Abstract
Mediterranean diet (MD) adherence has been associated with a large variety of health benefits. However, prospective studies investigating the relation between MD adherence and colorectal cancer risk had inconsistent results. In this analysis of the Netherlands Cohort Study (NLCS), we evaluated sex- and subsite-specific associations of MD adherence with colorectal cancer risk. In 1986, 120,852 subjects filled out the NLCS baseline questionnaire, which incorporated a 150-item food frequency questionnaire. MD adherence was estimated through alternate Mediterranean diet scores including and excluding alcohol (aMED and aMEDr, respectively). Using 20.3 year follow-up data, 1993 male and 1574 female colorectal cancer cases could be included in multivariable case-cohort analyses. aMEDr was not significantly associated with colorectal cancer risk, regardless of sex. Hazard ratios (95% confidence intervals) per two-point increment were 1.04 (0.95–1.13) for men and 0.97 (0.88–1.07) for women. Additionally, there was no evidence of an inverse association with any of the colorectal cancer subsites (colon, proximal colon, distal colon, and rectum). In women, the association between aMEDr and colorectal cancer risk was significantly modified by smoking status (Pinteraction = 0.015). Comparable results were obtained for the original aMED including alcohol. In conclusion, higher MD adherence was not associated with a reduced risk of colorectal cancer or anatomical subsites in the context of a Dutch population.
Electronic supplementary material
The online version of this article (10.1007/s10654-019-00549-8) contains supplementary material, which is available to authorized users.
Keywords: Mediterranean diet, Colorectal cancer, Subsites, Cohort study, Epidemiology, Prevention
Introduction
Globally, colorectal cancer was an important contributor to the total cancer burden in 2018, ranking third and second in terms of incidence and mortality, respectively [1]. The global burden of colorectal cancer is expected to increase even further in the next decade. In 2030, over 2.2 million people are estimated to be diagnosed with colorectal cancer, whereas more than 1.1 million people are expected to die from this disease [2]. Colorectal cancer is a slow-growing disease [3], which offers the opportunity to intervene during the disease development process using preventive measures. These preventive strategies could for instance focus on maintenance of a healthy diet.
The traditional Mediterranean diet (MD), typical for the olive-cultivating areas bordering the Mediterranean basin in the early 1960s, has been associated with a large variety of health benefits, including decreases in all-cause mortality as well as cardiovascular disease risk and mortality [4–8]. This dietary pattern is characterized by the consumption of large quantities of vegetables, legumes, fruits, nuts, whole grains, and olive oil (rich in monounsaturated fatty acids, MUFA). In contrast, intakes of foods from animal origin (e.g. dairy and meat) are low. Finally, wine is consumed in moderate amounts, particularly during meals [4, 5].
The relation between a priori defined MD adherence and colorectal cancer risk has been evaluated in a number of prospective studies so far, with mixed results. Though some studies reported MD adherence to be associated with a significantly reduced colorectal cancer risk [9–12], inverse associations were absent or only observed in specific subgroups in others [13–20]. Additionally, heterogeneity of associations across the sexes and colorectal cancer subsites was indicated [9, 10, 12, 13, 16, 17, 20].
The colorectum can anatomically be divided in the proximal colon, distal colon, and rectum. Depending on the anatomical subsite, colorectal tumors may develop through distinct molecular pathways and show varying patterns of (epi)genetic changes [21, 22]. Furthermore, differences have been shown in subsite-specific incidence trends and survival [21, 22]. Because of their potentially distinct etiologies, cancers of the proximal colon, distal colon, and rectum should initially be considered as separate endpoints in epidemiological studies.
In the present analysis, we aimed to investigate associations of MD adherence with risks of colorectal cancer and anatomical subsites (colon, proximal colon, distal colon, and rectum) in the prospective Netherlands Cohort Study (NLCS). The level of MD adherence was assessed using a priori defined MD scores with and without alcohol component. Moreover, associations were estimated separately for men and women.
Methods
Study population and cancer follow-up
The NLCS was conducted among 58,279 men and 62,573 women, who were aged 55–69 years [23–26]. At baseline (September 1986), information on diet and other cancer risk factors was gathered via a self-administered questionnaire. Data were processed and analysed using the case-cohort method, in which cases are derived from the entire cohort and person-years at risk are estimated based on a subcohort. Therefore, a random subcohort (N = 5000) was selected immediately after baseline and vital status information of subcohort members was acquired biennially [23, 26, 27]. Follow-up for cancer incidence was accomplished via annual record linkage with the Netherlands Cancer Registry and the nationwide Dutch Pathology Registry (PALGA) [24]. The NLCS was approved by institutional review boards from Maastricht University and the Netherlands Organization for Applied Scientific Research.
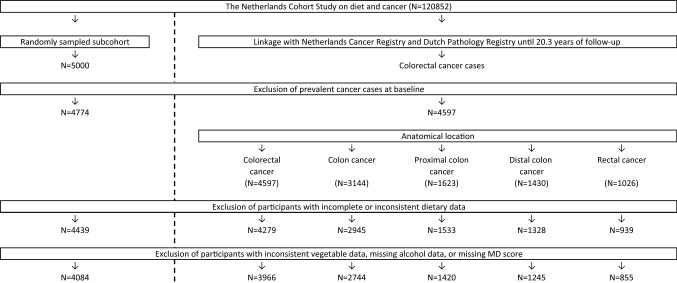
After 20.3 years of follow-up, 4084 subcohort members and 3966 cases with incident and microscopically confirmed colorectal cancer (ICD-O-3 codes: C18–C20) were eligible for inclusion in the present analyses (Fig. 1). Eligible study participants did not have a history of cancer at baseline (except skin cancer), had complete and consistent dietary data, and had data available on alcohol consumption and MD adherence.
Fig. 1.
Flow diagram of the number of participants of the Netherlands Cohort Study, who are eligible for inclusion in the analyses concerning colorectal cancer (case-cohort design). MD Mediterranean diet
Exposure assessment
Habitual dietary intake during the year preceding baseline was assessed using a 150-item, semi-quantitative food frequency questionnaire (FFQ) [25, 28]. Previously, it has been shown that this FFQ performed adequately and that dietary habits were reproducible for over at least 5 years [25, 28]. The 1986 Dutch food composition (NEVO) table was used to calculate nutrient intakes from the FFQ data [29].
Mediterranean diet adherence
MD adherence was assessed using the alternate Mediterranean diet score (aMED), which is a variant of the traditional Mediterranean diet score (tMED) developed by Trichopoulou et al., that was adapted for usage in the United States [30–33]. aMED assesses relative MD adherence based on energy-adjusted mean daily intakes of nine food groups with typically high or low consumption in the MD [32, 33]. Each food group is scored by 0 or 1 points, creating a sum score with a maximum value of 9 points (highest level of MD adherence). Subjects receive 1 point for: high intakes (≥ sex-specific median) of vegetables (excluding potatoes), legumes, fruits, nuts, whole grains, and fish; a high (≥ sex-specific median) MUFA to saturated fatty acid (SFA) ratio; a moderate alcohol intake (5–25 g/day); and a low intake (< sex-specific median) of red and processed meats [32, 33].
