Abstract
Background:
Previous studies identified distinct subgroups (“phenogroups”) of patients with heart failure with preserved ejection fraction (HFpEF).
Objective:
To assess if clinical phenogroups differ in comprehensive biomarker profiles, cardiac and arterial structure/function, and responses to spironolactone therapy.
Methods:
Among Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist Trial (TOPCAT) participants, we performed latent-class analysis to identify HFpEF phenogroups based on standard clinical features, and assessed differences in: (1) multiple biomarkers measured from frozen plasma; (2) cardiac and arterial structure/function measured with echocardiography and arterial tonometry; (3) prognosis, and; (4) response to spironolactone.
Results:
Three HFpEF phenogroups were identified. Phenogroup 1 (n=1,214) exhibited younger age, higher smoking prevalence, preserved functional class, and the least evidence of left ventricular (LV) hypertrophy and arterial stiffness. Phenogroup 2 (n=1,329) was older, with normotrophic concentric LV remodeling, atrial fibrillation, left atrial enlargement, large artery stiffening, and biomarkers of innate immunity and vascular calcification. Phenogroup 3 (n=899) demonstrated more functional impairment, obesity, diabetes, chronic kidney disease, concentric LV hypertrophy, high renin, and biomarkers of tumor necrosis factor-alpha-mediated inflammation, liver fibrosis, and tissue remodeling. Compared to phenogroup 1, phenogroup 3 exhibited the highest risk of the primary endpoint of cardiovascular death, heart failure hospitalization, or aborted cardiac arrest (HR=3.44; 95% CI=2.79–4.24); phenogroups 2 and 3 demonstrated similar all-cause mortality (phenotype 2 HR=2.36; 95% 0=1.89–2.95; phenotype 3 HR=2.26, 95% 0=1.77–2.87). Spironolactone randomized therapy was associated with a more pronounced reduction in the risk of the primary endpoint in phenogroup 3 (HR 0.75, 95% CI 0.59–0.95; P for interaction=0.016). Results were similar after excluding participants from Eastern Europe.
Conclusions:
We identified important differences in circulating biomarkers, cardiac/arterial characteristics, prognosis, and response to spironolactone across clinical HFpEF phenogroups. These findings suggest distinct underlying mechanisms across clinically-identifiable phenogroups of HFpEF, which may benefit from different targeted interventions.
Keywords: HFpEF, phenogroups, biomarkers, arterial stiffness, TOPCAT trial
INTRODUCTION
Heart failure with preserved ejection fraction (HFpEF), which affects approximately half of patients with heart failure, results in substantial morbidity, mortality, and impaired quality of life. Although several pharmacologic therapies are known to improve patient outcomes in HFrEF, no pharmacologic therapies have been clearly demonstrated to reduce adverse events in HFpEF.
HFpEF likely represents a heterogeneous group of disease processes. This heterogeneity may contribute to the difficulty identifying effective treatments for HFpEF. A wide range of clinical risk factors for HFpEF have been identified, including older age, female sex, history of hypertension, diabetes, obesity, atrial fibrillation (AF), and coronary artery disease, among others (1,2). Patients with HFpEF also have highly variable underlying cardiac structural and functional abnormalities (3). Accordingly, previous studies have proposed that several different phenotypes of HFpEF exist, (4) encompassing relatively discrete subgroups (“phenogroups”) with distinct clinical features (5). These studies demonstrated that HFpEF phenogroups may be linked to important differences in disease prognosis (4,5). However, little data exist regarding differences in underlying biologic processes or response to therapies between HFpEF phenogroups. Identifying biomarker profiles within each phenogroup may suggest phenogroup-specific mechanisms that can be targeted for therapeutic purposes. Moreover, recent data indicate that clustering techniques informed by standard clinical features can classify patients with HFrEF into phenogroups that exhibit differential responses to spironolactone (6,7). Whether differential responses to spironolactone are present between HFpEF clinical phenogroups is unknown.
In this study, our objective was to utilize data and frozen plasma samples from the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist Trial (TOPCAT) in order to: 1) Examine plasma protein profiles between clinical phenogroups, via de novo measurements of multiple biomarkers; 2) Examine differences in outcomes and response to spironolactone therapy between the phenogroups; 3) Further characterize relevant cardiac and vascular characteristics of phenogroups utilizing echocardiographic and arterial tonometry data.
METHODS
Please see the Supplemental Methods for additional details regarding the study design and statistical analyses.
Data Source and Study Population
We utilized data and biosamples from TOPCAT, obtained from the National Heart, Lung, and Blood Institute. TOPCAT was a large, multicenter, international trial evaluating the efficacy of spironolactone therapy in individuals older than 50 years with symptomatic HFpEF and a left ventricular (LV) ejection fraction ≥45% (8). The study was approved by the institutional review board at each participating site, and all participants provided written informed consent.
A total of 3,445 subjects were enrolled across the USA, Canada, Brazil, Argentina, Russia, and Georgia. Due to substantial differences regarding subject recruitment and study implementation in Russia and Georgia (9), we performed sub-analyses restricted to participants from the Americas (n=1,765), where feasible, accounting for statistical power. The primary endpoint of TOPCAT was a composite of cardiovascular death, heart failure hospitalization, or aborted cardiac arrest.
Using available frozen plasma samples from the baseline visit, we measured 49 protein analytes of key disease pathways/mechanisms (Tables S1–S2), using a Luminex® Bead-Based multiplexed assay (Bristol Myers-Squibb; Ewing Township, NJ). Analytes were chosen a priori to represent a diverse number of physiologic processes related to cardiovascular disease and downstream effects (Table S1). We also analyzed data from other ancillary sub-studies, including an echocardiographic study and an arterial tonometry study (see Supplemental Methods). The assay range, intra- and inter-assay coefficients of variation for each analyte in the Luminex multiplexed assay are shown in Table S2.
Assignment of Clinical Phenogroups
Latent class analysis (LCA) was performed to determine clusters of clinical phenotypes. LCA is a clustering statistical technique that uses finite mixture modeling to classify individuals into mutually exclusive and exhaustive subgroups, maximizing within-group similarities and between-group differences on the basis of multiple observed characteristics in a population (6,7,10,11). Participants were characterized based on age, sex, race, diabetes status, history of AF, obesity, severe heart failure symptoms (New York Heart Association class III or IV), and chronic kidney disease (CKD) status. These clinical covariates were selected a priori to incorporate widely available clinical covariates that can be easily obtained in routine practice, and taking into account known associations with adverse outcomes in HFpEF (3,4).
