Abstract
Although isolated local (LRs) and regional recurrences (RRs) constitute a minority of post-stereotactic ablative radiotherapy (SABR) relapses, their management is becoming increasingly important as the use of SABR continues to expand. However, few evidence-based strategies are available to guide treatment of these potentially curable recurrences. On behalf of the Advanced Radiation Technology Committee (ART) of the International Association for the Study of Lung Cancer (IASLC), this article was written to address management of recurrent disease. Topics discussed include diagnosis and workup, including the roles of volumetric and functional imaging as well as histopathologic methods; clinical outcomes after salvage therapy; patterns of recurrence after salvage therapy; and management options. Our main conclusions are that survival for patients with adequately salvaged LRs is similar to that for patients after primary SABR without recurrence, and survival for those with salvaged RRs (regardless of nodal burden or location) is similar to that of patients with de novo stage III disease. Although more than half of patients who undergo salvage do not develop a second relapse, the predominant pattern of second failure is distant, especially for RRs. Management requires rigorous multidisciplinary coordination. Isolated LRs can be managed with resection and nodal dissection, repeat SABR, thermal ablation, or systemic therapies. RRs can be treated with combined chemoradiotherapy, radiation or chemotherapy alone, or supportive services. Finally, regular and structured follow-up is recommended after post-SABR salvage therapy.
Keywords: stereotactic ablative radiotherapy, stereotactic body radiation therapy, non-small cell lung cancer, salvage, recurrence
INTRODUCTION
Stereotactic ablative radiotherapy (SABR), also known as stereotactic body radiation therapy (SBRT), has become the first-line therapy for medically inoperable early-stage non-small cell lung cancer (NSCLC). Ample evidence supports the efficacy and low toxicity associated with SABR.1–7 For these reasons, many anticipate that SABR may also be effective for medically operable disease. Preliminary comparisons of SABR vs. surgery for early-stage NSCLC indicate that cure rates are similar but SABR has considerably fewer side effects,2, 8–10 and several randomized trials comparing SABR to surgical resection continue to accrue patients (e.g., NCT02984761 NCT02468024, NCT02629458). However, concerns remain regarding SABR for first-line treatment of operable disease. For example, SABR cannot address potential disease in the remainder of the involved lobe or regional lymphatics. Moreover, a recent phase II trial of neoadjuvant SABR followed by lung resection for patients with operable disease has triggered debate regarding how best to define local recurrence after SABR.11 Nevertheless, recurrences after SABR are uncommon; the intra-lobar local disease control rate is in excess of 90% and the local/regional disease control rate is 85% or more.1–7,12 Findings from several phase II trials have shown rates of isolated local recurrences (iLRs) of up to 6%, and up to 8% for isolated regional recurrences (iRRs).3–4,6.
Salvage of iLRs and iRRs is crucial for several reasons. First, such failures are potentially curable, and untreated recurrences pose a mortality risk. Second, evidence-based clarification of the role of a particular modality for salvage would affect its use in clinical practice. As an example, relapses after initial resection have historically been treated with other resection techniques, but SABR may have a role in that setting, despite the general lack of data at this time.6,13–18 Likewise, use of salvage surgery or salvage SABR for post-SABR recurrences has also not been well defined. Third, documentation of the safety and effectiveness of various salvage approaches may affect their use in the primary setting. For example, in early-stage laryngeal or anal cancer, first-line organ-sparing approaches are standard of care, and surgical resection is reserved for salvage.19–20 This issue is also important for NSCLC, in that organ-sparing approaches may be desirable for patients who are frail, have comorbid conditions, are of advanced age, have compromised baseline cardiopulmonary function, and may have continued organ damage from persistent smoking.21–23
Regardless, the use of SABR will continue to increase as the number of elderly patients with comorbid conditions continues to grow, and forms a greater proportion of all patients with potentially inoperable early-stage lung cancer that would be appropriate for SABR therapy.24 From 2008 through 2013, use of SABR for early-stage NSCLC nearly tripled.25 As more patients undergo SABR, more patients will experience post-SABR recurrences that will require salvage, and evidence-based recommendations for the management of such cases become increasingly important. The perceived lack of options in these cases was captured in the current guidelines from the National Comprehensive Cancer Network (NCCN): “…Recurrent and metastatic disease have historically been regarded as incurable…However, selected limited locoregional recurrences may be treated with curative intent”.26 Notably, treatment for patients who develop distant disease after SABR should follow the principles of treatment for stage IV disease.
The purpose of this article is to highlight the nuances of diagnosing recurrent disease in patients treated with SABR, many of whom have complex comorbidities that led to their being referred for SABR in the first place. We offer insight into the rationale for salvage management, and to describe evidence for the various salvage options to direct clinical decision-making in this unique but important setting.
DIAGNOSIS AND WORKUP OF SUSPECTED RECURRENCE
Diagnosing recurrent disease after SABR, particularly suspected LRs or RRs, can be challenging, largely because benign processes (e.g., fibrosis and reactive lymphadenopathy) are difficult to distinguish from true recurrences. Therefore, a central principle for diagnosing recurrences is the need for thorough multidisciplinary evaluation and individualized diagnostic management.
Imaging
The evaluation process for suspected recurrence should commence with a complete interval history and physical examination, including an evaluation of risk factors for recurrent disease. Contrast-enhanced thoracic computed tomography (CT) scans should be obtained 3–6 months after curative SABR for early-stage NSCLC; evidence of suspicious lesions on those images should prompt positron emission tomography (PET)/CT to support the diagnosis of LR, identify areas for biopsy, and detect potential distant failures, the most common pattern of recurrence.1 Brain magnetic resonance imaging (MRI) should be considered for patients with regional or distant metastases, particularly in patients with driver mutations such as epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK). Thus, although brain MRI should be performed for patients with confirmed or highly suspected iRR, the rate of synchronous brain metastases is unknown for iLR. However, because the presence of intracranial disease would drastically change management, and because iLR would ideally require confirmation before aggressive consolidative approaches are pursued, brain MRI in the iLR setting can be considered judiciously.
Evaluation of post-SABR images should include close attention to the thorax, not only because it is the single most common site of post-SABR recurrence1 but also to detect second primary lung neoplasms. Imaging of the irradiated site after SABR remains among the most challenging aspects of follow-up, but the importance of multidisciplinary assessment of the findings cannot be understated. The following characteristics on CT have been identified as being predictors of LR: enlarging lesions with soft tissue density on multiple images obtained over a prolonged interval (e.g., >6 months); craniocaudal growth (because SABR often involves depositing dose in a coplanar ‘horizontal’ manner, the appearance of growth outside this ‘dose plane’ is particularly worrisome); bulging margins; and disappearance of the linear margin.27–28
Although PET/CT can be more sensitive than CT for staging primary NSCLC,29 its usefulness for detecting in-field recurrence after SABR remains challenging. False-positive findings from radiation-related inflammation and fibrosis are common, particularly on PET/CT images obtained within 6 months of SABR and those on which the tumor maximum standardized uptake value (SUVmax) is less than 5.30–31 PET/CT findings may be more reliable for detecting true recurrences if the tumor SUVmax is at least 5 and the scan is obtained at least 6 months after SABR.32–33 In any case, PET/CT should be considered on a case-by-case basis based on careful multidisciplinary evaluation of CT findings.
