Abstract
Patients with congestive heart failure (CHF) require complex medical management across the continuum of care. Electronic health records (EHRs) are currently used for traditional tasks of documentation, reviewing and managing test results, computerized order entry, and billing. Unfortunately many clinicians view EHRs merely digitized versions of paper charts, which create additional work and cognitive burden without improving quality or efficiency of care. In fact, EHRs are revolutionizing the care of chronic diseases such as CHF. In this article we describe how appropriate use of technologies offered by EHRs can help standardize CHF care, promote adherence to evidence-based guidelines, optimize workflow efficiency, improve performance metrics, and facilitate patient engagement. We discuss a number of tools including documentation templates, telehealth and telemedicine, health information exchange, order sets, clinical decision support, registries, and analytics. Where available, we present evidence of their potential utility in management of CHF. Together these EHR tools can also be used to enhance quality improvement, patient management and clinical research as part of a learning healthcare system model. We will describe how existing EHR tools can support patients, cardiologists, and care teams to deliver consistent, high quality, coordinated, patient-centered and guideline-concordant care of CHF.
Keywords: electronic health record, heart failure, clinical decision support, analytics
INTRODUCTION
Congestive heart failure (CHF) is a prevalent and costly disease with a grim prognosis. (1) Several interventions have proven beneficial in patients with a left ventricular ejection fraction (LVEF) ≤ 40%, yet utilization of these therapies is suboptimal. (2) Care for this vulnerable population is made challenging by multiple factors including delayed identification, complex comorbidity profiles, fragmented care, and lack of timely access to clinical resources.
Electronic health records (EHRs) are used widely to support clinical care through activities such as documentation, order entry, results reporting, and billing. (3) Despite great potential for improving care quality and efficiency, EHRs are often not used effectively for chronic disease management. We review EHR features that support CHF care by improving adherence to evidence-based guidelines, supporting efficient care coordination, and facilitating patient engagement. We present evidence of EHR tool efficacy where available and discuss how EHR tools can used in population health management, learning healthcare systems (LHS), and clinical research.
Clinical vignette
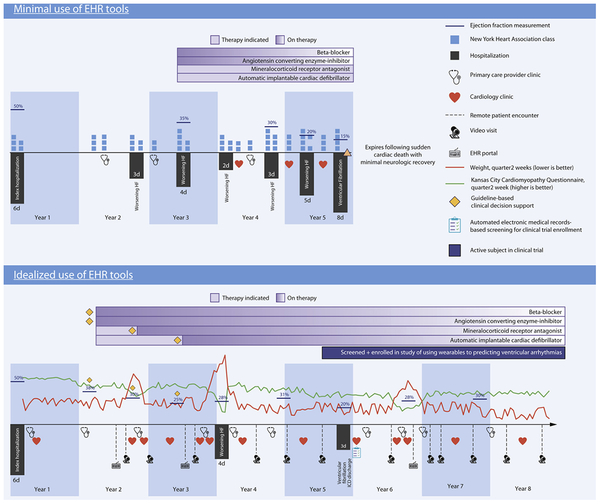
Throughout this review, we refer to the Central Illustration, which depicts the potential role of EHRs in CHF care. Timelines represent alternate clinical courses for a hypothetical 65 year-old woman named Donna following index hospitalization for CHF. The top timeline depicts minimal EHR use whereas the bottom depicts idealized EHR use.
PATIENT ENGAGEMENT
Patient engagement outside clinic visits has many potential benefits in chronic CHF care including opportunities for patient assessment, detection of deterioration, identification of barriers to care, and education to empower patients through self-management. EHRs are increasingly providing tools that facilitate remote interactions between patients and clinicians, and these tools are quickly becoming critical components of patient outreach.
