Abstract
Multidrug-resistant (MDR) and extended-spectrum β-lactamase (ESBL)-producing Klebsiella pneumoniae associated with nosocomial infections have caused serious problems in antibiotic management with limited therapeutic choices. This study aimed to determine the genotypic and phenotypic characteristics of K. pneumoniae strains isolated from a tertiary hospital in Malaysia. Ninety-seven clinical K. pneumoniae strains were analyzed for antimicrobial susceptibility, all of which were sensitive to amikacin and colistin (except one strain), while 31.9 % and 27.8 % were MDR and ESBL producers, respectively. PCR and DNA sequencing of the amplicons indicated that the majority of MDR strains (26/27) were positive for blaTEM, followed by blaSHV (24/27), blaCTX-M-1 group (23/27), blaCTX-M-9 group (2/27), and mcr-1 (1/27). Thirty-seven strains were hypervirulent and PCR detection of virulence genes showed 38.1 %, 22.7 %, and 16.5 % of the strains were positive for K1, wabG, and uge genes, respectively. Genotyping by pulsed-field gel electrophoresis (PFGE) and multilocus sequence typing (MLST) showed that these strains were genetically diverse and heterogeneous. Sequence types, ST23, ST22, and ST412 were the predominant genotypes. This is the first report of colistin-resistant K. pneumoniae among clinical strains associated with mcr-1 plasmid in Malaysia. The findings in this study have contributed to the effort in combating the increase in antimicrobial resistance by providing better understanding of genotypic characteristics and resistance mechanisms of the organisms.
Keywords: ESBL, Klebsiella pneumoniae, Multidrug resistance, MLST, PFGE
Introduction
Extended-spectrum β-lactamases (ESBLs) are enzymes produced by Gram-negative bacteria to confer resistance to aminopenicillins, cephalosporins (first-, second-, third-, and fourth-generation), and aztreonam which are inhibited by clavulanic acid [1, 2]. Members of TEM, SHV, and CTX-M groups are the common families of β-lactamases which are found in Escherichia coli, K. pneumoniae, and other Enterobacteriaceae worldwide [1, 2]. These resistance determinants are usually plasmid-encoded. Among the ESBL enzymes, CTX-M type has been increasingly reported [1, 3]. In Malaysia, Palasubramaniam et al. (2005) reported an association of blaSHV-5 ESBL gene in K. pneumoniae with a nosocomial outbreak, followed by several other reports [4–6].
Carbapenems have been used as the last-line antimicrobial drugs to treat serious infections caused by ESBL-producing Enterobacteriaceae; however, the emergence of carbapenemase-producing Enterobacteriaceae worldwide have left clinicians with very limited therapeutic antibiotic options [6, 7]. Therefore, early detection of ESBL- and carbapenemase-producing pathogens is the first step towards better infection control management [8]. ESBL-producing K. pneumoniae causing nosocomial infections such as pneumonia, urinary tract infections, septicemia, and soft tissue is a worldwide problem [1, 2]. The clonal complex 258 (CC258), which is the predominant sequence type 258 (ST258), and single-locus variants of ST258 such as ST340, ST437, ST11, and ST512 have been reported to produce ESBLs [9].
Since the increase in carbapenemase-producing K. pneumoniae worldwide, colistin and tigecyclin have been used for treating infections caused by these resistant organisms [10, 11]. Unfortunately, the overuse of colistin has also resulted in increased colistin-resistant strain worldwide [10]. The main cause of resistance to colistin and tigecyclin is lipopolysaccharide (LPS) modification and is associated with mutations in mgrB and the two-component systems, phoPQ and pmrAB [12].
The plasmid-mediated colistin resistance due to mcr-1 resistance gene was first reported by Liu et al. (2016) from food animals and patients recently in China which is followed by several reports in animals and humans worldwide [13–15].
In addition, several virulence factors have been detected in K. pneumoniae such as capsular serotypes, magA, K2, rmpA (the mucoid phenotype regulator) genes, kfu (responsible for an iron uptake system), wabG (responsible for biosynthesis of the outer core lipopolysaccharide), uge (responsible for biosynthesis of the capsule and smooth lipopolysaccharide), and allS which is associated with allantoin metabolism [16]. These virulence factors are associated with the ability to cause severe community-acquired infections such as liver abscesses, pneumonia, and meningitis in young healthy hosts and the ability to cause metastatic infections [16].
There is a need to have a more comprehensive data on the presence of ESBLs, virulence genes, and antimicrobial susceptibility trends of clinical K. pneumoniae strains in Malaysia to improve treatment options for a wide range of infections caused by this pathogen. Therefore, the objective of this study was to determine the antimicrobial susceptibility, resistance genes, virulence genes, and genetic diversity of clinical strains of K. pneumoniae isolated from a hospital in Johor Bahru, Malaysia.
