Abstract
miR-221 is overexpressed in several malignancies where it promotes tumor growth and survival by interfering with gene transcripts, including p27Kip1, PUMA, PTEN, and p57Kip2. We previously demonstrated that a novel 13-mer miR-221 inhibitor (locked nucleic acid [LNA]-i-miR-221) exerts antitumor activity against human cancer with a pilot-favorable pharmacokinetics and safety profile in mice and non-naive monkeys. In this study, we report a non-good laboratory practice (GLP)/GLP dose-finding investigation of LNA-i-miR-221 in Sprague-Dawley rats. The safety of the intravenous dose (125 mg/kg/day) for 4 consecutive days, two treatment cycles, was investigated by a first non-GLP study. The toxicokinetics profile of LNA-i-miR-221 was next explored in a GLP study at three different doses (5, 12.5, and 125 mg/kg/day). Slight changes in blood parameters and histological findings in kidney were observed at the highest dose. These effects were reversible and consistent with an in vivo antisense oligonucleotide (ASO) class effect. The no-observed-adverse-effect level (NOAEL) was established at 5 mg/kg/day. The plasma exposure of LNA-i-miR-221, based on C0 (estimated concentration at time 0 after bolus intravenous administration) and area under the curve (AUC), suggested no differential sex effect. Slight accumulation occurred between cycles 1 and 2 but was not observed after four consecutive administrations. Taken together, our findings demonstrate a safety profile of LNA-i-miR-221 in Sprague-Dawley rats and provide a reference translational framework and path for the development of other LNA miR inhibitors in phase I clinical study.
Keywords: miR-221, LNA-i-miR-221, non-coding RNAs, miRNA therapeutics, RNA therapeutics, LNA, pharmacokinetics, toxicity study, rat, antisense oligonucleotides
Introduction
RNA therapeutics is an emerging field and a provocative challenge for the next decade.1,2 Among novel strategies that may provide successful use of RNA-based therapeutics, the targeting of non-coding RNAs is creating a new valuable opportunity to be investigated in early clinical trials. MicroRNAs (miRNAs) are a class of highly conserved endogenous small non-coding RNAs (19–23 nt) that regulate gene expression by translational repression, mRNA cleavage, and mRNA decay, which translates in the modulation of several crucial cell pathways. In cancer, the role of miRNAs has been well depicted, since they may act as oncogenes, promoting tumor development by inhibiting tumor suppressor genes, or as tumor suppressors by regulating oncogenes and/or genes that control cell differentiation.3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
miR-221, a member of the miR-221/222 cluster gene, is located on the X chromosome and acts as an oncomiR in many human solid and hematologic malignancies. In these diseases, overexpression of miR-221 influences a large set of gene transcripts involved mainly in cell proliferation and apoptosis. We previously developed an original 13-mer oligonucleotide miR-221 inhibitor, named locked nucleic acid (LNA)-i-miR-221, which is a second-generation phosphorothioate (PS) antisense oligonucleotide (ASO), and takes advantages from LNA technology and PS backbone chemistry in terms of increased affinity to the target and resistance to nucleases. In the PS oligonucleotide, the sulfur atom replaces one of the non-bridging oxygen atoms in the internucleoside phosphate, increasing nuclease stability. We recently reported on the biological effects induced by LNA-i-miR-22115,16 by specific inhibition of miR-221 and consequent modulation of its canonical targets, including p27Kip1, PUMA, PTEN, and p57Kip2, regulators of the cell cycle and apoptosis.17 In vitro and in vivo studies demonstrated that LNA-i-miR-221 exerts strong antitumor activity, providing the first evidence of its efficacy against multiple myeloma (MM)15 and other tumors.18 Moreover, detectability of LNA-i-miR-221 in animals and tumor tissues as well as in plasma and urine specimens was demonstrated19 together with a favorable pilot pharmacokinetics profile and rapid wide tissue distribution in mice and non-naive monkeys.20
In the translational aim toward a first-in-human study, we investigated the suitability of LNA-i-miR-221 for clinical use by a non-GLP as well as a GLP dose-finding investigation of this new agent in Sprague-Dawley rats. Our data provide a formal framework for the definition of the optimal pharmacokinetics and safety profile of LNA-i-miR-221, which is essential to move to a phase I clinical study (EudraCT: 2017-002615-33). Our findings also provide a reference translational path for the clinical development of other LNA miR inhibitors.
Results
Rat Pilot Non-GLP Study
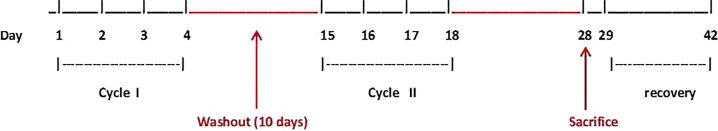
Rat toxicity studies were designed with the aim to evaluate the potential toxicity of LNA-i-miR-221. In a non-GLP study, LNA-i-miR-221 was administered at a high dose level of 125 mg/kg/day. This dose level was selected based on a previous monkey study and corresponds to the rat equivalent of maximum tolerated dose (MTD) of 8.75 mg/kg,20 where, however, no toxicity was observed. The intravenous (i.v.) route of injection was selected since it is the intended mode of injection in the first-in-human clinical study. As shown in Table 1, treatment with LNA-i-miR-221 changed the ratio of main organ weights as compared to controls, where they are noted from 8% onward. In particular, increased weight in male kidney, spleen, and liver ranged from 9% to 20% of absolute values, while decreased weight in female spleen, adrenals, and ovaries ranged from −11% to −21% of absolute values. Despite the low number of animals per group, a relationship to LNA-i-miR-221 could not be excluded in these organs. A complete macroscopic post-mortem examination performed on all principal animals (sacrificed on day 28) revealed only a tan discoloration in the kidneys from all treated males and two out of three females. This finding may correlate with the increased organ weights and has been related to LNA-i-miR-221 administration. No significant clinical signs have been related to the LNA-i-miR-221 administration. Under the experimental conditions of the study, the no-observed-adverse-effect level (NOAEL) has not been established. In addition, in this study an animal group was allocated only for pharmacokinetics (PK) investigations to evaluate the systemic exposure for sex differences and time course of the LNA-i-miR-221, following i.v. bolus administration at a dose level of 125 mg/kg/day during 4 consecutive days, for two cycles separated by a 10-day washout period. LNA-i-miR-221 was quantifiable in all plasma samples collected by blood sampling in both sexes. A low to moderate inter-animal plasma concentration variability was observed, with coefficient of variation (CV) values ranging from 4% to 40% and from 2% to 65% in males and females, respectively. LNA-i-miR-221 plasma concentration time profiles and all pharmacokinetics parameters are shown in Figure S1. The plasma exposure of LNA-i-miR-221, based on C0 and AUC values, after multiple administrations at the highest dose (125 mg/kg/day), showed in males an apparent trend vs higher exposure, due to potential LNA-i-miR-221 accumulation. Nevertheless, as no descriptive statistic was applicable in these cases, no definitive conclusion can be given regarding these trends. Accumulation was observed between days 1 and 18 in both sexes, but no clear accumulation was observed after four consecutive administrations (between days 1 and 4 or between days 15 and 18).
Table 1.
Main Organ Weights
| Males | Females | |
|---|---|---|
| Group | 2 | 2 |
| Dose level (mg/kg/day) | 125 | 125 |
| Examined animals | 3 | 3 |
| Final body weight | −2 | −7 |
| Adrenal Glands | ||
| Absolute | −22 | −13 |
| Relative | −20 | −8 |
| Kidneys | ||
| Absolute | +9 | +10 |
| Relative | +11 | +18 |
| Liver | ||
| Absolute | +20 | +20 |
| Relative | +22 | +29 |
| Ovaries | ||
| Absolute | NA | −21 |
| Relative | NA | −16 |
| Spleen | ||
| Absolute | +12 | −11 |
| Relative | +13 | −5 |
The differences in percent between treated and control animals are mentioned from 8% onward. The organs were weighed wet as soon as possible after dissection. The ratio of organ weight to body weight recorded immediately before sacrifice was calculated. There was a low number of animals per group (n = 3) for statistical analysis, and thus the significance of the organ weight changes was considered to be irrelevant. NA, not applicable.