Histopathology
The gold standard for the diagnosis of LR remains biopsy; core biopsy is preferred over fine-needle aspiration because the former can provide information such as molecular profiling, PDL1 expression, and other relevant markers. Moreover, fine-needle aspiration for recurrence has been associated with a higher rate of nondiagnostic findings (e.g., 20% vs. 3% for diagnosing primary disease34). Nevertheless, biopsy has some notable shortcomings that should be considered. First, pathologic diagnosis of irradiated tissue can be challenging when the post-SABR interval is short, and false-positive findings are likely.11 Also, tissue biopsies are sampling procedures; false-negative results remain a concern even with high-quality image guidance (e.g., CT), especially for lesions smaller than 1−1.5 cm.35–36 Thus, the presence of fibrosis as well as difficulties in distinguishing viable tumor from necrotic radiation-related changes can further increases the false-negative rate. Core biopsies can also be associated with complications, such as clinically significant hemoptysis, pulmonary hemorrhage, and pneumothorax, which could be more common in the presence of lung parenchymal fibrosis. One meta-analysis reported a complication rate of nearly 40% for core biopsy procedures, including major complications in 6%.37
Nodal sampling of suspected iRR (ideally guided by endobronchial ultrasonography or mediastinoscopy) can provide pathologic evidence of RR and its anatomic location, particularly when imaging findings are questionable. Nodal sampling can also be useful for delineating the specific stations to be covered with salvage radiotherapy, as LRs after SABR have been associated with occult nodal disease in 25%−33% of cases,38–40 suggesting that endobronchial sampling be considered on an individualized basis.41 Moreover, because the presence of distant relapses dictates management regardless of nodal status, pathologic nodal sampling procedures may not change the management strategy for patients with documented distant disease. Regardless of iLR or iRR status, as discussed below, such patients are at highest risk for distant failure (and in the case of iLR, for subsequent LR or RR events). As such, histopathology could identify actionable mutations for drug therapy which we argue should be considered in iLR and iRR cases given the patterns and rates of second failure. Overall, we propose that use of pathologic nodal assessment after SABR be limited to patients with locally (case-by-case) or regionally (all) recurrent disease (either proven or strongly suspected), no evidence of distant failure, and the fitness to tolerate these procedures with minimal complications.
SURVIVAL AND SUBSEQUENT DISEASE PROGRESSION AFTER SALVAGE
Review of existing data on outcomes of salvage therapies and patterns of subsequent failure is essential to identify optimal salvage strategies. At this time, the existing data are sparse and are mostly retrospective reviews of small numbers of patients with heterogeneous conditions, largely originating from centers that were early adopters of lung SABR. As a result, identifying patients for particular salvage strategies should be done on an individual basis and based on rigorous multidisciplinary assessment.
Survival after Salvage Therapy
The largest study of post-SABR salvage to date was published in 2018 by investigators at MD Anderson Cancer Center.12 That study reported survival outcomes for 102 patients whose iLRs or iRRs after SABR were salvaged with a variety of different treatment types, including repeat irradiation, surgery, thermal ablation, or systemic therapy.12 The median follow-up times in that study were 57 months from initial SABR and 39 months from recurrence. The most prominent finding was that the median overall survival (OS) time for patients who received salvage therapy was considerably longer than those who did not receive salvage (35.5 months vs. 7.3 months). Although patients who did not have salvage therapy often had poor performance status or could not tolerate further treatment, all such patients experienced progressive disease, suggesting that disease progression may have affected the OS findings. These findings supported those of a similar study by Senthi and colleagues, who noted that salvage therapy for limited recurrent disease was associated with prolonged survival.1 Although recurrent NSCLC has historically been associated with poor OS, the improved prognosis associated with “oligo-recurrent NSCLC” suggests that salvage therapy should be offered more proactively than has been done in the past.
In support of this argument was another finding that the OS for patients who received salvage for iLR was similar to the OS for patients without recurrence after primary SABR,12 which strongly implies that having an iLR is unlikely to affect OS as long as it is adequately salvaged. This in turn suggests that patients with locally recurrent disease should undergo curative-intent approaches (including local definitive therapy) whenever possible. It also reinforces the need for close post-SABR surveillance to identify salvageable iLR promptly.
Regarding RR (whether isolated or in combination with LR), the OS time for patients undergoing salvage therapy was similar to that for patients with newly diagnosed stage III (node-positive) disease,12 which corroborates the findings of another small study.42 This is noteworthy for multiple reasons. First, de novo diagnosis of stage III NSCLC in the contemporary era is not considered particularly unfavorable because treatment outcomes have improved.43 Second, RRs after SABR may reflect occult nodal disease at time of SABR, which would have been addressed during lobectomy.8 This phenomenon has been observed in surgical series, where nodal upstaging after lobectomy for cN0 disease led to OS similar to that of patients initially diagnosed with cN+ disease.44 Because unexpected discovery of pN+ disease after resection should prompt consideration of chemo(radio)therapy, post-SABR RRs should similarly prompt consideration of similar salvage regimens, as discussed in a subsequent section.
Patterns of Subsequent Progression
Despite the limited data available at this time, an understanding of post-salvage progression patterns (and the natural history thereof) has important implications for the choice of salvage therapy. In 50% to 60% of cases – the majority -- patients who have salvage therapy do not experience further recurrence.12, 38–40, 42, 45–46 Of those who do have a second recurrence, the predominant pattern is distant failure.12, 38–40, 42, 45–46 Distant failure rates are ~20% after LR,39–40, 42, 45–46 ~40% after RR that is not treated with chemotherapy,42 and less than 30% after salvage with chemotherapy.12 These findings, although preliminary, imply that systemic therapy could have a critical role for iRR, similar to that for nodal disease in the de novo setting.
Distant failure also seems to occur at different locations after salvage for iLR versus salvage for iRR. Although data are limited at this time, in one study about 90% of distant failures after salvaged iLR occur in other pulmonary lobes and are often amenable to further oligometastatic salvage.12 Conversely, almost half of distant failures after iRR are extrathoracic, which could contribute to the lower survival rates for patients with iRR relative to those with iLR. Nevertheless, the lower risk of extrathoracic dissemination provides another rationale for managing iLRs more aggressively than iRRs.
Second locoregional recurrences after salvage may also be different for those with iLR versus iRR.12, 46 Among patients with iRR, the overall rate of locoregional second recurrence is low (6%) owing to the preponderance of distant failure. However, among patients with iLR, the location of second recurrences tends to be distributed equally between local and regional sites.
Collectively, these findings suggest that LR and RR after initial SABR represent two distinct clinical entities and thus should be managed in ways that reflect the two distinct outcomes. Understanding the nature of post-salvage failures after LR versus those after RR should form the basis of salvage management, as described further below and in Figures 1 and 2.
