Abstract
Amoebiasis occurs worldwide and affects about 20–50 million people annually. Stool samples were collected from patients attending different rural clinics in Northern South Africa in the present study. Microscopic examination was performed for the initial detection of Entamoeba parasites. A multiplex PCR protocol based on the small subunit rRNA gene of E. moshkovskii, E. dispar, and E. histolytica, was used for the differential detection of the three Entamoeba species (collectively referred to as Entamoeba complex). A total of 170 participants were recruited in the study, with a mean age of 35.9 ± 17.8 years and a median of 37.0 years. The prevalence of Entamoeba species was found to be 34.7% and 33% by PCR and microscopy, respectively. E. histolytica had a prevalence of 4.1%, E. dispar 14.7% and E. moshkovskii 15.9%. Of the three species, only E. histolytica was significantly associated with diarrhoea and was more prevalent among HIV patients even in the absence of diarrhoea while the other two were not, although the difference was not significant (p > 0.05). This is the first study in South Africa to describe the prevalence of E. moshkovskii. E. dispar was significantly associated with abdominal pains (p = 0.003). Further studies are needed to clarify the role of E. moshkovskii and E. dispar in abdominal pain and diarrhoea.
Keywords: Diarrhoea, Entamoeba moshkovskii, Rural, South Africa
1. Introduction
Amoebiasis is a common public health problem particularly in developing countries and is caused by Entamoeba histolytica. With the occurrence of two other non-pathogenic species of Entamoeba namely E. dispar (W.H.O., 1997) and E. moshkovskii (Ali et al., 2003), the pathogenic E. histolytica is often inaccurately reported or diagnosed. Although several studies have been conducted on the prevalence of E. histolytica around the world, most of the studies did not differentiate between E. histolytica and E. dispar due to the diagnostic methods used; resulting in an overestimation of the prevalence of E. histolytica (Kebede et al., 2003). Identified recently as a cause of infection in humans, E. moshkovskii endemicity is yet to be evaluated in many parts of the globe. Following its description, several studies have been conducted to determine its prevalence in human communities in different countries (Fotedar et al., 2008). In Iran, molecular studies showed that E. dispar was the predominant species especially in the central and northern areas of the country and that infection with E. moshkovskii may be common among Iranians while amoebiasis due to E. histolytica appear to be a rare infection in that country (Hooshyar et al., 2012).
Humans are the major host of E. moshkovskii and there does not appear to be other meaningful animal reservoirs of this parasite (Ximenez et al., 2011). Although previously thought to be a non-pathogenic organism, recent studies have shown that E. moshkovskii is capable of causing diarrhoea, colitis, and weight loss in mice (Hamano et al., 2006). Moreover, acquisition of E. moshkovskii infection was associated with diarrhoea among Bangladeshi children (Shimokawa et al., 2012) thus seemingly influencing the burden of amoebiasis. Previous studies in the Vhembe Districts in South Africa have indicated that the prevalence of E. histolytica in hospitals and among school children was 18.8% and 2.1%, respectively, whereas 25.3% and 8.5% of these study populations had E. dispar, respectively (Samie et al., 2006). However, this study did not include E. moshkovskii. Recently identified as a cause of infection in humans, E. moshkovskii endemicity has not been evaluated in most epidemiological studies. Apart from the report by Ngobeni et al. (2017) in low-income South African populations in Giyani and Pretoria where no E. moshkovskii was detected (0%), no other study has investigated its presence in the country. Hence, in the present study, we carefully determined the occurrence of the three Entamoeba species in the Vhembe and Mopani Districts of South Africa.
2. Materials and methods
2.1. Ethical considerations
The study was approved by the Health and Ethics committee of the University of Venda and the Limpopo Department of Health in Polokwane, South Africa. Approval was also received from the District Department of Health of the Mopani District as well as the Chiefs of the respective villages in the Limpopo province, northern South Africa. The objectives of the study were clearly explained to the participants, and those who agreed were asked to sign a consent form. Written informed consent was obtained from the parents or guardians of children prior to enrolment of the children in the research, and good clinical practice was followed.
2.2. Study participants' recruitment and data collection
Participants were recruited (February 2012 to September 2012) from Nkomo Clinic and Donald Fraser Hospital situated in the Mopani District and Vhembe District, respectively in the northern part of South Africa. Outpatients attending the clinics with intestinal complaints were recruited upon arrival at the clinics and asked to complete a questionnaire. The questions comprised of demographic information of the study participants, clinical and health related questions, socio economic status, and diarrhoeal history of the study participants. The children's mothers were asked to indicate if the child recently had diarrhoea and to specify the consistency of stools passed by the child in the past days. Anthropometric measurements including body weight, age and height were assessed and the Body mass index (BMI) was calculated for all participants as weight (in kilograms) divided by the square of height (in meters) and expressed as kg/m2.
