Abstract
Background:
Growing evidence suggests that pesticide exposure may influence respiratory health, but data in young children are very limited. We examined the association of prenatal pesticide exposure with lower respiratory tract infections (LRTIs) and wheeze at one year of age in children from the Infants' Environmental Health Study (ISA) in Costa Rica.
Methods
We measured seven pesticide metabolites, including ethylenethiourea (ETU, metabolite of mancozeb), in maternal urine samples collected repeatedly during pregnancy. For each woman, we averaged pesticide concentrations during each half of pregnancy (≤20 and >20 weeks of gestation) and across repeated samples collected over the course of pregnancy. We collected information about LRTIs (n = 355) and wheezing (n = 272) during the first year of life from mothers when their children were 11-19 months old. We fit multivariable logistic regression models using high (quartile 4) vs. low (quartiles 1-3) urinary pesticide concentrations as exposures and adjusted models for maternal age, education, parity, gestational age at birth, and child sex.
Results
Ten percent of the children had at least one LRTI and 39% had at least one episode of wheezing during their first year of life. Median (25-75th percentile) specific gravity-corrected urinary ETU concentrations during the first half, second half, and over the course of pregnancy were 3.4 (2.1-5.0), 3.3 (2.2-4.7), and 3.4 (2.4-5.0) ng/mL, respectively. We observed that high urinary ETU concentrations during the first half of pregnancy were associated with increased odds of LRTI (OR = 2.45; 95% CI: 0.96, 6.26), whereas high urinary ETU concentrations during the second half of pregnancy were associated with decreased odds of wheezing (OR = 0.50; 95% CI: 0.26, 0.96). We found that the association between high urinary ETU concentrations during the first half of pregnancy and LRTIs persisted among mother-child pairs with either high or low ETU concentrations during the second half. In contrast, the association of high urinary ETU concentrations during the second half of pregnancy with wheezing was attenuated when we simultaneously adjusted for urinary ETU concentrations during the first half. We observed null associations between other pesticide metabolites measured during pregnancy and respiratory outcomes.
Conclusions
Our data indicate that exposure to mancozeb/ETU during the first half of pregnancy may be associated with respiratory outcomes in the first year of life.
Keywords: Pesticides, mancozeb, respiratory outcomes, infants, Costa Rica
1. Introduction1
Respiratory outcomes including infection and wheezing during infancy can have long-term consequences for respiratory health (Busse et al. 2010; Jackson et al. 2008; Jackson 2014; Jackson et al. 2016; Lemanske et al. 2005; Liu et al. 2017; Lodge et al. 2014; Rubner et al. 2017). For example, viral wheezing respiratory illnesses during infancy were associated with an increased risk of subsequent wheezing at preschool age (Lemanske et al. 2005) and asthma at school age (Jackson et al. 2008). Similarly, lower respiratory tract infections (LRTIs) in the first year of life have been associated with increased early transient wheeze (before age 3 years) and intermediate-onset wheeze (after age 2 years) in a high risk for allergy birth cohort (Lodge et al. 2014). To date, limited information is available for environmental factors that contribute to LRTIs or wheezing during the first year of life (Bloomberg 2011; Cupul-Uicab et al. 2014; Dick et al. 2014; Gascon et al. 2012; Gascon et al. 2013; Gascon et al. 2014; Hehua et al. 2017; McEvoy and Spindel 2017; Selby et al. 2018; Vanker et al. 2017).
Pesticides have been associated with adverse respiratory outcomes in young children, but the evidence is scarce, as the timing of exposure, pesticides assessed, and outcomes measured have varied among studies. Most analyses have focused on dichlorodiphenyldichloroethylene (DDE), the metabolite of dichlorodiphenyldichloroethane (DDT), an organochlorine pesticide that is both biologically and environmentally persistent. A meta-analysis of 10 European birth cohorts found that higher prenatal DDE concentrations were associated with an increased risk of bronchitis and wheezing in the first 18 months of life, though the magnitude of the observed association was small (Gascon et al. 2014). Prenatal exposure to DDE was also associated with an increased risk of LRTI and wheezing in 12-14-month-old children in Spain (Gascon et al. 2012). In contrast, prenatal DDE concentrations were not associated with LRTIs in 18-month-old Mexican boys (Cupul-Uicab et al. 2014).
Few studies have examined the associations between pesticides other than DDE/DDT and respiratory outcomes in children. In an analysis of National Health and Nutrition Examination Survey (NHANES) 1999-2008, organophosphate (OP) metabolite concentrations in school-age children were not associated with current wheeze (Perla et al. 2015). However, a cross-sectional analysis of school-age children with asthma living in an agricultural community in Washington State found that short-term exposure to OPs was associated with a higher risk of asthma morbidity (Benka-Coker et al. 2019). In addition, a birth cohort study of California children living near agricultural fields found these OP metabolite concentrations in the second half of pregnancy and in childhood to be associated with respiratory symptoms at ages 5 and 7 years (Raanan et al. 2015) and/or decreased lung function at 7 years (Raanan et al. 2016). Prenatal exposure to piperonyl butoxide, a synergist for residential pyrethroid insecticides, was associated with cough at age 5-6 years in a New York City birth cohort (Liu et al. 2012). In a cross-sectional study in France, higher urinary ETU concentrations, a marker of exposure to bisdithiocarbamate fungicides, were associated with asthma and rhinitis symptoms in children aged 3-10 years (Raherison et al. 2019). To our knowledge, no published study has examined the association of prenatal exposure to current-use pesticides with respiratory symptoms and infections during infancy.
In Costa Rica, residents living in banana growing regions are exposed to a variety of pesticides including fungicides (e.g., mancozeb), insecticides (e.g., OP chlorpyrifos, cypermethrin), and herbicides (e.g., 2,4-D). These exposures result in measurable concentrations of pesticides and their metabolites in the residents, including children (van Wendel de Joode et al. 2012) and pregnant women (van Wendel de Joode et al. 2014). Using biological monitoring data from the Infants' Environmental Health (‘Infantes y Salud Ambiental’, ISA) study, a prospective birth cohort study of pregnant women and their children living near banana plantations, we evaluated the impact of prenatal pesticide exposure on respiratory health in the first year of life.
2. Methods
2.1. Study population
Pregnant women were enrolled in the ISA study from March 2010 and June 2011 (Mora et al. 2014; van Wendel de Joode et al. 2014). Of 451 women enrolled in the study, 22 (5%) had a miscarriage or stillbirth and 69 (15%) were lost to follow-up before the one-year study visit. Of the remaining 360 mother-child pairs, 355 (99%) singleton liveborn infants had maternal urinary pesticide concentrations measured during pregnancy and available information on respiratory outcomes in the first year of life. Mother-child pairs included in these analyses (n = 355) did not differ significantly from the initial cohort (n = 451) (van Wendel de Joode et al. 2014) on their attributes, including maternal education, parity, household income, and prenatal specific gravity-corrected urinary pesticide concentrations.
