Abstract
Paracoccidioidomycosis (PCM) is a disease caused by fungi of the genus Paracoccidioides. The disease is responsible for high rates of premature deaths and socioeconomic repercussions. The limitations of antifungal agents against PCM have motivated the search for new compounds. In our ongoing exploration of Cerrado plants as potential sources of new antifungal agents, we selected Copaifera langsdorffii oil (Copaíba resin oil) in order to explore its bioactive potential and test a formulation to increase oil stability and solubilization employing Pluronic F-127 to obtain the nanoemulsion of the oil. We aim at testing both Copaíba resin oil and its nanoemulsion against four species of the Paracoccidioides genus. We performed cytotoxicity test in Balb/C3T3 cells, hemolytic activity and interaction of Copaíba resin oil and Copaíba resin oil nanoemulsion (CopaPlu) with the antifungal agents such as amphotericin B, co-trimoxazole, and itraconazole. Moreover, the Copaíba resin oil was analyzed by mass spectrometry to identify its chemical profile. Eventually, a new methodology to prepare the nanoemulsion is presented. The Copaíba resin oil and CopaPlu nanoemulsion inhibited Paracoccidioides sp. growth efficiently, and no cytotoxicity or hemolytic effect was observed at minimum inhibitory concentration (MIC). When combined with amphotericin B, Copaíba resin oil and its nanoemulsion showed an additive effect with reduction of MIC values. The Copaíba resin oil and CopaPlu nanoemulsion is a promising antifungal agent against Paracoccidioides.
Keywords: Copaifera langsdorffii, Copaíba resin oil, Nanoemulsion, Antifungal, Paracoccidioides spp.
Introduction
Fungal infections are responsible for high rates of morbidity and mortality worldwide. It is estimated that 1.2 billion people suffer from infections caused by pathogenic fungi [1]. Paracoccidioidomycosis (PCM) is a disease caused by dimorphic fungi of the genus Paracoccidioides. PCM is considered the eighth highest cause of mortality among the systemic mycoses being responsible for mortality rate of 1.45 cases per one million inhabitants. It especially affects rural workers and males aged between 30 and 60 years [2]. Brazil is responsible for the majority of PCM cases with 3360 new cases per year [3].
The therapeutic limitations of currently available antifungal agents against PCM have motivated the search for new compounds, the development of more effective drugs and with fewer side effects [4, 5]. In addition, nanotechnology has improved the performance of hydrophobic compounds increasing stability and solubility of molecules, decreasing cytotoxicity and undesired drug interactions [6].
Plants with therapeutic potential have contributed to the treatment of several diseases. Brazil has the largest biodiversity of flora on the planet, represented by biomes such as Amazon, Cerrado, Atlantic Forest, Caatinga, Pampa, and Pantanal [7]. Several species of plants from those ecosystems produce natural oils with high chemical diversity and pharmacological potential, which makes them promising medicinal herbs [8].
An increased interest in natural oils with complex chemical composition, such as Copaíba resin oil, is due to their great potential of developing phytoproducts for the application in pharmaceutical, cosmetic, sanitary, and food industries. The oil exudate from the leguminous trees Copaifera (Fabaceae family) is sold in Brazilian popular markets as a natural medicine to treat inflammation and infections associated with skin diseases such as eczema, psoriasis, and non-specific dermatitis [9]. Despite these unique properties, natural oils are water insoluble. Thus, in vivo and in vitro approaches and industrial applications related to them are a challenge. Conversely, nanotechnology has improved the performance of hydrophobic drugs. Encapsulation of such drugs in biodegradable polymers is easily absorbed, and it enhances biological effects, lowers cytotoxicity, increases stability of molecules, and decreases drug interactions [10].
In our ongoing exploration of Cerrado plants as potential sources of new antifungal agents, we selected Copaifera langsdorffii oil (Copaíba resin oil) in order to explore its bioactive potential and test a formulation to increase oil stability and solubilization. We tested the Copaíba resin oil and its nanoemulsion against Paracoccidioides lutzii (Pb01), Paracoccidioides brasiliensis (Pb18), Paracoccidioides americana (Pb03), and Paracoccidioides restrepiensis (EPM83). Additionally, we performed cytotoxicity test in Balb/C3T3 cells, hemolytic activity and interaction of Copaíba resin oil and Copaíba resin nanoemulsion (CopaPlu) with antifungal agents used in the treatment of PCM, such as amphotericin B, co-trimoxazole (sulfamethoxazole and trimethoprim), and itraconazole. The Copaíba resin oil was analyzed by HRESIMS direct infusion to identify its chemical profile, and eventually, a new methodology to prepare the nanoemulsion is presented.
Materials and methods
Chemicals and extraction of Copaíba resin oil
The natural Copaíba resin oil was collected from the trunk of C. langsdorffii, in the Zoobotanical Park, Federal University of Acre, Rio Branco (9° 57′ 41″ S; 67° 52′ 27″ W).
The antifungal agents were purchased; amphotericin B (Sigma-Aldrich, MO, USA); co-trimoxazole—sulfamethoxazole 400 mg and trimethoprim 80 mg (Prati Donaduzzi, Paraná, BRA); and itraconazole (Tokarski Com. & Ind. Ltd., Paraná, BRA). Pluronic F-127 (triblock copolymer [PEO100PPO70PEO100]) was purchased from Sigma-Aldrich and used without further purification.