Pivotal GLP Rat Toxicity Study
The formal GLP rat toxicity study was designed on the perspective of the proposed clinical trial of LNA-i-miR-221 in advanced cancer patients. Three groups of five principal and three satellite animals per sex (male and female Sprague-Dawley rats) received LNA-i-miR-221, during 4 consecutive days for two cycles with a 10-day washout, by i.v. bolus injection at dose levels of 5, 12.5, and 125 mg/kg/day. The rationale of two cycles is within our project to combine miR-221/222 inhibition with melphalan, which results in restoring of cell sensitivity to alkylating agents, in accordance with to our in vitro and in vivo findings in preclinical models of MM.21 Recently a renewed scientific interest on melphalan is emerging, and major efforts have been devoted to delineate the mechanisms underlying primary or acquired melphalan resistance. These efforts have already led to the design of novel regimens to overcome melphalan resistance or to improve its anti-tumor activity.22 With the aim of providing the rationale for clinical trials investigating LNA-i-miR-221 plus melphalan in drug-refractory MM, our study recapitulates the melphalan treatment schedule. We designed a protocol with at least two treatment cycles to translate in the clinical setting for a minimum chance of exploring not only safety but also induction of the clinical response. The dose levels were selected based on the results of previous studies.15,20,21 Based on the MM mouse xenograft models, we reported tumor growth inhibition at 25 mg/kg.15 This dose has been selected as a therapeutic dose and used to identify the relative equivalent species doses for our formal toxicity studies, according to the Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals S6 (R1), Parent Guideline, 1997.23 In the light of providing, with our findings, support for phase I clinical trial approval, the study was drawn with the same schedule established for the first-in-human study.
Clinical Examination Results
The appearance of treatment-related clinical signs was monitored in both sexes at all treatment doses. Clinical signs limited to hematoma on the tail, chromodacryorrhea, or alopecia as well as soft pale feces were considered due to the administration procedure, since it was observed in the control group and in both sexes unrelated to LNA-i-miR-221 treatment. At 5 and 12.5 mg/kg/day, in both sexes, there were no changes in mean body weight, when compared to control group, over the whole study period. At 125 mg/kg/day, a moderate lower mean body weight was observed in males from day 22 to day 42 (i.e., 37 g difference versus controls on day 28, reaching statistical significance, p < 0.05), whereas in females no relevant changes in mean body weight were observed. This decrease can be considered related to the LNA-i-miR-221 treatment but is of minor toxicological significance, and because it occurred in the absence of a decrease in food consumption and feeding efficiency, it did not occur in the female group, and it is not associated with signs of poor clinical condition (Supplemental Materials and Methods; Figure S2). Moreover, no changes were observed on breathing or on central nervous system (CNS) activity, and no ocular findings were noted at all doses. Detailed information about evaluations of clinical examinations are available in Supplemental Materials and Methods.
Laboratory Investigation Results
In two time points during the study, on days 19 and 43, blood sampling was taken for analytical evaluations, including hematology and biochemistry.
Hematology
No relevant changes in hematology parameters were observed at the end of the treatment (day 19) in animals exposed to doses of 5 and 12.5 mg/kg/day. In the group treated at the highest dose, statistically significant dose-related 49%–71% decreases of neutrophil and reticulocyte counts were observed in females. At the end of the recovery period (day 43), in males we observed 64%–78% decreases of neutrophils, large unstained cells (LUCs), and monocyte counts, and a slightly lower reticulocyte count was still observed in females, associated with a 64% decrease of LUCs. In a single animal a decrease in eosinophil counts occurred, which was considered as incidental. These changes were considered related to the LNA-i-miR-221, but they were of minor significance and small magnitude. No changes were seen in white and red blood cells, and no clinical conditions were associated. Moreover, no changes in coagulation parameters were observed at all doses in both sexes. Detailed data are reported in Table 2 and Supplemental Materials and Methods.
Table 2.
Blood Parameter Changes
| Day 19 |
Day 43 |
|||
|---|---|---|---|---|
| Vehicle ± SD | 125 mg/kg/day ± SD | Vehicle ± SD | 125 mg/kg/day ± SD | |
| Hematologic Parameter | ||||
| NeutroHephil | 0.95 ± 0.443* F | 0.37 ± 0.08 F | 1.81 ± 0.567 M | 0.76 ± 0.057 M |
| Reticulocytes | 2.88 ± 0.997** F | 1.46 ± 0.219 F | 2.39 ± 0.656 F | 1.39 ± 0.354 F |
| Monocytes | 0.55 ± 0.186 M | 0.14 ± 0.028 M | ||
| LUC | ||||
| Eosinophils | 0.08 ± 0.032 | 0.14 ± 0.045 | ||
| Biochemistry Parameter | ||||
| Creatinine | 28.40 ± 1.563 M | 35.58 ± 1.450 M | ||
| Urea | ||||
| Glucose | 5.76 ± 0.534 M | 7.75 ± 1.041** M | ||
| Triglyceride | 0.51 ± 0.151 M | 0.21 ± 0.037* M | 0.82 ± 0.339 M | 0.18 ± 0.071 M |
| ALAT | 39 ± 4.3 M | 59 ± 6.4* M | 47 ± 10.2 M | 34 ± 3.5 M |
Blood parameter changes were observed at the end of the treatment period (day 19, n = 5/sex/group) and at the end of the recovery period (day 43, n = 3/sex/group) as measured in the animal group treated with 125 mg/kg/day of LNA-i-miR-221 or in the control (vehicle) group. M, male; F, female. *p < 0.01, **p < 0.05.
Biochemistry
Regarding biochemistry on day 19, no relevant changes were observed in both sexes at 5 and 12.5 mg/kg/day, while at 125 mg/kg/day, increases of creatinine and urea concentrations, correlating with microscopic findings in the kidney (see below), were noted in both sexes with statistical significance. Specifically, changes in biochemistry included 1.2- to 1.6-fold higher creatinine levels in both sexes and a 1.6-fold higher urea concentration, a 1.4-fold higher glucose concentration, a 0.4-fold lower triglyceride concentration, and 1.5-fold higher alanine aminotransferase (ALAT) activity in males. Furthermore, biochemistry at the end of the recovery period (day 43) still evidenced an increase of glucose and urea concentrations in both sexes, while in males a lower triglyceride concentration and a higher creatinine concentration were detected. However, the creatinine concentration in females and the ALAT activity in males returned to normal levels (Table 2). These changes were considered LNA-i-miR-221 related but were not adverse, taking into account the slight magnitude of changes. The urinary parameters did not change either at the end of the treatment (day 19) or at the end of the recovery period (day 43), at all doses and in both animal sexes.
Pathology
Organ Weights
Measures of organ weight performed for all principal animals at the end of the second washout period (day 28) demonstrated that the LNA-i-miR-221 induced organ weight changes in liver, kidney, and spleen. Specifically, when compared with controls, minimal or slight increases in the mean absolute (+27; p < 0.01) and relative (+28; p < 0.01) weights of liver were seen in males and females at the highest dose. This increase correlated with foamy/granular Kupffer cells at microscopic examination. In addition, a minimal increase in the mean absolute and relative weights of kidney in males (+10; +11) and females (+11; +17), respectively, at the highest dose was recorded, even if not statistically significant. These increases correlated with microscopic tubular changes (basophilic granules, regeneration). Similarly, the slight increase of the absolute mean and weight of spleen in males (+23; +25) and decrease in females (−9; −4) at the highest dose correlated with the increased development of germinal centers observed microscopically. Finally, there were minimal increases in the mean absolute and relative weights of testes (+10; +12) in males treated at 125 mg/kg/day that correlated with the presence of interstitial granular macrophages observed at microscopic examination. Other organ weight changes were not considered to be related to LNA-i-miR-221, as they were small in amplitude, had no gross or microscopic correlates, and/or were not dose related in magnitude. Significant changes in mean absolute and relative organ weights in treated groups at the end of the second washout period are summarized in Table S1. Pathology examinations performed at the end of the recovery period in males treated at 125 mg/kg/day showed a minimal decrease of terminal body weight (−11% for the mean weight) as compared to controls, which could be considered related to LNA-i-miR-221. In females, a moderate increase of the absolute and relative weights of liver (+46%) and kidneys (+35%) were detected (Table S2). Only one male animal had a highest kidney weight (2.940 g) compared to controls, while in females a minimal increase in the absolute (+22%) and relative (+16%) weights of the spleen was noticed. In males, the slight increase in the mean relative organ weights for brain, epididymides, spleen, testes, and thyroids were considered to be due to reductions in body weight and not due to LNA-i-miR-221-related organ toxicity. Other differences in organ weights were minor and reflected the usual range of individual variations.
