight loss in our study was similar to the 96-h weight loss in CARRESS-HF (5.5kg), which utilized metolazone in 46% of patients.(14)
We utilized weight change as the primary outcome similar to CARRESS-HF and previous combination diuretic studies.(6,14) Weight change during AHF hospitalization has been independently associated with post-discharge mortality and readmission.(23,24) Neither treatment was superior to metolazone when compared by total urine output, which was employed in other diuretic trials.(22) Dyspnea scores did not differ between treatment arms, although weight loss and urine output correlate poorly with dyspnea relief.(24)
Potential pharmacokinetic advantages of IV chlorothiazide compared to metolazone include 100% bioavailability and immediate drug delivery to the site of action. Previous comparisons of oral and IV thiazides combined with loop diuretics in AHF have been small, retrospective analyses.(16,25,26) No differences were found between dosage forms but inferences are hindered by imbalances in: baseline characteristics, loop and thiazide doses, reliability of retrospective urine quantifications, and confounding by indication. In the first randomized controlled trial to compare oral and IV thiazides in AHF, we found no difference in urine output or weight loss at 24 or 48 hours. IV chlorothiazide produce greater natriuresis at 24 hours but was no longer significantly different at 48 hours. This profile matches the medications’ expected pharmacokinetics. Metolazone is slowly absorbed with a long half-life, while IV chlorothiazide bypasses absorption time.(8) Thus, early diuretic metrics are better with chlorothiazide, yet metolazone offers equivalent efficacy over time.
As the majority of DR in HF is reported to arise distal to the loop of Henle, we sought to investigate the addition of a non-thiazide distally acting diuretic. Tolvaptan antagonizes the vasopressin-2 channel in the collecting duct to reduce water absorption through aquaporin channels. Tolvaptan paradoxically trended toward more urine output but less weight loss than metolazone, although neither reached statistical significance. One explanation for this discrepancy could be increased thirst with greater unrecorded fluid intake despite restrictions.(27) The addition of tolvaptan produced less urinary sodium excretion than either thiazide. Urine sodium following loop diuretics alone predicts outcomes and is recommended as the diuretic efficacy metric by which loop diuretic therapy should be titrated.(28) Whether urine sodium retains its prognostic significance in the setting of different combination diuretic therapies requires further investigation.
Changes in serum chemistries were a notably different between tolvaptan and thiazide treatment arms. Approximately 25% of patients hospitalized with hypervolemic AHF develop hyponatremia/hypochloremia during decongestion, which is associated with poor outcomes.(29) Compared to loop diuretic therapy alone, combination therapy with metolazone substantially and independently increases the risk of hyponatremia.(9) Thiazides cause greater relative urinary sodium losses by blocking sodium reabsorption in distal nephron where water permeability is reduced.(30) Non-thiazide diuretics may be considered when hyponatremia is complicating decongestive goals.(30) Tolvaptan could be an equally effective alternative for water loss to thiazides in this setting with monitoring to ensure appropriate sodium correction rates.
Hypokalemia is a common and sometimes profound adverse effect from thiazide combination diuretic therapy.(6) Neither hypokalemia incidence nor supplemental potassium doses differed between study arms. Tolvaptan and chlorothiazide caused less urinary potassium losses and less cumulative potassium dosing than metolazone, but this was not statistically significant. These differences may become clinically significant with longer combination diuretic therapy durations.
Our study has important limitations. We conducted a single-center trial with patients younger than past diuretic trials.(12,14,22) Our findings should be investigated in a larger cohort across multiple centers. We were not powered for non-inferiority analysis and expected a difference in weight loss standard deviation of 1.6kg in 48-hour weight change. The observed standard deviation was 2.7kg thus resulting in a power of 40% to detect the hypothesized effect size. Similar to previous diuretic studies, we allowed the use of diuretics prior to randomization and titration of loop diuretics at 24 hours, which may have reduced the overall between group differences.(14,22) A longer study duration may detect additional differences between treatments. Outcomes at discharge were influenced by open-label diuretic therapy following study completion, which may have eliminated differences between treatment arms.
We did not identify significant differences in weight loss when adding either metolazone, IV chlorothiazide or tolvaptan to high-dose IV loop diuretics in patients with AHF and diuretic resistance. While all 3 strategies produced excellence weight loss in this moderate-sized trial, larger trials are warranted to determine treatment effect differences.
