Abstract
The incidence of lymphoma has gradually increased over previous decades, and it ranks among the ten most prevalent cancers worldwide. With the development of targeted therapeutic strategies, though a subset of lymphoma patients has become curable, the treatment of refractory and relapsed diseases remains challenging. Many efforts have been made to explore new targets and to develop corresponding therapies. In addition to novel antibodies targeting surface antigens and small molecular inhibitors targeting oncogenic signaling pathways and tumor suppressors, immune checkpoint inhibitors and chimeric antigen receptor T-cells have been rapidly developed to target the tumor microenvironment. Although these targeted agents have shown great success in treating lymphoma patients, adverse events should be noted. The selection of the most suitable candidates, optimal dosage, and effective combinations warrant further investigation. In this review, we systematically outlined the advances in targeted therapy for malignant lymphoma, providing a clinical rationale for mechanism-based lymphoma treatment in the era of precision medicine.
Subject terms: Haematological cancer, Cancer therapy
Introduction
Lymphoma is the most common lymphoid malignancy and is among the ten most prevalent cancers worldwide.1 Lymphoma is a heterogeneous entity and includes Hodgkin’s lymphoma (HL) and non-Hodgkin’s lymphoma (NHL). HL accounts for 10–15% of lymphoma and is characterized by the presence of Reed–Sternberg cells. NHL accounts for 80–85% of lymphoma, including B-cell NHLs (B-NHLs) expressing CD20 or CD19, T-cell NHLs (T-NHLs) expressing CD3, CD4, or CD8, and natural killer (NK)/T-cell NHLs expressing CD56. Chemotherapy is the standard of care for lymphoma patients. The introduction of monoclonal antibodies targeting surface antigens has greatly changed the therapeutic landscape of lymphoma. For example, rituximab, an anti-CD20 antibody targeting CD20 in B-NHLs and brentuximab vedotin targeting CD30 in classical HL and T-NHLs, have significantly improved the response rates and clinical outcomes of patients.2,3 In addition, growing insights into molecular biology and signaling pathways have led to the development of many innovative agents for lymphoma in recent years.4 More recently, with a better understanding of the crosstalk between malignant lymphocytes and the tumor microenvironment, chimeric antigen receptor T-cells (CAR-T cells) have been rapidly developed in treating relapse and refractory patients.5,6 Although the overall survival (OS) of lymphoma patients has been considerably improved by the new immunochemotherapeutic regimens, the selection of targeted agents and the optimal dosage are important due to treatment-related adverse events (AEs). In this review, we systematically outlined the advances in targeted therapy for malignant lymphoma that provide significant improvement in mechanism-based lymphoma treatment in the era of precision medicine.
Surface antigens and targeted therapies
Surface antigens are the most accessible part of lymphoma cells, and monoclonal antibodies (mAbs) targeting surface antigens have become important therapeutic strategies in many lymphoid malignancies. Cytotoxic to tumor cells, mAbs relatively spare normal tissues. The mechanisms of action include the induction of apoptosis, antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). In addition to “bare” antibodies, antibodies or their fragments may be linked with cell toxins, immunotoxins, or radioisotopes to increase clinical efficacy.
CD20
The CD20 molecule is a transmembrane protein involved in B-cell activation and differentiation and is present on all mature B-cells and most B-NHL cells.7 Moreover, without internalization or downregulation following antibody binding, CD20 functions as an ideal therapeutic target for most B-NHLs.8 Moreover, pro-B cells and antibody-producing plasma cells do not express CD20, so anti-CD20 treatment will not impair the healthy B-cell population.
Anti-CD20 mAbs are classified as type I and type II.9 Type I antibodies most effectively induce CDC, in which the binding of the mAb activates a complement cascade. Type I antibodies also induce ADCC, in which immune cells expressing Fc gamma receptor (FcγR) attack antibody-coated cells. Type II antibodies initiate ADCC as well as cell death through apoptotic or non-apoptotic mechanisms.
Rituximab was the first mAb to target CD20 and the first mAb approved to treat cancer patients. It is a chimeric antibody with a murine variable region and a human IgG1-kappa constant region,8 classified as a type I mAb. The significant anti-lymphoma activity of rituximab in early trials3,10–12 has led to its widespread use in most CD20+ B-NHLs.
The targeted agents and clinical trials related to mAbs are listed in Table 1. Ofatumumab is a fully humanized second-generation type I CD20 antibody that exhibits more potent CDC than rituximab in vitro.13 Ofatumumab is approved in combination with chlorambucil for chronic lymphocytic leukemia (CLL).14,15 Moreover, the results from a phase 2 trial (NCT00410163) suggested that ofatumumab in combination with fludarabine and cyclophosphamide was efficient in untreated CLL patients.16 The main AEs were infusion-related reactions and grade 1–2 infections.
Table 1.
Targeted agents and clinical trials related to monoclonal antibodies
| Drug | Disease | Trial name | Phase | Status | ORR/CR | NCT# | Reference |
|---|---|---|---|---|---|---|---|
| Anti-CD20 antibody | |||||||
| Ofatumumab | A fully humanized second-generation type I CD20 antibody | ||||||
| Ofatumumab, fludarabine, cyclophosphamide | CLL | Ofatumumab with fludarabine and cyclophosphamide in b-cell chronic lymphocytic leukemia patients | 2 | Completed | 500 mg, 77%/42%; 100 mg, 73%/50% | NCT00410163 | 16 |
| Obinutuzumab | A humanized type II CD20 antibody | ||||||
| Obinutuzumab | Relapsed or refractory DLBCL/MCL | A dose-escalating study of obinutuzumab in patients with b-lymphocyte antigen (CD20+) malignant disease (gauguin) | 1/2 | Completed | DLBCL, 28%/4%; MCL, 27%/13% | NCT00517530 | 19 |
| Obinutuzumab, bendamustine vs. bendamustine | Rituximab-refractory iNHLs | A study to investigate the efficacy and safety of bendamustine compared with bendamustine plus obinutuzumab in participants with rituximab-refractory, indolent non-Hodgkin’s lymphoma (GADOLIN) | 3 | Completed | Obinutuzumab plus bendamustine, 69%/11%; bendamustine monotherapy, 63%/12% | NCT01059630 | 20 |
| Obinutuzumab, CHOP/CVP/bendamustine vs. rituximab, CHOP/CVP/bendamustine | Untreated iNHLs | A study of obinutuzumab (RO5072759) plus chemotherapy in comparison with rituximab plus chemotherapy followed by obinutuzumab or rituximab maintenance in patients with untreated advanced indolent non-Hodgkin’s lymphoma (GALLIUM) | 3 | Active, not recruiting | FL: obinutuzumab group, 88.5%/19.5%; rituximab group, 86.9%/23.8% | NCT01332968 | 21 |
| Obinutuzumab, CHOP/FC/bendamustine | FL | A study of obinutuzumab in combination with chemotherapy in participants with CD20+ B-cell follicular non-Hodgkin’s lymphoma | 1 | Completed | G-CHOP, 96%/39%; G-FC, 93%/50% | NCT00825149 | 22 |
| G-Clb vs. Clb vs. R-Clb | Untreated CLL | CLL11: a study of obinutuzumab with chlorambucil in patients with previously untreated chronic lymphocytic leukemia (Stage 1a) | 3 | Completed | G-Clb, 77.3%/22.3%; Clb, 31.4%/0%; R-Clb, 65.7%/7.3% | NCT01010061 | 23 |
| Obinutuzumab, ibrutinib vs. obinutuzumab, chlorambucil | Untreated CLL/SLL | A multicenter study of ibrutinib in combination with obinutuzumab versus chlorambucil in combination with obinutuzumab in patients with treatment naïve CLL or SLL | 3 | Completed | Obinutuzumab plus ibrutinib, 91%/41%; obinutuzumab plus chlorambucil, 81%/16% | NCT02264574 | 24 |
| Ublituximab | A type I, chimeric, recombinant IgG1 monoclonal antibody targeting a unique epitope on the CD20 antigen, glycoengineered to enhance affinity for all FcRIIIa variants | ||||||
| Ublituximab, ibrutinib | CLL/MCL | Ublituximab plus ibrutinib in select B-cell malignancies | 1/2 | Completed | 88%/5% | NCT02013128 | 27 |
| Ublituximab, ibrutinib vs. ibrutinib | Previously treated high-risk CLL | Ublituximab in combination with ibrutinib versus ibrutinib alone in patients with previously treated high-risk chronic lymphocytic leukemia | 3 | Active, not recruiting | combination arm, 78%/7%; monotherapy, 45%/0% | NCT02301156 | 28 |
| Ublituximab, umbralisib vs. obinutuzumab, chlorambucil | CLL | Ublituximab plus umbralisib compared to obinutuzumab plus chlorambucil in patients with untreated and previously treated chronic lymphocytic leukemia | 3 | Active, not recruiting | – | NCT02612311 | – |
| Ublituximab, umbralisib; ublituximab, umbralisib, ibrutinib | B-NHLs, CLL | Ublituximab in combination with umbralisib +/− ibrutinib or bendamustine in patients with B-cell malignancies | 1 | Completed | Ublituximab, umbralisib, ibrutinib, 84%/30%; ublituximab, umbralisib, 46%/17% | NCT02006485 | 29,30 |
| Veltuzumab | A humanized type I anti-CD20 monoclonal antibody | ||||||
| Veltuzumab | Relapsed or refractory B-NHLs | Study of humanized anti-CD20 in patients with CD20+ non-Hodgkin’s lymphoma | 1/2 | Completed | FL, 44%/27%; MZL, 83%/33%; DLBCL, 43%/0% | NCT00285428 | 31 |
| Ocrelizumab | A humanized type I anti-CD20 monoclonal antibody | ||||||
| Ocrelizumab | Relapsed or refractory FL | An open-label, multicentre, dose-escalating phase 1/2 trial of 3-weekly ocrelizumab in patients with follicular non-Hodgkin’s lymphoma | 1/2 | Completed | 38%/15% | NCT02723071 | 32 |
| CT-P10 | A rituximab biosimilar | ||||||
| CT-P10 vs. rituximab | FL | To compare efficacy and safety between CT-P10 and rituxan in patients with low tumor burden follicular lymphoma | 3 | Active, not recruiting | CT-P10, 83%/28%; rituximab, 81%/34% | NCT02260804 | 33 |
| CT-P10, CVP vs. R-CVP | FL | To demonstrate equivalence of pharmacokinetics and noninferiority of efficacy for CT-P10 in comparison with rituxan | 3 | Completed | CT-P10, CVP, 97%/30%; R-CVP, 93%/22% | NCT02162771 | 34 |
| GP2013 | A rituximab biosimilar | ||||||
| GP2013, CVP vs. R-CVP | Untreated advanced-stage FL | GP2013 in The treatment of patients with previously untreated, advanced-stage follicular lymphoma | 3 | Completed | GP2013, CVP, 87%/15%; R-CVP, 88%/13% | NCT01419665 | 35 |
| PF-05280586 | A rituximab biosimilar | ||||||
| PF-05280586 vs.rituximab | FL | A study of PF-05280586 (Rituximab-Pfizer) or MabThera® (Rituximab-EU) for the First-Line treatment of patients with CD20+, low tumor burden, follicular lymphoma (REFLECTIONS B328-06) | 3 | Completed | PF-05280586, 76%/26%; rituximab, 71%/28% | NCT02213263 | 36 |
| ABP798 | A rituximab biosimilar | ||||||
| ABP798 vs. rituximab | B-NHLs | Study to assess if ABP798 Is safe and effective in treating non-Hodgkin’s lymphoma compared to rituximab | 3 | completed | NA | NCT02747043 | – |
| 90Y-ibritumomab tiuxetan | A radiolabeled anti-CD20 monoclonal antibody which targets the same epitope on the CD20 molecule like rituximab and chelates the radioactive particle Yttrium-90 | ||||||
| 90Y-ibritumomab tiuxetan | FL | 90Y-Ibritumomab tiuxetan first line in follicular lymphoma | 2 | Unknown status | 87%/56% | NCT00772655 | 41 |
| 90Y-ibritumomab tiuxetan | FL | Phase 2 study of fractionated 90Y-ibritumomab tiuxetan radioimmunotherapy as an initial therapy of follicular lymphoma | 2 | Completed | 95.8%/69.4% | NCT01493479 | 42 |
| 90Y-ibritumomab tiuxetan vs. no treatment | FL | Treatment with 90Y-ibritumomab tiuxetan versus no treatment in patients with follicular non-Hodgkin’s lymphoma (stage III or IV) having achieved a partial or complete pemission after first line chemotherapy | 3 | Completed | PR after induction therapy converted to a CR/CRu: consolidation arm, 77%; control arm, 17.5% | NCT00185393 | 43,44 |
| 90Y-ibritumomab tiuxetan, rituximab vs. rituximab | Untreated FL | Rituximab with or without 90Y-Ibritumomab tiuxetan in treating patients with untreated follicular lymphoma | 3 | Recruiting | – | NCT02320292 | – |
| 90Y-ibritumomab tiuxetan vs. ASCT | Relapsed or refractory FL | A phase 3 multicenter, randomized study comparing 90Y-Ibritumomab tiuxetan vs. ASCT in patients with relapsed or refractory FL | 3 | Recruiting | – | NCT01827605 | – |
| 90Y-ibritumomab tiuxetan, BEAM | FL/DLBCL/MCL/transformed lymphomas | Phase 2 trial of a transplantation regimen of 90Y-Ibritumomab tiuxetan and high-dose chemotherapy in patients with non-Hodgkin’s lymphoma | 2 | Completed | NA | NA | 45 |
| Anti-CD22 antibody | |||||||
| Epratuzumab | A humanized IgG1 monoclonal antibody targeting CD22 | ||||||
| Epratuzumab | Relapsed or refractory iNHLs | Phase 1/2 trial of epratuzumab in indolent non-Hodgkin’s lymphoma | 1/2 | Completed | all, 18%/6%; FL, 24%/8% | NA | 52 |
| Epratuzumab | Relapsed or refractory aggressive NHLs | Phase 1/2 trial of epratuzumab in patients with recurrent aggressive NHLs | 1/2 | Completed | all, 10%/6%; DLBCL, 15%/9% | NA | 53 |
| Epratuzumab, rituximab | Relapsed or refractory iNHLs | Phase 2 trial of rituximab plus epratuzumab in patients with relapsed or refractory, indolent non-Hodgkin’s lymphoma | 2 | Completed | FL, 54%/24%; SLL, 57%/43% | NA | 54 |
| Epratuzumab, rituximab | Untreated FL | Epratuzumab and rituximab in treating patients with previously untreated follicular non-Hodgkin’s lymphoma | 2 | Completed | 88.2%/42.4% | NCT00553501 | 55 |
| Epratuzumab, R-CHOP | DLBCL | Monoclonal antibody therapy and combination chemotherapy in treating patients with stage II, stage III, or stage IV diffuse large B-cell lymphoma | 2 | Completed | 96%/74% | NCT00301821 | 56 |
| Inotuzumab | A CD22-targeted ADC combining a humanized IgG4 anti-CD22 monoclonal antibody with calicheamicin, an enediyne antibiotic | ||||||
| Inotuzumab ozogamicin, rituximab | B-NHLs | Study evaluating inotuzumab ozogamicin administered in combination with rituximab in subjects with non-Hodgkin’s lymphoma | 1/2 | Completed | Relapsed FL, 87%/62%; relapsed DLBCL, 74%/50%; refractory aggressive NHLs, 20%/3% | NCT00299494 | 60 |
| R-InO vs. RB/RG | Relapsed or refractory aggressive NHLs | A study of inotuzumab ozogamicin plus rituximab for relapsed or refractory aggressive non-Hodgkin’s lymphoma patients who are not candidates for intensive high-dose chemotherapy | 3 | Terminated | R-InO, 41%/13%; RB/RG, 44%/13%; | NCT01232556 | 61 |
| Inotuzumab ozogamicin, R-CVP vs. R-G-CVP | DLBCL | Treatment of patients with diffuse large B-cell lymphoma who are not suitable for anthracycline containing chemotherapy | 2 | Active, not recruiting | – | NCT01679119 | – |
| Moxetumomab pasudotox | A recombinant immunotoxin consisting of the Fv portion of the anti-CD22 antibody and a fragment of pseudomonas exotoxin A | ||||||
| Moxetumomab pasudotox | Relapsed or refractory HCL | Safety study of moxetumomab pasudotox in patients with HCL with advance disease | 1 | Unknown | 86%/46% | NCT00462189 | 64 |
| Moxetumomab pasudotox | Relapsed or refractory HCL | Moxetumomab pasudotox for advanced HCL | 3 | Completed | 75%/41% | NCT01829711 | 65 |
| Anti-CD30 antibody | |||||||
| SGN-30 | A chimeric monoclonal antibody consisting of the variable region of an anti-CD30 murine monoclonal antibody with human gamma 1 heavy chain and kappa light chain constant regions | ||||||
| SGN-30 | Relapsed or refractory HL/ALCL | Phase 2 study of SGN-30 in Hodgkin’s lymphoma or systemic anaplastic large cell lymphoma | 2 | Completed | ALCL, 17%/5%; HL, 0%/0% | NA | 74 |
| SGN-30, GVD vs. placebo, GVD | Relapsed or refractory classical HL | Phase 2 trial of SGN-30 or placebo with GVD in patients with relapsed or refractory classical HL | 2 | Terminated | SGN-30, GVD, 65%/NA; GVD, 57%/NA | NA | 75 |
| BV | A CD30 ADC connecting an anti-CD30 antibody with the anti-mitotic agent MMAE via a valine-citrulline peptide linker | ||||||
| BV | HL/ALCL | Phase 1 open-label dose finding study of brentuximab vedotin for CD30+ hematologic malignancies | 1 | Completed | 38%/27% | NCT00430846 | 2 |
| BV | HL | A pivotal open-label Trial of brentuximab vedotin for Hodgkin’s lymphoma | 2 | Completed | 75%/34% | NCT00848926 | 79 |
| BV | ALCL | A phase 2 open-label trial of brentuximab vedotin for systemic anaplastic large cell lymphoma | 2 | Completed | 86%/57% | NCT00866047 | 80 |
| BV | Relapsed or refractory NHLs | A study of brentuximab vedotin in relapsed or refractory non-Hodgkin’s lymphoma | 2 | Completed | T-NHLs, 41%/24% | NCT01421667 | 81 |
| BV vs. methotrexate/bexarotene | CD30+ CTCL | A phase 3 trial of brentuximab vedotin versus physician’s choice (methotrexate or bexarotene) in participants with CD30+ cutaneous T-cell lymphoma (ALCANZA study) | 3 | Completed | BV, 56%/16%; methotrexate/bexarotene, 13%/2% | NCT01578499 | 83 |
| BV, AVD vs. ABVD | Advanced classical HL | A frontline therapy trial in participants with advanced classical Hodgkin’s lymphoma | 3 | Active, not recruiting | A+AVD, 86%/73%; ABVD, 83%/70% | NCT01712490 | 84 |
| BV, CHP, CHOP | CD30+ mature T-cell and NK-cell neoplasms | A phase 1 study of brentuximab vedotin given sequentially and combined with multi-agent chemotherapy for CD30+ mature T-cell and NK-cell neoplasms | 1 | Completed | sequential treatment, 85%/62%; combination treatment, 100%/88% | NCT01309789 | 85,86 |
| BV, CHP vs. CHOP | CD30+ mature T-cell lymphomas | ECHELON-2: A comparison of brentuximab vedotin and CHP with standard-of-care CHOP in the treatment of patients with CD30+ mature T-cell lymphomas | 3 | Active, not recruiting | BV, CHP, 83%/68%; CHOP, 72%/56% | NCT01777152 | 87 |
| Anti-CD52 antibody | |||||||
| Alemtuzumab | A humanized monoclonal antibody targeting CD52 | ||||||
| Alemtuzumab | Relapsed or refractory CLL | Phase 2 trial of slemtuzumab in patients with relapsed or refractory B-cell chronic lymphocytic leukemia exposed to alkylating agents and having failed fludarabine therapy | 2 | Completed | 33%/2% | NA | 91 |
| Alemtuzumab vs. chlorambucil | CLL | A phase 3 study to evaluate the efficacy and safety of frontline therapy with alemtuzumab vs. chlorambucil in patients with progressive B-cell chronic lymphocytic leukemia | 3 | Completed | Alemtuzumab, 83%/24%; chlorambucil, 55%/2% | NA | 92 |
| Alemtuzumab | Advanced MF/SS | Phase 2 study of alemtuzumab in patients with advanced mycosis fungoides/Sézary syndrome | 2 | Completed | 55%/32% | NA | 93 |
| Alemtuzumab | Relapsed or refractory PTCL | A pilot study of alemtuzumab therapy for patients with relapsed or chemotherapy-refractory peripheral T-cell lymphoma | 2 | Completed | 36%/21% | NA | 94 |
| Alemtuzumab, FC vs. FCR | CLL | Fludarabine, cyclophosphamide, and rituximab or alemtuzumab in treating CLL | 3 | Completed | FCCam, 90%/19.2%; FCR, 91%/33.75% | NCT00564512 | 95 |
| Subcutaneous alemtuzumab, bendamustine | Relapsed or refractory CLL | Bendamustine and subcutaneous alemtuzumab in relapsed or refractory chronic lymphocytic leukemia patients | 1/2 | Completed | 68%/24% | NA | 96 |
| Alemtuzumab, rituximab, pentostatin | Relapsed or refractory CLL/SLL | Pentostatin, alemtuzumab, and rituximab in treating patients with relapsed or refractory chronic lymphocytic leukemia or small lymphocytic lymphoma | 2 | Completed | 56%/28% | NCT00669318 | 97 |
| Alemtuzumab, CHOP | PTCL | A phase 2 study of alemtuzumab plus CHOP as frontline chemotherapy for patients with peripheral T-cell lymphoma | 2 | Completed | 80%/65% | NA | 98 |
| Alemtuzumab, CHOP | PTCL | GITIL trial of alemtuzumab and CHOP chemotherapy as first-line treatment of peripheral T-cell lymphoma | 2 | Completed | 75%/71% | NA | 99 |
| Alemtuzumab, CHOP | PTCL | Alemtuzumab, MabCampath® with 2-weekly CHOP chemotherapy for mature T-cell non-Hodgkin’s lymphoma | 2 | Completed | 90%/60% | NA | 100 |
| Alemtuzumab, CHOP14 vs.CHOP14 | PTCL | Alemtuzumab and CHOP in T-cell Lymphoma | 3 | Completed | ALZ-CHOP, NA/52%; CHOP, NA/42% | NCT00646854 | 101 |
| alemtuzumab, CHOP14 vs. CHOP14 | PTCL | Immunotherapy in peripheral T-cell lymphoma—the role of alemtuzumab in addition to dose dense CHOP | 3 | Unknown | ALZ-CHOP, NA/60%; CHOP, NA/43% | NCT00725231 | 102 |
| Anti-CD79 antibody | |||||||
| polatuzumab vedotin | An anti-CD79b monoclonal antibody conjugated to MMAE | ||||||
| Polatuzumab vedotin, rituximab | Relapsed or refractory B-NHLs/CLL | A study of escalating doses of polatuzumab vedotin in participants with relapsed or refractory B-cell non-Hodgkin’s lymphoma and chronic lymphocytic leukemia and polatuzumab vedotin in combination with rituximab in participants with relapsed or refractory B-cell non-Hodgkin’s lymphoma | 1 | Completed | single-agent polatuzumab vedotin: DLBCL, 56%/16%; iNHLs, 47%/20%; MCL, 100%/0%; CLL, 0%/0%; R-pola: 78%/22% | NCT01290549 | 106 |
| Pinatuzumab vedotin, obinutuzumab, polatuzumab vedotin, rituximab | Relapsed or refractory DLBCL/FL | A study of pinatuzumab vedotin combined with rituximab or polatuzumab vedotin combined with rituximab or obinutuzumab in participants with relapsed or refractory B-cell non-Hodgkin’s lymphoma | 1/2 | Completed | DLBCL: R-pina, 60%/26%; R-pola, 54%/21%; FL: R-pina, 60%/5%; R-pola, 70%/45% | NCT01691898 | 107 |
| Polatuzumab vedotin, rituximab vs. bendamustine, obinutuzumab | Relapsed or refractory DLBCL/FL | A study of polatuzumab vedotin in combination with rituximab or obinutuzumab plus bendamustine in participants with relapsed or refractory follicular or diffuse large B-cell lymphoma | 1/2 | Active, not recruiting | – | NCT02257567 | 108 |
| Polatuzumab vedotin, R-CHP vs. R-CHOP | DLBCL | A study comparing the efficacy and safety of polatuzumab vedotin with rituximab-cyclophosphamide, doxorubicin, and prednisone versus rituximab-cyclophosphamide, doxorubicin, vincristine, and prednisone in participants with diffuse large B-cell lymphoma | 3 | Recruiting | – | NCT03274492 | – |
| Anti-CD19 antibody | |||||||
| Inebilizumab | A CD19-targeted humanized monoclonal antibody | ||||||
| Inebilizumab | Relapsed or refractory advanced B-NHLs | A phase 1, dose-escalation study of inebilizumab in japanese adult patients with relapsed or refractory advanced B-cell malignancies | 1 | Completed | FL, 82%/55%; DLBCL, 50%/17% | NCT01957579 | 112 |
| Inebilizumab, rituximab | Relapsed or refractory B-NHLs | A clinical study using inebilizumab in adult subjects with relapsed or refractory advanced B-cell malignancies | 1/2 | Completed | NA | NCT00983619 | – |
| Inebilizumab, bendamustine vs. rituximab, bendamustine | Relapsed or refractory CLL | A phase 2, multicenter, open-label study of inebilizumab in adults with relapsed or refractory chronic lymphocytic leukemia | 2 | Completed | rituximab, bendamustine 59.7%/6.5%; inebilizumab 2mg/kg, bendamustine 52.8%/5.6%; inebilizumab 4mg/kg, bendamustine 63.9%/11.5% | NCT01466153 | – |
| Inebilizumab, ICE/DHAP vs. rituximab, ICE/DHAP | Relapsed or refractory DLBCL | A phase 2, multicenter, randomized, open-label study of inebilizumab in adults with relapsed or refractory diffuse large B-cell lymphoma | 2 | Completed | inebilizumab 2mg/kg, ICE/DHAP, 46.2%/NA; inebilizumab 4mg/kg, ICE/DHAP, 43.6%/NA; rituximab, ICE/DHAP, 47.5%/NA | NCT01453205 | – |
| Tafasitamab | A novel Fc-engineered, humanized, anti-CD19 antibody with enhanced ADCC | ||||||
| Tafasitamab | Relapsed or refractory NHLs | Study of Fc-optimized anti-CD19 antibody tafasitamab to treat non-Hodgkin’s lymphoma | 2 | Active, not recruiting | DLBCL, 26%/6%; FL, 29%/9%; iNHLs, 27%/18% | NCT01685008 | 114 |
| Tafasitamab, lenalidomide | Relapsed or refractory DLBCL | A study to evaluate the safety and efficacy of lenalidomide with tafasitamab in patients with relapsed or refractory DLBCL | 2 | Active, not recruiting | 58%/33% | NCT02399085 | 115 |
| Tafasitamab, lenalidomide | CLL/SLL, PLL | Phase 2 tafasitamab in combination with lenalidomide for patients with relapsed or refractory CLL/SLL or PLL or older patients with untreated CLL/SLL or PLL | 2 | Active, not recruiting | – | NCT02005289 | – |
| Tafasitamab, bendamustine vs. rituximab, bendamustine | Relapsed or refractory DLBCL | A trial to evaluate the efficacy and safety of tafasitamab with bendamustine versus rituximab with bendamustine in adult patients with relapsed or refractory diffuse large B-cell lymphoma | 2/3 | Recruiting | – | NCT02763319 | – |
| Coltuximab ravtansine | A CD19-targeted ADC consists of CD19 antibody and a cytotoxic maytansinoid, DM4, which is a potent inhibitor of tubulin polymerization and microtubule assembly | ||||||
| Coltuximab ravtansine | Relapsed or refractory DLBCL | Coltuximab ravtansine as single agent in relapsed or refractory diffuse large B-cell lymphoma patients | 2 | Completed | 43.9%/14.6% | NCT01472887 | 116 |
| loncastuximab tesirine | An ADC consisting of an anti-CD19 humanized monoclonal antibody conjugated to a cytotoxic, crosslinking agent pyrrolobenzodiazepine dimer | ||||||
| loncastuximab tesirine | Relapsed or refractory DLBCL | Study to evaluate the efficacy and safety of ioncastuximab tesirine in patients with relapsed or refractory diffuse large B-cell lymphoma | 2 | Active, not recruiting | – | NCT03589469 | – |
| loncastuximab tesirine | Relapsed or refractory B-NHLs | Study of ioncastuximab tesirine in patients with relapsed or refractory B-cell lineage non-Hodgkin’s lymphoma | 1 | Completed | NA | NCT02669017 | – |
| loncastuximab tesirine, ibrutinib | DLBCL/MCL | Safety and antitumor activity study of loncastuximab tesirine plus ibrutinib in diffuse large B-cell or mantle cell lymphoma | 1 | Recruiting | – | NCT03684694 | – |
| loncastuximab tesirine, durvalumab | DLBCL/MCL/FL | Safety and antitumor activity study of loncastuximab tesirine and durvalumab in diffuse large B-cell, mantle cell, or follicular lymphoma | 1 | Recruiting | – | NCT03685344 | – |
| Anti-CD37 antibody | |||||||
| Otlertuzumab | A humanized variant of SMIP-016 built on the ADAPTIR platform | ||||||
| Otlertuzumab | Relapsed or refractory NHL/CLL | Phase 1/1b study of otlertuzumab in patients with previously treated CLL or select subtypes of non-Hodgkin’s lymphoma | 1 | Completed | FL, 12.5%/0%; MCL, 0%/0%; WM, 25%/0%; CLL, 23%/0% | NCT00614042 | 123,124 |
| Otlertuzumab, bendamustine vs. bendamustine | Relapsed CLL | Safety and efficacy study of otlertuzumab plus bendamustine vs. bendamustine in relapsed chronic lymphocytic leukemia | 1/2 | Completed | Otlertuzumab and bendamustin, 69%/9%; bendamustin, 39%/3% | NCT01188681 | 125 |
| Otlertuzumab, bendamustine, rituximab | Relapsed iNHLs | A study of otlertuzumab in combination with rituximab and bendamustine in subjects with relapsed indolent lymphoma | 1 | Completed | 83%/32% | NCT01317901 | 126 |
| IMGN529 | Consisting of an anti-CD37 antibody coupled with the maytansine-derived anti-microtubule agent, DM1 | ||||||
| IMGN529 | Relapsed or refractory NHLs/CLL | IMGN529 in treating patients with relapsed or refractory non-Hodgkin’s lymphoma and chronic lymphocytic leukemia | 1 | Completed | DLBCL, 22.2%/5.6%; FL, 7.7%/0%; MCL, 0%/0%; MZL, 0%/0% | NCT01534715 | 129 |
| AGS67E | A fully human monoclonal IgG2 antibody conjugated via a protease-cleavable linker to MMAE | ||||||
| AGS67E | Relapsed or refractory lymphoid malignancy | A study to evaluate safety, tolerability, and pharmacokinetics of escalating doses of AGS67E given as monotherapy in subjects with refractory or relapsed lymphoid malignancies | 1 | Active, not recruiting | – | NCT02175433 | – |
| Betalutin | A novel ARC targeting the CD37 antigen | ||||||
| Betalutin | Relapsed or refractory NHLs | A Phase 1/2 study of betalutin for treatment of relapsed non-Hodgkin’s lymphoma | 1/2 | Recruiting | – | NCT01796171 | – |
| Betalutin | Relapsed or refractory DLBCL | Study of betalutin for treatment of relapsed or refractory non-Hodgkin’s lymphoma (LYMRIT-37-05) | 1 | Recruiting | – | NCT02658968 | – |
| Betalutin, rituximab | Relapsed or refractory FL | Study of safety and efficacy of betalutin and rituximab in patients with FL | 1 | Recruiting | – | NCT03806179 | – |
| Anti-CCR4 | |||||||
| Mogamulizumab | A defucosylated humanized monoclonal antibody directed against CCR4 | ||||||
| Mogamulizumab | ATLL | Phase 2 study of KW-0761 in subjects with CCR4+ adult T-cell leukemia/lymphoma | 2 | Completed | 50%/31% | NCT00920790 | 138 |
| Mogamulizumab, mLSG15 vs. mLSG15 | ATLL | Multicenter, randomized, open-label, parallel-group study to compare mLSG15 plus mogamulizumab to mLSG15 | 2 | Completed | Mogamulizumab, mLSG15, 86%/52%; mLSG15, 75%/33% | NCT01173887 | 139 |
| Mogamulizumab | PTCL | Safety study to evaluate monoclonal antibody mogamulizumab in subjects with peripheral T-cell lymphoma | 1/2 | Completed | 36.8%/7.9% | NCT00888927 | 140 |
| Mogamulizumab | PTCL | Study of mogamulizumab in subjects with CCR4+ T-cell lymphoma | 2 | Completed | 35%/14% | NCT01192984 | 141 |
| Mogamulizumab vs. vorinostat | Relapsed or refractory CTCL | Study of mogamulizumab versus vorinostat in relapsed or refractory CTCL | 3 | Active, not recruiting | Mogamulizumab, 28%/3%; vorinostat, 5%/0% | NCT01728805 | 142 |
| Anti-CD25 antibody | |||||||
| 90Y-daclizumab | A radiolabeled anti-CD25 antibody | ||||||
| 90Y-daclizumab | HL/NHLs | 90Y-Daclizumab to treat Hodgkin’s disease, non-Hodgkin’s lymphoma and lymphoid leukemia | 1/2 | Completed | Relapsed HL, 50%/30% | NCT00001575 | 145 |
| 90Y-basiliximab | A radiolabeled anti-CD25 antibody | ||||||
| 90Y-basiliximab, BEAM | Relapsed or refractory HL | Radiolabeled monoclonal antibody therapy and combination chemotherapy before stem cell transplant in treating patients with primary refractory or relapsed Hodgkin’s lymphoma | 1 | Active, not recruiting | – | NCT01476839 | – |
| 90Y-basiliximab, BEAM | Mature T-NHLs | 90Y-basiliximab and combination chemotherapy before stem cell transplant in treating patients with mature T-cell non-Hodgkin’s lymphoma | 1 | Recruiting | – | NCT02342782 | – |
| Camidanlumab tesitine | A CD25 antibody-drug conjugate | ||||||
| Camidanlumab tesitine | Relapsed or refractory HL/NHLs | Study of camidanlumab tesitine in patients with relapsed or refractory Hodgkin’s and non-Hodgkin’s lymphoma | 1 | Completed | NA | NCT02432235 | – |
| Anti-CD38 antibody | |||||||
| Daratumumab | An anti-CD38 monoclonal antibody | ||||||
| Daratumumab | Relapsed or refractory NKTCL, nasal type | A study to assess the clinical efficacy and safety of daratumumab in participants with relapsed or refractory NK/T-cell lymphoma, nasal type | 2 | Active, not recruiting | 35.7%/0% | NCT02927925 | 149 |
| Anti-CD40 antibody | |||||||
| Dacetuzumab | A humanized IgG1 monoclonal antibody targeting CD40 | ||||||
| Dacetuzumab | NHL | A safety study of dacetuzumab in patients with non-Hodgkin’s lymphoma | 1 | Completed | 12%/2% | NCT00103779 | 152 |
| Dacetuzumab | Relapsed DLBCL | Study of dacetuzumab in patients with relapsed diffuse large B-cell lymphoma | 2 | Completed | 9%/4% | NCT00435916 | 153 |
| Dacetuzumab, R-ICE vs. placebo, R-ICE | Relapsed DLBCL | A randomized phase 2 placebo-controlled study of R-ICE chemotherapy with and without dacetuzumab for patients with DLBCL | 2 | Terminated | Dacetuzumab, R-ICE, 66%/33%; placebo, R-ICE, 64%/36% | NCT00529503 | 154 |
| Anti-CD74 antibody | |||||||
| milatuzumab | A humanized antibody against CD74 | ||||||
| Milatuzumab, veltuzumab | Relapsed or refractory B-NHLs | Veltuzumab and milatuzumab in treating patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma | 1/2 | Completed | FL, 33%/7%; DLBCL, 0%/0%; MCL, 17%/0%; MZL, 100%/50%; WM, 0%/0% | NCT00989586 | 156 |
| Anti-CD80 antibody | |||||||
| Galiximab | An anti-CD80 monoclonal antibody | ||||||
| Galiximab | Relapsed or refractory HL | Galiximab in treating patients with relapsed or refractory Hodgkin’s lymphoma | 2 | Completed | 10.3%/NA | NCT00516217 | – |
| Galiximab | Relapsed or refractory FL | Phase 1/2 study of galiximab for relapsed or refractory follicular lymphoma | 1/2 | Completed | 11%/6% | – | 159 |
| Galiximab, rituximab | Relapsed or refractory FL | Safety and efficacy of galiximab in combination with rituxan in the treatment of non-Hodgkin’s lymphoma | 1/2 | Completed | 66%/19% | NCT00048555 | 160 |
| Anti-CD158k antibody | |||||||
| IPH4102 | An anti-CD158k monoclonal antibody | ||||||
| IPH4102 | Relapsed or refractory CTCL | Study of IPH4102 in patients with relapsed or refractory cutaneous T-cell lymphoma | 1 | Active, not recruiting | 45%/0% | NCT02593045 | 165 |
| IPH4102 vs. IPH4102, gemcitabine, oxaliplatin | Advanced T-NHLs | IPH4102 alone or in combination with chemotherapy in patients with advanced T-cell lymphoma | 2 | Recruiting | – | NCT03902184 | – |
| Bispecific T cell Engager | |||||||
| Blinatumomab | A CD19/CD3 Bispecific T cell Engager | ||||||
| Blinatumomab | Relapsed NHLs | Safety study of the bispecific T-cell engager blinatumomab in patients with relapsed NHLs | 1 | Completed | DLBCL, 55%/36%; MCL, 71%/43%; FL, 80%/40% | NCT00274742 | 169 |
| Blinatumomab | Relapsed or refractory DLBCL | Clinical study with blinatumomab in patients with relapsed or refractory diffuse large B-cell lymphoma | 2 | Completed | 43%/19% | NCT01741792 | 170 |
| Blinatumomab | Relapsed or refractory aggressive B-NHLs | Study to evaluate safety and efficacy of blinatumomab in subjects with relapsed or refractory aggressive B-cell NHL | 2 | Active, not recruiting | – | NCT02910063 | – |
| Mosunetuzumab | A CD20/CD3 Bispecific T cell Engager | ||||||
| Mosunetuzumab | DLBCL | A trial of mosunetuzumab as consolidation therapy in participants with diffuse large B-cell lymphoma following first-line immunochemotherapy and as therapy in participants with previously untreated diffuse large B-cell lymphoma who are unable to tolerate full-dose chemotherapy | 1/2 | Recruiting | – | NCT03677154 | – |
| Mosunetuzumab, polatuzumab vedotin | B-NHLs | A study to evaluate the safety and efficacy of mosunetuzumab in combination with polatuzumab vedotin in B-cell non-Hodgkin’s lymphoma | 1 | Recruiting | – | NCT03671018 | – |
| Mosunetuzumab, polatuzumab vedotin, CHP vs.mosunetuzumab, CHOP | B-NHLs | A phase 1/2 study investigating the safety, tolerability, pharmacokinetics, and efficacy of mosunetuzumab in combination With CHOP or CHP-polatuzumab vedotin in participants With B-cell non-Hodgkin’s lymphoma | 1/2 | Recruiting | – | NCT03677141 | – |
| RO7082859 | A CD20/CD3 Bispecific T cell Engager | ||||||
| RO7082859, obinutuzumab | Relapsed or refractory B-NHLs | A dose escalation study of RO7082859 as a single agent and in combination with obinutuzumab, administered after a fixed, single pre-treatment dose of obinutuzumab in participants with relapsed or refractory B-cell non-Hodgkin’s lymphoma | 1 | Recruiting | – | NCT03075696 | – |
| RO7082859, atezolizumab, obinutuzumab | Relapsed or refractory B-NHLs | An open-label phase 1b study of RO7082859 and atezolizumab in adult patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma | 1 | Recruiting | – | NCT03533283 | – |
| RO7082859, obinutuzumab/rituximab, CHOP | B-NHLs | A study of RO7082859 in combination with rituximab or obinutuzumab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in participants with non-Hodgkin’s lymphomas | 1 | Recruiting | – | NCT03467373 | – |
| REGN1979 | A CD20/CD3 Bispecific T cell Engager | ||||||
| REGN1979 | Relapsed or refractory FL | Assess the antitumor activity and safety of REGN1979 in patients with relapsed or refractory follicular lymphoma | 2 | Recruiting | – | NCT03888105 | – |
| REGN1979 | B-NHLs | A phase 1 study to investigate the safety and tolerability of REGN1979 in patients With CD20+ B-cell malignancies | 1 | Recruiting | – | NCT02290951 | – |
| REGN1979, REGN2810 | B-NHLs | Study of REGN2810 and REGN1979 in patients with lymphoma | 1 | Recruiting | – | NCT02651662 | – |
| XmAb13676 | A CD20/CD3 Bispecific T cell Engager | ||||||
| XmAb13676 | B-NHLs, CLL/SLL | Study to evaluate safety and tolerability of XmAb13676 in patients with CD20− expressing hematologic malignancies | 1 | Recruiting | – | NCT02924402 | – |
NA: ORR or CR are not available on the clinicaltrials.gov or from the published article although the trial has been completed
iNHLs indolent NHLs, CHOP cyclophosphamide, doxorubicin, vincristine, prednisolone, CVP cyclophosphamide, vincristine, and prednisolone, FC fludarabine and cyclophosphamide, G-CHOP obinutuzumab, cyclophosphamide, doxorubicin, vincristine and prednisone, G-FC obinutuzumab, fludarabine and cyclophosphamide, G-Clb obinutuzumab and chlorambucil, R-Clb rituximab and chlorambucil, BEAM carmustine, etoposide, cytarabine, melphalan chemotherapy, R-CHOP rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone, R-InO rituximab and inotuzumab ozogamicin, RB rituximab and bendamustine, RG rituximab and gemcitabine, R-G-CVP rituximab, gemcitabine, cyclophosphamide, vincristine and prednisolone, GVD gemcitabine, vinorelbine, and liposomal doxorubicin, BV brentuximab vedotin, A+AVD brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine, ABVD doxorubicin, bleomycin, vinblastine, and dacarbazine, CHP cyclophosphamide, doxorubicin and prednisone, FCCam fludarabine cyclophosphamide and alemtuzumab, FCR fludarabine cyclophosphamide and rituximab, CHOP14 cyclophosphamide, doxorubicin, vincristine, and prednisone every 14 days, ALZ-CHOP alemtuzumab, cyclophosphamide, doxorubicin, vincristine and prednisone, R-pola rituximab and polatuzumab vedotin, R-pina rituximab and pinatuzumab vedotin, R-CHP rituximab, cyclophosphamide, doxorubicin and prednisone, ICE ifosfamide, carboplatin, etoposide, DHAP dexamethasone, high-dose cytarabine, cisplatin, PLL prolymphocytic leukemia, mLSG15 a dose-intensified chemotherapy
Obinutuzumab (GA101, Gazyva™) is a humanized type II mAb that can induce ADCC and direct apoptosis both in vitro and in vivo.17,18 In a phase 1/2 study (NCT00517530), obinutuzumab as monotherapy showed clinical activity with an acceptable safety profile in aggressive B-NHLs.19 Moreover, clinical trials (NCT01059630, NCT01332968, and NCT00825149) of obinutuzumab in combination with other chemotherapy regimens showed promising results in relapsed or refractory indolent B-NHLs20,21 and untreated follicular lymphoma (FL).22 The most common nonhematologic AEs were grade 1-2 infusion-related reactions, and the most common hematologic AE was neutropenia. For CLL, the findings of a phase 3 study (NCT01010061) of naïve elderly patients suggested that obinutuzumab in combination with chlorambucil yields better response rates and longer progression-free survival (PFS) than rituximab with chlorambucil and chlorambucil; thus, obinutuzumab became the first drug with “breakthrough therapy designation” approved by the FDA for the treatment of untreated CLL in combination with chlorambucil.23 Recently, a multicenter, randomized, phase 3 trial (iLLUMINATE, NCT02264574) demonstrated the advantages of obinutuzumab plus ibrutinib over obinutuzumab plus chlorambucil as a first-line treatment for CLL.24
Ublituximab is another type I, chimeric, recombinant IgG1 mAb targeting a unique epitope on the CD20 antigen, glycoengineered to enhance affinity for all FcRIIIa variants, leading to greater ADCC than other anti-CD20 mAbs such as rituximab and ofatumumab.25 Ublituximab demonstrated efficacy and safety as a single agent in early clinical trials in patients with B-NHLs and CLL,25,26 and it was further investigated in combination regimens. A phase 2 study (NCT02013128) combining ublituximab with ibrutinib was carried out in relapsed or refractory CLL and obtained an overall response rate (ORR) of 88%. Of note, in high-risk patients bearing del17p, del11q, or TP53 mutations, the ORR was 95%.27 A phase 3 trial (GENUINE, NCT02301156) of ublituximab plus ibrutinib in high-risk relapsed or refractory CLL reported an ORR of 78% for the combination arm vs 45% for the monotherapy arm.28 The combination of ublituximab and umbralisib with/without ibrutinib had indicated tolerability and activity in patients with relapsed or refractory B-NHLs and CLL in a phase 1 study (NCT02006485).29,30
Other humanized type I anti-CD20 mAbs, such as veltuzumab (IMMU-106) and ocrelizumab (PRO70769), also showed efficacy in patients with relapsed or refractory B-NHLs and FL in phase 1/2 studies (NCT00285428 and NCT02723071).31,32 In addition, progress has been made in the study of biosimilars of rituximab. CT-P10 (CELLTRION) was the first mAb biosimilar anticancer drug to gain international regulatory approval following the results of phase 3 trials (NCT02260804 and NCT02162771) in FL.33,34 Other examples of rituximab biosimilars include GP2013, PF-05280586, and ABP798. GP2013 has also been approved in the European Union for its efficacy data from a phase 3 trial in FL (ASSIST-FL, NCT01419665).35 The phase 3 study (NCT02213263) of PF-05280586 displayed positive results as well.36 Moreover, ABP798 is currently under study (NCT02747043).