Materials and methods
Ninety-seven non-repeat clinical K. pneumoniae strains previously isolated from patients (males, n = 62 and female, n = 35) admitted to the hospital from September to December 2014 were analyzed. These strains were archived laboratory cultures previously collected from the hospital. There is no personal information about patients. The only information are sources and gender. The strains were cultured from blood (n = 25), bronchoscopic aspirates (BBA) (n = 24), wound tissue (n = 9), swab sample (n = 10), urine (n = 8), pus (n = 6), poc (n = 3), sputum (n = 8), fluid (n = 2), slough (n = 1), and bone (n = 1). Identification of K. pneumoniae was performed by PCR using specific primers [17]. The species were confirmed by PCR targetting the mdh housekeeping gene (http://bigsdb.web.pasteur.fr/klebsiella/primers_used.html). All strains were cultured in Luria-Bertani broth and kept in 50% glycerol at − 20 °C. The PCR amplicons were purified and sequenced for validation of their identity.
The susceptibility of the K. pneumoniae strains to 16 antimicrobial agents, including cefoperazone (30 μg), ciprofloxacin (5 μg), ampicillin (10 μg), aztreonam (30 μg), piperacillin/tazobactam (10 μg), imipenem (10 μg), amikacin (30 μg), ceftazidime (30 μg), gentamycin (10 μg), colistin (10 μg), tetracycline (30 μg), cefotaxime (30 μg), sulbactam-cefoperazone (150 μg), amoxicillin-clavulanate (20/10 μg), meropenem (10 μg), and cefixime (5 μg) (Oxoid Limited Basingstoke, Hampshire, England) were determined by the disk diffusion method according to the Clinical and Laboratory Standard Institute (CLSI) guidelines [18].
ESBL production was confirmed using disk diffusion method as described in the CLSI guideline [18] and E. coli strain ATCC 25922 and K. pneumoniae ATCC 700603 were used as quality control. Modified Hodge Test (MHT) was carried out to detect carbapenemase production in K. pneumoniae strains according to the CLSI guideline [19] and ATCC® BBA-1705 and ATCC® BBA-1706 were used as positive and negative controls, respectively. The minimum inhibitory concentration (MICs) for ceftazidime, amoxicillin-clavulanate, cefotaxime, meropenem, and imipenem (BioMerieux) was determined by E-test according to the CLSI guidelines and broth microdilution method (for colistin-resistant strain; KP2014C56) was performed for colistin. E. coli ATCC 25922 was used as a quality control strain [18].
Genomic DNA of all MDR K. pneumoniae strains were extracted by using DNA extraction kit (Yeastern Biotech Co., Ltd.) and subjected to PCR detection of β-lactamase genes including blaTEM, blaSHV, blaCTXM-1 group, blaCTXM-2 group, blaCTX-M-9 group, blaOXA-1, and blaOXA-9; carbapenem resistance genes blaKPC, blaNDM, blaVIM, blaIMP, and blaOXA-48; and colistin resistance genes mcr-1, mcr-2, mcr-3, mcr-4, and mcr-5 [13, 20, 21]. All PCR products were sequenced to validate their identity and the strains with the confirmed amplicons were used as positive controls for subsequent PCR analysis.
String test was performed for all 97 K. pneumoniae strains to distinguish hypervirulent K. pneumoniae (hvKP) from classical K. pneumoniae (cKP) [22]. Genomic DNA of all 97 K. pneumoniae strains were used for PCR detection of virulence genes including K1, K2, uge, wabG, fimH, magA, rmpA, and kfu [23]. Positive PCR-amplified products were sequenced to validate their identity and the strains with the correct amplicons were used as positive controls for subsequent PCR analysis.
Plasmid DNA from ESBL-producing K. pneumoniae strains were extracted using the alkaline lysis method which contains DNase (10 mg/mL) to avoid chromosomal DNA in the extracted plasmid. [24], followed by electrophoresis on a 1.5% agarose gel for 4.5 h at 80 V (3.2 V/cm). A 1 kb DNA ladder and lambda DNA/HindIII (Promega, Madison,WI USA) were used as DNA markers and plasmid extraction also was performed by using plasmid extraction kit (Qiagen), this experiment was repeated 3 times to confirm the results. The plasmid linearization and confirmation of the plasmid sizes were performed by S1 nuclease PFGE and lambda DNA; low-range DNA markers and E. coli V517 strain were used as standard reference [25]. PCR detection of selected β-lactamase genes (blaSHV, blaTEM, blaCTXM-1 group, and blaCTXM-9 group) and colistin resistance gene (mcr-1) was performed using extracted plasmid DNA. The PCR products were sequenced to confirm their identity [25, 26].