Moderate and heavy alcohol consumption have been associated with an increased colorectal cancer risk [34, 35]. Therefore, MD adherence was also assessed using a reduced variant of aMED (aMEDr) that does not include alcohol and ranges from 0 to 8 points. Because of the positive association between alcohol consumption and colorectal cancer risk, we will concentrate on results obtained using aMEDr in this article. MD score values were grouped into three MD adherence categories [low (0–3), middle (4–5), and high (6–8 (9))] and were continuously modelled per two-point increment [33].
Statistical analyses
Cox proportional hazards analyses were conducted to estimate hazards ratios (HRs) and 95% confidence intervals (95% CIs) for sex-specific associations of MD adherence with incidences of total colorectal cancer and anatomical subsites (colon, proximal colon, distal colon, and rectum). Duration of follow-up was used as time variable and person-years at risk of subcohort members were calculated from baseline until colorectal cancer diagnosis, death, emigration, loss to follow-up, or end of follow-up, whichever came first. To account for the increased variance inherent to the case-cohort design, we estimated standard errors using the robust Huber–White sandwich estimator [36]. Scaled Schoenfeld residuals tests and –ln(–ln) survival plots confirmed that it was appropriate to assume proportionality of hazards for the exposure variables [37].
MD scores were included as categorical and continuous terms in age- and multivariable-adjusted Cox models. Based on the literature, we included the following predefined confounders in the multivariable-adjusted models: age at baseline, cigarette smoking behaviour (status, frequency, and duration), body mass index (BMI), alcohol consumption (except for models containing the original aMED including alcohol), total daily energy intake, highest level of education, non-occupational physical activity, and family history of colorectal cancer. Other covariates considered were height, history of diabetes, history of chronic bowel irritation, use of hormone replacement therapy (women only), and long-term use of non-steroidal anti-inflammatory drugs. These factors did not change the HR estimates of aMEDr ≥ 10% and were therefore not included in the final model.
P values for trends over the MD adherence categories were obtained by appointing sex-specific median MD score values among subcohort members to each category and fitting these as continuous terms in Cox regression models. Performances of models including MD score variants with and without alcohol (aMED and aMEDr, respectively) were compared using Akaike’s Information Criterion (AIC) [38]. Statistical significance of differences in associations with aMEDr across the anatomical locations of colorectal cancer (colon, proximal colon, distal colon, and rectum) was tested using a competing risks procedure, by which standard errors were estimated using a bootstrapping method developed for the case-cohort design [39, 40].
Stratified analyses were performed to evaluate associations of aMEDr with colorectal cancer risk across levels of cigarette smoking status, alcohol consumption, BMI, educational level, and family history of colorectal cancer. Interaction terms between aMEDr and these potential effect modifiers were added to the models to test the statistical significance of potential differences. To test the sensitivity of our results, analyses were repeated excluding the first 2 years of follow-up. Furthermore, the total follow-up time was divided into three periods (≤ 2, > 2 to ≤ 10, and > 10 years).
As an additional sensitivity analysis, we compared the population-dependent aMED to the absolute WCRF/AICR diet score, which is based on the dietary recommendations for cancer prevention issued by the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) in 2007 [41]. Our WCRF/AICR diet score is based on the WCRF/AICR score developed in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort [42, 43] and operationalizes the recommendations concerning foods and drinks that promote weight gain, plant foods, red and processed meats, (alcohol), and salt. A detailed description of the calculation of the score has been published previously [44]. Score variants were created including and excluding the alcohol recommendation, resulting in sum scores ranging from 0 to 4 (or 5 when including alcohol) points with higher values reflecting closer adherence to the WCRF/AICR dietary recommendations. Cox regression analyses were performed to estimate multivariable-adjusted associations of the WCRF/AICR diet scores (per SD-increment) with risks of colorectal, colon, and rectal cancer. A similar approach was applied to the aMED indices to be able to compare model performances of both scores using AIC. Statistical analyses were conducted using Stata (version 15). Statistically significant results had a two-sided P value below 0.05.
Results
Sex-specific median daily intakes of the aMEDr components among subcohort members are displayed in Table 1. As expected, median daily intakes of beneficial components increased with higher levels of MD adherence, whereas the opposite was observed for the intake of red and processed meats. Alcohol consumption was constant over the aMEDr categories in men, whereas in women slightly higher intakes were observed with closer adherence to the MD. Distributions of potential (colorectal) cancer risk factors (e.g. smoking status, BMI, and physical activity) over the aMEDr categories in the NLCS subcohort have been described in detail previously [45]. Comparing the highest to the lowest aMEDr category, subcohort members adhering more closely to the MD were less likely to smoke at baseline, had a lower BMI, and were more physically active. Generally, comparable levels of MD adherence were observed among colorectal cancer cases and subcohort members of both sexes, with mean aMEDr values of approximately 4 (Table 2). Considering other baseline characteristics, male and female colorectal cancer cases were more often former smokers compared to subcohort members, but less often current smokers (except female rectal cancer cases, Table 2). Additionally, levels of physical activity and alcohol consumption were higher in male, but lower in female, colorectal cancer cases. Furthermore, colon cancer cases were more likely to be highly educated than subcohort members (men only), whereas the opposite was observed for rectal cancer cases. Finally, colorectal cancer cases of both sexes more frequently reported a family history of this disease.
Table 1.
Sex-specific median daily intakes of MD components (total and by aMEDr category) in subcohort members of the Netherlands Cohort Study
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| All | aMEDr category | All | aMEDr category | |||||
| 0–3 | 4–5 | 6–8 | 0–3 | 4–5 | 6–8 | |||
| N = 2057 | N = 855 | N = 887 | N = 315 | N = 2027 | N = 769 | N = 901 | N = 357 | |
| Vegetables (g) | 207 (124) | 177 (96) | 222 (128) | 266 (120) | 219 (121) | 179 (89) | 228 (113) | 272 (98) |
| Legumes (g) | 6 (16) | 0 (7) | 9 (19) | 13 (17) | 5 (12) | 0 (5) | 6 (13) | 11 (13) |
| Fruits (g) | 157 (157) | 120 (128) | 167 (159) | 230 (159) | 209 (177) | 161 (134) | 227 (168) | 284 (157) |
| Nuts (g) | 3 (11) | 1 (4) | 5 (12) | 9 (14) | 2 (6) | 0 (3) | 2 (7) | 5 (9) |
| Whole grains (g) | 0 (10) | 0 (0) | 0 (16) | 12 (41) | 0 (13) | 0 (0) | 2 (15) | 12 (23) |
| Fish (g) | 11 (23) | 6 (17) | 14 (21) | 22 (19) | 9 (22) | 3 (11) | 12 (23) | 20 (17) |
| Red and processed meats (g) | 125 (63) | 139 (64) | 120 (61) | 101 (51) | 106 (61) | 125 (62) | 101 (56) | 86 (45) |
| MUFA:SFA ratio | 0.98 (0.24) | 0.92 (0.21) | 1.01 (0.23) | 1.04 (0.14) | 0.94 (0.21) | 0.90 (0.19) | 0.96 (0.21) | 0.99 (0.20) |
| Alcohol (g) | 10 (21) | 10 (21) | 10 (22) | 9 (18) | 2 (8) | 1 (6) | 2 (9) | 3 (9) |
| Total energy (kcal) | 2126 (648) | 2125 (674) | 2171 (653) | 2057 (557) | 1655 (516) | 1670 (533) | 1636 (475) | 1664 (541) |
MD Mediterranean diet, aMEDr alternate Mediterranean diet score without the alcohol component, MUFA monounsaturated fatty acids, SFA saturated fatty acids
Median (IQR) daily values in subcohort members are reported
Table 2.