The latent class models were compared across successive numbers of subgroups (i.e. phenogroups). Several metrics were assessed to determine the optimal number of phenogroups: the parametric bootstrap likelihood ratio (LR) test, Akaike’s Information Criterion (AIC), Bayesian Information Criterion (BIC), and sample-size adjusted BIC (12,13).
Statistical Analyses of Clinical Outcomes
Patient characteristics were summarized using standard descriptive statistics. Given that we compared multiple biomarkers and echocardiographic/tonometry features of cardiac structure and function across the phenogroups, we also report corrected P values that account for multiple comparisons based on the number of underlying principal components (14,15). Given that biomarkers and echocardiographic parameters were available in only a subset of participants, there was insufficient power to perform these specific comparisons after restricting to participants enrolled in the Americas (16).
To compare outcomes between the phenogroups, Kaplan-Meier curves with log-rank testing for trend were applied to assess for equality of survival distributions for the primary composite endpoint, a composite endpoint of all-cause mortality or heart failure hospitalization, heart failure hospitalization alone, and all-cause mortality alone. Cox proportional hazards models were performed to estimate unadjusted and adjusted hazards ratios (HRs) and 95% confidence intervals (CIs). Exploratory models were adjusted for 1) age, sex, and race; and 2) Meta-Analysis Global Group in Chronic Heart Failure (MAGGIC) risk score (17). We assessed the effect of spironolactone treatment using analyses stratified by phenogroup, and formally tested effect modification using interaction terms of phenogroup membership and spironolactone treatment arm.
Statistical analyses were performed using STATA version 15.0 (Statacorp LP, College Station, TX) using the LCA Stata Plugin, version 1.2, and the LCA Bootstrap, version 1.0, (18,19) and the Matlab statistics and machine learning toolbox (Matlab 2016b, the Mathworks; Natwick, MA).
RESULTS
Clinical Characteristics of Identified Phenotypes
In the overall study population at baseline, the mean age was 69 years (SD 10), with 52% women and 9% black participants. CKD was present in 43% of participants, the overall mean eGFR was 65 mL/min/1.73m2(SD 19), 32% had diabetes mellitus, 55% were obese, 35% had a history of AF, and 10% were smokers.
The optimal number of clinical phenogroups was 3 (bootstrap LR test for 2 vs. 3 classes p-value=0.01; LR test for 3 vs. 4 classes p-value=0.16; AIC, BIC, and sample-size adjusted BIC also supported a 3-class solution). Figure S1 shows the distribution of participants included in the analysis, and Tables 1 and 2 show the clinical characteristics across the phenogroups for the overall study population and restricted to the Americas, respectively. Importantly, characteristics of the clinical phenogroups were similar in the overall cohort and after restricting to participants enrolled in the Americas. However, phenogroup 1 composed the majority of participants enrolled in Russia/Georgia (53.49%) but only a minority of participants enrolled in the Americas (17.96%; Figure S2).
Table 1.
Baseline characteristics across clinical phenogroups identified using latent class analysis
| Baseline Characteristic | Overall Study Population |
Phenogroup 1 |
Phenogroup 2 |
Phenogroup 3 |
P- value |
|---|---|---|---|---|---|
| N (%) | 3442 | 1214 (35%) | 1329 (39%) | 899 (26%) | |
| Mean age, years (SD) | 69 (10) | 61 (6) | 77 (5) | 66 (8) | <0.001 |
| Female sex, n (%) | 1774 (52%) | 557 (46%) | 741 (56%) | 476 (53%) | <0.001 |
| Black race, n (%) | 302 (9%) | 58 (5%) | 54 (4%) | 190 (21%) | <0.001 |
| Mean eGFR, mL/min/1.73m2 | 65 (19) | 76 (16) | 58 (16) | 61 (19) | <0.001 |
| CKD, n (%) | 1463 (43%) | 185 (15%) | 769 (58%) | 509 (57%) | <0.001 |
| Smoker, n (%) | 360 (10%) | 288 (24%) | 28 (2%) | 44 (5%) | <0.001 |
| Obese, n (%) | 1902 (55%) | 530 (44%) | 494 (37%) | 878 (98%) | <0.001 |
| Diabetes Mellitus, n (%) | 1118 (32%) | 106 (9%) | 222 (17%) | 790 (88%) | <0.001 |
| Insulin, n (%) | 427 (38%) | 25 (2%) | 60 (5%) | 342 (38%) | <0.001 |
| Atrial fibrillation, n (%) | 1213 (35%) | 290 (24%) | 645 (49%) | 278 (31%) | <0.001 |
| Systolic blood pressure >=140, n (%) | 872 (25%) | 305 (25%) | 300 (23%) | 267 (30%) | <0.001 |
| Diastolic blood pressure >=80, n (%) | 842 (24%) | 391 (32%) | 247 (19%) | 204 (23%) | <0.001 |
| Heart rate >=90, n (%) | 101 (3%) | 26 (2%) | 25 (2%) | 50 (6%) | <0.001 |
| Spironolactone treatment arm, n (%) | 1720 (50%) | 621 (51%) | 664 (50%) | 435 (48%) | 0.45 |
| ACEI/ARB treatment, n (%) | 2899 (84%) | 1055 (87%) | 1058 (80%) | 786 (87%) | <0.001 |
| Beta Blocker treatment, n (%) | 2676 (78%) | 958 (79%) | 996 (75%) | 722 (80%) | 0.006 |
| Aspirin, n (%) | 2250 (65%) | 824 (68%) | 814 (61%) | 612 (68%) | <0.001 |
| History of myocardial infarction, n (%) | 893 (26%) | 337 (28%) | 311 (23%) | 245 (27%) | 0.026 |
| History of stroke, n (%) | 17 (3%) | 70 (6%) | 103 (8%) | 92 (10%) | <0.001 |
| History of COPD, n (%) | 403 (12%) | 110 (9%) | 153 (12%) | 140 (16%) | <0.001 |
| NYHA Class 3–4, n (%) | 1136 (33%) | 254 (21%) | 430 (32%) | 452 (50%) | <0.001 |
| Edema, n (%) | 2065 (60%) | 626 (52%) | 809 (61%) | 630 (70%) | <0.001 |
| Depression | 382 (27%) | 64 (23%) | 121 (19%) | 197 (36%) | <0.001 |
| Selective serotonin reuptake inhibitor therapy, n (%) | 256 (7%) | 58 (5%) | 95 (7%) | 103 (11%) | <0.001 |
| Mean KCCQ QOL score (SD) | 50 (24) | 47 (21) | 55 (24) | 47 (25) | <0.001 |
| Mean KCCQ overall score (SD) | 55 (20) | 55 (18) | 58 (21) | 50 (22) | <0.001 |
| Mean MAGGIC risk score (SD) | 15 (6) | 10 (4) | 19 (4) | 15 (5) | <0.001 |
| Primary endpoint, n (%) | 671 (19%) | 130 (11%) | 273 (21%) | 268 (30%) | <0.001 |
| Death and HF admission, n (%) | 804 (23%) | 146 (12%) | 356 (27%) | 302 (34%) | <0.001 |
| HF admission, n (%) | 451 (13%) | 59 (5%) | 178 (13%) | V 214 (24%) | <0.001 |
| All-cause death, n (%) | 530 (15%) | 112 (9%) | 257 (19%) | 161 (18%) | <0.001 |
| Cardiac death, n (%) | 336 (10%) | 87 (7%) | 151 (11%) | 98 (11%) | <0.001 |
| Aborted cardiac arrest, n (%) | 8 (<1%) | 6 (<1%) | 0 (0%) | 2 (<1%) | 0.035 |
ACE/ARB=angiotensin converting enzyme inhibitor/angiotensin receptor blocker; CKD=chronic kidney disease; COPD=chronic obstructive pulmonary disease; eGFR=estimated glomerular filtrations rate; HF=Heart failure; KCCQ=Kansas City Cardiomyopathy Questionnaire; MAGGIC=Meta-Analysis Global Group in Chronic Heart Failure; NYHA=New York Heart Association; SD=standard deviation
Table 2.