Transfer of ESBL and mcr-1 genes by conjugation was performed in Luria-Bertani broth using nalidixic acid–resistant E. coli DH5α as the recipient strain and conjugation experiment was repeated 5 times to confirm the results. Transconjugants were selected on LB agar supplemented with nalidixic acid (100 mg/mL) and cefotaxime (2 mg/L) or colistin (1 mg/L) (Sigma Aldrich) [25, 26]. PCR detection of selected β-lactamase genes (blaSHV, blaTEM, blaCTXM-1 group, blaCTXM-9 group, and mcr-1) was performed using plasmid DNA extracted from transconjugants. The plasmid linearization was performed by S1 nuclease PFGE and lambda DNA; low-range DNA markers were used as the standard reference [25].
PFGE typing was carried out according to Lim et al. (2009) with minor modification [5]. In brief, equal volumes of the standardized cell suspensions (OD610 = 0.6) and 1% Seakem Gold agarose were mixed gently and allowed to solidify to form agarose plugs. The plugs were lysed with cell lysis buffer (50 mM Tris, 50 mM EDTA (pH = 8), 1% sarcosine, 1 mg/mL proteinase K (Promega, Madison, WI, USA)) and incubated for 4 h at 54 °C. The lysed plugs were then washed twice with sterile double distilled water and six times with TE buffer. The DNA agarose plugs were digested with 10 U of XbaI (Promega. Madison, WI USA) for 24 h at 37 °C and electrophoresed by using a CHEF-Mapper (BioRad, USA) with pulse times of 2.25–54.2 s at 6 V/cm for 24 h. Analysis of the PFGE banding patterns based on the unweighted pair group method was carried out using the BioNumerics 6.0 software; the DNA marker, Salmonella serotype Braenderup H9812 has been incorporated in the gel (first, middle, and last lanes) for linearization purpose with tolerance of 1.5.
Genotyping of 97 K. pneumoniae strains was determined by multilocus sequence typing (MLST) analysis. MLST was performed with seven housekeeping genes (tonB, rpoB, pgi, phoE, infB, mdh, and gapA). Alleles and sequence types were allocated by using the MLST database (http://bigsdb.web.pasteur.fr/perl/bigsdb/bigsdb.pl?db=pubmlst_klebsiella_seqdef_public).
eBURST V3 (http://eburst.mlst.net) analysis using the most stringent definition, where the STs were identical or shared at least six alleles, was used to detect clonal complex or BURST groups (BGs) among the STs in this study and the K. pneumoniae MLST database. The STs were then categorized as BG founders, single-locus variants (SLVs), double-locus variants, and singletons [27].
Results and discussion
All 97 K. pneumoniae were susceptible to amikacin and colistin except for one strain (KP2014C56) that showed resistance to colistin. This strain, from a swab sample of an infected wound, was detected as MDR and ESBL-producing (multiple drug-resistant to aztreonam, sulbactam-cefoperazone, tetracycline, ampicillin, ceftazidime, cefotaxime, cefixime, and amoxicillin-clavulanate), hypervirulent, and harbored resistance genes blaCTXM-15, blaTEM-1,blaSHV-11, and mcr-1, and virulence genes K1 and wabG. This strain had a unique pulstotype and belonged to ST65. In Malaysia, this is the first report of colistin-resistant K. pneumoniae among clinical strain associated with plasmidial mcr-1, which was recently reported in zoonotic K. pneumoniae from swine farms [20]. Polymyxin E (colistin) is effective against multidrug-resistant and carbapenemase-producing Gram-negative bacteria, but recently, colistin-resistant Enterobacteriaceae including K. pneumoniae has been reported worldwide [10, 12, 28]. The clinical K. pneumoniae strains from Greece (10.5–20%), Singapore (6.3%), South Korea (6.8%), and Canada (2.9%) showed highest resistance rate to colistin [10].
The percentages of antibiotic resistance for 97 K. pneumoniae strains are as follows: ampicillin (83.5%), cefotaxime (31.9%), tetracycline (30.9%), cefixime (29.8%), cefoperazone (27.8%), aztreonam (27.8%), ceftazidime (25.7%), amoxicillin-clavulanate (19.5%), tazobactam (11.3%), gentamycin (11.3%), sulbactam cefoperazone (8.2%), ciprofloxacin (7.2%), meropenem (6.1%), imipenem (2%), and colistin (1%). A majority of the K. pneumoniae strains (81/97) were resistant to ampicillin. There were 31 strains (31.9%) which showed resistance to more than three classes of antibiotics (qouinolone, monobactam, β-lactam, cephalosporin, polymyxin, aminoglycoside, tetracycline) and categorized as multidrug-resistant (MDR). Five strains showed carbapnem resistance based on AST and MIC results; KP2014C15 based on result of the disk diffusion method and MHT tests, 27 (27.8%) K. pneumoniae strains were ESBL producers and no carbapenemase-producing K. pneumoniae was detected.