Sex-specific baseline characteristics of subcohort members and cases of colorectal cancer in the Netherlands Cohort Study
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Subcohort | Colorectal cancer cases | Subcohort | Colorectal cancer cases | |||||
| All | Colon | Rectum | All | Colon | Rectum | |||
| N = 2057 | N = 2263 | N = 1469 | N = 549 | N = 2027 | N = 1703 | N = 1275 | N = 306 | |
| aMEDr | 3.9 (1.6) | 3.9 (1.6) | 3.9 (1.6) | 4.0 (1.5) | 4.0 (1.6) | 4.0 (1.6) | 4.0 (1.6) | 3.8 (1.6) |
| Age (years)a | 61 (7) | 61 (7) | 62 (7) | 61 (7) | 61 (7) | 62 (6) | 62 (6) | 62 (6) |
| Former cigarette smokers (%) | 52.1 | 58.2 | 59.5 | 56.1 | 21.0 | 22.7 | 22.3 | 22.2 |
| Current cigarette smokers (%) | 35.1 | 29.9 | 28.1 | 33.3 | 21.3 | 19.9 | 19.4 | 22.2 |
| Higher vocational education or university (%) | 19.3 | 21.0 | 23.7 | 16.1 | 9.5 | 9.5 | 9.5 | 7.3 |
| Alcohol consumption (g/day)a | 9.7 (20.9) | 11.3 (21.7) | 10.6 (21.3) | 11.4 (22.2) | 1.6 (7.8) | 1.4 (7.5) | 1.5 (7.5) | 1.3 (8.4) |
| Daily energy intake (kcal) | 2162 (501) | 2149 (483) | 2135 (489) | 2191 (473) | 1687 (392) | 1678 (374) | 1673 (379) | 1696 (339) |
| Body mass index (kg/m2) | 24.9 (2.6) | 25.2 (2.7) | 25.2 (2.7) | 25.1 (2.5) | 25.0 (3.5) | 25.0 (3.5) | 25.0 (3.5) | 25.0 (3.5) |
| Non-occupational physical activity (min/day)a | 62.1 (67.1) | 64.3 (64.3) | 64.3 (64.3) | 68.6 (60.0) | 54.3 (52.9) | 51.4 (53.6) | 51.4 (53.6) | 53.9 (50.0) |
| Family history of colorectal cancer (%) | 5.3 | 9.1 | 9.5 | 7.3 | 6.0 | 9.9 | 10.4 | 8.8 |
aMEDr alternate Mediterranean diet score without the alcohol component
The % missing values in the total eligible population was < 5% for all variables included in this table. Mean (SD) values are reported unless otherwise specified
aMedian (IQR) values are reported
Table 3 presents sex-specific and multivariable-adjusted associations of aMED, including and excluding alcohol, with risks of colorectal cancer and anatomical subsites. Age-adjusted associations can be found in Online Resource 1. Not all eligible subjects could be included in the Cox models because of missing information on covariates.
Table 3.
Multivariable-adjusted associations of aMED (including and excluding alcohol) with colorectal cancer risk for men and women in the Netherlands Cohort Study
| Colorectum | Colon | Proximal colon | Distal colon | Rectum | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PYsubcohort | Cases | HR (95% CI)a | Cases | HR (95% CI)a | Cases | HR (95% CI)a | Cases | HR (95% CI)a | Cases | HR (95% CI)a | |
| Men | |||||||||||
| aMEDr | |||||||||||
| 0–3 | 11,788 | 779 | 1.00 | 507 | 1.00 | 232 | 1.00 | 256 | 1.00 | 178 | 1.00 |
| 4–5 | 12,448 | 873 | 1.07 (0.92–1.24) | 566 | 1.07 (0.90–1.26) | 244 | 1.02 (0.82–1.26) | 307 | 1.13 (0.92–1.39) | 218 | 1.17 (0.93–1.47) |
| 6–8 | 4710 | 341 | 1.04 (0.85–1.28) | 223 | 1.00 (0.80–1.26) | 113 | 1.15 (0.87–1.52) | 105 | 0.89 (0.67–1.19) | 79 | 1.16 (0.85–1.59) |
| Ptrend | 0.654 | 0.938 | 0.339 | 0.484 | 0.319 | ||||||
| Continuous, per 2 pts | 28,946 | 1993 | 1.04 (0.95–1.13) | 1296 | 1.02 (0.92–1.12) | 589 | 1.06 (0.94–1.20) | 668 | 0.98 (0.87–1.11) | 475 | 1.11 (0.97–1.27) |
| aMEDb | |||||||||||
| 0–3 | 9167 | 613 | 1.00 | 396 | 1.00 | 183 | 1.00 | 198 | 1.00 | 143 | 1.00 |
| 4–5 | 12,891 | 883 | 1.04 (0.88–1.21) | 576 | 1.05 (0.88–1.25) | 256 | 1.02 (0.81–1.28) | 304 | 1.09 (0.88–1.36) | 214 | 1.07 (0.84–1.37) |
| 6–9 | 6889 | 497 | 1.07 (0.89–1.28) | 324 | 1.04 (0.84–1.28) | 150 | 1.06 (0.82–1.38) | 166 | 1.03 (0.79–1.33) | 118 | 1.16 (0.87–1.53) |
| Ptrend | 0.500 | 0.651 | 0.679 | 0.674 | 0.331 | ||||||
| Continuous, per 2 pts | 28,946 | 1993 | 1.03 (0.95–1.12) | 1296 | 1.01 (0.92–1.11) | 589 | 1.04 (0.92–1.17) | 668 | 0.99 (0.88–1.11) | 475 | 1.09 (0.96–1.23) |
| Women | |||||||||||
| aMEDr | |||||||||||
| 0–3 | 12,149 | 619 | 1.00 | 460 | 1.00 | 274 | 1.00 | 173 | 1.00 | 117 | 1.00 |
| 4–5 | 14,963 | 656 | 0.86 (0.73–1.00) | 498 | 0.87 (0.73–1.03) | 284 | 0.84 (0.68–1.03) | 201 | 0.92 (0.73–1.17) | 113 | 0.81 (0.60–1.08) |
| 6–8 | 6207 | 299 | 1.01 (0.82–1.23) | 229 | 1.03 (0.83–1.29) | 145 | 1.12 (0.87–1.45) | 78 | 0.92 (0.68–1.24) | 47 | 0.87 (0.60–1.27) |
