Abstract
Hydrogen sulfide (H2S), along with nitric oxide (NO) and carbon monoxide (CO), proved to have renoprotective effects in various renal diseases. Therefore, this study investigated the renoprotective effect of H2S, in a renal injury model, and its crosstalk with other gasotransmitters such as CO. Thirty-two adult rats were divided into four groups: control, gentamicin (GEN)-treated, GEN + sodium hydrosulfide (NaHS), and GEN + NaHS + zinc protoporphyrin (ZnPP) groups. GEN was used to induce renal injury, NaHS is a water-soluble H2S, and ZnPP is a selective heme oxygenase-1 (HO-1) inhibitor used to inhibit CO synthesis in vivo. NaHS improved kidney functions in the GEN group as evidenced by significantly lower levels of renal injury markers: serum urea, creatinine, uric acid, urinary albumin excretion, and urinary albumin/creatinine. Moreover, NaHS administration to the GEN-treated group significantly lowered renal levels of NO and tumor necrosis factor-α with an increase in total antioxidant, HO-1, and interleukin-10 levels. Furthermore, NaHS administration downregulated the GEN-induced overexpression of the renal inducible nitric oxide synthase (iNOS) and upregulated the suppression of endothelial nitric oxide synthase (eNOS) with improvement in the histological examination and periodic acid Schiff (PAS) staining. However, this improvement in kidney function produced by NaHS was reduced by combination with ZnPP but still improved as compared with the GEN-treated group. The renoprotective effects of H2S can be through its effects on renal tissue antioxidants, pro-inflammatory and anti-inflammatory cytokines, and expression of eNOS and iNOS which can be partially dependent on CO pathway via induction of HO-1 enzyme.
Keywords: Hydrogen sulfide, Carbon monoxide, Renal injury, Antioxidants, Anti-inflammatory
Introduction
Hydrogen sulfide (H2S) is one of the endogenous gaseous signaling molecules in mammalian tissues, which is synthesized enzymatically by cystathionine β-synthase (CBS), cystathionine γ-lyase (CGL), and 3-mercaptopyruvate sulfurtransferase (3-MST) (Kasinath 2014). These three enzymes are expressed in large quantities in the kidney and are involved in the renal H2S production (Song et al. 2014).
H2S has been considered historically as a toxic gas and an environmental pollutant. Lately, it has been considered as an important mediator of several cellular signal transductions and pathophysiological responses including energy production, angiogenesis, insulin release, cardioprotection, blood vessel relaxation, and neuromodulation, in addition to enhancement of the cytotoxic cellular responses, e.g., oxidative stress, apoptosis, inflammation, and necrosis (Kolluru et al. 2013).
Heme oxygenase 1 (HO-1) is a stress protein that catalyzes the rate-limiting step of heme degradation yielding equimolar amounts of biliverdin, carbon monoxide (CO, another endogenous gaseous signaling molecule), and iron, and the products are bioactive (Lever et al. 2016). It can be induced in a variety of kidney substructures in response to injury, including proximal tubules, glomeruli, and renal interstitium, as well as in renal mononuclear phagocytes. Following an acute injury, HO-1 is rapidly induced but its expression subsides before renal recovery fully occurs; such reduction in HO-1 expression may allow the continued expression of pro-inflammatory and fibrogenic genes (Nath 2014). HO-1 has been studied widely for its antioxidant and anti-inflammatory functions, as well as its abilities to maintain microcirculation and suppress immunological rejection (Du et al. 2019).
The kidney is a vital organ, playing a crucial role in health, disease, and overall growth and development. A number of environmental variables including certain drugs affect these functions. Renal injury can be induced by nephrotoxic substances which have negative effects on renal function; these substances can include arsenic and lead, antibiotics such as aminoglycosides, cancer therapeutics such as cisplatin, metals such as mercury, drugs of abuse such as cocaine, and fungi (Pendergraft et al. 2014).
Renal injury mechanisms include renal free radical production and accumulation, consumption of the antioxidant defense mechanisms, acute tubular necrosis, and glomerular congestion leading to diminished creatinine clearance and renal dysfunction (Otunctemur et al. 2014). One main sign of renal injury is a change in renal function as measured by the blood urea nitrogen, glomerular filtration rate, serum creatinine, or urine output (Barnett and Cummings 2018).
Drugs are considered a major contributor to acute kidney insult. Gentamicin (GEN) is an aminoglycoside wide-spectrum antibiotic used commonly to treat infections caused by Gram-negative bacteria (Krause et al. 2016). However, their usage is limited by their nephrotoxicity, which appears in about 10–25% of therapeutic courses. GEN-induced renal damage is a frequently used model for the induction of renal injury in experimental animals. The mechanism of GEN-induced renal injury is not completely understood. Oxidative stress is involved as the central mechanism of GEN-induced renal cell injury. Thus, amelioration of aminoglycoside renal injury by any means would be of great clinical significance (Virani et al. 2016).
There are many strategies dependent on nephroprotective drugs for co-treatment along with aminoglycosides. At the preclinical level, many substances have been shown to exert protective effects on drug-induced renal injury and especially on aminoglycoside nephrotoxicity. In particular, many studies have shown that antioxidants improve aminoglycoside nephrotoxicity (Casanova et al. 2017).
Since the biological actions of H2S are still increasing and its role in both renal physiology and pathology is still not well understood (van den Born et al. 2016), the present study is designed to shed light on the effects of H2S in a GEN-induced renal injury model, as well as its crosstalk with one of the other endogenous gasotransmitters (CO) that may be integrated in its effect. Different oxidative, inflammatory renal parameters and renal injury markers with renal histology and immunohistochemical examination of inducible nitric oxide synthase (iNOS) and endothelial nitric oxide synthase (eNOS) were evaluated in the different studied groups in an attempt to assess the possible mechanisms of H2S effect.
Material and methods
Ethical approval
The Minia University Faculty of Medicine, Research Ethics Committee (FMREC) approved this research proposal regarding the source of the animals, health status, inclusion criteria, exclusion criteria, caging, comfort, and the detailed experimental design and procedures. It was conducted in agreement with the NIH Guide for Care and Use of Laboratory Animals (Council 2010).
Animals
In the present study, thirty-two adult male albino (Sprague Dawley strain) rats, with an average weight of 150–200 g, about 4 months old, were used. Rats were bought from the National Research Center, Cairo, Egypt. They were housed in groups of eight in stainless steel cages that offered adequate space for free movement (40 cm × 40 cm × 25 cm) at room temperature with natural dark/light cycles and allowed free access to water and commercial rat’s diet (Nile Company, Egypt) for 2 weeks for acclimatization.
Experimental groups
Animals were randomly divided into four equal groups (8 animals each):
Control group: each rat received 0.5 ml of dimethyl sulfoxide (DMSO) (Sigma-Aldrich Co., Germany) intraperitoneally (IP) for 7 consecutive days.