Telemedicine
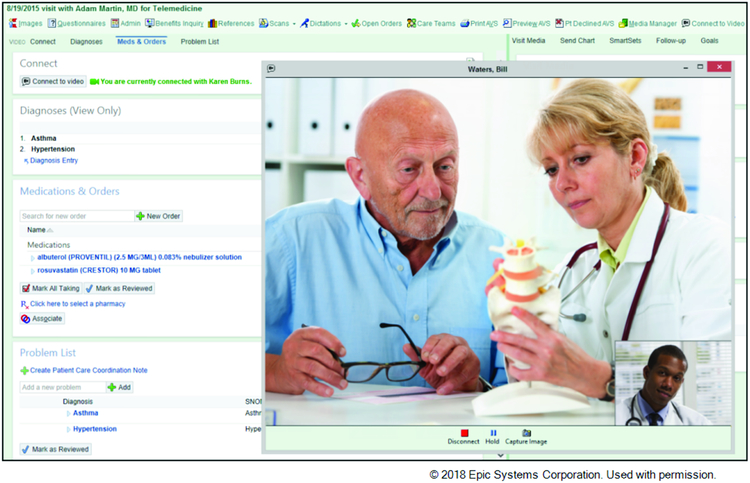
We define telemedicine as remote delivery of medical care using information and communication technologies. The most common mode for remote care delivery has historically been the telephone. Documentation of telephone interactions in the EMR provides a narrative, longitudinal record of status, management, and rationale for clinical decisions. Although these notes contain considerable information, their free-text structure limits ability to search and visualize trends. Patients and clinicians are increasingly using EHR patient portals, which enable communication between clinicians and patients using an e-mail format. (4) In addition to email messages, patients can upload images and videos to enable, for example, remote wound checks. Patient portals can also be used to disseminate education materials, assist patients with decisions such as advance directives, and provide behavior reinforcement reminders. Patient portals may increase outpatient visits and decrease emergency department visits and hospitalizations in patients with complex chronic conditions including HF, although evidence is limited. (4) Video visits are increasingly integrated into EHRs and reduce barriers for relatively immobile patients to be seen, discuss concerns, and remain connected to their clinician (Figure 1).
Figure 1: Video visit conducted and documented within the EHR.
- Integrated patient engagement tools improve access and frequency of assessment by care team.
Telemonitoring
We define telemonitoring as remote collection of patient data for surveillance and decision-making. Perhaps the most common method used presently is the patient portal, through which patients can enter discrete data such as questionairres and measurements like weight. There is growing interest in capturing patient-reported outcomes (PROs) as measures of quality of life and predictors of adverse outcomes, although there are few widely available EHR-based PRO tools. The EHR Access to Seamless Integration of Patient Reported Outcomes Measurement Information System (EASI-PROMIS) project makes the PROMIS tool available in multiple EHR platforms through app stores such as Epic’s App Orchard and Cerner’s App Gallery. There are institutional implementations of CHF-specific questionnaires such as the Kansas City Cardiomyopathy Questionnaire, but none are interoperable between EHRs. (5) Direct capture of biometric data such as heart rate, blood pressure, activity, or volume status is exploding with the advent of interconnected scales, implanted devices (e.g. biventricular pacemaker-defibrillators), and wearables (e.g. fitness trackers) that can transmit data directly to a central repository. (6) However these devices have historically not been integrated with EHRs, and data are often only accessible through external systems. Efforts are underway to import remote biometric data into EHRs, but they are not yet widely available.
With minimal EHR use, assessment of Donna’s health status using NYHA scores occurs only during clinic encounters once or twice per year or during hospitalizations. With idealized EHR use, telemonitoring captures her weight (red line) and health status (KCCQ, green line) at higher frequency. Patient portal messages and video visits increase frequency of timely interactions between Donna and her clinician, for example in response to a change in clinical status (e.g. increase in weight, decrease in KCCQ in years 2 and 6).
DOCUMENTATION
Documentation is an essential component of patient care, which chronicles all aspects of a patient’s clinical course. EHRs have largely replaced paper charts as the primary documentation tool given their considerable advantages in accessibility and security. Still, for many clinicians the transition from paper to EHR has seemed incomplete and even regressive by creating additional work while producing less useful, more confusing documentation. Narrative data, the story of the patient’s clinical course in prose, are often captured in scanned handwritten notes, hand-typed documents, and dictated paragraphs. They are hugely valuable to patients and clinicians but include minimal structured or computer-understandable data. Although familiar and expedient, if clinical documentation remains unstructured, EHRs may remain digital file cabinets and fail to realize their full potential. (7)
Capture of structured data enables many valuable EHR functions including ability to search for and import clinical data elements into notes, perform calculations, visualize trends and trigger clinical recommendations. For example a structured CHF-specific note might be prepopulated with recent LVEF values, weights, medications and risk scores. These tools can reduce clinician tedium and cognitive burden associated with manually assembling data from throughout the EHR and minimize unnecessary variations in care while providing patient-specific recommendations and improving documentation workflow efficiency. Speech recognition tools are imperfect, but they can accelerate data entry by replacing typing or dictation for transcription of narrative notes. Speech recognition tools can also be used in combination with natural language processing (NLP) to interpret narrative text and produce structured data without requiring additional data entry. For example, when a clinician writes or speaks the term “congestive heart failure”, NLP might detect the concept and request additional specifics (e.g. systolic vs. diastolic CHF) and add it to the patient’s problem list.