| Ptrend | 0.941 | 0.755 | 0.377 | 0.575 | 0.462 | ||||||
| Continuous, per 2 pts | 33,318 | 1574 | 0.97 (0.88–1.07) | 1187 | 0.99 (0.89–1.10) | 703 | 1.02 (0.90–1.15) | 452 | 0.96 (0.83–1.10) | 277 | 0.91 (0.76–1.08) |
| aMEDb | |||||||||||
| 0–3 | 10,637 | 543 | 1.00 | 406 | 1.00 | 240 | 1.00 | 155 | 1.00 | 101 | 1.00 |
| 4–5 | 14,551 | 640 | 0.86 (0.73–1.01) | 478 | 0.85 (0.72–1.02) | 281 | 0.85 (0.69–1.05) | 183 | 0.86 (0.67–1.10) | 114 | 0.83 (0.62–1.12) |
| 6–9 | 8130 | 391 | 0.97 (0.80–1.18) | 303 | 1.01 (0.82–1.24) | 182 | 1.03 (0.81–1.32) | 114 | 0.99 (0.75–1.31) | 62 | 0.86 (0.60–1.23) |
| Ptrend | 0.960 | 0.744 | 0.637 | 0.917 | 0.453 | ||||||
| Continuous, per 2 pts | 33,318 | 1574 | 0.97 (0.89–1.06) | 1187 | 0.98 (0.89–1.08) | 703 | 0.99 (0.88–1.12) | 452 | 0.98 (0.86–1.12) | 277 | 0.91 (0.77–1.07) |
aMED alternate Mediterranean diet score, PYsubcohort person-years in the subcohort, aMEDr alternate Mediterranean diet score without the alcohol component
aAdjusted for age at baseline (years), cigarette smoking status (never, former, current), cigarette smoking frequency (cigarettes smoked per day, centered), cigarette smoking duration (years, centered), body mass index (kg/m2), alcohol consumption (0, > 0 to < 5, ≥ 5 to < 15, ≥ 15 to < 30, ≥ 30 g/day), daily energy intake (kcal), highest level of education (primary school or lower vocational, secondary school or medium vocational, higher vocational or university), non-occupational physical activity (≤ 30, > 30 to ≤ 60, > 60 to ≤ 90, > 90 min/day), and family history of colorectal cancer (no, yes)
bNot adjusted for alcohol consumption
In men, aMEDr was not significantly associated with colorectal cancer risk in categorical and continuous analyses [HRper two-point increment (95% CI) 1.04 (0.95–1.13)] (Table 3). Subsite-specific HR estimates per two-point increment in aMEDr (all not statistically significant) ranged from 0.98 for distal colon cancer to 1.11 for rectal cancer and did not significantly differ [Pheterogeneity: 0.566 (proximal vs. distal colon) and 0.518 (colon vs. rectum)]. Similar to men, no significant association was observed between aMEDr and colorectal cancer risk in women [HRper two-point increment (95% CI) 0.97 (0.88–1.07)] (Table 3). However, middle vs. low aMEDr values were associated with a borderline significantly reduced colorectal cancer risk [HR (95% CI) 0.86 (0.73–1.00)]. Though subsite-specific associations were all not statistically significant and there was no evidence of heterogeneity [Pheterogeneity: 0.690 (proximal vs. distal colon) and 0.194 (colon vs. rectum)], results suggested a weak inverse association between aMEDr and rectal cancer risk in women [HRper two-point increment (95% CI) 0.91 (0.76–1.08)]. Comparable results were obtained for the original aMED including alcohol in both men and women (Table 3). For colorectal cancer risk, inclusion of alcohol in the MD score resulted in a worse model fit.
Associations of aMEDr with colorectal cancer risk in women differed statistically significantly across strata of smoking status (Pinteraction = 0.015, Table 4). In female ex-smokers, increasing aMEDr values were associated with a significantly reduced colorectal cancer risk [HRper two-point increment (95% CI) 0.78 (0.63–0.98)]. In contrast, a positive association was suggested in female current smokers, with a significant positive trend over the aMEDr categories (Ptrend = 0.04, data not shown). Finally, there was no evidence of an association in women who had never smoked. No significant interactions were observed between aMEDr and other potential colorectal cancer risk factors (alcohol consumption, BMI, educational level, and family history of colorectal cancer) in men and women, or smoking status in men (Table 4). Simultaneous inclusion of all aMEDr components as dichotomous variables in multivariable-adjusted models showed that none of the individual components was significantly associated with colorectal cancer risk (data not shown). Associations were comparable after exclusion of the first 2 years of follow-up and did not significantly differ across the three follow-up periods (data not shown).
Table 4.