Macroscopic Examination
Macroscopic examination of organs performed at the end of the first washout period did not show macroscopic findings. At the end of the second washout period, LNA-i-miR-221 induced tan discoloration of the kidneys at 12.5 and 125 mg/kg/day doses, as well as enlargement of lymph nodes in one animal at the highest dose group. Tan color of the kidneys was observed in three out of five males and two out of four females at 12.5 mg/kg/day, and in four out of five males and in all three females at a 125 mg/kg/day dose treatment. This finding correlated microscopically, as below reported, with the presence of basophilic granules, indicative of the LNA-i-miR-221 accumulation, along with tubular regenerative and/or vacuolar lesions at the highest dose level. At the lower dose of 5 mg/kg/day, tan color of the kidneys was also noted in two out of five males and one out of four females, but in the absence of microscopic correlates, so this could be considered incidental and unrelated to the LNA-i-miR-221 treatment. Enlargement of the iliac lymph nodes was noted in one out of five males at the highest dose and correlated with microscopic examination, which showed LNA-i-miR-221-related foamy/granular macrophages. A scab was noted at the tip of the tail (i.e., injection site) and correlated with serocellular crust and deep ulcer evidenced at microscopic examination. The other macroscopic findings had no histologic correlates or correlated with common histologic findings in control rats, and were considered to be incidental. Finally, at the end of the recovery period, tan discoloration of the kidneys was found in one out of two females at the highest dose treatment. The meaning of this finding remained unclear because no microscopic examinations were performed on the recovery animals group. The few other macroscopic findings noted at the end of the recovery period are commonly recorded in the rat, and none can be considered related to the LNA-i-miR-221 treatment.
Microscopic Findings
Microscopic observation performed at the end of the second washout period showed events related to the administration of LNA-i-miR-221 in the kidneys, liver, and lymph nodes mainly, and were considered consistent with findings observed after systemic administration of single-stranded oligonucleotides.24 These effects involved degeneration/regeneration and basophilic granules in the kidneys, as well as foamy/granular macrophages in most organs and tissues. Specifically, in the kidneys at doses of 12.5 and 125 mg/kg/day, basophilic granules, vacuolization, degeneration/necrosis, and regeneration of proximal tubular cells were found. Basophilic granules were characterized by variable sizes of dark blue/gray cytoplasmic granules located in proximal tubular cells, and they were generally associated with tubular cytoplasmic vacuolization (clear vacuoles) that was most prominent in females. Tubular regeneration was characterized by tubules lined by basophilic enlarged cells, with karyomegaly (i.e., large nuclei) and prominent nucleoli, and occasional mitotic figures. Degeneration/necrosis consisted of scattered necrotic cells and/or sloughed cells/debris in tubules, with rare casts. In addition, an occasional tubular dilation was observed. Incidence and severity of selected microscopic findings in the kidneys at the end of the second washout period are summarized in Table S3. Infiltrates of foamy and/or granular macrophages (i.e., with vacuolar and/or basophilic granular cytoplasm) were noted at the highest dose in most organs and tissues of LNA-i-miR-221-treated animals. The most pronounced changes were seen in the lymph nodes and in the liver (Table S4). Basophilic granular cytoplasm was characterized by small dark gray granules similar to those seen in renal proximal tubular cells. In all lymph nodes, large vacuolated and/or granular macrophages were seen in the sinusoids. In the liver, Kupffer cells were enlarged and had vacuolated and granular cytoplasm. There were also some aggregates of foamy/granular macrophages in portal tracts or around central veins. In two of three females, this was associated with a minimal increase in mitotic figures. Minimal or slight granular macrophages were seen in many other organs/tissues, e.g., interstitium of the bone marrow, testes and epididymides, ovaries, uterus, vagina, heart, adrenals, pancreas, salivary glands, Harderian glands, and joints. Finally, the spleen showed an increased development (i.e., increased numbers and size) of germinal centers (i.e., center of secondary lymphoid follicles, Table S5) in males and females at 125 mg/kg/day dose treatment when compared with controls. The few microscopic findings noted at the injection sites were considered to be related to the injection procedure and not to LNA-i-miR-221. Of note, at 125 mg/kg/day, one of three females showed minimal diffuse hypertrophy of the thyroid follicular cells. This isolated finding was considered to be most probably incidental and unrelated to LNA-i-miR-221. Other microscopic findings noted in treated animals were considered incidental changes, as they also occurred in controls, were of low incidence, had no dose relationship in incidence or severity and/or are common background findings for the rat.
Consequently, under this experimental conditions and based on the above-described evidence, the NOAEL was established at 5 mg/kg/day, which is the safety dose that ensures no adverse event.
Pharmacokinetics
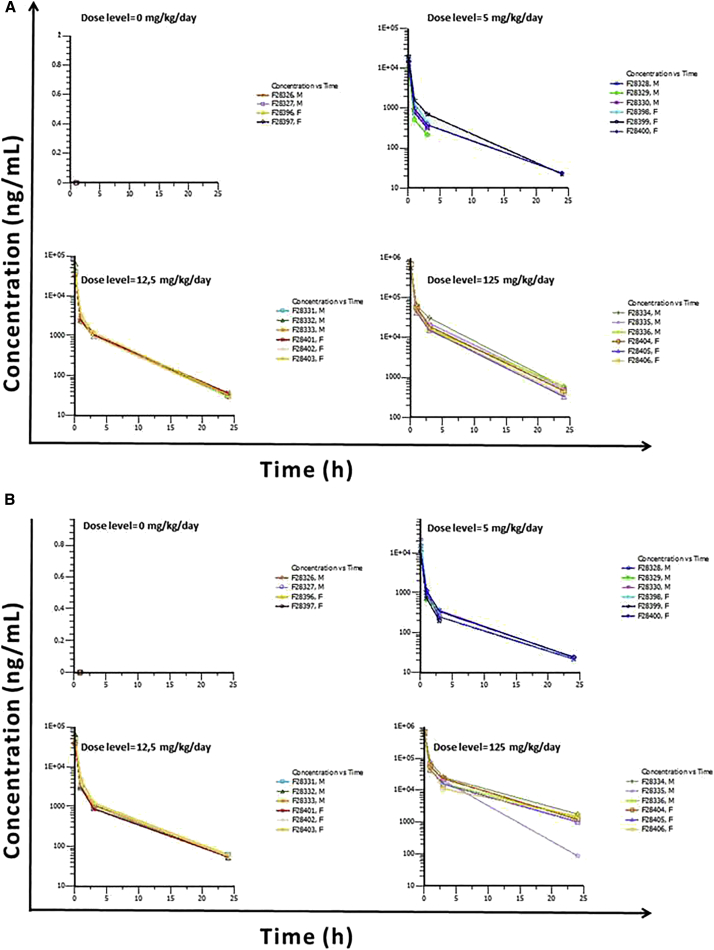
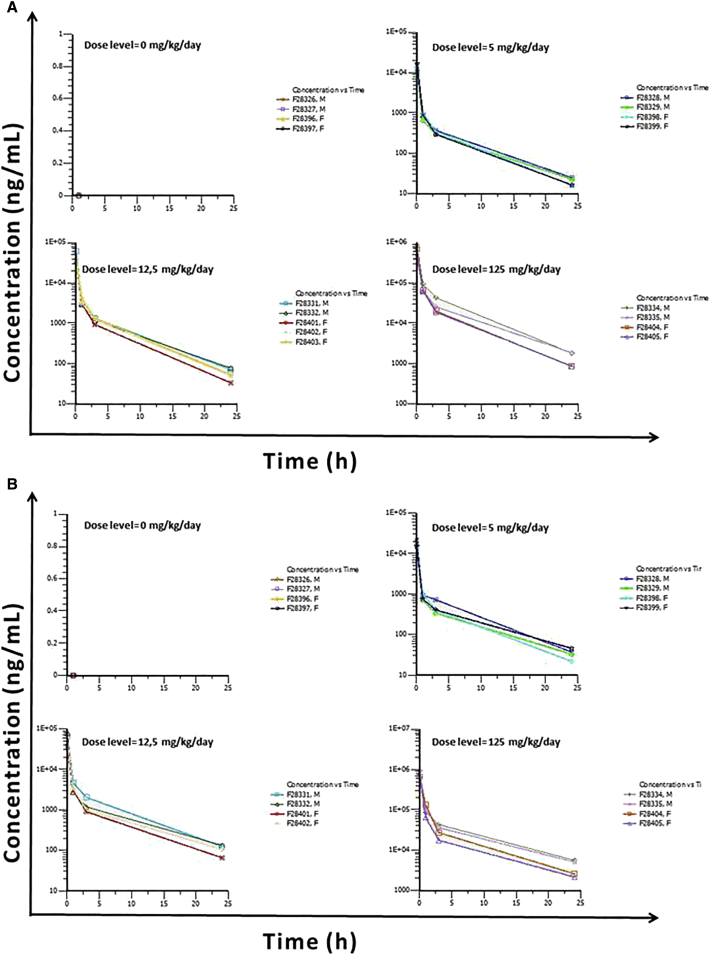