Chemical composition of Copaíba resin oil by mass spectrometry analysis
Fragmentation patterns in the mass spectra of the Copaíba resin oil were acquired using the high-resolution and high-accuracy Orbitrap equipment (Q-exactive) in positive and negative mode. The Copaíba resin oil samples were analyzed by APCI-HRMS/MS. The samples were solubilized in 1 mL of methanol and filtered by a cellulose acetate sieve (0.20 μm). We used the APCI-HRMS/MS system in order to monitor the compounds during the direct infusion experiments. The MS parameters used were as follows: spray voltage 4 kV, sheath gas flow rate 20, auxiliary gas flow rate 5, capillary temperature 320 °C, auxiliary gas heater temperature 250 °C, S-Lens RF parameter value 55, and mass range of 150–700 m/z. Data were processed using the software Xcalibur™.
Nanoemulsion preparation
The nanoemulsion sample containing Copaíba resin oil, in the final concentration of 2 mg/mL, was prepared by dissolving 120 mg of Pluronic F-127 and 10 mg of Copaíba resin oil in 5 mL of ethanol. The resultant solution was evaporated at 60 °C yielding a thin film, which was hydrated with hot (60 °C) demineralized water. The nanoemulsion spontaneously formed was maintained in an orbital shaker at room temperature for 12 h. Next, the sample was filtered using a 0.22-μm Nylon syringe filter under laminar flow conditions. The filtered nanoemulsion was freeze-dried and the powder obtained was stored at 4 °C. For the antifungal activity tests, aliquots of the powder were hydrated with sterilized phosphate buffer solution at a pH of 7.4. A sample for control containing only Pluronic micelles was prepared by dissolving 120 mg of Pluronic in 5 mL of alcohol. The solution was evaporated, and the film was hydrated with 5 mL of buffer solution yielding a Pluronic micelle dispersion in a concentration of 24 mg/mL.
Nanoemulsion characterization
Ultraviolet visible spectroscopy (UV-vis spectra) of the Copaíba resin oil diluted with alcohol and the nanoemulsion was obtained using a UV-Vis Lambda 45 spectrophotometer (PerkinElmer, Massachusetts, EUA). The spectra of nanoemulsion samples were measured before and after filtration. Aliquots were diluted with demineralized water or phosphate buffered saline (PBS) solution at a ratio of 1:100. To verify if components of the Copaíba resin oil were present in the aqueous phase, 20 μL of the oil was added to 10 mL of water. The mixture was shaken for 24 h and the aqueous phase was analyzed by mass spectrometry. The hydrodynamic diameter distribution of the Copaíba resin oil droplets into the nanoemulsion samples was measured by dynamic light scattering using a Zetasizer ZS-Nano (Malvern Instruments Ltd., Malvern, UK). Aliquots of the nanoemulsion were measured without any dilution. The morphology and size of nanoemulsion droplets were observed with a JEM 2100 transmission electron microscope (JEOL Ltd., Tokyo, Japan). For the analysis, a drop of the diluted nanoemulsion (1:10) was placed on a carbon coated copper grid and exposed to osmium tetroxide vapor for 20 min. Then, samples were dried at room temperature for 2 h before analysis.
Biological assay
Microorganism and culture conditions
P. lutzii (Pb01), P. brasiliensis (Pb18), P. americana (Pb03), and P. restrepiensis (EPM83) isolates were cultivated in liquid Fava-Netto medium (0.3% protease peptone, 1% peptone, 0.5% (w/v) meat extract, 0.5% (w/v) yeast extract, 4% glucose, 0.5% NaCl), pH 7.2 for 48 h at 37 °C under agitation at 120 rpm. The fungal cells were then transferred to the chemically defined medium Roswell Park Memorial Institute medium (RPMI 1640, Sigma-Aldrich) and maintained under incubation for 16 h.
Determination of minimum inhibitory concentration and minimum fungicidal concentration
The determination of MIC and minimum fungicidal concentration (MFC) was performed using the microdilution technique, as recommended by the Clinical and Laboratory Standards Institute (CLSI 2008) and Silva et al. 2018 [11]. The Copaíba resin oil was dissolved in 0.1% dimethylsulfoxide (DMSO, Sigma-Aldrich). Using a 96-well microdilution plate, 100 μL of the serial dilutions of the compounds was added to each well along with 100 μL of the dilution containing fungal cells (Pb01, Pb03, Pb18, and EPM83), in the final concentration of 1 × 105 cells/mL. To determine the maximum fungal growth (positive control), we added RPMI medium and 1 × 105 cells/mL. To obtain the maximum inhibition value (negative control), the antifungal itraconazole was added to specific wells. Plates were maintained at 37 °C under agitation at 150 rpm for 48 h. Then, 20 μL of the resazurin solution at 0.02% (a redox indicator) was added and incubated subsequently for 24 h. The MIC was determined visually, based on the reduction of resazurin (blue) to resorufin (pink) by NADH or NADPH reductase activity in mitochondria.
For the determination of MFC, after evaluating the minimum inhibitory concentration, 20 μL of sample from each well was transferred to petri dish with Fava-Netto medium complemented with agar. A sample of the positive control was also plated. The plates were incubated at 37 °C for 7 days until further visual assessment. The MFC was defined as the lowest concentration where fungal growth was not visualized.
Interaction assay with antifungals
Synergy evaluation of synthetic antifungals agents (itraconazole, amphotericin B or co-trimoxazole with Copaíba resin oil and CopaPlu nanoemulsion were determined using the checkerboard dilution method [12]. Initially, 50 μL of each antifungal in combination with 50 μL of compound was disposed in an orderly manner: concentrations of Copaíba resin oil and CopaPlu nanoemulsion decreased along the vertical direction and concentrations of the synthetic antifungal agents decreased along the horizontal direction. Then, 50 μL of Paracoccidioides sp. cells was added at the final concentration of 1 × 105 cells/mL. Samples were incubated at 37 °C and kept under agitation for 48 h. Resazurin solution (0.02%) was added to the samples and incubated for 24 h. Growth inhibition was determined by visual inspection. Fractional inhibitory concentration (FIC) was calculated using the formula: FICs = FICA + FICB, where FICA is the MIC of compound A combined divided by the MIC of compound A alone; FICB is MIC of compound B combined divided by MIC of compound B alone. The FICIs were interpreted as follows: synergic for FIC ≤ 0.5, additive for FIC > 0.5 to ≤ 1, indifferent effect for FIC > 1 to ≤ 4, antagonism for FIC > 4.