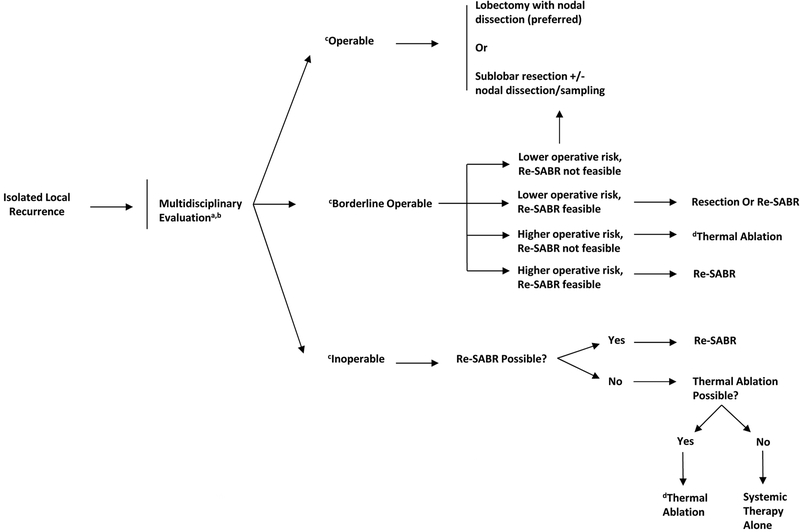
Figure 1.
Proposed management algorithm for isolated local recurrence after stereotactic ablative radiotherapy.
aMultidisciplinary evaluation including thoracic surgeons, radiation oncologists, medical oncologists, and interventional radiologists
bSystemic therapy may be carefully considered in conjunction with locally directed therapy for iLR or iRR given the rates of distant metastases observed
cOperable status pertains to both operability of disease (extent) and patient tolerance. All patients should be formally evaluated by a treating thoracic surgeon. Operability frequently pertains to sufficient pulmonary function (predicted postoperative diffusing capacity for carbon monoxide and forced expiratory volume in 1 second >40%) and patients deemed adequate risk candidates by a thoracic surgeon. However, in light of other less invasive and effective options (re-SABR and thermal ablation) the decision to operate needs to be weighed carefully in this select patient population.
dThermal ablation includes percutaneous destruction of tumor via minimally invasive catheter maneuvering by interventional radiologists or surgeons using radiofrequency ablation, ultrasound ablation, cryoablation, microwave ablation, or lase ablation.
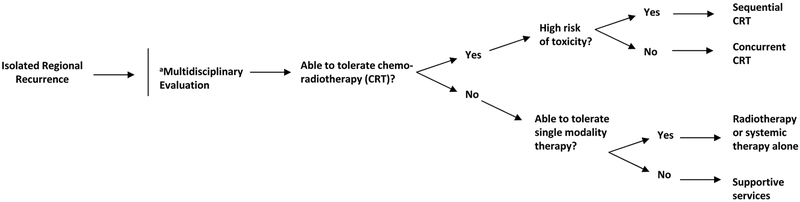
Figure 2.
Proposed management algorithm for isolated regional recurrence after stereotactic ablative radiotherapy.
aMultidisciplinary evaluation including thoracic surgeons, radiation oncologists, medical oncologists, and interventional radiologists
MANAGEMENT STRATEGIES FOR ISOLATED LOCAL RECURRENCES (Figure 1)
Surgery
Use of resection for salvage therapy has two major advantages. First, the presence of a LR implies that the original tumor had some resistance to radiation, and thus surgical resection may be beneficial to avoid treating potentially radioresistant disease with similar techniques. Surgery may be especially preferred if the LR is located directly within the SABR field or in a “high-risk” area (i.e., close to organs at risk in the mediastinum, which are common for central lesions), both of which would make repeat use of SABR challenging. Second, because recurrence after most iLRs are non-disseminated locoregional failures (and because 90% of distant failures occur within the thorax), the ability to remove tumor tissues and evaluate regional lymphatics is critical for potentially preventing further locoregional recurrence and for guiding adjuvant therapy. It might also be more beneficial for larger tumor recurrences where SABR and thermal ablation are known to be less effective in providing long-term control. The primary drawback to surgery is its feasibility for patients who had been poor candidates for surgery in the first place (thereby leading to the choice of SABR for primary treatment). The risk of complications for patients with previously irradiated tissue and poor tolerance for surgery may be higher as well.12, 38–40, 45, 47 Moreover, the presence of fibrotic tissue in about 50% of patients after SABR may complicate salvage resection by requiring open techniques or a greater extent of resection.38–40, 45, 47 However, for “fit” patients who can tolerate surgery, delivering SABR before resection does not increase the surgical risk or impair quality of life.11 Whether these findings can be extrapolated to “marginally fit” patients remains unclear.
Thus, judicious and careful examination of operative risks in the salvage setting is critical to ensure that treatment-related morbidities and mortality are minimized. Notably, if surgical assessment was done before SABR, it should be repeated in full because of the potential for disease that is initially inoperable (at the time of SABR) to convert to operable (at the time of recurrence), if comorbidities can be adequately managed.12, 38–40, 47 We strongly recommend that salvage surgery should be considered by experienced clinicians at high-volume centers with strong multidisciplinary coordination. Even at such institutions, postoperative complications remain relatively common, although in most studies these complications are associated with 0% 90-day mortality and high (approaching 90%) locoregional control rates over the short term.12,38–40,45,47–50] (Table 1).
Table 1.
