Abstract
The Wnt/β-catenin signaling pathway has a well-described role in liver pathobiology. Its suppression was recently shown to decrease bile acid (BA) synthesis, thus preventing the development of cholestatic liver injury and fibrosis after bile duct ligation (BDL). To generalize these observations, we suppressed β-catenin in Mdr2 knockout (KO) mice, which develop sclerosing cholangitis due to regurgitation of BA from leaky ducts. When β-catenin was knocked down (KD) in KO for 2 weeks, hepatic and biliary injury were exacerbated in comparison to KO given placebo, as shown by serum biochemistry, ductular reaction, inflammation, and fibrosis. Simultaneously, KO/KD livers displayed increased oxidative stress and senescence and an impaired regenerative response. Although total liver BA levels were similar between KO/KD and KO, there was significant dysregulation of BA transporters and BA detoxification/synthesis enzymes in KO/KD compared to KO alone. Multiphoton intravital microscopy revealed a mixing of blood and bile in the sinusoids, and validated the presence of increased serum BA in KO/KD mice. Although hepatocyte junctions were intact, KO/KD livers had significant canalicular defects, which resulted from loss of hepatocyte polarity. Thus, in contrast to the protective effect of β-catenin KD in BDL model, β-catenin KD in Mdr2 KO aggravated rather than alleviated injury by interfering with expression of BA transporters, hepatocyte polarity, canalicular structure, and the regenerative response. The resulting imbalance between ongoing injury and restitution led to worsening of the Mdr2 KO phenotype, suggesting caution in targeting β-catenin globally for all cholestatic conditions.
Keywords: cholestatic liver disease, ductular proliferation, biliary fibrosis, bile acids, adherens junctions, tight junctions, hepatocyte polarity
INTRODUCTION
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease characterized by inflammation and fibrosis of the intra- and extra-hepatic bile ducts, resulting in end-stage liver disease and reduced life expectancy. Because liver transplantation remains the only life-extending treatment option for PSC patients(1), effective medical therapies to halt PSC disease progression represent an area of significant unmet clinical need. Mice with a disruption of the Mdr2 gene (Mdr2 KO) spontaneously develop sclerosing cholangitis, characterized by pericholangitis, atypical ductular proliferation, and fibrosis. This phenotype is due to defective biliary phospholipid secretion, which renders bile toxic and injures cholangiocytes, leading to leakage of bile into the parenchyma. Infiltration of inflammatory cell populations exacerbates injury; ongoing inflammation ultimately results in activated myofibroblasts and onion-skin like periductal fibrosis(2). Because the Mdr2-deficient mouse recapitulates some of the morphological and pathogenic features of PSC(3, 4), it is a useful model for evaluating and validating the contribution of cell signaling pathways and for developing novel therapeutic strategies.
Among the potential contributors to the progression of cholestatic liver disease is the Wnt signaling pathway. β-catenin is the chief downstream effector of canonical Wnt signaling, and in combination with TCF/LEF transcription factors, it activates target genes important in proliferation and differentiation. β-catenin also forms a bridge between the actin cytoskeleton and E-cadherin present at the surface of hepatocytes, where it is part of adherens junctions (AJ). The Wnt/β-catenin pathway is crucial to normal liver development, adult tissue homeostasis, and liver regeneration, and it also plays an important role in pathophysiological processes such as diet-induced steatohepatitis and cancer (for a comprehensive review, see (5)). Loss of β-catenin also impacts bile acid (BA) metabolism at homeostasis, as mice that lack β-catenin in both hepatocytes and cholangiocytes exhibited modestly higher basal hepatic BA than controls, which was most likely due to sluggish bile flow as no defect in hepatic tight junctions (TJ) was noted(6, 7). Despite these changes, we have recently demonstrated that loss or inhibition of β-catenin in mice leads to improved outcomes after bile duct ligation (BDL) through regulation of BA synthesis and transport, providing evidence that suppression of this pathway during cholestasis may alleviate injury and progression of disease(8).
Based on these findings, we anticipated that inhibiting β-catenin in the hepatocytes of Mdr2 knockout (KO) mice would ameliorate hepatic damage, fibrosis, and cholestasis. Remarkably, we found that loss of β-catenin exacerbates the extent of cholestatic injury. Knockdown (KD) of β-catenin in Mdr2 KO led to increased inflammation, cellular senescence, oxidative stress, fibrosis, and impaired liver regeneration after injury. Although both AJ and TJ were intact in these mice, loss of β-catenin from hepatocytes resulted in upregulation of genes associated with a mesenchymal phenotype, which led to disrupted cell polarity during cholestasis. As a consequence, biliary canaliculi were distorted, and BA transporter expression was altered, leading to mixing of blood and bile in sinusoids and increased serum BA levels in the absence of β-catenin and Mdr2. This study highlights the essential role of β-catenin in maintaining structural integrity of the liver and facilitating hepatobiliary repair during cholestatic injury.
METHODS
Animals
Animal studies were performed in accordance with the guidelines of the Institutional Animal Use and Care Committee at the University of Pittsburgh School of Medicine and the National Institutes of Health (protocol number 17071066). Mdr2 KO mice and littermate controls (FVB background; JAX stock #002539) at 5 weeks of age were administered either a Dicer-substrate siRNA (DsiRNA) against β-catenin encapsulated in a lipid nanoparticle (LNP) or a scrambled DsiRNA control LNP intravenously at a concentration of 3mg/kg. Injections were repeated 1 week later, and mice were sacrificed 5 days after the final injection. Alternatively, Mdr2 KO mice and littermate controls were administered siRNA against β-catenin conjugated to N-acetylgalactosamine or PBS vehicle subcutaneously at a concentration of 5mg/kg. Injections were repeated 1 week later, and mice were sacrificed 7 days after the final injection. Mdr2 KO mice were also bred into a C57BL/6 background for >6 generations and crossed with Ctnnb1loxp/loxp to obtain Mdr2−/−; Ctnnb1loxp/loxp. At 5 weeks of age, mice were injected with 5×1011 viral particles of AAV8-TBG-cre (Penn Vector Core) to delete β-catenin from hepatocytes(9). Mice were sacrificed 2 weeks after injection. For all groups, blood was collected at the time of sacrifice and serum sent to University of Pittsburgh Medical Center Clinical Chemistry lab for biochemical analysis. Liver sections were fixed in 10% formalin and processed for paraffin embedding; the remaining liver was frozen in liquid nitrogen and stored at −80°C. All mice were maintained in ventilated cages under 12h light/dark cycles with access to enrichment, water and standard chow diet ad libitum.
Further experimental details are available in an online supplement.
RESULTS
Inhibition of β-catenin exacerbates hepatobiliary injury in Mdr2 KO mice.
