Abstract
Objective:
Cerebral arterial networks match blood flow delivery with neural activity. Neurovascular response begins with a stimulus and a focal change in vessel diameter, which by themselves is inconsequential to blood flow magnitude, until they spread and alter the contractile status of neighboring arterial segments. We sought to define the mechanisms underlying integrated vascular behavior and considered the role of intercellular electrical signalling in this phenomenon.
Approach and Results:
Electron microscopic and histochemical analysis revealed the structural coupling of cerebrovascular cells and the expression of gap junctional subunits at the cell interfaces, enabling intercellular signaling among vascular cells. Indeed, robust vasomotor conduction was detected in human and mice cerebral arteries after focal vessel stimulation; a response attributed to endothelial gap junctional communication, as its genetic alteration attenuated this behavior. Conducted responses was observed to ascend from the penetrating arterioles, influencing the contractile status of cortical surface vessels, in a simulated model of cerebral arterial network. Ascending responses recognised in vivo after whisker stimulation, were significantly attenuated in mice with altered endothelial gap junctional signalling confirming that gap junctional communication drives integrated vessel responses. The diminishment in vascular communication also impaired the critical ability of the cerebral vasculature to maintain blood flow homeostasis and hence tissue viability, after stroke.
Conclusions:
Our findings highlight the integral role of intercellular electrical signalling in transcribing focal stimuli into coordinated changes in cerebrovascular contractile activity and expose, a hitherto unknown mechanism for flow regulation after stroke.
Keywords: Cell Signalling, Conducted vascular response, Cerebral blood flow, Endothelial gap junctions, Stroke, Vascular Biology
Subject codes: Animal Models of Human Disease, Hemodynamics, Translational Studies, Vascular Biology, Ischemic Stroke, Physiology
Graphical Abstract
Introduction
Cerebral arteries are linked in series and parallel, forming an integrated network responsible for matching blood flow delivery with energetic demand (1, 2). The coupling process is commonly perceived as beginning with neural cells generating a stimulus and ending with its diffusion to a neighboring blood vessel, altering the contractile state of vascular smooth muscle. While focal changes in vasomotor tone can subtly tune blood flow distribution, they are unable to initiate larger changes in perfusion magnitude, which is needed to support robust neural activity. The latter response will only occur if vascular resistance changes in multiple arterial segments, upstream from the focal site of neural stimulation.
As vascular resistance is broadly distributed in network structures, the arterial wall must be encoded with mechanism(s) to convert focal neural stimuli into broader multi-segmental responses. The identity of these mechanisms is uncertain and a center point of investigation in the vascular field. Of note in the peripheral vasculature is the ability of varied but focal stimuli, to elicit responses that spread along arteries and across branch points (3, 4). The so called “conducted response” is independent of metabolite diffusion, hemodynamics and innervation, and requires a common signal to spread among thousands of vascular cells (5-7). The movement of ions (charge) via vascular gap junctions is that signal and it coordinates changes in membrane potential (VM), cytosolic [Ca2+] and myosin light chain phosphorylation (8), altering vessel contractility. Vascular gap junctions are comprised of two hemi-channels of six connexin (Cx) subunits (i.e. Cx37 (9-12), Cx40 (10-13) Cx43 (12,14) and Cx45 (14-17); each expressed at highly variable levels, depending on the type of cells they connect (18,19).
The process of intercellular conduction has escaped deep quantitative examination in the brain, a knowledge gap that compromises understanding of integrated blood flow control, in this critical organ. Consequently, we crafted a clear quantitative framework to rationalize conduction, its electrical underpinnings and how they integrate into and drive the control of brain blood flow. Foundational experiments focused on arterial structure, examining intercellular connections and Cx40 abundance, a key gap junctional subunit exclusive to endothelial cells, in mice (8, 20-22). In the ex vivo setting, robust conduction was observed, and this response was impaired upon the elimination of endothelium or Cx40. A virtual network, built from structural and electrical observations, revealed that the arterial wall is optimally designed to facilitate the “ascension” of vascular responses from penetrating arterioles into the cortical surface network. Multi-photon imaging of awake mice detected ascension after whisker stimulation, and deletion of Cx40 strikingly impaired the upward spread of vessel dilation. The process of ascension need not be limited to functional hyperemic responses but could, as in the case of stroke, aid in blood flow homeostasis and neuroprotection. This relationship was confirmed in vivo, with the deletion of Cx40 blunting the compensatory blood flow responses to stroke, expanding tissue injury. Vascular electrical signalling is integral in translating discrete neural events into broader multi-segmental responses, necessary to support neurovascular coupling and to maintain critical levels of perfusion during severe cerebrovascular challenges.
Materials and Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Animals and reagents
All procedures were approved by the Animal Care Committees at the Universities of Calgary and Western Ontario and complied with the Canadian Council on Animal Care and Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines. All animals (See “Supplemental Material, Major resource table I, II” for details) were male, 8-10 weeks of age and within 21-27 grams body weight; were group housed with a 12-hour light/dark cycle and with free access to food and water. Cx40−/− mice were obtained from Prof. Janis M. Burt (University of Arizona) and are available through The Jackson Laboratory (B6.129S4-Gja5tm1Paul/J) (Stock No: 025697). Female Cx40−/− mice were first bred with C57BL/6J males and the resulting heterozygous animals were then bred to create the first generation of homozygous Cx40−/− animals. Cx40−/− breeding pairs were subsequently established, and pups were backcrossed to parents for up to 4 generations. Quality control genotyping were done on randomly collected samples from every generation of experimental animals. Considering the minor, non-vascular effects of Cx40 and in order to minimise the breeding and use of animals as per the regulation of Animal care and use Committee, C57BL/6J mice were used as controls in all experiments. Animals within strains were randomly assigned into different experimental groups. One mouse (C57BL/6J group) died during stroke/MR imaging in the 3 h reperfusion experiments. Two mice were also lost (one each from C57BL/6J and Cx40−/− groups) during the 24 h reperfusion experiments. One brain could not be collected (Cx40−/− group) for cresyl violet staining, as the animal did not recover after the MR session. Experimental sample (n) numbers can be found in “Supplemental Material, Tables I & II”; all reagents were purchased from Sigma-Aldrich (Missouri, USA), unless otherwise stated.
Indirect calorimetry, activity and inactivity
Differences in oxygen and carbon dioxide consumption, respiratory exchange ratio, cumulative mass of food and water consumed, activity (number of infrared beam breaks) and sleep epochs were analysed in C57BL/6J and Cx40−/− mice, using the Comprehensive Lab Animal Monitoring System (CLAMS) metabolic chamber, interfaced using Oxymax software (Columbus Instruments, Columbus, Ohio, USA), as described (23). Chambers were maintained at 24 ± 1°C with airflow of 0.5 L/min and animals had ad libitum access to food and water. Metabolic parameters recorded every 10 minutes for 48 hours. To standardize data analysis, data from the same 12-hour interval were selected for each mouse.
Human tissue experiments
A total of 11 human cerebral arteries (3 for conduction assay; 2 for immunohistochemistry; 6 for quantitative polymerase chain reaction), were collected from 6 patients (3 males/3 females), (27-59 years of age) undergoing epileptic resection surgery. All experiments involving human tissue was approved by the Health Sciences Research Ethics Board at the University of Western Ontario (HSREB ID#107196). All samples were obtained after written, informed consent from patients. Resected brain sections were collected in a sealed container with saline solution, on ice and was transported to the lab in an insulated carrier bag. Freshly isolated cerebral arteries were used for conduction assay while isolated arteries were frozen in liquid nitrogen and stored at −80 for qPCR experiments. Data was analyzed using pooled male and female samples.
Myography, conduction assay and arterial membrane potential
Animals were euthanized by carbon dioxide asphyxiation and brains were carefully removed and placed in phosphate-buffered saline solution (pH 7.4) containing (in mM) 138 NaCl, 3 KCl, 10 Na2HPO4, 2 NaH2PO4, 5 glucose, 0.1 CaCl2, and 0.1 MgSO4 (24-26). First and second order middle cerebral arteries (MCAs) were dissected away from surrounding tissue and cut into 2.5 mm segments. The segments were then cannulated in a customized arteriograph and superfused with warm (37°C) physiological salt solution (PSS; pH 7.4) containing (in mM) 119 NaCl, 4.7 KCl, 1.7 KH2PO4, 1.2 MgSO4, 1.6 CaCl2, 10 glucose, and 20 NaHCO3. Arterial diameter was monitored using an automated edge detection system (IonOptix, Massachusetts, USA) coupled to a 10X objective. In a subset of experiments, an MCA with penetrating arteriole was isolated, triple cannulated and pressurized (50 mmHg) so that conduction experiments could be performed on the penetrating arteriole. Arteries were equilibrated for 30 minutes at 15 mmHg intravascular pressure and contractile responsiveness assessed by brief (~10s) exposure to 60 mM KCl in the bath solution. Following equilibration, intravascular pressure was raised to 50 mmHg (in vivo pressure). Maximal diameter was obtained by superfusing with a Ca2+ free PSS containing 2 mM EGTA. Small arterial branches were carefully closed by pinching with forceps, without damaging the main vessel segment. Small caliber MCAs were chosen, as a suitable length of vessel (2000 μm) could be isolated with minimal branching; this avoids (a) strong end effects where electrical events reflect back through the vessel and (b) the inclusion of large charge sinks that compromise charge spread.
The conducted response was assessed by placing a narrow bore (1-2 μm diameter) glass micropipette backfilled with KCl (500 mM) near one end of a pressurized human/mouse cerebral arteries (25, 26). Using pressure ejection (30 psi, 10s pulse), KCl was applied to a small portion of the artery inducing depolarization and a local constriction (25-30 μm) that represents ~50% the maximal constrictor capacity; within this range changes in membrane potential lead to a graded vasomotor response (25). Our approach yields a 15-20mV change of VM at the stimulation site (Figure 2e); a response that corresponds with a ~50 mM rise in local [K+] (27). Arterial diameter was monitored at sites 0, 450, 900, 1350 and 1800 μm distal to the point of agent application. Conducted dilatory responses were assessed after pressure injection of acetylcholine (1 mM) or K+ (25 mM); arterial diameter changes were monitored at the 0 and 450 μm sites on an isolated surface vessel or a penetrating arteriole coupled to a surface vessel (triple cannulation preparation). We chose to use small-bore pipettes backfilled with high stimulant concentrations as this approach minimizes the volume of fluid ejected onto the preparation. Superfusate flow then dilutes and crucially, keeps stimulants away from the conducted sites, positioned upstream from the site of agent application. Vasomotor responses were calculated as the difference in vessel diameter before and after stimulation with agonist. The role of the endothelium was investigated by comparing the conducted vasomotor responses prior to and following its removal, by passing an air bubble down the vessel lumen. Each group consisted of 7-10 vessels, each from a different animal (see “Supplemental Material, Table I” for details).