In the pivotal rat GLP study, systemic exposure was evaluated for sex difference and time course of LNA-i-miR-221 at low (5 mg/kg/day), middle (12.5 mg/kg/day), and high (125 mg/kg/day) doses following bolus injection for 4 consecutive days for two cycles with a 10-day washout (Figure 1). In addition, a recovery period ran from day 29 to day 42 to investigate the exposure of rats to LNA-i-miR-221. The dose levels were selected based on the results of previous equivalent monkey doses (2.5, 6.25, and 8.75 mg/kg) and non-GLP rat (125 mg/kg) studies. LNA-i-miR-221 was not quantifiable in the plasma from control animals, whereas in treated groups LNA-i-miR-221 was quantifiable from 0.08 to 24 h post-administration in all treated groups, except for the group treated with the lower dose, specifically three males on day 1 and two females on day 4, where LNA-i-miR-221 was quantifiable up to 3 h post-administration. After multiple administrations at the highest dose (125 mg/kg/day), based on AUC and C0, exposure increased slightly more than dose proportionally between 5 and 125 mg/kg/day in both sexes. The volume of distribution (Vd) and clearance (Cl) decreased slightly as the dose increased, especially between 12.5 and 125 mg/kg/day in both sexes (Table 3). No significant time effect pattern was observed, but a trend toward higher exposure in both sexes was observed at the highest dose level on days 15 and 18. Nevertheless, as no descriptive statistic was applicable in these cases, no definitive conclusion can be given regarding these trends. Certainly, the slight accumulation was observed in animals between cycles 1 and 2, with no clear accumulation during the four consecutive administrations (between the first day and the fourth day of administration of the first cycle or between the first day [day 15] and the fourth day [day 18] of the second cycle of administration). Specifically, on day 1 the AUC0–24h (h⋅ng/mL) in males was 177,070 and in females 190,000, while on day 18 the AUC0–24h (h⋅ng/mL) in males was 436,000 and in females 344,000. Logarithmic scale representations of LNA-i-miR-221 plasma concentration versus time profiles following single i.v. administration to male and female Sprague-Dawley rats at different days and dose are shown in Figures 2 and 3. LNA-i-miR-221 toxicokinetics (TK) parameters in plasma following i.v. (bolus) administration at a nominal dose level of 5, 12.5, and 125 mg/kg/day to male and female Sprague-Dawley rats are reported in Table 3. In the pivotal rat study, a comparison of the mean values of males and females within each dose group showed higher exposure to LNA-i-miR-221 in males, especially at higher doses. As reported in Table 3, on day 1 the increase of dose from 5 to 12.5 mg/kg (2.5-fold) established an AUC0–24h increase of ∼2.5-fold, as calculated by the mean values of combined females and males in each dose group. The increase of the dose from 5 to 125 (25-fold) and from 12.5 to 125 mg/kg (10-fold) established an increase in AUC0–24h of 39.5- and 15.9-fold, respectively. On day 18 (cycle 2), the AUC0–24h increase, from 5 to 12.5 mg/kg of dose, was ∼3.4-fold, while from 5 to 125 and 12.5 to 125 mg/kg it was 68.9- and 20.5-fold, respectively. In conclusion, the AUC0–24h increment from day 1 to 18 was similar in the different dose range evaluated: 1.4-fold from 5 to 12.5 mg/kg, 1.7-fold from 5 to 125 mg/kg, and 1.3-fold from 12.5 to 125 (10-fold) mg/kg. Similar results have been obtained by sex, as reported in Table S6, including that the male/female ratio was not significant. Based on these data, we conclude that, at the highest dose after multiple injections, in males, there is an apparent trend versus higher exposure, due to the potential LNA-i-miR-221 accumulation. Accumulation was limited to a 2-fold increase in the high-dose males between day 1 and day 18; no other accumulation was observed. Moreover, the estimated clearance of LNA-i-miR-221 decreased while the plasma half-life (generally ranged from 3.05 to 6.20 hours) increased with dose enhancement and time (day 18 versus day 1). Based on our findings, males appear to be more exposed than females to LNA-i-miR-221.
Figure 1.
Rat Treatment Timeline
LNA-i-miR-221 was administered for 4 consecutive days in two cycles: four administrations on days 1, 2, 3, and 4 for the first cycle, and four administrations on days 15, 16, 17, and 18 for the second cycle (eight administrations in total). A period of 10 days washout between the two cycles was considered. The day of sacrifice was on day 28. Day 1 corresponds to the first day of the treatment period. A recovery period ran from day 29 to day 42.
Table 3.
LNA-i-miR-221 Toxicokinetics Parameters
| Period | Sex | C0 (ng/mL) | AUC0–t (h⋅ng/mL) | AUC0–24ha (h⋅ng/mL) | Vd (mL/kg) | Cl (mL/h/kg) | t1/2 ± SD (h) |
|---|---|---|---|---|---|---|---|
| 5 mg/kg/day | |||||||
| Day 1 | F | 21,485 | 11,221 | 12,787 | 1,879 ± 1,393 | 457 ± 112 | 3.05 ± 2.14 |
| M | 18,150 | 6,325 | 9,382 | 658 ± 132 | 782 ± 166 | 0.585 ± 0.014 | |
| Day 4 | F | 18,112 | 7,856 | 9,801 | 1,612 ± 1,815 | 662 ± 220 | 1.91 ± 2.35 |
| M | 20,541 | 9,862 | 9,862 | 3,553 ± 861 | 514 ± 104 | 4.76 ± 0.217 | |
| Day 15 | F | 18,707 | 9,234 | 9,234 | 3,296 ± NA | 537 ± NA | 4.26 ± NA |
| M | 14,246 | 8,083 | 8,083 | 4,396 ± NA | 612 ± NA | 4.97 ± NA | |
| Day 18 |
F | 19,490 | 10,398 | 10,398 | 3,545 ± NA | 469 ± NA | 5.25 ± NA |
| M | 24,469 | 12,157 | 12,157 | 3,193 ± NA | 417 ± NA | 5.25 ± NA | |
| 12.5 mg/kg/day | |||||||
| Day 1 | F | 51,467 | 26,620 | 26,620 | 2,597 ± 445 | 473 ± 66.4 | 3.80 ± 0.168 |
| M | 60,092 | 27,016 | 27,016 | 2,654 ± 583 | 473 ± 93.5 | 3.187 ± 0.099 | |
| Day 4 | F | 57,188 | 31,388 | 31,388 | 2,456 ± 531 | 402 ± 70.8 | 4.22 ± 0.168 |
| M | 67,055 | 31,571 | 31,571 | 2,495 ± 362 | 395 ± 47.0 | 4.37 ± 0.206 | |
| Day 15 | F | 45,333 | 27,599 | 27,599 | 2,606 ± 316 | 454 ± 63.4 | 3.98 ± 0.142 |
| M | 65,562 | 32,812 | 32,812 | 2,539 ± NA | 379 ± NA | 4.64 ± NA | |
| Day 18 |
F | 55,025 | 29,363 | 29,363 | 2,998 ± NA | 423 ± NA | 4.94 ± NA |
| M | 95,080 | 45,242 | 45,242 | 2,053 ± NA | 271 ± NA | 5.21 ± NA | |
| 125 mg/kg/day | |||||||
| Day 1 | F | 79,9291 | 420,966 | 420,966 | 1,510 ± 169 | 298 ± 36.2 | 3.51 ± 0.035 |
| M | 63,3578 | 432,082 | 432,082 | 1,565 ± 307 | 290 ± 37.8 | 3.71 ± 0.241 | |
| Day 4 | F | 77,9878 | 450,242 | 450,242 | 1,916 ± 356 | 274 ± 29.2 | 4.82 ± 0.529 |
| M | 74,0797 | 470,791 | 470,791 | 1,613 ± 676 | 272 ± 63.0 | 4.15 ± 1.41 | |
| Day 15 | F | 69,0764 | 462,730 | 462,730 | 1,571 ± NA | 269 ± NA | 4.04 ± NA |
| M | 97,4027 | 694,482 | 694,482 | 1,210 ± NA | 181 ± NA | 4.57 ± NA | |
| Day 18 | F | 80,2382 | 620,686 | 620,686 | 1,474 ± NA | 200 ± NA | 5.07 ± NA |
| M | 1,150,506 | 900,137 | 900,137 | 1,180 ± NA | 132 ± NA | 6.20 ± NA | |
LNA-i-miR-221 toxicokinetics parameters in plasma following intravenous (bolus) administration at a nominal dose level of 5, 12.5, and 125 mg/kg/day to male and female Sprague-Dawley rats. SD was not calculated (NA [not applicable]) for groups with reduced animals (fewer than two). Vd, volume of distribution; Cl, clearance; AUC0–t, AUC from hour 0 to the time point of the last quantifiable concentration; t1/2, half time.