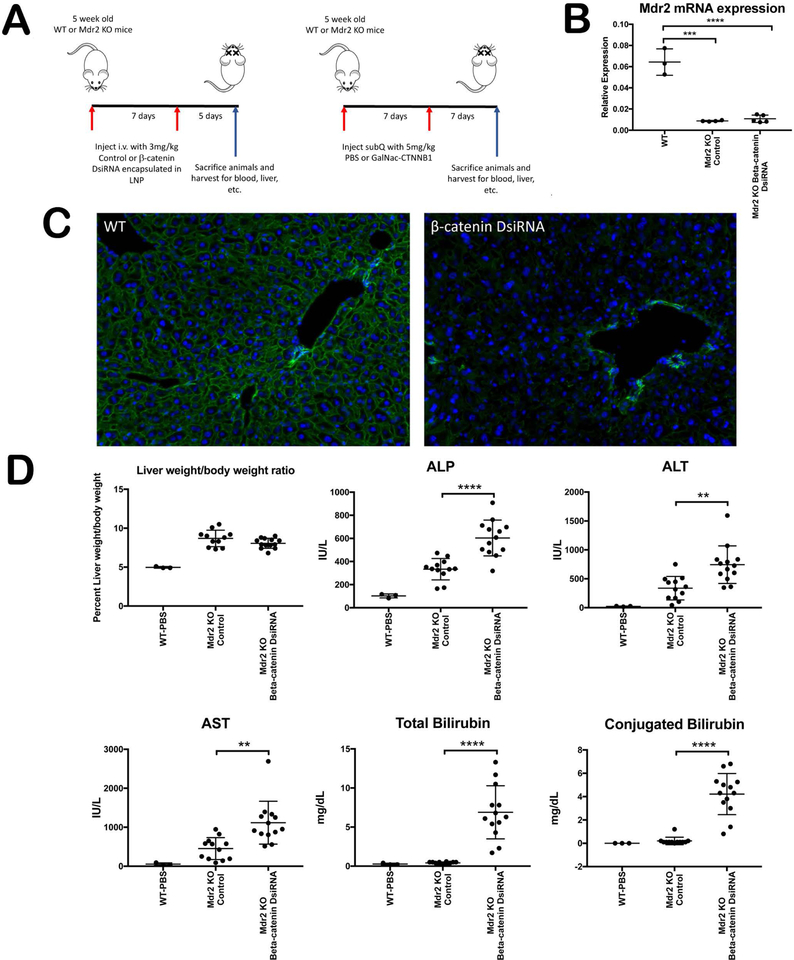
To assess whether inhibiting β-catenin would delay or prevent the onset of injury in a genetic model of cholestasis, 5 week old Mdr2 KO and WT littermates were administered a Dicer-substrate siRNA (DsiRNA) against β-catenin. To demonstrate reproducibility, we utilized two different formulations, both provided by Dicerna Pharmaceuticals: β-catenin DsiRNA encapsulated in a lipid nanoparticle (LNP), injected intravenously; and a β-catenin DsiRNA conjugated to N-acetylgalactosamine, injected subcutaneously. Different primary oligonucleotide sequences were used for both test articles. Both drugs were administered twice, and mice were sacrificed either 5 days or 7 days after the final dose (Figure 1A). We confirmed that Mdr2 expression in liver was dramatically decreased in KO livers as expected, as assessed by mRNA analysis (Figure 1B). We also confirmed efficacy of hepatocyte β-catenin suppression by immunofluorescence (IF) for β-catenin in WT livers (Figure 1C); similar suppression was achieved in both WT and Mdr2 KO mice using both inhibitors.
Figure 1: Knockdown of β-catenin aggravates hepatobiliary injury in Mdr2 KO mice.
(A) Schematic showing different delivery methods of β-catenin DsiRNA in Mdr2 KO mice. (B) qPCR shows significant decrease in Mdr2 gene expression in KO liver with and without β-catenin inhibition as compared to WT. (C) IF demonstrates almost complete reduction of β-catenin in hepatocytes in WT liver following its knockdown (200X). (D) Liver weight/body weight (LW/BW) ratio remains unchanged in KO/KD compared to Mdr2 KO. However, KO/KD had significantly higher serum ALP, ALT, AST, total bilirubin, and conjugated bilirubin levels compared to Mdr2 KO alone. **p<0.01; ****p<0.0001.
We next analyzed serum biochemistry parameters in Mdr2 KO mice treated with either placebo or the two β-catenin inhibitors; WT mice at baseline served as a reference range. As the phenotype of hepatocyte-specific β-catenin inhibition or deletion in WT mice has been previously described(8), the results are not shown for brevity; all parameters and endpoints for this study recapitulated previously reported findings. At the time of sacrifice, liver weight to body weight ratios did not differ between the Mdr2 KO and Mdr2 KO/β-catenin knockdown (KO/KD) livers using either formulation, although in all cases KO livers were larger than WT livers at the same time point (Figure 1D). Surprisingly, however, we found a worsening liver injury in KO/KD regardless of modality of inhibition (Figure 1D). Both hepatic injury (ALT, AST) and biliary injury (ALP, bilirubin) were significantly increased in KO in the absence of β-catenin. We also analyzed serum biochemistry in Mdr2 KO; Ctnnb1loxp/loxp mice that were sacrificed 2 weeks after AAV8-cre injection (Supplementary Figure 1A). These genetic double KO had a similar albeit slower progression of injury compared to siRNA-inhibited KO/KD mice, with non-significant increases in ALP and bilirubin compared to littermate Mdr2 KO mice (Supplementary Figure 1B). Thus, rather than affording protection from cholestasis, loss of hepatocyte β-catenin in the Mdr2 KO model worsens injury, independent of the mechanism of inhibition.
Parenchymal injury, ductular response, inflammation, and fibrosis are increased after β-catenin inhibition in Mdr2 KO mice.
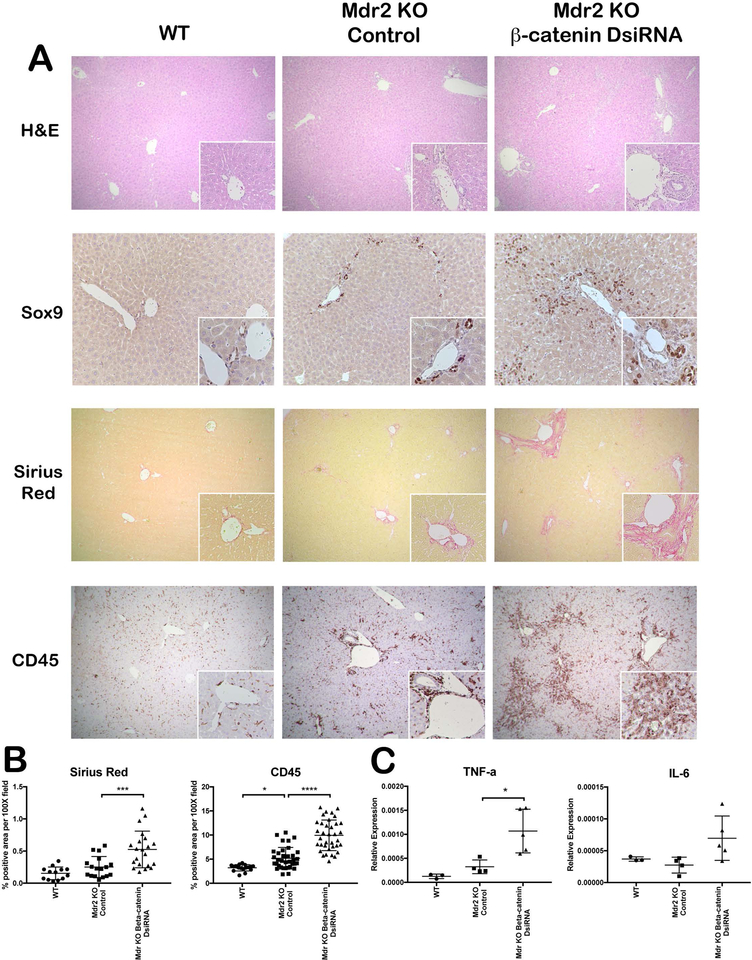
Histologically, Mdr2 KO mice had a mild but notable ductular response compared to WT, which was consistent with previous findings(4, 10) (Figure 2A). These changes were more pronounced in KO/KD livers, which had extensive portal tracts containing reactive ductules and inflammatory cells. Genetic Mdr2/β-catenin KO showed similar histological abnormalities (Supplementary Figure 1C). Sox9 immunohistochemistry (IHC) confirmed a significantly increased number of cholangiocytes in the portal tracts of Mdr2 KO/β-catenin inhibited mice (Figure 2A). Fibrosis was also exacerbated in livers of Mdr2 KO mice lacking β-catenin compared to Mdr2 KO alone, with multiple areas of onionskin-like fibrotic lesions and porto-portal bridging fibrosis per section in KO/KD (Figure 2A,B). CD45 IHC indicates a strong periductal and parenchymal inflammatory response in KO/KD, which is significantly increased over KO alone (Figure 2A,B). Pro-inflammatory cytokines IL-6 and TNF-α mRNA are also increased in KO/KD (Figure 2C). Taken together, these findings indicate accelerated disease progression in Mdr2 KO livers lacking β-catenin.
Figure 2: KO/KD liver show increased parenchymal injury, ductular response, fibrosis, and inflammation.
