Abstract
Antimicrobial peptides are considered to be one of the candidate antimicrobial agents for antibiotic-resistant bacterial infection in the future. The effects of antimicrobial peptide hBD3-CBD on Pseudomonas aeruginosa PA14 and PA14 ΔexsA were analyzed by the bactericidal effects, hemolysis assays, pyocyanin pigment productions, and virulence factor expressions (exoU, exoS, hcnA, and lasB). Pyocyanin production and virulence factor expressions are important features of the type III secretion system in Pseudomonas aeruginosa. HBD3-CBD killed PA14 and PA14 ΔexsA with similar efficiency; it lowered the hemolysis levels of PA14 and PA14 ΔexsA and reduced the pyocyanin production, biofilm formation, and exoU, exoS, and lasB expressions in PA14. Compared with PA14, PA14 ΔexsA showed a lower hemolysis effect, pyocyanin production, exoU, and lasB expressions. The effects of hBD3-CBD on the PA14 toxin secretion were similar to the changes in the type III secretion system mutant isolate PA14 ΔexsA. Our results demonstrated that the type III secretion system was involved in the biological functions on PA 14 from hBD3-CBD.
Keywords: Antimicrobial peptide, hBD3, Pseudomonas aeruginosa, T3SS
Antimicrobial peptides (AMPs) are polypeptides with broad-spectrum antimicrobial activity against bacteria, fungi, and other microorganisms [1]. Their potent activity at low concentrations against many pathogenic organisms, including multi-resistant strains, makes AMPs attractive candidates as antimicrobial agents to many inflammatory and infectious diseases [2]. Human beta-defensin-3 (hBD3) is a multifunctional peptide and widely expressed in oral and other tissues. The anti-infective effects of this polypeptide have been confirmed by several studies [3–5].
One of the bactericidal mechanisms of AMPs is their carbohydrate-binding ability through the carbohydrate-binding domains (CBDs) (or carbohydrate-binding proteins) in many AMPs. In our previous studies, we have confirmed better bactericidal levels in hBD3 combined with a CBD than hBD3 itself [6]. Better antimicrobial stability and affinity of AMPs combined with CBD or other domains may be focused on in future studies about not only infectious diseases and immune diseases but also tumor treatment [7].
Pseudomonas aeruginosa is one of the most dangerous opportunistic bacterial pathogens. The virulence and drug resistance of P. aeruginosa is a severe challenge in today’s clinical practice. At the same time, P. aeruginosa can also form a biofilm, which makes it more difficult to find an effective treatment [6]. Several studies have confirmed that AMPs have killing effects on P. aeruginosa and its biofilm, and may assist in the treatment of antibiotics [8, 9]. We have reported the bactericidal effects against P. aeruginosa PA14 (PA14) of hBD3-CBD with in vitro bactericidal test, individual gene expressions, biofilm formation assays, swimming, twitching, and swarming activities [7].
The type 3 secretion system (T3SS) is a macromolecular protein nano-syringe used by different bacterial pathogens to inject effectors into host cells [10]. There is a T3SS in P. aeruginosa, shared with many other Gram-negative bacteria, which transfer effector toxins directly from the bacterium into the host cell cytosol [11]. P. aeruginosa can repress AMP expression in both epithelial cells and macrophages by its T3SS in vitro [12]. ExsA is an essential transcriptional activator of T3SS in P. aeruginosa. In the current study, an exsA gene deleted strain P. aeruginosa PA14 ΔexsA (PA14 ΔexsA), and wild strain PA14 were used to evaluate the effects of peptide hBD3-CBD on T3SS in P. aeruginosa.
Materials and methods
The peptide, bacterial strains, and kits
Peptide hBD3-CBD (China Peptides Corporation Shanghai China) was produced and used in this study. The peptide sequence is GIINTLQKYYCRVRGGRCAVLSCLPKEEQIGKCSTRGRKCCRRKKGGGQHDGNFVVY (57 amino acid peptides composed of 45 amino acids of hBD3, nine amino acids of CBD, and a GGG linker). P. aeruginosa PA14 and PA14 ΔexsA were generous gifts from the University of Science and Technology of China. Bacterial RNA extraction, reverse transcription, and real-time quantitative polymerase chain reaction (Q-PCR) kits (Tiagen Biological Company, Beijing, China) and 96-well plates (BD Company, NJ, USA) were used in this study. Q-PCR reactions were operated on the ABI 7500 Fast machine (Applied Biosystems Inc., NY, USA).