Cytotoxicity assay
BALB/c 3T3 clone A31 (ATCC® CCL-163 ™) cells were cultured in Dulbecco’s Modified Eagle’s medium (DMEM, Sigma-Aldrich) and supplemented with 10% fetal bovine serum (Nutricell, São Paulo, BRA). A total of 1 × 105 cells/mL were incubated with different concentrations of Copaíba resin oil and CopaPlu nanoemulsion for 48 h at 37 °C, 5% CO2. Then, 20 μL of resazurin at 0.02% was added to each microwell and incubated for 24 h. The cytotoxic concentration (CC) was determined by reading the absorbance at 640 nm and 530 nm. The following expression was used to determine the selectivity index of the compounds:
Hemolytic potential
The study was submitted and approved by the research ethics committee of the Federal University of Goiás (Protocol No. 019/13). The human blood was collected in sterile vacutainer tubes containing the anticoagulant heparin and then washed three times with PBS at pH 7.4. Then, an erythrocyte solution (2%) was distributed in the microtubes and incubated with different concentrations of Copaíba resin oil and CopaPlu nanoemulsion. The positive hemolysis control was obtained using 1% Triton X-100, and a negative control for hemolysis was used with PBS (0.09% Na2HPO4, 0.02% KH2PO4, 0.8% NaCl, 0.02% KCl, pH 7.4). The microtubes were then incubated for 30 min at room temperature and centrifuged at 5000g for 10 min. The supernatant was transferred to a microplate, and the absorbance was read by spectrophotometry at 540 nm. Two independent experiments were performed and the samples were measured in quintuplicate. The percentage of hemolysis was calculated with the following equation:
Results
Chemical analysis
The direct infusion-tandem mass spectrometry analysis showed the most abundant ions present in the Copaíba resin oil (Table 1). MS/MS fragmentation pattern allowed us to confirm the presence of ions compatible with caryophyllene, kaurenoic acid, copalic acid, and sesquiterpenes. The terpenoid composition present in the Copaíba resin oil has been already reported [9].
Table 1.
Identification of chemical component by mass spectrometry
| m/z (error ppm) [M−H]− |
Molecular formula | MS/MS main fragments | Compound (isomer) |
|---|---|---|---|
| 301.21056 (4) | C20H30O2 | Kaurenoic acid | |
| 303.23074 (4) | C20H32O2 | 219, 99 | Copalic acid |
| 315.19595 (1) | C20H28O3 | 271 | Hardiwickii acid |
| 319.31922 (6) | C20H32O3 | 219, 217, 189 | Hydroxi-copalic acid |
| 333. 20,720 (2) | C20H30O4 | 289, 273, 233 | Agathic acid |
| [M+H]+ | – | – | – |
| 203.17889 (3) | C15H22 | 147, 119,133 | Spathullen |
| 205.19463 (2) | C15H24 | 149, 121,135 | Caryophyllene |
| 221.18948 (2) | C15H24O | 223, 207, 175, 161,147 | Caryophyllene oxide /spathulenol |
| 273.25696 (3) | C20H32 | Kauren-16-ene |
CopaPlu nanoemulsion
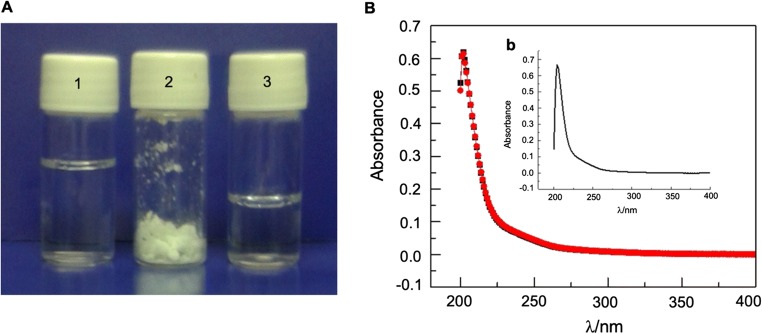
The methodology for preparing oil-in-water (O/W) nanoemulsion based on Copaíba resin oil stabilized by the triblock copolymer Pluronic F-127 was developed in our laboratory as an adaptation of the thin-film hydration method. The latter was proposed to encapsulate hydrophobic substances into block copolymer micelles [13]. The Pluronic F-127 stabilized Copaíba resin oil (O/W) nanoemulsion was named CopaPlu. Figure 1a, tube 1 presents a photography of a recently prepared CopaPlu aliquot, which is a transparent and colorless liquid. Due to its high degree of transparency, at first, we thought that the Copaíba resin oil was dissolved into the Pluronic micelles. This hypothesis was rejected after analyzing the oil droplets through electron microscopy. CopaPlu was easily sterilized by filtration with Nylon membrane 0.22 μm, without changes in the concentration of oil, as it was verified considering the UV-Vis spectra of CopaPlu obtained before and after filtration, presented in Fig. 1b. The intensity of the band at 201 nm, attributed to the aromatic components of the oil, did not change after filtration with Nylon membrane, which is an evidence that the oil droplets were not retained in the filtration process. The UV-visible spectra of alcoholic solution of Copaíba resin oil are also showed in the inserted graphic in Fig. 1b.