These rates were higher than the rates reported in previous study which was carried out in 2000–2004 from another urban general hospital (cefixime = 20.2%, cefotaxime = 18.1%, cefoperazone = 7.3%, and ceftazidime = 8%) [29]. However, it was lower than another study that was carried out in 2009 from five different hospitals in Peninsular Malaysia [5]. This could be due to different locations of the hospitals that serve different patient population. The current study only reported the resistance from a hospital in southern Malaysia while majority of the strains from previous studies were isolated from the central region.
The minimum inhibitory concentration (MIC) of cefotaxime and ceftazidime ranged from 16 to 256 μg/mL and from 8 to 256 μg/mL, respectively. Five strains of K. pneumoniae showed resistance to meropenem. All strains showed intermediate susceptibility and resistance to amoxicillin-clavulanate. Only one was resistant to colistin (16 μg/mL).
Multidrug-resistant and ESBL-producing Enterobacteriaceae of certain clonal complexes such as ST258 of K. pneumoniae have been disseminated worldwide [30]. The presence of virulence genes and drug resistance in these clonal strains of K. pneumoniae complicates treatment particularly among immunocompromised individuals [7, 31]. Previously, Low et al. (2017) reported the occurrence of carbapenem-resistant K. pneumoniae strains in another tertiary Malaysian hospital [6]. However, in this study, no carbapenemase-producing K. pneumoniae and no carbapenem resistance gene was detected.
Analysis of the DNA sequence of all the amplicons of ESBL-encoding genes in the 31 MDR K. pneumoniae strains showed that the majority (26/31) were positive for blaTEM, all of which were identified as β-lactamase producer of TEM-1 enzyme. TEM-1 is a common β-lactamase among Enterobacteriaceae family [1]. This was followed by blaSHV (24/31) which comprised of 9 blaSHV-11, 7 blaSHV-12, 6 blaSHV-28, and 2 blaSHV-61. Twenty-three MDR strains were blaCTX-M-1 group (11 blaCTX-M-15, 7 blaCTX-M-1, 5 blaCTX-M-28), two MDR had blaCTX-M-9 and one MDR colistin-resistant strain had mcr-1. No amplification was observed for blaCTX-M-2 group, blaOXA-1, blaOXA-9, blaKPC, blaNDM, blaVIM, blaIMP, and blaOXA-48 genes.
The CTX-M groups have been reported as predominant ESBL enzymes worldwide [1]. CTX-M-15 is one of the most common CTX-M-type ESBLs among the Enterobacteriaceae family. Nosocomial infections caused by CTX-M-15-producing K. pneumoniae have dramatically increased in recent years [1]. In Malaysia and other Asian countries, CTX-M-15 is the major ESBL enzyme reported [5, 32, 33].
According to antimicrobial susceptibility test and minimum inhibitory concentration results, five strains of K. pneumoniae showed resistance to meropenem ranged from 4 to 8 μg/mL (KP2014C15, KP2014C37, KP2014C62, KP2014C96, and KP2014C99) and only one strain (KP2014C96) showed resistance to imipenem (8 μg/mL). However, these 5 strains were MHT negative and carbapenemase genes were not detected. This finding suggested that the carbapenem resistance was due to different mechanisms and the possible mechanisms will be determined in future.
Thirty-seven strains (38.1%) were hypervirulent as they were positive for the string test, and all had the virulence gene, K1. Among 97 clinical strains, 22 (22.7%) and 16 (16.5%) were positive for wabG and uge genes, respectively. No fimH, magA, rmpA, kfu, and K2 gene was detected.
Liu et al. (2018) reported the occurrence of virulence genes K1 (34.4%), K2 (20.8%), rmpA1 (79.2%), rmpA2 (70.8%), and magA (80.2%) among hvKP in China [13]. Lin et al. (2014) also reported the occurrence of rmpA (100%), iuc (96%), and kfu (11.5%) in K. pneumoniae strains from Singapore, Hong Kong, and Taiwan [33].
Plasmid analysis showed that all 27 ESBL-producing K. pneumoniae strains had plasmids with sizes ranging from 1500 to 20,000 bp; PCR detection of selected β-lactamase and colistin genes (blaSHV, blaTEM, blaOXA-1, blaCTXM-1, and mcr-1) using extracted plasmid DNA as templates showed 21 strains had blaTEM in plasmids, 18 strains had blaSHV, 15 strains had blaCTXM-1, and one strain had mcr-1 (KP2014C56), that this strain had 4 plasmids with sizes of 2500, 6000, 8000, and 20,000 bp that harbored blaCTXM-15, blaTEM-1,blaSHV-11, and mcr-1. Conjugation was carried out for all ESBL-producing and nalidixic acid–sensitive K. pneumoniae strains which harbored plasmids and ESBLs associated. Nineteen out of 27 were ESBL-encoding transconjugants. Plasmid analysis of the transconjugants confirmed that plasmids with sizes of 1500, 2500, 3000, 6000, 8000, 10,000, and 20,000 bp were transferred from the donors to the recipient, with each donor transferring between 2 and 4 plasmids to the recipient. Conjugation was carried out for colistin-resistant and nalidixic acid–sensitive K. pneumoniae strain (KP2014C56) which harbored plasmids, colistin, and ESBL genes. This strain was detected as colistin-resistant transconjugants. Plasmid analysis of the transconjugants confirmed that 3 plasmids with sizes of 2500, 8000, and 20,000 bp were transferred from the donors to the recipient which contained blaCTXM-15, blaTEM-1,blaSHV-11, and mcr-1.