Selected studies of surgery as salvage after initial stereotactic ablative radiotherapy
| Study and Reference | No. of Patients | Population | Techniques | Median time, SABR to surgery (mo) | Median follow-up time, mo | Significant Findings | Outcomes | Toxic Effects/Complications |
|---|---|---|---|---|---|---|---|---|
| Neri et al, 2010 [48] | 2 | iLR in initially inoperable cases | VATS lobectomy, segmentectomy | 14 | 17 | Complete resection in both patients | Both alive and disease-free at 2 and 32 months after resection | Postoperative pulmonary fistula (n=1) treated with surgery and pleurodesis |
| Chen et al, 2010 [45] | 5 | iLR in initially operable cases | Lobectomy | 17 | 27 | No significant fibrosis/adhesions impairing dissection | All patients alive at last follow-up | None |
| Allibhai et al, 2012 [38] | 4 | iLR in initially inoperable cases | Lobectomy | 15 | 31 | Adhesions in all cases; required conversion to open procedure and partial chest wall resection (n=1 each) | All patients alive and disease-free at last follow-up | None |
| Taira et al, 2014 [49] | 2 | iLR in patients initially operable or inoperable | Wedge resection | 37 | -- | Neither case had viable tumor cells in resection specimen | Not reported | Not reported |
| Hamaji et al, 2015 [50] | 12 | iLR in patients initially operable (9/12) and inoperable (3/12) | Open lobectomy (n=6), VATS lobectomy (n=3), VATS segmentectomy (n=2), open wedge resection (n=1) | 15 | 55 | Nodal dissection done in 11 patients, two of which were positive and offered adjuvant chemotherapy | Median CSS and OS from salvage surgery were 83 months | Intraoperative bleeding (n=1) requiring conversion to open lobectomy; prolonged air leak (n=3) |
| Verstegen et al, 2016 [39] | 9 | iLR (n=7) in initially operable cases; remainder with regional (n=1) or single distant metastasis (n=1) | Open lobectomy (n=5), VATS lobectomy (n=1), sleeve lobectomy (n=1), wedge resection (n=1), pneumonectomy (n=1) | 22 | 19 | Adhesions limited (n=3) or extensive (n=2), one of which required conversion to pneumonectomy; complete resection in all but one patient | Median OS 26 months; two patients failed (one with regional/distant, the other distant alone) | Grade 2 infection (n=2), grade 3 air leak requiring new chest tube (n=1); no 30-day mortality and 11% (n=1) 90-day mortality |
| Antonoff et al, 2017 [47] | 21* | iLR in initially inoperable (18/21) cases | Open lobectomy (n=8), robot/VATS lobectomy (n=2), wedge resection (n=3), segmentectomy (n=1), pneumonectomy (n=1) | 16 | 17 | Complete resection in all; nodal dissection in all patients (n=2 pN1, n=1 pN2, n=1 pM1) | Median OS 14 months | Of all patients, any complication (n=6) and ICU admission (n=2). Prolonged air leak (n=2), atrial arrhythmia (n=3), pulmonary artery thrombosis requiring pneumonectomy (n=1); 30- and 90-day mortality 5% |
| Brooks et al, 2018 [12] | 10 | iLR in initially inoperable cases | Lobectomy (n=6), sublobar (n=4) | -- | 39 | Complete resection in all patients | 100% local control and 90% regional control | 40% with grade 3+ toxicities, including respiratory distress (n=1), pleural effusion (n=1), atrial fibrillation (n=1); no 90- day mortality |
15 with non-metastatic disease
Abbreviations: SABR, stereotactic ablative radiotherapy; iLR, isolated local recurrence; VATS, video-assisted thoracoscopic surgery; CSS, cancer-specific survival; OS, overall survival
Investigations of surgical salvage after SABR are summarized in Table 1. Lobectomy is considered the standard of care in the primary setting, as it has the advantage of removing sufficient tissue to avoid the potential for intralobar failure. However, many clinicians perform sublobar resections for select cases,26 pending the publication of randomized data ( NCT02468024). We recommend that sublobar resections should involve anatomic segmentectomies with systematic lymph node dissection whenever possible, rather than wedge resections.51 Sublobar techniques may be appropriate (1) if the risk of operative morbidity is deemed to be lower than that for lobectomy, (2) if the patient would benefit from resection but has borderline pulmonary function precluding lobectomy, or (3) if re-SABR would be technically challenging. If a patient is eligible for a sublobar resection and no contraindications for re-SABR are present, multidisciplinary evaluation, with patient preferences accounted for, should be used to choose between the two.
Repeat SABR
Although repeat SABR can be challenging owing to complicated treatment planning and theoretical concerns regarding whether radiation should be used to treat presumably radioresistant disease (specifically in-field), repeat SABR has the important advantage of avoiding the morbidity and complications of surgery. Some limited studies of repeat SABR used for LR are summarized in Table 2. Although variations in the extent of dose overlap (e.g., within 1 cm of the original field46,52 versus >1 cm12) among these studies are probably a source of selection bias, the principles of repeat SABR are similar to those for primary disease treatment, including the need to consider the size and location of the disease, tumor motion, high-quality image guidance, and delivery of biologically effective doses (BEDs) of >100 Gy (although whether higher BEDs are required for recurrent disease remains controversial).46,53,54 Overall, repeat SABR has led to excellent rates of short-term local control (nearly 90%), acceptable rates of regional control (>80%), and relatively low rates of grade ≥3 events (<10%)12,28,41,46,52,55–57 (Table 2).
Table 2.
Selected studies of radiation as salvage after initial stereotactic ablative radiotherapy*
| Study and Reference | No. of Patients | Population | Initial SABR | Median time between SABR and reSABR, mo | Median follow-up time, mo | Re-Treatment | Local (Regional) Control | Toxic Effects/Complications |
|---|---|---|---|---|---|---|---|---|
| Peulen et al, 2016 [28] | 29** | iLR of previously SABRed patients with NSCLC (n=10) or metastases (n=19); central (n=11) or peripheral (n=21) | 20–45 Gy in 1–5 fractions | 14 | 12 | SABR, 20–45 Gy in 1–5 fractions; chemo in 12 patients | 5m LC 52% | Twelve grade 3 events, most commonly cough/dyspnea; two grade 4 events including vena caval stenosis and tracheal fistula; three grade 5 bleeds; no grade >3 toxicities in peripheral re-SABR |
| Valakh et al, 2013 [55] | 9 | iLR of previously SABRed patients with primary NSCLC (n=8), all peripheral | 30–60 Gy in 3–5 fractions | 11 | 22 | SABR, 30–60 Gy in 3–5 fractions | 2y LC 75% | Three grade 3 events (dyspnea, chest wall pain); no grade 4–5 events |
| Hearn et al, 2014 [46] | 10 | iLR of previously SABRed patients; all but two peripheral | 30–50 Gy in 1–5 fractions | 15 | 14 | SABR, 50–60 Gy in 3–5 fractions | 60% LC at last follow-up | No grade 3+ events |
| Kennedy et al, 2019 [52] | 21 | iLR of previously SABRed patients; central (n=6) or peripheral (n=15) | 50–60 Gy in 3–5 fractions | 23 | 24 | SABR, 50–60 Gy in 3–5 fractions | 2y LC 81% | No grade 3+ events |
| Manabe et al, 2018 [56] | 26 | iRR of patients previously SABRed (n=14) or resected (n=12) | 48–60 Gy in 4–10 fractions | 12 | 35 | CFRT, 54–66 Gy in 27–33 fractions; chemo in 3 patients | 1y LRC 76% | One grade 3 toxicity (dermatitis), 1 grade 5 pneumonitis |
| Kilburn et al, 2014 [57] | 12 | iRR of patients previously SABRed (n=9), hypofractionated (n=2), or both (n=1) | 50–60 Gy in 3–5 fractions | 15 | 10 | CFRT, 60–70.2 Gy in 23–36 fractions; chemo in 2 patients | 2y LRC 100% | One grade 3 dyspnea; no grade 4–5 events |
| Ward et al, 2016 [41] | 15 | iRR of patients previously SABRed | 30–60 Gy in 1–10 fractions | 11 | -- | 45 Gy in 15 fractions (53%) or 50–60.4 Gy in 20–33 fractions (33%); chemo in 6 patients | 1y LRC 84% | No grade 3+ events |
| Brooks et al, 2018 [12] | 46 | iRR of patients previously SABRed | 50 Gy in 4 fractions or 70 Gy in 10 fractions | -- | 39 | CRT (n=26), most commonly (n=24) with 60–70 Gy in 30–35 fractions; chemo only (n=12), RT only (n=8) | 92% LRC (CRT) and 100% LRC (RT only) at last follow-up | Grade 3+ toxicity in 10 CRT patients (n=3 esophagitis, n=1 pneumonitis, n=4 hematologic); 4 chemotherapy only patients; 1 RT only patients |
Includes only studies of salvage after SABR, not salvage after mixed conventionally-fractionated radiotherapy and SABR.