GEN-treated group: rats were injected IP with GEN (Memphis Co., Egypt) to induce nephrotoxicity in a dose level of 100 mg/kg/day daily for 7 consecutive days (Chen et al. 2017) and received 0.5 ml of DMSO IP 1 h before the injection of GEN.
GEN + sodium hydrosulfide (NaHS)–treated group: rats were given NaHS (water-soluble H2S inducer) (Honeywell Fluka Co., China) intraperitoneally at 56 μmol/kg/day for 7 days immediately after injection of GEN (Otunctemur et al. 2014). In addition, each rat received 0.5 ml of DMSO IP 1 h before the NaHS injection.
GEN + NaHS + zinc protoporphyrin (ZnPP)–treated group: ZnPP is a selective HO-1 inhibitor, thus used to inhibit CO synthesis in vivo (Podkalicka et al. 2018), and dissolved in DMSO. GEN-treated rats were given NaHS by the same previous dose, and ZnPP by IP injection in a dose of 40 μmol/kg/day (25 mg/kg/day) for 7 consecutive days (Takagi et al. 2018).
Biochemical analyses
One day before the end of the study, each rat was housed separately in a metabolic cage for collection of 24-h urine to determine creatinine and albumin by colorimetric assay kits (Spinreact SAU, Spain) according to the manufacturer’s instructions. Measured creatinine with albumin was used to determine albumin/creatinine (A/C) ratio.
Subsequently, the rats were sacrificed by decapitation. The blood samples were immediately collected from the jugular veins in 10-ml Eppendorf tubes, allowed to clot, and then centrifuged at 3000 rpm for 20 min. The serum samples were separated in 2-ml Eppendorf tubes and stored at − 20 °C until use for estimation of the following parameters: urea (BioSystems S.A., Spain), creatinine (Spinreact SAU, Spain), and uric acid (Stanbio Lab., USA) by using an enzymatic colorimetric assay kits according to the manufacturer’s instructions.
The kidneys were also removed from each rat, and each organ was weighed and homogenized in potassium phosphate buffer 10 mM. The ratio of tissue weight to homogenization buffer was 1:10. The homogenates were centrifuged at 5000 rpm for 10 min at 4 °C. The resulting supernatant was used for determination of total antioxidant capacity (TAC) by colorimetric assay kit (LC Diagnostics Ltd., Korea), nitric oxide (NO) by colorimetric assay kit (Bio-diagnostic, Egypt), tumor necrosis factor-α (TNF-α) by ELISA kit (Boditech Med. Inc., Korea), interleukin-10 (IL-10) by ELISA kit (Elabscience Biotechnology Ltd., USA), and HO-1 by using rat HO-1 immunoassay kit (BioVendor, USA) according to the manufacturer’s instructions.
Histological examination
Tissue specimens (right kidney) for light microscopy were fixed in 10% formol saline and processed to obtain paraffin blocks. Five micrometer-thick sections were cut and stained with hematoxylin and eosin (H&E) stain for histological studies (Swisher et al. 2002), periodic acid Schiff (PAS) to record interstitial edema, and the possible separation of epithelial cells from the basement membranes (Martín-Solé et al. 2016). At the same time, immunohistochemical staining was performed for the recognition of expression of iNOS and eNOS. iNOS and eNOS monoclonal mouse antibodies were used (Lab Vision Laboratories) according to the manufacturer’s protocol. The slides were then counterstained, dehydrated, and mounted. The reaction is cytoplasmic (Côté 1993). For photography, Olympus light microscopy was used. Slides were photographed using an Olympus digital camera.
Morphometric analysis
Measurements were performed in five non-overlapping fields from five different sections of five different rats, in each group at × 400 magnifications, using the image analyzer (Leica Imaging System, Germany) to measure the following:
Color percentage of PAS-positive areas in PAS-stained sections
iNOS immunoreactive optical density
iNOS immunoreactive optical density
Statistical analysis
Data were represented as means ± standard errors of the mean (SEM). Statistical analysis was performed using GraphPad Prism 5 software, and a significant difference between groups was assessed by one-way ANOVA followed by the Tukey-Kramer post hoc test for multiple comparisons with a P value of ≤ 0.05 considered statistically significant.
Result
Assessment of the urinary parameters in the different experimental groups
The data of the present study clearly demonstrated that the urinary albumin level and A/C ratio were significantly higher in all the GEN-treated groups than in the control group. In addition, they were significantly lower in the GEN-treated + NaHS and GEN + NaHS + ZnPP–treated groups than in the GEN-treated group, with significantly higher levels in the GEN + NaHS + ZnPP–treated group than in the GEN + NaHS–treated group (Figs. 1 and 2, respectively).
Fig. 1.
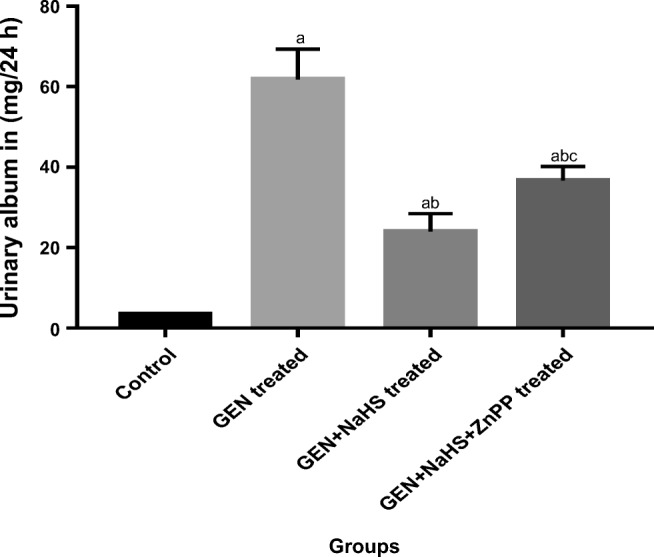
Changes in urinary albumin excretion in the different experimental groups. Data represent mean ± SEM of 8 rats. a Significantly different from the control group. b Significantly different from the GEN-treated group. c Significantly different from the GEN + NaHS–treated group, P ˂ 0.05. GEN gentamicin, NaHS sodium hydrosulfide, ZnPP zinc protoporphyrin IX
Fig. 2.