Plasmid analysis and transconjugation experiments showed that all 27 ESBL-producing strains harbored plasmids and most of the ESBL genes were plasmid-encoded and 19 strains had conjugative plasmids. These results concurred with previous studies on clinical K. pneumoniae which showed that ESBL genes carried on plasmids are transmissible [5, 34, 35]. Plasmid is one of the ways for the spread of ESBLs and other antibiotic resistance genes among microorganisms. Casper et al. (2017) reported the presence of ESBL genes and the mcr-1 gene on a unique plasmid [36].
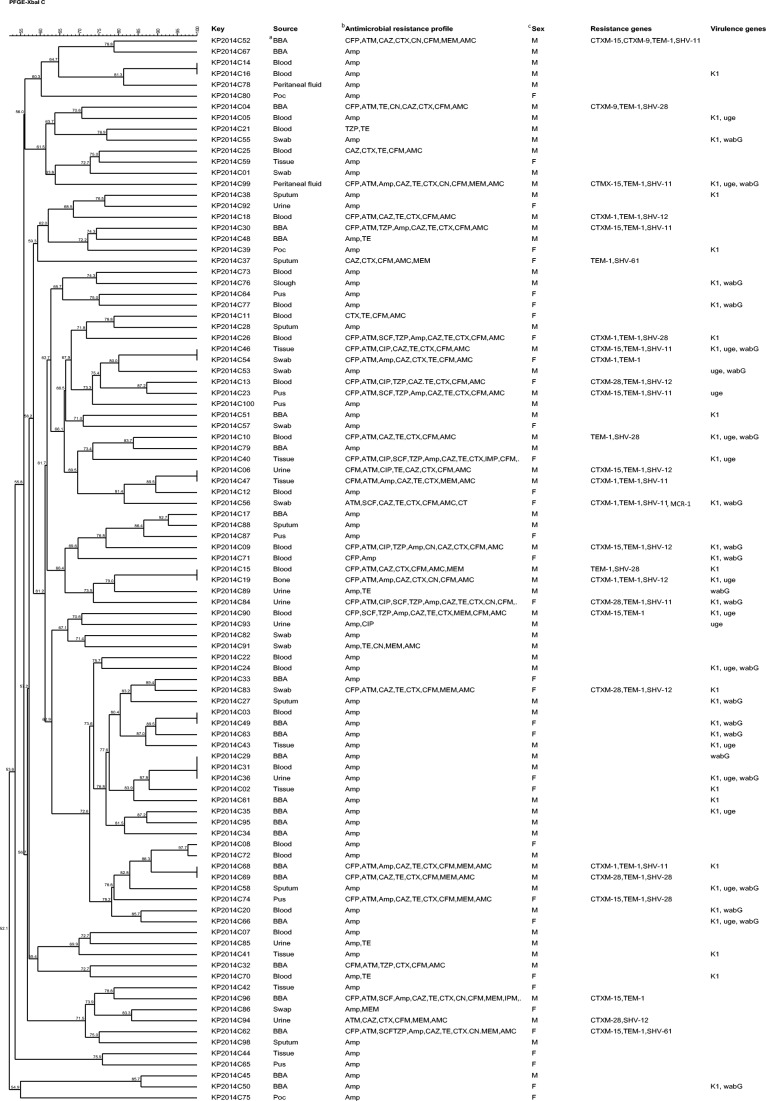
All the 97 strains were subtyped into 89 distinct pulsotypes comprising 14–27 restriction fragments. The genetic similarity of the strains ranged from 52.1 to 100%. The PFGE dendrogram showed 13 clusters (A to N) and 63 unique pulsotypes at 85% similarity cut off (Fig. 1). There was no direct association between pulsotypes with source of isolation (sampling source, kind of infection, and gender), virulence genes, and antimicrobial resistance phenotypes. This was not surprising as the strains were collected from different patients at different time points. Strains with high genetic similarity showed several antimicrobial susceptibility profiles. For instance, two strains in cluster B with identical pulsotypes but different antimicrobial resistance phenotypes. The 27 ESBL-producing K. pneumoniae strains yielded 23 unique pulsotypes. Generally, genotyping of 97 K. pneumoniae clinical strains showed high genetic diversity and heterogeneity.
Fig. 1.