Radioimmunotherapy (RIT) has also emerged as an important therapeutic strategy for B-NHLs. Ibritumomab tiuxetan (IDEC-Y2B8, Zevalin®) is a radiolabeled anti-CD20 mAb that targets the same epitope on the CD20 molecule as rituximab. This compound chelates the radioactive particle yttrium-90 (90Y), which delivers high beta energy to improve its ability to kill bulky, poorly vascularized tumors.37 Ibritumomab tiuxetan is effective in both rituximab-naïve and rituximab-resistant FL, as well as in transformed B-NHLs.38,39 Consequently, ibritumomab tiuxetan acquired FDA approval for rituximab-naïve relapsed or refractory low-grade B-NHLs and transformed NHLs. The long-term toxicity of developing myelodysplastic syndrome and acute myelogenous leukemia was observed.40 Furthermore, ibritumomab tiuxetan has shown promising results in the first-line treatment of untreated FL (NCT00772655 and NCT01493479).41,42 In addition, a phase 3 trial (FIT, NCT00185393) observed an improvement of efficacy through ibritumomab tiuxetan consolidation;43,44 thus, the FDA approved this agent for consolidation therapy in untreated FL patients who achieve partial response (PR) or complete response (CR) after first-line chemotherapy. A phase 3 study of rituximab with or without ibritumomab tiuxetan in untreated FL is ongoing (NCT02320292). Ibritumomab tiuxetan is also being evaluated as consolidation therapy in relapsed or refractory FL in a phase 3 study (NCT01827605). Additionally, ibritumomab tiuxetan combined with high-dose chemotherapy prior to autologous stem cell transplantation (ASCT) has also been proven to be safe with relative efficacy.45,46
CD22
CD22 is a single-spanning membrane glycoprotein with a molecular weight of 140,000 located on the surface of B-cells. It is mostly expressed in mature B-cells and many malignant B-cells.47,48 CD22 acts as a negative regulator of B-cell receptor (BCR)-induced signaling and plays a critical role in B-cell activation.47,49 The inhibitory function of CD22 and its restricted expression on B-cells make CD22 an ideal target in NHLs.
Epratuzumab is a humanized IgG1 mAb targeting CD22. The crosslinking of CD22 by epratuzumab triggers BCR signaling and caspase-dependent apoptosis in human lymphoma cells.50 Preclinical studies demonstrated that CD22 mAbs had independent lymphomacidal properties.51 Single-agent epratuzumab has been investigated in both indolent and aggressive NHLs. In an early phase 1/2 trial including 55 patients with recurrent NHLs, epratuzumab showed a response in FL (ORR 24%), while no response was observed in other indolent lymphomas.52 In another concurrent phase 1/2 trial, 15% of patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) responded to epratuzumab.53 The combination of epratuzumab with rituximab has been tested in a multicenter phase 2 trial and exhibited an ORR of 54% in FL and 57% in small lymphocytic lymphoma (SLL).54 Epratuzumab plus rituximab was also studied in untreated FL and obtained an ORR of 88.2% (NCT00553501).55 In aggressive lymphomas, a phase 2 trial (NCT00301821) showed that epratuzumab combined with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) achieved an ORR of 96% in DLBCL, with 3-year event-free survival (EFS) and OS rates of 70% and 80%, respectively.56
Conjugate antibodies utilize the direct conjugation of mAbs with cytotoxic agents, and there are two types of antibody-based conjugates: antibody-drug conjugates (ADCs) and immunotoxins.57 ADCs are mAbs connected to bioactive drugs by chemical linkers. Inotuzumab ozogamicin (InO, CMC-544) is a CD22-targeted ADC combining a humanized IgG4 anti-CD22 mAb with calicheamicin, an enediyne antibiotic, which causes DNA damage and cell apoptosis.58,59 The combination of InO with rituximab in a phase 1/2 study (NCT00299494) of relapsed FL, DLBCL, and refractory aggressive NHL induced ORRs of 87%, 74%, and 20%, respectively. The most common grade 3–4 AEs were thrombocytopenia (31%) and neutropenia (22%).60 However, InO plus rituximab failed to obtain positive results in a randomized phase 3 trial (NCT01232556) of relapsed or refractory CD22+ aggressive B-NHLs and FLs.61 A phase 2 trial (NCT01679119) of InO plus rituximab, cyclophosphamide, vincristine, and prednisolone (R-CVP) in chemotherapy-naïve DLBCL not suitable for anthracycline-based treatment is ongoing. An immunotoxin is a genetically engineered protein consisting of a targeting portion linked to a toxin. Moxetumomab pasudotox connects anti-CD22 to PE38, a fragment of Pseudomonas exotoxin A, and induces apoptosis through the inhibition of protein synthesis.62,63 A phase 1 study (NCT00462189) demonstrated an ORR of 86% in hairy cell leukemia (HCL) patients with no dose-limiting toxicity.64 Moreover, a pivotal phase 3 study (NCT01829711) for relapsed or refractory HCL obtained an ORR of 75%, with a CR rate of 41%.65 The FDA approved moxetumomab pasudotox (Lumoxiti) for the treatment of adult patients with relapsed or refractory HCL.
CD30
CD30 is a 120-kDa type I transmembrane receptor of the tumor necrosis factor receptor (TNFR) superfamily.66 The binding of CD30 with its ligand induces signal transduction through several downstream pathways, especially nuclear factor-κB (NF-κB).67 CD30 is normally expressed on activated B cells, T cells, and NK cells, as well as virally infected lymphocytes. In addition, CD30 is universally expressed in HL and anaplastic large cell lymphoma (ALCL).68,69 Other lymphoproliferative disorders, such as DLBCL, primary mediastinal B-cell lymphoma (PMBCL), peripheral T-cell lymphoma (PTCL), mycosis fungoides (MF), Sézary syndrome (SS) and adult T-cell leukemia/lymphoma (ATLL), can also express CD30 to various degrees.70–72
A chimeric mAb SGN-30, consisting of the variable region of an anti-CD30 murine mAb with human gamma 1 heavy chain and kappa light chain constant regions, promotes growth arrest and DNA fragmentation in vitro and exhibits antitumor activity in HL models.73 In a phase 2 study of relapsed or refractory HL or ALCL, SGN-30 showed only a modest effect in ALCL (2 CR and 5 PR in 41 ALCL patients).74 However, another phase 2 trial used a combination of SGN-30 with gemcitabine, vinorelbine, and liposomal doxorubicin in relapsed HL and showed an ORR of 65%, while grades 3–5 pneumonitis occurred in five patients, leading to the premature closure of the trial.75
Brentuximab vedotin (BV, Adcetris), a CD30 ADC, connects an anti-CD30 antibody with the anti-mitotic agent monomethyl auristatin E (MMAE) via a valine-citrulline peptide-linker. It showed strong activity against CD30+ tumor cell lines in vitro, as well as xenograft models of HL and ALCL.76 A phase 1 dose-escalation study (NCT00430846) of BV in 45 patients with relapsed or refractory CD30+ hematological malignancies (mainly HL) determined the optimal dose of BV as 1.8 mg/m2 intravenously every 3 weeks and showed an ORR of 38%.2 Common AEs of BV include fatigue, pyrexia, diarrhea, nausea, peripheral neuropathy, neutropenia, anemia, and arthralgias.2 Other AEs, such as anaphylaxis and acute pancreatitis, have also been reported.77,78 BV was granted FDA accelerated approval for the treatment of relapsed or refractory HL and ALCL based on the results of two phase 2 studies. NCT00848926 enrolled 102 relapsed or refractory HL patients and obtained an ORR of 75% (CR 34%) with a median duration of response (DoR) of 6.7 months.79 NCT00866047 showed an ORR of 86% (CR 57%) with a median DoR of 12.6 months in 58 patients with relapsed or refractory CD30+ ALCL.80 After approval, the FDA issued a boxed warning related to the risk of progressive multifocal leukoencephalopathy and added a contraindication warning for the concomitant use of BV and bleomycin due to pulmonary toxicity.
In addition to ALCL, BV has shown efficacy as a single agent in other T-NHLs (NCT01421667).81 In addition to systemic lymphomas, BV was also utilized in primary CD30+ cutaneous lymphomas and showed encouraging efficacy.82 A phase 3 randomized multicenter trial (ALCANZA, NCT01578499) was conducted to evaluate single-agent BV vs a control arm of the investigator’s choice of standard therapies in patients with CD30+ primary cutaneous ALCL or MF. ALCANZA demonstrated an improvement in ORR (ORR: 56.3% in the BV arm vs. 12.5% in the conventional therapy arm),83 leading to FDA approval for the treatment of adult patients with primary cutaneous ALCL or CD30+ MF.
For BV combined with chemotherapy, in a multicenter phase 3 trial (NCT01712490) involving patients with untreated stage III or IV HL, patients were randomized to receive BV, doxorubicin, vinblastine, and dacarbazine (A+AVD) or doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD). The results showed that at a median follow-up of 24.6 months, the 2-year modified PFS rates in the A+AVD and ABVD groups were 82.1% and 77.2%, respectively. Neutropenia and peripheral neuropathy were the most common AEs.84 Based on these promising clinical data, the FDA expanded the approval of BV for the first-line treatment of stage III or IV HL in combination with chemotherapy. A phase 1 study (NCT01309789) combining BV with cyclophosphamide, doxorubicin, and prednisolone in patients with CD30+ PTCL resulted in an objective response in all patients (CR 88%).85 Moreover, the five-year follow-up demonstrated durable remission in half of the patients after combination therapy.86 Therefore, a randomized phase 3 trial (ECHELON-2, NCT01777152) comparing BV plus cyclophosphamide, doxorubicin and prednisone (CHP) with CHOP was conducted in untreated patients and demonstrated a significant improvement in PFS and OS with a manageable safety profile when using BV plus CHP.87 The FDA thus approved BV in combination with chemotherapy for adults with untreated ALCL or other CD30+ PTCL.
CD52
The CD52 antigen is a small glycopeptide highly expressed on normal and malignant B and T lymphocytes. The exact function of CD52 remains undefined, but in vitro studies have proven that it is a costimulatory molecule for the induction of CD4+ regulatory T-cells.88
Alemtuzumab (Campath®) is a humanized mAb targeting CD52 that can induce complement-mediated lysis as well as caspase-independent cell death in malignant lymphoid cells.89,90 Single-agent alemtuzumab received accelerated approval by the FDA for CLL patients who had received alkylating agents and failed fludarabine therapy.91 A phase 3 randomized trial comparing alemtuzumab to chlorambucil as first-line treatment showed significantly improved PFS, time to alternative treatment, ORR and CR, with manageable toxicity in CLL.92 Alemtuzumab has also been evaluated as monotherapy in T-NHLs and exhibited efficacy in advanced MF, Sézary syndrome (SS), and relapsed or refractory PTCL,93,94 where hematological toxicity and cytomegalovirus (CMV) reactivation were the most common AEs.
Alemtuzumab-containing chemoimmunotherapy regimens can be effective but have been limited by their toxicities in CLL (NCT00564512).95 The bendamustine and subcutaneous alemtuzumab combination was proven to be as effective as the combination of fludarabine, cyclophosphamide, and cladribine and was safe in heavily pretreated and elderly patients.96 Other attempts at combining pentostatin, alemtuzumab, and low-dose rituximab (NCT00669318) also yielded efficacy and tolerability in relapsed or refractory 17p13-deleted CLL.97 The combination of alemtuzumab and CHOP-based chemotherapy was explored in untreated PTCL.98–100 Phase 3 randomized studies (NCT00646854 and NCT00725231) of alemtuzumab plus CHOP in either young or elderly PTCL patients achieved improved PFS or OS.101,102
CD79
CD79, composed of CD79A and CD79B components, is a main BCR signaling component and is expressed almost exclusively on B-cells and B-NHLs. CD79 expression precedes immunoglobulin heavy-chain gene rearrangement and CD20 expression during B-cell development but disappears in the late stage of B-cell differentiation.103 When BCR is cross-linked, CD79 is targeted to a lysosome-like compartment104 and induces cell apoptosis or triggers cell activation and division with rescue signals from T cells.105 Therefore, CD79 has become an attractive target for the use of ADCs, and preclinical studies found two stable-linker ADCs capable of killing NHL cell lines in vitro and in xenograft models.106
Polatuzumab vedotin (DCDS4501A) is an anti-CD79B mAb conjugated to MMAE. In a phase 1 study (NCT01290549) in relapsed or refractory B-NHLs and CLL, no objective response was observed in CLL, while at the recommended phase 2 dose of 2.4 mg/kg, objective responses were obtained in 23 of 42 patients with NHLs by polatuzumab vedotin monotherapy (56% in patients with DLBCL, 47% with indolent NHLs, and 100% with mantle cell lymphoma (MCL)) and in 7 of 9 patients by polatuzumab vedotin plus rituximab.106 Polatuzumab vedotin was further evaluated in a phase 2 trial (NCT01691898) in combination with rituximab in patients with relapsed or refractory NHLs. The results showed that the ORRs and CR rates were 54% and 21% in DLBCL and 70% and 45% in FL, respectively. Grade ≥3 AEs occurred in 77% of DLBCL patients and 50% of FL patients, mainly as neutropenia, anemia, and diarrhea.107 Furthermore, the findings of a phase 2 study (NCT02257567) pointed out that adding polatuzumab vedotin to bendamustine and rituximab (BR) treatment improved survival in patients with relapsed or refractory DLBCL.108 The combination of polatuzumab vedotin with rituximab, cyclophosphamide, doxorubicin and prednisone (R-CHP) vs R-CHOP in DLBCL is currently being investigated in a phase 3 study (POLARIX, NCT03274492).
CD19
CD19 is a B-cell-specific member of the immunoglobulin superfamily that augments signals by the pre-BCR/BCR and modulates B-cell fate decisions at multiple stages of development.109 CD19 is highly expressed in nearly all B-NHLs, making it an excellent target for immune-based therapies.110
Inebilizumab (MEDI-551) is a CD19-targeted humanized mAb that has potent ADCC activity in vitro and in vivo in preclinical studies.111 Inebilizumab monotherapy has been evaluated in phase 1 studies and showed acceptable toxicity and promising efficacy in patients with relapsed or refractory FL and DLBCL (NCT01957579).112 A phase 1/2 trial (NCT00983619) of inebilizumab alone and in combination with rituximab in FL, CLL, and DLBCL has recently been completed. Regarding inebilizumab in combination with chemotherapy, recent clinical trials did not yield promising results. A phase 2 trial (NCT01466153) comparing inebilizumab plus bendamustine and BR did not find any significant difference in the ORR between the two groups. Another randomized phase 2 study (NCT01453205) on rituximab plus ifosfamide, carboplatin, and etoposide (ICE)/dexamethasone, high-dose cytarabine, and cisplatin (DHAP) vs inebilizumab plus ICE/DHAP in patients with relapsed or refractory DLBCL did not show any significant difference in ORR, PFS, or OS.
Tafasitamab (MOR208, XmAb®5574) is a novel Fc-engineered, humanized, anti-CD19 antibody with enhanced ADCC, antibody-dependent cellular phagocytosis and apoptosis, as well as more potent antitumor activity in vivo than its IgG1 analog.113 These effects were achieved by increasing the affinity for FcγRIIIa on effector cells through the introduction of S239D and I332E amino acid substitutions to the Fc domain. Tafasitamab monotherapy exhibited promising clinical activity in patients with relapsed or refractory B-NHLs with a favorable safety profile. The ORRs were 26%, 29%, and 27% in DLBCL, FL, and other indolent NHLs, respectively, with 9% of patients experiencing grade 3–4 neutropenia (NCT01685008).114 Furthermore, combinations with lenalidomide and bendamustine are being evaluated in recent phase 2/3 clinical trials (NCT02399085, NCT02005289, and NCT02763319). Based on the preliminary data from a phase 2 study (L-MIND, NCT02399085) in combination with lenalidomide, this mAb was granted FDA breakthrough therapy and fast track designations for DLBCL. Eighty-one patients enrolled in the L-MIND study obtained an ORR of 58%, including 33% CR, with no unexpected toxicities observed. With a median follow-up of 12 months, the median PFS was 16.2 months.115
In addition, the CD19-targeted ADC coltuximab ravtansine (SAR3419) consists of a cytotoxic maytansinoid, DM4, which is a potent inhibitor of tubulin polymerization and microtubule assembly. In a phase 2 study (NCT01472887), this agent showed good tolerance and moderate clinical responses in pretreated patients with relapsed or refractory DLBCL (ORR 43.9%).116 A novel ADC based on coltuximab ravtansine showed promising preclinical data and may become an attractive candidate for clinical investigation.117
Loncastuximab tesirine (ADCT-402) is a novel CD19-targeted ADC that delivers SG3199, a highly cytotoxic pyrrolobenzodiazepine dimer, and showed highly targeted cytotoxicity in vitro and antitumor activity in vivo in preclinical studies.118 A pivotal phase 2 study (NCT03589469) is currently ongoing on relapsed or refractory DLBCL, as well as phase 1 studies (NCT02669017, NCT03684694, and NCT03685344) on relapsed or refractory B-NHLs.
CD37
CD37 is a heavily glycosylated transmembrane protein of the tetraspanin superfamily and represents one of the specific proteins for normal and malignant mature B-cells. The expression of CD37 is detected in CLL, Burkitt lymphoma (BL), MCL, and FL,119,120 and it is involved in various biological processes, such as cell adhesion, proliferation, differentiation, intercellular communication via exosomes and immune response.121
Small modular immunopharmaceuticals (SMIPs) are disulfide-linked single-chain proteins comprised of one antigen-binding region (VH/VL), a hinge, and an Fc domain of the human IgG1 region (CH2-CH3). Due to their smaller size, SMIPs may have better tissue penetration than mAbs. SMIP-016 is a homodimeric protein specially engineered to exhibit the full binding activity of an anti-CD37 antibody. Preclinical studies have demonstrated that SMIP-016 can induce apoptosis and ADCC in B-cell leukemia/lymphoma cell lines and primary CLL cells.122
Otlertuzumab (TRU-016) is a humanized variant of SMIP-016 built on the ADAPTIR (modular protein technology) platform. In a phase 1 study (NCT00614042), otlertuzumab was well tolerated and exhibited modest activity as monotherapy in CLL and select subtypes of relapsed or refractory NHLs. The ORR was 23% in CLL, with the most frequent grade ≥3 AEs being thrombocytopenia, neutropenia, anemia, fatigue, and hypophosphatemia.123 For patients with relapsed or refractory FL, MCL, and Waldenström’s macroglobulinemia (WM), a lymph node reduction of 50% or more was observed in 3 of 12 patients.124 The efficacy of this agent can be enhanced in combination with chemotherapy. A randomized phase 2 trial (NCT01188681) showed a significantly increased response rate and prolonged PFS of otlertuzumab in combination with bendamustine over single-agent bendamustine in relapsed CLL. The ORR of this combination therapy was 69%, with a median PFS of 15.9 months.125 Similarly, a phase 1 study (NCT01317901) combining otlertuzumab with BR in relapsed or refractory B-NHLs showed promising activity with no unexpected toxicity. The ORR was 83% (CR 32%).126
Anti-CD37 ADCs such as IMGN529 and AGS67E were also studied. IMGN529 couples an anti-CD37 antibody with the maytansine-derived anti-microtubule agent, DM1. IMGN529 has exhibited potent antitumor activity in preclinical models of CD37+ NHLs.127,128 A phase 1 trial (NCT01534715) of IMGN529 in relapsed or refractory NHLs and CLL has recently been reported, showing manageable safety profiles and preliminary evidence of activity, particularly in DLBCL.129 AGS67E is a fully human monoclonal IgG2 antibody conjugated via a protease-cleavable linker to MMAE. AGS67E has shown remarkable preclinical antitumor effects in NHLs and CLL cell lines and patient-derived xenograft models.130 Clinically, a phase 1 study (NCT02175433) of escalating doses of AGS67E as monotherapy in relapsed or refractory lymphoid malignancies is ongoing.
177Lu-lilotomab satetraxetan (177Lu-DOTA-HH1, Betalutin®) is a novel antibody radionuclide conjugate (ARC) targeting the CD37 antigen. This agent received fast channel assignment from the FDA based on the preliminary data of efficacy and safety in a phase 1/2 trial (LYMRIT 37-01, NCT01796171) in relapsed or refractory FL. It is currently in a pivotal phase 2 trial (PARADIGME) in third-line rituximab-resistant FL, while also being investigated as a single agent in a phase 1 study (NCT02658968) in relapsed or refractory DLBCL and in combination with rituximab in a phase 1 study (NCT03806179) in second-line FL treatment.
C-C chemokine receptor type 4
C-C chemokine receptor type 4 (CCR4) is a seven-transmembrane G-protein-coupled receptor principally expressed on Th2 cells and CD4+ regulatory T cells,131,132 as well as in various types of PTCLs, including MF and ATLL.133,134 Furthermore, CCR4 expression was found to be an independent and significant unfavorable prognostic factor in these diseases,133,134 which makes it a promising target in the treatment of PTCL and ATLL.
Mogamulizumab (KW-0761, Poteligeo) is the first defucosylated humanized mAb directed against CCR4; it has been proven to induce ADCC against CCR4+ malignant T cells135 and to reduce CCR4+ Treg cell numbers in cutaneous T-cell lymphoma (CTCL).136,137 Mogamulizumab was first approved for relapsed or refractory ATLL due to its promising efficacy (ORR 50%) and acceptable toxicities in a phase 2 study (NCT00920790).138 In a randomized phase 2 study (NCT01173887) of dose-intensified chemotherapy with or without mogamulizumab in untreated aggressive ATLL, the mogamulizumab-containing arm showed a higher CR rate with manageable toxicities.139 In addition to its application in ATLL, the efficacy of mogamulizumab in CTCL has also been confirmed. A phase 1/2 study (NCT00888927) of mogamulizumab was performed on 41 pretreated patients with CTCL and resulted in an ORR of 36.8% (47.1% in SS and 28.6% in MF). The most common AEs were nausea, chills, and infusion-related reactions.140 A multicenter phase 2 study (NCT01192984) of relapsed CCR4+ PTCL and CTCL patients in Japan obtained an ORR of 35% and a median PFS of 3 months. Lymphocytopenia, leukocytopenia, and neutropenia (19%) were the most common grade 3-4 AEs.141 Therefore, mogamulizumab was first approved for untreated ATLL as well as relapsed or refractory PTCL in Japan.
The final results of a phase 3, randomized, multicenter clinical trial of mogamulizumab vs vorinostat in previously treated CTCL (MAVORIC, NCT01728805) have been reported.142 The study included 372 patients and was the largest randomized trial in CTCL. Mogamulizumab resulted in a longer PFS than vorinostat (median 7.7 months vs. 3.1 months). The most common AEs of mogamulizumab were pyrexia and cellulitis. Mogamulizumab was granted approval in the European Union and the United States for the treatment of adult patients with relapsed or refractory MF or SS after at least one prior systemic therapy.143
Other surface antigens
CD25
CD25 (IL2R-α) is expressed on both HL and various NHLs and has been studied as a therapeutic target for over two decades. Denileukin diftitox (DD, ONTAK), a diphtheria exotoxin conjugated to an IL-2 fragment, was granted full FDA approval for the treatment of CTCL.144 Although the efficacy of the anti-CD25 antibodies basiliximab and daclizumab is limited, radiolabeled antibodies are promising. 90Y-daclizumab achieved responses in 50% of patients with relapsed HL (NCT00001575).145 90Y-basiliximab is being evaluated in combination with carmustine, etoposide, cytarabine, melphalan (BEAM) chemotherapy for ASCT in relapsed or refractory HL (NCT01476839), as well as T-NHLs (NCT02342782). Camidanlumab tesirine (ADCT-301), a CD25 ADC, has been investigated in a phase 1 trial (NCT02432235) in patients with CD25+ relapsed or refractory HL and NHLs.
CD38
The CD38 antigen is a type II transmembrane glycoprotein with receptor and enzyme functions that is expressed in a number of hematological malignancies, particularly in multiple myeloma (MM).146 In addition, its expression has also been reported in lymphomas such as MCL147 and NK/T-cell lymphoma (NKTCL).148 Daratumumab is a CD38 mAb approved for treating relapsed or refractory and untreated MM. In a phase 2 study (NCT02927925) of daratumumab in relapsed or refractory NKTCL, the ORR was 35.7% in 16 patients.149
CD40
CD40 is a type-I transmembrane protein that belongs to the TNFR family. CD40 is expressed on B cells, monocytes, dendritic cells, endothelial cells and epithelial cells and plays a critical role in the regulation of immune responses.150 In addition, CD40 is expressed on B-NHLs, leading to the modulation of tumor cell growth after binding with its natural ligand (CD40L).151 Dacetuzumab (SGN-40) is a humanized IgG1 mAb targeting CD40. Although dacetuzumab has previously demonstrated anti-lymphoma activity in a phase 1 study (NCT00103779),152 single-agent dacetuzumab showed only modest activity in patients with relapsed DLBCL (NCT00435916)153 and failed to obtain higher CR rates when combined with rituximab plus ICE (R-ICE) in relapsed DLBCL in a phase 2 study (NCT00529503).154
CD74
The humanized antibody milatuzumab (hLL1) is a mAb against CD74, which is involved in malignant B-cell proliferation and survival. Preclinical studies found that milatuzumab had promising antitumor activity in NHL in vitro and in tumor xenograft models.155 Moreover, a phase 1/2 study (NCT00989586) delivered the anti-CD20 mAb veltuzumab (200 mg/m2 weekly) and escalating doses of milatuzumab to relapsed or refractory B-NHL patients and reported an ORR of 24% and a median DoR of 12 months.156 Another preclinical study of the novel bispecific hexavalent Abs (HexAbs) veltuzumab and milatuzumab demonstrated enhanced antitumor activity in cell lines or primary patient samples of MCL and other CD20+/CD74+ malignancies.157
CD80
CD80 (B7-1), a cell-surface receptor, is implicated in the costimulation of T-cell function and expressed on B-NHLs. The anti-CD80 mAb galiximab (IDEC-114) can inhibit tumor cells of B-NHLs in vitro and in mouse models, either alone or combined with chemotherapy (fludarabine or doxorubicin).158 A phase 2 study (NCT00516217) evaluated galiximab in relapsed or refractory HL and reported an ORR of 10.3%. Moreover, a phase 1/2 study on galiximab in relapsed or refractory FL revealed an ORR of 11% (CR 6%).159 Another phase 1/2 trial (NCT00048555) of galiximab and rituximab reported an ORR of 66% (CR 19% and unconfirmed complete remission (CRu) 14%) in relapsed or refractory FL with rituximab-refractory patients excluded.160
CD158k
CD158k (KIR3DL2) is a member of the highly polymorphic family of killer-cell immunoglobulin-like receptors (KIRs) and is expressed on NK cells and a small proportion of CD8+ T cells, as well as CD4+ T cells in CTCL.161–163 The anti-CD158k mAb IPH4102 has been found to be potent and safe in preclinical studies.164 A phase 1 study (NCT02593045) demonstrated efficacy and safety in CTCL,165 with the expansion study ongoing. In addition, a phase 2 study (NCT03902184) of IPH4102 alone or in combination with chemotherapy is recruiting patients with advanced T-NHLs.
Bispecific T cell Engagers
Bispecific T cell Engagers (BiTEs) are engineered bispecific anti-CD3 antibodies consisting of the variable domains of two antibodies linked in a single chain. A BiTE antibody binds both CD3+ cytotoxic T cells and a target antigen to bring the two cells into proximity and thus triggers T cells to kill tumor cells via perforin-mediated apoptosis.166 Blinatumomab is a CD19/CD3 BiTE that shows remarkable anti-lymphoma activity both in vitro and in vivo.167,168 In a phase 1 dose-escalation study (NCT00274742) in patients with relapsed or refractory NHLs, 60 μg/m2/day was established as the maximum tolerated dose, with 22% of patients experiencing grade 3 neurologic events. For patients treated at 60 μg/m2/day, the ORR was 69% (DLBCL, 55%; MCL, 71%; FL, 80%), with a median DoR of 404 days.169 In another phase 2 study (NCT01741792) in patients with relapsed or refractory DLBCL comparing weekly step-up dosing with flat dosing, the ORR was 43%. However, neurological AEs are also common.170 A later phase 2 trial (NCT02910063) of blinatumomab in aggressive B-NHLs is ongoing.
In addition, trials on anti-CD20/CD3 bispecific antibodies, including mosunetuzumab (BTCT4465A, NCT03677154, NCT03671018 and NCT03677141), RO7082859 (NCT03075696, NCT03533283 and NCT03467373), REGN1979 (NCT03888105, NCT02290951, and NCT02651662) and XmAb13676 (NCT02924402) are currently ongoing.
In summary, therapies targeting the lymphoma surface antigen have made great progress. In general, mAbs are effective in the treatment of lymphoma, as evidenced by the FDA accelerated approval of many drugs. Moreover, mAbs as monotherapy have fewer adverse reactions and higher tolerance than conventional chemotherapy. However, mAbs also have limitations, such as off-target effects. In the future, more research on the precise mechanisms of the efficacy and resistance of mAbs is needed. The design of future clinical trials should focus on subgroups with specific pathogenic mechanisms. At the same time, attention should also be paid to the timing, duration, and dose optimization of mAbs, either alone or in combination with traditional chemotherapy.
Signaling transduction pathways and targeted therapies
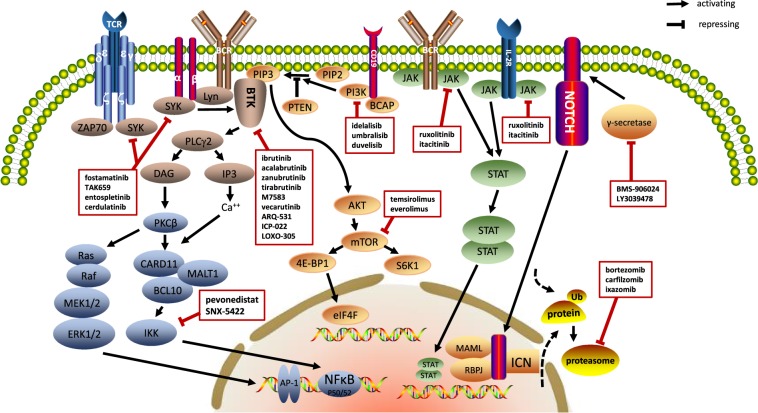
Signaling transduction pathways are critically involved in lymphoma progression. Inhibitors targeting key pathways, including spleen tyrosine kinase (SYK), Bruton’s tyrosine kinase (BTK), phosphoinositide 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR), Janus kinase-signal transducer and activator of transcription (JAK-STAT), NOTCH, NF-κB and ubiquitin-proteasome pathway (UPP), have been applied to treat lymphomas.