2.3. Sample collection and microscopic examination
Stool samples were collected using a wide mouthed stool container and transported in cooler boxes filled with ice to the laboratory of Microbiology at the University of Venda. All specimens were properly labelled with patient's code, and date of collection. The specimens were transported to the laboratory within 4 h of passing of the stool, since amoebic trophozoites die and become unrecognizable after longer periods of time. Precautions were taken to prevent the samples from being contaminated with urine or dirt particles. Two wet mount slides (saline and Lugol's iodine) were prepared directly from each sample to increase the chance of detecting protozoan trophozoites and cysts as well as red and white blood cells. The smears were covered with cover slips and examined under the microscope using 40× objective lens. Cysts and trophozoites were identified as per their characteristic morphometric features.
2.4. DNA extraction from stool samples and PCR assay
Extraction of parasite genomic DNA from faecal specimens was done by the QIAamp DNA stool Mini kit from Qiagen (Qiagen, GmbH, Hilden, Germany), with some modifications following the beads beating in order to break down the cysts. About 0.25 g of glass beads were weighed and placed in an eppendorf tube containing 0.25 g of the stool sample. The tubes were placed in a bead beater and run for 1 min. After a brief vortexing, the protocol was continued with the Qiagen mini kit following the manufacturer's instructions. The PCR amplification reaction was performed in a final volume of 20 μl in a 0.2 ml PCR tubes by the use of a thermal cycler (G storm, Gene technologies, Braintree UK) following a previously described method (Hamzah et al., 2006). Reaction conditions were optimised to combine the forward primer (EntaF) with each of the three reverse primers (Ehr, Emr & Edr) in a single reaction mixture and under the same conditions.
The reaction mixture contained 0.2 μM of each forward and reverse primer, 10 μl dream Taq, a volume of 2.6 μl nuclease free water and 5 μl of extracted DNA samples. Amplification of each species-specific DNA fragment started with initial denaturation at 94 °C for 3 min, followed by 40 cycles of 94 °C for 1 min, 58 °C for 1 min, and extension at 72 °C for 1 min, with a final extension at 72 °C for 7 min. The samples were stored at 4 °C. Amplified products were visualised with ethidium bromide staining after electrophoresis on 1.5% agarose gel and a picture of the gel was taken using the gel documentation system from Genesnap.
2.5. Statistical analysis
The results of the study were analysed using the SPSS software, version 19. The chi square (χ2) test was used to determine the potential correlation between different demographic characteristics of the study participants who provided the samples and Entamoeba infections as well as other parameters such as clinical characteristics. The difference was considered significant when the p value was <0.05.
3. Results
3.1. Microscopic examination and PCR amplification
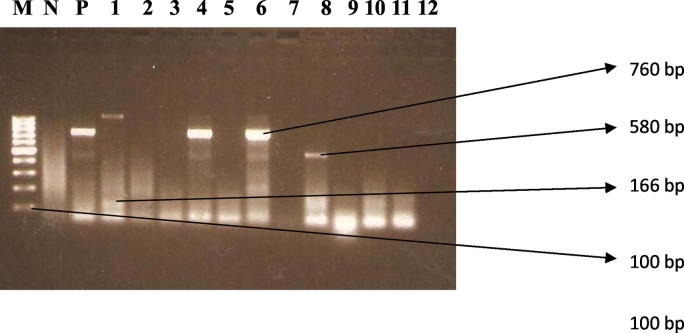
A total of 170 samples were collected from the same number of patients and microscopically examined by saline and iodine wet mount microscopy. Vegetative and/or cysts forms of E. histolytica, E. dispar and E. moshkovskii (collectively referred to as Entamoeba complex, as they are morphologically indistinguishable) were found in 57 (33%) samples. The extraction of DNA from the stool samples, followed by differential detection for the presence of E. histolytica, E. dispar, and E. moshkovskii by the PCR assay, with the use of species specific primers (EntaF and one of Emr, Edr, or Ehr) detected Entamoeba in 59 (34.7%) samples. The samples showed bands at 166 bp, 580 bp and/or 760 bp, which indicates E. histolytica, E. moshkovskii, or E. dispar, respectively (Fig. 1).
Fig. 1.
Representative images showing the agarose gel electrophoresis of the amplification of the Entamoeba DNA. The 166-, 580- and 760-bp bands depict specific amplification of DNA from E. histolytica, E. moshkovskii, or E. dispar, respectively. Lane “M” is the 100 bp ladder molecular weight maker. Lane “N” is no stool sample (negative control), P is positive control. Lanes 1–12 are representative clinical samples.