Disease-specific templates can reduce the time required for documentation, reduce errors in data entry (e.g. copying lab values), and standardize note formats for ease of review by other clinicians. Availability of structured, computer-understandable data can improve outcomes diabetes mellitus and coronary artery disease, (8) although it has not yet been evaluated in CHF. Development of documentation tools is a process that requires iterative evaluation and revision according to clinician feedback. Close collaboration between clinicians and developers is critical to integrate documentation frameworks with institutional workflows, meet practice-specific needs, and accommodate clinician preferences. As data capture technology evolves, one can foresee a future when the EHR records audio of a clinic visit in real-time, transcribes the encounter, catalogs structured data, and generates recommendations without requiring the clinician to write a note (https://www.youtube.com/watch?v=VHMJaV7zJxE).
With minimal EHR use, documentation of Donna’s clinical deterioration in a narrative clinic or telephone note may be missed by clinicians resulting in a failure to follow-up resulting in hospitalization for decompensated heart failure. Additionally, findings like LVEF and NYHA class that represent indications for guideline-directed medical therapy (GDMT) may be overlooked. With idealized EHR use, Donna has frequent encounters with her clinicians via patient portal messages, video visits and face-to-face encounters during episodes of EHR-detected clinical deterioration. Alerts triggered by structured data such as weight or symptom frequency prompt intensification of medical therapy and timely re-evaluation thereby averting hospitalization. When stable, surveillance of structured data detects that Donna’s LVEF declined to < 40%, prompting her care team to initiate GDMT and other evidence-based interventions such as primary prevention implantable cardiac defibrillator placement (ICD, years 2–3).
ANALYTICS AND REPORTING
Analytics represent critical functionality that is vastly superior in EHRs compared with paper charts. Historically, medical record analytics required manual data abstraction from narrative notes, which was slow, error prone, and not scalable. EHR-based analytics are orders of magnitude faster, less prone to error in data transfer (e.g. manually copying lab results), and reusable for multiple needs. Additionally, the explosion of predictive modeling for patient risk stratification and targeting therapy is made possible by increasingly sophisticated EHR capabilities.
RETROSPECTIVE ANALYTICS
Retrospective analytics summarize historical data to demonstrate trends either in an individual patient’s clinical course or in populations. Algorithms applied to retrospective data can identify patient cohorts such as those hospitalized for CHF in order to identify and enroll patients in studies and registries, target interventions, monitor outcomes, and assess performance/quality measures. (9) Reports summarizing care of either an individual cardiologist’s patient panel or a whole clinic’s patient population have been shown to improve adherence to GDMT in CHF when included as part of multi-disciplinary quality improvement. (10) Reports may also be used to automatically extract data contributions to national initiatives such as the National Cardiovascular Data Registry.
PREDICTIVE ANALYTICS
Algorithms that predict patient outcomes such as new-onset disease, adverse events, and treatment response are increasingly used in many settings. One well-studied example is automated early identification of both outpatients (11) and inpatients with CHF. (9) Timely ascertainment of hospitalized CHF patients can trigger cardiology, pharmacy or case manager consultation, and targeted EHR alerts can promote adherence to evidence-based guidelines. (12) A second example of predictive analytics is risk stratification of patients at risk for hospital readmission. (13) Sorting CHF patients into risk groups facilitates optimal allocation of limited resources (e.g. case managers). (12) EHR vendors are now creating real-time predictive applications such as complex early warning scores for clinical deterioration. (14) However complex predictive models require significant resources for development and validation, so those developed by EHR vendors are generally chosen based on strategic considerations that apply to a large proportion of their customer base. Consequently, EHR vendors typically do not build applications that address institution-specific needs. Instead, institutions must build custom applications when their needs do not align with vendor priorities. To facilitate custom application development, some EHR vendors have created app store ecosystems such as Epic’s App Orchard and Cerner’s App Gallery, which assist customers and third-party vendors by providing application performing interfaces (APIs) and technical support. Once published, apps can be licensed by other EHR users with revenue potential for the developers. These ecosystems may ultimately accelerate availability of tools for CHF management but currently remain nascent in terms of number and diversity of apps.
Implementation of customized real-time predictive analytics in an EHR is challenging. Predictive models that perform well enough for clinical applications often use statistical methods such as logistic regression, Cox proportional hazards models, and machine learning, which are not available in most EHRs. Consequently applications may require integration of EHRs with external resources like local external servers or cloud-based platforms. Data exchange between EHRs and external systems is increasingly supported using standards such as Fast Healthcare Interoperability Resources and Substitutable Medical Applications, Reusable Technologies (SMART on FHIR). Using this approach, predictive models run in an external environment using patient data sent from the EHR via FHIR, after which results are returned to the EHR. We provide an example of this in the upcoming section on Advanced Clinical Decision Support (CDS).