Sex-specific and multivariable-adjusted associations of aMEDr (per two-point increment) with colorectal cancer risk for various subgroups in the Netherlands Cohort Study
| Colorectal cancer | ||||
|---|---|---|---|---|
| Men | Women | |||
| Cases | HR (95% CI)a,b | Cases | HR (95% CI)a,b | |
| Cigarette smoking statusc | ||||
| Never | 256 | 1.11 (0.87–1.41) | 915 | 1.00 (0.88–1.13) |
| Former | 1184 | 1.03 (0.92–1.15) | 350 | 0.78 (0.63–0.98) |
| Current | 553 | 1.02 (0.85–1.22) | 309 | 1.21 (0.96–1.51) |
| Pinteractiond | 0.714 | 0.015 | ||
| Alcohol consumptione | ||||
| 0 g/day | 235 | 1.14 (0.90–1.46) | 489 | 1.04 (0.86–1.25) |
| > 0 to < 15.0 g/day | 934 | 1.05 (0.92–1.19) | 865 | 0.97 (0.86–1.10) |
| ≥ 15.0 g/day | 824 | 0.98 (0.85–1.13) | 220 | 0.88 (0.66–1.17) |
| Pinteractiond | 0.731 | 0.539 | ||
| Body mass indexf | ||||
| ≥ 18.5 to < 25.0 kg/m2 | 970 | 1.04 (0.92–1.17) | 848 | 1.03 (0.90–1.17) |
| ≥ 25.0 kg/m2 | 1018 | 1.05 (0.92–1.19) | 707 | 0.90 (0.78–1.04) |
| Pinteractiond | 0.876 | 0.232 | ||
| Highest level of educationg | ||||
| Primary school or lower vocational | 863 | 0.97 (0.84–1.12) | 841 | 0.95 (0.84–1.08) |
| Secondary school or medium vocational | 706 | 1.04 (0.90–1.19) | 579 | 1.00 (0.84–1.19) |
| Higher vocational or university | 424 | 1.21 (0.98–1.50) | 154 | 1.08 (0.79–1.47) |
| Pinteractiond | 0.133 | 0.920 | ||
| Family history of colorectal cancerh | ||||
| No | 1811 | 1.06 (0.97–1.16) | 1412 | 0.99 (0.89–1.09) |
| Yes | 182 | 0.82 (0.55–1.23) | 162 | 0.90 (0.59–1.39) |
| Pinteractiond | 0.204 | 0.423 | ||
aMEDr alternate Mediterranean diet score without the alcohol component
aAll HRs were estimated per two-point increment in aMEDr
bAdjusted for age at baseline (years), cigarette smoking status (never, former, current), cigarette smoking frequency (cigarettes smoked per day, centered), cigarette smoking duration (years, centered), body mass index (kg/m2), alcohol consumption (0, > 0 to < 5, ≥ 5 to < 15, ≥ 15 to < 30, ≥ 30 g/day), daily energy intake (kcal), highest level of education (primary school or lower vocational, secondary school or medium vocational, higher vocational or university), non-occupational physical activity (≤ 30, > 30 to ≤ 60, > 60 to ≤ 90, > 90 min/day), and family history of colorectal cancer (no, yes)
cNot adjusted for cigarette smoking status
dP values for interaction were obtained by testing the statistical significance of interaction terms between aMEDr and the stratifying covariates in multivariable-adjusted models
eNot adjusted for alcohol consumption
fNot adjusted for body mass index
gNot adjusted for highest level of education
hNot adjusted for family history of colorectal cancer
Like the population-dependent aMED indices, the absolute WCRF/AICR diet scores (including and excluding alcohol) were not significantly associated with risks of colorectal, colon, and rectal cancer in men and women (Table 5). Performances of models containing aMED indices and WCRF/AICR diet scores were mostly comparable.
Table 5.
Sex-specific and multivariable-adjusted associations of the absolute WCRF/AICR diet score and aMED (per SD-increment) with colorectal cancer risk in the Netherlands Cohort Study
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Colorectum | Colon | Rectum | Colorectum | Colon | Rectum | |
| HRSD (95% CI)a,b | HRSD (95% CI)a,b | HRSD (95% CI)a,b | HRSD (95% CI)a,b | HRSD (95% CI)a,b | HRSD (95% CI)a,b | |
| PYsubcohort/casesc | 28,304/1933 | 28,304/1256 | 28,304/462 | 32,678/1545 | 32,678/1166 | 32,678/270 |
| Excluding alcohol | ||||||
| WCRF/AICR diet scored | 0.99 (0.93–1.07) | 0.97 (0.90–1.05) | 1.03 (0.92–1.14) | 0.99 (0.92–1.07) | 1.01 (0.93–1.10) | 0.97 (0.85–1.12) |
| aMEDr | 1.03 (0.96–1.10) | 1.01 (0.93–1.09) | 1.09 (0.98–1.21) | 0.98 (0.91–1.05) | 0.99 (0.91–1.07) | 0.93 (0.81–1.07) |
| Including alcohol | ||||||
| WCRF/AICR diet scored,e | 0.95 (0.88–1.02) | 0.96 (0.88–1.04) | 0.93 (0.83–1.04) | 1.00 (0.92–1.07) | 1.01 (0.93–1.10) | 0.96 (0.83–1.11) |
| aMEDe | 1.02 (0.96–1.10) | 1.01 (0.93–1.09) | 1.07 (0.97–1.19) | 0.97 (0.90–1.05) | 0.98 (0.90–1.07) | 0.92 (0.80–1.06) |
WCRF/AICR World Cancer Research Fund/American Institute for Cancer Research, aMED alternate Mediterranean diet score, PYsubcohort person-years in the subcohort, aMEDr alternate Mediterranean diet score without the alcohol component
aHRs were estimated per SD-increment in the scores
bAdjusted for age at baseline (years), cigarette smoking status (never, former, current), cigarette smoking frequency (cigarettes smoked per day, centered), cigarette smoking duration (years, centered), body mass index (kg/m2), alcohol consumption (0, > 0 to < 5, ≥ 5 to < 15, ≥ 15 to < 30, ≥ 30 g/day), daily energy intake (kcal), highest level of education (primary school or lower vocational, secondary school or medium vocational, higher vocational or university), non-occupational physical activity (≤ 30, > 30 to ≤ 60, > 60 to ≤ 90, > 90 min/day), and family history of colorectal cancer (no, yes)
cA lower number of subjects could be included in these analyses as a result of missing values for salt intake
dScore based on the WCRF/AICR dietary recommendations to prevent cancer issued in 2007
eNot adjusted for alcohol consumption
Discussion
In this prospective cohort study, a priori defined MD adherence, assessed by aMEDr, was not significantly associated with colorectal cancer risk. Associations were absent for all investigated anatomical subsites and in both men and women. The association between aMEDr and colorectal cancer risk in women was significantly modified by smoking status (Pinteraction = 0.015). A significant inverse association was observed in female ex-smokers, whereas a positive association was suggested in female current smokers. For colorectal cancer risk, the best model performance was obtained when alcohol intake was not included in the MD score.