3.2. The prevalence of Entamoeba infections in the study population
A total of 170 samples were tested for Entamoeba by PCR. Of these, 27 (15.9%) samples were positive for E. moshkovskii, and 25 (14.7%) were positive for E. dispar and 7 (4.1%) were positive for E. histolytica. E. histolytica and E. dispar were found to be more prevalent in males (11.1% and 27.8% respectively) compared to females (5% and 19.8% respectively), while E. moshkovskii was more prevalent in the females (23.8%) compared to males (16.7%), but the difference was not statistically significant (p > 0.05). E. moshkovskii was more common in the age group 26–45 (27.5%), while E. dispar was more prevalent in the age group 0–25 with 23.3% occurrence and E. histolytica was more prevalent in the age group 26–45 with 7.5% occurrence than the other age groups, although the differences were not significant (p > 0.05). Individuals who were single had the highest prevalence of the parasites, E. moshkovskii (25.5%), E. dispar (25%) and E. histolytica (8.5%). All three Entamoeba species were more prevalent in patients who were not underweight compared to those who were [E. moshkovskii (6.9%), E. dispar (22.5%), and E. histolytica (23.5%)], although the difference was not significant (p > 0.05). E. histolytica was significantly more prevalent in the Vhembe district (p = 0.039). E. moshkovskii (27.3%) was significantly more prevalent in those who were not taking any medication at the moment, than those who were taking some form of medication (p < 0.05). The results are recorded in Table 1.
Table 1.
The prevalence of Entamoeba infections in the study population as determined by PCR.
| Characteristics of the population | E. moshkovskii | E. dispar | E. histolytica | |
|---|---|---|---|---|
| Sex | Male | 3(16.7%) | 5(27,8%) | 2(11,1%) |
| Female | 24 (23.8%) | 20 (19.8%) | 5 (5.0%) | |
| χ2 | 0.439 | 0.586 | 1.047 | |
| p value | 0.508 | 0.444 | 0.306 | |
| Age group | 0–25 | 10 (23.3%) | 10 (23.3%) | 3 (7.0%) |
| 26–45 | 11(27.5%) | 8(20%) | 3 (7.5%) | |
| >45 | 6(16.7%) | 7(19.4%) | 1 (2.8%) | |
| χ2 | 1.280 | 0.208 | ||
| p value | 0.527 | 0.901 | ||
| Marital status | Divorced | 0 | 0 | 0 |
| Married | 4(20.9%) | 13 (19.4%) | 3(4.5) | |
| Single | 12 (25.5%) | 12 (25.5%) | 4(8.5%) | |
| Widowed | 1 (25.0%) | 0 | 0 | |
| BMI | Not Underweight | 7(6.9%) | 23(22.5%) | 24(23.5%) |
| Underweight | 0 | 2(11.8%) | 3(17.6%) | |
| χ2 | 0.287 | 1.021 | 1.240 | |
| p value | 0.592 | 0.312 | 0.266 | |
| District | Mopani | 27(65.9%) | 25(65.8%) | 7(46.7%) |
| Vhembe | 14(34.1%) | 13(34.2%) | 8(53.3%) | |
| χ2 | 0.442 | 0.413 | 4.265 | |
| p value | 0.506 | 0.520 | 0.039 | |
| Medication (drugs or antibiotics) | No | 6(6.8%) | 19(21.6%) | 24(27.3%) |
| Yes | 1(3.2%) | 6(19.4%) | 3(9.7%) | |
| χ2 | 4.046 | 0.069 | 0.534 | |
| p value | 0.044 | 0.793 | 0.465 | |
3.3. The demographic characteristics of the study population
A total of 132 of the 170 participants (77.5%) were females while 34 (20%) were males. Of the participants 89 (52.4%) were married, while 3 (1.8%) were divorced. The age of the study participants varied from 1 year to 76 years old. The mean age was 35.968 ± 17.81 years while the median age was 37.00 years. They were grouped into 3 age groups (0–25, 26–45, >45 years old), and most of the patients were aged between 26 and 45 years old (37.1%). Of the 170 participants, 72 (42.4%) indicated that they kept animals in their households. Of these 30 (41.7%) kept cattle, while only 4 (5.6%) had cats.
3.4. The occurrence of diarrhoea and related ailments among the study participants
Of the 170 study participants, 46 (27.1%) were taking antibiotics at the time of recruitment, and 32 (18.8%) had experienced some diarrhoea during the past 3 months. Of these 18 (56.3%) passed loose stools and 3 (9.3%) passed bloody stools. Of the 32 patients who experienced diarrhoea, 24 (75%) experienced acute diarrhoeal episodes while 8 (25%) had chronic diarrhoea. Abdominal pains were experienced by 79 (46.1%) of the study population. Of the 170 participants, 53 (31.2%) were coughing at the time of recruitment, and 39 (23%) indicated that they had difficulties in breathing. Among the study participants, 35 (20.6%) had some vision problems.