CLINICAL DECISION SUPPORT
CDS refers to technologies that provide knowledge or data to aid clinicians in making specific management decisions. The volume of data associated with each patient in the EHR combined with rapidly evolving guidelines and institutional workflows make it impossible for a clinician to consider all of a patients’s EHR data when making clinical decisions. EHR-based CDS can address these challenges by rapidly retrieving relevant patient data, summarizing relevant, up-to-date evidence, and recommending actions based on institutional directives or evidence-based guidelines. Designed properly, CDS makes the recommended course of action the quickest and most efficient for the clinician (i.e. “makes the right thing easy”). Data regarding effectiveness of EHRs for improving adherence to CHF guidelines shows some evidence that CDS that promotes adherence to guidelines improves clinician confidence in CHF management (15) and can improve survival in severe CHF in part through timely referral for advanced therapies. (16)
Order sets
Prespecified order sets address key clinical recommendations for patients with specific conditions in order to standardize care and optimize adherence to selected guidelines. Order sets can promote guideline adherence by improving workflow efficiency when placing recommended orders. CHF order sets might offer evidence-based beta-blockers (e.g. metoprolol succinate, carvedilol, or bisoprolol) as streamlined one-click orders, whereas choosing a non-evidence-based beta-blocker (e.g. atenolol) would require multiple steps. Order sets can also be triggered by specific, customized criteria to improve specificity and reduce fatigue. For instance, the presence of CHF on a problem list, an elevated BNP, or use of IV diuretics make a hospital discharge diagnosis of CHF likely (11). These criteria can then be used at admission and/or discharge to prompt the clinician to use CHF order sets that might suggest medication initiation, CHF education and LVEF assessment. (17, 18) In combination with other CDS technologies, order sets appear effective in increasing compliance with GDMT (19) and possibly in reducing inpatient mortality. (20)
Triggered Alerts
Triggered alerts are activated by a specific combination of patient-specific factors. Alert triggers can be diverse (e.g. ‘if LVEF ≤ 40%’, or “if taking beta blocker and heart rate < 50 bpm”), and timing of activation within clinicians’ workflow is customizable. For example, if a patient with CHF has not had a documented LVEF in the past 365 days, upon entering the ‘wrap up’ section of the chart during a clinic encounter, a triggered alert could suggest an order for an LVEF assessment and present pre-populated orders for an echocardiogram. Thoughtful trigger selection in collaboration with clinicians results in fewer false alarms and improved signal-to-noise ratio. This enhances physician uptake by prompting a clinician at the proper time in their workflow and ideally only when a recommendation is not already being followed.
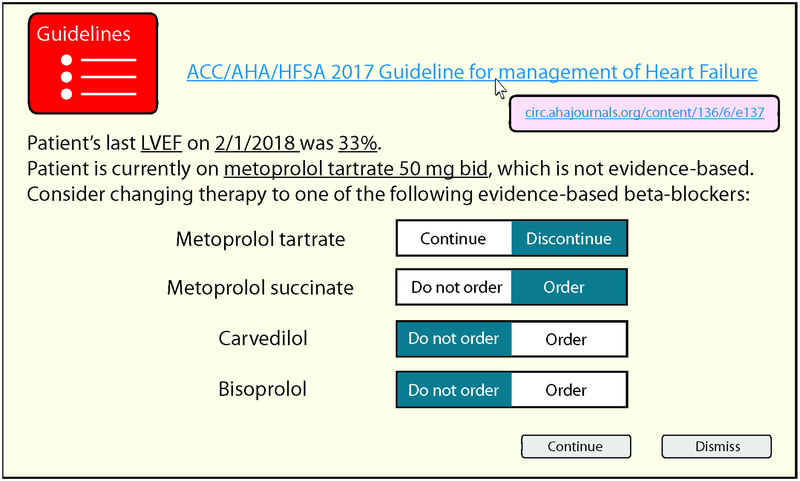
Triggered alerts are either passive or active. Passive alerts are displayed in the patient’s chart but do not interrupt the user. For example, prior to discharge, the clinician may review a ‘menu’ of passive alerts, which might indicate the patient is due for an echocardiogram or is a candidate for an ICD. Passive alerts are minimally disruptive to clinician workflow but may be easily overlooked, particularly if the clinician is unaware of their existence. (21) Active alerts, often referred to as “pop-ups”, interrupt the user who cannot proceed without responding. An active alert might detect metoprolol tartrate on the medication list in a patient with an LVEF ≤ 40% and offer metoprolol succinate, bisoprolol, or carvedilol instead (Figure 2). Active alerts are effective for altering clinician behavior on an individual application basis, but excessive active alerts, especially those with low specificity or low clinical value can produce ‘alert fatigue,’ whereby clinicians are less likely to respond to additional alerts. (22) As accuracy of predictive analytics improves, triggered alerts will become increasingly specific, which should minimize alert fatigue.
Figure 2: Example alert suggesting guideline-based beta-blocker therapy for HFrEF.
- EHRs can assess a clinician’s order and prepopulate corrective orders to redirect the clinician to recommended action.