Peptide hBD3-CBD killing curves
Peptide hBD3-CBD killing curves were generated as described in the previous study [7]. In this direct bactericidal test, hBD3-CBD was in addition to 3 mL phosphate buffer saline (PBS) of PA14 and PA14 ΔexsA (105 CFU/mL) with different final concentrations of 0, 4, 8, 16, 32, and 64 μg/mL. All tubes were incubated at 37 °C for 3 h. The bactericidal activities were analyzed by plating serial dilutions of the incubation mixture and determined by the CFUs per milliliter on the following day.
Hemolysis assays of hBD3-CBD, PA14, and PA14 ΔexsA
Hemolytic potentials of peptide hBD3-CBD and bacterial culture supernatants were measured as previously described [13] and Rossignol’s study [14]. There were six groups in this test: control, 32 μg/mL peptide (AMP), PA14, and PA14 ΔexsA with or without 32 μg/mL peptide. Briefly, sheep erythrocytes were washed three times in PBS and resuspended to a final concentration of 2%. After a 3 h peptide hBD3-CBD treatment, bacterial supernatants were collected and sterilized by a Millipore filter with 0.22 μm pores. Then, 500 μL red blood cells (RBCs) and 500 μL of supernatants were combined and incubated for 1 h at 37 °C. Lastly, the suspension was centrifuged at 8000 g for 10 min at 4 °C, and hemoglobin release was assessed by determining the absorbance at 540 nm.
Polypeptide hBD3-CBD’s effects on the pyocyanin pigment production
PA14 and PA14 ΔexsA were grown under type III inducing conditions (LB containing 5 mM EGTA) [15]. A low concentration of hBD3-CBD (8 μg/mL) was added to PA14 and PA14 ΔexsA to evaluate its effect on pyocyanin production. After 18 h incubation at 37 °C in LB broth, pyocyanin was extracted from the cell-free filtrate using chloroform and hydrochloric acid (HCl) according to the procedure previously described [16]. Pyocyanin was quantified based on measuring the absorbance of pyocyanin in the acidic form at 520 nm.
Crystal violet assay for biofilm formation test
P. aeruginosa PA 14 and PA14 ΔexsA were grown overnight in LB medium at 37 °C. The cultures were subsequently diluted with tryptone broth (TB) to an OD600 of approximately 0.02, and 10 μL of the diluted culture was added to 96-well flat-bottom tissue culture plates containing 200 μL of LB diluted 1:50. Each strain was added to six wells (blank control wells contained medium only), with and without 5 μg/mL hBD3-CBD, the plates were incubated as static cultures at 37 °C for 3 days. The unattached bacteria were removed by gently washing the plates with phosphate-buffered saline (PBS), while the remaining bacteria were stained for 20 min with 0.1% crystal violet. The wells were rinsed with PBS to remove nonspecific staining. Adherent dye was dissolved in a solution consisting of 95% ethanol, and the absorbance was detected at 570 nm.
Peptide hBD3-CBD’s effects on the virulence factor expressions in PA14 and PA14 ΔexsA
PA14 and PA14 ΔexsA strains were grown overnight in LB broth. Bacterial cells were collected, and messenger RNA was extracted by the use of the RNAprep pure Cell/Bacteria Kit (Tiagen, Beijing China). T3SS-related virulence factor expressions (exoU, exoS, hcnA, and lasB) were measured by FastQuant RT SuperMix and SuperReal PreMix Color Kit (Tiagen, Beijing China). The primers are shown in Table 1.
Table 1.
Primers used in this study
| Gene | Primers’ sequence | Source |
|---|---|---|
| exoU |
GCTAAGGCTTGGCGGAATA AGATCACACCCAGCGGTAAC-3 |
[17] |
| exoS |
GGAGCTGGATGCGGGACA GGCCGCCTCTTCGAGAAC |
[17] |
| hcnA |
CAACGTGCTCAATGCCGTG CTGGTCGAAGCGGTTGCTTT |
This study |
| lasB |
CGCAAGACCGAGAATGACA AGACCAGTTGGGCGATGTT |
[18] |
| 16sRNA |
CGGTCCAGACTCCTACGGGAGGCAGCA GCGTGGACTACCAGGGTATCTAATCC |
[19] |
Statistical analysis
Bacterial killing, hemolysis response, segment production, biofilm formation, and gene expression in variable groups were compared with the control or PA14 group and were analyzed by Student–Newman–Keuls t test. Data were presented as means ± SD. Differences at P < 0.05 were considered significant. The statistical software GraphPad Prism 7.1 was employed in this study.