3.5. The health statuses of the study participants
Of the 170 participants, 22 (12.9%) were underweight according to Mchiza et al. (2015). A total of 58 (34.1%) reported to be HIV positive, of whom 30 (51.7%) were on anti-retroviral (ARV) treatment. Fourteen (8.2%) of the patients indicated that they had made use of alternative treatment, of whom 11 (78.6%) and 3 (21.4%) had received treatment from spiritual healers and traditional healers, respectively.
3.6. Relationship between the prevalence of Entamoeba infections and diarrhoea history of the patients
E. moshkovskii and E. histolytica were found to be more prevalent (25.2% and 11.5% respectively) in people who have a diarrhoea history than those who did not, while E. dispar was more prevalent in people without diarrhoea history (23.7%), although the difference was not significant (p > 0.05). E. moshkovskii and E. dispar were commonly found in watery stools (50% and 25% respectively) in patients who were experiencing diarrhoea, although the difference was not significant (p > 0.05). E. histolytica was more associated with mucous stools (50%) than the other types of stools, and the difference was significant (p < 0.05). E. moshkovskii was more associated with chronic diarrhoea (60%), than acute diarrhoea (9.5%), with a significant difference (p < 0.05). E. dispar and E. histolytica were associated more with acute diarrhoea than chronic, although the difference was not significant (p > 0.05). E. dispar was significantly associated with abdominal pains by 30.4% (p = 0.039). E. histolytica and E. moshkovskii were also associated with abdominal pains, but the difference was not significant (p > 0.05). The results are recorded in Table 2.
Table 2.
The prevalence of Entamoeba infections according to history of diarrhoea among study participants.
| Characteristics | E. moshkovskii | E. dispar | E. histolytica | |
|---|---|---|---|---|
| Diarrhoea the last 3 months | No | 22 (23.7%) | 22(23.7%) | 4 (4.3%) |
| Yes | 5 (25.2%) | 3 (11.5%) | 3(11.5%) | |
| χ2 | 0.227 | 1.798 | 1.922 | |
| p value | 0.634 | 0.180 | 0.166 | |
| Consistency | Bloody | 0 (0%) | 0(0%) | 0(0%) |
| Loose | 3(18.8%) | 2(12.5%) | 1(6.3%) | |
| Mucous | 0(0%) | 0(0%) | 2(50.0%) | |
| Watery | 2(50.0%) | 1(25.0%) | 0(0%) | |
| χ2 | 3.653 | 2.725 | 14.861 | |
| p value | 0.455 | 0.605 | 0.005 | |
| Type of diarrhoea | Acute | 2(9.5%) | 2(9.5%) | 2(9.5%) |
| Chronic | 3(60.0%) | 1(20.0%) | 1(20.0%) | |
| χ2 | 6.093 | 2.065 | 2.723 | |
| p value | 0.048 | 0.356 | 0.256 | |
| Abdominal pains | No | 9(18.0%) | 4(8.0%) | 3(6.0%) |
| Yes | 18(26.1%) | 21(30.4%) | 4(5.8%) | |
| χ2 | 1.081 | 8.793 | 0.002 | |
| p value | 0.298 | 0.003 | 0.562 | |
3.7. Relationship between the prevalence of Entamoeba infections and the clinical manifestations of the study participants
The prevalence of E. moshkovskii and E. dispar was high in the study participants who did not have any skin infections (25.5% and 21.6% respectively) as compared to those who did, although the difference was not significant (>0.05). Entamoeba dispar and E. moshkovskii were found to be more prevalent in the study participants who were currently having respiratory infections (71.4% and 57.1% respectively) than those who were not (p > 0.05). E. histolytica was significantly more prevalent in participants who were not coughing than those who were (p < 0.039). The prevalence of E. moshkovskii was higher in HIV negative participants (24.1%) than those who were positive (p > 0.05). E. dispar and E. histolytica were more prevalent in HIV positive patients (28.6% and 14.6%, respectively) than those who were HIV negative (p > 0.05).
3.8. Relationship between the prevalence of Entamoeba infections and animals in the households of the participants
Animals in the houses of the study participants were not associated with any infection, all Entamoeba species were found to be more prevalent in houses with no animals than those with animals, although the difference was not significant (p > 0.05). Donkeys were mostly associated with the presence of E. moshkovskii (33.3%) and E. dispar (33.3%), however the difference was not significant (p > 0.05). Goats were mostly associated with the presence of E. histolytica (8.3%), although the difference was not significant (p > 0.05).