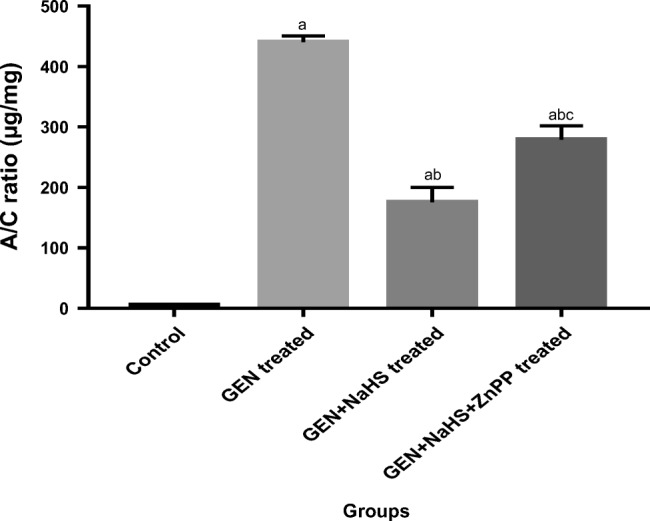
Changes in urinary albumin/creatinine (A/C) ratio in the different experimental groups. Data represent mean ± SEM of 8 rats. a Significantly different from the control group. b Significantly different from the GEN-treated group. c Significantly different from the GEN + NaHS treated group. P ˂ 0.05. GEN gentamicin, NaHS sodium hydrosulfide, ZnPP zinc protoporphyrin IX
Assessment of the serum parameters in the different experimental groups
The data presented in Table 1 show that serum urea, creatinine, and uric acid were significantly higher in all the GEN-treated groups than in the control group. Furthermore, they were significantly lower in the GEN + NaHS–treated and GEN + NaHS + ZnPP–treated groups than in the GEN-treated group, with significantly higher levels in the GEN + NaHS + ZnPP–treated group than in the GEN + NaHS–treated group.
Table 1.
Changes in the serum parameters in the different experimental groups
| Group parameters | Control | GEN-treated | GEN + NaHS–treated | GEN + NaHS + ZnPP–treated |
|---|---|---|---|---|
| Serum urea (mg%) | 20.9 ± 1.48 | 76.8 ± 1.02 a | 31.96 ± 0.54 ab | 47 ± 1.19 abc |
| Serum creatinine (mg%) | 0.19 ± 0.01 | 1.73 ± 0.02 a | 0.61 ± 0.01 ab | 0.87 ± 0.03 abc |
| Serum uric acid (mg%) | 1.17 ± 0.12 | 7.14 ± 0.12 a | 2.53 ± 0.1 ab | 3.72 ± 0.05 abc |
Data represent mean ± SEM of 8 rats
GEN gentamicin, NaHS sodium hydrosulfide, ZnPP zinc protoporphyrin IX
aSignificantly different from the control group
bSignificantly different from the GEN-treated group
cSignificantly different from the GEN + NaHS–treated group (P ˂ 0.05)
Assessment of the renal tissue parameters in the different experimental groups
The data presented in Table 2 show that the renal tissue TAC was significantly higher in the control group than in all other groups. Furthermore, they were significantly lower in the GEN-treated group than in all other GEN-treated groups, with significantly higher levels in the GEN + NaHS–treated group than in the GEN + NaHS + ZnPP–treated group.
Table 2.
Changes in the renal tissue parameters in the different experimental groups
| Group parameters | Control | GEN-treated | GEN + NaHS–treated | GEN + NaHS + ZnPP–treated |
|---|---|---|---|---|
| Renal TAC (mmol/L) | 0.21 ± 0.02 | 0.04 ± 0.01 a | 0.14 ± 0.01 ab | 0.09 ± 0.01 abc |
| Renal NO (nmol/g tissue) | 2.71 ± 0.03 | 26.11 ± 1.23 a | 8.95 ± 0.62 ab | 16.34 ± 0.58 abc |
| Renal TNF-α (pg/g tissue) | 198.4 ± 2.1 | 523.5 ± 2.8 a | 243.9 ± 1.5 ab | 343.1 ± 6.3 abc |
| Renal IL-10 (pg/g tissue) | 130 ± 3.8 | 100.6 ± 5.2 a | 185.3 ± 3.5 ab | 130.9 ± 3.8 bc |
Data represent mean ± SEM of 8 rats
GEN gentamicin, NaHS sodium hydrosulfide, ZnPP zinc protoporphyrin IX, TAC total antioxidant capacity, NO nitric oxide, TNF-α tumor necrosis factor-α, IL-10 interleukin-10
aSignificantly different from the control group
bSignificantly different from the GEN-treated group
cSignificantly different from the GEN + NaHS–treated group (P ˂ 0.05)
As regards renal NO level, the data presented in Table 2 show that it was significantly higher in all the GEN-treated groups than in the control group, with significantly lower levels in the GEN + NaHS–treated group than in the GEN-treated and GEN + NaHS + ZnPP–treated groups.
The present data show also that the renal tissue TNF-α levels were significantly lower in the control group than in the other GEN-treated groups, with significantly higher levels in the GEN-treated group alone than in the GEN groups treated with NaHS or NaHS + ZnPP.
As regards renal IL-10 level, the data show that there was no significant change between the control and the GEN + NaHS + ZnPP–treated group but that it was significantly lower in the GEN-treated group than in the control group. However, it was significantly higher in the GEN + NaHS–treated group than in all the other groups.
In the present study, renal HO-1 level was not significantly altered by gentamicin administration; however, it was significantly increased in renal tissues of the GEN + NaHS–treated group compared with the control and GEN-treated groups. Additionally, the increased HO-1 level by NaHS was eliminated by concomitant administration of the HO-1 selective inhibitor (ZnPP) (Fig. 3).
Fig. 3.
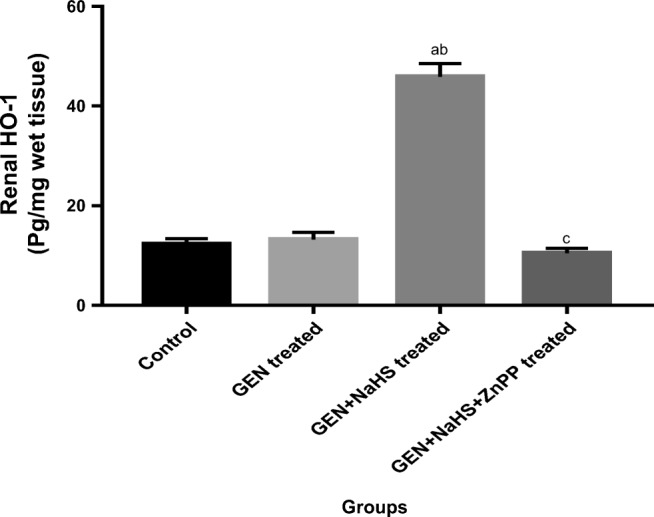
Changes in renal HO-1 level in the different experimental groups. Data represent mean ± SEM of 8 rats. a Significantly different from the control group. b Significantly different from the GEN-treated group. c Significantly different from the GEN + NaHS–treated group. P ˂ 0.05. GEN gentamicin, NaHS sodium hydrosulfide, ZnPP zinc protoporphyrin IX, HO-1 heme oxygenase 1
Assessment of the histological examination of the renal tissue in the different experimental groups
Light microscopic study
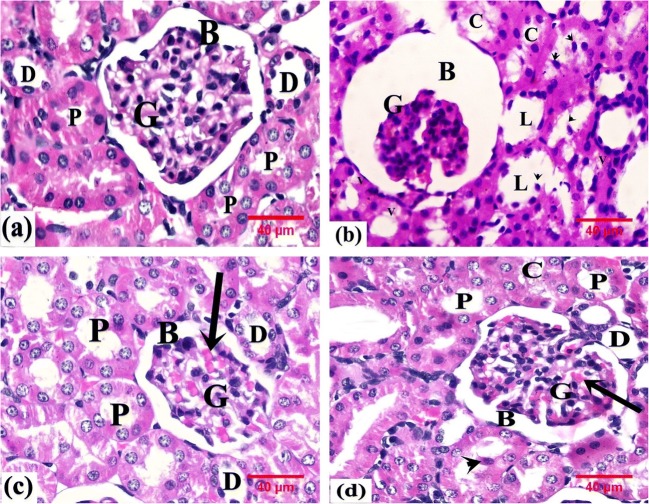
Histological examination of the kidney of control rats revealed the renal cortex with a normal architecture. Each renal corpuscle was formed of a glomerular tuft of capillaries encircled by Bowman’s capsule with a capsular space in between the visceral layer adherent to the capillary tuft and the parietal layer. The cells of proximal convoluted tubules (PCTs) were cuboidal epithelial cells and had an irregular narrow lumen, whereas the distal convoluted tubules (DCTs) had a wider lumen and were lined by low cuboidal epithelial cells (Fig. 4a).