Results
Peptide hBD3-CBD killing curves
In the direct bactericidal test, the bactericidal effects of hBD3-CBD on PA14 and PA14 ΔexsA in different concentrations were identified. There was no significant difference in the bactericidal activity in vitro on these two isolates. The deletion of a critical gene exsA in T3SS did nothing on the resistant to antimicrobial peptide hBD3-CBD in PA14 (Fig. 1).
Fig. 1.
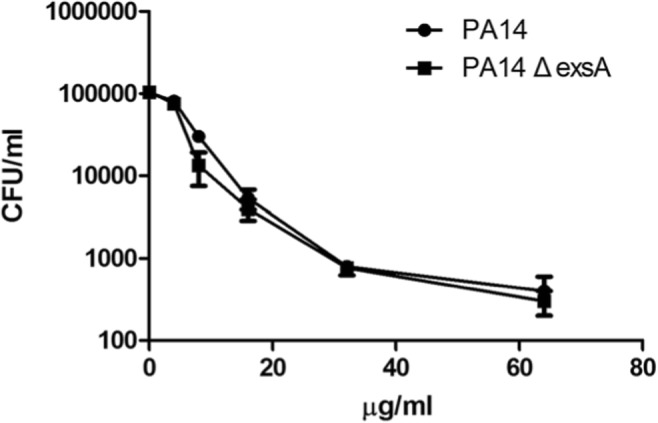
Bactericidal activity in vitro of the peptide against P. aeruginosa PA14 and PA14 ΔexsA. Killing curves at different peptide concentrations (0, 4, 8, 16, 32, and 64 μg/mL) were performed using PA14 and PA14 ΔexsA. Data are presented as the mean ± standard deviation of three independent experiments. Statistical analysis comparing groups was performed by Student–Newman–Keuls t test. Significance was accepted when the P value was < 0.05. There was no difference between the bactericidal activities on these two bacteria
Hemolysis assays of hBD3-CBD, PA14, and PA14 ΔexsA
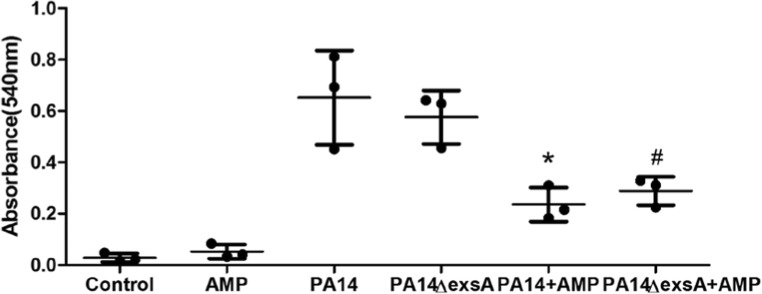
We measured the effects on hemolysis activities of hBD3-CBD with PA14 and PA14 ΔexsA. Compared with the PBS group, there was no difference in optical density in the hBD3-CBD group (Fig. 2). Peptide hBD3-CBD significantly reduced the hemolysis activities of PA14 and PA14 ΔexsA. However, we did not find any difference in hemolysis activities between the PA14 group and PA14 ΔexsA group.
Fig. 2.
Peptide hBD3-CBD’s effects on the hemolysis effects of P. aeruginosa PA14 and PA14 ΔexsA. Sheep erythrocytes were used in the hemolysis analysis, and the hemoglobin release was assessed by the absorbances at 540 nm. Peptide hBD3-CBD significantly reduced the hemolysis activities of PA14 and PA14 ΔexsA. Data are presented as the mean ± standard deviation of three independent experiments. Statistical analysis comparing groups was performed by Student–Newman–Keuls t test. Significance was accepted when the P value was < 0.05. * means a significant difference to PA14; # means a significant difference to PA14 ΔexsA
Peptide hBD3-CBD’s effects on pyocyanin pigment production and biofilm formation
Pyocyanin production is one of the natural features and virulence factors of PA14. Here we used a colorimetry method to illustrate the segment production of PA14 and PA14 ΔexsA. Compared with PA14, there were lower pyocyanin secretions in PA14 ΔexsA. Peptide hBD3-CBD also decreased the pyocyanin productions in PA14 (Fig. 3a). However, there was no difference between the pyocyanin productions in PA14 ΔexsA and hBD3-CBD-treated PA14 ΔexsA group.
Fig. 3.