(A) H&E staining shows increased parenchymal injury in KO/KD liver compared to WT or KO alone (50X; 100X inset). IHC for Sox-9 exhibits increased ductular mass in the periportal area of KO/KD liver (100X; 200X inset). Sirius red (50X; 100X inset) and CD45 IHC (100X; 200X inset) demonstrate increased liver fibrosis and inflammation in KO/KD liver compared to WT or KO alone. (B) Quantitative analysis of Sirius red and CD45 further confirms histological interpretations. (C) qPCR analysis shows increased mRNA level of proinflammatory cytokines IL-6 and TNFα in KO/KD liver. *p<0.05; ***p<0.001; ****p<0.0001.
Both hepatocyte and cholangiocyte senescence is increased in KO/KD livers.
Abnormally proliferating cholangiocytes can become senescent and release pro-fibroinflammatory factors that contribute to the pathogenesis of PSC(11). We assessed p21, a marker and mediator of cellular senescence, by IHC and found expression in cholangiocytes and rare periportal hepatocytes in Mdr2 KO, which is consistent with previous reports of biliary senescence in this model(12, 13). Inhibiting β-catenin in the background of Mdr2 KO resulted in an increased number of p21-positive cholangiocytes and expansion of staining to most of the hepatocytes surrounding the ducts (Supplementary Figure 2A). Co-localization with HNF4a and CK19 confirms an increased number of p21-positive hepatocytes and cholangiocytes, respectively, in KO/KD livers (Supplementary Figure 2B). qPCR also shows a significant upregulation of p21 expression in KO/KD livers, while p16INK4a, another regulator of senescence, remains unchanged (Supplementary Figure 2C). Thus, exacerbated ductular response in KO/KD livers correlates with induction of widespread cholangiocyte and hepatocyte senescence.
Oxidative stress response genes are dysregulated in KO/KD livers.
Because β-catenin has been shown to regulate cellular redox homeostasis in liver, we next examined oxidative stress in KO after β-catenin inhibition(14). Several genes involved in the protective response against oxidative stress are dysregulated in KO/KD, with some (such as glutathione peroxidase (GPX) and superoxide dismutase (SOD1)) that are downregulated, while others (glutathione-S-transferase (GST), NAD(P)H:quinone oxidoreductase 1 (NQO1), and heme oxygenase (HO-1)) are increased relative to WT and KO alone (Supplementary Figure 3A). KO/KD mice also have increased lipid peroxidation, which was present in multiple hepatocytes adjacent to reactive ductules, as assessed by malondialdehyde (MDA) staining (Supplementary Figure 3B). Oxidative stress could thus be an additional mechanism contributing to accelerated injury in the KO/KD mice.
Mdr2 KO mice lacking β-catenin have an impaired regenerative response which leads to the appearance of β-catenin positive hepatocyte clusters.
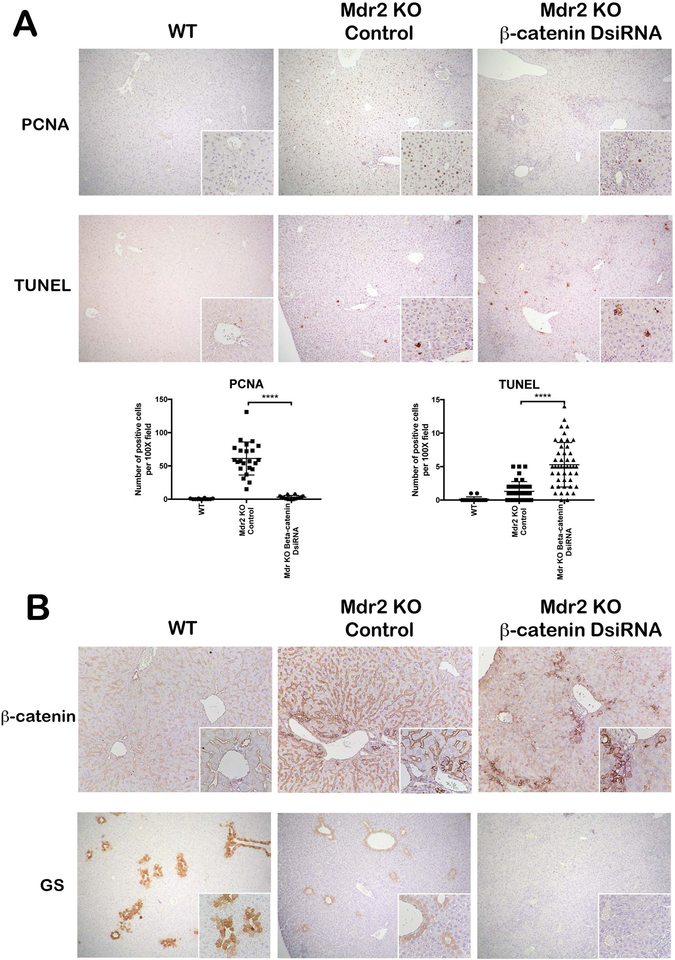
In livers that are subject to chronic insult, the ability of hepatocytes to regenerate is crucial to ensure continued liver function. We analyzed hepatocyte proliferation by PCNA IHC and noted a robust proliferative response in Mdr2 KO mice, which was significantly depressed in KO/KD livers (Figure 3A). TUNEL staining also revealed increased cell death in these livers compared to Mdr2 KO alone (Figure 3A). Thus, the balance between injury and repair in Mdr2 KO is altered when β-catenin, an important regulator of hepatocyte proliferation through cyclin D1 transcription(15), is inhibited.
Figure 3: Simultaneous loss of Mdr2 and β-catenin leads to impaired regenerative response in liver.
IHC for PCNA shows increased proliferative response in Mdr2 KO mice, which was significantly reduced in KO/KD liver (50X; 100X inset). TUNEL staining revealed increased cell death in KO/KD liver compared to Mdr2 KO alone (50X; 100X inset). Quantification of PCNA and TUNEL staining further confirms the IHC interpretation. (B) IHC for β-catenin showed significantly reduced expression in KO/KD hepatocytes compared to WT or KO alone (100X; inset 200X). GS staining is reduced in Mdr2 KO as compared to WT and absent in KO/KD liver (50X; 100X inset). ****p<0.0001.
Loss of β-catenin from hepatocytes in KO/KD livers was confirmed by IHC, though expression of β-catenin was retained in cholangiocytes (Figure 3B). Notably, there was a small subset of hepatocytes mainly located in the periportal region that also stained positive for β-catenin, despite efficient knockdown of β-catenin in the absence of injury (Figure 1C). These hepatocytes tended to be small in size with a high nuclear/cytoplasmic ratio (Figure 3B, inset), and do not appear to be fully differentiated, as expression of glutamine synthetase (GS), a transcriptional target of β-catenin and a marker of mature hepatocytes(16), was absent in KO/KD livers (Figure 3B).
Loss of β-catenin in KO/KD is compensated for by γ-catenin at adherens junctions (AJ).