The Ethical and Scientific Committee of the Universidad Nacional in Costa Rica approved all study protocols. All mothers provided written informed consent at enrollment and additional informed consent was obtained from the parents or legal guardians of participants aged <18 years.
2.2. Data collection
We interviewed women during pregnancy (one to three times depending on their gestational age at enrollment; median at the first, second, and third visit = 19, 30, and 33 weeks of gestation, respectively), shortly after delivery (median = 7 weeks’ postpartum), and when their children were 11-19 months old (median = 13.2 months; one-year study visit). We collected socio-demographic data, such as maternal age, education, parity, and household income, at the baseline interview. We also gathered information on maternal occupational status, smoking habits, medical conditions, medications, and obstetric ultrasounds at each interview. We abstracted data completed by hospital/clinic personnel from prenatal (e.g., ultrasounds) and delivery (e.g., length of gestation) medical records provided to the study participants. We estimated gestational age at birth using the last menstrual period date, information from early ultrasounds (<14 weeks of gestation), and medical record estimates (Mora et al. 2015).
2.3. Respiratory outcomes
We evaluated two respiratory outcomes: physician- or nurse-confirmed diagnosis of LRTIs and wheeze during the first year of life. Information about these outcomes was obtained from mothers through questionnaires when children were 11-19 months old. Occurrence of a LRTI episode was defined as a positive answer to one of the following two questions: “Has a doctor or nurse ever told you that your child has pneumonia?” or “Has a doctor or nurse ever told you that your child has bronchiolitis or bronchitis?”. Children with negative answers to both questions were defined as not having LRTI. Wheezing during the first year of life was defined as a positive answer to the question “Since he/she were born, has your child ever experienced whistling or wheezing from the chest?”. Questions were extracted from Spanish version of the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire (ISAAC 1998) and have been previously used in other cohort studies (Gascon et al. 2012). LRTI questions were administered to mothers of all infants who were assessed at the one-year study visit (n = 355), whereas the wheezing question was administered to only 272 mothers (because it was added to the questionnaire after the study visits had already started).
2.4. Urinary pesticide metabolites measurements
We collected maternal urine samples one to three times during pregnancy (at the same time as pregnancy interviews) in 100 mL beakers (Vacuette®, sterile), aliquoted them into 15 mL tubes (PerformR™ Centrifuge tubes, Labcon®, sterile), and then stored them at −20°C until shipment to the Division of Occupational and Environmental Medicine at Lund University, Sweden, for analysis. A total of 93 women provided three samples during pregnancy, 222 women provided two samples, and 40 provided only one. Samples were analyzed for metabolites of fungicides [ethylenethiourea (ETU, metabolite of mancozeb), hydroxypyrimethanil (OH-PYR, metabolite of pyrimethanil), and 5-hydroxythiabendazole (5-OH-TBZ, metabolite of thiabendazole)] and OP insecticides [3,5,6-trichloro-2-pyridinol (TCPy, metabolite of chlorpyrifos) used in banana plantations (Table S1). Urine specimens were also analyzed for metabolites of common synthetic pyrethroids used in vector control programs and at home, but not in banana: 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylic acid (DCCA, metabolite of permethrin, cypermethrin, and cyfluthrin); and 3-phenoxybenzoic acid (3PBA, metabolite of permethrin, cypermethrin, cyfluthrin, deltamethrin, allethrin, resmethrin, and fenvalerate). The herbicide 2,4-D, used to control broadleaf in pasture and rice, was also measured in urine samples.
Urinary metabolites were measured using a liquid chromatography mass spectrometer (LC-MS/MS; UFLCRX; Shimadzu Corporation) with a triple quadrupole linear ion trap (QTRAP 5500; AB Sciex) (Ekman et al. 2013; Ekman et al. 2014; Faniband et al. 2019). For ETU analyses, duplicate urine samples were added with internal standards, hydrolyzed using basic buffer, and then analyzed using two-dimensional LC-MS/MS methodology (Ekman et al. 2013). For all other analyses, duplicate urine specimens were added with internal standards and hydrolyzed using sulfatase/glucuronidase enzyme thereafter the metabolites were extracted from the urinary matrix using solid phase extraction (Norén et al. 2020). Average concentrations of the duplicate samples were used in further calculations. Between-run and between-batch precisions were 4-18% and 8-19%, respectively.
Pesticide metabolite concentrations were normalized for dilution using the formula MSG = M × [(1.017 – 1) – (SG –1)], where MSG is the specific gravity-corrected metabolite concentration (μg/L), M is the observed metabolite concentration (μg/L), SG is the specific gravity of the urine sample, and 1.017 kg/L is the average specific gravity for all urine samples included in these analyses (n = 763). Urinary specific gravity (kg/L) was determined using a hand refractometer.
2.5. Statistical analyses
We calculated descriptive statistics and distributional plots for all variables. We then estimated bivariate associations between biomarkers of exposure, outcomes, and covariates using t-tests and chi-square tests. We also estimated correlations between specific gravity-corrected urinary pesticide metabolite concentrations using Spearman’s correlation coefficients (rs).
We examined associations of maternal urinary pesticide metabolite concentrations with respiratory outcomes using multivariable logistic regression models. We examined three windows of exposure: (i) first half of pregnancy (≤20 weeks of gestation, n = 194), (ii) second half of pregnancy (>20 weeks of gestation, n = 343), and (iii) average over the course of pregnancy (n = 355). Our primary analyses focused on evaluating those in the top quartile [≥75th percentile (P75)] vs. all other concentrations (<P75), but we also ran our models with our exposures modeled as continuous variables (i.e., log10-transformed specific gravity-corrected urinary pesticide metabolite concentrations). We selected our covariates a priori using directed acyclic graphs based on previous literature (Cupul-Uicab et al. 2014; Gascon et al. 2012; Gascon et al. 2014): maternal age, maternal education, parity, gestational age at birth, and infant sex. We imputed missing values for covariates (all <5% missing) using data from the nearest available study visit. If values for a missing covariate were not available from an earlier or later study visit, we randomly selected a value from the dataset (n = 2 participants missing parity; n = 2 participants missing maternal smoking during pregnancy for sensitivity analyses).