SYK
SYK, a nonreceptor tyrosine kinase, plays an important role in BCR and T-cell receptor (TCR) signaling. The phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) in the Igα (CD79A)/Igβ (CD79B) cytoplasm region recruits SYK and induces SYK activation, BTK recruitment, and phospholipase Cγ2 (PLCγ2) activation.171 In TCR signaling, phosphorylated CD3 and ζ subunits of the TCR complex by the Src-related kinases LCK and FYN recruit zeta-chain-associated protein kinase 70 (ZAP-70) and SYK (Fig. 1).172
Fig. 1.
Signaling transduction pathways in lymphoma cells
The activated B cell-like subtype of DLBCL (ABC-DLBCL) is characterized by antigen-driven BCR signaling,173,174 while germinal center B cell-like (GCB)-DLBCL features tonic, antigen-independent BCR signaling.175,176 BL is also characterized by tonic BCR signaling and mostly relies on SYK.177 In T-NHLs, aberrant SYK expression was reported in monomorphic epitheliotropic intestinal T-cell lymphomas (MEITL, type II EATL),178 the follicular variant of PTCL, not otherwise specified (PTCL-NOS), and angioimmunoblastic T-cell lymphoma (AITL) due to t(5;9)(q33;q22) ITK/SYK translocation.179–181
The targeted agents and clinical trials related to SYK and BTK are listed in Table 2. Fostamatinib disodium, the first approved oral SYK inhibitor, was evaluated in a phase 1/2 trial (NCT00446095) of recurrent B-NHLs, showing an ORR of 22% in DLBCL, 10% in FL, and 11% in MCL.182 TAK-659 is being studied in a phase 2 trial in relapsed or refractory DLBCL (NCT03123393) alone, in combination with venetoclax in NHLs in a phase 1 trial (NCT03357627), and in combination with R-CHOP in DLBCL in a phase 1 trial (NCT03742258). The efficacy of entospletinib (GS-9973) is being explored in a phase 2 trial (NCT01799889) in relapsed or refractory hematologic malignancies alone as well as in combination with obinutuzumab in a phase 1/2 trial in NHLs (NCT03010358). Another phase 2 study (NCT01796470) of entospletinib combined with idelalisib in relapsed or refractory NHLs and CLL underwent early termination due to treatment-emergent pneumonitis in 18% of patients.183 Cerdulatinib (PRT-062070), a dual SYK/JAK inhibitor, was reported to have a greater capacity to suppress cell proliferation and induce apoptosis than PRT-060318, an SYK-selective inhibitor, in ATLL-derived cell lines and murine models.184 A phase 1/2 trial (NCT01994382) of cerdulatinib in NHLs and CLL/SLL and a phase 2 trial (NCT04021082) of cerdulatinib in relapsed or refractory PTCL are ongoing.
Table 2.
Targeted drugs and clinical trials related to SYK and BTK
| Drug | Disease | Trial name | Phase | Status | ORR/CR | NCT# | Reference |
|---|---|---|---|---|---|---|---|
| SYK inhibitor | |||||||
| Fostamatinib | A SYK inhibitor | ||||||
| Fostamatinib | Relapsed or refractory B-NHLs | Efficacy and safety study of fostamatinib tablets to treat B-cell lymphoma | 1/2 | Completed | DLBCL, 22%; FL, 10%; MCL, 11% | NCT00446095 | 182 |
| TAK-659 | A SYK inhibitor | ||||||
| TAK-659 | Relapsed or refractory DLBCL | TAK-659 in participants with relapsed or refractory diffuse large B-cell lymphoma | 2 | Active, not recruiting | – | NCT03123393 | – |
| TAK-659, venetoclax | Relapsed or refractory NHL | A study of TAK-659 in combination with venetoclax for adult participants with previously treated non-Hodgkin’s lymphoma | 1 | Active, not recruiting | – | NCT03357627 | – |
| TAK-659, R-CHOP | High-risk DLBCL | Combination chemotherapy and TAK-659 as frontline treatment in treating patients with high-risk diffuse large B-cell lymphoma | 1 | Recruiting | – | NCT03742258 | – |
| Entospletinib | A SYK inhibitor | ||||||
| Entospletinib | Relapsed or refractory hematologic malignancies | Entospletinib in adults with relapsed or refractory hematologic malignancies | 2 | Active, not recruiting | – | NCT01799889 | – |
| Entospletinib, obinutuzumab | Relapsed or refractory CLL/SLL, NHL | Entospletinib and obinutuzumab in treating patients with relapsed chronic lymphocytic leukemia, small lymphocytic lymphoma, or non-Hodgkin’s lymphoma | 1/2 | Recruiting | – | NCT03010358 | – |
| Entospletinib, idelalisib | Relapsed or refractory hematologic malignancies | Entospletinib in combination with idelalisib in adults with relapsed or refractory hematologic malignancies | 2 | Terminated | – | NCT01796470 | 183 |
| Cerdulatinib | A dual SYK/JAK inhibitor | ||||||
| Cerdulatinib | CLL/SLL, NHL | Phase 1/2 dose-escalation study in CLL/SLL or NHL | 1/2 | Recruiting | – | NCT01994382 | – |
| Cerdulatinib | Relapsed or refractory PTCL | CELTIC-1: a phase 2/3 study of cerdulatinib in patients with relapsed or refractory peripheral T-cell lymphoma | 2/3 | Not yet recruiting | – | NCT04021082 | – |
| BTK inhibitor | |||||||
| Ibrutinib | Suppressing BTK enzymatic activity through a irreversible covalent bond with a cysteine residue in the BTK active site | ||||||
| Ibrutinib | Relapsed or refractory B-NHLs | Study of the safety and tolerability of ibrutinib in patients with recurrent B-cell lymphoma | 1/2 | Completed | 60%/16% | NCT00849654 | 193 |
| Ibrutinib | Relapsed or refractory DLBCL | Safety and efficacy study of a Bruton’s tyrosine kinase inhibitor in subjects with relapsed or refractory diffuse large B-cell lymphoma | 1/2 | Completed | ABC-DLBCL, 37%/16%; GCB-DLBCL, 5%/0% | NCT01325701 | 194 |
| Ibrutinib | Relapsed or refractory FL | Ibrutinib in treating patients with relapsed or refractory follicular lymphoma | 2 | Active, not recruiting | 37.5%/12.5% | NCT01849263 | 195 |
| Ibrutinib | Relapsed or refractory MZL | Study of the Bruton’s tyrosine kinase inhibitor in subjects with relapsed or refractory marginal zone lymphoma | 2 | Completed | 48%/3% | NCT01980628 | 196 |
| Ibrutinib | Relapsed or refractory MCL | Safety and efficacy of ibrutinib in participants with relapsed or refractory mantle cell lymphoma | 2 | Completed | 68%/21% | NCT01236391 | 197 |
| Ibrutinib, nivolumab | Relapsed or refractory B-NHLs, CLL/SLL | A study to evaluate safety, pharmacokinetics, pharmacodynamics and preliminary efficacy of the combination of ibrutinib with nivolumab in participants with hematologic malignancies | 1/2 | Active, not recruiting | DLBCL, 36%/16%; FL, 33%/10%; CLL/SLL, 61%/0% | NCT02329847 | 198 |
| Ibrutinib, venetoclax | MCL | Venetoclax plus ibrutinib in mantle cell lymphoma (AIM) | 2 | Completed | 71%/62% | NCT02471391 | 199 |
| Ibrutinib, lenalidomide, rituximab | Untreated and unfit elderly DLBCL | Study evaluating the safety and efficacy of ibrutinib, lenalidomide, and rituximab in untreated and unfit elderly patients with DLBCL | 2 | Recruiting | – | NCT03949062 | – |
| Ibrutinib, lenalidomide, rituximab | Untreated FL | Ibrutinib, lenalidomide, and rituximab in treating patients with previously untreated stage II–IV follicular lymphoma | 1 | Active, not recruiting | 95%/NA | NCT01829568 | 200 |
| Ibrutinib, lenalidomide, rituximab | Relapsed or refractory MCL | A trial of ibrutinib, lenalidomide, and rituximab for patients with relapsed or refractory mantle cell lymphoma (PHILEMON) | 2 | Recruiting | 76%/56% | NCT02460276 | 201 |
| Ibrutinib, R-CHOP | Untreated CD20+ B-NHLs | A study combining ibrutinib with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone in patients With CD20+ B-cell non-Hodgkin’s lymphoma | 1 | Completed | 100%/NA | NCT01569750 | 202 |
| Ibrutinib, R-CHOP vs. placebo, R-CHOP | Untreated non-GCB DLBCL | A study of the Bruton’s tyrosine kinase inhibitor, ibrutinib, in combination with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone in patients with newly diagnosed non-germinal center B-cell subtype of diffuse large B-cell lymphoma | 3 | Active, not recruiting | Ibrutinib, R-CHOP, NA/67.3%; placebo, R-CHOP, NA/68.0% | NCT01855750 | – |
| Ibrutinib, high-dose methotrexate, rituximab | Relapsed or refractory CNSL | Bruton’s tyrosine kinase inhibitor, ibrutinib, in patients with relapsed or refractory primary central nervous system lymphoma and relapsed or refractory secondary central nervous system lymphoma | 1/2 | Active, not recruiting | Phase 1 part: 80%/53% | NCT02315326 | 203 |
| Acalabrutinib | A new, irreversible and second-generation BTK inhibitor with enhanced efficacy and improved off-target effect | ||||||
| Acalabrutinib | Relapsed or refractory MCL | An open-label, phase 2 study of acalabrutinib in subjects with mantle cell lymphoma | 2 | Active, not recruiting | 81%/40% | NCT02213926 | 205 |
| Acalabrutinib | Relapsed CLL | Acalabrutinib, a novel bruton tyrosine kinase inhibitor, for treatment of chronic lymphocytic leukemia | 1/2 | Active, not recruiting | 95%/0% | NCT02029443 | 206 |
| Acalabrutinib vs. ibrutinib | Previously treated high-risk CLL | Study of acalabrutinib versus ibrutinib in previously treated subjects with high-risk CLL | 3 | Active, not recruiting | – | NCT02477696 | – |
| Acalabrutinib, pembrolizumab | Hematologic malignancies | Acalabrutinib in combination with pembrolizumab, for treatment of hematologic malignancies (KEYNOTE145) | 1/2 | Active, not recruiting | – | NCT02362035 | – |
| Acarabrutinib, venetoclax | Relapsed or refractory MCL | Acalabrutinib and venetoclax in treating patients with relapsed or refractory mantle cell lymphoma | 2 | Recruiting | – | NCT03946878 | – |
| Acalabrutinib, BR vs. placebo, BR | Untreated MCL | A study of bendamustine and rituximab alone versus in combination with acalabrutinib in subjects with previously untreated mantle cell lymphoma | 3 | Recruiting | – | NCT02972840 | – |
| Acalabrutinib, R-CHOP | Untreated DLBCL | A combination of acalabrutinib with R-CHOP for patient with diffuse large B-cell lymphoma (ACCEPT) | 1/2 | Recruiting | – | NCT03571308 | – |
| acalabrutinib, R-ICE | relapsed or refractory DLBCL | Acalabrutinib plus R-ICE for relapsed or refractory diffuse large B-cell lymphoma | 2 | Not yet recruiting | – | NCT03736616 | – |
| Zanubrutinib | A second-generation BTK inhibitor showing distinguished kinase selectivity and lower side effect | ||||||
| Zanubrutinib | B-cell lymphoid malignancies | Study of the safety and pharmacokinetics of zanubrutinib in subjects with B-cell lymphoid malignancies | 1 | Active, not recruiting | total, 96.2%/2.6%; treatment-naïve, 100%/4.5%; relapsed or refractory, 94.6%/1.8% | NCT02343120 | 207 |
| Zanubrutinib | Relapsed or refractory non-GCB DLBCL | Study of BTK inhibitor zanubrutinib in subjects with relapsed or refractory non-GCB type diffuse large B-cell lymphoma | 2 | Active, not recruiting | – | NCT03145064 | – |
| Zanubrutinib | Relapsed or refractory MZL | Study of zanubrutinib in patients with marginal zone lymphoma | 2 | Recruiting | – | NCT03846427 | – |
| Zanubrutinib | Relapsed or refractory MCL | Study to evaluate efficacy and safety of zanubrutinib in subjects with relapsed or refractory mantle cell lymphoma | 2 | Active, not recruiting | – | NCT03206970 | – |
| Zanubrutinib vs. ibrutinib | Relapsed or refractory CLL | A study of zanubrutinib versus ibrutinib in patients with relapsed or refractory chronic lymphocytic leukemia (ALPINE) | 3 | Recruiting | – | NCT03734016 | – |
| Zanubrutinib vs. ibrutinib | WM | A study comparing zanubrutinib and ibrutinib in subjects with Waldenström’s macroglobulinemia | 3 | Active, not recruiting | – | NCT03053440 | – |
| Tirabrutinib | A highly selective irreversible BTK inhibitor | ||||||
| Tirabrutinib | relapsed or refractory NHLs/CLL | Phase 1 study of tirabrutinib given as monotherapy in patients with relapsed or refractory NHLs and CLL | 1 | Completed | ABC-DLBCL, 35%/9.7%; MCL, 92%/46%; CLL, 96%/NA | NCT01659255 | 208 |
| M7583 | A novel irreversible BTK inhibitor | ||||||
| M7583 | B-cell malignancies | BTK inhibitor in B-cell malignancies | 1/2 | Active, not recruiting | – | NCT02825836 | – |
| Vecarutinib | A noncovalent or reversible BTK inhibitor | ||||||
| Vecarutinib | B-NHLs | Safety and antitumor activity of vecabrutinib in B-lymphoid cancers | 1/2 | Recruiting | – | NCT03037645 | – |
| ARQ-531 | A reversible BTK inhibitor with off-target activity against Src and Tec family of protein tyrosine kinases | ||||||
| ARQ-531 | Hematologic malignancies | Safety and antitumor activity of ARQ-531 in hematologic malignancies | 1 | Recruiting | – | NCT03162536 | – |
| ICP-022 | A novel BTK inhibitor | ||||||
| ICP-022 | Relapsed or refractory B-cell malignancies | Dose escalation of ICP-022 in patients with relapsed or refractory B-cell malignancies | 1 | Recruiting | – | NCT04014205 | – |
| LOXO-305 | A novel, selective noncovalent or reversible BTK inhibitor | ||||||
| LOXO-305 | CLL/SLL, NHLs | A study of oral LOXO-305 in patients with previously treated CLL/SLL or NHLs | 1/2 | Recruiting | – | NCT03740529 | – |
NA: ORR or CR are not available on the clinicaltrials.gov or from the published article although the trial has been completed
R-CHOP rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone, BR bendamustine and rituximab, R-ICE rituximab, ifosfamide, carboplatin, etoposide
BCR-BTK
The activation of BCR leads to the phosphorylation of LYN and SYK, which phosphorylate tyrosine residues in the cytoplasmic part of CD19 and B-cell adaptor for PI3K (BCAP), inducing PI3K activation, phosphatidylinositol 4,5-bisphosphate (PIP2) transformation to phosphatidylinositol 3,4,5-trisphosphate (PIP3), and BTK recruitment. BTK activation leads to PLCγ2 phosphorylation, which could further hydrolyze PIP2 to produce 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG).185 IP3 is involved in intracellular calcium regulation and nuclear factor of activated T cells (NFAT) transcription, and DAG is associated with protein kinase Cβ (PKCβ) and mitogen-activated protein kinase (MAPK) family activation.186 PKCβ also participates in the NF-κB pathway through a scaffold complex including CARD11, BCL-10, and MALT1. BTK plays a key role in the tonic BCR signaling pathway through the positive regulation of AKT phosphorylation (Fig. 1).187 The inhibition of BTK decreased BTKY223 phosphorylation and anti-apoptotic protein expression (BCL-2, BCL-XL, and MCL-1), resulting in increased apoptosis in MCL cell lines.188 Moreover, recurrent gene mutations of the BCR-BTK signaling pathway are frequently found in ABC-DLBCL, FL, and marginal zone lymphoma (MZL).4,189–192
Ibrutinib is an irreversible BTK inhibitor that suppresses BTK enzymatic activity through a covalent bond with a cysteine residue in the BTK active site. A phase 1/2 study (NCT00849654) of ibrutinib enrolled patients with relapsed or refractory B-NHLs and reported promising safety and response (ORR 60% and CR 16%).193 In a phase 1/2 trial (NCT01325701) of relapsed or refractory DLBCL, ibrutinib induced an ORR of 37% in ABC-DLBCL but only an ORR of 5% in GCB-DLBCL.194 A phase 2 trial (NCT01849263) of ibrutinib in relapsed or refractory FL reported an ORR of 37.5% (CR 12.5%).195 Ibrutinib has also been actively investigated in other relapsed or refractory B-NHLs and has shown clinical efficacy (NCT01980628 and NCT01236391).196,197 A phase 1/2 trial (NCT02329847) of ibrutinib in combination with nivolumab in relapsed or refractory B-cell malignancies revealed an ORR of 36% in DLBCL (CR 16%), 33% in FL (CR 10%), and 61% in CLL/SLL (CR 0%).198 Moreover, a phase 2 study (NCT02471391) of ibrutinib combined with venetoclax in MCL reported an ORR of 71% (CR 62%).199 The combination of ibrutinib, lenalidomide, and rituximab is being explored in a phase 2 trial (NCT03949062) to evaluate its efficacy and safety in untreated and unfit elderly DLBCL patients. This combination also induced an ORR of 95% in untreated FL in a phase 1 trial (NCT01829568), as well as an ORR of 76% (CR 56%) in relapsed or refractory MCL in a phase 2 trial (NCT02460276).200,201 In untreated CD20+ B-NHLs, ibrutinib plus R-CHOP achieved an ORR of 100% in a phase 1 study (NCT01569750).202 In addition, in a phase 3 study (NCT01855750) in untreated non-GCB DLBCL, ibrutinib plus R-CHOP produced a CR rate of 67.3%, and placebo plus R-CHOP produced a CR rate of 68.0%, with no statistically significant difference. Moreover, the sequential combination of ibrutinib with high-dose methotrexate and rituximab was studied in patients with primary central nervous system lymphoma (PCNSL) (NCT02315326).203
Acalabrutinib (ACP-196) is a BTK inhibitor that has been proven to have a more enhanced efficacy than ibrutinib in canine studies.204 A phase 2 study (NCT02213926) reported an ORR of 81% (CR 40%) in relapsed or refractory MCL.205 The FDA has approved acalabrutinib for treating relapsed or refractory MCL. Moreover, in a phase 1/2 trial (NCT02029443) of acalabrutinib in relapsed CLL, the ORR was 95%, and a 100% ORR was obtained among patients with chromosome 17p13.1 deletion.206 A phase 3 trial (NCT02477696) of acalabrutinib vs ibrutinib in high-risk CLL is ongoing. Trials on acalabrutinib in combination with pembrolizumab (NCT02362035), venetoclax (NCT03946878), BR (NCT02972840), R-CHOP (NCT03571308), or R-ICE (NCT03736616) in hematological malignancies are ongoing. Zanubrutinib (BGB-3111) is a second-generation BTK inhibitor that has a promising ORR (96.2%) with low toxicity in CLL/SLL patients in a phase 1 trial (NCT02343120).207 Phase 2 trials of zanubrutinib in relapsed or refractory DLBCL (NCT03145064), MZL (NCT03846427), and MCL (NCT03206970), as well as phase 3 trials (NCT03734016 and NCT03053440) comparing zanubrutinib with ibrutinib in patients with relapsed or refractory CLL or WM, are ongoing. Tirabrutinib (ONO/GS-4059), a highly selective irreversible BTK inhibitor, achieved a response of 35%, 92%, and 96% in relapsed or refractory ABC-DLBCL, MCL, and CLL patients, respectively, in a phase 1 trial (NCT01659255).208 M7583, a novel irreversible BTK inhibitor, is being explored in a phase 1/2 trial (NCT02825836) in patients with relapsed or refractory B-cell malignancies. Vecabrutinib (SNS-062), a noncovalent or reversible BTK inhibitor, suppresses both wild-type and C481S-mutated BTK activity and is being investigated in a phase 1/2 trial (NCT03037645) in B-NHLs. ARQ-531 is another reversible BTK inhibitor with off-target activity against the Src and Tec family of protein tyrosine kinases. Compared with ibrutinib, ARQ-531 has a better capacity to reduce CLL cell viability in mice.209 In addition, a phase 1 trial (NCT03162536) of ARQ-531 in patients with hematological malignancies is ongoing. Trials on ICP-022 and LOXO-305, which are also novel BTK inhibitors, are recruiting patients with refractory B-cell malignancies (NCT04014205 and NCT03740529).
PI3K-AKT-mTOR
The PI3K-AKT-mTOR pathway is an important regulator in normal myeloid and lymphoid development.210 Upon activation, BCAP is upregulated, and the catalytic subunit of PI3K (referred to as p110α, p110β, p110γ, and p110δ for the four different isoforms) triggers PIP3 and recruits the serine/threonine kinase AKT to the plasma membrane.211 AKT can subsequently activate mTOR, which encompasses two different multiprotein complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). mTORC1 phosphorylates 4E-BP1 and S6K1 to activate key drivers of protein translation (Fig. 1).212 Downstream signaling of BCR is largely dependent on p110δ, and mutations in PIK3CA (the gene encoding p110α) were found in approximately 1–8% of DLBCL.213,214 In T-NHLs, p110δ and p110γ are vital kinases of TCR signaling and chemokine receptor signaling, respectively.215–217
The targeted drugs and clinical trials related to the PI3K-AKT-mTOR, JAK-STAT, NOTCH, and NF-κB signaling pathways are listed in Table 3. Idelalisib (CAL-101, GS-1101), a p110δ-selective inhibitor, is the first FDA-approved PI3K inhibitor in treating relapsed FL and SLL. A phase 2 trial (NCT01393106) demonstrated that idelalisib was tolerable and had modest single-agent activity in relapsed or refractory HL (ORR 68% and CR 4%).218 Another phase 2 trial (NCT01282424) of idelalisib treated indolent NHLs including FL, SLL, MZL, and lymphoplasmacytic lymphoma (LPL) with or without WM and showed antitumor activity with an acceptable safety profile (ORR 57% and CR 6%).219 Moreover, combinations of idelalisib with other novel agents may improve the response rate and DoR. Studies of idelalisib in combination with obinutuzumab in relapsed or refractory FL (NCT03890289) and in combination with BR in indolent B-NHLs and MCL (NCT01088048 and NCT01090414) are ongoing. However, two phase 1 trials of idelalisib and lenalidomide in patients with recurrent FL (NCT01644799) and MCL (NCT01838434) showed emerging toxicities as new combinations.220 Umbralisib (TGR-1202) and parsaclisib (INCB050465) are also p110δ inhibitors with different chemical structures.221 A phase 1 trial (NCT02268851) of umbralisib and ibrutinib showed an ORR of 67% (CR 19%) in relapsed or refractory MCL.222 Duvelisib (IPI-145/INK1197), which is an inhibitor of both p110δ and p110γ, showed efficacy in various types of lymphomas, including DLBCL and MCL, in preclinical studies.223,224 A phase 2 trial (NCT01882803) of duvelisib monotherapy in relapsed or refractory indolent NHLs demonstrated an ORR of 46% (41% in FL, 33% in MZL, and 68% in SLL).225
Table 3.
Targeted drugs and clinical trials related to the PI3K-AKT-mTOR, JAK-STAT, NOTCH, and NF-κB signaling pathways
| Drug | Disease | Trial name | Phase | Status | ORR/CR | NCT# | Reference |
|---|---|---|---|---|---|---|---|
| PI3k-AKT-mTOR pathway | |||||||
| Idelalisib | A p110δ-selective inhibitor | ||||||
| Idelalisib | Relapsed or refractory HL | Safety and efficacy of idelalisib in relapsed or refractory Hodgkin’s lymphoma | 2 | Completed | 68%/4% | NCT01393106 | 218 |
| Idelalisib | Idolent B-NHLs | Efficacy and safety study of idelalisib in subjects with indolent B-cell non-Hodgkin’s lymphoma (DELTA) | 2 | Completed | 57%/6% | NCT01282424 | 219 |
| Idelalisib, obinutuzumab | Relapsed or refractory FL | Idelalisib plus obinutuzumab in patients with relapsed or refractory follicular lymphoma (GAUDEALIS) | 2 | Not yet recruiting | – | NCT03890289 | – |
| Idelalisib, BR | Relapsed or refractory indolent B-NHLs/MCL/CLL | Study to investigate idelalisib in combination with chemotherapeutic agents, immunomodulatory agents and anti-CD20 monoclonal antibody in subjects with relapsed or refractory indolent B-cell non-Hodgkin’s lymphoma, mantle cell lymphoma or chronic lymphocytic leukemia | 1 | Completed | 81%/32% | NCT01088048, NCT01090414 | – |
| Idelalisib, lenalidomide | Recurrent FL | Lenalidomide and idelalisib in treating patients with recurrent follicular lymphoma | 1 | Completed | NA | NCT01644799 | 220 |
| Idelalisib, lenalidomide | Relapsed or refractory MCL | Lenalidomide with or without idelalisib in treating patients with relapsed or refractory mantle cell lymphoma | 1 | Completed | NA | NCT01838434 | 220 |
| Umbralisib | A second-generation p110δ-selective inhibitor | ||||||
| Umbralisib, ibrutinib | CLL/MCL | A phase 1 safety and efficacy study of the PI3K-delta Inhibitor umbralisib and ibrutinib in patients with CLL or MCL | 1 | Completed | MCL, 67%/19%; CLL, 90%/29% | NCT02268851 | 222 |
| Duvelisib | A PI3Kδ/γ inhibitor | ||||||
| Duvelisib | Refractory iNHLs | A phase 2 study of duvelisib in subjects with refractory indolent non-Hodgkin’s lymphoma (DYNAMO) | 2 | Active, not recruiting | 46%/NA | NCT01882803 | 225 |
| Temsirolimus | A mTOR inhibitor | ||||||
| Temsirolimus | MCL | Study evaluating temsirolimus in mantle cell lymphoma (OPTIMAL) | 3 | Completed | 38%/3% | NCT00117598 | 226 |
| Temsirolimus vs. ibrutinib | Relapsed or refractory MCL | Study of ibrutinib versus temsirolimus in patients with relapsed or refractory mantle cell lymphoma who have received at least one prior therapy | 3 | Completed | NA | NCT01646021 | 227 |
| Temsirolimus, rituximab, DHAP | Relapsed or refractory DLBCL | Temsirolimus, rituximab, and DHAP for relapsed or refractory diffuse large B-cell lymphoma (STORM) | 2 | Unknown | – | NCT01653067 | 228 |
| Everolimus | An oral mTOR inhibitor | ||||||
| Everolimus, itacitinib | HL | Everolimus plus itacitinib in Hodgkin’s lymphoma | 1/2 | Recruiting | – | NCT03697408 | – |
| Everolimus, panobinostat | Relapsed or refractory lymphoma | Everolimus plus panobinostat in patients with relapsed or refractory lymphoma | 1/2 | Completed | NA | NCT00967044 | – |
| JAK-STAT pathway | |||||||
| Ruxolitinib | A JAK1/2 inhibitor | ||||||
| Ruxolitinib | Relapsed or refractory cHL | A phase 2 study of oral JAK1/JAK2 inhibitor ruxolitinib in adult patients with relapsed or refractory classical Hodgkin’s lymphoma (HIJAK) | 2 | Completed | 9.4%/0% | NCT01877005 | 245 |
| Ruxolitinib, nivolumab | Relapsed or refractory cHL | Nivolumab with ruxolitinib in relapsed or refractory classical Hodgkin’s lymphoma | 1 | Recruiting | – | NCT03681561 | – |
| Itacitinib | A JAK1 selective inhibitor | ||||||
| Itacitinib, everolimus | HL | Itacitinib plus everolimus in Hodgkin’s lymphoma | 1/2 | Recruiting | – | NCT03697408 | – |
| Itacitinib, ibrutinib | Relapsed or refractory DLBCL | A study of itacitinib in combination with ibrutinib in subjects with relapsed or refractory diffuse large B-cell lymphoma | 1/2 | Active, not recruiting | – | NCT02760485 | – |
| NOTCH signaling pathway | |||||||
| BMS-906024 | A γ-secretase inhibitor | ||||||
| BMS-906024, dexamethasone | T-ALL/T-LBL | Study to evaluate the safety and tolerability of weekly intravenous doses of BMS-906024 in subjects with acute T-cell lymphoblastic leukemia or T-cell lymphoblastic lymphoma | 1 | Completed | NA | NCT01363817 | – |
| LY3039478 | A γ-secretase inhibitor | ||||||
| LY3039478, dexamethasone | T-ALL/T-LBL | A study of LY3039478 in combination with dexamethasone in participants with acute T-cell lymphoblastic leukemia or T-cell lymphoblastic lymphoma | 1/2 | Completed | – | NCT02518113 | – |
| CB-103 | A pan-NOTCH inhibitor | ||||||
| CB-103 | Solid tumors/NHLs | Study of CB-103 in adult patients with advanced or metastatic solid tumors and hematological malignancies | 1/2 | Recruiting | – | NCT03422679 | – |
| NF-κB pathway | |||||||
| Pevonedistat | A NEDD8-activating enzyme inhibitor | ||||||
| Pevonedistat, ibrutinib | Relapsed or refractory CLL/NHL | Pevonedistat and ibrutinib in treating participants with relapsed or refractory chronic lymphocytic leukemia or non-Hodgkin’s lymphoma | 1 | Recruiting | – | NCT03479268 | – |
| SNX-5422 | A synthetic, novel, small-molecule HSP90 Inhibitor | ||||||
| SNX-5422 | Solid tumors/lymphomas | Safety study of SNX-5422 to treat solid tumor cancers and lymphomas | 1 | Completed | NA | NCT00647764 | – |
| SNX-5422 | Solid tumors/lymphomas | SNX-5422 to treat solid tumor cancers and lymphoma | 1 | Completed | NA | NCT00644072 | – |
NA: ORR or CR are not available on the clinicaltrials.gov or from the published article, although the trial has been completed
DHAP dexamethasone, high-dose cytarabine, cisplatin, T-ALL acute T-cell lymphoblastic leukemia
Temsirolimus (CCI-779) is a derivative of rapamycin, and a phase 2 trial (NCT00117598) of temsirolimus as a single agent in relapsed MCL showed an ORR of 38% (CR 3%).226 In a randomized phase 3 trial (NCT01646021) enrolling patients with relapsed or refractory MCL, significant improvement in PFS and better tolerance were observed in patients treated with ibrutinib vs temsirolimus.227 Another ongoing study (NCT01653067) is evaluating temsirolimus in combination with DHAP in patients with relapsed or refractory DLBCL.228 Everolimus (RAD001) is an oral mTOR inhibitor that has been used as a single agent in relapsed or refractory aggressive and indolent NHLs as well as HL.229–231 Clinical trials (NCT03697408 and NCT00967044) to assess everolimus combined with other agents, such as itacitinib and panobinostat, are recruiting patients.
JAK-STAT
The JAK-STAT pathway is activated by extracellular cytokines such as interferons, IL-2, IL-6 and growth factors, which regulate cell survival, proliferation, differentiation, and apoptosis.232,233 There are four cytoplasmic JAK kinases: JAK1, JAK2, JAK3, and TYK2. JAK1/JAK3 are prone to immunoregulation, while JAK2 is associated with erythrocyte and platelet formation.234,235 JAKs lead to STAT phosphorylation, homodimerization, and nuclear translocation (Fig. 1).233,236 There are seven STAT proteins (STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6).234,235 The activation of the JAK/STAT signaling pathway, as assessed by STAT3 or STAT5B phosphorylation, was present in T-NHLs, including anaplastic lymphoma kinase (ALK)-positive and ALK-negative ALCL,237,238 HTLV-1-associated ATLL,239,240 and NKTCL.241,242 Twenty percent of ALK-negative ALCL patients present mutations of the JAK1 and/or STAT3 genes,237 and approximately 10% of NKTCL patients present STAT3 mutations.243
Ruxolitinib (INCB018424) is a JAK1/2 inhibitor approved by the FDA to treat myelofibrosis. Ruxolitinib significantly enhanced apoptosis in HL and PMBCL in vitro and promoted survival in a lymphoma xenograft murine model.244 A phase 2 study (NCT01877005) of ruxolitinib in advanced relapsed or refractory HL showed poor efficacy as monotherapy (ORR 9.4% and CR 0%).245 Ruxolitinib and navitoclax, a Bcl-2/Bcl-XL inhibitor, reduced the tumor burden and prolonged survival in an ATLL xenograft murine model.246 A phase 1 study (NCT03681561) of ruxolitinib in combination with nivolumab in relapsed or refractory HL is currently recruiting patients. However, ruxolitinib has off-target effects due to JAK2 inhibition, which may lead to thrombocytopenia, anemia, and neutropenia.247 Therefore, agents that can selectively inhibit JAK1, such as itacitinib (INCB039110), are expected to better treat lymphomas in view of the risk-benefit ratio. A phase 1/2 study (NCT03697408) of itacitinib in combination with everolimus in relapsed or refractory HL is ongoing. In addition, a phase 1/2 trial (NCT02760485) of itacitinib in combination with ibrutinib in subjects with relapsed or refractory DLBCL is also active.
NOTCH
NOTCH receptors are single-pass type I transmembrane proteins. Four receptors (NOTCH1-4) are expressed in mammals and share a common structure. Among them, NOTCH1 and NOTCH2 are the most widely expressed receptors and play a role in cell growth, proliferation, survival, and differentiation.248 NOTCH is cleaved in the transmembrane region by the γ-secretase complex, which can be inhibited by small-molecule γ-secretase inhibitors (GSIs). After release from the membrane, the intracellular portion of the NOTCH receptor translocates to the nucleus, where it interacts with the RBPJ DNA-binding protein and recruits the MAML1 transcriptional coactivator to assemble the transcriptional complex and start transcription. The signal can be terminated by the proteasome (Fig. 1).249 Mutations of NOTCH1 and NOTCH2 have been reported to mediate the differentiation of B- or T-cell lineages.250 In T-cell lymphoblastic lymphoma (T-LBL), NOTCH1 mutations vary from 30% to 80%.251 In DLBCL, NOTCH1 mutations are classified into the N1 subtype, which accounts for 6.1% of ABC DLBCL cases and is associated with poor prognosis.252 Activation of the NOTCH1 pathway was also observed in MCL, HL and BL.253–255 NOTCH2 mutations are present in approximately 25% of patients with splenic marginal zone lymphoma (SMZL) and approximately 5% of patients with non-splenic MZL253 and are related to adverse clinical outcomes.253,256,257 In addition, a similar gene profile has been found in FL.258 In DLBCL, the BN2 subtype is characterized by BCL6 fusions and NOTCH2 mutations and presents a relatively good prognosis.252
For targeted agents of the NOTCH pathway, GSIs, as well as antibodies against NOTCH, Delta/Jagged ligands, or other extracellular components involved in the NOTCH signaling cascade, have been tested in multiple clinical trials.259 GSIs can suppress the release of ICN1 from the membrane and effectively abrogate the activation of NOTCH1 transcriptional programs in cell lines.260 A phase 1 trial (NCT01363817) evaluating the safety and tolerability of BMS-906024 in subjects with T-LBL was completed. Another study showed strong synergy between glucocorticoids and GSIs.261 A phase 1/2 trial (NCT02518113) to evaluate LY3039478 in combination with dexamethasone in T-LBL patients was also completed. However, GSIs demonstrated dose-limiting goblet cell hyperplasia of the gut, mainly due to the inhibition of both NOTCH1 and NOTCH2 expression on these tissues.262 In addition, a phase 1/2 trial (NCT03422679) to investigate the safety, tolerability, and preliminary efficacy of CB-103, a pan-NOTCH inhibitor, is recruiting patients. More research and clinical trials are needed to better understand targeted therapy of the NOTCH pathway.
NF-κB
The NF-κB pathway is one of the key signaling pathways implicated in physiological cellular functions and neoplastic processes.263,264 Core components of the NF-κB pathway are inhibitors of NF-κB (IκB) proteins, the IκB kinase (IKK) complex, and NF-κB transcription factors, which include RelA/p65, RelB, c-Rel/Rel, p50, and p52.265 B-cell associated kinases (BAKs), such as BTK or PI3Kδ, are critical signaling transducers of BCR signaling and can trigger a cascade reaction to form a multiprotein CARD11-BCL-10-MALT1 (CBM) complex.266 This complex interacts with IKK, the upstream molecule of NF-κB, and promotes NF-κB activation (Fig. 1).267–270 The constitutive activation of NF-κB is common in most types of B-NHLs.269 In DLBCL, NF-κB activity is upregulated in PMBCL and ABC-DLBCL but not in GCB-DLBCL.173 BCR-dependent NF-κB activation was the highest in the MCD subtype (based on the cooccurrence of MYD88 L265P and CD79B mutations) and BN2 subtype.252 In CLL, the NF-κB pathway is usually activated through BCR and TLRs.271 For mucosa-associated lymphoid tissue (MALT) lymphomas, intrinsic BCR activation is associated with an advanced stage.
The NF-κB pathway can be inhibited by directly or indirectly targeting NF-κB components. As a direct targeting agent, pevonedistat (TAK-924/MLN4924), a NEDD8-activating enzyme (NAE) inhibitor, suppresses NF-κB activity by blocking phospho-IκBα degradation.272 A phase 1 study (NCT03479268) of relapsed or refractory CLL and NHLs is ongoing. HSP90 is a component of the IKK complex and prevents the proteasomal degradation of IKKα and IKKβ.270 Two phase 1 trials (NCT00647764 and NCT00644072) of the HSP90 inhibitor SNX-5422 in patients with lymphomas were completed.