Arterial membrane potential (VM) was monitored with a glass microelectrode backfilled with 1M KCl (tip resistance = 75-100 MΩ) inserted into the vessel wall. Cerebral arteries were pressurized to 50 mmHg and VM was assessed in the presence of nifedipine (200 nM) to minimise changes in vasomotor tone. A successful impalement consisted of: 1) a sharp negative deflection in VM upon insertion; 2) a stable recording for at least one minute; and 3) a sharp return to baseline upon retraction.
Quantitative polymerase chain reaction (qPCR)
Cerebral arteries were collected from resected brain tissue (6 arteries/4 patients) and MCAs were collected from C57BL/6J and Cx40−/− animals (6-7 animals per group, details in “Supplemental Material, Table I & II”). Total ribonucleic acid (RNA) was isolated using RNeasy plus micro kit (Qiagen, ON, Canada) and reverse transcription was performed with the Quantinova reverse transcription kit (Qiagen); reaction mixtures lacking reverse transcriptase was used as negative control. Following standardization to the reference genes, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (human) or ɑ-actin (mouse); test genes (vascular connexins) in human vessels were normalized relative to human whole heart (RNA purchased from Clontech) and in mouse; were expressed relative to C57 (Cx40+/+) vessels, using the relative expression software tool (REST) (29).
Real-Time PCR was performed using Prime Time qPCR primers (Integrated DNA Technologies, IA, USA) (“Supplemental Material, Table IV”), the Kapa SYBR Fast Universal qPCR kit (Kapa Biosystems, MA, USA) and cDNA template from cerebral arteries (1ng of template per reaction). The running protocol employed an Eppendorf Realplex 4 Mastercycler (ON, Canada) and was 45 cycles in length (95°C for 5 seconds, 55°C for 15 seconds and 72°C for 10 seconds). PCR specificity was confirmed by dissociation curve analysis and template controls which yielded no detectable fluorescence. Primer efficiencies were determined experimentally and ranged from (80-100%).
Electron microscopy and connexin immunohistochemistry
For electron microscopic examinations, animals were perfusion fixed in 2% paraformaldehyde, 3% glutaraldehyde in 0.1 M sodium cacodylate, with 10 mM betaine and 0.1 M sucrose. Vessel segments, ~3-5 mm long, were embedded in Araldite 502 using standard procedures and sectioned longitudinally. Sections were imaged at 4 MP on a transmission electron microscope (JEOL (Australasia) Pty. Ltd.).
For Cx immunohistochemistry, animals were perfusion fixed in 2% paraformaldehyde in 0.1 M PBS. MCAs were dissected, opened and pinned (en face preparation) on Sylgard (WPI, USA); penetrating arterioles were also dissected and mounted. Sections were compared against appropriate controls (without primary antibody, Cx40 binding peptide). Propidium iodide or DAPI were used to label nuclei as needed. A list of primary/secondary antibodies used can be found in “Supplemental Material, Major resource table III”). Arterial segments were washed with PBS and incubated for 2 h at room temperature in a blocking buffer containing 1% BSA and 0.2% Triton-X in PBS. Segments were then incubated overnight with primary antibody against Cx37, Cx40 and Cx43 at 4°C followed by incubation with secondary antibody at room temperature for 2 h. Tissue was then mounted in anti-fade mounting media and examined under a FV1000 confocal microscope (Nikon Instruments Inc.) with uniform settings.
Computational modeling
A single vessel and an arterial network model were constructed using a generative modeling approach (30). A single vessel model of defined length and diameter was comprised of one layer of endothelial and two layers of smooth muscle; each was represented 3D space with cells of a particular volume. Homocellular gap junction couplings are defined using a pattern generator that specify interacting cells (a principle that only specifies links among model objects and not specific cell coordinates). Each endothelial and smooth muscle cell was given 6 homocellular couplings and conductance was set according to past calculations (3, 30). Ionic conductance was represented by polynomial fits of whole cell IV curves from cerebral arterial endothelial and smooth muscle cells (“Supplemental Material, Fig. III”). While IV curves are generally linear over the physiological VM range, a number of the underlying channels do display voltage dependent properties which could impact conduction (26). Arterial VM was subsequently coupled to diameter through a sigmoid function (“Supplemental Material, Fig. IV”). Using a best fit approach, this computational model was used to determine the endothelial-to-endothelial coupling conductance from data collected in Figure 2.
In order to create a 3D network, coordinates of vessel bifurcations and network ends were obtained from Shih et al 2009 (30). Assuming vessels to be straight cylinders, vessel lengths and connectivity were calculated (meandering vessels were approximated using piecewise linear curve fits (27). Each surface vessel was covered with two smooth muscle layers and was assumed to have a resting diameter of 75 μm, whereas penetrating arterioles were 400 μm long, covered with a single layer of smooth muscle and assumed to have a resting diameter of 30 μm. At each bifurcation, endothelial cells at the end of an incoming branch were divided into two sets; the number of ECs in each set being dependent on the ratio of ECs in the two opposing branches. Endothelial cells within each set were coupled to endothelial cells from a single opposing branch while smooth muscle cells, owing to their circumferential orientation, were coupled to at least one smooth muscle cell from each opposing branch, at the bifurcation (30).
Current-voltage (IV) curves of cerebral endothelial and smooth muscle cells were obtained by patch clamping of isolated vascular cells in whole-cell mode, in physiological solutions (26). Polynomial fits (Supplemental Fig. III) were described by:
A VMrest of −40 mV was achieved by simple coordinate transformation of the VM-axis. Following simulation of VM within each cell of the vascular network, the electrical response was translated into a change in inner diameter using the sigmoid function:
where VMrest is resting potential that corresponds to the electrical model. For penetrating arterioles, Dmax = 50μm and for surface vessels Dmax = 120μm.
To estimate Km, a constant determining the dynamic range of excitation-contraction coupling, a minimal diameter, Dmin was added to the sigmoid and this function was fitted to the observed relationship between simultaneously measured VM and outer vessel diameter in cerebral vessels (see Supplemental Fig. IV); from the fit, Km = 0.134.
Neurovascular coupling experiments and Two-photon microscopy
Animal surgery and training were performed as described previously (31). Briefly, mice were anesthetized with isoflurane (5% for induction and 1-1.5% for maintenance), mounted on a stereotaxic frame and body temperature maintained using a feedback control system for all surgeries. One week before imaging, a head bar was implanted over the interparietal bone and glued in place with cyanoacrylate without creating a cranial window. An observation well was progressively built around the future cranial window using dental cement, for a water dipping objective. After two days of rest, all animals were given two training sessions (30-45 minutes) on a spherical air-supported treadmill and were habituated to a 5s air puff to the whiskers.
After at least two more rest days, craniotomy was performed to generate a cranial window (3×3 mm square) over the barrel cortex. Prior to surgery, animals were treated with Dexamethasone 21-phosphate disodium salt (anti-inflammatory drug, 4 mg/ml, Sigma) and Buprenorphine (analgesic). Bone and underlying dura mater were removed. A drop of artificial cerebrospinal fluid was then applied to the surface of the cortex; the open window was covered with a cover glass and sealed using cyanoacrylate and dental cement. FITC-dextran was injected through a tail artery cannula to label the vasculature. Animals were positioned on the spherical treadmill with the head immobilised using the head bar and activity was constantly monitored using a video camera. Imaging of the microvascular network was performed on active, awake animals that displayed signs of alertness (vigorous running) and had natural whisker movements. This experimental setup best mirrors a normal physiological state and allows the animals to control key cardiovascular parameters including respiration, heart rate and blood flow. A 5 sec air puff was delivered to the contralateral side using just enough air to deflect all the whiskers while vascular responses were imaged using a custom built in vivo two-photon microscope equipped with a Ti:sapphire laser (Coherent Chameleon, Ultra II, ~4 W, 670-1080 nm, ~80 MHz, 140 fs pulse width) and controlled by Scan Image software. Vasomotor responses of the pial and penetrating arterioles were monitored using xy raster scanning (0.98 to 7.8Hz) while the animals were running. Peak vasomotor response (ρD) was calculated as the difference in vessel diameter at rest (2 sec time averaged) and following an air puff stimulation. 3D rendering of the microvascular network was done using the 3D viewer plugin (31).
Mouse model of ischemic stroke
Animals were anesthetized using 1.5% isoflurane (30% O2, remainder N2O) and body temperature was maintained using a feedback-controlled heating system. Focal cerebral ischemia was induced using an intraluminal technique (32). Briefly, a midline neck incision was made, and the left common and external carotid arteries were isolated and ligated. A microvascular clip was temporarily placed on the internal carotid artery and an 8-0 silicon-coated nylon monofilament (Doccol, USA) was directed through the internal carotid artery until the origin of MCA. After induction of ischemia, animals were transferred to the MRI system for imaging. Following 60 minutes of ischemia, the monofilament was withdrawn, and reperfusion was monitored at 1, 2 and 3 hours. In a subset of animals, stroke was induced/monitored, and reperfusion was extended to 24 hours during which a repeat scan was performed. Sham controls underwent anesthesia and midline incision, without manipulation of the arteries. Anesthesia was maintained by monitoring the heart rate and respiration, throughout the experimental procedure.
Magnetic resonance imaging and analysis
MR imaging and analysis were performed on a 9.4T/21 cm horizontal bore magnet (Magnex, UK), a Bruker BGA-12S gradient system and an Avance II hardware (Bruker, Germany) as described (33). The animal’s head was secured in a 35mm quadrature volume radiofrequency coil, positioned at the centre of the magnet. While inside the magnet, anesthesia and key physiological parameters (respiration, body temperature, heart rate) were carefully monitored and maintained throughout. Each scanning session acquired a set of T2 weighted (T2W) images and a Perfusion-Weighted (PW) map, for investigating the changes in tissue injury and cerebral blood flow, respectively (34, 35). T1 Weighted (T1W) maps were also acquired from corresponding slice location as PW scans.
Cerebral perfusion was calculated in a total of 13 Regions of Interests (ROI’s) as:
where T1 was calculated from T1 weighted images and partition coefficient of water (λ) was assumed to be 0.9 (36). Mcon is the intensity of control images and Msat is the intensity of the images acquired after arterial inversion (34).