We conducted sensitivity analyses to assess the robustness of our results and better understand our exposure-outcome associations. Because maternal smoking during pregnancy has been identified as a risk factor for infant wheezing and LRTI (McEvoy and Spindel 2017) but the low prevalence of maternal smoking during pregnancy in our study population (n = 15) prevented us from conducting stratified analyses and adjusting for this variable, we excluded these mothers from our models. In addition, we ran our main models only with mothers who had exposure data for both the first and second half of pregnancy (n = 182 for LRTI and 161 for wheeze). We also clustered mother-infant pairs into four groups, based on urinary ETU concentrations during the first and second half of pregnancy dichotomized at the P75 of the two distributions: a) low ETU during first half/low ETU during second half, representing concordant low exposures; b) high ETU during first half/low ETU during second half; c) low ETU during first half/high ETU during second half; and d) high ETU during first half/high ETU during second half, representing concordant high exposures. Using multivariable regression models, we estimated the associations of this categorical variable with LRTI and wheezing during the first year of life. All statistical analyses were performed using Stata (version 14.2; StataCorp LLC) and R (version 3.1.2; R Development Core Team).
3. Results
Ten percent of the children had at least one LRTI and 39% had at least one episode of wheezing during their first year of life (Table 1). About 71% of children with history of a LRTI also had wheezing. LTRI and wheezing were both associated with maternal smoking both during pregnancy and during the first year of life. Mothers included in our analyses were relatively young at the time of enrollment (median age = 22.3 years; range = 15-44), predominantly Costa Rican-born (83%) and multiparous (64%), had no history of asthma (87%), and lived below the Costa Rican poverty line at the time of enrollment (59%). Only 4% of mothers reported smoking during pregnancy. Pesticides were detected in the urine of all women, with ETU, TCPy, 3PBA, and 2,4-D detected in all samples (Table 2). Median (P25-P75) specific gravity-corrected urinary ETU, TCPy, and 3PBA concentrations averaged during pregnancy were 3.3 (2.4-4.9) ng/mL, 1.8 (1.3-2.5) ng/mL, and 0.8 (0.5-1.3) ng/mL, respectively. Urinary pesticide metabolites measured during the same window of exposure were weakly to moderately correlated (rs for first half of pregnancy = 0.03-0.34; rs for second half of pregnancy = 0.01-0.20; rs for pregnancy average = 0-0.24), except for pyrethroid metabolites 3PBA and DCCA, which were highly correlated during all exposure periods (rs = 0.79-0.84; Table S2).
Table 1.
Characteristics of study population by respiratory outcomes at one year of age, ISA study, Matina County, 2010-2013 [n (%) or median (P25-P75)].
| LRTI (n = 355) |
Wheeze (n = 272)a |
|||
|---|---|---|---|---|
| Never | Ever | Never | Ever | |
| All children | 318 (89.6) | 37 (10.4) | 166 (61.0) | 106 (39.0) |
|
Child characteristics Child's sex | ||||
| Boy | 154 (48.4) | 24 (64.9) | 85 (51.2) | 54 (50.9) |
| Girl | 164 (51.6) | 13 (35.1) | 81 (48.8) | 52 (49.1) |
| Low birth weight (<2,500 grams) | ||||
| No | 305 (95.9) | 35 (94.6) | 161 (97.0) | 100 (94.4) |
| Yes | 8 (2.5) | 2 (5.4) | 4 (2.4) | 3 (2.8) |
| Missing | 5 (1.6) | 0 (0.0) | 1 (0.6) | 3 (2.8) |
| Preterm birth (<37 weeks) | ||||
| No | 298 (93.7) | 34 (91.9) | 154 (92.8) | 103 (97.2) |
| Yes | 20 (6.3) | 3 (8.1) | 12 (7.2) | 3 (2.8) |
| Breastfeeding duration | ||||
| ≤6 months | 71 (22.3) | 11 (29.7) | 44 (26.5) | 24 (22.6) |
| >6 months | 247 (77.7) | 26 (70.3) | 122 (73.5) | 82 (77.4) |
| Age at outcome assessment (months) | 13.1 (12.4-14.7) | 13.0 (12.2-14.7) | 13.3 (12.5-15.3) | 13.8 (12.6-15.1) |
| Maternal characteristics | ||||
| Age at enrollment (years) | 22.1 (19.1-28.2) | 23.5 (20.9-28.6) | 21.4 (18.5-25.8) | 23.0 (19.7-29.1) |
| Country of birth | ||||
| Costa Rica | 266 (83.7) | 30 (81.1) | 134 (80.7) | 90 (84.9) |
| Other | 52 (16.3) | 7 (18.9) | 32 (19.3) | 16 (15.1) |
| Education | ||||
| ≤6th grade | 169 (53.1) | 12 (32.4) | 82 (49.4) | 57 (53.8) |
| >6th grade | 149 (46.9) | 25 (67.6) | 84 (50.6) | 49 (46.2) |
| Parity | ||||
| 0 | 117 (36.8) | 9 (24.3) | 66 (39.8) | 34 (32.1) |
| ≥1 | 199 (62.6) | 28 (75.7) | 99 (59.6) | 72 (67.9) |
| Missing | 2 (0.6) | 0 (0.0) | 1 (0.6) | 0 (0.0) |
| Agricultural work at enrollment | ||||
| No | 289 (90.9) | 35 (94.6) | 155 (93.4) | 93 (87.7) |
| Yes | 29 (9.1) | 2 (5.4) | 11 (6.6) | 13 (12.3) |
| Agricultural work at one-year visit | ||||
| No | 241 (75.8) | 28 (75.7) | 130 (78.3) | 75 (70.8) |
| Yes | 77 (24.2) | 9 (24.3) | 36 (21.7) | 31 (29.2) |
| History of asthma | ||||
| No | 274 (86.2) | 34 (91.9) | 144 (86.8) | 91 (85.9) |
| Yes | 44 (13.8) | 3 (8.1) | 22 (13.2) | 15 (14.1) |
| Smoking during pregnancy | ||||
| No | 306 (96.2) | 33 (89.2) | 163 (98.2) | 95 (89.6) |
| Yes | 11 (3.5) | 4 (10.8) | 3 (1.8) | 10 (9.4) |
| Missing | 9 (0.3) | 0 (0.0) | 0 (0.0) | 1 (1.0) |
| Cotinine levels during pregnancy | ||||
| <LOD (1 ng/mL) | 259 (81.4) | 30 (81.1) | 137 (82.5) | 83 (78.3) |
| ≥LOD | 48 (15.1) | 7 (18.9) | 25 (15.0) | 17 (16.0) |
| Missing | 11 (3.5) | 0 (0.0) | 4 (2.4) | 6 (5.7) |
| Smoking during the year after delivery | ||||
| No | 309 (97.2) | 34 (91.9) | 164 (98.8) | 98 (92.5) |
| Yes | 9 (2.8) | 3 (8.1) | 2 (1.2) | 8 (7.5) |
| Household characteristics | ||||
| Income at enrollmentb | ||||
| >Poverty line | 125 (39.3) | 17 (46.0) | 73 (44.0) | 41 (38.7) |
| <Poverty line and >extreme poverty line | 129 (40.6) | 12 (32.4) | 62 (37.3) | 43 (40.6) |
| <Extreme poverty line | 60 (18.9) | 8 (21.6) | 29 (17.5) | 21 (19.8) |
| Missing | 4 (1.2) | 0 (0.0) | 2 (1.2) | 1 (0.9) |
Abbreviations: LOD, limit of detection; LRTI, lower respiratory tract infection; n, number of children; P25, 25th percentile; P75, 75th percentile.