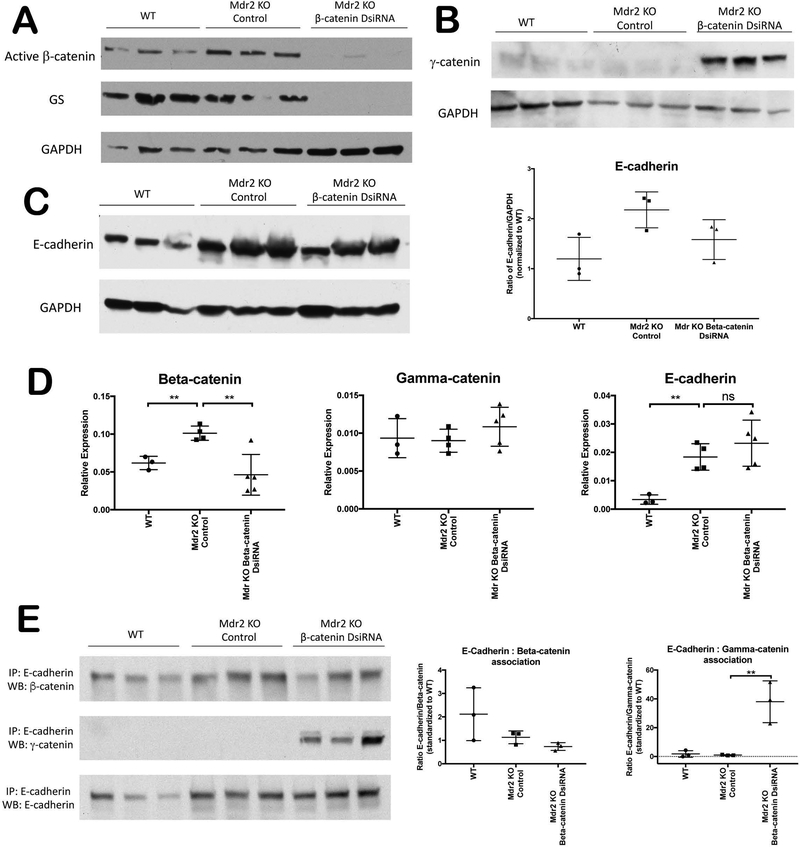
Since cholestasis alters the structure and function of hepatocyte junctions(17), and β-catenin is a structural component of AJ, we next sought to examine the junctional integrity of KO/KD livers. Western blot (WB) confirms loss of hepatocyte β-catenin in KO/KD, as assessed by active β-catenin and GS (Figure 4A). Intriguingly, however, β-catenin protein and mRNA expression increases in Mdr2 KO alone relative to WT (Figure 4A, D). Previous work has shown that γ-catenin, which is normally a component of desmosomes, can compensate for loss of β-catenin at AJ to maintain cell-cell adhesion(18, 19). Accordingly, γ-catenin protein, which is expressed at low levels in WT and Mdr2 KO, is significantly increased in KO/KD livers (Figure 4B). qPCR demonstrates that increased γ-catenin expression is regulated by post-translational modifications, as described previously(18) (Figure 4D). E-cadherin was modestly increased in Mdr2 KO compared to WT, but was insignificantly changed from KO in KO/KD, as assessed by WB and qPCR (Figure 4C, D). Immunoprecipitation with E-cadherin confirms that γ-catenin associates with E-cadherin in the absence of β-catenin in KO/KD livers (Figure 4E). Thus, loss of hepatocyte β-catenin does not affect integrity of AJ in KO/KD livers.
Figure 4: No significant changes in AJ components in KO/KD liver.
(A) WB for active (hypo-phosphorylated) β-catenin and GS confirms loss of both β-catenin activity and GS in KO/KD. (B) WB for γ-catenin exhibits significant upregulation in KO/KD liver compared to either WT or Mdr2 KO. (C) WB and corresponding densitometric quantification shows upregulation of E-cadherin protein level in both Mdr2 KO and KO/KD liver. (D) mRNA expression of γ-catenin and E-cadherin does not differ significantly between KO/KD and KO alone, while β-catenin mRNA increases in Mdr2 KO alone and decreases in KO/KD. (E) Immunoprecipitation of E-cadherin from whole liver lysates followed by WB and densitometric validation shows its association with β-catenin is diminished in KO/KD liver, whereas increased association of E-cadherin and γ-catenin is observed. GAPDH shown as loading control; **p<0.01.
Hepatocyte tight junctions (TJ) are intact in KO/KD livers.
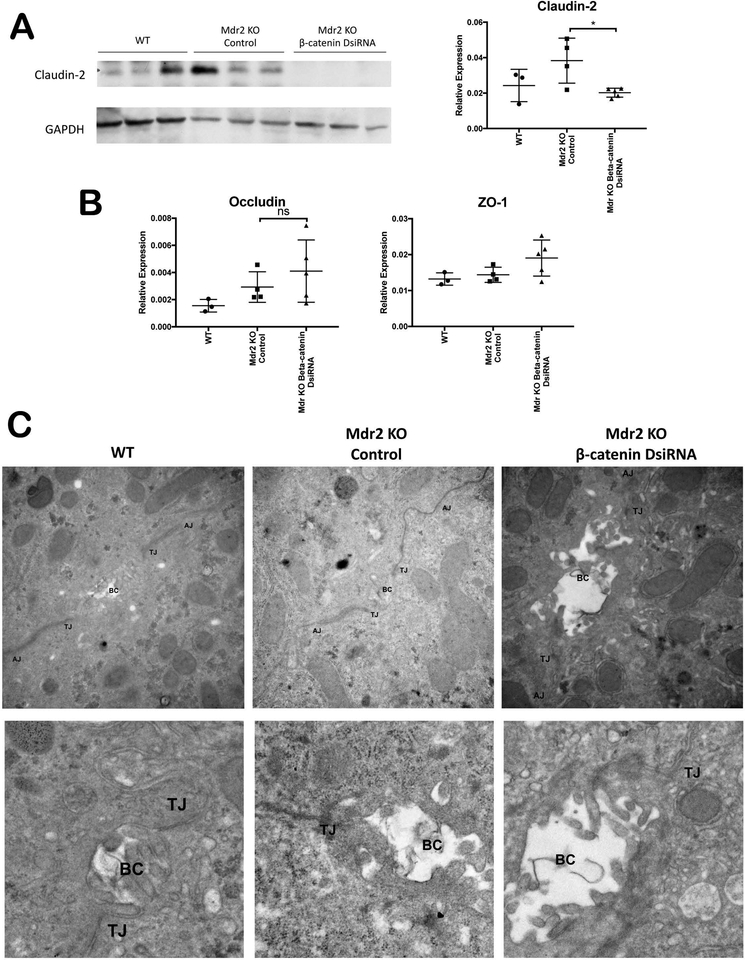
We further analyzed integrity of hepatocyte TJ in Mdr2 KO with and without β-catenin inhibition. Claudin-2, a transcriptional target of β-catenin(18), was absent in KO/KD livers, as assessed by WB and qPCR (Figure 5A). However, Claudin-3 and Claudin-5, which act as barrier-forming TJ proteins, are expressed at equivalent levels in KO/KD and KO livers (Supplementary Figure 4). Other TJ proteins such as Occludin and ZO-1 were mildly but insignificantly increased after β-catenin KD (Figure 5B). Transmission electron microscopy (TEM) also demonstrated no obvious defects in either AJ or TJ in either Mdr2 KO alone or KO/KD, although biliary canaliculi appeared to be dilated in KO/KD compared to WT or KO alone (Figure 5C). Thus, hepatocyte junctional permeability does not contribute to exacerbated injury in the KO/KD model.
Figure 5: TJ are intact in KO/KD liver.
(A) WB shows loss of Claudin-2 in KO/KD liver. Densitometric analysis validates the findings. (B) Analysis of mRNA expression shows TJ proteins occludin and ZO-1 are mildly but insignificantly increased in KO/KD compared to KO. (C) TEM demonstrates that TJ structure is intact in KO/KD liver, although biliary canaliculi appear dilated. For the top panel, images were taken at 30,000X; for the bottom panel, the images were taken 20,000–30,000X and cropped to zoom in on the bile canalicular region. *p<0.05.
BA synthesis, metabolism, and transporter genes are dysregulated in KO/KD mice compared to KO alone, which results in stabilization of BA levels.