The T2W scan (Repetition time of 4s) was acquired with a field of view of 2cm2, a 128 X 128 matrix and thirty-two continuous slices (0.8mm thick) with an inter-echo spacing of 10 ms. PW map was obtained in a single slice (1 mm) using a continuous arterial spin labelling technique targeted at the mid-striatum region (anatomical centre of MCA supplied region) as described previously (31, 32). A 2s long radiolabelling pulse was applied to the neck vessels in the presence of a 2G/cm gradient followed with a delay of 400 ms by a HASTE (Half-Fourier Acquisition Single Shot Turbo Spin Echo) sequence using a repetition time of 3000 ms, Effective Echo Time of 13.3 ms and a RARE factor of 36, for a total of 16 averages. Magnetization in unsaturated control images were obtained by using the same radiofrequency excitation applied symmetrically, opposite to the labeling plane, to help eliminate magnetization transfer effects.
Absolute blood flow values obtained as below zero were considered as no flow regions (0 ml/100g/min), in analysis. All MR images were analysed using Paravision 5.1 software and four anatomical (two cortical and two striatal) regions were studied, by forming averages from multiple ROI’s. Cortical region-1 represented the watershed region of the cortex supplied by MCA and ACA while cortical region 2 was demarcated in the MCA supplied territory of the cortex and were formed from averages of two and five ROI’s, respectively. Similarly, striatal region 1 included the watershed area between MCA and PCA while striatal region 2 was located in the core of MCA supplied territory and was formed from averages of three ROI’s, each (Fig. 5a).
Histology
After MR imaging (24 h reperfusion endpoint), animals were euthanized and transcardially perfused, first with heparinized saline and then with normal saline (37). Brains were removed, frozen on dry ice and cut into 20 μm coronal sections using a cryostat. Brain sections 1 mm apart were stained with cresyl violet and image analysis software (ImageJ; NIH) was used to outline the border between infarcted and healthy tissue (32). Infarcted area was quantified by subtracting the area of non-lesioned ipsilateral hemisphere from that of the contralateral hemisphere. Infarct volume was calculated by integrating infarcted areas from 6 equally spaced rostrocaudal sections. Edema or brain swelling was calculated as absolute difference between ischemic and non-ischemic hemispheres (32, 37, 38). An examiner blinded to tissue identity performed the analysis.
Blood pressure and heart rate assessment
The non-invasive assessment of blood pressure was performed (between 10.00am and 1.00pm) on conscious C57BL/6J and Cx40−/− animals (10 animals per group) using the non-invasive CODA tail-cuff system (Kent Scientific, Connecticut, USA) as previously described (39). To minimize anxiety, animals were properly acclimatized, and body temperature was maintained with a warming platform. Systolic blood pressure and heart rate was averaged from two experiments, each consisting of up to 15 individual trials.
Statistical analysis
Data sets are expressed as box and whisker plots and P < 0.05 was considered statistically significant. Power analysis was done to calculate sample size sufficient for obtaining statistical significance and n numbers reported in previous publications were considered, in experiments involving vessels and animals. Significant results are indicated in the graphs or as bold text (“Supplemental Material, Table III”), after animal groups were evaluated and compared for differences using non-parametric Mann-Whitney U test (single tailed) (GraphPad PRISM 4.0, California, USA), performed at individual time points. To assess differences in conduction decay in vivo, individual data sets were fitted to an exponential function f(x) = k*exp(−x/lambda), using the nonlinear least-squares Marquardt-Levenberg algorithm (Gnuplot version 5.2). A single tailed- t-test compare the summative decay constant from wild type and Cx40−/− mice. Experimental design and data presentation are reported in compliance with the ARRIVE guidelines.
Results
The cerebral arterial wall is structurally encoded for conduction
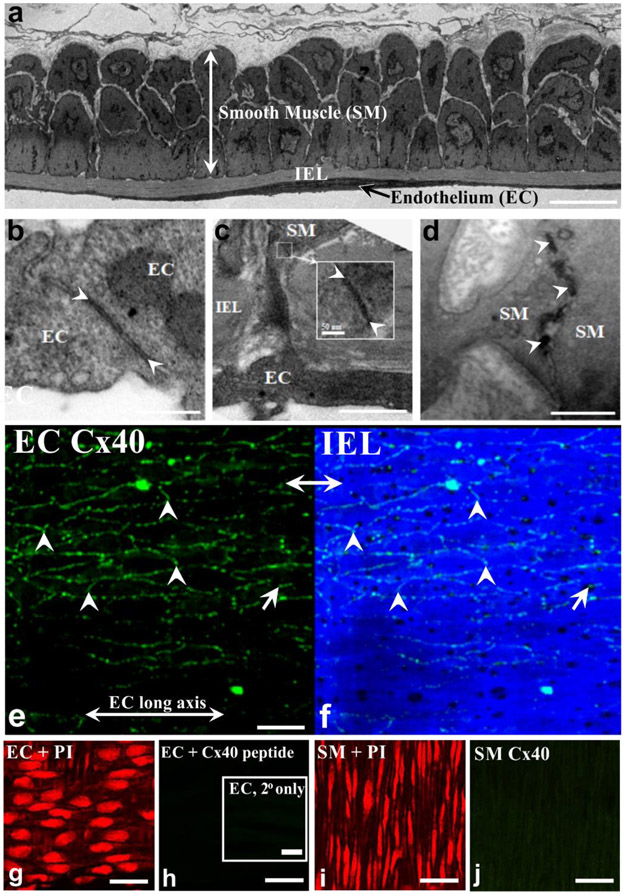
The spread of electrical signals along cerebral arteries requires intercellular contacts and the presence of gap junctions. Mouse MCAs analyzed using electron microscopy revealed robust cellular contact sites, principally among neighboring endothelial cells (Fig. 1a,b). Contact sites were sparser among smooth muscle cells and between the two cell layers, with the latter requiring projections to form and extend through a connective tissue layer (19) (Fig. 1c,d). Endothelial-to-smooth muscle projections are particularly striking given the thickness of the internal elastic lamina (IEL) and the distance between the two cell layers. Contact sites irrespective of the location were electron dense with regions of pentalaminar membrane, suggestive of Cx expression and the presence of gap junctions (19). In this regard, Cx40 was detected between longitudinally oriented endothelial cells (Fig. 1e,f; arrowheads, g), indicative of its potential role in longitudinal charge spread. Note, punctate Cx40 was observed near ‘IEL holes’ (Fig. 1f (arrow)), consistent with Cx40 localization at the: 1) endothelial-smooth muscle interface; or 2) in the endoplasmic reticulum, found at the base of projections. Cx40 labelling was absent; in control tissues treated with secondary antibody alone or with a Cx40 peptide that competes for the primary antibody binding site (Fig. 1h(inset)) and in the smooth muscle layer (Fig.1i, j). Subsequently, Cx40 expression was also confirmed in the endothelial layer of human cerebral arteries (“Supplemental Material, Fig.I”). A broader immunohistochemical investigation revealed the abundance of Cx37, Cx40 and Cx43 in the endothelial cell layer of mouse MCAs (“Supplemental Material, Fig.II a-c”). Punctate Cx37 and Cx43 labelling was also observed near IEL holes indicating its localization near the endothelial-smooth muscle interface (“Supplemental Material, Fig.II a, c”).
Figure 1: Vascular cells in cerebral arteries connect to one another and express Cx40.
(a) Electron microscopy reveals that mouse middle cerebral arteries are composed of smooth muscle (SM), internal elastic lamina (IEL) and endothelial cell (EC) layers. Bar = 5 μm. (b-d) Contact sites (arrowheads) between endothelial cells (b, bar = 0.25 μm), between smooth muscle and endothelium (c, scale bar 0.25 μm), and between smooth muscle cells (d, bar = 0.5 μm). (e) Cx40 (green), a key gap junctional protein was identified by immunohistochemistry in middle cerebral arteries isolated from mice. (f) IEL was delineated by 488 nm autofluorescence (g,i) Propidium iodide labeling verified EC and SMC patency. (h) Endothelial Cx40 signal was absent in sections incubated without the primary antibody (inset) and in endothelial cells treated with a Cx40 peptide that competes for the primary antibody binding site. (j) Cx40 staining was also absent in the smooth muscle cell layer. Bar, e-j = 25 μm
Endothelial/gap junctional communication drives conducted responses
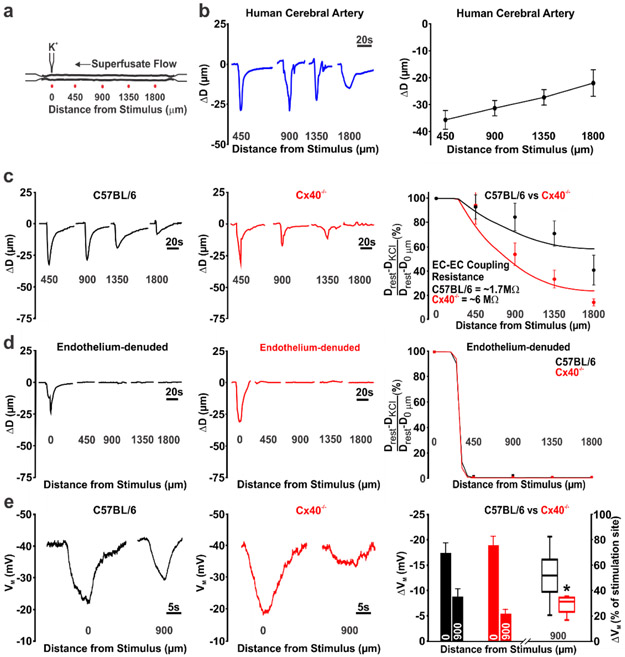
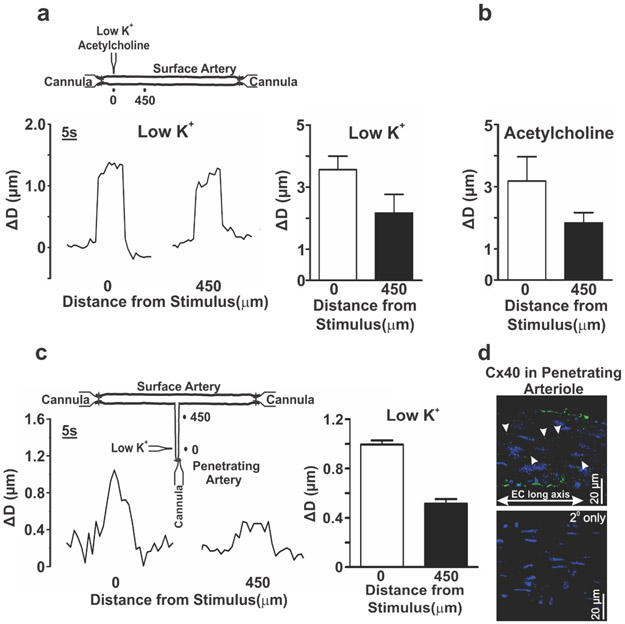
Mouse and human cerebral arteries (~100 μm diameter) were mounted in a pressure myograph for ex vivo conduction experiments (Fig. 2a). The discrete application of a depolarizing stimulus (high pipette [K+]) to one end of a human or a mouse (C57BL/6J) cerebral artery, elicited a focal constriction that robustly conducted (within ~300ms) to the distal 1800 μm site in an electrotonic manner (Fig. 2b, c). Note, robust conduction was observed in human cerebral arteries despite past observations of infiltrated leucocytes in resected brain tissue from epileptic patients (40). Consistent with the endothelium operating as the primary pathway for longitudinal charge spread (7), air bubble disruption eliminated conduction beyond the focal site of stimulation (Fig. 2d). Genetic ablation of Cx40 diminished but did not eliminate conducted vasomotor responses (Fig. 2c; middle column) consistent with an increase in endothelial-to-endothelial coupling resistance. We subsequently calculated the change in endothelial-to-endothelial coupling resistance using a validated model of electrical communication that accounted for the structural, gap junctional and ionic properties of vascular cells. The latter entailed incorporating polynomial fits of IV curves from isolated endothelial and smooth muscle cells (“Supplemental Material, Fig. III”). A nonlinear voltage-diameter relationship generated from cerebral arterial observations was used to convert VM into a vasomotor response (27) (“Supplemental Material, Fig. IV”). This approach yielded endothelial-to-endothelial coupling resistances of 1.7 MΩ and 6 MΩ in C57BL/6J and Cx40−/− mice, respectively (Fig. 2c, right column). Our modelling data also predicted the absence of conduction in endothelial denuded arteries (Fig. 2d, right column) and that the rise in endothelial-to-endothelial coupling resistance in Cx40−/− arteries would starkly diminish electrical spread and vasomotor response, a 1000 μm from the point of stimulation (“Supplemental Material, Fig. V”). Arterial VM measurements confirmed that spreading electrical responses were indeed attenuated, 900 μm from the site of agent application in Cx40−/− arteries (Fig 2e). A subset of experiments also revealed that hyperpolarizing stimuli (lower pipet [K+], acetylcholine) could induce a small but consistent conducted vasodilation that decayed along surface vessels and penetrating arterioles (Fig. 3a-c). Cx40 expression was also observed in the endothelial cell layer of penetrating arterioles in mice (Fig. 3d).