Various prospective cohorts have investigated the relation of a priori defined MD adherence with colorectal cancer risk and indicated disparate associations for men and women. In men, higher MD adherence has fairly consistently been associated with a reduced colorectal cancer risk (but not always significant) [9, 10, 13, 16, 17, 20]. For example, in male participants of the Multiethnic Cohort Study (MEC), the National Institutes of Health (NIH)-AARP Diet and Health Study, and the Health Professionals Follow-up Study (HPFS), statistically significant HR estimates of 0.84, 0.72, and 0.80, respectively, were obtained when comparing high to low aMED values [13, 16, 20]. Furthermore, high compared to low MD adherence (modified Mediterranean diet score) was associated with a non-significantly reduced colorectal cancer risk in the male part of the EPIC cohort [HR (95% CI) 0.89 (0.76–1.04)] [10]. With some exceptions [9, 12], studies in women did not support the presence of an inverse association between MD adherence and colorectal cancer risk [10, 13, 15–18, 20]. For comparison, non-significant HR estimates of 0.96 (MEC), 0.89 (NIH-AARP), 0.99 (Nurses’ Health Study, NHS), and 0.88 (EPIC), were reported for high vs. low MD adherence in female participants of the abovementioned studies [10, 13, 16, 20]. In the present analysis of the NLCS, a priori defined MD adherence was not associated with a significantly decreased risk of colorectal cancer in both sexes. Similar to our analysis, the majority of the previously conducted studies used aMED (variants) to assess MD adherence. However, the particular food items included in the aMED components may have differed between studies, which could (partly) explain the contrasting results that we observed for men in our cohort. Additionally, the more homogenous nature of the NLCS study population may have resulted in relatively small contrasts in absolute food intakes between subjects in the highest and lowest adherence categories making it more difficult to detect potentially beneficial effects of the MD on health outcomes. Median daily intakes among male NLCS subcohort members in the highest and lowest aMEDr categories were for example 266 g and 177 g for vegetables, 230 g and 120 g for fruits, and 101 g and 139 g for red and processed meats, respectively. In male participants of the HPFS [20], the mean numbers of servings per day in the highest and lowest aMED quintiles were 4.9 and 2.0 for vegetables, 2.6 and 0.8 for fruits, and 0.7 and 1.2 for red and processed meats. We calculated ratios comparing median/mean intakes in the highest and lowest MD categories. The ratios showed clearly higher contrasts in intakes of vegetables and fruits in the HPFS [vegetables: 1.5 (NLCS) vs. 2.5 (HPFS), fruits: 1.9 (NLCS) vs. 3.3 (HPFS)]. The contrast in the intake of red and processed meats was comparable in both cohorts [0.7 (NLCS) vs. 0.6 (HPFS)]. We were forced to compare median daily intakes in the NLCS with mean numbers of servings per day in the HPFS, because there were no other data available. Despite our relatively homogeneous study population, we previously detected significant inverse associations between aMEDr and risks of esophageal squamous cell carcinoma, gastric cardia adenocarcinoma (GCA), and gastric non-cardia adenocarcinoma (GNCA) in men in the NLCS [46], suggesting sufficient contrast. In female NLCS participants, associations of aMEDr with risks of GCA and GNCA were also inverse, but did not reach statistical significance.
None of the aMEDr components was individually associated with colorectal cancer risk in the present study. Possibly, the individual effects of the aMEDr components were too weak to be detected. Combining these components into a dietary pattern score increases the likelihood that the potentially weak individual effects are being detected. Furthermore, by investigating the effect of a dietary pattern, one allows for synergistic or antagonistic interactions between the dietary components, and solves confounding and collinearity problems associated with the analysis of single food groups. Finally, the contrast within the study population in terms of overall healthiness of the diet is possibly increased when considering the MD as a whole, which increases the chance of detecting true effects, if present [31, 47, 48].
The potentially distinct etiological backgrounds of tumors arising in the proximal colon, distal colon, and rectum and varying exposures to (carcinogens in) fecal matter across subsites may cause heterogeneous susceptibilities to (lifestyle) risk factors [21, 49]. Subsite-specific analyses in the NIH-AARP and HPFS cohorts demonstrated that the inverse association between MD adherence and colorectal cancer risk in men was particularly pronounced for distal colon cancer and rectal cancer [13, 20]. However, associations did not seem to differ across the subsites in men in our study. Additionally, there was no clear evidence for heterogeneity across the anatomical subsites in women, both in our cohort and in most previous studies [13, 15, 20].
In women in our cohort, associations between MD adherence and colorectal cancer risk significantly differed across strata of smoking status, with oppositely directed associations being observed in former smokers (inverse) and current smokers (positive). Smoking status did not significantly interact with MD adherence in female participants of the NHS [20]. However, this study did not differentiate between former and current smokers. A possible explanation for the interaction with smoking status that we observed is chance, considering the large number of tests performed. We recommend that the potentially modifying role of smoking status in the association between MD adherence and colorectal cancer risk, as well as underlying mechanisms, are investigated in future studies. Preferably, these studies should be performed separately for men and women, and distinguish between former and current smokers.
Colorectal cancers usually develop slowly over the course of 10–15 years [3], making the prospective design and long duration of follow-up major strengths of the present study. The large number of cases diagnosed during follow-up facilitated the performance of sex-specific analyses for cancers of the colorectum, colon, proximal colon, distal colon, and rectum with acceptable statistical power, while adjusting for relevant confounders. Additionally, associations were estimated within strata of colorectal cancer risk factors, separately for men and women. Since the national population screening program for colorectal cancer in the Netherlands started after the end of follow-up of our study [50], it could not have influenced the results. Despite the high quality of the dietary information, possible measurement error may have attenuated associations. Another limitation is the single measurement of diet and lifestyle factors at baseline. Changes in diet and lifestyle factors during follow-up may have led to non-differential misclassification and attenuated associations. However, the baseline assessment of the NLCS-FFQ has been shown to be capable of ranking subjects according to their nutrient intakes relatively well for over at least 5 years [28]. Furthermore, associations between aMEDr and colorectal cancer risk were largely similar, and did not significantly differ, across the three periods of follow-up (≤ 2, > 2 to ≤ 10, and > 10 years). Residual confounding by unmeasured factors also cannot be excluded. Lastly, aMEDr assesses the relative level of MD adherence using population-based cut-offs. Therefore, subjects with high scores do not necessarily adhere closely to a traditional MD, particularly in non-Mediterranean study populations. Comparison of diets of the Netherlands and Greece using previously reported intake data from the EPIC cohort [51] showed that mean daily intakes of food groups typically consumed in large amounts in the MD, such as vegetables, fruits, and legumes, were lower in participants of the Dutch EPIC cohorts (EPIC-NL) compared to participants of the Greek EPIC cohort (EPIC-Greece). Mean daily intakes of vegetables, fruits, and legumes among men were 131 g, 156 g, and 6 g in EPIC-NL and 269 g, 234 g, and 33 g in EPIC-Greece, respectively. Among female participants of EPIC-NL and EPIC-Greece, mean daily intakes were 128 g and 211 g for vegetables, 183 g and 218 g for fruits, and 4 g and 21 g for legumes, respectively. As expected, meat consumption was higher in Dutch subjects [EPIC-NL: 141 g (men) and 80 g (women), EPIC-Greece: 68 g (men) and 35 g (women)] [51]. Regardless of its use of population-based cut-offs, the model fit of aMEDr was generally comparable to that of the absolute WCRF/AICR diet score in our study.
In conclusion, results of this large prospective cohort study do not support the hypothesis that higher MD adherence is associated with a reduced risk of colorectal cancer. MD adherence was not significantly associated with the risk of any of the colorectal cancer subsites in both men and women.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are indebted to the participants of the Netherlands Cohort Study (NLCS) and further wish to thank the Netherlands Cancer Registry and the Dutch Pathology Registry. Additionally, NLCS staff members are acknowledged for their valuable assistance and advice.