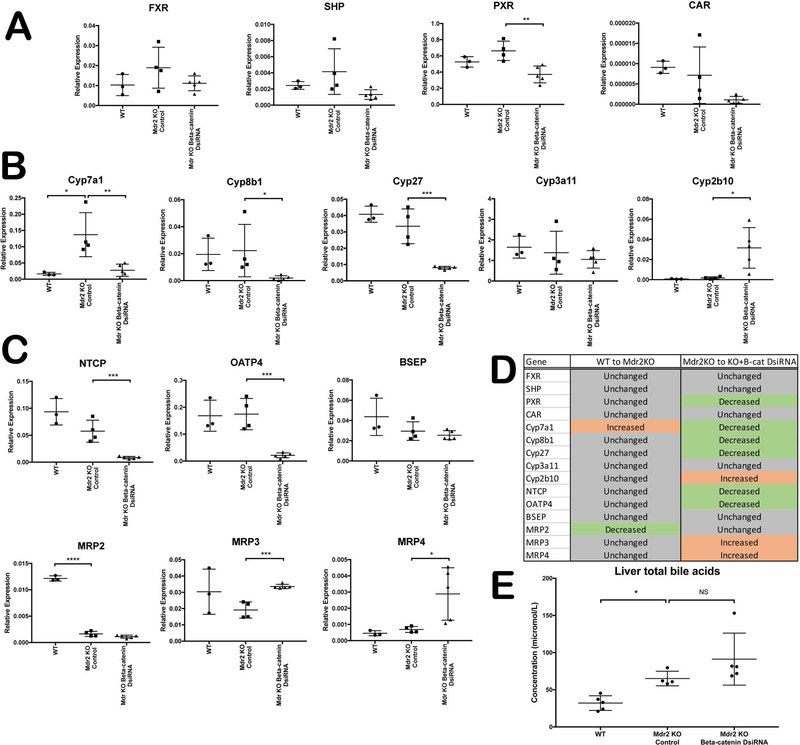
Since BA homeostasis is disrupted in Mdr2 KO mice(4), we next assessed the expression of BA synthesis and transport genes to identify any additional dysregulation in the absence of β-catenin. Expression of farnesoid X receptor (FXR), a master regulator of BA synthesis, is unchanged in Mdr2 KO with or without β-catenin inhibition (Figure 6A). FXR in turn induces expression of small heterodimer protein (SHP), which inhibits Cyp7a1, suppressing BA synthesis(20). In the absence of β-catenin, SHP mRNA expression decreases compared to Mdr2 KO alone, albeit insignificantly. Likewise, pregnane X receptor (PXR) and constitutive androstane receptor (CAR), which participate in detoxifying and transporting BA(21), are suppressed in KO/KD mice compared to KO (Figure 6A).
Figure 6: Dysregulation of BA synthesis, metabolism and transport genes in KO/KD liver.
(A) qPCR analysis shows decreased expression of several BA-regulated nuclear receptors in KO/KD liver. (B) Analysis of P450 expression shows that synthesis of BA is suppressed in KO/KD liver, while detoxification is increased. (C) BA uptake is suppressed, and basolateral efflux is increased, in KO/KD liver, as assessed by qPCR. (D) Tabular representation of the qPCR analysis shown in A, B, and C. (E) Levels of hepatic BA are unchanged between KO/KD and KO alone. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
Accordingly, many of the cytochrome P450 (Cyp) genes that are regulated by these nuclear receptors are also dysregulated in the absence of β-catenin. Both Cyp7a1 and Cyp8b1, which are involved in the synthesis of BA from cholesterol, are suppressed in the KO/KD mice compared to Mdr2 KO alone (Figure 6B). Likewise, Cyp27, the initiator of alternative BA synthesis, is also decreased in the absence of β-catenin. Cyp3a11, a target of PXR that detoxifies hydrophobic BA, is unchanged in either KO or KO/KD; however, Cyp2b10, a target of CAR involved in BA detoxification, is significantly increased after β-catenin inhibition (Figure 6B). Together, these findings indicate a net suppression of BA synthesis and an increase in detoxification, although this effect appears to be FXR-independent, as SHP is not induced in KO/KD livers. Rather, it is likely that decreased Cyp27 and Cyp7a1 is due to loss of β-catenin transcriptional activity, which directly or indirectly regulates expression of these enzymes(8, 22).
BA transport was also altered in Mdr2 KO mice lacking hepatocyte β-catenin. Basolateral uptake transporters sodium-taurocholate cotransporting polypeptide
(NTCP) and organic anion transporting polypeptide-4 (OATP4) were significantly suppressed in KO/KD (Figure 6C). There was no change in expression of apical efflux transporters such as multidrug resistance-associated protein-2 (MRP2) and bile salt export pump (BSEP) compared to KO alone. However, efflux transporters MRP3 and MRP4, which are expressed on the basolateral membrane and are induced under cholestatic conditions(23), are notably upregulated in KO/KD livers (Figure 6C). As a net result of these alterations in BA metabolism and transport (Figure 6D), total BA levels in the liver are unchanged after β-catenin inhibition (Figure 6E). Thus, the increased injury in KO/KD mice is not due to increased liver BA load.
Intravital microscopy demonstrates mixing of blood and bile in sinusoids of KO/KD livers, concomitant with increased serum BA levels.
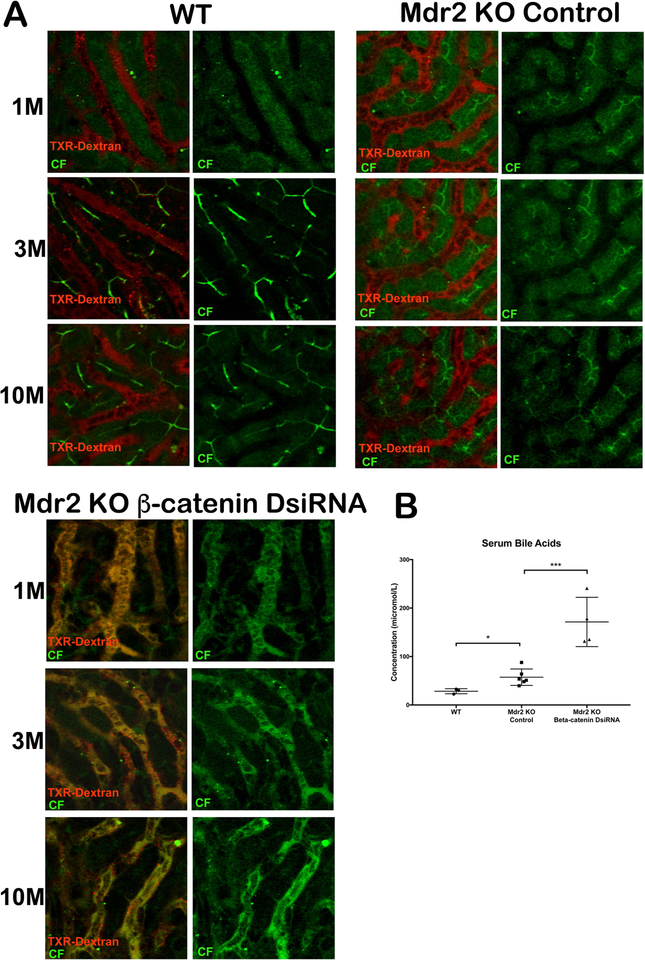
To confirm that TJ were intact and determine the effect of transporter dysregulation on BA uptake and efflux in KO/KD, we utilized multiphoton intravital microscopy to visualize the flow of blood and bile in live mice(24). In WT and Mdr2 KO mice, the probe molecule 6-carboxyfluorescein diacetate (6-CFDA) is taken up by transporters, hydrolyzed by the hepatocyte to fluorescent carboxyfluorescein (CF), and secreted into the biliary canaliculi as early as two minutes after injection. Blood and bile flow are also restricted to sinusoids and biliary canaliculi, respectively (Figure 7A; Supporting Movie S1–6). In KO/KD livers, on the other hand, CF is effluxed from the basolateral surface of hepatocytes almost immediately after uptake and hydrolysis, causing blood and bile to mix in the sinusoids; very little if any CF is transported into the biliary canaliculi (Figure 7A; Supporting Movie S7–9). Analysis of BA levels in blood confirm these findings, as KO/KD animals have significantly elevated levels of serum BA (Figure 7B). However, lack of paracellular leaks between hepatocytes affirms that TJ are intact in KO/KD. These result show that regurgitation of bile into blood occurs rapidly in KO/KD, perhaps due to increased MRP3 and MRP4 expression; this, combined with decreased basolateral BA uptake from blood, results in higher levels of serum BA in these mice.
Figure 7: Dual loss of β-catenin and Mdr2 in hepatocytes leads to extrusion of bile into hepatic sinusoids.
(A)Time series images (1m, 3m and 10m) of WT liver shows localization of CF (green) and Texas-red dextran (red) in bile canaliculi and sinusoids, respectively. Mdr2 KO alone showed similar localization of CF and TXR-dextran. However, KO/KD liver shows co-localization of CF and Texas-red dextran in sinusoids and failure of secretion into bile canaliculi at all time points after injection of dyes. Scale bars: 50μm. (B) Levels of serum BA are significantly higher in KO/KD compared to KO alone *p<0.05; ***p<0.001.