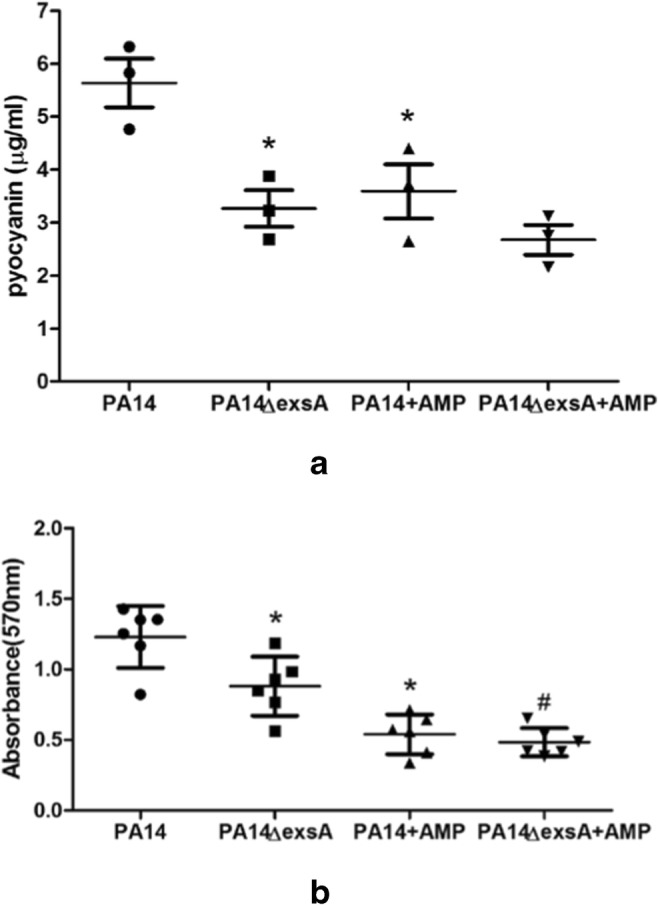
Peptide hBD3-CBD’s effects on the pyocyanin productions (a) and biofilm formation (b) of P. aeruginosa PA14 and PA14 ΔexsA. Pyocyanin productions and biofilm formation in type III inducing conditions were detected by the absorbances at 520 nm and 570 nm, respectively. Peptide hBD3-CBD decreased the pyocyanin productions and the biofilm formation in PA14 and PA14 ΔexsA. Data are presented as the mean ± standard deviation of three independent experiments. Statistical analysis comparing groups was performed by Student–Newman–Keuls t test. Significance was accepted when the P value was < 0.05. * means a significant difference to PA14; # means a significant difference to PA14 ΔexsA
We used crystal violet staining to detect the biofilm formations of PA14, PA14 ΔexsA, and the effects on biofilm formation of hBD3-CBD (Fig. 3b). Peptide hBD3-CBD can decrease the biofilm formation in PA14 and PA14 ΔexsA group. Deleted ΔexsA can cut down the biofilm formation in PA14.
Peptide hBD3-CBD’s effects on the virulence factor expressions of PA14 and PA14 ΔexsA
We detected virulence-associated gene expressions in this study (Fig. 4). They were exoU, exoS, hcnA, and lasB. Compared with the PA14 group, exoU, exoS, and lasB were downregulated in peptide hBD3-CBD–treated PA14 group; exoU and lasB were decreased and promoted in PA14 ΔexsA group, respectively. When PA14 ΔexsA was treated with peptide hBD3-CBD, we found the expressions in all four genes were less than the PA14 ΔexsA group.
Fig. 4.
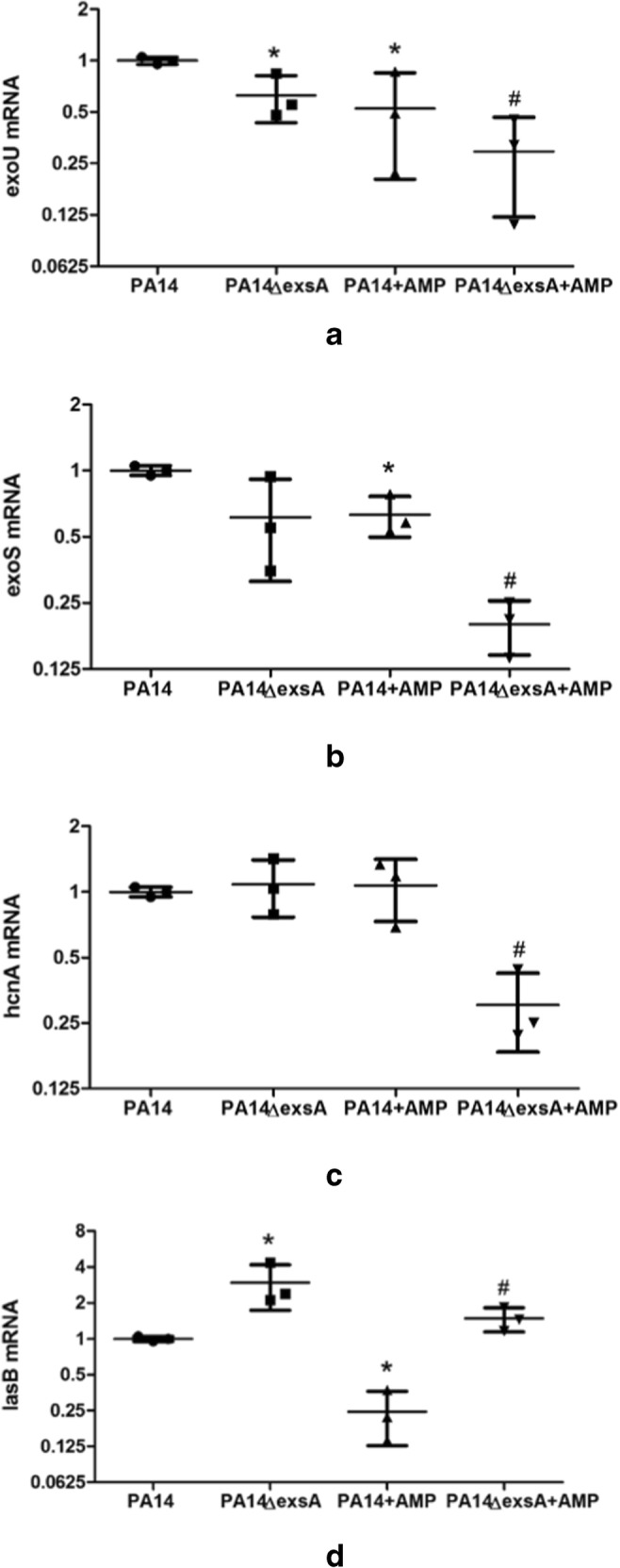
Gene expressions of peptide-treated P. aeruginosa PA 14 and PA14 ΔexsA. T3SS-related gene exoU (a), exoS (b), hcnA (c), and lasB (d) were detected by quantitative real-time PCR. Gene expression levels were showed as the ratio to the expression in PA14. Peptide hBD3-CBD treatment can decrease the expressions of exoU, exoS, and lasB in the PA14 group and all four genes in the PA14 ΔexsA group. Gene lasB was high-regulated in the PA14 ΔexsA group than the PA14 group. Data are presented as the mean ± standard deviation of three independent experiments. Statistical analysis comparing groups was performed by Student–Newman–Keuls t test. Significance was accepted when the P value was < 0.05. * means a significant difference to PA14; # means a significant difference to PA14 ΔexsA