Funding
This study was funded by Wereld Kanker Onderzoek Fonds Nederland (WCRF-NL), as part of the World Cancer Research Fund International grant program (Grant No. 2015/1390 to P.A. van den Brandt). WCRF-NL had no role in the design, analysis, or writing of this article.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Individuals invited to participate in the NLCS received an invitation letter with details on the study and they received the baseline questionnaire, which included an envelope for returning toenail clippings alongside with the questionnaire. Individuals agreed to participate in the NLCS by means of returning the baseline questionnaire (response rate 35.5%).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 3.Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, et al. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065. doi: 10.1038/nrdp.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, Helsing E, et al. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr. 1995;61(6 Suppl):1402S–1406S. doi: 10.1093/ajcn/61.6.1402S. [DOI] [PubMed] [Google Scholar]
- 5.Trichopoulou A, Lagiou P. Healthy traditional Mediterranean diet: an expression of culture, history, and lifestyle. Nutr Rev. 1997;55(11 Pt 1):383–389. doi: 10.1111/j.1753-4887.1997.tb01578.x. [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Gonzalez MA, Bes-Rastrollo M. Dietary patterns, Mediterranean diet, and cardiovascular disease. Curr Opin Lipidol. 2014;25(1):20–26. doi: 10.1097/MOL.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 7.Sofi F, Macchi C, Abbate R, Gensini GF, Casini A. Mediterranean diet and health status: an updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2014;17(12):2769–2782. doi: 10.1017/s1368980013003169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grosso G, Marventano S, Yang J, Micek A, Pajak A, Scalfi L, et al. A comprehensive meta-analysis on evidence of Mediterranean diet and cardiovascular disease: are individual components equal? Crit Rev Food Sci Nutr. 2017;57(15):3218–3232. doi: 10.1080/10408398.2015.1107021. [DOI] [PubMed] [Google Scholar]
- 9.Agnoli C, Grioni S, Sieri S, Palli D, Masala G, Sacerdote C, et al. Italian Mediterranean Index and risk of colorectal cancer in the Italian section of the EPIC cohort. Int J Cancer. 2013;132(6):1404–1411. doi: 10.1002/ijc.27740. [DOI] [PubMed] [Google Scholar]
- 10.Bamia C, Lagiou P, Buckland G, Grioni S, Agnoli C, Taylor AJ, et al. Mediterranean diet and colorectal cancer risk: results from a European cohort. Eur J Epidemiol. 2013;28(4):317–328. doi: 10.1007/s10654-013-9795-x. [DOI] [PubMed] [Google Scholar]
- 11.Fasanelli F, Zugna D, Giraudo MT, Krogh V, Grioni S, Panico S, et al. Abdominal adiposity is not a mediator of the protective effect of Mediterranean diet on colorectal cancer. Int J Cancer. 2017;140(10):2265–2271. doi: 10.1002/ijc.30653. [DOI] [PubMed] [Google Scholar]
- 12.Jones P, Cade JE, Evans CEL, Hancock N, Greenwood DC. The Mediterranean diet and risk of colorectal cancer in the UK Women’s Cohort Study. Int J Epidemiol. 2017;46(6):1786–1796. doi: 10.1093/ije/dyx155. [DOI] [PubMed] [Google Scholar]
- 13.Reedy J, Mitrou PN, Krebs-Smith SM, Wirfalt E, Flood A, Kipnis V, et al. Index-based dietary patterns and risk of colorectal cancer: the NIH-AARP Diet and Health Study. Am J Epidemiol. 2008;168(1):38–48. doi: 10.1093/aje/kwn097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fung TT, Hu FB, Wu K, Chiuve SE, Fuchs CS, Giovannucci E. The Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets and colorectal cancer. Am J Clin Nutr. 2010;92(6):1429–1435. doi: 10.3945/ajcn.2010.29242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vargas AJ, Neuhouser ML, George SM, Thomson CA, Ho GY, Rohan TE, et al. Diet quality and colorectal cancer risk in the Women’s Health Initiative Observational Study. Am J Epidemiol. 2016;184(1):23–32. doi: 10.1093/aje/kwv304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park SY, Boushey CJ, Wilkens LR, Haiman CA, Le Marchand L. High-quality diets associate with reduced risk of colorectal cancer: analyses of diet quality indexes in the Multiethnic Cohort. Gastroenterology. 2017;153(2):386–394. doi: 10.1053/j.gastro.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stone RAT, Waring ME, Cutrona SL, Kiefe CI, Allison J, Doubeni CA. The association of dietary quality with colorectal cancer among normal weight, overweight and obese men and women: a prospective longitudinal study in the USA. BMJ Open. 2017;7(6):e015619. doi: 10.1136/bmjopen-2016-015619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng E, Um CY, Prizment AE, Lazovich D, Bostick RM. Evolutionary-concordance lifestyle and diet and Mediterranean diet pattern scores and risk of incident colorectal cancer in Iowa women. Cancer Epidemiol Biomarkers Prev. 2018;27(10):1195–1202. doi: 10.1158/1055-9965.EPI-17-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lavalette C, Adjibade M, Srour B, Sellem L, Fiolet T, Hercberg S, et al. Cancer-specific and general nutritional scores and cancer risk: results from the prospective NutriNet-Sante cohort. Cancer Res. 2018;78(15):4427–4435. doi: 10.1158/0008-5472.CAN-18-0155. [DOI] [PubMed] [Google Scholar]
- 20.Petimar J, Smith-Warner SA, Fung TT, Rosner B, Chan AT, Hu FB, et al. Recommendation-based dietary indexes and risk of colorectal cancer in the Nurses’ Health Study and Health Professionals Follow-up Study. Am J Clin Nutr. 2018;108(5):1092–1103. doi: 10.1093/ajcn/nqy171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee GH, Malietzis G, Askari A, Bernardo D, Al-Hassi HO, Clark SK. Is right-sided colon cancer different to left-sided colorectal cancer? A systematic review. Eur J Surg Oncol. 2015;41(3):300–308. doi: 10.1016/j.ejso.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Paschke S, Jafarov S, Staib L, Kreuser ED, Maulbecker-Armstrong C, Roitman M, et al. Are colon and rectal cancer two different tumor entities? A proposal to abandon the term colorectal cancer. Int J Mol Sci. 2018;19(9):2577. doi: 10.3390/ijms19092577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van den Brandt PA, Goldbohm RA, van ’t Veer P, Volovics A, Hermus RJ, Sturmans F. A large-scale prospective cohort study on diet and cancer in The Netherlands. J Clin Epidemiol. 1990;43(3):285–295. doi: 10.1016/0895-4356(90)90009-E. [DOI] [PubMed] [Google Scholar]
- 24.van den Brandt PA, Schouten LJ, Goldbohm RA, Dorant E, Hunen PM. Development of a record linkage protocol for use in the Dutch Cancer Registry for Epidemiological Research. Int J Epidemiol. 1990;19(3):553–558. doi: 10.1093/ije/19.3.553. [DOI] [PubMed] [Google Scholar]
- 25.Goldbohm RA, van den Brandt PA, Brants HA, van ’t Veer P, Al M, Sturmans F, et al. Validation of a dietary questionnaire used in a large-scale prospective cohort study on diet and cancer. Eur J Clin Nutr. 1994;48(4):253–265. [PubMed] [Google Scholar]
- 26.Volovics A, van den Brandt PA. Methods for the analyses of case-cohort studies. Biom J. 1997;39(2):195–214. doi: 10.1002/bimj.4710390208. [DOI] [Google Scholar]
- 27.Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73(1):1–11. doi: 10.1093/biomet/73.1.1. [DOI] [Google Scholar]
- 28.Goldbohm RA, van ’t Veer P, van den Brandt PA, van ’t Hof MA, Brants HA, Sturmans F, et al. Reproducibility of a food frequency questionnaire and stability of dietary habits determined from five annually repeated measurements. Eur J Clin Nutr. 1995;49(6):420–429. [PubMed] [Google Scholar]
- 29.NEVO table. Dutch food composition table 1986–1987. The Hague, the Netherlands: Voorlichtingsbureau voor de Voeding; 1986.
