Abstract
Inflammation and proliferation of vascular smooth muscle cells (VSMCs) are the key events in intimal hyperplasia. This study aimed to explore the mechanism by which long non-coding RNA (lncRNA) KCNQ1OT1 affects VSMC inflammation and proliferation in this context. A vein graft (VG) model was established in mice to introduce intimal hyperplasia. Isolated normal VSMCs were induced with platelet-derived growth factor type BB (PDGF-BB), and the cell proliferation, migration, and secretion of inflammatory factors were determined. The results showed that KCNQ1OT1 was downregulated in the VSMCs from mice with intimal hyperplasia and in the PDGF-BB-treated VSMCs, and such downregulation of KCNQ1OT1 resulted from the increased methylation level in the KCNQ1OT1 promoter. Overexpressing KCNQ1OT1 suppressed PDFG-BB-induced VSMC proliferation, migration, and secretion of inflammatory factors. In VSMCs, KCNQ1OT1 bound to the nuclear transcription factor kappa Ba (IκBa) protein and increased the cellular IκBa level by reducing phosphorylation and promoting ubiquitination of the IκBa protein. Meanwhile, KCNQ1OT1 promoted the expression of IκBa by sponging miR-221. The effects of KCNQ1OT1 knockdown on promoting VSMC proliferation, migration, and secretion of inflammatory factors were abolished by IκBa overexpression. The roles of KCNQ1OT1 in reducing the intimal area and inhibiting IκBa expression were proved in the VG mouse model after KCNQ1OT1 overexpression. In conclusion, KCNQ1OT1 attenuated intimal hyperplasia by suppressing the inflammation and proliferation of VSMCs, in which the mechanism upregulated IκBa expression by binding to the IκBa protein and sponging miR-221.
Keywords: vascular smooth muscle cells, intimal hyperplasia, KCNQ1OT1, IκBa, proliferation, inflammation, ubiquitination, miR221, methylation
Introduction
Intimal hyperplasia is a common phenomenon that occurs in the process of artery remodeling after vascular injury, and it is often observed in the treatment of various vascular diseases, such as atherosclerosis, angioplasty, stent implantation, and bypass operation.1,2 In response to vascular injury, vascular smooth muscle cells (VSMCs) can undergo increased inflammation, proliferation, and migration, as well as decreased expression of smooth muscle markers, developing into intimal hyperplasia.3 Therefore, inhibiting the proliferation and inflammation of VSMCs is a crucial step in delaying vein graft (VG) intimal hyperplasia, and research on the regulatory mechanism of the function and phenotype of VSMCs is desperately needed. Platelet-derived growth factor type BB (PDGF-BB) is a strong stimulant for VSMC proliferation and migration,4 and it is generally used in intimal hyperplasia-related study.
Nuclear transcription factor κB (NF-κB) is a key transcription factor implicated in the control of inflammation, cell migration, proliferation, and apoptosis, and it is reported to be greatly involved in intimal hyperplasia.1 The inhibitors of NF-κB (IκB), including IκBa, IκBb, IκBg, and IκBe, comprise a protein family that binds with NF-κB to prevent its nuclear translocation.5 Once the NF-κB-bound IκB is degraded, which may result from phosphorylation and the subsequent ubiquitination-dependent degradation, NF-κB is released and able to translocate to the nucleus; at this point, the NF-κB pathway is activated.6 NF-κB is a critical regulator of VSMC proliferation under inflammation.7 It has been reported that the phosphorylation of IκB plays a key role in human VSMC proliferation,8 and the overexpression of IκBa inhibits VSMC proliferation and intimal hyperplasia formation.9 However, the regulation of PDGF-induced downregulation of IκB is not fully elucidated. Interestingly, microRNA-221 (miR-221) has been identified to be transcriptionally induced upon PDGF treatment in primary VSMCs, and it is critical for cell proliferation,10 while the decrease in the expression and activity of miR-221 represses neointimal hyperplasia in VGs.3 Importantly, we predicted complementary bases between miR-221 and IκBa (encoded by the NFKBIA gene) using the bioinformatics method (TargetScan), implying potential binding between them. Therefore, we speculated that miR-221 may affect VSMC proliferation and intimal hyperplasia development by targeting IκBa.
Long non-coding RNAs (lncRNAs), serving as the sponge of the miRNAs, have garnered extensive attention.11 Increasingly, lncRNAs like RNCR312 and ANRIL13 have been noted to play a role in regulating the VSMCs’ proliferation or growth. We used an online database (DIANA tools) to search for the candidate lncRNAs and found that lncRNA KCNQ1OT1 was predicted to have binding sites with miR-221. Meanwhile, by using RNA pull-down assay and mass spectrometry, we found that KCNQ1OT1 could bind with IκBa protein in VSMCs. Notably, KCNQ1OT1 is involved in cardiac development, and KCNQ1OT1 gene variants could be associated with the risk of developing long QT syndrome or a prolonged QT interval,14,15 suggesting that KCNQ1OT1 may play a role in cardiovascular diseases. Taken together, we inferred that KCNQ1OT1 may regulate the expression of IκBa by binding the protein and targeting miR-221, resulting in the inflammation and proliferation of VSMCs and intimal hyperplasia pathogenesis. This study aimed to clarify this hypothesis and explore the impact of KCNQ1OT1 on intimal hyperplasia progression.
Results
KCNQ1OT1 Is Downregulated in the VSMCs of Mice with Intimal Hyperplasia and in the Process of VSMC Proliferation
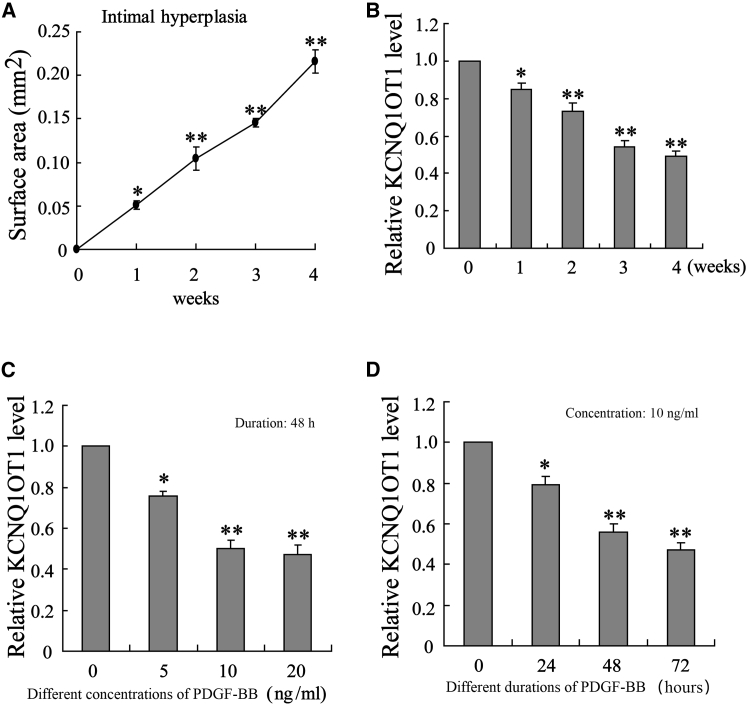
First, the VG model was constructed in mice (VG, n = 25) to introduce the intimal hyperplasia. At 0, 1, 2, 3, and 4 weeks (n = 5 at each time point), detection on the intimal area indicated that the surface area was increased in a time-dependent manner (Figure 1A). At the same time, the VSMCs were isolated from the model mice at 0, 1, 2, 3, and 4 weeks, and it was interesting to find that the expression of KCNQ1OT1 in VSMCs declined in a time-dependent way (Figure 1B). We assumed that KCNQ1OT1 could be implicated in the pathogenesis of intimal hyperplasia.