Discussion
Antimicrobial peptides are multipurpose effector molecules of the innate immune system. AMPs are involved in many physiological processes and are most closely related to infection and immunity. AMPs have a broad antimicrobial spectrum and lyse microbial cells with very high efficiency. The expression and function of AMPs can be independent of NF-kappaB-mediated inflammatory responses [20]. The mechanism of the bactericidal capabilities of AMPs mainly includes the destructive effects on bacterial cell walls and membranes. Besides their direct antimicrobial function on susceptible bacteria, AMPs have multiple roles as mediators in the inflammations and other diseases [21]. The expression and regulation of AMPs and their applications have attracted much attention [22].
P. aeruginosa is an opportunistic pathogen that causes chronic infections in individuals suffering from cystic fibrosis [23]. The epidemiology, virulence and antibiotic susceptibility, and biofilm formation activity of P. aeruginosa attracted the most attention these days. ExsA is a critical regulatory factor in T3SS, the essential secretory system of Gram-negative bacteria, in PA14 [24].
PA14 ΔexsA and its homologous strain PA14 were included in this study. There is no difference in the hBD3-CBD killing curves between PA14 and PA14 ΔexsA. It seems this gene was not directly engaged in the bactericidal effects of hBD3-CBD. In the hemolysis test, no difference was detected between PA14 and PA14 ΔexsA. However, peptide-treated groups showed significantly lower hemolysis activity than PBS-treated groups in PA14 and PA14 ΔexsA, while peptide hBD3-CBD itself did not cause hemolysis. Hemolysis testing is the basis of the practical application of antibacterial agents and other medical preparations. For hBD3-CBD and other AMPs, many of them showed perfect performance in hemolysis testing [25]. As we have demonstrated in previous studies, combined peptides can do much better in bactericidal performance and stability [7, 13, 26].