Fig. 4.
Photomicrographs of rats’ renal tissue. a From the control group showing the renal corpuscle containing the glomerulus (G) and surrounded by Bowman’s space (B) and proximal (P) and distal (D) convoluted tubules. b From the GEN group showing atrophied glomerulus (G) and dilated Bowman’s space (B). Some renal tubules display vacuolated cells (V) and shed nuclei (arrowhead); others display dilated lumen (L) and hyaline cast (C). c From the GEN+ NaHS–treated group showing the glomerulus (G), Bowman’s space (B), and proximal (P) and distal (D) convoluted tubules with apparently normal structure. Note the congested capillary tuft (arrow). d From the GEN+ NaHS + ZnPP–treated group showing the glomerulus (G), Bowman’s space (B), and proximal (P) and distal (D) convoluted tubules with apparently normal structure. Note the congested capillary tuft (arrow), the shed nuclei (arrowhead), and hyaline cast (C). H&E, × 400
In this study, the renal cortex of GEN-treated rats showed disorganized renal cortical structure, partially atrophied glomerulus, and dilated Bowman’s space. The renal tubules exhibited marked degeneration with flat epithelial lining, vacuolated cytoplasm, and dilated lumen containing hyaline cast or shed nuclei (Fig. 4b).
However, the renal cortex of GEN + NaHS–treated rats kept the generally normal structure without any indication of major morphological injury for many renal corpuscles, PCTs, and DCTs; only congested glomerular capillaries were found (Fig. 4c).
The renal cortex of GEN + NaHS + ZnPP–treated rats revealed morphological changes as compared to controls. Some renal corpuscles showed congested glomerular capillaries. Some renal tubules with nuclear shedding and luminal hyaline casts were observed (Fig. 4d).
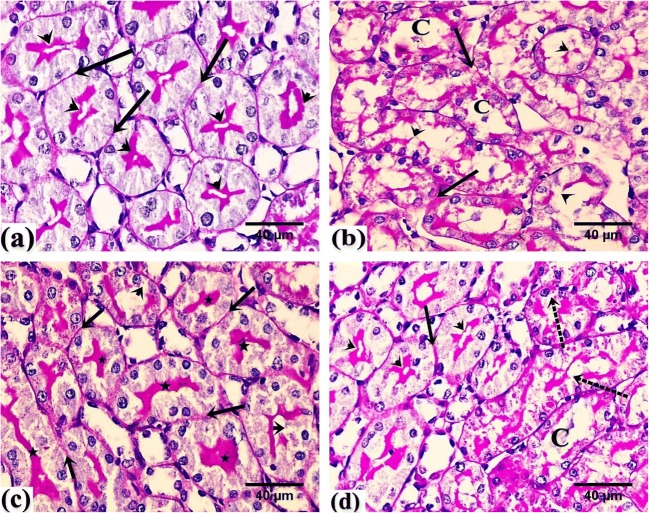
PAS stain
PAS-stained sections of the renal cortex of control rats showed a PAS-positive stain along the regular basement membrane and the apical brush border of the PCT (Fig. 5a), while sections of the renal cortex of GEN-treated rats showed faint PAS reaction along the basement membrane and the disrupted brush border of renal tubules. The renal tubules displayed dilated lumen with hyaline cast (Fig. 5b).
Fig. 5.
Photomicrographs of rats’ renal tissue. a From the control group showing positive PAS reaction along the regular basement membrane (arrow) and the intact brush borders (arrowhead) of renal PCTs. b From the GEN group showing faint PAS reaction along the basement membrane (arrow) and along the disrupted brush border of renal tubules (arrowhead). Note the stained cast within the tubules (C). c From the GEN + NaHS–treated group showing positive PAS reaction along the regular basement membrane (arrow) and the intact brush borders (star) of renal PCTs. Note the disrupted brush border of renal tubules (arrowhead). d From the GEN + NaHS + ZnPP–treated group showing positive PAS reaction along the regular basement membrane (arrow) and the intact brush borders (arrowhead) of renal PCTs. Note the disrupted brush border of renal tubules (dotted arrow) and the stained cast within the tubules (C). PAS, counterstained with H, × 400
However, the renal cortex of GEN + NaHS–treated rats revealed a PAS-positive reaction along the basement membrane and the intact brush borders of many PCTs (Fig. 5c).
In contrast, the renal cortex of GEN + NaHS + ZnPP–treated rats displayed some renal PCTs with positive PAS reaction along the regular basement membrane and intact brush borders. Others displayed a disrupted brush border and a stained cast within the lumen (Fig. 5d).
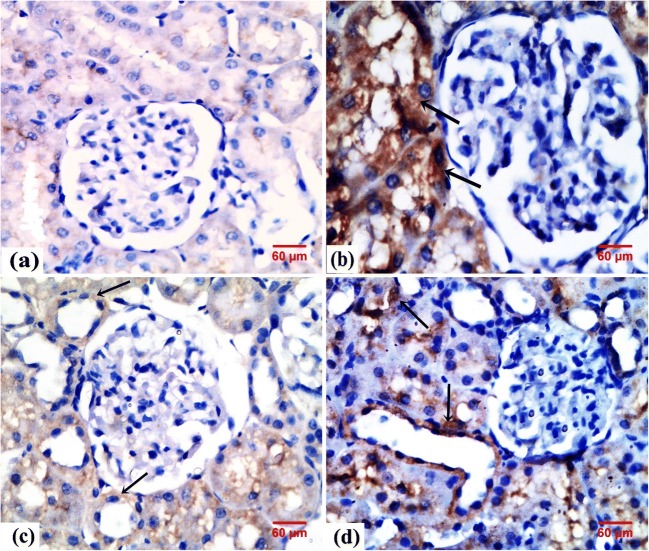
Immunohistochemical result
Sections of the control rats displayed negative immunoreaction for iNOS in the cytoplasm of tubular cells (Fig. 6a). Meanwhile, sections of the GEN-treated rats displayed highly expressed iNOS reaction in the cytoplasm of tubular cells (Fig. 6b). In contrast, the renal cortex of GEN + NaHS–treated rats and GEN + NaHS + ZnPP–treated rats revealed faint expression and positive immunoreaction for iNOS in the cytoplasm of tubular cells, respectively (Fig. 6c, d).