Assumed to be equal to AUC0–∞.
Figure 2.
LNA-i-miR-221 Plasma Concentrations and Time Profiles on Days 1 and 4
(A and B) LNA-i-miR-221 plasma concentrations (mg/mL)/time (h) profiles following a single intravenous bolus administration at 0, 5, 12.5, and 125 mg/kg/day to males and females on day 1 (A) and on day 4 (B) plotted on a semi-logarithmic scale are shown.
Figure 3.
LNA-i-miR-221 Plasma Concentrations and Time Profiles on Days 15 and 18
(A and B) LNA-i-miR-221 plasma concentrations (mg/mL)/time (h) profiles following a single intravenous bolus administration at 0, 5, 12.5, and 125 mg/kg/day to males and females on day 15 (A) and on day 18 (B) plotted on a semi-logarithmic scale are shown.
Discussion
It is well established that miR-221 promotes tumorigenesis by inhibiting tumor suppressor genes involved in cancer hallmarks, including growth, resistance to cell death, invasion, metastasis, and immune escape. The cell cycle regulator p27kip1 has been identified as the main miR-221 target in different tumors. It has been shown that in prostate carcinoma expression of p27kip1 and miR-221 is inversely correlated.25 Two target sites for miR-221/222 were identified in the 3′ UTR of p27 mRNA, which explains p27 downregulation following ectopic miR-221 expression and enhanced proliferation and G1 to S cell cycle phase transition. These results were also confirmed in neurological tumors,26,27 breast cancer,28 hepatocellular carcinoma (HCC)29, lung cancer,30 and myeloma.17 In liver, CDKN1C/p57 was also described as a direct target of miR-221, suggesting that miR-221 has oncogenic function in HCC through negative regulation of cell cycle progression inhibitors.31 Moreover, miR-221 antagonizes PTEN expression leading to activation of AKT, indicating that inhibition of miR-221 might play an important therapeutic role by PTEN upregulation in cancer cells.32 Finally, downregulation of proapoptotic PUMA/BBC3 has been associated with drug resistance,33,34 while its upregulation induced by miR-221/222 inhibition translates to sensitizing activity to temozolamide35 and melphalan.21
So far, while in vitro and in vivo findings demonstrated therapeutic activity of miRNA inhibition, no clinical attempts have been made in cancer patients to evaluate the potential activity of non-coding RNA inhibitors. Among available anti-miRNAs strategies, LNA antisense oligonucleotides may represent one of the most promising paths for their chemistry features36, 37, 38, 39, 40, 41 and have been successfully used to efficiently inhibit endogenous non-coding RNAs. Recently, systemic injection of LNA-miR-122 inhibitor (miravirsen, Santaris Pharma/Roche) has been investigated in patients carrying hepatitis C virus (HCV) infection up to a phase II clinical trial,42 demonstrating drug-like biologic properties of LNA oligonucleotides together with very low systemic toxicity, therefore suggesting their suitability for human use. So far, however, to our knowledge, no advanced formal GLP pre-clinical pharmacokinetics investigation for targeting oncogenic miRNAs or clinical attempts in cancer patients has been reported.
Using an original 13-mer LNA inhibitor, named LNA-i-miR-221, we previously demonstrated that direct miR-221 inhibition upregulates canonical miR-221 targets, including p27kip1, in vitro and in vivo, translating as significant anti-tumor activity.15,21 This new agent was also associated with a favorable PK profile in pilot studies in mice and monkeys,20 making it a promising molecule for clinical translation. Toward this aim, we presented herein a non-GLP and a GLP dose-finding investigation in Sprague-Dawley rats as a crucial step to move into a first-in-human study. The rat species was chosen as a relevant toxicology investigation species based on the sequence retention of miR-22143 and as rodent species accepted by regulatory authorities for this type of study.
Our formal rat GLP study was designed to evaluate the potential toxicity of LNA-i-miR-221 with three different doses (low, middle, high). A recovery group of animals was also included to determine the exposure during the period of treatment. In the principal animal group, according to experimental design, the observation until 10 days after the last treatment did not demonstrate any clinical signs or effects on food consumption. The slightly lower mean body weight recorded only in the male group treated with the highest dose was considered a minor side effect, since the mean food consumption and clinical conditions were not affected. In this group, slight changes in hematology parameters, including a decrease of neutrophils and reticulocytes counts, were considered related to LNA-i-miR-221 with minor toxicological significance, since they were not associated with changes in the absolute number of white or red blood cells or in clinical conditions. The increases of creatinine in both sexes and urea concentration in males were correlated with microscopic findings in kidneys and, consistent with other reports,44 were considered as class effects induced by systemic administration of single-stranded oligonucleotides. Macrophage infiltrates, following treatment with the highest dose, correlated with an increase in liver weight and enzyme concentrations. Moreover, the granular and/or foamy cytoplasm observed in macrophages was considered to be due to the accumulation of oligonucleotides as well as cellular activation and cytokine production, as already described.44 In addition, the minimal increase in mitotic figures associated with foamy/granular Kupffer cells could be related to the release of cytokines by the activated Kupffer cells, while the basophilic granules are reflective of accumulation of drug-related material and/or lysosomal degradation products. Finally, the increased development of splenic germinal centers was suggestive of lymphoid stimulation.
The PK profiles defined by both non-GLP and GLP rat studies indicate no sex differences for the plasma exposure of LNA-i-miR-221, with similar C0 and AUC values in males and females. No accumulation (defined as a ≥2-fold increase in AUC) was observed between cycle 1 and cycle 2. However, accumulation was observed between day 1 and day 18 at 125 mg/kg/day. This effect can be due to inter-individual variability and may not be clearly sex related, taking into account that other ASOs do not show sex-related effects.45 LNA-i-miR-221 decreased in rat plasma following biphasic elimination kinetic, with a rapid tissue distribution phase, and low to moderate inter-individual variability of concentrations. Exposure increases slightly more than dose proportionally between 5 and 125 mg/kg/day in both sexes.
Certainly, the PK profile was similar in all animal species tested20 and to other oligonucleotides with a PS backbone.46 The bioavailability of LNA-i-miR-221 likely relies on the systemic distribution and retention by tissues after a process of internalization and excretion favored by surface protein interactions and endocytosis similarly to the other PS ASO class of molecules.24,47 We can speculate that, similar to other ASOs, LNA-i-miR-221 binds to plasma proteins and transfers rapidly from blood to tissues, with a short distribution half-life and a low urine recovery, as previously reported.20 To predict an acceptable human plasma clearance applying appropriate multiple allometric interspecies scaling approaches,48 we developed a PK model by non-compartmental analysis methods. This allowed us to identify that measured exposure AUC in rat at the NOAEL and the predicted human exposure at human equivalent dose (HED) are comparable and consistent with safe exposure in rats and monkeys. To draw inferences about safe human plasma levels in the absence of prior human data, we then applied this approach to predict LNA-i-miR-221 clearance in humans by the use of HEDs.49 The geometric mean of the different estimates was finally used to predict the clearance of LNA-i-miR-221 and its exposure in humans, according to rat NOAEL conversion in HED calculations recommended in the US Food and Drug Administration (FDA) guidance.50
Finally, we can speculate that LNA-i-miR-221 was not associated with clinical changes or irreversible alterations in rats. Pathological findings were dose-dependent and detected at the middle dose and high dose by tissue examination (Table S3). All of these effects are chargeable to ASO class and considered reversible based on available studies.24 Our work defined the NOAEL at 5 mg/kg/day, which is relevant for establishment of the first dose in the subsequent first-in-human study, according to guidelines (EMEA/CHMP/SWP/28367/07).23 In detail, after the conversion of NOAEL to HED, on the basis of body surface area and correction factor (Km), we considered that the use of the conventional safety factor (10-fold de-escalation) would lead to a starting dose far from the presumable therapeutic dose, which does not fulfill our ethical standard. In fact, taking into consideration the therapeutic dose in mice (25 mg/kg), which is equivalent to 2.02 mg/kg in humans, a low starting dose (0.078 mg/kg = 1/10 of NOAEL/HED) would lead to the need to explore several escalating dose levels before approaching the potential therapeutic window, according to the conventional Fibonacci escalation flow. Therefore, we decided on a starting dose at 0.5 mg/kg, equivalent to 64.1% of the HED (0.78 mg/kg). Modeling of PK data strongly supports this dose selection, demonstrating that the predicted human exposure is consistent with safe exposure in rats and monkeys. In fact, the measured exposure AUC in rats at the NOAEL (females at 10,398 h⋅ng/mL and males at 12,157 h⋅ng/mL on day 18) and the predicted human exposure at HED (9,849 h⋅ng/mL) are comparable. According to the different allometric scaling approaches used, the predicted exposure at HED (0.78 mg/kg) ranged from 6,741 to 12,686 h⋅ng/mL. These data were also supported by the inclusion of a plasma protein binding correction that did not modify the prediction.49 Moreover, as previously demonstrated,20 vital organs, including liver and bone marrow, are sites of major LNA-i-miR-221 uptake. This pattern of distribution may represent an advantage for the treatment of solid tumors of a primary or secondary involvement of these sites as well as hematopoietic malignancies, such as MM. In conclusion, our formal rat study indicates the suitability of LNA-i-miR-221 for clinical use and provides a reference translational framework and path for the development of LNA-miRNA-based therapeutics in human cancers.