Baseline characteristics by clinical phenogroup identified using latent class analysis, restricted to participants enrolled in the Americas
| Americas Study Population |
Phenogroup 1 |
Phenogroup 2 |
Phenogroup 3 |
P- value |
|
|---|---|---|---|---|---|
| N (%) | 1765 | 389 (22%) | 826 (47%) | 550 (31%) | |
| Mean age, years (SD) | 72 (10) | 62 (7) | 79 (6) | 67 (8) | <0.001 |
| Female sex, n (%) | 882 (50%) | 200 (51%) | 427 (52%) | 255 (46%) | 0.12 |
| Black race, n (%) | 302 (17%) | 136 (35%) | 47 (6%) | 119 (22%) | <0.001 |
| Mean eGFR, ml/min/1.73m2 (SD) | 61 (19) | 76 (19) | 55 (16) | 58 (19) | <0.001 |
| CKD, n (%) | 941 (53%) | 70 (18%) | 528 (64%) | 343 (62%) | <0.001 |
| Smoker, n (%) | 117 (7%) | 96 (25%) | 13 (2%) | 8 (1%) | <0.001 |
| Obese, n (%) | 1135 (65%) | 236 (61%) | 355 (43%) | 544 (99%) | <0.001 |
| Diabetes Mellitus, n (%) | 778 (45%) | 86 (22%) | 172 (21%) | 530 (96%) | <0.001 |
| Insulin, n (%) | 379 (21%) | 36 (9%) | 56 (7%) | 287 (52%) | <0.001 |
| Atrial fibrillation, n (%) | 742 (42%) | 90 (23%) | 481 (58%) | 171 (31%) | <0.001 |
| Systolic blood pressure >=140, n (%) | 403 (23%) | 96 (25%) | 157 (19%) | 150 (27%) | 0.006 |
| Diastolic blood pressure >=80, n (%) | 345 (20%) < | 124 (32%) | 129 (16%) | 92 (17%) | <0.001 |
| Heart rate >=90, n (%) | 75 (4%) | 21 (5%) | 21 (3%) | 33 (6%) | 0.004 |
| Spironolactone treatment arm, n (%) | 879 (50%) | 194 (50%) | 411 (50%) | 274 (50%) | 1.00 |
| ACEI/ARB treatment at baseline, n (%) | 1394 (79%) | 316 (81%) | 605 (73%) | 473 (86%) | <0.001 |
| Beta Blocker treatment at baseline, n (%) | 1387 (79%) | 301 (77%) | 627 (76%) | 459 (83%) | 0.003 |
| Aspirin, n (%) | 1027 (58%) | 217 (56%) | 434 (53%) | 376 (68%) | <0.001 |
| History of myocardial infarction, n (%) | 359 (20%) | 60 (15%) | 167 (20%) | 132 (24%) | 0.01 |
| History of stroke, n (%) | 158 (9%) | 29 (7%) | 73 (9%) | 56 (10%) | 0.35 |
| History of COPD, n (%) | 291 (17%) | 66 (17%) | 126 (15%) | 99 (18%) | 0.39 |
| NYHA Class 3–4, n (%) | 620 (35%) | 80 (21%) | 245 (30%) | 295 (54%) | <0.001 |
| Edema, n (%) | 1264 (72%) | 260 (67%) | 566 (69%) | 438 (80%) | <0.001 |
| Selective serotonin reuptake inhibitor therapy, n (%) | 254 (14%) | 66 (17%) | 97 (12%) | 91 (17%) | 0.012 |
| Mean KCCQ QOL score (SD) | 56 (26) | 53 (26) | 61(25) | 48 (25) | <0.001 |
| Mean KCCQ overall score (SD) | 58(23) | 58 (23) | 63 (22) | 50 (23) | <0.001 |
| Mean MAGGIC risk score (SD) | 17 (6) | 11 (5) | 20 (4) | 16 (5) | <0.001 |
| Primary endpoint, n (%) | 522 (30%) | 90 (23%) | 230 (28%) | 202 (37%) | <0.001 |
| Death and HF admission, n (%) | 627 (36%) | 95 (24%) | 304 (37%) | 228 (41%) | <0.001 |
| HF admission, n (%) | 400 (23%) | 61 (16%) | 170 (21%) | 169 (31%) | <0.001 |
| All-cause death, n (%) | 387 (22%) | 53 (14%) | 215 (26%) | 119 (22%) | <0.001 |
| Cardiac death, n (%) | 223 (13%) | 39 (10%) | 118 (14%) | 66 (12%) | 0.098 |
| Aborted cardiac arrest, n (%) | 6 (<1%) | 4 (1%) | 0 (0%) | 2 (<1%) | 0.016 |
ACE/ARB=angiotensin converting enzyme inhibitor/angiotensin receptor blocker; CKD=chronic kidney disease; COPD=chronic obstructive pulmonary disease; eGFR=estimated glomerular filtrations rate; HF=Heart failure; KCCQ=Kansas City Cardiomyopathy Questionnaire; MAGGIC=Meta-Analysis Global Group in Chronic Heart Failure; NYHA=New York Heart Association; SD=standard deviation
Phenogroup 1 was comprised of younger individuals (mean age 61±6 years) with relatively preserved functional class, the highest prevalence of smoking (24%) among the groups, along with relatively preserved renal function (mean eGFR 76±16 mL/min/1.73m2) and a low prevalence of diabetes (9%). Phenogroup 2 was characterized by older age (mean age 77±5 years), the highest proportion of women (56%), a high prevalence of AFib (49%) and CKD (mean eGFR 58±16 mL/min/1.73m2), but a low prevalence of diabetes and obesity. Phenogroup 3 exhibited intermediate age (mean age 66±8 years) with a very high prevalence of obesity (98%), diabetes mellitus (88%), and prominently impaired functional class; it also exhibited a high prevalence of CKD (57%), depression (36%) and a higher proportion of participants of black race (21%). All three phenotype groups had similar rates of spironolactone treatment assignment and other antihypertensive drug use.