Fig. 6.
Photomicrographs of rats’ renal tissue. a From the control group showing negative immunoreaction for iNOS. b From the GEN group showing highly expressed cytoplasmic immunoreaction for iNOS in the tubular cells (arrow). c From the GEN + NaHS–treated group showing faint cytoplasmic immunoreaction for iNOS in the tubular cells (arrow). d From the GEN + NaHS + ZnPP–treated group showing positive cytoplasmic immunoreaction for iNOS in the tubular cells (arrow). iNOS immunostaining, × 400
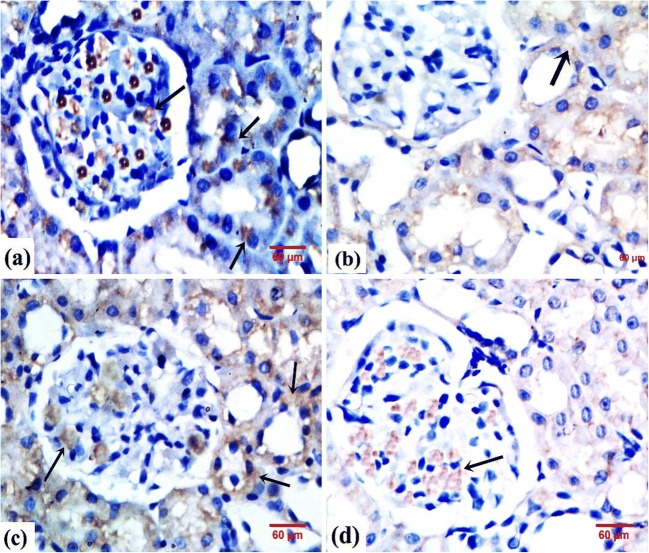
Regarding eNOS immunoreactivity, the control group demonstrated intense eNOS immunoreaction in the PCTs, DCTs, and the capillary tufts of glomeruli (Fig. 7a).
Fig. 7.
Photomicrographs of rats’ renal tissue showing eNOS expression in glomeruli and renal tubules. a From the control group showing intense cytoplasmic immunoreaction (arrow). b From the GEN group showing faint cytoplasmic immunoreaction (arrow). c From the GEN + NaHS–treated group showing moderate cytoplasmic immunoreaction (arrow). d From the GEN + NaHS + ZnPP–treated group showing weak cytoplasmic immunoreaction. eNOS immunostaining, × 400
The GEN-treated rats demonstrated faint eNOS immunoreaction in the glomerular tufts and tubules (Fig. 7b). Meanwhile in the GEN + NaHS–treated rats, most glomeruli were immunoreactive. Both the PCTs and DCTs showed positive immunoreaction (Fig. 7c).
In contrast, the renal cortex of GEN + NaHS + ZnPP–treated rats revealed variable degrees of immunoreaction. Most glomeruli were immunoreactive. Some tubules demonstrated weak cytoplasmic reaction. Other tubules demonstrated faint reaction. Nonreactive tubules could also be observed (Fig. 7d).
Morphometric results
The mean area % for PAS and the mean optical density of iNOS and eNOS immunostaining as evaluated displayed a statistically significant variance between the groups (P < 0.05). The results are displayed in Table 3. The scores of iNOS immunostaining were significantly higher for the GEN-treated group as contrasted with the other groups (P < 0.05). Scores of the mean color area percentage of PAS and eNOS immunostaining were significantly lower for the GEN-treated group when compared with the other groups (P < 0.05).
Table 3.
Morphometric analysis in the studied groups
| Group parameters | Control | GEN-treated | GEN + NaHS–treated | GEN + NaHS + ZnPP–treated |
|---|---|---|---|---|
| The mean color area percentage of PAS | 5.06 ± 2.44 | 0.88 ± 0.28 a | 3.33 ± 0.78 ab | 2.28 ± 0.68 ab |
| The mean optical density of iNOS immunostaining | 00.00 ± 00.00 | 98.09 ± 2.57 a | 72.51 ± 2.65 abc | 80.19 ± 2.56 abc |
| The mean optical density of eNOS immunostaining | 92.08 ± 2.56 | 60.51 ± 6.12 a | 87.02 ± 4.48 ab | 71.54 ± 2.64 abc |
Data represent mean ± SEM of 8 rats
GEN gentamicin, NaHS sodium hydrosulfide, ZnPP zinc protoporphyrin IX, PAS periodic acid Schiff, iNOS inducible nitric oxide synthase, eNOS endothelial nitric oxide synthase
aSignificantly different from the control group
bSignificantly different from the GEN-treated group
cSignificantly different from the GEN + NaHS–treated group (P ˂ 0.05)
Discussion
On studying the effect of GEN on the kidney, the results obtained in the present study showed its nephrotoxic effect; the renal injury markers serum urea, creatinine, and uric acid were significantly higher in the GEN-treated group than in the control group. Moreover, the urinary albumin excretion (UAE) and urinary A/C ratio were significantly higher in the GEN-treated group than in the control group with extensive renal damage as shown in the histological examination and PAS staining. These results are in line with previous studies that reported that urinary albumin and urinary A/C ratio was significantly increased in GEN-induced nephrotoxicity (Chen et al. 2017; Hasanvand et al. 2018).
The kidney receives nearly 25% of total blood supply and is rich in mitochondria that render it liable to damage from reactive oxygen species (ROS) and consequent development of acute kidney damage (Sureshbabu et al. 2015). An association between nephrotoxicity and oxidative stress has been established in various experimental models as shown in Kakalij et al. (2014).
Renal NO has a “double face” in renal physiology. At physiological concentrations produced by eNOS, its “good face” appears as a major vasodilator agent regulating renal macrovascular and microvascular hemodynamics (Park et al. 2018). At toxic concentrations produced by iNOS, its “bad face” is revealed in renal inflammatory conditions through the formation of peroxynitrite (ONOO−), a pro-inflammatory cytokine, by reacting with superoxide anion (O2·−) (Ozturk et al. 2018).
In the present study, GEN administration led to deficits in the antioxidant defense mechanisms, which were likely to have contributed to increased lipid peroxidation in the kidney. Thus, the toxicity of GEN that elevated renal injury markers can be related to the generation of ROS in the kidney. Mehan et al. (2017) reported that GEN acts as an iron chelator and the iron-GEN complex is a potent catalyst of radical generation, and ROS can damage some macromolecules to induce necrosis and cellular injury via numerous mechanisms including protein denaturation, peroxidation of membrane lipids, and DNA damage.