32 lesions
Abbreviations: SABR, stereotactic ablative radiotherapy; re-SABR, repeat stereotactic ablative radiotherapy; iLR, isolated local recurrence; NSCLC, non-small cell lung cancer; Gy, Gray; LC, local control; iRR, isolated regional recurrence; CFRT, conventionally fractionated radiation therapy; LRC, locoregional control; CRT, chemoradiotherapy
The dosimetric objectives, however, for repeat SABR remain somewhat unclear. Toxic effects seem to be correlated with the composite dose to mediastinal structures and unirradiated lung, rather than the dose received by lung volumes previously treated to high doses.54–55 This observation likely stems from the high-dose lung volumes being relatively nonfunctional,58 but the interval between SABR and repeat SABR is likely important as well. Although most LRs appear more than 1 year after SABR, more rapid recurrences of NSCLC (e.g., ≤16 months) may reflect disease that is radioresistant to SABR and thus may not respond as well to reirradiation.59 As such, the other definitive local therapies (surgery and thermal ablation) may be considered.
If repeat SABR is to be used, several strategies can be used to provide high BED (>100Gy) while minimizing the risk of toxic effects, based on lessons learned from primary SABR. These strategies include use of extended hypofractionated regimens (e.g., 8- to 10-fractions),60 non-daily delivery to allow normal tissue repair,61,62 simultaneous integrated boosting to deliver a lower dose to the planning target volume while maintaining higher doses to gross disease,63 methods of reducing tumor motion with respiration, and avoiding hypofractionation in and around the mediastinum which can damage major vessels and cause deadly bleeding (surgery or other techniques may be considered in these cases).64
Thermal Ablation
Thermal ablation refers to a heterogeneous set of procedures often performed by interventional radiologists (radiofrequency, microwave, and cryoablation); the vast majority of reports involve radiofrequency ablation. Thermal ablation treatments are more invasive than SABR but are generally less invasive than resection; both general anesthesia and conscious sedation can be used.65–68 Although thermal ablation is not often used for primary disease, it may be indicated in some cases of recurrent disease for patients who are not candidates for surgery or repeat SABR. Thermal ablation may be most attractive for in-field SABR recurrences (i.e., potentially radioresistant disease), especially those that cannot be resected with surgery.
Use of thermal ablation is guided by several principles.65–68 First, patients must be able to tolerate a small pneumothorax, which can occur in up to 40% of cases, although fewer than 20% require chest tube placement. Second, thermal ablation can be used to target tumors 1 cm or more from critical central thoracic structures; however, for lesions less than 1 cm from mediastinal critical structures, thermal ablation carries risks of excessive toxicity and potentially a lack of efficacy owing to thermal energy being carried away via convection by the great vessels. Third, thermal ablation results in suboptimal local control for tumors ≥3 cm in diameter.
A small study comparing rates of 90-day mortality and grade ≥3 adverse events for 31 patients treated with SABR (n=15), surgery (n=10), or thermal ablation (n=6) for LR reported rates of 40% after surgery, 7% after SABR, and 0% after thermal ablation.12 Rates of subsequent LR seemed to be similar among all three groups, but the numbers of patients in each group were too small for reliable survival or other comparisons.
Systemic Therapies
Because the most common location of relapse after salvage for iLR is distant (followed by regional), post-salvage systemic therapy should be considered in such cases. Although use of adjuvant systemic therapy may reduce relapse after primary treatment,69 no studies of repeat SABR for iLR reported to date have included systemic therapy, and hence firm recommendations cannot be made. However, an accruing randomized trial is evaluating SABR versus nivolumab+SABR for both primary and iLR disease ( NCT03110978), which is important because immunotherapy for relapsed NSCLC is not approved for post-SABR recurrence, only for post-chemotherapy recurrence.
Systemic therapy alone is an option for salvage if local therapies are contraindicated, but this approach is usually not considered curative.70 Nevertheless, national guidelines recommend a variety of potential agents, including cytotoxic therapy (e.g., platinum doublets, pemetrexed, or other acceptable agents), immunotherapy (e.g., anti-PD-1/PD-L1), or targeted therapy (e.g., EGFR or ALK inhibitors), as well as enrollment in a clinical trial.26 Notably, many of the aforementioned agents require biopsy to establish the molecular profile or PD-L1 expression, which may not be possible in the salvage setting but provides rationale for its use.
MANAGEMENT STRATEGIES FOR REGIONAL RECURRENCES (Figure 2)
Chemoradiotherapy
Because survival for patients receiving salvage treatment for RR (either in isolation or with LR) can be similar to those with de novo stage III disease,12,42 and because distant failure is common in such cases, salvage therapy should ideally mimic that for de novo unresected stage III NSCLC (i.e., chemoradiotherapy with a platinum doublet). Although concurrent chemoradiotherapy improves survival over sequential chemoradiotherapy for primary disease,71 this may not be true for salvage therapy, when the overall disease bulk is much smaller and toxicity may be amplified (as is evidenced in studies on RR comparing radiation alone versus chemoradiation).12, 42 Thus, clinicians should carefully consider the risks and benefits of concurrent versus sequential therapy on an individual basis for each patient.41 Moreover, although giving durvalumab after chemoradiotherapy is now the standard of care for de novo stage III NSCLC,43 no data exist at present to support its use after RR; as such, individualized multidisciplinary discussion is recommended to consider off-label indications of expensive immunotherapeutic agents.72,73
Mediastinal radiotherapy for RR after SABR has been even less well studied. Elective nodal irradiation has no role in locally advanced disease,74 as corroborated by the low rate of out-of-field RRs after salvage.12, 41 Thus, elective nodal irradiation is not recommended for RR after SABR, and providers should be aware of the need to balance potential oncologic gain with the risk of treatment-related toxic effects. With regard to dose and fractionation, the two most common regimens are conventionally fractionated (i.e., 60–70 Gy in 2-Gy fractions, or a simultaneous integrated boost to gross disease with the planning target volume kept to 60 Gy in 30 fractions)12, 41 and mildly hypofractionated (45–60 Gy in 15–20 fractions).12, 41 Due to toxicity, concurrent chemotherapy or other systemic drugs are not routinely recommended with hypofractionated courses but can be given beforehand or afterwards depending on patient tolerance.
Finally, if a RR is accompanied by local failure, treatment options include fractionated radiotherapy that encompasses the LR in the treatment volume or, alternatively, managing the RR independently, with local therapy given for the LR. If surgery is used for local therapy, another option to address mediastinal disease is a therapeutic nodal dissection (provided that doing so does not increase operative risks). However, some form of adjuvant management would be required regardless of whether a dissection takes place, for the reasons noted above.
Radiotherapy or Systemic Therapy Alone
Delivery of either radiation or chemotherapy alone should be considered suboptimal therapy, but this approach may be required if patients are not candidates to receive combined chemoradiotherapy. As noted previously, chemotherapy alone is not considered curative and should not be used as a substitute for local therapy. Similarly, radiotherapy alone is inadequate for locally advanced disease given the rate of micrometastatic disease at presentation.75 These principles should be used in counseling patients who are ineligible for chemoradiotherapy.