Wheezing question was administered to only 272 mother-child pairs because it was added to the questionnaire after the one-year visits had already started.
Costa Rican poverty and extreme poverty lines in 2011: US$141 and US$69 per capita per month.
Table 2.
Distribution of prenatal pesticide metabolites (specific gravity-adjusted, ng/mL) concentrations in the study population, ISA study, 2010-2013.
| Pesticide metabolite | %>LOD | Min | Percentile |
Max | ||
|---|---|---|---|---|---|---|
| 25th | 50th | 75th | ||||
| 1st half of pregnancy (n = 194) | ||||||
| ETU | 100 | 0.58 | 2.14 | 3.40 | 4.97 | 31.06 |
| TCPy | 100 | 0.28 | 1.06 | 1.62 | 2.46 | 50.01 |
| 3PBA | 100 | 0.07 | 0.42 | 0.72 | 1.26 | 32.61 |
| 2,4-D | 100 | 0.04 | 0.17 | 0.27 | 0.48 | 3.50 |
| DCCA | 100 | 0.06 | 0.60 | 1.16 | 2.02 | 45.77 |
| OH-PYR | 85 | <LOD | 0.15 | 0.32 | 0.86 | 946.36 |
| 5-OH-TBZ | 63 | <LOD | 0.02 | 0.06 | 0.29 | 144.73 |
| 2nd half of pregnancy (n = 343) | ||||||
| ETU | 100 | 0.65 | 2.21 | 3.25 | 4.70 | 127.38 |
| TCPy | 100 | 0.41 | 1.21 | 1.77 | 2.59 | 91.10 |
| 3PBA | 100 | 0.06 | 0.44 | 0.73 | 1.34 | 16.81 |
| 2,4-D | 100 | 0.05 | 0.22 | 0.31 | 0.55 | 159.21 |
| DCCA | 99 | 0.13 | 0.68 | 1.20 | 2.21 | 18.06 |
| OH-PYR | 92 | <LOD | 0.19 | 0.49 | 1.25 | 368.55 |
| 5-OH-TBZ | 72 | <LOD | 0.02 | 0.09 | 0.47 | 644.46 |
| Pregnancy average (n = 355) | ||||||
| ETU | 100 | 0.81 | 2.38 | 3.35 | 4.90 | 127.38 |
| TCPy | 100 | 0.41 | 1.31 | 1.75 | 2.54 | 62.96 |
| 3PBA | 100 | 0.10 | 0.49 | 0.79 | 1.31 | 16.96 |
| 2,4-D | 100 | 0.09 | 0.23 | 0.33 | 0.53 | 79.76 |
| DCCA | 100 | 0.15 | 0.75 | 1.30 | 2.30 | 23.56 |
| OH-PYR | 94 | <LOD | 0.21 | 0.56 | 1.33 | 368.55 |
| 5-OH-TBZ | 76 | <LOD | 0.03 | 0.11 | 0.58 | 339.00 |
Abbreviations: 2,4-D, 2,4-dichlorophenoxyacetic acid; DCCA, 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylic acid; ETU, ethylenethiourea; ICC, intraclass correlation coefficient; LOD, limit of detection; n, number of children; OH-P, 3-hydroxypyrimetanil; 5-OH-TBZ, 5-hydroxythiabendazol; 3-PBA, 3-phenoxybenzoic acid; SD, standard deviation; TCPy, 3,5,6-trichloro-2-pyridinol.
We observed that most associations of the seven prenatal urinary pesticide metabolites [categorized in high (≥P75) and low (<P75)] with respiratory outcomes during the first year of life hovered around the null (Table 3). However, we found that high urinary ETU concentrations during the first half of pregnancy were associated with increased odds of LRTI (OR = 2.45; 95% CI: 0.96, 6.26). We also observed that high urinary ETU concentrations during the second half of pregnancy were associated with decreased odds of wheezing (OR = 0.50; 95% CI: 0.26, 0.96). We observed similar associations when we ran our models with our exposures modeled as log10-transformed specific gravity-corrected urinary pesticide metabolite concentrations (OR for ETU concentrations during the first half of pregnancy and LRTI = 8.20; 95% CI: 1.66, 40.59; OR for ETU concentrations during the second half of pregnancy and wheezing = 0.37; 95% CI: 0.13, 1.02; Table S3). Our effects estimates did not change appreciably when we excluded mothers who reported smoking during pregnancy (Table S4).
Table 3.