Figure 2: Conducted responses in cerebral arteries require intact endothelium and gap junctions.
(a) General conduction paradigm: An isolated, pressurised middle cerebral artery was stimulated by pressure injecting KCl (500 mM, 10s, 30 PSI) through a micropipette; vasomotor responses monitored 0, 450, 900, 1350 and 1800 μm, distal to the point of stimulation. (b) K+ induced conducted responses were observed in cerebral arteries isolated from resected human tissue. (c) Genetic deletion of Cx40 attenuated K+ induced conduction in mouse cerebral arteries. Conducted responses were similar among C57BL/6 and Cx40−/− mice near the point of K+ application (450 μm site); enhanced conduction decay was observed in Cx40−/− mice at the 900 μm (U =18.50, P<0.01, Mann-Whitney U test; control, n=10 vessels; Cx40−/−, n=10 vessels), 1350 μm (U =11, P<0.01, Mann-Whitney U test; C57BL/6, n = 9 vessels; Cx40−/−, n = 10 vessels), and 1800 μm (U =13, P<0.01, Mann-Whitney U test; C57BL/6, n = 8 vessels; Cx40−/−, n = 10 vessels) sites. Data in (c, right column) were fitted using a validated computational model to calculate endothelial-to-endothelial (EC-EC) coupling resistance. (d) The endothelium’s role in conduction was tested by passing air bubbles through the lumen to denude this layer. Data in (d, right column) were fitted using a validated computational model in which the endothelium had been electrically uncoupled. (e) The spreading membrane potential (VM) response in Cx40−/− arteries was diminished at the conducted site (900 μm). Summary data (right column) is presented in absolute terms or with the 900 μm site expressed relative to the stimulation site (U =4, P<0.05, Mann-Whitney U test; control, n=6 vessels; Cx40−/−, n=5 vessels).
Figure 3. Conducted vasodilation in middle cerebral arteries and penetrating arterioles from C57BL/6 mice.
(a-b) Middle cerebral arteries were isolated and pressurized to 50 mmHg (Inset). A micropipette containing ‘low pipette [K+]’ (25mM) or acetylcholine (1 mM) was positioned next to the arterial wall. The solution was pressure ejected (10 secs, 30 psi) and diameter was measured at the local application site (0 μm) and one conducted site (450 μm). Representative tracing and summary data of the local and conducted vasodilatory response (resting diameter for the ‘low pipette [K+]’ (n=4 vessels) and acetylcholine groups (n=3 vessels) were 91+14 and 76+8, respectively). (c) A middle cerebral artery with penetrating arteriole was isolated and triple cannulated (Inset). The preparation was pressurized to 50 mmHg; a pipet containing a 25 mM K+ saline solution was placed next to the arterial wall and pressure ejected (10 secs, 30 psi). Diameter was measured at the local (0 μm) and conducted site (450 μm). Summary data (n=4 vessels; resting diameter 47+5) of the local and conducted vasodilatory responses. (d) Cx40 (green) was identified by immunohistochemistry in the endothelial layer of penetrating arterioles from C57BL/6 mice.
Control qPCR experiments confirmed the presence of Cx37, Cx40, Cx43 and Cx45 mRNA in human and mice cerebral arteries (“Supplemental Material, Fig. VI”). Cx40 mRNA was particularly abundant in human cerebral arteries (“Supplemental Material, Fig. VI a”). Immunohistochemical analysis of Cx40−/− mice revealed an absence of Cx40 labeling in the endothelium and a marked reduction in Cx37 and Cx43 expression (“Supplemental Material, Fig. VII a-c”). The decrease in Cx37 and Cx43 expression was surprising as quantitative PCR analysis revealed a significantly higher Cx37 mRNA and a comparable expression of Cx43 in Cx40−/− mice, compared to C57BL/6J animals (“Supplemental Material, Fig. VI b”). Myogenic tone and vessel reactivity to 60 mM K+ was similar among vessels isolated from C57BL/6J and Cx40−/− mice (“Supplemental Material, Fig. VIII a, b”).
Virtual cerebral arterial network predicts the ascension of conducted responses
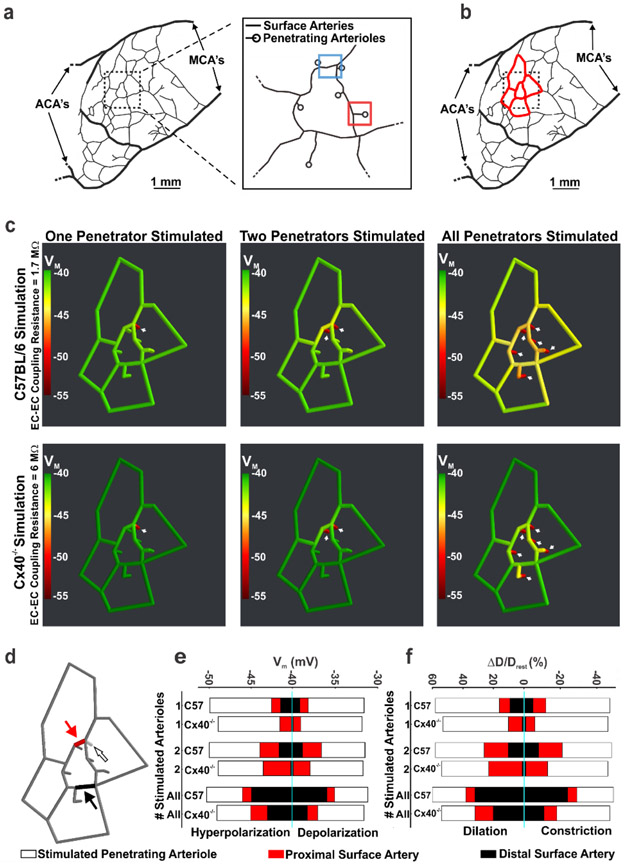
To assess the impact of conduction on integrated network function, we established and utilized an in silico model of vascular communication. Network architecture based on observations from Shih et al. (2009) (30) consisted of a surface vessel plexus, adjoined to six penetrating arterioles (Fig. 4a,b); each composed of smooth muscle and endothelial cells with defined structural and electrical properties. Simulations entailed voltage clamping an endothelial segment in one, two or all penetrating arterioles while monitoring responses throughout the entire network structure (Fig. 4c,d). As the number of stimulated penetrating arterioles increased, the electrical (Fig. 4c,e) and vasomotor (Fig. 4f and “Supplemental Material, Fig. IX”) responses progressively ascended into the surface vessel network, where their impact on blood flow delivery would be greater. The ascension process was diminished but remained intact when the voltage clamp was reduced from −55 to −45 mV (“Supplemental Material, Fig. X”). Simulating the increase in endothelial-to-endothelial coupling resistance in Cx40−/− arteries, impaired the ascension of electrical/vasomotor responses (Fig. 4c,e,f and “Supplemental Material, Figs. XI, XII”). This attenuation was observed irrespective of whether the distal arteriolar segments were hyperpolarized or depolarized, as quantitated in smooth muscle cells located at one site in the penetrating arteriole and at two sites in the cortical surface vessels (Fig. 4d-f and “Supplemental Material, Figs. IX-XII”). Furthermore, our in silico network simulations also equated varying endothelial-to-endothelial coupling resistance to direct changes in ascension of electrical and vasomotor responses (“Supplemental Material, Figs. XI, XII”).
Figure 4: Vascular cells retain biophysical properties that support the ascension of responses from penetrating arterioles to surface vessels.