Figure 1.
KCNQ1OT1 Is Downregulated in the VSMCs of Mice with Intimal Hyperplasia and in the Process of VSMC Proliferation
The vein graft model (VG) was constructed in mice (n = 25) to introduce intimal hyperplasia. (A and B) After 0, 1, 2, 3, and 4 weeks (n = 5 at each time point), (A) the intimal area was calculated by subtracting the luminal area from the area within the internal elastic lamina, and (B) the expression of KCNQ1OT1 in isolated VSMCs was detected using quantitative real-time PCR. VSMCs were isolated from the normal mice and stimulated with PDGF-BB with an increased concentration gradient (0, 5, 10, and 20 ng/mL) for 48 h. (C) The expression of KCNQ1OT1 in VSMCs was determined using quantitative real-time PCR. VSMCs were treated with PDGF-BB (10 ng/mL) for different durations (24, 48, and 72 h). (D) The expression of KCNQ1OT1 was examined by quantitative real-time PCR. *p < 0.05 and **p < 0.01 compared with the 0 time point or without PDGF-BB.
For investigating the expression level of KCNQ1OT1 during the proliferation of VSMCs, we used PDGF-BB to stimulate the VSMCs isolated from the normal mice. With the concentration of PDGF-BB increased in a gradient (0, 5, 10, and 20 ng/mL), the expression of KCNQ1OT1 at 48 h in VSMCs was reduced in a dose-dependent way (Figure 1C). In addition, when treated with PDGF-BB (10 ng/mL) for different durations (24, 48, and 72 h), the expression of KCNQ1OT1 in VSMCs was decreased in a time-dependent manner (Figure 1D). These data implied some relationship between KCNQ1OT1 expression and VSMC proliferation induced by PDGF-BB.
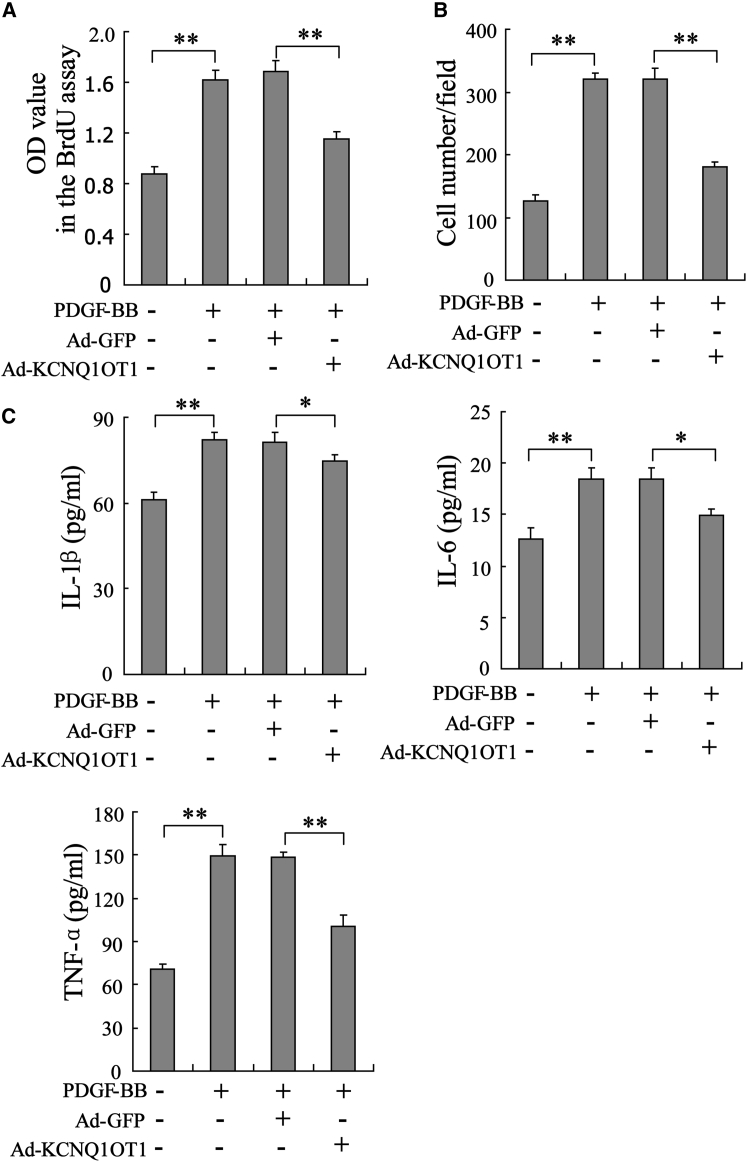
Overexpression of KCNQ1OT1 Suppresses VSMC Proliferation, Migration, and Secretion of Inflammatory Factors
To clarify the potential role of KCNQ1OT1 in affecting VSMC proliferation, we overexpressed KCNQ1OT1 in VSMCs treated with PDGF-BB (10 ng/mL) by transfecting the Ad-KCNQ1OT1 vector, with the Ad-GFP acting as the negative control. As shown in Figure 2A, the proliferation of VSMCs was promoted by PDGF-BB, but it was attenuated by KCNQ1OT1 overexpression. The cell migration enhanced by PDGF-BB was also almost abolished by Ad-KCNQ1OT1 transfection (Figure 2B). In VSMCs, the secretion of inflammatory factors, such as interleukin-1β (IL-1β), IL-6, and tumor necrosis factor alpha (TNF-α), was markedly increased with PDGF-BB treatment, while overexpression of KCNQ1OT1 repressed the secretion of these inflammatory factors (Figure 2C). Collectively, we demonstrated that overexpression of KCNQ1OT1 abolished the effects of PDGF-BB on stimulating VSMCs proliferation, migration, and secretion of inflammatory factors.
Figure 2.
Overexpression of KCNQ1OT1 Suppresses VSMC Proliferation, Migration, and Secretion of Inflammatory Factors
In VSMCs treated with PDGF-BB (10 ng/mL, 48 h), KCNQ1OT1 was overexpressed by transfecting the Ad-KCNQ1OT1 vector, with Ad-GFP acting as the negative control. (A) The proliferation of VSMCs was evaluated using the BrdU staining method. (B) VSMC migration was assessed by transwell assay. (C) The level of inflammatory factors, such as IL-1β, IL-6, and TNF-α, in VSMC culture supernatant was determined using ELISA. *p < 0.05 and **p < 0.01.