Circulating Biomarkers Across Clinical Phenotypes
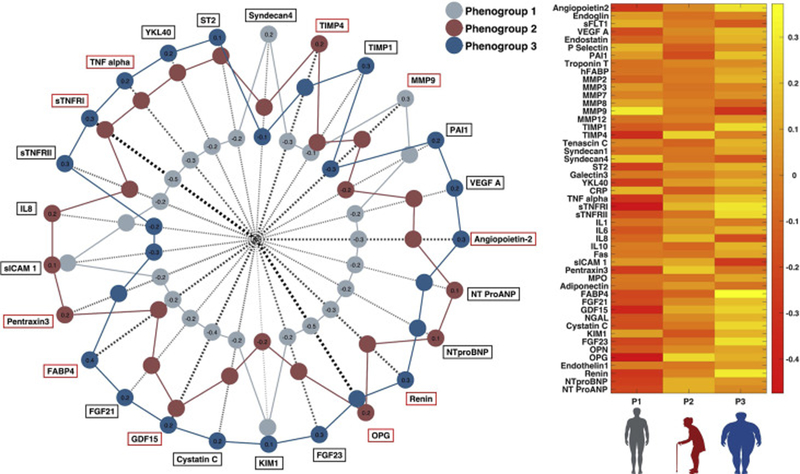
Differences in the examined biomarkers across the clinical phenotypes are shown in Figure 1, Table S3, and Figure S3. Phenogroup 1 demonstrated the lowest NT-proBNP levels and much higher levels of metalloproteinase (MMP)-9 (an extracellular turnover marker) compared to the other groups. This phenogroup also exhibited high levels of syndecan-4 (a cell-matrix interaction marker) compared to the other clinical phenogroups. Phenogroup 2 exhibited the highest levels of osteoprotegerin (a regulator of mineral metabolism and tissue calcification), tissue inhibitor of MMP (TIMP)-4 (a marker of extracellular turnover) and inflammatory biomarkers related to the innate immune response (interleukin-8, pentraxin-3, soluble intercellular adhesion molecule-1). Phenogroup 3 exhibited the highest levels of biomarkers of tumor necrosis factor (TNF)-mediated inflammation (TNF-alpha, soluble TNF receptors type 1 and 2), intermediary metabolism (fatty-acid binding protein 4, fibroblast growth factor [FGF]-21 and GDF-15), YKL-40 (liver fibrosis), plasma renin, kidney injury (cystatin C and kidney injury molecule-1), mineral metabolism/calcification (FGF-23 and osteoprotegerin), angiogenesis (angiopoietin and vascular endothelial growth factor A) and tissue remodeling (sST2).
Figure 1. Differences in biomarkers across clinical phenogroups.
Red boxes surrounding the biomarker title on the radar plot represent biomarkers that met statistical significance accounting for multiplicity correction based on the number of underlying principal components
Echocardiographic Parameters and Large Artery Stiffness Across Clinical Phenotypes
Echocardiographic findings across the clinical phenotypes in the 935 subjects who took part in the echocardiographic ancillary study are shown in Figure 2 and Table S4. Phenogroup 1 exhibited the least concentric LVs, with largest LV cavities, and the lowest absolute and relative wall thickness, LA volumes, and mitral septal and lateral E/e’ ratios (both septal and lateral). This group also exhibited the lowest values of resistive arterial load (systemic vascular resistance), pulsatile arterial load (total arterial compliance), and large artery stiffness (carotid-femoral pulse wave velocity). Phenogroup 2 demonstrated a distinct pattern characterized by small, concentric LVs with the lowest LV mass among the groups, the largest left atria, the lowest mitral annular tissue velocities, the stiffest large arteries (highest carotid-femoral pulse wave velocity), and the highest pulsatile and resistive arterial load. Finally, phenogroup 3 exhibited a distinct pattern of concentric LV hypertrophy, with the highest values of LV wall thickness, LV mass and LV mass indexed for height (but not of LV mass indexed for body surface area), and E/e’ ratios. This phenogroup exhibited relatively low values of resistive arterial load, but high pulsatile arterial load indexed for body size (total arterial compliance index).
Figure 2. Differences in echocardiographic findings and tonometry across clinical phenogroups.