3.9. Relationship between the prevalence of Entamoeba infections and the quality of drinking water used by participants
Drilled ground wells was the source of water which was most associated with the prevalence of E. moshkovskii (27%) and E. histolytica (6.3%) compared to all water sources, although the difference was not significant (p > 0.05). E. moshkovskii, E. dispar, and E. histolytica were found to be more prevalent in the patients who stored water for <3 days and the least affected were those who stored water for 3–4 days, although the difference was not significant (p > 0.05) (Table 3).
Table 3.
Prevalence of Entamoeba infections according to drinking water sources and storage.
| Characteristics | E. moshkovskii | E. dispar | E. histolytica | |
|---|---|---|---|---|
| Source of drinking water | Borehole | 17(27.0%) | 12(19%) | 4(6.3%) |
| Communal tap | 5(14.7%) | 5(14.7%) | 2(5.9%) | |
| Direct from the river | 0(0%) | 0(0%) | 0(0%) | |
| Tap in the house | 5(23.8%) | 8(38.1%) | 1(4.8%) | |
| χ2 | 2.206 | 4.920 | 0.135 | |
| p value | 0.531 | 0.178 | 0.987 | |
| Water storage | <3 days | 6(24.0%) | 6(24.0%) | 2(8.0%) |
| 3–4 | 5(20.8%) | 5(20.8%) | 1(4.2%) | |
| >5 | 16(22.9%) | 14(20.0%) | 4(5.7%) | |
| χ2 | 0.073 | 0.178 | 0.334 | |
| p value | 0.964 | 0.915 | 0.846 | |
3.10. Relationship between the socio-economic status of the patients and the prevalence of Entamoeba infections
E. moshkovskii was prevalent in patients who earned between R3001 (“R”, South African Rand; 1 Rand equal to 0.092 US Dollars as of on January 2014) and R4999, although the difference was not significant (p > 0.05). E. histolytica was highly prevalent in those with no dependents at all, although the difference was not significant (p > 0.05). Albeit all the educational levels were affected, E. histolytica and E. dispar were prevalent in those who had tertiary education (20% and 60% respectively), however the difference was not significant (p > 0.05) (Table 4).
Table 4.
The prevalence of Entamoeba infections according to the socio-economic status of the study participants.
| Characteristics | E. moshkovskii | E. dispar | E. histolytica | |
|---|---|---|---|---|
| Income range | R1000 | 4(20.0%) | 6(30.0%) | 0(0%) |
| R1001-R3000 | 10(17.2%) | 10(17.2%) | 5(8.6%) | |
| R3001-R4999 | 10(35.7%) | 5(17.9%) | 1(3.6%) | |
| R5000 & above | 3(23.1%) | 4(30.8%) | 1(7.7%) | |
| χ2 | 3.773 | 2.384 | 2.383 | |
| p value | 0.287 | 0.497 | 0.497 | |
| Number of dependents | 0 | 0(0%) | 0(0%) | 1(33.3%) |
| 1 | 6(20%) | 4(13.3%) | 1(3.3%) | |
| 2–5 | 14(22.2%) | 18(28.6%) | 4(6.3%) | |
| 6 & above | 7(30.4%) | 3(13.0%) | 1(4.3%) | |
| χ2 | 1.799 | 4.914 | 4.558 | |
| p value | 0.615 | 0.178 | 0.207 | |
| Education | Illiterate | 4(22.2%) | 4(22.2%) | 1(5.6%) |
| Primary | 2(18.2%) | 4(36.4%) | 1(9.1%) | |
| Secondary | 20(23.5%) | 14(16.5%) | 4(4.7%) | |
| Tertiary | 1(20.0%) | 3(60%) | 1(20%) | |
| χ2 | 0.184 | 7.214 | 2.221 | |
| p value | 0.980 | 0.065 | 0.528 | |
4. Discussion
The present study indicated a high prevalence of E. moshkovskii compared to E. dispar and E. histolytica in stool samples from Mopani and Vhembe Districts in the Limpopo Province, South Africa (Samie et al., 2006). Microscopic examination of the samples showed a prevalence of 33% for all the Entamoeba species while PCR detected an overall prevalence of 34.7% of which most were E. moshkovskii. A similar study in Cote d' Ivoire reported a lower prevalence of E. histolytica/dispar (18.8%) among primary school children using microscopy (Ouattara et al., 2010). Unlike the present study, in Saudi Arabia a higher prevalence of Entamoeba complex (64.8%) was reported by microscopy among diarrheic samples revealing wide variation between different regions of the world (Al-Harthi and Jamjoom, 2007). Contrary to the Saudi study, the present study was not limited on diarrhoeal patients only but included general out-patients complaining of abdominal pains visiting the two clinics.