Supportive Services
A diagnosis of recurrent cancer often weighs heavily on patients, both emotionally and physically. Despite encouraging survival after multidisciplinary salvage approaches, some patients may refuse or be ineligible for curative-intent therapy. Compassionate counseling and timely coordination of services such as case management, palliative care, and onco-psychology may be of great importance to these patients, and may well improve quality of life and patient-specific outcomes.76 These professionals may also be helpful to encourage patients to be more accepting of therapeutic options (oncologic or non-oncologic). Nevertheless, clinicians and ancillary staff should respect patient autonomy and seek to make the appropriate referrals based on the patient’s reaction to the diagnosis and recommended management.
FOLLOW-UP CONSIDERATIONS
Follow-up after primary SABR usually includes an interval history and physical examination along with thoracic CT but can vary based on institutional and clinician preference. Such follow-up is generally recommended every 3–6 months for the first 1–2 years, every 6–12 months for the next 3–5 years, and annually thereafter. NCCN guidelines recommend follow-up every 3–6 months for the first 3 years, every 6 months for the next 2 years, and annually thereafter.26 The European Society for Medical Oncology recommends biannual follow-up for 3 years followed by annually thereafter.77 The International Association for the Study of Lung Cancer endorses imaging every 3–6 months for the first year, followed by every 6–12 months for the next 3 years, and annually thereafter.78 A recent consensus panel has proposed follow-up at 3 and 6 months, then every 6 months until the end of year 2, and annually thereafter.79.
Follow-up after salvage requires particular attention. Because second recurrences often occur within a year of salvage therapy,12, 46 close follow-up (e.g., every 3 months) during this period is critical to ensure that second recurrences are captured before distant dissemination of disease. Notably, this close follow-up should be independent of the pre-salvage follow-up schedule. For example, if a patient is being followed up every 6 months when an LR/RR is detected, then more frequent follow-up thereafter is indicated. How long the post-salvage follow-up should be remains an open issue because of the rarity of experiencing a second recurrence and the limited follow-up data available for such patients.
Acknowledgements:
The authors gratefully acknowledge the editorial contributions of C. F. Wogan, MS, ELS, of the MD Anderson Division of Radiation Oncology in editorial assistance of this report.
Funding: Supported in part by Cancer Center Support (Core) Grant CA016672 from the National Cancer Institute, National Institutes of Health, to The University of Texas MD Anderson Cancer Center.
REFERENCES
- 1.Senthi S, Lagerwaard FJ, Haasbeek CJ, et al. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol 2012;13:802–809. [DOI] [PubMed] [Google Scholar]
- 2.Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 2009;27:3290–3296. [DOI] [PubMed] [Google Scholar]
- 4.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagata Y, Hiraoka M, Shibata T, et al. Prospective Trial of Stereotactic Body Radiation Therapy for Both Operable and Inoperable T1N0M0 Non-Small Cell Lung Cancer: Japan Clinical Oncology Group Study JCOG0403. Int J Radiat Oncol Biol Phys 2015;93:989–996. [DOI] [PubMed] [Google Scholar]
- 6.Sun B, Brooks ED, Komaki RU, et al. 7-year follow-up after stereotactic ablative radiotherapy for patients with stage I non–small cell lung cancer: Results of a phase 2 clinical trial. Cancer 2017;123:3031–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ball D, Mai GT, Vinod S, et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial. Lancet Oncol 2019;20:494–503. [DOI] [PubMed] [Google Scholar]
- 8.Treasure T, Rintoul RC, Macbeth F. SABR in early operable lung cancer: time for evidence. Lancet Oncol 2015;16,597–598. [DOI] [PubMed] [Google Scholar]
- 9.Verma V. Stereotactic radiotherapy versus surgery for early-stage operable lung cancer: More questions than answers. J Natl Compr Canc Netw 2015;13:1293–1295. [DOI] [PubMed] [Google Scholar]
- 10.Cao C, D’Amico T, Demmy T, et al. Surgery versus SABR for resectable non-small-cell lung cancer. Lancet Oncol 2015;16: e370–e371. [DOI] [PubMed] [Google Scholar]
- 11.Brooks ED, Verma V, Chang JY. Does pathologic response equate to clinical response following SABR for early-stage NSCLC? Front Oncol 2019;9:551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brooks ED, Sun B, Feng L, et al. Association of long-term outcomes and survival with multidisciplinary salvage treatment for local and regional recurrence after stereotactic ablative radiotherapy for early-stage lung cancer. JAMA Netw Open 2018;1:e181390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiong W, Xu Q, Xu Y, et al. Stereotactic body radiation therapy for post-pulmonary lobectomy isolated lung metastasis of thoracic tumor: survival and side effects. BMC Cancer 2014;14:719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gill BS, Clump DA, Burton SA, et al. Salvage stereotactic body radiotherapy for locally recurrent non-small cell lung cancer after sublobar resection and I(125) vicryl mesh brachytherapy. Front Oncol 2015;5:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agolli L, Valeriani M, Carnevale A, et al. Role of salvage stereotactic body radiation therapy in post-surgical locoregional recurrence in a selected population of non-small cell lung cancer patients. Anticancer Res 2015;35:1783–1789. [PubMed] [Google Scholar]
- 16.Sun B, Brooks ED, Komaki R, et al. Long-Term outcomes of salvage stereotactic ablative radiotherapy for isolated lung recurrence of Non–Small cell lung cancer: a phase II clinical trial. J Thorac Oncol 2017;12: 983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He C, Liu YM, Cerra-Franco A, et al. Long-term survival after salvage SBRT for recurrent or secondary non-small cell lung cancer after prior surgery or radiation therapy. J Clin Oncol 2018;36:abstr 8558. [Google Scholar]
- 18.Hou Y, Hermann G, Lewis JH, et al. Clinical Outcomes After Lung Stereotactic Body Radiation Therapy in Patients With or Without a Prior Lung Resection. Am J Clin Oncol 2018;41:695–701. [DOI] [PubMed] [Google Scholar]
- 19.National Comprehensive Cancer Network. Head and Neck Cancers. Version 1.2019. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Accessed January 2, 2019. [DOI] [PMC free article] [PubMed]
- 20.National Comprehensive Cancer Network. Anal Cancer. Version 1.2019. https://www.nccn.org/professionals/physician_gls/pdf/anal.pdf. Accessed January 2, 2019.
- 21.Tammemagi CM, Neslund-Dudas C, Simoff M, Kvale P. Impact of comorbidity on lung cancer survival. Int J Cancer 2003;103:792–802. [DOI] [PubMed] [Google Scholar]
- 22.Tammemagi CM, Neslund-Dudas C, Simoff M, Kvale P. Smoking and lung cancer survival: the role of comorbidity and treatment. Chest 2004;125: 27–37. [DOI] [PubMed] [Google Scholar]
- 23.`Tammemagi CM, Neslund-Dudas C, Simoff M, Kvale P. In lung cancer patients, age, race-ethnicity, gender and smoking predict adverse comorbidity, which in turn predicts treatment and survival. J Clin Epidemiol 2004;57:597–609. [DOI] [PubMed] [Google Scholar]
- 24.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol 2009;27:2758–2765. [DOI] [PubMed] [Google Scholar]
- 25.Holmes JA, Zagar TM, Chen RC. Adoption of Stereotactic Body Radiotherapy for Stage IA Non–Small Cell Lung Cancer Across the United States. JNCI Cancer Spectrum 2017;1:pkx003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Comprehensive Cancer Network. Non-small cell lung cancer. Version 7.2019. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed September 9, 2019.