Proteasome
UPP is a choreographed system that degrades misfolded proteins in all eukaryotic cells. It plays a role in the processes of cell apoptosis, cell-cycle progression, antigen presentation, and DNA repair.273–276 The first step of protein degradation is polyubiquitination, and the proteasome binds the polyubiquitin chain and mediates deubiquitination and then degrades the target proteins to oligopeptides less than 25 amino acids (Fig. 1).277,278 Inhibition of the pro-survival NF-κB pathway is the main antitumor mechanism of proteasome inhibitors in lymphoma.279
The targeted drugs and clinical trials related to the proteasome are listed in Table 4. Currently, three proteasome inhibitors (bortezomib, carfilzomib, and ixazomib) are approved for MM or MCL. Bortezomib, a reversible proteasome inhibitor, binds primarily with β5 and, to a lesser extent, with β2 and β1 of the 20S proteasome particle.280 A phase 2 trial (NCT00063713) of bortezomib in relapsed or refractory MCL reported an ORR of 31% (CR 8%).281 Another phase 2 trial (NCT00901147) of bortezomib and panobinostat showed an ORR of 43% (CR 22%) in relapsed or refractory PTCL patients.282 Bortezomib in combination with other agents, such as ibrutinib in MCL (NCT02356458), dexamethasone in CTCL (NCT03487133), and chemotherapeutic regimens, such as gemcitabine, dexamethasone, and cisplatin (GDP) in DLBCL (NCT02542111) and CHOP in T-NHLs (NCT00374699), are currently ongoing. A randomized phase 3 trial (NCT00722137) compared the efficacy of R-CHOP with bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) in untreated MCL, showing an improved median PFS but increased hematologic toxicity.283 Moreover, a phase 2 trial of bortezomib, low-dose dexamethasone, and rituximab (NCT00981708) presented an ORR of 85% (CR 3%) in untreated WM.284 A phase 3 trial (NCT01788020) conducted in WM patients to evaluate bortezomib in combination with dexamethasone, cyclophosphamide, and rituximab is ongoing. Other proteasome inhibitors, including the irreversible carfilzomib and the reversible oral inhibitor ixazomib, have been studied in a variety of clinical trials. Trials of carfilzomib (NCT01336920) alone or in combination with other agents including vorinostat (NCT01276717), romidepsin (NCT03141203), umbralisib (NCT02867618), rituximab (NCT03269552), BR (NCT02187133), R-CHOP (NCT02073097) and R-ICE (NCT01959698) in relapsed or refractory lymphoma are ongoing. Phase 2 trials of ixazomib showed an ORR of 8.3% (CR 0%) in relapsed or refractory FL (NCT01939899) and an ORR of 67% in relapsed or refractory CTCL/PTCL (NCT02158975). Ixazomib in combination with rituximab (NCT02339922) or with ibrutinib (NCT03323151) is currently under evaluation in indolent B-NHLs and MCL. Phase 1/2 trials of ixazomib combined with romidepsin (NCT03547700) in refractory PTCL and with rituximab and lenalidomide as frontline therapy in high-risk indolent B-NHLs (NCT02898259) are ongoing.
Table 4.
Targeted drugs and clinical trials related to the proteasome
| Drug | Disease | Trial name | Phase | Status | ORR/CR | NCT# | Reference |
|---|---|---|---|---|---|---|---|
| Proteasome inhibitor | |||||||
| Bortezomib | A reversible proteasome inhibitor binding primarily with β5 and to a lesser extent, with β2 and β1 of the 20S proteasome particle | ||||||
| Bortezomib | Relapsed or refractory MCL | Bortezomib in subjects with relapsed or refractory mantle cell lymphoma | 2 | Completed | 31%/8% | NCT00063713 | 281 |
| Bortezomib, panobinostat | Relapsed or refractory PTCL | Study of bortezomib and panobinostat in treating patients with relapsed or refractory peripheral T-cell lymphoma | 2 | Completed | 43%/22% | NCT00901147 | 282 |
| Bortezomib, ibrutinib | MCL | Combination of ibrutinib and bortezomib to treat patients with mantle cell lymphoma | 1/2 | Recruiting | – | NCT02356458 | – |
| Bortezomib, dexamethasone | Relapsed or refractory CTCL | Bortezomib plus dexamethasone therapy in patients with relapsed or refractory cutaneous T-cell lymphoma | 2 | Recruiting | – | NCT03487133 | – |
| Bortezomib, GDP | Non-GCB DLBCL | A study of bortezomib plus GDP in the treatment of relapsed or refractory non-GCB DLBCL | 2 | Unknown | – | NCT02542111 | – |
| Bortezomib, CHOP | Advanced aggressive T-NHLs/NKTCL | Bortezomib and CHOP in patients with advanced-stage aggressive T-cell or NK/T-cell lymphoma | 1/2 | Completed | NA | NCT00374699 | – |
| VR-CAP vs. R-CHOP | Untreated MCL | Study of the combination of rituximab, cyclophosphamide, doxorubicin, bortezomib, and prednisone or rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone in patients with newly diagnosed mantle cell lymphoma | 3 | Completed | NA | NCT00722137 | 283 |
| Bortezomib, dexamethasone, rituximab | Untreated WM | Bortezomib, low-dose dexamethasone, and rituximab in untreated Waldenström’s macroglobulinemia | 2 | Completed | 85%/3% | NCT00981708 | 284 |
| Bortezomib, dexamethasone, rituximab, and cyclophosphamide | WM | Efficacy of First-Line Dexamethasone, Rituximab, and Cyclophosphamide +/− Bortezomib for Patients With Waldenström’s Macroglobulinemia | 3 | Active, not recruiting | – | NCT01788020 | – |
| Carfilzomib | A second-generation irreversible proteasome inhibitor binding to the β5 subunit of the 20S proteasome particle | ||||||
| Carfilzomib | Relapsed or refractory T-NHLs | Carfilzomib in treating patients with relapsed or refractory T-cell lymphoma | 1 | Completed | NA | NCT01336920 | – |
| Carfilzomib, vorinostat | Relapsed or refractory lymphoma | Study of carfilzomib and vorinostat for relapsed or refractory lymphoma | 1 | Completed | NA | NCT01276717 | – |
| Carfilzomib, romidepsin | Relapsed or refractory PTCL | Evaluation of the combination of romidepsin and carfilzomib in relapsed or refractory peripheral T-cell lymphoma patients | 1/2 | Recruiting | – | NCT03141203 | – |
| Carfilzomib, umbralisib | Relapsed or refractory lymphoma | Carfilzomib and umbralisib in treatment of relapsed or refractory lymphoma | 1/2 | Recruiting | – | NCT02867618 | – |
| Carfilzomib, rituximab | WM/MZL | Carfilzomib with or without rituximab in the treatment of Waldenström’s macroglobulinemia or marginal zone lymphoma | 2 | Completed | NA | NCT03269552 | – |
| Carfilzomib, bendamustine, rituximab | Relapsed or refractory NHLs | Carfilzomib with bendamustine and rituximab in patients with relapsed or refractory non-Hodgkin’s lymphoma | 1 | Recruiting | – | NCT02187133 | – |
| Carfilzomib, R-CHOP | DLBCL | Carfilzomib, rituximab, and combination chemotherapy in treating patients with diffuse large B-cell lymphoma | 1/2 | Recruiting | – | NCT02073097 | – |
| Carfilzomib, R-ICE | Relapsed or refractory DLBCL | Carfilzomib, rituximab, ifosfamide, carboplatin, and etoposide in treating patients with relapsed or refractory stage I-IV diffuse large B-cell lymphoma | 1/2 | Recruiting | – | NCT01959698 | – |
| Ixazomib | A reversible proteasome inhibitor binding to the β5 subunit of the 20S proteasome particle | ||||||
| Ixazomib | Relapsed or refractory FL | Phase 2 study of oral ixazomib in adult patients with relapsed or refractory follicular lymphoma | 2 | Completed | PSMB1 positice, 8.3%/0%; PSMB1 negative, 0%/0% | NCT01939899 | – |
| Ixazomib | Relapsed or refractory CTCL/PTCL | Open-label, phase 2 study of ixazomib in patients with relapsed or refractory cutaneous and peripheral T-cell lymphoma | 2 | Completed | 67%/NA | NCT02158975 | – |
| Ixazomib, rituximab | Indolent B-NHLs | Ixazomib and rituximab in treating patients with indolent B-cell non-Hodgkin’s lymphoma | 2 | Recruiting | – | NCT02339922 | – |
| Ixazomib, ibrutinib | Relapsed or refractory MCL | A study of ixazomib and ibrutinib in relapsed or refractory mantle cell lymphoma | 1/2 | Recruiting | – | NCT03323151 | – |
| Ixazomib, romidepsin | Relapsed or refractory PTCL | Study of ixazomib and romidepsin in peripheral T-cell lymphoma | 1/2 | Recruiting | – | NCT03547700 | – |
| lenalidomide, ixazomib, rituximab | High-risk indolent B-NHLs | Lenalidomide, ixazomib, and rituximab as frontline therapy for high-risk indolent B-cell lymphoma | 1/2 | Active, not recruiting | – | NCT02898259 | – |
NA: ORR or CR are not available on the clinicaltrials.gov or from the published article, although the trial has been completed
GDP gemcitabine, dexamethasone, and cisplatin, CHOP cyclophosphamide, doxorubicin, vincristine, prednisolone, VR-CAP bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone, R-CHOP rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone, R-ICE rituximab, ifosfamide, carboplatin, etoposide, PSMB1 proteasome subunit beta type-1
Directly targeting signaling pathways and off-target effects remain a major issue of signaling pathway inhibitors. For example, AEs of ibrutinib, such as atrial fibrillation and bleeding-related events, were connected with the irreversible targeting of ibrutinib on BTK signaling in cardiac myocytes and platelets.285,286 The off-target inhibition of kinases containing an analogous cysteine residue with BTKC481 may also be crucial to the side effects of ibrutinib.287 Moreover, drug resistance reduces the clinical efficacy, warranting further investigation on combined treatment and dual inhibitors. BTKC481S in the ibrutinib binding site is associated with ibrutinib resistance288,289 but can be overcome in combination with venetoclax.290 mTOR inhibitors show limited long-term effectiveness due to feedback PI3K/AKT activation, while dual PI3K/mTOR inhibitors could be better alternatives.
Epigenetic regulation and targeted therapy
Epigenetic regulation mainly includes DNA methylation, histone acetylation and methylation. Histone acetylation and methylation regulate the chromatin state. In the active status, chromatin is accessible to transcription factors, which is represented by the enrichment of H3K27 acetylation and H3K4 methylation. In the repressive status, chromatin is compact and inaccessible to transcription factors, which is characterized by the enrichment of H3K36, H3K27 and H3K9 trimethylation (Fig. 2).291 Epigenetic dysregulation plays an important role in both B- and T-NHLs and represents potential therapeutic targets according to preclinical data and clinical trials.
Fig. 2.
Epigenetic modifications in lymphoma cells
DNA methylation and targeted therapy
DNMT
The main type of DNA methylation observed in mammals is the methylation of CpG dinucleotides.292 DNA methyltransferases (DNMTs) mediate this process and induce transcriptional repression. DNMT1 maintains DNA methylation on hemimethylated CpG sites, whereas DNMT3A and DNMT3B are involved in DNA methylation on unmethylated CpG sites. In vitro, the molecular silencing of DNMT1 decreased the expression of cell-cycle genes, such as CDK1, CCNA2, and E2F2, in GCB-DLBCL-derived cell lines.293 Analysis of DLBCL patients reported the overexpression of DNMT1, DNMT3A, and DNMT3B in 48%, 13%, and 45% of patients, respectively.294 Moreover, DNMT1 loss induced altered methylation levels and impaired tumor cell proliferation in mice with T-NHLs.295 Almost all T-NHL subtypes harbor mutations of DNMT3A.296
The targeted drugs and clinical trials related to epigenetic modifications are listed in Table 5. Azacitidine, a demethylating agent, inhibits DNMTs by incorporating into RNA and DNA through covalent bonding to DNMTs. A phase 1/2 trial (NCT01120834) showed that azacitidine in combination with vorinostat induced an ORR of 6.7% in patients with relapsed or refractory DLBCL. Azacitidine was also studied in combination with R-CHOP in a phase 1/2 trial (NCT01004991) that reported a CR rate of 91.7% in 12 untreated DLBCL patients. In addition, there are some other trials investigating azacitidine plus R-ICE (NCT03450343) or rituximab and GDP (R-GDP) (NCT03719989) in relapsed or refractory DLBCL and azacitidine with CHOP (NCT03542266) in untreated PTCL patients. Decitabine, a DNMT inhibitor, inhibits DNMTs by incorporating into DNA and reversing DNA methylation and transcriptional repression. A phase 1 trial of low-dose decitabine in NHL and CLL reported dose-limiting myelosuppression.297 Decitabine combined with R-CHOP is being studied in a phase 1/2 trial (NCT02951728) of untreated DLBCL patients with International Prognostic Index (IPI) >1. Moreover, there is a recruiting phase 4 trial (NCT03579082) exploring the efficacy and safety of decitabine, rituximab, with/without DHAP in relapsed or refractory DLBCL. A phase 3 randomized trial (NCT03553537) is comparing the efficacy and safety of decitabine plus CHOP (D-CHOP) vs CHOP alone in patients with untreated PTCL.
Table 5.
Targeted drugs and clinical trials related to epigenetic modifications
| Drug | Disease | Trial name | Phase | Status | ORR/CR | NCT# | Reference |
|---|---|---|---|---|---|---|---|
| DNMT inhibitor | |||||||
| Azacitidine | A DNMT inhibitor which is incorporated into RNA and, to a lesser extent, into DNA, and inhibits DNMTs | ||||||
| Azacitidine, vorinostat | Relapsed or refractory DLBCL | Study of azacitidine in combination with vorinostat in patients with relapsed or refractory diffuse large B-cell lymphoma | 1/2 | Completed | 6.7%/NA | NCT01120834 | – |
| Azacitidine, R-CHOP | Untreated DLBCL | Phase 1/2 trial of R-CHOP plus azacytidine in diffuse large B-cell lymphoma | 1/2 | Completed | NA/91.7% | NCT01004991 | – |
| Azacitidine, R-ICE | Relapsed or refractory DLBCL | Oral azacitidine plus salvage chemotherapy in relapsed or refractory diffuse large B-cell lymphoma | 1 | Recruiting | – | NCT03450343 | – |
| Azacitidine, R-GDP | Relapsed or refractory DLBCL | Azacitidine and R-GDP in patients with relapsed or refractory diffuse large B-cell lymphoma (EPIC) | 2 | Not yet recruiting | – | NCT03719989 | – |
| Azacitidine, CHOP | Untreated PTCL | Azacitidine plus CHOP in patients with untreated peripheral T-cell lymphoma | 2 | Recruiting | – | NCT03542266 | – |
| Decitabine | A DNMT inhibitor which is incorporated into DNA and inhibits DNMTs through disrupting the interaction between DNA and DNMTs | ||||||
| Decitabine, R-CHOP | Untreated DLBCL with IPI > 1 | Decitabine plus R-CHOP in diffuse large B-cell lymphoma | 1/2 | Active, not recruiting | – | NCT02951728 | – |
| Decitabine combined with R±DHAP | Relapsed or refractory DLBCL | A clinical trial of decitabine in relapse or refractory diffuse large B-cell lymphoma | 4 | Recruiting | – | NCT03579082 | – |
| Decitabine plus CHOP vs. CHOP | Untreated PTCL | Efficacy and safety of decitabine plus CHOP vs. CHOP in patients with untreated peripheral T-cell lymphoma | 3 | Not yet recruiting | – | NCT03553537 | – |
| IDH2 inhibitor | |||||||
| Enasidenib | An IDH2 inhibitor | ||||||
| Enasidenib | AITL | Study of orally administered enasidenib in subjects with advanced solid tumors, including glioma, and with angioimmunoblastic T-cell lymphoma, with an IDH2 mutation | 1/2 | Completed | NA | NCT02273739 | – |
| EZH2 inhibitor | |||||||
| Tazemetostat | A selective inhibitor of EZH2 | ||||||
| Tazemetostat | Relapsed or refractory B-NHLs | Open-label, multicenter, phase 1/2 study of tazemetostat as a single agent in subjects with advanced solid tumors or with B-cell lymphomas and tazemetostat in combination with prednisolone in subjects with DLBCL | 1/2 | Active, not recruiting | Phase 1 part, 38%/14% | NCT01897571 | 324 |
| SHR2554 | A novel EZH2 inhibitor | ||||||
| SHR2554 | Relapsed or refractory mature lymphoid neoplasms | A phase 1 study of SHR2554 in subjects with relapsed or refractory mature lymphoid neoplasms | 1 | Recruiting | – | NCT03603951 | – |
| PF-06821497 | A novel EZH2 inhibitor | ||||||
| PF-06821497 | Relapsed or refractory FL | PF-06821497 treatment of relapsed or refractory small cell lung cancer, castration resistant prostate cancer, and follicular lymphoma | 1 | Recruiting | – | NCT03460977 | – |
| HDAC inhibitor | |||||||
| Vorinostat | A pan-HDAC inhibitor | ||||||
| Vorinostat | Relapsed or refractory DLBCL | An investigational drug study with vorinostat in relapsed diffuse large B-cell lymphoma (0683-013) | 2 | Completed | 5.6%/5.6% | NCT00097929 | 334 |
| Vorinostat | Relapsed or refractory FL/other subtypes of indolent B-NHLs/MCL | Vorinostat in treating patients with low-grade non-Hodgkin’s lymphoma | 2 | Completed | FL, 47%/23.5%; MZL, 22%/11%; MCL, 0%/0% | NCT00253630 | 335 |
| Vorinostat | Relapsed and refractory CTCL | Oral vorinostat in advanced cutaneous T-cell lymphoma (0683-001) | 2 | Completed | 29.7%/0% | NCT00091559 | 336 |
| Vorinostat, rituximab | NHLs | Vorinostat and rituximab in treating patients with indolent non-Hodgkin’s lymphoma | 2 | Completed | FL, 50%/40.9%; MZL, 50%/50%; MCL, 33.3%/0%; LPL, 0%/0% | NCT00720876 | 337 |
| Vorinostat, R-CHOP | Newly diagnosed advanced-stage DLBCL | Vorinostat, rituximab, and combination chemotherapy in treating patients with newly diagnosed stage II, stage III, or stage IV diffuse large B-cell lymphoma | 1/2 | Completed | 81%/52% | NCT00972478 | 338 |
| Vorinostat, R-ICE | Relapsed or refractory NHLs | Vorinostat, rituximab, ifosfamide, carboplatin, and etoposide in treating patients with relapsed or refractory lymphoma | 1 | Completed | 70%/29.6% | NCT00601718 | 339 |
| Vorinostat, CHOP | Relapsed or refractory PTCL | Phase I study of vorinostat in combination with standard CHOP in patients with newly diagnosed peripheral T-cell lymphoma | 1 | Completed | 93%/93% | – | 340 |
| Belinostat | A pan-HDAC inhibitor | ||||||
| Belinostat | Relapsed or refractory aggressive B-NHLs | Belinostat in treating patients with relapsed or refractory aggressive B-cell non-Hodgkin’s lymphoma | 2 | Completed | 10.5%/0% | NCT00303953 | 341 |
| Belinostat | Relapsed or refractory PTCL/CTCL | A phase II clinical trial of belinostat in patients with relapsed or refractory peripheral and cutaneous T-cell lymphomas (PXD101-CLN-6) | 2 | Completed | PTCL, 25%/8.3%; CTCL, 14%/10.3% | NCT00274651 | 342 |
| Belinostat, carfilzomib | Relapsed or refractory NHLs | Carfilzomib plus belinostat in relapsed or refractory NHL | 1 | Completed | NA | NCT02142530 | – |
| Panobinostat | A pan-HDAC inhibitor | ||||||
| Panobinostat | Relapsed or refractory NHLs | Panobinostat in treating patients with relapsed or refractory non-Hodgkin’s lymphoma | 2 | Active, not recruiting | 21%/NA | NCT01261247 | – |
| Panobinostat, lenalidomide | Relapsed or refractory HL | A phase 2 trial of panobinostat and lenalidomide in patients with relapsed or refractory Hodgkin’s lymphoma | 2 | Completed | 16.7%/8.3% | NCT01460940 | – |
| Panobinostat, ICE vs. ICE | Relapsed or refractory HL | Panobinostat plus ifosfamide, carboplatin, and etoposide compared with ifosfamide, carboplatin, and etoposide for relapsed or refractory Hodgkin’s lymphoma | 1/2 | Completed | NA | NCT01169636 | – |
| Romidepsin | A selective HDAC I inhibitor | ||||||
| Romidepsin | Relapsed or refractory PTCL/CTCL | Romidepsin to treat patients with peripheral T-cell lymphoma and cutaneous T-Cell lymphoma | 2 | Completed | PTCL, 38%/18%; CTCL, 34%/5.6% | NCT00007345 | 343,344 |
| Romidepsin, alisertib | Relapsed or refractory lymphoma | Alisertib and romidepsin in treating patients with relapsed or refractory B-cell or T-cell lymphoma | 1 | Completed | NA | NCT01897012 | – |
| Romidepsin, duvelisib; bortezomib, duvelisib | Relapsed or refractory T-NHLs | Trial of duvelisib in combination with either romidepsin or bortezomib in relapsed or refractory T-cell lymphoma | 1 | Recruiting | – | NCT02783625 | – |
| Romidepsin, lenalidomide | Relapsed or refractory NHLs/MM | Romidepsin in combination with lenalidomide in adults with relapsed or refractory lymphomas and myeloma | 1/2 | Active, not recruiting | – | NCT01755975 | – |
| Romidepsin, pralatrexate | Lymphoid malignancies | Romidepsin plus pralatrexate in relapsed or refractory lymphoid malignancies | 1/2 | Recruiting | – | NCT01947140 | – |
| Romidepsin, ixazomib | Relapsed or refractory PTCL | Study of ixazomib and romidepsin in peripheral T-cell lymphoma | 1/2 | Recruiting | – | NCT03547700 | – |
| Romidepsin, carfilzomib | Relapsed or refractory PTCL | Evaluation of the combination of romidepsin and carfilzomib in relapsed or refractory peripheral T-cell lymphoma patients | 1/2 | Recruiting | – | NCT03141203 | – |
| Romidepsin, pembrolizumab | Relapsed or refractory PTCL | Study of pembrolizumab in combination with romidepsin | 1/2 | Recruiting | – | NCT03278782 | – |
| Romidepsin, azacitidine | Relapsed or refractory lymphoma | Romidepsin plus azacitidine in relapsed or refractory lymphoid malignancies | 1/2 | Active, not recruiting | – | NCT01998035 | – |
| Romidepsin, gemcitabine | Relapsed or refractory PTCL | Phase 2 study of romidepsin plus gemcitabine in the relapsed or refractory peripheral T-cell lymphoma patients | 2 | Completed | 30%/15% | NCT01822886 | 345 |
| Romidepsin, ICE | Relapsed or refractory PTCL | Romidepsin, ifosfamide, carboplatin, and etoposide in treating participants with relapsed or refractory peripheral T-cell lymphoma | 1 | Completed | 93%/80% | NCT01590732 | 346 |
| Romidepsin, CHOP | Untreated PTCL | A study of escalating doses of romidepsin in association with CHOP in the treatment of peripheral T-cell lymphoma | 1/2 | Completed | 68%/51% | NCT01280526 | 347 |
| Romidepsin, CHOP vs. CHOP | Untreated PTCL | Efficacy and safety of romidepsin plus CHOP vs. CHOP in patients with untreated peripheral T-cell lymphoma | 3 | Active, not recruiting | – | NCT01796002 | – |
| Chidamide | A selective HDAC I inhibitor | ||||||
| Chidamide | Relapsed or refractory B-NHLs | Study of chidamide as a single-agent treatment for patients with relapse or refractory B-NHLs | 2 | Unknown | – | NCT03245905 | – |
| Chidamide | Relapsed or refractory DLBCL/FL | Chidamide for patients with relapse or refractory diffuse large B-cell lymphoma and follicular lymphoma | 2 | Not yet recruiting | – | NCT03410004 | – |
| Chidamide | Relapsed or refractpry PTCL | A multicenter, open-label, pivotal phase 2 study of chidamide in relapsed or refractory peripheral T-cell lymphoma | 2 | Completed | 28%/14% | – | 348 |
| Chidamide, sintilimab | Relapsed or refractory ENKTCL | Chidamide in combination with sintilimab in relapsed or refractory ENKTCL | 1/2 | Recruiting | – | NCT03820596 | – |
| Chidamide, DICE | Relapsed or refractory B-NHLs | Chidamide plus DICE regimen for patients with relapse or refractory B-cell non-Hodgkin’s lymphoma | 2 | Unknown | – | NCT03105596 | – |
| Chidamide, VDDT | Relapsed or refractory DLBCL | Chidamide combined with VDDT regimen in the relapse or refractory diffuse large B-cell lymphoma | 2 | Recruiting | – | NCT02733380 | – |
| Chidamide, R-GDP | Relapsed or refractory DLBCL | Chidamide combined with R-GDP in treating patients with relapsed or refractory diffuse large B-cell lymphoma | 2 | Recruiting | – | NCT03373019 | – |
| Chidamide, R-CHOP | Relapsed or refractory DLBCL | Chidamide with R-CHOP regimen for DLBCL patients | 2 | Recruiting | – | NCT03201471 | – |
| Chidamide, R-CHOP | Elderly DLBCL | Chidamide plus R-CHOP in elderly DLBCL | 2 | Completed | NA | NCT02753647 | – |
| Chidamide, CHOP | Untreated PTCL | Clinical trial of chidamide combined with CHOP in peripheral T-cell lymphoma patients | 1 | Completed | NA | NCT02809573 | – |
| Chidamide, CHOP | Untreated AITL | Study evaluating the safety and efficacy of chidamide plus CHOP in untreated subjects with angioimmunoblastic T-cell lymphoma | 2 | Recruiting | – | NCT03853044 | – |
| Chidamide, CHOEP | PTCL | Chidamide combined with CHOEP regimen for peripheral T-cell lymphoma patients | 2 | Recruiting | – | NCT03617432 | – |
| Chidamide, CPT | Relapsed or refractory PTCL | Chidamide combined with cyclophosphamide, prednisone, thalidomide in treatment of fragile patients with relapse or refractory peripheral T-cell lymphoma | 2 | Recruiting | – | NCT02879526 | – |
| Chidamide, PET | AITL | Chidamide with prednisone, etoposide, and thalidomide regimen for angioimmunoblastic T-cell lymphoma | 2 | Unknown | – | NCT03273452 | – |
| Chidamide, PECM | Relapsed or refractory PTCL | Chidamide combined with PECM in relapsed or refractory peripheral T-cell lymphoma | 2 | Recruiting | – | NCT03321890 | – |
NA: ORR or CR are not available on the clinicaltrials.gov or from the published article, although the trial has been completed
R-CHOP rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone, R-ICE rituximab, ifosfamide, carboplatin, etoposide, R-GDP rituximab, gemcitabine, dexamethasone, cisplatin, CHOP cyclophosphamide, doxorubicin, vincristine, prednisolone, DICE dexamethasone, ifosfamide, cisplatin, etoposide, VDDT vinorelbine, liposomal doxorubicin, dexamethasone and thalidomide, CHOEP cyclophosphamide, doxorubicin, vincristine, etoposide, prednisone, CPT cyclophosphamide, prednisone, thalidomide, PET prednisone, etoposide, thalidomide, PECM prednisone, cyclophosphamide, etoposide, methotrexate
TET2
TET2 mediates the oxidation process of 5-methylcytosine (5mC) in gene bodies to 5-hydroxymethylcytosine (5hmC), which plays an important role in transcriptional activation (Fig. 2).298–301 Experimentally, TET2 deletion decreased DNA hydroxymethylation at enhancers and reduced the expression of a set of genes in GC B cells associated with GC exit and plasma cell differentiation.302–307 TET2 was mutated in 12% of DLBCL patients, predominantly in the GCB subtype.308 TET2 mutations occur more frequently in T-cell lymphomas, including 47% of AITL and 38% of PTCL-NOS.309–311 A retrospective study indicated that TET2 mutations in PTCL were associated with advanced-stage disease and high-risk IPI.310 To date, there are no specific TET2 inhibitors in clinical application. However, the growth inhibition of TET2-knockdown DLBCL cells was observed after treatment with a histone deacetylase 3 (HDAC3) inhibitor in vitro.312 Clinically, AITL patients with TET2 mutations were reported to have an objective response to azacitidine treatment.313
IDH2
The isocitrate dehydrogenase (IDH) family, including IDH1, IDH2, and IDH3, catalyzes the oxidative decarboxylation process that transduces isocitrate to α-ketoglutarate.314 Gain-of-function mutations of IDH2R172 result in the production of 2-hydroxyglutarate (2HG), which inhibits TET enzymes and histone-lysine demethylases and induces the epigenetic modification of DNA.315–317 Altered DNA methylation and downregulated Th1 cell differentiation-associated genes were observed in IDH2R172-mutant AITL.316 IDH2R172/TET2 double mutations were found in AITL and correlated with increased follicular T-helper-associated gene expression.316 For targeted therapy, a phase 1/2 trial (NCT02273739) of enasidenib (also known as AG-221) in subjects with AITL that harbor IDH2 mutations has been completed.
Histone methylation and targeted therapy
EZH2
Enhancer of zeste homolog 2 (EZH2) functions as a histone methyltransferase and induces transcriptional repression via the trimethylation of H3K27. EZH2 in GC B-cells represses the expression of a set of genes involved in terminal differentiation, such as PRDM1, IRF4, and XBP1, as well as in the negative regulation of cell-cycle progression, such as CDKN1A and CDKN1B.318–320 Mutations of EZH2 occur in 25% of FL and 21.7% of GCB-DLBCL but not in ABC-DLBCL.319,321 A strong association between EZH2 mutations and the loss of MHC-I or MHC-II expression was found in DLBCL, especially in GCB-DLBCL.322 A higher level of H3K27me3 at promoters of NLRC5 and CIITA (MHC-I and MHC-II transactivators) was also found in EZH2-mutant cells,322 indicating the underlying mechanisms of EZH2 mutation on MHC expression. EZH2 mutations also occur in T-NHLs. Tazemetostat, a selective inhibitor of EZH2, can effectively block H3K27 methylation and inhibit mutant lymphoma cells.323 The phase 1 part of a phase 1/2 study (NCT01897571) of tazemetostat in relapsed or refractory B-NHLs was completed and demonstrated acceptable safety and potential antitumor activity (ORR 38% and CR 14%).324 Additionally, phase 1 studies are assessing the novel EZH2 inhibitors SHR2554 and PF-06821497 in lymphoma (NCT03603951 and NCT03460977).
KMT2D
Histone-lysine N-methyltransferase 2D (KMT2D), also called MLL2, is a member of the SET1 family of histone methyltransferases and modulates transcription by H3K4 methylation. Integrative genomic analysis identified that KMT2D-targeted genes included TNFAIP3, TNFRSF14, and SOCS3, which suppress tumorigenesis, and genes involved in cell signaling pathways such as JAK-STAT and BCR.325 The incidence of inactivating mutations of KMT2D is observed in 72% of FL319 and 30% of DLBCL.326 KMT2D missense mutations lead to a significant reduction in H3K4 methylation in vitro.327 Recent studies in mice showed that the loss of KMT2D resulted in decreased H3K4 methylation and increased tumor development.325,327 Though there are no targeted agents for KMT2D, the histone deacetylase inhibitors (HDACis) romidepsin and chidamide showed the ability to restore H3K4me3 levels in KMT2D mutant cells in vitro.328 Chidamide combined with decitabine was observed to induce the apoptosis of Jurkat cells bearing KMT2D mutations in vitro and in vivo.328
Histone acetylation
CREBBP/EP300
The balance between histone acetyltransferases (HATs, including CREBBP and EP300) and HDACs is critical to maintain a normal histone acetylation status in cells. CREBBP and EP300, as histone acetyltransferases, regulate gene transcription by catalyzing the acetylation of the lysine residues of histones. Inactivating mutations in CREBBP and EP300 in GC B-cells decrease p53-mediated tumor suppression and enhance the proto-oncogenic activity of BCL-6.329,330 CREBBP mutation is also associated with reduced MHC-II expression, which is a key element in antigen presentation, thereby promoting tumor escape from the immune system.331 CREBBP and EP300 mutations were found in 65% and 15% of FL, respectively. CREBBP is mutated in DLBCL, with a significantly higher incidence in the GCB subtype (32% in GCB-DLBCL vs. 13% in ABC-DLBCL). Mutations of EP300 were observed in 10% of DLBCL.329 In PTCL-NOS, CREBBP and EP300 are mutated in 4% and 8% of patients, respectively.328 In NKTCL, EP300 is mutated in approximately 3.8% of patients.243
HDACs
HDACs are divided into four groups: HDAC I (HDAC 1, 2, 3, and 8), HDAC II (HDAC 4, 5, 6, 7, 9, and 10), HDAC III and HDAC IV.332 There are three types of HDACis under clinical development: pan-HDACis (vorinostat, belinostat, and panobinostat), selective HDACis (HDAC I inhibitors including romidepsin, chidamide, and entinostat; the HDAC6 inhibitor ricolinostat) and multipharmacological HDACis.333
Vorinostat (suberoylanilide hydroxamic acid, SAHA), the first HDACi approved by the FDA for treating CTCL, inhibits both HDAC I and HDAC II. A phase 2 trial (NCT00097929) of vorinostat in relapsed DLBCL presented an ORR of 5.6% (CR 5.6%), suggesting that vorinostat monotherapy has limited antitumor activity in relapsed DLBCL. Common AEs were grade 1/2 diarrhea, fatigue, nausea, anemia and vomiting, and grade ≥ 3 AEs including thrombocytopenia and asthenia occurred in 16.7% and 11.1% of the patients, respectively.334 Another phase 2 trial (NCT00253630) of vorinostat enrolled relapsed or refractory patients with B-NHLs and showed an ORR of 47% (CR 23.5%) in FL, 22% (CR 11%) in MZL, and no response in MCL. Grade ≥ 3 AEs were thrombocytopenia (39%), anemia (11%), leucopenia (11%), and fatigue (9%).335 Vorinostat in relapsed or refractory CTCL (NCT00091559) had an ORR of 29.7% (CR 0%).336 A phase 2 trial (NCT00720876) studied the efficacy and safety of vorinostat plus rituximab in NHLs, showing an ORR of 50% (CR 40.9%) in FL, 50% (CR 50%) in MZL, 33% (CR 0%) in MCL and no response in LPL.337 Vorinostat plus R-CHOP was explored in a phase 1/2 study (NCT00972478) and showed a tendency to improve R-CHOP in untreated advanced-stage DLBCL (ORR 81% and CR 52%); 38% febrile neutropenia and 19% sepsis were reported.338 Vorinostat in combination with R-ICE was applied in patients with relapsed or refractory NHLs (NCT00601718), and an ORR of 70% (CR 29.6%) was reported. Grade ≥ 3 AEs included febrile neutropenia (27%), infection (27%), and hypophosphatemia (27%) in patients treated at the maximum tolerated dose.339 A phase 1 trial investigated vorinostat in combination with standard CHOP in untreated PTCL patients and presented an ORR of 93% (CR 93%). Grade ≥ 3 AEs were neutropenia (50%), anemia (17%), and diarrhea (17%) in patients receiving 300 mg three times daily on days 2 to 3.340
Another pan-HDACi, belinostat (PXD101), was approved by the FDA to treat PTCL. A phase 2 trial (NCT00303953) of belinostat in relapsed or refractory aggressive B-NHLs reported an ORR of 10.5% (CR 0%).341 Another phase 2 trial (NCT00274651) explored belinostat in relapsed or refractory PTCL or CTCL with an ORR of 25% (CR 8.3%) in PTCL and an ORR of 14% (CR 10.3%) in CTCL. Treatment-related AEs were found in 77% of patients, including nausea (43%), vomiting (21%), infusion site pain (13%), and dizziness (11%).342 A trial of belinostat combined with carfilzomib in relapsed or refractory NHLs (NCT02142530) is ongoing.
Panobinostat, a pan-HDACi, showed an ORR of 21% in relapsed NHLs (NCT01261247). In relapsed or refractory HL, panobinostat in combination with lenalidomide (NCT01460940) had an ORR of 16.7% (CR 8.3%), while its effect in combination with ICE (NCT01169636) is currently under evaluation.
Romidepsin (FK228), a selective HDAC inhibitor, was approved by the FDA for treating CTCL. A phase 2 trial (NCT00007345) reported an ORR of 38% (CR 18%) in relapsed or refractory PTCL and an ORR of 34% (CR 5.6%) in relapsed or refractory CTCL. Common AEs included nausea, fatigue, transient thrombocytopenia and granulocytopenia.343,344 Trials of the combined treatment of romidepsin with other targeted agents, such as alisertib (NCT01897012), duvelisib (NCT02783625), lenalidomide (NCT01755975), pralatrexate (NCT01947140), ixazomib (NCT03547700), carfilzomib (NCT03141203), pembrolizumab (NCT03278782), and azacytidine (NCT01998035), in relapsed or refractory NHLs are ongoing. A phase 2 trial (NCT01822886) of romidepsin plus gemcitabine in relapsed or refractory PTCL showed an ORR of 30% (CR 15%).345 A phase 1 trial (NCT01590732) of romidepsin plus ICE in relapsed or refractory PTCL had an ORR of 93% (CR 80%), and the most common grade ≥ 3 AEs were thrombocytopenia (83%), anemia (50%), neutropenia (44%), fatigue (33%), nausea or vomiting (33%), infections (28%), dyspnea (17%), and transaminitis (11%).346 Of note, a phase 1/2 trial (NCT01280526) of romidepsin plus CHOP induced an ORR of 68% (CR 51%) in untreated PTCL.347 Thus, a randomized phase 3 trial (NCT01796002) of romidepsin plus CHOP vs CHOP in untreated PTCL is ongoing.
Chidamide, a selective HDAC I inhibitor, is being evaluated in relapsed or refractory B-NHLs (NCT03245905 and NCT03410004). In a phase 2 trial of relapsed or refractory PTCL, chidamide showed an ORR of 28% (CR 14%). Grade ≥ 3 AEs were thrombocytopenia (22%), leucopenia (13%), and neutropenia (11%).348 A trial of chidamide in combination with sintilimab is ongoing in relapsed or refractory NKTCL (NCT03820596). Phase 2 trials of chidamide in combination with chemotherapy, such as dexamethasone, ifosfamide, cisplatin, and etoposide (DICE) (NCT03105596), vinorelbine, liposomal doxorubicin, dexamethasone and thalidomide (VDDT) (NCT02733380), R-GDP (NCT03373019), and R-CHOP (NCT03201471) in relapsed or refractory B-NHLs, as well as R-CHOP (NCT02753647) in untreated elderly DLBCL patients, are ongoing. In T-NHLs, the efficacy of chidamide combined with CHOP (NCT02809573 and NCT03853044); cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone (CHOEP) (NCT03617432); cyclophosphamide, prednisone, and thalidomide (CPT) (NCT02879526); prednisone, etoposide, and thalidomide (PET) (NCT03273452); and prednisone, etoposide, cyclophosphamide, and methotrexate (PECM) (NCT03321890) are under evaluation.