Pyocyanin production is a standard test in the exploration and analysis of T3SS. Many studies illustrated the effects on bacterial virulence and the antibacterial efficacy of biological agents with pyocyanin and other T3SS members’ production [27]. In this study, PA14 ΔexsA showed lower pyocyanin production than wild PA14. When these bacteria were treated with hBD3-CBD, pyocyanin was significantly decreased in PA14 rather than in PA14 ΔexsA. Our results illustrated that peptide hBD3-CBD and exsA deletion can interfere with pyocyanin’s formation and secretion, but we did not find any synergism between them. Pyocyanin is a major virulence factor of P. aeruginosa, and the inhibition of pyocyanin from hBD3-CBD prompted the potential protective effects of bacterial infection in many AMPs.
Biofilm is an important pathogenic factor for many Gram-negative and Gram-positive bacteria, including klebsiella and pseudomonas. Biofilm also gives bacteria members the ability of antibiotic resistance. The destructive effect on biofilm is one of the important goals in the research and development of new antimicrobial agents. Here we found hBD3-CBD could lyse pseudomonas biofilm in both PA14 and PA14 ΔexsA. A combination of multiple antimicrobial agents is an essential option for future drug resistance and biofilm-forming bacterial infections.
We selected four virulence genes, exoU, exoS, hcnA, and lasB, to evaluate hBD3-CBD effects on T3SS in PA14 and PA14 ΔexsA. ExoU and exoS are important toxins regulated by T3SS. ExoU is a phospholipase and correlated with acute cytotoxicity in epithelial cells and macrophages, and contributes to injury in models systems; exoS is one of the two bi-functional type III cytotoxins of P. aeruginosa [28]. HcnA belongs to the transcriptional control of the hydrogen cyanide biosynthetic genes hcnABC, and lasB is a critical gene in the quorum-sensing system in P. aeruginosa [29, 30]. Firstly, exoU, exoS, and lasB were decreased in peptide hBD3-CBD–treated PA14 group than PA14 group. It suggests that this peptide is not only killing PA14 directly but also interrupting its virulence. Secondly, compared with the PA14 group, exoU and lasB were down- and upregulated in PA14 ΔexsA group, respectively. Our results showed that lasB expressions were improved in PA14 ΔexsA groups than PA14 group, whether hBD3-CBD exist or not. It indicated that the destruction of pseudomonas biofilm by antimicrobial peptides is not dependent on the effect of lasB. The role of lasB in the biofilm formation process is still controversial [31–33]. Thirdly, only the hBD3-CBD-treated PA14 ΔexsA group showed a lower hcnA expression. HcnA is an electron carrier and iron-sulfur cluster binding site in PA14, for which we did not find a homologous change in hBD3-CBD-treated PA14. Future studies will aim to clarify the role of hcnA in exsA deletion and hBD3-CBD treatment.
Quorum sensing systems and T3SS are extraordinary virulence and pathogenic mechanisms in bacterial pathogens. As the primary regulator in T3SS, exsA’s function was explained in many studies [34, 35]. The role of exsA in AMP application is worthy of more attention. Moreover, there are difficulties and challenges in the clinical usage of AMPs, such as high manufacturing costs, limited stability, loss of activity in physiological conditions, unwanted local or systemic reactions, and interference with normal flora that may change when using on peptides as antibacterial agents [36].
Several groups proposed and applied AMPs and other novel agents to treat and control the infection of P. aeruginosa and other similar pathogens [25, 37, 38]. More attention should be paid on the relationship between the bactericidal effects, quorum sensing system, and T3SS and their functions in bacterial infection.
Funding information
This study was funded by the National Natural Science Foundation of China (#81201334).
Compliance with ethical standards
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ke Dong, Email: kiwidong@126.com.
Qingtian Li, Email: qingtianli@sjtu.edu.cn.