Supplementary Material
Clinical Perspectives.
Diuretic resistance can limit decongestion in heart failure. We compared three combination diuretic strategies to overcome diuretic resistance. Combined with high-dose intravenous furosemide, neither chlorothiazide nor tolvaptan was better than metolazone by metrics of weight loss or urine output. Each strategy restored diuretic efficacy and facilitated decongestion.
Translational Outlook.
Future research should investigate the significance of urine sodium output with combination diuretic therapies to ascertain if the same poor prognostic relationship exists as with loop diuretic monotherapy or if restoration of diuretic response is paramount.
Acknowledgments
Financial Support: This work was supported by a grant from Otsuka Pharmaceuticals and by CTSA award No. UL1 TR002243 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Abbreviations List:
- AHF
Acute heart failure
- DR
Diuretic resistance
- eGFR
Estimated glomerular filtration rate
- FE
Furosemide equivalent
- FENa
Fractional excretion of sodium
- HFrEF
Heart failure reduced ejection fraction
- HFpEF
Heart failure preserved ejection fraction
- IV
Intravenous
- LVEF
Left ventricular ejection fraction
- Scr
Serum creatinine
Footnotes
Clinical Trial Registration: NCT 02606253
Disclosures: Dr. Cox reports research support from Otsuka Pharmaceuticals. Dr. Testani reports research support from Otsuka Pharmaceuticals and Honoraria for DSMB participation form Bayer. The authors have no other relevant conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Testani JM, Brisco MA, Turner JM et al. Loop diuretic efficiency: a metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circulation Heart failure 2014;7:261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rao VS, Planavsky N, Hanberg JS et al. Compensatory Distal Reabsorption Drives Diuretic Resistance in Human Heart Failure. Journal of the American Society of Nephrology: JASN 2017; 28:3414–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ter Maaten JM, Rao VS, Hanberg JS et al. Renal tubular resistance is the primary driver for loop diuretic resistance in acute heart failure. European journal of heart failure 2017; 19:1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yancy CW, Jessup M, Bozkurt B et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology 2013;62:1495–1539. [DOI] [PubMed] [Google Scholar]
- 5.Ponikowski P, Voors AA, Anker SD et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European heart journal 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 6.Jentzer JC, DeWald TA, Hernandez AF. Combination of loop diuretics with thiazide-type diuretics in heart failure. Journal of the American College of Cardiology 2010;56:1527–34. [DOI] [PubMed] [Google Scholar]
- 7.Goodman LS, Brunton LL, Chabner B, Knollmann BrC. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011. [Google Scholar]
- 8.Ernst ME, Moser M. Use of diuretics in patients with hypertension. The New England journal of medicine 2009;361: 2153–64. [DOI] [PubMed] [Google Scholar]
- 9.Brisco-Bacik MA, Ter Maaten JM, Houser SR et al. Outcomes Associated With a Strategy of Adjuvant Metolazone or High-Dose Loop Diuretics in Acute Decompensated Heart Failure: A Propensity Analysis. J Am Heart Assoc 2018;7:e009149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felker GM, Mentz RJ, Cole RT et al. Efficacy and Safety of Tolvaptan in Patients Hospitalized With Acute Heart Failure. Journal of the American College of Cardiology 2017;69:1399–1406. [DOI] [PubMed] [Google Scholar]
- 11.Konstam MA, Kiernan M, Chandler A et al. Short-Term Effects of Tolvaptan in Patients With Acute Heart Failure and Volume Overload. Journal of the American College of Cardiology 2017;69:1409–1419. [DOI] [PubMed] [Google Scholar]
- 12.Felker GM, Lee KL, Bull DA et al. Diuretic strategies in patients with acute decompensated heart failure. The New England journal of medicine 2011;364:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borhani NO. Chlorothiazide and hydrochlorothiazide: a comparative study of their hypotensive, saluretic and hyperuricemic action. Annals of internal medicine 1960;53:342–58. [DOI] [PubMed] [Google Scholar]