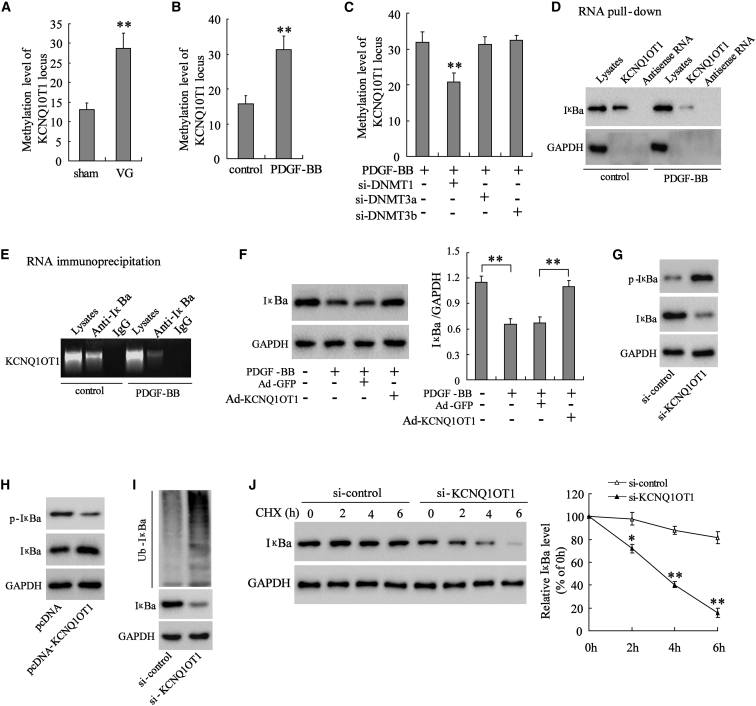
Hypermethylation Represses KCNQ1OT1 Expression and KCNQ1OT1 Binds to IκBa Protein to Impede Its Degradation
To explain the downregulation of KCNQ1OT1 in the VSMCs of mice with intimal hyperplasia and the PDGF-BB-treated VSMCs from normal mice, we determined the methylation level of its promoter. Compared with the VSMCs from the mice in the sham group (n = 7), the methylation level of KCNQ1OT1 promoter in the VSMCs of mice that underwent VG (n = 7) was notably elevated (Figure 3A). In the VSMCs isolated from normal mice, PDGF-BB treatment clearly augmented the methylation level in the promoter of KCNQ1OT1 (Figure 3B). However, the high level of methylation in the promoter of KCNQ1OT1 induced by PDGF-BB was diminished after DNA methyltransferase 1 (DNMT1) knockdown, while silencing of DNMT3a or DNMT3b had no influence on the methylation level (Figure 3C). Herein, we revealed that the downregulation of KCNQ1OT1 in the VSMCs of mice with intimal hyperplasia and the PDGF-BB-treated normal VSMCs could be attributed to the increased methylation level in its promoter.
Figure 3.
Hypermethylation Represses KCNQ1OT1 Expression and KCNQ1OT1 Binds to IκBa Protein to Impede Its Degradation
(A) For mice undergoing vein graft (VG, n = 7) and those in the sham group (n = 7), the VSMCs were isolated and the methylation level of the KCNQ1OT1 promoter was detected. In the VSMCs isolated from normal mice, PDGF-BB (10 ng/mL, 48 h) was used, and (B) the methylation level in the promoter of KCNQ1OT1 was measured. (C) DNMT1, DNMT3a, or DNMT3b was silenced in PDGF-BB-induced VSMCs to detect the level of methylation in the promoter of KCNQ1OT1. (D and E) RNA pull-down (D) and RIP assay (E) were performed to assess the binding and interaction between KCNQ1OT1 and IκBa protein in VSMCs. VSMCs were divided into control, PDGF-BB, PDGF-BB+Ad-GFP, and PDGF-BB+Ad-KCNQ1OT1, and (F) the level of IκBa protein in VSMCs was analyzed by western blot. (G) Via transfecting si-KCNQ1OT1 or si-control in VSMCs, the expression level of the p-IκBa and IκBa proteins was determined by western blot. (H) VSMCs were transfected with pcDNA-KCNQ1OT1 or pcDNA, and the p-IκBa and IκBa protein levels were measured by western blot. (I and J) In VSMCs transfected with si-KCNQ1OT1 or si-control, (I) the ubiquitination of IκBa protein was assessed, and (J) the degeneration of IκBa protein was investigated by CHX assay. *p < 0.05, **p < 0.01 compared with the sham, control, or PDGF-BB group.
Next, we used RNA pull-down and mass spectrometry to screen for proteins that may bind to KCNQ1OT1 in VSMCs, and we found that the IκBa protein was expressed in the pull-down compounds (data not shown). Then, RNA pull-down and RNA immunoprecipitation (RIP) were employed to confirm the binding and the interaction between KCNQ1OT1 and IκBa protein. VSMCs were transfected with biotinylated KCNQ1OT1 or the antisense RNA probe, and RNA pull-down was carried out, followed by western blot with the IκBa antibody. Figure 3D validates the binding between biotinylated KCNQ1OT1 and IκBa rather than between the antisense RNA probe and IκBa; however, the binding between biotinylated KCNQ1OT1 and IκBa was reduced after PDGF-BB treatment. In the RIP assay, abundant KCNQ1OT1 was detected in the complex precipitated by the antibody against IκBa, suggesting the binding between KCNQ1OT1 and IκBa, which was diminished in cells treated with PDGF-BB (Figure 3E). Then, we illustrated that the level of IκBa protein was reduced in VSMCs treated with PDGF-BB; however, the overexpression of KCNQ1OT1 abolished this inhibitory effect on IκBa protein level (Figure 3F). These findings proved that KCNQ1OT1 upregulated the expression of IκBa protein by binding with it, while PDGF-BB lowered the IκBa level through downregulating KCNQ1OT1.
Except for binding with IκBa protein, the other pathways by which KCNQ1OT1 regulated the IκBa level were also explored. Via transfecting si-KCNQ1OT1 in VSMCs, KCNQ1OT1 expression was inhibited and p-IκBa was upregulated, whereas IκBa was significantly downregulated (Figure 3G), indicating the increased phosphorylation level of IκBa. In contrast, overexpression of KCNQ1OT1 boosted the p-IκBa expression but decreased the expression of IκBa (Figure 3H), suggesting that the phosphorylation level of IκBa was lowered. It could be deduced that the expression of KCNQ1OT1 inhibited the phosphorylation of IκBa. Afterward, we illustrated that knockdown of KCNQ1OT1 promoted the ubiquitination of IκBa protein (Figure 3I), and the CHX assay further verified that KCNQ1OT1 downregulation facilitated the degeneration of IκBa protein (Figure 3J). Taken together, it could be elucidated that KCNQ1OT1 restrained the ubiquitination-dependent degradation of IκBa protein.