Materials and Methods
LNA-i-miR-221 and Vehicle
A unique GLP batch 178722 (Exiqon, Sweden)–236504 (BioSpring, Germany) of LNA-i-miR-221 was used for our study. Chemical analysis of the formulation was performed at Aptuit (Verona, Italy). The formulation batch achieved the acceptance criteria and was used for animal treatments. Prior to the first administration, the pH, density, and osmolality were determined for the LNA-i-miR-221 formulation and vehicle. More details on the materials are reported on Supplemental Materials and Methods.
Rat Pilot Non-GLP Study
A pilot non-GLP rat study was first designed for the evaluation of the potential toxicity of LNA-i-miR-221, following 4 consecutive days for two cycles i.v. administration (bolus injection) with a washout period of 10 days (total of eight injections) (Figure 1). A total of 42 rats (21 males and 21 females) were used at CiToxLAB (France). The strain of rats was Sprague-Dawley, Crl CD (SD) IGS BR (Charles River Laboratories, Italy). The LNA-i-miR-221 dose injected to each animal was adjusted according to the body weight and in a steady volume of 1.0 mL/kg. Control animals received 0.9% NaCl as vehicle, under the same conditions (Table S7). The study also included a group intended as satellite animals treated with the same dose (125 mg/kg/day) and treatment schedule of the principal group, for pharmacokinetic investigation only (see also Supplemental Materials and Methods).
Pivotal GLP Rat Toxicity Study
The pivotal study was performed according to International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guideline M3 to evaluate the toxicity of LNA-i-miR-221. A total of 72 Sprague-Dawley rats (36 males and 36 females) were used for this GLP study. The treatment schedule repeated the non-GLP study (Figure 1). In total, three groups of five principal and three satellite animals per sex received LNA-i-miR-221 at 5, 12.5, and 125 mg/kg/day (Table S8). Each animal was checked for clinical signs, body weight, food consumption, ophthalmology status, breathing, and CNS activity using the functional observation battery (FOB) and biochemical and hematological parameters.
Laboratory analyses were performed on animals at the end of the second treatment period (day 19) and at the end of the recovery period (day 43). The histological tissue preparation was performed in compliance with GLP procedures at Novaxia (France). Tissues were preserved in 10% buffered formalin, except for the eyes with optic nerves and Harderian glands, and the testes and epididymides, which were fixed in modified Davidson’s fixative. Tissue peer review was performed for at least 30% of the histological slides from the highest dose group per sex and on an adequate number of slides from identified target organs to confirm that findings recorded by the study pathologist were consistent and accurate. More details on this study are available in Supplemental Materials and Methods.
TK Evaluation
LNA-i-miR-221 quantification was performed using the rat plasma by LC-MS/MS analysis, as previously described.19,20 The TK evaluation was performed using non-compartmental analysis on Phoenix WinNonlin software, version 6.4 (Pharsight, Mountain View, CA, USA) at CiToxLAB. TK parameters were determined from the mean concentration of the matrix samples collected from different animals at each time point (sparse sampling model). A separate TK analysis was performed for each sex and sampling occasion. The standard deviation (SD) and CV were calculated to assess inter-individual variability. The absence of quantifiable levels of LNA-i-miR-221 at pre-dose (before the first administration) and in control animals was evaluated (see also Supplemental Materials and Methods).
Statistical Analysis
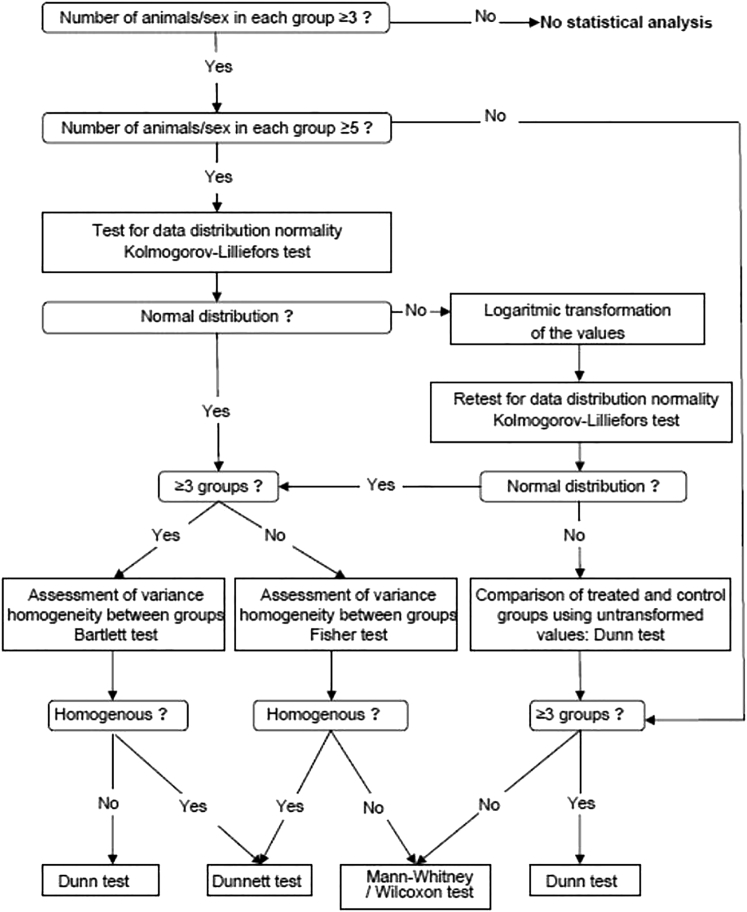
Statistical analysis of body weight, food consumption, hematology, blood biochemistry, and urinalysis data were performed at CiToxLAB according to the sequence illustrated in Figure 4. PathData software was used to perform the statistical analysis of organ weight data (level of significance, 0.05 or 0.01) according to the sequence illustrated in Figure S3.
Figure 4.
Statistical Analyses Flowchart
CiToxLAB software was used to perform the statistical analyses of body weight, food consumption, hematology, blood biochemistry, and urinalysis data; different software was applied according to the sequence depicted.
Chemical Analysis of the Dose Formulations
Analysis was performed at Aptuit to determine the concentration of the LNA-i-miR-221. For each determination, two samples of 1 mL per formulation were taken from control and LNA-i-miR-221 dose solution formulations on day 1 and kept at −80°C and protected from light. The analytical method as well as stability data were validated at Aptuit prior to dose formulation analysis. Acceptance criteria were fixed on the measured concentration equal to nominal concentration ±10%. Prior to the first administration, the pH, density, and osmolality were determined for the vehicle and the LNA-i-miR-221 formulations.
Author Contributions
M.T.D.M., P. Tagliaferri, and P. Tassone designed the experimental work and wrote the manuscript; M.T.D.M. and M.A. evaluated results; F.S. and D.C. revised results.
Acknowledgments
This work was supported by the Italian Association for Cancer Research (AIRC) (principal investigator, P.Tassone) “Special Program Molecular Clinical Oncology – 5 per Mille” no. 9980 (2010/15) and its Extension Program no. 9980 (2016/17).