Red boxes surrounding the measurement title on the radar plot represent parameters that met statistical significance accounting for multiplicity correction based on the number of underlying principal components
Prognostic Relationship between Clinical Phenotypes and Patient Outcomes
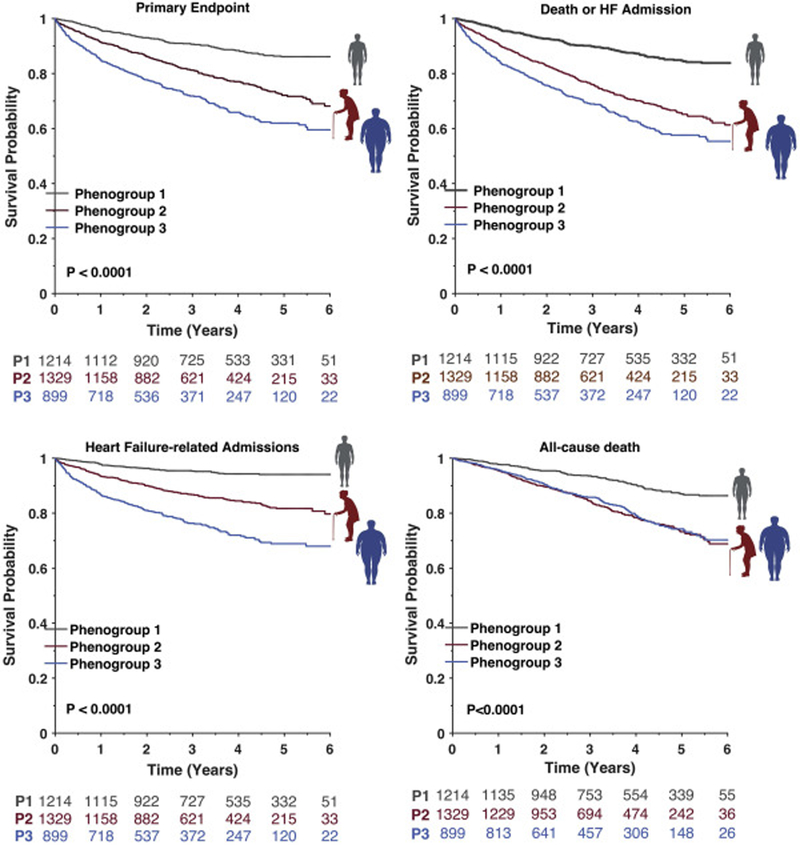
Compared to phenogroup 1 (“Younger with mild symptoms and normal LV geometry”), participants in phenogroup 2 (“Older with Stiff Arteries and small ventricles”) exhibited a significantly higher risk of the combined primary endpoint (HR 2.17, 95% CI 1.76–2.68) and heart failure hospitalization alone (HR 3.07, 95% CI 2.28–4.12), with phenogroup 3 (“Older Obese Diabetics with LVH”) demonstrating the highest risk of both outcomes (primary endpoint HR 3.44, 95% CI 2.79–4.24; heart failure hospitalization HR 5.91, 95% CI 4.42–7.89; Figure 3 and Table S5). Both phenogroup 2 and phenogroup 3 demonstrated similarly increased risk of combined all-cause mortality or heart failure hospitalization (phenogroup 2 HR 2.59, 95% CI 2.04–3.28; phenogroup 3 HR 3.04, 95% CI 2.36–3.91) and all-cause mortality (phenogroup 2 HR 2.36, 95% CI 1.89–2.95; phenogroup 3 HR 2.26, 95% CI 1.77–2.87). In exploratory analyses adjusting for age, sex, and race (Table S5), phenogroup 2 and phenogroup 3 both exhibited an increased risk of each of the endpoints compared to phenogroup 1. In analyses adjusting for the MAGGIC risk score, phenogroup 3 continued to be significantly associated with increased risk of the primary endpoint, combined all-cause mortality or heart failure hospitalization, and heart failure hospitalization alone.
Figure 3.
Kaplan-Meier curves for patient outcomes by clinical phenogroup
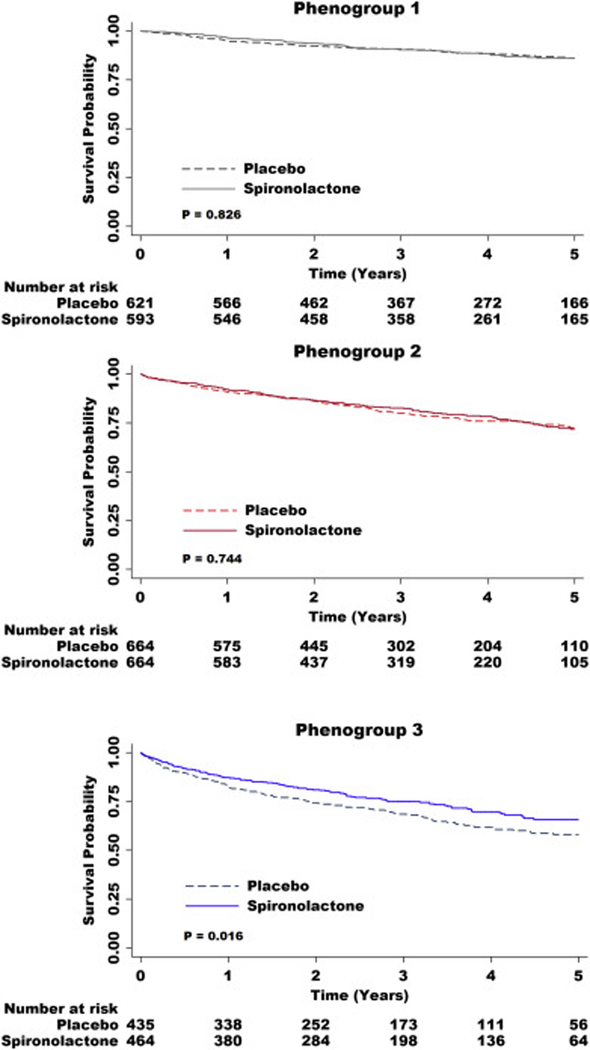
Spironolactone randomized therapy was associated with a more pronounced reduction in the risk of the primary endpoint (p-value for interaction 0.016) and heart failure hospitalization (p-value for interaction 0.007) in phenogroup 3. In stratified analyses, randomized spironolactone treatment was associated with a significantly lower risk of the primary endpoint (HR 0.75, 95% CI 0.59–0.95), and heart failure hospitalization (HR 0.69, 95% CI 0.53–0.90) in phenogroup 3, but did not appear to substantially benefit the other phenogroups (Table 3 and Figure 4).
Table 3.
Cox proportional hazards models for spironolactone vs. placebo, stratified by clinical phenogroup
| Phenogroup 1 | P- value |
Phenogroup 2 | P- value |
Phenogroup 3 | P- value |
||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | ||||
| Overall cohort | |||||||||
| Primary endpoint | 0.97 | 0.69–1.37 | 0.865 | 0.96 | 0.76–1.22 | 0.741 | 0.75 | 0.59–0.95 | 0.016 |
| All-Cause Mortality or Heart Failure Hospitalization | 1.10 | 0.74–1.64 | 0.626 | 0.94 | 0.72–1.22 | 0.641 | 0.82 | 0.60–1.12 | 0.214 |
| Heart Failure Hospitalizations | 0.76 | 0.45–1.27 | 0.301 | 0.96 | 0.72–1.29 | 0.799 | 0.67 | 0.53–0.90 | 0.007 |
| All-Cause Mortality | 1.09 | 0.75–1.58 | 0.663 | 0.88 | 0.69–1.12 | 0.294 | 0.89 | 0.65–1.21 | 0.448 |
| Restricted to participants enrolled in the Americas | |||||||||
| Primary endpoint | 0.86 | 0.57–1.30 | 0.470 | 0.88 | 0.68–1.14 | 0.327 | 0.74 | 0.56–0.97 | 0.031 |
| All-Cause Mortality or Heart Failure Hospitalization | 1.06 | 0.61–1.87 | 0.827 | 0.85 | 0.64–1.13 | 0.270 | 0.90 | 0.63–1.29 | 0.573 |
| Heart Failure Hospitalizations | 0.81 | 0.49–1.34 | 0.418 | 0.93 | 0.69–1.26 | 0.640 | 0.70 | 0.52–0.95 | 0.023 |
| All-Cause Mortality | 1.19 | 0.69–2.05 | 0.526 | 0.80 | 0.61–1.05 | 0.104 | 0.86 | 0.60–1.23 | 0.410 |
CI=confidence interval; HR=hazard ratio
Figure 4.