- 14.Bart BA, Goldsmith SR, Lee KL et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. The New England journal of medicine 2012;367:2296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trullas JC, Morales-Rull JL, Casado J et al. Rationale and Design of the "Safety and Efficacy of the Combination of Loop with Thiazide-type Diuretics in Patients with Decompensated Heart Failure (CLOROTIC) Trial:" A Double-Blind, Randomized, Placebo-Controlled Study to Determine the Effect of Combined Diuretic Therapy (Loop Diuretics With Thiazide-Type Diuretics) Among Patients With Decompensated Heart Failure. Journal of cardiac failure 2016;22:529–36. [DOI] [PubMed] [Google Scholar]
- 16.Kissling KT, Pickworth KK. Comparison of the effects of combination diuretic therapy with oral hydrochlorothiazide or intravenous chlorothiazide in patients receiving intravenous furosemide therapy for the treatment of heart failure. Pharmacotherapy 2014;34:882–7. [DOI] [PubMed] [Google Scholar]
- 17.Channer KS, McLean KA, Lawson-Matthew P, Richardson M. Combination diuretic treatment in severe heart failure: a randomised controlled trial. British heart journal 1994;71:146–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chioncel O, Mebazaa A, Maggioni AP et al. Acute heart failure congestion and perfusion status - impact of the clinical classification on in-hospital and long-term outcomes; insights from the ESC-EORP-HFA Heart Failure Long-Term Registry. European journal of heart failure 2019. [DOI] [PubMed] [Google Scholar]
- 19.Lala A, McNulty SE, Mentz RJ et al. Relief and Recurrence of Congestion During and After Hospitalization for Acute Heart Failure: Insights From Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure (DOSE-AHF) and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARESS-HF). Circulation Heart failure 2015;8:741–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmad T, Jackson K, Rao VS et al. Worsening Renal Function in Patients With Acute Heart Failure Undergoing Aggressive Diuresis Is Not Associated With Tubular Injury. Circulation 2018;137:2016–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiernan MS, Stevens SR, Tang WHW et al. Determinants of Diuretic Responsiveness and Associated Outcomes During Acute Heart Failure Hospitalization: An Analysis From the NHLBI Heart Failure Network Clinical Trials. Journal of cardiac failure 2018;24:428–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen HH, Anstrom KJ, Givertz MM et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA : the journal of the American Medical Association 2013;310:2533–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ambrosy AP, Cerbin LP, Armstrong PW et al. Body Weight Change During and After Hospitalization for Acute Heart Failure: Patient Characteristics, Markers of Congestion, and Outcomes: Findings From the ASCEND-HF Trial. J AmColl Cardiol HF 2017;5:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kociol RD, McNulty SE, Hernandez AF et al. Markers of decongestion, dyspnea relief, and clinical outcomes among patients hospitalized with acute heart failure. Circulation Heart failure 2013;6:240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shulenberger CE, Jiang A, Devabhakthuni S, Ivaturi V, Liu T, Reed BN. Efficacy and Safety of Intravenous Chlorothiazide versus Oral Metolazone in Patients with Acute Decompensated Heart Failure and Loop Diuretic Resistance. Pharmacotherapy 2016;36:852–60. [DOI] [PubMed] [Google Scholar]
- 26.Cardinale M, Altshuler J, Testani JM. Efficacy of Intravenous Chlorothiazide for Refractory Acute Decompensated Heart Failure Unresponsive to Adjunct Metolazone. Pharmacotherapy 2016;36:843–51. [DOI] [PubMed] [Google Scholar]
- 27.Konstam MA, Gheorghiade M, Burnett JC Jr., et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA : the journal of the American Medical Association 2007;297:1319–31. [DOI] [PubMed] [Google Scholar]
- 28.Mullens W, Damman K, Harjola VP et al. The use of diuretics in heart failure with congestion - a position statement from the Heart Failure Association of the European Society of Cardiology. European journal of heart failure 2019;21:137–155. [DOI] [PubMed] [Google Scholar]
- 29.Shchekochikhin DY, Schrier RW, Lindenfeld J, Price LL, Jaber BL, Madias NE. Outcome differences in community- versus hospital-acquired hyponatremia in patients with a diagnosis of heart failure. Circulation Heart failure 2013;6:379–86. [DOI] [PubMed] [Google Scholar]
- 30.Verbrugge FH, Steels P, Grieten L, Nijst P, Tang WH, Mullens W. Hyponatremia in acute decompensated heart failure: depletion versus dilution. Journal of the American College of Cardiology 2015;65:480–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.