Although epigenetic alterations show clinical significance, modulators specifically targeting these alterations remain to be developed. Demethylation agents and HDACis have presented clinical efficacy in many lymphoma subtypes. However, the exact mechanisms of action remain unclear, and biomarkers to predict clinical effects need to be further explored. Moreover, monotherapy with epigenetic agents may have limited efficacy in lymphoma in early phase studies. Trials in combination with chemotherapy or other small molecules have demonstrated potent efficacy and acceptable safety and warrant further investigation.
Tumor microenvironment and checkpoint-related targeted therapy
In addition to tumor cells themselves, the tumor microenvironment plays an important role in lymphoma progression. Immunotherapeutic agents can effectively activate the immune system, leading to tumor regression, and have improved clinical outcomes in lymphoma patients.349–352 In addition, checkpoint inhibitors combined with CAR-T therapy, epigenetic modulators, radiotherapy, and BTK inhibitors have shown striking efficacy in refractory lymphoma.353–355
PD-1/PD-L1
Programmed cell death-1 (PD-1, also known as CD279) is a member of the immunoglobulin superfamily and functions as an important immune checkpoint that suppresses excessive immune responses.6 PD-1 is mainly expressed on activated T cells and a small number of B cells, NK cells, activated monocytes, and dendritic cells but is not expressed on naïve T cells. The persistent stimulation of PD-1 on T cells can lead to T-cell exhaustion.356,357 The ligands of PD-1 include PD-L1 (also known as B7-H1, CD274) and PD-L2 (also known as B7-DC, CD273).358,359 PD-L1 is expressed on B cells, T cells, dendritic cells, and macrophages. PD-L2 is expressed mainly on dendritic cells, macrophages, mast cells, and certain B cells in response to IL-4 and IFN.358,359 In addition to those immune cells, PD-L1 is expressed on tumor cells and protects them from immune surveillance; a high level of PD-L1 on tumor cells is associated with poor prognosis in patients.360–363 Therefore, PD-1/PD-L1 pathway blockade can promote T-cell activation and cytokine production and preserve the antitumor capacity of T cells in the treatment of lymphomas.364
PD-1 is overexpressed in the tumor-infiltrating lymphocytes (TILs) of HL,365 and 94–100% of refractory or relapsed HL cases are positive for PD-L1.353,366 The 9p24.1 amplification is frequently detected in HL, resulting in increased PD-L1 and PD-L2 expression on Hodgkin and Reed–Sternberg (HRS) cells.367 Moreover, the amplified 9p24.1 region contains the JAK2 locus, further enhancing PD-L1 expression in HRS cells.367 In FL, though PD-1 expression on TILs is abundant, PD-L1 expression on lymphoma cells is low (0–5%).368–375 In DLBCL, the positive rate of PD-1 was 39.5–68.6%,376–380 and the positive rate of PD-L1 was 24–75%.375,380–382 Moreover, the number of PD-1+ TILs is higher in the GCB subtype, and patients with PD-L1+ tumor cells have inferior OS compared to those with PD-L1- tumor cells.379 Soluble PD-L1 (sPD-L1), independent of IPI, has been reported to be an adverse prognostic factor for DLBCL. Similar to PD-1, sPD-L1 is elevated in DLBCL patients at diagnosis and returns to normal when patients achieve CR. Thus, sPD-L1 is an effective predictor of DLBCL.382 In PTCL, PD-1 is positive in 70% and 61% of AITL and PTCL-NOS, respectively, and PD-1 is rarely detected in ALCL. PD-L1 is expressed in 46% of ALK+ ALCL and in 46% of ALK- ALCL. In contrast, there is no PD-L1 expression in AITL and PTCL-NOS.383
The targeted drugs and clinical trials related to PD-1 are shown in Table 6. Nivolumab and pembrolizumab were approved by the FDA to treat relapsed or refractory HL.366,377,384,385 In a phase 1 trial (NCT01592370) of nivolumab in relapsed or refractory HL, the ORR was 87% (CR 17%), and the 24-week PFS was 86%.349 In a phase 2 trial (NCT02181738), the efficacy of nivolumab was evaluated in relapsed or refractory HL. At a median follow-up of 8.9 months, the ORR was 66.3% (CR 9%), and the 6-month PFS and OS were 77% and 99%, respectively.350 In relapsed or refractory NHLs, a phase 1 trial of nivolumab (NCT01592370) showed an ORR of 40% (CR 10%) in FL, 36% (CR 18%) in DLBCL, and 17% (CR 0%) in T-NHLs.386 Another phase 2 trial (NCT02038933) of nivolumab in ASCT-failed DLBCL showed an ORR of 10.3% (CR 3.4%). Nivolumab in relapsed or refractory ALK+ ALCL (NCT03703050) and PTCL (NCT03075553) is currently under clinical evaluation in phase 2 trials. In relapsed or refractory PCNSL and testicular lymphoma (NCT02857426), nivolumab showed an ORR of 100% (CR 80%).351 In addition, nivolumab combined with BV (NCT02572167) in relapsed or refractory HL had a reported ORR of 82% (CR 16%),387 and this combination in NHLs (NCT02581631) is ongoing. Nivolumab combinations with other targeted agents such as lenalidomide in relapsed or refractory lymphoma (NCT03015896), rituximab in FL (NCT03245021), cabiralizumab in PTCL (NCT03927105), and in combination with chemotherapy, such as rituximab, gemcitabine, and oxaliplatin (R-GemOx) in elderly lymphoma patients (NCT03366272), R-CHOP (NCT03704714), and rituximab, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (DA-R-EPOCH) (NCT03749018) in aggressive NHLs are ongoing.
Table 6.
Targeted drugs and clinical trials related to PD-1
| Drug | Disease | Trial name | Phase | Status | ORR/CR | NCT# | Reference |
|---|---|---|---|---|---|---|---|
| Nivolumab | A human monoclonal antibody that blocks the interaction between PD-1 and its ligands, PD-L1 and PD-L2 | ||||||
| Nivolumab | Relapsed or refractory HL | PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma | 1 | Completed | 87%/17% | NCT01592370 | 349 |
| Nivolumab | Relapsed or refractory HL | Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicenter, multicohort, single-arm phase 2 trial | 2 | Completed | 66.3%/9% | NCT02181738 | 350 |
| Nivolumab | Relapsed or refractory NHLs/MM | Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase 1 study | 1 | Completed | FL, 40%/10%; DLBCL, 36%/18%; T-NHLs, 17%/0% | NCT01592370 | 386 |
| Nivolumab | Relapsed or refractory DLBCL (failed or not eligible for ASCT) | Study of nivolumab in patients with relapsed or refractory diffuse large B-cell lymphoma that have either failed or are not eligible for autologous stem cell transplant | 2 | Completed | ASCT-failed, 10.3%/2.9%; ASCT ineligible, 3.4%/0% | NCT02038933 | – |
| Nivolumab | Relapsed or refractory ALK+ ALCL | Phase 2 trial of nivolumab for pediatric and adult relapsed or refractory ALK+ anaplastic large cell lymphoma, for evaluation of response in patients with progressive disease (cohort 1) or as consolidative immunotherapy in patients in complete remission after relapse (cohort 2) | 2 | Recruiting | – | NCT03703050 | – |
| Nivolumab | Relapsed or refractory PTCL | Nivolumab in treating patients with relapsed or refractory peripheral T-cell lymphoma | 2 | Active, not recruiting | – | NCT03075553 | – |
| Nivolumab | Relapsed or refractory PCNSL/primary testicular lymphoma | PD-1 blockade with nivolumab in relapsed or refractory primary central nervous system and testicular lymphoma | 2 | Completed | 100%/80% | NCT02857426 | 351 |
| Nivolumab, BV | Relapsed or refractory HL | Interim results of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin’s lymphoma | 1/2 | Completed | 82%/61% | NCT02572167 | 387 |
| Nivolumab, BV | NHLs | An investigational immunotherapy effectiveness and safety study of nivolumab in combination with brentuximab vedotin to treat non-Hodgkin’s lymphomas | 1/2 | Active, not recruiting | – | NCT02581631 | – |
| Nivolumab, lenalidomide | Relapsed or refractory NHLs/HL | Nivolumab and lenalidomide in treating patients with relapsed or refractory non-Hodgkin’s or Hodgkin’s lymphoma | 1/2 | Recruiting | – | NCT03015896 | – |
| Nivolumab, rituximab | FL | Nivolumab plus rituximab in first-line follicular lymphoma grade 1-3A | 1 | Recruiting | – | NCT03245021 | – |
| Nivolumab, cabiralizumab | PTCL | Nivolumab and the antagonistic CSF-1R monoclonal antibody cabiralizumab in patients with relapsed or refractory peripheral T-cell lymphoma | 2 | Recruiting | – | NCT03927105 | – |
| Nivolumab, rituximab, gemcitabine, oxaliplatin | NHLs (elderly patients) | Nivolumab with gemcitabine, oxaliplatin, rituximab in relapsed or refractory elderly lymphoma patients | 2/3 | Recruiting | – | NCT03366272 | – |
| Nivolumab, R-CHOP | Aggressive NHLs | Nivolumab and combination chemotherapy in treating participants with diffuse large B-cell lymphoma | 1/2 | Recruiting | – | NCT03704714 | – |
| Nivolumab, DA-R-EPOCH | Aggressive NHLs | Nivolumab with DA-REPOCH chemotherapy regimen in treating patients with aggressive B-cell non-Hodgkin’s lymphoma | 2 | Recruiting | – | NCT03749018 | – |
| Pembrolizumab | A humanized monoclonal antibody that blocks the interaction between PD-1 and its ligands, PD-L1 and PD-L2 | ||||||
| Pembrolizumab | Relapsed or refractory HL | PD-1 blockade with pembrolizumab in patients with classical Hodgkin’s lymphoma after brentuximab vedotin failure | 1 | Completed | 65%/16% | NCT01953692 | 353 |
| Pembrolizumab | Relapsed or refractory HL | Phase 2 study of the efficacy and safety of pembrolizumab for relapsed or refractory classic Hodgkin’s lymphoma | 2 | Completed | 69%/22.4% | NCT02453594 | 366 |
| Pembrolizumab | Transformed DLBCL/relapsed or refractory CLL | Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL | 2 | Completed | transformed DLBCL, 41%/11%; CLL, 0%/0% | NCT02332980 | 388 |
| Pembrolizumab | Relapsed or refractory PMBCL | Safety and tolerability of pembrolizumab in patients with relapsed or refractory primary mediastinal large B-cell lymphoma | 1 | Completed | 41%/11.8% | NCT01953692 | 389 |
| Pembrolizumab | Relapsed or refractory GZL/extranodal DLBCL | Pembrolizumab in relapsed or refractory gray-zone lymphoma, primary central nervous system lymphoma, and other extranodal diffuse large B-cell lymphomas | 2 | Recruiting | – | NCT03255018 | – |
| Pembrolizumab | Untreated B-NHLs | Pembrolizumab in untreated B-cell non-Hodgkin’s lymphoproliferative diseases | 2 | Recruiting | – | NCT03498612 | – |
| Pembrolizumab | Relapsed or refractory stage IB-IVB MF/SS | A phase 2 study of pembrolizumab for the treatment of relapsed or refractory mycosis fungoides/Sézary syndrome | 2 | Completed | 37.5%/8.3% | NCT02243579 | – |
| Pembrolizumab | Stage IB-IV MF | Pembrolizumab in treating patients with stage IB-IV mycosis fungoides | 2 | Recruiting | – | NCT03695471 | – |
| Pembrolizumab | Early stage NKTCL, nasal type | Study of pembrolizumab in patients with early stage NK/T-Cell lymphoma, nasal type | 2 | Recruiting | – | NCT03728972 | – |
| Pembrolizumab, umbralisib | Relapsed or refractory B-NHLs/CLL | Combination of pembrolizumab with umbralisib in patients with relapsed or refractory CLL and B-NHLs | 1 | Recruiting | – | NCT03283137 | – |
| Pembrolizumab, lenalidomide | Relapsed NHLs/HL | Efficacy and safety study of combination of pembrolizumab and lenalidomide, in patients with relapsed non-Hodgkin’s and Hodgkin’s lymphoma | 1/2 | Active, not recruiting | – | NCT02875067 | – |
| Pembrolizumab, mogamulizumab | Relapsed or refractory NHLs/HL | Pembrolizumab and mogamulizumab in treating patients with relapsed or refractory lymphomas | 1/2 | Recruiting | – | NCT03309878 | – |
| Pembrolizumab, rituximab | Relapsed or refractory DLBCL/FL | Pembrolizumab and rituximab in treating patients with relapsed or refractory diffuse large B-cell lymphoma or follicular lymphoma | 2 | Recruiting | – | NCT03401853 | – |
| Pralatrexate | Relapsed or refractory mature T- and NK-cell NHLs/MF | Pembrolizumab and pralatrexate in treating participants with relapsed or refractory peripheral T-cell lymphoma | 1/2 | Recruiting | – | NCT03598998 | – |
| Pembrolizumab, tisagenlecleucel | Relapsed or refractory DLBCL | Study of pembrolizumab in combination with tisagenlecleucel in relapsed or refractory diffuse large B-cell lymphoma patients | 1 | Recruiting | – | NCT03630159 | – |
| Pembrolizumab, EBRT | Relapsed or refractory NHLs | Pembrolizumab and external beam radiation therapy in treating participants with relapsed or refractory non-Hodgkin lymphomas | 2 | Recruiting | – | NCT03210662 | – |
| Pembrolizumab, radiotherapy | Relapsed or refractory MF/SS | A trial assessing the effect of pembrolizumab combined with radiotherapy in patients with relapsed or refractory, specified stages of cutaneous T-cell lymphoma mycosis fungoides/Sézary syndrome (PORT) | 2 | Recruiting | – | NCT03385226 | – |
NA: ORR or CR are not available on the clinicaltrials.gov or from the published article although the trial has been completed
BV brentuximab vedotin, R-CHOP rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone, DA-R-EPOCH rituximab, dose-adjusted EPOCH, GZL gray-zone lymphoma, EBRT external beam radiotherapy
Pembrolizumab, a humanized mAb of PD-1, showed an ORR of 65% (CR 16%) in a phase 1 trial (KEYNOTE-013, NCT01953692) of relapsed or refractory HL353 and an ORR of 69% (CR 22.4%) in a phase 2 trial (KEYNOTE-087, NCT02453594).366 Pembrolizumab induced an ORR of 41% (CR 11%) in transformed DLBCL and showed no response in relapsed or refractory CLL in a phase 2 trial (NCT02332980).388 In a phase 1 trial (NCT01953692), pembrolizumab was evaluated in relapsed or refractory PMBCL, and the ORR was 41% (CR 11.8%).389 Trials of pembrolizumab in relapsed or refractory gray-zone lymphoma and PCNSL (NCT03255018) and in untreated B-NHLs (NCT03498612) are ongoing. In a study of relapsed or refractory NKTCL patients who failed asparaginase treatment or ASCT, pembrolizumab presented an ORR of 100% (CR 71.4%).352 In a phase 2 trial (NCT02243579) of pembrolizumab in advanced relapsed or refractory MF and SS, the ORR was 37.5% (CR 8.3%). Thus, trials of pembrolizumab in MF (NCT03695471) and NKTCL (NCT03728972) are ongoing. Pembrolizumab in combination with other targeted agents, such as umbralisib (NCT03283137), lenalidomide (NCT02875067), mogamulizumab (NCT03309878), rituximab (NCT03401853), pralatrexate (NCT03598998), CAR-T (tisagenlecleucel, NCT03630159), and radiation (NCT03210662 and NCT03385226), are under evaluation.
CTLA-4
CTLA-4, a member of the immunoglobulin family receptors, together with CD28, are homologous receptors of CD4+ and CD8+ T cells. Both receptors share a pair of ligands (CD80/CD86) expressed on the surface of antigen-presenting cells (APCs). In contrast with CD28, the signal of CTLA-4 suppresses the activation of T cells, and the affinity of CTLA-4 and CD80 is higher than that of CD28 and CD80. In addition to APCs, CTLA-4 is also present in resting T cells in the form of intracellular vesicles and expressed on the cell membrane surface when T cells are activated.390 The CTLA4-CD86 protein recruits and activates Tyk2, leading to STAT3 activation and the expression of genes involved in immune suppression and tumor growth. Although the CTLA-4 antibody ipilimumab391 has become a first-line therapy in metastatic melanoma,392,393 the application of the CTLA-4 antibody still needs to be explored in hematological malignancies.394 The phase 1 part of a phase 1/2 trial (NCT00089076) of ipilimumab in relapsed or refractory B-NHLs induced an ORR of 11.1% (CR 5.6%).395 Trials of ipilimumab combined with other agents, such as nivolumab (NCT02408861) in HIV-associated HL, are ongoing. A trial of tremelimumab, another CTLA-4 mAb, in combination with durvalumab in relapsed or refractory DLBCL (NCT02549651) was completed.
CD47/SIRPα
CD47 is a new immune checkpoint that is expressed in normal cells and upregulated in various tumors.396–398 Its ligand SIRPα is expressed on myeloid cells (monocytes, macrophages, and myeloid dendritic cells). CD47/SIRPα mainly regulates innate immune cell activity and sends out a “do not eat me” signal to escape the attack of innate immune cells.399 MYC can upregulate the expression of the CD47 gene by binding to the promoter of CD47. The downregulation of MYC gene expression in a murine model led to decreased CD47 expression.400 CD47 is upregulated in various NHLs (DLBCL, MCL, FL, and CLL) and is associated with poor clinical outcomes in patients.401 Targeting CD47 can reduce liver and central nervous system metastasis in Raji-engrafted mice,402 suggesting the association of CD47 with the extranodal metastasis of lymphoma cells. TTI-621 (an anti-CD47 antibody) enhances macrophage-mediated phagocytosis and can effectively control B-NHL growth in xenograft murine models. Another anti-CD47 antibody, Hu5F9-G4, combined with rituximab, is effective in the treatment of NHLs. In the phase 1 part of a phase 1/2 trial (NCT02953509), 22 relapsed or refractory DLBCL and FL patients were enrolled. The ORRs and CR rates were 40% and 33% in DLBCL and 71% and 43% in FL, respectively. The most common AEs were anemia and infusion reactions.403
OX40/OX40L
OX40 is a member of the TNFR superfamily. Under physiological conditions, it is mainly expressed in activated T cells and is more abundant in CD4+ T cells than in CD8+ T cells. OX40L, the ligand of OX40, is a type II transmembrane protein and is expressed in a variety of APCs (B-cells, dendritic cells, and macrophages), activated T cells, vascular endothelial cells and mast cells.404–406 The OX40L-OX40 signaling pathway is the basis for effector T-cell proliferation and memory T-cell development. However, the OX40L-OX40 axis can promote immune escape and tumor growth.
Experimentally, an OX40 agonist showed antitumor activity in combination with other drugs. Intratumoral injection of anti-CTLA-4 and OX40 agonists depleted tumor-infiltrating Tregs in murine lymphoma models.407 A phase 1 clinical study (NCT03636503) of PF-04518600 (the OX40 agonist) in combination with utomilumab (4-1BB agonist) and rituximab or in combination with avelumab (anti-PD-L1) and rituximab is ongoing in aggressive B-NHLs.
Other immune checkpoint molecules
T-cell immunoglobulin and ITIM domain (TIGIT) is a coinhibitory receptor that is expressed on NK cells and different types of T cells, including effector and memory T cells and Tregs.408–410 The ligands of TIGIT, CD155 (PVR) and CD112 (PVRL2, nectin-2) are expressed on APCs, T cells, and tumor cells.411,412 In NHL, TIGIT and PD-1 are frequently coexpressed on TILs. Approximately 78–83% of CD8+ and 69–70% of CD4+ T effector memory cells (TEMs) are simultaneously positive for these two inhibitory molecules, and these TEMs have limited capability for IL-2, IFN-γ, and TNF-α secretion.413 In FL, TIGIT is mainly expressed by CD8+ effector and memory T cells and is related to advanced disease stage.414 TIM-3 inhibits Th1 cell responses,415 and its antibodies have been found to potently enhance antitumor immunity.416 An increased number of TIM-3+ T cells is related to the unfavorable prognosis of FL patients.414 TIM-3 is preferentially expressed on the microvascular endothelial cells of lymphoma, suppresses the activation of CD4+ T lymphocytes and facilitates the progression of lymphoma by mediating immune evasion.417
Indoleamine 2,3-dioxygenase (IDO), a known immune suppressor, plays a role in human mesenchymal stromal cells (MSCs) to regulate immunity in the tumor microenvironment. IDO+ MSCs can inhibit T-cell proliferation in vitro. In a lymphoma murine model, IDO+ MSCs could enhance tumor growth, which could be reversed by the IDO inhibitor D-1-methyl-tryptophan (D1-MT).418 Since MSCs secrete IDO to further suppress T-cell immune responses, umbilical cord-derived MSCs genetically secrete TandAb (a tetravalent bispecific antibody with two CD3 and two CD19 binding sites). In vitro, TandAb can induce the specific lysis of CD19+ cell lines in the presence of T cells, and an IDO inhibitor could enhance the cytotoxicity of T cells triggered by MSC-TandAb.419 Clinical studies of IDO inhibitors in lymphomas are still lacking. V-domain immunoglobulin suppressor of T-cell activation (VISTA) is another checkpoint molecule that has a strong inhibitory influence on T cells.420 VISTA is constitutively expressed in CD11bhigh myeloid cells and is expressed at a low level on T cells and Foxp3+CD4+ Treg cells.421 In animal models with solid tumors, myeloid-derived suppressor cells (MDSCs) infiltrating tumors were found to highly express VISTA compared to peripheral blood cells.422,423 In a murine model of squamous cancer, anti-VISTA monotherapy increased the infiltration and activation of T cells. A clinical trial (NCT02812875) evaluating the efficacy and safety of CA-107 (targeting PD-L1, PD-L2, and VISTA) for the treatment of lymphoma is ongoing.
Adoptive T/NK-cell therapy
Adoptive T-cell transfer is an emerging immunotherapy in a variety of tumors, particularly CAR-T therapy. In 2017, the FDA approved tisagenlecleucel (a CD19-specific 4-1BB-CAR construct) for the treatment of relapsed or refractory B-ALL, and in 2018, the FDA approved axicabtagene ciloleucel (a CD19-specific CD28-CAR construct) for the treatment of relapsed or refractory DLBCL. Another CD19 CAR-T cell line, lisocabtagene maraleucel (a CD19-specific 4-1BB-CAR construct), is also undergoing evaluation.
CAR-T therapy in lymphoma
In a single-arm, multicenter clinical trial (NCT02348216) for relapsed or refractory DLBCL, transformed FL, and PMBCL, axicabtagene ciloleucel had an ORR of 83% (CR 58%) and median PFS of 5.9 months.424 In another clinical trial (NCT02030834) of relapsed or refractory B-NHLs, tisagenlecleucel induced an ORR of 64.3% (CR 57.1%). Moreover, all CR patients were still in remission at 6 months.425 In a phase 2 trial (NCT02445248) of tisagenlecleucel in relapsed or refractory DLBCL, the ORR was 52% (CR 40%), with a 1-year RFS of 65%.426
In addition to axicabtagene ciloleucel and tisagenlecleucel, lisocabtagene maraleucel was tested in relapsed or refractory DLBCL, PMBCL, FL, and MCL (TRANSCEND, NCT02631044) and showed a CR rate of 80% in high-grade B-cell lymphoma (double/triple hit) and DLBCL.427,428 A phase 1 dose-escalation study (NCT03355859) of anti-CD19 JWCAR029 was conducted in refractory B-NHLs, and the ORR was 100%, with 6 of 9 (66.7%) evaluable patients achieving CR. In this study, core needle biopsy was performed on tumor samples on day 11 after CAR-T cell infusion. Further RNA sequencing of these tumor samples identified gene expression signatures differentially enriched in complete and partial remission patients. Increased tumor-associated macrophage infiltration was negatively associated with remission status.429
In addition to studies targeting CD19 CAR-T cells, studies on CD20, CD22, and CD30 CAR-T cell therapy have also been carried out. In a phase 2 study (NCT01735604) of anti-CD20 CAR-T therapy, the ORR was 81.8% (CR 54.5%).430 In a phase 1 trial (NCT02315612), anti-CD22 CAR-T cells were evaluated in patients with B-cell malignancies resistant to CD19 CAR-T cells and showed a CR rate of 73%, with a median remission duration of 6 months.431 A phase 1 trial (NCT01306146) of anti-CD30 CAR-T cells showed a CR rate of 28.6% in relapsed HL and a CR rate of 50% in ALCL.432
Anti-CD4 CAR-T cells could control the growth of tumors in a xenograft murine model of ALCL.433 However, this therapy also faces the challenge of CAR-T cells sharing antigens with normal T cells and can recognize and kill three types of cells: tumor T cells, normal T cells, and CAR-T cells. This problem can lead to the “auto-phase killing” of CAR-T cells, while CAR-T cells targeting normal T cells may lead to severe infection in patients.434 Therefore, reducing the side effects of CAR-T cells in T-NHLs has become the focus of research. Moreover, using CRISPR/Cas9 gene-editing technology, generating CAR-T cells (also known as UCART7) that lack CD7 and TCR alpha-chain expression could target CD7+ T-cell malignancies and reduce mutual attacks between CAR-T cells.435
Although CAR-T therapy has been successful in the treatment of hematological malignancies, there are still patients who do not respond to the treatment, as well as some patients presenting signs of AEs such as severe cytokine release syndrome (CRS), infection, and neurotoxicity. Therefore, identifying patients who may respond to CAR-T therapy and patients who may have serious side effects during treatment has become a research hotspot.
CAR-NK therapy
With the continuous development of CAR-T therapy, CAR-NK cells have also become a focus of attention. NK cells are cytotoxic immune cells that form a small fraction of normal lymphocytes and can trigger the innate immune response against tumor cells and virus-infected cells.436 Studies have shown that NK cells have a nonnegligible role in tumor monitoring, and loss of NK cells leads to tumor progression.437–439 Because NK and T cells are functionally similar, NK cells can also be used to attack tumors. Many researchers hope that CAR-NK cells can achieve results in tumor treatment similar to CAR-T cells. Compared with T cells, NK cells kill tumor cells in a nonantigen-dependent manner. Moreover, NK cells express CD56 and CD7 but lack the expression of CD3, TCR, and CD5.440 When used in the treatment of T-NHLs, the fratricide of CAR-NK cells was reduced.441
Anti-CD19 cord blood (CB)-derived NK cells were evaluated in a xenograft lymphoma murine model and significantly prolonged the survival of mice.442 In another study, anti-CD5 CAR-NKs had potent antitumor activity against a variety of T-NHLs and primary tumor cells in vitro and in a murine model.443 Clinical trials (NCT03383965, NCT029170083 and NCT03049449) of CAR-NK cells targeting CD19, CD20, and CD22 have begun for the treatment of B-NHLs. In addition, a phase 1 trial (NCT02742727) of anti-CD7 CAR-NK for the treatment of T-cell malignancies is ongoing.
Specific oncogenes and proteins related to targeted therapy
Specific oncogenes, such as MYC, BCL-2, and BCL-6, converge proliferation, differentiation, and anti-apoptotic signaling in lymphoma cells and play critical roles in lymphomas. Moreover, lymphomas that have a concomitant translocation of MYC and BCL-2 or BCL-6 represent high-grade B-cell lymphoma and are resistant to conventional R-CHOP chemotherapy. The tumor suppressor gene p53 is involved in the process of DNA repair, and the depletion or mutation of p53 promotes lymphoma progression and drug resistance. The t(2;5)(p23;q35) translocation results in the NPM1/ALK fusion protein and then activates the downstream oncogenic transcription factor STAT3, enhancing lymphoma cell proliferation and growth. Thus, these specific oncogenes are greatly involved in lymphoma genesis and progression, and targeting these genes and their downstream pathways might retard tumor progression and improve patient survival.
MYC
MYC is a family of three proto-oncogenes that function as important regulators of cell proliferation, growth, differentiation, and apoptosis. They encode the related transcription factors MYC, MYCN, and MYCL, also known as c-MYC, N-MYC, and L-MYC, respectively.444 The Ig-MYC translocation is the most common type of MYC alteration and can cause MYC overexpression.445 MYC is expressed at the pro-B and pre-B-cell stages and in a minority of GC B-cells.446–448 Additionally, MYC is frequently overexpressed in lymphomas of GC origin. In BL, the t(8;14) translocation is found in approximately 80% of all patients.449 In DLBCL, MYC overexpression is shown in 30–50% of patients.450,451 MYC translocations preferentially occur in GCB-DLBCL over ABC-DLBCL (17.7% vs. 6.7%).450 A high level of MYC is associated with a low treatment response and poor prognosis in DLBCL patients treated with R-CHOP and may also lead to an increased relapse rate in the central nervous system.452,453
Studies have shown the potential effect of MYC-associated agents, including targeting cell-cycle-associated vulnerabilities, transcription, RNA processing and turnover, ribosome biogenesis and translation, as well as MYC-induced metabolic perturbations.454 The mitotic spindle-regulatory kinases Aurora-A and Aurora-B are both overexpressed in MYC-associated B-cells, and Aurora-A promotes the stabilization of MYC and MYCN.455,456 The targeted drugs and clinical trials related to specific oncogenes and proteins are shown in Table 7. A phase 1 trial (NCT01897012) of alisertib combined with romidepsin in relapsed or refractory NHLs is ongoing.
Table 7.
Targeted drugs and clinical trials related to specific oncogenes and proteins
| Drug | Disease | Trail name | Phase | Status | ORR/CR | NCT# | Reference |
|---|---|---|---|---|---|---|---|
| MYC | |||||||
| Alisertib | a selective Aurora-A inhibitor | ||||||
| Alisertib, romidepsin | Relapsed or refractory NHLs | Alisertib and romidepsin in treating patients with relapsed or refractory B-cell or T-cell lymphomas | 1 | Completed | NA | NCT01897012 | – |
| BCL-2 | |||||||
| Navitoclax | A BCL-2, BCL-XL, and BCL-w inhibitor | ||||||
| Navitoclax, rituximab | Lymphoid cancers | Safety study of navitoclax in combination with rituximab in lymphoid cancers | 1 | Active, not recruiting | – | NCT00788684 | – |
| Venetoclax | A highly selective BH3 mimetic | ||||||
| Venetoclax | relapsed or refractory NHLs/CLL | A phase 1 study evaluating the safety and pharmacokinetics of venetoclax in subjects with relapsed or refractory non-Hodgkin’s lymphoma and chronic lymphocytic leukemia | 1 | Active, not recruiting | MCL, 75%/21%; FL, 38%/14%; DLBCL, 18%/12%; MZL, 67%/0% | NCT01328626 | 472 |
| Venetoclax, bendamustine, rituximab | Relapsed or refractory FL | A study evaluating the safety and efficacy of venetoclax plus bendamustine and rituximab in comparison with bendamustine plus rituximab or venetoclax plus rituximab in participants with relapsed and refractory FL | 2 | Completed | Venetoclax, rituximab, 32.7%/13.2%; venetoclax, BR, 45.1%/27.5%; BR, 51%/23.5% | NCT02187861 | – |
| Venetoclax, ibrutinib | MCL | Study of venetoclax combined with ibrutinib in subjects with mantle cell lymphoma (SYMPATICO) | 3 | Active, not recruiting | – | NCT03112174 | – |
| Venetoclax, RO6870810, rituximab | Relapsed or refractory DLBCL/high-grade B-cell lymphoma | A study to evaluate safety, pharmacokinetics, and clinical activity of combination of venetoclax and RO6870810, With or without rituximab, in participants with relapsed or refractory DLBCL and high-grade B-cell lymphoma | 1 | Active, not recruiting | – | NCT03255096 | – |
| Venetoclax, R-CHOP/G-CHOP | B-NHLs | A safety and pharmacokinetics study of venetoclax in participants with non-Hodgkin’s lymphoma | 1/2 | Completed | Phase 1 part: venetoclax, R-CHOP, 87.5%/79.2%; venetoclax, G-CHOP, 87.5%/78.1% | NCT02055820 | 473 |
| Venetoclax, DA-R-EPOCH | Aggressive B-NHLs | Study of venetoclax plus DA-R-EPOCH for the treatment of aggressive B-cell lymphomas | 1 | Active, not recruiting | – | NCT03036904 | – |
| TP53 | |||||||
| Idasanutlin | A potent and selective MDM2 antagonist | ||||||
| Idasanutlin, obinutuzumab/rituximab, venetoclax | Relapsed or refractory FL/DLBCL | A study of obinutuzumab in combination with idasanutlin and venetoclax in participants with relapsed or refractory follicular lymphoma or rituximab in combination with idasanutlin and venetoclax in participants with relapsed or refractory diffuse large B-cell lymphoma | 1/2 | Active, not recruiting | – | NCT03135262 | – |
| Selinexor | An inhibitor of exportin 1 | ||||||
| Selinexor | Advanced hematological cancer | Safety study of the selective inhibitor of nuclear export selinexor in patients with advanced hematological cancer | 1 | Completed | 31%/6% | NCT01607892 | 489 |
| Selinexor, chemotherapy | Advanced B-NHLs | Selinexor plus chemotherapy in treating patients with advanced B-cell non-Hodgkin’s lymphoma | 1/2 | Recruiting | – | NCT03147885 | – |
| ALK | |||||||
| Crizotinib | The first-generation ALK tyrosine kinase inhibitor | ||||||
| Crizotinib | Relapsed ALK+ lymphomas | Pilot study of crizotinib in relapsed ALK+ Lymphomas | 2 | Recruiting | – | NCT02419287 | – |
| Brigatinib | The second-generation ALK tyrosine kinase inhibitor | ||||||
| Brigatinib | Relapsed or refractory ALK+ ALCL | Brigatinib in relapsed or refractory ALK+ anaplastic large cell lymphoma | 2 | Recruiting | – | NCT03719898 | – |
| Lorlatinib | The third-generation ALK tyrosine kinase inhibitor | ||||||
| Lorlatinib | Relapsed ALK+ lymphoma | A study of oral lorlatinib in patients with relapsed ALK+ lymphoma (CRU3) | 2 | Recruiting | – | NCT03505554 | – |
NA: ORR or CR are not available on the clinicaltrials.gov or from the published article although the trial has been completed
R-CHOP rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone, G-CHOP obinutuzumab, cyclophosphamide, doxorubicin, vincristine and prednisone, DA-R-EPOCH rituximab, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin
BCL-2
The BCL-2 family of proteins regulates the intrinsic pathway of mitochondrial apoptosis457 and can be divided into three groups: anti-apoptotic proteins (BH1-4 domains), multi-BH domain pro-apoptotic proteins (BH1-3 domains), and BH3-only pro-apoptotic proteins. The t(14;18)(q32;q21) translocation is a common type of BCL-2 translocation.458 Mutated BCL-2 affects cells in several aspects, such as proliferation, apoptosis, angiogenesis, and metastasis, resulting in the development of hematological malignancies.459,460 BCL-2 translocation is the major hallmark of FL (>80% of samples); it occurs in bone marrow pre-B cells and leads to high BCL-2 protein expression.461 Chromosome 18q21 amplification leads to BCL-2 overexpression and is observed in patients with MCL.462 BCL-2 overexpression is also detected in approximately 30% of DLBCL.463 The term double-hit lymphoma (DHL) refers to a subset of DLBCLs that present concurrent rearrangements of MYC and BCL-2 (sometimes BCL-6).464 DHL is present in 5–10% of DLBCL and is mostly classified as the GCB subtype, with highly aggressive clinical behavior and poor response to frontline regimens.465,466 The term double-expressor lymphoma (DEL) refers to a subset of DLBCLs that show the coexpression of MYC (>40%) and BCL2 (>50%) by immunohistochemistry in the absence of chromosomal translocations. DEL is present in 25–30% of DLBCL and is mostly classified as the ABC subtype, which is also associated with poor clinical outcomes.466,467
ABT-737, which binds to BCL-2, BCL-XL, and BCL-w with high affinity, had promising preclinical effects in CLL.468,469 Navitoclax (ABT-263), the orally available derivative of ABT-737,470 was shown to provoke transient thrombocytopenia in phase 2 trials of patients with B-NHLs due to the importance of BCL-XL for the survival of platelets.471 A phase 1 trial of navitoclax combined with rituximab (NCT00788684) in lymphoid cancers is ongoing. Venetoclax (ABT-199), a highly selective BH3 mimetic, is designed to treat lymphomas with BCL-2 translocations. A phase 1 trial (NCT01328626) of venetoclax in relapsed or refractory NHLs showed an ORR of 75% (CR 21%) in MCL, an ORR of 38% (CR 14%) in FL, an ORR of 18% (CR 12%) in DLBCL and an ORR of 67% (CR 0%) in MCL.472 A phase 2 study (NCT02187861) of venetoclax plus rituximab vs. venetoclax plus BR in patients with relapsed or refractory FL was completed. The results showed an ORR of 32.7% (CR 13.2%) in the venetoclax plus rituximab group, an ORR of 45.1% (CR 27.5%) in the venetoclax plus BR group, and an ORR of 51% (CR 23.5%) in the BR group. Many clinical trials on combination therapy of venetoclax and chemotherapy or other targeted agents are active. In MCL, a phase 3 randomized, double-blind study (NCT03112174) to compare the efficacy and safety of the combination of ibrutinib and venetoclax vs. ibrutinib and placebo is ongoing. A phase 1 study (NCT03255096) on the combination of RO6870810 (a bromodomain inhibitor) and venetoclax, with or without rituximab, in relapsed or refractory DLBCL and high-grade B-cell lymphoma is ongoing. To test the effect of venetoclax in combination with chemotherapy, a study (NCT02055820) of venetoclax in combination with R-CHOP or obinutuzumab plus CHOP (G-CHOP) in previously untreated DLBCL was performed, and the results demonstrated an ORR of 87.5% (CR 79.2%) in the venetoclax plus R-CHOP group and an ORR of 87.5% (CR 78.1%) in the venetoclax plus G-CHOP group. Moreover, 87.5% of DEL patients achieved CR.473 Another phase 1 trial (NCT03036904) of venetoclax plus DA-R-EPOCH is also active for aggressive B-NHLs.