With minimal EHR use, Donna experiences several gaps in care. Failure to reassess LVEF after year 1 suggests a missed opportunity to start GDMT. When she does start on a beta-blocker and an angiotensin converting enzyme-inhibitor, infrequent visits and clinician inaction result in never starting a mineralocorticoid receptor antagonist (MRA). Finally, there is a missed opportunity to place an ICD, which may have aborted her terminal event of sudden cardiac death. With idealized EHR use, when Donna’s cardiac function worsens, timely CDS alerts ensure appropriate LVEF monitoring, initiation of GDMT and referral for ICD placement.
COMPLEX APPLICATIONS
Advanced CDS
Complex applications may use several tools to address a single scenario from multiple perspectives. Patient-specific clinical data can therefore be used to assess the relevance of guideline-based care recommendations to the patient and generate documentation, active and passive alerts, and analytics. For example at our institution we built a tool to guide statin therapy based on current United States Preventative Task Force Guidelines. (23) The application retrieves patient data needed to calculate the American College of Cardiology/American Heart Association Atherosclerotic Cardiovascular Disease 10-year risk score (e.g. lab values, demographics, and vital signs), detects the presence of diagnoses such as diabetes mellitus, coronary artery disease or hypertension and determines whether statin therapy is indicated. If so, the application compares the recommended statin therapy intensity to the intensity of the patient’s current statin therapy if any, based on their active medication list. If the recommended statin therapy is more intense than the patient’s current statin therapy the clinician is offered orders to start or change medications, for example discontinuing pravastatin 10 mg daily (low intensity) and initiating rosuvastatin 20 mg daily (high intensity). Standardized text is generated, which imports all of the patient data used to generate the statin recommendation into the encounter note. Although not CHF-specific, this application illustrates the potential value of combining multiple EHR tools and external resources to produce a comprehensive approach to promoting guideline-based care.
Care pathways
Care pathways generally consist of a sequence of decision points and actions to aid clinicians in management of clinical scenarios such as CHF exacerbation, chest pain, or neutropenic fever. By presenting guidelines in an intuitive manner within the context of clinical workflow, care pathways improve adherence to guideline recommendations as well as outcomes. (24) Traditionally, care pathways were printed step-by-step instructions or paper flowcharts. Care pathways are increasingly available within the EHR and directly integrated with clinicians’ workflow with the ability to place, modify or discontinue orders, perform calculations, and view test results. Well-designed pathways save clinician time by making guidelines clear, assembling patient data necessary for decision-making, presenting recommended orders for the clinician to implement while simultaneously generating standardized progress notes. Using this framework, the recommended clinical approach becomes the ‘easiest’ through streamlined order entry and documentation. (25)
Registries and population health
Population health is the process of optimizing the health of a targeted group of individuals who share a disease condition such CHF. The shift in healthcare from volume- to value-based care necessitates a population health strategy beyond clinic visits and hospitalizations for management of chronic conditions like CHF. (26, 27) As currently conceived, population health management requires construction of registries, which help track and optimize care of patients. (26) Registries serve three distinct functions: 1) identify patients with a target condition; 2) assemble relevant clinical data (e.g. EHR, insurance claims), and 3) monitor patient status in terms of care delivery goals and identifies those with gaps (e.g. HFrEF patients not on GDMT).
EHRs primarily capture care data from only one organization, but a registry should incorporate as many data sources as possible to generate comprehensive patient records. EHR platform-based health information exchanges (e.g. Epic’s Care Everywhere) and health information exchanges aggregate clinical data such as encounters, notes, test results, and medications across EHR networks or regional collaboratives. For example, some pharmacy benefit companies such as Surescripts™ can aggregate prescriptions written for a patient and can identify those taking CHF medications. All-payer claims databases (aggregations of paid health insurance claims for clinical services) can provide a more complete regional picture of when and where a patient receives care.