Adjusted associations [OR (95% CI)] for prenatal pesticide metabolites (high vs. low)a and respiratory outcomes at one year of age, ISA study,2010-2013
| Pesticide metabolites | LRTI | Wheezing |
|---|---|---|
| First half of pregnancy | n = 194 | n = 172 |
| ETU | 2.45 (0.96, 6.26) | 1.01 (0.48, 2.12) |
| TCPy | 1.36 (0.50, 3.68) | 0.73 (0.34, 1.56) |
| 3PBA | 1.47 (0.54, 4.01) | 1.52 (0.73, 3.16) |
| 2,4-D | 1.21 (0.43, 3.40) | 0.79 (0.37, 1.68) |
| DCCA | 1.90 (0.72, 5.01) | 1.71 (0.82, 3.57) |
| OH-PYR | 1.60 (0.58, 4.38) | 1.02 (0.48, 2.16) |
| 5-OH-TBZ | 0.34 (0.09, 1.26) | 1.50 (0.74, 3.03) |
| Second half of pregnancy | n = 343 | n = 261 |
| ETU | 0.87 (0.37, 2.05) | 0.50 (0.26, 0.96) |
| TCPy | 0.60 (0.24, 1.53) | 0.82 (0.45, 1.50) |
| 3PBA | 1.40 (0.64, 3.05) | 1.23 (0.68, 2.22) |
| 2,4-D | 1.45 (0.67, 3.11) | 0.87 (0.47, 1.60) |
| DCCA | 0.92 (0.40, 2.10) | 0.76 (0.40, 1.47) |
| OH-PYR | 0.93 (0.41, 2.13) | 0.71 (0.37, 1.33) |
| 5-OH-TBZ | 1.03 (0.46, 2.30) | 0.69 (0.38, 1.28) |
| Pregnancy average | n = 355 | n = 272 |
| ETU | 1.50 (0.70, 3.19) | 0.69 (0.37, 1.28) |
| TCPy | 0.84 (0.36, 1.95) | 0.86 (0.48, 1.54) |
| 3PBA | 1.64 (0.78, 3.47) | 1.07 (0.60, 1.90) |
| 2,4-D | 1.48 (0.69, 3.14) | 0.87 (0.48, 1.59) |
| DCCA | 1.07 (0.49, 2.36) | 0.74 (0.39, 1.38) |
| OH-PYR | 1.49 (0.70, 3.18) | 0.83 (0.45, 1.53) |
| 5-OH-TBZ | 0.98 (0.45, 2.16) | 0.80 (0.45, 1.45) |
Abbreviations: 2,4-D, 2,4-dichlorophenoxyacetic acid; DCCA, 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylic acid; ETU, ethylenethiourea; LRTI, lower respiratory tract infection; n, number of children; OH-P, 3-hydroxypyrimetanil; 5-OH-TBZ, 5-hydroxythiabendazol; OR, odds ratio; 3-PBA, 3-phenoxybenzoic acid; TCPy, 3,5,6-trichloro-2-pyridinol.
Models adjusted for maternal age, education, parity, gestational age at birth, and child sex.
Pesticide exposure categorized as high (≥75th percentile) and low (≥75th percentile, reference category).
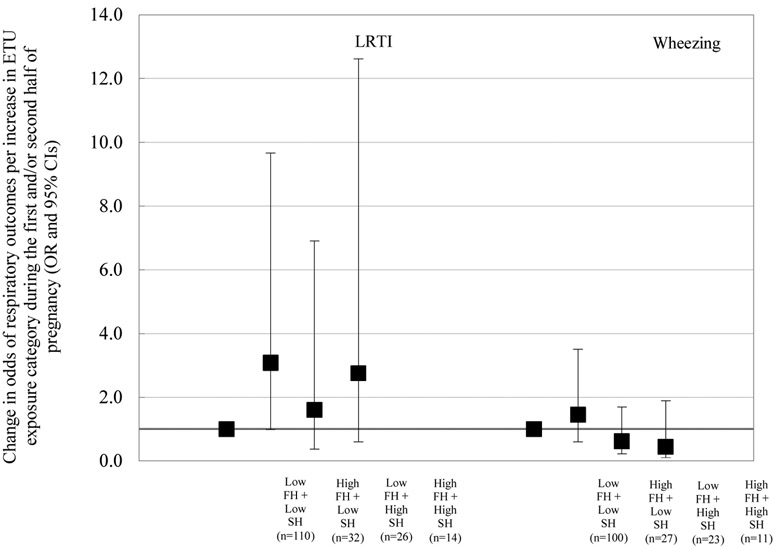
When we ran our main models only with mothers who had exposure data for both the first and second half of pregnancy, we found that high urinary ETU concentrations during the first half of pregnancy remained associated with increased odds of LRTI (OR = 2.69; 95% CI: 1.03, 7.05; n = 182; data not shown in tables or figures). We also observed that high urinary ETU concentrations during the second half of pregnancy were no longer associated with decreased odds of wheezing among these women (OR = 0.51; 95% CI: 0.22, 1.21; n = 161). Similarly, when we clustered mother-infant pairs into four groups based on their urinary ETU concentrations during the first and second half of pregnancy, we observed that pairs with high ETU during first half/low ETU during second half had increased odds of LRTI during the first year of life, compared to mother-child pairs with low ETU during first half/low ETU during second half of pregnancy (OR = 3.08; 95% CI: 0.98, 9.67; Figure 1). Odds of wheezing were not associated with urinary ETU concentrations in our four-group analyses (e.g., OR for mother-infant pairs with low ETU during first half/high ETU during second half compared to the low concordant exposure group = 0.62; 95% CI: 0.22, 1.70; Figure 1).
Figure 1.
Adjusted associations of urinary ETU concentrations during the first (FH) and second (SH) half of pregnancy with respiratory outcomes at one year of age, ISA study, 2010-2013. Models adjusted for maternal age, education, parity, gestational age at birth, and child sex. High indicates above or at the 75th percentile (P75); low indicates below P75. P75 for ETU concentrations during the first half of pregnancy = 3.40 ng/mL; P75 for ETU concentrations during the second half of pregnancy = 3.25 ng/mL. Low FH + Low SH: reference category.
Abbreviations: ETU, ethylenethiourea; LRTI, low respiratory tract infections.
4. Discussion
Our findings suggest that prenatal exposure to mancozeb, as indicated by urinary ETU concentrations in women during pregnancy, is associated with respiratory outcomes in the first year of life. We observed that infants in the highest quartile of maternal urinary ETU concentrations during the first half of pregnancy had increased odds of LRTI, compared to those in the lowest three quartiles. We also found that infants in the highest quartile of maternal urinary ETU concentrations during the second half of pregnancy had decreased odds of wheezing, compared to those in the lowest three quartiles, but this association was attenuated when we included in our analyses only mothers with complete exposure information and when we accounted for maternal ETU concentrations during the first half of pregnancy. It could be possible that exposure to current-use pesticides during the first half of pregnancy is more important than exposure during the second half; however, further research is warranted to assess the possibility of susceptible period(s) during pregnancy and the mechanisms by which exposure to current-use pesticides may affect respiratory system development.