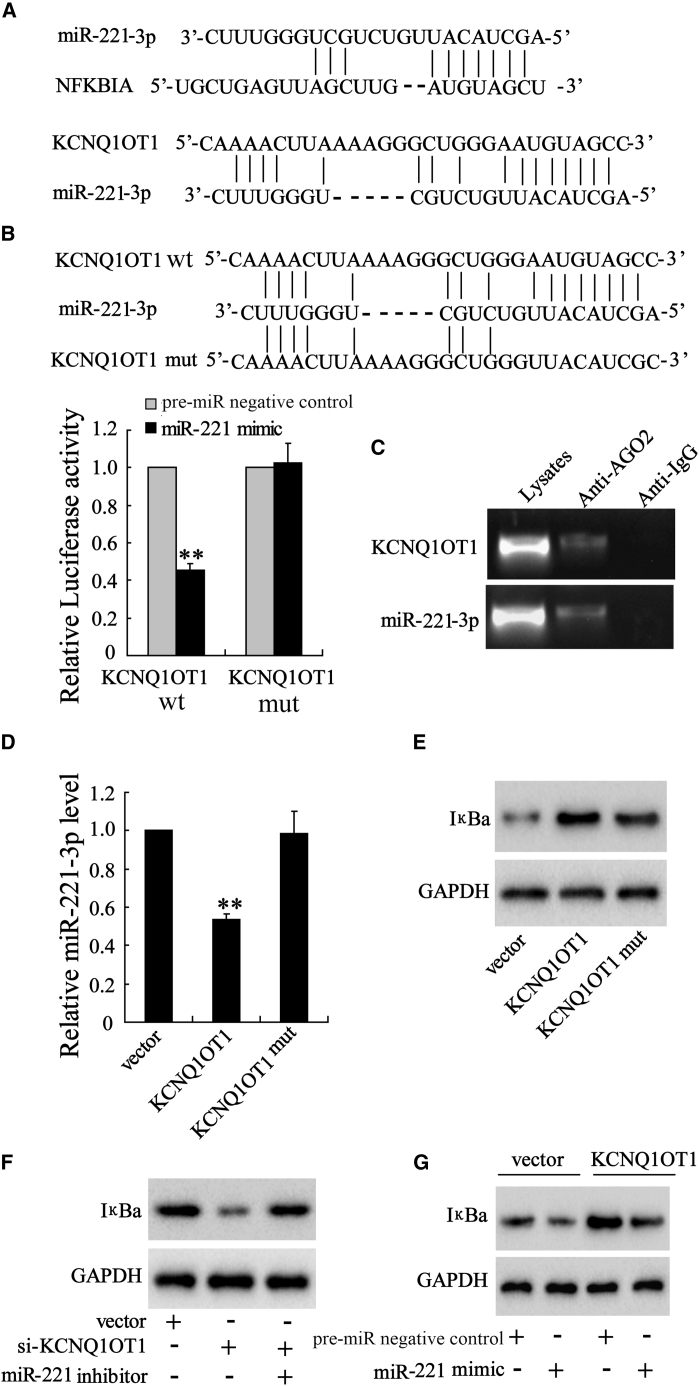
KCNQ1OT1 Upregulates IκBa Level by Sponging miR-221
Via bioinformatics software, the binding sites of miR-221 (miR-221-3p) on the IκBa 3′ untranslated region (3′ UTR) were predicted (TargetScan), as well as the complementary bases between KCNQ1OT1 and miR-221 (DIANA tools; Figure 4A). There was a total of 25 binding sites between KCNQ1OT1 and miR-221, and Figure 4A showed the site at 32403-32427. By co-transfecting pGL3-KCNQ1OT1-WT (wild-type) or pGL3-KCNQ1OT1-mut (mutant) and the miR-221 mimic into 293 cells, we found that overexpression of miR-221 inhibited the luciferase activity in KCNQ1OT1-WT-transfected cells, while it had no influence on that in KCNQ1OT1-mut-transfected cells (Figure 4B). The RNA-binding protein AGO2 participates in the maturation of miRNAs and mediates the binding between miRNAs and their target RNAs. The result of the RIP assay using the anti-AGO2 showed that AGO2 could bind with KCNQ1OT1 and miR-221 in VSMCs (Figure 4C). Moreover, overexpression of KCNQ1OT1 in VSMCs downregulated miR-221 (Figure 4D) but increased the expression of IκBa protein (Figure 4E). Knockdown of KCNQ1OT1 in VSMCs suppressed the IκBa protein level, which was reversed by miR-221 inhibition (Figure 4F); in contrast, the upregulation of IκBa protein caused by KCNQ1OT1 overexpression was reversed by miR-221 expression (Figure 4G). These data proved that KCNQ1OT1 sponged miR-221 to elevate the expression of its target, IκBa.
Figure 4.
KCNQ1OT1 Upregulates IκBa Levels by Sponging miR-221
(A) Via bioinformatics software, the binding sites of miR-221 (miR-221-3p) on the IκBa 3′ UTR were predicted (TargetScan), as were the complementary bases between KCNQ1OT1 and miR-221 (DIANA tools). (B) Luciferase reporter assay was performed to assess the binding between KCNQ1OT1 and miR-221. (C) AGO2-RIP assay was conducted to validate the interaction between KCNQ1OT1 and miR-221. (D and E) The expressions of (D) miR-221 and (E) IκBa protein were detected in VSMCs in which KCNQ1OT1 was overexpressed. (F and G) In VSMCs transfected with vector, si-KCNQ1OT1, si-KCNQ1OT1+miR-221 inhibitor (F), or vector + pre-miR negative control/miR-221 mimic, KCNQ1OT1 + pre-miR negative control/miR-221 mimic (G), the IκBa protein level was determined by western blot. **p < 0.01 compared with the pre-miR negative control or vector.
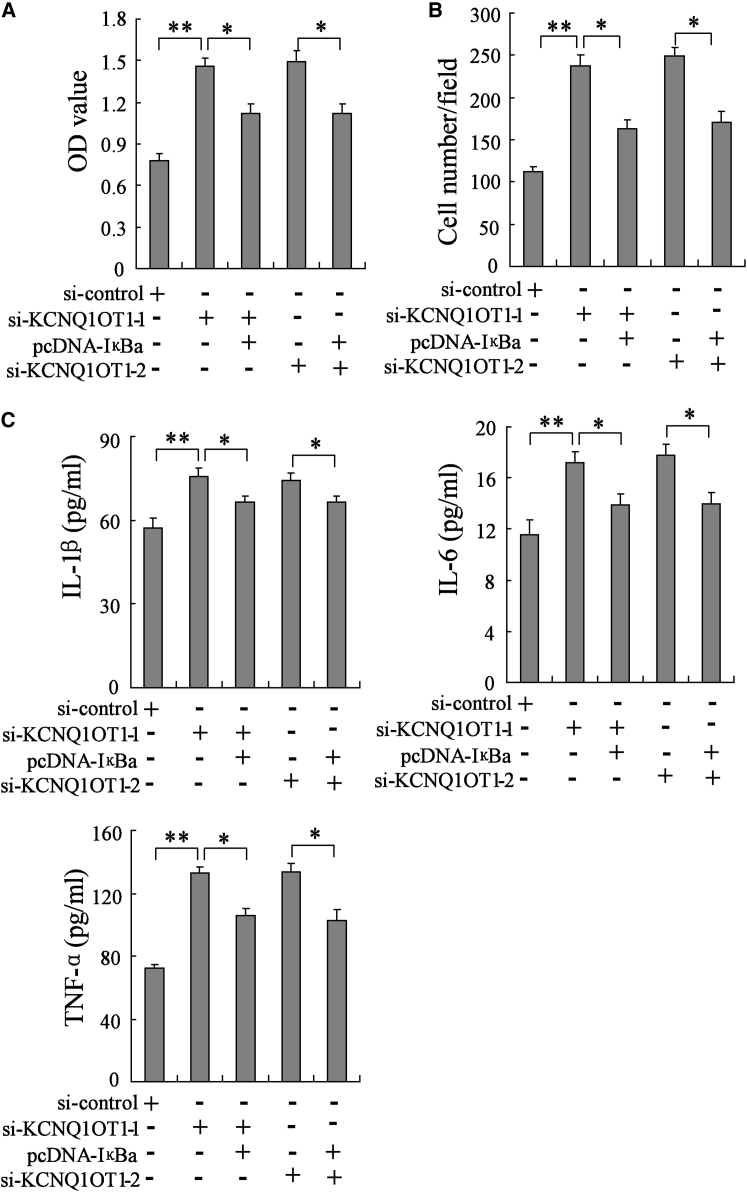
KCNQ1OT1 Suppresses VSMC Proliferation, Migration, and Secretion of Inflammatory Factors via Upregulating IκBa
For exploring the mechanism by which KCNQ1OT1 affected VSMC proliferation, migration, and inflammation, knockdown of KCNQ1OT1 and simultaneous upregulation of IκBa were performed in VSMCs. VSMCs were allocated into several groups, which were as follows: si-control, si-KCNQ1OT1-1, si-KCNQ1OT1-1+pcDNA-IκBa, si-KCNQ1OT1-2, and si-KCNQ1OT1-2+pcDNA-IκBa. We illustrated that silencing of KCNQ1OT1 in VSMCs promoted cell proliferation (Figure 5A) and migration (Figure 5B), which were largely abolished with IκBa overexpression. In addition, interference of KCNQ1OT1 enhanced the secretion of inflammatory factors in VSMCs, including IL-1β, IL-6, and TNF-α, but the secretion of inflammatory factors was inhibited by overexpression of IκBa (Figure 5C). The results revealed that KCNQ1OT1 suppressed VSMC proliferation, migration, and secretion of inflammatory factors via upregulating IκBa.