KO/KD livers have bile canalicular morphologic abnormalities and loss of hepatocyte polarity.
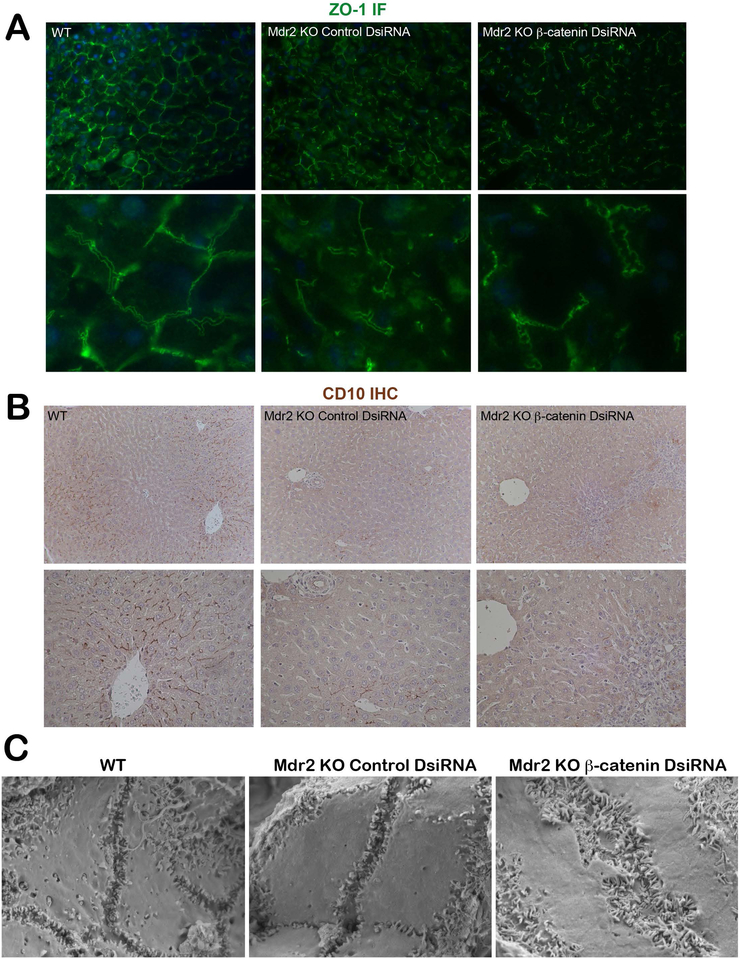
Because cholestasis can also be exacerbated by polarity defects, we performed IF for ZO-1, which directs epithelial polarization and lumen formation(25). WT livers showed typical pericanalicular localization, visible as a pair of parallel lines on either side of the canaliculi(17) (Figure 8A). This train-track morphology is similar in Mdr2 KO mice despite leaky bile ducts. In KO/KD livers, however, we noted a corkscrew-shaped ZO-1 staining pattern indicative of distorted bile canaliculi. IHC for CD10, another canalicular marker, shows a distinct pattern of membranous staining between adjacent hepatocytes in WT liver primarily in zone 1(26) (Figure 8B). Mdr2 KO livers showed a similar albeit less extensive pattern of staining, while KO/KD showed near-complete loss of canalicular CD10 localization (Figure 8B). Scanning electron micrographs (SEM) also confirm significant canalicular defects in the absence of both Mdr2 KO and β-catenin. Instead of the small narrow channels seen in WT and Mdr2 KO, the canaliculi appear enlarged, tortuous, and convoluted, although microvilli number and structure were unchanged compared to WT or Mdr2 KO. Finally, expression of vimentin, TGFβ, Snail, and Slug, which are genes associated with loss of polarity and epithelial-to-mesenchymal transition (EMT)(27) are increased in KO/KD compared to WT and KO alone (Supplementary Figure 5A). Genetic Mdr2/β-catenin KO, which have slower kinetics of injury progression, also show a trend toward increased expression of vimentin, TGFβ, and TNFα compared to Mdr2 KO littermates 2 weeks after AAV8-cre injection (Supplementary Figure 5B).
Figure 8: KO/KD livers have abnormal biliary canaliculi and defective hepatocyte polarity.
(A) ZO-1 IF shows a typical pericanalicular localization pattern in WT and in Mdr2 KO alone; however, in KO/KD liver ZO-1 IF highlights the tortuous and corkscrew shape of the biliary canaliculi (200X; 1000X inset). (B) CD10 IHC shows a distinct thin, dark brown staining pattern between WT hepatocytes outlining the biliary canaliculi. This pattern is also present in Mdr2 KO mice, although less extensively, whereas in KO/KD, CD10 staining is almost completely absent. (C) SEM images show that KO/KD livers have distorted and enlarged biliary canaliculi compared to WT and Mdr2 KO, which have narrow, clearly defined channels (30,000X).
Loss of β-catenin in Hep3B cells increases expression of genes involved in loss of polarity.
To determine whether the polarity defect in KO/KD was due to loss of β-catenin or secondary to progressive cholestatic injury, we treated hepatocyte-like Hep3B cells with control or β-catenin siRNA. qPCR analysis showed successful knockdown of β-catenin, as well as target genes GS and cyclin D1, after 48 hours of siRNA treatment (Supplementary Figure 6A). Conversely, expression of vimentin and TGFβ are increased, suggesting that loss of β-catenin from hepatocytes adversely affects polarity even before the onset of cholestatic injury (Supplementary Figure 6B). Increased expression of hepatocyte TNFα, which is likely due to activation of NF-κB in the absence of β-catenin(28), is also significantly upregulated. Interestingly, knockdown of β-catenin induces expression of MRP3 but not MRP4 even in the absence of BA (Supplementary Figure 6C). Altogether, these results suggest that loss of β-catenin predisposes hepatocytes to disruption of hepatocyte polarity and BA transporter expression, and thus is a primary driver of the progressive cholestatic phenotype in KO/KD mice.
DISCUSSION
Despite previous work showing a beneficial effect of inhibiting β-catenin expression after BDL, we show here that loss of β-catenin in a genetic model of cholestasis is deleterious. In order to understand these findings in context, it is important to note the differences between this and our previous study. In the BDL model, complete obstruction of bile flow leads to biliary stasis in the liver; thus, suppression of BA synthesis, which is enhanced by loss of β-catenin, is beneficial in this model. In Mdr2 KO, on the other hand, the phenotype is mainly driven by the toxicity of phospholipid-absent BA; therefore, reducing injury, improving the regenerative response, and/or maintaining bile flow to prevent stasis are critical to maintain liver function in these mice. Since β-catenin has been shown to play a role in all of these processes, it therefore follows that loss of β-catenin in this model would result in decompensation and accelerated injury. Notably, we found that both expression and activation of β-catenin was increased in Mdr2 KO compared to WT (Figure 4), supporting the hypothesis that active β-catenin is required for maintenance of homeostasis in the absence of Mdr2.
β-catenin is an important component of adherens junctions, and although its loss is normal liver is adequately compensated by γ-catenin, recent reports suggest that loss of β-catenin, γ-catenin, or both catenins predisposes livers to intrahepatic cholestasis secondary to junctional defects(7, 18, 24, 29). Additionally, the pore-forming TJ protein Claudin-2, a downstream target of β-catenin, is important for proper bile flow, although loss of Claudin-2 from hepatocytes did not cause a defect in TJ(30). However, both TJ and AJ were intact in KO/KD, as assessed by WB, IP, ultrastructural analysis, and IVM, likely due to increased expression of γ-catenin and maintained expression of plaque-forming claudins. Notably, however, significant abnormalities in biliary canalicular structure were noted in KO/KD, as demonstrated by TEM and ZO-1 IF.