- 30.Trichopoulou A, Kouris-Blazos A, Wahlqvist ML, Gnardellis C, Lagiou P, Polychronopoulos E, et al. Diet and overall survival in elderly people. BMJ. 1995;311(7018):1457–1460. doi: 10.1136/bmj.311.7018.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348(26):2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 32.Fung TT, McCullough ML, Newby PK, Manson JE, Meigs JB, Rifai N, et al. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2005;82(1):163–173. doi: 10.1093/ajcn/82.1.163. [DOI] [PubMed] [Google Scholar]
- 33.Mitrou PN, Kipnis V, Thiebaut AC, Reedy J, Subar AF, Wirfalt E, et al. Mediterranean dietary pattern and prediction of all-cause mortality in a US population: results from the NIH-AARP Diet and Health Study. Arch Intern Med. 2007;167(22):2461–2468. doi: 10.1001/archinte.167.22.2461. [DOI] [PubMed] [Google Scholar]
- 34.Fedirko V, Tramacere I, Bagnardi V, Rota M, Scotti L, Islami F, et al. Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Ann Oncol. 2011;22(9):1958–1972. doi: 10.1093/annonc/mdq653. [DOI] [PubMed] [Google Scholar]
- 35.World Cancer Research Fund/American Institute for Cancer Research. Continuous update project expert report 2018. Diet, nutrition, physical activity and colorectal cancer. 2018. https://www.wcrf.org/dietandcancer.
- 36.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84(408):1074–1078. doi: 10.2307/2290085. [DOI] [Google Scholar]
- 37.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–526. doi: 10.1093/biomet/81.3.515. [DOI] [Google Scholar]
- 38.Akaike H. A new look at the statistical model identification. IEEE Trans Autom Control. 1974;AC-19(6):716–723. doi: 10.1109/TAC.1974.1100705. [DOI] [Google Scholar]
- 39.Wacholder S, Gail MH, Pee D, Brookmeyer R. Alternative variance and efficiency calculations for the case-cohort design. Biometrika. 1989;76(1):117–123. doi: 10.1093/biomet/76.1.117. [DOI] [Google Scholar]
- 40.de Vogel S, Bongaerts BW, Wouters KA, Kester AD, Schouten LJ, de Goeij AF, et al. Associations of dietary methyl donor intake with MLH1 promoter hypermethylation and related molecular phenotypes in sporadic colorectal cancer. Carcinogenesis. 2008;29(9):1765–1773. doi: 10.1093/carcin/bgn074. [DOI] [PubMed] [Google Scholar]
- 41.World Cancer Research Fund/American Institute for Cancer Research . Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington DC: American Institute for Cancer Research; 2007. [Google Scholar]
- 42.Romaguera D, Vergnaud AC, Peeters PH, van Gils CH, Chan DS, Ferrari P, et al. Is concordance with World Cancer Research Fund/American Institute for Cancer Research guidelines for cancer prevention related to subsequent risk of cancer? Results from the EPIC study. Am J Clin Nutr. 2012;96(1):150–163. doi: 10.3945/ajcn.111.031674. [DOI] [PubMed] [Google Scholar]
- 43.Vergnaud AC, Romaguera D, Peeters PH, van Gils CH, Chan DS, Romieu I, et al. Adherence to the World Cancer Research Fund/American Institute for Cancer Research guidelines and risk of death in Europe: results from the European Prospective Investigation into Nutrition and Cancer cohort study. Am J Clin Nutr. 2013;97(5):1107–1120. doi: 10.3945/ajcn.112.049569. [DOI] [PubMed] [Google Scholar]
- 44.van den Brandt PA, Schulpen M. Mediterranean diet adherence and risk of postmenopausal breast cancer: results of a cohort study and meta-analysis. Int J Cancer. 2017;140(10):2220–2231. doi: 10.1002/ijc.30654. [DOI] [PubMed] [Google Scholar]
- 45.Schulpen M, van den Brandt PA. Adherence to the Mediterranean diet and risk of lung cancer in the Netherlands Cohort Study. Br J Nutr. 2018;119(6):674–684. doi: 10.1017/S0007114517003737. [DOI] [PubMed] [Google Scholar]
- 46.Schulpen M, Peeters PH, van den Brandt PA. Mediterranean diet adherence and risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Gastric Cancer. 2019;22(4):663–674. doi: 10.1007/s10120-019-00927-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jacques PF, Tucker KL. Are dietary patterns useful for understanding the role of diet in chronic disease? Am J Clin Nutr. 2001;73(1):1–2. doi: 10.1093/ajcn/73.1.1. [DOI] [PubMed] [Google Scholar]
- 48.Verberne L, Bach-Faig A, Buckland G, Serra-Majem L. Association between the Mediterranean diet and cancer risk: a review of observational studies. Nutr Cancer. 2010;62(7):860–870. doi: 10.1080/01635581.2010.509834. [DOI] [PubMed] [Google Scholar]
- 49.Wei EK, Giovannucci E, Wu K, Rosner B, Fuchs CS, Willett WC, et al. Comparison of risk factors for colon and rectal cancer. Int J Cancer. 2004;108(3):433–442. doi: 10.1002/ijc.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elferink MAG, Toes-Zoutendijk E, Vink GR, Lansdorp-Vogelaar I, Meijer GA, Dekker E, et al. National population screening for colorectal carcinoma in the Netherlands: results of the first years since the implementation in 2014. Ned Tijdschr Geneeskd. 2018;162:D2283. [PubMed] [Google Scholar]
- 51.Slimani N, Fahey M, Welch AA, Wirfalt E, Stripp C, Bergstrom E, et al. Diversity of dietary patterns observed in the European Prospective Investigation into Cancer and Nutrition (EPIC) project. Public Health Nutr. 2002;5(6B):1311–1328. doi: 10.1079/PHN2002407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.