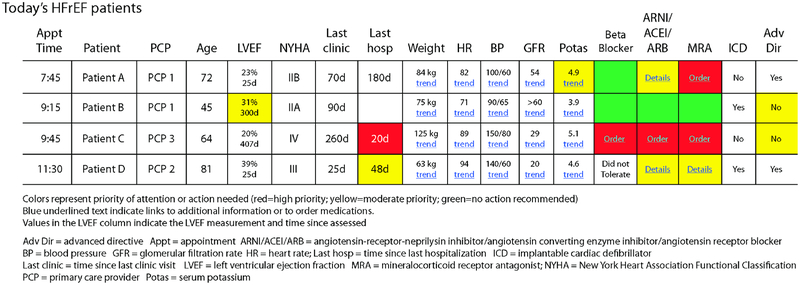
Each member of a population health care team has a unique role (e.g. physicians vs. case managers vs. pharmacists), and integrating role-specific tools into the EHR can improve adherence to disease-specific recommendations. (28) In IMPROVE-HF, adherence to 5/7 CHF quality measures including medication management, cardiac resynchronization therapy and ICD referral increased with EHR-facilitated performance improvement. (29) The complexity and variety of data sources required for effective population health management make EHR-based tools such as documentation (structured note templates and retrieval of health information exchange data), telemedicine/health (patient portals to connect patients with clinicians), dashboards and analytics (to identify care gaps), and CDS are critical to deliver efficient, effective care. Concise tools that combine multiple functionalities reduce clinician cognitive burden and focus attention on care gaps and adherence to appropriate clinical guidelines (Figure 3). (26) For example, at hospital discharge, clinicians can use a CHF discharge order set that contacts a care manager via secure messaging, orders a follow-up echocardiogram, and sets up PCP and cardiology clinic visits. Care managers can view a worklist and contact patients overdue for LVEF testing or who are not taking an MRA when they meet criteria. At one institution, a CHF dashboard demonstrated significant improvements in completion of indicated interventions and reductions in 30-day CHF readmission rates CHF (14.1% to 10%; p=0.001). (9)
Figure 3 -. Clinic dashboard for HFrEF patients.
– EHRs provide the capacity to rapidly visualize disparate types of data and triage findings that require attention.
With minimal EHR use, all changes to Donna’s CHF management occur during face-to-face encounters, usually hospital admissions. This introduces delays in GDMT optimization and poor adherence to non-pharmacologic guidelines, in this case resulting in premature death when a primary prevention ICD may have been life-saving. With idealized EHR use, patients like Donna are automatically identified as CHF patients and enrolled in the institution’s CHF registry. Registry tools such as dashboards and retrospective analytics assist the care team in coordinating routine monitoring (e.g. annual LVEF assessment), initiation and long-term management of GDMT (year 2), and eligibility for ICD (year 3), which resulted in successful treatment of her episode of ventricular fibrillation (year 5). Reports can function as screening for clinical trials, which leads to Donna’s participation in the trial of a wearable device to detect ventricular arrhythmias (years 6–8).
Learning healthcare systems
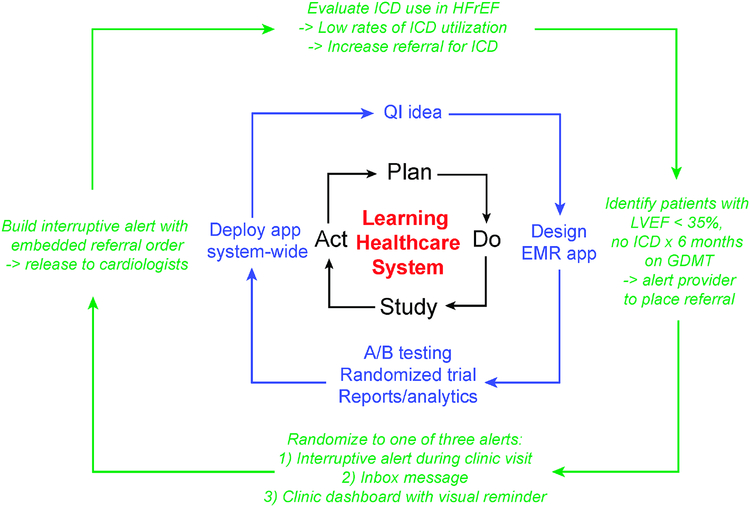
In an LHS, clinical science, informatics, and other factors are combined to enable continuous improvement, innovation, and discovery in health care delivery. Identification of patient cohorts, documentation of clinical course, retrospective and predictive analytics, and implementation of changes in care delivery via CDS all enable the virtuous cycle of an LHS. Health data analytics and care delivery tools are critical components of realizing an LHS by identifying patients, characterizing patterns of CHF care, and identifying opportunities to improve care quality such as by maximizing referrals for primary prevention ICDs (Figure 4). (30) First-time interventions are not always effective, and EHR tools allow for rapid refinement and re-assessment of care processes. Rapid assessment of effectiveness, revision of design, and implementation of the revised application can lead to rapid improvements in EHR-based interventions.
Figure 4 -. EHR-based learning health care system to optimize HF care.
- Analytics, structured documentation, CDS, and population applications can be used to rapidly develop closed-loop systems to maximize use of guideline-based care such as ICD.
Clinical research
The EHR tools used in an LHS model serve research efforts as well including streamlined, population-wide screening for clinical trials, creation of registries, tracking of research patients with visual dashboards, and EHR reminders and scheduling tools to improve clinical trial enrollment and retention. EHRs can reduce research staff requirements through automated screening for eligible patients, management of consent forms and extraction of structured data for case report forms rather than by manual entry. Automated EHR-based patient selection and data extraction will facilitated by adoption of common, widely-accepted CHF-specific case report forms with specific consensus-based structured elements. (31) Initiatives such as the Observational Health Data Sciences and Informatics program, and the Informatics for Integrating Biology & the Bedside tranSMART foundation enable data aggregation from multiple, which can be used for a number of research-related purposes including patient cohort identification. (32) The power of the EHR to support clinical research is not fully realized, but many of the tools we have described will likely significantly improve the speed of study enrollment and quality of data capture while reducing operational costs.