ZO-1 is also an important regulator of cell polarity, which is compromised in cholestatic injury(31, 32). Hepatocyte polarity can be divided into structural and functional components(33). While structural polarity refers to integrity of TJ, functional polarity is determined by the function of ATP binding cassette (ABC) transporters. Dysregulated expression of these transporters can compromise the multipolar epithelial phenotype. Although canalicular function is initially maintained in functional polarity, over time the canaliculi become damaged as a consequence of bile acid exposure. Further examination of the canalicular structure in KO/KD by SEM and CD10 IHC showed enlarged and convoluted canaliculi that lacked CD10 on the hepatocyte canalicular membrane. We also found EMT markers such as TGFβ, vimentin, Snail, and Slug to be significantly increased in KO/KD, suggesting acquisition of a mesenchymal-like identity. As canalicular abnormalities are also found in genetic β-catenin KO mice, it is likely that loss of β-catenin from hepatocytes is the primary driver of this phenotype(7). Indeed, knockdown of β-catenin in Hep3B cells also resulted in increased expression of TGFβ and vimentin, suggesting that compromised hepatocyte polarity is a direct result of loss of β-catenin from hepatocytes. In genetic β-catenin KO, there are likely to be compensatory mechanisms that maintain homeostasis and normal liver function. In the Mdr2 KO, however, maintenance of bile flow is already compromised, and further defects in canalicular structure, hepatocyte polarization, and transporter expression caused by acute loss of β-catenin cannot be counteracted.
Compromised polarity also affects proper transporter sorting to the appropriate membrane domains. Several types of Progressive Familial Intrahepatic Cholestasis are characterized by inherited defects in canalicular membrane transporter proteins. Although inhibition of hepatocyte β-catenin appears to be responsible for upregulation of MRP3, other BA transporters may be overexpressed or suppressed in KO/KD due to loss of polarity-induced mislocalization, with the result being aberrant BA transport as shown in IVM. The BA transporter profile of KO/KD may also contribute to injury through increased efflux and retention of BA in the sinusoids. Although normally BA are rapidly cleared from the blood, in KO/KD the decreased uptake and increased efflux results in a net higher level of serum BA than in KO alone. KO/KD hepatocytes are thus exposed to increased BA through both increased biliary pressure as well as increased systemic circulation, which may amplify the BA-induced inflammatory, oxidative stress, or apoptotic responses(33).
In addition to its pleiotropic roles in fibrosis and EMT, TGFβ also induces hepatocyte senescence in acute liver injury(34). Loss of hepatocyte β-catenin causes upregulation of TGFβ, loss of polarity, and increased inflammation, all of which contribute to an aberrant ductular response, a major cause of cellular senescence. Likewise, β-catenin KO mice are more susceptible to various types of oxidative stressors, such as ischemia/reperfusion injury(6, 14). This failure to adapt to oxidative stress, coupled with the increased exposure to BA, which themselves generate reactive oxygen species(35), induces lipid peroxidation and hepatocyte apoptosis. Although not primary drivers of disease, senescence and oxidative stress likely contributes to inflammation and fibrosis by participating in a feed-forward cycle that accelerates injury progression.
Compensatory hepatocyte proliferation to replace damaged parenchymal cells occurs in cholestatic liver diseases, and may offer some protection against chronic injury in Mdr2 KO(36). β-catenin plays an important role in the regenerative response through its regulation of cyclin D1 expression(15). Thus, one of the mechanisms contributing to accelerated injury in KO/KD mice may be lack of compensatory regeneration after hepatocyte damage. On the other hand, this chronic cycle of injury and repair ultimately results in the development of HCC in Mdr2 KO mice. Previous analysis found that these tumors were characterized by the absence of β-catenin activation(37). Further studies will be needed to address the long-term implication of β-catenin loss on the impact of tumor development in this model. Interestingly, recent studies have shown that mice lacking β-catenin in hepatocytes have impaired proliferation during severe injury, which induces cholangiocytes to transdifferentiate into hepatocytes to repair and repopulate the liver(38). In the KO/KD model described here, we also observed clusters of β-catenin-positive hepatocytes in the periportal region, concomitant with a defective proliferative response. It is possible that loss of β-catenin and the subsequent inability of hepatocytes to regenerate in the KO/KD liver creates a selective pressure for cholangiocytes in the biliary compartment to undergo phenotypic reprogramming and become more hepatocyte-like to facilitate hepatic repair.
In light of our findings, we believe that β-catenin is performing some essential compensatory function in the absence of Mdr2, whether it be to increase regeneration, maintain hepatocyte polarity, preserve bile flow, or all three. Therapeutically, these results advise against an unrestricted inhibition of β-catenin in all types of cholestatic liver diseases. When high hepatic BA are the primary cause of cholestatic injury, such inhibition may be beneficial, as activation of FXR, suppression of BA synthesis, and increased BA efflux are the eventual consequences of inhibiting β-catenin. However, in situations where BA toxicity or leakage is at least partially compensated, β-catenin may be playing a protective role, meaning that inhibition of this pathway can result in exacerbation rather than improvement of injury. Elucidating the role of Wnt/β-catenin signaling in other experimental models will further improve our understanding of its varying functions during the pathogenesis of cholestasis, and inform the use of inhibitors or activators of this pathway for targeted therapeutics.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Marc Abrams from Dicerna Pharmaceuticals for generously providing essential reagents for this study.
Funding: This study was funded by NIH grant 1R01DK103775 to KNB, and NIH training grant 5T32DK63922-13 to Tirthadipa Pradhan-Sundd. The Nikon multiphoton-excitation microscope was funded by NIH grant 1S10RR028478-01.
Abbreviations:
- KO
Mdr2 knockout
- WT
littermate controls
- KD
β-catenin knockdown
- BDL
bile duct ligation
- BA
bile acids
- PSC
primary sclerosing cholangitis
- WB
Western blot
- DsiRNA
Dicer-substrate siRNA
- LNP
lipid nanoparticle
- IHC
immunohistochemistry
- IF
immunofluorescence
- H&E
hematoxylin and eosin
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
- PCNA
proliferating cell nuclear antigen
- ALT
Alanine aminotransferase
- AST
Aspartate animotransferase
- ALP
alkaline phosphatase
- GS
Glutamine synthetase
- GPX
glutathione peroxidase
- SOD1
superoxide dismutase
- GST
glutathione-S-transferase
- NQO1
NAD(P)H:quinone oxidoreductase 1
- HO-1
heme oxygenase
- MDA
malondialdehyde
- FXR
farnesoid X receptor
- SHP
small heterodimer protein
- PXR
pregnane X receptor
- CAR
constitutive androstane receptor
- Cyp
cytochrome P450
- NTCP
sodium-taurocholate cotransporting polypeptide
- OATP4
organic anion transporting polypeptide-4
- MRP2
multidrug resistance-associated protein-2
- BSEP
bile salt export pump
- ZO-1
zonula occludens-1
- 6-CFDA
6-carboxyfluorescein diacetate
- CF
carboxyfluorescein
- AJ
adherens junctions
- TJ
tight junctions
Footnotes
Conflict of Interest Statement: The authors declare that they have no conflicts to disclose.