The results of the present study indicated that the recently discovered E. moshkovskii and the non-invasive E. dispar were more prevalent than the invasive E. histolytica. These findings are consistent with previous studies conducted on Entamoeba species (Pinheiro et al., 2004; Yakoob et al., 2012). It is important to point out that a much lower rate of infection with E. histolytica (4.8%) was observed in the present study than that in a previous study in the same region by Samie et al. (2006). This difference could be due to the type of population studied and the origin of the study participants. Most patients originated from Giyani while the previous study was conducted exclusively in the Vhembe region. In fact, the Mopani District represented by Giyani is dry with much higher numbers of diarrhoea compared to the Vhembe District (Samie et al., 2006).
The current study is the first to reveal the presence of E. moshkovskii (15.9%) in this region. A similar study in Yemen reported an E. moshkovskii prevalence of 18.2% (Al-Areeqi et al., 2017), while a higher frequency of E. moshkovskii (25.4%) was described from a rural area from Central Colombia (López et al., 2015). Interestingly, E. moshkovskii was found to be more prevalent than E. histolytica in our study.
Numerous studies reported the prevalence of Entamoeba complex among HIV+ and HIV−patients. Moran et al. (2005) reported a prevalence of 5.9% and 2.9% of E. histolytica/dispar among HIV+ and HIV− patients respectively using microscopy in Mexico City. On the other hand, higher prevalences of E. histolytica were detected (25.3% in HIV+ and 18.5% in HIV− patients) in the same Mexican study when the samples were retested by sensitive molecular tools (Moran et al., 2005). These results are consistent with ours which showed E. dispar and E. histolytica with a prevalence of 28.6% and 14.3% respectively in HIV+ patients and a prevalence of 20.5% and 5.4% respectively in HIV negative patients. These results are consistent with findings from our study. The prevalence of E. dispar and E. histolytica among HIV+ patients in the present report were 28.6% and 14.3%, respectively whereas in HIV negative patients a less prevalence was noted (20.5% and 5.4%, respectively). The prevalence of E. moshkovskii was higher in HIV negative participants (24.1%) than those who were positive (p > 0.05). Intriguingly, unlike a study by Al-Areeqi et al. (2017), neither age nor gender were significant risk factors for E. moshkovskii in the present report.
Similar to a study by Tasawar et al. (2010), no significant difference was noted in the present study between the prevalence of E. histolytica in males (11.1%) and females (5%). Moreover, no association between animal ownership and economic status of the participants and the prevalence of Entamoeba complex was observed. Previous studies in other countries have indicated that contact with animals and low personal hygiene are risk factors for infections with intestinal protozoa including E. histolytica (Alyousefi et al., 2011; Ismail et al., 2010). These results indicate the widespread occurrence of the cysts in the environment and the farms involved, which may serve as infective agents for the parasites in humans.
No significant difference was noted between the prevalence of E. moshkovskii and E. dispar in people with and without diarrhoea. However, the prevalence of E. histolytica was higher in patients who indicated that they had diarrhoea over the last three months. These findings are different from those obtained by Fadeyi et al. (2009), who found that E. histolytica and other intestinal parasites, were rare in acute and persistent diarrhoeic patients attending Ilorin hospitals by ELISA antigen detection method. The results of the current study are dissimilar to those obtained by Mojarad and colleagues in a study investigating the circulation of E. moshkovskii in patients with gastrointestinal disorders in Iran where a low prevalence (3.45%) was reported. On the other hand, like the current study the prevalence of E. histolytica (3.45%) was comparable to our result (Mojarad et al., 2010). In another study conducted in Turkey, E. moshkovskii was detected in two patients with diarrhoea and co-infected with E. histolytica (Tanyuksel et al., 2007).
Although E. dispar is known to be non-pathogenic, it was nevertheless significantly associated with abdominal pain (p = 0.003) in the present study, indicating the possibility that E. dispar might have some pathogenic characteristics, or these abdominal pains may be due to other causes. These results are similar to those obtained in a study conducted to determine the production of amoebic liver abscess by E. dispar xenic culture (Dolabella et al., 2012). In that study a culture of E. dispar strain (ICB-ADO) produced liver abscesses and tissue damage distinct from the frequently known non-pathogenic E. dispar (SAW 760) strain. These results are an indication that some E. dispar strains may be pathogenic. It is important to note that while no association existed between Entamoeba infections and the quality of drinking water used in the current study, sources of drinking water was a significant risk factor for E. dispar infection (Al-Areeqi et al., 2017).
This is the first study to identify E. moshkovskii in the region. Another African study reported an infection rate of 13% with E. moshkovskii in a cohort of HIV-suspected or confirmed inpatients from Tanzania (Beck et al., 2008). Moreover, this report found that E. moshkovskii was the most commonly species identified, yet it was not associated with neither diarrhoea nor abdominal pain. Unlike in the previous study conducted in the Vhembe District, the present survey showed a higher prevalence of E. histolytica in HIV positive patients even though the difference was not statistically significant. E. histolytica was more common among patients with diarrhoea. The present study also indicated that E. dispar was more common among participants who had abdominal pain. This may be an indication that E. dispar contributes to pathogenicity. However, further studies are needed using a larger study population in order to confirm this hypothesis.