CONCLUSION
EHRs offer a broad range of tools shown to reduce variation in CHF patient management leading to improved patient outcomes. Such tools improve efficiency of care delivery while encouraging adherence to guideline recommendations. As analytic engines, EHRs can support rapid development of customized applications through an LHS model and generate new knowledge through clinical research. For a cardiologist considering EHR tools to improve CHF management, evidence is strongest supporting the use of analytics for case identification and risk stratification as well as HF-specific dashboards.
Central illustration:
Hypothetical clinical course of a chronic heart failure patient according to utilization of EHR tools
HIGHLIGHTS.
Modern electronic health records (EHRs) provide many tools that can improve quality and efficiency of heart failure management, but these tools are underutilized.
EHR tools can support remote patient engagement, effective documentation, multifaceted analytics, and point-of-care education and decision support.
Close collaboration between clinicians and developers will increase awareness, convenience, utilization, and effectiveness of EHR tools, which in turn has the potential to revolutionize CHF care delivery and clinical research.
Acknowledgments:
The authors acknowledge Esther Langmack, MD, for editorial assistance.
Funding:
Dr. Kao: NHLBI K08HL125725 (Kao)
ABBREVIATIONS
- CDS
Clinical Decision Support
- CHF
Congestive Heart Failure
- EHR
Electronic Health Record
- GDMT
Guideline-Directed Medical Therapy
- HL-7
Health Level-7
- HFrEF
Heart Failure with Reduced Ejection Fraction
- LVEF
Left Ventricular Ejection Fraction
- PCP
Primary care provider
- PROMIS
Patient Reported Outcomes Measurement Information System
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Dr. Kao: Consultant fees: Medtronic (modest)
Dr. Trinkley: Contract: Pfizer
Dr. Lin: none
References
- 1.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128:1810–52. [DOI] [PubMed] [Google Scholar]
- 2.Walker S, Spackman E, Conrad N, et al. Impact of missed treatment opportunities on outcomes in hospitalised patients with heart failure. Open Hear. 2017;4:e000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walsh MN, Albert NM, Curtis AB, et al. Lack of association between electronic health record systems and improvement in use of evidence-based heart failure therapies in outpatient cardiology practices. Clin Cardiol 2012;35:187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reed ME, Huang J, Brand RJ, et al. Patients with complex chronic conditions: Health care use and clinical events associated with access to a patient portal. PLoS One 2019;14:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stehlik J, Rodriguez-correa C, Spertus JA, et al. Implementation of Real-Time Assessment of Patient-Reported Outcomes in a Heart Failure Clinic: A Feasibility Study. J. Card. Fail 2017;23:813–816. [DOI] [PubMed] [Google Scholar]
- 6.Anker SD, Koehler F, Abraham WT. Telemedicine and remote management of patients with heart failure. Lancet 2011;378:731–739. [DOI] [PubMed] [Google Scholar]
- 7.Johnson SB, Bakken S, Dine D, et al. An Electronic Health Record Based on Structured Narrative. J Am Med Inf. Assoc 2008;15:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linder JA, Schnipper JL, Middleton B. Method of electronic health record documentation and quality of primary care. J. Am. Med. Informatics Assoc 2012;19:1019–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banerjee D, Thompson C, Kell C, et al. An informatics-based approach to reducing heart failure all-cause readmissions: The Stanford heart failure dashboard. J. Am. Med. Informatics Assoc 2017;24:550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellrodt G, Glasener R, Cadorette B, et al. Multidisciplinary rounds (MDR): An implementation system for sustained improvement in the American Heart Association’s get with the guidelines program. Crit. Pathw. Cardiol 2007;6:106–116. [DOI] [PubMed] [Google Scholar]