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtn.2020.01.036.
Contributor Information
Maria Teresa Di Martino, Email: teresadm@unicz.it.
Pierfrancesco Tassone, Email: tassone@unicz.it.
Supplemental Information
References
- 1.Caracciolo D., Montesano M., Altomare E., Scionti F., Di Martino M.T., Tagliaferri P., Tassone P. The potential role of miRNAs in multiple myeloma therapy. Expert Rev. Hematol. 2018;11:793–803. doi: 10.1080/17474086.2018.1517041. [DOI] [PubMed] [Google Scholar]
- 2.Rupaimoole R., Slack F.J. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 3.Di Martino M.T., Campani V., Misso G., Gallo Cantafio M.E., Gullà A., Foresta U., Guzzi P.H., Castellano M., Grimaldi A., Gigantino V. In vivo activity of miR-34a mimics delivered by stable nucleic acid lipid particles (SNALPs) against multiple myeloma. PLoS ONE. 2014;9:e90005. doi: 10.1371/journal.pone.0090005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caracciolo D., Di Martino M.T., Amodio N., Morelli E., Montesano M., Botta C., Scionti F., Talarico D., Altomare E., Gallo Cantafio M.E. miR-22 suppresses DNA ligase III addiction in multiple myeloma. Leukemia. 2019;33:487–498. doi: 10.1038/s41375-018-0238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morelli E., Biamonte L., Federico C., Amodio N., Di Martino M.T., Gallo Cantafio M.E., Manzoni M., Scionti F., Samur M.K., Gullà A. Therapeutic vulnerability of multiple myeloma to MIR17PTi, a first-in-class inhibitor of pri-mir-17-92. Blood. 2018;132:1050–1063. doi: 10.1182/blood-2018-03-836601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pitari M.R., Rossi M., Amodio N., Botta C., Morelli E., Federico C., Gullà A., Caracciolo D., Di Martino M.T., Arbitrio M. Inhibition of miR-21 restores RANKL/OPG ratio in multiple myeloma-derived bone marrow stromal cells and impairs the resorbing activity of mature osteoclasts. Oncotarget. 2015;6:27343–27358. doi: 10.18632/oncotarget.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stamato M.A., Juli G., Romeo E., Ronchetti D., Arbitrio M., Caracciolo D., Neri A., Tagliaferri P., Tassone P., Amodio N. Inhibition of EZH2 triggers the tumor suppressive miR-29b network in multiple myeloma. Oncotarget. 2017;8:106527–106537. doi: 10.18632/oncotarget.22507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossi M., Amodio N., Di Martino M.T., Caracciolo D., Tagliaferri P., Tassone P. From target therapy to miRNA therapeutics of human multiple myeloma: theoretical and technological issues in the evolving scenario. Curr. Drug Targets. 2013;14:1144–1149. doi: 10.2174/13894501113149990186. [DOI] [PubMed] [Google Scholar]
- 9.Morelli E., Leone E., Cantafio M.E., Di Martino M.T., Amodio N., Biamonte L., Gullà A., Foresta U., Pitari M.R., Botta C. Selective targeting of IRF4 by synthetic microRNA-125b-5p mimics induces anti-multiple myeloma activity in vitro and in vivo. Leukemia. 2015;29:2173–2183. doi: 10.1038/leu.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amodio N., Di Martino M.T., Foresta U., Leone E., Lionetti M., Leotta M., Gullà A.M., Pitari M.R., Conforti F., Rossi M. miR-29b sensitizes multiple myeloma cells to bortezomib-induced apoptosis through the activation of a feedback loop with the transcription factor Sp1. Cell Death Dis. 2012;3:e436. doi: 10.1038/cddis.2012.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rossi M., Amodio N., Di Martino M.T., Tagliaferri P., Tassone P., Cho W.C. MicroRNA and multiple myeloma: from laboratory findings to translational therapeutic approaches. Curr. Pharm. Biotechnol. 2014;15:459–467. doi: 10.2174/1389201015666140519104743. [DOI] [PubMed] [Google Scholar]
- 12.Di Martino M.T., Guzzi P.H., Caracciolo D., Agnelli L., Neri A., Walker B.A., Morgan G.J., Cannataro M., Tassone P., Tagliaferri P. Integrated analysis of microRNAs, transcription factors and target genes expression discloses a specific molecular architecture of hyperdiploid multiple myeloma. Oncotarget. 2015;6:19132–19147. doi: 10.18632/oncotarget.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Misso G., Zappavigna S., Castellano M., De Rosa G., Di Martino M.T., Tagliaferri P., Tassone P., Caraglia M. Emerging pathways as individualized therapeutic target of multiple myeloma. Expert Opin. Biol. Ther. 2013;13(Suppl 1):S95–S109. doi: 10.1517/14712598.2013.807338. [DOI] [PubMed] [Google Scholar]
- 14.Amodio N., Di Martino M.T., Neri A., Tagliaferri P., Tassone P. Non-coding RNA: a novel opportunity for the personalized treatment of multiple myeloma. Expert Opin. Biol. Ther. 2013;13(Suppl 1):S125–S137. doi: 10.1517/14712598.2013.796356. [DOI] [PubMed] [Google Scholar]
- 15.Di Martino M.T., Gullà A., Gallo Cantafio M.E., Altomare E., Amodio N., Leone E., Morelli E., Lio S.G., Caracciolo D., Rossi M. In vitro and in vivo activity of a novel locked nucleic acid (LNA)-inhibitor-miR-221 against multiple myeloma cells. PLoS ONE. 2014;9:e89659. doi: 10.1371/journal.pone.0089659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Martino M.T., Rossi M., Caracciolo D., Gullà A., Tagliaferri P., Tassone P. miR-221/222 are promising targets for innovative anticancer therapy. Expert Opin. Ther. Targets. 2016;20:1099–1108. doi: 10.1517/14728222.2016.1164693. [DOI] [PubMed] [Google Scholar]
- 17.Di Martino M.T., Gullà A., Cantafio M.E., Lionetti M., Leone E., Amodio N., Guzzi P.H., Foresta U., Conforti F., Cannataro M. In vitro and in vivo anti-tumor activity of miR-221/222 inhibitors in multiple myeloma. Oncotarget. 2013;4:242–255. doi: 10.18632/oncotarget.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santolla M.F., Lappano R., Cirillo F., Rigiracciolo D.C., Sebastiani A., Abonante S., Tassone P., Tagliaferri P., Di Martino M.T., Maggiolini M., Vivacqua A. miR-221 stimulates breast cancer cells and cancer-associated fibroblasts (CAFs) through selective interference with the A20/c-Rel/CTGF signaling. J. Exp. Clin. Cancer Res. 2018;37:94. doi: 10.1186/s13046-018-0767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franzoni S., Vezzelli A., Turtoro A., Solazzo L., Greco A., Tassone P., Di Martino M.T., Breda M. Development and validation of a bioanalytical method for quantification of LNA-i-miR-221, a 13-mer oligonucleotide, in rat plasma using LC-MS/MS. J. Pharm. Biomed. Anal. 2018;150:300–307. doi: 10.1016/j.jpba.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 20.Gallo Cantafio M.E., Nielsen B.S., Mignogna C., Arbitrio M., Botta C., Frandsen N.M., Rolfo C., Tagliaferri P., Tassone P., Di Martino M.T. Pharmacokinetics and Pharmacodynamics of a 13-mer LNA-inhibitor-miR-221 in Mice and Non-human Primates. Mol. Ther. Nucleic Acids. 2016;5:e3361. doi: 10.1038/mtna.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gullà A., Di Martino M.T., Gallo Cantafio M.E., Morelli E., Amodio N., Botta C., Pitari M.R., Lio S.G., Britti D., Stamato M.A. A 13 mer LNA-i-miR-221 inhibitor restores drug sensitivity in melphalan-refractory multiple myeloma cells. Clin. Cancer Res. 2016;22:1222–1233. doi: 10.1158/1078-0432.CCR-15-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chauhan D., Ray A., Viktorsson K., Spira J., Paba-Prada C., Munshi N., Richardson P., Lewensohn R., Anderson K.C. In vitro and in vivo antitumor activity of a novel alkylating agent, melphalan-flufenamide, against multiple myeloma cells. Clin. Cancer Res. 2013;19:3019–3031. doi: 10.1158/1078-0432.CCR-12-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.European Medicines Agency . 2007. Guideline on strategies to identify and mitigate risks for first-in-human and early clinical trials with investigational medicinal products.https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-strategies-identify-mitigate-risks-first-human-early-clinical-trials-investigational_en.pdf EMEA/CHMP/SWP/28367/07, July 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geary R.S., Norris D., Yu R., Bennett C.F. Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv. Drug Deliv. Rev. 2015;87:46–51. doi: 10.1016/j.addr.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Galardi S., Mercatelli N., Giorda E., Massalini S., Frajese G.V., Ciafrè S.A., Farace M.G. miR-221 and miR-222 expression affects the proliferation potential of human prostate carcinoma cell lines by targeting p27Kip1. J. Biol. Chem. 2007;282:23716–23724. doi: 10.1074/jbc.M701805200. [DOI] [PubMed] [Google Scholar]