(a, b) Using observations from Shih et al., 2009, a network model of surface arteries and penetrating arterioles was built to study the spread of electrical/vasomotor responses. The in silico model consisted of an interconnected network of surface arteries and six adjoining penetrating arterioles. Simulations entailed voltage clamping (15 mV positive or negative to resting VM, 500 ms) one distal segment in one, two or all penetrating arterioles and resolving the voltage/vasomotor response throughout the network. (c) The ascension of hyperpolarization was color mapped along the network; endothelial-to-endothelial coupling resistance was set to 1.7 (C57BL/6 control) or 6 (Cx40−/−) MΩ (calculated in Figure 2). (d-f) One distal segment in one, two or all penetrating arterioles was/were stimulated and steady state responses (smooth muscle VM and diameter) plotted at 3 sites in the network. Those included the penetrating arteriole (200 μm upstream from the stimulation site) and two surface vessel sites. (f) D and Drest represent peak response and resting diameters, respectively. MCAs = Middle cerebral arteries; ACA’s = Anterior cerebral arteries. Figures 4 a, b are reused after permission from SAGE Publishing Ltd.
Conducted responses are integral to neurovascular coupling function
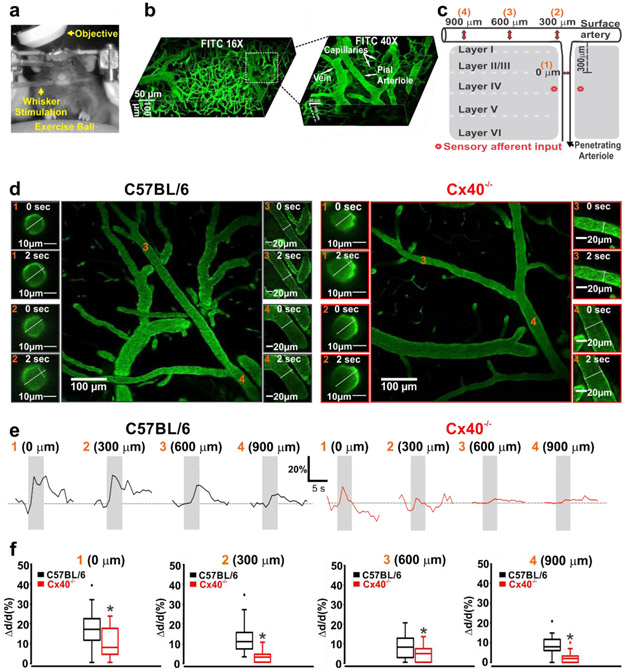
Past studies of functional hyperemia have implied that conduction plays a central role in coupling blood flow delivery with energetic demand (41-44). To rigorously test this perspective and to place it within our structured biophysical framework, mice were implanted with cranial windows, and trained to run on an exercise ball with their head fixed to an external apparatus. The cerebral vasculature was then labeled with fluorescein isothiocyanate and imaged by 2-photon microscopy while the barrel cortex was activated by whisker stimulation (Fig. 5a-c). The resulting dilatory response was monitored every 300 μm, starting in a penetrating arteriole (upstream of the barrel cortex) and into the adjoining cortical surface artery (Fig. 5c,d). Whisker stimulation in C57BL/6J mice initiated a marked dilation of penetrating arterioles that ascended upstream, with its magnitude decreasing as it advanced along the surface vessel (Fig. 5e,f). The spreading vasomotor response was strikingly reduced in Cx40−/− mice; changes at the 600 or 900 μm sites were minimal compared to controls (Fig. 5e,f). A non-linear regression analysis of the sequential measurement sites revealed that the rate constant of decay, decreased significantly in Cx40−/− mice (λ=501±269) compared to C57BL/6J mice (λ=1385±304; “Supplemental Material, Fig. XIII”). These findings highlight that conduction is an integral aspect of cerebrovascular control and neurovascular coupling responses to deep brain activity. Note that Cx40 is not a neuronal gap junctional subunit in adult mice and that Cx40−/− animals displayed comparable general development, feeding and breeding behaviours, to controls. Analysis of metabolic parameters revealed similar calorimetric properties in C57BL/6J and Cx40−/− mice, while exposed a modest, but statistically significant decrease in activity, in the Cx40−/− mice strain (“Supplemental Material, Table III”).
Figure 5: Compromised vascular conduction impairs neurovascular coupling responses.
(a,b) Awake mice were positioned on an exercise ball with their head fixed to an external apparatus, to facilitate two-photon imaging of the microvasculature labeled with FITC Dextran. c) Vasomotor responses to whisker stimulation were monitored at 4 sites upstream (orange numbers) near to the point of barrel cortex activation. (d) Photomicrographs of functional dilation at four vessel sites, prior to (0 s) and during (2 s) whisker stimulation in C57BL/6 and Cx40−/− mice. (e,f) Representative tracings and summary data of the functional vasomotor response to whisker stimulation. Measurements show significant attenuation of conducted responses in the penetrating arterioles and cortical surface vessels of Cx40−/− mice (0 μm: (U =85, P<0.05, Mann-Whitney U test; C57BL/6, n = 21 vessels; Cx40−/−, n = 14 vessels)), (300 μm: (U =24, P<0.05, Mann-Whitney U test; C57BL/6, n = 21 vessels; Cx40−/−, n = 14 vessels)), (600 μm: (U =126, P<0.05, Mann-Whitney U test; C57BL/6, n = 19 vessels; Cx40−/−, n = 21 vessels)), (900 μm: (U =22.50, P<0.05, Mann-Whitney U test; C57BL/6, n = 9 vessels; Cx40−/−, n = 16 vessels)). * denotes significant difference between groups.
Conduction is crucial for blood flow homeostasis during cerebral ischemia
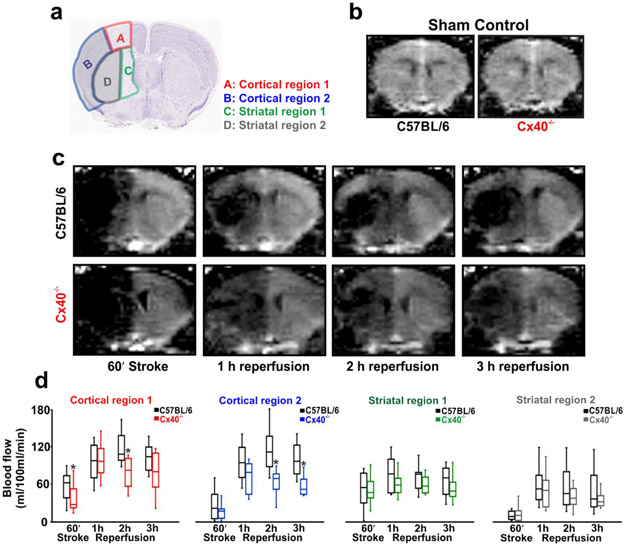
Surface vessel dilation occurs under pathophysiological conditions such as stroke to reroute blood flow to penumbral tissue. This response is observable in humans and animals alike and the redirection of blood notably improves recovery and tissue salvage (45). We hypothesized that vascular conduction is key to surface vessel dilation and consequently, a strategy was implemented to test this concept. Focal cerebral ischemia was induced by occluding the MCA and change in perfusion was monitored using Arterial spin labelling-magnetic resonance imaging (ASL-MRI) in C57BL/6J and Cx40−/− mice. Blood flow changes were quantified in two cortical and two striatal regions; Cortical/striatal region 1 demarks the watershed region supplied by middle/anterior or middle/posterior cerebral arteries while cortical/striatal region 2 represents the area supplied by the MCA (Fig. 6a). In sham controls, cortical/striatal blood flow averaged between 175-195 ml/100g/min and was similar in C57BL/6J and Cx40−/− mice (Fig. 6b). Occluding the MCA in C57BL/6J mice starkly reduced cortical and striatal blood flow (Fig 6c,d) and a partial but robust recovery was observed during short term reperfusion (3h) (Fig. 6c,d). In comparison, the blood flow response to stroke was notably lower in Cx40−/− mice, especially in the cortical/striatal watershed regions, achieving statistical significance only in cortical region 1 (Fig. 6c,d). Similarly, flow responses during reperfusion were also compromised in Cx40−/− animals, notably in the cortical regions and in watershed striatal region 1 (Fig. 6c,d). Diminished blood flow delivery in Cx40−/− mice cannot be explained by anatomical differences in cerebral vasculature or altered hemodynamics. Indeed, Cx40−/− animals are shown to have a similar number of pial surface vessels (46) and our analysis revealed identical heart rates (C57BL/6J; 608±97 vs Cx40−/−; 609±84), in comparison to controls. Systolic blood pressure was modestly higher in Cx40−/− mice (166.7±4.3 vs 140.4±3.6; U =7, P<0.05), Mann-Whitney U test; control, n=10 animals; Cx40−/−, n=10 animals), a physiological variable otherwise linked to better perfusion responses during and after stroke (47, 48).
Figure 6: The initial blood flow response to stroke is attenuated in Cx40−/− mice.
(a) Perfusion was monitored during and following stroke in four regions of interest: cortical region 1 (A), cortical region 2 (B), striatal region 1 (C) and striatal region 2 (D) (Image credit: Allen Institute). Anatomically, regions B and D are supplied by the middle cerebral artery whereas regions A and C are watershed regions supplied by the middle, and the anterior or the posterior cerebral arteries. (b) ASL-MR perfusion images demonstrate that blood flow is similar in the cortex of sham controlled C57BL/6 and Cx40−/− mice (U =2, P>0.05, Mann-Whitney U test; C57BL/6, n = 4 mice; Cx40−/−, n = 4 mice) and in striatum (U =3, P>0.05, Mann-Whitney U test; C57BL/6, n = 4 mice; Cx40−/−, n = 4 mice). (c) ASL-MR perfusion images from C57BL/6 and Cx40−/− mice during stroke and reperfusion. (d) Summary data and quantitative non-parametric analysis of blood flow responses to stroke and reperfusion in C57BL/6 and Cx40−/− mice. In comparison to control mice, a significant decline in blood flow was observed in Cx40−/− mice, during stroke in cortical region 1: (U = 24, P<0.05, Mann-Whitney U test; C57BL/6, n = 9 mice; Cx40−/−, n = 10 mice), during 2 h reperfusion in cortical region 1: (U = 13, P<0.05, Mann-Whitney U test; C57BL/6, n = 9 mice; Cx40−/−, n = 10 mice); cortical region 2 (U = 5, P<0.05, Mann-Whitney U test; C57BL/6, n = 9 mice; Cx40−/−, n = 10 mice) and during 3h reperfusion in cortical region 2 (U = 5, P<0.05, Mann-Whitney U test; C57BL/6, n = 9 mice; Cx40−/−, n = 10 mice). * denotes significant difference between groups.