- 27.Huang K, Senthi S, Palma DA, et al. High-risk CT features for detection of local recurrence after stereotactic ablative radiotherapy for lung cancer. Radiother Oncol 2013;109:51–57. [DOI] [PubMed] [Google Scholar]
- 28.Peulen H, Mantel F, Guckenberger M, et al. Validation of High-Risk Computed Tomography Features for Detection of Local Recurrence After Stereotactic Body Radiation Therapy for Early-Stage Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2016;96:134–141. [DOI] [PubMed] [Google Scholar]
- 29.Fischer B, Lassen U, Mortensen J, et al. Preoperative Staging of Lung Cancer with Combined PET–CT. N Engl J Med 2009;361:32–39. [DOI] [PubMed] [Google Scholar]
- 30.Takeda A, Yokosuka N, Ohashi T, et al. The maximum standardized uptake value (SUVmax) on FDG-PET is a strong predictor of local recurrence for localized non-small-cell lung cancer after stereotactic body radiotherapy (SBRT). Radiother Oncol 2011;101:291–297. [DOI] [PubMed] [Google Scholar]
- 31.Bollineni VR, Widder J, Pruim J, et al. Residual 18F-FDG-PET uptake 12 weeks after stereotactic ablative radiotherapy for stage I non-small-cell lung cancer predicts local control. Int J Radiat Oncol Biol Phys 2012;83:e551–e555. [DOI] [PubMed] [Google Scholar]
- 32.Huang K, Dahele M, Senan S, et al. Radiographic changes after lung stereotactic ablative radiotherapy (SABR)–can we distinguish recurrence from fibrosis? A systematic review of the literature. Radiother Oncol 2012;102:335–342. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X, Liu H, Balter P, Allen PK, et al. Positron emission tomography for assessing local failure after stereotactic body radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2012;83:1558–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beslic S, Zukic F, Milisic S. Percutaneous transthoracic CT guided biopsies of lung lesions; fine needle aspiration biopsy versus core biopsy. Radiol Oncol 2012;46:19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montaudon M, Latrabe V, Pariente A, et al. Factors influencing accuracy of CT-guided percutaneous biopsies of pulmonary lesions. Eur Radiol 2004;14:1234–1240. [DOI] [PubMed] [Google Scholar]
- 36.Yeow KM, Tsay PK, Cheung YC, et al. Factors affecting diagnostic accuracy of CT-guided coaxial cutting needle lung biopsy: retrospective analysis of 631 procedures. J Vasc Interv Radiol 2003;14:581–588. [DOI] [PubMed] [Google Scholar]
- 37.Heerink WJ, de Bock GH, de Jonge GJ, et al. Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol 2017;27:138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allibhai Z, Cho BCJ, Taremi M, et al. Surgical salvage following stereotactic body radiotherapy for early-stage NSCLC. Eur Resp J 2012;39:1039–1042. [DOI] [PubMed] [Google Scholar]
- 39.Verstegen NE, Maat AP, Lagerwaard FJ, et al. Salvage surgery for local failures after stereotactic ablative radiotherapy for early stage non-small cell lung cancer. Radiat Oncol 2016;11:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verstegen NE, Lagerwaard FJ, Hashemi SM, et al. Patterns of disease recurrence after SABR for early stage non–small-cell lung cancer: optimizing follow-up schedules for salvage therapy. J Thorac Oncol 2015;10:1195–1200. [DOI] [PubMed] [Google Scholar]
- 41.Ward MC, Oh SC, Pham YD, et al. Isolated nodal failure after stereotactic body radiotherapy for lung cancer: the role for salvage mediastinal radiotherapy. J Thorac Oncol 2016;11:1558–1564. [DOI] [PubMed] [Google Scholar]
- 42.Kilburn JM, Lester SC, Lucas JT Jr, et al. Management of mediastinal relapse after treatment with stereotactic body radiotherapy or accelerated hypofractionated radiotherapy for stage I/II non–small-cell lung cancer. J Thorac Oncol 2014:9:572–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Antonia SJ, Villegas A, Daniel D, et al. overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018;379:2342–2350. [DOI] [PubMed] [Google Scholar]
- 44.Yang CFJ, Kumar A, Gulack BC, et al. Long-term outcomes after lobectomy for non–small cell lung cancer when unsuspected pN2 disease is found: A National Cancer Data Base analysis. J Thorac Cardiovasc Surg 2016;151:1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen F, Matsuo Y, Yoshizawa A, et al. Salvage lung resection for non-small cell lung cancer after stereotactic body radiotherapy in initially operable patients. J Thorac Oncol 2010;5:1999–2002. [DOI] [PubMed] [Google Scholar]
- 46.Hearn JW, Videtic GM, Djemil T, Stephans KL. Salvage stereotactic body radiation therapy (SBRT) for local failure after primary lung SBRT. Int J Radiat Oncol Biol Phys 2014;90: 402–406. [DOI] [PubMed] [Google Scholar]
- 47.Antonoff MB, Correa AM, Sepesi B, et al. Salvage pulmonary resection after stereotactic body radiotherapy: A feasible and safe option for local failure in selected patients. J Thorac Cardiovasc Surg 2017;154,689–699. [DOI] [PubMed] [Google Scholar]
- 48.Neri S, Takahashi Y, Terashi T, Hamakawa H, Tomii K, Katakami N, Kokubo M. Surgical treatment of local recurrence after stereotactic body radiotherapy for primary and metastatic lung cancers. J Thorac Oncol. 2010. December;5(12):2003–7. doi: 10.1097/JTO.0b013e3181f8a785.49. [DOI] [PubMed] [Google Scholar]
- 49.Taira N, Kawabata T, Ichi T, Kushi K, Yohena T, Kawasaki H, Ishikawa K, Kato S. Salvage operation for late recurrence after stereotactic body radiotherapy for lung cancer: two patients with no viable cancer cells. Ann Thorac Surg. 2014. June;97(6):2167–71. doi: 10.1016/j.athoracsur.2013.07.123. [DOI] [PubMed] [Google Scholar]
- 50.Hamaji M, Chen F, Matsuo Y, Ueki N, Hiraoka M, Date H. Treatment and prognosis of isolated local relapse after stereotactic body radiotherapy for clinical stage I non-small-cell lung cancer: importance of salvage surgery. J Thorac Oncol. 2015. November;10(11):1616–24. doi: 10.1097/JTO.0000000000000662. [DOI] [PubMed] [Google Scholar]
- 51.Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S–e313S. [DOI] [PubMed] [Google Scholar]