BCL-6
BCL-6 was initially discovered as an oncogene in B-NHLs. The BCL6 protein is an evolutionarily conserved zinc finger transcription factor with an N-terminal broad-complex, tram track and bric-a-brac/Pox virus and zinc finger (BTB/POZ) domain and functions as a transcriptional repressor.474 Transcription factors, transcriptional corepressors, signaling mediators, and catalytic enzymes can be regulated by BCL-6. Studies have shown that BCL-6 overexpression inhibits reactive oxygen species (ROS) generation and represses the apoptosis induced by chemotherapy in B-NHL cells.475,476 Similar to BCL-2, BCL-6 is the key factor for the development and maintenance of GCs within lymphoid follicles. Once GC B-cells begin their differentiation into memory B-cells and PCs with an appropriate affinity for the inciting antigen, BCL-6 will be phosphorylated and subsequently degraded by the proteasome.476 Moreover, BCL-6 regulates TFH cell differentiation.477,478 BCL-6 translocations are found in 40% of DLBCL, 48% of nodular lymphocyte-predominant Hodgkin lymphoma, and 5-10% of FL.445,479,480 ABC-DLBCL patients have more BCL6 translocations than GCB-DLBCL patients (24% vs. 10%). In T-NHLs, BCL-6 is detectable in some types of PTCL, especially ALK+ ALCL and lymphomas derived from TFH cells, particularly AITL.481,482 Oncogene addiction is switched to BCL-2 and BCL-XL in the context of BCL-6 inhibition.483 To solve this problem, a combined treatment of RI-BPI (a BCL-6 inhibitor) and ABT-737 might be a choice but needs more experimental verification.
p53
The p53 transcription factor plays an important role in regulating cell survival by activating gene transcription that is involved in apoptosis and other biological functions.484 Notably, p53 can interact with the BCL-2 pathway by directly and indirectly regulating the anti-apoptotic activity of the BCL-2 family of proteins.485 With a negative feedback response, the E3 ubiquitin ligase MDM2 can bind p53 for degradation, maintaining a low expression level of p53 under normal conditions.486 The dysregulation of p53 can be found in many types of lymphomas, including DLBCL (16–30%), MCL (21–45%), FL (9–29%), and MZL (8–12%).458 It is often regarded as an independent prognostic factor for poor outcomes and a signal for chemotherapy resistance.487 Targeting p53 can potentially restart apoptosis and trigger cell death. Idasanutlin (RG7388), a potent and selective MDM2 antagonist, when combined with obinutuzumab and venetoclax, showed significant antitumor activity in xenograft models.488 A phase 1/2 trial (NCT03135262) of idasanutlin in combination with rituximab and venetoclax in relapsed or refractory DLBCL patients is ongoing. Selinexor, an inhibitor of exportin 1 (XPO1), inhibits the nuclear export of p53 and restores p53 nuclear localization. A phase 1 study (NCT01607892) of selinexor showed an ORR of 31% (CR 6%) in advanced NHLs.489 A study of selinexor combined with chemotherapy (NCT03147885) in advanced B-NHLs is ongoing.
ALK
ALK+ ALCL is characterized by the expression of ALK fusion proteins.464 The major type of ALK fusion is the t(2;5)(p23;q35) translocation, which is detectable in approximately 75–85% of ALK+ ALCL.490,491 All fusion proteins can activate the downstream oncogenic transcription factor STAT3 and promote proliferation and growth in cancer cells.492 Many inhibitors are clinically available for targeting ALK tyrosine kinase activity, including crizotinib as a first-generation agent, ceritinib and brigatinib as second-generation agents and lorlatinib as a third-generation agent.493 The study of crizotinib in relapsed and refractory ALK+ lymphomas showed an ORR of 90.5%, with a 2-year PFS of 63.7% and a 2-year OS of 72.7%.494 Thus, a phase 2 trial of crizotinib (NCT02419287) and brigatinib (NCT03719898) in relapsed ALK+ lymphomas is ongoing. Moreover, because of acquired resistance from first- and second-generation agents, a phase 2 study (NCT03505554) to define the ORR of lorlatinib in patients with ALK+ lymphomas resistant or refractory to ALK inhibitors is ongoing.
Conclusions
With the understanding of the biological function of surface markers, signaling transduction pathways, and epigenetic modulations as well as the orchestration of the microenvironment with lymphoma cells in lymphoma progression, many novel agents and immune therapeutic strategies have been developed. These therapies enable clinicians to perform precision medicine and significantly improve the prognosis of patients. However, many questions remain to be answered, such as treatment scheduling, optimized dosage and combinations with other agents. The identification of potential biomarkers that can predict the clinical responses and toxicities of these targeted therapies is challenging. In conclusion, mechanism-based targeted therapy is a promising strategy to eventually make lymphoma a curable disease.
Supplementary information
Acknowledgements
This study was supported, in part, by research funding from the National Natural Science Foundation of China (81520108003, 81830007, and 81670176), the Chang Jiang Scholars Program, the Shanghai Commission of Science and Technology (16JC1405800), the Shanghai Municipal Education Commission Gaofeng Clinical Medicine Grant Support (20152206 and 20152208), the Clinical Research Plan of Shanghai Hospital Development Center (SHDC, 16CR2017A), the Multicenter Clinical Research Project by Shanghai Jiao Tong University School of Medicine (DLY201601), the Collaborative Innovation Center of Systems Biomedicine, the Samuel Waxman Cancer Research Foundation, the innovative research team of high-level local universities in Shanghai, and the shared Research Project Grants, University of Sydney and SJTU.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Li Wang, Wei Qin
Supplementary information
The online version of this article (10.1038/s41392-020-0113-2) contains supplementary material, which is available to authorised users.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J. Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Coiffier B, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N. Engl. J. Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 3.Younes A, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N. Engl. J. Med. 2010;363:1812–1821. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 4.Krysiak K, et al. Recurrent somatic mutations affecting B-cell receptor signaling pathway genes in follicular lymphoma. Blood. 2017;129:473–483. doi: 10.1182/blood-2016-07-729954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neelapu SS, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, et al. Structural and functional analysis of the costimulatory receptor programmed death-1. Immunity. 2004;20:337–347. doi: 10.1016/S1074-7613(04)00051-2. [DOI] [PubMed] [Google Scholar]
- 7.Tedder TF, Engel P. CD20: a regulator of cell-cycle progression of B lymphocytes. Immunol. Today. 1994;15:450–454. doi: 10.1016/0167-5699(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 8.Liu AY, et al. Production of a mouse-human chimeric monoclonal antibody to CD20 with potent Fc-dependent biologic activity. J. Immunol. 1987;139:3521–3526. [PubMed] [Google Scholar]
- 9.Beers SA, Chan CH, French RR, Cragg MS, Glennie MJ. CD20 as a target for therapeutic type I and II monoclonal antibodies. Semin. Hematol. 2010;47:107–114. doi: 10.1053/j.seminhematol.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Feugier P, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J. Clin. Oncol. 2005;23:4117–4126. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 11.Pfreundschuh M, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 12.Salles G, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011;377:42–51. doi: 10.1016/S0140-6736(10)62175-7. [DOI] [PubMed] [Google Scholar]
- 13.Teeling JL, et al. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood. 2004;104:1793–1800. doi: 10.1182/blood-2004-01-0039. [DOI] [PubMed] [Google Scholar]
- 14.Wierda WG, et al. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J. Clin. Oncol. 2010;28:1749–1755. doi: 10.1200/JCO.2009.25.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wierda WG, et al. Ofatumumab is active in patients with fludarabine-refractory CLL irrespective of prior rituximab: results from the phase 2 international study. Blood. 2011;118:5126–5129. doi: 10.1182/blood-2011-04-348656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wierda WG, et al. Chemoimmunotherapy with O-FC in previously untreated patients with chronic lymphocytic leukemia. Blood. 2011;117:6450–6458. doi: 10.1182/blood-2010-12-323980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golay J, et al. Glycoengineered CD20 antibody obinutuzumab activates neutrophils and mediates phagocytosis through CD16B more efficiently than rituximab. Blood. 2013;122:3482–3491. doi: 10.1182/blood-2013-05-504043. [DOI] [PubMed] [Google Scholar]
- 18.Herter S, et al. Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Mol. Cancer Ther. 2013;12:2031–2042. doi: 10.1158/1535-7163.MCT-12-1182. [DOI] [PubMed] [Google Scholar]
- 19.Morschhauser FA, et al. Obinutuzumab (GA101) monotherapy in relapsed/refractory diffuse large b-cell lymphoma or mantle-cell lymphoma: results from the phase II GAUGUIN study. J. Clin. Oncol. 2013;31:2912–2919. doi: 10.1200/JCO.2012.46.9585. [DOI] [PubMed] [Google Scholar]
- 20.Sehn LH, et al. Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab-refractory indolent non-Hodgkin lymphoma (GADOLIN): a randomised, controlled, open-label, multicentre, phase 3 trial. Lancet Oncol. 2016;17:1081–1093. doi: 10.1016/S1470-2045(16)30097-3. [DOI] [PubMed] [Google Scholar]
- 21.Radford J, et al. Obinutuzumab (GA101) plus CHOP or FC in relapsed/refractory follicular lymphoma: results of the GAUDI study (BO21000) Blood. 2013;122:1137–1143. doi: 10.1182/blood-2013-01-481341. [DOI] [PubMed] [Google Scholar]
- 22.Marcus R, et al. Obinutuzumab for the first-line treatment of follicular lymphoma. N. Engl. J. Med. 2017;377:1331–1344. doi: 10.1056/NEJMoa1614598. [DOI] [PubMed] [Google Scholar]
- 23.Goede V, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N. Engl. J. Med. 2014;370:1101–1110. doi: 10.1056/NEJMoa1313984. [DOI] [PubMed] [Google Scholar]
- 24.Moreno C, et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:43–56. doi: 10.1016/S1470-2045(18)30788-5. [DOI] [PubMed] [Google Scholar]
- 25.Le Garff-Tavernier M, et al. Antibody-dependent cellular cytotoxicity of the optimized anti-CD20 monoclonal antibody ublituximab on chronic lymphocytic leukemia cells with the 17p deletion. Leukemia. 2014;28:230–233. doi: 10.1038/leu.2013.240. [DOI] [PubMed] [Google Scholar]
- 26.Sawas A, et al. A phase 1/2 trial of ublituximab, a novel anti-CD20 monoclonal antibody, in patients with B-cell non-Hodgkin lymphoma or chronic lymphocytic leukaemia previously exposed to rituximab. Br. J. Haematol. 2017;177:243–253. doi: 10.1111/bjh.14534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharman JP, et al. Ublituximab (TG-1101), a novel glycoengineered anti-CD20 antibody, in combination with ibrutinib is safe and highly active in patients with relapsed and/or refractory chronic lymphocytic leukaemia: results of a phase 2 trial. Br. J. Haematol. 2017;176:412–420. doi: 10.1111/bjh.14447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharman JP, Brander DM, Mato A, Kambhampati S, Flinn IW. Ublituximab and ibrutinib for previously treated genetically high-risk chronic lymphocytic leukemia: results of the genuine phase 3 stUDY. Hematol. Oncol. 2017;35:111–112. doi: 10.1002/hon.2437_100. [DOI] [Google Scholar]
- 29.Nastoupil LJ, et al. Tolerability and activity of ublituximab, umbralisib, and ibrutinib in patients with chronic lymphocytic leukaemia and non-Hodgkin lymphoma: a phase 1 dose escalation and expansion trial. Lancet Haematol. 2019;6:e100–e109. doi: 10.1016/S2352-3026(18)30216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lunning M, et al. Ublituximab and umbralisib in relapsed/refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2019;134:1811–1820. doi: 10.1182/blood.2019002118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morschhauser F, et al. Humanized anti-CD20 antibody, veltuzumab, in refractory/recurrent non-Hodgkin’s lymphoma: phase I/II results. J. Clin. Oncol. 2009;27:3346–3353. doi: 10.1200/JCO.2008.19.9117. [DOI] [PubMed] [Google Scholar]
- 32.Morschhauser F, et al. Results of a phase I/II study of ocrelizumab, a fully humanized anti-CD20 mAb, in patients with relapsed/refractory follicular lymphoma. Ann. Oncol. 2010;21:1870–1876. doi: 10.1093/annonc/mdq027. [DOI] [PubMed] [Google Scholar]
- 33.Ogura M, et al. Efficacy, pharmacokinetics, and safety of the biosimilar CT-P10 in comparison with rituximab in patients with previously untreated low-tumour-burden follicular lymphoma: a randomised, double-blind, parallel-group, phase 3 trial. Lancet Haematol. 2018;5:e543–e553. doi: 10.1016/S2352-3026(18)30157-1. [DOI] [PubMed] [Google Scholar]
- 34.Kim WS, et al. Efficacy, pharmacokinetics, and safety of the biosimilar CT-P10 compared with rituximab in patients with previously untreated advanced-stage follicular lymphoma: a randomised, double-blind, parallel-group, non-inferiority phase 3 trial. Lancet Haematol. 2017;4:e362–e373. doi: 10.1016/S2352-3026(17)30120-5. [DOI] [PubMed] [Google Scholar]
- 35.Jurczak W, et al. Rituximab biosimilar and reference rituximab in patients with previously untreated advanced follicular lymphoma (ASSIST-FL): primary results from a confirmatory phase 3, double-blind, randomised, controlled study. Lancet Haematol. 2017;4:e350–e361. doi: 10.1016/S2352-3026(17)30106-0. [DOI] [PubMed] [Google Scholar]
- 36.Sharman, J. P. et al. A randomized, double-blind, efficacy and safety study of PF-05280586 (a rituximab biosimilar) compared with rituximab reference product (MabThera®) in subjects with previously untreated CD20-positive, low-tumor-burden follicular lymphoma (LTB-FL). BioDrugs: clinical immunotherapeutics, biopharmaceuticals and gene therapy. 10.1007/s40259-019-00398-7 (2019). [DOI] [PMC free article] [PubMed]
- 37.Knox SJ, et al. Yttrium-90-labeled anti-CD20 monoclonal antibody therapy of recurrent B-cell lymphoma. Clin. Cancer Res. 1996;2:457–470. [PubMed] [Google Scholar]
- 38.Witzig TE, et al. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma. J. Clin. Oncol. 2002;20:2453–2463. doi: 10.1200/JCO.2002.11.076. [DOI] [PubMed] [Google Scholar]
- 39.Emmanouilides C, et al. Treatment with yttrium 90 ibritumomab tiuxetan at early relapse is safe and effective in patients with previously treated B-cell non-Hodgkin’s lymphoma. Leuk. Lymphoma. 2006;47:629–636. doi: 10.1080/10428190500376076. [DOI] [PubMed] [Google Scholar]
- 40.Guidetti A, et al. Myeloablative doses of yttrium-90-ibritumomab tiuxetan and the risk of secondary myelodysplasia/acute myelogenous leukemia. Cancer. 2011;117:5074–5084. doi: 10.1002/cncr.26182. [DOI] [PubMed] [Google Scholar]
- 41.Scholz CW, et al. (90)Yttrium-ibritumomab-tiuxetan as first-line treatment for follicular lymphoma: 30 months of follow-up data from an international multicenter phase II clinical trial. J. Clin. Oncol. 2013;31:308–313. doi: 10.1200/JCO.2011.41.1553. [DOI] [PubMed] [Google Scholar]
- 42.Illidge TM, et al. Fractionated 90Y-ibritumomab tiuxetan radioimmunotherapy as an initial therapy of follicular lymphoma: an international phase II study in patients requiring treatment according to GELF/BNLI criteria. J. Clin. Oncol. 2014;32:212. doi: 10.1200/JCO.2013.50.3110. [DOI] [PubMed] [Google Scholar]
- 43.Morschhauser F, et al. Phase III trial of consolidation therapy with yttrium-90-ibritumomab tiuxetan compared with no additional therapy after first remission in advanced follicular lymphoma. J. Clin. Oncol. 2008;26:5156–5164. doi: 10.1200/JCO.2008.17.2015. [DOI] [PubMed] [Google Scholar]
- 44.Morschhauser F, et al. 90Yttrium-ibritumomab tiuxetan consolidation of first remission in advanced-stage follicular non-Hodgkin lymphoma: updated results after a median follow-up of 7.3 years from the International, randomized, phase III First-LineIndolent trial. J. Clin. Oncol. 2013;31:1977–1983. doi: 10.1200/JCO.2012.45.6400. [DOI] [PubMed] [Google Scholar]
- 45.Krishnan A, et al. Phase II trial of a transplantation regimen of yttrium-90 ibritumomab tiuxetan and high-dose chemotherapy in patients with non-Hodgkin’s lymphoma. J. Clin. Oncol. 2008;26:90–95. doi: 10.1200/JCO.2007.11.9248. [DOI] [PubMed] [Google Scholar]
- 46.Briones J, et al. Autologous stem cell transplantation after conditioning with yttrium-90 ibritumomab tiuxetan plus BEAM in refractory non-Hodgkin diffuse large B-cell lymphoma: results of a prospective, multicenter, phase II clinical trial. Haematologica. 2014;99:505–510. doi: 10.3324/haematol.2013.093450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nitschke L, Carsetti R, Ocker B, Kohler G, Lamers MC. CD22 is a negative regulator of B-cell receptor signalling. Curr. Biol. 1997;7:133–143. doi: 10.1016/S0960-9822(06)00057-1. [DOI] [PubMed] [Google Scholar]
- 48.Sullivan-Chang L, O’Donnell RT, Tuscano JM. Targeting CD22 in B-cell malignancies: current status and clinical outlook. Biodrugs. 2013;27:293–304. doi: 10.1007/s40259-013-0016-7. [DOI] [PubMed] [Google Scholar]
- 49.Cyster JG, Goodnow CC. Tuning antigen receptor signaling by CD22: integrating cues from antigens and the microenvironment. Immunity. 1997;6:509–517. doi: 10.1016/S1074-7613(00)80339-8. [DOI] [PubMed] [Google Scholar]
- 50.Chang CH, Wang Y, Gupta P, Goldenberg DM. Extensive crosslinking of CD22 by epratuzumab triggers BCR signaling and caspase-dependent apoptosis in human lymphoma cells. MAbs. 2015;7:199–211. doi: 10.4161/19420862.2014.979081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tuscano JM, et al. Anti-CD22 ligand-blocking antibody HB22.7 has independent lymphomacidal properties and augments the efficacy of 90Y-DOTA-peptide-Lym-1 in lymphoma xenografts. Blood. 2003;101:3641–3647. doi: 10.1182/blood-2002-08-2629. [DOI] [PubMed] [Google Scholar]
- 52.Leonard JP, et al. Phase I/II trial of epratuzumab (humanized anti-CD22 antibody) in indolent non-Hodgkin’s lymphoma. J. Clin. Oncol. 2003;21:3051–3059. doi: 10.1200/JCO.2003.01.082. [DOI] [PubMed] [Google Scholar]
- 53.Leonard JP, et al. Epratuzumab, a humanized anti-CD22 antibody, in aggressive non-Hodgkin’s lymphoma: phase I/II clinical trial results. Clin. Cancer Res. 2004;10:5327–5334. doi: 10.1158/1078-0432.CCR-04-0294. [DOI] [PubMed] [Google Scholar]
- 54.Leonard JP, et al. Durable complete responses from therapy with combined epratuzumab and rituximab: final results from an international multicenter, phase 2 study in recurrent, indolent, non-Hodgkin lymphoma. Cancer. 2008;113:2714–2723. doi: 10.1002/cncr.23890. [DOI] [PubMed] [Google Scholar]
- 55.Grant BW, et al. A phase 2 trial of extended induction epratuzumab and rituximab for previously untreated follicular lymphoma: CALGB 50701. Cancer. 2013;119:3797–3804. doi: 10.1002/cncr.28299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Micallef IN, et al. Epratuzumab with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy in patients with previously untreated diffuse large B-cell lymphoma. Blood. 2011;118:4053–4061. doi: 10.1182/blood-2011-02-336990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.FitzGerald DJ, Wayne AS, Kreitman RJ, Pastan I. Treatment of hematologic malignancies with immunotoxins and antibody-drug conjugates. Cancer Res. 2011;71:6300–6309. doi: 10.1158/0008-5472.CAN-11-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DiJoseph JF, et al. Antibody-targeted chemotherapy of B-cell lymphoma using calicheamicin conjugated to murine or humanized antibody against CD22. Cancer Immunol. Immunother. 2005;54:11–24. doi: 10.1007/s00262-004-0572-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DiJoseph JF, et al. Antibody-targeted chemotherapy with CMC-544: a CD22-targeted immunoconjugate of calicheamicin for the treatment of B-lymphoid malignancies. Blood. 2004;103:1807–1814. doi: 10.1182/blood-2003-07-2466. [DOI] [PubMed] [Google Scholar]
- 60.Fayad L, et al. Safety and clinical activity of a combination therapy comprising two antibody-based targeting agents for the treatment of non-Hodgkin lymphoma: results of a phase I/II study evaluating the immunoconjugate inotuzumab ozogamicin with rituximab. J. Clin. Oncol. 2013;31:573–583. doi: 10.1200/JCO.2012.42.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dang NH, et al. Randomized, phase 3 trial of inotuzumab ozogamicin plus rituximab versus chemotherapy plus rituximab for relapsed/refractory aggressive B-cell non-Hodgkin lymphoma. Br. J. Haematol. 2018;182:583–586. doi: 10.1111/bjh.14820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salvatore G, Beers R, Margulies I, Kreitman RJ, Pastan I. Improved cytotoxic activity toward cell lines and fresh leukemia cells of a mutant anti-CD22 immunotoxin obtained by antibody phage display. Clin. Cancer Res. 2002;8:995–1002. [PubMed] [Google Scholar]
- 63.Mansfield E, Pastan I, FitzGerald DJ. Characterization of RFB4-Pseudomonas exotoxin A immunotoxins targeted to CD22 on B-cell malignancies. Bioconjug. Chem. 1996;7:557–563. doi: 10.1021/bc960043y. [DOI] [PubMed] [Google Scholar]
- 64.Kreitman RJ, et al. Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J. Clin. Oncol. 2012;30:1822–1828. doi: 10.1200/JCO.2011.38.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kreitman RJ, et al. Moxetumomab pasudotox in relapsed/refractory hairy cell leukemia. Leukemia. 2018;32:1768–1777. doi: 10.1038/s41375-018-0210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Younes A, Kadin ME. Emerging applications of the tumor necrosis factor family of ligands and receptors in cancer therapy. J. Clin. Oncol. 2003;21:3526–3534. doi: 10.1200/JCO.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 67.Aizawa S, et al. Tumor necrosis factor receptor-associated factor (TRAF) 5 and TRAF2 are involved in CD30-mediated NFkappaB activation. J. Biol. Chem. 1997;272:2042–2045. doi: 10.1074/jbc.272.4.2042. [DOI] [PubMed] [Google Scholar]
- 68.Schwab U, et al. Production of a monoclonal antibody specific for Hodgkin and Sternberg-Reed cells of Hodgkin’s disease and a subset of normal lymphoid cells. Nature. 1982;299:65–67. doi: 10.1038/299065a0. [DOI] [PubMed] [Google Scholar]
- 69.Mir SS, Richter BW, Duckett CS. Differential effects of CD30 activation in anaplastic large cell lymphoma and Hodgkin disease cells. Blood. 2000;96:4307–4312. doi: 10.1182/blood.V96.13.4307. [DOI] [PubMed] [Google Scholar]
- 70.Slack GW, Steidl C, Sehn LH, Gascoyne RD. CD30 expression in de novo diffuse large B-cell lymphoma: a population-based study from British Columbia. Br. J. Haematol. 2014;167:608–617. doi: 10.1111/bjh.13085. [DOI] [PubMed] [Google Scholar]
- 71.Sabattini E, et al. CD30 expression in peripheral T-cell lymphomas. Haematologica. 2013;98:e81–e82. doi: 10.3324/haematol.2013.084913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pierce JM, Mehta A. Diagnostic, prognostic and therapeutic role of CD30 in lymphoma. Expert Rev. Hematol. 2017;10:29–37. doi: 10.1080/17474086.2017.1270202. [DOI] [PubMed] [Google Scholar]
- 73.Wahl AF, et al. The anti-CD30 monoclonal antibody SGN-30 promotes growth arrest and DNA fragmentation in vitro and affects antitumor activity in models of Hodgkin’s disease. Cancer Res. 2002;62:3736–3742. [PubMed] [Google Scholar]
- 74.Forero-Torres A, et al. A Phase II study of SGN-30 (anti-CD30 mAb) in Hodgkin lymphoma or systemic anaplastic large cell lymphoma. Br. J. Haematol. 2009;146:171–179. doi: 10.1111/j.1365-2141.2009.07740.x. [DOI] [PubMed] [Google Scholar]
- 75.Blum KA, et al. Serious pulmonary toxicity in patients with Hodgkin’s lymphoma with SGN-30, gemcitabine, vinorelbine, and liposomal doxorubicin is associated with an FcgammaRIIIa-158 V/F polymorphism. Ann. Oncol. 2010;21:2246–2254. doi: 10.1093/annonc/mdq211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Francisco JA, et al. cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood. 2003;102:1458–1465. doi: 10.1182/blood-2003-01-0039. [DOI] [PubMed] [Google Scholar]
- 77.Story SK, Petrov AA, Geskin LJ. Successful desensitization to brentuximab vedotin after hypersensitivity reaction. J. Drugs Dermatol. 2014;13:749–751. [PubMed] [Google Scholar]
- 78.Gandhi MD, et al. Pancreatitis in patients treated with brentuximab vedotin: a previously unrecognized serious adverse event. Blood. 2014;123:2895–2897. doi: 10.1182/blood-2014-03-561878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Younes A, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J. Clin. Oncol. 2012;30:2183–2189. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pro B, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J. Clin. Oncol. 2012;30:2190–2196. doi: 10.1200/JCO.2011.38.0402. [DOI] [PubMed] [Google Scholar]
- 81.Horwitz SM, et al. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood. 2014;123:3095–3100. doi: 10.1182/blood-2013-12-542142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim YH, et al. Phase II investigator-initiated study of brentuximab vedotin in mycosis fungoides and sezary syndrome with variable CD30 expression level: a Multi-Institution Collaborative Project. J. Clin. Oncol. 2015;33:3750–3758. doi: 10.1200/JCO.2014.60.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Prince HM, et al. Brentuximab vedotin or physician’s choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open-label, randomised, phase 3, multicentre trial. Lancet. 2017;390:555–566. doi: 10.1016/S0140-6736(17)31266-7. [DOI] [PubMed] [Google Scholar]
- 84.Connors JM, et al. Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin’s Lymphoma. N. Engl. J. Med. 2018;378:331–344. doi: 10.1056/NEJMoa1708984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fanale MA, et al. Brentuximab vedotin in the front-line treatment of patients with CD30+ peripheral T-cell lymphomas: results of a phase I study. J. Clin. Oncol. 2014;32:3137–3143. doi: 10.1200/JCO.2013.54.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fanale MA, et al. Five-year outcomes for frontline brentuximab vedotin with CHP for CD30-expressing peripheral T-cell lymphomas. Blood. 2018;131:2120–2124. doi: 10.1182/blood-2017-12-821009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Horwitz S, et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet. 2019;393:229–240. doi: 10.1016/S0140-6736(18)32984-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Watanabe T, et al. CD52 is a novel costimulatory molecule for induction of CD4+ regulatory T cells. Clin. Immunol. 2006;120:247–259. doi: 10.1016/j.clim.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 89.Xia MQ, Hale G, Waldmann H. Efficient complement-mediated lysis of cells containing the CAMPATH-1 (CDw52) antigen. Mol. Immunol. 1993;30:1089–1096. doi: 10.1016/0161-5890(93)90155-5. [DOI] [PubMed] [Google Scholar]
- 90.Mone AP, et al. Alemtuzumab induces caspase-independent cell death in human chronic lymphocytic leukemia cells through a lipid raft-dependent mechanism. Leukemia. 2006;20:272–279. doi: 10.1038/sj.leu.2404014. [DOI] [PubMed] [Google Scholar]
- 91.Keating MJ, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood. 2002;99:3554–3561. doi: 10.1182/blood.V99.10.3554. [DOI] [PubMed] [Google Scholar]
- 92.Hillmen P, et al. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J. Clin. Oncol. 2007;25:5616–5623. doi: 10.1200/JCO.2007.12.9098. [DOI] [PubMed] [Google Scholar]
- 93.Lundin J, et al. Phase 2 study of alemtuzumab (anti-CD52 monoclonal antibody) in patients with advanced mycosis fungoides/Sezary syndrome. Blood. 2003;101:4267–4272. doi: 10.1182/blood-2002-09-2802. [DOI] [PubMed] [Google Scholar]
- 94.Enblad G, et al. A pilot study of alemtuzumab (anti-CD52 monoclonal antibody) therapy for patients with relapsed or chemotherapy-refractory peripheral T-cell lymphomas. Blood. 2004;103:2920–2924. doi: 10.1182/blood-2003-10-3389. [DOI] [PubMed] [Google Scholar]
- 95.Lepretre S, et al. Excess mortality after treatment with fludarabine and cyclophosphamide in combination with alemtuzumab in previously untreated patients with chronic lymphocytic leukemia in a randomized phase 3 trial. Blood. 2012;119:5104–5110. doi: 10.1182/blood-2011-07-365437. [DOI] [PubMed] [Google Scholar]
- 96.Montillo M, et al. Bendamustine and subcutaneous alemtuzumab combination is an effective treatment in relapsed/refractory chronic lymphocytic leukemia patients. Haematologica. 2014;99:e159–e161. doi: 10.3324/haematol.2014.106740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zent CS, et al. Chemoimmunotherapy for relapsed/refractory and progressive 17p13-deleted chronic lymphocytic leukemia (CLL) combining pentostatin, alemtuzumab, and low-dose rituximab is effective and tolerable and limits loss of CD20 expression by circulating CLL cells. Am. J. Hematol. 2014;89:757–765. doi: 10.1002/ajh.23737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim JG, et al. Alemtuzumab plus CHOP as front-line chemotherapy for patients with peripheral T-cell lymphomas: a phase II study. Cancer Chemother. Pharmacol. 2007;60:129–134. doi: 10.1007/s00280-007-0469-9. [DOI] [PubMed] [Google Scholar]
- 99.Gallamini A, et al. Alemtuzumab (Campath-1H) and CHOP chemotherapy as first-line treatment of peripheral T-cell lymphoma: results of a GITIL (Gruppo Italiano Terapie Innovative nei Linfomi) prospective multicenter trial. Blood. 2007;110:2316–2323. doi: 10.1182/blood-2007-02-074641. [DOI] [PubMed] [Google Scholar]
- 100.Kluin-Nelemans HC, et al. Intensified alemtuzumab-CHOP therapy for peripheral T-cell lymphoma. Ann. Oncol. 2011;22:1595–1600. doi: 10.1093/annonc/mdq635. [DOI] [PubMed] [Google Scholar]
- 101.d’Amore F, et al. Final analysis of the front-line phase III randomized ACT-1 trial in younger patients with systemic peripheral T-cell lymphoma treated with CHOP chemotherapy with or without alemtuzumab and consolidated by autologous hematopoietic stem cell transplant. Blood. 2018;132:998–998. doi: 10.1182/blood-2018-99-110429. [DOI] [Google Scholar]
- 102.Trumper, L. H., Wulf, G., Ziepert, M. & D’Amore, F. Alemtuzumab added to CHOP for treatment of peripheral T-cell lymphoma (pTNHL) of the elderly: final results of 116 patients treated in the international ACT-2 phase III trial. In American Society of Clinical Oncology 2016 Annual Meeting. abstract 7500 (2016).
- 103.Chu PG, Arber DA. CD79: a review. Appl. Immunohistochem. Mol. Morphol. 2001;9:97–106. doi: 10.1097/00129039-200106000-00001. [DOI] [PubMed] [Google Scholar]
- 104.Drake JR, Lewis TA, Condon KB, Mitchell RN, Webster P. Involvement of MIIC-like late endosomes in B cell receptor-mediated antigen processing in murine B cells. J. Immunol. 1999;162:1150–1155. [PubMed] [Google Scholar]
- 105.Niiro H, Clark EA. Regulation of B-cell fate by antigen-receptor signals. Nat. Rev. Immunol. 2002;2:945–956. doi: 10.1038/nri955. [DOI] [PubMed] [Google Scholar]
- 106.Polson AG, et al. Antibody-drug conjugates targeted to CD79 for the treatment of non-Hodgkin lymphoma. Blood. 2007;110:616–623. doi: 10.1182/blood-2007-01-066704. [DOI] [PubMed] [Google Scholar]
- 107.Morschhauser F, et al. Polatuzumab vedotin or pinatuzumab vedotin plus rituximab in patients with relapsed or refractory non-Hodgkin lymphoma: final results from a phase 2 randomised study (ROMULUS) Lancet Haematol. 2019;6:e254–e265. doi: 10.1016/S2352-3026(19)30026-2. [DOI] [PubMed] [Google Scholar]
- 108.Sehn, L. H., Kamdar, M. & Herrara, A. F. Adding polatuzumab vedotin to bendamustine and rituximab treatment improves survival in patients with relapsed/refractory DLBCL: results of a phase II clinical trial. In 2018 EHA Congress. abstract S802 (2018).