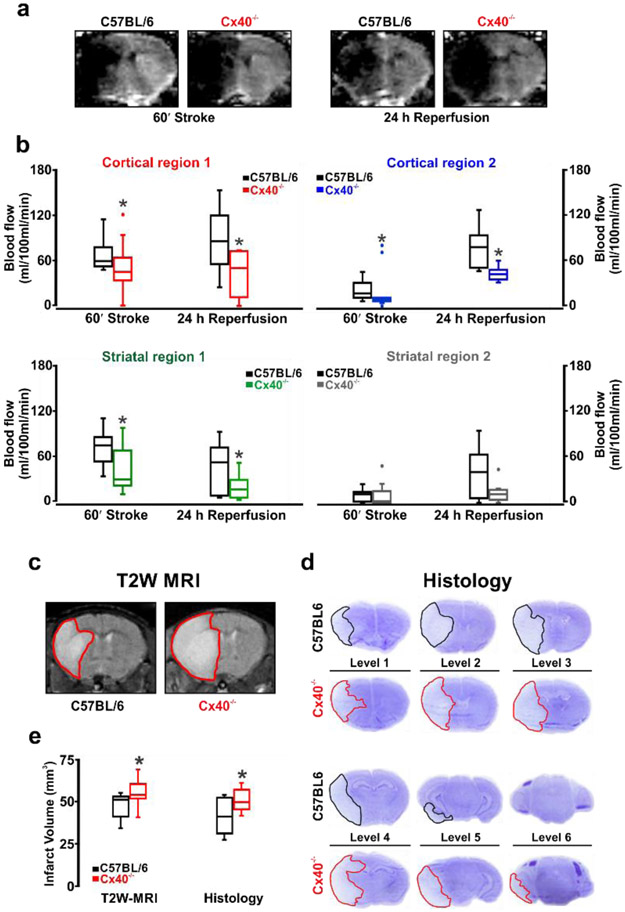
Stroke injury evolves during the acute phase and if perfusion is sustained, brain injury will be moderated. To test the linkage between conduction, blood flow and stroke injury, the reperfusion period was extended to 24 hours in a subset of animals, and measures of perfusion were complemented with assessments of tissue injury. Consistent with our previous observations, brain perfusion was decidedly lower in Cx40−/− mice compared to C57BL/6J controls. This difference persisted after 24 hours of reperfusion, particularly in the cortical regions and in watershed striatal region 1 (Fig. 7a,b). Blood flow in striatal region 2, a region fed by the MCA and encompassing the core of ischemic damage, was similar in C57BL/6J and Cx40−/− mice during stroke and early reperfusion although there was a trend of flow recovery in the C57BL/6J mice after 24 hours of reperfusion compared to Cx40−/− animals (Fig. 7a,b). The marked reduction in perfusion translated to augmented tissue injury in the Cx40−/− animals, as MR and histological examinations confirmed an increase in stroke volume (Fig. 7c-e). Interestingly, ischemic damage was similar in the core MCA territory but showed augmentation in adjacent brain levels, indicative of greater penumbral tissue loss in Cx40−/− mice (Fig. 7d). Collectively, these data confirm the decisive role for vascular conduction in vessel responses to stroke and in the preservation of salvageable brain tissue.
Figure 7: The prolonged blood flow response to stroke is attenuated in Cx40−/− mice, augmenting tissue injury.
Perfusion was monitored by ASL-MR during stroke (60 min) and reperfusion (24 h) in four regions of interest: cortical region 1&2 and striatal region 1&2 (see Figure 5). (a) ASL-MR perfusion images from C57BL/6 and Cx40−/− mice during stroke and 24 h reperfusion. (b) Summary data and quantitative non-parametric analysis of blood flow responses to stroke and 24h reperfusion in C57BL/6 and Cx40−/− mice. A significant decline in blood flow was observed in Cx40−/− mice, during stroke in cortical region 1 (U = 21, P<0.05, Mann-Whitney U test; C57BL/6, n = 9 mice; Cx40−/−, n = 9 mice); in cortical region 2 (U = 17, P<0.05, Mann-Whitney U test; C57BL/6, n = 9 mice; Cx40−/−, n = 9 mice); in striatal region 1 (U = 21, P<0.05, Mann-Whitney U test; C57BL/6, n = 9 mice; Cx40−/−, n = 9 mice) and during 24 h reperfusion in cortical region 1 (U = 17, P<0.05, Mann-Whitney U test; C57BL/6, n = 9 mice; Cx40−/−, n = 9 mice); in cortical region 2 (U = 6, P<0.05, Mann-Whitney U test; C57BL/6, n = 9 mice; Cx40−/−, n = 9 mice) and in striatal region 1 (U = 20, P<0.05, Mann-Whitney U test; C57BL/6, n = 9 mice; Cx40−/−, n = 9 mice). (c, d) T2 weighted MR imaging and cresyl violet staining of brain injury following 24 h reperfusion in C57BL/6 and Cx40−/− mice. (e) Summary data and non-parametric analysis of tissue injury in animals subjected to stroke and 24 h reperfusion (T2 weighted imaging: U = 19.50, P<0.05, Mann-Whitney U test; C57BL/6, n = 9 mice; Cx40−/−, n = 9 mice), (cresyl violet: U = 18, P<0.05, Mann-Whitney U test; C57BL/6, n = 9 mice; Cx40−/−, n = 8 mice). Edema volume was not different between the two animal groups. * denotes significant difference between groups.
Discussion
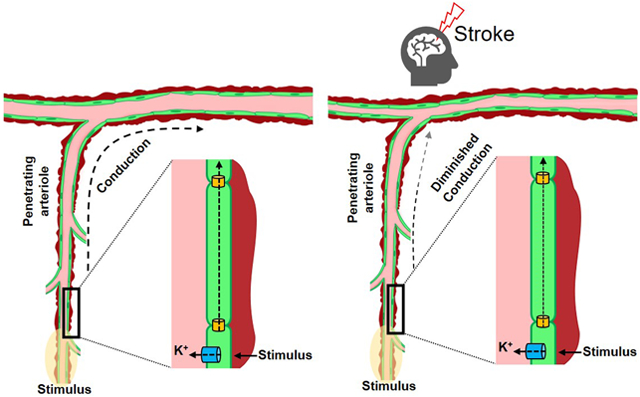
Matching blood flow delivery to neural activity requires the cerebral arterial network to respond in an integrated manner (1, 2). The coupling process begins with a neural-derived stimulus and a focal vasomotor response, which must subsequently spread along arteries, to induce the broader changes in resistance needed to impact blood flow magnitude. Spreading vasomotor responses are largely independent of metabolite diffusion or pressure/flow change (7) but require the sharing of a common signal among neighbouring vascular cells (4, 5). This study illustrates that charge is that signal and its conduction, first along the endothelium and then to the overlying smooth muscle, is the mechanism that coordinates contractile activity. This perspective is grounded in the knowledge that cerebrovascular cells are interconnected, express gap junctional Cx subunits (14-16), and that vasomotor responses can robustly spread along, both human and mice cerebral arteries. Building on this foundation, we initiated a quantitative in silico analysis to show how the structural/electrical properties of vascular cells are ideally designed to enable the ascension of vasomotor responses from penetrating arterioles to the surface vessel network. Vasomotor ascension was observed in awake mice subjected to barrel cortex stimulation, a response markedly compromised in mice lacking Cx40, a key gap junctional subunit between neighbouring endothelial cells. Impairing the ability of electrical/vasomotor responses to ascend into surface arteries also weakened flow regulation during/following stroke, promoting brain injury. We conclude that conducted responses are an integral part of the coupling mechanisms, needed to optimize the delivery of oxygenated blood to active neural cells, under a range of functional paradigms.
Conduction is a functional necessity and is enabled by structural connectivity
The study of neurovascular coupling is often reduced to identifying stimuli and the cells from which they are released. This focus routinely overlooks significant conceptual issues, the most important being that vascular resistance is broadly distributed within an arterial tree (2). As such, stimuli that are discretely released and of limited diffusional range cannot mobilize a sufficient proportion of the vascular structure to augment perfusion magnitude (5-7). The vasculature must therefore be encoded with a mechanism to transcribe local neural activity and the stimuli they produce into coordinated multi-segmental responses. While vascular coordination has been traditionally attributed to presumptive changes in pressure and flow, the shortcomings of this logic are clear: upstream change in hemodynamics due to focal stimulation do not need to be substantive or sustained (49). It is within this context that intercellular signalling is considered, with gap junctions enabling electrical, and their corresponding vasomotor responses to conduct along arteries and branch points.
For electrical responses to conduct along cerebral arteries, a defined structural arrangement must exist to facilitate signal exchange among neighboring cells. Using longitudinally sectioned mouse cerebral arteries and electron microscopy, this study documented sites of contact between endothelial cells, between smooth muscle cells, and between the two cell layers (Fig. 1). Endothelial-to-endothelial contacts were particularly abundant with electron dense pentalaminar membranes, an observation indicative of robust gap junctional protein expression. In contrast, sites of contact between smooth muscle cells and the two cell layers were modest in number, with the latter typically observed as a projection extending through “holes” in the internal elastic lamina layer (18-19). We subsequently observed Cx40 expression in the endothelial layer, most notably at homologous contact sites, consistent with their role in charge conduction. Cx37 and Cx43 were also expressed at endothelial cell borders and near IEL holes (“Supplemental Material, Fig. II”). Translational investigations detected varying levels of Cx37, Cx40, Cx43 and Cx45 mRNA in human cerebral arteries and confirmed the presence of Cx40 protein in the endothelial cell layer (“Supplemental Material, Figs. I, VI”). Overall, our observations align well with findings from the peripheral vasculature and are important in two aspects (18-20). First, they support the postulation that coupling resistance is markedly lower between endothelial cells compared to other contact sites and secondly, that Cx40 deletion will preferentially increase endothelial-to-endothelial coupling resistance.