Kaplan-Meier curves for the primary outcome by spironolactone treatment status, stratified by clinical phenogroup
Analyses Restricted to the Americas
In analyses restricted to individuals enrolled in the Americas, three clinical phenotypes of HFpEF were also identified (bootstrap LR test for 2 vs. 3 classes p-value = 0.01; LR test for 3 vs. 4 classes p=0.24), with very similar clinical characteristics across the groups as observed in the overall study population (Table 2).
In Cox proportional hazards models, phenogroup 3 consistently demonstrated poorer prognosis than phenogroup 1 (Table S6, Figure S4); phenogroup 2 demonstrated higher risk of the composite endpoint of all-cause mortality or heart failure hospitalization (HR 1.63, 95% CI 1.19–2.23) and all-cause mortality (HR 1.68, 95% CI 1.24–2.27) compared to phenogroup 1, but no difference in the primary endpoint and heart failure hospitalization.
Similar to the overall cohort, in analyses stratified by phenogroup, assignment to the spironolactone treatment arm was associated with significantly lower risk of the primary endpoint (HR 0.74, 95% CI 0.56–0.97, p-value for interaction 0.031) and heart failure hospitalization (HR 0.70, 95% CI 0.52–0.95, p-value for interaction 0.023) in phenogroup 3, but not in the other phenogroups (Table 3 and Figure S5).
DISCUSSION
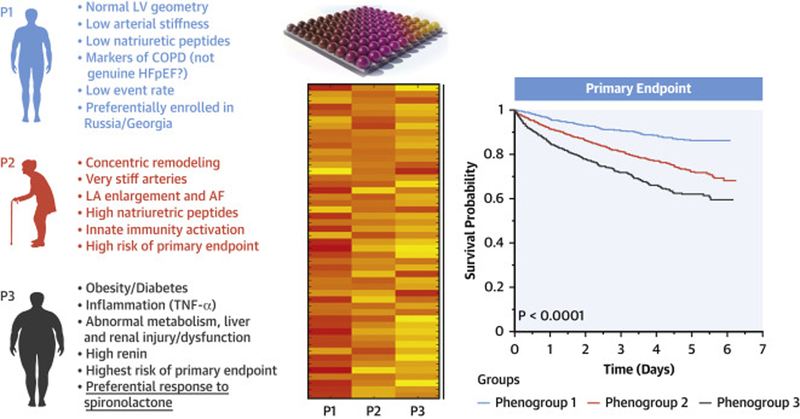
In this analysis of the TOPCAT trial, we identified three phenogroups of HFpEF based on standard clinical features using LCA, and characterized circulating biomarker profiles, cardiac and vascular phenotypes, outcomes, and response to spironolactone therapy between these phenotypic groups (summarized in Figure 5 and the Central Illustration). We demonstrate important differences in the levels of multiple biomarkers, suggesting distinct underlying pathophysiologic processes in these phenogroups. We also found pronounced differences in large artery stiffness, pulsatile and resistive arterial load, and echocardiographic parameters of LV and LA structure and function between the groups. Importantly, we identified the phenogroup that best responded to spironolactone randomized therapy in TOPCAT. Our findings support the existence of distinct clinical HFpEF phenogroups, and contribute to a better understanding of the heterogeneity between these phenogroups in biomarker profiles, cardiac/arterial structure and function, prognosis, and response to spironolactone.
Figure 5. Summary of biomarker, echocardiographic, vascular, and clinical differences across the three identified phenogroups.
Phenogroups were identified using LCA based on age, sex, race, diabetes status, history of AF, obesity, severe heart failure symptoms, and CKD status
Central Illustration. Clinical phenogroups in HFpEF.
Three clinical phenogroups were identified in TOPCAT. Biomarkers of key pathways were measured in available frozen samples from trial participants. Key circulating biomarker, cardiac and vascular features were found, indicating distinct patterns. The phenogroups exhibited different prognosis and differential response to spironolactone.
Comparison with HFpEF phenogroups identified in previous studies
Previous studies have identified subgroups of clinical characteristics in HFpEF that were associated with differences in clinical outcomes (4,5). In a post hoc analysis of data from the Irbesartan in Heart Failure with Preserved Ejection Fraction Study (I-PRESERVE) and Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM)-Preserved study, (4) Kao et al. used latent class analysis to identify six clinical subgroups of patients with HFpEF. Two of the subgroups, predominantly composed of 1) obese diabetics and 2) older individuals with the highest prevalence of AF, were consistent with our phenotypes 3 and 2, respectively, with regard to clinical characteristics and prognosis. There was no detailed echocardiographic or biomarker data available in this previous study (only LV ejection fraction and n-terminal B-type natriuretic peptide). Although we identified a different number of subgroups by LCA, this study only used unadjusted BIC to determine the optimal number of subgroups, which has been demonstrated to be inferior to the bootstrap LR test used in the current study (corroborated with AIC, BIC, and sample-size adjusted BIC) (12,13).
In a previous single-center study of 397 patients with HFpEF, (5) Shah et al. used hierarchical modeling to identify three phenotypes characterized by 1) younger age with lower B-type natriuretic peptide, the least concentric LVs, smallest LA volumes, and lowest E/e’ ratio; 2) higher prevalence of diabetes and obesity, with large LV mass; and 3) older age with the highest prevalence of AF and LA dilation. The authors found a similarly elevated risk of cardiac hospitalization, heart failure hospitalization, death, and a composite of the three individual endpoints among their second and third phenotypes compared to the first phenotype. The phenogroups found in our study are in general consistent with the findings of Shah et al. Our study was further strengthened by the use of data from a multi-center, international study population with robust biomarker data to facilitate exploration of underlying pathophysiologic differences and differences in treatment response across the clinical phenotypes.
Phenogroup 1 (“Younger with mild symptoms ”)
Previous evidence suggests that younger individuals with confirmed HFpEF have similar LV filling pressures and prevalence of LV hypertrophy as older individuals with HFpEF, (20) which we did not observe. Thus, it is unclear if phenogroup 1 constituted individuals with genuine HFpEF, or if it is confounded by individuals with non-cardiac causes of dyspnea. This hypothesis is supported by the observation that phenogroup 1 had the highest prevalence of smoking and highest levels of MMP-9 among the phenogroups. MMP-9 is involved in respiratory tract remodeling, and is elevated in individuals with asthma and chronic obstructive pulmonary disease (21). Interestingly, phenogroup 1 had the highest rates of enrollment in Russia and Georgia. Our findings are thus consistent with previous work questioning the validity of HFpEF diagnosis among Eastern-European participants of TOPCAT (9). Although the proportion of patients in this phenogroup in the Americas was small, the results were similar after restricting to subjects enrolled in the Americas. Our findings emphasize the importance of careful echocardiographic assessment and pulmonary function testing when evaluating individuals for possible HFpEF (22).