Declaration of competing interest
The authors declare that there are no financial and commercial conflicts of interest.
Acknowledgements
The present study was funded by the National Research Foundation of South Africa through the South-Africa Japan bilateral collaboration to S. A. and by Japan Science and Technology Agency [Grant-in-Aid on Strategic International Research Cooperative Program (SICP)] to T. Nozaki. The authors are thankful to the Department of Health Limpopo as well as the Hospitals and the patients for their cooperation.
References
- Al-Areeqi M.A., Sady H., Al-Mekhlafi H.M., Anuar T.S., Al-Adhroey A.H., Atroosh W.M., Dawaki S., Elyana F.N., Nasr N.A., Ithoi I., Lau Y.L., Surin J. First molecular epidemiology of Entamoeba histolytica, E. dispar and E. moshkovskii infections in Yemen: different species-specific associated risk factors. Trop. Med. Int. Health. 2017;22:493–504. doi: 10.1111/tmi.12848. [DOI] [PubMed] [Google Scholar]
- Al-Harthi S.A., Jamjoom M.B. Enteroparasitic occurrence in stools from residents in Southwestern region of Saudi Arabia before and during Umrah season. Saudi Med. J. 2007;28:386–389. [PubMed] [Google Scholar]
- Ali I.K., Hossain M.B., Roy S., Ayeh-Kumi P.F., Petri W.A., Jr., Haque R., Clark C.G. Entamoeba moshkovskii infections in children, Bangladesh. Emerg. Infect. Dis. 2003;9:580–584. doi: 10.3201/eid0905.020548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alyousefi N.A., Mahdy M.A., Mahmud R., Lim Y.A. Factors associated with high prevalence of intestinal protozoan infections among patients in Sana'a City, Yemen. PLoS One. 2011;6 doi: 10.1371/journal.pone.0022044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck D.L., Doğan N., Maro V., Sam N.E., Shao J., Houpt E.R. High prevalence of Entamoeba moshkovskii in a Tanzanian HIV population. Acta Trop. 2008;107:48–49. doi: 10.1016/j.actatropica.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolabella S.S., Serrano-Luna J., Navarro-Garcia F., Cerritos R., Ximenez C., Galván-Moroyoqui J.M., Silva E.F., Tsutsumi V., Shibayama M. Amoebic liver abscess production by E. dispar. Ann. Hepatol. 2012;11:107–117. [PubMed] [Google Scholar]
- Fadeyi A., Nwabuisi C., Adegboro B., Akanbii A.A., II, Adeola F., Odimayo M.S. Apparent rarity of E. histolytica and other intestinal parasites in acute and persistent diarrhoeic patients attending Ilorin hospitals: time for ELISA antigen based Amebiasis. Eur. J. Sci. Res. 2009;31:388–397. [Google Scholar]
- Fotedar R., Stark D., Marriott D., Ellis J., Harkness J. Entamoeba moshkovskii infections in Sydney, Australia. Eur. J. Clin. Microbiol. Infect. Dis. 2008;27:133–137. doi: 10.1007/s10096-007-0399-9. [DOI] [PubMed] [Google Scholar]
- Hamano S., Asgharpour A., Stroup S.E., Wynn T.A., Leiter E.H., Houpt E. Resistance of C57BL/6 mice to amoebiasis is mediated by nonhemopoietic cells but requires hemopoietic IL-10 production. J. Immunol. 2006;177:1208–1213. doi: 10.4049/jimmunol.177.2.1208. [DOI] [PubMed] [Google Scholar]
- Hamzah Z., Petmitr S., Mungthin M., Leelayoova S., Chavalitshewinkoon-Petmitr P. Differential detection of Entamoeba histolytica, Entamoeba dispar, and Entamoeba moshkovskii by a single round PCR assay. J. Clin. Microbiol. 2006;44:95–137. doi: 10.1128/JCM.00778-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooshyar H., Rostamkhani P., Rezaian M. Molecular epidemiology of human intestinal amoebas in Iran. Iran. J. Public Health. 2012;41:10–17. [PMC free article] [PubMed] [Google Scholar]
- Ismail H.A., Jeon H.K., Yu Y.M., Do C., Lee Y.H. Intestinal parasite infections in pigs and beef cattle in rural areas of Chungcheongnam-do, Korea. Korean J. Parasitol. 2010;48:347–349. doi: 10.3347/kjp.2010.48.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebede A., Verweij J., Dorigo-Zetsma W., Sanders E., Messele T., van Lieshout L., Petros B., Polderman T. Overdiagnosis of amoebiasis in the absence of Entamoeba histolytica among patients presenting with diarrhoea in Wonji and Akaki, Ethiopia. Trans. R. Soc. Trop. Med. Hyg. 2003;97:305–307. doi: 10.1016/s0035-9203(03)90153-2. [DOI] [PubMed] [Google Scholar]