- 109.Del Nagro CJ, et al. CD19 function in central and peripheral B-cell development. Immunol. Res. 2005;31:119–131. doi: 10.1385/IR:31:2:119. [DOI] [PubMed] [Google Scholar]
- 110.Watkins MP, Bartlett NL. CD19-targeted immunotherapies for treatment of patients with non-Hodgkin B-cell lymphomas. Expert Opin. Investig. Drugs. 2018;27:601–611. doi: 10.1080/13543784.2018.1492549. [DOI] [PubMed] [Google Scholar]
- 111.Ward E, et al. A glycoengineered anti-CD19 antibody with potent antibody-dependent cellular cytotoxicity activity in vitro and lymphoma growth inhibition in vivo. Br. J. Haematol. 2011;155:426–437. doi: 10.1111/j.1365-2141.2011.08857.x. [DOI] [PubMed] [Google Scholar]
- 112.Ohmachi K, et al. A multicenter phase I study of inebilizumab, a humanized anti-CD19 monoclonal antibody, in Japanese patients with relapsed or refractory B-cell lymphoma and multiple myeloma. Int. J. Hematol. 2019;109:657–664. doi: 10.1007/s12185-019-02635-9. [DOI] [PubMed] [Google Scholar]
- 113.Horton HM, et al. Potent in vitro and in vivo activity of an Fc-engineered anti-CD19 monoclonal antibody against lymphoma and leukemia. Cancer Res. 2008;68:8049–8057. doi: 10.1158/0008-5472.CAN-08-2268. [DOI] [PubMed] [Google Scholar]
- 114.Jurczak W, et al. Phase IIa study of the CD19 antibody MOR208 in patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma. Ann. Oncol. 2018;29:1266–1272. doi: 10.1093/annonc/mdy056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Salles GA, et al. Single-arm phase II study of MOR208 combined with lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma: L-mind. Blood. 2018;132:227–227. doi: 10.1182/blood-2018-99-113399. [DOI] [Google Scholar]
- 116.Trneny M, et al. A phase II multicenter study of the anti-CD19 antibody drug conjugate coltuximab ravtansine (SAR3419) in patients with relapsed or refractory diffuse large B-cell lymphoma previously treated with rituximab-based immunotherapy. Haematologica. 2018;103:1351–1358. doi: 10.3324/haematol.2017.168401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hicks SW, et al. The novel CD19-targeting antibody-drug conjugate huB4-DGN462 shows improved anti-tumor activity compared to SAR3419 in CD19-positive lymphoma and leukemia models. Haematologica. 2019;104:1633–1639. doi: 10.3324/haematol.2018.211011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zammarchi F, et al. ADCT-402, a PBD dimer-containing antibody drug conjugate targeting CD19-expressing malignancies. Blood. 2018;131:1094–1105. doi: 10.1182/blood-2017-10-813493. [DOI] [PubMed] [Google Scholar]
- 119.Moore K, Cooper SA, Jones DB. Use of the monoclonal antibody WR17, identifying the CD37 gp40-45 Kd antigen complex, in the diagnosis of B-lymphoid malignancy. J. Pathol. 1987;152:13–21. doi: 10.1002/path.1711520103. [DOI] [PubMed] [Google Scholar]
- 120.Press OW, Howell-Clark J, Anderson S, Bernstein I. Retention of B-cell-specific monoclonal antibodies by human lymphoma cells. Blood. 1994;83:1390–1397. doi: 10.1182/blood.V83.5.1390.1390. [DOI] [PubMed] [Google Scholar]
- 121.Payandeh Z, et al. Anti-CD37 targeted immunotherapy of B-Cell malignancies. Biotechnol. Lett. 2018;40:1459–1466. doi: 10.1007/s10529-018-2612-6. [DOI] [PubMed] [Google Scholar]
- 122.Zhao X, et al. Targeting CD37-positive lymphoid malignancies with a novel engineered small modular immunopharmaceutical. Blood. 2007;110:2569–2577. doi: 10.1182/blood-2006-12-062927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Byrd JC, et al. A phase 1 study evaluating the safety and tolerability of otlertuzumab, an anti-CD37 mono-specific ADAPTIR therapeutic protein in chronic lymphocytic leukemia. Blood. 2014;123:1302–1308. doi: 10.1182/blood-2013-07-512137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pagel JM, et al. Otlertuzumab (TRU-016), an anti-CD37 monospecific ADAPTIR() therapeutic protein, for relapsed or refractory NHL patients. Br. J. Haematol. 2015;168:38–45. doi: 10.1111/bjh.13099. [DOI] [PubMed] [Google Scholar]
- 125.Robak T, et al. Randomized phase 2 study of otlertuzumab and bendamustine versus bendamustine in patients with relapsed chronic lymphocytic leukaemia. Br. J. Haematol. 2017;176:618–628. doi: 10.1111/bjh.14464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gopal AK, et al. Phase 1b study of otlertuzumab (TRU-016), an anti-CD37 monospecific ADAPTIR therapeutic protein, in combination with rituximab and bendamustine in relapsed indolent lymphoma patients. Invest. N. Drugs. 2014;32:1213–1225. doi: 10.1007/s10637-014-0125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Deckert J, et al. A novel anti-CD37 antibody-drug conjugate with multiple anti-tumor mechanisms for the treatment of B-cell malignancies. Blood. 2013;122:3500–3510. doi: 10.1182/blood-2013-05-505685. [DOI] [PubMed] [Google Scholar]
- 128.Beckwith KA, et al. The CD37-targeted antibody-drug conjugate IMGN529 is highly active against human CLL and in a novel CD37 transgenic murine leukemia model. Leukemia. 2014;28:1501–1510. doi: 10.1038/leu.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stathis A, et al. Safety, tolerability, and preliminary activity of IMGN529, a CD37-targeted antibody-drug conjugate, in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: a dose-escalation, phase I study. Invest. N. Drugs. 2018;36:869–876. doi: 10.1007/s10637-018-0570-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pereira DS, et al. AGS67E, an anti-CD37 monomethyl auristatin E antibody-drug conjugate as a potential therapeutic for B/T-cell malignancies and AML: a new role for CD37 in AML. Mol. Cancer Ther. 2015;14:1650–1660. doi: 10.1158/1535-7163.MCT-15-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.D’Ambrosio D, et al. Selective up-regulation of chemokine receptors CCR4 and CCR8 upon activation of polarized human type 2 Th cells. J. Immunol. 1998;161:5111–5115. [PubMed] [Google Scholar]
- 132.Iellem A, et al. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J. Exp. Med. 2001;194:847–853. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ishida T, et al. CXC chemokine receptor 3 and CC chemokine receptor 4 expression in T-cell and NK-cell lymphomas with special reference to clinicopathological significance for peripheral T-cell lymphoma, unspecified. Clin. Cancer Res. 2004;10:5494–5500. doi: 10.1158/1078-0432.CCR-04-0371. [DOI] [PubMed] [Google Scholar]
- 134.Ishida T, et al. Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: its close association with skin involvement and unfavorable outcome. Clin. Cancer Res. 2003;9:3625–3634. [PubMed] [Google Scholar]
- 135.Pease JE, Horuk R. Recent progress in the development of antagonists to the chemokine receptors CCR3 and CCR4. Expert Opin. Drug Discov. 2014;9:467–483. doi: 10.1517/17460441.2014.897324. [DOI] [PubMed] [Google Scholar]
- 136.Ni X, et al. Reduction of regulatory T cells by mogamulizumab, a defucosylated anti-CC chemokine receptor 4 antibody, in patients with aggressive/refractory mycosis fungoides and Sezary syndrome. Clin. Cancer Res. 2015;21:274–285. doi: 10.1158/1078-0432.CCR-14-0830. [DOI] [PubMed] [Google Scholar]
- 137.Makita S, Tobinai K. Mogamulizumab for the treatment of T-cell lymphoma. Expert Opin. Biol. Ther. 2017;17:1145–1153. doi: 10.1080/14712598.2017.1347634. [DOI] [PubMed] [Google Scholar]
- 138.Ishida T, et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J. Clin. Oncol. 2012;30:837–842. doi: 10.1200/JCO.2011.37.3472. [DOI] [PubMed] [Google Scholar]
- 139.Ishida T, et al. Dose-intensified chemotherapy alone or in combination with mogamulizumab in newly diagnosed aggressive adult T-cell leukaemia-lymphoma: a randomized phase II study. Br. J. Haematol. 2015;169:672–682. doi: 10.1111/bjh.13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Duvic M, et al. Phase 1/2 study of mogamulizumab, a defucosylated anti-CCR4 antibody, in previously treated patients with cutaneous T-cell lymphoma. Blood. 2015;125:1883–1889. doi: 10.1182/blood-2014-09-600924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ogura M, et al. Multicenter phase II study of mogamulizumab (KW-0761), a defucosylated anti-cc chemokine receptor 4 antibody, in patients with relapsed peripheral T-cell lymphoma and cutaneous T-cell lymphoma. J. Clin. Oncol. 2014;32:1157–1163. doi: 10.1200/JCO.2013.52.0924. [DOI] [PubMed] [Google Scholar]
- 142.Kim YH, et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19:1192–1204. doi: 10.1016/S1470-2045(18)30379-6. [DOI] [PubMed] [Google Scholar]
- 143.Kaplon H, Reichert JM. Antibodies to watch in 2019. MAbs. 2019;11:219–238. doi: 10.1080/19420862.2018.1556465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Foss F. Clinical experience with denileukin diftitox (ONTAK) Semin. Oncol. 2006;33:S11–S16. doi: 10.1053/j.seminoncol.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 145.Janik JE, et al. 90Y-daclizumab, an anti-CD25 monoclonal antibody, provided responses in 50% of patients with relapsed Hodgkin’s lymphoma. Proc. Natl Acad. Sci. U.S.A. 2015;112:13045–13050. doi: 10.1073/pnas.1516107112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lin P, Owens R, Tricot G, Wilson CS. Flow cytometric immunophenotypic analysis of 306 cases of multiple myeloma. Am. J. Clin. Pathol. 2004;121:482–488. doi: 10.1309/74R4TB90BUWH27JX. [DOI] [PubMed] [Google Scholar]
- 147.Parry-Jones N, et al. Cytogenetic abnormalities additional to t(11;14) correlate with clinical features in leukaemic presentation of mantle cell lymphoma, and may influence prognosis: a study of 60 cases by FISH. Br. J. Haematol. 2007;137:117–124. doi: 10.1111/j.1365-2141.2007.06526.x. [DOI] [PubMed] [Google Scholar]
- 148.Wang L, et al. CD38 expression predicts poor prognosis and might be a potential therapy target in extranodal NK/T cell lymphoma, nasal type. Ann. Hematol. 2015;94:1381–1388. doi: 10.1007/s00277-015-2359-2. [DOI] [PubMed] [Google Scholar]
- 149.Kim W-S, et al. Daratumumab monotherapy for patients with relapsed or refractory (R/R) natural killer/T-cell lymphoma (NKTCL), nasal type: an open-label, single-arm, multicenter phase 2 study. Blood. 2018;132:1617–1617. doi: 10.1182/blood-2018-99-111885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.van Kooten C, Banchereau J. Functions of CD40 on B cells, dendritic cells and other cells. Curr. Opin. Immunol. 1997;9:330–337. doi: 10.1016/S0952-7915(97)80078-7. [DOI] [PubMed] [Google Scholar]
- 151.Gruss HJ, et al. CD40/CD40 ligand interactions in normal, reactive and malignant lympho-hematopoietic tissues. Leuk. Lymphoma. 1997;24:393–422. doi: 10.3109/10428199709055580. [DOI] [PubMed] [Google Scholar]
- 152.Advani R, et al. Phase I study of the humanized anti-CD40 monoclonal antibody dacetuzumab in refractory or recurrent non-Hodgkin’s lymphoma. J. Clin. Oncol. 2009;27:4371–4377. doi: 10.1200/JCO.2008.21.3017. [DOI] [PubMed] [Google Scholar]
- 153.de Vos S, et al. A phase II study of dacetuzumab (SGN-40) in patients with relapsed diffuse large B-cell lymphoma (DLBCL) and correlative analyses of patient-specific factors. J. Hematol. Oncol. 2014;7:44. doi: 10.1186/1756-8722-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fayad L, et al. Dacetuzumab plus rituximab, ifosfamide, carboplatin and etoposide as salvage therapy for patients with diffuse large B-cell lymphoma relapsing after rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone: a randomized, double-blind, placebo-controlled phase 2b trial. Leuk. Lymphoma. 2015;56:2569–2578. doi: 10.3109/10428194.2015.1007504. [DOI] [PubMed] [Google Scholar]
- 155.Stein R, et al. CD74: a new candidate target for the immunotherapy of B-cell neoplasms. Clin. Cancer Res. 2007;13:5556s–5563s. doi: 10.1158/1078-0432.CCR-07-1167. [DOI] [PubMed] [Google Scholar]
- 156.Christian BA, et al. The combination of milatuzumab, a humanized anti-CD74 antibody, and veltuzumab, a humanized anti-CD20 antibody, demonstrates activity in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Br. J. Haematol. 2015;169:701–710. doi: 10.1111/bjh.13354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gupta P, et al. Dual-targeting immunotherapy of lymphoma: potent cytotoxicity of anti-CD20/CD74 bispecific antibodies in mantle cell and other lymphomas. Blood. 2012;119:3767–3778. doi: 10.1182/blood-2011-09-381988. [DOI] [PubMed] [Google Scholar]
- 158.Hariharan K, et al. Galiximab (anti-CD80)-induced growth inhibition and prolongation of survival in vivo of B-NHL tumor xenografts and potentiation by the combination with fludarabine. Int. J. Oncol. 2013;43:670–676. doi: 10.3892/ijo.2013.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Czuczman MS, et al. Phase I/II study of galiximab, an anti-CD80 antibody, for relapsed or refractory follicular lymphoma. J. Clin. Oncol. 2005;23:4390–4398. doi: 10.1200/JCO.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 160.Leonard JP, et al. A phase I/II study of galiximab (an anti-CD80 monoclonal antibody) in combination with rituximab for relapsed or refractory, follicular lymphoma. Ann. Oncol. 2007;18:1216–1223. doi: 10.1093/annonc/mdm114. [DOI] [PubMed] [Google Scholar]
- 161.Ortonne N, et al. CD158k/KIR3DL2 and NKp46 are frequently expressed in transformed mycosis fungoides. Exp. Dermatol. 2012;21:461–463. doi: 10.1111/j.1600-0625.2012.01489.x. [DOI] [PubMed] [Google Scholar]
- 162.Battistella M, et al. KIR3DL2 (CD158k) is a potential therapeutic target in primary cutaneous anaplastic large-cell lymphoma. Br. J. Dermatol. 2016;175:325–333. doi: 10.1111/bjd.14626. [DOI] [PubMed] [Google Scholar]
- 163.Battistella M, et al. KIR3DL2 expression in cutaneous T-cell lymphomas: expanding the spectrum for KIR3DL2 targeting. Blood. 2017;130:2900–2902. doi: 10.1182/blood-2017-06-792382. [DOI] [PubMed] [Google Scholar]
- 164.Sicard H, et al. A novel targeted immunotherapy for CTCL is on its way: Anti-KIR3DL2 mAb IPH4102 is potent and safe in non-clinical studies. Oncoimmunology. 2015;4:e1022306. doi: 10.1080/2162402X.2015.1022306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bagot M, Porcu P, Ram-Wolff C, Khodadoust M, Kim YH. Phase I study of IPH4102, anti-KIR3DL2 mab, in relapsed/refractory cutaneous T-cell lymphomas (CTCL): dose-escalation safety, biomarker and clinical activity results. Hematol. Oncol. 2017;35:48–49. doi: 10.1002/hon.2437_31. [DOI] [Google Scholar]
- 166.Baeuerle PA, Reinhardt C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res. 2009;69:4941–4944. doi: 10.1158/0008-5472.CAN-09-0547. [DOI] [PubMed] [Google Scholar]
- 167.Gruen M, Bommert K, Bargou RC. T-cell-mediated lysis of B cells induced by a CD19xCD3 bispecific single-chain antibody is perforin dependent and death receptor independent. Cancer Immunol. Immunother. 2004;53:625–632. doi: 10.1007/s00262-003-0496-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dreier T, et al. T cell costimulus-independent and very efficacious inhibition of tumor growth in mice bearing subcutaneous or leukemic human B cell lymphoma xenografts by a CD19-/CD3- bispecific single-chain antibody construct. J. Immunol. 2003;170:4397–4402. doi: 10.4049/jimmunol.170.8.4397. [DOI] [PubMed] [Google Scholar]
- 169.Goebeler ME, et al. Bispecific T-cell engager (BiTE) antibody construct blinatumomab for the treatment of patients with relapsed/refractory non-Hodgkin lymphoma: final results from a phase I study. J. Clin. Oncol. 2016;34:1104–1111. doi: 10.1200/JCO.2014.59.1586. [DOI] [PubMed] [Google Scholar]
- 170.Viardot A, et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood. 2016;127:1410–1416. doi: 10.1182/blood-2015-06-651380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Brezski RJ, Monroe JG. B-cell receptor. Adv. Exp. Med. Biol. 2008;640:12–21. doi: 10.1007/978-0-387-09789-3_2. [DOI] [PubMed] [Google Scholar]
- 172.Latour S, Fournel M, Veillette A. Regulation of T-cell antigen receptor signalling by Syk tyrosine protein kinase. Mol. Cell. Biol. 1997;17:4434–4441. doi: 10.1128/MCB.17.8.4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Davis RE, Brown KD, Siebenlist U, Staudt LM. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J. Exp. Med. 2001;194:1861–1874. doi: 10.1084/jem.194.12.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Davis RE, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463:88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen L, et al. SYK inhibition modulates distinct PI3K/AKT- dependent survival pathways and cholesterol biosynthesis in diffuse large B cell lymphomas. Cancer Cell. 2013;23:826–838. doi: 10.1016/j.ccr.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Szydlowski M, et al. FOXO1 activation is an effector of SYK and AKT inhibition in tonic BCR signal-dependent diffuse large B-cell lymphomas. Blood. 2016;127:739–748. doi: 10.1182/blood-2015-06-654111. [DOI] [PubMed] [Google Scholar]
- 177.Schmitz R, et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature. 2012;490:116–120. doi: 10.1038/nature11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mutzbauer G, et al. SYK expression in monomorphic epitheliotropic intestinal T-cell lymphoma. Mod. Pathol. 2018;31:505–516. doi: 10.1038/modpathol.2017.145. [DOI] [PubMed] [Google Scholar]
- 179.Attygalle AD, Feldman AL, Dogan A. ITK/SYK translocation in angioimmunoblastic T-cell lymphoma. Am. J. Surg. Pathol. 2013;37:1456–1457. doi: 10.1097/PAS.0b013e3182991415. [DOI] [PubMed] [Google Scholar]
- 180.Feldman AL, et al. Overexpression of Syk tyrosine kinase in peripheral T-cell lymphomas. Leukemia. 2008;22:1139–1143. doi: 10.1038/leu.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Streubel B, Vinatzer U, Willheim M, Raderer M, Chott A. Novel t(5;9)(q33;q22) fuses ITK to SYK in unspecified peripheral T-cell lymphoma. Leukemia. 2006;20:313–318. doi: 10.1038/sj.leu.2404045. [DOI] [PubMed] [Google Scholar]
- 182.Friedberg JW, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010;115:2578–2585. doi: 10.1182/blood-2009-08-236471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Barr PM, et al. Phase 2 study of idelalisib and entospletinib: pneumonitis limits combination therapy in relapsed refractory CLL and NHL. Blood. 2016;127:2411–2415. doi: 10.1182/blood-2015-12-683516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ishikawa C, Senba M, Mori N. Anti-adult Tcell leukemia/lymphoma activity of cerdulatinib, a dual SYK/JAK kinase inhibitor. Int. J. Oncol. 2018;53:1681–1690. doi: 10.3892/ijo.2018.4513. [DOI] [PubMed] [Google Scholar]
- 185.Kim YJ, Sekiya F, Poulin B, Bae YS, Rhee SG. Mechanism of B-cell receptor-induced phosphorylation and activation of phospholipase C-gamma2. Mol. Cell. Biol. 2004;24:9986–9999. doi: 10.1128/MCB.24.22.9986-9999.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hashimoto A, et al. Involvement of guanosine triphosphatases and phospholipase C-gamma2 in extracellular signal-regulated kinase, c-Jun NH2-terminal kinase, and p38 mitogen-activated protein kinase activation by the B cell antigen receptor. J. Exp. Med. 1998;188:1287–1295. doi: 10.1084/jem.188.7.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Craxton A, Jiang A, Kurosaki T, Clark EA. Syk and Bruton’s tyrosine kinase are required for B cell antigen receptor-mediated activation of the kinase Akt. J. Biol. Chem. 1999;274:30644–30650. doi: 10.1074/jbc.274.43.30644. [DOI] [PubMed] [Google Scholar]
- 188.Cinar M, et al. Bruton tyrosine kinase is commonly overexpressed in mantle cell lymphoma and its attenuation by Ibrutinib induces apoptosis. Leuk. Res. 2013;37:1271–1277. doi: 10.1016/j.leukres.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 189.Clipson A, et al. KLF2 mutation is the most frequent somatic change in splenic marginal zone lymphoma and identifies a subset with distinct genotype. Leukemia. 2015;29:1177–1185. doi: 10.1038/leu.2014.330. [DOI] [PubMed] [Google Scholar]
- 190.Compagno M, et al. Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature. 2009;459:717–721. doi: 10.1038/nature07968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lenz G, et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science. 2008;319:1676–1679. doi: 10.1126/science.1153629. [DOI] [PubMed] [Google Scholar]
- 192.Ngo VN, et al. A loss-of-function RNA interference screen for molecular targets in cancer. Nature. 2006;441:106–110. doi: 10.1038/nature04687. [DOI] [PubMed] [Google Scholar]
- 193.Advani RH, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J. Clin. Oncol. 2013;31:88–94. doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wilson WH, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat. Med. 2015;21:922–926. doi: 10.1038/nm.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bartlett NL, et al. Single-agent ibrutinib in relapsed or refractory follicular lymphoma: a phase 2 consortium trial. Blood. 2018;131:182–190. doi: 10.1182/blood-2017-09-804641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Noy A, et al. Targeting Bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood. 2017;129:2224–2232. doi: 10.1182/blood-2016-10-747345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wang ML, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 2013;369:507–516. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Younes A, et al. Safety and activity of ibrutinib in combination with nivolumab in patients with relapsed non-Hodgkin lymphoma or chronic lymphocytic leukaemia: a phase 1/2a study. Lancet Haematol. 2019;6:e67–e78. doi: 10.1016/S2352-3026(18)30217-5. [DOI] [PubMed] [Google Scholar]
- 199.Tam CS, et al. Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N. Engl. J. Med. 2018;378:1211–1223. doi: 10.1056/NEJMoa1715519. [DOI] [PubMed] [Google Scholar]
- 200.Ujjani CS, et al. Phase 1 trial of rituximab, lenalidomide, and ibrutinib in previously untreated follicular lymphoma: alliance A051103. Blood. 2016;128:2510–2516. doi: 10.1182/blood-2016-06-718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jerkeman M, et al. Ibrutinib, lenalidomide, and rituximab in relapsed or refractory mantle cell lymphoma (PHILEMON): a multicentre, open-label, single-arm, phase 2 trial. Lancet Haematol. 2018;5:e109–e116. doi: 10.1016/S2352-3026(18)30018-8. [DOI] [PubMed] [Google Scholar]
- 202.Younes A, et al. Combination of ibrutinib with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) for treatment-naive patients with CD20-positive B-cell non-Hodgkin lymphoma: a non-randomised, phase 1b study. Lancet Oncol. 2014;15:1019–1026. doi: 10.1016/S1470-2045(14)70311-0. [DOI] [PubMed] [Google Scholar]
- 203.Grommes C, et al. Phase 1b trial of an ibrutinib-based combination therapy in recurrent/refractory CNS lymphoma. Blood. 2019;133:436–445. doi: 10.1182/blood-2018-09-875732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Harrington BK, et al. Preclinical evaluation of the novel BTK Inhibitor acalabrutinib in canine models of B-cell non-Hodgkin lymphoma. PLoS ONE. 2016;11:e0159607. doi: 10.1371/journal.pone.0159607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wang M, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial. Lancet. 2018;391:659–667. doi: 10.1016/S0140-6736(17)33108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Byrd JC, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 2016;374:323–332. doi: 10.1056/NEJMoa1509981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Tam CS, et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019;134:851–859. doi: 10.1182/blood.2019001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Walter HS, et al. A phase 1 clinical trial of the selective BTK inhibitor ONO/GS-4059 in relapsed and refractory mature B-cell malignancies. Blood. 2016;127:411–419. doi: 10.1182/blood-2015-08-664086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Reiff SD, et al. The BTK inhibitor ARQ 531 targets ibrutinib-resistant CLL and Richter transformation. Cancer Discov. 2018;8:1300–1315. doi: 10.1158/2159-8290.CD-17-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Goodman LS, et al. Nitrogen mustard therapy; use of methyl-bis (beta-chloroethyl) amine hydrochloride and tris (beta-chloroethyl) amine hydrochloride for Hodgkin’s disease, lymphosarcoma, leukemia and certain allied and miscellaneous disorders. J. Am. Med. Assoc. 1946;132:126–132. doi: 10.1001/jama.1946.02870380008004. [DOI] [PubMed] [Google Scholar]
- 211.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 2010;11:329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 212.Populo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int. J. Mol. Sci. 2012;13:1886–1918. doi: 10.3390/ijms13021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Abubaker J, et al. PIK3CA mutations are mutually exclusive with PTEN loss in diffuse large B-cell lymphoma. Leukemia. 2007;21:2368–2370. doi: 10.1038/sj.leu.2404873. [DOI] [PubMed] [Google Scholar]
- 214.Baohua Y, Xiaoyan Z, Tiecheng Z, Tao Q, Daren S. Mutations of the PIK3CA gene in diffuse large B cell lymphoma. Diagn. Mol. Pathol. 2008;17:159–165. doi: 10.1097/PDM.0b013e31815d0588. [DOI] [PubMed] [Google Scholar]
- 215.Okkenhaug K, et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 216.Garcon F, et al. CD28 provides T-cell costimulation and enhances PI3K activity at the immune synapse independently of its capacity to interact with the p85/p110 heterodimer. Blood. 2008;111:1464–1471. doi: 10.1182/blood-2007-08-108050. [DOI] [PubMed] [Google Scholar]
- 217.Janas ML, et al. Thymic development beyond beta-selection requires phosphatidylinositol 3-kinase activation by CXCR4. J. Exp. Med. 2010;207:247–261. doi: 10.1084/jem.20091430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Gopal AK, et al. Phase II study of idelalisib, a selective inhibitor of PI3Kdelta, for relapsed/refractory classical Hodgkin lymphoma. Ann. Oncol. 2017;28:1057–1063. doi: 10.1093/annonc/mdx028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Gopal AK, et al. PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. N. Engl. J. Med. 2014;370:1008–1018. doi: 10.1056/NEJMoa1314583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Smith SM, et al. Safety and tolerability of idelalisib, lenalidomide, and rituximab in relapsed and refractory lymphoma: the alliance for clinical trials in oncology A051201 and A051202 phase 1 trials. Lancet Haematol. 2017;4:e176–e182. doi: 10.1016/S2352-3026(17)30028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Terwilliger T, Abdul-Hay M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. 2017;7:e577. doi: 10.1038/bcj.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Davids MS, et al. Umbralisib in combination with ibrutinib in patients with relapsed or refractory chronic lymphocytic leukaemia or mantle cell lymphoma: a multicentre phase 1-1b study. Lancet Haematol. 2019;6:e38–e47. doi: 10.1016/S2352-3026(18)30196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Campbell V, et al. The potent PI3K-δ,γ inhibitor IPI-145 exhibits differential activity in diffuse large B-cell lymphoma (DLBCL) cell lines. Blood. 2013;122:1832–1832. doi: 10.1182/blood.V122.21.1832.1832. [DOI] [Google Scholar]
- 224.Wang J, et al. The effects of PI3K-δ/γ inhibitor, duvelisib, in mantle cell lymphoma in vitro and in patient-derived xenograft studies. Blood. 2016;128:3016–3016. doi: 10.1182/blood.V128.22.3016.3016. [DOI] [Google Scholar]
- 225.Zinzani P, et al. Dynamo: a phase 2 study demonstrating clinical activity duvelisib patients double-refractory indolent non-hodgkin lymphoma. Hematol. Oncol. 2017;35:69–70. doi: 10.1002/hon.2437_57. [DOI] [Google Scholar]
- 226.Witzig TE, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J. Clin. Oncol. 2005;23:5347–5356. doi: 10.1200/JCO.2005.13.466. [DOI] [PubMed] [Google Scholar]
- 227.Dreyling M, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet. 2016;387:770–778. doi: 10.1016/S0140-6736(15)00667-4. [DOI] [PubMed] [Google Scholar]
- 228.Witzens-Harig M, Memmer ML, Dreyling M, Hess G. A phase I/II trial to evaluate the safety, feasibility and activity of salvage therapy consisting of the mTOR inhibitor Temsirolimus added to standard therapy of Rituximab and DHAP for the treatment of patients with relapsed or refractory diffuse large cell B-Cell lymphoma—the STORM trial. BMC Cancer. 2013;13:308. doi: 10.1186/1471-2407-13-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Smith SM, et al. Temsirolimus has activity in non-mantle cell non-Hodgkin’s lymphoma subtypes: The University of Chicago phase II consortium. J. Clin. Oncol. 2010;28:4740–4746. doi: 10.1200/JCO.2010.29.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Balmant NV, de Souza Reis R, de Oliveira Santos M, Pinto Oliveira J, de Camargo B. Trends in cancer mortality among adolescents and young adults in Brazil. J. Adolesc. Young. Adult Oncol. 2017;6:341–347. doi: 10.1089/jayao.2016.0042. [DOI] [PubMed] [Google Scholar]
- 231.Johnston PB, et al. A Phase II trial of the oral mTOR inhibitor everolimus in relapsed Hodgkin lymphoma. Am. J. Hematol. 2010;85:320–324. doi: 10.1002/ajh.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Jones SA, Scheller J, Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J. Clin. Invest. 2011;121:3375–3383. doi: 10.1172/JCI57158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sansone P, Bromberg J. Targeting the interleukin-6/Jak/stat pathway in human malignancies. J. Clin. Oncol. 2012;30:1005–1014. doi: 10.1200/JCO.2010.31.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Chen E, Staudt LM, Green AR. Janus kinase deregulation in leukemia and lymphoma. Immunity. 2012;36:529–541. doi: 10.1016/j.immuni.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Rui L, et al. Cooperative epigenetic modulation by cancer amplicon genes. Cancer Cell. 2010;18:590–605. doi: 10.1016/j.ccr.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Yan J, et al. EZH2 phosphorylation by JAK3 mediates a switch to noncanonical function in natural killer/T-cell lymphoma. Blood. 2016;128:948–958. doi: 10.1182/blood-2016-01-690701. [DOI] [PubMed] [Google Scholar]
- 237.Crescenzo R, et al. Convergent mutations and kinase fusions lead to oncogenic STAT3 activation in anaplastic large cell lymphoma. Cancer Cell. 2015;27:516–532. doi: 10.1016/j.ccell.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Khoury JD, et al. Differential expression and clinical significance of tyrosine-phosphorylated STAT3 in ALK+ and ALK- anaplastic large cell lymphoma. Clin. Cancer Res. 2003;9:3692–3699. [PubMed] [Google Scholar]
- 239.Kataoka K, et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat. Genet. 2015;47:1304–1315. doi: 10.1038/ng.3415. [DOI] [PubMed] [Google Scholar]
- 240.Takemoto S, et al. Proliferation of adult T cell leukemia/lymphoma cells is associated with the constitutive activation of JAK/STAT proteins. Proc. Natl Acad. Sci. U. S. A. 1997;94:13897–13902. doi: 10.1073/pnas.94.25.13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Bouchekioua A, et al. JAK3 deregulation by activating mutations confers invasive growth advantage in extranodal nasal-type natural killer cell lymphoma. Leukemia. 2014;28:338–348. doi: 10.1038/leu.2013.157. [DOI] [PubMed] [Google Scholar]
- 242.Koo GC, et al. Janus kinase 3-activating mutations identified in natural killer/T-cell lymphoma. Cancer Discov. 2012;2:591–597. doi: 10.1158/2159-8290.CD-12-0028. [DOI] [PubMed] [Google Scholar]
- 243.Jiang L, et al. Exome sequencing identifies somatic mutations of DDX3X in natural killer/T-cell lymphoma. Nat. Genet. 2015;47:1061–1066. doi: 10.1038/ng.3358. [DOI] [PubMed] [Google Scholar]
- 244.Lee S, et al. Ruxolitinib significantly enhances in vitro apoptosis in Hodgkin lymphoma and primary mediastinal B-cell lymphoma and survival in a lymphoma xenograft murine model. Oncotarget. 2018;9:9776–9788. doi: 10.18632/oncotarget.24267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Van Den Neste E, et al. A phase II study of the oral JAK1/JAK2 inhibitor ruxolitinib in advanced relapsed/refractory Hodgkin lymphoma. Haematologica. 2018;103:840–848. doi: 10.3324/haematol.2017.180554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zhang M, et al. Selective targeting of JAK/STAT signaling is potentiated by Bcl-xL blockade in IL-2-dependent adult T-cell leukemia. Proc. Natl Acad. Sci. U. S. A. 2015;112:12480–12485. doi: 10.1073/pnas.1516208112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.O’Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N. Engl. J. Med. 2013;368:161–170. doi: 10.1056/NEJMra1202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Andersson ER, Sandberg R, Lendahl U. Notch signaling: simplicity in design, versatility in function. Development. 2011;138:3593–3612. doi: 10.1242/dev.063610. [DOI] [PubMed] [Google Scholar]
- 249.Sanchez-Martin M, Ferrando A. The NOTCH1-MYC highway toward T-cell acute lymphoblastic leukemia. Blood. 2017;129:1124–1133. doi: 10.1182/blood-2016-09-692582. [DOI] [PubMed] [Google Scholar]
- 250.Robey EA, Bluestone JA. Notch signaling in lymphocyte development and function. Curr. Opin. Immunol. 2004;16:360–366. doi: 10.1016/j.coi.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 251.Aster JC, Blacklow SC, Pear WS. Notch signalling in T-cell lymphoblastic leukaemia/lymphoma and other haematological malignancies. J. Pathol. 2011;223:262–273. doi: 10.1002/path.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Schmitz R, et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N. Engl. J. Med. 2018;378:1396–1407. doi: 10.1056/NEJMoa1801445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Kridel R, et al. Whole transcriptome sequencing reveals recurrent NOTCH1 mutations in mantle cell lymphoma. Blood. 2012;119:1963–1971. doi: 10.1182/blood-2011-11-391474. [DOI] [PubMed] [Google Scholar]
- 254.Cao Z, et al. Angiocrine factors deployed by tumor vascular niche induce B cell lymphoma invasiveness and chemoresistance. Cancer Cell. 2014;25:350–365. doi: 10.1016/j.ccr.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kochert K, et al. High-level expression of mastermind-like 2 contributes to aberrant activation of the NOTCH signaling pathway in human lymphomas. Oncogene. 2011;30:1831–1840. doi: 10.1038/onc.2010.544. [DOI] [PubMed] [Google Scholar]
- 256.Rossi D, et al. The coding genome of splenic marginal zone lymphoma: activation of NOTCH2 and other pathways regulating marginal zone development. J. Exp. Med. 2012;209:1537–1551. doi: 10.1084/jem.20120904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Spina V, et al. The genetics of nodal marginal zone lymphoma. Blood. 2016;128:1362–1373. doi: 10.1182/blood-2016-02-696757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Karube K, et al. Recurrent mutations of NOTCH genes in follicular lymphoma identify a distinctive subset of tumours. J. Pathol. 2014;234:423–430. doi: 10.1002/path.4428. [DOI] [PubMed] [Google Scholar]
- 259.Groth C, Fortini ME. Therapeutic approaches to modulating notch signaling: current challenges and future prospects. Semin. Cell Dev. Biol. 2012;23:465–472. doi: 10.1016/j.semcdb.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Berezovska O, et al. Aspartate mutations in presenilin and gamma-secretase inhibitors both impair notch1 proteolysis and nuclear translocation with relative preservation of notch1 signaling. J. Neurochem. 2000;75:583–593. doi: 10.1046/j.1471-4159.2000.0750583.x. [DOI] [PubMed] [Google Scholar]
- 261.Real PJ, et al. Gamma-secretase inhibitors reverse glucocorticoid resistance in T cell acute lymphoblastic leukemia. Nat. Med. 2009;15:50–58. doi: 10.1038/nm.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Riccio O, et al. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep. 2008;9:377–383. doi: 10.1038/embor.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat. Immunol. 2011;12:715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 264.Scott DW, Gascoyne RD. The tumour microenvironment in B cell lymphomas. Nat. Rev. Cancer. 2014;14:517–534. doi: 10.1038/nrc3774. [DOI] [PubMed] [Google Scholar]
- 265.Huang DB, Vu D, Ghosh G. NF-kappaB RelB forms an intertwined homodimer. Structure. 2005;13:1365–1373. doi: 10.1016/j.str.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 266.Mohamed AJ, et al. Bruton’s tyrosine kinase (Btk): function, regulation, and transformation with special emphasis on the PH domain. Immunol. Rev. 2009;228:58–73. doi: 10.1111/j.1600-065X.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- 267.Eliopoulos AG, et al. Epstein-Barr virus-encoded latent infection membrane protein 1 regulates the processing of p100 NF-kappaB2 to p52 via an IKKgamma/NEMO-independent signalling pathway. Oncogene. 2003;22:7557–7569. doi: 10.1038/sj.onc.1207120. [DOI] [PubMed] [Google Scholar]
- 268.Liu F, Xia Y, Parker AS, Verma IM. IKK biology. Immunol. Rev. 2012;246:239–253. doi: 10.1111/j.1600-065X.2012.01107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Baldwin AS. Regulation of cell death and autophagy by IKK and NF-kappaB: critical mechanisms in immune function and cancer. Immunol. Rev. 2012;246:327–345. doi: 10.1111/j.1600-065X.2012.01095.x. [DOI] [PubMed] [Google Scholar]
- 270.Broemer M, Krappmann D, Scheidereit C. Requirement of Hsp90 activity for IkappaB kinase (IKK) biosynthesis and for constitutive and inducible IKK and NF-kappaB activation. Oncogene. 2004;23:5378–5386. doi: 10.1038/sj.onc.1207705. [DOI] [PubMed] [Google Scholar]
- 271.Puente XS, et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature. 2011;475:101–105. doi: 10.1038/nature10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Milhollen MA, et al. MLN4924, a NEDD8-activating enzyme inhibitor, is active in diffuse large B-cell lymphoma models: rationale for treatment of NF-{kappa}B-dependent lymphoma. Blood. 2010;116:1515–1523. doi: 10.1182/blood-2010-03-272567. [DOI] [PubMed] [Google Scholar]
- 273.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol. Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 274.Nunes AT, Annunziata CM. Proteasome inhibitors: structure and function. Semin. Oncol. 2017;44:377–380. doi: 10.1053/j.seminoncol.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Orlowski M, Michaud C. Pituitary multicatalytic proteinase complex. Specificity of components and aspects of proteolytic activity. Biochemistry. 1989;28:9270–9278. doi: 10.1021/bi00450a006. [DOI] [PubMed] [Google Scholar]
- 276.Schwartz AL, Ciechanover A. Targeting proteins for destruction by the ubiquitin system: implications for human pathobiology. Annu. Rev. Pharmacol. Toxicol. 2009;49:73–96. doi: 10.1146/annurev.pharmtox.051208.165340. [DOI] [PubMed] [Google Scholar]
- 277.Adams J. The proteasome: structure, function, and role in the cell. Cancer Treat. Rev. 2003;29(Suppl 1):3–9. doi: 10.1016/S0305-7372(03)00081-1. [DOI] [PubMed] [Google Scholar]
- 278.King RW, Deshaies RJ, Peters JM, Kirschner MW. How proteolysis drives the cell cycle. Science. 1996;274:1652–1659. doi: 10.1126/science.274.5293.1652. [DOI] [PubMed] [Google Scholar]
- 279.Palombella VJ, Rando OJ, Goldberg AL, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell. 1994;78:773–785. doi: 10.1016/S0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- 280.Orlowski M, Wilk S. Catalytic activities of the 20 S proteasome, a multicatalytic proteinase complex. Arch. Biochem. Biophys. 2000;383:1–16. doi: 10.1006/abbi.2000.2036. [DOI] [PubMed] [Google Scholar]
- 281.Fisher RI, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J. Clin. Oncol. 2006;24:4867–4874. doi: 10.1200/JCO.2006.07.9665. [DOI] [PubMed] [Google Scholar]
- 282.Tan D, et al. Panobinostat in combination with bortezomib in patients with relapsed or refractory peripheral T-cell lymphoma: an open-label, multicentre phase 2 trial. Lancet Haematol. 2015;2:e326–e333. doi: 10.1016/S2352-3026(15)00097-6. [DOI] [PubMed] [Google Scholar]
- 283.Robak T, et al. Bortezomib-based therapy for newly diagnosed mantle-cell lymphoma. N. Engl. J. Med. 2015;372:944–953. doi: 10.1056/NEJMoa1412096. [DOI] [PubMed] [Google Scholar]
- 284.Dimopoulos MA, et al. Primary therapy of Waldenstrom macroglobulinemia (WM) with weekly bortezomib, low-dose dexamethasone, and rituximab (BDR): long-term results of a phase 2 study of the European Myeloma Network (EMN) Blood. 2013;122:3276–3282. doi: 10.1182/blood-2013-05-503862. [DOI] [PubMed] [Google Scholar]
- 285.McMullen JR, et al. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood. 2014;124:3829–3830. doi: 10.1182/blood-2014-10-604272. [DOI] [PubMed] [Google Scholar]
- 286.Quek LS, Bolen J, Watson SP. A role for Bruton’s tyrosine kinase (Btk) in platelet activation by collagen. Curr. Biol. 1998;8:1137–1140. doi: 10.1016/S0960-9822(98)70471-3. [DOI] [PubMed] [Google Scholar]
- 287.Pal Singh S, Dammeijer F, Hendriks RW. Role of Bruton’s tyrosine kinase in B cells and malignancies. Mol. Cancer. 2018;17:57. doi: 10.1186/s12943-018-0779-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Kadri S, et al. Clonal evolution underlying leukemia progression and Richter transformation in patients with ibrutinib-relapsed CLL. Blood Adv. 2017;1:715–727. doi: 10.1182/bloodadvances.2016003632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Woyach JA, et al. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N. Engl. J. Med. 2014;370:2286–2294. doi: 10.1056/NEJMoa1400029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Paulus A, et al. Waldenstrom macroglobulinemia cells devoid of BTK(C481S) or CXCR4(WHIM-like) mutations acquire resistance to ibrutinib through upregulation of Bcl-2 and AKT resulting in vulnerability towards venetoclax or MK2206 treatment. Blood Cancer J. 2017;7:e565. doi: 10.1038/bcj.2017.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Flavahan, W. A., Gaskell, E. & Bernstein, B. E. Epigenetic plasticity and the hallmarks of cancer. Science357, eaal2380 (2017). [DOI] [PMC free article] [PubMed]