Figure 5.
KCNQ1OT1 Suppresses VSMC Proliferation, Migration, and Secretion of Inflammatory Factors via Upregulating IκBa
VSMCs were allocated into the following five groups: si-control, si-KCNQ1OT1-1, si-KCNQ1OT1-1+pcDNA-IκBa, si-KCNQ1OT1-2, and si-KCNQ1OT1-2+pcDNA-IκBa. (A) The proliferation of VSMCs was evaluated using the BrdU staining method. (B) VSMC migration was assessed via transwell assay. (C) The level of inflammatory factors, including IL-1β, IL-6, and TNF-α, in VSMC culture supernatant was determined by ELISA. *p < 0.05 and **p < 0.01.
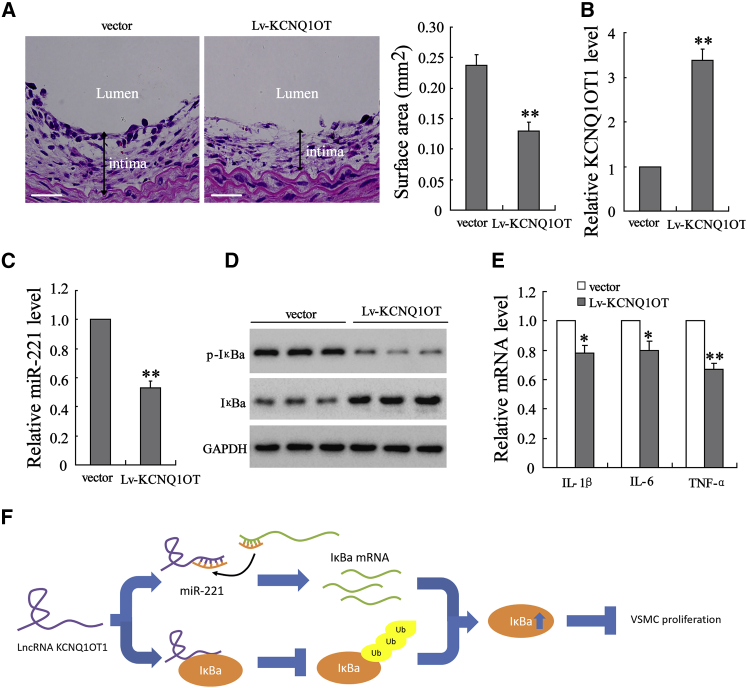
Overexpression of KCNQ1OT1 Reduces Intimal Area in a VG Mouse Model
Finally, we validated the function of KCNQ1OT1 in affecting intimal hyperplasia in mice undergoing VG. Figure 6A showed that the thickness and surface area of the intima was reduced in mice injected with lentivirus vector expressing KCNQ1OT1 (Lv-KCNQ1OT1) (n = 7) compared with those injected with empty vector (n = 7). The upregulation of KCNQ1OT1 in VSMCs isolated from the Lv-KCNQ1OT1-injected mice was verified (Figure 6B). Furthermore, the expression of miR-221 was reduced (Figure 6C), while p-IκBa protein was reduced and IκBa protein was increased after overexpressing KCNQ1OT1 (Figure 6D). The overexpression of KCNQ1OT1 also reduced the mRNA levels of IL-1β, IL-6, and TNF-α (Figure 6E), suggesting that KCNQ1OT1 overexpression could attenuate the inflammatory response. These findings demonstrated that overexpression of KCNQ1OT1 attenuated intimal hyperplasia in mice undergoing VG.
Figure 6.
Overexpression of KCNQ1OT1 Attenuated Intimal Hyperplasia in a VG Mouse Model
The lentivirus vector expressing KCNQ1OT1 (Lv-KCNQ1OT1) or empty vector (vector, 108 pfu/mL) was subcutaneously injected into mice with VG (n = 7 in each group). (A) After 4 weeks, the graft vein was collected, and the intimal thickness was observed and intimal surface area was calculated. Scale bar represents 20 μm. (B and C) The VSMCs were isolated, and (B) the expressions of KCNQ1OT1 and (C) miR-221 were detected using quantitative real-time PCR. (D and E) p-IκBa and IκBa protein levels were determined using western blot (D) and mRNA levels of IL-1β, IL-6, and TNF-α were detected using quantitative real-time PCR (E). (F) KCNQ1OT1 upregulates IκBa, suppressing the inflammation and proliferation of VSMCs and attenuating intimal hyperplasia. *p < 0.05, **p < 0.01 compared with vector.
Discussion
In this study, we considered the regulatory mechanism of lncRNA KCNQ1OT1 on the inflammation and proliferation of VSMCs and investigated its influence on the development and progression of intimal hyperplasia. The results revealed that KCNQ1OT1 upregulates IκBa, suppressing the inflammation and proliferation of VSMCs; this results in a reduced intimal area (Figure 6F). Our study provides a promising therapeutic target for the treatment of VG intimal hyperplasia.
VSMCs, which have a major presence in the media of vessels, are the dominant cellular constituents of arteries and the crucial determinants in vascular disorders.16 In response to vascular injury, VSMCs will engage in phenotypic regulation featuring augmented inflammation, proliferation, and migration; thus, they form a neointima.1 Proinflammatory cytokines are pivotal regulators of arterial inflammation and intimal hyperplasia, and they participate in pathological vascular remodeling.17 The present study demonstrated that PDGF-BB induced VSMC proliferation and migration, accompanied by increased secretion of inflammatory factors, including IL-1β, IL-6, and TNF-α, which was consistent with the previous study by Pi et al.18 However, these noticeable facilitating effects on the VSMC proliferative phenotype and inflammation were largely abolished by KCNQ1OT1 overexpression, implying an enormous potential of KCNQ1OT1 as an effective target in the treatment of intimal hyperplasia.