- 52.Kennedy WR, Gabani P, Nikitas J, et al. Repeat stereotactic body radiation therapy (SBRT) for salvage of isolated local recurrence after definitive lung SBRT. Radiother Oncol 2019;doi: 10.1016/j.radonc.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peulen H, Karlsson K, Lindberg K, et al. Toxicity after reirradiation of pulmonary tumours with stereotactic body radiotherapy. Radiother Oncol 2011;101:260–266. [DOI] [PubMed] [Google Scholar]
- 54.Meijneke TR, Petit SF, Wentzler D, Hoogeman M, Nuyttens JJ. Reirradiation and stereotactic radiotherapy for tumors in the lung: dose summation and toxicity. Radiother Oncol 2013;107:423–427. [DOI] [PubMed] [Google Scholar]
- 55.Valakh V, Miyamoto C, Micaily B, Chan P, Neicu T, Li S. Repeat stereotactic body radiation therapy for patients with pulmonary malignancies who had previously received SBRT to the same or an adjacent tumor site. J Cancer Res Ther 2013;9(4):680–685. [DOI] [PubMed] [Google Scholar]
- 56.Manabe Y, Shibamoto Y, Baba F, Yanagi T, Iwata H, Miyakawa A, Murai T, Okuda K. Definitive radiotherapy for hilar and/or mediastinal lymph node metastases after stereotactic body radiotherapy or surgery for stage I non-small cell lung cancer: 5-year results. Jpn J Radiol 2018;36(12):719–725. [DOI] [PubMed] [Google Scholar]
- 57.Kilburn JM, Kuremsky JG, Blackstock AW, et al. Thoracic re-irradiation using stereotactic body radiotherapy (SBRT) techniques as first or second course of treatment. Radiother Oncol 2014;110:505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kelly P, Balter PA, Rebueno N, et al. Stereotactic body radiation therapy for patients with lung cancer previously treated with thoracic radiation. Int J Radiat Oncol Biol Phys;2010:78:1387–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trakul N, Harris JP, Le QT, et al. Stereotactic ablative radiotherapy for reirradiation of locally recurrent lung tumors. J Thorac Oncol 2012;7:1462–1465. [DOI] [PubMed] [Google Scholar]
- 60.Li Q, Swanick CW, Allen PK, et al. Stereotactic ablative radiotherapy (SABR) using 70 Gy in 10 fractions for non-small cell lung cancer: exploration of clinical indications. Radiother Oncol 2014;112:256–261. [DOI] [PubMed] [Google Scholar]
- 61.Verma V, Shostrom VK, Zhen W, et al. Influence of Fractionation Scheme and Tumor Location on Toxicities After Stereotactic Body Radiation Therapy for Large (≥5 cm) Non-Small Cell Lung Cancer: A Multi-institutional Analysis. Int J Radiat Oncol Biol Phys 2017;97:778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verma V, Shostrom VK, Kumar SS, et al. Multi-institutional experience of stereotactic body radiotherapy for large (≥5 centimeters) non-small cell lung tumors. Cancer 2017;123:686–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xia T, Li H, Sun Q, et al. Promising clinical outcome of stereotactic body radiation therapy for patients with inoperable Stage I/II non–small-cell lung cancer. Int J Radiat Oncol Biol Phys 2006;66:117–125. [DOI] [PubMed] [Google Scholar]
- 64.Finazzi T, Palacios MA, Spoelstra FO, et al. Role of on-table plan adaptation in MR-guided ablative radiation therapy for central lung tumors. Int J Radiat Oncol Biol Phys 2019;doi: 10.1016/j.ijrobp.2019.03.035. [DOI] [PubMed] [Google Scholar]
- 65.Dupuy DE, Zagoria RJ, Akerley W, et al. Percutaneous radiofrequency ablation of malignancies in the lung. Am J Roentgenol 2000;174:57–59. [DOI] [PubMed] [Google Scholar]
- 66.Lee JM, Jin GY, Goldberg SN, et al. Percutaneous radiofrequency ablation for inoperable non–small cell lung cancer and metastases: preliminary report. Radiology 2004;230:125–134. [DOI] [PubMed] [Google Scholar]
- 67.Fernando HC, De Hoyos A, Landreneau RJ, et al. Radiofrequency ablation for the treatment of non–small cell lung cancer in marginal surgical candidates. J Thorac Cardiovasc Surg 2005;129:639–644. [DOI] [PubMed] [Google Scholar]
- 68.de Baère TD, Palussiere J, Aupérin A, et al. Midterm local efficacy and survival after radiofrequency ablation of lung tumors with minimum follow-up of 1 year: prospective evaluation. Radiology 2006;240:587–596. [DOI] [PubMed] [Google Scholar]
- 69.Kann BH, Miccio JA, Stahl JM, et al. Stereotactic body radiotherapy with adjuvant systemic therapy for early-stage non-small cell lung carcinoma: A multi-institutional analysis. Radiother Oncol 2019;132:188–196. [DOI] [PubMed] [Google Scholar]
- 70.Milton DT, Miller VA. Advances in cytotoxic chemotherapy for the treatment of metastatic or recurrent non-small cell lung cancer. Semin Oncol 2005;32:299–314. [DOI] [PubMed] [Google Scholar]
- 71.Auperin A, Le Pechoux C, Pignon JP, et al. Concomitant radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (NSCLC): a meta-analysis of individual data from 1764 patients. Ann Oncol 2006;17:473–483. [DOI] [PubMed] [Google Scholar]
- 72.Verma V, Sprave T, Haque W, et al. A systematic review of the cost and cost-effectiveness studies of immune checkpoint inhibitors. J Immunother Cancer 2018;6:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Verma V. Economic sustainability of immune-checkpoint inhibitors: the looming threat. Nat Rev Clin Oncol 2018;15:721–722. [DOI] [PubMed] [Google Scholar]
- 74.Rosenzweig KE, Sura S, Jackson A, Yorke E. Involved-field radiation therapy for inoperable non-small-cell lung cancer. J Clin Oncol 2007;25:5557–5561. [DOI] [PubMed] [Google Scholar]
- 75.Dillman RO, Seagren SL, Propert KJ, et al. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancer. N Engl J Med 1990;323:940–945. [DOI] [PubMed] [Google Scholar]
- 76.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med 2010;363:733–742. [DOI] [PubMed] [Google Scholar]
- 77.Vansteenkiste J, Crino L, Dooms C, et al. 2nd ESMO Consensus Conference on Lung Cancer: early-stage non-small-cell lung cancer consensus on diagnosis, treatment and follow-up. Ann Oncol 2014;25:1462–1474. [DOI] [PubMed] [Google Scholar]
- 78.Huang K, Palma DA, IASLC Advanced Radiation Technology Committee. Follow-up of patients after stereotactic radiation for lung cancer: a primer for the nonradiation oncologist. J Thorac Oncol 2015;10:412–419. [DOI] [PubMed] [Google Scholar]
- 79.Nguyen TK, Senan S, Bradley JD, et al. Optimal imaging surveillance after stereotactic ablative radiation therapy for early-stage non-small cell lung cancer: Findings of an International Delphi Consensus Study. Pract Radiat Oncol 2018;8:e71–e78. [DOI] [PubMed] [Google Scholar]