Our findings on ETU and respiratory health are somewhat consistent to the only published study to date on the association of bisdithiocarbamates fungicides exposure and respiratory outcomes in children (Raherison et al. 2019). This cross-sectional study conducted in France found that higher urinary ETU concentrations were associated with asthma and rhinitis symptoms in children aged 3-10 years. This was a relatively small study (n = 66) that examined health effects in older children (mean age = 7.5 years). The creatinine-corrected urinary ETU concentrations in their study population (medians in Phases I and II =0.4 and 0.7 μg/g creatinine, respectively; n = 96) were lower than those observed in the present study (median throughout pregnancy = 3.1 μg/g creatinine), but were higher than those observed in the US general population sample of children aged 6-11 years from the NHANES 2007-2008 study (median <LOD, P95 = 1.0 μg/g creatinine, n = 382) (Centers for Disease Control and Prevention (CDC) 2019). The potential mechanism of bisdithiocarbamates exposure on respiratory health is not well understood; nevertheless, evidence from occupational studies suggests that these fungicides could affect respiratory function by modulating the immune system, inducing macrophage activation, and enhancing the inflammatory response (Colosio et al. 1996; Corsini et al. 2005; Weis et al. 2019). Given the widespread use of mancozeb worldwide, more research studies are needed to understand better the specific mechanisms by which bisdithiocarbamates interfere with the immune and respiratory systems.
Although no published study has assessed the association of exposure to current-use pesticides other than bisdithiocarbamate fungicides with respiratory health in the first year of life, a few studies have examined the association of OP exposure with respiratory outcomes in school-age children (Benka-Coker et al. 2019; Perla et al. 2015; Raanan et al. 2015; Raanan et al. 2016). These studies have reported inconsistent results, possibly due to differences in their study design (e.g., cross-sectional vs. prospective cohort), sources of pesticide exposure (e.g., diet vs. drift from agricultural fields), windows of exposure (e.g., prenatal vs. childhood), and/or exposure assessment methods (e.g., non-specific vs. OP-specific metabolites). For example, in a cross-sectional study of U.S. children aged 5-16 years, OP exposure, as indicated by non-specific dialkyl phosphate (DAP) metabolites in urine, was not associated with parent-reported asthma (n = 2,777) (Perla et al. 2015). In contrast, a birth cohort study of children living near agricultural fields in California observed that higher urinary DAP metabolites in the second half of pregnancy and early childhood (0.5-5 years) were associated with parent-reported respiratory symptoms at ages 5 and 7 years (n = 359) (Raanan et al. 2015) and/or decreased lung function at 7 years (n = 279) (Raanan et al. 2016). Additionally, a very small cross-sectional study of children with asthma aged 6-16 years and living in an agricultural community in Washington State (n = 16), found that higher urinary DAPs metabolites were associated with increased urinary leukotriene E4, a marker of asthma exacerbation (Benka-Coker et al. 2019). In our study, we did not measure urinary DAP metabolites. We measured urinary TCPy, a metabolite specific to OP insecticide chlorpyrifos, in maternal samples collected during pregnancy, but did not find an association with respiratory outcomes in the first year of life; nor did we observe associations of prenatal exposure to pyrimethanil, thiabendazole, common synthetic pyrethroids, and 2,4-D with LRTI and wheezing during the first year of life. In the present study, we did not assess exposure to elemental sulfur or piperonyl butoxide both pesticides and/or pesticide ingredients that have been previously associated with respiratory outcomes in children (Liu et al. 2012; Raanan et al. 2017).
Our study has limitations, mostly related to the assessment of respiratory outcomes. We did not obtain medical records to corroborate the physician- or nurse-confirmed diagnosis of LRTI or wheezing episodes that mothers reported via questionnaire; thus, non-differential outcome misclassification may have occurred. In addition, we examined respiratory outcomes during the first year of life, which may be too early to identify long-lasting respiratory effects of prenatal exposures to environmental toxicants. At the present time, further respiratory assessments of the ISA study participants are being conducted to determine if the exposure-outcome associations observed during the first year of life persist throughout childhood. In our study, we cannot rule out the possibility that selection bias could have arisen from loss to follow-up. Lastly, we recognize that we conducted multiple comparisons, which could have led to statistically significant associations by chance, but we tried to look for patterns in our results rather than to highlight isolated findings.
The present study has considerable strengths, perhaps most notable among them being its longitudinal nature. We measured urinary pesticide metabolites concentrations in maternal samples collected repeatedly during pregnancy – which is a strength considering that the metabolites that we measured reflect short-term pesticide exposures (Lindh et al. 2008; Nolan et al. 1984) -- and evaluated respiratory outcomes at age 1. As in any epidemiologic study, the exposure-outcome associations that we found in our study could be attributable to uncontrolled confounders, but we were able to assess or adjust for several important factors, including maternal characteristics.
To our knowledge, ours is the first study to examine the association of prenatal exposure to current-use pesticides with respiratory symptoms and infections during the first year of life. Early life respiratory outcomes can have long-term consequences for respiratory health (Busse et al. 2010; Jackson et al. 2008; Jackson 2014; Jackson et al. 2016; Lemanske et al. 2005; Liu et al. 2017; Lodge et al. 2014; Rubner et al. 2017). For example, viral wheezing respiratory illnesses in infancy and early childhood life have been associated with an increased risk of asthma at school age (Jackson et al. 2008) and adolescence (Rubner et al. 2017). Early life viral wheezing illnesses have also been associated with decreased lung function at age 8 years (Guilbert et al. 2011). Previous studies have shown that the respiratory and allergic disease risk profiles in Costa Rica are similar to those reported in industrialized countries with a Western lifestyle (Celedon et al. 2002) and that children in Costa Rica have one of the highest prevalence of wheeze worldwide (Lai et al. 2009). In our study population, 39% of children had at least one episode of wheezing during their first year of life, whereas 27% of children from eight European cohorts (Gascon et al. 2014) and 26% of children from a New York City birth cohort (Donohue et al. 2008) experienced wheezing by ages 1.5 and 3 years, respectively. In contrast, only 10% of the children included in our study had developed at least one LRTI episode during their first year of life, whereas 35% of the children from a large Spanish birth cohort (Gascon et al. 2012) and 19% of the children from a Mexican birth cohort (Cupul-Uicab et al. 2014) experienced at least one LRTI by ages 12-14 and 18 months, respectively. Further studies should examine how prevalence differences in respiratory outcomes during infancy and early-life exposure to pesticides can influence long-term respiratory health effects.
5. Conclusions
Prenatal exposure to mancozeb, but not to other current-use pesticides, was associated with respiratory outcomes during the first year of life in infants living near banana plantations in Costa Rica. Our results are biologically plausible, given the immunomodulatory effects of bisdithiocarbamate fungicides observed in occupational studies, and are, to some degree, consistent with the only published study to date on the association of bisdithiocarbamates fungicides exposure and respiratory outcomes in children. Our findings provide additional evidence supporting an association between prenatal pesticide exposure and respiratory health in children and are important due to the widespread use of pesticides in agriculture (Food and Agriculture Organization of the United Nations (FAO) 2019) and increasing prevalence of chronic respiratory diseases in children worldwide (Pearce et al. 2007; Zar and Ferkol 2014).