References
- 1.Izadpanah A, Gallo RL. Antimicrobial peptides. J Am Acad Dermatol. 2005;52:381–390. doi: 10.1016/j.jaad.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 2.Harder J, Glaser R, Schroder JM. The role and potential therapeutical applications of antimicrobial proteins in infectious and inflammatory diseases. Endocr Metab Immune Disord Drug Targets. 2007;7:75–82. doi: 10.2174/187153007780832091. [DOI] [PubMed] [Google Scholar]
- 3.Dunsche A, Açil Y, Dommisch H, Siebert R, Schröder JM, Jepsen S. The novel human beta-defensin-3 is widely expressed in oral tissues. Eur J Oral Sci. 2002;110:121–124. doi: 10.1034/j.1600-0722.2002.11186.x. [DOI] [PubMed] [Google Scholar]
- 4.Dhople V, Krukemeyer A, Ramamoorthy A. The human beta-defensin-3, an antibacterial peptide with multiple biological functions. Biochim Biophys Acta. 2006;1758:1499–1512. doi: 10.1016/j.bbamem.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276:5707–5713. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 6.Bassetti M, Vena A, Croxatto A, et al. How to manage Pseudomonas aeruginosa infections. Drugs Context. 2018;7:212527. doi: 10.7573/dic.212527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin P, Li Y, Dong K, Li Q. The antibacterial effects of an antimicrobial peptide human beta-defensin 3 fused with carbohydrate-binding domain on Pseudomonas aeruginosa PA14. Curr Microbiol. 2015;71:170–176. doi: 10.1007/s00284-015-0814-x. [DOI] [PubMed] [Google Scholar]
- 8.Beaudoin T, Stone TA, Glibowicka M, et al. Activity of a novel antimicrobial peptide against Pseudomonas aeruginosa biofilms. Sci Rep. 2018;8:14728. doi: 10.1038/s41598-018-33016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohammed I, Said DG, Nubile M, et al. Cathelicidin-derived synthetic peptide improves therapeutic potential of vancomycin against. Front Microbiol. 2019;10:2190. doi: 10.3389/fmicb.2019.02190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lombardi C, Tolchard J, Bouillot S, et al. Structural and functional characterization of the type three secretion system (T3SS) needle of Pseudomonas aeruginosa. Front Microbiol. 2019;10:573. doi: 10.3389/fmicb.2019.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galle M, Carpentier I, Beyaert R. Structure and function of the type III secretion system of Pseudomonas aeruginosa. Curr Protein Pept Sci. 2012;13:831–842. doi: 10.2174/138920312804871210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma P, Guha S, Garg P et al (2018) Differential expression of antimicrobial peptides in corneal infection and regulation of antimicrobial peptides and reactive oxygen species by type III secretion system of Pseudomonas aeruginosa. Pathog Dis 76(1). 10.1093/femspd/fty001 [DOI] [PubMed]
- 13.Li Q, Zhou Y, Dong K, et al. Potential therapeutic efficacy of a bactericidal-immunomodulatory fusion peptide against methicillin-resistant Staphylococcus aureus skin infection. Appl Microbiol Biotechnol. 2010;86:305–309. doi: 10.1007/s00253-009-2313-0. [DOI] [PubMed] [Google Scholar]
- 14.Rossignol G, Merieau A, Guerillon J, et al. Involvement of a phospholipase C in the hemolytic activity of a clinical strain of Pseudomonas fluorescens. BMC Microbiol. 2008;8:189. doi: 10.1186/1471-2180-8-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang H, Shan Z, Kim J, Wu W, Lian W, Zeng L, Xing L, Jin S. Regulatory role of PopN and its interacting partners in type III secretion of Pseudomonas aeruginosa. J Bacteriol. 2007;189:2599–2609. doi: 10.1128/JB.01680-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Essar DW, Eberly L, Hadero A, Crawford IP. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J Bacteriol. 1990;172:884–900. doi: 10.1128/JB.172.2.884-900.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berthelot P, Attree I, Plesiat P, et al. Genotypic and phenotypic analysis of type III secretion system in a cohort of Pseudomonas aeruginosa bacteremia isolates: evidence for a possible association between O serotypes and exo genes. J Infect Dis. 2003;188:512–518. doi: 10.1086/377000. [DOI] [PubMed] [Google Scholar]
- 18.Gokalsin B, Sesal NC. Lichen secondary metabolite evernic acid as potential quorum sensing inhibitor against Pseudomonas aeruginosa. World J Microbiol Biotechnol. 2016;32:150. doi: 10.1007/s11274-016-2105-5. [DOI] [PubMed] [Google Scholar]
- 19.Eleaume H, Jabbouri S. Comparison of two standardisation methods in real-time quantitative RT-PCR to follow Staphylococcus aureus genes expression during in vitro growth. J Microbiol Methods. 2004;59:363–370. doi: 10.1016/j.mimet.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Gao N, Kumar A, Jyot J, et al. Flagellin-induced corneal antimicrobial peptide production and wound repair involve a novel NF-kappaB-independent and EGFR-dependent pathway. PLoS One. 2010;5:e9351. doi: 10.1371/journal.pone.0009351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beisswenger C, Bals R. Functions of antimicrobial peptides in host defense and immunity. Curr Protein Pept Sci. 2005;6:255–264. doi: 10.2174/1389203054065428. [DOI] [PubMed] [Google Scholar]