Cluster analysis of PFGE profiles for 97 K. pneumoniae strains used in this study. aBronchoscopic aspirates (BBA). bCefoperazone (CFP), aztreonam (ATM), amikacin (AMK), sulbactam cefoperazone (SCF), meropenem (MEM), cefixime (CFM), amoxicillin-clavulanate (AMC), ciprofloxacin (CIP), ampicillin (Amp), tazobactam (TZP), imipenem (IMP), ceftazidime (CAZ), colistin (CT), cefotaxime (CTX), gentamycin (GEN), and tetracycline (TET). cFemale (F) or male (M)
MLST analysis of the K. pneumoniae strains yielded 24 different STs based on genetic variation in seven housekeeping genes. ST23 (n = 20) was a common ST among these strains. Other strains belonged to ST22 (n = 7), ST412 (n = 7), ST845 (n = 6), ST37 (n = 5), ST685(n = 5) and ST336 (n = 5), ST1896 (n = 4), ST268 (n = 4), ST86 (n = 4), ST17 (n = 3), ST65 (n = 3), ST40 (n = 3), ST929 (n = 3), ST52 (n = 3), ST714 (n = 2), ST20 (n = 2), ST420 (n = 2), ST161 (n = 2), ST644 (n = 2), and ST29 (n = 2) and 3 unique STs such as ST426, ST592, and ST584. In comparison with the global K. pneumoniae MLST database by using eBURST V3, the Malaysian K. pneumoniae strains, ST22, ST23, ST17, ST20, and ST29, were international predicted founders. ST65 and ST37 were SLVs, while ST412, ST845, ST685, ST336, ST896, ST268, ST86, ST40, ST 929, ST52, ST714, ST420, ST161, ST644, ST426, ST592, and ST584 were singletons.
In conclusion, this study reports 31.9% MDR and 27.8% ESBL producers among 97 clinical K. pneumoniae strains. This is the first report of colistin resistance among clinical K. pneumoniae strains in Malaysia. No carbapenemase-producing K. pneumoniae strain was found, and blaSHV and blaCTXM-1 were the predominant ESBL-encoding genes detected. PFGE typing showed diver subtypes circulating in the hospital. ST23, ST22, and ST412 and wabG, uge, and K1 were the predominant STs and virulence genes, respectively. These data indicated presence of diverse virulent MDR strains which might be useful for controlling and preventing the spread of antibiotic-resistant infections.
Acknowledgments
We thank the University of Malaya for the financial support and facilities.
Funding information
This work was supported by the University of Malaya Postgraduate Research Grant (PG072-2014B) and High Impact Research (HIR) Grant (UM.C/625/1/HIR/MOHE/CHAN/11/02).
Compliance with ethical standards
Conflict of interest
The authors declare they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.ur Rahman Sadeeq, Ali Tariq, Ali Ijaz, Khan Nazir Ahmad, Han Bo, Gao Jian. The Growing Genetic and Functional Diversity of Extended Spectrum Beta-Lactamases. BioMed Research International. 2018;2018:1–14. doi: 10.1155/2018/9519718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magiorakos AP, Srinivasan A, Carey R, Carmeli Y, Falagas M, Giske C, Harbarth S, Hindler J, Kahlmeter G, Olsson-Liljequist B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 3.Iovleva A, Bonomo RA. The ecology of extended-spectrum β-lactamases (ESBLs) in the developed world. J Travel Med. 2017;1;24(suppl_1):S44–S51. doi: 10.1093/jtm/taw102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palasubramaniam S, Subramaniam G, Muniandy S, Parasakthi N. SHV-5 extended-spectrum beta-lactamase from Klebsiella pneumoniae associated with a nosocomial outbreak in a paediatric oncology unit in Malaysia. Int J Infect Dis. 2005;9:170–172. doi: 10.1016/j.ijid.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Lim KT, Yeo CC, Yasin RM, Balan G, Thong KL. Characterization of multidrug-resistant and extended-spectrum β-lactamase-producing Klebsiella pneumoniae strains from Malaysian hospitals. J Med Microbiol. 2009;58:1463–1469. doi: 10.1099/jmm.0.011114-0. [DOI] [PubMed] [Google Scholar]