Abnormal expression of imprinted genes is often associated with various human disorders with complex mutations and phenotypic defects. As an imprinted gene, KCNQ1OT1 is generally methylated in its promoter, which has been proven to be associated with embryonic developmental failure19 and the risk of symptomatic cardiac long QT.14 In the current study, the downregulation of KCNQ1OT1 in the VSMCs from mice with intimal hyperplasia and in the PDGF-BB-induced VSMCs was attributed to the increase of methylation level at its promoter, indicating some inner link between KCNQ1OT1 expression and intimal hyperplasia pathogenesis. Except in the abnormal expression change of KCNQ1OT1 itself, it is devoted to modulating the expression of ubiquitous genes to participate in diverse biological processes. For example, Mohammad et al.20 found that KCNQ1OT1 mediates transcriptional gene silencing by interacting with the DNMT1, and they confirmed the direct interaction between KCNQ1OT1 and DNMT1 via RIP assay. Interestingly, our study showed that the knockdown of DNMT1 reduced the methylation level of the KCNQ1OT1 promoter, affecting the KCNQ1OT1 expression. These data indicated that there may be feedback regulation between KCNQ1OT1 and DNMT1, although more evidence on this is still needed. Another study conducted by Chen et al.21 indicated that KCNQ1OT1 promotes lens epithelial cell proliferation and the epithelial-mesenchymal transition via upregulating SMAD4. Although they did not elucidate the specific interactions between KCNQ1OT1 and SMAD4, they determined that KCNQ1OT1 positively regulated SMAD4 at both the mRNA and protein levels. Consistent with their study, our research explains the regulation of KCNQ1OT1 on IκBa expression at both the mRNA and protein levels. On the one hand, KCNQ1OT1 directly binds to IκBa protein, which is similar to its effect on β-catenin protein,22 to restrain the phosphorylation and ubiquitination-dependent degradation of IκBa protein. On the other, KCNQ1OT1 functions as a competing endogenous RNA (ceRNA) of miR-221, leading to the upregulation of its target gene, IκBa.
The influence of miR-221 on VSMC proliferation and intimal hyperplasia was previously reported.3,10 However, it is surprising that we identified IκBa as a novel target of miR-221 in this study, which further elucidates the action mechanism of miR-221 contributing to VSMC proliferation and intimal hyperplasia occurrence. NF-κB is a key transcription factor that is implicated in the control of inflammation, cell migration, proliferation, and apoptosis; hence, its inhibitory subunit IκBa is also widely focused on. Recently, it has been claimed that phosphorylation and degradation of IκBa abolishes PDGF-BB-evoked NF-κB nuclear translocation and influences VSMC phenotypic switching, proliferation, migration, and neointima formation.4 VSMC migration and vascular injury-induced intimal hyperplasia are also accompanied by IκBa phosphorylation and degradation.16 Our study demonstrated that the increase of IκBa induced by KCNQ1OT1 suppresses VSMC proliferation, migration, and secretion of inflammatory factors, further confirming the intrinsic function of IκBa in controlling VSMC proliferation, migration, and inflammation via the inhibition of NF-κB activation.
Taken together, the study results highlight the significance and mechanism of KCNQ1OT1 involved in the VSMC proliferative phenotype and inflammation, and the subsequent intimal hyperplasia, which provides a novel insight into the prevention and treatment of vascular inflammatory disorders following VG.
Materials and Methods
Surgical Procedures of VG
Animal housing and procedures were approved by the local Animal Care and Use Committee at the First Affiliated Hospital of Wenzhou Medical University and complied with humane animal care standards. VG surgeries were performed with a VG introduced via end-to-end anastomoses.23 Prior to surgery, all the mice were anesthetized with intraperitoneal pentobarbital (50 mg/kg). After an aseptic incision made in the ventral neck, the posterior facial branch of the jugular vein was exposed and veins were harvested from donor mice and placed in isotonic saline. For recipient mice, the femoral arteries were aseptically exposed through an inguinal incision; they were then dissected and occluded temporarily using nontraumatic clamps. After resection of a small portion of the artery, a VG was introduced via end-to-end anastomoses using 8–10 interrupted stitches of 11-0 nylon suture for each anastomosis. After blood flow was restored, the clamps were removed and skin wounds were closed using 5-0 nylon suture. Heparin (100 U/mL) in 0.2 mL of saline was used locally during the grafting procedure. Patency was verified 3 days following grafting. Five mice each were sacrificed at the time points of 0, 1, 2, 3, and 4 weeks after VG.
Measurement of Intimal Surface Area
The intimal surface area was calculated by subtracting the luminal area from the area within the internal elastic lamina.24 Briefly, the VG was harvested and embedded in paraffin. Cross-sections (5 μm thick) were cut from three levels (70 μm between levels) of the VG midportion, mounted on slides, and stained with hematoxylin and eosin for area quantification. Microscopic images were captured and transferred to the computer, and Image-Pro Plus software (Media Cybernetics, Silver Spring, MD, USA) was applied to draw the boundaries of the lumen-vascular wall and tunica media-adventitia. The difference between the two drawn cross-sectional areas was defined as the neointimal area. The mean neointimal area for each VG was calculated. The mean intimal thickness of each cross-section was obtained by measuring at the 3-, 6-, 9-, and 12-o’clock positions; the mean intimal thickness of each VG was calculated by measuring the cross-sections from three levels.
Isolation of VSMCs and Cell Culture
4 weeks following VG, VSMCs were isolated from the inferior vena cava of the mice, as described previously.25 After the fat tissue and loose adventitia were carefully removed, the vein was digested with collagenase. The venous VSMCs were cultured and passaged in DMEM supplemented with 10% fetal bovine serum (FBS; HyClone, USA) maintained in 37°C incubators with 5% CO2. VSMCs were passaged fewer than five times before being used in this study.
Quantitative Real-Time PCR
We extracted total RNA from VSMCs using the RNeasy Mini Kit (QIAGEN) according to the manufacturer’s instructions. For quantitative detection of lncRNAs and mRNAs, 1 μg of RNA was subjected to reverse transcription for the synthesis of cDNAs with the iScript Select cDNA synthesis kit (Bio-Rad). Quantitative real-time PCR analysis was performed with the SYBR Green Mix (Bio-Rad) on a real-time PCR machine (iQ5, Bio-Rad). The PCR conditions were 94°C for 3 min and 40 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 45 s. Gene-specific expression levels were normalized to the levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or U6, and data were analyzed using the comparative CT method. The primers were as follows: KCNQ1OT1: 5′-GCACTCTGGGTCCTGTTCTC-3′ (F), 5′-CACTTCCCTGCCTCCTACAC-3′ (R); miR-221: 5′-GGGAAGCTACATTGTCTGC-3′ (F), 5′-CAGTGCGTGTCGTGGAGT-3′ (R); GAPDH: 5′-ATCACTGCCACCCAGAAGAC-3′ (F), 5′-TTTCTAGACGGCAGGTCAGG-3′ (R); and U6: 5′-CTCGCTTCGGCAGCACATATACT-3′ (F), 5′-ACGCTTCACGAATTTGCGTGTC-3′ (R).
Adenoviral Constructions and Infection
The adenoviruses harboring KCNQ1OT1 (Ad-KCNQ1OT1) and control viruses expressing GFP (Ad-GFP) were constructed using the Adeno-X expression system kit (Clontech, CA, USA) according to the manufacturer’s protocols. In brief, the entire mouse KCNQ1OT1 gene open reading frame was obtained by PCR, cloned into the pAdTrack-CMV vector, and ligated into a shuttle plasmid. Subsequently, the shuttle plasmid and adenoviral backbone plasmid were co-transfected into HEK293A cells to produce the recombinant adenoviral vector Ad-KCNQ1OT1. For the infection of VSMCs, cells were incubated with Ad-KCNQ1OT1 or Ad-GFP for 48 h.
VSMC Treatment and Cell Transfection
To induce VSMC proliferation and migration, we used PDGF-BB at different concentrations (0, 5, 10, and 20 ng/mL) to treat VSMCs isolated from normal mice or mice with VGs for 48 h. In addition, VSMCs were treated with PDGF-BB (10 ng/mL) for different durations (24, 48, and 72 h), and then the expression changes of KCNQ1OT1 were analyzed.