The alteration in kidney function induced by lipid peroxidation is a primary event in the injury cascade of GEN-mediated nephrotoxicity as observed in the present study by significantly increasing the renal tissue NO and inflammatory cytokines (TNF-α) with a reduction of anti-inflammatory cytokines (IL-10). These results are in line with Erjaee et al. (2015) who reported that unbalanced or increased ROS production and oxidative stress mediate the inflammatory response mediated by GEN. Nuclear factor kappa-B (NF-кB), activated by superoxide anions and hydrogen peroxides, is a key mediator for several inflammatory pathways, induces the expression of pro-inflammatory cytokines such as interleukin-6 (IL-6) and TNF-α, and also increases iNOS leading to increased production of NO (Mehan et al. 2017). Furthermore, the iNOS-dependent inhibition of eNOS deteriorates endothelial function further, creating a triangle among NO, ROS, and oxygen in the pathophysiology of oxidative stress and acute kidney injury (Pavlakou et al. 2017), and this is in line with the present immunohistological findings. In addition, Abd-Elhamid et al. (2018) reported that there was a negative correlation between eNOS immunoexpression and the degree of renal pathology in experimental models.
Collectively, the results of the present study verified that GEN induced renal injury, as demonstrated by extensive renal histological damage with an increase in the renal injury markers through increasing inflammatory mechanisms, oxidative stress mechanisms, and renal tissue NO as proved by overexpression of iNOS with suppression of eNOS.
On studying the effect of H2S on the GEN-induced renal injury, the present results showed that the treatment of GEN-treated rats with NaHS led to a significant decrease in the measured renal injury markers (serum urea, creatinine, and uric acid levels) compared to the GEN-treated group with a reduction in severe renal damages observed by the present histological examination and PAS staining. These results are in line with Otunctemur et al. (2014) who reported that NaHS significantly reduced the serum creatinine level in GEN-induced nephrotoxicity. In contrast, Liu et al. (2016) reported that treatment with GYY4137, a novel slow-releasing H2S donor, exacerbated cisplatin-induced nephrotoxicity in mice possibly through promoting oxidative stress, inflammation, and apoptotic response.
The present study demonstrated that the treatment of GEN-treated rats with NaHS led to a significant decrease in UAE and urinary A/C ratio compared to the GEN-treated group. These results are in line with Karimi and Absalan (2017) who reported that the NaHS significantly reduced albuminuria in cisplatin-induced nephrotoxicity and these results verified the ability of NaHS to improve or normalize renal function. H2S contributes in controlling renal function and enhances urinary sodium excretion through both tubular actions and renal vasculature. In addition, both albuminuria and A/C ratio are correlated inversely with urinary sulfate concentration, the metabolic end product of H2S (van den Born et al. 2016).
Moreover, the anti-inflammatory effects of NaHS, as observed in the present study by a significant reduction in the renal TNF-α level and the significant increase in the IL-10 level, are probably the cause of urinary protein reduction, thus preserving the endothelial barrier in the glomeruli (Zhou et al. 2014). H2S has been shown to have an anti-inflammatory effect by mechanisms which limit neutrophil adhesion and activation in response to the inflammatory stimuli, as well as by suppressing the release of pro-inflammatory cytokines (Ibrahim et al. 2015). In addition, H2S downregulated the adhesion molecules, and pro-inflammatory cytokines, and the inflammatory cytokines/chemokines by a mechanism involving the downregulation of NF-κB (Wu et al. 2017).
Increasing evidence indicates that ROS are important mediators of GEN-induced renal injury (Wu et al. 2017). In the present study, NaHS treatment significantly elevated TAC level in the GEN-treated group. The protective effect of H2S against oxidative stress is thought to be mediated by a simultaneous increase in the antioxidant activities of GSH, catalase, and superoxide dismutase and by its ability to lower ROS production by suppression of the expression of the ROS-generating enzyme, NADPH oxidase (Ibrahim et al. 2015; Yuan et al. 2017). Moreover, NaHS has the ability to activate the nuclear factor erythroid 2–related factor-2 (Nrf2) that protects against tissue injury by increasing the antioxidant and the detoxification responses to oxidative stress (Zheng et al. 2015) as shown in the present study by increasing concentrations of renal HO-1 because Nrf2 is an important regulator of HO-1 transcription in response to oxidative stress (Lever et al. 2016).
The results obtained in the present study showed also that the treatment of GEN-treated rats with NaHS led to a significant decrease in renal tissue total NO level compared to that of the GEN-treated group. This can be explained by the upregulation of eNOS under the effect of H2S (King et al. 2014). This is supported by Yilmaz et al. (2019) whose study revealed that NaHS treatment restored reduced protein expression of eNOS and H2S levels. The present study concluded that treating rats with NaHS enhanced eNOS immunoexpression in glomeruli and tubular cells compared to control rats. In addition, Ibrahim et al. (2015) reported that H2S decreased the expression of iNOS while it prevented the eNOS degradation and induced eNOS phosphorylation as shown in the present study. This leads to subsequent NO production with inhibition of its cytotoxic effects, probably through sulfinyl nitrite formation. This novel product of H2S and ONOO− has the potential to release NO in physiological concentrations that are sufficient to dilate blood vessels and increase blood flow, but at the same time, to suppress the formation of peroxynitrite with its toxic oxidative and pro-apoptotic effects. These observations indicate that H2S can mediate its effects through a NO-dependent pathway.
It is becoming increasingly clear that there are important interactions among the gasotransmitters (NO, CO, and H2S). Previous studies reported that there is a possible crosstalk between them (Abdel-Zaher et al. 2019). In order to investigate whether the renoprotective effect of H2S in the case of GEN-induced renal injury is dependent on CO pathways or not, we treated the GEN-treated rats with NaHS and ZnPP (HO-1 inhibitor).
The results obtained in the present study showed that the treatment of GEN-treated rats with NaHS and ZnPP led to a significant increase in renal injury markers compared to that of the GEN group treated with NaHS alone that was confirmed also by the present histological examination and PAS staining. This may be due to the fact that the oxidative stress enhanced by ZnPP can be explained by the inhibition of HO-1 as evidenced in the present study that confers a transient resistance against oxidative damage through the activation of Nrf2 pathway, which is the key element in the regulation of the cellular response to the oxidative stress (Wang et al. 2018).
The results obtained in the present study showed also that the treatment of GEN-treated rats with NaHS and ZnPP led to a significant increase in renal tissue total NO level compared to that of the GEN-treated group with NaHS alone. The NO-increasing effect of ZnPP can be explained by inhibition of HO-1 with a concomitant decrease in CO level, since CO can inhibit iNOS expression by preventing the activation of NF-κB (Taye and Ibrahim 2013) which upregulates the transcription of the iNOS gene. This is in line with the present immunohistological findings. In addition, CO triggers the Nrf2 to increase the expression and function of a series of antioxidant enzymes (Al-Kahtani et al. 2014). Moreover, Liu et al. (2015) reported that the induction of the heme oxygenase enzyme increases the expression of eNOS to produce renal protection as HO-1 controls the availability of cellular heme, an essential co-factor for NOS and CBS (H2S enzyme) (Wesseling et al. 2015).