Fig. 1.
a Photography of CopaPlu samples. 1—CopaPlu as prepared; 2—CopaPlu freeze-dried powder; 3—CopaPlu reconstituted from freeze-dried process. b UV-visible spectra: black—CopaPlu as prepared; red—CopaPlu sterilized by filtration; inset graphic—alcoholic solution of Copaifera langsdorffii oil
We have also shown that CopaPlu can be freeze-dried to yield a white powder (Fig. 1a, tube 2) and be reconstituted maintaining a similar aspect to that presented before the freezing process (Fig. 1C, tube 3).
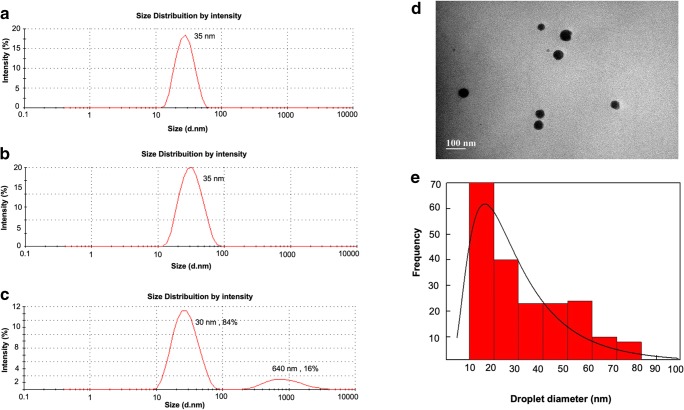
The hydrodynamic diameter of the CopaPlu droplets presented unimodal distribution with mean diameter of 34.97 nm and polydispersity index (PDI) 0.12 (Fig. 2a). After filtration (sterilization), the sample preserved the unimodal distribution and the mean size (34.75 nm and PDI 0.09) (Fig. 2b). Reconstituted CopaPlu presented a similar hydrodynamic diameter and a bimodal distribution with a mean diameter of 30.98 nm (PDI 0.30) (Fig. 2c). The sample presented a population of droplets (85%) with mean diameter of 29.98 nm and a small population of droplets (16%) with diameter in the range of 200–1200 nm (mean size 641.3 nm). Larger droplet size is attributed to coalescence during the freezing process. Figure 2d shows TEM (transmission electron microscopy) images of isolated droplets from the reconstituted droplets. The nanoemulsion droplets presented mean diameter of 25.54 nm ± 2.90 nm, which are in accordance with the mean sizes observed by DLS (dynamic light scattering) (Fig. 2e).
Fig. 2.
Hydrodynamic diameter size distribution of CopaPlu samples: a as prepared; b filtered; c reconstituted after freeze-drying. d Transmission electron microscopy images of droplets from reconstituted CopaPlu. e Droplet size distribution histogram, in which the solid line represents the best curve fitting using the log-normal distribution function
Antifungal and cytotoxic activity
The antifungal activity of Copaíba resin oil and CopaPlu was evaluated at different concentrations against Paracoccidioides spp. after 72 h of treatment. The MIC, MFC, CC, and selectivity index (SI) are showed in Table 2. Copaíba resin oil inhibited the growth of all isolates, showing similar MIC (62.5 μg/mL) and MFC (62.5 μg/mL). The CopaPlu also showed similar MIC (125 μg/mL) and MFC (125 μg/mL) for all the species under study. The cytotoxicity of Copaíba resin oil and CopaPLu was evaluated in Balb/C 3T3 cells, with CC value of 250 μg/mL and 500 μg/mL respectively. To calculate SI, the CC values were divided by MIC values. Copaíba resin oil and CopaPlu presented similar SI results (SI = 4).
Table 2.
Biological activity of Copaíba resin oil and CopaPlu nanoemulsion
| Samples | Pb isolates | MIC100 μg/mL | MFC | CC100 μg/mL | Selectivity index (SI) | PH* (%) |
|---|---|---|---|---|---|---|
| Copaíba resin oil | Pb01 | 62.5 | 62.5 | 250 | 4 | 0.24 |
| Pb03 | 62.5 | 62.5 | 4 | |||
| Pb18 | 62.5 | 62.5 | 4 | |||
| PbEPM83 | 62.5 | 62.5 | 4 | |||
| CopaPlu nanoemulsion | Pb01 | 125 | 125 | 500 | 4 | 0.25 |
| Pb03 | 125 | 125 | 4 | |||
| Pb18 | 125 | 125 | 4 | |||
| PbEPM83 | 125 | 125 | 4 |
MIC minimal inhibitory concentration, MFC minimum fungicidal concentration, CC cytotoxic concentration, SI selectivity index. Hemolytic concentration. *Percentage Hemolysis. (PH) percentage of hemolytic activity relative to minimal inhibitory concentration. Paracoccidioides lutzii (Pb01), Paracoccidioides brasiliensis (Pb18), Paracoccidioides americana (Pb03). Copaifera langsdorffii oil (Copaíba resin oil)
The hemolytic activity of Copaíba resin oil and CopaPlu was dose-dependent to the concentrations tested. The MIC value of Copaíba resin oil (62.5 μg/mL) led to 0.14% of hemolytic activity, while MIC value of CopaPlu (125 μg/mL) led to 0.25% (Table 2). No cytotoxicity or hemolytic effect was observed at MIC concentrations.
The Copaíba resin oil and amphotericin B combination resulted in an additive interaction. It decreased the MIC values from 0.75 to 0.18 μg/mL and from 62.5 to 31.25 μg/mL of amphotericin B and Copaíba resin oil, respectively (Table 3). Copaíba resin oil/co-trimoxazole and Copaíba resin oil/itraconazole combinations resulted in an indifferent interaction. Similar effects were found for CopaPlu that also presented an additive interaction only with amphotericin B.