VSMC Proliferation
VSMCs (5,000 cells/well) were placed in 96-well plates with growth medium. After 24 h, 10 mM bromodeoxyuridine (BrdU) was added to the culture medium for incorporation into the DNA of replicating cells. Following fixation and permeabilization, the cells were incubated with a monoclonal BrdU antibody for 45 min, with tetramethylbenzidine as a substrate. BrdU incorporation was measured as the absorbance of the converted substrate at 405 nm.
VSMC Migration
For the transwell assay, cells were seeded in 24-well transwell plates (5 × 104 cells/well) and incubated for 24 h. Cells were re-suspended in serum-free DMEM and maintained in the upper chambers with Matrigel-coated membrane (BD Bioscience, USA), with 500 μL of DMEM containing 10% FBS added to the lower chambers. Cells were cultured for 24 h to allow invading through the 8-μm polyethylene terephthalate membrane. Cells that passed through the membrane were fixed in 4% formaldehyde, stained with 0.1% crystal violet, and analyzed using a cell migration assay kit.26
Detection of the Methylation Level
We checked the methylation status of the KCNQ1OT1 promoter by bisulfite mutagenesis and sequencing.14 Approximately 200 ng of genomic DNA was treated with bisulfite and purified, and then used for duplex PCR. Five independent duplex PCRs were followed by gene-specific nested PCR, and the PCR products were used for sequencing. The DNA sequence was analyzed for the methylation status of the CpG dinucleotide.
Western Blot
Proteins were extracted from VSMCs by lysing cells with radioimmunoprecipitation assay (RIPA) buffer containing 1% PMSF, then subjected to electrophoresis on 10% SDS-PAGE gels. With proteins transferred onto polyvinylidene fluoride (PVDF) membranes, the membranes were blocked with 5% dry milk in tris-buffered saline (TBS) for 2 h at 37°C, followed by incubation overnight at 4°C with the following primary antibodies: anti-IκBa and anti-GAPDH. Following incubation with the appropriate secondary antibody, the blots were visualized and imaged using enhanced chemiluminescence (ECL) substrates.
RNA Pull-Down
The biotinylated KCNQ1OT1 or antisense RNA probe was synthesized and dissolved in 500 μL of wash/binding buffer. The probes were incubated with streptavidin-coated magnetic beads (Sigma) at 25°C for 2 h to generate probe-coated magnetic beads. Whole VSMC lysates were incubated with purified biotinylated KCNQ1OT1 at room temperature for 1 h, and complexes were isolated with streptavidin agarose beads (Invitrogen, USA). The complexes bound to the beads were eluted and extracted for western blot analysis.
RIP
RIP assay was conducted to verify the relationship between KCNQ1OT1 and IκBa using an RNA Binding Protein Immunoprecipitation Kit (Millipore) along with the AGO2 antibody. Briefly, cultured VSMCs were rinsed with PBS and lysed in RIP lysis buffer containing protease inhibitor and RNase inhibitor. Following this, 100 μL of cell lysis solution was taken to incubate with the RIP buffer containing magnetic bead-bound human anti-AGO2 antibody (Millipore) or the negative control (immunoglobulin G, IgG; Millipore). After incubation with proteinase K to digest protein, the immunoprecipitated RNA was isolated and subjected to quantitative real-time PCR analysis.
Ubiquitination and Cycloheximide (CHX) Experiment
To detect the influence of KCNQ1OT1 on IκBa ubiquitination, we co-transfected VSMCs with si-control or si-KCNQ1OT1 and hemagglutinin (HA)-tagged ubiquitin (HA-Ub). At 36 h following transfection, cells were harvested, and the cell pellets were lysed in RIPA buffer. Bead-bound proteins were then eluted in SDS-PAGE. Ubiquitinated products were detected by immunoblotting analysis with a Ub-specific antibody. The same membrane was stripped and re-probed with anti-IκBa polyclonal antibody. For the cycloheximide (CHX) experiment, the cells in each group were mixed with 12.5 μg/mL of CHX, an inhibitor of protein synthesis, after the transfection of si-KCNQ1OT1 or si-control. The expression of IκBa protein was determined using western blot analysis at 0, 2, 4, and 6 h.
Luciferase Reporter Gene Assay
The KCNQ1OT1 full-length sequence (WT) and KCNQ1OT1 mut (with only the putative miR-221 binding sites mutated) were synthesized (Promega, USA) and amplified by PCR. The PCR fragment was cloned into the BamHI and XhoI sites downstream of the pGL3-luciferase vector. The 293 cells were seeded into 24-well plates in triplicate, and then pGL3-KCNQ1OT1-WT or pGL3-KCNQ1OT1-mut recombinant plasmid was co-transfected with miR-221 mimic or mimic control (pre-miR negative control) into 293 cells using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions. Luciferase activities in cell lysates were determined using the dual-luciferase reporter gene system (Promega) according to the manufacturer’s protocol.
Enzyme-Linked Immunosorbent Assay (ELISA)
The levels of inflammatory factors, including IL-1β, IL-6, and TNF-α in the VSMC culture supernatants were detected using the commercially available DuoSet Enzyme ELISA kits (R&D Systems, USA) following the protocol recommended by the manufacturer.
Overexpression of KCNQ1OT1 in VG Mice
The Lv-KCNQ1OT1 or the empty vector (108 pfu/mL) was subcutaneously injected into mice with VG (n = 7 in each group). After 4 weeks, the graft vein was collected and the intimal surface area was calculated.
Statistical Analysis
All experiments were performed in triplicate, and the data were presented as mean ± standard deviation (SD). SPSS 22.0 software was used to analyze statistical difference. The Student’s t test was used for comparing the significant differences between two groups, and one-way analysis of variance (ANOVA) used for more groups. A p value < 0.05 was considered statistically significant. The experiments were done in triplicate, including technical and experimental replicates.
Author Contributions
B.Y., Z.-h.W., and T.Y.T. put forward the concept of the study, designed the study, prepared the manuscript and contributed to the statistical analysis. X.-t.Z. contributed to the concept of the study, designed the study. B.-f.Z. and X.S. analyzed the data and interpretation. Y.-h.Q. edited the manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 81770409 and 81970694), the Natural Science Foundation of Zhejiang Province of China (no. LY17H020011), and Science and Technology Project of Wenzhou Science & Technology Bureau of China (no. Y20170081).