Phenogroup 2 (“Older with stiff arteries, small LVs and AF”)
Phenogroup 2 was characterized by the oldest mean age, highest prevalence of women and AF, and intermediate E/e’ ratios. This phenogroup is consistent with the phenotype of HFpEF in older adults reported by Tromp et al (23). We found that this phenogroup also exhibited normotrophic concentric LV remodeling, LA enlargement, and large artery stiffness. Phenogroup 2 demonstrated the highest levels of several markers of innate immunity, which have been previously associated with vascular injury as well as aging (24). This group also exhibited the highest levels of osteoprotegerin, which is a regulator of tissue calcification that has been linked with increased large artery stiffness, independent of atherosclerotic disease (25). Aortic stiffness is associated with faster wave transit time from the heart to reflection sites and back to the aorta (26). These premature wave reflections increase the mid-to-late systolic LV workload, which is in turn associated with diastolic dysfunction (27). Additionally, pulsatile LV load, aortic stiffness, and AF are each associated with the increased LA remodeling and dysfunction (28), which is consistent with the marked LA enlargement in this phenogroup.
Phenogroup 3 (“Obese, diabetic with advanced symptoms ”)
Phenogroup 3 exhibited the worst overall prognosis even after adjustment for comorbid diseases and the lowest risk associated with spironolactone therapy vs. placebo. This phenogroup also exhibited the highest levels of biomarkers in multiple pathways, including high renin, TNF-alpha mediated inflammation, markers of renal injury/dysfunction, dysregulated intermediary metabolism, liver fibrosis, angiogenesis and FGF-23 (a regulator of mineral metabolism that also promotes LV hypertrophy (29)). These elevated biomarkers are consistent with known links between metabolic dysregulation in obesity with inflammation, elevated renin-angiotensin-aldosterone system activity, nonalcoholic fatty liver disease (resulting in hepatic fibrosis), and CKD (30). Individuals with obesity have impaired natriuresis due to upregulation of renin-angiotensin-aldosterone system activity, which is exacerbated by HFpEF, likely driving the increased fluid retention and resulting hospitalizations observed in phenogroup 3. Related to these mechanisms, in HFrEF, mineralocorticoid antagonist therapy yields more pronounced reduction in adverse outcomes in obese compared to non-obese individuals (31). This pathophysiologic evidence is consistent with the lower risk of adverse outcomes we observed in individuals in the spironolactone treatment arm in phenogroup 3. This phenogroup also exhibited the highest prevalence of depression. Depression and HF have also been associated with overlapping underlying biological mechanisms, including sympathetic activation (32) and elevated pro-inflammatory cytokines such as interleukin-6 and TNF-alpha (33). Therefore, the additive effects of inflammation among patients with both HF and depression may impact disease severity and progression.
Strengths and Limitations
Strengths of our study include the inclusion of a well-characterized multicentric HFpEF cohort, the use of multiple biomarkers, the use of detailed phenotypes of cardiac and arterial structure/function, and the application of robust and well-established clustering techniques. Our study also has several limitations. We did not have plasma samples, echocardiographic or tonometry data from all TOPCAT trial participants, and had to restrict aspects of the study to subpopulations with available samples or data.
CONCLUSIONS
Our study demonstrates meaningful differences in biomarkers as well as cardiac and arterial structure across three distinct clinically-identifiable phenogroups: phenogroup 1 (“Younger with mild symptoms”), phenogroup 2 (“Older with stiff arteries, small LVs and AF”), and phenogroup 3 (“Obese, diabetic with advanced symptoms”). The 2 latter phenotypes, which constitute genuine high-risk HFpEF, exhibit distinct abnormalities in biomarkers, cardiac/arterial structure and function, and differential response to spironolactone therapy. In contrast, Phenogroup 1 represents a low-risk group which may not represent genuine HFpEF and may be confounded by lung disease, which in turn explains geographic differences in TOPCAT. Our findings provide important insights into potential driving factors behind differences in prognosis and response to treatment across the clinical phenogroups. In the absence of clear therapies to improve prognosis in HFpEF, separation of individuals into clinically-identifiable subgroups can help to identify patients who have the potential to benefit most from targeted interventions. Specifically, phenogroup 3 had the poorest prognosis and exhibited a reduction in adverse outcomes associated with randomized treatment with spironolactone. Given the heterogeneity of risk factors and outcomes in patients with HFpEF, future trials should focus on different interventions in distinct phenogroups of individuals with HFpEF.
Supplementary Material
Clinical Perspectives:
Heart failure with preserved ejection fraction (HFpEF) represents a heterogenous group of disease processes. We characterized three clinical phenogroups in HFpEF, one of which (characterized by obesity, diabetes, high renin, renal injury and liver fibrosis) appears to respond better to spironolactone.
Translational Outlook:
Future research should emphasize targeted interventions for HFpEF based on different underlying disease mechanisms across HFpEF phenogroups.
Acknowledgements:
This manuscript was prepared using TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist Trial) research materials obtained from the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the TOPCAT Trial or the National Heart, Lung, and Blood Institute. We appreciate the technical support from Terrye Delmonte and Karl Kammerhoff from BMS Biorepository.
Sources of Funding: This work was funded by a research grant from Bristol-Myers Squibb (J.A.C.). J.B.C. is supported by K23-HL133843. P.Z. is supported by K23-HL-130551.
Disclosures: J.A.C. has received consulting honoraria from Sanifit, Microsoft, Fukuda-Denshi, Bristol-Myers Squibb, OPKO Healthcare, Ironwood Pharmaceuticals, Pfizer, Akros Pharma, Merck and Bayer. He received research grants from National Institutes of Health, American College of Radiology Network, Fukuda Denshi, Bristol-Myers Squibb and Microsoft. J.A.C. is named as inventor in a University of Pennsylvania patent application for the use of inorganic nitrates/nitrites for the treatment of HFpEF and a patent application for novel neoepitope biomarkers of tissue fibrosis in HFpEF. K.B.M. has received research funding from Glaxo Smith Kline, Astra-Zeneca, Merck Sharp and Dohme, Sanofi-Aventis and American Reagent Phamaceuticals (formerly Luitpold) and consulting honoraria from American Reagent and MyoKardia, Inc. T.P.C. has received research funding from BG Medicine.