- López M.C., León C.M., Fonseca J., Reyes P., Moncada L., Olivera M.J., Ramírez J.D. Molecular epidemiology of Entamoeba: first description of Entamoeba moshkovskii in a rural area from Central Colombia. PLoS One. 2015;10 doi: 10.1371/journal.pone.0140302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mchiza Z.J., Parker W., Makoae M., Sewpaul R., Kupamupindi T., Labadarios D. Body image and weight control in South Africans 15 years or older: SANHANES-1. BMC Public Health. 2015;15:992. doi: 10.1186/s12889-015-2324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojarad E.N., Nochi Z., Sahebekhtiari N., Dabiri H., Zali M.R., Kazemi B., Haghighi A. Discrimination of E. moshkovskii in patients with gastrointestinal disorders by single round PCR. Jpn. J. Infect. Dis. 2010;63:136–138. [PubMed] [Google Scholar]
- Moran P., Ramos F., Ramiro M., Curiel G., Gonzalez E., Valadez A., Gomez A., Garcia G., Melendro E.L., Ximenez C. Entamoeba histolytica & E. dispar: infection frequency in HIV+/AIDS patients in Mexico City. Exp. Parasitol. 2005;110:3331–3334. doi: 10.1016/j.exppara.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Ngobeni R., Samie A., Moonah S., Watanabe K., Petri W.A., Jr., Gilchrist C. Entamoeba species in South Africa: correlations with the host microbiome, parasite burdens, and first description of Entamoeba bangladeshi outside of Asia. J. Infect. Dis. 2017;216(12):1592–1600. doi: 10.1093/infdis/jix535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouattara M., N'Guessan N.A., Yapi A., N'goran E.K. Prevalence and spatial distribution of Entamoeba histolytica/ dispar and Giardia lamblia among school children in Agboville (Cote d’ Ivoire) PLoS Negl. Trop. Dis. 2010;4 doi: 10.1371/journal.pntd.0000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro S.M.B., Carneiro R.M., Aca I.S., Irmão J.I., Morais M.A., Jr., Coimbra M.R., Carvalho L.B., Jr. Determination of the prevalence of Entamoeba histolytica and E. dispar in the Pernambuco State of North-eastern Brazil by a PCR. Am. J. Trop. Med. 2004;70:221–224. [PubMed] [Google Scholar]
- Samie A., Obi L.C., Bessong P.O., Stroup S., Houpt E., Guerrant R.L. Prevalence and species distribution of E. histolytica and E. dispar in the Venda region, Limpopo, South Africa. Am. J. Trop. Med. Hyg. 2006;75:565–567. [PubMed] [Google Scholar]
- Shimokawa C., Kabir M., Taniuchi M., Mondal D., Kobayashi S., Ali I.K., Sobuz S.U., Senba M., Houpt E., Haque R., Petri W.A., Jr., Hamano S. Entamoeba moshkovskii is associated with diarrhea in infants and causes diarrhea and colitis in mice. J. Infect. Dis. 2012;206:744–751. doi: 10.1093/infdis/jis414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanyuksel M., Ulukanligil M., Guclu Z., Araz E., Koru O., Petri W.A., Jr. Two cases of rarely recognised infection with E. moshkovskii. Am. J. Trop. Med. Hyg. 2007;76:723–724. [PubMed] [Google Scholar]
- Tasawar Z., Kausar S., Lashari M.H. Prevalence of Entamoeba histolytica in humans. Pak. J. Pharm. Sci. 2010;23:344–348. [PubMed] [Google Scholar]
- World Health Organization W.H.O World Health Organization/Pan American Health Organization/UNESCO report of a consultation of experts on amoebiasis. Wkly Epidemiol. Rec. 1997;72:97–100. [Google Scholar]
- Ximenez C., Moran P., Rojas L., Valadez A., Gómez A., Ramiro M., Cerritos R., González E., Hernández E., Oswaldo P. Novelties on amoebiasis: a neglected tropical disease. J. Glob. Infect. Dis. 2011;3:166–174. doi: 10.4103/0974-777X.81695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakoob J., Abbas Z., Beg M.A., Naz S., Khan R., Jafri W. Entamoeba species associated with chronic diarrhoea in Pakistan. Epidemiol. Infect. 2012;140:323–328. doi: 10.1017/S0950268811000215. [DOI] [PubMed] [Google Scholar]