Table 3.
Interaction of Copaíba resin oil and CopaPlu with amphotericin B, co-trimoxazole and itraconazole
| Pb01 | Pb03 | Pb18 | EPM83 | |
|---|---|---|---|---|
| Copaíba resin oil + amphotericin B | ||||
| MICCopaíba resin oil | 62.50 | 62.50 | 62.50 | 62.50 |
| MICamphotericin B | 0.75 | 0.75 | 0.75 | 0.75 |
| MICCopaíba resin oil combined | 31.25 | 31.25 | 31.25 | 31.25 |
| MICamphotericin B combined | 0.18 | 0.18 | 0.18 | 0.18 |
| FIC index | 0.74 | 0.74 | 0.74 | 0.74 |
| Interaction | Additive | Additive | Additive | Additive |
| Copaíba resin oil + co-trimoxazole | ||||
| MICCopaíba resin oil | 62.50 | 62.50 | 62.50 | 62.50 |
| MICco-trimoxazole | 2.32 | 2.32 | 2.32 | 2.32 |
| MICCopaíba resin oil combined | 31.25 | 31.25 | 31.25 | 31.25 |
| MICco-trimoxazole combined | 1.16 | 1.16 | 1.16 | 1.16 |
| FIC index | 1.00 | 1.00 | 1.00 | 1.00 |
| Interaction | Indifferent | Indifferent | Indifferent | Indifferent |
| Copaíba resin oil + itraconazole | ||||
| MICCopaíba resin oil | 62.50 | 62.50 | 62.50 | 62.50 |
| MICitraconazole | 0.001 | 0.003 | 0.003 | 0.003 |
| MICCopaíba resin oil combined | 31.25 | 31.25 | 31.25 | 31.25 |
| MICitraconazole combined | 0.0005 | 0.001 | 0.001 | 0.001 |
| FIC index | 1.50 | 1.50 | 1.50 | 1.50 |
| Interaction | Indifferent | Indifferent | Indifferent | Indifferent |
| CopaPlu + amphotericin B | ||||
| MICCopaPLu | 125 | 125 | 125 | 125 |
| MICamphotericin B | 0.75 | 0.75 | 0.75 | 0.75 |
| MICCopaPLu combined | 62.50 | 62.50 | 62.50 | 62.50 |
| MICamphotericin B combined | 0.18 | 0.18 | 0.18 | 0.18 |
| FIC index | 0.74 | 0.74 | 0.74 | 0.74 |
| Interaction | Additive | Additive | Additive | Additive |
| CopaPlu + co-trimoxazole | ||||
| MICCopaPlu | 125 | 125 | 125 | 125 |
| MICco-trimoxazole | 2.32 | 2.32 | 2.32 | 2.32 |
| MICCopaPlu combined | 125 | 125 | 125 | 125 |
| MICco-trimoxazole combined | 0.07 | 0.07 | 0.07 | 0.07 |
| FIC index | 1.03 | 1.03 | 1.03 | 1.03 |
| Interaction | Indifferent | Indifferent | Indifferent | Indifferent |
| CopaPlu + itraconazole | ||||
| MICCopaPlu | 125 | 125 | 125 | 125 |
| MICitraconazole | 0.001 | 0.003 | 0.003 | 0.003 |
| MICCopaPlu combined | 125 | 125 | 125 | 125 |
| MICitraconazole combined | 0.005 | 0.001 | 0.001 | 0.001 |
| FIC index | 1.05 | 1.05 | 1.05 | 1.05 |
| Interaction | Indifferent | Indifferent | Indifferent | Indifferent |
The inhibitory concentrations are shown in μg/mL. Paracoccidioides lutzii (Pb01), Paracoccidioides brasiliensis (Pb18), Paracoccidioides americana (Pb03). Copaifera langsdorffii oil (Copaíba resin oil)
Discussion
Several problems related to the current antifungal have been evidenced, mainly related to long time of treatment and resistant strains [3, 14]. The search for new anti-microbial compounds, especially those derived from plants, has increased over the years due to their availability and popular usage and often ensures less toxicity to host cells [15].
The therapeutic effects of Copaifera spp. have been reported, and the most common are anti-inflammatory [16], antineoplastic [17], antimicrobial [18], and antifungal [19]. The oils from C. martii, C. reticulata, and C. officinalis showed activity in Trichophyton rubrum with MIC values ranging from 250 to 500 μg/mL. The C. multijuga oil showed fungistatic activity against Aspergillus flavus and Candida parapsilosis with MIC values of 80 μg/mL and 100 μg/mL, respectively [20]. C. langsdorffii showed fungicidal activity against Trichophyton mentagrophyte (MIC and MFC = 170 μg/mL) leading to altered membrane structure and cell death due to changes in membrane permeability, similarly to antifungal agents belonging to the class of polyenes [19].
Despite several reports of antifungal activity of Copaíba resin oil, its activity on Paracoccidioides spp. is not known. In addition, no interaction study of this oil with commercial antifungal was performed. Thus, the investigations of its bioactive potential, safety profile, and interaction with other antifungal agents were performed here. Copaíba resin oil showed efficient antifungal activity (MIC and MFC = 62.5 μg/mL) compared with other natural compounds against Paracoccidioides spp., such as oenotein B, isolated from the Cerrado plant Eugenia uniflora, and that presented a MIC value of 500 μg/mL against P. lutzii [4]. In addition, Copaíba resin oil showed a fungicidal effect on Paracoccidioides sp. cells. Among commercial drugs used against Paracoccidioides spp., amphotericin B has a fungicidal effect, but its use is limited due to its low selectivity that culminates in potentially fatal side effects [21].