- 6.Low YM, Yap PS, Jabar KA, Ponnampalavanar S, Karunakaran R, Velayuthan R, Chong CW, Bakar SA, Yusof MY, Teh CS. The emergence of carbapenem resistant Klebsiella pneumoniae in Malaysia: correlation between microbiological trends with host characteristics and clinical factors. Antimicrob Res Infect Cont. 2017;6(1):5. doi: 10.1186/s13756-016-0164-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodrigues C, Machado E, Ramos H, Peixe L, Novais Â. Expansion of ESBL-producing Klebsiella pneumoniae in hospitalized patients: a successful story of international clones (ST15, ST147, ST336) and epidemic plasmids (IncR, IncFII K) Int J Med Microbiol. 2014;304:1100–1108. doi: 10.1016/j.ijmm.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Hamzan NI, Yean CY, Rahman RA, Hasan H, Rahman ZA. Detection of blaIMP4 and blaNDM1 harboring Klebsiella pneumoniae isolates in a university hospital in Malaysia. Emerg Health Threat J. 2015;1;8(1):26011. doi: 10.3402/ehtj.v8.26011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woodford N, Turton JF, Livermore DM. Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol Rev. 2011;35:736–755. doi: 10.1111/j.1574-6976.2011.00268.x. [DOI] [PubMed] [Google Scholar]
- 10.Ah YM, Kim AJ, Lee JY. Colistin resistance in Klebsiella pneumoniae. Int J Antimicrob Agents. 2014;1;44(1):8–15. doi: 10.1016/j.ijantimicag.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis. 2011;53(1):60–67. doi: 10.1093/cid/cir202. [DOI] [PubMed] [Google Scholar]
- 12.Olaitan AO, Morand S, Rolain JM. Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Front Microbio. 2014;26(5):643. doi: 10.3389/fmicb.2014.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu C, Shi J, Guo J. High prevalence of hypervirulent Klebsiella pneumoniae infection in the genetic background of elderly patients in two teaching hospitals in China. Infect Drug Res J. 2018;11:1031–1041. doi: 10.2147/IDR.S161075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olaitan AO, Diene SM, Kempf M, Berrazeg M, Bakour S, Gupta SK, Thongmalayvong B, Akkhavong K, Somphavong S, Paboriboune P. Worldwide emergence of colistin resistance in Klebsiella pneumoniae from healthy humans and patients in Lao PDR, Thailand, Israel, Nigeria and France owing to inactivation of the PhoP/PhoQ regulator mgrB: an epidemiological and molecular study. Int J Antimicrob Agents. 2014;44:500–507. doi: 10.1016/j.ijantimicag.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Hasman H, Hammerum AM, Hansen F, Hendriksen RS, Olesen B, Agersø Y, Zankari E, Leekitcharoenphon P, Stegger M, Kaas RS. Detection of mcr-1 encoding plasmid-mediated colistin-resistant Escherichia coli isolates from human bloodstream infection and imported chicken meat, Denmark. Euro Surveill. 2015;20(49):261–262. doi: 10.2807/1560-7917.ES.2015.20.49.30085. [DOI] [PubMed] [Google Scholar]
- 16.Yu WL, Ko WC, Cheng KC, Lee CC, Lai CC, Chuang YC. Comparison of prevalence of virulence factors for Klebsiella pneumoniae liver abscesses between isolates with capsular K1/K2 and non-K1/K2 serotypes. Diagn Microbiol Infect Dis. 2008;62:1–6. doi: 10.1016/j.diagmicrobio.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Diancourt L, Virginie P, Jan V, Patrick ADG, Sylvain B. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J Clin Microbiol. 2005;43(8):4178–4182. doi: 10.1128/JCM.43.8.4178-4182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CLSI . Performance standards for antimicrobial susceptibility testing; 28th informational supplement, document M100-S28. Wayne: Clinical and Laboratory Standards Institute; 2018. [Google Scholar]
- 19.CLSI . Performance standards for antimicrobial susceptibility testing; 26th informational supplement, document M100-S26. Wayne: Clinical and Laboratory Standards Institute; 2016. [Google Scholar]
- 20.Mobasseri Golnaz, Teh Cindy Shuan Ju, Ooi Peck Toung, Thong Kwai Lin. The emergence of colistin-resistant Klebsiella pneumoniae strains from swine in Malaysia. Journal of Global Antimicrobial Resistance. 2019;17:227–232. doi: 10.1016/j.jgar.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 21.Rebelo AR, Bortolaia V, Kjeldgaard JS, Pedersen SK, Leekitcharoenphon P, Hansen IM, Guerra B, Malorny B, Borowiak M, Hammerl JA, Battisti A. Multiplex PCR for detection of plasmid-mediated colistin resistance determinants, mcr-1, mcr-2, mcr-3, mcr-4 and mcr-5 for surveillance purposes. Euro Surveill. 2018;23(6):17–00672. doi: 10.2807/1560-7917.ES.2018.23.6.17-00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qu TT, Zhou JC, Jiang Y, Shi KR, Li B, Shen P, Wei ZQ, Yu YS. Clinical and microbiological characteristics of Klebsiella pneumoniae liver abscess in East China. BMC Infect Dis. 2015;15(1):161. doi: 10.1186/s12879-015-0899-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiong C, Zou JW, Qiu CN, Wang MM, Wang XJ, Ruan Z, Fan JZ. Antimicrobial susceptibility and microbiological and epidemiological characteristics of hypermucoviscous Klebsiella pneumoniae strains in a tertiary hospital in Hangzhou, China. J Glob Antimicrob Resist. 2018;15:61–64. doi: 10.1016/j.jgar.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 24.Feliciello I, Chinali G. A modified alkaline lysis method for the preparation of highly purified plasmid DNA from Escherichia coli. Anal Biochem. 1993;212:394–401. doi: 10.1006/abio.1993.1346. [DOI] [PubMed] [Google Scholar]