REFERENCES
- 1.Lazaridis KN, LaRusso NF. Primary Sclerosing Cholangitis. N Engl J Med 2016;375:2501–2502. [DOI] [PubMed] [Google Scholar]
- 2.Popov Y, Patsenker E, Fickert P, Trauner M, Schuppan D. Mdr2 (Abcb4)−/− mice spontaneously develop severe biliary fibrosis via massive dysregulation of pro- and antifibrogenic genes. J Hepatol 2005;43:1045–1054. [DOI] [PubMed] [Google Scholar]
- 3.Smit JJ, Schinkel AH, Oude Elferink RP, Groen AK, Wagenaar E, van Deemter L, Mol CA, et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell 1993;75:451–462. [DOI] [PubMed] [Google Scholar]
- 4.Fickert P, Fuchsbichler A, Wagner M, Zollner G, Kaser A, Tilg H, Krause R, et al. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology 2004;127:261–274. [DOI] [PubMed] [Google Scholar]
- 5.Monga SP. beta-Catenin Signaling and Roles in Liver Homeostasis, Injury, and Tumorigenesis. Gastroenterology 2015;148:1294–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behari J, Yeh TH, Krauland L, Otruba W, Cieply B, Hauth B, Apte U, et al. Liver-specific beta-catenin knockout mice exhibit defective bile acid and cholesterol homeostasis and increased susceptibility to diet-induced steatohepatitis. Am J Pathol 2010;176:744–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeh TH, Krauland L, Singh V, Zou B, Devaraj P, Stolz DB, Franks J, et al. Liver-specific beta-catenin knockout mice have bile canalicular abnormalities, bile secretory defect, and intrahepatic cholestasis. Hepatology 2010;52:1410–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson MD, Moghe A, Cornuet P, Marino R, Tian J, Wang P, Ma X, et al. beta-Catenin regulation of farnesoid X receptor signaling and bile acid metabolism during murine cholestasis. Hepatology 2018;67:955–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yanger K, Zong Y, Maggs LR, Shapira SN, Maddipati R, Aiello NM, Thung SN, et al. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev 2013;27:719–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fickert P, Zollner G, Fuchsbichler A, Stumptner C, Weiglein AH, Lammert F, Marschall HU, et al. Ursodeoxycholic acid aggravates bile infarcts in bile duct-ligated and Mdr2 knockout mice via disruption of cholangioles. Gastroenterology 2002;123:1238–1251. [DOI] [PubMed] [Google Scholar]
- 11.Tabibian JH, O’Hara SP, Splinter PL, Trussoni CE, LaRusso NF. Cholangiocyte senescence by way of N-ras activation is a characteristic of primary sclerosing cholangitis. Hepatology 2014;59:2263–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou T, Wu N, Meng F, Venter J, Giang TK, Francis H, Kyritsi K, et al. Knockout of secretin receptor reduces biliary damage and liver fibrosis in Mdr2(−/−) mice by diminishing senescence of cholangiocytes. Lab Invest 2018;98:1449–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moncsek A, Al-Suraih MS, Trussoni CE, O’Hara SP, Splinter PL, Zuber C, Patsenker E, et al. Targeting senescent cholangiocytes and activated fibroblasts with B-cell lymphoma-extra large inhibitors ameliorates fibrosis in multidrug resistance 2 gene knockout (Mdr2(−/−) ) mice. Hepatology 2018;67:247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehwald N, Tao GZ, Jang KY, Sorkin M, Knoefel WT, Sylvester KG. Wnt-beta-catenin signaling protects against hepatic ischemia and reperfusion injury in mice. Gastroenterology 2011;141:707–718, 718 e701–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torre C, Benhamouche S, Mitchell C, Godard C, Veber P, Letourneur F, Cagnard N, et al. The transforming growth factor-alpha and cyclin D1 genes are direct targets of beta-catenin signaling in hepatocyte proliferation. J Hepatol 2011;55:86–95. [DOI] [PubMed] [Google Scholar]
- 16.Sekine S, Lan BY, Bedolli M, Feng S, Hebrok M. Liver-specific loss of beta-catenin blocks glutamine synthesis pathway activity and cytochrome p450 expression in mice. Hepatology 2006;43:817–825. [DOI] [PubMed] [Google Scholar]
- 17.Fallon MB, Brecher AR, Balda MS, Matter K, Anderson JM. Altered hepatic localization and expression of occludin after common bile duct ligation. Am J Physiol 1995;269:C1057–1062. [DOI] [PubMed] [Google Scholar]
- 18.Wickline ED, Awuah PK, Behari J, Ross M, Stolz DB, Monga SP. Hepatocyte gamma-catenin compensates for conditionally deleted beta-catenin at adherens junctions. J Hepatol 2011;55:1256–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wickline ED, Du Y, Stolz DB, Kahn M, Monga SP. gamma-Catenin at adherens junctions: mechanism and biologic implications in hepatocellular cancer after beta-catenin knockdown. Neoplasia 2013;15:421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell 2000;6:517–526. [DOI] [PubMed] [Google Scholar]
- 21.Guo GL, Lambert G, Negishi M, Ward JM, Brewer HB Jr, Kliewer SA, Gonzalez FJ, et al. Complementary roles of farnesoid X receptor, pregnane X receptor, and constitutive androstane receptor in protection against bile acid toxicity. J Biol Chem 2003;278:45062–45071. [DOI] [PubMed] [Google Scholar]
- 22.Gougelet A, Torre C, Veber P, Sartor C, Bachelot L, Denechaud PD, Godard C, et al. T-cell factor 4 and beta-catenin chromatin occupancies pattern zonal liver metabolism in mice. Hepatology 2014;59:2344–2357. [DOI] [PubMed] [Google Scholar]
- 23.Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev 2003;83:633–671. [DOI] [PubMed] [Google Scholar]
- 24.Pradhan-Sundd T, Zhou L, Vats R, Jiang A, Molina L, Singh S, Poddar M, et al. Dual catenin loss in murine liver causes tight junctional deregulation and progressive intrahepatic cholestasis. Hepatology 2018;67:2320–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Odenwald MA, Choi W, Buckley A, Shashikanth N, Joseph NE, Wang Y, Warren MH, et al. ZO-1 interactions with F-actin and occludin direct epithelial polarization and single lumen specification in 3D culture. J Cell Sci 2017;130:243–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shousha S, Gadir F, Peston D, Bansi D, Thillainaygam AV, Murray-Lyon IM. CD10 immunostaining of bile canaliculi in liver biopsies: change of staining pattern with the development of cirrhosis. Histopathology 2004;45:335–342. [DOI] [PubMed] [Google Scholar]
- 27.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res 2009;19:156–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nejak-Bowen KN, Zeng G, Tan X, Cieply B, Monga SP. Beta-catenin regulates vitamin C biosynthesis and cell survival in murine liver. J Biol Chem 2009;284:28115–28127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou L, Pradhan-Sundd T, Poddar M, Singh S, Kikuchi A, Stolz DB, Shou W, et al. Mice with Hepatic Loss of the Desmosomal Protein gamma-Catenin Are Prone to Cholestatic Injury and Chemical Carcinogenesis. Am J Pathol 2015;185:3274–3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsumoto K, Imasato M, Yamazaki Y, Tanaka H, Watanabe M, Eguchi H, Nagano H, et al. Claudin 2 deficiency reduces bile flow and increases susceptibility to cholesterol gallstone disease in mice. Gastroenterology 2014;147:1134–1145 e1110. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Boyer JL. The maintenance and generation of membrane polarity in hepatocytes. Hepatology 2004;39:892–899. [DOI] [PubMed] [Google Scholar]
- 32.Ikenouchi J, Umeda K, Tsukita S, Furuse M, Tsukita S. Requirement of ZO-1 for the formation of belt-like adherens junctions during epithelial cell polarization. J Cell Biol 2007;176:779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gissen P, Arias IM. Structural and functional hepatocyte polarity and liver disease. J Hepatol 2015;63:1023–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bird TG, Muller M, Boulter L, Vincent DF, Ridgway RA, Lopez-Guadamillas E, Lu WY, et al. TGFbeta inhibition restores a regenerative response in acute liver injury by suppressing paracrine senescence. Sci Transl Med 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sokol RJ, Winklhofer-Roob BM, Devereaux MW, McKim JM, Jr. Generation of hydroperoxides in isolated rat hepatocytes and hepatic mitochondria exposed to hydrophobic bile acids. Gastroenterology 1995;109:1249–1256. [DOI] [PubMed] [Google Scholar]
- 36.De Vree JM, Ottenhoff R, Bosma PJ, Smith AJ, Aten J, Oude Elferink RP. Correction of liver disease by hepatocyte transplantation in a mouse model of progressive familial intrahepatic cholestasis. Gastroenterology 2000;119:1720–1730. [DOI] [PubMed] [Google Scholar]
- 37.Katzenellenbogen M, Mizrahi L, Pappo O, Klopstock N, Olam D, Jacob-Hirsch J, Amariglio N, et al. Molecular mechanisms of liver carcinogenesis in the mdr2-knockout mice. Mol Cancer Res 2007;5:1159–1170. [DOI] [PubMed] [Google Scholar]
- 38.Russell JO, Lu WY, Okabe H, Abrams M, Oertel M, Poddar M, Singh S, et al. Hepatocyte-specific beta-catenin deletion during severe liver injury provokes cholangiocytes to differentiate into hepatocytes. Hepatology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.