The emulsification of natural oils has been known to potentiate its effects against resistant microorganisms that infect the population. Svetlichny et al. 2015 [22] demonstrated that the oil of C. multijuga in emulsion potentiated the action against the pathogenic fungi C. krusei, C. parapsilosis, and T. rubrum. In addition, Alencar et al. 2015 [23] demonstrated the antimicrobial activity of Copaíba resin oil of C. langsdorffii in emulsions against azole-resistant Staphylococcus spp. and Candida spp.
Pluronic® F-127 is an amphiphilic triblock copolymer formed by a hydrophilic (polyethylene oxide) and a hydrophobic (polypropylene oxide) segment that show a significant potential for drug delivery. Interestingly, Pluronic® F-127 self-assemble into nanosize micelles within aqueous solutions, increasing the stability and solubility of a hydrophobic drug [24]. The efficiency of Pluronic-F127 to allow the spontaneous emulsification of Copaíba resin oil in aqueous phase, yielding an emulsion with droplets size in the nanometer range, and presenting high colloidal stability, as presented in this work, is understood considering the previous dissolution of both components, the oil and the Pluronic F-127, in alcohol. The complete solubilization of the components allows the interaction of the hydrophobic blocs of Pluronic (PPO, poly(propylene oxide)) with the oil components at molecular level. After the alcohol evaporation, the hydrophobic chains of Pluronic remain attached to the oil phase [25]. The high affinity of the hydrophilic blocs of Pluronic (PEO, poly(ethylene oxide)) with the aqueous phase insures the oil droplets’ dispersion into the aqueous phase, due to the solvation of the PEO chains [26]. It is worth to mention that no components of the oil were detected by mass spectrometry analysis of an aliquot of water that was shaken in contact with a portion of oil, for 24 h. This finding allows us to state that into the nanoemulsion, all oil components are into the droplets involved by the copolymer.
CopaPlu also presented antifungal activity (MIC and MFC = 125 μg/mL), but it is lower than that presented by pure oil. The activity presented by the pure oil could be justified considering the direct interaction of the oil with the hydrophobic structures of the fungi membrane, similarly to what have been proposed in the literature for the antibacterial activity of essential oils [27]. When the oil droplets are involved by the amphiphilic copolymer, as in the nanoemulsion, the hydrophilic block is the first to interact with the fungi membrane when the droplets reach it, which could hinder the oil access to the membrane, yielding a higher MIC as was observed.
During the drug development flow, the rejection of a compound in the final stages leads to a major financial loss. In addition, it is necessary to propose molecules with fewer side effects. Thus, regarding drug safety, it is important to perform a strict quality control in the early stages of drug development. The results showed that the selectivity index of Copaíba resin oils and CopaPlu was satisfactory (SI = 4). In addition, the Copaíba resin oil and CopaPlu at the concentration of 62.5 μg/mL and 125 μg/mL, respectively, did not cause hemolysis (Table 2). Following parameters presented in the literature [28], values PH < 10% may not be dangerous while PH values > 25% could lead to hemolysis. The hemolytic effect induces anemia through osmotic lysis caused by increased permeability of the plasma membrane, oxidation of hemoglobin, and loss of lipids of the erythrocyte plasma membrane [29]. The hemolysis and cytotoxicity tests showed that Copaíba resin oils and CopaPlu have low toxicity to the mammalian cells, even though they should not be used indiscriminately.
One of the major goals of combining antifungal agents is to achieve a synergistic effect, to reduce the amount of antifungal administrated to patients and to reduce toxicity. However, antagonistic effects and reduction of the efficacy of both antifungals have been reported [30]. In this scenario, techniques that evaluate combinatorial bioactive compounds, as checkerboard, have been employed. Checkerboard assays are based on the distribution of compounds on a microdilution plate and allow to test combinations of different concentrations of two drugs in a single test. Thus, this method was used to elucidate the type of interaction that Copaíba resin oil and CopaPlu would have with the commercial antifungal agents used in the treatment of PCM.
Both Copaíba resin oil and CopaPlu showed a high additive effect in combination with the antifungal amphotericin B, reducing the MIC values of amphotericin B in the same extension, from 0.75 to 0.18 μg/mL (Table 3). In addition, MIC values of Copaíba resin oil and CopaPlu also decreased in about 50%. These findings make Copaíba resin oil and CopaPlu even more promising as antifungal, since reducing the concentration of amphotericin B would lead to fewer toxic effects. The other antifungals did not have any additive effects when combined with the Copaíba resin oil and CopaPlu. Additive effects have been reported for natural oils and antifungals, such as thyme oil and amphotericin B against C. albicans and A. niger; cinnamon oil also acted synergistically with amphotericin B against C. albicans [31].
Thus, the Copaíba resin oil and CopaPlu nanoemulsion have been shown to be promising as antifungal against Paracoccidioides spp., since they inhibited Paracoccidioides sp. growth efficiently. When combined with amphotericin B, Copaíba resin oil and CopaPlu showed a similar and additive effect with reduction of their MIC values. In addition, it is worth to mention that both the oil and the CopaPlu have decreased the MIC of amphotericin B of fourfold. This suggests that Copaíba resin oil and CopaPlu may be employed together with amphotericin B in the treatment of PCM.
The formulation of Copaíba resin oil as nanoemulsion represents an advantage compared with the pure oil, because it can be administrated for different routes as inhalation and intravenous. Besides, the possibility of stocking CopaPlu as a powder allowing the reconstitution of the nanoemulsion before use, preserving its original characteristic, is an attractive aspect for future applications.