Supplementary Material
Acknowledgments
We gratefully acknowledge the ISA families, staff, and community partners. We would also like to thank R. Quesada, C. Hernandez, J. Peñaloza Castaneda, Marie Bengtsson, Daniela Pineda, and Margaretha Maxe for their fieldwork, laboratory analyses, and/or data management assistance.
Funding
This publication was made possible by research supported by grant numbers: PO1 105296-001 (IDRC); 6807-05-2011/7300127 (Health Canada); 2010-1211, 2009-2070, and 2014-01095 (Swedish Research Council Formas); and R21 ES025374 (NIEHS).
Footnotes
Competing financial interests
None of the other authors declares any actual or potential competing financial interests.
Abbreviations: 2,4-D, 2,4-dichlorophenoxyacetic acid; DAP, dialkyl phosphate; DCCA, 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylic acid; DDE, dichlorodiphenyldichloroethylene; DDT, dichlorodiphenyldichloroethane; ETU, ethylenethiourea; ISA, Infants' Environmental Health (‘Infantes y Salud Ambiental’); ICC, intraclass correlation coefficient; ISAAC, International Study of Asthma and Allergies in Childhood; LOD, limit of detection; LC-MS/MS, liquid chromatography mass spectrometer; LRTI, lower respiratory tract infection; OH-P, 3-hydroxypyrimetanil; 5-OH-TBZ, 5-hydroxythiabendazol; OP, organophosphate; OR, odds ratio; 3-PBA, 3-phenoxybenzoic acid; P75, 75th percentile; SD, standard deviation; TCPy, 3,5,6-trichloro-2-pyridinol.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benka-Coker W, Loftus C, Karr C, Magzamen S. 2019. Association of organophosphate pesticide exposure and a marker of asthma morbidity in an agricultural community. J Agromedicine:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloomberg GR. 2011. The influence of environment, as represented by diet and air pollution, upon incidence and prevalence of wheezing illnesses in young children. Curr Opin Allergy Clin Immunol 11:144–149. [DOI] [PubMed] [Google Scholar]
- 3.Busse WW, Lemanske RF Jr., Gern JE. 2010. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet 376:826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celedon JC, Soto-Quiros ME, Hanson LA, Weiss ST. 2002. The relationship among markers of allergy, asthma, allergic rhinitis, and eczema in Costa Rica. Pediatr Allergy Immunol 13:91–97. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC). 2019. Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, January 2019. Available: https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume1_Jan2019-508.pdf [accessed August 10, 2019].
- 6.Colosio C, Barcellini W, Maroni M, Alcini D, Bersani M, Cavallo D, et al. 1996. Immunomodulatory effects of occupational exposure to mancozeb. Arch Environ Health 51:445–451. [DOI] [PubMed] [Google Scholar]
- 7.Corsini E, Birindelli S, Fustinoni S, De Paschale G, Mammone T, Visentin S, et al. 2005. Immunomodulatory effects of the fungicide mancozeb in agricultural workers. Toxicol Appl Pharmacol 208:178–185. [DOI] [PubMed] [Google Scholar]
- 8.Cupul-Uicab LA, Terrazas-Medina EA, Hernandez-Avila M, Longnecker MP. 2014. Prenatal exposure to p,p'-DDE and p,p'-DDT in relation to lower respiratory tract infections in boys from a highly exposed area of Mexico. Environ Res 132:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dick S, Friend A, Dynes K, AlKandari F, Doust E, Cowie H, et al. 2014. A systematic review of associations between environmental exposures and development of asthma in children aged up to 9 years. BMJ Open 4:e006554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donohue KM, Al-alem U, Perzanowski MS, Chew GL, Johnson A, Divjan A, et al. 2008. Anti-cockroach and anti-mouse IgE are associated with early wheeze and atopy in an inner-city birth cohort. J Allergy Clin Immunol 122:914–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ekman E, Maxe M, Littorin M, Jonsson BA, Lindh CH. 2013. High-throughput method for the analysis of ethylenethiourea with direct injection of hydrolysed urine using online on-column extraction liquid chromatography and triple quadrupole mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 934:53–59. [DOI] [PubMed] [Google Scholar]
- 12.Ekman E, Faniband MH, Littorin M, Maxe M, Jonsson BA, Lindh CH. 2014. Determination of 5-hydroxythiabendazole in human urine as a biomarker of exposure to thiabendazole using LC/MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 973C:61–67. [DOI] [PubMed] [Google Scholar]
- 13.Faniband M, Ekman E, Littorin M, Maxe M, Larsson E, Lindh CH. 2019. Biomarkers of exposure to pyrimethanil after controlled human experiments. J Anal Toxicol 43:277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Food and Agriculture Organization of the United Nations (FAO). 2019. Statistics and Database of the Food and Agriculture Organization of the United Nations (FAOSTAT). Available: http://www.fao.org/faostat/en/ [accessed August 12, 2019 2019].