As regards the renal tissue anti-inflammatory cytokines, the results obtained in the present study showed that treatment of GEN-treated rats with NaHS and ZnPP led to a significant decrease in renal tissue IL-10 level and an increase in renal tissue TNF-α level compared to the GEN-treated group with NaHS alone. The pro-inflammatory effects of ZnPP can be explained by the inhibition of HO-1 as shown in the present study with a concomitant decrease in CO levels. CO can suppress the expression of TNF-α and increase IL-10 via selective activation of mitogen-activated protein kinase p38 (p38MAPK) signaling (Lee and Chau 2002; Taye and Ibrahim 2013); p38MAPK is an important intracellular kinase that is activated by cellular stress that links inflammatory as well as environmental stress to transcription factors (Cuadrado and Nebreda 2010). In contrast, there was no significant difference in renal tissue IL-10 levels between the GEN-treated groups with NaHS and ZnPP and the control group. These results indicate that the effect of H2S on IL-10 depends mainly on the CO pathway.
From the abovementioned results, the renoprotective effects of H2S through its effects on renal tissue antioxidants, pro-inflammatory and anti-inflammatory cytokines, and NO level that was evidenced by iNOS and eNOS expression can be partially dependent on the CO pathway via induction of HO-1. This study demonstrates the effects of H2S therapy in renal protection on a renal injury–induced model, and this will open the door for future studies, using multiple dosing groups and genetically modified animals. Moreover, further studies may show that the involvement of apoptotic markers in the renoprotective mechanisms of sulfide and its link with endogenous CO or NO is needed. Finally, a similar study in human subjects is desirable; however, the odor of H2S is clearly unacceptable for humans so new formulations that release H2S must be available for future testing.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the present experimental study were in accordance with the ethical standards of Minia University Faculty of Medicine, Research Ethics Committee (FMREC) at which the studies were conducted (IRB approval number 151-2/2019).
Informed consent
No informed consent since it is an experimental study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abd-Elhamid TH, Elgamal DA, Ali SS, Ali FEM, Hassanein EHM, el-Shoura EAM, Hemeida RAM. Reno-protective effects of ursodeoxycholic acid against gentamicin-induced nephrotoxicity through modulation of NF-kappaB, eNOS and caspase-3 expressions. Cell Tissue Res. 2018;374(2):367–387. doi: 10.1007/s00441-018-2886-y. [DOI] [PubMed] [Google Scholar]
- Abdel-Zaher AO, et al. The interrelationship between gasotransmitters and lead-induced renal toxicity in rats. Toxicol Lett. 2019;310:39–50. doi: 10.1016/j.toxlet.2019.04.012. [DOI] [PubMed] [Google Scholar]
- Al-Kahtani MA, et al. Hemin attenuates cisplatin-induced acute renal injury in male rats. Oxidative Med Cell Longev. 2014;2014:476430. doi: 10.1155/2014/476430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett LMA, Cummings BS. Nephrotoxicity and renal pathophysiology: a contemporary perspective. Toxicol Sci. 2018;164(2):379–390. doi: 10.1093/toxsci/kfy159. [DOI] [PubMed] [Google Scholar]
- Casanova AG, Vicente-Vicente L, Hernández-Sánchez MT, Pescador M, Prieto M, Martínez-Salgado C, Morales AI, López-Hernández FJ. Key role of oxidative stress in animal models of aminoglycoside nephrotoxicity revealed by a systematic analysis of the antioxidant-to-nephroprotective correlation. Toxicology. 2017;385:10–17. doi: 10.1016/j.tox.2017.04.015. [DOI] [PubMed] [Google Scholar]
- Chen Q, Cui Y, Ding G, Jia Z, Zhang Y, Zhang A, Huang S. PEA3 protects against gentamicin nephrotoxicity: role of mitochondrial dysfunction. Am J Transl Res. 2017;9(5):2153–2162. [PMC free article] [PubMed] [Google Scholar]
- Côté, S, 1993 Current protocol for light microscopy immunocytochemistry. Immunohistochemistry, II:148–167
- Council, National Research (2010) Guide for the care and use of laboratory animals. National Academies Press
- Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429(3):403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- Du Y, et al. Hydrogen sulfide treatment protects against renal ischemia-reperfusion injury via induction of heat shock proteins in rats. Iran J Basic Med Sci. 2019;22(1):99–105. doi: 10.22038/ijbms.2018.29706.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erjaee H, Azma F, Nazifi S (2015) Effect of caraway on gentamicin-induced oxidative stress, inflammation and nephrotoxicity in rats. Vet Sci Dev 5(2)
- Hasanvand A, et al. Ameliorative effect of ferulic acid on gentamicininduced nephrotoxicity in a rat model; role of antioxidant effects. J Renal Inj Prev. 2018;7(2):73–77. doi: 10.15171/jrip.2018.18. [DOI] [Google Scholar]
- Ibrahim MY, Aziz NM, Kamel MY, Rifaai RA. Sodium hydrosulphide against renal ischemia/reperfusion and the possible contribution of nitric oxide in adult male albino rats. Bratislavske Lek Listy. 2015;116(11):681–688. doi: 10.4149/bll_2015_133. [DOI] [PubMed] [Google Scholar]
- Kakalij RM, Alla CP, Kshirsagar RP, Kumar BH, Mutha SS, Diwan PV. Ameliorative effect of Elaeocarpus ganitrus on gentamicin-induced nephrotoxicity in rats. Indian J Pharmacol. 2014;46(3):298–302. doi: 10.4103/0253-7613.132163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi A, Absalan F. Sodium hydrogen sulfide (NaHS) ameliorates alterations caused by cisplatin in filtration slit diaphragm and podocyte cytoskeletal in rat kidney. J Nephropathol. 2017;6(3):150–156. doi: 10.15171/jnp.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasinath BS. Hydrogen sulfide to the rescue in obstructive kidney injury. Kidney Int. 2014;85(6):1255–1258. doi: 10.1038/ki.2013.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AL, et al. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc Natl Acad Sci. 2014;111(8):3182–3187. doi: 10.1073/pnas.1321871111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolluru GK, Shen X, Bir SC, Kevil CG. Hydrogen sulfide chemical biology: pathophysiological roles and detection. Nitric Oxide: Biol Ch. 2013;35:5–20. doi: 10.1016/j.niox.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause KM, et al. Aminoglycosides: an overview. Cold Spring Harb Perspect Med. 2016;6(6):a027029. doi: 10.1101/cshperspect.a027029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T-S, Chau L-Y. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8(3):240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- Lever JM, Boddu R, George JF, Agarwal A. Heme oxygenase-1 in kidney health and disease. Antioxid Redox Signal. 2016;25(3):165–183. doi: 10.1089/ars.2016.6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Mi, et al. 2016 A H2S donor GYY4137 exacerbates cisplatin-induced nephrotoxicity in mice. Mediators of Inflammation 2016 [DOI] [PMC free article] [PubMed]