Author contribution
Each author has contributed significantly to this work. FSE and AFR performed the extraction and identification of Copaíba resin oil. LCS and MACM performed biological analysis and share the first authorship. JVF, SFAF, and ECOL performed the preparation and analysis of the nanoemulsion Copaíba resin. LK, APT, and CMAO performed the characterization of the components of the Copaíba resin oil, and LCS, MACM, CMAS, MP, CMAO, and LK analyzed the data and wrote the paper.
Funding information
This work was performed at Universidade Federal de Goiás supported by MCTI/CNPq (Ministério da Ciência e Tecnologia/Conselho Nacional de Desenvolvimento Científico e Tecnológico), FNDCT (Fundo Nacional de Desenvolvimento Científico e Tecnológico), FAPEG (Fundação de Amparo à Pesquisa do Estado de Goiás), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior/ Finance Code 001), FINEP (Financiadora de Estudos e Projetos), PRONEX (Programa de Apoio a Núcleos de Excelência) and INCT-IF (Instituto Nacional de Ciência e Tecnologia para Inovação Farmacêutica). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Denning DW, Bromley MJ. How to bolster the antifungal pipeline. Science. 2015;347:1414–1416. doi: 10.1126/science.aaa6097. [DOI] [PubMed] [Google Scholar]
- 2.Shikanai-Yasuda MA, Mendes RP, Colombo AL, Queiroz-Telles F de, Kono ASG, Paniago AMM, Nathan A, do Valle ACF , Bagagli E, Benard G, Ferreira MS, de Teixeira MM, Silva-Vergara ML, Pereira RM, Cavalcante R de S, Hahn R, Durlacher RR, Khoury Z, Camargo ZP de, Moretti ML, Martinez R. (2017). Brazilian guidelines for the clinical management of paracoccidioidomycosis. Rev Soc Bras Med Trop 50: 715–740. 10.5123/s1679-49742018000500001 [DOI] [PubMed]
- 3.Martinez R. Epidemiology of Paracoccidioidomycosis. Rev Inst Med Trop São Paulo. 2015;57:11–20. doi: 10.1590/S0036-46652015000700004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zambuzzi-Carvalho P, Tomazett P, Santos S, Ferri P, Borges C, Martins W, de Almeida Soares C, Pereira M. Transcriptional profile of Paracoccidioides induced by oenothein B, a potential antifungal agent from the Brazilian Cerrado plant Eugenia uniflora. BMC Microbiol. 2013;13:227. doi: 10.1186/1471-2180-13-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Do Carmo LS, Tamayo Ossa DP, da Castro SVC, Bringel Pires L, de Alves Oliveira CM, da Conceição Silva C, Coelho NP, Bailão AM, Parente-Rocha JA, de Soares CMA, Ruiz OH, Ochoa JGM, Pereira M. Transcriptome Profile of the Response of Paracoccidioides spp. to a Camphene Thiosemicarbazide Derivative. PLoS One. 2015;10:e0130703. doi: 10.1371/journal.pone.0130703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amaral AC, Bocca AL, Ribeiro AM, Nunes J, Peixoto DLG, Simioni AR, Primo FL, Lacava ZGM, Bentes R, Titze-de-Almeida R, Tedesco AC, Morais PC, Felipe MSS. Amphotericin B in poly(lactic-co-glycolic acid) (PLGA) and dimercaptosuccinic acid (DMSA) nanoparticles against paracoccidioidomycosis. J Antimicrob Chemother. 2009;63:526–533. doi: 10.1093/jac/dkn539. [DOI] [PubMed] [Google Scholar]
- 7.Overbeck G, Muller S, Fidelis A, Pfadenhauer J, Pillar V, Blanco C, Boldrini I, Both R, Forneck E. Brazil’s neglected biome: the South Brazilian Campos. Persp Plant Ecol Evol System. 2007;9:101116. doi: 10.1016/j.ppees.2007.07.005. [DOI] [Google Scholar]
- 8.Dutra RC, Campos MM, Santos ARS, Calixto JB. Medicinal plants in Brazil: pharmacological studies, drug discovery, challenges and perspectives. Pharm Res. 2016;112:4–29. doi: 10.1016/j.phrs.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 9.da Trindade R, da Silva J, Setzer W. Copaifera of the Neotropics: a review of the phytochemistry and pharmacology. Int J Mol Sci. 2018;19:1511. doi: 10.3390/ijms19051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jahangirian H, Ghasemian Lemraski E, Webster TJ, Rafiee-Moghaddam R, Abdollahi Y. A review of drug delivery systems based on nanotechnology and green chemistry: green nanomedicine. Int J Nanomedicine. 2017;12:2957–2978. doi: 10.2147/IJN.S127683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva LC, Neves BJ, Gomes MN, Melo-Filho CC, Soares CM, Andrade CH, Pereira M. Computer-aided identification of novel anti-paracoccidioidomycosis compounds. Future Microbiol. 2018;13:1523–1535. doi: 10.2217/fmb-2018-0175. [DOI] [PubMed] [Google Scholar]
- 12.Johnson MD, MacDougall C, Ostrosky-Zeichner L, Perfect JR, Rex JH. Combination antifungal therapy. Antimicrob Agents Chemother. 2004;48:693–715. doi: 10.1128/AAC.48.3.693-715.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu H, Zhong Q, Zhong R, Huang H, Xia Z, Ke Z, Zhang Z, Song J, Jia X. Preparation and antitumor evaluation of self-assembling oleanolic acid-loaded Pluronic P105/D-α-tocopheryl polyethylene glycol succinate mixed micelles for non-small-cell lung cancer treatment. Int J Nanomedicine. 2016;11:6337–6352. doi: 10.2147/IJN.S119839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hahn RC, Morato Conceição YT, Santos NL, Ferreira JF, Hamdan JS. Disseminated paracoccidioidomycosis: correlation between clinical and in vitro resistance to ketoconazole and trimethoprim sulphamethoxazole. Mycoses. 2003;46:342–347. doi: 10.1046/j.1439-0507.2003.00901.x. [DOI] [PubMed] [Google Scholar]