Endothelial gap junctions facilitate conduction of vasomotor responses
Building upon structural observations, ex-vivo experiments subsequently provided clear evidence that discrete electrical events, either depolarizing or hyperpolarizing in nature, can elicit conducting vasomotor responses in cerebral vasculature. In detail, we observed in both human and mouse cerebral arteries that the discrete application of ‘high pipette [K+]’ stimulus elicited a localized depolarization and constriction that conducted along the entire length of the cannulated artery (Fig. 2). A ‘high pipette [K+]’ stimulus consistently initiates robust conduction as it shifts EK strongly rightward (~15-20 mV depolarization). A ‘low pipette [K+]’ stimulus and acetylcholine both induced a comparably small but consistent conducted vasodilation that decayed along surface vessels and penetrating arterioles, both shown to express endothelial Cx40 (Fig. 1 & 3; “Supplemental Material, Fig. I”). While small in magnitude, this response aligns with biophysical expectations. In a confined structure of coupled cells, subtle changes in resting K+ channel activity won’t be able to generate sufficient current to initiate a robust endothelial VM response. Robust responses could be initiated if an agent were to: 1) profoundly activate a silent population of K+ channels (e.g. small/intermediate conductance Ca2+ activated K+ channels); or 2) stimulate an enhanced number of endothelial cells. The latter concept is intriguing when considered in the context of physiological stimuli diffusing to interconnected structures (e.g. capillaries) and influencing a greater mass of endothelial cells.
Similar to vessels from peripheral circulatory beds (4, 20), the endothelium was critical for the longitudinal charge spread, as its elimination prevented vasomotor responses from conducting beyond the point of agent application. The genetic deletion of Cx40, a Cx subunit expressed exclusively in the endothelium, impaired but did not abolish conduction; endothelial Cx37 and Cx43 were also downregulated in these mice (“Supplemental Material, Fig. VII”); an effect that could also contribute to the impairment in conduction. This deletion-induced impairment did not affect vessel reactivity to a global K+ challenge nor did influence myogenic reactivity of cerebral arteries (“Supplemental Material, Fig. VIII”). Consecutively, VM arterial measurements confirmed the attenuation of charge spread along the artery in the absence of Cx40−/− in the endothelium. Changes in endothelial-to-endothelial coupling resistance were subsequently calculated using a validated model of electrical communication, modified to convert diameter responses into voltage changes (“Supplemental Material, Fig. III & IV”). Best fit analysis yielded endothelial-to-endothelial coupling resistances of 1.7 MΩ and 6 MΩ in C57BL/6J and Cx40−/− mice, respectively. The former aligns surprisingly well with direct electrical measurements performed on mice microvascular endothelial cells (50).
Intercellular signalling drives ascending neurovascular responses
The preceding experiments provided essential knowledge needed to advance a deeper understanding of conduction in integrated network function. In this regard, gap junctional resistance values were incorporated into a virtual network that consisted of a previously described surface vessel network with six adjoining penetrating arterioles (28). With endothelial-to-endothelial coupling resistance set at 1.7 MΩ (C57BL/6J, control mice), simulations revealed that stimulating one endothelial segment in a single penetrating arteriole was sufficient to initiate changes in VM and vasomotor tone along the arteriole and marginally in the cortical surface vessels (Fig. 4; “Supplemental Material, Fig. IX”). The robustness of this ascending response, albeit hyperpolarization or depolarization, increased substantially as the number of stimulated penetrating arterioles were increased. A similar pattern of ascension was also observed when the strength of the initial hyperpolarizing stimulus was decreased (“Supplemental Material, Fig. X”). This grading of upstream vasomotor behavior is biologically important; as in its absence blood flow delivery cannot be properly matched with varying neural activity. Elevating endothelial-to-endothelial coupling resistance to 6 MΩ (Cx40−/− mice) markedly attenuated the ability of electrical/vasomotor responses to ascend through the arterial tree. These findings establish the importance of endothelial-to-endothelial cell coupling in vascular conduction and illustrates, similar to functional studies (41-44), that compromised coupling would decisively impact functional hyperemic responses. In fact, by varying endothelial-to-endothelial cell coupling, in silico network also indicated its direct correlation to ascending electrical and vasomotor responses (“Supplemental Material, Figs. XI & XII”). We examined this idea experimentally by implanting cranial windows in awake mice and monitoring vasomotor activity prior to and following barrel cortex stimulation (Fig. 5). Notably in control mice, whisker stimulation elicited a dilation that ascended in an electronic fashion from penetrating arterioles to pial arteries. Consistent with conduction driving this upstream response in an endothelial/gap junctional dependent manner, the deletion of Cx40−/− attenuated this functional hyperemic response. Given the mildly hypertensive phenotype of Cx40−/− mice, we investigated the changes in the rate of decay of vascular responses along sequential measurement sites (“Supplemental Material, Fig. XIII”) which substantiated the role of gap junctional signalling, independent of this phenotype.
These findings confirm that neurovascular coupling is a two-step process, consisting of a stimulus initially driving a focal electrical/vasomotor response, followed by charge spread via gap junctions, altering contractile activity along an extended portion of the arterial network.
Conducted response is vital to perfusion homeostasis during stroke
The necessity of conduction to maintain vasomotor activity and perfusion is a new, unexplored concept in the cerebral circulation. While logically framed in the context of functional hyperemia, conduction may also be important in pathophysiological scenarios. Case-in-point is stroke where occlusion of a major cerebral artery dilates surface vessels, rerouting blood from other branches to the tissue at risk (45). This rerouting of perfusion is observable in humans and rodents alike and limits tissue injury, particularly in penumbral (salvageable) regions outside the ischemic core (45, 47). The mechanism underlying surface vessel dilation is unknown, but it follows that conduction, given the external positioning of surface vessels, is a likely player. We tested this hypothesis by subjecting mice to MCA occlusion and monitoring perfusion during stroke and reperfusion (Figs. 6 & 7). Compared to C57BL/6J controls, perfusion was impaired in Cx40−/− mice during stroke and during the early reperfusion phase. This diminution was particularly evident in the cortex and was observed despite a modest rise in blood pressure, predisposing Cx40−/− mice to enhanced blood flow during stroke. As stroke injury development likely depends on sustained blood flow regulation in the surface vessel network (51), we subsequently extended the reperfusion period and paired perfusion data with measurements of tissue injury. The attenuated blood flow responses in Cx40−/− mice during the short reperfusion period were consistent, after 24 hours of reperfusion and even became more pronounced. These differences correlated positively with augmented tissue injury in Cx40−/− mice, as revealed by T2 weighted imaging and cresyl violet histology. Notwithstanding the presence of mechanisms that influence cerebral blood flow independent of electrical communication (e.g. flow mediated responses); our findings establish the importance of intercellular signalling and vascular conduction for the maintenance of perfusion during stroke, where the brain is critically vulnerable to injury.
In considering the preceding experiments, it is important to recognize that our work was performed on young, healthy, male mice. As conducted responses are central to electromechanical coupling, these findings are expected to cross species and gender. This study did not, however, perform a gender comparison, as the known link between biological sex and stroke outcome (52) would have confounded our primary analysis. Lastly, vascular comorbidities (atherosclerosis, diabetes) and other physiological variables (age) can impinge upon conduction and this could translate into dysfunctional blood flow homeostasis and a predisposition to stroke injury (22, 25).
Summary
The optimization of neural function requires cerebral arterial networks to tune how much and where blood flow is distributed within the brain (1,2). In this regard, investigative efforts have largely focused on identifying key vasoactive agents (e.g. prostaglandins, epoxyeicosatrienoic acids, nitric oxide, adenosine, K+ etc.) and the cellular structures from which they originate (2). Deeper discussions of biophysical constraints have been lacking in these examinations, the most important being that focally derived vasoactive agents have a limited diffusional range. Thus, they alone cannot alter tone in a sufficient portion of the arterial tree to effect a sizable change in blood flow magnitude. Our study illustrates that this constraint is overcome in the cerebral arterial networks where focal responses can conduct along arteries and across branch points. The conduction process is facilitated by gap junctions and as our quantitative work show, impairing communication diminishes perfusion and vasomotor control over a range of metabolic challenges. Without electrical signalling to drive conducted vasomotor responses, blood flow delivery cannot be effectively matched to metabolic demand, depleting brain’s energy sources and compromising its function.
Supplementary Material
Highlights.
Large changes in brain perfusion magnitude are achieved through electrical communication along its vascular cells.
Coordination of intercellular signals optimizes blood flow delivery, supporting brain function.
Functional hyperemic responses and blood flow homeostasis after stroke are critically dependant on vascular electrical signalling.
Acknowledgements
a) The authors would like to acknowledge the excellent, general technical support of Suzanne Brett (University of Western Ontario); Tadeusz Foniok and David Rushforth (Experimental Imaging Center, University of Calgary) for assistance with MR experiments; Dr. Frank Visser (HBI Molecular Core Facility, University of Calgary) for assistance with qPCR experiments; Dr. Robert Gros (Robarts Research Institute, University of Western Ontario) for assistance in blood pressure/heart rate experiments. Cx40−/− animals were a kind gift from Dr. Janis M. Burt (University of Arizona).
b) Sources of Funding: This work was supported by operating grants from the Canadian Institute of Health Research (CIHR) to D.G.W (377412) and G.R.J.G (130233). D.G.W is the Rorabeck Chair in Molecular Neuroscience and Vascular Biology at the University of Western Ontario; G.R.J.G is a Canada Research Chair (Tier 2). A.Z was supported by postdoctoral fellowships from the University of Calgary (Eyes High), Alberta Innovates Health Solutions (AIHS) and CIHR. C.H.T-T was supported by a postdoctoral fellowship from AIHS and COBRE 1P20GM130459 (NIH). S.L.S was supported by the Brain Foundation and Diabetes Trust, Australia.
Nonstandard Abbreviations and Acronyms
- ASL-MRI
arterial spin labeling-magnetic resonance imaging
- Cx
connexin
- EC
endothelial cell
- IEL
internal elastic lamina
- MCAs
middle cerebral arteries
- SMC
smooth muscle cell
- VM
membrane potential
Footnotes
c) Disclosure: None.