- Liu X, Zang P, Han F, Hou N, Sun X. Renal protective effects of induction of haem oxygenase-1 combined with increased adiponectin on the glomerular vascular endothelial growth factor-nitric oxide axis in obese rats. Exp Physiol. 2015;100(7):865–876. doi: 10.1113/EP085116. [DOI] [PubMed] [Google Scholar]
- Martín-Solé O, Rodó J, García-Aparicio L, Blanch J, Cusí V, Albert A. Effects of platelet-rich plasma (PRP) on a model of renal ischemia-reperfusion in rats. PLoS One. 2016;11(8):e0160703. doi: 10.1371/journal.pone.0160703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehan S, Dudi R, Kalra S. Renoprotective effect of corosolic acid in gentamicin-induced nephrotoxicity and renal dysfunction in experimental rats. Ann Pharmacol Pharm. 2017;2(12):1065. [Google Scholar]
- Nath KA. Heme oxygenase-1 and acute kidney injury. Curr Opin Nephrol Hypertens. 2014;23(1):17–24. doi: 10.1097/01.mnh.0000437613.88158.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otunctemur A, Ozbek E, Dursun M, Sahin S, Besiroglu H, Ozsoy OD, Cekmen M, Somay A, Ozbay N. Protective effect of hydrogen sulfide on gentamicin-induced renal injury. Ren Fail. 2014;36(6):925–931. doi: 10.3109/0886022X.2014.900599. [DOI] [PubMed] [Google Scholar]
- Ozturk H, Cetinkaya A, Duzcu SE, Tekce BK, Ozturk H. Carvacrol attenuates histopathogic and functional impairments induced by bilateral renal ischemia/reperfusion in rats. Biomed Pharmacother. 2018;98:656–661. doi: 10.1016/j.biopha.2017.12.060. [DOI] [PubMed] [Google Scholar]
- Park S, Bivona BJ, Harrison-Bernard LM. Lack of contribution of nitric oxide synthase to cholinergic vasodilation in murine renal afferent arterioles. Am J Physiol Renal Physiol. 2018;314(6):F1197–f1204. doi: 10.1152/ajprenal.00433.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlakou P et al (2017) Oxidative stress and acute kidney injury in critical illness: pathophysiologic mechanisms—biomarkers—interventions, and future perspectives. Oxidative Med Cell Longev 2017 [DOI] [PMC free article] [PubMed]
- Pendergraft WF, 3rd, et al. Nephrotoxic effects of common and emerging drugs of abuse. Clin J Am Soc Nephrol: CJASN. 2014;9(11):1996–2005. doi: 10.2215/CJN.00360114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podkalicka P, Mucha O, Józkowicz A, Dulak J, Łoboda A. Heme oxygenase inhibition in cancers: possible tools and targets. Contemp Oncol (Pozn) 2018;22(1a):23–32. doi: 10.5114/wo.2018.73879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Wang F, Li Q, Shi YB, Zheng HF, Peng H, Shen HY, Liu CF, Hu LF. Hydrogen sulfide inhibits the renal fibrosis of obstructive nephropathy. Kidney Int. 2014;85(6):1318–1329. doi: 10.1038/ki.2013.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sureshbabu A, Ryter SW, Choi ME. Oxidative stress and autophagy: crucial modulators of kidney injury. Redox Biol. 2015;4:208–214. doi: 10.1016/j.redox.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swisher B, Bancroft JD, Gamble M. Microorganisms. In: Bancroft JD, Gamble M, editors. Theory and practice of histological techniques. 5. London: Churchill Livingstone; 2002. p. 337. [Google Scholar]
- Takagi T et al (2018) Heme oxygenase-1 prevents murine intestinal inflammation. J Clin Biochem Nutr:17–133 [DOI] [PMC free article] [PubMed]
- Taye A, Ibrahim BM. Activation of renal haeme oxygenase-1 alleviates gentamicin-induced acute nephrotoxicity in rats. J Pharm Pharmacol. 2013;65(7):995–1004. doi: 10.1111/jphp.12067. [DOI] [PubMed] [Google Scholar]
- van den Born JC, et al. High urinary sulfate concentration is associated with reduced risk of renal disease progression in type 2 diabetes. Nitric Oxide. 2016;55:18–24. doi: 10.1016/j.niox.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Virani S, et al. Aloe vera attenuates gentamicin-induced nephrotoxicity in Wistar albino rats: histo-pathological and biochemical changes. Asian J Pharm Clin Res. 2016;9(1):113–117. [Google Scholar]
- Wang J et al (2018) Sitagliptin improves renal function in diabetic nephropathy in male Sprague Dawley rats through upregulating heme oxygenase-1 expression. Endocrine:1–9 [DOI] [PubMed]
- Wesseling S, Fledderus JO, Verhaar MC, Joles JA. Beneficial effects of diminished production of hydrogen sulfide or carbon monoxide on hypertension and renal injury induced by NO withdrawal. Br J Pharmacol. 2015;172(6):1607–1619. doi: 10.1111/bph.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Luo N, Wang L, Zhao Z, Bu H, Xu G, Yan Y, Che X, Jiao Z, Zhao T, Chen J, Ji A, Li Y, Lee GD. Hydrogen sulfide ameliorates chronic renal failure in rats by inhibiting apoptosis and inflammation through ROS/MAPK and NF-κB signaling pathways. Sci Rep. 2017;7(1):455. doi: 10.1038/s41598-017-00557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz E, Kaya-Sezginer E, Yilmaz-Oral D, Cengiz T, Bayatli N, Gur S. Effects of hydrogen sulphide donor, sodium hydrosulphide treatment on the erectile dysfunction in L-NAME-induced hypertensive rats. Andrologia. 2019;51(5):e13240. doi: 10.1111/and.13240. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Zheng J, Zhao T, Tang X, Hu N. Hydrogen sulfide alleviates uranium-induced acute hepatotoxicity in rats: role of antioxidant and antiapoptotic signaling. Environ Toxicol. 2017;32(2):581–593. doi: 10.1002/tox.22261. [DOI] [PubMed] [Google Scholar]
- Zheng J, Zhao T, Yuan Y, Hu N, Tang X. Hydrogen sulfide (H2S) attenuates uranium-induced acute nephrotoxicity through oxidative stress and inflammatory response via Nrf2-NF-κB pathways. Chem Biol Interact. 2015;242:353–362. doi: 10.1016/j.cbi.2015.10.021. [DOI] [PubMed] [Google Scholar]
- Zhou X, Feng Y, Zhan Z, Chen J. Hydrogen sulfide alleviates diabetic nephropathy in a streptozotocin-induced diabetic rat model. J Biol Chem. 2014;289(42):28827–28834. doi: 10.1074/jbc.M114.596593. [DOI] [PMC free article] [PubMed] [Google Scholar]