- 15.Gupta D, Jain D. Chalcone derivatives as potential antifungal agents: synthesis, and antifungal activity. J Adv Pharm Tech Res. 2015;6:114. doi: 10.4103/2231-4040.161507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veiga Junior VF, Rosas EC, Carvalho MV, Henriques MGMO, Pinto AC. Chemical composition and anti-inflammatory activity of copaiba oils from Copaifera cearensis Huber ex Ducke, Copaifera reticulata Ducke and Copaifera multijuga Hayne--a comparative study. J Ethnopharmacol. 2007;112:248–254. doi: 10.1016/j.jep.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 17.de Gomes NM, de Rezende CM, Fontes SP, Hovell AMC, Landgraf RG, Matheus ME, da Pinto AC, Fernandes PD. Antineoplastic activity of Copaifera multijuga oil and fractions against ascitic and solid Ehrlich tumor. J Ethnopharmacol. 2008;119:179–184. doi: 10.1016/j.JEP.2008.06.033. [DOI] [PubMed] [Google Scholar]
- 18.Abrão F, de Araújo Costa LD, Alves JM, Senedese JM, de Castro PT, Ambrósio SR, Veneziani RCS, Bastos JK, Tavares DC, Martins CHG. Copaifera langsdorffii oleoresin and its isolated compounds: antibacterial effect and antiproliferative activity in cancer cell lines. BMC Compl Alternative Med. 2015;15:443. doi: 10.1186/s12906-015-0961-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zimmermam-Franco D, Bolutari E, Polonini H, do Carmo A, Graças AM, das Chaves M, Raposo N. Antifungal activity of Copaifera langsdorffii Desf oleoresin against dermatophytes. Molecules. 2013;18:12561–12570. doi: 10.3390/molecules181012561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deus RJ, Alves C, Arruda MS. Avaliação do efeito antifúngico do óleo resina e do óleo essencial de copaíba (Copaifera multijuga Hayne) Rev Bras Plant Med. 2011;13:01–07. doi: 10.1590/S1516-05722011000100001. [DOI] [Google Scholar]
- 21.Harmsen S, McLaren AC, Pauken C, McLemore R. Amphotericin B is cytotoxic at locally delivered concentrations. Clin Orth Related Res. 2011;469:3016–3021. doi: 10.1007/s11999-011-1890-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svetlichny G, Külkamp-Guerreiro IC, Cunha SL, Silva FEK, Bueno K, Pohlmann AR, Fuentefria AM, Guterres SS. Solid lipid nanoparticles containing copaiba oil and allantoin: development and role of nanoencapsulation on the antifungal activity. Pharmazie. 2015;70:155–164. doi: 10.1691/ph.2015.4116. [DOI] [PubMed] [Google Scholar]
- 23.Alencar ÉN, Xavier-Júnior FH, Morais ARV, Dantas TRF, Dantas-Santos N, Verissimo LM, Rehder VLG, Chaves GM, Oliveira AG, Egito EST. Chemical characterization and antimicrobial activity evaluation of natural oil nanostructured emulsions. J Nanoscience Nanotech. 2015;15:880–888. doi: 10.1166/jnn.2015.9187. [DOI] [PubMed] [Google Scholar]
- 24.Khattak SF, Bhatia SR, Roberts SC. Pluronic F127 as a cell encapsulation material: utilization of membrane-stabilizing agents. Tissue Eng. 2005;11:974–983. doi: 10.1089/ten.2005.11.974. [DOI] [PubMed] [Google Scholar]
- 25.Ai X, Zhong L, Niu H, He Z. Thin-film hydration preparation method and stability test of DOX-loaded disulfide-linked polyethylene glycol 5000-lysine-di-tocopherol succinate nanomicelles. Asian J Pharm Sciences. 2014;9:244–250. doi: 10.1016/j.ajps.2014.06.006. [DOI] [Google Scholar]
- 26.Shar JA, Obey TM, Cosgrove T. Adsorption studies of polyethers Part 1. Adsorption onto hydrophobic surfaces. Colloids Surf A: Phys Engin Aspects. 1998;136:2133. doi: 10.1016/S0927-7757(97)00182-9. [DOI] [Google Scholar]
- 27.Burt S. Essential oils: their antibacterial properties and potential applications in foods − a review. Int J Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 28.Amin K, Dannenfelser RM. In vitro hemolysis: guidance for the pharmaceutical scientist. J Pharm Sci. 2006;95:1173–1176. doi: 10.1002/jps.20627. [DOI] [PubMed] [Google Scholar]
- 29.Aparicio RM, José García-Celma M, Pilar Vinardell M, Mitjans M. In vitro studies of the hemolytic activity of microemulsions in human erythrocytes. J Pharm Biomed Anal. 2005;39:1063–1067. doi: 10.1016/j.jpba.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 30.Cuenca-Estrella M. Combinations of antifungal agents in therapy-what value are they? J Antimicrob Chemother. 2004;54:854869. doi: 10.1093/jac/dkh434. [DOI] [PubMed] [Google Scholar]
- 31.Sherweit E-A, Mohamed E-S, Rola M. The synergetic efficacy of the combination of amphotericin B and certain essential oils against selected fungal clinical isolates. J Applied Pharm Science. 2013;3:26–30. doi: 10.7324/JAPS.2013.3404. [DOI] [Google Scholar]