References
- 1.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat. Neurosci 2007;101369–1376. [DOI] [PubMed] [Google Scholar]
- 2.Longden TA, Dabertrand F, Koide M, Gonzales AL, Tykocki NR, Brayden JE, Hill-Eubanks D, Nelson MT. Capillary K+-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat Neurosci. 2017;20:717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tran CH, Vigmond EJ, Plane F, Welsh DG. Mechanistic basis of differential conduction in skeletal muscle arteries. J Physiol. 2009;587:1301–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagher P, Segal SS. Regulation of blood flow in the microcirculation: role of conducted vasodilation. Acta Physiol (Oxf). 2011;202:271–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Segal SS, Duling BR. Flow control among microvessels coordinated by intercellular conduction. Science. 1986;234:868–70. [DOI] [PubMed] [Google Scholar]
- 6.Segal SS, Duling BR. Conduction of vasomotor responses in arterioles: a role for cell-to-cell coupling? Am J Physiol. 1989;256:H838–45. [DOI] [PubMed] [Google Scholar]
- 7.Segal SS. Regulation of blood flow in the microcirculation. Microcirculation. 2005;12:33–45. [DOI] [PubMed] [Google Scholar]
- 8.Figueroa XF, Paul DL, Simon AM, Goodenough DA, Day KH, Damon DN, Duling BR. Central role of connexin40 in the propagation of electrically activated vasodilation in mouse cremasteric arterioles in vivo. Circ Res. 2003;92:793–800. [DOI] [PubMed] [Google Scholar]
- 9.Figueroa XF, Duling BR. Gap junctions in the control of vascular function. Antioxid. Redox Signal. 2009;11:251–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabriels JE, Paul DL. Connexin43 is highly localized to sites of disturbed flow in rat aortic endothelium but connexin37 and connexin40 are more uniformly distributed. Circ Res. 1998;83:636–43. [DOI] [PubMed] [Google Scholar]
- 11.Severs NJ. Immunocytochemical analysis of connexin expression in the healthy and diseased cardiovascular system. Microsc Res Tech. 2001;52:301–22. [DOI] [PubMed] [Google Scholar]
- 12.van Kempen MJ, Jongsma HJ. Distribution of connexin37, connexin40 and connexin43 in the aorta and coronary artery of several mammals. Histochem Cell Biol. 1999;112:479–86. [DOI] [PubMed] [Google Scholar]
- 13.Little TL. Beyer EC, Duling BR. Connexin 43 and connexin 40 gap junctional proteins are present in arteriolar smooth muscle and endothelium in vivo. Am J Physiol 1995;268:H729–39. [DOI] [PubMed] [Google Scholar]
- 14.Yang X, Chu H, Tang Y, Dong Q. The role of connexin43 in hemorrhagic transformation after thrombolysis in vivo and in vitro. Neuroscience. 2016;329:54–65. [DOI] [PubMed] [Google Scholar]
- 15.Krüger O, Plum A, Kim JS, Winterhager E, Maxeiner S, Hallas G, Kirchhoff S, Traub O, Lamers WH, Willecke K. Defective vascular development in connexin 45-deficient mice. Development. 2000;127:4179–93. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Simard JM. Connexin45 gap junction channels in rat cerebral vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2001;281: H1890–8. [DOI] [PubMed] [Google Scholar]
- 17.Rummery NM, Hickey H, McGurk G, Hill CE. Connexin37 is the major connexin expressed in the media of caudal artery. Arterioscler Thromb Vasc Biol. 2002;22:1427–32. [DOI] [PubMed] [Google Scholar]
- 18.Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res. 2000;86:341–6. [DOI] [PubMed] [Google Scholar]
- 19.Sandow SL, Senadheera S, Bertrand PP, Murphy TV, Tare M. Myoendothelial contacts, gap junctions, and microdomains: anatomical links to function? Microcirculation. 2012;19:403–415. [DOI] [PubMed] [Google Scholar]
- 20.de Wit C, Roos F, Bolz SS, Kirchhoff S, Krüger O, Willecke K, Pohl U. Impaired conduction of vasodilation along arterioles in connexin40-deficient mice. Circ Res. 2000;86:649–55. [DOI] [PubMed] [Google Scholar]
- 21.Hwan Seul K, Beyer EC. Heterogeneous localization of connexin40 in the renal vasculature. Microvasc Res. 2000;59:140–8. [DOI] [PubMed] [Google Scholar]
- 22.Kwak BR, Mulhaupt F, Veillard N, Gros DB, Mach F. Altered pattern of vascular connexin expression in atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2002;22:225–30. [DOI] [PubMed] [Google Scholar]
- 23.Guzman MS, De Jaeger X, Drangova M, Prado MA, Gros R, Prado VF. Mice with selective elimination of striatal acetylcholine release are lean, show altered energy homeostasis and changed sleep/wake cycle. J Neurochem. 2013;124(5):658–69. [DOI] [PubMed] [Google Scholar]
- 24.Mufti RE, Zechariah A, Sancho M, Mazumdar N, Brett SE, Welsh DG. Implications of αvβ3 Integrin Signaling in the Regulation of Ca2+ Waves and Myogenic Tone in Cerebral Arteries. Arterioscler Thromb Vasc Biol. 2015;35:2571–2578. [DOI] [PubMed] [Google Scholar]
- 25.Kurjiaka DT, Bender SB, Nye DD, Wiehler WB, Welsh DG. Hypertension attenuates cell-to-cell communication in hamster retractor muscle feed arteries. Am J Physiol Heart Circ Physiol. 2008;288:H861–H870. [DOI] [PubMed] [Google Scholar]
- 26.Sancho M, Samson NC, Hald BO, Hashad AM, Marrelli SP, Brett SE, Welsh DG. KIR channels tune electrical communication in cerebral arteries. J Cereb Blood Flow Metab. 2017;37:2171–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol. 1998;508:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hald BO. A generative modeling approach to connectivity-Electrical conduction in vascular networks. J Theor Biol. 2016;399:1–12. [DOI] [PubMed] [Google Scholar]
- 30.Shih AY, Friedman B, Drew PJ, Tsai PS, Lyden PD, Kleinfeld D. Active dilation of penetrating arterioles restores red blood cell flux to penumbral neocortex after focal stroke. J Cereb Blood Flow Metab. 2009;29:738–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tran CH-T, Gordon GR. Acute two-photon imaging of the neurovascular unit in the cortex of active mice. Front Cell Neurosci. 2015;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zechariah A, Elali A, Hermann DM. Combination of tissue-plasminogen activator with erythropoietin induces blood-brain barrier permeability, extracellular matrix disaggregation, and DNA fragmentation after focal cerebral ischemia in mice. Stroke. 2010;41:1008–1012. [DOI] [PubMed] [Google Scholar]
- 33.Tuor UI, Deng Q, Rushforth D, Foniok T, Qiao M. Model of minor stroke with mild peri-infarct ischemic injury. J. Neurosci. Methods 2016;268:56–65. [DOI] [PubMed] [Google Scholar]
- 34.Qiao M, Meng S, Scobie K, Foniok T, Tuor UI. Magnetic resonance imaging of differential gray versus white matter injury following a mild or moderate hypoxic-ischemic insult in neonatal rats. Neurosci. Lett 2004;368:332–336. [DOI] [PubMed] [Google Scholar]
- 35.Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. PNAS. 1992;89:212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herscovitch P, Raichle ME. What is the correct value for the brain-blood partition coefficient for water? J Cereb Blood Flow Metab. 1985;5:65–69. [DOI] [PubMed] [Google Scholar]
- 37.Zechariah A, ElAli A, Doeppner TR, Jin F, Hasan MR, Helfrich I, Mies G, Hermann DM. Vascular endothelial growth factor promotes pericyte coverage of brain capillaries, improves cerebral blood flow during subsequent focal cerebral ischemia, and preserves the metabolic penumbra. Stroke. 2013;44:1690–1697. [DOI] [PubMed] [Google Scholar]
- 38.Zechariah A, ElAli A, Hagemann N, Jin F, Doeppner TR, Helfrich I, Mies G, Hermann DM. Hyperlipidemia attenuates vascular endothelial growth factor-induced angiogenesis, impairs cerebral blood flow, and disturbs stroke recovery via decreased pericyte coverage of brain endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33:1561–1567. [DOI] [PubMed] [Google Scholar]
- 39.Feng M, DiPetrillo K. Non-invasive Blood Pressure Measurement in Mice. Methods Mol Biol. 2009;573:45–55. [DOI] [PubMed] [Google Scholar]
- 40.Sancho M, Gao Y, Hald BO, Yin H, Boulton M, Steven DA, MacDougall KW, Parrent AG, Pickering JG, Welsh DG. An assessment of KIR channel function in human cerebral arteries. Am J Physiol Heart Circ Physiol. 2019;316(4):H794–H800. [DOI] [PubMed] [Google Scholar]
- 41.Iadecola C, Yang G, Ebner TJ, Chen G. Local and propagated vascular responses evoked by focal synaptic activity in cerebellar cortex. J Neurophysiol. 1997;78:651–9. [DOI] [PubMed] [Google Scholar]
- 42.O’Herron P, Chhatbar PY, Levy M, Shen Z, Schramm AE, Lu Z, Kara P. Neural correlates of single-vessel haemodynamic responses in vivo. Nature. 2016;16; 534:378–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen BR, Kozberg MG, Bouchard MB, Shaik MA, Hillman EM. A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J Am Heart Assoc. 2014;12;3(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rungta RL, Chaigneau E, Osmanski BF, Charpak S. Vascular compartmentalization of functional hyperemia from the synapse to the pia. Neuron. 2018;25;99(2):362–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liebeskind DS. Collateral circulation. Stroke. 2003;34:2279–84. [DOI] [PubMed] [Google Scholar]
- 46.Fang JS, Angelov SN, Simon AM, Burt JM. Compromised regulation of tissue perfusion and arteriogenesis limit, in an AT1R-independent fashion, recovery of ischemic tissue in Cx40(−/−) mice. Am J Physiol Heart Circ Physiol. 2013;304:H816–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Effect of blood pressure and diabetes on stroke in progression. Lancet. 1994;344:156–9. [DOI] [PubMed] [Google Scholar]
- 48.Schwarz S, Georgiadis D, Aschoff A, Schwab S. Effects of induced hypertension on intracranial pressure and flow velocities of the middle cerebral arteries in patients with large hemispheric stroke. Stroke. 2002;33:998–1004. [DOI] [PubMed] [Google Scholar]
- 49.Arciero JC, Carlson BE, Secomb TW. Theoretical model of metabolic blood flow regulation: roles of ATP release by red blood cells and conducted responses. Am J Physiol Heart Circ Physiol. 2008;295:H1562–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McKinnon RL, Bolon ML, Wang HX, Swarbreck S, Kidder GM, Simon AM, Tyml K. Reduction of electrical coupling between microvascular endothelial cells by NO depends on connexin37. Am J Physiol Heart Circ Physiol. 2009;297:H93–H101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Armitage GA, Todd KG, Shuaib A, Winship IR. Laser speckle contrast imaging of collateral blood flow during acute ischemic stroke. J Cereb Blood Flow Metab. 2010;30:1432–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hurn PD, Brass LM. Estrogen and stroke: a balanced analysis. Stroke. 2003;34(2):338–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.