- 25.Lai K, Ma Y, Guo L, An J, Ye L, Yang J. Molecular characterization of clinical IMP-producing Klebsiella pneumoniae isolates from a Chinese tertiary hospital. Ann Clin Microbiol Antimicrob. 2017;16(1):42. doi: 10.1186/s12941-017-0218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anjum MF, Duggett NA, AbuOun M, Randall L, Nunez-Garcia J, Ellis RJ, Rogers J, Horton R, Brena C, Williamson S. Colistin resistance in Salmonella and Escherichia coli isolates from a pig farm in Great Britain. J Antimicrob Chemother. 2016;71(8):2306–2313. doi: 10.1093/jac/dkw149. [DOI] [PubMed] [Google Scholar]
- 27.Spratt BG, Hanage WP, Li B, Aanensen DM, Feil EJ. Displaying the relatedness among isolates of bacterial species–the eBURST approach. FEMS Microbiol Lett. 2004;1:241(2):129–134. doi: 10.1016/j.femsle.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Capone A, Giannella M, Fortini D, Giordano A, Meledandri M, Ballardini M, Venditti M, Bordi E, Capozzi D, Balice MP. High rate of colistin resistance among patients with carbapenem-resistant Klebsiella pneumoniae infection accounts for an excess of mortality. Clin Microbiol Infec. 2013;19:E23–E30. doi: 10.1111/1469-0691.12070. [DOI] [PubMed] [Google Scholar]
- 29.Loh LC, Chin H, Chong Y, Jeyaratnam A, Raman S, Vijayasingham P, Thayaparan T, Kumar S. Klebsiella pneumoniae respiratory isolates from 2000 to 2004 in a Malaysian hospital: characteristics and relation to hospital antibiotics consumption. Singap Med J. 2007;48(9):813–818. [PubMed] [Google Scholar]
- 30.Bonnedahl J, Hernandez J, Stedt J, Waldenström J, Olsen B, Drobni M. Extended-spectrum β-lactamases in Escherichia coli and Klebsiella pneumoniae in gulls, Alaska, USA. Emerg Infect Dis. 2014;20:897–899. doi: 10.3201/eid2005.130325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramos PIP, Picão RC, de Almeida LGP, Lima NCB, Girardello R, Vivan ACP, Xavier DE, Barcellos FG, Pelisson M, Vespero EC. Comparative analysis of the complete genome of KPC-2-producing Klebsiella pneumoniae Kp13 reveals remarkable genome plasticity and a wide repertoire of virulence and resistance mechanisms. BMC Genomics. 2014;15:1–16. doi: 10.1186/1471-2164-15-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee MY, Kwan SK, Cheol-In K, Doo RC, Kyong RP, Jae HS. High prevalence of CTX-M-15-producing Klebsiella pneumoniae isolates in Asian countries: diverse clones and clonal dissemination. Int J Antimicrob Agents. 2011;38:160–163. doi: 10.1016/j.ijantimicag.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 33.Mohd Helmi U, Desa M, Taib N, Tengku Jamaluddin T, Masri S. Multiple ambler class A ESBL genes among Klebsiella pneumoniae isolates in a Malaysian district hospital. Trop Biomed. 2016;33(1):109–119. [PubMed] [Google Scholar]
- 34.Göttig S, Gruber TM, Stecher B, Wichelhaus TA, Kempf VAJ. In vivo horizontal gene transfer of the carbapenemase OXA-48 during a nosocomial outbreak. Clin Infect Dis. 2015;60(12):1808–1815. doi: 10.1093/cid/civ191. [DOI] [PubMed] [Google Scholar]
- 35.Ho WS, Yap KP, Yeo CC, Rajasekaram G, Thong KL. The complete sequence and comparative analysis of a multidrug-resistance and virulence multireplicon IncFII plasmid pEC302/04 from an extraintestinal pathogenic Escherichia coli EC302/04 indicate extensive diversity of IncFII plasmids. Front Microbiol. 2016;11(6):1547. doi: 10.3389/fmicb.2015.01547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caspar Y, Maillet M, Pavese P, Francony G, Brion JP, Mallaret M-R, Bonnet R, Robin F, Beyrouthy R, Maurin M. Mcr-1 colistin resistance in ESBL-producing Klebsiella pneumoniae, France. Emerg Infect Dis. 2017;23(5):874–876. doi: 10.3201/eid2305.161942. [DOI] [PMC free article] [PubMed] [Google Scholar]