- 11.Blecker S, Katz SD, Horwitz LI, et al. Comparison of Approaches for Heart Failure Case Identification From Electronic Health Record Data. JAMA Cardiol. 2016;1:1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amarasingham R, Patel PC, Toto K, et al. Allocating scarce resources in real-time to reduce heart failure readmissions: a prospective, controlled study. BMJ Qual Saf 2013;22:998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans RS, Benuzillo J, Horne BD, et al. Automated identification and predictive tools to help identify high-risk heart failure patients: pilot evaluation. J. Am. Med. Informatics Assoc 2016;23:872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimabukuro DW, Barton CW, Feldman MD, Mataraso SJ, Das R. Effect of a machine learning-based severe sepsis prediction algorithm on patient survival and hospital length of stay : a randomised clinical trial. 2017;880:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toth-Pal E, Wårdh I, Strender L-EE, Nilsson G. A guideline-based computerised decision support system (CDSS) to influence general practitioners management of chronic heart failure. Inform. Prim. Care 2008;16:29–39. [DOI] [PubMed] [Google Scholar]
- 16.Evans RS, Kfoury AG, Horne BD, et al. Clinical Decision Support to Efficiently Identify Patients Eligible for Advanced Heart Failure Therapies. J Card Fail 2017;23:719–26. [DOI] [PubMed] [Google Scholar]
- 17.Ballard DJ, Ogola G, Fleming NS, et al. Impact of a standardized heart failure order set on mortality, readmission, and quality and costs of care. Int. J. Qual. Heal. Care 2010;22:437–444. [DOI] [PubMed] [Google Scholar]
- 18.Krive J, Shoolin JS, Zink SD. Effectiveness of Evidence-Based Congestive Heart Failure (CHF) CPOE Order Sets Measured by Health Outcomes In: AMIA Annual Symposium proceedings.Vol 2014. American Medical Informatics Association, 2014:815–24. [PMC free article] [PubMed] [Google Scholar]
- 19.Riggio JM, Sorokin R, Moxey ED, Mather P, Gould S, Kane GC. Effectiveness of a clinical-decision-support system in improving compliance with cardiac-care quality measures and supporting resident training. Acad Med 2009;84:1719–1726. [DOI] [PubMed] [Google Scholar]
- 20.Krive J, Shoolin JS, Zink SD. Effectiveness of Evidence-Based Congestive Heart Failure (CHF) CPOE Order Sets Measured by Health Outcomes. AMIA … Annu. Symp. proceedings AMIA Symp; 2014;2014:815–24. [PMC free article] [PubMed] [Google Scholar]
- 21.Scheepers-Hoeks A-MJ, Grouls RJ, Neef C, Ackerman EW, Korsten EH. Physicians’ responses to clinical decision support on an intensive care unit—Comparison of four different alerting methods. Artif. Intell. Med 2013;59:33–38. [DOI] [PubMed] [Google Scholar]
- 22.Baysari MT, Westbrook JI, Richardson K, Day RO. Optimising computerised alerts within electronic medication management systems: A synthesis of four years of research. Stud. Health Technol. Inform 2014;204:1–6. [PubMed] [Google Scholar]
- 23.US Preventive Services Task Force. Statin use for the primary prevention of cardiovascular disease in adults - US Preventive Services Task Force recommendation statement. JAMA 2016;316:1997–2007. [DOI] [PubMed] [Google Scholar]
- 24.Dykes PC, Acevedo K, Boldrighini J, et al. Clinical practice guideline adherence before and after implementation of the heartfelt heart failure effectiveness & leadership team intervention. J. Cardiovasc. Nurs 2005;20:306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardetto NJ, Greaney K, Arai L, et al. Critical pathway for the management of acute heart failure at the veterans affairs san diego healthcare system: Transforming performance measures into cardiac care. Crit. Pathw. Cardiol 2008;7:153–172. [DOI] [PubMed] [Google Scholar]
- 26.Handmaker K, Hart J. 9 steps to effective population health management. Healthc. Financ. Manage 2015;69:70–6. [PubMed] [Google Scholar]
- 27.Heidenreich P Heart Failure Prevention and Team-based Interventions. Heart Fail. Clin 2015;11:349–358. [DOI] [PubMed] [Google Scholar]
- 28.Zhou YY, Unitan R, Wang JJ, et al. Improving Population Care with an Integrated Electronic Panel Support Tool. Popul. Health Manag 2011;14:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fonarow GC, Albert NM, Curtis AB, et al. Improving evidence-based care for heart failure in outpatient cardiology practices: primary results of the Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF). Circulation 2010;122:585–596. [DOI] [PubMed] [Google Scholar]
- 30.Maddox TM, Albert NM, Borden WB, et al. The Learning Healthcare System and Cardiovascular Care: A Scientific Statement From the American Heart Association. Circulation 2017;135:e826–e857. [DOI] [PubMed] [Google Scholar]
- 31.Psotka MA, Fiuzat M, Carson PE, et al. Design of a “Lean” Case Report Form for Heart Failure Device Development. JACC Hear. Fail 2019;Divi:1–9. [DOI] [PubMed] [Google Scholar]
- 32.Weeks J, Pardee R. Learning to Share Health Care Data: A Brief Timeline of Influential Common Data Models and Distributed Health Data Networks in U.S. Health Care Research. eGEMs (Generating Evid. Methods to Improv. patient outcomes) 2019;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]