Abbreviations:
- HFpEF
Heart failure with preserved ejection fraction
- HFrEF
Heart failure with reduced ejection fraction
- AF
Atrial fibrillation
- TOPCAT
Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist Trial
- LCA
Latent class analysis
- CKD
Chronic kidney disease
- MAGGIC
Meta-Analysis Global Group in Chronic Heart Failure
- MMP
Metalloproteinase
- TIMP
Tissue inhibitor of metalloproteinase
- TNF
Tumor necrosis factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251–9. [DOI] [PubMed] [Google Scholar]
- 2.Lee DS, Gona P, Vasan RS et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the framingham heart study of the national heart, lung, and blood institute. Circulation 2009;119:3070–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah AM, Pfeffer MA. The many faces of heart failure with preserved ejection fraction. Nat Rev Cardiol 2012;9:555–6. [DOI] [PubMed] [Google Scholar]
- 4.Kao DP, Lewsey JD, Anand IS et al. Characterization of subgroups of heart failure patients with preserved ejection fraction with possible implications for prognosis and treatment response. Eur J Heart Fail 2015;17:925–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah SJ, Katz DH, Selvaraj S et al. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation 2015;131:269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferreira JP, Duarte K, McMurray JJV et al. Data-Driven Approach to Identify Subgroups of Heart Failure With Reduced Ejection Fraction Patients With Different Prognoses and Aldosterone Antagonist Response Patterns. Circ Heart Fail 2018;11 :e004926. [DOI] [PubMed] [Google Scholar]
- 7.Chirinos JA, Lanfear DE. Embracing the Long Road to Precision Medicine. Circ Heart Fail 2018;11:e005089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pitt B, Pfeffer MA, Assmann SF et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014;370:1383–92. [DOI] [PubMed] [Google Scholar]
- 9.Pfeffer MA, Claggett B, Assmann SF et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015;131:34–42. [DOI] [PubMed] [Google Scholar]
- 10.Lanza ST, Rhoades BL. Latent class analysis: an alternative perspective on subgroup analysis in prevention and treatment. Prev Sci 2013;14:157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schrauben SJ, Hsu JY, Rosas SE et al. CKD Self-management: Phenotypes and Associations With Clinical Outcomes. Am J Kidney Dis 2018;72:360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nylund KL, Asparouhov T, Muthen BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Struct Equ Modeling 2007;14:435–469. [Google Scholar]
- 13.Tein JY, Coxe S, Cham H. Statistical Power to Detect the Correct Number of Classes in Latent Profile Analysis. Struct Equ Modeling 2013;20:640–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao X, Starmer J, Martin ER. A multiple testing correction method for genetic association studies using correlated single nucleotide polymorphisms. Genet Epidemiol 2008;32:361–9. [DOI] [PubMed] [Google Scholar]
- 15.Tromp J, Khan MA, Klip IT et al. Biomarker Profiles in Heart Failure Patients With Preserved and Reduced Ejection Fraction. J Am Heart Assoc 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu JC. Multiple Comparisons: Theory and Methods. London: Chapman & Hall, 1996. [Google Scholar]
- 17.Pocock SJ, Ariti CA, McMurray JJ et al. Meta-Analysis Global Group in Chronic Heart Failure. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J 2013;34:1404–13. [DOI] [PubMed] [Google Scholar]
- 18.Lanza ST, Dziak JJ, Huang L, Wanger AT, Collins LM. LCA STATA Plugin Users’ Guide (version 1.2). University Park, PA: The Methodology Center, Penn State; 2015. http://methodology.psu.edu Accessed March 19, 2019. [Google Scholar]
- 19.Huang L, Dziak JJ, Wagner AT, Lanza ST. LCA Bootstrap STATA Function Users’ Guide (version 1.0). University Park, PA: The Methodology Center, Penn State; 2016. http://methodology.psu.edu Accessed March 19. 2019. [Google Scholar]
- 20.Tromp J, MacDonald MR, Tay WT et al. Heart Failure With Preserved Ejection Fraction in the Young. Circulation 2018;138:2763–2773. [DOI] [PubMed] [Google Scholar]
- 21.Grzela K, Litwiniuk M, Zagorska W, Grzela T. Airway Remodeling in Chronic Obstructive Pulmonary Disease and Asthma: the Role of Matrix Metalloproteinase-9. Arch Immunol Ther Exp (Warsz) 2016;64:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minasian AG, van den Elshout FJ, Dekhuijzen PR et al. Serial pulmonary function tests to diagnose COPD in chronic heart failure. Transl Respir Med 2014;2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tromp J, Shen L, Jhund PS et al. Age-Related Characteristics and Outcomes of Patients With Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol 2019;74:601–612. [DOI] [PubMed] [Google Scholar]
- 24.Puntmann VO, Taylor PC, Mayr M. Coupling vascular and myocardial inflammatory injury into a common phenotype of cardiovascular dysfunction: systemic inflammation and aging - a mini-review. Gerontology 2011;57:295–303. [DOI] [PubMed] [Google Scholar]
- 25.Zagura M, Serg M, Kampus P et al. Association of osteoprotegerin with aortic stiffness in patients with symptomatic peripheral artery disease and in healthy subjects. Am J Hypertens 2010;23:586–91. [DOI] [PubMed] [Google Scholar]
- 26.Phan TS, Li JK, Segers P et al. Aging is Associated With an Earlier Arrival of Reflected Waves Without a Distal Shift in Reflection Sites. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chirinos JA. Deciphering Systolic-Diastolic Coupling in the Intact Heart. Hypertension 2017;69:575–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chirinos JA, Phan TS, Syed AA et al. Late Systolic Myocardial Loading Is Associated With Left Atrial Dysfunction in Hypertension. Circ Cardiovasc Imaging 2017;10:e006023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scialla JJ, Wolf M. Roles of phosphate and fibroblast growth factor 23 in cardiovascular disease. Nat Rev Nephrol 2014;10:268–78. [DOI] [PubMed] [Google Scholar]
- 30.Cohen JB. Hypertension in Obesity and the Impact of Weight Loss. Curr Cardiol Rep 2017;19:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olivier A, Pitt B, Girerd N et al. Effect of eplerenone in patients with heart failure and reduced ejection fraction: potential effect modification by abdominal obesity. Insight from the EMPHASIS-HF trial. Eur J Heart Fail 2017;19:1186–1197. [DOI] [PubMed] [Google Scholar]
- 32.McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res 2000;886:172–189. [DOI] [PubMed] [Google Scholar]
- 33.Irwin M. Psychoneuroimmunology of depression: clinical implications. Brain Behav Immun 2002;16:1–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.