References
- 1.He M., Wang C., Sun J.H., Liu Y., Wang H., Zhao J.S., Li Y.F., Chang H., Hou J.M., Song J.N. Roscovitine attenuates intimal hyperplasia via inhibiting NF-κB and STAT3 activation induced by TNF-α in vascular smooth muscle cells. Biochem. Pharmacol. 2017;137:51–60. doi: 10.1016/j.bcp.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 2.Subbotin V.M. Excessive intimal hyperplasia in human coronary arteries before intimal lipid depositions is the initiation of coronary atherosclerosis and constitutes a therapeutic target. Drug Discov. Today. 2016;21:1578–1595. doi: 10.1016/j.drudis.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Wang X.W., He X.J., Lee K.C., Huang C., Hu J.B., Zhou R., Xiang X.Y., Feng B., Lu Z.Q. MicroRNA-221 sponge therapy attenuates neointimal hyperplasia and improves blood flows in vein grafts. Int. J. Cardiol. 2016;208:79–86. doi: 10.1016/j.ijcard.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Lu Q.B., Wan M.Y., Wang P.Y., Zhang C.X., Xu D.Y., Liao X., Sun H.J. Chicoric acid prevents PDGF-BB-induced VSMC dedifferentiation, proliferation and migration by suppressing ROS/NFκB/mTOR/P70S6K signaling cascade. Redox Biol. 2018;14:656–668. doi: 10.1016/j.redox.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Napetschnig J., Wu H. Molecular basis of NF-κB signaling. Annu. Rev. Biophys. 2013;42:443–468. doi: 10.1146/annurev-biophys-083012-130338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell S., Vargas J., Hoffmann A. Signaling via the NFκB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016;8:227–241. doi: 10.1002/wsbm.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang D., Sun C., Zhang J., Lin S., Zhao L., Wang L., Lin R., Lv J., Xin S. Proliferation of vascular smooth muscle cells under inflammation is regulated by NF-κB p65/microRNA-17/RB pathway activation. Int. J. Mol. Med. 2018;41:43–50. doi: 10.3892/ijmm.2017.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sasu S., Beasley D. Essential roles of IkappaB kinases alpha and beta in serum- and IL-1-induced human VSMC proliferation. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H1823–H1831. doi: 10.1152/ajpheart.2000.278.6.H1823. [DOI] [PubMed] [Google Scholar]
- 9.Zuckerbraun B.S., McCloskey C.A., Mahidhara R.S., Kim P.K., Taylor B.S., Tzeng E. Overexpression of mutated IkappaBalpha inhibits vascular smooth muscle cell proliferation and intimal hyperplasia formation. J. Vasc. Surg. 2003;38:812–819. doi: 10.1016/s0741-5214(03)00427-0. [DOI] [PubMed] [Google Scholar]
- 10.Davis B.N., Hilyard A.C., Nguyen P.H., Lagna G., Hata A. Induction of microRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. J. Biol. Chem. 2009;284:3728–3738. doi: 10.1074/jbc.M808788200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y., Wu B. Knockdown of Long Non-coding RNA TUG1 Suppresses Osteoblast Apoptosis in Particle-induced Osteolysis by Up-regulating BMP-7. Clinical Surgery Research Communications. 2018;2:19–25. [Google Scholar]
- 12.Shan K., Jiang Q., Wang X.Q., Wang Y.N., Yang H., Yao M.D., Liu C., Li X.M., Yao J., Liu B. Role of long non-coding RNA-RNCR3 in atherosclerosis-related vascular dysfunction. Cell Death Dis. 2016;7:e2248. doi: 10.1038/cddis.2016.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Congrains A., Kamide K., Oguro R., Yasuda O., Miyata K., Yamamoto E., Kawai T., Kusunoki H., Yamamoto H., Takeya Y. Genetic variants at the 9p21 locus contribute to atherosclerosis through modulation of ANRIL and CDKN2A/B. Atherosclerosis. 2012;220:449–455. doi: 10.1016/j.atherosclerosis.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Coto E., Calvo D., Reguero J.R., Morís C., Rubín J.M., Díaz-Corte C., Gil-Peña H., Alosno B., Iglesias S., Gómez J. Differential methylation of lncRNA KCNQ1OT1 promoter polymorphism was associated with symptomatic cardiac long QT. Epigenomics. 2017;9:1049–1057. doi: 10.2217/epi-2017-0024. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Y., Du W., Chu Q. Downregulation of Long Non-Coding RNA Kcnq1ot1: An Important Mechanism of Arsenic Trioxide-Induced Long QT Syndrome. Cell Physiol Biochem. 2018;45:192–202. doi: 10.1159/000486357. [DOI] [PubMed] [Google Scholar]
- 16.Sun H.J., Zhao M.X., Ren X.S., Liu T.Y., Chen Q., Li Y.H., Kang Y.M., Wang J.J., Zhu G.Q. Salusin-β Promotes Vascular Smooth Muscle Cell Migration and Intimal Hyperplasia After Vascular Injury via ROS/NFκB/MMP-9 Pathway. Antioxid. Redox Signal. 2016;24:1045–1057. doi: 10.1089/ars.2015.6475. [DOI] [PubMed] [Google Scholar]
- 17.Li Y.Q., Wang J.Y., Qian Z.Q., Li Y.L., Li W.N., Gao Y., Yang D.L. Osthole inhibits intimal hyperplasia by regulating the NF-κB and TGF-β1/Smad2 signalling pathways in the rat carotid artery after balloon injury. Eur. J. Pharmacol. 2017;811:232–239. doi: 10.1016/j.ejphar.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 18.Pi Y., Zhang L.L., Li B.H., Guo L., Cao X.J., Gao C.Y., Li J.C. Inhibition of reactive oxygen species generation attenuates TLR4-mediated proinflammatory and proliferative phenotype of vascular smooth muscle cells. Lab. Invest. 2013;93:880–887. doi: 10.1038/labinvest.2013.79. [DOI] [PubMed] [Google Scholar]
- 19.Khoueiry R., Ibala-Romdhane S., Al-Khtib M., Blachère T., Lornage J., Guérin J.F., Lefèvre A. Abnormal methylation of KCNQ1OT1 and differential methylation of H19 imprinting control regions in human ICSI embryos. Zygote. 2013;21:129–138. doi: 10.1017/S0967199411000694. [DOI] [PubMed] [Google Scholar]
- 20.Mohammad F., Mondal T., Guseva N., Pandey G.K., Kanduri C. Kcnq1ot1 noncoding RNA mediates transcriptional gene silencing by interacting with Dnmt1. Development. 2010;137:2493–2499. doi: 10.1242/dev.048181. [DOI] [PubMed] [Google Scholar]
- 21.Chen B., Ma J., Li C., Wang Y. Long noncoding RNA KCNQ1OT1 promotes proliferation and epithelial-mesenchymal transition by regulation of SMAD4 expression in lens epithelial cells. Mol. Med. Rep. 2018;18:16–24. doi: 10.3892/mmr.2018.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao X., Ge J., Li W., Zhou W., Xu L. LncRNA KCNQ1OT1 promotes osteogenic differentiation to relieve osteolysis via Wnt/β-catenin activation. Cell Biosci. 2018;8:19. doi: 10.1186/s13578-018-0216-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooley B.C., Nevado J., Mellad J., Yang D., St Hilaire C., Negro A., Fang F., Chen G., San H., Walts A.D. TGF-β signaling mediates endothelial-to-mesenchymal transition (EndMT) during vein graft remodeling. Sci. Transl. Med. 2014;6:227ra34. doi: 10.1126/scitranslmed.3006927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong C.Y., de Vries M.R., Wang Y., van der Vorst J.R., Vahrmeijer A.L., van Zonneveld A.J., Roy-Chaudhury P., Rabelink T.J., Quax P.H., Rotmans J.I. Vascular remodeling and intimal hyperplasia in a novel murine model of arteriovenous fistula failure. J. Vasc. Surg. 2014;59:192–201 e191. doi: 10.1016/j.jvs.2013.02.242. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L., Jin H., Huang J., Lu H., Guan Y., Chen X., Sun H. Local delivery of pravastatin inhibits intimal formation in a mouse vein graft model. Can. J. Cardiol. 2012;28:750–757. doi: 10.1016/j.cjca.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 26.Li K., Pan J., Wang J., Liu F., Wang L. MiR-665 regulates VSMCs proliferation via targeting FGF9 and MEF2D and modulating activities of Wnt/β-catenin signaling. Am. J. Transl. Res. 2017;9:4402–4414. [PMC free article] [PubMed] [Google Scholar]