- 292.Laisne, M., Gupta, N., Kirsh, O., Pradhan, S. & Defossez, P. A. Mechanisms of DNA methyltransferase recruitment in mammals. Genes (Basel)9, 617 (2018). [DOI] [PMC free article] [PubMed]
- 293.Loo SK, Ab Hamid SS, Musa M, Wong KK. DNMT1 is associated with cell cycle and DNA replication gene sets in diffuse large B-cell lymphoma. Pathol. Res. Pract. 2018;214:134–143. doi: 10.1016/j.prp.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 294.Amara K, et al. DNA methyltransferase DNMT3b protein overexpression as a prognostic factor in patients with diffuse large B-cell lymphomas. Cancer Sci. 2010;101:1722–1730. doi: 10.1111/j.1349-7006.2010.01569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Peters SL, et al. Essential role for Dnmt1 in the prevention and maintenance of MYC-induced T-cell lymphomas. Mol. Cell. Biol. 2013;33:4321–4333. doi: 10.1128/MCB.00776-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Van Arnam JS, Lim MS, Elenitoba-Johnson KSJ. Novel insights into the pathogenesis of T-cell lymphomas. Blood. 2018;131:2320–2330. doi: 10.1182/blood-2017-11-764357. [DOI] [PubMed] [Google Scholar]
- 297.Blum KA, et al. Phase I trial of low dose decitabine targeting DNA hypermethylation in patients with chronic lymphocytic leukaemia and non-Hodgkin lymphoma: dose-limiting myelosuppression without evidence of DNA hypomethylation. Br. J. Haematol. 2010;150:189–195. doi: 10.1111/j.1365-2141.2010.08213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Cimmino L, Abdel-Wahab O, Levine RL, Aifantis I. TET family proteins and their role in stem cell differentiation and transformation. Cell Stem Cell. 2011;9:193–204. doi: 10.1016/j.stem.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Ito S, et al. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science. 2011;333:1300–1303. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Rampal R, et al. DNA hydroxymethylation profiling reveals that WT1 mutations result in loss of TET2 function in acute myeloid leukemia. Cell Rep. 2014;9:1841–1855. doi: 10.1016/j.celrep.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Wu H, et al. Genome-wide analysis of 5-hydroxymethylcytosine distribution reveals its dual function in transcriptional regulation in mouse embryonic stem cells. Genes Dev. 2011;25:679–684. doi: 10.1101/gad.2036011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Bogdanovic O, et al. Active DNA demethylation at enhancers during the vertebrate phylotypic period. Nat. Genet. 2016;48:417–426. doi: 10.1038/ng.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Figueroa ME, et al. DNA methylation signatures identify biologically distinct subtypes in acute myeloid leukemia. Cancer Cell. 2010;17:13–27. doi: 10.1016/j.ccr.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Hon GC, et al. 5mC oxidation by Tet2 modulates enhancer activity and timing of transcriptome reprogramming during differentiation. Mol. Cell. 2014;56:286–297. doi: 10.1016/j.molcel.2014.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Mahe EA, et al. Cytosine modifications modulate the chromatin architecture of transcriptional enhancers. Genome Res. 2017;27:947–958. doi: 10.1101/gr.211466.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Yamazaki J, et al. TET2 Mutations affect non-CpG island DNA methylation at enhancers and transcription factor-binding sites in chronic myelomonocytic leukemia. Cancer Res. 2015;75:2833–2843. doi: 10.1158/0008-5472.CAN-14-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Zhang YW, et al. Acetylation enhances TET2 function in protecting against abnormal DNA methylation during oxidative stress. Mol. Cell. 2017;65:323–335. doi: 10.1016/j.molcel.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Asmar F, et al. Genome-wide profiling identifies a DNA methylation signature that associates with TET2 mutations in diffuse large B-cell lymphoma. Haematologica. 2013;98:1912–1920. doi: 10.3324/haematol.2013.088740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Couronne L, Bastard C, Bernard OA. TET2 and DNMT3A mutations in human T-cell lymphoma. N. Engl. J. Med. 2012;366:95–96. doi: 10.1056/NEJMc1111708. [DOI] [PubMed] [Google Scholar]
- 310.Lemonnier F, et al. Recurrent TET2 mutations in peripheral T-cell lymphomas correlate with TFH-like features and adverse clinical parameters. Blood. 2012;120:1466–1469. doi: 10.1182/blood-2012-02-408542. [DOI] [PubMed] [Google Scholar]
- 311.Quivoron C, et al. TET2 inactivation results in pleiotropic hematopoietic abnormalities in mouse and is a recurrent event during human lymphomagenesis. Cancer Cell. 2011;20:25–38. doi: 10.1016/j.ccr.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 312.Dominguez PM, et al. TET2 deficiency causes germinal center hyperplasia, impairs plasma cell differentiation, and promotes B-cell lymphomagenesis. Cancer Discov. 2018;8:1632–1653. doi: 10.1158/2159-8290.CD-18-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Delarue R, et al. Treatment with hypomethylating agent 5-azacytidine induces sustained response in angioimmunoblastic T cell lymphomas. Blood. 2016;128:4164–4164. doi: 10.1182/blood.V128.22.4164.4164. [DOI] [Google Scholar]
- 314.Yang H, Ye D, Guan KL, Xiong Y. IDH1 and IDH2 mutations in tumorigenesis: mechanistic insights and clinical perspectives. Clin. Cancer Res. 2012;18:5562–5571. doi: 10.1158/1078-0432.CCR-12-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Miletic AV, et al. Vav links the T cell antigen receptor to the actin cytoskeleton and T cell activation independently of intrinsic Guanine nucleotide exchange activity. PLoS ONE. 2009;4:e6599. doi: 10.1371/journal.pone.0006599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Wang C, et al. IDH2R172 mutations define a unique subgroup of patients with angioimmunoblastic T-cell lymphoma. Blood. 2015;126:1741–1752. doi: 10.1182/blood-2015-05-644591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Yu B, et al. Structural and energetic mechanisms of cooperative autoinhibition and activation of Vav1. Cell. 2010;140:246–256. doi: 10.1016/j.cell.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Beguelin W, et al. EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell. 2013;23:677–692. doi: 10.1016/j.ccr.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Green MR. Chromatin modifying gene mutations in follicular lymphoma. Blood. 2018;131:595–604. doi: 10.1182/blood-2017-08-737361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Souroullas GP, et al. An oncogenic Ezh2 mutation induces tumors through global redistribution of histone 3 lysine 27 trimethylation. Nat. Med. 2016;22:632–640. doi: 10.1038/nm.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Green MR, et al. Mutations in early follicular lymphoma progenitors are associated with suppressed antigen presentation. Proc. Natl Acad. Sci. U. S. A. 2015;112:E1116–E1125. doi: 10.1073/pnas.1501199112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Ennishi D, et al. Molecular and genetic characterization of MHC deficiency identifies EZH2 as therapeutic target for enhancing immune recognition. Cancer Discov. 2019;9:546–563. doi: 10.1158/2159-8290.CD-18-1090. [DOI] [PubMed] [Google Scholar]
- 323.Knutson SK, et al. A selective inhibitor of EZH2 blocks H3K27 methylation and kills mutant lymphoma cells. Nat. Chem. Biol. 2012;8:890–896. doi: 10.1038/nchembio.1084. [DOI] [PubMed] [Google Scholar]
- 324.Italiano A, et al. Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study. Lancet Oncol. 2018;19:649–659. doi: 10.1016/S1470-2045(18)30145-1. [DOI] [PubMed] [Google Scholar]
- 325.Ortega-Molina A, et al. The histone lysine methyltransferase KMT2D sustains a gene expression program that represses B cell lymphoma development. Nat. Med. 2015;21:1199–1208. doi: 10.1038/nm.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Pasqualucci L, et al. Analysis of the coding genome of diffuse large B-cell lymphoma. Nat. Genet. 2011;43:830–837. doi: 10.1038/ng.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Zhang J, et al. Disruption of KMT2D perturbs germinal center B cell development and promotes lymphomagenesis. Nat. Med. 2015;21:1190–1198. doi: 10.1038/nm.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Ji MM, et al. Histone modifier gene mutations in peripheral T-cell lymphoma not otherwise specified. Haematologica. 2018;103:679–687. doi: 10.3324/haematol.2017.182444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Pasqualucci L, et al. Inactivating mutations of acetyltransferase genes in B-cell lymphoma. Nature. 2011;471:189–195. doi: 10.1038/nature09730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Zhang J, et al. The CREBBP acetyltransferase is a haploinsufficient tumor suppressor in B-cell lymphoma. Cancer Discov. 2017;7:322–337. doi: 10.1158/2159-8290.CD-16-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Hashwah H, et al. Inactivation of CREBBP expands the germinal center B cell compartment, down-regulates MHCII expression and promotes DLBCL growth. Proc. Natl Acad. Sci. U. S. A. 2017;114:9701–9706. doi: 10.1073/pnas.1619555114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Zhang Q, Wang S, Chen J, Yu Z. Histone deacetylases (HDACs) guided novel therapies for T-cell lymphomas. Int. J. Med. Sci. 2019;16:424–442. doi: 10.7150/ijms.30154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Li, Y. & Seto, E. HDACs and HDAC inhibitors in cancer development and therapy. Cold Spring Harb. Perspect. Med. 6, a026831 (2016). [DOI] [PMC free article] [PubMed]
- 334.Crump M, et al. Phase II trial of oral vorinostat (suberoylanilide hydroxamic acid) in relapsed diffuse large-B-cell lymphoma. Ann. Oncol. 2008;19:964–969. doi: 10.1093/annonc/mdn031. [DOI] [PubMed] [Google Scholar]
- 335.Kirschbaum M, et al. Phase II study of vorinostat for treatment of relapsed or refractory indolent non-Hodgkin’s lymphoma and mantle cell lymphoma. J. Clin. Oncol. 2011;29:1198–1203. doi: 10.1200/JCO.2010.32.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Olsen EA, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J. Clin. Oncol. 2007;25:3109–3115. doi: 10.1200/JCO.2006.10.2434. [DOI] [PubMed] [Google Scholar]
- 337.Chen R, et al. A phase II study of vorinostat and rituximab for treatment of newly diagnosed and relapsed/refractory indolent non-Hodgkin lymphoma. Haematologica. 2015;100:357–362. doi: 10.3324/haematol.2014.117473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Persky DO, et al. A phase I/II trial of vorinostat (SAHA) in combination with rituximab-CHOP in patients with newly diagnosed advanced stage diffuse large B-cell lymphoma (DLBCL): SWOG S0806. Am. J. Hematol. 2018;93:486–493. doi: 10.1002/ajh.25010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Budde LE, et al. A phase I study of pulse high-dose vorinostat (V) plus rituximab (R), ifosphamide, carboplatin, and etoposide (ICE) in patients with relapsed lymphoma. Br. J. Haematol. 2013;161:183–191. doi: 10.1111/bjh.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Oki Y, et al. Phase I study of vorinostat in combination with standard CHOP in patients with newly diagnosed peripheral T-cell lymphoma. Br. J. Haematol. 2013;162:138–141. doi: 10.1111/bjh.12326. [DOI] [PubMed] [Google Scholar]
- 341.Puvvada SD, et al. A phase II study of belinostat (PXD101) in relapsed and refractory aggressive B-cell lymphomas: SWOG S0520. Leuk. Lymphoma. 2016;57:2359–2369. doi: 10.3109/10428194.2015.1135431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Foss F, et al. A Phase II trial of Belinostat (PXD101) in patients with relapsed or refractory peripheral or cutaneous T-cell lymphoma. Br. J. Haematol. 2015;168:811–819. doi: 10.1111/bjh.13222. [DOI] [PubMed] [Google Scholar]
- 343.Piekarz RL, et al. Phase 2 trial of romidepsin in patients with peripheral T-cell lymphoma. Blood. 2011;117:5827–5834. doi: 10.1182/blood-2010-10-312603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Piekarz RL, et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J. Clin. Oncol. 2009;27:5410–5417. doi: 10.1200/JCO.2008.21.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Pellegrini C, et al. A phase II study on the role of gemcitabine plus romidepsin (GEMRO regimen) in the treatment of relapsed/refractory peripheral T-cell lymphoma patients. J. Hematol. Oncol. 2016;9:38. doi: 10.1186/s13045-016-0266-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Strati P, et al. A phase I study of romidepsin and ifosfamide, carboplatin, etoposide for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma. Haematologica. 2018;103:e416–e418. doi: 10.3324/haematol.2018.187617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Dupuis J, et al. Combination of romidepsin with cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated patients with peripheral T-cell lymphoma: a non-randomised, phase 1b/2 study. Lancet Haematol. 2015;2:e160–e165. doi: 10.1016/S2352-3026(15)00023-X. [DOI] [PubMed] [Google Scholar]
- 348.Shi Y, et al. Results from a multicenter, open-label, pivotal phase II study of chidamide in relapsed or refractory peripheral T-cell lymphoma. Ann. Oncol. 2015;26:1766–1771. doi: 10.1093/annonc/mdv237. [DOI] [PubMed] [Google Scholar]
- 349.Ansell SM, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Younes A, et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17:1283–1294. doi: 10.1016/S1470-2045(16)30167-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Nayak L, et al. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood. 2017;129:3071–3073. doi: 10.1182/blood-2017-01-764209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Kwong YL, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. 2017;129:2437–2442. doi: 10.1182/blood-2016-12-756841. [DOI] [PubMed] [Google Scholar]
- 353.Armand P, et al. Programmed death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. J. Clin. Oncol. 2016;34:3733–3739. doi: 10.1200/JCO.2016.67.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Sasse S, et al. Programmed cell death protein-1 (PD-1)-expression in the microenvironment of classical Hodgkin lymphoma at relapse during anti-PD-1-treatment. Haematologica. 2019;104:e21–e24. doi: 10.3324/haematol.2018.196279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Hill JA, et al. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood. 2018;131:121–130. doi: 10.1182/blood-2017-07-793760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 358.Freeman GJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Latchman Y, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 360.Sabatier R, et al. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget. 2015;6:5449–5464. doi: 10.18632/oncotarget.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Tamura T, et al. Programmed death-1 ligand-1 (PDL1) expression is associated with the prognosis of patients with stage II/III gastric cancer. Anticancer Res. 2015;35:5369–5376. [PubMed] [Google Scholar]
- 362.Cimino-Mathews A, et al. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum. Pathol. 2016;47:52–63. doi: 10.1016/j.humpath.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Tseng YH, et al. PD-L1 expression of tumor cells, macrophages, and immune cells in non-small cell lung cancer patients with malignant pleural effusion. J. Thorac. Oncol. 2018;13:447–453. doi: 10.1016/j.jtho.2017.10.034. [DOI] [PubMed] [Google Scholar]
- 364.Bardhan K, Anagnostou T, Boussiotis VA. The PD1:PD-L1/2 pathway from discovery to clinical implementation. Front. Immunol. 2016;7:550. doi: 10.3389/fimmu.2016.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Yamamoto R, et al. PD-1-PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood. 2008;111:3220–3224. doi: 10.1182/blood-2007-05-085159. [DOI] [PubMed] [Google Scholar]
- 366.Chen R, et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J. Clin. Oncol. 2017;35:2125–2132. doi: 10.1200/JCO.2016.72.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Green MR, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116:3268–3277. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Carreras J, et al. High numbers of tumor-infiltrating programmed cell death 1-positive regulatory lymphocytes are associated with improved overall survival in follicular lymphoma. J. Clin. Oncol. 2009;27:1470–1476. doi: 10.1200/JCO.2008.18.0513. [DOI] [PubMed] [Google Scholar]
- 369.Huang Y, et al. Peripheral T-cell lymphomas with a follicular growth pattern are derived from follicular helper T cells (TFH) and may show overlapping features with angioimmunoblastic T-cell lymphomas. Am. J. Surg. Pathol. 2009;33:682–690. doi: 10.1097/PAS.0b013e3181971591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Wahlin BE, et al. A unifying microenvironment model in follicular lymphoma: outcome is predicted by programmed death-1–positive, regulatory, cytotoxic, and helper T cells and macrophages. Clin. Cancer Res. 2010;16:637–650. doi: 10.1158/1078-0432.CCR-09-2487. [DOI] [PubMed] [Google Scholar]
- 371.Myklebust JH, et al. High PD-1 expression and suppressed cytokine signaling distinguish T cells infiltrating follicular lymphoma tumors from peripheral T cells. Blood. 2013;121:1367–1376. doi: 10.1182/blood-2012-04-421826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Smeltzer JP, et al. Pattern of CD14+ follicular dendritic cells and PD1+ T cells independently predicts time to transformation in follicular lymphoma. Clin. Cancer Res. 2014;20:2862–2872. doi: 10.1158/1078-0432.CCR-13-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Yang ZZ, et al. PD-1 expression defines two distinct T-cell sub-populations in follicular lymphoma that differentially impact patient survival. Blood Cancer J. 2015;5:e281. doi: 10.1038/bcj.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Kwiecinska A, et al. CD274 (PD-L1)/PDCD1 (PD-1) expression in de novo and transformed diffuse large B-cell lymphoma. Br. J. Haematol. 2018;180:744–748. doi: 10.1111/bjh.14432. [DOI] [PubMed] [Google Scholar]
- 375.Menter T, Bodmer-Haecki A, Dirnhofer S, Tzankov A. Evaluation of the diagnostic and prognostic value of PDL1 expression in Hodgkin and B-cell lymphomas. Hum. Pathol. 2016;54:17–24. doi: 10.1016/j.humpath.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 376.Kwon D, et al. Clinicopathological analysis of programmed cell death 1 and programmed cell death ligand 1 expression in the tumour microenvironments of diffuse large B cell lymphomas. Histopathology. 2016;68:1079–1089. doi: 10.1111/his.12882. [DOI] [PubMed] [Google Scholar]
- 377.Xu-Monette ZY, Zhou J, Young KH. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood. 2018;131:68–83. doi: 10.1182/blood-2017-07-740993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Fang X, et al. The expression and clinical relevance of PD-1, PD-L1, and TP63 in patients with diffuse large B-cell lymphoma. Med. (Baltim.) 2017;96:e6398. doi: 10.1097/MD.0000000000006398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Kiyasu J, et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood. 2015;126:2193–2201. doi: 10.1182/blood-2015-02-629600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Xu-Monette ZY, et al. Immune profiling and quantitative analysis decipher the clinical role of immune-checkpoint expression in the tumor immune microenvironment of DLBCL. Cancer Immunol. Res. 2019;7:644–657. doi: 10.1158/2326-6066.CIR-18-0439. [DOI] [PubMed] [Google Scholar]
- 381.Andorsky DJ, et al. Programmed death ligand 1 is expressed by non-hodgkin lymphomas and inhibits the activity of tumor-associated T cells. Clin. Cancer Res. 2011;17:4232–4244. doi: 10.1158/1078-0432.CCR-10-2660. [DOI] [PubMed] [Google Scholar]
- 382.Rossille D, et al. High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: results from a French multicenter clinical trial. Leukemia. 2014;28:2367–2375. doi: 10.1038/leu.2014.137. [DOI] [PubMed] [Google Scholar]
- 383.Zaja F, et al. CD38, BCL-2, PD-1, and PD-1L expression in nodal peripheral T-cell lymphoma: possible biomarkers for novel targeted therapies? Am. J. Hematol. 2017;92:E1–e2. doi: 10.1002/ajh.24571. [DOI] [PubMed] [Google Scholar]
- 384.Haverkos BM, et al. PD-1 blockade for relapsed lymphoma post-allogeneic hematopoietic cell transplant: high response rate but frequent GVHD. Blood. 2017;130:221–228. doi: 10.1182/blood-2017-01-761346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Ansell SM. Nivolumab in the treatment of Hodgkin lymphoma. Clin. Cancer Res. 2017;23:1623–1626. doi: 10.1158/1078-0432.CCR-16-1387. [DOI] [PubMed] [Google Scholar]
- 386.Lesokhin AM, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase ib study. J. Clin. Oncol. 2016;34:2698–2704. doi: 10.1200/JCO.2015.65.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Herrera AF, et al. Interim results of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2018;131:1183–1194. doi: 10.1182/blood-2017-10-811224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Ding W, et al. Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood. 2017;129:3419–3427. doi: 10.1182/blood-2017-02-765685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Zinzani PL, et al. Safety and tolerability of pembrolizumab in patients with relapsed/refractory primary mediastinal large B-cell lymphoma. Blood. 2017;130:267–270. doi: 10.1182/blood-2016-12-758383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Rowshanravan B, Halliday N, Sansom DM. CTLA-4: a moving target in immunotherapy. Blood. 2018;131:58–67. doi: 10.1182/blood-2017-06-741033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Robert C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 394.Robert C, et al. Pembrolizumab versus Ipilimumab in advanced melanoma. N. Engl. J. Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 395.Ansell SM, et al. Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin. Cancer Res. 2009;15:6446–6453. doi: 10.1158/1078-0432.CCR-09-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Majeti R, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Willingham SB, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl Acad. Sci. U. S. A. 2012;109:6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Brightwell RM, et al. The CD47 “don’t eat me signal” is highly expressed in human ovarian cancer. Gynecol. Oncol. 2016;143:393–397. doi: 10.1016/j.ygyno.2016.08.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Matlung HL, Szilagyi K, Barclay NA, van den Berg TK. The CD47-SIRPalpha signaling axis as an innate immune checkpoint in cancer. Immunol. Rev. 2017;276:145–164. doi: 10.1111/imr.12527. [DOI] [PubMed] [Google Scholar]
- 400.Casey SC, et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352:227–231. doi: 10.1126/science.aac9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Chao MP, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Chao MP, et al. Extranodal dissemination of non-Hodgkin lymphoma requires CD47 and is inhibited by anti-CD47 antibody therapy. Blood. 2011;118:4890–4901. doi: 10.1182/blood-2011-02-338020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Advani R, et al. CD47 Blockade by Hu5F9-G4 and rituximab in non-Hodgkin’s lymphoma. N. Engl. J. Med. 2018;379:1711–1721. doi: 10.1056/NEJMoa1807315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Wang Q, et al. Characterization and functional study of five novel monoclonal antibodies against human OX40L highlight reverse signalling: enhancement of IgG production of B cells and promotion of maturation of DCs. Tissue Antigens. 2004;64:566–574. doi: 10.1111/j.1399-0039.2004.00300.x. [DOI] [PubMed] [Google Scholar]
- 405.Ito T, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Karulf M, Kelly A, Weinberg AD, Gold JA. OX40 ligand regulates inflammation and mortality in the innate immune response to sepsis. J. Immunol. 2010;185:4856–4862. doi: 10.4049/jimmunol.1000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Marabelle A, et al. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J. Clin. Invest. 2013;123:2447–2463. doi: 10.1172/JCI64859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Stanietsky N, et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc. Natl Acad. Sci. U. S. A. 2009;106:17858–17863. doi: 10.1073/pnas.0903474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Yu X, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 2009;10:48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- 410.Joller N, et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity. 2014;40:569–581. doi: 10.1016/j.immuni.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Casado JG, et al. Expression of adhesion molecules and ligands for activating and costimulatory receptors involved in cell-mediated cytotoxicity in a large panel of human melanoma cell lines. Cancer Immunol. Immunother. 2009;58:1517–1526. doi: 10.1007/s00262-009-0682-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Mendelsohn CL, Wimmer E, Racaniello VR. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989;56:855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- 413.Josefsson SE, et al. TIGIT and PD-1 mark intratumoral T cells with reduced effector function in B-cell non-Hodgkin lymphoma. Cancer Immunol. Res. 2019;7:355–362. doi: 10.1158/2326-6066.CIR-18-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Yang ZZ, et al. IL-12 upregulates TIM-3 expression and induces T cell exhaustion in patients with follicular B cell non-Hodgkin lymphoma. J. Clin. Invest. 2012;122:1271–1282. doi: 10.1172/JCI59806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Zhu C, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 416.Ngiow SF, et al. Anti-TIM3 antibody promotes T cell IFN-gamma-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011;71:3540–3551. doi: 10.1158/0008-5472.CAN-11-0096. [DOI] [PubMed] [Google Scholar]
- 417.Huang X, et al. Lymphoma endothelium preferentially expresses Tim-3 and facilitates the progression of lymphoma by mediating immune evasion. J. Exp. Med. 2010;207:505–520. doi: 10.1084/jem.20090397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Ling W, et al. Mesenchymal stem cells use IDO to regulate immunity in tumor microenvironment. Cancer Res. 2014;74:1576–1587. doi: 10.1158/0008-5472.CAN-13-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Zhang X, et al. Mesenchymal stromal cells as vehicles of tetravalent bispecific Tandab (CD3/CD19) for the treatment of B cell lymphoma combined with IDO pathway inhibitor D-1-methyl-tryptophan. J. Hematol. Oncol. 2017;10:56. doi: 10.1186/s13045-017-0397-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Nowak EC, et al. Immunoregulatory functions of VISTA. Immunol. Rev. 2017;276:66–79. doi: 10.1111/imr.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Wang L, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J. Exp. Med. 2011;208:577–592. doi: 10.1084/jem.20100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Le Mercier I, et al. VISTA regulates the development of protective antitumor immunity. Cancer Res. 2014;74:1933–1944. doi: 10.1158/0008-5472.CAN-13-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Deng J, et al. Hypoxia-induced VISTA promotes the suppressive function of myeloid-derived suppressor cells in the tumor microenvironment. Cancer Immunol. Res. 2019;7:1079–1090. doi: 10.1158/2326-6066.CIR-18-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Locke FL, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20:31–42. doi: 10.1016/S1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Schuster SJ, et al. Chimeric antigen receptor T cells in refractory B-Cell lymphomas. N. Engl. J. Med. 2017;377:2545–2554. doi: 10.1056/NEJMoa1708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Schuster SJ, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-Cell lymphoma. N. Engl. J. Med. 2019;380:45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 427.Abramson, J. S. et al. High durable CR rates in relapsed/refractory (R/R) Aggressive B-NHL treated with the CD19-directed CAR T cell product JCAR017 (TRANSCEND NHL 001): Defined composition allows for Dose-Finding and Definition of Pivotal Cohort. In 59th Annual Meeting of the American-Society-of-Hematology (ASH). Blood130, abstract 581 (2017).
- 428.Abramson, J. S. et al. Updated safety and long term clinical outcomes in TRANSCEND NHL 001, pivotal trial of lisocabtagene maraleucel (JCAR017) in R/R aggressive NHL. In 2018 American Society of Clinical Oncology (ASCO) Annual Meeting. J. Clin. Oncol. 36, abstract S7505 (2018).
- 429.Yan ZX, et al. Clinical efficacy and tumor microenvironment influence in a dose-escalation study of anti-CD19 chimeric antigen receptor T cells in refractory B-Cell non-Hodgkin’s lymphoma. Clin. Cancer Res. 2019;25:6995–7003. doi: 10.1158/1078-0432.CCR-19-0101. [DOI] [PubMed] [Google Scholar]
- 430.Zhang WY, et al. Treatment of CD20-directed chimeric antigen receptor-modified T cells in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: an early phase IIa trial report. Signal Transduct. Target Ther. 2016;1:16002. doi: 10.1038/sigtrans.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Fry TJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 2018;24:20–28. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Ramos CA, et al. Clinical and immunological responses after CD30-specific chimeric antigen receptor-redirected lymphocytes. J. Clin. Invest. 2017;127:3462–3471. doi: 10.1172/JCI94306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Pinz K, et al. Preclinical targeting of human T-cell malignancies using CD4-specific chimeric antigen receptor (CAR)-engineered T cells. Leukemia. 2016;30:701–707. doi: 10.1038/leu.2015.311. [DOI] [PubMed] [Google Scholar]
- 434.Alcantara M, Tesio M, June CH, Houot R. CAR T-cells for T-cell malignancies: challenges in distinguishing between therapeutic, normal, and neoplastic T-cells. Leukemia. 2018;32:2307–2315. doi: 10.1038/s41375-018-0285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Cooper ML, et al. An “off-the-shelf” fratricide-resistant CAR-T for the treatment of T cell hematologic malignancies. Leukemia. 2018;32:1970–1983. doi: 10.1038/s41375-018-0065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Campbell KS, Hasegawa J. Natural killer cell biology: an update and future directions. J. Allergy Clin. Immunol. 2013;132:536–544. doi: 10.1016/j.jaci.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Gross E, Sunwoo JB, Bui JD. Cancer immunosurveillance and immunoediting by natural killer cells. Cancer J. 2013;19:483–489. doi: 10.1097/PPO.0000000000000005. [DOI] [PubMed] [Google Scholar]
- 438.Orange JS. Natural killer cell deficiency. J. Allergy Clin. Immunol. 2013;132:515–525. doi: 10.1016/j.jaci.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Rouce RH, et al. The TGF-beta/SMAD pathway is an important mechanism for NK cell immune evasion in childhood B-acute lymphoblastic leukemia. Leukemia. 2016;30:800–811. doi: 10.1038/leu.2015.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Daher M, Rezvani K. Next generation natural killer cells for cancer immunotherapy: the promise of genetic engineering. Curr. Opin. Immunol. 2018;51:146–153. doi: 10.1016/j.coi.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Liu E, et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia. 2018;32:520–531. doi: 10.1038/leu.2017.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Chen KH, et al. Preclinical targeting of aggressive T-cell malignancies using anti-CD5 chimeric antigen receptor. Leukemia. 2017;31:2151–2160. doi: 10.1038/leu.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Kress TR, Sabo A, Amati B. MYC: connecting selective transcriptional control to global RNA production. Nat. Rev. Cancer. 2015;15:593–607. doi: 10.1038/nrc3984. [DOI] [PubMed] [Google Scholar]
- 445.Basso K, Dalla-Favera R. Germinal centres and B cell lymphomagenesis. Nat. Rev. Immunol. 2015;15:172–184. doi: 10.1038/nri3814. [DOI] [PubMed] [Google Scholar]
- 446.Huang CY, Bredemeyer AL, Walker LM, Bassing CH, Sleckman BP. Dynamic regulation of c-Myc proto-oncogene expression during lymphocyte development revealed by a GFP-c-Myc knock-in mouse. Eur. J. Immunol. 2008;38:342–349. doi: 10.1002/eji.200737972. [DOI] [PubMed] [Google Scholar]
- 447.Sabo A, et al. Selective transcriptional regulation by Myc in cellular growth control and lymphomagenesis. Nature. 2014;511:488–492. doi: 10.1038/nature13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Calado DP, et al. The cell-cycle regulator c-Myc is essential for the formation and maintenance of germinal centers. Nat. Immunol. 2012;13:1092–1100. doi: 10.1038/ni.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Chong LC, et al. High-resolution architecture and partner genes of MYC rearrangements in lymphoma with DLBCL morphology. Blood Adv. 2018;2:2755–2765. doi: 10.1182/bloodadvances.2018023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Chisholm KM, et al. Expression profiles of MYC protein and MYC gene rearrangement in lymphomas. Am. J. Surg. Pathol. 2015;39:294–303. doi: 10.1097/PAS.0000000000000365. [DOI] [PubMed] [Google Scholar]
- 451.Karube K, Campo E. MYC alterations in diffuse large B-cell lymphomas. Semin. Hematol. 2015;52:97–106. doi: 10.1053/j.seminhematol.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 452.Feng SM, et al. Repair and sensory reconstruction of the children’s finger pulp defects with perforator pedicled propeller flap in proper digital artery. Eur. Rev. Med. Pharmacol. Sci. 2017;21:3533–3537. [PubMed] [Google Scholar]
- 453.Savage KJ, et al. Impact of dual expression of MYC and BCL2 by immunohistochemistry on the risk of CNS relapse in DLBCL. Blood. 2016;127:2182–2188. doi: 10.1182/blood-2015-10-676700. [DOI] [PubMed] [Google Scholar]
- 454.Bisso A, Sabo A, Amati B. MYC in germinal center-derived lymphomas: mechanisms and therapeutic opportunities. Immunol. Rev. 2019;288:178–197. doi: 10.1111/imr.12734. [DOI] [PubMed] [Google Scholar]
- 455.Brockmann M, et al. Small molecule inhibitors of aurora-a induce proteasomal degradation of N-myc in childhood neuroblastoma. Cancer Cell. 2013;24:75–89. doi: 10.1016/j.ccr.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.den Hollander J, et al. Aurora kinases A and B are up-regulated by Myc and are essential for maintenance of the malignant state. Blood. 2010;116:1498–1505. doi: 10.1182/blood-2009-11-251074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Rosenquist R, Bea S, Du MQ, Nadel B, Pan-Hammarstrom Q. Genetic landscape and deregulated pathways in B-cell lymphoid malignancies. J. Intern. Med. 2017;282:371–394. doi: 10.1111/joim.12633. [DOI] [PubMed] [Google Scholar]
- 459.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 460.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 461.Pasqualucci L, et al. Genetics of follicular lymphoma transformation. Cell Rep. 2014;6:130–140. doi: 10.1016/j.celrep.2013.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Bentz M, et al. t(11;14)-positive mantle cell lymphomas exhibit complex karyotypes and share similarities with B-cell chronic lymphocytic leukemia. Genes Chromosomes Cancer. 2000;27:285–294. doi: 10.1002/(SICI)1098-2264(200003)27:3<285::AID-GCC9>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 463.Huang JZ, et al. The t(14;18) defines a unique subset of diffuse large B-cell lymphoma with a germinal center B-cell gene expression profile. Blood. 2002;99:2285–2290. doi: 10.1182/blood.V99.7.2285. [DOI] [PubMed] [Google Scholar]
- 464.Swerdlow SH, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Maddocks KJ, et al. Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol. 2015;1:80–87. doi: 10.1001/jamaoncol.2014.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Sarkozy C, Traverse-Glehen A, Coiffier B. Double-hit and double-protein-expression lymphomas: aggressive and refractory lymphomas. Lancet Oncol. 2015;16:e555–e567. doi: 10.1016/S1470-2045(15)00005-4. [DOI] [PubMed] [Google Scholar]
- 467.Johnson NA, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J. Clin. Oncol. 2012;30:3452–3459. doi: 10.1200/JCO.2011.41.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Oltersdorf T, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 469.Del Gaizo Moore V, Schlis KD, Sallan SE, Armstrong SA, Letai A. BCL-2 dependence and ABT-737 sensitivity in acute lymphoblastic leukemia. Blood. 2008;111:2300–2309. doi: 10.1182/blood-2007-06-098012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Tse C, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 471.Davids MS, Letai A. Targeting the B-cell lymphoma/leukemia 2 family in cancer. J. Clin. Oncol. 2012;30:3127–3135. doi: 10.1200/JCO.2011.37.0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Davids MS, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J. Clin. Oncol. 2017;35:826–833. doi: 10.1200/JCO.2016.70.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Zelenetz AD, et al. Venetoclax plus R- or G-CHOP in non-Hodgkin lymphoma: results from the CAVALLI phase 1b trial. Blood. 2019;133:1964–1976. doi: 10.1182/blood-2018-11-880526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Basso K, et al. Integrated biochemical and computational approach identifies BCL6 direct target genes controlling multiple pathways in normal germinal center B cells. Blood. 2010;115:975–984. doi: 10.1182/blood-2009-06-227017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Miles RR, Crockett DK, Lim MS, Elenitoba-Johnson KS. Analysis of BCL6-interacting proteins by tandem mass spectrometry. Mol. Cell. Proteom. 2005;4:1898–1909. doi: 10.1074/mcp.M500112-MCP200. [DOI] [PubMed] [Google Scholar]
- 476.Niu H, Ye BH, Dalla-Favera R. Antigen receptor signaling induces MAP kinase-mediated phosphorylation and degradation of the BCL-6 transcription factor. Genes Dev. 1998;12:1953–1961. doi: 10.1101/gad.12.13.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Liu X, et al. Bcl6 expression specifies the T follicular helper cell program in vivo. J. Exp. Med. 2012;209:1841–1852. doi: 10.1084/jem.20120219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Nurieva RI, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Wlodarska I, et al. Frequent occurrence of BCL6 rearrangements in nodular lymphocyte predominance Hodgkin lymphoma but not in classical Hodgkin lymphoma. Blood. 2003;101:706–710. doi: 10.1182/blood-2002-05-1592. [DOI] [PubMed] [Google Scholar]
- 480.Ye BH, et al. Alterations of a zinc finger-encoding gene, BCL-6, in diffuse large-cell lymphoma. Science. 1993;262:747–750. doi: 10.1126/science.8235596. [DOI] [PubMed] [Google Scholar]
- 481.Bunting KL, Melnick AM. New effector functions and regulatory mechanisms of BCL6 in normal and malignant lymphocytes. Curr. Opin. Immunol. 2013;25:339–346. doi: 10.1016/j.coi.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Lamant L, et al. Gene-expression profiling of systemic anaplastic large-cell lymphoma reveals differences based on ALK status and two distinct morphologic ALK+ subtypes. Blood. 2007;109:2156–2164. doi: 10.1182/blood-2006-06-028969. [DOI] [PubMed] [Google Scholar]
- 483.Dupont T, et al. Selective targeting of BCL6 induces oncogene addiction switching to BCL2 in B-cell lymphoma. Oncotarget. 2016;7:3520–3532. doi: 10.18632/oncotarget.6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Mello SS, Attardi LD. Deciphering p53 signaling in tumor suppression. Curr. Opin. Cell Biol. 2018;51:65–72. doi: 10.1016/j.ceb.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Mihara M, et al. p53 has a direct apoptogenic role at the mitochondria. Mol. Cell. 2003;11:577–590. doi: 10.1016/S1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 486.Oliner JD, et al. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature. 1993;362:857–860. doi: 10.1038/362857a0. [DOI] [PubMed] [Google Scholar]
- 487.Zenz T, et al. TP53 mutation and survival in aggressive B cell lymphoma. Int. J. Cancer. 2017;141:1381–1388. doi: 10.1002/ijc.30838. [DOI] [PubMed] [Google Scholar]
- 488.Herting F, Friess T, Umana P, Middleton S, Klein C. Chemotherapy-free, triple combination of obinutuzumab, venetoclax and idasanutlin: antitumor activity in xenograft models of non-Hodgkin lymphoma. Leuk. Lymphoma. 2018;59:1482–1485. doi: 10.1080/10428194.2017.1376740. [DOI] [PubMed] [Google Scholar]
- 489.Kuruvilla J, et al. Selective inhibition of nuclear export with selinexor in patients with non-Hodgkin lymphoma. Blood. 2017;129:3175–3183. doi: 10.1182/blood-2016-11-750174. [DOI] [PubMed] [Google Scholar]
- 490.Morris SW, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263:1281–1284. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 491.Feldman AL, et al. Novel TRAF1-ALK fusion identified by deep RNA sequencing of anaplastic large cell lymphoma. Genes Chromosomes Cancer. 2013;52:1097–1102. doi: 10.1002/gcc.22104. [DOI] [PubMed] [Google Scholar]
- 492.Martinengo C, et al. ALK-dependent control of hypoxia-inducible factors mediates tumor growth and metastasis. Cancer Res. 2014;74:6094–6106. doi: 10.1158/0008-5472.CAN-14-0268. [DOI] [PubMed] [Google Scholar]
- 493.Sgambato A, Casaluce F, Maione P, Gridelli C. Targeted therapies in non-small cell lung cancer: a focus on ALK/ROS1 tyrosine kinase inhibitors. Expert Rev. Anticancer Ther. 2018;18:71–80. doi: 10.1080/14737140.2018.1412260. [DOI] [PubMed] [Google Scholar]
- 494.Gambacorti Passerini C, et al. Crizotinib in advanced, chemoresistant anaplastic lymphoma kinase-positive lymphoma patients. J. Natl Cancer Inst. 2014;106:djt378. doi: 10.1093/jnci/djt378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.