- 26.Zhang C.Z., Zhang J.X., Zhang A.L., Shi Z.D., Han L., Jia Z.F., Yang W.D., Wang G.X., Jiang T., You Y.P. miR-221 and miR-222 target PUMA to induce cell survival in glioblastoma. Mol. Cancer. 2010;9:229. doi: 10.1186/1476-4598-9-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He X.Y., Tan Z.L., Mou Q., Liu F.J., Liu S., Yu C.W., Zhu J., Lv L.Y., Zhang J., Wang S. MicroRNA-221 enhances MYCN via targeting nemo-like kinase and functions as an oncogene related to poor prognosis in neuroblastoma. Clin. Cancer Res. 2017;23:2905–2918. doi: 10.1158/1078-0432.CCR-16-1591. [DOI] [PubMed] [Google Scholar]
- 28.Miller T.E., Ghoshal K., Ramaswamy B., Roy S., Datta J., Shapiro C.L., Jacob S., Majumder S. MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27Kip1. J. Biol. Chem. 2008;283:29897–29903. doi: 10.1074/jbc.M804612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Callegari E., Elamin B.K., Giannone F., Milazzo M., Altavilla G., Fornari F., Giacomelli L., D’Abundo L., Ferracin M., Bassi C. Liver tumorigenicity promoted by microRNA-221 in a mouse transgenic model. Hepatology. 2012;56:1025–1033. doi: 10.1002/hep.25747. [DOI] [PubMed] [Google Scholar]
- 30.Garofalo M., Quintavalle C., Di Leva G., Zanca C., Romano G., Taccioli C., Liu C.G., Croce C.M., Condorelli G. MicroRNA signatures of TRAIL resistance in human non-small cell lung cancer. Oncogene. 2008;27:3845–3855. doi: 10.1038/onc.2008.6. [DOI] [PubMed] [Google Scholar]
- 31.Fornari F., Gramantieri L., Ferracin M., Veronese A., Sabbioni S., Calin G.A., Grazi G.L., Giovannini C., Croce C.M., Bolondi L., Negrini M. miR-221 controls CDKN1C/p57 and CDKN1B/p27 expression in human hepatocellular carcinoma. Oncogene. 2008;27:5651–5661. doi: 10.1038/onc.2008.178. [DOI] [PubMed] [Google Scholar]
- 32.Li W., Guo F., Wang P., Hong S., Zhang C. miR-221/222 confers radioresistance in glioblastoma cells through activating Akt independent of PTEN status. Curr. Mol. Med. 2014;14:185–195. doi: 10.2174/1566524013666131203103147. [DOI] [PubMed] [Google Scholar]
- 33.Zhang L.N., Li J.Y., Xu W. A review of the role of Puma, Noxa and Bim in the tumorigenesis, therapy and drug resistance of chronic lymphocytic leukemia. Cancer Gene Ther. 2013;20:1–7. doi: 10.1038/cgt.2012.84. [DOI] [PubMed] [Google Scholar]
- 34.Yu J., Zhang L. No PUMA, no death: implications for p53-dependent apoptosis. Cancer Cell. 2003;4:248–249. doi: 10.1016/s1535-6108(03)00249-6. [DOI] [PubMed] [Google Scholar]
- 35.Chen L., Zhang J., Han L., Zhang A., Zhang C., Zheng Y., Jiang T., Pu P., Jiang C., Kang C. Downregulation of miR-221/222 sensitizes glioma cells to temozolomide by regulating apoptosis independently of p53 status. Oncol. Rep. 2012;27:854–860. doi: 10.3892/or.2011.1535. [DOI] [PubMed] [Google Scholar]
- 36.Yu R.Z., Grundy J.S., Geary R.S. Clinical pharmacokinetics of second generation antisense oligonucleotides. Expert Opin. Drug Metab. Toxicol. 2013;9:169–182. doi: 10.1517/17425255.2013.737320. [DOI] [PubMed] [Google Scholar]
- 37.Lundin K.E., Højland T., Hansen B.R., Persson R., Bramsen J.B., Kjems J., Koch T., Wengel J., Smith C.I. Biological activity and biotechnological aspects of locked nucleic acids. Adv. Genet. 2013;82:47–107. doi: 10.1016/B978-0-12-407676-1.00002-0. [DOI] [PubMed] [Google Scholar]
- 38.Koller E., Vincent T.M., Chappell A., De S., Manoharan M., Bennett C.F. Mechanisms of single-stranded phosphorothioate modified antisense oligonucleotide accumulation in hepatocytes. Nucleic Acids Res. 2011;39:4795–4807. doi: 10.1093/nar/gkr089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fluiter K., ten Asbroek A.L., de Wissel M.B., Jakobs M.E., Wissenbach M., Olsson H., Olsen O., Oerum H., Baas F. In vivo tumor growth inhibition and biodistribution studies of locked nucleic acid (LNA) antisense oligonucleotides. Nucleic Acids Res. 2003;31:953–962. doi: 10.1093/nar/gkg185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurreck J., Wyszko E., Gillen C., Erdmann V.A. Design of antisense oligonucleotides stabilized by locked nucleic acids. Nucleic Acids Res. 2002;30:1911–1918. doi: 10.1093/nar/30.9.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elmén J., Thonberg H., Ljungberg K., Frieden M., Westergaard M., Xu Y., Wahren B., Liang Z., Ørum H., Koch T., Wahlestedt C. Locked nucleic acid (LNA) mediated improvements in siRNA stability and functionality. Nucleic Acids Res. 2005;33:439–447. doi: 10.1093/nar/gki193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janssen H.L., Reesink H.W., Lawitz E.J., Zeuzem S., Rodriguez-Torres M., Patel K., van der Meer A.J., Patick A.K., Chen A., Zhou Y. Treatment of HCV infection by targeting microRNA. N. Engl. J. Med. 2013;368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 43.Friedman R.C., Farh K.K., Burge C.B., Bartel D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frazier K.S. Antisense oligonucleotide therapies: the promise and the challenges from a toxicologic pathologist’s perspective. Toxicol. Pathol. 2015;43:78–89. doi: 10.1177/0192623314551840. [DOI] [PubMed] [Google Scholar]
- 45.Geary R.S., Baker B.F., Crooke S.T. Clinical and preclinical pharmacokinetics and pharmacodynamics of mipomersen (Kynamro®): a second-generation antisense oligonucleotide inhibitor of apolipoprotein B. Clin. Pharmacokinet. 2015;54:133–146. doi: 10.1007/s40262-014-0224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geary R.S. Antisense oligonucleotide pharmacokinetics and metabolism. Expert Opin. Drug Metab. Toxicol. 2009;5:381–391. doi: 10.1517/17425250902877680. [DOI] [PubMed] [Google Scholar]
- 47.Linnane E., Davey P., Zhang P., Puri S., Edbrooke M., Chiarparin E., Revenko A.S., Macleod A.R., Norman J.C., Ross S.J. Differential uptake, kinetics and mechanisms of intracellular trafficking of next-generation antisense oligonucleotides across human cancer cell lines. Nucleic Acids Res. 2019;47:4375–4392. doi: 10.1093/nar/gkz214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahmood I. Pharmacokinetic allometric scaling of oligonucleotides. Nucleic Acid Ther. 2011;21:315–321. doi: 10.1089/nat.2011.0299. [DOI] [PubMed] [Google Scholar]
- 49.Di Martino M.T., Arbitrio M., Fonsi M., Erratico C.A., Scionti F., Caracciolo D., Tagliaferri P., Tassone P. Allometric scaling approaches for predicting human pharmacokinetic of a locked nucleic acid oligonucleotide targeting cancer-associated miR-221. Cancers (Basel) 2019;12:E27. doi: 10.3390/cancers12010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.US Food and Drug Administration . U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) 2005. Guidance for Industry: Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers.https://www.fda.gov/media/72309/download [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.