- 22.Sechet E, Telford E, Bonamy C, et al. Natural molecules induce and synergize to boost expression of the human antimicrobial peptide β-defensin-3. Proc Natl Acad Sci U S A. 2018;115:E9869–e9878. doi: 10.1073/pnas.1805298115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cody WL, Pritchett CL, Jones AK, et al. Pseudomonas aeruginosa AlgR controls cyanide production in an AlgZ-dependent manner. J Bacteriol. 2009;191:2993–3002. doi: 10.1128/JB.01156-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng X, Li M, Pan X, et al. Fis regulates type III secretion system by influencing the transcription of exsA in Pseudomonas aeruginosa strain PA14. Front Microbiol. 2017;8:669. doi: 10.3389/fmicb.2017.00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Memariani H, Shahbazzadeh D, Ranjbar R, et al. Design and characterization of short hybrid antimicrobial peptides from pEM-2, mastoparan-VT1, and mastoparan-B. Chem Biol Drug Des. 2017;89:327–338. doi: 10.1111/cbdd.12864. [DOI] [PubMed] [Google Scholar]
- 26.Li Q, Huang J, Guo H, et al. Bactericidal activity against meticillin-resistant Staphylococcus aureus of a novel eukaryotic therapeutic recombinant antimicrobial peptide. Int J Antimicrob Agents. 2012;39:496–499. doi: 10.1016/j.ijantimicag.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Ho Sui SJ, Lo R, Fernandes AR, et al. Raloxifene attenuates Pseudomonas aeruginosa pyocyanin production and virulence. Int J Antimicrob Agents. 2012;40:246–251. doi: 10.1016/j.ijantimicag.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sawa T, Shimizu M, Moriyama K, et al. Association between Pseudomonas aeruginosa type III secretion, antibiotic resistance, and clinical outcome: a review. Crit Care. 2014;18:668. doi: 10.1186/s13054-014-0668-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chowdhury N, Bagchi A. Molecular insight into the activity of LasR protein from Pseudomonas aeruginosa in the regulation of virulence gene expression by this organism. Gene. 2016;580:80–87. doi: 10.1016/j.gene.2015.12.067. [DOI] [PubMed] [Google Scholar]
- 30.Rangel SM, Logan LK, Hauser AR. The ADP-ribosyltransferase domain of the effector protein ExoS inhibits phagocytosis of Pseudomonas aeruginosa during pneumonia. MBio. 2014;5:e01080–e01014. doi: 10.1128/mBio.01080-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roshani-Asl P, Rashidi N, Shokoohizadeh L, Zarei J. Relationship among antibiotic resistance, biofilm formation and lasB gene in Pseudomonas Aeruginosa isolated from burn patients. Clin Lab. 2018;64:1477–1484. doi: 10.7754/Clin.Lab.2018.180331. [DOI] [PubMed] [Google Scholar]
- 32.Yu H, He X, Xie W, Xiong J, Sheng H, Guo S, Huang C, Zhang D, Zhang K. Elastase LasB of Pseudomonas aeruginosa promotes biofilm formation partly through rhamnolipid-mediated regulation. Can J Microbiol. 2014;60:227–235. doi: 10.1139/cjm-2013-0667. [DOI] [PubMed] [Google Scholar]
- 33.Tettmann B, Niewerth C, Kirschhöfer F, et al. Enzyme-mediated quenching of the quinolone signal (PQS) promotes biofilm formation of by increasing Iron availability. Front Microbiol. 2016;7:1978. doi: 10.3389/fmicb.2016.01978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brutinel ED, Vakulskas CA, Yahr TL. Functional domains of ExsA, the transcriptional activator of the Pseudomonas aeruginosa type III secretion system. J Bacteriol. 2009;191:3811–3821. doi: 10.1128/JB.00002-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shrestha M, Xiao Y, Robinson H, et al. Structural analysis of the regulatory domain of ExsA, a key transcriptional regulator of the type three secretion system in Pseudomonas aeruginosa. PLoS One. 2015;10:e0136533. doi: 10.1371/journal.pone.0136533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaiou M. Multifunctional antimicrobial peptides: therapeutic targets in several human diseases. J Mol Med. 2007;85:317–329. doi: 10.1007/s00109-006-0143-4. [DOI] [PubMed] [Google Scholar]
- 37.Wu X, Li Z, Li X, et al. Synergistic effects of antimicrobial peptide DP7 combined with antibiotics against multidrug-resistant bacteria. Drug Des Devel Ther. 2017;11:939–946. doi: 10.2147/DDDT.S107195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li G, Lu S, Shen M, et al. Characterization and interstrain transfer of prophage pp3 of Pseudomonas aeruginosa. PLoS One. 2017;12:e0174429. doi: 10.1371/journal.pone.0174429. [DOI] [PMC free article] [PubMed] [Google Scholar]