- 15.Gascon M, Vrijheid M, Martinez D, Ballester F, Basterrechea M, Blarduni E, et al. 2012. Prenatal exposure to dichlorodiphenyldichloroethylene and infant lower respiratory tract infections and wheeze. Eur Respir J 39:1188–1196. [DOI] [PubMed] [Google Scholar]
- 16.Gascon M, Morales E, Sunyer J, Vrijheid M. 2013. Effects of persistent organic pollutants on the developing respiratory and immune systems: A systematic review. Environ Int 52:51–65. [DOI] [PubMed] [Google Scholar]
- 17.Gascon M, Sunyer J, Casas M, Martinez D, Ballester F, Basterrechea M, et al. 2014. Prenatal exposure to DDE and PCB 153 and respiratory health in early childhood: A meta-analysis. Epidemiology 25:544–553. [DOI] [PubMed] [Google Scholar]
- 18.Guilbert TW, Singh AM, Danov Z, Evans MD, Jackson DJ, Burton R, et al. 2011. Decreased lung function after preschool wheezing rhinovirus illnesses in children at risk to develop asthma. J Allergy Clin Immunol 128:532–538.e531-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hehua Z, Qing C, Shanyan G, Qijun W, Yuhong Z. 2017. The impact of prenatal exposure to air pollution on childhood wheezing and asthma: A systematic review. Environ Res 159:519–530. [DOI] [PubMed] [Google Scholar]
- 20.ISAAC. 1998. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet 351:1225–1232. [PubMed] [Google Scholar]
- 21.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, et al. 2008. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med 178:667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson DJ. 2014. Early-life viral infections and the development of asthma: A target for asthma prevention? Curr Opin Allergy Clin Immunol 14:131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson DJ, Gern JE, Lemanske RF Jr., 2016. The contributions of allergic sensitization and respiratory pathogens to asthma inception. J Allergy Clin Immunol 137:659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai CK, Beasley R, Crane J, Foliaki S, Shah J, Weiland S, et al. 2009. Global variation in the prevalence and severity of asthma symptoms: Phase Three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 64:476–483. [DOI] [PubMed] [Google Scholar]
- 25.Lemanske RF Jr., Jackson DJ, Gangnon RE, Evans MD, Li Z, Shult PA, et al. 2005. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol 116:571–577. [DOI] [PubMed] [Google Scholar]
- 26.Lindh CH, Littorin M, Johannesson G, Jonsson BA. 2008. Analysis of ethylenethiourea as a biomarker in human urine using liquid chromatography/triple quadrupole mass spectrometry. Rapid Commun Mass Spectrom 22:2573–2579. [DOI] [PubMed] [Google Scholar]
- 27.Liu B, Jung KH, Horton MK, Camann DE, Liu X, Reardon AM, et al. 2012. Prenatal exposure to pesticide ingredient piperonyl butoxide and childhood cough in an urban cohort. Environ Int 48:156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu W, Huang C, Wang X, Cai J, Hu Y, Zou Z, et al. 2017. Multimorbidities of asthma, allergies, and airway illnesses in childhood: Chance or not chance? J Asthma 54:687–698. [DOI] [PubMed] [Google Scholar]
- 29.Lodge CJ, Zaloumis S, Lowe AJ, Gurrin LC, Matheson MC, Axelrad C, et al. 2014. Early-life risk factors for childhood wheeze phenotypes in a high-risk birth cohort. J Pediatr 164:289–294.e281-282. [DOI] [PubMed] [Google Scholar]
- 30.McEvoy CT, Spindel ER. 2017. Pulmonary effects of maternal smoking on the fetus and child: Effects on lung development, respiratory morbidities, and life long lung health. Paediatr Respir Rev 21:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mora AM, van Wendel de Joode B, Mergler D, Cordoba L, Cano C, Quesada R, et al. 2014. Blood and hair manganese concentrations in pregnant women from the Infants' Environmental Health study (ISA) in Costa Rica. Environ Sci Technol 48:3467–3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mora AM, van Wendel de Joode B, Mergler D, Cordoba L, Cano C, Quesada R, et al. 2015. Maternal blood and hair manganese concentrations, fetal growth, and length of gestation in the ISA cohort in Costa Rica. Environ Res 136:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nolan RJ, Rick DL, Freshour NL, Saunders JH. 1984. Chlorpyrifos: Pharmacokinetics in human volunteers. Toxicol Appl Pharmacol 73:8–15. [DOI] [PubMed] [Google Scholar]
- 34.Norén E, Lindh CH, Rylander L, Glynn A, Axelsson J, Littorin M, et al. 2020. Concentrations and temporal trends in pesticide biomarkers in urine of Swedish adolescents, 2000-2017. J Expo Sci Environ Epidemiol. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pearce N, Ait-Khaled N, Beasley R, Mallol J, Keil U, Mitchell E, et al. 2007. Worldwide trends in the prevalence of asthma symptoms: Phase III of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 62:758–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perla ME, Rue T, Cheadle A, Krieger J, Karr CJ. 2015. Biomarkers of insecticide exposure and asthma in children: A National Health and Nutrition Examination Survey (NHANES) 1999-2008 analysis. Arch Environ Occup Health 70:309–322. [DOI] [PubMed] [Google Scholar]
- 37.Raanan R, Harley KG, Balmes JR, Bradman A, Lipsett M, Eskenazi B. 2015. Early-life exposure to organophosphate pesticides and pediatric respiratory symptoms in the CHAMACOS cohort. Environ Health Perspect 123:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raanan R, Balmes JR, Harley KG, Gunier RB, Magzamen S, Bradman A, et al. 2016. Decreased lung function in 7-year-old children with early-life organophosphate exposure. Thorax 71:148–153. [DOI] [PubMed] [Google Scholar]
- 39.Raanan R, Gunier RB, Balmes JR, Beltran AJ, Harley KG, Bradman A, et al. 2017. Elemental sulfur use and associations with pediatric lung function and respiratory symptoms in an agricultural community (California, USA). Environ Health Perspect 125:087007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raherison C, Baldi I, Pouquet M, Berteaud E, Moesch C, Bouvier G, et al. 2019. Pesticides exposure by air in vineyard rural area and respiratory health in children: A pilot study. Environ Res 169:189–195. [DOI] [PubMed] [Google Scholar]
- 41.Rubner FJ, Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Pappas TE, et al. 2017. Early life rhinovirus wheezing, allergic sensitization, and asthma risk at adolescence. J Allergy Clin Immunol 139:501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Selby A, Munro A, Grimshaw KE, Cornelius V, Keil T, Grabenhenrich L, et al. 2018. Prevalence estimates and risk factors for early childhood wheeze across Europe: The EUROPREVALL birth cohort. Thorax 73:1049–1061. [DOI] [PubMed] [Google Scholar]
- 43.van Wendel de Joode B, Barraza D, Ruepert C, Mora AM, Cordoba L, Oberg M, et al. 2012. Indigenous children living nearby plantations with chlorpyrifos-treated bags have elevated 3,5,6-trichloro-2-pyridinol (TCPy) urinary concentrations. Environ Res 117:17–26. [DOI] [PubMed] [Google Scholar]
- 44.van Wendel de Joode B, Mora AM, Cordoba L, Cano JC, Quesada R, Faniband M, et al. 2014. Aerial application of mancozeb and urinary ethylene thiourea (ETU) concentrations among pregnant women in Costa Rica: The Infants' Environmental Health study (ISA). Environ Health Perspect 122:1321–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vanker A, Gie RP, Zar HJ. 2017. The association between environmental tobacco smoke exposure and childhood respiratory disease: A review. Expert Rev Respir Med 11:661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weis GCC, Assmann CE, Cadona FC, Bonadiman B, Alves AO, Machado AK, et al. 2019. Immunomodulatory effect of mancozeb, chlorothalonil, and thiophanate methyl pesticides on macrophage cells. Ecotoxicol Environ Saf 182:109420. [DOI] [PubMed] [Google Scholar]
- 47.Zar HJ, Ferkol TW. 2014. The global burden of respiratory disease-impact on child health. Pediatr Pulmonol 49:430–434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.